O-GlcNAcylation in Gli1+ Mesenchymal Stem Cells Is Indispensable for Bone Formation and Fracture Healing
Abstract
1. Introduction
2. Results
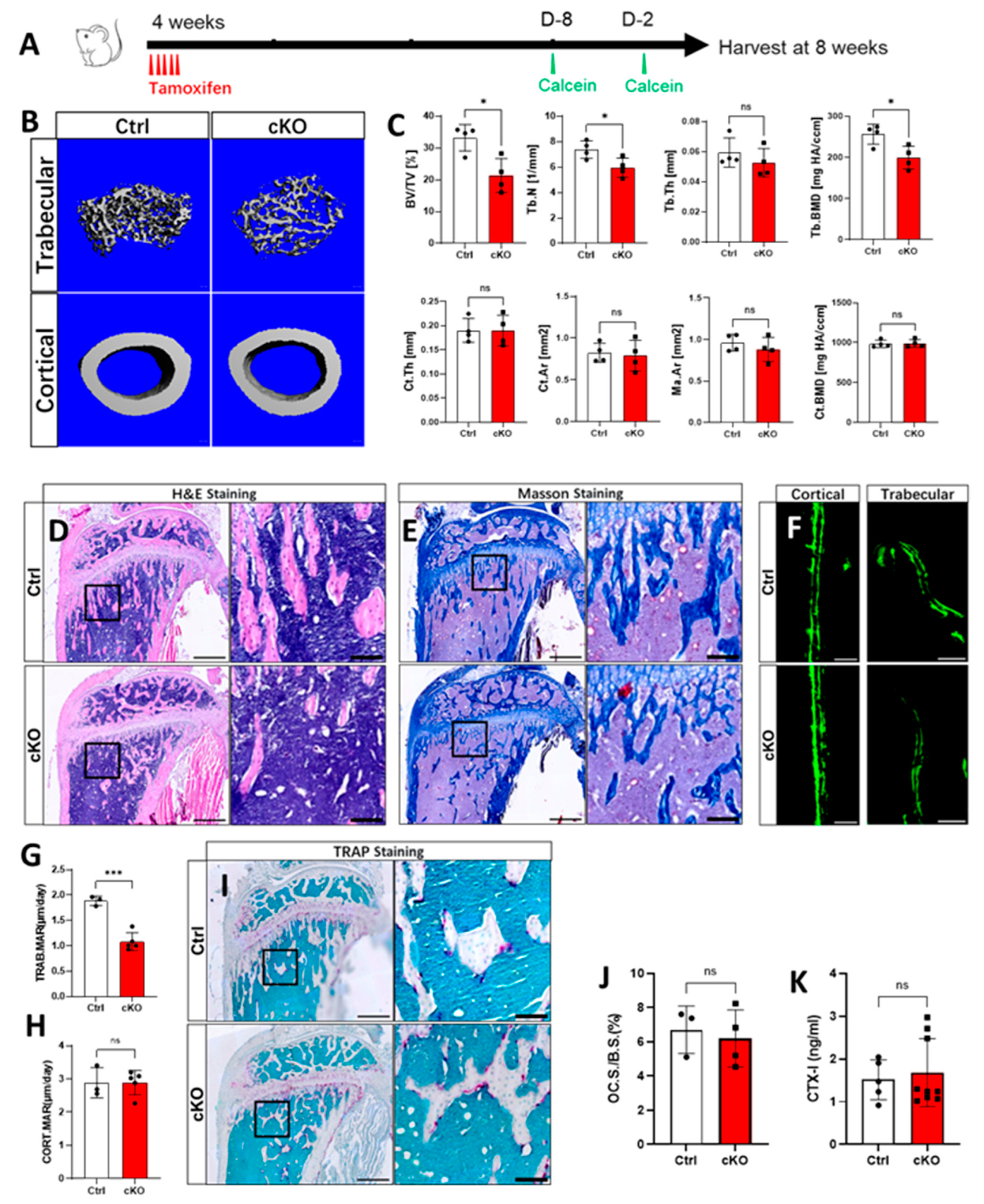
2.1. Lack of OGT in Gli1+ MSCs Decreased Trabecular Bone Formation in Postnatal Mice
2.2. OGT Depletion Led to the Depletion of the Gli1+ MSCs Pool
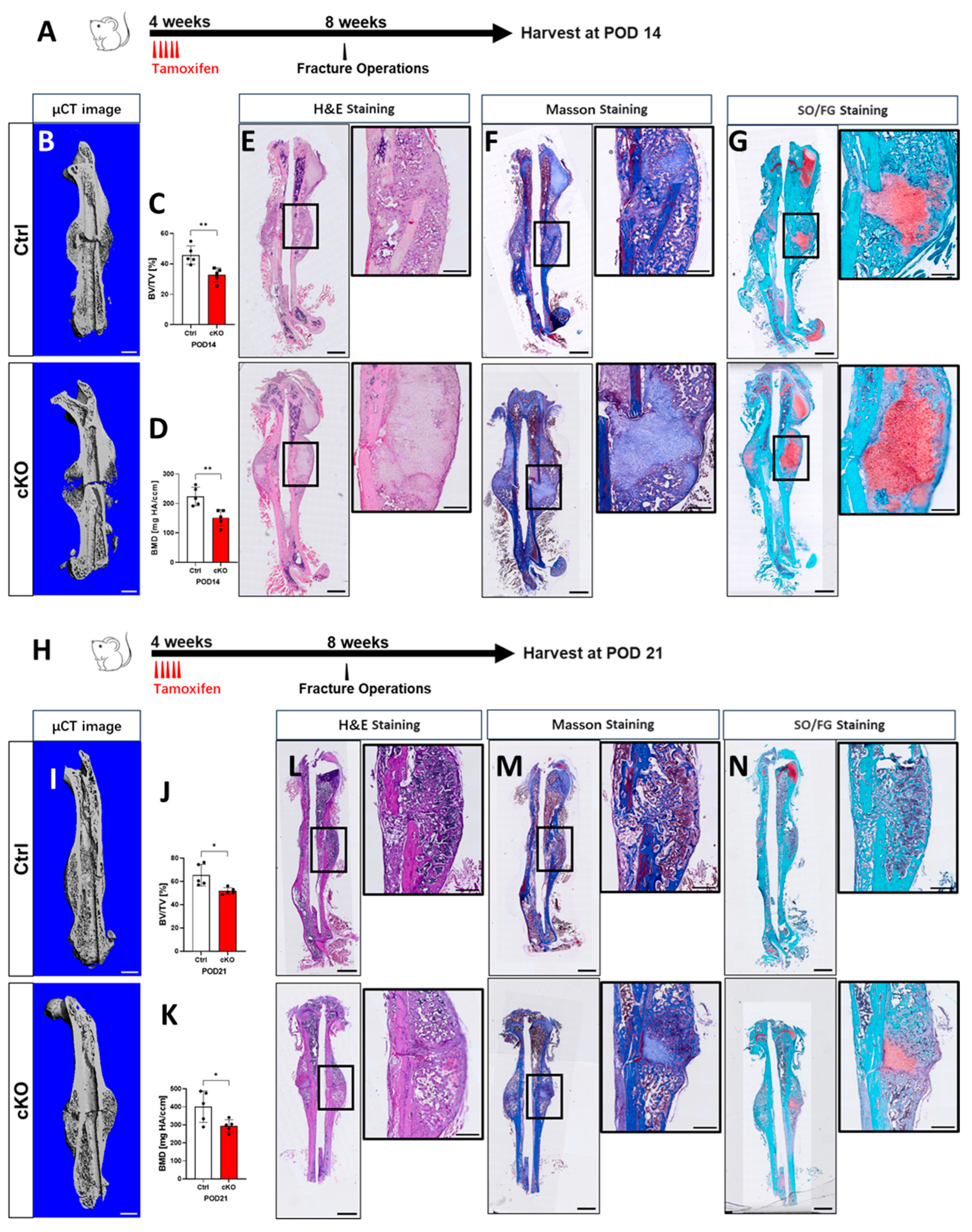
2.3. OGT Deletion in Gli1+ MSCs Delays Fracture Repair
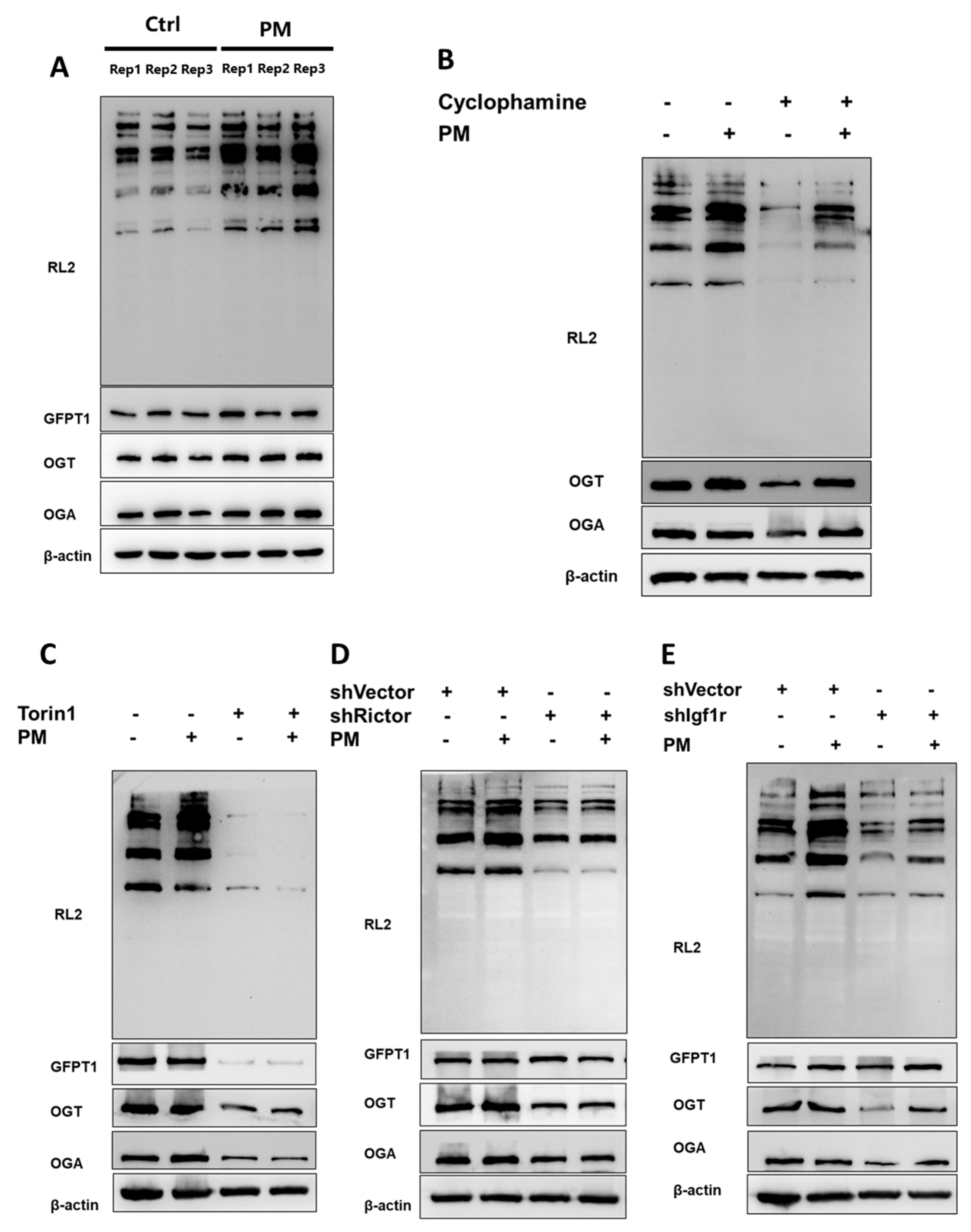
2.4. Hh Modulated O-GlcNAcylation Through the Igf-mTORC2 Signaling Cascade
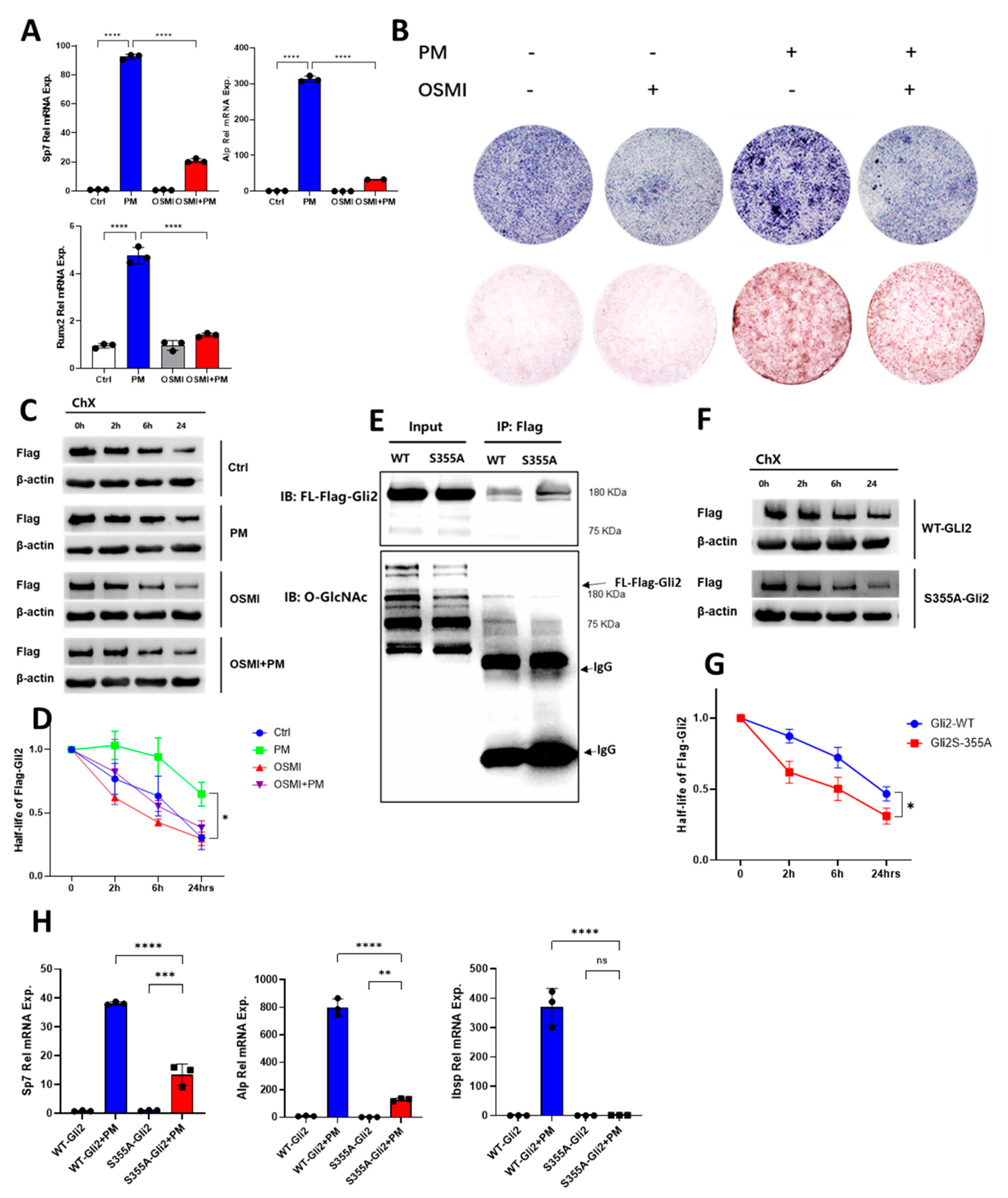
2.5. O-GlcNAcylation of Gli2 Was Required for Hh-Induced Osteogenesis
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. TAM Administration
4.3. Immunofluorescence Staining
4.4. Bone Fracture
4.5. Histology and Histomorphometry
4.6. MicroCT
4.7. Serum CTX-I Assays
4.8. Proliferation Assay
4.9. Cell Culture
4.10. Western Blot Analyses
4.11. Co-Immunoprecipitation
4.12. Quantitative PCR
4.13. O-GlcNAc Sites Prediction
4.14. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briscoe, J.; Thérond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S. Multiple roles for lipids in the Hedgehog signalling pathway. Nat. Rev. Mol. Cell Biol. 2008, 9, 437–445. [Google Scholar] [CrossRef]
