Abstract
Obesity represents a complex, multifactorial syndrome that represents a high burden for public health systems worldwide. Serotonin is an important factor in feeding behavior and weight regulation and their interplay implies multiple mechanisms that could explain the correlation with obesity, so understanding these interconnections is essential for developing targeted therapeutic strategies. A systematic review of the literature was conducted using PubMed and Scopus databases, using articles published between 1 January 2015 and 1 December 2024, based on predefined inclusion and exclusion criteria. After the selection process, 22 studies were selected for detailed analysis, focusing on the role of serotonin in obesity. Serotonin significantly influences appetite control and energy homeostasis through multiples pathways, including insulin resistance, high-fat diets, gut microbiota, low-grade inflammation, interferences with tryptophan metabolism, psychiatric modifications, genetic alterations of serotonin receptors, serotonin implications in eating behavior, and neurohormonal regulation of appetite. This review highlights the multidimensional characteristics of the serotonin–obesity association, along with its significance in metabolic and psychiatric pathologies. In order to develop more efficient methods for managing obesity, future studies should concentrate on serotonergic regulation and complex management strategies involving the neurohormonal axis.
1. Introduction
Obesity is a complex, heterogeneous, chronic, latent, and progressive disease, which causes multiple organ damages, especially regarding the liver, the heart, kidneys, and the pancreas, alongside with respiratory dysfunction [,,,]. Moreover, it is already known that obesity is a worldwide problem, an “epidemic disease” as declared by The World Health Organization. Now, several directions about obesity are studied, including pathophysiology pathways, in order to obtain complex and suitable therapeutic targets for more efficient management.
Although improvements in the diagnosis and treatment of patients with obesity have been recorded, its incidence trend continues to increase. Obesity represents a cumulative disease that appears through genetic factors, and interaction between modern lifestyle and chemical exposure [,,].
One of the recently researched mechanistic pathways implies serotonin involvement in obesity. In the last decade, this correlation has added to the existing knowledge about obesity, including eating behavior influences, such as the activation of the anorexigenic α-melanocyte stimulating hormone, as well as orexigenic neuropeptide Y (NPY) and Agouti-related peptide inhibition, centered by tryptophan (TRP) metabolism [,,].
Serotonin (5-hydroxytryptamine, 5-HT) is a biogenic amine, known as a neurotransmitter and recognized for its multiple functions in gastrointestinal motility, vascular tonus maintenance, and platelet function, and for its role in metabolic homeostasis.
Serotonin biosynthesis from TRP occurs in enterochromaffin cells of the digestive tract and neurons in the central nervous system []. Functionally, serotonin has a major localization of 90–95% in the gastrointestinal tract of the human body. It is stored in granules at the basal and apical ends of the cells [,]. In platelets, serotonin is a signal molecule that is involved in the response to damage endothelium and ischemia [].
The smaller part of serotonin (less than 1%) is secreted in the central nervous system, where it is released and implicated in synapse functions, as well as in emotional responses, including sleep, temperature regulation, cognition, anxiety, and appetite [,,,].
Regarding serotonin receptors, fourteen distinct 5-HT receptors have been described []. In terms of the insulin secretion and glucose metabolism implication, 5-HT1ARs, 5-HT1DRs, 5-HT1FRs, 5-HT2BRs, 5-HT3Rs, and 5-HT5ARs are present at this level, which act as an autocrine signal modulating β-cells’ function and proliferation [,,,,,].
Normal serotonin secretion is important for proper appetite and body-weight regulation, and increased secretion is responsible for weight gain and dysregulation processes that lead to obesity development [,,].
So, we can conclude that serotonin is involved in regulating energy homeostasis through its involvement in feeding behavior, which could be targeted as an optimal therapeutic option in obesity management [,,,,]. The worldwide impact of obesity and the necessity of establishing optimal strategies are the reasons that prompted us to summarize this pathway, aiming to contribute to centralized information that could help in developing new therapeutic strategies.
2. Methods
We conducted a systematic review of the literature regarding the involvement of serotonin in the obesity mechanism, reporting relevant items about this topic. We registered the review protocol under the CRD42025650050 number on Prospero, and, furthermore, using the Population, Intervention, Comparison, Outcome, and Study Design, we developed the strategy that guided our study rationale.
2.1. Research Question and Search Strategy
We conducted a qualitative systematic search of the literature, based on the PubMed and Scopus databases, using the “serotonin AND obesity” search criteria. We used Boolean operators such as “AND” in the search process, targeting free full articles written in English and studies carried out on adults, and we excluded preprints. Also, we limited our search to keywords, titles, and abstracts, and without specific searching due to the existence of exclusion criteria.
We obtained 68 articles from PubMed and 159 articles from Scopus.
2.2. Inclusion Criteria
The inclusion criteria were original full-text articles, namely randomized controlled trials and clinical trials that were published in English from 1 January 2015 to 1 December 2024 conducted on human populations that reported information about the role of serotonin in the pathogenesis of obesity.
2.3. Exclusion Criteria
The exclusion criteria were articles that were case reports, reviews, meta-analyses, letters to the editors, or duplicates, that lacked originality, that were published in languages other than English, that were conducted on non-human populations or only on cell cultures or cell lines, or evaluated the diagnosis and therapeutic processes; we also excluded child, adolescent, psychology, mental disease, and drug influences.
2.4. Study Selection
Studies that met the eligibility criteria (1) included human patients with obesity and serotonin assessment; (2) evaluated the relationship between serotonin involvement and obesity; and (3) provided sufficient information such as 95% confidence intervals or, at least, p-values. Studies were excluded if they (1) were redundant publications; (2) provided insufficient or incomplete data; or (3) were case reports, letters to the editor, meeting abstracts, expert opinions, or reviews.
