Abstract
Like phytohormones, peptide hormones participate in many cellular processes, participate in intercellular communications, and are involved in signal transmission. The system of intercellular communications based on peptide–receptor interactions plays a critical role in the development and functioning of plants. One of the most important molecules are reactive oxygen species (ROS). ROS participate in signaling processes and intercellular communications, including the development of the root system. ROS are recognized as active regulators of cell division and differentiation, which depend on the oxidation–reduction balance. The stem cell niche and the size of the root meristem are maintained by the intercellular interactions and signaling networks of peptide hormone and ROS. Therefore, peptides and ROS can interact with each other both directly and indirectly and function as regulators of cellular processes. Peptides and ROS regulate cell division and stem cell differentiation through a negative feedback mechanism. In this review, we focused on the molecular mechanisms regulating the development of the main root, lateral roots, and nodules, in which peptides and ROS participate.
1. Introduction
The root is the main organ responsible for the absorption of nutrients. In a longitudinal section through the apical part of the root, four characteristic zones are distinguished: the root cap, the apical meristem, the elongation zone, the zone of cell differentiation, and the formation of permanent tissues (Figure 1A).
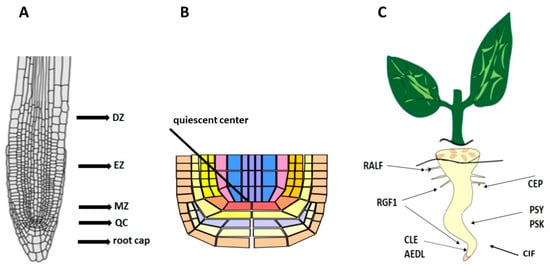
Figure 1.
(A) Root zones. QC—quiescent center, MZ—meristematic zone, EZ—elongation zone, DZ—differential zone. (B) Quiescent center. (C) Localization of peptides in root zones.
The specialization of the root as an organ of absorption, accumulation, conduction of substances, and anchoring of the plant in the substrate is associated, first of all, with an increase in the surface in direct contact with the soil. This is achieved by its branching, increasing the power of the root system, and the formation of many root hairs, which significantly increase the absorbent surface of the roots. During the development of the rudiment of the lateral root, a local growth of the meristem in thickness occurs. A further increase in its size is accompanied by the isolation of a group of small, actively dividing cells of the future apical meristem at the end of the root. Continuous growth of the root system is possible only in the presence of a pool of stem cells, which replenishes and restores the pool of meristem cells. A very important feature of the root apical meristem is that the initial cells themselves, under normal conditions, divide very rarely, forming a quiescent center (QC) [1], consisting of mitotically inactive stem cells that can replace root cells after their damage [2,3]. In most dicots, the initial cells are arranged in three layers. The derivatives of the upper layer subsequently form the central cylinder, the cells of the middle layer give rise to the primary cortex, and those of the lower layer give rise to the cells of the cap and rhizoderm. Root growth occurs due to controlled cell division in the meristematic zone. In the meristems, stem cells receive maintenance signals from QC cells [1]. Stem cell division results in the formation of rapidly dividing cells that migrate out of the meristem and then differentiate into specialized cell types or tissues [4]. After division, most cells increase in size in the elongation zone and mature in the differentiation zone. The size of these developmental zones is determined by internal and external signals. The root system architecture is determined by complex interactions between internal root development and external environmental signals. These feedback mechanisms are mediated through signaling molecules such as ROS, transcription factors, and secreted peptides [5].
Peptide hormones play key roles in many physiological processes by coordinating developmental and environmental signals between different cells (Figure 1C) [6,7,8]. Peptide hormones are recognized by their receptors, which transmit signals to downstream targets and interact with several pathways to fine-tune plant growth. Functional studies on the mechanisms of peptide action in roots are scarce. As numerous studies have shown, reactive oxygen species (ROS) are active participants in all cellular processes regulating plant development. In this review, we focus on recent advances in understanding the mechanisms of regulation of plant root development by peptide hormones, and their relationship with receptors and ROS.
2. ROS and Role of ROS in Root Development
ROS are a product of redox reactions that occur in all cells during plant life [9]. In plant cells, redox homeostasis is a normal state [10]. Under normal conditions, ROS production and levels are safe for normal cellular function, including proliferation, differentiation, signaling, and intercellular communication. The interplay between ROS and redox changes/regulation in cells is commonly referred to as redox biology and is thought to play an important role in ROS cellular signaling and metabolic regulation [11,12,13,14]. Since the membrane acts as a barrier to redox levels, each intracellular compartment can contain its own redox state, corresponding to its steady-state ROS levels, contributing to the formation of specific intracellular ROS signatures during abiotic stress [15]. The level of ROS is strictly controlled in space and time as a result of changes in the composition of ROS production and its detoxification [16]. At low levels in the cell, ROS act as positive signaling molecules, actively participating in many biologically important processes. At elevated concentrations, ROS can act as highly toxic molecules. The accumulation of ROS in a cell depends on many parameters. The complex process of plant adaptation includes a whole range of mechanisms of interaction between oxidants and antioxidants, capable of flexibly changing redox signals [17].
ROS are divided into two groups: ROS generated as a result of metabolic dysfunction (metabolic ROS) and ROS generated for signaling purposes as part of a signaling network in response to abiotic stress (signaling ROS). Metabolic ROS can counteract the effects of stress by directly altering the redox state of rate-limiting enzymes and controlling cellular metabolic fluxes, thereby altering various metabolic reactions [18]. In addition, ROS-induced redox modifications can affect transcription and translation by altering the functions of key regulatory proteins [11,12].
ROS metabolism is a very complex multicomponent system. Changing one of its elements may not lead to the desired effect due to the variety of enzyme isoforms. ROS metabolism can be influenced by environmental factors, such as bright light, ion imbalance, and damage. An increase in ROS levels is often observed under the influence of all these factors. It has been noted that disruption of the mitochondrial complex, which led to oxidative stress in the mitochondria, affected ROS metabolism in the apoplast [18]. ROS generation in mitochondria or chloroplasts during metabolic fluctuations can cause ROS production in the extracellular space.
Recent studies demonstrate the ever-increasing role of reactive oxygen species (ROS) as signaling molecules regulating development and various physiological processes in plants [19,20]. ROS include numerous small molecules such as singlet oxygen, as well as superoxide (O2•−), which oxidize lipids, proteins, and DNA. Hydrogen peroxide (H2O2), being a relatively stable molecule capable of penetrating cell membranes, is considered a key ROS involved in intercellular communication [21]. ROS interact with many other signaling molecules, which leads to a variety of cellular responses. Modern transcriptomic methods have identified important stress-sensitive signaling molecules such as mitogen-activated protein kinase (MAPK) and phosphatase, which play a role in ROS metabolism [22]. MAPK signaling networks that recognize stress stimuli through cell surface receptors and sensors are transduced [23]. Ca2+ ions are also actively involved in intracellular and extracellular signaling networks [24]. There is a two-way interaction between Ca2+ and ROS: Ca2+ is required for ROS production, and ROS, in turn, regulate cellular Ca2+ levels [25,26]. Plant cells exposed to stress factors transmit signals through redox-sensitive proteins through reversible oxidation–reduction reactions [27]. In addition, thiols, iron- and sulfur-containing compounds, and proteins are targets of ROS [28].
ROS are key regulators of subcellular, cellular, and systemic signals. They function in plants as an integral part of a variety of hormonal, physiological, and developmental processes, and play a critical role in defense and adaptive responses to various biotic and abiotic conditions [29]. ROS signals are integrated through intracellular networks and coordinate plant physiological responses [30]. ROS mediate a rapid, whole-plant systemic signal called the “ROS wave”, required to induce a state of systemic acquired acclimation or a systemic wound response. The authors show that the wound-induced systemic ROS surge in Arabidopsis thaliana is accompanied by a rapid systemic “redox surge”, which in turn can induce transcriptional, metabolic, and proteomic changes that lead to acclimation and/or systemic wound responses [31]. Intercellular communication and long-range signaling play an important role in plant responses to extreme environmental conditions. ROS is involved in organelle-to-organelle and cell-to-cell signaling. Signal propagation was accompanied by ROS accumulation in the extracellular spaces between cells and was inhibited by the suppression of ROS accumulation at sites distant from the initiation site [28,32,33].
Thus, ROS are key signaling molecules that allow cells to respond quickly to various stimuli. In plants, ROS play a critical role in recognizing abiotic and biotic stresses, integrating various environmental signals and activating stress response networks, thereby promoting the development of defense mechanisms and increasing plant resilience.
There is increasing evidence that redox regulation can significantly affect root development. Reactive oxygen species (ROS) act as key signaling molecules by interacting with antioxidants such as ascorbic acid (ASC) and glutathione (GSH) [34]. Studies have shown that ASC oxidase plays a role in root cell maintenance. Mutants of the rml1 gene, which is involved in the synthesis of GSH, an important molecule for cellular redox homeostasis and meristem maintenance, and other mutants have been widely used to demonstrate the roles of ROS, ASC, and GSH in root meristem function [35,36]. rml1 mutants block root cell division, which prevents the formation of an active meristem; exogenous GSH can correct the rml1-associated defect. Furthermore, impaired GSH function inhibits cell division during the G1 to S transition [36]. A mutant of UPB1, a bHLH transcription factor that regulates the transition from cell proliferation to differentiation in the elongation zone, exhibits a delay in the differentiation process [37]. These results highlight the importance of redox regulation in maintaining the root meristem structure. This is further supported by the fact that O2•− and H2O2 are unevenly distributed in roots, which affects their growth and differentiation [38]; O2•− and H2O2 have different functions and accumulate in the root tips, with the former mainly located in the meristematic zone and the latter in the differentiation zone, participating in growth restriction and root hair formation [38].
3. Glutathione and Role of Glutathione in Root Development
The tripeptide glutathione (γ-glutamylcysteinylglycine) is one of the most abundant low-molecular compounds in plant tissues. GSH is a unique peptide that forms a peptide bond between the amino group of cysteine and the carboxyl group of the side chain of glutamic acid. Since GSH performs many functions in cells, it is found in almost all cellular compartments: the cytosol, endoplasmic reticulum, vacuoles, and mitochondria [39]. GSH is a water-soluble antioxidant that is involved in the scavenging of reactive oxygen species via the glutathione–ascorbic acid cycle and acts as an electron donor for glutathione peroxidase (GPX). GSH is the primary form in which sulfur is stored, and reduced sulfur is transported over long distances. GSH is capable of binding to heavy metals. It acts as a precursor of phytochelatin, which is a polymer of glutathione [40,41]. In addition, GSH is involved in the process of detoxification of harmful substances [5], together with glutathione transferase (GST) [42], which catalyzes the formation of covalent bonds between the sulfur atom in the cysteine residue of glutathione and electrophilic compounds [43]. GST catalyzes the reduction of H2O2 due to GSH, resulting in the formation of the oxidized form GSSG [44]. GSH is involved in signaling through the formation of disulfide bonds with various proteins. Changes in the antioxidant activity of glutathione are accompanied by changes in its participation in cellular signaling pathways and interaction with various glutathione-dependent enzymes [45].
GSH contains a cysteine thiol group and functions as a proton donor (Figure 2). As a result, GSH can undergo reversible oxidation, leading to the formation of glutathione disulfide (GSSG), which is then reduced by glutathione reductase (GR). Under control conditions, when there is no stress, the GSH/GSSG ratio is 20:1. However, in the presence of stress, this ratio changes. Comparison of the reduced and oxidized forms of glutathione (GSH/GSSG) allows us to assess the level of oxidative stress, which is one of the key parameters stabilizing the cell state. The oxidation–reduction potential depends on the concentration of GSH. Even if the GSH/GSSG ratio remains unchanged, but the GSH concentration increases, this will lead to a decrease in the potential [46]. A distinctive feature of GSH from other primary and secondary metabolites, which can also react with ROS, is the rapid reduction of its oxidized form [47]. GSH in the cell is contained in millimolar concentrations. Such high levels of GSH and the rapid rate of reduction of its oxidized form GSSG provide it with an indispensable role in the redox homeostasis of the cell. GSH in the cell is contained in millimolar concentrations. Such high levels of GSH and the rapid rate of reduction of its oxidized form GSSG provide it with an indispensable role in the redox homeostasis of the cell. The reduction of the oxidized form of GSSG is carried out by the enzyme glutathione reductase (GR) [48].
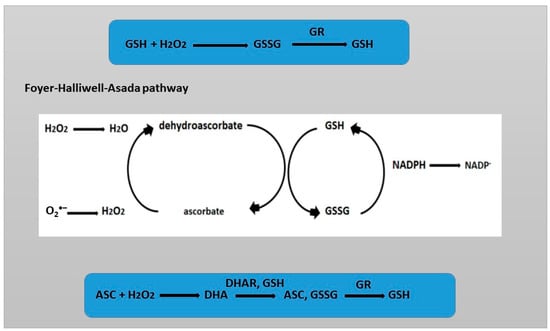
Figure 2.
Redox reactions of GSH and ASC. Foyer–Halliwell–Asads pathway.
