Abstract
The largest area in the adult mammalian brain that contains stem and progenitor cells at different stages of differentiation is the subventricular zone located along the lateral wall of the lateral ventricle. We have previously shown in adult monkeys that transient global cerebral ischemia upregulates the expression of hundreds of genes in this zone, including genes known to be related to stemness in the rodent brain. Here, we analyzed the immunophenotype of two of these genes, TNC and GJA1, by co-expression experiments, applying a panel of known stem/progenitor-cell-related markers. We found that both TNC and GJA1 were expressed in the perivascular region. They were localized not to the endothelial cells but to the periendothelial adventitial cells, which was consistent with our previous electron-microscopic data suggesting periendothelial cells as a source of progenitors. We report that the expression of GJA1 was high in quiescent progenitors, while TNC was mostly present in progenitors in the transition from a quiescent to an active state. Our data suggest that TNC and GJA1 can be used as markers for stem/progenitor cells in the largest stem cell area of the adult primate brain.
1. Introduction
The process by which new neurons are produced by stem/progenitor cells in the adult brain is known as adult neurogenesis. This process occurs in specialized histological domains known as neurogenic niches: the subventricular zone of the lateral ventricle (SVZ) and the subgranular zone (SGZ) of the dentate gyrus [1,2,3]. The presence of proliferating and differentiating cells in these niches is widely studied in rodents and less studied in primates. The neural stem cells (NSCs) in the brain of mammals exist in different stages of development, such as active NSCs (aNSCs) or quiescent NSCs (qNSCs) [4,5]. Rarely dividing and inactive under physiological conditions, qNSCs can transform into aNSCs after stimulation, and as aNSCs, become highly proliferative and exhibit distinct differences in their immunophenotypic characteristics [6]. In particular, aNSCs begin to express the Epidermal Growth Factor Receptor (EGFR). qNSCs do not express proliferation indicator proteins such as Ki67 (a marker expressed during the G1 phase of the cell cycle) [7]. The investigation of the presence and proliferation of neural stem cells and their progeny in the adult primate, including humans, brain is slowed down by the lack of reliable markers for these cells [8,9,10,11,12]. Thus, environmental triggers that can stimulate qNSC to adopt an activated state are useful when studying the qNSC-to-aNSC transition. Ischemic stroke is caused by the thromboembolic blockage of a major cerebral blood vessel or one of its branches. The occlusion of a cerebral vessel leads to blood and oxygen deprivation. This condition is an established trigger of increased brain progenitor cell proliferation in primates [13,14]. A public digital database (www.monkey-niche.org) shows the expression changes in 150 genes that accompany the post-ischemic increase in NSC proliferation in the adult primate SVZ [15]. Here, we visually inspected the images of the 150 genes included in the database and selected 2 candidate genes: TNC and GJA1. We provide evidence suggesting that TNC and GJA1 are candidate genes that mark stem and progenitor cells in the normal adult monkey anterior SVZ (SVZa).
TNC (Tenascin-C) is an extracellular protein found around neurons and glia in the CNS and also in stem cell niches in the intestinal crypts, bone marrow, and hair follicles. Its presence has been described in various pathological conditions, namely, tumor masses, inflammation, and mechanical and chemical injuries, with its expression being enhanced during tissue regeneration [16,17]. As part of the extracellular matrix (ECM), TNC is directly involved in adhesion to other ECM proteins like fibronectin, integrin, collagen, periostin, fibrillin-2, and others [16,17]. TNC has the ability to induce EGFR expression, thus stimulating cell proliferation [18]. TNC interacts with factors like TGFβ (Transforming Growth Factor beta), Wnt3a (Wingless 3a), and VEGF (Vascular Endothelial Growth Factor). TNC is known to be expressed in the rodent neurogenic niche, limited to the subependymal layer (SEL) of the SVZ and rostral migratory stream (RMS) [17]. In the rodent SVZ, TNC expression was detected in GFAP+ cells, most likely qNSCs, but not in cells expressing markers like PSA-NCAM (Polysialylated -–Neural Cell Adhesion Molecule), marking neuroblasts, and Ascl1 (Achaete-scute homolog 1), marking transit-amplifying progenitor cells (TAPs) [19].
GJA1 (Connexin 43) is a transmembrane protein that plays a crucial role in intercellular communication. It is expressed in various tissues and organs, including the brain. GJA1 is expressed in both embryonic and adult subventricular zones, where it plays a role in maintaining the proliferative abilities of NSCs. The elevated expression of GJA1 is sufficient to stimulate the formation of functional extracellular channels, which is an important condition for maintaining and proliferating NSCs. Additionally, the levels of GJA1 decrease as NSCs begin to differentiate into neurons [20,21].