- Ingham, P.W.; McMahon, A.P. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001, 15, 3059–3087. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.M.Y.; Curran, T. The Hedgehog’s tale: Developing strategies for targeting cancer. Nat. Rev. Cancer 2011, 11, 493–501. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Hanna, A.; Lama-Sherpa, T.; Metge, B.; Kammerud, S.C.; Benavides, G.A.; Kumar, A.; Alsheikh, H.A.; Mota, M.; Chen, D.; et al. Hedgehog Signaling Regulates Metabolism and Polarization of Mammary Tumor-Associated Macrophages. Cancer Res. 2021, 81, 5425–5437. [Google Scholar] [CrossRef] [PubMed]
- Beachy, P.A.; Hymowitz, S.G.; Lazarus, R.A.; Leahy, D.J.; Siebold, C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010, 24, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.F.X.; Sircar, R.; Miller, P.S.; Hedger, G.; Luchetti, G.; Nachtergaele, S.; Tully, M.D.; Mydock-McGrane, L.; Covey, D.F.; Rambo, R.P.; et al. Structural basis of Smoothened regulation by its extracellular domains. Nature 2016, 535, 517–522. [Google Scholar] [CrossRef]
- Kong, J.H.; Siebold, C.; Rohatgi, R. Biochemical mechanisms of vertebrate hedgehog signaling. Development 2019, 146, dev166892. [Google Scholar] [CrossRef]
- Petrov, K.; Wierbowski, B.M.; Salic, A. Sending and Receiving Hedgehog Signals. Annu. Rev. Cell Dev. Biol. 2017, 33, 145–168. [Google Scholar] [CrossRef]
- Wu, X.; Walker, J.; Zhang, J.; Ding, S.; Schultz, P.G. Purmorphamine Induces Osteogenesis by Activation of the Hedgehog Signaling Pathway. Chem. Biol. 2004, 11, 1229–1238. [Google Scholar] [CrossRef]