The study selection process is detailed in Figure 1. From the 232 studies initially selected, 8 were excluded due to lack of originality, having a publication date more than 10 years ago, being conducted on non-human study populations, or being written in a language other than English. Next, 25 duplicate studies and 6 meta-analyses, study protocols, or reports on unrelated outcomes were removed. From the remaining 193 studies, 171 were excluded due to insufficient data or being pharmacological studies.
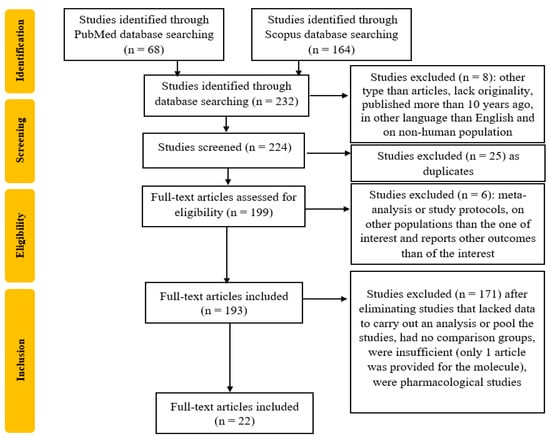
Figure 1.
Flowchart of the study selection process.
2.5. Data Extractions
Two researchers extracted the studies’ titles and abstracts, screened them for relevance to the present study theme, searched for the presence of at least one analysis of a human population with obesity, and selected the relevant ones by performing cross-screening. This process was performed using a standardized form, which included information about publication year, study type, study aim, inclusion and exclusion criteria, outcomes, and conclusions. If any disagreements occurred in the selection process, these were settled by a third reviewer.
We also performed a manual search of the databases to identify other potentially useful articles missed by our search strategy and identified an article.
2.6. Risk of Bias
Assessment bias assessment was carried out using the Newcastle-Ottawa scale (NOS) for cross-sectional studies and Risk of Bias 2 scale (RoB2) for randomized controlled studies (RCT). NOS and RoB2 were conducted by three reviewers. They independently assessed the quality of the chosen studies using a star rating system that evaluated the selection, compatibility, and outcome criteria for articles. The results are shown in Table 1 and Table 2.

Table 1.
Newcastle-Ottawa scale analysis of the included articles.

Table 2.
RoB2—risk of bias for RCT.
From the twenty-two selected articles, we collected data regarding their specific information that was presented, which is centralized in Table 3.

Table 3.
Characteristics of included studies in the systematic review.
2.7. Strategy of Data Synthesis
After the selection process and the articles’ evaluation by the reviewers, 22 articles were included. The process is summarized in Figure 1.
Furthermore, a narrative synthesis of the findings from the studies centered on serotonin’s involvement in human populations with obesity and its relationship with the disease was realized. All the studies included in this systematic review were screened for statistical significance according to serotonin’s implication in obesity; for that matter, all the correlations between serotonin and anthropometric measurements, diet habits, metabolic factors, inflammatory biomarkers, gut microbiota, genetic polymorphisms, and neurotransmitters that had statistical significance were extracted, and are synthesized in Table 4. Because the studies were expected to be heterogeneous in terms of study design, quality and screening methods, interventions, and outcomes described, a narrative synthesis was performed using text and tables in order to provide a descriptive summary and explanation of the study characteristics and findings. The current article refers to approximately the last 10 years of developments in understanding serotonin’s involvement in the pathogenic mechanisms in human populations with obesity, reviewing recent pathophysiology mechanisms that have been established and described on this topic.

Table 4.
A structured synthesis of elements found in the relevant selected articles.
3. Results and Discussion
In the last decades, researchers have investigated the role of serotonin in metabolic pathways, trying to find its reciprocation in weight regulation disturbances. As can be observed, there are many studies, some of them being conclusive about the influence of this neurotransmitter and its correlation in obesity, through lifestyle, genetic profiles, metabolic features, and other neurotransmitters that influence the normal way of weight maintenance.
3.1. Correlation Between Metabolic Factors, Serotonin and Obesity
TRP is an essential amino acid that cannot be synthesized by the human body, and is involved in protein synthesis and inflammation. Its metabolites are associated with indoleamine 2,3-dioxygenase (IDO-1) activity, which is increased by serotonin reduction.
Moreover, TRP is oxidized via kynurenine (KYN) and indole pathways, leading to metabolites that cross the blood–brain barrier and interfere with emotions, cognition, and appetite, among other neurological processes.
Increased pro-inflammatory cytokine levels brought on by inflammatory diseases like obesity can activate IDO-1, an enzyme that catalyzes the conversion of TRP to KYN. Serotonin availability is reduced as a result of this activation. According to studies, people who are obese have higher KYN/TRP ratios, which suggests that they have less serotonin production and more IDO-1 activity. Peripheral serotonin does not cross the blood–brain barrier, but can interfere with central serotonin production via vagal signaling.
Depressive symptoms have been linked to elevated levels of kynurenine and its metabolites, which further affect eating habits and lead to obesity. This cycle produces a feedback loop in which inflammation is made worse by obesity, which further diminishes serotonin and increases food intake.
Insulin resistance induced by high-fat diets involves multiple mechanisms, such as phosphorylation of insulin receptor substrate 1, a process determined by branched-chain amino acids (BCAAs) through the rapamycin (also known as the “mTOR pathway”—Mammalian Targets of Rapamycin) and S6-kinase 1 pathways, accumulation of oxidized BCAA metabolites and mitochondrial dysfunctions, or the accumulation of xanthurenic acid and kynurenic acid, metabolites of the indole pathway resulting from adipose tissue catabolism of tryptophan.