Glutathione is able to protect proteins from denaturation as a result of the oxidation of cysteine residues during exposure to stressors (Figure 3). The protection of thiol groups in proteins occurs during the formation of a disulfide bond between GSH and the protein, which is temporary and is restored by GR. In addition, these short-lived disulfide bonds that arise between GSH and proteins can be a pair for the transfer of information [45].
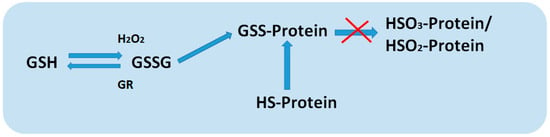
Figure 3.
Mechanism of protection of protein thiol groups from oxidation.
The central role of GSH in antioxidant defense is due to its ability to reduce another potent water-soluble antioxidant, ascorbic acid, through the ascorbate–glutathione cycle [49]. ROS such as superoxide ion and hydroxyl are able to directly oxidize ASC and GSH at a high rate. The importance of ASC and GSH is determined by the fact that there are specific enzymes that carry out catalytic conversions into oxidized, which are stable, and reduced forms [39]. The alternation of redox states of GSH helps maintain the redox balance of ASC or glutaredoxin/thioredoxin [39,49,50]. Together with its oxidized form (GSSG), glutathione maintains the redox balance in cellular compartments. In addition, the reduced form of GSH, as an electron donor, is capable of reducing dehydroascorbate as one of the stages of the ASC—GSH cycle [49].
Thus, ASC and GSH, interacting together, are part of a very complex antioxidant system in plants [51]. However, these two compounds are not interchangeable and can perform different functions [52,53]. By maintaining a pool of reduced ASC, GSH is involved in the regulation of the cell cycle [54,55,56,57] and cell elongation [58]. Work with GSH-deficient Arabidopsis mutants has shown that glutathione performs critical functions in embryo and meristem development [47,59].
As a result of various negative environmental influences, plant development processes slow down [60]. Using Arabidopsis mutants as an example, it has been shown that GSH is involved in the regulation of plant growth and adaptation to biotic and abiotic environmental conditions [61,62]. A significant increase in the redox potential leads to damage to compartments sensitive to redox effects, which is ultimately accompanied by growth arrest and/or even death [63]. It is known that a high oxidative status is maintained in the root dormancy center responsible for root cell elongation [64,65]. However, in addition to partial cell death caused by high redox potential, it also affects processes associated with the fate of stem cells [66]. Genetic studies using mutants deficient in GSH and its biosynthesis inhibitor, buthionine sulfoximine, have shown that GSH plays an important role in the development of the primary root and lateral root (LR) [59,67]. One possible mechanism of GSH action on the development of these roots is to suppress the expression of PIN-forming proteins, which in turn regulates auxin transport [59,67]. The PIN4 protein is located in the plasma membrane of the quiescent center and surrounding cells, which may contribute to a decrease in auxin concentration in this center [68,69]. The proper functioning of PIN proteins is necessary for the maintenance of the root terminal meristem [69] and the formation of the lateral root primordium [70].
Figure 4 shows the mechanisms of regulation of the formation and stabilization of the embryonic root primordium with the participation of PIN proteins and the transcription factor PLETHORA (PLT) at different stages of embryogenesis. At an early stage, PIN proteins inhibit PLT expression in the basal region of the embryo and initiate the formation of the root primordium. At a later stage of embryogenesis, PLT genes maintain PIN transcription and stabilize the distal stem cell niche. In postembryonic roots, PIN-mediated auxin transport stabilizes stem cells and regulates cell division in the meristem zone and cell expansion in the elongation zone. On the other hand, GSH is able to regulate auxin activity through inhibition of PIN protein synthesis. Thus, a feedback loop is formed that controls the auxin maximum and the stem cell niche and regulates the final cell size [70].
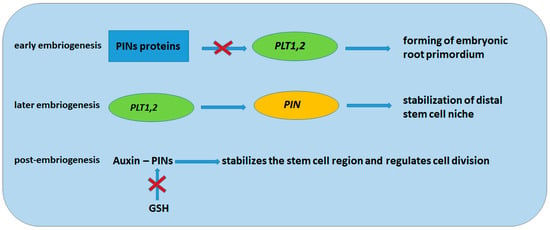
Figure 4.
Mechanisms of embryonic root primordium formation and stabilization.
While the positive role of GSH in cell proliferation is well established in other studies, a negative role of GSH in root growth has been found by some researchers [59,71,72,73]. Pasternak et al. [74] found that such involvement of GSH in root development suggested that this discrepancy is explained by the indirect effect of GSH on the pH of the growth medium: although GSH is a weakly acidic acid, it can affect the pH as a result of high GSH content. Thus, it has been suggested that the inhibition of root growth is associated with a more acidic environment that inhibits cell proliferation, highlighting the important role of GSH in this process [74,75]. ROS can oxidize GSH but are also able to increase its concentration by activating its biosynthesis [76,77]. GSH regulation is supported by ROS, and the active participation of GSH in cell proliferation and its interaction with auxin indicate a possible role of GSH in regulatory mechanisms [74].
4. Thiols and Role Thiols in Root Development
Changes in the oxidation–reduction status of enzymes (transcription proteins) are carried out mainly through the sulfo groups of the amino acid residues of cysteine and methionine, as well as through sulfated tyrosine residues [78].
Cysteine is the end product of sulfate assimilation, the process by which plants acquire sulfur. It acts as a major metabolite that provides sulfur for the synthesis of methionine, iron–sulfur clusters, vitamins such as thiamine and biotin, lipoic acid, coenzyme A, glutathione, and thiol-containing proteins [79]. Cysteine residues in macromolecules are highly susceptible to oxidative modifications associated with the formation of reactive oxygen species (Figure 5). These residues can undergo a three-step oxidation, first converting to cysteine sulfenic acid (Cys-SOH), which is reversible, and then to cysteine sulfinic acid (Cys-SO2H) and cysteine sulfonate (Cys-SO3H), which is irreversible (Figure 5) [80]. The formation of cystine (cysteine disulfide; CySS) probably occurs in plants via cysteine reductase [81]. It has also been proposed that in Arabidopsis, sulfhydride proteins may act as sulfinic acid reductases, reducing oxidized forms of cysteine [82,83]. Researchers have suggested [84,85] that molecules such as glutathione and enzymes such as glutathione reductase, thioredoxin reductase, and glutaredoxins may be involved in the reduction of CySS [86,87]. One group characterized the product as an oxidized protein with a Cys-SOH group that was glutathionized by reaction with GSH or an enzymatic catalyst. Cys residues can be reduced via reaction with GRX, which is highly dependent on GSH concentration and GR activity. The glutathionylation of proteins can be considered a post-translational regulatory mechanism that significantly affects the activity of various enzymes and transcription factors [88]. It may play an important role in the interaction of sulfated peptides and peptides containing cysteine residues with GSH. Such interactions may indicate the involvement of these peptides in redox balance and signaling [89]. Like cysteine, methionine can also undergo reactive oxygen species-induced oxidation to methionine sulfoxide (MetO), which can lead to changes in protein conformation and activity. The conversion of MetO back to methionine is accomplished by methionine sulfoxide reductase, an enzyme involved in the repair of oxidative damage [90,91,92].
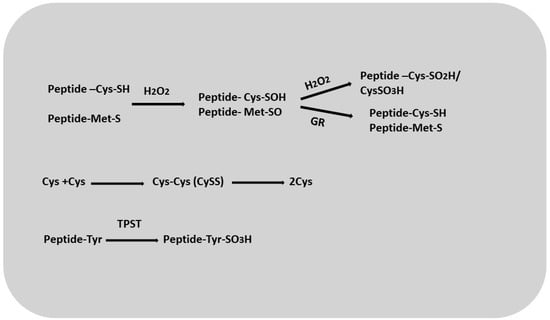
Figure 5.
Оxidation–reduction reactions of sulfate groups of proteins and peptides.
Tyrosine sulfation links peptide hormone synthesis to sulfate metabolism via a common intermediate, 3-phosphoadenosine-5-phosphosulfate (PAPS). The sulfate moiety provides stability to the peptide hormone secreted by the apoplast, and specificity for receptor recognition [93,94]. Tyrosine sulfation in plants is carried out by a unique sulfotransferase, tyrosyl protein sulfotransferase (TPST) [95]. Arabidopsis TPST functions as a single enzyme responsible for peptide sulfation, and its mutant has been shown to exhibit various pleiotropic phenotypes such as growth retardation, abnormal pollen tube and grain development, reduced primary root length accompanied by increased lateral density, aberrant vascular development, and impaired casparian stripe formation [96,97,98,99,100,101,102]. These phenotypic changes are closely associated with four peptide families: RGF, PSK, PSY, and CIF [103]. In this review, we will mainly focus on the functions of these four sulfated peptide families, namely PSK, PSY, RGF, and CIF, which have been extensively studied in plants.
5. Peptide RGF1 and the Role of RGF1 in Root Development
Plant root growth is maintained by continuous cell division in the meristematic tissue. In the root meristematic tissue, the quiescent center (QC) cells (four in Arabidopsis thaliana), which are inactive in division, together with the surrounding stem cells, constitute the root stem cell niche and are the source of cells for all root tissues [1,104]. The root apical meristem, a region of the root tip composed of undifferentiated cells, gives rise to various root cell types, thereby playing a vital role in regulating root patterning and adaptation to environmental stimuli [93]. RGF peptides positively influence the maintenance of the root stem cell niche. The root stem cell niche and the size of the root meristematic tissue are maintained by cell–cell interactions and signaling networks of the peptide hormone root meristematic growth factor 1 (RGF1) [105]. RGFs are required for the maintenance of the root stem cell niche and promote the proliferation of transitional cells in Arabidopsis thaliana. RGF1 determines the expression levels and patterns of multiple stem cell transcription factors, primarily at the posttranscriptional level [105]. RGF1, the best-studied peptide of the RGF family, is a secreted sulfated 13-membered peptide [105]. RGF1 is maturated from a ≈ 100-membered precursor peptide through post-translational sulfation and proteolytic processing. RGF1 is specifically expressed primarily in the stem cell region and the innermost layer of the central columella, and it diffuses into the meristematic region via the apoplast. Four additional RGF family members are also expressed in the root stem cell region, suggesting a redundant role for them in the root apical meristem.
Many RGF genes are expressed in roots, but some members are also expressed in shoots, reproductive organs, and even in specific cells or cell types [106,107], suggesting that RGF functions have diversified throughout plant evolution. RGF signaling targets PLETHORA (PLT) transcription factors, which are expressed in the root meristem and mediate the patterning of the root stem cell niche [105,108]. PLT proteins exhibit a gradient distribution that is highest in the stem cell region, with high PLT levels supporting stem cells, intermediate levels promoting cell division and enhancing transit, and low levels promoting cellular differentiation [109]. Since PLT1 and PLT2’s protein expression and gradient sizes are significantly reduced in tpst-1 roots but restored by RGF peptide application, it is an important regulator of proximal meristematic tissue activity via the PLT pathway [105].
The mechanism of regulation of root meristem development is carried out via the RGF1-RGFR receptor signaling pathway, which belongs to the LRR-RK family [93,110]. Five RGF peptide receptors are known [93,110,111]. Since different RGFR receptors, by binding to RGF, regulate different downstream targets involved in plant growth and development, a mutant of mitogen-activated protein kinase MKK was used to complement the RGF1-RGFR signaling cascade, which is responsible for the maintenance of the root meristem. MKK functions as a downstream signaling element in the RGF1-RGFR system and regulates the expression of PLT1 and PLT2 genes, which is important for the development of the root meristem. Studies [112,113] have shown that the RGF1-RGFR signaling pathway affects the distribution of reactive oxygen species (ROS) in the root growth region of Arabidopsis thaliana and contributes to an increase in the stability of the PLT2 protein [114]. Genetic data also indicate that RGF1 receptor signaling is required to maintain an appropriate PLT protein gradient in the proximal meristem [105]. The use of rgf mutants suggests that RGF stabilizes PLT protein at the protein level rather than at the transcriptional level, possibly suggesting that it determines PLT localization in root meristematic tissues [105]. In addition, RGF1 receptors affect the stability of PLT2 proteins. Transcriptome analysis revealed significant changes in the expression of reactive oxygen species-related genes, suggesting that RGF1 may signal through reactive oxygen species intermediates and regulate the size of the mitotic zone. After treatment with exogenous RGF1, O2•– levels increased in the meristematic zone, while H2O2 content decreased in the elongation and differentiation zone [114]. Furthermore, RGF1 treatment affects meristem size: the RGF1 receptor pathway regulates the distribution of reactive oxygen species along the root development zone in Arabidopsis. Whitford et al. [115] showed that RGF1 signaling and reactive oxygen species redistribution along the root zone are involved in this process. They also identified a novel transcription factor, named RGF1-INDUCED TRANSCRIPTION FACTOR 1 (RITF1), which is putatively involved in reactive oxygen species redistribution. Changes in their distribution contribute to an increase in the stability of PLETHORA2 (PLT2), a key regulator of root stem cells. Thus, there is a link between the RGF1 signaling cascade and reactive oxygen species signaling in the regulation of PLT2 protein stability, which changes depending on the O2•− and H2O2 levels under the influence of RGF1, as well as on the size of the root meristem [116].