Our previous transcriptomic analysis revealed the significant upregulation of TNC and GJA1 in the SVZ following transient global cerebral ischemia. This selective upregulation points to their relevance in the primate SVZ microenvironment and their potential as key markers for progenitor cell identification [15]. Furthermore, we utilized our bioinformatic analysis of the transcriptome data and compared it to available SVZ transcriptomes. When making this comparison with Dulken et al., we found that GJA1 is enriched in the categories of astrocytes and qNSC-like cells, while TNC was enriched within the astrocyte category [7]. When comparing our data with Llorens-Bobadilla et al.’s dataset, we found that GJA1 was enriched in primed qNSCs [5]. Colorimetric in situ hybridization (ISH) staining showed that these two genes were markedly induced in the subependymal region of the SVZa that hosts the stem progenitor cells in the primate brain [15]. In particular, TNC and GJA1 expression was common in perivascular adventitial cells that represent putative progenitors in the primate SGZ neurogenic niche [22]. In the present study, we performed fluorescent in situ hybridization (FISH) for each of the selected genes followed by the immunohistochemical co-labeling of markers for proliferation and known cell types in order to probe the putative expression of TNC and GJA1 in stem/progenitor cells in intact monkey brains. We studied the expression of these genes before and after cerebral ischemia, which is known to induce SVZ progenitor cell proliferation, and combined these expression studies with immunolabeling for known markers of brain cell types. Our selection of TNC and GJA1 as candidate markers for neural stem/progenitor cells in the primate SVZ is supported by their distinct functional roles, spatial localization, and relevance to neurogenic processes. By highlighting these markers’ specific contributions to identifying and characterizing progenitor populations, we aim to advance the understanding of stem cell biology in the adult primate brain and bridge interspecies differences in neurogenesis research. Our results demonstrate that a combinatorial labeling strategy may prove an effective method in the visualization of neural stem/progenitor cells in the adult primate brain.
2. Results
2.1. TNC and GJA1 Expression in the SVZa Before or After Cerebral Ischemia
Utilizing a publicly available database (www.monkey-niche.org accessed on 18 February 2024) of gene expression before or after brain ischemia in the adult monkey SVZ, we noticed that TNC and GJA1 were markedly increased after ischemia in the subependymal region that hosts the stem/progenitor cells in the primate brain [15]. Moreover, the selected two genes also demonstrated a marked increase in expression with a distinct clustering of the cells in the SVZ and, more notably, perivascularly (Figure 1), which is characteristic of progenitors [22]. It should be noted that the endothelial cells on the SVZ blood vessels (BVs) did not seem to be positive for either TNC or GJA1.
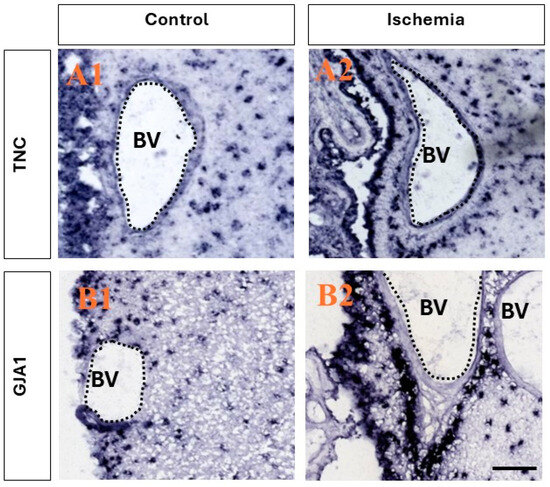
Figure 1.
Ischemia-induced upregulation of the expression of TNC and GJA1 in adult monkey neurogenic niche. (A1,A2) Comparison between the expression of TNC before and after ischemia. After ischemia, a stronger presence is seen in both the ependymal layers and in the SVZ, and the clustering of positive TNC+ cells is observed in close association with the blood vessels in the region, while little to no expression is seen in the epithelial cells lining the blood vessels. (B1,B2) Comparison between the expression of GJA1 before and after ischemia. After ischemic injury, an increase in expression in the ependymal layers and SVZ is observed, and intense staining is observed in close association with the blood vessels, with no staining in the epithelial cells lining the blood vessels. Images derived from www.monkey-niche.org. Scale bar—200 μm. BV—blood vessel.
2.2. Immunophenotype of TNC-Expressing Cells
Immunohistochemical staining for GFAP, VIM, and BrdU was conducted in combination with FISH for TNC. First, we calculated the fraction of double-positive (TNC+/marker+) cells of all SVZ cells (marked by DAPI). Our staining showed that TNC+/GFAP+ cells and TNC+/VIM+ cells represented approximately a quarter of the cells in the SVZa, while TNC+/BrdU+ were only 1.4% of the cells in the SVZa.
