The Prognostic Significance of Epidermal Growth Factor Receptor Amplification and Epidermal Growth Factor Receptor Variant III Mutation in Glioblastoma: A Systematic Review and Meta-Analysis with Implications for Targeted Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment and Risk of Bias Evaluation
2.5. Statistical Analysis
3. Results
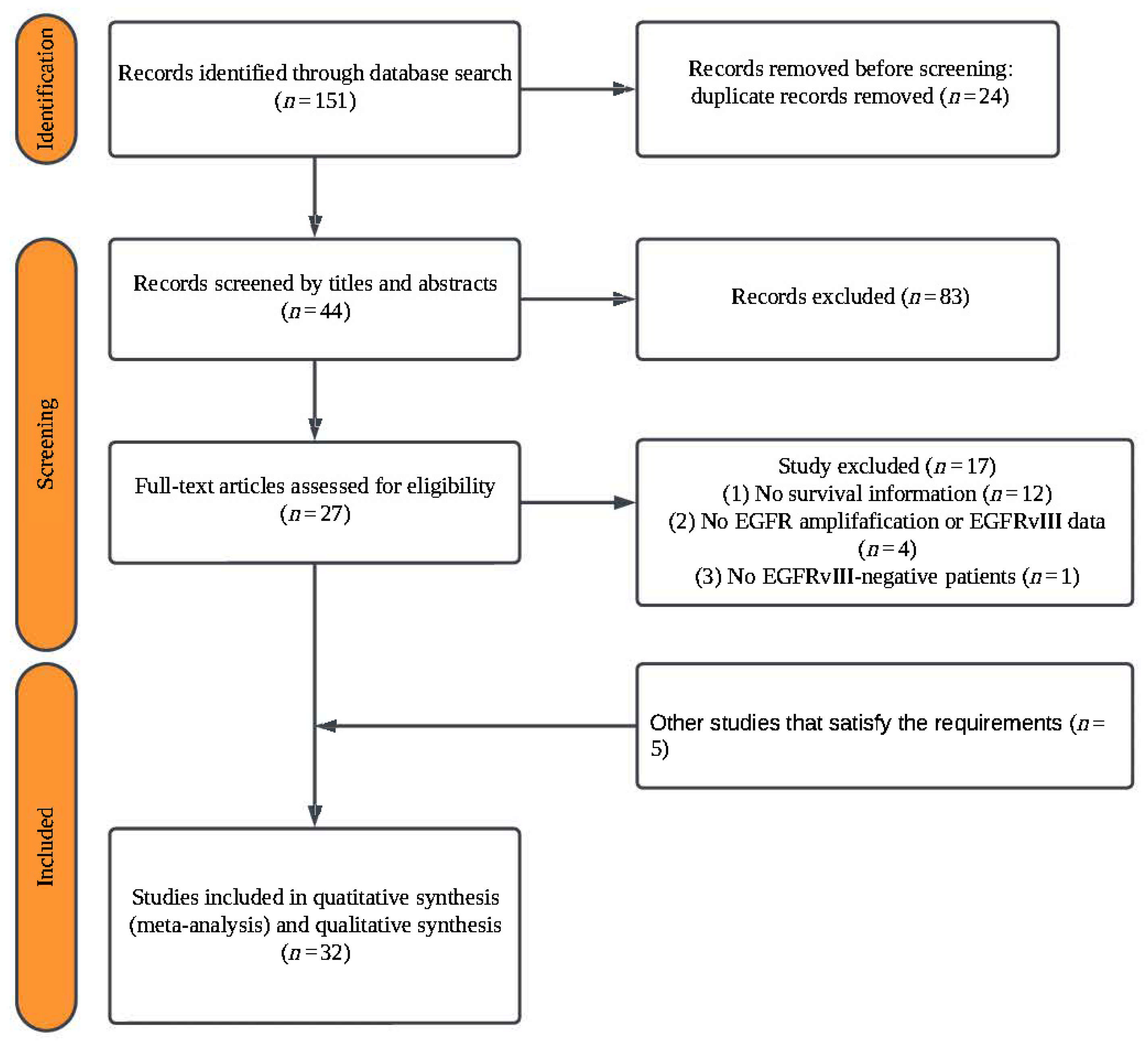
3.1. Literature Screening
3.2. Data Extraction and Quality Assessment
3.3. Meta Analysis
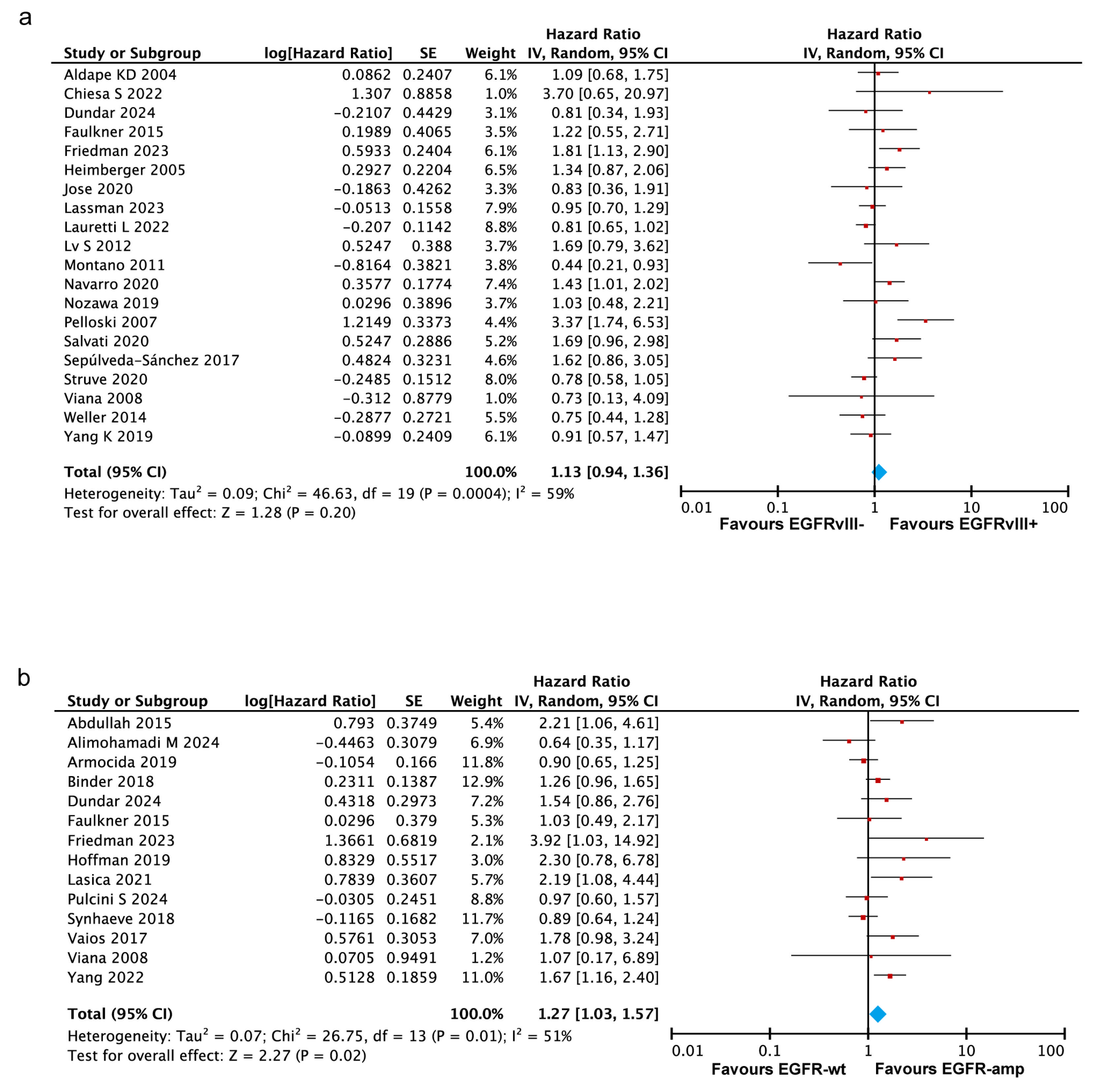
3.3.1. No Significant Association Between EGFRvIII Status and OS in GBM
3.3.2. EGFR Amplification Correlates with Worse Prognosis in GBM
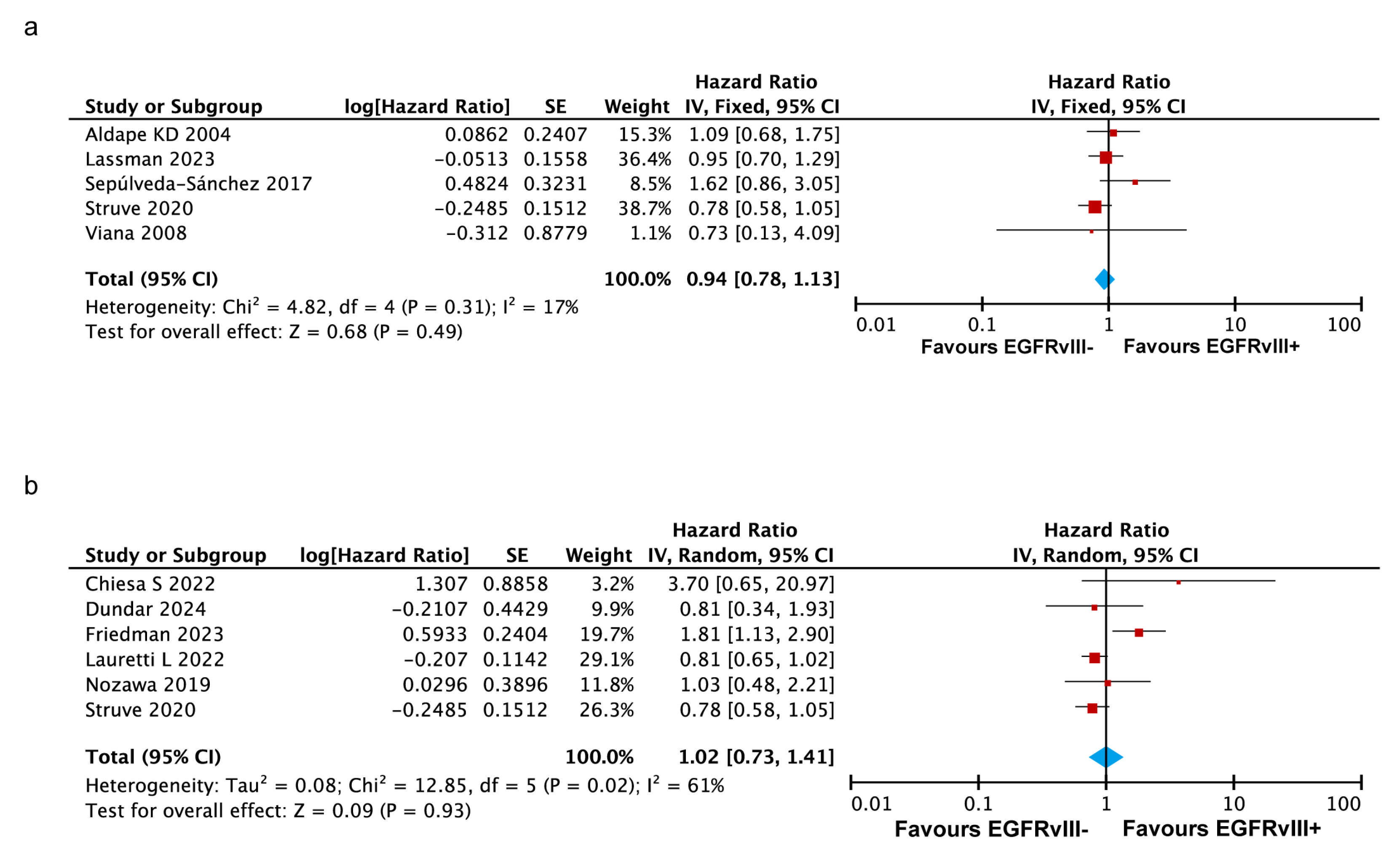
3.3.3. EGFRvIII Status or EGFR Amplification Show No Significant Association with PFS in GBM
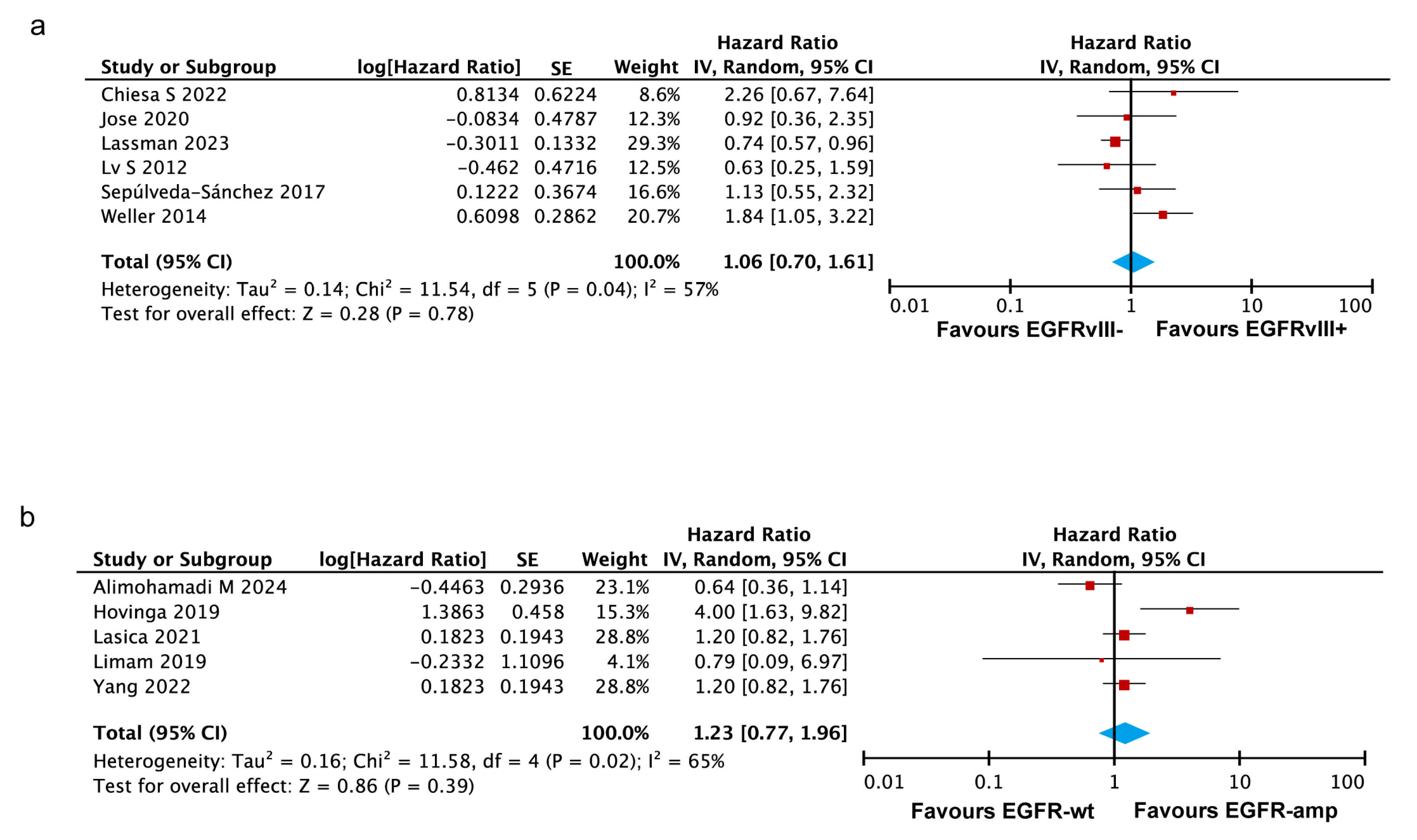
3.3.4. No Significant Impact of Co-Occurring EGFR Amplification and EGFRvIII Mutation on OS in GBM
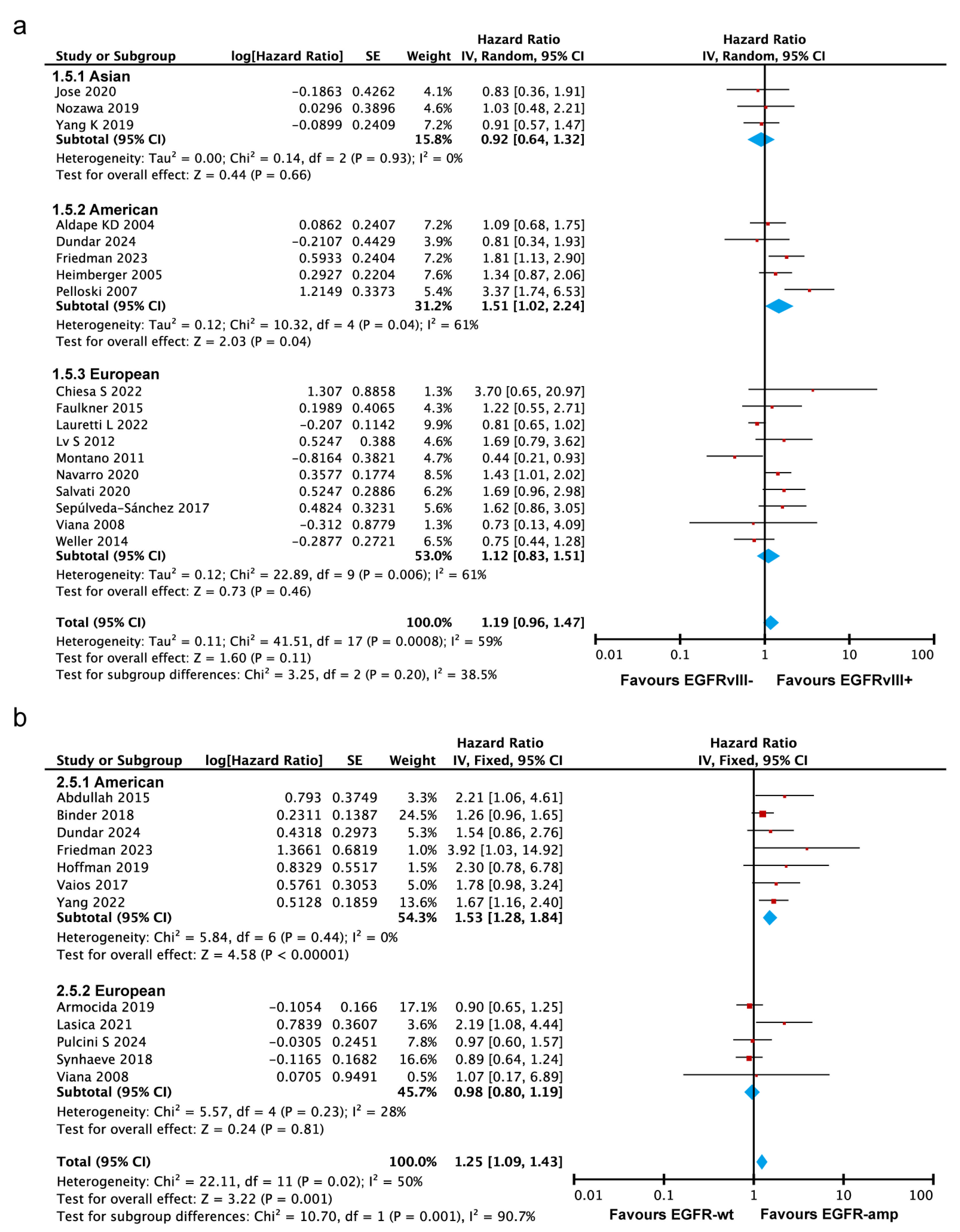
3.3.5. EGFRvIII Mutant and EGFR Amplification Are Associated with Poorer Survival in the Americas, but Not in Asia or Europe
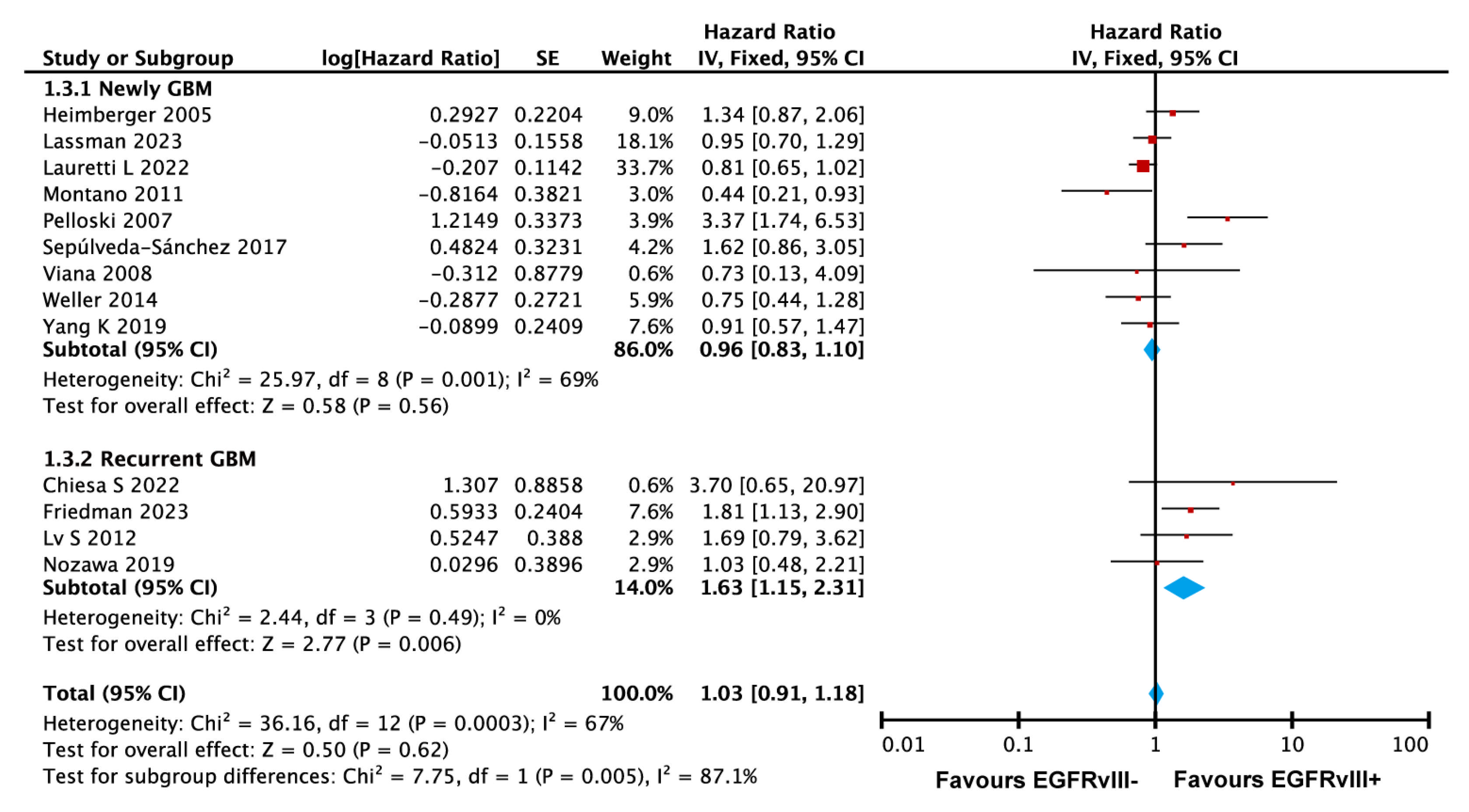
3.3.6. EGFRvIII Mutation Associated with Poorer Survival in Recurrent GBM
3.3.7. Sensitivity Analysis
3.3.8. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma Multiforme: An Overview of Emerging Therapeutic Targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Jezierzanski, M.; Nafalska, N.; Stopyra, M.; Furgol, T.; Miciak, M.; Kabut, J.; Gisterek-Grocholska, I. Temozolomide (TMZ) in the Treatment of Glioblastoma Multiforme-A Literature Review and Clinical Outcomes. Curr. Oncol. 2024, 31, 3994–4002. [Google Scholar] [CrossRef] [PubMed]
- Snuderl, M.; Fazlollahi, L.; Le, L.P.; Nitta, M.; Zhelyazkova, B.H.; Davidson, C.J.; Akhavanfard, S.; Cahill, D.P.; Aldape, K.D.; Betensky, R.A.; et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 2011, 20, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, F.S.; Mahfouz, R.; Assi, H.I. EGFR as a clinical marker in glioblastomas and other gliomas. Int. J. Biol. Markers 2018, 33, 22–32. [Google Scholar] [CrossRef]
- Mukherjee, J.; DeSouza, L.V.; Micallef, J.; Karim, Z.; Croul, S.; Siu, K.W.; Guha, A. Loss of collapsin response mediator Protein1, as detected by iTRAQ analysis, promotes invasion of human gliomas expressing mutant EGFRvIII. Cancer Res. 2009, 69, 8545–8554. [Google Scholar] [CrossRef]
- Stec, W.J.; Rosiak, K.; Siejka, P.; Peciak, J.; Popeda, M.; Banaszczyk, M.; Pawlowska, R.; Treda, C.; Hulas-Bigoszewska, K.; Piaskowski, S.; et al. Cell line with endogenous EGFRvIII expression is a suitable model for research and drug development purposes. Oncotarget 2016, 7, 31907–31925. [Google Scholar] [CrossRef][Green Version]
- Gangoso, E.; Southgate, B.; Bradley, L.; Rus, S.; Galvez-Cancino, F.; McGivern, N.; Guc, E.; Kapourani, C.A.; Byron, A.; Ferguson, K.M.; et al. Glioblastomas acquire myeloid-affiliated transcriptional programs via epigenetic immunoediting to elicit immune evasion. Cell 2021, 184, 2454–2470.e26. [Google Scholar] [CrossRef]
- Fan, Q.W.; Cheng, C.K.; Gustafson, W.C.; Charron, E.; Zipper, P.; Wong, R.A.; Chen, J.; Lau, J.; Knobbe-Thomsen, C.; Weller, M.; et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell 2013, 24, 438–449. [Google Scholar] [CrossRef]
- An, Z.; Fan, Q.W.; Wang, L.; Yoda, H.; Barata, M.J.; Jimenez-Morales, D.; Phillips, J.J.; Swaney, D.L.; Stevenson, E.; Lee, E.; et al. EGFR and EGFRvIII coopt host defense pathways promoting progression in glioblastoma. Neuro-Oncology 2025, 27, 383–397. [Google Scholar] [CrossRef]