Based on the data, the following scheme for the involvement of RGFs in the maintenance of the root stem cell niche was proposed (Figure 6). RGFs are secreted peptides and mature through proteolytic processing of their precursor proteins and TPST-catalyzed post-translational tyrosine sulfation, which are required for the biological activity of RGFs [109]. We found that TPST expression is positively regulated by auxin and that mutations in this gene affect the auxin distribution by reducing the expression levels of several PIN genes and auxin biosynthesis genes in the stem cell niche region. TPST-dependent RGF sulfation leads to an interaction between auxin and PLT in regulating root stem cell niche maintenance.
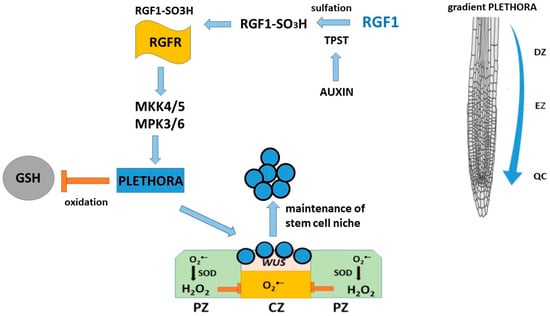
Figure 6.
A positive regulation of stem cell niche maintenance by RGF1.
In addition to their activity in the root apical meristem, RGFs control root gravitropism [115,117], lateral root development [117,118], and root hair development [106], and are sensitive to phosphate deprivation [118]. The effect of RGFs on plant gravitropism was demonstrated using synthetic peptides [105]. Gravistimulation assays showed that these peptides influenced the root gravitropic response. Moreover, plant roots treated with synthetic RGF peptides showed significantly increased RAM compared to controls.
6. Peptide PSK and PSK’s Role in Root Development
Phytosulfokine-α (PSK) is a secreted pentapeptide containing two sulfated tyrosines [119]. Transfer of the sulfate group from PAPS to the tyrosine side chain alters the chemical properties of the peptide by increasing hydrophilic binding. This alters the affinity for proteins that bind sulfated peptides and act as peptide receptors. The binding of PSK to its receptor PSKR1 (PSK RECEPTOR1) occurs primarily through hydrogen bonding, whereby the sulfate moieties of PSK interact with specific residues in the perception domain of PSKR1 [94]. Therefore, it is not surprising that full biological activity of PSK requires both amino acid scaffolding [120,121] and sulfation of tyrosine residues [119,122,123,124].
PSK has been shown to have several physiological functions. It stimulates somatic embryogenesis [125] and promotes adventitious root formation in cucumber hypocotyls [126]. In addition, PSK promotes the differentiation of Zinnia elegans mesophyll cells into tracheal elements in cell cultures [127] by repressing stress response genes early in the transdifferentiation process [128]. In tpst-1 knockout mutants and PSK receptor null mutants, the number of metaxylem cells is increased, suggesting a role for PSK in controlling xylem cell fate [100]. In plant reproduction, PSK promotes pollen germination [129] and pollen tube elongation [97], and it acts as a short-range signal that helps guide the pollen tube from the transmission tract along the funiculus to the embryo sac. Plants lacking PSK receptors produce fewer seeds [97]. Using synthetic PSK and the genetic knockout of PSK receptors, PSK signaling has been shown to promote hypocotyl elongation [119], leaf growth [130], and root growth [131], as well as cotton fiber cell elongation. PSK is involved in QC cell division and the distal regulation of stem cell differentiation [3,132].
Tyrosine sulfation is required for activation of the PSK peptide. Sulfur, which is taken up by the plant as inorganic sulfate, is activated by ATP sulfurylase (ATPS, encoded by AtATPS1-4 in Arabidopsis) to adenosine-5-phosphosulfate (APS) (Figure 7). ATS kinases located in plastids (AtAPK1/2/4) or in the cytosol (APK3) phosphorylate APS to 3-phosphoadenosine-5-phosphosulfate (PAPS). The fact that none of the apk single mutants have a phenotype, whereas the apk1 and apk2 double mutants have a dwarf phenotype [133], suggests that APK3 and APK4 contribute only marginally to PAPS synthesis.
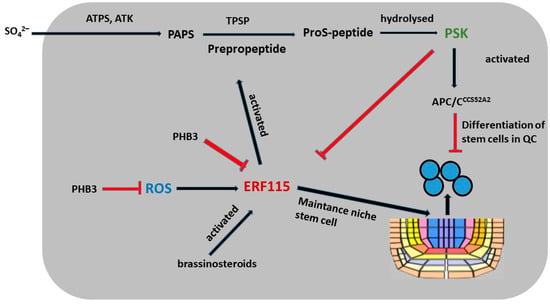
Figure 7.
Positive regulation of stem cell niche maintenance by PSK.
The Arabidopsis PSK peptide receptors (PSKRs) belong to the leucine-rich repeat receptor kinase family LRR-RK. This kinase consists of an N-terminal hydrophobic signal sequence, an extracellular repeat sequence containing 21 leucine-rich repeats (LRRs), a 36-amino acid island domain that serves as a binding site for the PSK peptide, a transmembrane domain, and a cytoplasmic kinase domain [134,135]. The kinase domain of PSKR1 is the catalytic center of guanylate cyclase and contributes to both in vivo and in vitro activity [136].
Further studies indicate that the PSK peptide may interact with other hormones to regulate plant growth. In Arabidopsis, PSK5 activates the APC/CCCS52A2 complex in the QC, resulting in decreased levels of ERF115 transcription factors and a reduced expression of target genes, thereby inhibiting QC cell division in roots [3]. In contrast, elevated brassinosteroid (BR) concentrations and higher temperatures promote increased ERF115 expression, which in turn leads to increased PSK5 transcription and mitotic cell cycle activation (Figure 7) [3]. The overexpression of PSK4 enhances the expression of elongation-encoding genes involved in cell wall weakening but it reduces fertility levels [137]. Arabidopsis prohibitin (PHB3) plays a key role in maintaining stem cell identity by restricting the expression of ethylene response factors (ERF109, ERF114, and ERF115), which also respond to ROS in roots and have been shown to have important functions (Figure 7) [132]. PHB3 mutants exhibit increased ROS accumulation in association with increased NADH dehydrogenase activity, suggesting that PHB3 is involved in the regulation of ROS homeostasis [132]. Studies have shown that ERF115 plays a role in BR signaling by controlling cell division in the QC zone through the transcriptional activation of PSK5 [3]. Furthermore, PSK2 and PSK5 have been identified as direct targets of ERF109 and ERF114. These data indicate that ERF109, ERF114, and ERF115 participate in PSK-mediated signaling regulated by PHB3 and control the maintenance of the stem cell niche [132].
7. RALF and Role of RALF in Root Development
In contrast to the RGF, PSK, PSY, and CIF peptides, the RALF (Rapid ALkalinization Factor) family of peptides belongs to the Cys-rich peptides that do not require post-translational sulfation. The RALF1 peptide was first identified in tobacco cell culture [138]. RALF is a peptide with a molecular weight of about 5 kDa, a peptide containing four cysteine residues that form two disulfide bridges between the Cys-18 and -28 and Cys-41 and -47 residues, which are important for the organization of the RALF structure [138]. The RALF family, consisting of more than 30 peptides, has a high homology of N-terminal amino acids in all plants [139]. RALF peptides were found to be factors that cause rapid alkalization of the environment [138]. They play important roles in various processes such as root growth and development [140,141,142], root nodule formation [143], fertilization [144,145], fruit ripening [146], and plant–pathogen interactions [147,148]. RALF has been identified in tobacco leaves as a cysteine-rich polypeptide that also causes an increase in pH [149]. Overexpression of AtRALF1 and AtRALF23 results in the shortening and bushiness of Arabidopsis plants [150,151]. AtRALF1 is mainly expressed in roots, and knockout plants of this gene exhibit increased root length, a higher number of lateral roots, hypocotyl, and cell elongation [141,142,152]. The RALF34 peptide from Arabidopsis (AtRALF34) has been proposed to be involved in the regulatory network controlling lateral root formation [141]. AtRALF34, which is regulated by auxin and probably ethylene, is expressed prior to the first asymmetric division of pericycle cells at the xylem pole [141] and before the expression of AtGATA23, the earliest known marker of lateral root initiation that is responsible for founder cell identity [153]. In Arabidopsis, RALF34 acts as a negative regulator of lateral root initiation, probably preventing it from existing near primordia [141], acting extracellularly and autonomously, similarly to CLE peptides [154,155]. Signaling involving RALF peptides and their receptors involves many different signaling pathways and molecules [8,156,157]. However, the role of each molecular component in the RALF signaling pathway is not yet fully understood.
The initial discovery of RALF proteins showed that one of its important functions is the suppression of root elongation [138]. Overexpression of AtRALF1, which is considered the best-studied peptide in the RALF family, was found to result in bushy, semi-dwarf plants with small leaves, short roots, and a reduced number of lateral roots and small cells in the roots [140,158]. This fact was confirmed using mutants with a reduced expression or knockout of the AtRALF1 gene, which showed the opposite phenotype: plants with long roots and long hypocotyls, an increased number of lateral roots, and large root cells [158].
The RALF1 peptide is recognized by the FERONIA receptor (FER) (Figure 8), which is a member of the Catharanthus roseus RLK1-like (CrRLK1L) family. FER was first discovered as a regulator of interactions between male and female gametes during fertilization [159,160,161]. Binding of RALF1 to FER was confirmed using various methods, including immunoprecipitation, in vitro binding assays, and phosphoproteomic studies [159]. However, the fer-4 mutant does not exhibit complete insensitivity to high concentrations of RALF peptides, suggesting that RALF1 may interact with other receptors. Interestingly, PSY1 and RALF peptides exert opposing effects on plant growth, positively and negatively regulating membrane AHA2, respectively [159,162].
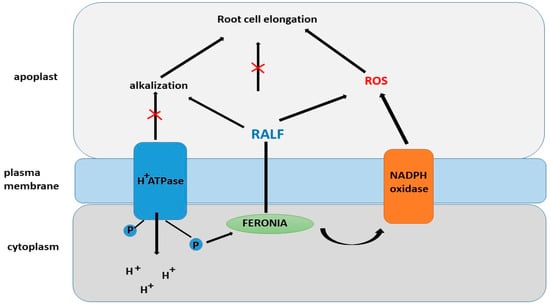
Figure 8.
Regulation of root cell elongation by RALF and ROS.
FER is also regulated at the transcriptional or post-translational level by many intrinsic and extrinsic factors. FER is induced by BR [163] and ethylene [164] treatment. At the post-translational level, FER is modified by several factors. For example, both RALF and ABA treatment promote FER phosphorylation and activation [165].
One of the mechanisms explaining the role of FER in root hair development is its regulation through RHO GTPase (RAC/ROP) and NADPH oxidase, which leads to an accumulation of reactive oxygen species (ROS) due to the interaction with the guanine nucleotide exchange factor RHO GTPase (ROPGEF) [166,167,168,169]. RAC/ROP, acting as an antiviral agent, promotes the activation of NADPH oxidase, which in turn initiates the generation of ROS and ROS-dependent processes such as root hair development [166,170,171]. FER interacts with the GTPase ROP2 to control ROS accumulation and auxin-dependent root hair growth. It is suggested that other FER-related factors may also be involved in this process [171]. Studies indicate that RALF peptides exert effects on hormonal regulation [166]. These peptides have been shown to be involved in modifying the dynamics of jasmonic acid, ethylene, abscisic acid, and brassinolides in plants via the receptor-like kinase FER [172].
Another mechanism of regulation of root hair development by the RALF peptide was discussed in a study [173]. RALF negatively regulates root hair formation by forming a negative feedback loop with RSL4 [174]. Numerous studies have shown that RSL4 controls the expression of hundreds of genes. Taken together, these properties of RSL4 allow us to identify it as a master regulator of RH growth and, consequently, the final cell size. It is known that the phytohormone auxin is a key regulator of RH growth and induces cell growth in situ. It was found that auxin increases RSL4 expression several-fold [175].
As mentioned above, RALF peptides are rapid alkalinizing factors [138]. Not all RALF family members have been found to have alkalinizing activity [176,177,178]. However, alkalinization of the extracellular environment or apoplast by AtRALF may be a prerequisite for binding to their receptors, as a possible dependence of binding on pH has been shown [179].
Stegmann et al. [177] demonstrated that several AtRALF proteins are involved in regulating intracellular ROS levels. Their study demonstrated a decrease in the relative levels of NADH dehydrogenase, complex I of the respiratory chain, and succinyl-CoA synthase, the enzyme that converts succinyl-CoA to succinate. These results indicate that RALF plays an important role in the functioning of the respiratory chain. In addition, RALF negatively affects the activity of complex III. A decrease in electron flow throughout the respiratory chain leads to a decrease in the rate of superoxide formation [150,151]. Roots with increased RALF expression also showed a decrease in H2O2 levels compared to controls. This could be explained by the inhibition of electron transfer in the respiratory chain, since superoxide anions are converted to H2O2 by superoxide dismutase. Thus, it has been suggested that RALF is involved in the regulation of reactive oxygen species homeostasis by initiating the controlled production of hydroxyl radicals in root cells and possibly interacting with intracellular signaling. This suggestion is consistent with the work of Song et al. [180], who showed that mutations in the FER gene, the RALF receptor in Arabidopsis thaliana, are associated with decreased ROS production. It has also been suggested that RALF may have a direct effect on ROS production [181].