We studied the fraction of GFAP+ cells expressing TNC. GFAP is a marker for qNSC/ parenchymal astrocytes [4,7]. We discovered that while two-thirds of the GFAP+ cells co-expressed TNC (95% CI 60.45–74.42%), only one-third of the TNC+ cells were co-labeled for GFAP (95% CI 28.15–37.87%) (a total of 1158 counted cells) (Figure 2). We also studied the fraction of VIM+ cells that were co-labeled for TNC. VIM is a marker for aNSCs, TAPs, and blood vessels [15]. We found that 27% of the TNC+ cells were positive for VIM (95% CI 29.96–43.52%), while 81% of the VIM+ cells expressed TNC (95% CI 74.45–90.42%) (a total of 716 counted cells). To track and quantify de novo generated cells in this area, we injected monkeys with BrdU [13,14] followed by immunostaining. Double-labeling TNC/BrdU experiments showed that 3% of all TNC+ cells were BrdU-positive (95% CI 1.67–3.61%), while 56% of all BrdU+ cells were TNC-positive (95% CI 43.52–71.93%) (a total of 4244 counted cells). To differentiate between qNSCs and aNSCs, we used triple staining, including GFAP and BrdU in combination with TNC. This revealed the existence of a subpopulation of TNC+/GFAP+/BrdU+, 1.3% of all DAPI+ cells, representing NSCs initiating proliferation (Figure 2(D4)). In contrast, the non-proliferative (quiescent) qNSC fraction (TNC+/GFAP+/BrdU−) was estimated to be 21.4% of all SVZa cells (Figure 2(D4)).
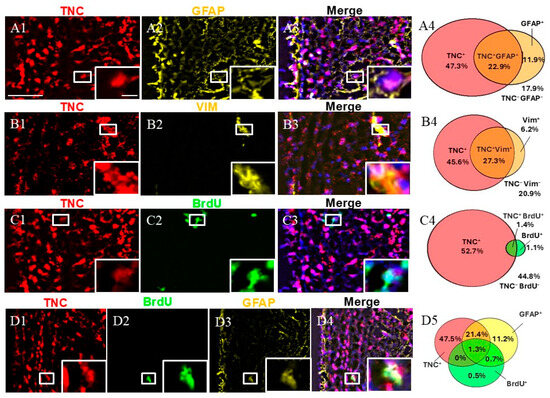
Figure 2.
Expression pattern of TNC+ cells in adult monkey SVZa. (A1–A3) Dual staining for TNC (red) and GFAP (yellow) counterstained with DAPI. TNC/GFAP co-staining demonstrates the presence of TNC+/GFAP+ cells (insert). (B1–B3) Dual staining for TNC (red) and VIM (yellow). TNC/VIM co-staining reveals the presence of TNC+/VIM+ cells (insert). (C1–C3) Dual staining for TNC (red) and BrdU (green). TNC/VIM co-staining shows the presence of TNC+/BrdU+ cells (insert). (D1–D4) Triple staining for TNC (red), GFAP (yellow), and BrdU (green) demonstrates the presence of the proliferative NSCs (TNC+/GFAP+/BrdU+; insert). (A4,B4,C4,D5) Venn diagrams depicting the share of different cell subpopulations. The percentages reflect the fraction of a specific cellular expression pattern of all cells in the SVZ as stained by DAPI. Scale bar—100 μm. Scale bar insert—20 μm.
2.3. Immunophenotype of GJA1-Expressing Cells
An immunophenotypic analysis of GJA1+ cells was performed using the markers GFAP, VIM, and BrdU (Figure 3). Dual labeling revealed that while GJA1+/GFAP+ represented approximately a quarter of the SVZ cells (Figure 3(A4)), GJA1+/VIM+ and GJA1+/BrdU+ were less than 10% of the SVZ cells (Figure 3(B4,C4)).
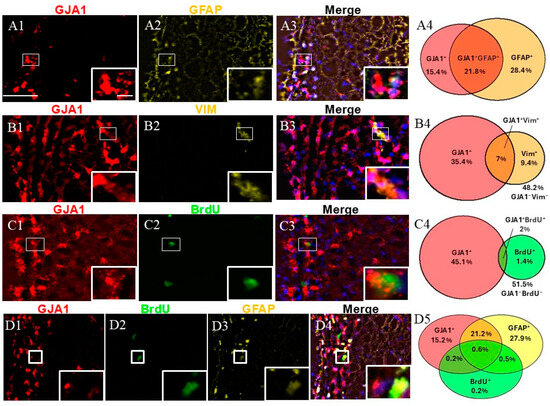
Figure 3.
Cell identity of GJA1+ cells in adult monkey rostral SVZ. (A1–A3) Dual staining for GJA1 (red) and GFAP (yellow). GJA1/GFAP co-staining demonstrates the presence of GJA1+/GFAP+ cells (insert). (B1–B3) Dual staining for GJA1 (red) and VIM (yellow) reveals the presence of GJA1+/VIM+ cells (insert). (C1–C3) Dual staining for GJA1 (red) and BrdU (green). GJA1/BrdU co-staining reveals the presence of proliferative cells GJA1+/BrdU+ (insert). (D1–D4) Triple staining for GJA1 (red), GFAP (yellow), and BrdU (green). GJA1/GFAP/BrdU co-staining demonstrates the presence of proliferative NSCs (GJA1+GFAP+BrdU+, insert). (A4,B4,C4,D5) Venn diagrams depicting the different cell subpopulations. The percentages reflect the fraction of a specific cellular expression pattern of all cells in the SVZ as stained by DAPI. Scale bar—100 μm. Scale bar insert—20 μm.
