Abstract
Prostate cancer (PCa) is the second most prevalent cancer among men and a major cause of cancer-related mortality worldwide. Despite an initial favorable response to hormone-based therapies, many patients ultimately develop an advanced and lethal form of the disease, referred to as castration-resistant PCa (CRPC). CRPC is associated with poor prognosis and a lack of effective curative treatments. As a result, new alternatives or improved therapeutic strategies to combat this life-threatening condition are urgently needed. Chalcones, also referred to as 1,3-diphenyl-2-propen-1-ones, have attracted significant attention because of their potent antitumor properties. Owing to their distinctive chemical structure and diverse biological activities, these compounds are promising candidates for treating various cancers, including PCa. Both naturally occurring and synthetically derived chalcones have demonstrated anticancer potential by modulating key cellular processes, including apoptosis, cell cycle regulation, cell migration, invasion, metastasis and angiogenesis, as well as major signaling pathways, such as PI3K/Akt/mTOR, androgen signaling, and NF-κB. This review aims to outline the recent advances in the therapeutic potential of chalcone derivatives in prostate cancer, with a focus on their molecular targets, mechanisms of action, and translational relevance.
1. Introduction
Globally, prostate cancer (PCa) ranks as the second most commonly diagnosed cancer and a major cause of cancer-related mortality in males [1,2]. In 2022, 1,467,854 individuals were diagnosed with PCa, and 397,430 died from the disease [1]. PCa is more common in Western countries, with incidence rates in developed nations being over 25-fold higher than those in developing countries [3].
The onset and development of PCa are associated with several genetic and environmental risk factors, including age, race, genetic mutations, and family history [4]. The risk of PCa increases dramatically with age, where approximately 60% of PCa cases are diagnosed in men older than 65 years [5]. Furthermore, Asians exhibit a lower probability of developing PCa, compared to African Americans, who exhibit a significantly higher incidence and mortality rates, approximately twice that of other ethnic groups [5,6]. Family history is a notable risk factor for PCa, as having a relative with the disease doubles an individual’s likelihood of developing it. The risk increases further if multiple family members are affected or if the diagnosis occurs before the age of 65 [7,8]. Genetic mutations associated with an increased PCa susceptibility are classified as prevalent high-risk mutations and infrequent low-risk mutations [9]. One significant example of a rare but influential mutation occurs in the breast cancer susceptibility gene 2 (BRCA2), which is present in approximately 1% of early-onset PCa patients [10]. Carriers of this mutation are 5 to 7 times more likely to develop PCa than non-carriers [9,11].
In recent years, progress in early diagnosis, along with the introduction of new treatments, has significantly improved the overall survival rates for PCa patients. However, despite these improvements, PCa continues to pose a major health burden and remains among the primary causes of cancer-related deaths [12]. For those with metastatic PCa, prognosis remains poor, with a markedly low 5-year survival rate of approximately 30% [5]. Furthermore, more than 20% of individuals diagnosed with localized PCa eventually experience disease recurrence and progression to metastatic PCa, a stage for which curative treatment options remain unavailable [13].
Considering the high prevalence of PCa and the low survival rates in metastatic cases, there is an urgent need for innovative and effective curative therapies. Chalcone derivatives have emerged as a promising therapeutic target for treating PCa and other cancers. Chalcones, also referred to as benzylideneacetophenones, are open-chain flavonoids found in many plant species [14]. Given their attractive biological activities, plants rich in chalcones have been used in folk medicine for centuries [15]. These derivatives exhibit a wide array of biological activities, including anti-diabetic, anti-hypertensive, antiviral and antibacterial effects [16,17,18,19]. Additionally, epidemiological studies on diets rich in polyphenols, such as chalcones, suggest an association with reduced cancer incidence [20]. Consequently, chalcones may hold promise as novel anticancer therapies.
To the best of our knowledge, no comprehensive reviews have focused specifically on the role of chalcone derivatives in PCa. By integrating the most recent research and bringing together various viewpoints, this review aims to address a significant gap in the literature. This study also provides a distinct and valuable perspective on the potential therapeutic opportunities that chalcone derivatives may offer in the management of PCa.
2. Prostate Cancer (PCa)
The majority of newly diagnosed untreated PCa patients remain responsive to androgens and depend on androgen for their development [21]. As a result, androgen deprivation therapy (ADT) is the primary therapy for metastatic PCa [22,23]. ADT aims to lower testosterone levels in serum, therefore reducing the binding of androgen to the androgen receptor (AR), which in turn diminishes AR-mediated transcriptional activity. Androgen deprivation can be attained through surgical castration (orchiectomy, which removes the testicles) or reversible medical castration via the use of drugs such as anti-androgens or luteinizing hormone-releasing hormone (LHRH) agonists or antagonists [22,23]. Despite their effectiveness, the response to these therapies is generally temporary, and ultimately, all PCa patients develop resistance, leading to progression to a more advanced and often fatal form known as castration-resistant prostate cancer (CRPC) [24].
Metastatic CRPC (mCRPC) continues to present a significant concern despite the introduction of numerous innovative treatments. The use of chemotherapy for the treatment of mCRPC was established in 2004 with the approval of docetaxel (DTX), a cytotoxic anti-microtubule agent, by the US Food and Drug Administration (FDA) for this purpose [24,25]. While various cytotoxic agents, including 5-fluorouracil, cisplatin, doxorubicin, and capecitabine, have been investigated in earlier trials, none have demonstrated considerable survival benefits, with therapeutic responses observed in only 10–20% of patients [26]. The approval of DTX was based on phase III trials, which revealed a slight survival advantage of up to 3 months for mCRPC patients [27,28]. Since then, DTX has remained the standard first-line therapy for mCRPC and was the sole drug proven to increase survival until 2010. Nevertheless, its survival advantage is temporary, and therapy resistance is unavoidable [25]. Moreover, DTX is a potent drug with a narrow therapeutic index, often resulting in severe side effects such as hypersensitivity reactions, anemia, and neutropenia [29,30].
In the past decade, the FDA has approved a few drugs for the treatment of mCRPC. Despite these advancements, these therapies have not been able to significantly extend overall survival, with improvements generally limited to approximately five months [31,32]. Furthermore, many patients exhibit intrinsic resistance, resulting in a lack of response to these treatments. The newly approved therapies comprise non-endocrine-based agents such as sipuleucel-T, alpharadin and cabazitaxel (CBZ), as well as androgen receptor axis-targeted (ARAT) therapies, such as darolutamide, enzalutamide, abiraterone and apalutamide.
CBZ received approval in 2010 for the management of DTX-resistant mCRPC [33,34]. In 2017, the FIRSTANA trial reported that CBZ was non-inferior to DTX, positioning it as a viable treatment for mCRPC patients with no prior exposure to chemotherapy [35].
That same year, sipuleucel-T became the first immunotherapy to gain approval for the treatment of asymptomatic or minimally symptomatic mCRPC patients [35]. Sipuleucel-T, a cancer vaccine and form of autologous immunotherapy, functions by directing dendritic cells to attack prostatic acid phosphatase, a protein present in 95% of PCa tissues [36,37]. However, its application is restricted to patients with gradually advancing disease, for whom an immediate clinical improvement is not essential [32].
Radium-223 is a recently approved therapy that offers benefits for certain patients with mCRPC. As a calcium-mimetic radioisotope, radium-223 specifically targets metastases in bone tissues, which are the most prevalent site of metastasis in PCa [38]. Consequently, it is recommended only for patients exhibiting symptomatic bone metastases without any indication of visceral metastasis [35]. Although this treatment has a survival benefit of 2 to 4 months, it does not offer a cure, and most patients will eventually develop resistance [31,39,40].
Recent published studies have highlighted the persistent importance of AR signaling in the progression of several mCRPC cases [41]. While ADT can lower systemic androgen levels by 90–95%, it only reduces intra-tumoral androgen levels by approximately 50% [42,43]. In light of these findings and an enhanced comprehension of AR signaling, second-generation ARAT therapies have been developed to attain more effective suppression of AR signaling [44]. Among these, abiraterone and enzalutamide have improved overall survival and have gained FDA approval for the management of mCRPC. Abiraterone works by reducing intra-tumoral androgens through the inhibition of cytochrome P450 17A1 (CYP17A1), an enzyme required for testosterone synthesis [13,45]. Alternatively, enzalutamide is a nonsteroidal anti-androgen that inhibits AR signaling at multiple levels. Like first-generation anti-androgens, enzalutamide functions as a competitive antagonist of the AR by binding to its ligand-binding domain (LBD), preventing androgen attachment. Its binding affinity is 5–8 times stronger than that of earlier anti-androgens [21,46]. Moreover, enzalutamide inhibits AR activity by blocking the translocation of AR to the nucleus and its subsequent attachment to DNA, which in turn suppresses the transcription of tumor-related genes [46]. Both abiraterone and enzalutamide have substantially improved overall survival, extending life by up to 4.8 months [47]. However, up to 40% of patients experience primary resistance and do not benefit from these treatments [48,49]. For patients who respond, the benefits are often short-lived, with secondary resistance developing within a few months. Additionally, the restricted therapeutic alternatives available for refractory PCa patients and the uncertain survival benefits they provide highlight the challenges posed by ARAT agents, which can dramatically alter cancer cell biology, complicating the future management of PCa [50].
The FDA granted approval for rucaparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, in May 2020 to treat mCRPC patients who have BRCA mutations and have previously undergone ADT or chemotherapy [51]. Compared with non-carriers, patients with BRCA mutations represent 5.3% of mCRPC cases and generally experience less favorable outcomes and reduced overall survival [52]. The same month, olaparib, an additional PARP inhibitor, was approved for use in mCRPC patients who have homologous recombination repair (HRR) defects, including individuals with BRCA mutations [53]. Furthermore, in 2017, pembrolizumab, a PD-1 inhibitor, gained approval for use in advanced solid tumors that are associated with mismatch repair deficiencies or microsatellite instability [32,54]. The NCCN guidelines indicate that mismatch repair mutations are present in 2–5% of mCRPC patients [35].
Although therapeutic alternatives for mCRPC have markedly increased over the last 10 years, the disease continues to be largely incurable, with only limited improvements in survival rates. A substantial number of mCRPC patients exhibit resistance to available therapies, and among those who respond, resistance frequently develops in a relatively short time. The prognosis for mCRPC patients remains poor, with a short median overall survival of approximately 1–2 years [32]. Therefore, there is an urgent need for the development of innovative and more effective treatment strategies.
Early strategies focused on targeting alternative pathways in combination with AR inhibitors are being proposed to inhibit the development of aggressive disease forms during PCa treatment [55,56,57]. An increasing number of studies suggest that the current strategy of sequentially using AR-targeted therapies may lead to cross-resistance, extending beyond the specific agents used and potentially affecting all available therapies, including taxanes [57,58,59]. These findings underscore the pressing necessity for innovative medicines targeting other pathways, which could serve as potential treatments for resistant PCa patients or as complementary approaches to improve the efficacy of AR-targeted therapies. Chalcones, a promising class of compounds, are currently under investigation for their potential as multitarget anticancer agents.