- Shi, Y.; He, G.; Lee, W.-C.; McKenzie, J.A.; Silva, M.J.; Long, F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat. Commun. 2017, 8, 2043. [Google Scholar] [CrossRef]
- Men, Y.; Wang, Y.; Yi, Y.; Jing, D.; Luo, W.; Shen, B.; Stenberg, W.; Chai, Y.; Ge, W.-P.; Feng, J.Q.; et al. Gli1+ Periodontium Stem Cells Are Regulated by Osteocytes and Occlusal Force. Dev. Cell 2020, 54, 639–654.e6. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xu, C.; Zhao, H.; Wang, J.; Feng, J.Q. A Biphasic Feature of Gli1+-Mesenchymal Progenitors during Cementogenesis That Is Positively Controlled by Wnt/β-Catenin Signaling. J. Dent. Res. 2021, 100, 1289–1298. [Google Scholar] [CrossRef]
- Yi, Y.; Stenberg, W.; Luo, W.; Feng, J.Q.; Zhao, H. Alveolar Bone Marrow Gli1+ Stem Cells Support Implant Osseointegration. J. Dent. Res. 2021, 101, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, P.; Shen, F.; You, C.; Wu, F.; Shi, Y.; Ye, L. Gli1 labels progenitors during chondrogenesis in postnatal mice. EMBO Rep. 2024, 25, 1773–1791. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liao, X.; Long, J.Y.; Yao, L.; Chen, J.; Yin, B.; Lou, F.; He, G.; Ye, L.; Qin, L.; et al. Gli1+ progenitors mediate bone anabolic function of teriparatide via Hh and Igf signaling. Cell Rep. 2021, 36, 109542. [Google Scholar] [CrossRef]