- Yang, P.H.; Tao, Y.; Luo, J.; Paturu, M.; Lu, H.-C.; Ramkissoon, S.; Heusel, J.W.; Leuthardt, E.C.; Chicoine, M.R.; Dowling, J.L.; et al. Multivariate analysis of associations between clinical sequencing and outcome in glioblastoma. Neuro-Oncol. Adv. 2022, 4, vdac002. [Google Scholar] [CrossRef]
- Alimohamadi, M.; Larijani, A.; Pour-Rashidi, A.; Farzin, M.; Ebrahimi, H.; Rahmani, M.; Hendi, K.; Yarandi, K.K.; Aghajanian, S.; Shirani, M. Comparative Analysis of the Prognostic Significance of IDH, TERT, EGFR and MGMT Status in Patients with Adult Non-H3-Altered Grade 4 Gliomas: A Prospective Cohort Study. World Neurosurg. 2024, 181, e628–e639. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2021. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 27 November 2024).
- Abdullah, K.G.; Ramayya, A.; Thawani, J.P.; Macyszyn, L.; Martinez-Lage, M.; O’Rourke, D.M.; Brem, S. Factors Associated with Increased Survival after Surgical Resection of Glioblastoma in Octogenarians. PLoS ONE 2015, 10, e0127202. [Google Scholar] [CrossRef]
- Aldape, K.D.; Ballman, K.; Furth, A.; Buckner, J.C.; Giannini, C.; Burger, P.C.; Scheithauer, B.W.; Jenkins, R.B.; James, C.D. Immunohistochemical Detection of EGFRvIII in High Malignancy Grade Astrocytomas and Evaluation of Prognostic Significance. J. Neuropathol. Exp. Neurol. 2004, 63, 700–707. [Google Scholar] [CrossRef]
- Armocida, D.; Pesce, A.; Di Giammarco, F.; Frati, A.; Santoro, A.; Salvati, M. Long Term Survival in Patients Suffering from Glio-blastoma Multiforme: A Single-Center Observational Cohort Study. Diagnostics 2019, 9, 209. [Google Scholar] [CrossRef]
- Binder, Z.A.; Thorne, A.H.; Bakas, S.; Wileyto, E.P.; Bilello, M.; Akbari, H.; Rathore, S.; Ha, S.M.; Zhang, L.; Ferguson, C.J.; et al. Epidermal Growth Factor Receptor Extracellular Domain Mutations in Glioblastoma Present Opportunities for Clinical Imaging and Therapeutic Development. Cancer Cell 2018, 34, 163–177.e7. [Google Scholar] [CrossRef]
- Chiesa, S.; Mangraviti, A.; Martini, M.; Cenci, T.; Mazzarella, C.; Gaudino, S.; Bracci, S.; Martino, A.; Della Pepa, G.M.; Offi, M.; et al. Clinical and NGS predictors of response to regorafenib in recurrent glioblastoma. Sci. Rep. 2022, 12, 16265. [Google Scholar] [CrossRef]
- Dundar, B.; Alsawas, M.; Masaadeh, A.; Conway, K.; Snow, A.N.; Sompallae, R.R.; Bossler, A.D.; Ma, D.; Lopes Abath Neto, O. Molecular characterization and survival analysis of a cohort of glioblastoma, IDH-wildtype. Pathol. Res. Pract. 2024, 257, 155272. [Google Scholar] [CrossRef]
- Faulkner, C.; Palmer, A.; Williams, H.; Wragg, C.; Haynes, H.R.; White, P.; DeSouza, R.-M.; Williams, M.; Hopkins, K.; Kurian, K.M. EGFR and EGFRvIII analysis in glioblastoma as therapeutic biomarkers. Br. J. Neurosurg. 2015, 29, 23–29. [Google Scholar] [CrossRef]
- Friedman, J.S.; Jun, T.; Rashidipour, O.; Huang, K.-L.; Ellis, E.; Kadaba, P.; Belani, P.; Nael, K.; Tsankova, N.M.; Sebra, R.; et al. Using EGFR amplification to stratify recurrent glioblastoma treated with immune checkpoint inhibitors. Cancer Immunol. Immunother. 2023, 72, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Heimberger, A.B.; Hlatky, R.; Suki, D.; Yang, D.; Weinberg, J.; Gilbert, M.; Sawaya, R.; Aldape, K. Prognostic Effect of Epidermal Growth Factor Receptor and EGFRvIII in Glioblastoma Multiforme Patients. Clin. Cancer Res. 2005, 11, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.I.; Abdullah, K.G.; McCoskey, M.; Binder, Z.A.; O’Rourke, D.M.; Desai, A.S.; Nasrallah, M.P.; Bigdeli, A.; Morrissette, J.J.D.; Brem, S.; et al. Negative prognostic impact of epidermal growth factor receptor copy number gain in young adults with isocitrate dehydrogenase wild-type glioblastoma. J. Neuro-Oncol. 2019, 145, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Hovinga, K.E.; McCrea, H.J.; Brennan, C.; Huse, J.; Zheng, J.; Esquenazi, Y.; Panageas, K.S.; Tabar, V. EGFR amplification and classical subtype are associated with a poor response to bevacizumab in recurrent glioblastoma. J. Neuro-Oncol. 2019, 142, 337–345. [Google Scholar] [CrossRef]