3. Chalcones
Chalcones, chemically referred to as benzylideneacetophenones or 1,3-phenyl-2-propenones, are a class of open-chain flavonoids widely distributed across numerous plant species. Structurally, chalcones are composed of two phenyl rings joined by a three-carbon α, β-unsaturated carbonyl linker [60]. These compounds can exist in either cis or trans configurations, with the trans isomer exhibiting higher thermodynamic stability, making it the dominant form [61,62].
While chalcones primarily serve as floral pigments in plants, they are also present in the bark, heartwood, fruits, leaves and roots of several plants [60]. They possess a unique chemical structure that enables a broad array of biological activities, including antidiabetic, anti-inflammatory, antimicrobial [17], antioxidant [63], antihypertensive [18], and anticancer properties [64,65]. The diversity of these biological activities is attributed to the distinctive nature of the chalcone structure, which contains substitutable hydrogens, allowing the creation of various derivatives with differing reactivities and specificities toward biological targets [66]. The α, β-unsaturated ketone group in chalcones serves as a Michael acceptor, enabling the formation of covalent bonds with the sulfhydryl groups in proteins, thereby influencing protein functionality [14]. The capacity of chalcones to function as Michael acceptors is determined by the type of electron-withdrawing or electron-donating functional groups present in each derivative, which influences the electron density of the aromatic rings, thereby altering the reactivity of the enone group [14,67]. Chalcones are highly valued by medicinal chemists due to their broad range of biological effects, ease of synthesis, potential for extensive structural modification, minimal engagement with DNA, and low mutagenic risk [68,69]. Consequently, both natural and synthetic chalcones have been extensively investigated in the fields of drug design and discovery.
Natural chalcones act as essential intermediates in the biosynthesis of flavonoids and isoflavonoids, and are naturally produced via the shikimate or acetate metabolic pathways [70]. As secondary metabolites, chalcones play essential roles in plants, contributing to their defense and regulation. These functions include facilitating pollination, protecting against pathogens, shielding against UV radiation, and deterring herbivorous insects [71,72]. Furthermore, chalcones offer notable health benefits and are recognized for their nutraceutical properties, which are linked to a range of significant biological activities [70]. As a result, plants rich in chalcones, such as Angelica, Camellia sinensis (green tea) and Piper have been utilized in folk medicine worldwide for centuries [68,73].
Numerous pure chalcones have been extracted from plants and approved for use in humans (Figure 1). One such example is metochalcone, a chalcone derived from Pterocarpus marsupium, which has been used as a diuretic and choleretic drug [74]. Similarly, sofalcone, obtained from Sophora tonkinensis, is recognized for its anti-ulcer properties, as it promotes mucosal prostaglandin secretion and provides protective effects against Helicobacter pylori [75]. Furthermore, hesperidin methyl chalcone has been evaluated in clinical trials for treating venous lymphatic insufficiency and is marketed as part of a combination product with two additional ingredients [76,77].
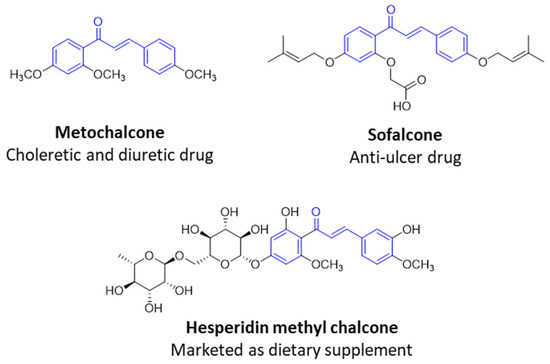
Figure 1.
Examples of clinically approved chalcone derivatives. The basic chalcone scaffold is highlighted in blue, while all additional substituent functional groups are shown in black.
While plants serve as a primary source of chalcones, the extraction of these compounds from natural sources poses significant challenges, often involving complex procedures that yield low quantities. Consequently, the chemical synthesis of chalcones offers a practical alternative to the resource-intensive natural product extraction. This approach simplifies the process, reduces time and cost, and enhances yield, enabling the production of a variety of chalcone analogs that may not occur naturally.
Chalcones are commonly synthesized via the Claisen-Schmidt condensation reaction, wherein a substituted benzaldehyde reacts with a substituted acetophenone under either basic or acidic conditions [64]. To increase yields and promote environmentally sustainable practices in chalcone synthesis, alternative synthetic methods to the traditional Claisen-Schmidt condensation have been proposed. These include the Suzuki Miyaura reaction, ultrasound-assisted synthesis, microwave-assisted synthesis and the Friedel-Crafts method [78]. Additionally, eco-friendly synthesis techniques such as solvent-free approaches have also been explored [70,79].
The effective utilization of natural chalcones as remedies for numerous conditions has motivated medicinal chemists to design new chalcone analogs with enhanced biological activities. Traditionally, modifications to the phenyl rings have been the main strategy for structural alterations [66]. By replacing one or more protons on the aromatic rings with different functional groups such as aryl, alkyl, halogen, nitro, amino and carboxylic groups, a range of chalcones can be synthesized [66]. The nature and position of these functional groups strongly influence their biological effects on different targets, mirroring the activity of naturally occurring chalcones [80].
Recent progress in chalcone design has resulted in the development of chalcone hybrids, in which the traditional phenyl rings are substituted with heterocyclic rings or other structural frameworks [81]. This strategy is rooted in molecular hybridization, which combines the pharmacophoric moieties of different active compounds to create new hybrids with enhanced pharmacological effects, selectivity, pharmacokinetic features, safety profiles, and minimal susceptibility to therapeutic resistance [82]. A range of hybrid chalcones including pyrrole-, coumarin-, pyrimidine-, tetralone-, and pyridine-, based derivatives have been synthesized by connecting the chalcone scaffold with compounds known to exhibit anticancer properties. These hybrids have shown either additive or synergistic pharmacological effects [81]. Molecular hybridization has demonstrated success as a valuable approach for discovering new derivatives with enhanced anticancer activity, positioning hybrid molecules as a promising avenue in modern drug discovery.
4. Chalcones and PCa
Many chalcones, whether synthetically or naturally derived, have shown promising anticancer properties by targeting signaling cascades and processes involved in various stages of PCa development. Chalcones influence multiple biological functions, such as cell proliferation, angiogenesis, apoptosis, the cell cycle, and metastasis. The antiproliferative activity of these compounds is often associated with their impact on signaling pathways associated with carcinogenesis, such as the β-catenin/Wnt, nuclear factor kappa B (NF-κB), and PI3k/Akt/mTOR pathways. In addition, chalcones demonstrate cytotoxicity against metastatic cancer cells, including those resistant to conventional therapies [83]. As chalcones are found in various edible plants, including kava (Piper methysticum), licorice (Glycyrrhiza spp.), hops (Humulus lupulus) and hop-derived products, as well as several fruits, vegetables and spices, their ability to target multiple pathways suggests that they may possess a relatively broad therapeutic window [80,84]. Chalcone analogs could thus emerge as effective anticancer agents, offering favorable efficacy and safety profiles for PCa treatment (Figure 2). Population-based studies further support this idea, revealing a significant negative correlation between cancer occurrence and the use of chalcone-rich kava plant extract [85]. Table 1 presents studies evaluating the effects of chalcone derivatives on different PCa models. The key cellular functions and signaling pathways that chalcones may target in PCa are outlined below.
4.1. Apoptosis
Apoptosis, commonly known as programmed cell death, is a precisely controlled biological process critical for sustaining tissue homeostasis by eradicating dysfunctional or aged cells [86]. When this defense mechanism is impaired, it disrupts the equilibrium between cellular survival and apoptosis, providing conditions that promote malignant transformation and tumor progression [87]. Consequently, evasion of apoptosis is recognized as a hallmark of cancer and a major contributor to treatment resistance. The execution of apoptosis primarily depends on the activation of cysteine proteases called caspases, which are activated via two main pathways: the intrinsic (mitochondrial) and the extrinsic (death receptor) pathways [88]. The extrinsic pathway begins with the interaction of death receptors on the cell surface with pro-apoptotic ligands, such as Fas ligand (FasL), the tumor necrosis factor-α (TNFα) and TNF-related apoptosis-inducing ligand (TRAIL) [89]. In contrast, the intrinsic pathway is initiated by the release of cytochrome c from the mitochondria, a process regulated by the Bcl-2 family of proteins [90]. Members of this protein family are divided into pro-apoptotic (e.g., Bax, Bcl-xS, Bad, Bim and Bid) and anti-apoptotic (e.g., Bcl-XL, Bcl-2, and Mcl-1) categories [88]. Despite these distinct signaling cascades, both pathways often involve caspase-3 activation, resulting in the breakdown of critical cellular structures such as cytoskeletal proteins and DNA, ultimately causing cell death [91].
Dysregulation of apoptotic proteins is frequently observed in tumors, enabling the evasion of apoptosis. In PCa, these disruptions are associated with ADT resistance and the progression to more aggressive disease stages [92,93]. For instance, Bcl-2 is often overexpressed in androgen-independent PCa, facilitating cell survival in environments with low androgen levels [90]. Conversely, the expression patterns of caspase-1 and caspase-3 are significantly lower in PCa tissues than in normal prostate tissue [94]. Additionally, PCa cells often resist TRAIL-induced apoptosis because of genetic alterations in the 8p21-22 chromosomal region, which harbors the gene encoding the TRAIL receptor DR5, or because of elevated levels of anti-apoptotic proteins [95,96]. Since many anticancer therapies rely on triggering apoptosis to destroy cancer cells, disruptions to these signaling pathways allow malignant cells to avoid cell death, promoting uncontrolled growth and resistance to treatments [89]. However, these deficiencies in apoptotic pathways may also present opportunities for developing innovative cancer therapies, making them promising areas for targeted intervention [97].
Chalcone derivatives have demonstrated considerable promise in selectively inducing apoptosis in PCa cells by targeting distinct apoptotic signaling pathways (Figure 3). Many chalcones disrupt the mitochondrial membrane potential, effectively triggering apoptosis [98,99,100]. This disruption is frequently linked to the activation of caspase-3 and caspase-9 [101], upregulation of pro-apoptotic proteins such as Bid and Bax [102,103], and downregulation of anti-apoptotic proteins such as Bcl-xl and Bcl-2 [99,102]. Additionally, some chalcones were shown to trigger apoptosis through the extrinsic pathway, specifically by enhancing TRAIL-induced apoptosis [104,105,106,107]. TRAIL is an endogenous cytokine that exhibits anticancer activity and selectively targets cancer cells for apoptosis while sparing healthy cells [108]. However, TRAIL resistance is observed in certain PCa cell lines [104]. Notably, 2-hydroxy-4-methylsulfonyl chalcone has demonstrated the ability to effectively restore sensitivity to TRAIL-induced apoptosis in resistant PCa cell lines [106]. This sensitization was mediated by enhanced activation of the TRAIL death receptor (DR5) and reduced expression of Bcl-2 [106]. Similarly, another study demonstrated a synergistic activity between the prenylated chalcone, xanthohumol, and TRAIL in triggering apoptosis in LNCaP PCa cell line [102]. Individual treatments with xanthohumol or TRAIL induced apoptosis rates of 11.1% and 12.57%, respectively; however, their combination markedly enhanced apoptosis, up to 76.58% [102]. Collectively, these results underscore the potential use of chalcones as both standalone and adjuvant therapeutic agents for PCa, either by directly inducing apoptosis or by promoting the response of resistant PCa cells to other treatments.