- Vocadlo, D.J.; Hang, H.C.; Kim, E.-J.; Hanover, J.A.; Bertozzi, C.R. A chemical approach for identifying O-GlcNAcmodified proteins in cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9116–9121. [Google Scholar] [CrossRef]
- Torres, C.R.; Hart, G.W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984, 259, 3308–3317. [Google Scholar] [CrossRef]
- Ye, L.; Ding, W.; Xiao, D.; Jia, Y.; Zhao, Z.; Ao, X.; Wang, J. O-GlcNAcylation: Cellular physiology and therapeutic target for human diseases. MedComm 2023, 4, e456. [Google Scholar] [CrossRef]
- Lee, J.M.; Hammarén, H.M.; Savitski, M.M.; Baek, S.H. Control of protein stability by post-translational modifications. Nat. Commun. 2023, 14, 201. [Google Scholar] [CrossRef]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, Z.; Awad, M.; Elsalanty, M.; Cray, J.; Ball, L.E.; Maynard, J.C.; Burlingame, A.L.; Zeng, H.; Mansky, K.C.; et al. O-GlcNAc glycosylation orchestrates fate decision and niche function of bone marrow stromal progenitors. eLife 2023, 12, e85464. [Google Scholar]
- You, C.; Shen, F.; Yang, P.; Cui, J.; Ren, Q.; Liu, M.; Hu, Y.; Li, B.; Ye, L.; Shi, Y. O-GlcNAcylation mediates Wnt-stimulated bone formation by rewiring aerobic glycolysis. EMBO Rep. 2024, 25, 4465–4487. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.C.; Mann, T.L.A.; Pool, J.A.; Zhao, Z.; Morrison, S.J. Bone marrow and periosteal skeletal stem/progenitor cells make distinct contributions to bone maintenance and repair. Cell Stem Cell 2022, 29, 1547–1561.e6. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Karner, C.M.; Long, F. Hedgehog signaling activates a positive feedback mechanism involving insulin-like growth factors to induce osteoblast differentiation. Proc. Natl. Acad. Sci. USA 2015, 112, 4678–4683. [Google Scholar] [CrossRef]
- Huangfu, D.; Anderson, K.V. Signaling from Smo to Ci/Gli: Conservation and divergence of Hedgehog pathways from Drosophilato vertebrates. Development 2006, 133, 3–14. [Google Scholar] [CrossRef]
- Das, S.; Bailey, S.K.; Metge, B.J.; Hanna, A.; Hinshaw, D.C.; Mota, M.; Forero-Torres, A.; Chatham, J.C.; Samant, R.S.; Shevde, L.A. O-GlcNAcylation of GLI transcription factors in hyperglycemic conditions augments Hedgehog activity. Lab. Investig. 2019, 99, 260–270. [Google Scholar] [CrossRef]
- Mak, K.K.; Bi, Y.; Wan, C.; Chuang, P.-T.; Clemens, T.; Young, M.; Yang, Y. Hedgehog Signaling in Mature Osteoblasts Regulates Bone Formation and Resorption by Controlling PTHrP and RANKL Expression. Dev. Cell 2008, 14, 674–688. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, X.; Ni, L.; Liu, C.; Zheng, Y.; You, H.; Li, M.; Xiu, C.; Zhang, L.; Gong, T.; et al. Hedgehog Signaling Controls Bone Homeostasis by Regulating Osteogenic/Adipogenic Fate of Skeletal Stem/Progenitor Cells in Mice. J. Bone Miner. Res. 2020, 37, 559–576. [Google Scholar] [CrossRef]