- Jose, W.M.; Munirathnam, V.; Narendranath, V.; Philip, A.; Keechilat, P. Frequency and Prognosis of Epidermal Growth Factor Receptor Variant III Mutations in Glioblastoma Multiforme among Indian Patients: A Single-Institution Study. South Asian J. Cancer 2020, 9, 126–129. [Google Scholar] [CrossRef]
- Lasica, A.B.; Jaunmuktane, Z.; Fersht, N.; Kirkman, M.A.; Dixon, L.; Hoskote, C.; Brandner, S.; Samandouras, G. Genomic Prognosticators and Extent of Resection in Molecularly Subtyped World Health Organization Grade II and III Gliomas—A Single-Institution, Nine-Year Data. World Neurosurg. 2021, 151, e217–e233. [Google Scholar] [CrossRef]
- Lassman, A.B.; Pugh, S.L.; Wang, T.J.C.; Aldape, K.; Gan, H.K.; Preusser, M.; Vogelbaum, M.A.; Sulman, E.P.; Won, M.; Zhang, P.; et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: A phase III randomized clinical trial. Neuro-Oncology 2023, 25, 339–350. [Google Scholar] [CrossRef]
- Lauretti, L.; Cenci, T.; Montano, N.; Offi, M.; Giordano, M.; Caccavella, V.M.; Mangraviti, A.; Agostini, L.; Olivi, A.; Gabriele, L.; et al. Molecular Analysis in a Glioblastoma Cohort—Results of a Prospective Analysis. J. Pers. Med. 2022, 12, 685. [Google Scholar] [CrossRef]
- Limam, S.; Missaoui, N.; Abdessayed, N.; Mestiri, S.; Selmi, B.; Mokni, M.; Yacoubi, M.T. Prognostic significance of MGMT methylation and expression of MGMT, P53, EGFR, MDM2 and PTEN in glioblastoma multiforme. Ann. Biol. Clin. 2019, 77, 307–317. [Google Scholar] [CrossRef]
- Lv, S.; Teugels, E.; Sadones, J.; De Brakeleer, S.; Duerinck, J.; Du Four, S.; Michotte, A.; De Grève, J.; Neyns, B. Correlation of EGFR, IDH1 and PTEN status with the outcome of patients with recurrent glioblastoma treated in a phase II clinical trial with the EGFR-blocking monoclonal antibody cetuximab. Int. J. Oncol. 2012, 41, 1029–1035. [Google Scholar] [CrossRef]
- Montano, N.; Cenci, T.; Martini, M.; D’Alessandris, Q.G.; Pelacchi, F.; Ricci-Vitiani, L.; Maira, G.; Maria, R.D.; Larocca, L.M.; Pallini, R. Expression of EGFRvIII in Glioblastoma: Prognostic Significance Revisited. Neoplasia 2011, 13, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; San-Miguel, T.; Megías, J.; Santonja, N.; Calabuig, S.; Muñoz-Hidalgo, L.; Roldán, P.; Cerdá-Nicolás, M.; López-Ginés, C. Identification of New Genetic Clusters in Glioblastoma Multiforme: EGFR Status and ADD3 Losses Influence Prognosis. Cells 2020, 9, 2429. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, T.; Okada, M.; Natsumeda, M.; Eda, T.; Abe, H.; Tsukamoto, Y.; Okamoto, K.; Oishi, M.; Takahashi, H.; Fujii, Y.; et al. EGFRvIII Is Expressed in Cellular Areas of Tumor in a Subset of Glioblastoma. Neurol. Med. Chir. 2019, 59, 89–97. [Google Scholar] [CrossRef]
- Pelloski, C.E.; Ballman, K.V.; Furth, A.F.; Zhang, L.; Lin, E.; Sulman, E.P.; Bhat, K.; McDonald, J.M.; Yung, W.K.A.; Colman, H.; et al. Epidermal Growth Factor Receptor Variant III Status Defines Clinically Distinct Subtypes of Glioblastoma. J. Clin. Oncol. 2007, 25, 2288–2294. [Google Scholar] [CrossRef]
- Pulcini, S.; Beaussire-Trouvay, L.; Marguet, F.; Viailly, P.-J.; Langlois, O.; Alexandru, C.; Tennevet, I.; Di Fiore, F.; Sarafan-Vasseur, N.; Fontanilles, M. The clinical impact of EGFR alterations in elderly glioblastoma patients: Results from a real-life cohort. J. Neuro-Oncol. 2024, 171, 619–628. [Google Scholar] [CrossRef]