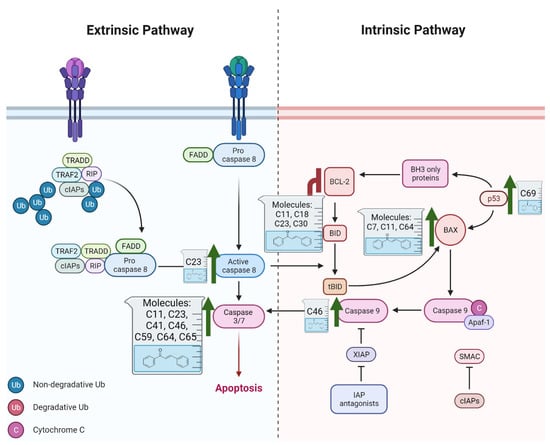
Figure 3.
Role of Chalcones in modulating intrinsic and extrinsic apoptosis pathways in prostate cancer (PCa). Compound numbers shown in the figure correspond to the numbering used in Table 1. Arrows indicate activation or signaling progression, whereas T-shaped bars indicate inhibition or suppression of the indicated target.
4.2. Cell Cycle
The cell cycle is a structured and tightly regulated process essential for cellular growth and division. It comprises four sequential phases: Gap 1 (G1), DNA synthesis (S), Gap 2 (G2), and mitosis (M), with checkpoints built into each phase to control growth and maintain the integrity of genetic material [109]. The progression of the cell cycle is primarily directed by cyclin-dependent kinases (CDKs) and their associated cyclins. CDKs are kinases that pair with cyclins to phosphorylate key proteins, promoting progression through the cell cycle phases [110]. Cyclin-CDK complexes are carefully controlled by cyclin-dependent kinase inhibitors (CDKIs) such as P21CIP1, P27KIP1, and P57KIP2, which function as negative regulators of CDK activity [111]. CDK activity is stimulated by mitogenic signals and inhibited when DNA damage is sensed via cell cycle checkpoints [109]. Checkpoints then activate inhibitory signals to halt the cell cycle, allowing time for the damage to be repaired. If the damage is irreparable, cells are directed toward senescence or programmed cell death [112]. Dysregulation of cell-cycle control mechanisms disrupts these regulatory checkpoints, leading to unrestrained cell division and the development of neoplastic transformations [112]. These aberrations are fundamental to cancer progression, as they allow cells to bypass normal growth controls and proliferate uncontrollably.
Nearly all malignancies involve direct or indirect disruptions to the cell cycle, making these alterations a hallmark of cancer [113]. Abnormalities in cell cycle regulation have been linked to almost all regulatory proteins that are involved in this process [112]. For example, Cyclin D1 is notably overexpressed in androgen-independent PCa, and is associated with tumor metastasis [114,115,116]. Similarly, the absence of p27 (a key CDK inhibitor) is observed in around 16–68% of patients diagnosed with PCa and strongly correlates with enhanced tumor development [117]. Owing to the common occurrence of cell cycle dysregulation in PCa and its critical role in tumors, targeting the cell cycle process represents a promising strategy for novel cancer therapies. These therapeutic approaches could address the uncontrolled proliferation that underpins cancer progression, offering hope for more effective PCa treatments.
Studies have consistently demonstrated that the antiproliferative activity of chalcones against PCa cells is repeatedly linked to their impact on cell cycle regulation (Figure 4) [83,118,119,120,121,122]. For example, licochalcone-A has been found to decrease cyclin B1 and Cdc2 levels in PC3 cell line, resulting in G2/M phase cell cycle arrest [123]. Similarly, a chalcone derivative containing dithiocarbonates induced G2/M arrest by upregulating the CDK inhibitor p21, while downregulating cyclin B1 and CDK1 [124]. Although G2/M arrest is the predominant effect of most chalcone derivatives, some studies have reported G1 phase arrest. For example, Sun et al. have shown that a methoxy-chalcone analog elicited time- and concentration-dependent G1 phase arrest in PC3 cell lines [125]. This was associated with a decrease in multiple G1 modulators, including cyclin E, cyclin D1, CDK2, CDK4, phosphorylated retinoblastoma (Rb) protein, Cdc25A, and E2F-1 [125]. Interestingly, the effects of chalcone derivatives on the cell cycle can vary depending on the cell line or treatment duration. Isoliquiritigenin was observed to result in G1 phase arrest in DU145 cells after 2 h of treatment, shifting to G2/M arrest after 4 h [126]. Furthermore, a methoxy chalcone derivative caused G2/M arrest in cell lines harboring a p53 mutation but induced G0/G1 arrest in cell lines with wild-type p53 [127]. Together, these results indicate that, irrespective of the specific cell cycle phase targeted, chalcone-induced cell cycle arrest consistently culminates in apoptosis.
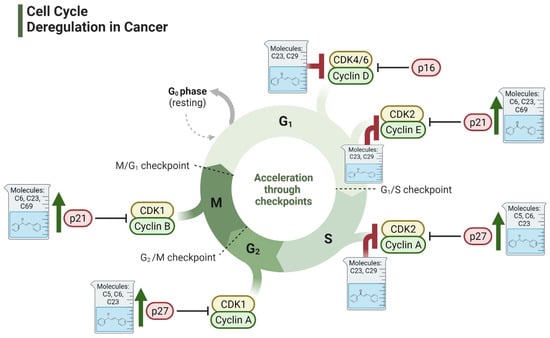
Figure 4.
Effect of chalcones on modulating the cell cycle and its regulators in PCa cells. Compound numbers shown in the figure correspond to the numbering used in Table 1. Arrows indicate activation or signaling progression, whereas T-shaped bars indicate inhibition or suppression of the indicated target.
One potential mechanism by which chalcones block cell cycle progression is through their binding to tubulin and the subsequent disruption of microtubules [66,128]. Like other antimitotic agents, chalcones can impact microtubules by either inhibiting tubulin polymerization comparable to colchicine, or via stabilizing microtubules and promoting polymerization similar to taxanes [129]. In fact, using chalcones as inhibitors of microtubule assembly is one of the earliest therapeutic applications evaluated for these compounds [129]. Several studies have demonstrated that chalcones inhibit tubulin polymerization through binding to the colchicine binding site, as confirmed through docking analyses [130,131,132]. A library of 1,2,3-triazole-based chalcones was developed and assessed their cytotoxic effects on DU145 PCa cell line [131]. These derivatives significantly suppressed tubulin polymerization and promoted the arrest at the G2/M phase. In contrast, other reports revealed that chalcones can enhance tubulin polymerization. For example, an isoprenylated chalcone derived from Dalea frutescens was found to exhibit antitumor activity against AR-positive (AR+) PCa cells [133]. Mechanistic studies revealed that this compound increased tubulin polymerization rates and caused G2/M phase arrest [133]. Regardless of whether chalcones inhibit or promote tubulin polymerization, their interaction with tubulin consistently leads to G2/M phase arrest and antiproliferative activity, underscoring their potential as antimitotic agents.
4.3. Cancer Cell Invasion and Migration
Typically, localized PCa is associated with favorable prognosis; however, the 5-year survival rate declines sharply to 30.2% once the disease progresses to metastatic stages [5]. Metastatic cancer spreads through several steps: breaking tight cell junctions, detaching from the original cancer location, migrating to nearby tissues, invading blood or lymphatic vessels, exiting these vessels (extravasation), and metastasizing to distant sites [134]. At the molecular level, epithelial-to-mesenchymal transition (EMT) plays a critical role in facilitating metastasis [135]. During EMT, epithelial cells undergo significant morphological alterations, transitioning from a cuboidal to a spindle-shaped form [136]. This process is characterized by the upregulation of mesenchymal markers, such as N-cadherin, fibronectin, and vimentin, alongside the downregulation of epithelial markers, such as E-cadherin and occludins [137]. These changes result in the loss of cell–cell adhesion and an increase in stem-like properties, which promote the invasive behavior necessary for metastasis [138]. EMT is regulated by several signaling pathways, such as the TGF-β, EGF, Wnt/β-catenin, and NF-κB pathway, either directly or indirectly [136,138].
Several chalcone derivatives have been shown to inhibit EMT and, as a result, reduce the invasion of various cancer types [64,139,140]. While the role of chalcones in modulating EMT in PCa remains relatively underexplored, one study documented their ability to suppress EMT-associated metastatic behavior in PCa [124]. They investigated a new set of dithiocarbonate-based chalcones and reported that they suppress cancer cell migration and EMT by increasing E-cadherin expression, reducing N-cadherin, and suppressing vimentin, MMP2, and MMP9 in PC3 cell lines [124]. Although not directly investigated, EMT inhibition could be an important mechanism in underlying the antiproliferative activity of chalcones against PCa. Various chalcone derivatives have been demonstrated to reduce PCa cell invasion and migration by impacting EMT-related pathways and effectors, including TGF-β, VEGF, MMP, and NF-κB [141,142,143]. These studies collectively indicate that chalcones are capable of preventing PCa cell migration, invasion, and metastasis by suppressing EMT.
4.4. PI3k/Akt/mTOR Pathway
The PI3K/AKT/mTOR signaling pathway plays crucial roles in regulating cell metabolism, survival, and proliferation [144]. Elevated activity of this pathway has been reported in PCa, with a particularly significant upregulation observed in CRPC [145,146]. Alterations in PI3K pathway genes have been reported to be highly prevalent in PCa, occurring in approximately 42% of primary and 100% of metastatic PCa cases [147,148,149]. Therefore, chalcones have emerged as promising therapeutic agents for targeting the PI3K/AKT/mTOR signaling pathway in PCa [100,106,125,150,151,152,153]. For example, a chalcone molecule with methyl sulfonyl and hydroxy substituents has been demonstrated to overcome TRAIL resistance in PCa cell lines. The compound enhanced TRAIL-induced apoptosis through the downregulation of Bcl-2, PI3K/AKT, COX-2, and NF-κB signaling pathways [106]. Similarly, isoliquiritigenin, another chalcone, has been reported to modulate PI3K/AKT/mTOR cellular function, particularly by diminishing the expression of the ErbB3 gene [150]. This inhibition prevented the phosphorylation of ErbB3 and reduced the engagement of the p85 subunit of PI3K, which in turn inhibited Akt phosphorylation [150]. While these findings are promising, they are limited by the lack of adequate in vivo studies. Therefore, further in vivo research is necessary to validate these mechanisms of action and to evaluate their therapeutic potential in PCa management.
4.5. Angiogenesis Pathway
Angiogenesis is the formation of new blood vessels from existing blood vessels. Uncontrolled angiogenesis is a key driver of cancer progression and metastasis [154]. Cancer cells can provoke angiogenesis by disrupting the physiological balance of pro-angiogenic and anti-angiogenic signals, thus facilitating the growth of new blood vessels to supply the growing tumors with oxygen and nutrients [155]. Vascular endothelial growth factor (VEGF) is a key activator of angiogenesis, which enhances proliferation of endothelial cells and increases vascular permeability [156]. In PCa, the overexpression of VEGF has been linked to enhanced metastasis and resistance to therapy [157,158]. Owing to its pivotal role in tumor growth and spread, angiogenesis is considered one of the hallmarks of cancer, making angiogenesis inhibition a promising therapeutic target for cancer.