8. Peptides CLE and Role CLE in Root Development
In contrast to sulfated peptides (RGF, PSK, PSY, and CIF), CLE CLAVATA3/ESR-related peptides negatively regulate root development [182]. The largest group of peptides identified to date is thought to be the CLE, named after the CLAVATA/embryonic region-related gene family [182]. The Arabidopsis genome contains more than 40 CLE genes. CLE genes have been found to be expressed in almost all tissues, indicating their broad biological functions [183,184]. All CLE peptides have been shown to be formed by the hydrolytic processing of larger precursor proteins [182]. The precursors of CLE proteins have a signal peptide at the N-terminus and a conserved 14-amino acid motif at the C-terminus. After hydrolysis of the precursor and post-translational modifications, the mature CLE peptide is formed [185]. The CLE peptide also undergoes post-translational modifications, including proline hydroxylation, hydroxyproline arabinosylation, and tyrosine sulfation [186,187]. It is suggested that arabinosylation causes conformational distortion of the peptide chain in a strictly defined direction, which significantly increases the affinity for the corresponding receptors [188].
CLE peptides play an important role in various cellular processes, such as the regulation of shoot and root apical meristem structure, stem cell activity, vascular tissue differentiation, lateral root and node formation, early embryogenesis, stomatitis development, and response to various external factors. The shoot apical meristem (SAM) utilizes a mechanism of direct communication between it and the overlying stem cells that ensures an optimal balance between stem cell maintenance and differentiation. In particular, the homeodomain transcription factor WUSCHEL (WUS) is expressed in the organizing center (OC) and translocates to stem cells, promoting their development and activation of the 13-amino acid peptide CLAVATA3 (CLV3), which is secreted by stem cells. CLV3 penetrates the plasma membrane and cytoplasmic bridges between adjacent cells. CLV3 interacts with the receptor kinase CLV1, the LRR receptor protein—CLV2 and the membrane pseudokinase CORYNE (CRN), which are recognized by several receptor kinases on the plasma membrane, resulting in a decrease in the level of WUS in the OC. The interaction between WUS and CLV3 leads to a decrease in the number of stem cells and cellular differentiation in the meristematic tissue of the shoot. A negative feedback loop is formed, which helps maintain the balance [186,187,188,189,190,191,192,193]. The CLE peptide negatively affects the development of the root meristem (RAM). Studies have shown that the use of synthetic CLE peptides stops the growth of the root meristem. Two functional groups of CLE peptides have been identified: type A peptides, which inhibit the development of the root meristem, and type B peptides, which do not affect its growth [194]. WUSCHEL RELATED HOMEOBOX 5 (WOX5), a homolog of WUS, is expressed in the RAM [195] and promotes stem cell maintenance in a non-autonomous manner, similarly to WUS [195]. The peptide CLAVATA3/ESR-RELATED 40 (CLE40), which is synthesized and secreted by progenitor cells, promotes CSC differentiation. The ARABIDOPSIS CRINKLY4 receptor-like kinase (ACR4) is actively involved in this process. Both CLE40 and ACR4 are expressed in the distal root meristem, and plants with mutations in cle40 and acr4 fail to restrict CSC fate to a single cell layer. Furthermore, acr4 mutants are resistant to exogenous addition of the CLE40 peptide (CLE40p), indicating that ACR4 must sense the CLE40 signal to limit CSC maintenance [196].Thus, CLE40 is required to ensure sufficient numbers of daughter stem cells and acts as a feedback signal to limit stem cell activity (Figure 9). Based on the similarity with shoot stem cell regulation, it has been proposed that WOX5 expression in the QC is a downstream target of the CLE40/ACR4 signaling module. This is supported by the fact that introduction of CLE40p into root meristematic tissue not only promotes DSC differentiation but also redistributes the WOX5 expression domain from the QC to the vascular primordium [196]. In addition to ACR4, CLV1 has also been shown to be involved in DSC regulation. This is because clv1 mutants (as well as acr4 and cle40 mutants) have more DSC layers compared to wild-type plants (Arabidopsis Columbia [Col-0] ecotype). Interestingly, CLV1 and ACR4 form distinct heteromeric complexes in the plasma membrane concentrated in the PZ.
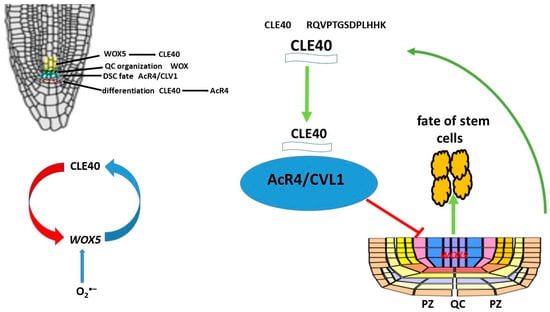
Figure 9.
CLE40 peptide forms a negative feedback loop CLE40-AcR4-WOX5 in RAM.
A negative feedback loop model of stem cell activity has been proposed, with the ACR4 and CLV1 receptor kinases located in the PZ restricting the intercellular movement of stemness factors between the QC and adjacent stem cells [197]. Activation occurs as a result of exposure to the secreted peptide CLE40. WOX5 protein has been shown to translocate from QC to DSC, and this translocation is critical for DSC maintenance [198,199]. Thus, CLE40 and WOX5 are negative regulators of DSC differentiation. Long-term exposure to high levels of CLE40 results in a loss of QC identity and the formation of new QCs. Changes in WOX5 expression and localization are secondary consequences, and loss of QC identity may trigger DSC differentiation. WOX5, like many other transcription factors, is mobile, allowing it to move between PZ-bound cells, but its mobility does not affect the formation or activation of the CLE40/CLV1/ACR4 complex. WOX5 functions within QCs to maintain DSC identity and regulates their maintenance by suppressing cell division. CLE40 antagonizes QC activity, and increased CLE40 signaling results in a loss of QC identity and the formation of new QCs in place of angioblasts. Local expressions of different receptor kinases, ACR4 and CLV1 in the distal stem cell niche and CLV2 and CRN in the proximal compartment, allows for specific interpretation of the CLE40 signal and serves as feedback from differentiated cells to fine-tune QC activity and location.
No data exist on the direct effect of ROS on the CLE40 peptide. However, it can be assumed that O2•− localized in QC regulates the activity of WOX5, the activation of which contributes to the suppression of QC cell division. On the other hand, WOX5 promotes the activation of the synthesis of the CLE40 peptide. Thus, O2•− is involved in the feedback loop CLE40-AcR4-WOX5 and regulation of the proliferation and differentiation processes of stem cells (Figure 9).
9. Peptides CEP and CEP’s Role in Root Development
In legumes, nitrogen-fixing root nodules and lateral roots (LRs) are established by the architecture of the root system. Competent cells responsible for the formation of root nodules and LRs are located close to the root apical meristem (RAM) [200]. N2-fixing nodules formed by rhizobia allow legumes to allocate nitrogen and enable them to grow in nitrogen-poor environments [201,202]. C-terminal peptides (CEPs) control essential functions of root development [203,204,205,206]. CEP peptides are signaling molecules that positively regulate nodule formation and negatively regulate LR occurrence in some legumes [205,206]. The MtCEP1 gene of Medicago truncatula has been shown to be induced under low-nitrogen conditions and is expressed during nodule formation and in LRs [205]. The MtCEP1 peptide is a mixture of 15-member hydroxylated peptides. The level of nodule and LR formation is strongly dependent on the hydroxylation pattern of the MtCEP1 peptides [205]. The mechanisms by which CEP peptides regulate both root nodule and LR formation are unknown, but early developmental stages of LR and nodules may share developmental genes [207,208] and morphological features such as reactivation of cortical, pericyclic, and endodermal cell divisions [200,205,206,209].
Legumes have developed mechanisms that control the simultaneous development, emergence, and growth of rhizoids and lateral roots. The formation of lateral roots depends on internal developmental factors and external environmental factors such as nitrogen availability. Previous studies have shown that the MtCEP1 peptide and its receptor MtCRA2 play unexpectedly important roles in regulating both LR and nodule formation [204,205,206,210]. Lateral root formation depends on intrinsic developmental factors and extrinsic environmental factors such as nitrogen availability. Previous studies have shown that the MtCEP1 peptide and its receptor MtCRA2 play important roles in regulating lateral roots and nodes [204,205,206]. A common feature of the development of LRs and concretions in M. truncatula is that these lateral organs arise from root cells located close to the RAM (Figure 10). MtCEP1 affects the development of legumes and concretions, but not the growth of the primary root. Therefore, this region of the root, located near the working memory, is particularly sensitive to MtCEP1 peptides in M. truncatula [205]. The possible involvement of MtCRA2 and its descending pathways in the regulation of root nodules and lateral root development, and hence root structure, by MtCEP1 peptides has not been fully studied, but MtCEP1 and MtCRA2 are expressed near the root apex, suggesting the possibility of direct interaction between them [205,210]. MtCRA2 was also found to be expressed in the shoot. This is a prerequisite for its involvement in the systemic control of the root [210].
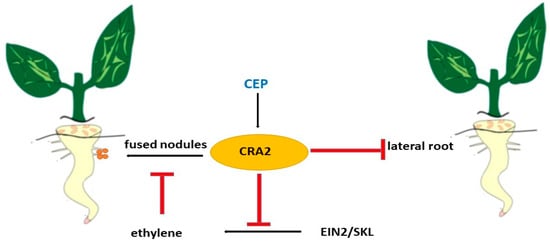
Figure 10.
Regulation of lateral roots and fused nodules by CEP peptide.
Ethylene in the M. truncatula node, as shown in the ethylene-insensitive mutant MtEIN2-deficient sickle ski [208,211], is known to play a negative role in nodule formation. In contrast, the use of ethylene synthesis inhibitors such as aminoethoxyvinylglycine promotes the formation of protostigma columns and fused nodes [212] and increases the number of infection threads and nodes [209,212]. In contrast, the ethylene precursor 1-aminocyclopropane-1-carboxylic acid reduces nodule numbers and at high concentrations, suppresses calcium oscillations, and inhibits nodule formation [211,213]. Recently, Mtein2/skl mutants were generated through comprehensive transcriptome profiling revealed a complex negative regulation of MtEIN2-dependent NF-mediated responses [208]. Since MtCEP1 peptide treatment resulted in increased nodulation over a wider area of roots, it was suggested that MtCEP1 enhances the ability of roots to form nodules.
10. AEDL and AEDL’s Role in Root Development
The very low native concentration of peptide hormones complicates their isolation and proteomic identification. Identification of their biological functions and establishment of their place and role in the mechanism of cascade signals is also difficult. These problems can be solved using synthetic molecules, both identical to native peptide hormones and modified or new in structure molecules.
The AEDL peptide, like the CLE40 peptide that negatively regulates WUS expression, is proposed to be involved in stem cell fate determination by forming a negative feedback loop with the WUS transcription factor expressed in QC cells (Figure 11). It has been previously shown that FITC-AEDL does not penetrate the meristem zone and suppresses WUS activity [214,215,216]; loss or impairment of WUS function leads to desiccation and the death of plant stem cells. In contrast, the AEDL peptide binds to the AcR4/CLV1 receptors located at the border of the stem cell niche, which prevents AEDL penetration and suppresses WUS activity. AEDL also stimulates GSH synthesis [216]. As is known, a high redox balance is necessary for normal stem cell homeostasis. In roots, there is a multidimensional gradient of H2O2 and O2•− (Figure 11). High concentrations of GSH disrupt the distribution of ROS, which leads to significant changes in plant development; it can be speculated that GSH tripeptides bind to the AcR4/CLV1 receptor, preventing GSH entry into the QC and neutralizing H2O2; an increase in H2O2 suppresses the expression of WUS in stem cells niche. It can also be speculated that the AEDL-AcR4/CLV1 complex prevents GSH entry into the QC and neutralizes H2O2.
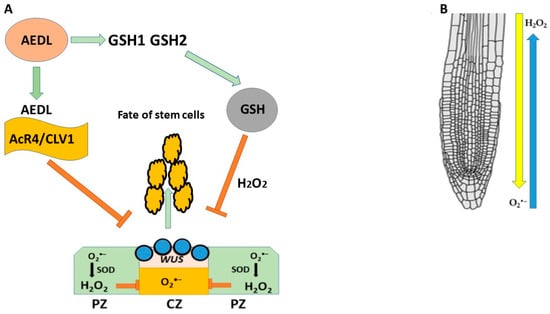
Figure 11.
(A) Negative feedback loop peptide AEDL-AcR4-WOX5 in RAM. (B) Opposite distribution of H2O2 and O2•− in root.
11. Conclusions and Prospects
Peptide hormones are widespread in all organs and tissues of plants. By binding to different receptors, they can participate in many cellular processes. The mechanisms of regulation of root system development in plants are very complex, and numerous cascades of compounds participate in them. In addition to peptide hormones, their receptors, numerous transcription factors, phytohormones, and metal ions participate in the regulation of root development. A high oxidative balance is necessary for the activation/inhibition of stem cell proliferation/differentiation processes, which are primarily controlled by super oxide dismutases and GSH. Thus, ROS are indirectly involved in the process of regulating root development. Peptide hormones containing Cys residues and sulfated peptides can directly interact with GSH. It was shown that the synthetic peptide AEDL, which does not have a sulfate group, can indirectly interact with GSH and affect the redox balance. Although there are currently no data on the influence of ROS on root development regulatory loops with peptides such as CLE and CEP, further studies may reveal a relationship between them.
