Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by cognitive decline, synaptic dysfunction, and the accumulation of amyloid plaques and neurofibrillary tangles. While prior research has focused mainly on protein aggregation and neuroinflammation, emerging evidence suggests that ionic imbalances, particularly involving sodium (Na+) and potassium (K+), may contribute to AD progression. Na+ and K+ are critical for maintaining neuronal membrane potential, regulating action potential firing, and supporting neurotransmitter function. Although studies primarily focused on absolute Na+ concentrations, the Na+/K+ ratio may provide a more sensitive marker of ionic dysregulation. Given that the Na+/K+ gradient is actively maintained by the Na+/K+-ATPase pump—a target known to be vulnerable in AD—we hypothesized that the Na+/K+ ratio is altered in AD. We analyzed postmortem tissue from the prefrontal cortex, thalamus, and cerebrospinal fluid (CSF) of 97 human subjects (67 AD, 30 controls). AD cases exhibited a significant increase in the Na+/K+ ratio in the thalamus and CSF, driven primarily by elevated Na+ levels. The Na+/K+ ratio positively correlated with Braak tangle stage, suggesting an association with AD progression. These findings provide novel insights into ionic dysregulation in AD and suggest that the Na+/K+ ratio in the CSF may serve as a valuable biomarker for disease severity and progression. Future research should explore the potential of targeting ionic homeostasis as a therapeutic strategy in AD.
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, synaptic dysfunction, and the accumulation of amyloidβ (Aβ) plaques and neurofibrillary tangles [1,2]. While significant research has focused on Aβ aggregation, tau pathology, and neuroinflammation in AD [3,4], accumulating evidence suggests that dysregulation of ion homeostasis, particularly involving sodium (Na+) and potassium (K+), may be an important but underexplored contributor to disease progression [5,6,7].
Na+ and K+ ions are critical for maintaining neuronal membrane potential, supporting action potential generation, regulating synaptic transmission, and sustaining metabolic homeostasis [8,9,10,11,12,13,14]. The steep transmembrane gradients of Na+ and K+ are actively maintained by the Na+/K+-ATPase pump, a high energy-demanding enzyme known to be vulnerable to oxidative stress and metabolic dysfunction, which are prevalent in AD brains [15,16,17,18,19]. Even subtle perturbations in Na+ and K+ levels can lead to impaired neuronal excitability, calcium overload, and ultimately cell death [20,21,22,23,24,25]. Previous studies have reported elevations in Na+ levels in the post-mortem brains of AD patients [26] that correlate with Braak tangle stage, a widely used neuropathological marker of disease severity. However, prior research has largely focused on absolute Na+ concentration [Na+], without considering the Na+/K+ ratio.
In this study, we investigated [Na+], [K+], and Na+/K+ ratio in homogenates made from post-mortem brain tissues (prefrontal cortex and thalamus) and cerebrospinal fluid (CSF) from people who died with a diagnosis of AD or non-dementia. Our findings reveal a significant increase in the Na+/K+ ratio in AD brains and CSF compared to non-dementia controls, suggesting dysregulation of ion balance in AD pathophysiology. Furthermore, our study highlights the alterations in Na+/K+ ratio in AD, shedding new light on mechanisms that may underlie neurodegeneration. Given the emerging recognition of the Na+/K+ ratio as a superior biomarker in other conditions, such as hypertension [27,28,29], these findings may stimulate further studies focused on ionic imbalance in AD risk assessment and progression.
2. Results
2.1. Study Subject Characteristics
All study subject characteristics are detailed in Table 1, which includes a summary of sex, race, age, and history of diagnosed hypertension. All patients were clinically diagnosed with AD. These were confirmed by neuropathological assessment post-mortem for the presence (Patients) or absence (Controls) of AD-related pathological abnormalities. Control and AD subjects were similar with respect to sex (p = 1), race (p = 0.369), and prior history of diagnosed hypertension (p = 0.27), whereas the average age of death of subjects was significantly younger in the AD (70 ± 10 years) compared with the Control (84 ± 8) group (p < 0.001).

Table 1.
Characteristics of study subjects.
2.2. Increased [Na+] and Na+/K+ Ratio in CSF and Brain Tissues of AD Patients
The Na+/K+ ratio is commonly used as an indicator of cardiovascular disease risk, including hypertension [27,30]. Accordingly, we assessed the levels of Na+ and K+ in the prefrontal cortex, thalamus, and CSF of AD patients (n = 67) and controls (n = 30). [Na+] in the prefrontal cortex was similar between AD patients and controls, whereas [K+] was lower in AD patients compared with control subjects without hypertension (HTN) (Figure 1A,B). Despite the absence of a significant difference in [Na+], the Na+/K+ ratio in the prefrontal cortex was significantly higher in AD subjects with or without HTN (Figure 1C). In the thalamus tissue (Figure 1D–F), [Na+] was significantly higher in hypertensive AD subjects compared with hypertensive controls, and tended to be higher in normotensive AD subjects, but this difference was not statistically significant. The [K+] was similar among all groups, resulting in a significantly higher Na+/K+ ratio in hypertensive AD subjects than in hypertensive controls (Figure 1F). In the CSF (Figure 1G–I), [Na+] was markedly higher and [K+] was markedly lower in AD subjects compared with their controls, regardless of HTN or not; consequently, the Na+/K+ ratio was significantly higher in AD patients compared with control subjects (Figure 1I).