- Salvati, M.; Bruzzaniti, P.; Relucenti, M.; Nizzola, M.; Familiari, P.; Giugliano, M.; Scafa, A.K.; Galletta, S.; Li, X.; Chen, R.; et al. Retrospective and Randomized Analysis of Influence and Correlation of Clinical and Molecular Prognostic Factors in a Mono-Operative Series of 122 Patients with Glioblastoma Treated with STR or GTR. Brain Sci. 2020, 10, 91. [Google Scholar] [CrossRef]
- Sepúlveda-Sánchez, J.M.; Vaz, M.Á.; Balañá, C.; Gil-Gil, M.; Reynés, G.; Gallego, Ó.; Martínez-García, M.; Vicente, E.; Quindós, M.; Luque, R.; et al. Phase II trial of dacomitinib, a pan–human EGFR tyrosine kinase inhibitor, in recurrent glioblastoma patients with EGFR amplification. Neuro-Oncology 2017, 19, 1522–1531. [Google Scholar] [CrossRef]
- Struve, N.; Binder, Z.A.; Stead, L.F.; Brend, T.; Bagley, S.J.; Faulkner, C.; Ott, L.; Müller-Goebel, J.; Weik, A.-S.; Hoffer, K.; et al. EGFRvIII upregulates DNA mismatch repair resulting in increased temozolomide sensitivity of MGMT promoter methylated glioblastoma. Oncogene 2020, 39, 3041–3055. [Google Scholar] [CrossRef]
- Synhaeve, N.E.; van den Bent, M.J.; French, P.J.; Dinjens, W.N.M.; Atmodimedjo, P.N.; Kros, J.M.; Verdijk, R.; Dirven, C.M.F.; Dubbink, H.J. Clinical evaluation of a dedicated next generation sequencing panel for routine glioma diagnostics. Acta Neuropathol. Commun. 2018, 6, 126. [Google Scholar] [CrossRef]
- Vaios, E.J.; Nahed, B.V.; Muzikansky, A.; Fathi, A.T.; Dietrich, J. Bone marrow response as a potential biomarker of outcomes in glioblastoma patients. J. Neurosurg. 2017, 127, 132–138. [Google Scholar] [CrossRef]
- Viana-Pereira, M.; Lopes, M.; Little, S.; Milanezi, F.; Basto, D.; Pardal, F.; Jones, C.; Reis, R.M. Analysis of EGFR Overexpression, EGFR Gene Amplification and the EGFRvIII Mutation in Portuguese High-grade Gliomas. Anticancer Res. 2008, 28, 913–920. [Google Scholar] [PubMed]
- Weller, M.; Kaulich, K.; Hentschel, B.; Felsberg, J.; Gramatzki, D.; Pietsch, T.; Simon, M.; Westphal, M.; Schackert, G.; Tonn, J.C.; et al. Assessment and prognostic significance of the epidermal growth factor receptor vIII mutation in glioblastoma patients treated with concurrent and adjuvant temozolomide radiochemotherapy. Int. J. Cancer 2014, 134, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ren, X.; Tao, L.; Wang, P.; Jiang, H.; Shen, L.; Zhao, Y.; Cui, Y.; Li, M.; Lin, S. Prognostic implications of epidermal growth factor receptor variant III expression and nuclear translocation in Chinese human gliomas. Chin. J. Cancer Res. 2019, 31, 188–202. [Google Scholar] [CrossRef]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- El Khayari, A.; Bouchmaa, N.; Taib, B.; Wei, Z.; Zeng, A.; El Fatimy, R. Metabolic Rewiring in Glioblastoma Cancer: EGFR, IDH and Beyond. Front. Oncol. 2022, 12, 901951. [Google Scholar] [CrossRef]
- Hafezi, F.; Perez Bercoff, D. The Solo Play of TERT Promoter Mutations. Cells 2020, 9, 749. [Google Scholar] [CrossRef]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: Refining the approach based on emerging evidence and current challenges. Neuro-Oncology 2019, 21, 167–178. [Google Scholar] [CrossRef]
- Li, F.; Wu, H.; Du, X.; Sun, Y.; Rausseo, B.N.; Talukder, A.; Katailiha, A.; Elzohary, L.; Wang, Y.; Wang, Z.; et al. Epidermal Growth Factor Receptor-Targeted Neoantigen Peptide Vaccination for the Treatment of Non-Small Cell Lung Cancer and Glioblastoma. Vaccines 2023, 11, 1460. [Google Scholar] [CrossRef]