- Joeng, K.S.; Long, F. The Gli2 transcriptional activator is a crucial effector for Ihh signaling in osteoblast development and cartilage vascularization. Development 2009, 136, 4177–4185. [Google Scholar] [CrossRef]
- Hilton, M.J.; Tu, X.; Cook, J.; Hu, H.; Long, F. Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development 2005, 132, 4339–4351. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, A.; Wada, M.; Ikeda, F.; Hata, K.; Matsubara, T.; Nifuji, A.; Noda, M.; Amano, K.; Yamaguchi, A.; Nishimura, R.; et al. Ihh/Gli2 Signaling Promotes Osteoblast Differentiation by Regulating Runx2 Expression and Function. Mol. Biol. Cell 2007, 18, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hou, C.; Wu, C. Demystifying the O-GlcNAc Code: A Systems View. Chem. Rev. 2022, 122, 15822–15864. [Google Scholar] [CrossRef]
- Hu, J.; Xia, W.; Zeng, S.; Lim, Y.-J.; Tao, Y.; Sun, Y.; Zhao, L.; Wang, H.; Le, W.; Li, D.; et al. Phosphorylation and O-GlcNAcylation at the same α-synuclein site generate distinct fibril structures. Nat. Commun. 2024, 15, 2677. [Google Scholar] [CrossRef]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Wulff-Fuentes, E.; Berendt, R.R.; Massman, L.; Danner, L.; Malard, F.; Vora, J.; Kahsay, R.; Olivier-Van Stichelen, S. The human O-GlcNAcome database and meta-analysis. Sci. Data 2021, 8, 25. [Google Scholar] [CrossRef]