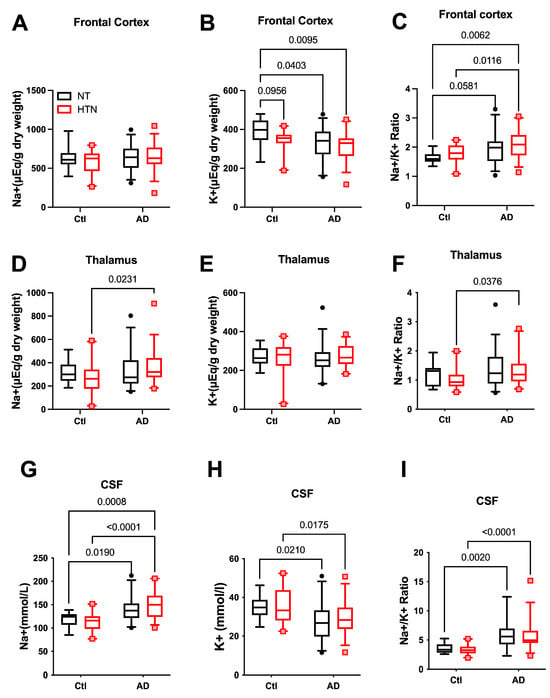
Figure 1.
Na+, K+, and Na+/K+ ratio in hypertensive and normotensive humans, with or without Alzheimer’s disease. (A–C): Na+ (A), K+ (B), and Na+/K+ ratio (C) in the frontal cortex. (D–F): Na+ (D), K+ (E), and Na+/K+ ratio (F) in the thalamus. (G–I): Na+ (G), K+ (H), and Na+/K+ ratio (I) in cerebrospinal fluid (CSF). NT-Ctl: n = 10; HTN-Ctl: n = 20; AD-NT: n = 32; AD-HTN: n = 35. Data analyzed by two-way ANOVA with mixed-effects model followed by Fisher’s LSD post hoc test. Two-tailed; p < 0.05 considered statistically significant. Graphs were plotted in Box and Whiskers, with Whiskers at the 5th–95th percentile. AD: Alzheimer’s disease. NT: normotensive; HTN: hypertension.
Although considerable number of subjects who were clinically diagnosed with hypertension in both the AD and Control groups, and the Na+ and K+ balance is important in hypertension [27,28,31,32,33], there was no statistically significant difference in [Na+], [K+], or Na+/K+ ratio in the prefrontal cortex, thalamus, or CSF samples in the hypertensive group compared with the normotensive group in our cohort.
2.3. Relationship Between Brain Tissue and CSF [Na+] and [K+]
To assess whether Na+ and K+ levels in CSF reflect those in brain tissue, we performed linear regression analyses comparing CSF [Na+] and [K+] with levels in the frontal cortex and thalamus across all subjects (Figure 2). In the frontal cortex (Figure 2A,B), CSF [Na+] showed no correlation with tissue [Na+], whereas CSF [K+] was positively correlated with tissue [K+] (p = 0.0071, R2 = 0.07). In the thalamus (Figure 2C,D), CSF [Na+] was not correlated with tissue [Na+], while CSF [K+] was negatively correlated with tissue [K+] (p = 0.03, R2 = 0.05). Despite statistical significance, the R2 values were very low, suggesting the correlations between CSF [Na+] or [K+] and corresponding tissue levels explain a negligible fraction of variance and therefore do not represent meaningful biological relationships. To evaluate whether these correlations were influenced by AD or hypertension, we repeated the linear regression analyses in these subgroups; similarly, no significant correlations were observed (Figures S1 and S2).
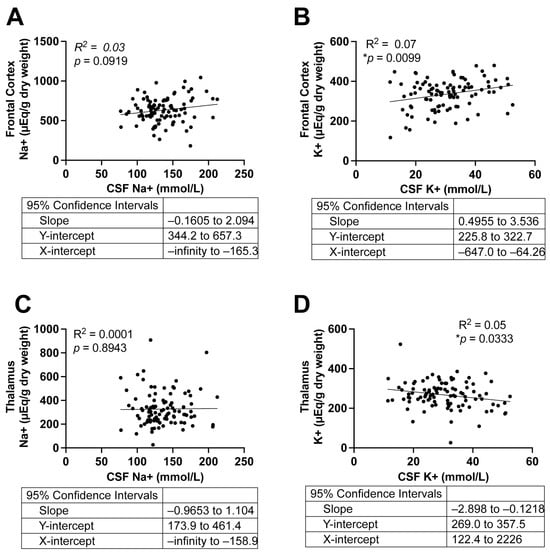
Figure 2.
Correlations between tissue and CSF Na+ and K+ concentrations in all subjects. (A,B): Correlations between Na+ (A) and K+ (B) levels in the frontal cortex and CSF. (C,D): Correlations between Na+ (C) and K+ (D) levels in the thalamus and CSF. Simple Linear Regression, n = 97. Two-tailed, and differences were considered statistically significant at p-values < 0.05. *p < 0.01.
2.4. [Na+] and Na+/K+ Ratios Are Associated with the Severity of Braak Stage in AD Patients
Braak stage is used as a basis for making a post-mortem diagnosis of AD and in research as an indicator of the relatively predictable progression of tau-based neurofibrillary tangle pathology in AD [34,35]. Age is considered the most significant non-modifiable risk factor for AD [36,37], whereas sex differences have also been reported in the incidence and severity of AD [38]. As shown in Table 1, AD subjects used in this study were younger than controls, although we did not see noticeable sex differences in our cohort. To accurately determine correlations between [Na+], [K+], or Na+/K+ ratio with Braak stage severity (0–VI), we performed multiple regression analyses, controlling for sex and age.