- Lin, B.; Ziebro, J.; Smithberger, E.; Skinner, K.R.; Zhao, E.; Cloughesy, T.F.; Binder, Z.A.; O’Rourke, D.M.; Nathanson, D.A.; Furnari, F.B.; et al. EGFR, the Lazarus target for precision oncology in glioblastoma. Neuro-Oncology 2022, 24, 2035–2062. [Google Scholar] [CrossRef]
- Deschenes-Simard, X.; Kottakis, F.; Meloche, S.; Ferbeyre, G. ERKs in cancer: Friends or foes? Cancer Res. 2014, 74, 412–419. [Google Scholar] [CrossRef]
- Kolch, W.; Halasz, M.; Granovskaya, M.; Kholodenko, B.N. The dynamic control of signal transduction networks in cancer cells. Nat. Rev. Cancer 2015, 15, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Ko, Y.T. Small molecule tyrosine kinase inhibitors in glioblastoma. Arch. Pharm. Res. 2020, 43, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Park, O.K.; Schaefer, T.S.; Nathans, D. In vitro activation of Stat3 by epidermal growth factor receptor kinase. Proc. Natl. Acad. Sci. USA 1996, 93, 13704–13708. [Google Scholar] [CrossRef]
- Sareen, H.; Ma, Y.; Becker, T.M.; Roberts, T.L.; de Souza, P.; Powter, B. Molecular Biomarkers in Glioblastoma: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 8835. [Google Scholar] [CrossRef]
- Chen, J.R.; Xu, H.Z.; Yao, Y.; Qin, Z.Y. Prognostic value of epidermal growth factor receptor amplification and EGFRvIII in glioblastoma: Meta-analysis. Acta Neurol. Scand. 2015, 132, 310–322. [Google Scholar] [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Bagley, S.J.; Binder, Z.A.; Lamrani, L.; Marinari, E.; Desai, A.S.; Nasrallah, M.P.; Maloney, E.; Brem, S.; Lustig, R.A.; Kurtz, G.; et al. Repeated peripheral infusions of anti-EGFRvIII CAR T cells in combination with pembrolizumab show no efficacy in glioblastoma: A phase 1 trial. Nat. Cancer 2024, 5, 517–531. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Dubrow, R.; Darefsky, A.S. Demographic variation in incidence of adult glioma by subtype, United States, 1992-2007. BMC Cancer 2011, 11, 325. [Google Scholar] [CrossRef]
- World Health Organization; International Agency for Reserch on Cancer. Cancer Today. 2022. Available online: https://gco.iarc.who.int/today/en (accessed on 6 November 2024).
- Minniti, G.; Niyazi, M.; Alongi, F.; Navarria, P.; Belka, C. Current status and recent advances in reirradiation of glioblastoma. Radiat. Oncol. 2021, 16, 36. [Google Scholar] [CrossRef]
- Tomar, M.S.; Kumar, A.; Srivastava, C.; Shrivastava, A. Elucidating the mechanisms of Temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188616. [Google Scholar] [CrossRef] [PubMed]
- Levantini, E.; Maroni, G.; Del Re, M.; Tenen, D.G. EGFR signaling pathway as therapeutic target in human cancers. Semin. Cancer Biol. 2022, 85, 253–275. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Fu, Y.; Zhou, W.; Gui, G.; Lal, B.; Li, Y.; Xia, S.; Ji, H.; Eberhart, C.G.; Laterra, J.; et al. EGFR Activates a TAZ-Driven Oncogenic Program in Glioblastoma. Cancer Res. 2021, 81, 3580–3592. [Google Scholar] [CrossRef]
- Solomon, M.T.; Miranda, N.; Jorrin, E.; Chon, I.; Marinello, J.J.; Alert, J.; Lorenzo-Luaces, P.; Crombet, T. Nimotuzumab in combination with radiotherapy in high grade glioma patients: A single institution experience. Cancer Biol. Ther. 2014, 15, 504–509. [Google Scholar] [CrossRef]
- Hasselbalch, B.; Lassen, U.; Hansen, S.; Holmberg, M.; Sorensen, M.; Kosteljanetz, M.; Broholm, H.; Stockhausen, M.T.; Poulsen, H.S. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: A phase II trial. Neuro-Oncology 2010, 12, 508–516. [Google Scholar] [CrossRef]