Obese individuals have markedly changed aromatic and BCAA pathways, which may have implications for serotonin activity and levels. Dysregulation of these pathways could make it more difficult for the body to regulate hunger and energy expenditure, which may lead to the development of obesity.
Patients with obesity have an increased production of proinflammatory cytokines, with both obesity and high-fat diets maintaining a low-grade inflammatory state.
Moreover, serotonin is involved in β-cell mass expansion during puberty through the growth hormone, its receptor, and insulin-like growth factor-1, which are associated with insulin resistance, increasing the risk of late-onset metabolic syndrome later in life.
As is well known, lower serotonin levels are linked to higher rates of anxiety and depression, but this effect might cause overeating as a coping strategy. However, this can lead to a vicious cycle whereby eating more food further lowers serotonin levels, worsening psychological distress and obesity.
There are several hormones involved in appetite regulation, such as leptin, ghrelin, peptide YY, glucagon-like peptide 1, insulin, and glucagon. Their release is modulated by food composition, fasted eating, and low-grade inflammation, and they modulate the serotonergic and dopaminergic systems.
3.2. Correlation Between Gut Microbiota, Serotonin, and Obesity
Increased levels of Prevotella spp. in the microbiome lead to dysregulations and insulin resistance through BCAA formation. This specific gut microbiota population in obese patients, rich in Prevotella spp. and Bacteroides spp., influences the conversion of TRP into serotonin, thereby diminishing serotonin levels, leading to increased appetite signaling and weight gain.
Dietary factors, particularly increased protein intake, lead to high levels of TRP in plasma, suggesting that dysbiosis induced by dietary habits interfere with serotonin levels through the gut microbiota.
Fiber-rich diets can increase the synthesis of short-chain fatty acids (SCFAs), such as butyrate, which has been demonstrated to improve insulin sensitivity and serotonin signaling. This implies that dietary changes targeted at enhancing gut health may positively impact serotonin levels, which in turn may influence hunger control and weight management.
Additionally, the location and function of enterochromaffin cells (ECs) may be changed in obese people, which can result in dysregulated hormone release and difficulties in controlling eating. Furthermore, the human microbiota promotes serotonin biosynthesis from ECs []. Thus, weight gain can result from EC dysfunction, which can increase hunger and food intake.
3.3. Correlation Between Genetics, Serotonin, and Obesity
Numerous eating disorders, which are frequently linked to obesity, have been associated with variations in the serotonin transporter gene, such as SLC6A4, which codes for the serotonin transporter (SERT) and interferes with serotonin reuptake in the brain. It has been demonstrated that SERT availability in the prefrontal cortex is associated with reward and decision-making, which interferes with food intake.
Genetic polymorphisms that impact serotonin transmission, such as rs6265 for brain-derived neurotrophic factor (BDNF) or SLC6A4 for 5-HTTLPR, may contribute to the development of obesity by increasing food intake, mood disturbances, and cognitive impairment.
Reduced SERT expression has been associated with the presence of the 5-HTTLPR short allele. This could result in elevated synaptic serotonin levels and altered reward processing in relation to food intake. Moreover, serotonin signaling can also be significantly influenced by epigenetic changes, such as DNA methylation of 5-HTTLPR. Higher 5-HTTLPR methylation rates have been linked to lower SERT availability in the prefrontal cortex, a part of the brain involved in eating behavior decision-making and impulse control.
All these alterations in epigenetics suggest that serotonin signaling plays an important role in reward-related behaviors, influencing weight gain.
3.4. Correlation Between Lifestyle, Serotonin and Obesity
From the total of 22 included articles, 6 presented traits about lifestyle. They revealed the intricate and multifaceted connection between obesity, serotonin levels, and weight regulation through lifestyle influences. For example, in a cohort study published in 2023, elevated body weight and obesity-related parameters were strongly associated with reduced serotonin levels, which in turn correlated with poorer sleep quality. After one month of initiated treatment, the levels of serotonin moderately increased. Over a six-month period, significant improvements were observed in Body Mass Index (BMI), mental health, sleep patterns, and metabolic markers, particularly in groups showing notable increases in serotonin levels. However, a negative correlation was observed between serotonin and obesity, suggesting that while serotonin plays a role in weight regulation, no direct correlation with obesity could be ascertained. In another study conducted on 37 patients, which also profiled the hormonal background, it was revealed that low serotonin levels linked to obesity might result in compensatory behaviors like overeating to raise serotonin and dopamine levels. Patients exhibited significant psychosocial and behavioral issues, such as depression and anxiety, linked to low serotonin levels and lifestyle factors.
Moreover, in a cross-sectional study that included 46 nurses that work in a night-shift program, it was once again highlighted that for this employee category, the BMI was higher and the serotonin level was lower.
That suggests that serotonin secretion is influenced by many other factors like psychosocial stressors and disruptions to circadian rhythms.
Thus, a reciprocal relationship was observed between BMI and serotonin. In order to treat obesity, patients received patient-centered motivational counseling about maintaining a healthy diet and lifestyle, exercising, maintaining good sleep hygiene, and adjusting risk factors in compliance with current clinical guidelines. Dietary adjustments were recommended to achieve efficient results in improving their health.
3.5. Correlation Between Neurotransmitters, Serotonin, and Obesity
Changes in BMI are closely linked to alterations in the structure and function of the brain, especially in the reward and cognitive control systems. Dopamine and serotonin imbalances affect signaling pathways and enhance craving and food intake, particularly of high-calorie foods. According to neuroimaging studies, patients with obesity exhibit abnormalities in areas like the cingulate cortex and frontal lobe, which are involved in cognitive control and eating-related decision-making processes. Reduced SERT availability in the diencephalon lead to glucose metabolism impairments [].