Across the frontal cortex and thalamus, as well as within CSF, [Na+] and Na+/K+ ratios were consistently and positively correlated with Braak stage (Table 2). Neither frontal cortex nor thalamus [K+] was correlated with Braak stage, whereas [K+] in the CSF remained negatively correlated with Braak stage. [Na+], [K+], and Na+/K+ ratios in the different Braak stages (0–VI), visualized by plotting them as bar graphs (Figure 3), showed that Na+ and Na+/K+ ratios gradually increased with the progression of Braak stage from 0 to VI. Collectively, these data indicate that [Na+] and Na+/K+ ratio in CSF and the brain tissues examined are positively correlated with AD severity, determined by Braak stage.

Table 2.
Multivariate linear regression between Na+, K+, or Na+/K+ ratio with Braak stage, controlling for sex and age in the frontal cortex, thalamus, and CSF.
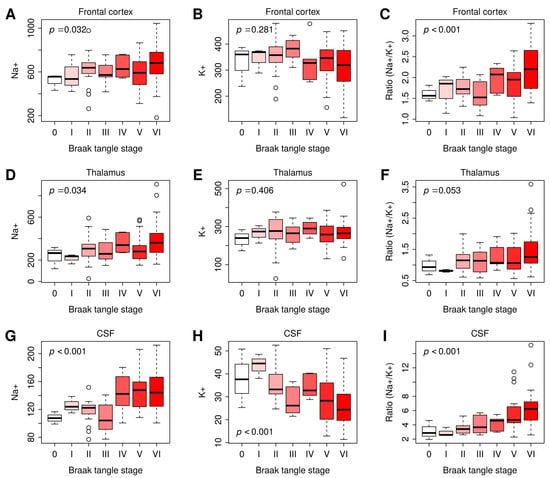
Figure 3.
Na+, K+, or Na+/K+ ratio in the frontal cortex, thalamus, and CSF at Braak stages 0–VI. (A–C): The Na+ (A), K+ (B), or Na+/K+ ratio (C) in the frontal cortex at Braak tangle stage I–VI. (D–F): The Na+ (D), K+ (E), or Na+/K+ ratio (F) in the thalamus at Braak tangle stage I–VI. (G–I): The Na+ (G), K+ (H), or Na+/K+ ratio (I) in the CSF at Braak tangle stage I–VI. Braak stage 0: n = 3; Braak stage I: n = 3; Braak stage II: n = 19; Braak stage III: n = 8; Braak stage VI: n = 6, Braak stage V: n = 27; Braak stage VI: n = 31. The p-values were computed by multivariate linear regression of Na+, K+, and Na+/K+ ratio on Braak stage, controlling for sex and age. Differences were considered statistically significant at p-values < 0.05. Graphs were plotted in Box and Whiskers with Whiskers at the 5th–95th percentile.
3. Discussion
Epidemiological studies, clinical trials, and experiments in animal models provide compelling evidence that high dietary salt intake is causally linked to impaired cognitive function in AD and vascular dementia [39,40,41,42]. Nevertheless, data on whether Na+ accumulation is elevated in brain tissues and CSF of AD patients remains scarce. In this study, we measured [Na+] and [K+] in the prefrontal cortex, thalamus, and CSF in post-mortem samples from human subjects. Several exciting observations were made: (1) [Na+] was increased in the CSF and thalamus of AD subjects; (2) the Na+/K+ ratio was significantly higher in brain tissues (prefrontal cortex and thalamus) and CSF of AD subjects; (3) the Na+/K+ ratio was significantly correlated with Braak stage. The higher the Na+/K+ ratio, the higher the Braak Stage (i.e., severity of AD tau pathology) in brain tissues and CSF, suggesting a strong association with disease progression.
A key finding of this study is elevated [Na+] in the CSF and thalamus of AD subjects, suggesting a potential disruption in Na+ homeostasis. These results align with the findings of Kerl et al. [43], who reported higher Na+ signal intensity in the CSF of early-stage AD patients compared with controls. This is despite methodological differences (23Na magnetic resonance imaging) of measurement and the living patients compared to our study of post-mortem brain tissue. Interestingly, our CSF–tissue correlation analysis revealed no significant relationship between CSF [Na+] and Na+ content in the thalamus or frontal cortex in both AD and control subjects. This suggests that brain tissue Na+ homeostasis is not simply a result of passive diffusion from the CSF but rather reflects active regulation, because passive diffusion would have resulted in a significant correlation between CSF and tissue ion levels. These findings also support previous studies indicating that dysfunction of Na+ transporters—responsible for maintaining physiological [Na+] in the CSF—may contribute to AD pathology [5,19,43,44]. One limitation of this study was the unavailability of plasma/serum samples from the same subjects, which is a challenge in post-mortem tissue studies because the bio sampling would need to be close to the time of death. Thus, we were unable to determine whether elevated plasma [Na+] contributes to increased CSF or brain tissue [Na+]. However, previous studies [45] have reported no significant correlation between CSF and plasma Na+ levels, reinforcing the concept of compartmentalized Na+ regulation in the brain. Another limitation of this study is that we were unable to evaluate the impact of potential confounders, such as post-mortem interval, time to freezing, storage duration, or the use of antihypertensives or corticosteroids, which may influence the study’s conclusions.