We studied the fraction of GFAP+ cells expressing GJA1. More than half of the GJA1+ fraction co-expressed GFAP (95% confidence interval: 45.92–60.64%), and again, nearly half of the GFAP+ cells co-expressed GJA1 (95% confidence interval: 34.26–47.04%) (a total of 1033 counted cells). The evaluation of GJA1/VIM showed that 16.4% of all GJA1+ cells co-expressed VIM (95% confidence interval: 14.01–20.60%), and nearly half (42%) of the VIM+ cells co-expressed GJA1 (95% confidence interval: 33.40–53.78%) (a total of 1250 counted cells). Additionally, 4% of all BrdU+ cells co-expressed GJA1 (95% confidence interval: 2.46–5.72), whereas more than half (57.6%) of all GJA1+ cells co-expressed BrdU (95% confidence interval: 43.33–73.52%) (a total of 2717 counted cells). Finally, when we evaluated a triple combination of BrdU, GFAP, and GJA1, our results revealed that 0.6% of all cells were triple-positive (GJA1+/GFAP+/BrdU+), representing NSCs in a proliferative state, while 21.1% of the cells were GJA1+/GFAP+/BrdU−, thus representing a quiescent state (a total of 1033 counted cells).
2.4. Dual Labeling for TNC and GJA1
Next, we investigated the cells expressing both TNC and GJA1 (Figure 4). To this end, we utilized dual FISH staining for TNC and GJA1 and found that 27.2% of all studied SVZ cells were TNC+/GJA1+, while 21.3% were TNC+/GJA1− and 16.5% were TNC−/GJA1+ (Figure 4(A1–A4)) (a total of 2540 counted cells). To investigate deeper the expression pattern of these cell subpopulations, we combined TNC/GJA1 dual FISH with immunohistochemical staining for BrdU or VIM (Figure 4(B1–C5)). We discovered that triple-positive TNC+/GJA1+/BrdU+ cells represented a very small (~1%) fraction of the SVZ cells (Figure 4(B5)) (a total of 1041 counted cells), while the TNC+/GJA1+/VIM+ cells accounted for 7–8% of the SVZ cells (Figure 4(C5)) (a total of 716 counted cells). Interestingly, we noticed the presence of a significant subpopulation of SVZ cells that were GJA1+/TNC−(~25% of the SVZ cells), and these cells expressed neither BrdU (Figure 4(B5)) nor VIM (Figure 4(C5)).
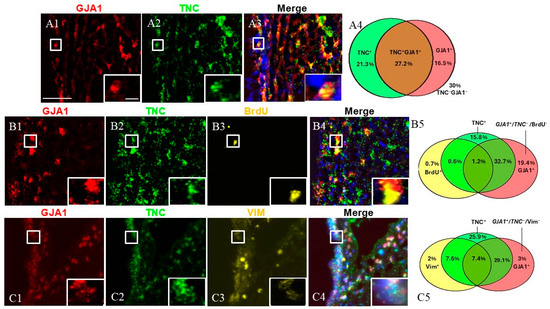
Figure 4.
SVZa cell subpopulations expressing TNC and GJA1. (A1–A3) Dual FISH staining for TNC (green) and GJA1 (red). A TNC+/GJA1+ cell is shown in the insert. (B1–B4) Dual FISH staining for TNC (green) and GJA1 (red) in combination with BrdU (yellow) demonstrates the presence of a GJA1+/TNC−/BrdU− population (C1–C4). Dual FISH staining for TNC (green) and GJA1 (red) in combination with VIM (yellow) demonstrates the presence of a GJA1+/TNC−/VIM−population (insert). (A4,B5,C5) Venn diagrams depicting the different cell subpopulations. The percentages reflect the fraction of a specific cellular pattern of all SVZa cells (stained by DAPI). Scale bar: 100 μm. Scale bar insert: 20 μm.
3. Discussion
The level of neurogenesis varies among species, with it being lower in primates compared to the commonly used rodent models [23]. Furthermore, research on adult neurogenesis in non-human primates offers limitations due to technological and ethical constraints [24,25,26]. Of note, the structural organization of the NSC niche shows interspecies differences between rodents and primates [27]. Thus, the identification of markers of progenitor cell populations in the stem cell niche of primates is of pivotal importance in tracking these cells and being able to identify them in their complex environment in the niche [28,29].
Aging is a critical factor influencing neurogenesis and the expression of key markers in the primate SVZ. Studies have shown that TNC expression decreases with age in tissues such as skin, cartilage, and the cardiovascular system. This downregulation is linked to reduced regenerative capacity and extracellular matrix remodeling in conditions like fibrosis and cardiovascular diseases [30,31]. This response may exacerbate maladaptive remodeling and inflammation. Aging is also associated with decreased GJA1 expression in many tissues, impairing gap-junction-mediated communication [32]. Aging impacts the phosphorylation, localization, and turnover of GJA1, reducing its effectiveness [33]. Age-related oxidative stress and inflammation exacerbate GJA1 dysfunction, particularly in the nervous system [34].