SERT levels are frequently lower in obese people, which may result in less serotonin being available in the central nervous system. The brain’s capacity to properly control food intake may be compromised by this decrease in SERT, which could result in overeating and obesity. Furthermore, certain receptors, such as the 5-HT1B and 5-HT2C receptors or the 5-HT6 receptor, mediate the anorexic effects of serotonin.
Fasting typically increases SERT availability, but in obese individuals, this effect is diminished, indicating a long-term disruption in serotonergic transmission that could be a factor in their ongoing overeating and weight gain. Serotonergic signaling plays a key role in the striatum, a crucial region involved in reward processing. Decreased serotonergic activity may promote overeating by intensifying cravings for high-calorie foods. Increased serotonergic transmission, on the other hand, has been linked to reduced food intake and improved satiety, suggesting that serotonin is involved in both reward-driven and homeostatic eating behaviors.
Higher levels of glycosylated hemoglobin (HbA1c), a sign of persistently elevated blood glucose, are inversely correlated with serotonin transporters availability in the hippocampus, a brain region responsible for hunger regulation. Given that lower levels of BDNF have been associated with both an increased risk of obesity and decreased cognitive performance, the association between SERT availability and BDNF implies that BDNF may be involved in influencing serotonin signaling and energy metabolism. Impaired hippocampal function due to serotonin dysregulation can result in learning and memory deficits related to satiety and food cues.
Enteroendocrine cells (EECs), part of the nervous system of the gastrointestinal tract, release different hormones and neurotransmitter, in response to luminal content. Peripheral serotonin, released by enterochromaffin cells, activates the gastrointestinal tract, leading to motility and secretion of molecules that activate the vagal system and induce the increase in central serotonin and loss of appetite, this leading to weight loss.
3.6. Serotonin Pathways in Obesity Development
Serotonin levels are influenced by various factors, with studies supporting its influence in mood regulation, metabolic processes, and appetite control. Recent research highlights 5-HT’s role in obesity development, where it mediates inflammatory pathways, food intake and energy expenditure (Figure 2).
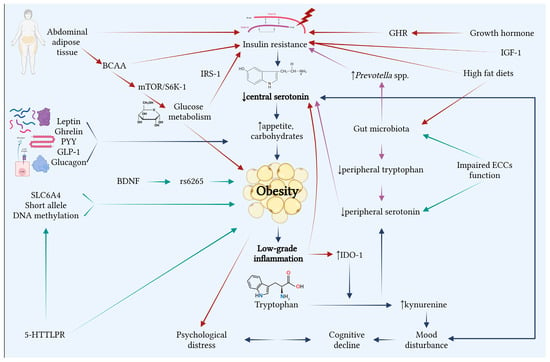
Figure 2.
Interrelations between serotonin and obesity (Created with BioRenders.com). Numerous factors, including genetics, neurotransmitters, gut microbiota, metabolism, and psychological disorders, connect obesity to serotonin. The primary contributor is low central serotonin levels, which increase appetite, particularly for carbohydrates, and contribute to and perpetuate obesity by causing low-grade inflammation. Obesity and psychological distress have a reciprocal relationship that results in mood disorders and cognitive deterioration. The gut microbiome is crucial because low tryptophan levels cause peripheral serotonin levels to drop, which maintain the low concentrations of central serotonin, the vagal signaling responsible for the central secretion being diminished. Increased hunger and low-grade inflammation driven by obesity and insulin resistance, as well as the interference of abdominal adipose tissue with many processes that sustain obesity, are the mechanisms by which hormones and serotonin are linked. Obesity is also caused by mutations in the genome, the most significant of which are 5-HTTLPR genetic variations. The various facets of obesity are shown by the connections between all of these linkages.
The primary mechanism involved is chronic inflammation, which is induced by obesity itself but also driven by psychological distress [], diet type [], gut microbiota composition [], and alterations in the brain network activity [].
Studies have shown that serum 5-HT levels are lower in patients with obesity compared with lean individuals [,]. Moreover, the work schedule, especially night shifts, may influence 5-HT levels []. Interestingly, higher 5-HT levels were observed in male patients with hypertriglyceridemia [], following weight loss [] or after 6 months of patient-centered motivational approach [].
TRP’s metabolism via KYN pathway, assessed by increased KYN levels and an elevated TRP/KYN ratio (as a surrogate for IDO-1 pathway’s activation), has been positively correlated with BMI [,]. Additionally, decreased fecal TRP levels, alongside increased Prevotella/Bacteroides ratio were associated with higher BMI [].
The interplay between lipid and glucose metabolisms affects the SERT availability. However, findings on the relationship between SERT availability and BMI are contradictory [,,], but one possible connection between glucose metabolism and SERT function could be BDNF []. Furthermore, lower SERT binding was associated with insulin resistance [] and increased levels of HbA1c [], suggesting that glucose dysregulation may contribute to cognitive impairment.
Although gut microbiota alteration appears to impact DAT binding rather than SERT binding, the results from studies show that patients with obesity exhibited alteration in brain connectivity, especially in reward-related regions [], with a negative correlation between BMI and SERT availability []. Also, patients with obesity showed no response to fasting compared to lean individuals regarding hypothalamic SERT levels [].
Furthermore, the metabolite profile shows certain BCAAs and AAAs involved in obesity mechanisms, correlating with VAT’s implication in metabolic pathways in obesity [].
5-HT plays an important role in obesity through its influence on energy homeostasis and chronic inflammation. The included studies suggest 5-HT’s implication in obesity development, but future research is required to assess the pathways involved and to develop targeted therapeutic approaches to manage weight and obesity-related risk factors.