Numerous antiangiogenic therapies have been clinically tested for therapeutic management of PCa; nonetheless, the outcomes have been inconclusive and unsatisfactory [159]. For instance, the addition of bevacizumab to hormone therapy in hormone-sensitive PCa has been linked to improved relapse-free survival, highlighting the role of angiogenesis in PCa progression [160]. Nevertheless, overall survival did not improve when a combined therapy of bevacizumab, prednisone and DTX was used in CRPC, and this combination was associated with an increased rate of treatment-related deaths [161]. Similarly, other antiangiogenic agents, such as aflibercept, sunitinib, and lenalidomide, have not been shown to improve overall survival in patients with CRPC patients [162,163,164]. Additionally, antiangiogenic therapies are frequently used in conjunction with other treatments, as they primarily limit tumor growth by inhibiting blood vessel formation rather than directly eradicating the tumor. Thus, further research should explore novel antiangiogenic therapies that are more effective and safer for combating cancer by targeting multiple mechanisms.
As multi-target agents with exceptional antiangiogenic effects, chalcones have the potential to serve as alternatives to current antiangiogenic therapies. A chalcone derivative, 3,4,2′,4′-tetrahydroxychalcone, was shown in one study to significantly suppress angiogenesis by attenuating the expression of VEGF and MMP-9 [141]. In our laboratory, we explored the antiangiogenic activity of newly developed potent chalcone analogs and found that their antitumor activity was associated with the potent suppression of angiogenesis, as evidenced by the in ovo chicken embryo chorioallantoic membrane (CAM) model [64,165]. Similarly, other investigators designed a new 3′,5′-diprenylated chalcone and reported that it substantially downregulated VEGF, a key player in the modulation of angiogenesis, in PCa cell lines [142]. Furthermore, this chalcone reduced the growth of PCa cells in vivo, suggesting that its antiangiogenic effects contribute to its anticancer activity [166]. Collectively, these findings support the idea that chalcones possess promising antiangiogenic therapeutic potential for PCa.
4.6. Androgen Receptor Signaling
Mutations or alterations in AR are central to the development and progression of PCa [167]. Thus, targeting AR through blockade by specific drugs has the potential to inhibit PCa progression. However, resistance to these therapies often develops after several years, leading to the progression of CRPC. In CRPC, cancer cells adapt to survive at low androgen levels through mechanisms such as AR overexpression, AR point mutations, alterations in androgen biosynthesis, and changes in androgen cofactors [168]. Therefore, targeting the AR remains critical for treating PCa, especially in hormone-refractory or advanced stages.
Several studies have explored the potential of chalcones in targeting the AR in PCa [169,170,171]. Jackson et al. investigated the antitumor activities of dibenzoylmethane, a chalcone analog, and found that it significantly downregulated AR protein and gene expression in a dose-dependent manner. Furthermore, this compound suppressed the secretion of prostate-specific antigen (PSA), a tumor marker regulated by AR [169]. A similar effect was observed with another chalcone derivative, isoliquiritigenin, further confirming chalcones as potential AR-targeting agents [170]. Moreover, an ionone-based synthetic chalcone demonstrated potent inhibitory effects (IC50 of 0.74 μM) in the LNCaP PCa cell line and successfully blocked dihydrotestosterone (DHT) from activating the wild-type AR. Notably, this compound also exhibited activity against clinically relevant AR mutations, including W741C, H874Y, and T877A [171]. These data collectively indicate that chalcone analogs have potential as treatments for AR-naïve, mutant and advanced forms of PCa.
4.7. Inflammatory Pathways
The link between inflammation and PCa development and progression has been extensively studied. Altered levels of reactive oxygen species (ROS), cytokines, chemokines, and other transcription factors have been linked to the initiation and growth of various malignancies, including PCa [172,173]. In particular, inflammation and oxidative stress play crucial roles in regulating the AR, a key receptor involved in the progression of PCa to CRPC [174,175]. Additionally, NF-κB activation is closely linked to inflammation and plays a significant role in PCa tumorigenesis and the development of CRPC [176,177]. Specifically, NF-κB transcription factors were found to enhance cell proliferation, invasion, and survival [176,177]. Among the various NF-κB subunits, p52, RelA, RelB, and c-Rel have been shown to be particularly important in PCa [178]. Additionally, several NF-κB target genes, such as VEGF, caspase-8, Bcl-2, Bax, and MMP-9, have been implicated in the pathogenesis of PCa [177]. Numerous natural and synthetic chalcone-based compounds have been shown to target NF-κB signaling [100,141,179,180]. Among these, butein has been demonstrated to suppress NF-κB activity by downregulating the expression of MMP-9 and VEGF. This compound also induced G2/M phase cell cycle arrest [141]. Since these target genes are also involved in angiogenesis and metastasis, these results indicate that chalcone derivatives may act as effective therapeutic agents for inhibiting angiogenesis, cell growth, and invasion in PCa.
4.8. Cancer Stem Cells
Physiologically, embryonic and adult stem cells are characterized as a small population of cells capable of self-renewal, differentiation, and the reconstitution of various tissues into mature cell types that constitute each organ. In cancer, a similar rare subpopulation of cells, known as cancer stem cells (CSCs), has been identified. These CSCs exhibit self-renewal capabilities and stem cell-like properties, which are crucial for cancer initiation, progression, resistance to therapy, recurrence, and metastasis [181,182]. Several signaling pathways that are involved in regulating normal stem cell behavior, were reported to be frequently deregulated in cancers [181]. Among these pathways are NF-κB, Wnt/β-catenin, TGF-β, and Hedgehog [183,184].
Studies on PCa have demonstrated that prostate cancer stem-like cells (PCSCs) are characterized by low or absent AR and PSA expression. These cells contribute to both the initiation of PCa and its progression to CRPC [185]. Given the role of PCSCs in PCa, targeted therapies against these cells represent a promising strategy for the eradication of PCa. One chalcone analog, 2′-hydroxy-2,4,4′,5,6′-penta-methoxychalcone, has been explored for its effects on PCSCs, particularly in combination with taxane therapy for both PCa and taxane-resistant (TXR) PCa [186,187]. This compound significantly enhanced the effects of paclitaxel and DTX when used together in the treatment of PCa and TXR PCa. Wen et al. reported that this molecule sensitized paclitaxel-resistant PCa cells to paclitaxel by upregulating the expression of miR-34a (a tumor suppressor gene) and reversing the expression of its downstream target genes [186]. The combination of paclitaxel and chalcone effectively inhibited the proliferation, migration, and growth of PCSCs, highlighting the role of chalcones in targeting CSCs. When this combination was loaded into micelles, PC3-TXR PCa cell viability decreased as the paclitaxel concentration increased. In addition, treatment of PC3 and PC3-TXR cells with paclitaxel resulted in IC50 values of 55.6 and 2580 nmol/L, respectively. However, dual therapy improved these effects, reducing the IC50 to 49.8 and 93.2 nmol/L, respectively, demonstrating the ability of chalcones to reverse paclitaxel resistance. Compared with paclitaxel monotherapy, in vivo testing of the micelle-based dual therapy significantly inhibited prostate tumor growth in nude mice. This effect was linked to the reversal of SIRT1, cyclin D1, E-cadherin, and miR-34a expression [186]. These results emphasize the potential of chalcone derivatives in reducing chemoresistance to conventional therapies and inhibiting PCSC growth.
5. Toxicity and Safety Profile of Chalcones
Despite the extensive evidence supporting the anticancer potential of chalcone derivatives, their toxicity and overall safety profile remain inadequately characterized. Among the studies reporting the anticancer effects of chalcones in PCa models, only a limited number have directly compared their cytotoxicity in cancer cells versus normal cells. The reported selectivity varies markedly depending on the specific derivative and the experimental model used. While some studies describe a high selectivity index, with certain derivatives exhibiting more than 100-fold selectivity toward cancer cells [188], others demonstrated only moderate or no selectivity (Table 1) [189,190]. Similarly, although the in vivo antitumor potential of chalcones has been extensively investigated, data on their systemic toxicity are still limited [191]. In a breast cancer xenograft model, Li et al. reported a favorable safety profile for a quinoline-chalcone derivative with a median lethal dose (LD50) of 665.62 mg/kg, while a dose of 20 mg/kg reduced tumors by 68.9% without observable toxic effects [192,193]. On the other hand, Zhou et al. reported severe liver damage upon oral consumption of flavokawain B in BALB/C mice [194], whereas dietary feeding with flavokawain A did not induce detectable adverse effects or organ dysfunction. Therefore, more comprehensive in vivo toxicological studies, including LD50 determination and acute toxicity assessment, are needed to better define the therapeutic window of promising chalcone analogs, particularly in PCa models.
Interestingly, several strategies designed to enhance the selectivity of chalcones have been reported and show promise in improving their anticancer potential. Structural modifications, particularly in the α,β-unsaturated carbonyl moiety, can markedly influence the selectivity [195,196,197]. Additionally, nanoparticle-based delivery systems have been employed in a few studies to encapsulate poorly soluble chalcones, enabling lower effective doses and decreased exposure of normal tissues [198,199]. Other targeted delivery strategies, such as glycosyl conjugates, where shown to exploit overexpressed transporters and receptors on cancer cells to enhance selective uptake of chalcone conjugates [200,201]. Collectively, these approaches offer promising avenues to enhance antitumor efficacy and reduce systemic toxicity and should be more systematically explored for PCa–directed chalcones.
6. Insights into Structural Perspectives
Among the 73 lead analogs covered in this review (Table 1), 19 are of natural origin, whereas the remainder are synthetic derivatives. Particularly, butein, isoliquiritigenin, flavokawain A, flavokawain B, cardamonin, licochalcone A, xanthohumol, and isobavachalcone are among the most frequently investigated anticancer chalcone derivatives. Natural chalcones with reported anticancer activity typically incorporate hydroxy groups and/or prenyl/isoprenyl substituents and methoxy groups. Both hydroxy and methoxy groups have been shown in multiple studies to modulate anticancer activity [202,203], whereas prenyl substituents were shown to substantially increase lipophilicity, potentially enhancing cellular uptake [204,205]. Additionally, natural chalcones may exert anticancer effects either directly or indirectly through their metabolites. For example, xanthohumol can undergo isomerization to isoxanthohumol in the stomach [206], and isoxanthohumol itself has also been reported to display anticancer activity [207]. On the other hand, synthetic chalcones encompass a broader range of electron-donating and electron-withdrawing groups, as well as diverse heteroaromatic rings, including pyrimidine, thiazole, indole, pyrazole, triazole, and benzofuran moieties. Owing to the variability in cytotoxicity assays, cell lines, and exposure times used across studies, a rigorous quantitative SAR analysis was not feasible in this review. Nevertheless, synthetic chalcone derivatives have repeatedly succeeded in lowering the half-maximal inhibitory concentration (IC50) values against PCa cell lines to the low micromolar or even nanomolar range; in some cases exhibiting greater potency than their natural counterparts.