Our study identified an elevation of the Na+/K+ ratio in brain tissues (prefrontal cortex and thalamus) and CSF of AD subjects. Using the Na+/K+ ratio as a measure helps to avoid the potential confounding effects of fluid volume in tissues on Na+ and K+ content. For example, fluctuations in brain fluid volume caused by edema can impact measurements of [Na+], which might not accurately reflect the actual Na+ content in the tissue (mEq/mg dry weight). The Na+/K+ ratio could therefore be a better marker for evaluating Na+ and K+ homeostasis.
The elevated Na+/K+ ratio in AD brain tissues aligns with the previous finding that AD is associated with Na+/K+-ATPase impairment [5,46]. Because of its electrogenic properties, Na+/K+-ATPase, which actively transports three Na+ ions out of the cell for every two K+ ions pumped in, plays a critical role in maintaining neuronal resting membrane potential and excitability. Dysfunction in this transporter can lead to intracellular Na+ accumulation and excessive K+ efflux, resulting in elevated intracellular [Na+] and reduced [K+] [47,48,49]. In other words, such dysfunction might give rise to an increased Na+/K+ ratio. Additionally, Na+/K+-ATPase in the choroid plexus is known to influence CSF Na+ levels in salt-sensitive hypertension models, likely due to increased Na+/K+-ATPase activity [50,51]. However, whether Na+/K+-ATPase activity is altered in the choroid plexus in AD remains unclear. The observed increase in CSF [Na+] and Na+/K+ ratio supports a hypothesis that elevated Na+/K+-ATPase activity in the choroid plexus may be present in AD pathology, though further research is needed to confirm this and to try and determine whether it might be a mediator or consequence of the disease.
Our study demonstrated that both [Na+] and the Na+/K+ ratio in CSF and tissues (thalamus and frontal cortex) of the brain are positively correlated with the severity of Braak tangle stage, suggesting the potential clinical relevance of these parameters in AD progression. Previous studies have reported similar correlations for Na+ levels and Braak stage [43,52]. For instance, Graham et al. measured Na+ in post-mortem brain tissue of AD patients using ICP-MS and found a positive correlation between Na+ and Braak tangle stage [26]. Likewise, Mohamed et al. [52] used 23Na MRI and demonstrated a positive correlation between Na+ signal intensity in the hippocampus and the visual atrophy level of the medial temporal lobe, implying that Na+ increases with AD progression. Kerl et al. also observed a correlation between Na+ signal intensity and atrophy levels in the hippocampus and amygdala using 23Na MRI [43]. However, none of these previous studies assessed the Na+/K+ ratio.
Our study highlights the potential importance of this ratio in evaluating AD progression, providing a novel perspective on electrolyte balance in neurodegeneration. An interesting observation in our study is that higher Na+/K+ ratios were detected in both the prefrontal cortex and thalamus in AD, whereas no meaningful Na+ elevation was observed. This suggests that the Na+/K+ ratio may be a more sensitive indicator than absolute Na+ or K+, as it reflects changes in either or both ions (i.e., higher Na+ and/or lower K+ levels). Recent studies have suggested that the Na+/K+ ratio is a superior marker compared with [Na+] or [K+] alone in predicting blood pressure outcomes and incident hypertension [27,28,29]. Given our findings, it is possible that the Na+/K+ ratio could also serve as a valuable biomarker for assessing AD risk and progression.
4. Materials and Methods
4.1. Human Subjects
Brain prefrontal lobe cortex and thalamus tissues, as well as subject-matched CSF samples, from a total of 97 human subjects with (n = 67) and without (n = 30) AD were collected by post-mortem autopsy, frozen, and stored at −80 °C before being shipped on dry ice. Tissues were taken from subjects selected according to their clinical and pathological diagnosis, categorizing them as either AD or normal by South West Dementia Brain Bank, according to the detailed protocols of updated National Institute on Aging/Reagan Institute of the Alzheimer Association Consensus Recommendations for the Post-mortem Diagnosis of AD (also known as the NIA-Reagan Criteria) that are widely accepted and internationally recognized consensus criteria [53]. The tissues were obtained from the Human Tissue Authority-licensed South West Dementia Brain Bank (https://www.bristol.ac.uk/translational-health-sciences/research/neurosciences/research/south-west-dementia-brain-bank/), accessed on 24 March 2020. Based on their clinical diagnosis from the NIA-Reagan Criteria [53], subjects were first categorized into two groups: control (age 50 years and older, with no or age-associated neuropathological abnormalities; Tau-related Braak tangle Stages 0–III) and AD (Braak tangle stage of IV–VI). Among these subjects, a considerable number were also diagnosed with having had a history of hypertension (HTN) according to the medical records. Thus, data were also categorized into normotension (NT) and HTN for analyses. Data for this study were collected between 1989 and 2015. Clinical and pathological reports, which include data on patient history, diagnosis, and de-identified personal information, were available for retrospective analysis. The South West Dementia Brain Bank is a Research Tissue Bank with ethical approval from the South West–Central Bristol Research Ethics Committee. The Research Integrity Offices at the University of Nevada, Reno, and the University of Bristol have determined that this project complies with human research protection oversight by the Institutional Review Board.