3.7. Limits
The current article is a systematic review of research conducted during the last decade regarding the involvement of 5-HT in obesity development. Based on the selected studies and their analysis, our study has the following limitations: (a) the majority of the studies are cross-sectional, observational studies, which demonstrated the correlation between 5-HT and BMI, along with metabolic dysregulation and brain activity changes; (b) all the studies had different inclusion and exclusion criteria, and the results cannot be generalized in the population; (c) most of the studies had a small sample size; (d) no follow-up of the patients was conducted; and (e) self-reported food diaries, sleep, and mental health data could be a source of bias.
3.8. Research Future Directions and Perspective Targeted Treatments
From health systems’ perspectives, it is essential to track shifts in the nutritional status of individuals and explore the possible causes and consequences of these changes. Obesity eradication strategies include lifestyle modifications, pharmacological interventions, and metabolic surgeries [].
In the human body, the hypothalamus regulates body weight by combining neuronal inputs to regulate the appetite using signals like leptin and insulin [,]. It is known that leptin concentrations increase proportionally with fat tissue []. Reduced levels of insulin and leptin are accompanied by increased concentrations of glucagon, growth hormone, and catecholamines []. Functionally, leptin inhibits diencephalic nitric oxide synthase activity and increases brain serotonin metabolism []. The complex interactions between the brain, gut, pancreatic islets, and adipose tissue in regulating appetite and the reward center may alter the insulin/serotonin (5-HT)/leptin axis in obesity, potentially impacting the density, function, or affinity of SERT in distinct ways [,].
Another recently described mechanism for obesity is represented by gut microbiota intervention. Studies have demonstrated that typical ratios between specific human gastrointestinal microbiota, like the Prevotella/Bacteroides ratio, are inversely proportional to fecal tryptophan. The administration of prebiotics and probiotics, and fecal microbiota transplantation, could be possible effective therapies used for efficient management of obesity [,].
The location and function of EECs may be changed in obese people, which can result in dysregulated hormone release and difficulties controlling eating. Weight gain can result from EEC dysfunction, which can increase hunger and food intake.
Increased serotonin levels brought on by changes in the circadian rhythm may cause weight gain by causing symptoms of depression to manifest. So, national programs of psychotherapy for motivational counseling have led to improvements in weight loss, physical activity, and sleep quality, and the persistence of emotional eating tendencies should be under control in this way. These highlight the need for targeted strategies to address this behavior, in association with drug therapy.
Additionally, genetically targeted treatments for obesity are probably the most expected treatments for this illness, which is a hopeless situation for the majority patients.
In summary, future research regarding 5-HT’s involvement in obesity should include (1) larger studies to investigate the role of 5-HT in metabolic dysregulation; (2) studies of the influence of gut microbiota on TRP metabolism and the involvement of the IDO-1 pathway in metabolic alterations; (3) studies of the inflammatory diet’s effect on the cognitive impairment of eating disorders, along with 5-HT metabolism in this population; (4) development of more specific SERT evaluation in obese individuals; and (5) studies of the involvement of psychological distress in gut microbiota changes and 5-HT levels. Understanding all these pathways could represent therapeutic target areas in obesity management.
4. Conclusions
There is a complex and multidimensional relationship between obesity, serotonin levels, and weight regulation.
Regarding the correlations between the hypothalamus, leptin concentration, involved hormones, neurotransmitter reciprocations (especially that of serotonin), gut microbiota, and genetic predisposition, future targeted therapy should cover this complex axis, for an equilibrated balance in normal weight recovery or preservation, in the event of predisposing factors without an apparent clinical impact. For this purpose, ample and extensive studies are necessary to understand the complete mechanisms with the purpose of creating cost-effective molecular drug therapies for complete, reproducible treatment results.
Author Contributions
Conceptualization, R.-C.C., L.-M.M. and R.-E.A.; methodology, A.C.; software, A.B. and A.C.; validation, E.-M.C., I.-G.D. and L.Ș.; formal analysis, D.F.; investigation, R.-C.C., L.-M.M., I.-G.D., A.B. and R.-E.A.; resources, R.-C.C., L.-M.M. and R.-E.A.; data curation, D.F. and A.C.; writing—original draft preparation, R.-C.C. and R.-E.A.; writing—review and editing, D.F. and L.Ș.; visualization, L.Ș.; supervision, L.-D.S.; project administration, R.-C.C.; funding acquisition, L.-D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Medicine and Pharmacy of Craiova.