- Choi, B.D.; Kuan, C.T.; Cai, M.; Archer, G.E.; Mitchell, D.A.; Gedeon, P.C.; Sanchez-Perez, L.; Pastan, I.; Bigner, D.D.; Sampson, J.H. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc. Natl. Acad. Sci. USA 2013, 110, 270–275. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Li, W.; Gao, P.; Zhang, N. HER2-targeting CAR-T cells show highly efficient anti-tumor activity against glioblastoma both in vitro and in vivo. Genes Immun. 2024, 25, 201–208. [Google Scholar] [CrossRef]
- Chuntova, P.; Hou, Y.; Naka, R.; Yamamichi, A.; Chen, T.; Goretsky, Y.; Hatae, R.; Nejo, T.; Kohanbash, G.; Mende, A.L.; et al. Novel EGFRvIII-CAR transgenic mice for rigorous preclinical studies in syngeneic mice. Neuro-Oncology 2022, 24, 259–272. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Stichel, D.; Ebrahimi, A.; Reuss, D.; Schrimpf, D.; Ono, T.; Shirahata, M.; Reifenberger, G.; Weller, M.; Hanggi, D.; Wick, W.; et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018, 136, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, J.; Li, M.; Li, K.; Pu, X.; Guo, Y. Immune landscape-based machine-learning-assisted subclassification, prognosis, and immunotherapy prediction for glioblastoma. Front. Immunol. 2022, 13, 1027631. [Google Scholar] [CrossRef] [PubMed]
- Sibilia, M.; Steinbach, J.P.; Stingl, L.; Aguzzi, A.; Wagner, E.F. A strain-independent postnatal neurodegeneration in mice lacking the EGF receptor. EMBO J. 1998, 17, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Chen, Y.; Zhao, C.; Wang, H.; He, D.; Xu, L.; Wang, J.; He, X.; Deng, Y.; Lu, E.E.; et al. Olig2-Dependent Reciprocal Shift in PDGF and EGF Receptor Signaling Regulates Tumor Phenotype and Mitotic Growth in Malignant Glioma. Cancer Cell. 2016, 29, 669–683. [Google Scholar] [CrossRef]
- Park, A.K.; Kim, P.; Ballester, L.Y.; Esquenazi, Y.; Zhao, Z. Subtype-specific signaling pathways and genomic aberrations associated with prognosis of glioblastoma. Neuro-Oncology 2019, 21, 59–70. [Google Scholar] [CrossRef]
| Patients Number | Outcomes (HR 95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study Name | EGFR-amp * | Year | Country | Recurrent/Newly Diagnosed GBM | Intervention | Study Design | EGFRvIII+ | EGFRvIII– | OS | PFS |
| Aldape 2004 [16] | Yes | NA | U.S.A. | NA | NA | Retrospective Cohort Study | 22 | 77 | 1.09 [0.68–1.76] | NA |
| Chiesa 2022 [19] | No | NA | Italy | Recurrent GBM | Regorafenib | Prospective Cohort Study | 14 | 14 | 3.695 [0.651–20.963] | 2.2556 [0.666–9.814] |
| Dundar 2024 [20] | No | 2020–2021 | U.S.A. | NA | NA | Retrospective Cohort Study | 19 | 25 | 0.81 [0.34–1.96] | NA |
| Faulkner 2015 [21] | No | NA | U.K. | NA | NA | Retrospective Cohort Study | 16 | 35 | 1.22 [0.55–2.69] | NA |
| Friedman 2023 [22] | No | 2014–2019 | U.S.A. | Recurrent GBM | Bev/Niv/Pem | Retrospective Cohort Study | NA | NA | 1.81 [1.13–2.89] | NA |
| Heimberger 2005 [23] | No | NA | U.S.A. | New GBM | RT | Prospective Cohort Study | 61 | 44 | 1.34 [0.87–2.07] | NA |
| Jose 2020 [26] | NA | 2014–2015 | India | NA | RT + TMZ | Retrospective Cohort Study | 23 | 17 | 0.83 [0.36–1.94] | 0.92 [0.36–2.30] |
| Lassman 2023 [28] | Yes | 2015–2018 | Global | New GBM | RT + TMZ | Phase III Randomized Clinical Trial | 168 | 148 | 0.95 [0.70–1.29] | 0.74 [0.57–0.97] |
| Lauretti 2022 [29] | NA | 2012–2017 | Italy | New GBM | RT + TMZ | Prospective Cohort Study | 184 | 171 | 0.813 [0.650–1.016] | NA |
| Lv 2012 [31] | No | NA | Belgium | Recurrent GBM | Cetuximab | Retrospective Cohort Study | 11 | 24 | 1.69 [0.79–3.61] | 0.63 [0.25–1.60] |
| Montano 2011 [32] | NA | NA | Italy | New GBM | RT + TMZ | Prospective Cohort Study | 32 | 41 | 0.442 [0.209–0.714] | NA |