4.2. Flame Photometry Measurement of [Na+] and [K+] in the CSF and Brain Tissues
For the extraction of Na+ and K+ from the prefrontal lobe cortex and thalamus, 100 mg of wet tissues was heated first at 95 °C for 48 h and then at 105 °C for an additional 48 h, as previously described [54]. Dried tissue samples were digested in 2 mL HCl (1 mol/L) for 1 week. Then, each sample solution was centrifuged at 4 °C at 10,000× g and the supernatant was collected and diluted 10 times with distilled water. Na+ and K+ in the CSF and supernatants extracted from tissues were measured with a flame photometer (BWB BIO 943 Flame Photometer) [45] according to the manufacturer’s specifications, and their concentrations were determined by referring to a five-point calibration curve with inclusion of quality controls. [Na+] and [K+] in tissues were expressed as the standardized unit, μEq/g dry weight, representing the amount of Na+ or K+ per gram of dried brain tissue.
4.3. Statistical Analysis
Data are presented as means ± SEM and were analyzed and plotted using Prism 10 software (GraphPad, La Jolla, CA, USA). Age demographics were assessed with an unpaired Student’s t-test. The chi-square test was used to test for sex and hypertension diagnosis. Differences in race demographics were assessed using Fischer’s test. The significance of differences between replicate means was assessed using two-way analysis of variance (ANOVA) with Fisher’s LSD post hoc analysis (multiple groups), where appropriate. Multivariate linear regressions were performed using the “lm” function in the R programming platform. All statistical tests were two-tailed, and differences were considered statistically significant at p-values < 0.05.
5. Conclusions and Perspectives
Our study provides compelling evidence that Na+ dysregulation and an elevated Na+/K+ ratio are associated with AD and its progression. Importantly, the Na+/K+ ratio, which we believe to be a more meaningful marker of Na+ and K+ homeostasis, was consistently higher in AD subjects across all examined regions and positively correlated with Braak tangle stage, a widely used neuropathological marker of disease severity. These findings highlight the potential clinical relevance of ionic imbalances in AD and suggest that the Na+/K+ ratio, perhaps measured in CSF, may serve as a novel biomarker for disease progression.
One surprising observation of this study was the lack of significant differences in [Na+], [K+], and Na+/K+ ratio between hypertensive and normotensive subjects. This is despite the well-established role of Na+ and K+ homeostasis in hypertension and cardiovascular diseases.
One limitation of this study is the lack of detailed information regarding antihypertensive treatment status and medication exposure among subjects diagnosed with hypertension. As such, future studies with dedicated funding and access to comprehensive clinical records will be required to determine the influence of antihypertensive therapy on brain and CSF ionic homeostasis in Alzheimer’s disease. Importantly, the central findings of the study, namely altered Na+ levels and elevated Na+/K+ ratios in AD brain tissue and CSF as well as their association with Braak stage, remain robust and independent of treatment-related interpretation, reinforcing the concept that ionic dysregulation accompanies neurodegeneration in Alzheimer’s disease.
These findings open several avenues for future research. First, further investigation into the mechanisms underlying Na+/K+-ATPase dysfunction, in conjunction with the measurement of levels of Na+ and K+ in AD is warranted to assess if impairment of this key ion transporter may explain the intracellular Na+ accumulation and K+ depletion. Additionally, studies examining whether CSF [Na+] and Na+/K+ ratio could serve as early diagnostic markers for AD patients are needed, perhaps alongside existing CSF Amyloid-Tao-Neurodegeneration biomarker approaches used already in clinical cohorts, but also in longitudinal cohorts tracking disease progression [55,56,57].
Recent evidence, including the study by Sasahara et al. suggests that amyloid β (Aβ) assemblies directly bind to and inhibit Na+/K+-ATPase, disrupting ionic gradients and contributing to AD pathogenesis [58,59,60]. Thus, another critical area for future research is the potential therapeutic implications of the modulation of Na+/K+ ratio in AD given that Na+/K+-ATPase is essential for maintaining neuronal membrane potential and cellular homeostasis. Targeting Na+ and K+ transporters such as Na+/K+-ATPase may offer new therapeutic strategies, aimed at enhancing its activity, rather than inhibiting it, which may prove beneficial in AD. Future therapeutic development might include small molecules that allosterically enhance ATPase activity, agents that prevent Aβ binding to the pump, or interventions that increase expression of specific isoforms of the ATPase more resistant to Aβ-induced inhibition.
Collectively, the results of our study highlight the importance of ionic balance in AD pathology and underscore the potential of the Na+/K+ ratio as a biomarker of disease severity. Understanding the mechanisms that drive Na+/K+ ratio dysregulation could provide valuable insights into AD progression and open new therapeutic avenues aimed at restoring ionic homeostasis in the aging brain.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms27031247/s1.
Author Contributions
Y.M.: Writing—review and editing, Writing—original draft, Methodology, Data curation, Investigation. S.P.: Writing—review and editing, Investigation, Methodology. T.Z.: Writing—review and editing, Formal Analysis. P.G.K.: Writing—review and editing, Conceptualization. Y.F.E.: Writing—review and editing, Conceptualization, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by grants from the National Institutes of Health National Heart Lung Blood Institute (R01HL122770 and R35HL155008), National Institute of Diabetes and Digestive and Kidney Diseases (R01DK135621) to Y. Feng Earley. P.G. Kehoe, a University of Bristol alumnus, is supported by a Fellowship from the Sigmund Gestetner Foundation. The funders had no role in study design, data collection, analysis, interpretation, or manuscript writing.