- McBride-Gagyi, S.H.; McKenzie, J.A.; Buettmann, E.G.; Gardner, M.J.; Silva, M.J. Bmp2 conditional knockout in osteoblasts and endothelial cells does not impair bone formation after injury or mechanical loading in adult mice. Bone 2015, 81, 533–543. [Google Scholar] [CrossRef]
- Yang, P.; Shen, F.; You, C.; Lou, F.; Shi, Y. Gli1+ Progenitors Mediate Glucocorticoid-Induced Osteoporosis In Vivo. Int. J. Mol. Sci. 2024, 25, 4371. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Hu, Y.; You, C.; Xiong, D.; Ye, L.; Shi, Y. O-GlcNAcylation in Gli1+ Mesenchymal Stem Cells Is Indispensable for Bone Formation and Fracture Healing. Int. J. Mol. Sci. 2025, 26, 2712. https://doi.org/10.3390/ijms26062712
Liu M, Hu Y, You C, Xiong D, Ye L, Shi Y. O-GlcNAcylation in Gli1+ Mesenchymal Stem Cells Is Indispensable for Bone Formation and Fracture Healing. International Journal of Molecular Sciences. 2025; 26(6):2712. https://doi.org/10.3390/ijms26062712
Chicago/Turabian StyleLiu, Moyu, Yujie Hu, Chengjia You, Ding Xiong, Ling Ye, and Yu Shi. 2025. "O-GlcNAcylation in Gli1+ Mesenchymal Stem Cells Is Indispensable for Bone Formation and Fracture Healing" International Journal of Molecular Sciences 26, no. 6: 2712. https://doi.org/10.3390/ijms26062712
APA StyleLiu, M., Hu, Y., You, C., Xiong, D., Ye, L., & Shi, Y. (2025). O-GlcNAcylation in Gli1+ Mesenchymal Stem Cells Is Indispensable for Bone Formation and Fracture Healing. International Journal of Molecular Sciences, 26(6), 2712. https://doi.org/10.3390/ijms26062712