7. Conclusions and Future Perspective
The exploration of novel anticancer agents, both natural and synthetic, is a rapidly advancing field. Chalcones, a class of organic compounds derived from either natural or synthetic sources, have attracted considerable attention for their anticancer properties. Numerous studies have demonstrated that chalcone derivatives and analogs exhibit potent anticancer activities across various PCa types, including CRPC. Extensive evidence underscores their capacity to influence various signaling pathways and cancer-related processes, enhancing their chemo-preventive potential. Furthermore, when used in combination with conventional chemotherapy, chalcones have shown promise in overcoming drug resistance and enhancing the therapeutic efficacy of existing treatments. Nevertheless, further in vivo studies are needed to provide a comprehensive understanding of the anticancer effects of chalcones in PCa. Additionally, pharmacokinetic studies are needed to determine the absorption, distribution, metabolism, and excretion (ADME) properties of these molecules and to further identify the best formulation and drug delivery systems for administering them. Furthermore, in vivo results must be validated through clinical trials to support the use of chalcones in patients with PCa. Since the safety of these molecules has not been extensively studied, determining their safety profile is of vital importance. Overall, the pharmacological effects of chalcones in PCa are noteworthy and offer promising potential to address the shortcomings of existing treatment strategies.


Table 1.
Comprehensive list of chalcones with reported anti-prostate cancer (PCa) activity, including origin, experimental models, inhibitory concentrations (IC50), biological effects, and associated molecular targets/pathways.
Table 1.
Comprehensive list of chalcones with reported anti-prostate cancer (PCa) activity, including origin, experimental models, inhibitory concentrations (IC50), biological effects, and associated molecular targets/pathways.
| No. | Chemical Structure | Origin | Research Model | Inhibitory Concentration (IC50) | Biological Effect | Target Pathway/Protein | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 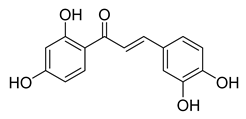 | Natural | Cell-line(s): CWR22Rν1, LNCaP, PC3, and DU145 Animal model: xenograft mice model | N/A |
|
| [141,208,209] |
| 2 | 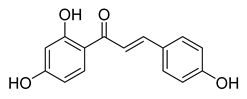 | Natural | Cell-line(s): PC-3 DU145 LNCaP 22RV1 | 19.6 µM 23.3 µM 15.7 µM 36.6 µM |
|
| [98,126,143,150,170,210,211,212] |
| Animal model: PC-3 xenograft mice model | |||||||
| 3 | 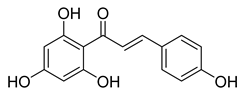 | Natural | Cell-line(s): PC3 22RV1 PNT1A | For all cell lines ≥ 1 µM |
|
| [213] |
| 4 | 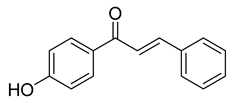 | Synthetic | Cell-line(s): PC-3 | 6.19 μM |
|
| [214] |
| 5 | 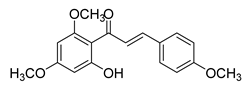 | Natural | Cell-line(s): PC3, DU145 22Rv1 PrECs PrSCs Animal models: TRAMP mice model; FVB/N mice | 22.86 μM N/A N/A >80 μM >80 μM |
|
| [132,215,216,217] |
| 6 | 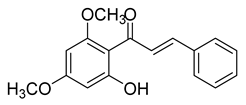 | Natural | Cell-line(s): LNCaP DU145 PC-3 LAPC4 | 48.3 µM 3.9 µM 6.2 µM 32 µM |
|
| [101,218] |
| Animal model: DU145 xenograft mice model | |||||||
| 7 | 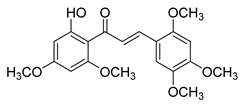 | Natural | Cell-line(s): PC3 DU145 LNCaP RWPE-1 C4-2 PC3-PTX DU145-PTX | >50 µM in all cell lines |
|
| [186] |
| Animal model: Nude mice | |||||||
| 8 | 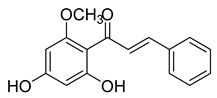 | Natural | Cell-line(s): PC3 DU145 LNCaP | 41.9 µM N/A N/A |
|
| [180,219,220] |
| 9 | 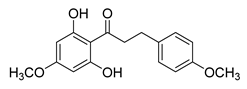 | Natural | Cell-line(s): LNCaP | N/A |
|
| [104] |
| 10 | 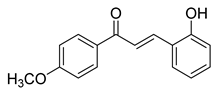 | Synthetic | Cell-line(s): PC3 | 23.14 µM |
|
| [221] |
| 11 | 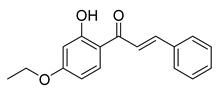 | Synthetic | Cell-line(s): PC3 | 8.2 µM |
|
| [99] |
| 12 | 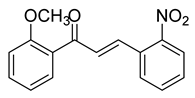 | Synthetic | Cell-line(s): LNCaP | 3.4 μM |
|
| [222] |
| 13 | 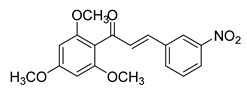 | Synthetic | Cell-line(s): 22Rv1 | N/A |
|
| [223] |
| Animal model: CRPC (22Rv1) xenograft mice model | |||||||
| 14 | 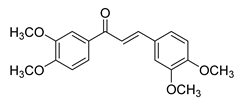 | Synthetic | Cell-line(s): PC3 LNCaP | N/A |
|
| [153] |
| 15 | 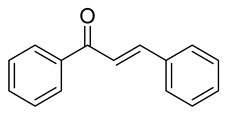 | Synthetic | Cell-line(s): PC3 RWPE-1 | 1.1 µM 34.7 µM |
|
| [224] |
| Animal model: Nude mice | |||||||
| 16 | 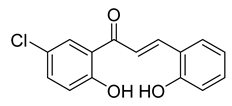 | Synthetic | Cell-line(s): LNCaP PC3 DU145 Animal model: PC-3 xenograft mice model | 3.74 µM 1.52 µM 4.5 µM |
|
| [130] |
| 17 | 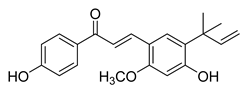 | Natural | Cell-line(s): PC3 LNCaP | N/A |
|
| [123,152] |
| 18 | 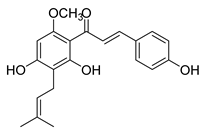 | Natural | Cell-line(s): PC3 DU145 LNCaP C4-2 MCF-10a HLMEC | 13.2 μM 12.3 μM N/A N/A |
|
| [100,102,188,195,225] |
| 19 | 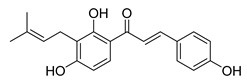 | Natural | Cell-line(s): PC3 LNCaP | 19.25 µM N/A |
|
| [105,226] |
| 20 | 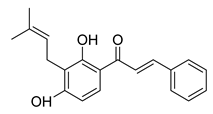 | Natural | Cell-line(s): PC3 | N/A |
|
| [227] |
| 21 | 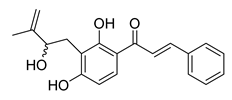 | Natural | Cell-line(s): PC3 DU145 | 11 μM 7 μM |
|
| [133] |
| 22 | 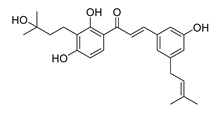 | Natural | Cell-line(s): DU145 | >10 µM |
|
| [228] |
| 23 | 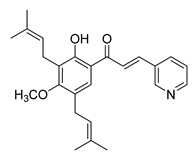 | Synthetic | Cell-line(s): PC3 DU145 RWPE-1 | 4.67 µM 6.56 µM 5.00 µM |
|
| [142,166,229] |
| Animal model: PC3 xenograft mice mode | |||||||
| 24 | 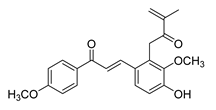 | Natural | Cell-line(s): PC3 | 1.9 μM |
|
| [230] |
| 25 | 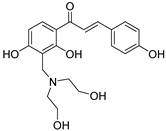 | Synthetic | Cell-line(s): PC3 | 35.14 μM |
|
| [231] |
| 26 | 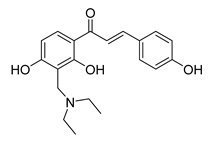 | Synthetic | Cell-line(s): PC3 | 3.9 μM |
|
| [232] |
| 27 | 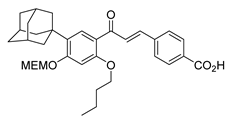 | Synthetic | Cell-line(s): PC3 | 0.74 μM |
|
| [233] |
| 28 | 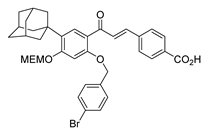 | Synthetic | Cell-line(s): PC3 | N/A |
|
| [234] |
| 29 | 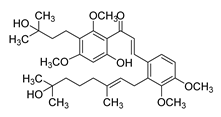 | Synthetic | Cell-line(s): LNCaP PC3 DU-145 | 5.8 µM 9.2 µM 2.2 µM |
|
| [125] |
| 30 | 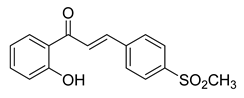 | Synthetic | Cell-line(s): LNCaP PC3 DU145 | 15 μM 15 μM 20 μM |
|
| [106,179] |
| 31 | 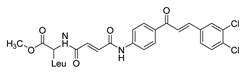 | Synthetic | Cell-line(s): PC-3 LNCaP Animal model: mouse xenografts | 29.5 μg/mL 21.4 μg/ml |
|
| [235] |
| 32 | 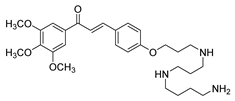 | Synthetic | Cell-line(s): PC3 DU145 | 13.73 µM 8.86 µM |
|
| [122] |
| 33 | 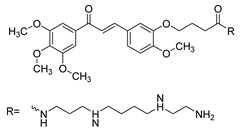 | Synthetic | Cell-line(s): PC3 DU145 | 31.8 µM 28.5 µM |
|
| [236] |
| 34 | 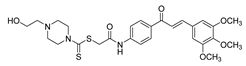 | Synthetic | Cell-line(s): PC3 | 1.05 µM |
|
| [124] |
| 35 | 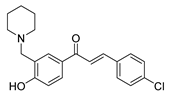 | Synthetic | Cell-line(s): PC3 | 3.7 μM |
|
| [237] |
| 36 | 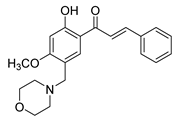 | Synthetic | Cell-line(s): PC3 | 0.78 μg/mL |
|
| [238] |
| 37 | 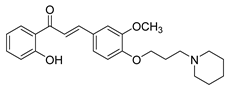 | Synthetic | Cell-line(s): PC3 DU145 | 1.95 µM 2.73 µM |
|
| [239] |
| 38 | 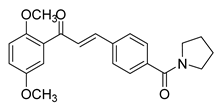 | Synthetic | Cell-line(s): PC3 | 0.53 μM |
|
| [240] |
| 39 | 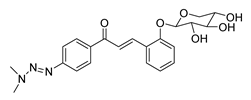 | Synthetic | Cell-line(s): PC3 | 28.2 µM |
|
| [241] |
| 40 | 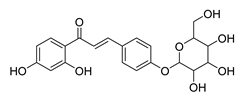 | Natural | Cell-line(s): LNCaP PC3 | 19.35 µM N/A |
|
| [119] |