In addition to the expression of TNC and GJA1, various signaling pathways, such as BMP, WNT, and NOTCH, are critical in regulating neurogenesis and progenitor cell behavior in the adult brain, particularly in response to injury or aging [35,36]. NOTCH signaling, known for maintaining progenitor cell quiescence, likely plays a role in regulating the activation of cells expressing TNC and GJA1 [37]. BMP signaling, which is involved in cell differentiation and repair, might modulate the transition of progenitors from quiescence to an active state [38]. Meanwhile, WNT signaling is crucial for progenitor cell proliferation and migration, which could be particularly relevant in ischemic injury models where tissue regeneration is required [39]. The interactions between these pathways, particularly in the context of TNC and GJA1 expression, might dictate the behavior of progenitor cells and their response to injury, including ischemia.
Further studies exploring how these pathways interact with TNC and GJA1 in the primate SVZ will be important to understand how cellular behaviors such as activation, proliferation, and differentiation are regulated in the stem cell niche of primates.
Here, we report for the first time the cellular immunophenotypic characteristics of cells expressing TNC and GJA1 in the adult primate SVZ. Our findings show that in the normal adult monkey SVZ, TNC and GJA1 are expressed by both qNSCs and aNSCs. Our combined FISH/immunofluorescent analyses revealed two subpopulations of TNC+ cells: GFAP+/TNC+/BrdU− (putative qNSCs) and GFAP+/TNC+/BrdU+ (putative aNSCs). Thus, while TNC is expressed by stem cells in both rodents and monkeys, in monkeys, TNC is also expressed by the aNSC/TAP (TNC+/VIM+) population of progenitors. In the rodent SVZ, TNC expression is restricted to GFAP+ cells [19], and thus, our data showing that TNC expression is maintained in a primate aNSC/TAP population suggest an interspecies difference.
Similarly to TNC, we detected two subpopulations of GJA1+ cells: GFAP+/GJA1+/BrdU− (putative qNSCs) and GFAP+/GJA1+/BrdU+ (putative aNSCs). GJA1 has a role in maintaining the proliferation and self-renewal of NSCs in mice. This is in line with our data, which may represent an interspecies similarity in the stem cell biology of the SVZ. We also observed a distinct decrease in gene expression when cells gradually differentiated from qNSCs to aNSCs in the two examined genes.
Additionally, using co-staining with TNC and GJA1, we discovered a subpopulation of GJA1+/TNC− cells, which was negative for both VIM and BrdU (Figure 4). The GJA1+/TNC− expression pattern most likely corresponds to a quiescent progenitor or to niche astrocytes.
A present limitation in brain stem cell research is the fact that the currently used markers can label more than one type of cellular subpopulation. For example, GFAP can label both qNSCs and niche astrocytes. Similarly, VIM and BrdU can label more than one cell population. In the current study, we chose a different starting point when focusing on the genes of interest: we used cerebral ischemia as a tool to trigger stem cell activation, and, at the same time, we searched for genes with a specific perivascular expression pattern, as such a pattern was proved to label progenitors in the hippocampal niche [22]. Furthermore, we employed a triple-labeling combining FISH with dual immunofluorescence approach to combine the gene of interest with the expression of not one but two different markers, in combinations. This combinatorial labeling allowed us to distinguish between qNSCs (GFAP+/VIM−/BrdU−) or aNSCs (GFAP−/VIM+/BrdU+) and define the fractions of these progenitors that express TNC and GJA1. An important step in differentiating qNSCs from niche astrocytes emerged from our effort to better characterize the molecular features of TNC− and GJA1− expressing cells. One putative fraction representing the niche astrocytes is the population of GJA1+/TNC− cells that we observed. Future research may clarify the molecular mechanisms by which cerebral ischemia regulates the expression of TNC and GJA1.