Author Contributions
L.I.F. wrote, designed, and finalized the manuscript; N.V.K. wrote and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The reported study was supported by FGUM-2025-0003 of the Ministry of Science and Higher Education of the Russian Federation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACR4 | Arabidopsis CRINKLY4, receptor-like kinase |
| AEDL | tetrapeptide AlaAspGluLeu |
| AHA2 | H+-ATPhase |
| ASC | ascorbic acid |
| CEP | C-terminally encoded peptide |
| CIF | CASPIAN STRIP INTEGRITY FACTORS |
| CLE | CLAVATA/embryo surrounding region (ESR), peptide |
| CLV1 | CLAVATA, leucine repeat-rich receptor |
| CRA2 | receptor |
| CZ | central zone |
| DSC | distal stem cell |
| DZ | differential zone, |
| ERF | ETHYLENE RESPONSE FACTOS |
| EZ | elongation zone |
| FER | FERONIA receptor like-kinase |
| GSH | glutathione |
| LR | lateral root |
| LRR-RK | leucine-rich repeat receptor kinase |
| MZ | meristematic zone |
| NADP | nicotinamide adenine dinucleotide phosphate |
| PAPS | 3-phosphoadenosine-5-phosphosulfate |
| PIN | auxin transport proteins |
| PLT | PLETHORA, транскрипциoнный фактoр |
| PSK | phytosulfokine, peptide |
| PSKR1 | PSK RECEPTOR1 |
| PSY1 | sulfated tyrosine 1, peptide |
| PZ | peripheral zone |
| QC | quiescent center in RAM |
| RALF | rapid alkalinization factor, peptide |
| RAM | root apical meristem, |
| RGF | root meristem growth factor, peptide |
| RGFR1 | RGF receptor 1 |
| ROS | reactive oxygen species |
| SAM | shoot apical meristem, |
| TPST | tyrosyl protein sulfotransferase |
| WOX5 | WUSCHEL RELATED HOMEOBOX 5 |
| WUS | wuschel homeobox, transcription factor |
References
- Aichinger, E.; Kornet, N.; Friedrich, T.; Laux, T. Plant stem cell niches. Annu. Rev. Plant Biol. 2012, 63, 615–636. [Google Scholar] [PubMed]
- Choe, G.; Lee, J.-Y. Push–pull strategy in the regulation of postembryonic root development. Curr. Opin. Plant Biol. 2017, 35, 158–164. [Google Scholar]
- Heyman, J.; Cools, T.; Vandenbussche, F.; Heyndrickx, K.S.; Van Leene, J.; Vercauteren, I.; Vanderauwera, S.; Vandepoele, K.; De Jaeger, G.; Van Der Straeten, D.; et al. ERF115 Controls Root Quiescent Center Cell Division and Stem Cell Replenishment. Science 2013, 342, 860–863. [Google Scholar] [PubMed]
- Greb, T.; Lohmann, J.U. Plant stem cells. Curr. Biol. 2016, 26, R816–R821. [Google Scholar] [PubMed]
- Pierre-Jerome, E.; Drapek, C.; Benfey, P.N. Regulation of division and differentiation of plant stem cells. Annu. Rev. Cell Dev. Biol. 2018, 34, 289–310. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Yamada, М. The Roles of Peptide Hormones and Their Receptors during Plant Root Development. Genes 2020, 12, 22. [Google Scholar] [CrossRef]
- Selby, R.; Jones, D.S. Complex peptide hormone signaling in plant stem cells. Curr. Opin. Plant Biol. 2023, 75, 102442. [Google Scholar]
- Blackburn, M.R.; Miyoshi Haruta, M.; Moura, D.S. Twenty Years of Progress in Physiological and Biochemical Investigation of RALF Peptides. Plant Physiol. 2020, 182, 1657–1666. [Google Scholar]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the balance right: ROS homeostasis and redox signalling in fruit. Front. Plant Sci. 2019, 10, 1091. [Google Scholar]
- Paciolla, C.; Paradiso, A.; de Pinto, M. Cellular redox homeostasis as central modulator in plant stress response. In Redox State as a Central Regulator of Plant-Cell Stress Responses; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Berlin, Germany, 2016; pp. 1–23. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar]
- Foyer, C.H.; Noctor, G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J. Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J. Exp. Bot. 2015, 66, 2401–2414. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J. Thiol-based peroxidases and ascorbate peroxidases: Why plants rely on multiple peroxidase systems in the photosynthesizing chloroplast? Mol. Cells 2016, 39, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Zeng, J.; Dong, Z.; Wu, H.; Tian, Z.; Zhao, Z. Redox regulation of plant stem cell fate. EMBO J. 2017, 36, 2844–2855. [Google Scholar] [CrossRef]
- Podgórska, A.; Szal, B. The role of reactive oxygen species under ammonium nutrition. In Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants; Gupta, K.J., Igamberdiev, A.U., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 133–153. [Google Scholar]
- Gapper, C.; Dolan, L. Control of Plant Development by Reactive Oxygen Species. Plant Physiol. 2006, 141, 341–345. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Mangano, S.; Juárez, S.P.D.; Estevez, J.M. ROS Regulation of Polar Growth in Plant Cells. Plant Physiol. 2016, 171, 1593–1605. [Google Scholar] [CrossRef]
- Kawasaki, T.; Yamada, K.; Yoshimura, S.; Yamaguchi, K. Chitin receptor-mediated activation of MAP kinases and ROS production in rice and Arabidopsis. Plant Signal. Behav. 2017, 12, e1361076. [Google Scholar] [CrossRef]
- Taj, G.; Agarwal, P.; Grant, M.; Kumar, A. MAPK machinery in plants. Plant Signal. Behav. 2010, 5, 1370–1378. [Google Scholar] [PubMed]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium transport across plant membranes: Mechanisms and functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [PubMed]
- Gaupels, F.; Durner, J.; Kogel, K.-H. Production, amplification and systemic propagation of redox messengers in plants? The phloem can do it all! New Phytol. 2017, 214, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-G.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 2017, 90, 698–707. [Google Scholar]
- Spoel, S.H.; van Ooijen, G. Circadian Redox Signaling in Plant Immunity and Abiotic Stress. Antioxid. Redox Signal. 2014, 20, 3024–3039. [Google Scholar]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Breusegem, F.V. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar]
- Fichman, Y.; Miller, G.; Mittler, R. Whole-plant live imaging of reactive oxygen species. Mol. Plant 2019, 12, 1203–1210. [Google Scholar]
- Pitzschke, A.; Forzani, C.; Hirt, H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal. 2006, 8, 1757–1764. [Google Scholar]
- Fichman, Y.; Linda Rowland, L.; Melvin, J.; Oliver, M.J.; Mittler, R. ROS are evolutionary conserved cell-to-cell stress signals. Proc. Natl. Acad. Sci. USA 2023, 120, e2305496120. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar]
- Fichman, Y.; Mittler, R. Integration of electric, calcium, reactive oxygen species and hydraulic signals during rapid systemic signaling in plants. Plant J. 2021, 107, 7–20. [Google Scholar] [PubMed]
- De Tullio, M.C.; Jiang, K.; Feldman, L.J. Redox regulation of root apical meristem organization: Connecting root development to its environment. Plant Physiol. Biochem. 2010, 48, 328–336. [Google Scholar]
- Cobbett, C.S.; May, M.J.; Howden, R.; Rolls, B.D. The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J. 1998, 16, 73–78. [Google Scholar] [PubMed]
- Vernoux, T.; Wilson, R.C.; Seeley, K.A.; Reichheld, J.-P.; Muroy, S.; Brown, S.; Maughan, S.C.; Cobbett, C.S.; Van Montagu, M.; Inzé, D.; et al. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 Gene Defines a Glutathione-Dependent Pathway Involved in Initiation and Maintenance of Cell Division during Postembryonic Root Development. Plant Cell 2000, 12, 97–109. [Google Scholar] [PubMed]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional Regulation of ROS Controls Transition from Proliferation to Differentiation in the Root. Cell 2010, 143, 606–616. [Google Scholar]
- Dunand, C.; Crèvecoeur, M.; Penel, C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: Possible interaction with peroxidases. New Phytol. 2007, 174, 332–341. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxifaction and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar]
- Cazalé, A.C.; Clemens, S. Arabidopsis thaliana expresses a second functional phytochelatin synthase. FEBS Lett. 2001, 507, 215–219. [Google Scholar]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione metabolism in plants under stress: Beyond reactive oxygen species toxification. Metab 2021, 11, 641. [Google Scholar]
- Dixon, D.P.; Edwards, R. GlutathioneS-transferases. Arab. Book 2010, 8, e0131. [Google Scholar]
- Chi, Y.; Cheng, Y.; Vanitha, J.; Kumar, N.; Ramamoorthy, R.; Ramachandran, S.; Jiang, S.Y. Expansion mechanisms and functional divergence of the glutathione S-transferase family in Sorghum and other higher plants. DNA Res. 2011, 18, 1–16. [Google Scholar]
- Noctor, G.; Gomez, L.; Vanacker, H.; Foyer, C.H. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J. Exp. Bot. 2002, 53, 1283–1304. [Google Scholar]
- Cairns, N.G.; Pasternak, M.; Wachter, A.; Cobbett, C.S.; Meyer, A.J. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol. 2006, 141, 446–455. [Google Scholar]
- Noctor, G.; Arisi, A.; Jouanin, L.; Kunert, K.; Rennenberg, H.; Foyer, C. Glutathione: Biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot. 1998, 49, 623–647. [Google Scholar]
- Cosio, C.; Dunand, C. Specific functions of individual class III peroxidase genes. J. Exp. Bot. 2009, 60, 391–408. [Google Scholar]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar]
- Potters, G.; Horemans, N.; Jansen, M. The cellular redox state in plant stress biology—A charging concept. Plant Physiol. Biochem. 2010, 48, 292–300. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar]
- Potters, G.; De Gara, L.; Asard, H.; Horemans, N. Ascorbate and glutathione: Guardians of the cell cycle, partners in crime? Plant Physiol. Biochem. 2002, 40, 537–548. [Google Scholar]
- Noctor, G. Metabolic signalling in defence and stress: The central roles of soluble redox couples. Plant Cell Environ. 2006, 29, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Müller-Schüssele, S.J.; Wang, R.; Gütle, D.D.; Romer, J.; Rodriguez-Franco, M.; Scholz, M.; Buchert, F.; Lüth, V.M.; Kopriva, S.; Dörmann, P.; et al. Chloroplasts require glutathione reductase to balance reactive oxygen species and maintain efficient photosynthesis. Plant J. 2020, 103, 1140–1154. [Google Scholar] [CrossRef]
- Marty, L.; Bausewein, D.; Müller, C.; Bangash, S.A.K.; Moseler, A.; Schwarzländer, M.; Müller-Schüssele, S.J.; Zechmann, B.; Riondet, C.; Balk, J.; et al. Arabidopsis glutathione reductase 2 is indispensable in plastids, while mitochondrial glutathione is safeguarded by additional reduction and transport systems. New Phytol. 2019, 224, 1569–1584. [Google Scholar] [CrossRef] [PubMed]
- Kataya, A.M.R.; Reumann, S. Arabidopsis glutathione reductase 1 is dually targeted to peroxisomes and the cytosol. Plant Signal. Behav. 2010, 5, 171–175. [Google Scholar] [CrossRef]
- Ding, S.H.; Lu, Q.T.; Zhang, Y.; Yang, Z.P.; Wen, X.G.; Zhang, L.X.; Lu, C.M. Enhanced sensitivity to oxidative stress in transgenic tobacco plants with decreased glutathione reductase activity leads to a decrease in ascorbate pool and ascorbate redox state. Plant Mol. Biol. 2009, 69, 577–592. [Google Scholar] [CrossRef]
- Frottin, F.; Espagne, C.; Traverso, J.A.; Mauve, C.; Valot, B.; Lelarge-Trouverie, C.; Zivy, M.; Noctor, G.; Meinnel, T.; Giglione, C. Cotranslational proteolysis dominates glutathione homeostasis to support proper growth and development. Plant Cell 2009, 21, 3296–3314. [Google Scholar] [CrossRef]