Acknowledgments
Radu-Cristian Cîmpeanu and Laurențiu Șorodoc share equal contributions and status as the main/first authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lingvay, I.; Cohen, R.V.; Roux, C.W.L.; Sumithran, P. Obesity in adults. Lancet 2024, 404, 972–987. [Google Scholar] [CrossRef] [PubMed]
- Schunkert, H. Obesity and target organ damage: The heart. Int. J. Obes. Relat. Metab. Disord. 2002, 26, S15–S20. [Google Scholar] [CrossRef]
- de Jong, P.E.; Verhave, J.C.; Pinto-Sietsma, S.J.; Hillege, H.L.; PREVEND study group. Obesity and target organ damage: The kidney. Int. J. Obes. Relat. Metab. Disord. 2002, 26, S21–S24. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.A.; Esler, M.D.; Schlaich, M.P.; Dixon, J.; Eikelis, N.; Lambert, G.W. Obesity-Associated Organ Damage and Sympathetic Nervous Activity. Hypertension 2019, 73, 1150–1159. [Google Scholar] [CrossRef]
- Lustig, R.H.; Collier, D.; Kassotis, C.; Roepke, T.A.; Kim, M.J.; Blanc, E.; Barouki, R.; Bansal, A.; Cave, M.C.; Chatterjee, S.; et al. Obesity I: Overview and molecular and biochemical mechanisms. Biochem. Pharmacol. 2022, 199, 115012. [Google Scholar] [CrossRef]
- Heindel, J.J.; Howard, S.; Agay-Shay, K.; Arrebola, J.P.; Audouze, K.; Babin, P.J.; Barouki, R.; Bansal, A.; Blanc, E.; Cave, M.C.; et al. Obesity II: Establishing causal links between chemical exposures and obesity. Biochem. Pharmacol. 2022, 199, 115015, Erratum in Biochem. Pharmacol. 2022, 202, 115144. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Vom Saal, F.S.; Babin, P.J.; Lagadic-Gossmann, D.; Le Mentec, H.; Blumberg, B.; Mohajer, N.; Legrand, A.; Munic Kos, V.; Martin-Chouly, C.; et al. Obesity III: Obesogen assays: Limitations, strengths, and new directions. Biochem. Pharmacol. 2022, 199, 115014, Erratum in Biochem. Pharmacol. 2022, 202, 115145. [Google Scholar] [CrossRef]
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; Ter Horst, K.W.; Serlie, M.J. The pathogenesis of obesity. Metabolism 2019, 92, 26–36. [Google Scholar] [CrossRef]
- Caron, A.; Jane Michael, N. New Horizons: Is Obesity a Disorder of Neurotransmission? J. Clin. Endocrinol. Metab. 2021, 106, e4872–e4886. [Google Scholar] [CrossRef]
- Jonnakuty, C.; Gragnoli, C. What do we know about serotonin? J. Cell Physiol. 2008, 17, 301–306. [Google Scholar] [CrossRef]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Szeitz, A.; Bandiera, S.M. Analysis and measurement of serotonin. Biomed. Chromatogr. 2018, 32, e4135. [Google Scholar] [CrossRef]
- Hornung, J.P. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 2003, 26, 331–343. [Google Scholar] [CrossRef]
- Chen, X.; Ye, R.; Gargus, J.J.; Blakely, R.D.; Dobrenis, K.; Sze, J.Y. Disruption of Transient Serotonin Accumulation by Non-Serotonin-Producing Neurons Impairs Cortical Map Development. Cell Rep. 2015, 10, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Lucki, I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry 1998, 44, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, T.; Lyons, D.; Heisler, L.K. Role of serotonin in body weight, insulin secretion and glycaemic control. J. Neuroendocr. 2021, 33, e12960. [Google Scholar] [CrossRef]
- Almaça, J.; Molina, J.; Menegaz, D.; Pronin, A.N.; Tamayo, A.; Slepak, V.; Berggren, P.-O.; Caicedo, A. Human beta cells produce and release serotonin to inhibit glucagon secretion from alpha cells. Cell Rep. 2016, 17, 3281–3291. [Google Scholar]
- Kim, K.; Oh, C.M.; Ohara-Imaizumi, M.; Park, S.; Namkung, J.; Yadav, V.K.; Tamarina, N.A.; Roe, M.W.; Philipson, L.H.; Karsenty, G.; et al. Functional role of sero tonin in insulin secretion in a diet- induced insulin- resistant state. Endocrinology 2015, 156, 444–452. [Google Scholar]
- Ohara-Imaizumi, M.; Kim, H.; Yoshida, M.; Fujiwara, T.; Aoyagi, K.; Toyofuku, Y.; Nakamichi, Y.; Nishiwaki, C.; Okamura, T.; Uchida, T.; et al. Serotonin regulates glucose- stimulated insulin secretion from pancreatic β cells during pregnancy. Proc. Natl. Acad. Sci. USA 2013, 110, 19420–19425. [Google Scholar]
- Bennet, H.; Mollet, I.G.; Balhuizen, A.; Medina, A.; Nagorny, C.; Bagge, A.; Fadista, J.; Ottosson-Laakso, E.; Vikman, P.; Dekker-Nitert, M.; et al. Serotonin (5- HT) receptor 2b activation augments glucose- stimulated insulin se cretion in human and mouse islets of Langerhans. Diabetologia 2016, 59, 744–754. [Google Scholar]
- Blodgett, D.M.; Nowosielska, A.; Afik, S.; Pechhold, S.; Cura, A.J.; Kennedy, N.J.; Kim, S.; Kucukural, A.; Davis, R.J.; Kent, S.C.; et al. Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 2015, 64, 3172–3181. [Google Scholar] [CrossRef] [PubMed]
- Breisch, S.T.; Zemlan, F.P.; Hoebel, B.G.; Url, S. Hyperphagia and obesity following serotonin depletion by intraventricular p-Chlorophenylalanine Hyperphagia and obesity following serotonin depletion by Intraventricular p-Chlorophenylalanine. Science 1976, 192, 382–385. [Google Scholar] [CrossRef]