| Animal model: SCID mice | |||||||
| 41 | 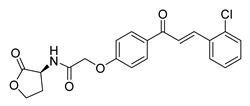 | Synthetic | Cell-line(s): PC3 DU145 Normal GES-1 | 4.61 µM 3.24 µM 13.37 µM |
|
| [107] |
| 42 | 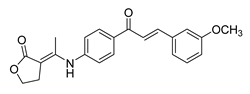 | Synthetic | Cell-line(s): PC3 FL normal cells | 69.92 µM 86.45 µM |
|
| [189] |
| 43 | 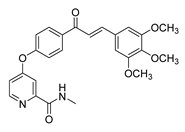 | Synthetic | Cell-line(s): PC3 | 3.15 μM |
|
| [242] |
| 44 | 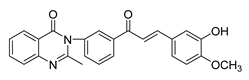 | Synthetic | Cell-line(s): PC3 Animal model: Non PCa | 54 μM |
|
| [243] |
| 45 | 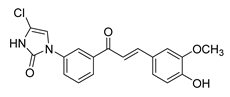 | Synthetic | Cell-line(s): PC3 | 1.95 µM |
|
| [244] |
| 46 | 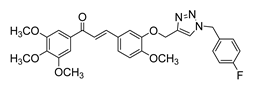 | Synthetic | Cell-line(s): DU145 | 1.3 μM |
|
| [131] |
| 47 | 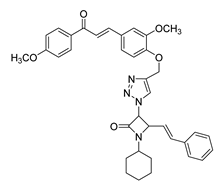 | Synthetic | Cell-line(s): PC-3 | 67.1 μM |
|
| [245] |
| 48 | 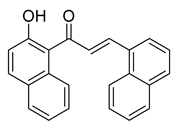 | Synthetic | Cell-line(s): PC3 DU145 | 0.45 µM 0.64 µM |
|
| [118] |
| 49 | 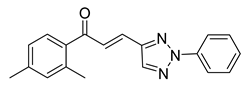 | Synthetic | Cell-line(s): PC3 | 15.64 µM |
|
| [246] |
| 50 | 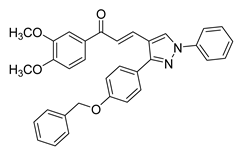 | Synthetic | Cell-line(s): PC3 | 4.46 µM |
|
| [128] |
| 51 | 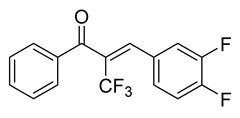 | Synthetic | Cell-line(s): PC3 DU145 LNCaP Drug resistant cell lines Animal model: SCID mice | 0.15 µM 0.19 µM N/A |
|
| [120,121] |
| 52 | 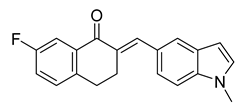 | Synthetic | Cell-line(s): PC3 | 0.147 |
|
| [247] |
| 53 | 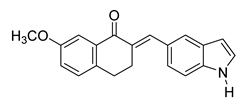 | Synthetic | Cell-line(s): PC3 Normal lung bronchial epithelial cells | PC3 3 μM >10 µM |
|
| [248] |
| 54 | 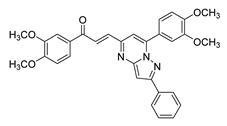 | Synthetic | Cell-line(s): DU145 | 7.2 µM |
|
| [249] |
| 55 | 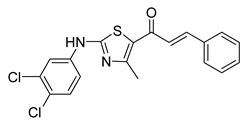 | Synthetic | Cell-line(s): PC-3 Animal model: ICR mice bearing sarcoma 180 | 7.99 μM |
|
| [250] |
| 56 | 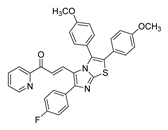 | Synthetic | Cell-line(s): DU145 | 1.05 μM |
|
| [251] |
| 57 | 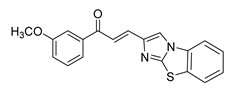 | Synthetic | Cell-line(s): DU145 | 2.7 µM |
|
| [252] |
| 58 | 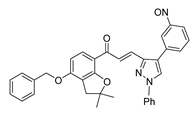 | Synthetic | Cell-line model: PC3 | 5.9 μM |
|
| [253] |
| 59 | 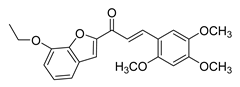 | Synthetic | Cell-line(s): PC3 | N/A |
|
| [254] |
| 60 | 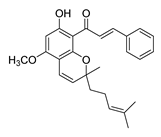 | Natural | Cell-line(s): PC3 | 27.95 µM |
|
| [255] |
| 61 | 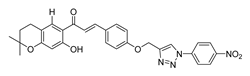 | Synthetic | Cell-line(s): DU145 | 29.9 μM |
|
| [256] |
| 62 | 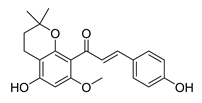 | Natural | Cell-line(s): PC3 | 10.7 µM |
|
| [257] |
| 63 | 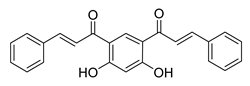 | Synthetic | Cell-line(s): DU145 | 1.70 μM |
|
| [258] |
| 64 | 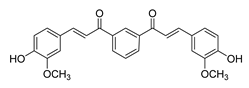 | Synthetic | Cell-line(s): LNCaP PC3 | 5.04 μM 4.15 μM |
|
| [103] |
| 65 | 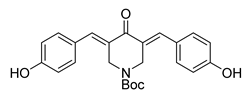 | Synthetic | Cell-line(s): PC3 22RV1 | 22.9 µg/mL 17.1 µg/mL |
|
| [190] |
| 66 | 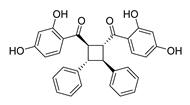 | Natural | Cell-line(s): PC3 | 3.5 µM |
|
| [259] |
| 67 | 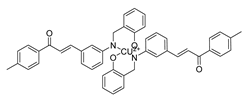 | Synthetic | Cell-line(s): PC3 | 5.95 μM |
|
| [260] |
| 68 | 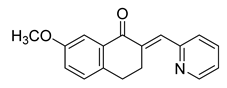 | Synthetic | Cell-line(s): PC3 DU145 | >10 µM <10 µM |
|
| [261] |
| 69 | 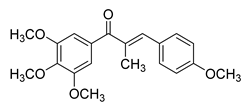 | Synthetic | Cell-line(s): LNCaP PC3 DU-145 22Rv1 C4-2 Animal model: 22Rv1 xenograft model in male Nu/Nu nude mice | 14–40 nM |
|
| [127] |
| 70 | 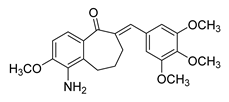 | Synthetic | Cell-line(s): DU145 HUVEC | 0.237 µM N/A |
|
| [262] |
| 71 | 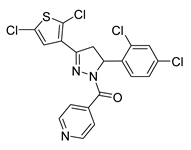 | Synthetic | Cell-line(s): DU145 | 4.95 µM |
|
| [263] |
Abbreviations: PCa, prostate cancer; CRPC, castration-resistant prostate cancer; ADT, androgen-deprivation therapy; AR, androgen receptor; PSA, prostate-specific antigen; CSCs, cancer stem cells; IC50, half-maximal inhibitory concentration; EMT, epithelial–mesenchymal transition; VEGF, vascular endothelial growth factor; MMP, matrix metalloproteinase; NF-κB, nuclear factor kappa-B; TRAIL, TNF-related apoptosis-inducing ligand; DR5, death receptor 5; ROS, reactive oxygen species; CAM, chorioallantoic membrane; SI, selectivity index; N/A, not available or not assessed. Symbols: ↑ indicates upregulation/activation or increase; ↓ indicates downregulation/inhibition or decrease, → indicates a resulting effect, association, or progression from one event to another.
Author Contributions
O.J.H. carried out the conceptualization and the writing—original draft, preparation, and editing; D.E. carried out the writing—original draft preparation; A.F.H. carried out the writing—reviewing and editing; F.A., A.-E.A.M. and A.K. carried out the conceptualization, supervision, and writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest. Ala-Eddin Al Moustafa is the Founder and CEO of ABS Research Review & Consultation. This company had no role in the preparation of this manuscript or in the decision to publish, and it provided no funding or other support for this work.
References
- GLOBOCAN. Global Cancer Observatory (GCO): International Agency for Research on Cancer. 2022. Available online: https://gco.iarc.fr/ (accessed on 15 August 2025).
- Dasgupta, P.; Baade, P.D.; Aitken, J.F.; Ralph, N.; Chambers, S.K.; Dunn, J. Geographical Variations in Prostate Cancer Outcomes: A Systematic Review of International Evidence. Front. Oncol. 2019, 9, 238. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef]
- Sartor, A.O. Risk Factors for Prostate Cancer UpToDate: Wolters Kluwer. 2025. Available online: https://0-www.uptodate.com.mylibrary.qu.edu.qa/contents/risk-factors-for-prostate-cancer?search=prostate%20cancer%20risk%20factors&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H41 (accessed on 15 August 2025).
- SEER. Cancer Stat Facts: Prostate Cancer: National Cancer Institute. 2025. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 13 August 2025).
- Haiman, C.A.; Chen, G.K.; Blot, W.J.; Strom, S.S.; Berndt, S.I.; Kittles, R.A.; Rybicki, B.A.; Isaacs, W.B.; Ingles, S.A.; Stanford, J.L.; et al. Characterizing Genetic Risk at Known Prostate Cancer Susceptibility Loci in African Americans. PLoS Genet. 2011, 7, e1001387. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.; Gerke, T.; Markt, S.C.; Peisch, S.F.; Wilson, K.M.; Ahearn, T.; Giovannucci, E.; Parmigiani, G.; Mucci, L.A. Family history of breast or prostate cancer and prostate cancer risk. Clin. Cancer Res. 2018, 24, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Kalish, L.A.; McDougal, W.S.; McKinlay, J.B. Family history and the risk of prostate cancer. Urology 2000, 56, 803–806. [Google Scholar] [CrossRef]
- Attard, G.; Parker, C.; Eeles, R.A.; Schröder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Kote-Jarai, Z.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Castro, E.; Mahmud, N.; Guy, M.; Edwards, S.; O’Brien, L.; Sawyer, E.; et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: Implications for genetic testing in prostate cancer patients. Br. J. Cancer 2011, 105, 1230–1234. [Google Scholar] [CrossRef]
- Thompson, D.; Easton, D. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am. J. Hum. Genet. 2001, 68, 410–419. [Google Scholar] [CrossRef]
- SEER. Cancer Stat Facts: Common Cancer Sites: National Cancer Institute. 2020. Available online: https://seer.cancer.gov/statfacts/html/common.html (accessed on 15 August 2020).