| Navarro 2020 [33] | NA | 1995–2010 | Spain | NA | RT + TMZ | Retrospective Cohort Study | 45 | 83 | 1.43 [1.01–2.03] | NA |
| Nozawa 2019 [34] | NA | NA | Japan | Recurrent GBM | RT/TMZ/Bev | Cohort Study | 13 | 54 | 1.03 [0.48–2.21] | NA |
| Pelloski 2007 [35] | NA | 1992–2003 | U.S.A. | New GBM | RT | Cohort Study | 84 | 184 | 3.37 [1.74,6.50] | NA |
| Salvati 2020 [37] | NA | 2013–2017 | Italy | NA | RT + TMZ | Retrospective Cohort Study | 61 | 61 | 1.69 [0.96–2.98] | NA |
| Sepúlveda-Sánchez 2017 [38] | Yes | 2012–2015 | Spain | New GBM | Dacomitinib | Open-Label Phase II Clinical Trial | 19 | 30 | 1.62 [0.86–3.07] | 1.13 [0.55–2.31] |
| Struve 2020 [39] | Yes | NA | NA | NA | RT + TMZ | Prospective Cohort Study | 92 | 244 | 0.78 [0.58–1.06] | NA |
| Viana-Pereira 2008 [42] | Yes | NA | Portugal | New GBM | NA | Prospective Cohort Study | 8 | 23 | 0.732 [0.131–4.095] | NA |
| Weller 2014 [43] | No | NA | Germany | New GBM | RT + TMZ | Prospective Cohort Study | 85 | 99 | 0.75 [0.44–1.29] | 1.84 [1.05–3.21] |
| Yang 2019 [44] | NA | 2011–2015 | China | New GBM | RT + TMZ | Retrospective Cohort Study | 73 | 167 | 0.914 [0.570–1.467] | NA |
| Patients Number | Outcomes (HR 95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Name | Year | Country | Recurrent/Newly Diagnosed GBM | Intervention | Study Design | EGFR-amp | EGFR-wt | OS | PFS |
| Abdullah 2015 [15] | 2003–2013 | U.S.A. | Recurrent GBM | Surgical resection | Retrospective Cohort Study | 20 | 27 | 2.21 [1.06–4.60] | NA |
| Alimohamadi 2024 [11] | 2019–2021 | Iran | New GBM | RT/TMZ | Prospective Cohort study | 17 | 14 | 0.64 [0.35–1.16] | 0.64 [0.36–1.14] |
| Armocida 2019 [17] | 2014–2016 | Italy | New GBM | RT/TMZ | Retrospective Cohort Study | 9 | 21 | 0.90 [0.65–1.24] | NA |
| Binder 2018 [18] | 2013–2016 | U.S.A. | New GBM | RT/TMZ | Retrospective Cohort Study | 97 | 160 | 1.26 [0.96–1.66] | NA |
| Dundar 2024 [20] | 2020–2021 | U.S.A. | NA | NA | Retrospective Cohort Study | 127 | 233 | 1.54 [0.86–2.75] | NA |
| Faulkner 2015 [21] | NA | U.K. | NA | NA | Retrospective Cohort Study | 22 | 29 | 1.03 [0.49–2.18] | NA |
| Friedman 2023 [22] | 2014–2019 | U.S.A. | Recurrent GBM | Bev/Niv/Pem | Retrospective Cohort Study | 5 | 10 | 3.92 [1.03–14.9] | NA |
| Hoffman 2019 [24] | 2014–2018 | U.S.A. | New GBM | RT/TMZ | Retrospective Cohort Study | 10 | 18 | 2.30 [0.78–6.73] | NA |
| Hovinga 2019 [25] | 2006–2014 | U.S.A. | Recurrent GBM | Bev | Retrospective Cohort Study | 28 | 37 | NA | 4.00 [1.63–9.77] |
| Lasica 2021 [27] | 2010–2019 | U.K. | NA | NA | Retrospective Cohort Study | 47 | 149 | 2.19 [1.08–4.44] | 1.20 [0.82–1.73] |
| Limam 2019 [30] | 2009–2015 | Tunisia | NA | NA | Retrospective Cohort Study | 59 | 15 | NA | 0.792 [0.090–6.955] |
| Pulcini 2024 [36] | 2013–2022 | France | NA | RT/TMZ | Retrospective Cohort Study | 30 | 43 | 0.97 [0.6–1.57] | NA |
| Synhaeve 2018 [40] | 2013–2017 | Netherlands | NA | NA | Retrospective Cohort Study | 83 | 97 | 0.89 [0.64–1.22] | NA |
| Vaios 2017 [41] | 2007–2014 | U.S.A. | New GBM | RT/TMZ | Retrospective Cohort Study | 37 | 37 | 1.779 [0.978–3.247] | NA |
| Viana-Pereira 2008 [42] | NA | Portugal | New GBM | NA | Retrospective Cohort Study | 10 | 9 | 1.073 [0.167–6.885] | NA |
| Yang 2022 [10] | 2015–2020 | U.S.A. | New GBM | RT/TMZ | Retrospective Cohort Study | 66 | 185 | 1.67 [1.16–2.42] | 1.20 [0.82–1.73] |
| Selection | Outcome Assessment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | 1 | 2 | 3 | 4 | Comparability | 1 | 2 | 3 | Score |
| Abdullah 2015 [15] | ★ | ★ | ★ | ☆ | ★★ | ★ | ★ | ☆ | 7 |
| Aldape 2004 [16] | ★ | ★ | ☆ | ☆ | ★★ | ★ | ★ | ★ | 7 |
| Alimohamadi 2024 [11] | ★ | ★ | ☆ | ★ | ★★ | ★ | ★ | ☆ | 7 |