- Bashandy, T.; Guilleminot, J.; Vernoux, T.; Caparros-Ruiz, D.; Ljung, K.; Meyer, Y.; Reichheld, J.P. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 2010, 22, 376–391. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Tausz, M.; Sircelj, H.; Grill, D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004, 55, 1955–1962. [Google Scholar] [CrossRef]
- Ogawa, K. Glutathione-associated regulation of plant growth and stress responses. Antioxid. Redox Signal. 2005, 7, 973–981. [Google Scholar] [PubMed]
- Kranner, I.; Birtic, S.; Anderson, K.M.; Pritchard, H.W. Glutathione half-cell reduction potential: A universal stress marker and modulator of programmed cell death. Free Radic. Biol. Med. 2006, 40, 2155–2165. [Google Scholar] [PubMed]
- Kranner, I.; Beckett, R.P.; Wornik, S.; Zorn, M.; Pfeifhofer, H.W. Revival of a resurrection plant correlates with its antioxidant status. Plant J. 2002, 31, 13–24. [Google Scholar]
- Enyedi, B.; Várnai, P.; Geiszt, M. Redox state of the endoplasmic reticulum is controlled by Ero1L-alpha and intraluminal calcium. Antioxid. Redox Signal. 2010, 13, 721–729. [Google Scholar]
- Jiang, K.; Feldman, L. Positioning of the auxin maximum affects the character of cells occupying the root stem cell niche. Plant Signal. Behav. 2010, 5, 202–204. [Google Scholar]
- Koprivova, A.; Mugford, S.; Kopriva, S. Arabidopsis root growth dependence on glutathione is linked to auxin transport. Plant Cell Rep. 2010, 29, 1157–1167. [Google Scholar]
- Friml, J.; Benkova, E.; Blilou, I.; Wisniewska, J.; Hamann, T.; Ljung, K.; Woody, S.; Sandberg, G.; Scheres, B.; Jurgens, G.; et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 2002, 108, 661–673. [Google Scholar]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, Efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar]
- Espunya, M.C.; Díaz, M.; Moreno-Romero, J.; Martínez, M.C. Modification of intracellular levels of glutathione-dependent formaldehyde dehydrogenase alters glutathione homeostasis and root development. Plant Cell Environ. 2006, 29, 1002–1011. [Google Scholar]
- Nongmaithem, S.; Ponukumatla, R.; Sreelakshmi, Y.; Frasse, P.; Bouzayen, M.; Sharma, R. Enhanced polar auxin transport in tomato polycotyledon mutant seems to be related to glutathione levels. J. Plant Growth Regul. 2020, 40, 761–773. [Google Scholar] [CrossRef]
- Pasternak, T.; Palme, K.; Paponov, I.A. Glutathione Enhances Auxin Sensitivity in Arabidopsis Roots. Biomolecules 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fernandez, R.; Fricker, M.; Corben, L.B.; White, N.S.; Sheard, N.; Leaver, C.J.; Van Montagu, M.; Inzé, D.; May, M.J. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proc. Natl. Acad. Sci. USA 1997, 94, 2745–2750. [Google Scholar] [PubMed]
- Kocsy, G.; Szalai, G.; Galiba, G. Induction of glutathione synthesis and glutathione reductase activity by abiotic stresses in maize and wheat. Sci. World J. 2002, 2, 1726–1732. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Blumwald, E. Salinity-induced glutathione synthesis in Brassica napus. Planta 2002, 214, 965–969. [Google Scholar]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R.O.N. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar]
- Hell, R.; Wirtz, M. Molecular biology, biochemistry and cellular physiology of cysteine metabolism in Arabidopsis thaliana. Arab. Book 2011, 9, e0154. [Google Scholar]
- Bashir, H.; Ahmad, J.; Bagheri, R.; Nauman, M.; Qureshi, M.I. Limited sulfur resource forces Arabidopsis thaliana of shift towards non-sulfur tolerance under cadmium stress. Environ. Exp. Bot. 2013, 94, 19–32. [Google Scholar]
- Romano, A.H.; Nickerson, W.J. Cystine reductase of pea seeds and yeasts. J. Biol. Chem. 1954, 208, 409–416. [Google Scholar]
- Rampitsch, C.; Bykova, N.V. Proteomics and plant disease: Advances in combating a major threat to the global food supply. Proteomics 2012, 12, 673–690. [Google Scholar]
- Chi, Y.H.; Kim, S.Y.; Jung, I.J.; Shin, M.R.; Jung, Y.J.; Park, J.H.; Lee, E.S.; Maibam, P.; Kim, K.S.; Park, J.H.; et al. Dual functions of Arabidopsis sulfiredoxin: Acting as a redox-dependent sulfinic acid reductase and as a redox-independent nuclease enzyme. FEBS Lett. 2012, 586, 3493–3499. [Google Scholar]
- Olm, E.; Fernandes, A.P.; Hebert, C.; Rundlöf, A.K.; Larsen, E.H.; Danielsson, O.; Björnstedt, M. Extracellular thiol-assisted selenium uptake dependent on the x(c)–cystine transporter explains the cancer-specific cytotoxicity of selenite. Proc. Natl. Acad. Sci. USA 2009, 106, 11400–11405. [Google Scholar] [PubMed]
- Kumar, B.; Singla-Paree, S.L.; Sopory, S.K. Glutathione Homeostasis: Crucial for Abiotic Stress Tolerance in Plants. In Abiotic Stress Adaptation in Plants; Pareek, A., Sopory, S.K., Bohnert, H.J., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 263–282. [Google Scholar]
- Lemaire, S.D.; Guillon, B.; Maréchal, P.; Keryer, E.; Miginiac-Maslow, M.; Decottignies, P. New thioredoxin targets in the unicellular photosynthetic eukaryote Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2004, 101, 7475–7480. [Google Scholar] [PubMed]
- Zaffagnini, M.; Bedhomme, M.; Marchand, C.H.; Couturier, J.; Gao, X.H.; Rouh, N.; Trost, P.; Lemaire, S.D. Glutaredoxins12: Unique properties for redox signaling. Antioxid. Redox Signal. 2012, 16, 17–32. [Google Scholar] [PubMed]
- Dos Santos, C.V.; Cuiné, S.; Rouhier, N.; Rey, P. The Arabidopsis plastidic methionine sulfoxide reductase B proteins. Sequence and activity characteristics, comparison of the expression with plastidic methionine sulfoxide reductase A, and induction by photooxidative stress. Plant Physiol. 2005, 13, 909–922. [Google Scholar]
- Cabreiro, F.; Picot, C.R.; Friguet, B.; Petropoulos, I. Methionine sulfoxide reductases. Ann. N. Y. Acad. Sci. 2006, 1067, 37–44. [Google Scholar]
- Good, A.G.; Zaplachinski, S.T. The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus. Physiol. Plant. 1994, 90, 9–14. [Google Scholar]
- Gzik, A. Accumulation of proline and pattern of alpha-amino acids in sugar beet plants in response to osmotic, water and salt stress. Environ. Exp. Bot. 1996, 36, 29–38. [Google Scholar]
- Hacham, Y.; Matityahu, I.; Schuster, G.; Amir, R. Overexpression of mutated forms of aspartate kinase and cystathionine γ-synthase in tobacco leaves resulted in the high accumulation of methionine and threonine. Plant J. 2008, 54, 260–271. [Google Scholar]
- Song, W.; Liu, L.; Wang, J.; Wu, Z.; Zhang, Y.; Tang, J.; Lin, G.; Wang, Y.; Wen, X.; Li, W.; et al. Signature motif-guided identification of receptors for peptide hormones essential for root meristem growth. Cell Res. 2016, 26, 674–685. [Google Scholar]
- Wang, J.; Li, H.; Han, Z.; Zhang, H.; Wang, T.; Lin, G.; Chang, J.; Yang, W.; Chai, J. Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature 2015, 525, 265–268. [Google Scholar] [PubMed]
- Moore, K.L. The biology and enzymology of protein tyrosine O-sulfation. J. Biol. Chem. 2003, 278, 24243–24246. [Google Scholar] [PubMed]
- Komori, R.; Amano, Y.; Ogawa-Ohnishi, M.; Matsubayashi, Y. Identification of tyrosylprotein sulfotransferase in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 5067–15072. [Google Scholar]
- Stührwohldt, N.; Dahlke, R.I.; Kutschmar, A.; Peng, X.; Sun, M.X.; Sauter, M. Phytosulfokine peptide signaling controls pollen tube growth and funicular pollen tube guidance in Arabidopsis thaliana. Physiol. Plant. 2015, 153, 643–653. [Google Scholar]
- Doblas, V.G.; Smakowska-Luzan, E.; Fujita, S.; Alassimone, J.; Barberon, M.; Madalinski, M.; Belkhadir, Y.; Geldner, N. Root diffusion barrier control by a vasculature-derived peptide binding to the SGN3 receptor. Science 2017, 355, 280–284. [Google Scholar]
- Nakayama, T.; Shinohara, H.; Tanaka, M.; Baba, K.; Ogawa-Ohnishi, M.; Matsubayashi, Y. A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots. Science 2017, 355, 284–286. [Google Scholar]
- Holzwart, E.; Huerta, A.I.; Glöckner, N.; Garnelo Gómez, B.; Wanke, F.; Augustin, S.; Askani, J.C.; Schürholz, A.-K.; Harter, K.; Wolf, S. BRI1 controls vascular cell fate in the Arabidopsis root through RLP44 and phytosulfokine signaling. Proc. Natl. Acad. Sci. USA 2018, 115, 11838–11843. [Google Scholar]
- Kaufmann, C.; Nils Stührwohldt, N.; Sauter, M. Tyrosylprotein sulfotransferase-dependent and -independent regulation of root development and signaling by PSK LRR receptor kinases in Arabidopsis. J. Exp. Bot. 2021, 72, 5508–5521. [Google Scholar]
- Truskina, J.; Brück, S.; Stintzi, A.; Boeuf, S.; Doll, N.M.; Fujita, S.; Geldner, N.; Schaller, A.; Ingram, G.C. A peptide-mediated, multilateral molecular dialogue for the coordination of pollen wall formation. Proc. Natl. Acad. Sci. USA 2022, 119, 2201446119. [Google Scholar]
- Kaufmann, C.; Sauter, M. Sulfated plant peptide hormones. J. Exp. Bot. 2019, 70, 4267–4277. [Google Scholar]
- Scheres, B. Stem-cell niches: Nursery rhymes across kingdoms. Nat. Rev. Mol. Cell Biol. 2007, 8, 345–354. [Google Scholar] [PubMed]
- Matsuzaki, Y.; Ogawa-Ohnishi, M.; Mori, A.; Matsubayashi, Y. Secreted peptide signals required for maintenance of root stem cell niche in Arabidopsis. Science 2010, 329, 1065–1067. [Google Scholar] [PubMed]
- Fernandez, A.; Drozdzecki, A.; Hoogewijs, K.; Nguyen, A.; Beeckman, T.; Madder, A.; Hilson, P. Transcriptional and functional classification of the GOLVEN/ROOT GROWTH FACTOR/CLE-like signaling peptides reveals their role in lateral root and hair formation. Plant Physiol. 2013, 161, 954–970. [Google Scholar] [PubMed]
- Fernandez, A.; Hilson, P.; Beeckman, T. GOLVEN peptides as important regulatory signalling molecules of plant development. J. Exp. Bot. 2013, 64, 5263–5268. [Google Scholar]
- Zhou, W.; Wei, L.; Xu, J.; Zhai, Q.; Jiang, H.; Chen, R.; Chen, Q.; Sun, J.; Chu, J.; Zhu, L.; et al. Arabidopsis Tyrosylprotein sulfotransferase acts in the auxin/PLETHORA pathway in regulating postembryonic maintenance of the root stem cell niche. Plant Cell 2010, 22, 3692–3709. [Google Scholar]
- Galinha, C.; Hofhuis, H.; Luijten, M.; Willemsen, V.; Blilou, I.; Heidstra, R.; Scheres, B. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 2007, 449, 1053–1057. [Google Scholar]
- Shinohara, H.; Mori, A.; Yasue, N.; Sumida, K.; Matsubayashi, Y. Identification of three LRR-RKs involved in perception of root meristem growth factor in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 3897–3902. [Google Scholar]
- .Ou, Y.; Lu, X.; Zi, Q.; Xun, Q.; Zhang, J.; Wu, Y.; Shi, H.; Wei, Z.; Zhao, B.; Zhang, X.; et al. RGF1 INSENSITIVE 1 to 5, a group of LRR receptor-like kinases, are essential for the perception of root meristem growth factor 1 in Arabidopsis thaliana. Cell Res. 2016, 26, 686–698. [Google Scholar]
- Lu, X.; Shi, H.; Ou, Y.; Cui, Y.; Chang, J.; Peng, L.; Gou, X.; He, K.; Li, J. RGF1-RGI1, a Peptide-Receptor Complex, Regulates Arabidopsis Root Meristem Development via a MAPK Signaling Cascade. Mol. Plant 2020, 13, 1594–1607. [Google Scholar]