4. Materials and Methods
For our experiments, we used tissues from 4 adult (four–six years old at the time of the experiment) macaque monkeys (Macaca fuscata). The tissues were derived following an experimental procedure approved by the Animal Care and Ethics Committee of Kanazawa University, Japan (approval protocols #AP-031498 and #AP-080920). Ischemia was induced using surgical procedures described previously [13,14]. To induce global ischemia, each monkey was anesthetized (ketamine at a dose of 2–5 mg/kg, i.m.), intubated, and connected to a ventilator. During the surgical procedures, the monkeys were additionally anesthetized via inhalation (1% halothane, gas mixture 40% O2/60% N2O). Arterial blood pressure, pulse, pupil diameter, and response were monitored. During the operation, the animals’ body temperature was maintained within 37 ± 0.5 °C. The surgical procedure for global ischemia was performed under sterile conditions in the following sequence: anterior median thoracotomy, the dissection of the skin and subcutaneous tissue, sternotomy, the dissection of soft tissues, and the visualization of the left subclavian artery and brachiocephalic trunk. One of the four monkeys received 5-bromo-2’-deoxyuridine (BrdU 100 mg/kg, i.v., from Sigma-Aldrich Corp., St. Louis, MO, USA) for 5 days and was sacrificed 2 h after the last BrdU application. Coronal sections of the anterior subventricular zone (SVZa) were cut on a freezing microtome (Leica CM3050 S, Leica Biosystems, Deer Park, IL, USA) at 20 micrometers. The non-radioactive in situ hybridization (ISH) staining procedure and the combinatorial labeling protocol with cell type markers have been published previously [15]. Briefly, macaque monkey-specific templates, 600–900 nucleotides long, were synthesized using RNA isolation, cDNA synthesis, and PCR amplification. The templates were generated using the following specific primers—TNC Forward: CAGAG-GAAGGAGCTCGCTA; TNC Reverse: GACACCAGGTTCTCCAGCTC; GJA1 Forward: AGCCTACTCAACTGCTGGAG; and GJA1 Reverse: TCGCCAGTAACCAGCTTGTA. The primer and template sequences used for the ISH of the genes TNC and GJA1 were identical to those published at www.monkey-niche.org [20]. In vitro transcription was used to generate Digoxigenin-tagged riboprobes. Hybridized probes were identified using a two-step chromogenic catalyzed reporter deposition method. For FISH staining, the TNC and GJA1 hybridized probe was detected with fluorochrome-labeled reagents. Following fluorescent ISH (FISH), sections were subjected to antigen retrieval (Dako PT Link, Agilent Technologies, Santa Clara, CA, USA) with a citrate buffer (pH 6) and blocked with Bovine serum albumin diluted 1:10 in 1% of Triton X-100/PBS. We used the following primary antibodies: anti-BrdU (1:100, Cat. No Ab6326, Abcam, Cambridge, UK), anti-GFAP (1:400 Cat. No M0761, Dako-Agilent Technologies GmbH, Hamburg, Germany), anti-GFAP (1:1000, Cat. No AB5541, Merck Millipore, Burlington, MA, USA), and anti-VIM (1:1000, Cat. No MAB3400, Merck Millipore, Burlington, MA, USA). The primary antibodies were visualized by species-specific secondary antibodies, counterstained with DAPI and covered with mountant media (Invitrogen™ ProLong™ Gold Antifade Mountant, Thermo Fisher Scientific, Waltham, MA, USA). A high-resolution mosaic image of the periventricular tissues was captured using an EC Plan-Neofluar 20×/0.50 objective with a lateral resolution of 0.65 µm/px. These images serve as a reference database and are intended for future analyses. A series of 3 to 5 z-stacks per microscope slide were obtained from the SVZ using an EC Plan-Neofluar 40×/0.75 objective, with a lateral resolution of 0.325 µm/px and an axial resolution (z-distance) ranging from 0.125 to 0.55 µm. The camera was set to a binning factor of 2 × 2 to reduce both scanning time and camera noise. The z-stacks from the striatal SVZ were captured sequentially, starting from the dorsolateral edge of the ventricle and progressing medially. The co-localization quantification of cells labeled by fluorescent immunohistochemistry was conducted as described previously [15]. Briefly, using a semi-automated digital workflow, we processed the acquired images by channel extraction and denoising followed by the top-hat filtering, channel thresholding, classification, and counting of the cell in a region of interest (1000 cells per specimen).
Author Contributions
Conceptualization, M.N.I. and A.B.T.; methodology, M.N.I. and A.B.T.; investigation, M.N.I., D.S.S., L.V.V., A.M.M. and S.P.P.; resources, M.N.I., A.B.T. and T.Y.; writing—original draft preparation, M.N.I. and A.B.T.; writing—review and editing, M.N.I., A.B.T. and T.Y.; visualization, M.N.I.; supervision, A.B.T.; funding acquisition, M.N.I. and A.B.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an intramural grant of the Medical University-Varna (21020/2021) (M.N.I and A.B.T) and The European Commission Horizon 2020 Framework Program (Project 856871—TRANSTEM) (A.B.T).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Ethics Committee of Kanazawa University, Japan (protocol codes AP-031498 and AP-080920).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article.