- Crona, D.J.; Whang, Y.E. Androgen Receptor-Dependent and -Independent Mechanisms Involved in Prostate Cancer Therapy Resistance. Cancers 2017, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics Applications and Chemistry of Chalcone Derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef] [PubMed]
- Isaac, A.T.; Du, L.; Chowdhury, A.; Xiaoke, G.; Lu, Q.; Yin, X. Signaling pathways and proteins targeted by antidiabetic chalcones. Life Sci. 2020, 284, 118982. [Google Scholar]
- Yadav, P.; Lal, K.; Kumar, A. Antimicrobial Screening, in Silico Studies and QSAR of Chalcone-based 1,4-disubstituted 1,2,3-triazole Hybrids. Drug Res. 2020, 71, 149–156. [Google Scholar] [CrossRef]
- Bukhari, S.N.; Butt, A.M.; Amjad, M.W.; Ahmad, W.; Shah, V.H.; Trivedi, A.R. Synthesis and evaluation of chalcone analogues based pyrimidines as angiotensin converting enzyme inhibitors. Pak. J. Biol. Sci. 2013, 16, 1368–1372. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Al-Hashimi, I.; Al Moustafa, A.E.; Khalil, A. A comprehensive review on the antiviral activities of chalcones. J. Drug Target. 2021, 29, 403–419. [Google Scholar] [CrossRef]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes. Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Feng, Q.; He, B. Androgen Receptor Signaling in the Development of Castration-Resistant Prostate Cancer. Front. Oncol. 2019, 9, 858. [Google Scholar] [CrossRef]
- Loblaw, D.A.; Virgo, K.S.; Nam, R.; Somerfield, M.R.; Ben-Josef, E.; Mendelson, D.S.; Middleton, R.; Sharp, S.A.; Smith, T.J.; Talcott, J.; et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American society of clinical oncology practice guideline. J. Clin. Oncol. 2007, 25, 1596–1605. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Frieling, J.S.; Basanta, D.; Lynch, C.C. Current and emerging therapies for bone metastatic castration-resistant prostate cancer. Cancer Control 2015, 22, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, E.Y. Castrate-resistant prostate cancer: Postdocetaxel management. Curr. Opin. Urol. 2013, 23, 201–207. [Google Scholar] [CrossRef]
- Hussain, A.; Dawson, M.A. Chemotherapy in Advanced Castration-Resistant Prostate Cancer UpToDate: Wolters Kluwer. 2025. Available online: https://0-www.uptodate.com.mylibrary.qu.edu.qa/contents/chemotherapy-in-advanced-castration-resistant-prostate-cancer?sectionName=Chemotherapy-na%C3%AFve%20patients&search=castration%20resistant&topicRef=112896&anchor=H8&source=see_link#H8 (accessed on 15 August 2025).
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.A.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef]
- Crombag, M.B.S.; de Vries Schultink, A.H.M.; van Doremalen, J.G.C.; Otten, H.M.; Bergman, A.M.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R. Age-Associated Hematological Toxicity in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Docetaxel in Clinical Practice. Drugs Aging 2019, 36, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Mackey, J.R. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag. Res. 2014, 6, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Elshan, N.; Rettig, M.B.; Jung, M.E. Molecules targeting the androgen receptor (AR) signaling axis beyond the AR-Ligand binding domain. Med. Res. Rev. 2019, 39, 910–960. [Google Scholar] [CrossRef]
- Dawson, N.A.; Leger, P. Overview of the Treatment of Castration-Resistant Prostate Cancer (CRPC) UpToDate: Wolters Kluwer. 2025. Available online: https://0-www.uptodate.com.mylibrary.qu.edu.qa/contents/overview-of-the-treatment-of-castration-resistant-prostate-cancer-crpc?search=castration-resistant%20prostate%20cancer&source=search_result&selectedTitle=1~64&usage_type=default&display_rank=1 (accessed on 15 August 2025).
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- (FDA) USFaDA. 2010 Notifications FDA2010. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/2010-notifications (accessed on 15 August 2025).
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Prostate Cancer: NCCN. 2019. Available online: https://www2.tri-kobe.org/nccn/guideline/urological/english/prostate.pdf (accessed on 15 August 2025).
- Lexicomp. Sipuleucel-T: Drug Information: Wolters Kluwer. 2020. Available online: https://0-www.uptodate.com.mylibrary.qu.edu.qa/contents/sipuleucel-t-drug-information?search=castration-resistant%20prostate%20cancer&topicRef=112896&source=see_link (accessed on 26 August 2020).
- Goldstein, N.S. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. Am. J. Clin. Pathol. 2002, 117, 471–477. [Google Scholar] [CrossRef]
- Lexicomp. Radium-223: Drug Information: Wolters Kluwer. 2020. Available online: https://www.google.com/search?q=wolters+kluwer&oq=wolter&aqs=chrome.0.69i59j69i57j0l3j46j69i60j69i61.1928j0j7&sourceid=chrome&ie=UTF-8 (accessed on 26 August 2020).
- Oudard, S.; Fizazi, K.; Sengeløv, L.; Daugaard, G.; Saad, F.; Hansen, S.; Hjälm-Eriksson, M.; Jassem, J.; Thiery-Vuillemin, A.; Caffo, O.; et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: A randomized phase III trial-FIRSTANA. J. Clin. Oncol. 2017, 35, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Pozas, J.; Álvarez Rodríguez, S.; Fernández, V.A.; Burgos, J.; Santoni, M.; Manneh Kopp, R.; Molina-Cerrillo, J.; Alonso-Gordoa, T. Androgen Receptor Signaling Inhibition in Advanced Castration Resistance Prostate Cancer: What Is Expected for the Near Future? Cancers 2022, 14, 6071. [Google Scholar] [CrossRef]
- Montgomery, R.B.; Mostaghel, E.A.; Vessella, R.; Hess, D.L.; Kalhorn, T.F.; Higano, C.S.; True, L.D.; Nelson, P.S. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008, 68, 4447–4454. [Google Scholar] [CrossRef]
- Labrie, F. Mechanism of action and pure antiandrogenic properties of flutamide. Cancer 1993, 72, 3816–3827. [Google Scholar] [CrossRef]
- Longo, D.L. New Therapies for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.A.; Barrie, S.E.; Jarman, M.; Rowlands, M.G. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): Potential agents for the treatment of prostatic cancer. J. Med. Chem. 1995, 38, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- Thomsen, F.B.; Røder, M.A.; Rathenborg, P.; Brasso, K.; Borre, M.; Iversen, P. Enzalutamide treatment in patients with metastatic castration-resistant prostate cancer progressing after chemotherapy and abiraterone acetate. Scand. J. Urol. 2014, 48, 268–275. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Kita, Y.; Goto, T.; Akamatsu, S.; Yamasaki, T.; Inoue, T.; Ogawa, O.; Kobayashi, T. Castration-resistant prostate cancer refractory to second-generation androgen receptor axis-targeted agents: Opportunities and challenges. Cancers 2018, 10, 345. [Google Scholar] [CrossRef]
- (FDA) USFaDA. FDA Grants Accelerated Approval to Rucaparib for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer. 2020. Available online: https://www.fda.gov/drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate (accessed on 15 August 2025).
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- (FDA) USFaDA. FDA Approves Olaparib for HRR Gene-Mutated Metastatic Castration-Resistant Prostate Cancer. 2020. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer (accessed on 15 August 2025).
- (FDA) USFaDA. FDA Approves First Cancer Treatment for Any Solid Tumor with a Specific Genetic Feature. 2017. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-cancer-treatment-any-solid-tumor-specific-genetic-feature (accessed on 15 August 2020).
- Nelson, P.S. Beyond the Androgen Receptor: Targeting Actionable Drivers of Prostate Cancer. JCO Precis. Oncol. 2017, 1, 1–3. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Zhou, X.C.; Wang, H.; Bassi, S.; Carducci, M.A.; Eisenberger, M.A.; Antonarakis, E.S. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur. Urol. 2014, 66, 646–652. [Google Scholar] [CrossRef]
- Cheng, H.H.; Gulati, R.; Azad, A.; Nadal, R.; Twardowski, P.; Vaishampayan, U.N.; Agarwal, N.; Heath, E.I.; Pal, S.K.; Rehman, H.T.; et al. Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis. 2015, 18, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Mezynski, J.; Pezaro, C.; Bianchini, D.; Zivi, A.; Sandhu, S.; Thompson, E.; Hunt, J.; Sheridan, E.; Baikady, B.; Sarvadikar, A.; et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: Clinical evidence for cross-resistance? Ann. Oncol. 2012, 23, 2943–2947. [Google Scholar] [CrossRef] [PubMed]
- Mah, S.H. Chalcones in Diets. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2019; pp. 1–52. [Google Scholar]
- Aksöz, B.E.; Ertan, R. Chemical and Structural Properties of Chalcones I. FABAD J. Pharm. Sci. 2014, 36, 223–242. [Google Scholar]
- Batovska, D.I.; Todorova, I.T. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmacol. 2010, 5, 1–29. [Google Scholar] [CrossRef]
- Guazelli, C.F.S.; Fattori, V.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Biol. Interact. 2021, 333, 109315. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Siddique, A.B.; Qusa, M.; Cyprian, F.S.; El Sayed, K.; Alali, F.; Al Moustafa, A.E.; Khalil, A. Design, synthesis, and validation of novel nitrogen-based chalcone analogs against triple negative breast cancer. Eur. J. Med. Chem. 2020, 187, 111954. [Google Scholar] [CrossRef]
- Hassan, A.F.; Hussein, O.; Al-Barazenji, T.; Allouch, A.; Kamareddine, L.; Malki, A.; Moustafa, A.E.A.; Khalil, A. The effect of novel nitrogen-based chalcone analogs on colorectal cancer cells: Insight into the molecular pathways. Heliyon 2024, 10, e27002. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef]
- Srinivasan, B.; Johnson, T.E.; Lad, R.; Xing, C. Structure-activity relationship studies of chalcone leading to 3-hydroxy-4,3′,4′,5′-tetramethoxychalcone and its analogues as potent nuclear factor kappaB inhibitors and their anticancer activities. J. Med. Chem. 2009, 52, 7228–7235. [Google Scholar] [CrossRef]
- Das, M.; Manna, K. Chalcone Scaffold in Anticancer Armamentarium: A Molecular Insight. J. Toxicol. 2016, 2016, 7651047. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kumar, V.; Kumar, P. Heterocyclic chalcone analogues as potential anticancer agents. Anticancer Agents Med. Chem. 2013, 13, 422–432. [Google Scholar]