- Shao, Y.; Yu, X.; Xu, X.; Li, Y.; Yuan, W.; Xu, Y.; Mao, C.; Zhang, S.; Xu, J. The YDA-MKK4/MKK5-MPK3/MPK6 cascade functions downstream of the RGF1-RGI ligand-receptor pair in regulating mitotic activity in the root apical meristem. Mol. Plant 2020, 13, 1608–1623. [Google Scholar]
- Yamada, M.; Han, X.; Benfey, P.N. RGF1 controls root meristem size through ROS signalling. Nature 2020, 577, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Whitford, R.; Fernandez, A.; Tejos, R.; Pérez, A.C.; Kleine-Vehn, J.; Vanneste, S.; Drozdzecki, A.; Leitner, J.; Abas, L.; Aerts, M.; et al. GOLVEN secretory peptides regulate auxin carrier turnover during plant gravitropic responses. Dev. Cell 2012, 22, 678–685. [Google Scholar] [CrossRef] [PubMed]
- .Li, S.; Yamada, M.; Han, X.; Ohler, U.; Benfey, P.N. High-Resolution Expression Map of the Arabidopsis Root Reveals Alternative Splicing and lincRNA Regulation. Dev. Cell 2016, 39, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Drozdzecki, A.; Hoogewijs, K.; Vassileva, V.; Madder, A.; Beeckman, T.; Hilson, P. The GLV6/RGF8/CLEL2 peptide regulates early pericycle divisions during lateral root initiation. J. Exp. Bot. 2015, 66, 5245–5256. [Google Scholar] [CrossRef]
- Cederholm, H.M.; Benfey, P.N. Distinct sensitivities to phosphate deprivation suggest that RGF peptides play disparate roles in Arabidopsis thaliana root development. New Phytol. 2015, 207, 683–691. [Google Scholar] [CrossRef]
- Stührwohldt, N.; Dahlke, R.I.; Steffens, B.; Johnson, A.; Sauter, M. Phytosulfokine-α controls hypocotyl length and cell expansion in Arabidopsis thaliana through phytosulfokine receptor. PLoS ONE 2011, 6, e21054. [Google Scholar] [CrossRef]
- Bahyrycz, A.; Matsubayashi, Y.; Ogawa, M.; Sakagami, Y.; Konopińska, D. Plant peptide hormone phytosulfokine (PSK-alpha): Synthesis of new analogues and their biological evaluation. J. Pept. Sci. 2004, 10, 462–469. [Google Scholar] [CrossRef]
- Bahyrycz, A.; Matsubayashi, Y.; Ogawa, M.; Sakagami, Y.; Konopińska, D. Further analogues of plant peptide hormonephytosulfokine-alpha (PSK-alpha) and their biological evaluation. J. Pept. Sci. 2005, 11, 589–592. [Google Scholar] [CrossRef]
- Sauter, M.J. Phytosulfokine peptide signalling. Exp. Bot. 2015, 66, 5161–5169. [Google Scholar] [CrossRef]
- Igarashi, D.; Tsuda, K.; Katagiri, F. The peptide growth factor, phytosulfokine, attenuates pattern-triggered immunity. Plant J. 2012, 71, 194–204. [Google Scholar] [CrossRef]
- Mosher, S.; Seybold, H.; Rodriguez, P.; Stahl, M.; Kelli, A.; Davies, K.A.; Dayaratne, S.; Morillo, S.A.; Wierzba, M.; Favery, B.; et al. The tyrosine-sulfated peptide receptors PSKR1 and PSY1R modify the immunity of Arabidopsis to biotrophic and necrotrophic pathogens in an antagonistic manner. Plant J. 2013, 73, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Igasaki, T.; Akashi, N.; Ujino-Ihara, T.; Matsubayashi, Y.; Sakagami, Y.; Shinohara, K. Phytosulfokine stimulates somatic embryogenesis in Cryptomeria japonica. Plant Cell Physiol. 2003, 44, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, S.; Sakuta, C.; Matsubayashi, Y.; Sakagami, Y.; Kamada, H.; Satoh, S. The promotive effects of a peptidyl plant growth factor, phytosulfokine-α, on the formation of adventitious roots and expression of a gene for a root-specific cystatin in cucumber hypocotyls. J. Plant Res. 1998, 111, 453–458. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Takagi, L.; Omura, N.; Morita, A.; Sakagami, Y. The endogenous sulfated pentapeptide phytosulfokine-alpha stimulates tracheary element differentiation of isolated mesophyll cells of zinnia. Plant Physiol. 1999, 120, 1043–1048. [Google Scholar]
- Motose, H.; Iwamoto, K.; Endo, S.; Demura, T.; Sakagami, Y.; Matsubayashi, Y.; Moore, K.L.; Fukuda, H. Involvement of phytosulfokine in the attenuation of stress response during the transdifferentiation of zinnia mesophyll cells into tracheary elements. Plant Physiol. 2009, 150, 437–447. [Google Scholar] [CrossRef]
- Chen, Y.F.; Matsubayashi, Y.; Sakagami, Y. Peptide growth factor phytosulfokine-alpha contributes to the pollen population effect. Planta 2000, 211, 752–755. [Google Scholar] [CrossRef]
- Hartmann, J.; Fischer, C.; Dietrich, P.; Sauter, M. Kinase activity and calmodulin binding are essential for growth signaling by the phytosulfokine receptor PSKR. Plant J. 2014, 78, 192–202. [Google Scholar] [CrossRef]
- Kutschmar, A.; Rzewuski, G.; Stührwohldt, N.; Beemster, G.T.; Inzé, D.; Sauter, M. PSK-α promotes root growth in Arabidopsis. New Phytol. 2009, 181, 820–831. [Google Scholar] [CrossRef]
- Kong, X.; Tian, H.; Yu, Q.; Zhang, F.; Wang, R.; Gao, S.; Xu, W.; Liu, J.; Shani, E.; Fu, C.; et al. PHB3 maintains root stem cell niche identity through ROS-responsive AP2/ERF transcription factors in Arabidopsis. Cell Rep. 2018, 22, 1350–1363. [Google Scholar] [CrossRef]
- Mugford, S.G.; Yoshimoto, N.; Reichelt, M.; Wirtz, M.; Hill, L.; Mugford, S.T.; Nakazato, Y.; Noji, M.; Takahashi, H.; Kramell, R.; et al. Disruption of adenosine-5′-phosphosulfate kinase in Arabidopsis reduces levels of sulfated secondary metabolites. Plant Cell 2009, 21, 910–927. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Ogawa, M.; Morita, A.; Sakagami, Y. An LRR receptor kinase involved in perception of a peptide plant hormone, phytosulfokine. Science 2002, 296, 1470–1472. [Google Scholar] [PubMed]
- Matsubayashi, Y.; Ogawa, M.; Kihara, H.; Niwa, M.; Sakagami, Y. Disruption and overexpression of Arabidopsis phytosulfokine receptor gene affects cellular longevity and potential for growth. Plant Physiol. 2006, 42, 45–53. [Google Scholar]
- Kwezi, L.; Ruzvidzo, O.; Wheeler, J.I.; Govender, K.; Iacuone, S.; Thompson, P.E.; Gehring, C.; Irving, H.R. The phytosulfokine (PSK) receptor is capable of guanylate cyclase activity and enabling cyclic GMP-dependent signaling in plants. J. Biol. Chem. 2011, 286, 22580–22588. [Google Scholar]
- Yu, L.; Liu, Y.; Liu, Y.; Li, Q.; Tang, G.; Luo, L. Overexpression of phytosulfokine-alpha induces male sterility and cell growth by regulating cell wall development in Arabidopsis. Plant Cell Rep. 2016, 35, 2503–2512. [Google Scholar]
- Pearce, G.; Moura, D.S.; Stratmann, J.; Ryan, C.A. Jr RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc. Natl. Acad. Sci. USA 2001, 98, 12843–12847. [Google Scholar]
- Olsen, A.N.; Mundy, J.; Skriver, K. Peptomics, identification of novel cationic Arabidopsis peptides with conserved sequence motifs. Silico Biol. 2002, 2, 441–451. [Google Scholar]
- Bergonci, T.; Ribeiro, B.; Ceciliato, P.H.O.; Guerrero-Abad, J.C.; Silva-Filho, M.C.; Moura, D.S. Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J. Exp. Bot. 2014, 65, 2219–2230. [Google Scholar]
- Murphy, E.; Vu, L.D.; Van den Broeck, L.; Lin, Z.; Ramakrishna, P.; van de Cotte, B.; Gaudinier, A.; Goh, T.; Slane, D.; Beeckman, T.; et al. RALFL34 regulates formative cell divisions in Arabidopsis pericycle during lateral root initiation. J. Exp. Bot. 2016, 67, 4863–4875. [Google Scholar]
- Abarca, A.; Franck, C.M.; Zipfel, C. Family-wide evaluation of RAPID ALKALINIZATION FACTOR peptides. Plant Physiol. 2021, 187, 996–1010. [Google Scholar]
- Combier, J.-P.; Küster, H.; Journet, E.-P.; Hohnjec, N.; Gamas, P.; Niebel, A. Evidence for the involvement in nodulation of the two small putative regulatory peptide-encoding genes MtRALFL1 and MtDVL1. Mol. Plant-Microbe Interact. 2008, 21, 1118–1127. [Google Scholar]
- Ge, Z.; Bergonci, T.; Zhao, Y.; Zou, Y.; Du, S.; Liu, M.-C.; Luo, X.; Ruan, H.; García-Valencia, L.E.; Zhong, S.; et al. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 2017, 358, 1596–1600. [Google Scholar] [PubMed]
- .Zhong, S.; Li, L.; Wang, Z.; Ge, Z.; Li, Q.; Bleckmann, A.; Wang, J.; Song, Z.; Shi, Y.; Liu, T.; et al. RALF peptide signaling controls the polytubey block in Arabidopsis. Science 2022, 375, 290–296. [Google Scholar] [PubMed]
- Germain, H.; Chevalier, É.; Caron, S.; Matton, D.P. Characterization of five RALF-like genes from Solanum chacoense provides support for a developmental role in plants. Planta 2005, 220, 447–454. [Google Scholar] [PubMed]
- Negrini, F.; O’Grady, K.; Hyvӧnen, M.; Folta, K.; Baraldi, E. Genomic structure and transcript analysis of the Rapid Alkalinization Factor (RALF) gene family during host-pathogen crosstalk in Fragaria vesca and Fragaria x ananassa strawberry. PLoS ONE 2020, 15, e0226448. [Google Scholar]
- Zhang, X.; Peng, H.; Zhu, S.; Xing, J.; Li, X.; Zhu, Z.; Zheng, J.; Wang, L.; Wang, B.; Chen, J.; et al. Nematode-encoded RALF peptide mimics facilitate parasitism of plants through the FERONIA receptor kinase. Mol. Plant 2020, 13, 1434–1454. [Google Scholar]
- Haruta, M.; Constabe, C.P. Rapid alkalinization factors in poplar cell cultures. Peptide isolation, cDNA cloning, and differential expression in leaves and methyl jasmonate-treated cells. Plant Physiol. 2003, 131, 814–823. [Google Scholar]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of drought stress research: Experimental setup and physiological characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef]
- Muthukumar, K.; Rajakumar, S.; Sarkar, M.N.; Nachiappan, V. Glutathione peroxidase3 of Saccharomyces cerevisiae protects phospholipids during cadmium-induced oxidative stress. Antonie Van Leeuwenhoek. J. Microbiol. 2011, 99, 761–771. [Google Scholar]
- De Rybel, B.; Vassileva, V.; Parizot, B.; Demeulenaere, M.; Grunewald, W.; Audenaert, D.; Van Campenhout, J.; Overvoorde, P.; Jansen, L.; Vanneste, S.; et al. A novel Aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr. Biol. 2010, 20, 1697–1706. [Google Scholar]
- Hirakawa, Y. Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc. Natl. Acad. Sci. USA 2008, 105, 15208–15213. [Google Scholar] [CrossRef]
- Chevalier, E.; Loubert-Hudon, A.; Matton, D.P. ScRALF3, a secreted RALF-like peptide involved in cell-cell communication between the sporophyte and the female gametophyte in a solanaceous species. Plant J. 2013, 73, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Sun, P.; Li, Z.; Zhang, F.; You, C.; Zhang, Z. FERONIA receptor kinase integrates with hormone signaling to regulate plant growth, development, and responses to environmental stimuli. Int. J. Mol. Sci. 2022, 23, 3730. [Google Scholar] [CrossRef] [PubMed]
- Baez, L.A.; Tichá, T.; Hamann, T. Cell wall integrity regulation across plant species. Plant Mol. Biol. 2022, 109, 483–504. [Google Scholar] [CrossRef]
- Matos, J.L.; Fiori, C.S.; Silva-Filho, M.C.; Moura, D.S. A conserved dibasic site is essential for correct processing of the peptide hormone AtRALF1 in Arabidopsis thaliana. FEBS Lett. 2008, 582, 3343–3347. [Google Scholar] [CrossRef]
- Haruta, M.; Sabat, G.; Stecker, K.; Minkoff, B.B.; Sussman, M.R. A Peptide Hormone and Its Receptor Protein Kinase Regulate Plant Cell Expansion. Science 2014, 343, 408–411. [Google Scholar] [CrossRef]
- Ghorbani, S.; Lin, Y.-C.; Parizot, B.; Fernandez, A.; Njo, M.F.; Van De Peer, Y.; Beeckman, T.; Hilson, P. Expanding the repertoire of secretory peptides controlling root development with comparative genome analysis and functional assays. J. Exp. Bot. 2015, 66, 5257–5269. [Google Scholar] [CrossRef]