Acknowledgments
We are grateful for the technical assistance of Velina Kenovska.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ming, G.-L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Bond, A.M.; Ming, G.L.; Song, H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, W. Neural Stem Cell Niche and Adult Neurogenesis. Neuroscientist 2021, 27, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Codega, P.; Silva-Vargas, V.; Paul, A.; Maldonado-Soto, A.R.; Deleo, A.M.; Pastrana, E.; Doetsch, F. Prospective Identification and Purification of Quiescent Adult Neural Stem Cells from Their In Vivo Niche. Neuron 2014, 82, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Bobadilla, E.; Zhao, S.; Baser, A.; Saiz-Castro, G.; Zwadlo, K.; Martin-Villalba, A. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells That Become Activated upon Brain Injury. Cell Stem Cell 2015, 17, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Chaker, Z.; Codega, P.; Doetsch, F. A Mosaic World: Puzzles Revealed by Adult Neural Stem Cell Heterogeneity. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 640–658. [Google Scholar] [CrossRef]
- Dulken, B.W.; Leeman, D.S.; Boutet, S.C.; Hebestreit, K.; Brunet, A. Single-Cell Transcriptomic Analysis Defines Heterogeneity and Transcriptional Dynamics in the Adult Neural Stem Cell Lineage. Cell Rep. 2017, 18, 777–790. [Google Scholar] [CrossRef]
- Flor-García, M.; Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Ávila, J.; Rábano, A.; Llorens-Martín, M. Unraveling Human Adult Hippocampal Neurogenesis. Nat. Protoc. 2020, 15, 668–693. [Google Scholar] [CrossRef]
- Liu, Y.W.J.; Curtis, M.A.; Gibbons, H.M.; Mee, E.W.; Bergin, P.S.; Teoh, H.H.; Connor, B.; Dragunow, M.; Faull, R.L.M. Doublecortin Expression in the Normal and Epileptic Adult Human Brain. Eur. J. Neurosci. 2008, 28, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Terreros-Roncal, J.; Flor-García, M.; Rábano, A.; Llorens-Martín, M. Evidences for Adult Hippocampal Neurogenesis in Humans. J. Neurosci. 2021, 41, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Verwer, R.W.H.; Sluiter, A.A.; Balesar, R.A.; Baayen, J.C.; Noske, D.P.; Dirven, C.M.F.; Wouda, J.; Van Dam, A.M.; Lucassen, P.J.; Swaab, D.F. Mature Astrocytes in the Adult Human Neocortex Express the Early Neuronal Marker Doublecortin. Brain 2007, 130, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, J.; Lier, J.; Franke, H.; Hammer, N.; Matschke, J.; Trautz, F.; Tse, R.; Ondruschka, B. GFAP Positivity in Neurons Following Traumatic Brain Injuries. Int. J. Legal Med. 2021, 135, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Tonchev, A.B.; Yamashima, T.; Zhao, L.; Okano, H.J.; Okano, H. Proliferation of Neural and Neuronal Progenitors after Global Brain Ischemia in Young Adult Macaque Monkeys. Mol. Cell. Neurosci. 2003, 23, 292–301. [Google Scholar] [CrossRef]
- Tonchev, A.B.; Yamashima, T.; Sawamoto, K.; Okano, H. Enhanced Proliferation of Progenitor Cells in the Subventricular Zone and Limited Neuronal Production in the Striatum and Neocortex of Adult Macaque Monkeys after Global Cerebral Ischemia. J. Neurosci. Res. 2005, 81, 776–788. [Google Scholar] [CrossRef]
- Chongtham, M.C.; Wang, H.; Thaller, C.; Hsiao, N.-H.; Vachkov, I.H.; Pavlov, S.P.; Williamson, L.H.; Yamashima, T.; Stoykova, A.; Yan, J.; et al. Transcriptome Response and Spatial Pattern of Gene Expression in the Primate Subventricular Zone Neurogenic Niche After Cerebral Ischemia. Front. Cell Dev. Biol. 2020, 8, 584314. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a Glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef]
- Tucić, M.; Stamenković, V.; Andjus, P. The Extracellular Matrix Glycoprotein Tenascin C and Adult Neurogenesis. Front. Cell Dev. Biol. 2021, 9, 674199. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Halilagic, A.; Faissner, A.; ffrench-Constant, C. Generation of an Environmental Niche for Neural Stem Cell Development by the Extracellular Matrix Molecule Tenascin C. Development 2004, 131, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Kazanis, I.; Belhadi, A.; Faissner, A.; Ffrench-Constant, C. The Adult Mouse Subependymal Zone Regenerates Efficiently in the Absence of Tenascin-C. J. Neurosci. 2007, 27, 13991–13996. [Google Scholar] [CrossRef]