Institutional Review Board Statement
The Human Tissue Authority-licensed South West Dementia Brain Bank, University of Bristol, which performed the post-mortem analysis, with tissue bank ethics approval from the South West–Central Bristol Research Ethics Committee (981105-1, approval date: 3 November 2016). The Research Integrity Offices at the University of Nevada, Reno, and the University of Bristol have determined that this project complies with human research protection oversight by the Institutional Review Board.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We especially thank Laura Palmer for tissue processing and facilitating access to clinical data, as well as the South West Dementia Brain Bank (SWDBB), their donors, and the donor’s families for donating brain tissue for this study. Tissue for this study was provided with support from the BDR program, jointly funded by Alzheimer’s Research UK and Alzheimer’s Society, as well as BRACE.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- King, A.; Bodi, I.; Troakes, C. The Neuropathological Diagnosis of Alzheimer’s Disease-The Challenges of Pathological Mimics and Concomitant Pathology. Brain Sci. 2020, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Irvine, G.B.; El-Agnaf, O.M.; Shankar, G.M.; Walsh, D.M. Protein aggregation in the brain: The molecular basis for Alzheimer’s and Parkinson’s diseases. Mol. Med. 2008, 14, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Vitvitsky, V.M.; Garg, S.K.; Keep, R.F.; Albin, R.L.; Banerjee, R. Na+ and K+ ion imbalances in Alzheimer’s disease. Biochim. Biophys. Acta 2012, 1822, 1671–1681. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, Z.B.; Liu, X.P.; Mao, Z.Q.; Alzheimer’s Disease Neuroimaging, I. Elevated serum sodium is linked to increased amyloid-dependent tau pathology, neurodegeneration, and cognitive impairment in Alzheimer’s disease. J. Neurochem. 2025, 169, e16257. [Google Scholar] [CrossRef]
- Roberts, B.R.; Doecke, J.D.; Rembach, A.; Yevenes, L.F.; Fowler, C.J.; McLean, C.A.; Lind, M.; Volitakis, I.; Masters, C.L.; Bush, A.I.; et al. Rubidium and potassium levels are altered in Alzheimer’s disease brain and blood but not in cerebrospinal fluid. Acta Neuropathol. Commun. 2016, 4, 119. [Google Scholar] [CrossRef]
- Sadegh-Zadeh, S.-A.; Kambhampati, C.; Davis, D.N. Ionic Imbalances and Coupling in Synchronization of Responses in Neurons. J 2019, 2, 17–40. [Google Scholar] [CrossRef]
- McCormick, D.A. CHAPTER 5—Membrane Potential and Action Potential. In From Molecules to Networks; Byrne, J.H., Roberts, J.L., Eds.; Academic Press: Burlington, MA, USA, 2004; pp. 115–140. [Google Scholar]
- Clausen, M.J.V.; Poulsen, H. Sodium/Potassium Homeostasis in the Cell. In Metallomics and the Cell; Banci, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 41–67. [Google Scholar]
- Pohl, H.R.; Wheeler, J.S.; Murray, H.E. Sodium and Potassium in Health and Disease. In Interrelations Between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 29–47. [Google Scholar]
- Stone, M.S.; Martyn, L.; Weaver, C.M. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients 2016, 8, 444. [Google Scholar] [CrossRef]
- Talal, E.; Mahmood, F.; Mohammed, G.; Merie, G.; Saadi, A. The Medical Importance of Sodium and Potassium. Int. J. Pharma Growth Res. Rev. 2025, 2, 1–10. [Google Scholar] [CrossRef]
- Huxley, A.F.; Stampfli, R. Effect of potassium and sodium on resting and action potentials of single myelinated nerve fibers. J. Physiol. 1951, 112, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Dobrota, D.; Matejovicova, M.; Kurella, E.G.; Boldyrev, A.A. Na/K-ATPase under oxidative stress: Molecular mechanisms of injury. Cell. Mol. Neurobiol. 1999, 19, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lee, W.; Bian, J.S. Recent Advances in the Study of Na+/K+-ATPase in Neurodegenerative Diseases. Cells 2022, 11, 4075. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Bulygina, E.R.; Kramarenko, G.G. Is Na,K-ATPase the target of oxidative stress? Bull. Exp. Biol. Med. 1996, 121, 275–278. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Gluvic, Z.; Banjac, K.; Rizzo, M.; Isenovic, E.R. The Na+/K+-ATPase: A potential therapeutic target in cardiometabolic diseases. Front. Endocrinol. 2023, 14, 1150171. [Google Scholar] [CrossRef]
- Zhang, L.N.; Sun, Y.J.; Pan, S.; Li, J.X.; Qu, Y.E.; Li, Y.; Wang, Y.L.; Gao, Z.B. Na+/K+-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam. Clin. Pharmacol. 2013, 27, 96–103. [Google Scholar] [CrossRef]