- Saller, C.F.; Stricker, E.M. Hyperphagia and increased growth in rats after intraventricular injection of 5,7- dihydroxytryptamine. Science 1976, 191, 385–387. [Google Scholar] [CrossRef]
- Miller, G.D. Appetite Regulation: Hormones, Peptides, and Neurotransmitters and Their Role in Obesity. Am. J. Lifestyle Med. 2017, 13, 586–601. [Google Scholar] [CrossRef]
- Nonogaki, K.; Strack, A.M.; Dallman, M.F.; Tecott, L.H. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat. Med. 1998, 4, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Berglund, E.D.; Sohn, J.W.; Holland, W.L.; Chuang, J.-C.; Fukuda, M.; Rossi, J.; Williams, K.W.; E Jones, J.; Zigman, J.M.; et al. 5-HT2CRs expressed by pro opiomelanocortin neurons regulate insulin sensitivity in liver. Nat. Neurosci. 2010, 13, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jones, J.E.; Kohno, D.; Williams, K.W.; Lee, C.E.; Choi, M.J.; Anderson, J.G.; Heisler, L.K.; Zigman, J.M.; Lowell, B.B.; et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 2008, 60, 582–589. [Google Scholar] [CrossRef]
- Ho, A.J.; Raji, C.A.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Lee, S.; Hibar, D.; Dinov, I.D.; Stein, J.L.; et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol. Aging 2010, 31, 1326–1339. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Shen, J.; Zhao, T.; Xiao, R.; Ma, W. Dietary Inflammatory Index and Cognitive Function: Findings from a Cross-Sectional Study in Obese Chinese Township Population from 45 to 75 Years. J. Inflamm. Res. 2024, 17, 2365–2382. [Google Scholar] [CrossRef]
- Kim, C.Y.; Park, Y.; Namgung, J.Y.; Park, Y.; Park, B.Y. The macroscale routing mechanism of structural brain connectivity related to body mass index. Hum. Brain Mapp. 2024, 45, e70019. [Google Scholar] [CrossRef]
- Liikonen, V.; Näätänen, M.; Kårlund, A.; Hanhineva, K.; Karhunen, L.; Kolehmainen, M. Association between whole-grain consumption, tryptophan metabolism and psychological distress: A secondary analysis of a randomised controlled trial. Br. J. Nutr. 2024, 132, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Borroni, E.; Frigerio, G.; Polledri, E.; Mercadante, R.; Maggioni, C.; Fedrizzi, L.; Pesatori, A.C.; Fustinoni, S.; Carugno, M. Metabolomic profiles in night shift workers: A cross-sectional study on hospital female nurses. Front. Public. Health 2023, 11, 1082074. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, V.; Bagro, T. The Correlation Between Body Weight, Serotonin Levels, Mental Health Status, Sleep Disorders and Metabolism in Patients with Obesity. Int. J. Endocrinol. (Ukr.) 2023, 19, 354–362. [Google Scholar] [CrossRef]
- Tkachenko, V.; Bagro, T. Effectiveness of Motivational Counseling for Lifestyle Modification in Obese Patients Using a Patient-centered Approach. Fam. Med. Eur. Pract. 2023, 1, 20–27. [Google Scholar] [CrossRef]
- Castell, A.L.; Goubault, C.; Ethier, M.; Fergusson, G.; Tremblay, C.; Baltz, M.; Dal Soglio, D.; Ghislain, J.; Poitout, V. β Cell mass expansion during puberty involves serotonin signaling and determines glucose homeostasis in adulthood. JCI Insight 2022, 7, e160854. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Guan, M.; Mayer, E.A.; Stains, J.; Liu, C.; Vora, P.; Jacobs, J.P.; Lagishetty, V.; Chang, L.; Barry, R.L.; et al. Obesity is associated with a distinct brain-gut microbiome signature that connects Prevotella and Bacteroides to the brain’s reward center. Gut Microbes 2022, 14, 2051999. [Google Scholar] [CrossRef]
- Orozco-Ruiz, X.; Anesi, A.; Mattivi, F.; Breteler, M.M.B. Branched-Chain and Aromatic Amino Acids Related to Visceral Adipose Tissue Impact Metabolic Health Risk Markers. J. Clin. Endocrinol. Metab. 2022, 107, e2896–e2905. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, C.S.; Yang, Y.K.; Shen, L.H.; Yao, W.J. Comparison of brain serotonin transporter using [I-123]-ADAM between obese and non-obese young adults without an eating disorder. PLoS ONE 2017, 12, e0170886. [Google Scholar] [CrossRef]
- Baumard, L.; Weerts, Z.Z.R.M.; Masclee, A.A.M.; Keszthelyi, D.; Michael-Titus, A.T.; Peiris, M. Effect of Obesity on the Expression of Nutrient Receptors and Satiety Hormones in the Human Colon. Nutrients 2021, 13, 1271. [Google Scholar] [CrossRef]
- van Galen, K.A.; Booij, J.; Schrantee, A.; Adriaanse, S.M.; Unmehopa, U.A.; Fliers, E.; Schwartz, G.J.; DiLeone, R.J.; Ter Horst, K.W.; la Fleur, S.E.; et al. The response to prolonged fasting in hypothalamic serotonin transporter availability is blunted in obesity. Metabolism 2021, 123, 154839. [Google Scholar] [CrossRef]
- Hartstra, A.V.; Schüppel, V.; Imangaliyev, S.; Schrantee, A.; Prodan, A.; Collard, D.; Levin, E.; Dallinga-Thie, G.; Ackermans, M.T.; Winkelmeijer, M.; et al. Infusion of donor feces affects the gut-brain axis in humans with metabolic syndrome. Mol. Metab. 2020, 42, 101076. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, L.; Cunha, M.; Padez, C.; Alvarez, M.; Pinto-Gouveia, J.; Manco, L. Association study of variants in genes FTO, SLC6A4, DRD2, BDNF and GHRL with binge eating disorder (BED) in Portuguese women. Psychiatry Res. 2019, 273, 309–311. [Google Scholar] [CrossRef]