- Cheng, A.; Tang, H.; Cai, J.; Zhu, M.; Zhang, X.; Rao, M.; Mattson, M.P. Gap Junctional Communication Is Required to Maintain Mouse Cortical Neural Progenitor Cells in a Proliferative State. Dev. Biol. 2004, 272, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Duval, N.; Gomès, D.; Calaora, V.; Calabrese, A.; Meda, P.; Bruzzone, R. Nathalie Duval Cell Coupling and Cx43 Expression in Embryonic Mouse Neural Progenitor Cells. J. Cell Sci. 2002, 115, 3241–3251. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Tonchev, A.B.; Vachkov, I.H.; Popivanova, B.K.; Seki, T.; Sawamoto, K.; Okano, H. Vascular Adventitia Generates Neuronal Progenitors in the Monkey Hippocampus after Ischemia. Hippocampus 2004, 14, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Alunni, A.; Bally-Cuif, L. A Comparative View of Regenerative Neurogenesis in Vertebrates. Dev. Camb. 2016, 143, 741–753. [Google Scholar] [CrossRef]
- Bonfanti, L.; Peretto, P. Adult Neurogenesis in Mammals—A Theme with Many Variations. Eur. J. Neurosci. 2011, 34, 930–950. [Google Scholar] [CrossRef]
- Shook, B.A.; Manz, D.H.; Peters, J.J.; Kang, S.; Conover, J.C. Spatiotemporal Changes to the Subventricular Zone Stem Cell Pool through Aging. J. Neurosci. 2012, 32, 6947–6956. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Zhang, Z.; Kang, G.; Pastor-Alonso, O.; Biagiotti, S.; Page, C.E.; Sandova, K.; Knox, A.; Connolly, A.; et al. Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus. J. Neurosci. 2021, 41, 2554–2565. [Google Scholar] [CrossRef] [PubMed]
- Quiñones-Hinojosa, A.; Sanai, N.; Gonzalez-Perez, O.; Garcia-Verdugo, J.M. The Human Brain Subventricular Zone: Stem Cells in This Niche and Its Organization. Neurosurg. Clin. N. Am. 2007, 18, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Donega, V.; van der Geest, A.T.; Sluijs, J.A.; van Dijk, R.E.; Wang, C.C.; Basak, O.; Pasterkamp, R.J.; Hol, E.M. Single-Cell Profiling of Human Subventricular Zone Progenitors Identifies SFRP1 as a Target to Re-Activate Progenitors. Nat. Commun. 2022, 13, 1036. [Google Scholar] [CrossRef]
- Beckervordersandforth, R.; Tripathi, P.; Ninkovic, J.; Bayam, E.; Lepier, A.; Stempfhuber, B.; Kirchhoff, F.; Hirrlinger, J.; Haslinger, A.; Lie, D.C.; et al. In Vivo Fate Mapping and Expression Analysis Reveals Molecular Hallmarks of Prospectively Isolated Adult Neural Stem Cells. Cell Stem Cell 2010, 7, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, Y.H.; Lee, D.H.; Chung, J.H.; Lee, S.-T. Expression of Tenascin-C Is down-Regulated during Intrinsic Skin Aging. J. Dermatol. Sci. 2017, 86, e92–e93. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Wang, W.; Morales-Nebreda, L.; Feng, G.; Wu, M.; Zhou, X.; Lafyatis, R.; Lee, J.; Hinchcliff, M.; Feghali-Bostwick, C.; et al. Tenascin-C Drives Persistence of Organ Fibrosis. Nat. Commun. 2016, 7, 11703. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-I.; Chang, H.-M.; Lu, W.-W.; Lee, Y.-N.; Ko, Y.-S.; Severs, N.J.; Tsai, C.-H. Age-Related Alteration of Gap Junction Distribution and Connexin Expression in Rat Aortic Endothelium. J. Histochem. Cytochem. 2000, 48, 1377–1389. [Google Scholar] [CrossRef]
- Jansen, J.A.; Van Veen, T.A.B.; De Jong, S.; Van Der Nagel, R.; Van Stuijvenberg, L.; Driessen, H.; Labzowski, R.; Oefner, C.M.; Bosch, A.A.; Nguyen, T.Q.; et al. Reduced Cx43 Expression Triggers Increased Fibrosis Due to Enhanced Fibroblast Activity. Circ. Arrhythmia Electrophysiol. 2012, 5, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Spitale, F.M.; Vicario, N.; Rosa, M.D.; Tibullo, D.; Vecchio, M.; Gulino, R.; Parenti, R. Increased Expression of Connexin 43 in a Mouse Model of Spinal Motoneuronal Loss. Aging 2020, 12, 12598–12608. [Google Scholar] [CrossRef]
- Yousef, H.; Morgenthaler, A.; Schlesinger, C.; Bugaj, L.; Conboy, I.M.; Schaffer, D.V. Age-Associated Increase in BMP Signaling Inhibits Hippocampal Neurogenesis. Stem Cells 2015, 33, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Armenteros, T.; Andreu, Z.; Hortigüela, R.; Lie, D.C.; Mira, H. BMP and WNT Signalling Cooperate through LEF1 in the Neuronal Specification of Adult Hippocampal Neural Stem and Progenitor Cells. Sci. Rep. 2018, 8, 9241. [Google Scholar] [CrossRef]
- Sarkar, S.; Mirzaei, R.; Zemp, F.J.; Wei, W.; Senger, D.L.; Robbins, S.M.; Yong, V.W. Activation of NOTCH Signaling by Tenascin-C Promotes Growth of Human Brain Tumor-Initiating Cells. Cancer Res. 2017, 77, 3231–3243. [Google Scholar] [CrossRef]
- Bond, A.M.; Peng, C.-Y.; Meyers, E.A.; McGuire, T.; Ewaleifoh, O.; Kessler, J.A. BMP Signaling Regulates the Tempo of Adult Hippocampal Progenitor Maturation at Multiple Stages of the Lineage. Stem Cells 2014, 32, 2201–2214. [Google Scholar] [CrossRef]
- Shruster, A.; Ben-Zur, T.; Melamed, E.; Offen, D. Wnt Signaling Enhances Neurogenesis and Improves Neurological Function after Focal Ischemic Injury. PLoS ONE 2012, 7, e40843. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).