- Aronsen, J.M.; Skogestad, J.; Lewalle, A.; Louch, W.E.; Hougen, K.; Stokke, M.K.; Swift, F.; Niederer, S.; Smith, N.P.; Sejersted, O.M.; et al. Hypokalaemia induces Ca2+ overload and Ca2+ waves in ventricular myocytes by reducing Na+/K+-ATPase alpha(2) activity. J. Physiol. 2015, 593, 1509–1521. [Google Scholar] [CrossRef]
- Grinwald, P.M.; Brosnahan, C. Sodium imbalance as a cause of calcium overload in post-hypoxic reoxygenation injury. J. Mol. Cell. Cardiol. 1987, 19, 487–495. [Google Scholar] [CrossRef]
- Ding, F.; Sun, Q.; Long, C.; Rasmussen, R.N.; Peng, S.; Xu, Q.; Kang, N.; Song, W.; Weikop, P.; Goldman, S.A.; et al. Dysregulation of extracellular potassium distinguishes healthy ageing from neurodegeneration. Brain 2024, 147, 1726–1739. [Google Scholar] [CrossRef]
- Barros, L.F.; Castro, J.; Bittner, C.X. Ion movements in cell death: From protection to execution. Biol. Res. 2002, 35, 209–214. [Google Scholar] [CrossRef]
- Pasantes-Morales, H.; Tuz, K. Volume changes in neurons: Hyperexcitability and neuronal death. Contrib. Nephrol. 2006, 152, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Cressman, J.R., Jr.; Ullah, G.; Ziburkus, J.; Schiff, S.J.; Barreto, E. The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states: I. Single neuron dynamics. J. Comput. Neurosci. 2009, 26, 159–170, Erratum in J. Comput. Neurosci. 2011, 30, 781. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.F.; Nasarauddin, M.B.; Carey, M.; McGuinness, B.; Holscher, C.; Kehoe, P.G.; Love, S.; Passmore, A.P.; Elliott, C.T.; Meharg, A.; et al. Quantitative measurement of [Na+] and [K+] in postmortem human brain tissue indicates disturbances in subjects with Alzheimer’s disease and dementia with Lewy bodies. J. Alzheimers Dis. 2015, 44, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.; Chang, E.T. Sodium-to-potassium ratio and blood pressure, hypertension, and related factors. Adv. Nutr. 2014, 5, 712–741. [Google Scholar] [CrossRef]
- Kogure, M.; Nakaya, N.; Hirata, T.; Tsuchiya, N.; Nakamura, T.; Narita, A.; Suto, Y.; Honma, Y.; Sasaki, H.; Miyagawa, K.; et al. Sodium/potassium ratio change was associated with blood pressure change: Possibility of population approach for sodium/potassium ratio reduction in health checkup. Hypertens. Res. 2021, 44, 225–231, Erratum in Hypertens. Res. 2021, 44, 262. [Google Scholar] [CrossRef]
- Ndanuko, R.N.; Ibrahim, R.; Hapsari, R.A.; Neale, E.P.; Raubenheimer, D.; Charlton, K.E. Association between the Urinary Sodium to Potassium Ratio and Blood Pressure in Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1751–1767. [Google Scholar] [CrossRef]
- Ma, Y.; He, F.J.; Sun, Q.; Yuan, C.; Kieneker, L.M.; Curhan, G.C.; MacGregor, G.A.; Bakker, S.J.L.; Campbell, N.R.C.; Wang, M.; et al. 24-Hour Urinary Sodium and Potassium Excretion and Cardiovascular Risk. N. Engl. J. Med. 2022, 386, 252–263. [Google Scholar] [CrossRef]
- Brobak, K.M.; Melsom, T.; Eriksen, B.O.; Hoieggen, A.; Norvik, J.V.; Solbu, M.D. The Association between Urinary Sodium-Potassium Ratio, Kidney Function, and Blood Pressure in a Cohort from the General Population. Kidney Blood Press. Res. 2024, 49, 184–195. [Google Scholar] [CrossRef]
- Abe, T.; Endo, T.; Hamano, T.; Okuyama, K.; Yano, S. Changes in the Urinary Sodium-to-Potassium Ratio Are Associated with Blood Pressure Change in Older Japanese Adults: A 7-Year Longitudinal Study. J. Clin. Med. 2022, 11, 5093. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Kogure, M.; Tabara, Y.; Hozawa, A.; Sakima, A.; Tsuchihashi, T.; Yoshita, K.; Hayabuchi, H.; Node, K.; Takemi, Y.; et al. Practical use and target value of urine sodium-to-potassium ratio in assessment of hypertension risk for Japanese: Consensus Statement by the Japanese Society of Hypertension Working Group on Urine Sodium-to-Potassium Ratio. Hypertens. Res. 2024, 47, 3288–3302. [Google Scholar] [CrossRef]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Lowe, V.J.; Wiste, H.J.; Senjem, M.L.; Weigand, S.D.; Therneau, T.M.; Boeve, B.F.; Josephs, K.A.; Fang, P.; Pandey, M.K.; Murray, M.E.; et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 2018, 141, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Jiang, Q.; McDermott, J.; Han, J.J. Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell 2018, 17, e12802. [Google Scholar] [CrossRef]
- Gong, J.; Harris, K.; Lipnicki, D.M.; Castro-Costa, E.; Lima-Costa, M.F.; Diniz, B.S.; Xiao, S.; Lipton, R.B.; Katz, M.J.; Wang, C.; et al. Sex differences in dementia risk and risk factors: Individual-participant data analysis using 21 cohorts across six continents from the COSMIC consortium. Alzheimers Dement. 2023, 19, 3365–3378. [Google Scholar] [CrossRef]