| Armocida 2019 [17] | ★ | ★ | ☆ | ☆ | ★★ | ☆ | ★ | ☆ | 5 |
| Binder 2018 [18] | ★ | ★ | ★ | ☆ | ★ | ★ | ★ | ☆ | 6 |
| Chiesa 2022 [19] | ★ | ★ | ☆ | ★ | ★★ | ★ | ★ | ★ | 8 |
| Dundar 2024 [20] | ★ | ★ | ☆ | ☆ | ★★ | ★ | ★ | ☆ | 6 |
| Faulkner 2015 [21] | ★ | ★ | ☆ | ☆ | ★★ | ★ | ★ | ☆ | 6 |
| Friedman 2023 [22] | ☆ | ★ | ☆ | ☆ | ★★ | ★ | ★ | ★ | 6 |
| Heimberger 2005 [23] | ★ | ★ | ★ | ☆ | ★★ | ★ | ★ | ☆ | 7 |
| Hoffman 2019 [24] | ★ | ★ | ★ | ☆ | ★★ | ★ | ★ | ☆ | 7 |
| Hovinga 2019 [25] | ★ | ★ | ★ | ☆ | ★★ | ★ | ★ | ☆ | 7 |
| Jose 2020 [26] | ★ | ★ | ☆ | ☆ | ★★ | ★ | ★ | ★ | 7 |
| Lasica 2021 [27] | ☆ | ★ | ★ | ☆ | ★★ | ★ | ★ | ☆ | 6 |
| Lauretti 2022 [29] | ★ | ★ | ☆ | ★ | ★★ | ☆ | ★ | ☆ | 6 |
| Limam 2019 [30] | ★ | ★ | ★ | ☆ | ★ | ★ | ★ | ☆ | 6 |
| Lv 2012 [31] | ★ | ★ | ☆ | ☆ | ★★ | ★ | ★ | ★ | 7 |
| Montano 2011 [32] | ★ | ★ | ☆ | ★ | ★★ | ☆ | ★ | ★ | 7 |
| Navarro 2020 [33] | ★ | ★ | ☆ | ☆ | ★ | ☆ | ★ | ★ | 5 |
| Nozawa 2019 [34] | ★ | ★ | ☆ | ☆ | ★★ | ☆ | ★ | ★ | 6 |
| Pelloski 2007 [35] | ★ | ★ | ☆ | ☆ | ★★ | ☆ | ★ | ☆ | 5 |
| Pulcini 2024 [36] | ★ | ★ | ☆ | ☆ | ★★ | ★ | ★ | ☆ | 6 |
| Salvati 2020 [37] | ★ | ★ | ☆ | ☆ | ★★ | ★ | ★ | ☆ | 6 |
| Struve 2020 [39] | ★ | ★ | ☆ | ☆ | ★ | ★ | ★ | ☆ | 5 |
| Synhaeve 2018 [40] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 |
| Vaios 2017 [41] | ★ | ★ | ★ | ☆ | ★★ | ★ | ★ | ☆ | 7 |
| Viana-Pereira 2008 [42] | ★ | ★ | ★ | ☆ | ★★ | ★ | ★ | ★ | 8 |
| Weller 2014 [43] | ★ | ★ | ☆ | ★ | ★★ | ☆ | ★ | ☆ | 6 |
| Yang 2022 [10] | ★ | ★ | ★ | ☆ | ★★ | ★ | ★ | ★ | 8 |
| Yang 2019 [44] | ☆ | ★ | ☆ | ☆ | ★★ | ★ | ★ | ☆ | 5 |
| Study Name | Randomization Process | Bias Due to Deviations from Intended Interventions | Missing Outcome Data | The Bias in the Measurement of the Outcome | The Bias in the Selection of the Reported Result |
|---|---|---|---|---|---|
| Lassman 2023 [28] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Sepúlveda-Sánchez 2017 [38] | High Risk | High Risk | Moderate risk | Moderate risk | Moderate risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.; Qiu, J.; Ye, H.; Su, W.; Wang, R.; Fu, Y. The Prognostic Significance of Epidermal Growth Factor Receptor Amplification and Epidermal Growth Factor Receptor Variant III Mutation in Glioblastoma: A Systematic Review and Meta-Analysis with Implications for Targeted Therapy. Int. J. Mol. Sci. 2025, 26, 3539. https://doi.org/10.3390/ijms26083539
Zhu F, Qiu J, Ye H, Su W, Wang R, Fu Y. The Prognostic Significance of Epidermal Growth Factor Receptor Amplification and Epidermal Growth Factor Receptor Variant III Mutation in Glioblastoma: A Systematic Review and Meta-Analysis with Implications for Targeted Therapy. International Journal of Molecular Sciences. 2025; 26(8):3539. https://doi.org/10.3390/ijms26083539
Chicago/Turabian StyleZhu, Fangge, Jinming Qiu, Haoyuan Ye, Wenting Su, Renxi Wang, and Yi Fu. 2025. "The Prognostic Significance of Epidermal Growth Factor Receptor Amplification and Epidermal Growth Factor Receptor Variant III Mutation in Glioblastoma: A Systematic Review and Meta-Analysis with Implications for Targeted Therapy" International Journal of Molecular Sciences 26, no. 8: 3539. https://doi.org/10.3390/ijms26083539
APA StyleZhu, F., Qiu, J., Ye, H., Su, W., Wang, R., & Fu, Y. (2025). The Prognostic Significance of Epidermal Growth Factor Receptor Amplification and Epidermal Growth Factor Receptor Variant III Mutation in Glioblastoma: A Systematic Review and Meta-Analysis with Implications for Targeted Therapy. International Journal of Molecular Sciences, 26(8), 3539. https://doi.org/10.3390/ijms26083539