- Escobar-Restrepo, J.-M.; Huck, N.; Kessler, S.; Gagliardini, V.; Gheyselinck, J.; Yang, W.-C.; Grossniklaus, U. The FERONIA Receptor-like Kinase Mediates Male-Female Interactions During Pollen Tube Reception. Science 2007, 317, 656–660. [Google Scholar] [CrossRef]
- Fuglsang, A.T.; Kristensen, A.; Cuin, T.; Schulze, W.X.; Persson, J.; Thuesen, K.H.; Ytting, C.K.; Oehlenschlaeger, C.B.; Mahmood, K.; Sondergaard, T.E.; et al. Receptor kinase-mediated control of primary active proton pumping at the plasma membrane. Plant J. 2014, 80, 951–964. [Google Scholar] [CrossRef]
- Guo, H.; Li, L.; Ye, H.; Yu, X.; Algreen, A.; Yin, Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2009, 106, 7648–7653. [Google Scholar] [CrossRef]
- Deslauriers, S.D.; Larsen, P.B. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol. Plant 2010, 3, 626–640. [Google Scholar] [PubMed]
- Chen, J.; Yu, F.; Liu, Y.; Du, C.; Li, X.; Zhu, S.; Wang, X.; Lan, W.; Rodriguez, P.L.; Liu, X.; et al. FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, E5519–E5527. [Google Scholar] [PubMed]
- Duan, Q.; Kita, D.; Li, C.; Cheung, A.Y.; Wu, H.M. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. USA 2010, 107, 17821–17826. [Google Scholar] [PubMed]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar]
- Carol, R.J.; Takeda, S.; Linstead, P.; Durrant, M.C.; Kakesova, H.; Derbyshire, P.; Dream, S.; Zarsky, V.; Dolan, L. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature 2005, 438, 1013–1016. [Google Scholar]
- Jones, M.A.; Raymond, M.J.; Yang, Z.; Smirnoff, N. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J. Exp. Bot. 2007, 58, 1261–1270. [Google Scholar]
- Berken, A.; Thomas, C.; Wittinghofer, A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 2005, 436, 1176–1180. [Google Scholar]
- Li, C.; Yeh, F.-L.; Cheung, A.Y.; Duan, Q.; Kita, D.; Liu, M.C.; Maman, J.; Luu, E.J.; Wu, B.W.; Gates, L.; et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife 2015, 4, e06587. [Google Scholar]
- Huang, G.-Q.; Li, E.; Ge, F.-R.; Li, S.; Wang, Q.; Zhang, C.-Q.; Zhang, Y. Arabidopsis RopGEF4 and RopGEF10 are important for FERONIA-mediated developmental but not environmental regulation of root hair growth. New Phytol. 2013, 200, 1089–1101. [Google Scholar]
- Datta, S.; Prescott, H.; Dolan, L. Intensity of a pulse of RSL4 transcription factor synthesis determines Arabidopsis. Nat. Plants 2015, 138, 15138. [Google Scholar]
- Hwang, Y.; Choi, H.; Cho, H.; Cho, H. Tracheophytes contain conserved orthologs of a basic helix-loop-helix transcription factor to modulate ROOT HAIR SPECIFIC genes. Plant Cell 2017, 29, 39–53. [Google Scholar] [PubMed]
- Zhu, S.; Pacheco, J.M.; Estevez, J.M.; Yu, F. Autocrine regulation of root hair size by the RALF-FERONIA-RSL4 signaling pathway. New Phytol. 2020, 227, 45–49. [Google Scholar] [PubMed]
- Dressano, K.; Ceciliato, P.H.O.; Silva, A.L.; Guerrero-Abad, J.C.; Bergonci, T.; Ortiz-Morea, F.A.; Bürger, M.; Silva-Filho, M.C.; Moura, D.S. BAK1 is involved in AtRALF1-induced inhibition of root cell expansion. PLoS Genet. 2017, 13, e1007053. [Google Scholar]
- Stegmann, M.; Monaghan, J.; Smakowska-Luzan, E.; Rovenich, H.; Lehner, A.; Holton, N.; Belkhadir, Y.; Zipfel, C. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 2017, 355, 287–289. [Google Scholar]
- Campos, W.F.; Dressano, K.; Ceciliato, P.H.O.; Guerrero-Abad, J.C.; Silva, A.L.; Fiori, C.S.; Morato do Canto, A.; Bergonci, T.; Claus, L.A.N.; Silva-Filho, M.C.; et al. Arabidopsis thaliana rapid alkalinization factor 1-mediated root growth inhibition is dependent on calmodulin-like protein J. Biol. Chem. 2018, 293, 2159–2171. [Google Scholar]
- Gonneau, M.; Desprez, T.; Martin, M.; Doblas, V.G.; Bacete, L.; Miart, F.; Sormani, R.; Hématy, K.; Renou, J.; Landrein, B.; et al. Receptor kinase THESEUS1 is a rapid alkalinization factor 34 receptor in Arabidopsis. Curr. Biol. 2018, 28, 2452–2458.e4. [Google Scholar] [CrossRef]
- Song, Y.; Wilson, A.J.; Zhang, X.C.; Thoms, D.; Sohrabi, R.; Song, S.; Geissmann, Q.; Liu, Y.; Walgren, L.; He, S.Y.; et al. FERONIA restricts Pseudomonas in the rhizosphere microbiome via regulation of reactive oxygen species. Nat. Plants 2021, 7, 644–654. [Google Scholar]
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis calcium-dependent protein kinases (CDPKs) and their roles in plant growth regulation and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef]
- Fiers, M.; Golemiec, E.; van der Schors, R.; van der Geest, L.; Li, K.W.; Stiekema, W.J.; Liu, C.-M. The CLAVATA3/ESR motif of CLAVATA3 is functionally independent from the nonconserved flanking sequences. Plant Physiol. 2006, 141, 1284–1292. [Google Scholar]
- Jun, J.; Fiume, E.; Roeder, A.H.; Meng, L.; Sharma, V.K.; Osmont, K.S.; Baker, C.; Ha, C.M.; Meyerowitz, E.M.; Feldman, L.J.; et al. Comprehensive analysis of CLE polypeptide signaling gene expression and overexpression activity in Arabidopsis. Plant Physiol. 2010, 154, 1721–1736. [Google Scholar]
- Gancheva, M.S.; Losev, M.R.; Gurina, A.A.; Poliushkevich, L.O.; Dodueva, I.E.; Lutova, L.A. Polymorphism of CLE gene sequences in potato. Vavilovskii Zhurnal Genet. Sel. 2021, 25, 746–753. [Google Scholar]
- Morita, J.; Kato, K.; Nakane, T.; Kondo, Y.; Fukuda, H.; Nishimasu, H.; Ishitani, R.; Nureki, O. Crystal structure of the plant receptor-like kinase TDR in complex with the TDIF peptide. Nat. Commun. 2016, 7, 12383. [Google Scholar] [PubMed]
- Ohyama, K.; Shinohara, H.; Ogawa-Ohnishi, M.; Matsubayashi, Y. A glycopeptide regulating stem cell fate in Arabidopsis thaliana. Nat. Chem. Biol. 2009, 5, 578–580. [Google Scholar] [PubMed]
- Whitewoods, C.D. Evolution of CLE peptide signalling. Semin Cell Dev Biol. 2021, 109, 12–19. [Google Scholar]
- Shinohara, H.; Matsubayashi, Y. Chemical synthesis of Arabidopsis CLV3 glycopeptide reveals the impact of hydroxyproline arabinosylation on peptide conformation and activity. Plant Cell Physiol. 2013, 54, 369–374. [Google Scholar]
- Yamaguchi, Y.L.; Ishida, T.; Sawa, S. CLE peptides and their signaling pathways in plant development. J. Exp. Bot. 2016, 67, 4813–4826. [Google Scholar]
- Fletcher, J.C. Recent advances in Arabidopsis CLE peptide signaling. Trends Plant Sci. 2020, 25, 1005–1016. [Google Scholar]
- Betsuyaku, S.; Sawa, S.; Yamada, M. The function of the CLE peptides in plant development and plant-microbe interactions. Arab. Book 2011, 9, e0149. [Google Scholar]
- Daum, G.; Medzihradszky, A.; Suzaki, T.; Lohmann, J.U. A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14619–14624. [Google Scholar]
- Yadav, R.K.; Perales, M.; Gruel, J.; Girke, T.; Jönsson, H.; Reddy, G.V. WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev. 2011, 25, 2025–2030. [Google Scholar]
- Whitford, R.; Fernandez, A.; De Groodt, R.; Ortega, E.; Hilson, P. Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells. Proc. Natl. Acad. Sci. USA 2008, 105, 18625–18630. [Google Scholar] [PubMed]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [PubMed]
- Stahl, Y.; Wink, R.H.; Ingram, G.C.; Simon, R. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr. Biol. 2009, 19, 909–914. [Google Scholar] [PubMed]
- Stahl, Y.; Simon, R. Gated communities: Apoplastic and symplastic signals converge at plasmodesmata to control cell fates. J. Exp. Bot. 2013, 64, 5237–5241. [Google Scholar] [PubMed]
- Pi, L.; Aichinger, E.; van der Graaff, E.; Llavata-Peris, C.I.; Weijers, D.; Hennig, L.; Groot, E.; Laux, T. Organizer-derived WOX5 signal maintains root columella stem cells through chromatin-mediated repression of CDF4 expression. Dev. Cell 2015, 33, 576–588. [Google Scholar]
- Berckmans, B.; Kirschner, G.; Gerlitz, N.; Stadler, R.; Simon, R. CLE40 Signaling Regulates Root Stem Cell Fate. Plant Physiol. 2019, 182, 1776–1792. [Google Scholar]
- Herrbach, V.; Remblière, C.; Gough, C.; Bensmihen, S. Lateral root formation and patterning in Medicago truncatula. J. Plant Physiol. 2014, 171, 301–310. [Google Scholar]
- Jensen, E.; Peoples, M.; Boddey, R.; Gresshoff, P.; Hauggaard-Nielsen, H.; Alves, B.; Morrison, M. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil. 2008, 311, 1–18. [Google Scholar]
- Ohyama, K.; Ogawa, M.; Matsubayashi, Y. Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC-MS-based structure analysis. Plant J. 2008, 55, 152–160. [Google Scholar]
- Djordjevic, M.A.; Mohd-Radzman, N.A.; Imin, N. Small-peptide signals that control root nodule number, development, and symbiosis. J. Exp. Bot. 2015, 66, 5171–5181. [Google Scholar] [PubMed]
- Imin, N.; Mohd-Radzman, N.A.; Ogilvie, H.A.; Djordjevic, M.A. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J. Exp. Bot. 2013, 64, 5395–5409. [Google Scholar] [PubMed]
- Mohd-Radzman, N.A.; Binos, S.; Truong, T.T.; Imin, N.; Mariani, M.; Djordjevic, M.A. Novel MtCEP1 peptides produced in vivo differentially regulate root development in Medicago truncatula. J. Exp. Bot. 2015, 66, 5289–5300. [Google Scholar]
- Franssen, H.J.; Xiao, T.T.; Kulikova, O.; Wan, X.; Bisseling, T.; Scheres, B.; Heidstra, R. Root developmental programs shape the Medicago truncatula nodule meristem. Development 2015, 142, 2941–2950. [Google Scholar]
- Larrainzar, E.; Riely, B.K.; Kim, S.C.; Carrasquilla-Garcia, N.; Yu, H.J.; Hwang, H.J.; Oh, M.; Kim, G.B.; Surendrarao, A.K.; Chasman, D.; et al. Deep sequencing of the Medicago truncatula root transcriptome reveals a massive and early interaction between Nod factor and ethylene signals. Plant Physiol. 2015, 169, 233–265. [Google Scholar]
- Xiao, T.T.; Schilderink, S.; Moling, S.; Deinum, E.E.; Kondorosi, E.; Franssen, H.; Kulikova, O.; Niebel, A.; Bisseling, T. Fate map of Medicago truncatula root nodules. Development 2014, 141, 3517–3528. [Google Scholar]
- Huault, E.; Laffont, C.; Wen, J.; Mysore, K.S.; Ratet, P.; Duc, G.; Frugier, F. Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. PLoS Genet. 2014, 10, e1004891. [Google Scholar]
- Penmetsa, R.V.; Uribe, P.; Anderson, J.; Lichtenzveig, J.; Gish, J.-C.; Nam, Y.W.; Engstrom, E.; Xu, K.; Sckisel, G.; Pereira, M.; et al. The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 2008, 55, 580–595. [Google Scholar]
- Heidstra, R.; Yang, W.C.; Yalcin, Y.; Peck, S.; Emons, A.M.; van Kammen, A.; Bisseling, T. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development 1997, 124, 1781–1787. [Google Scholar]
- Oldroyd, G.E.; Engstrom, E.M.; Long, S.R. Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell 2001, 13, 1835–1849. [Google Scholar]
- Fedoreyeva, L.I.; Vanyushin, B.F.; Baranova, E.N. Peptide AEDL alters chromatin conformation via binding. AIMS Biophys. 2020, 7, 1–16. [Google Scholar]
- Fedoreyeva, L.I.; Baranova, E.N.; Chaban, I.A.; Dilovarova, T.A.; Vanyushin, B.F.; Kononenko, N.V. Elongating Effect of the Peptide AEDL on the Root of Nicotiana tabacum under Salinity. Plants 2022, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, N.V.; Fedoreyeva, L.I. Peptide AEDL and Glutathione Stimulates Root Development Nicotiana tabacum. Int. J. Mol. Sci. 2025, 26, 289. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).