- Faraco, G.; Hochrainer, K.; Segarra, S.G.; Schaeffer, S.; Santisteban, M.M.; Menon, A.; Jiang, H.; Holtzman, D.M.; Anrather, J.; Iadecola, C. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 2019, 574, 686–690, Erratum in Nature 2020, 578, E9. [Google Scholar] [CrossRef]
- Liu, W.; Xing, S.; Wei, F.; Yao, Y.; Zhang, H.; Li, Y.C.; Liu, Z. Excessive Dietary Salt Intake Exacerbates Cognitive Impairment Progression and Increases Dementia Risk in Older Adults. J. Am. Med. Dir. Assoc. 2023, 24, 125–129 e124. [Google Scholar] [CrossRef]
- Mohan, D.; Yap, K.H.; Reidpath, D.; Soh, Y.C.; McGrattan, A.; Stephan, B.C.M.; Robinson, L.; Chaiyakunapruk, N.; Siervo, M.; DePEC team. Link Between Dietary Sodium Intake, Cognitive Function, and Dementia Risk in Middle-Aged and Older Adults: A Systematic Review. J. Alzheimers Dis. 2020, 76, 1347–1373. [Google Scholar] [CrossRef]
- Shi, K.; Yu, Y.; Li, Z.; Hou, M.; Li, X. Causal relationship between dietary salt intake and dementia risk: Mendelian randomization study. Genes Nutr. 2024, 19, 6. [Google Scholar] [CrossRef]
- Kerl, H.U.; Baazaoui, H.; Herrmann, K.; Adlung, A.; Ludwig, N.K.; Hausner, L.; Frolich, L.; Schad, L.; Groden, C.; Mohamed, S.A. Sodium signal intensity of CSF using 1H-guided 23Na-MRI as a potential noninvasive biomarker in Alzheimer’s disease. J. Neuroimaging 2024, 34, 619–626. [Google Scholar] [CrossRef]
- Kairane, C.; Roots, K.; Uusma, T.; Bogdanovic, N.; Karelson, E.; Koks, S.; Zilmer, M. Regulation of the frontocortical sodium pump by Na+ in Alzheimer’s disease: Difference from the age-matched control but similarity to the rat model. FEBS Lett. 2002, 531, 241–244. [Google Scholar] [CrossRef]
- Souza, L.A.C.; Trebak, F.; Kumar, V.; Satou, R.; Kehoe, P.G.; Yang, W.; Wharton, W.; Feng Earley, Y. Elevated cerebrospinal fluid sodium in hypertensive human subjects with a family history of Alzheimer’s disease. Physiol. Genom. 2020, 52, 133–142. [Google Scholar] [CrossRef]
- Hattori, N.; Kitagawa, K.; Higashida, T.; Yagyu, K.; Shimohama, S.; Wataya, T.; Perry, G.; Smith, M.A.; Inagaki, C. CI-ATPase and Na+/K+-ATPase activities in Alzheimer’s disease brains. Neurosci. Lett. 1998, 254, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M. Na, K-ATPase: Ubiquitous Multifunctional Transmembrane Protein and its Relevance to Various Pathophysiological Conditions. J. Clin. Med. Res. 2010, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Elimban, V.; Adameova, A.D. Role of Na+/K+ ATPase Alterations in the Development of Heart Failure. Int. J. Mol. Sci. 2024, 25, 10807. [Google Scholar] [CrossRef]
- Huang, B.S.; Van Vliet, B.N.; Leenen, F.H. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1160–H1166. [Google Scholar] [CrossRef]
- Amin, M.S.; Reza, E.; Wang, H.; Leenen, F.H. Sodium transport in the choroid plexus and salt-sensitive hypertension. Hypertension 2009, 54, 860–867. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Herrmann, K.; Adlung, A.; Paschke, N.; Hausner, L.; FrOlich, L.; Schad, L.; Groden, C.; Kerl, H.U. Evaluation of Sodium (23Na) MR-imaging as a Biomarker and Predictor for Neurodegenerative Changes in Patients with Alzheimer’s Disease. Vivo 2021, 35, 429–435. [Google Scholar] [CrossRef]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Carrillo, M.C.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef]
- Sulyok, E.; Pal, J.; Vajda, Z.; Steier, R.; Doczi, T. Benzamil prevents brain water accumulation in hyponatraemic rats. Acta Neurochir. 2009, 151, 1121–1125. [Google Scholar] [CrossRef]
- Heinzinger, N.; Maass, A.; Berron, D.; Yakupov, R.; Peters, O.; Fiebach, J.; Villringer, K.; Preis, L.; Priller, J.; Spruth, E.J.; et al. Exploring the ATN classification system using brain morphology. Alzheimers Res. Ther. 2023, 15, 50. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Sasahara, T.; Satomura, K.; Tada, M.; Kakita, A.; Hoshi, M. Alzheimer’s Abeta assembly binds sodium pump and blocks endothelial NOS activity via ROS-PKC pathway in brain vascular endothelial cells. iScience 2021, 24, 102936. [Google Scholar] [CrossRef]
- Petrushanko, I.Y.; Mitkevich, V.A.; Anashkina, A.A.; Adzhubei, A.A.; Burnysheva, K.M.; Lakunina, V.A.; Kamanina, Y.V.; Dergousova, E.A.; Lopina, O.D.; Ogunshola, O.O.; et al. Direct interaction of beta-amyloid with Na,K-ATPase as a putative regulator of the enzyme function. Sci. Rep. 2016, 6, 27738. [Google Scholar] [CrossRef]
- Ohnishi, T.; Yanazawa, M.; Sasahara, T.; Kitamura, Y.; Hiroaki, H.; Fukazawa, Y.; Kii, I.; Nishiyama, T.; Kakita, A.; Takeda, H.; et al. Na, K-ATPase alpha3 is a death target of Alzheimer patient amyloid-beta assembly. Proc. Natl. Acad. Sci. USA 2015, 112, E4465–E4474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.