- Fakhry, J.; Stebbing, M.J.; Hunne, B.; Bayguinov, Y.; Ward, S.M.; Sasse, K.C.; Callaghan, B.; McQuade, R.M.; Furness, J.B. Relationships of endocrine cells to each other and to other cell types in the human gastric fundus and corpus. Cell Tissue Res. 2019, 376, 37–49. [Google Scholar] [CrossRef]
- Nam, S.B.; Kim, K.; Kim, B.S.; Im, H.J.; Lee, S.H.; Kim, S.J.; Kim, I.J.; Pak, K. The Effect of Obesity on the Availabilities of Dopamine and Serotonin Transporters. Sci. Rep. 2018, 8, 4924. [Google Scholar] [CrossRef]
- Groer, M.; Fuchs, D.; Duffy, A.; Louis-Jacques, A.; D’Agata, A.; Postolache, T.T. Associations Among Obesity, Inflammation, and Tryptophan Catabolism in Pregnancy. Biol. Res. Nurs. 2018, 20, 284–291. [Google Scholar] [CrossRef]
- Drabe, M.; Rullmann, M.; Luthardt, J.; Boettcher, Y.; Regenthal, R.; Ploetz, T.; Becker, G.A.; Patt, M.; Schinke, C.; Bergh, F.T.; et al. Serotonin transporter gene promoter methylation status correlates with in vivo prefrontal 5-HTT availability and reward function in human obesity. Transl. Psychiatry 2017, 7, e1167. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, S.; Williams, A.L. Preliminary fMRI findings concerning the influence of 5-HTP on food selection. Brain Behav. 2016, 7, e00594. [Google Scholar] [CrossRef]
- Frigerio, G.; Favero, C.; Savino, D.; Mercadante, R.; Albetti, B.; Dioni, L.; Vigna, L.; Bollati, V.; Pesatori, A.C.; Fustinoni, S. Plasma Metabolomic Profiling in 1391 Subjects with Overweight and Obesity from the SPHERE Study. Metabolites 2021, 11, 194. [Google Scholar] [CrossRef]
- Grundmann, R.; Rullmann, M.; Luthardt, J.; Zientek, F.; Becker, G.A.; Patt, M.; Hankir, M.K.; Blüher, M.; Sabri, O.; Hesse, S. Higher HbA1c levels associate with lower hippocampal serotonin transporter availability in non-diabetic adults with obesity. Sci. Rep. 2020, 10, 21383. [Google Scholar] [CrossRef]
- Versteeg, R.I.; Koopman, K.E.; Booij, J.; Ackermans, M.T.; Unmehopa, U.A.; Fliers, E.; la Fleur, S.E.; Serlie, M.J. Serotonin Transporter Binding in the Diencephalon Is Reduced in Insulin-Resistant Obese Humans. Neuroendocrinology 2017, 105, 141–149. [Google Scholar] [CrossRef]
- Bollati, V.; Iodice, S.; Favero, C.; Angelici, L.; Albetti, B.; Cacace, R.; Cantone, L.; Carugno, M.; Cavalleri, T.; De Giorgio, B.; et al. Susceptibility to particle health effects, miRNA and exosomes: Rationale and study protocol of the SPHERE study. BMC Public Health 2014, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276, Erratum in Cell 2015, 163, 258. [Google Scholar] [CrossRef]
- Salmen, T.; Pietrosel, V.-A.; Reurean-Pintilei, D.; Iancu, M.A.; Cimpeanu, R.C.; Bica, I.-C.; Dumitriu-Stan, R.-I.; Potcovaru, C.-G.; Salmen, B.-M.; Diaconu, C.-C.; et al. Assessing Cardiovascular Target Attainment in Type 2 Diabetes Mellitus Patients in Tertiary Diabetes Center in Romania. Pharmaceuticals 2024, 17, 1249. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Ding, Y.; Cui, B.; Li, R.-R.; Qu, X.-R.; Zhou, H.; Au, K.-H.; Fan, X.-D.; Xie, J.-C.; Huang, Y.; et al. Navigating obesity: A comprehensive review of epidemiology, pathophysiology, complications and management strategies. Innov. Med. 2024, 2, 100090. [Google Scholar] [CrossRef]
- Sui, S.X.; Pasco, J.A. Obesity and Brain Function: The Brain-Body Crosstalk. Medicina 2020, 56, 499. [Google Scholar] [CrossRef]
- Picó, C.; Palou, M.; Pomar, C.A.; Rodríguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022, 23, 13–30. [Google Scholar] [CrossRef]
- van Galen, K.A.; Ter Horst, K.W.; Serlie, M.J. Serotonin, food intake, and obesity. Obes. Rev. 2021, 22, e13210. [Google Scholar] [CrossRef]
- Calapai, G.; Corica, F.; Corsonello, A.; Sautebin, L.; Di Rosa, M.; Campo, G.M.; Buemi, M.; Mauro, V.N.; Caputi, A.P. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J. Clin. Investig. 1999, 104, 975–982. [Google Scholar] [CrossRef]
- Marazziti, D.; Betti, L.; Baroni, S.; Palego, L.; Mucci, F.; Carpita, B.; Cremone, I.M.; Santini, F.; Fabbrini, L.; Pelosini, C.; et al. The complex interactions among serotonin, insulin, leptin, and glycolipid metabolic parameters in human obesity. CNS Spectr. 2022, 27, 99–108. [Google Scholar] [CrossRef]
- Reurean-Pintilei, D.; Pantea Stoian, A.; Potcovaru, C.-G.; Salmen, T.; Cinteză, D.; Stoica, R.-A.; Lazăr, S.; Timar, B. Skin Autofluorescence as a Potential Adjunctive Marker for Cardiovascular Risk Assessment in Type 2 Diabetes: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3889. [Google Scholar] [CrossRef]
- van Son, J.; Koekkoek, L.L.; La Fleur, S.E.; Serlie, M.J.; Nieuwdorp, M. The Role of the Gut Microbiota in the Gut-Brain Axis in Obesity: Mechanisms and Future Implications. Int. J. Mol. Sci. 2021, 22, 2993. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).