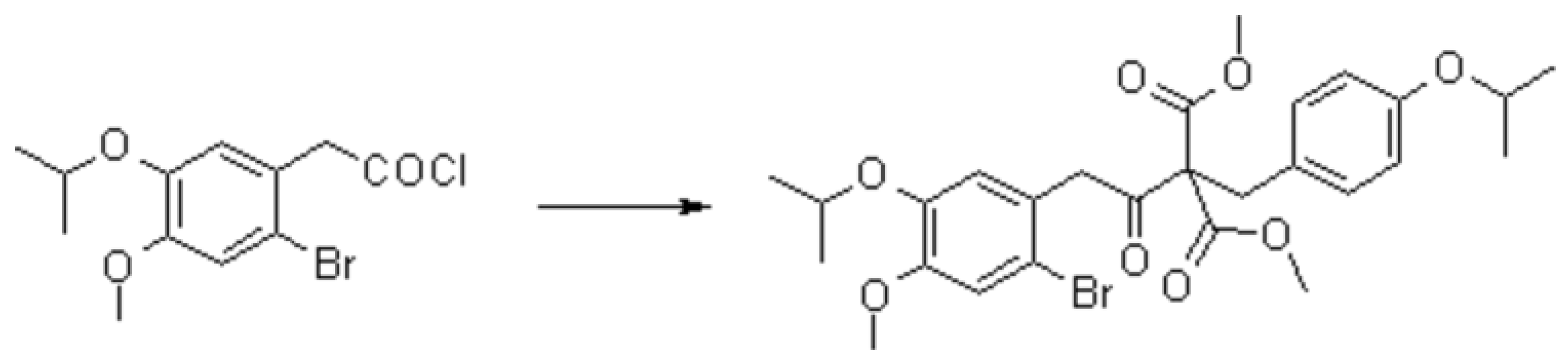
2-[2-[2-Bromo-4-methoxy-5-(1-methylethoxy)phenyl]-1-oxoethyl]-2-[4-(1-methylethoxy)phenylmethyl]propanedioic Acid Dimethyl Ester
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Jordis, U.; Froehlich, J.; Treu, M.; Hirnschall, M.; Czollner, L.; Kaelz, B.; Welzig, St. Preparation of galanthamine analogs for pharmaceutical use as acetyl- and butyrylcholinesterase inhibitors. WO 0174820, 2001. [Chemical Abstracts Nr.: 135:304054]. [Google Scholar]
- Treu, M.; Jordis, U. Molbank 2002, M296.
- Samples Availability: Available from the authors.
© 2003 MDPI. All rights reserved.
Share and Cite
Treu, M.; Jordis, U. 2-[2-[2-Bromo-4-methoxy-5-(1-methylethoxy)phenyl]-1-oxoethyl]-2-[4-(1-methylethoxy)phenylmethyl]propanedioic Acid Dimethyl Ester. Molbank 2002, 2002, M297. https://doi.org/10.3390/M297
Treu M, Jordis U. 2-[2-[2-Bromo-4-methoxy-5-(1-methylethoxy)phenyl]-1-oxoethyl]-2-[4-(1-methylethoxy)phenylmethyl]propanedioic Acid Dimethyl Ester. Molbank. 2002; 2002(1):M297. https://doi.org/10.3390/M297
Chicago/Turabian StyleTreu, Matthias, and Ulrich Jordis. 2002. "2-[2-[2-Bromo-4-methoxy-5-(1-methylethoxy)phenyl]-1-oxoethyl]-2-[4-(1-methylethoxy)phenylmethyl]propanedioic Acid Dimethyl Ester" Molbank 2002, no. 1: M297. https://doi.org/10.3390/M297
APA StyleTreu, M., & Jordis, U. (2002). 2-[2-[2-Bromo-4-methoxy-5-(1-methylethoxy)phenyl]-1-oxoethyl]-2-[4-(1-methylethoxy)phenylmethyl]propanedioic Acid Dimethyl Ester. Molbank, 2002(1), M297. https://doi.org/10.3390/M297