- Cazarolli, L.H.; Kappel, V.D.; Zanatta, A.P.; Suzuki, D.O.H.; Yunes, R.A.; Nunes, R.J.; Pizzolatti, M.G.; Silva, F.R.M.B. Chapter 2—Natural and Synthetic Chalcones: Tools for the Study of Targets of Action—Insulin Secretagogue or Insulin Mimetic? In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 39, pp. 47–89. [Google Scholar]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Hahlbrock, K.; Scheel, D. Physiology and Molecular Biology of Phenylpropanoid Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Banoth, R.; Thatikonda, A. A Review on Natural Chalcones as Update. Int. J. Pharm. Sci. Res. 2020, 11, 546–555. [Google Scholar]
- Ni, L.; Meng, C.Q.; Sikorski, J.A. Recent advances in therapeutic chalcones. Expert. Opin. Ther. Pat. 2004, 14, 1669–1691. [Google Scholar] [CrossRef]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef]
- Guex, J.J.; Avril, L.; Enrici, E.; Enriquez, E.; Lis, C.; Taïeb, C. Quality of life improvement in Latin American patients suffering from chronic venous disorder using a combination of Ruscus aculeatus and hesperidin methyl-chalcone and ascorbic acid (quality study). Int. Angiol. 2010, 29, 525–532. [Google Scholar]
- Allaert, F.A. Combination of Ruscus aculeatus extract, hesperidin methyl chalcone and ascorbic acid: A comprehensive review of their pharmacological and clinical effects and of the pathophysiology of chronic venous disease. Int. Angiol. 2016, 35, 111–116. [Google Scholar]
- Kumar, A.; Sharma, S.; Tripathi, V.D.; Srivastava, S. Synthesis of chalcones and flavanones using Julia–Kocienski olefination. Tetrahedron 2010, 66, 9445–9449. [Google Scholar] [CrossRef]
- Kar Mahapatra, D.; Asati, V.; Bharti, S.K. An updated patent review of therapeutic applications of chalcone derivatives (2014–present). Expert. Opin. Ther. Pat. 2019, 29, 385–406. [Google Scholar] [CrossRef]
- Jandial, D.D.; Blair, C.A.; Zhang, S.; Krill, L.S.; Zhang, Y.B.; Zi, X. Molecular targeted approaches to cancer therapy and prevention using chalcones. Curr. Cancer Drug Targets 2014, 14, 181–200. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Moorthy, N.S.; Ramasamy, S.; Vanam, U.; Manivannan, E.; Karunagaran, D.; Trivedi, P. Advances in chalcones with anticancer activities. Recent. Pat. Anticancer Drug Discov. 2015, 10, 97–115. [Google Scholar] [CrossRef]
- Gao, F.; Huang, G.; Xiao, J. Chalcone hybrids as potential anticancer agents: Current development, mechanism of action, and structure-activity relationship. Med. Res. Rev. 2020, 40, 2049–2084. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, Z.; Wang, Q.; Zhou, C.; Lu, F.; Xiao, Y. Chalcone Derivatives Suppress Proliferation and Migration of Castration-resistant Prostate Cancer Cells Through FAK-mediated DNA Damage. Anticancer Res. 2023, 43, 389–403. [Google Scholar] [CrossRef]
- Michalkova, R.; Mirossay, L.; Kello, M.; Mojzisova, G.; Baloghova, J.; Podracka, A.; Mojzis, J. Anticancer Potential of Natural Chalcones: In Vitro and In Vivo Evidence. Int. J. Mol. Sci. 2023, 24, 10354. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.G. The correlation between cancer incidence and kava consumption. Hawaii Med. J. 2000, 59, 420–422. [Google Scholar] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Plati, J.; Bucur, O.; Khosravi-Far, R. Dysregulation of apoptotic signaling in cancer: Molecular mechanisms and therapeutic opportunities. J. Cell. Biochem. 2008, 104, 1124–1149. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.M.; Mita, A.C.; Tolcher, A.W. Apoptosis: Mechanisms and implications for cancer therapeutics. Target. Oncol. 2006, 1, 197–214. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Kajiwara, T.; Takeuchi, T.; Ueki, T.; Moriyama, N.; Ueki, K.; Kakizoe, T.; Kawabe, K. Effect of Bcl-2 overexpression in human prostate cancer cells in vitro and in vivo. Int. J. Urol. 1999, 6, 520–525. [Google Scholar] [CrossRef]
- Cryns, V.; Yuan, J. Proteases to die for. Genes Dev. 1998, 12, 1551–1570. [Google Scholar] [CrossRef]
- Uzzo, R.G.; Haas, N.B.; Crispen, P.L.; Kolenko, V.M. Mechanisms of apoptosis resistance and treatment strategies to overcome them in hormone-refractory prostate cancer. Cancer 2008, 112, 1660–1671. [Google Scholar] [CrossRef]
- McKenzie, S.; Kyprianou, N. Apoptosis evasion: The role of survival pathways in prostate cancer progression and therapeutic resistance. J. Cell. Biochem. 2006, 97, 18–32. [Google Scholar] [CrossRef]
- Winter, R.N.; Kramer, A.; Borkowski, A.; Kyprianou, N. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 2001, 61, 1227–1232. [Google Scholar]
- Kagan, J.; Stein, J.; Babaian, R.J.; Joe, Y.S.; Pisters, L.L.; Glassman, A.B.; von Eschenbach, A.C.; Troncoso, P. Homozygous deletions at 8p22 and 8p21 in prostate cancer implicate these regions as the sites for candidate tumor suppressor genes. Oncogene 1995, 11, 2121–2126. [Google Scholar] [PubMed]
- Hernandez-Cueto, A.; Hernandez-Cueto, D.; Antonio-Andres, G.; Mendoza-Marin, M.; Jimenez-Gutierrez, C.; Sandoval-Mejia, A.L.; Mora-Campos, R.; Gonzalez-Bonilla, C.; Vega, M.I.; Bonavida, B.; et al. Death receptor 5 expression is inversely correlated with prostate cancer progression. Mol. Med. Rep. 2014, 10, 2279–2286. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Lim, S.S.; Choi, H.J.; Cho, H.J.; Shin, H.K.; Kim, E.J.; Chung, W.Y.; Park, K.K.; Park, J.H. Isoliquiritigenin induces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. J. Nutr. Biochem. 2006, 17, 689–696. [Google Scholar] [CrossRef]
- Marquina, S.; Maldonado-Santiago, M.; Sánchez-Carranza, J.N.; Antúnez-Mojica, M.; González-Maya, L.; Razo-Hernández, R.S.; Alvarez, L. Design, synthesis and QSAR study of 2′-hydroxy-4′-alkoxy chalcone derivatives that exert cytotoxic activity by the mitochondrial apoptotic pathway. Bioorganic Med. Chem. 2019, 27, 43–54. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Jiang, H.; Arbab, A.S.; Dulchavsky, S.A.; Gautam, S.C. Growth inhibitory and apoptosis-inducing effects of xanthohumol, a prenylated chalone present in hops, in human prostate cancer cells. Anticancer Res. 2010, 30, 3333–3339. [Google Scholar]
- Li, X.; Pham, V.; Tippin, M.; Fu, D.; Rendon, R.; Song, L.; Uchio, E.; Hoang, B.H.; Zi, X. Flavokawain B targets protein neddylation for enhancing the anti-prostate cancer effect of Bortezomib via Skp2 degradation. Cell Commun. Signal. 2019, 17, 25. [Google Scholar] [CrossRef]
- Kłósek, M.; Mertas, A.; Król, W.; Jaworska, D.; Szymszal, J.; Szliszka, E. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in prostate cancer cells after treatment with Xanthohumol-A natural compound present in Humulus lupulus L. Int. J. Mol. Sci. 2016, 17, 837. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yun, J.; Jung, J.C.; Oh, S.; Jung, Y.S. Anti-tumor activity of benzylideneacetophenone derivatives via proteasomal inhibition in prostate cancer cells. Pharmazie 2016, 71, 274–279. [Google Scholar]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Paradysz, A.; Krol, W. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules 2010, 15, 5336–5353. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Mazur, B.; Sedek, L.; Paradysz, A.; Krol, W. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int. J. Mol. Sci. 2009, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Fagnere, C.; Limami, Y.; Ghezali, L.; Pouget, C.; Fidanzi, C.; Ouk, C.; Gueye, R.; Beneytout, J.L.; Duroux, J.L.; et al. 2′-Hydroxy-4-methylsulfonylchalcone enhances TRAIL-induced apoptosis in prostate cancer cells. Anticancer Drugs 2015, 26, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, H.; Kong, X.; Chen, X.; Wu, C. Synthesis of new chalcone-based homoserine lactones and their antiproliferative activity evaluation. Eur. J. Med. Chem. 2019, 163, 500–511. [Google Scholar] [CrossRef]
- Thorburn, A.; Behbakht, K.; Ford, H. TRAIL receptor-targeted therapeutics: Resistance mechanisms and strategies to avoid them. Drug Resist. Updat. 2008, 11, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef]
- Diaz-Moralli, S.; Tarrado-Castellarnau, M.; Miranda, A.; Cascante, M. Targeting cell cycle regulation in cancer therapy. Pharmacol. Ther. 2013, 138, 255–271. [Google Scholar] [CrossRef]
- Carnero, A. Targeting the cell cycle for cancer therapy. Br. J. Cancer 2002, 87, 129–133. [Google Scholar] [CrossRef]
- Aaltomaa, S.; Eskelinen, M.; Lipponen, P. Expression of cyclin A and D proteins in prostate cancer and their relation to clinopathological variables and patient survival. Prostate 1999, 38, 175–182. [Google Scholar] [CrossRef]
- Comstock, C.E.S.; Revelo, M.P.; Buncher, C.R.; Knudsen, K.E. Impact of differential cyclin D1 expression and localisation in prostate cancer. Br. J. Cancer 2007, 96, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Chen, X.; Xu, Y.; Guo, F.; Ji, J.; Xu, H.; He, J.; Sun, Y.; Wang, F. Differential Expression and Prognostic Value of Cytoplasmic and Nuclear Cyclin D1 in Prostate Cancer. BioMed Res. Int. 2020, 2020, 1692658. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, O.J.; Haukaas, S.A.; Akslen, L.A. Combined Loss of PTEN and p27 Expression Is Associated with Tumor Cell Proliferation by Ki-67 and Increased Risk of Recurrent Disease in Localized Prostate Cancer. Clin. Cancer Res. 2003, 9, 1474–1479. [Google Scholar]
- Saito, Y.; Mizokami, A.; Maeda, S.; Takahashi, K.; Izumi, K.; Goto, M.; Nakagawa-Goto, K. Bicyclic Chalcones as Mitotic Inhibitors for Overcoming Androgen Receptor-Independent and Multidrug-Resistant Prostate Cancer. ACS Omega 2021, 6, 4842–4849. [Google Scholar] [CrossRef]
- Chen, C.; Shao, R.; Li, B.; Zhai, Y.; Wang, T.; Li, X.; Miao, L.; Huang, J.; Liu, R.; Liu, E.; et al. Neoisoliquiritin exerts tumor suppressive effects on prostate cancer by repressing androgen receptor activity. Phytomedicine 2021, 85, 153514. [Google Scholar] [CrossRef]
- Shimada, T.; Naito, R.; Toriumi, R.; Nakagawa, R.; Aoyama, S.; Kamijima, T.; Kano, H.; Kadomoto, S.; Iwamoto, H.; Yaegashi, H.; et al. Novel α-Trifluoromethyl Chalcone Exerts Antitumor Effects Against Prostate Cancer Cells. Anticancer Res. 2023, 43, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Mizokami, A.; Izumi, K.; Naito, R.; Goto, M.; Nakagawa-Goto, K. α-Trifluoromethyl Chalcones as Potent Anticancer Agents for Androgen Receptor-Independent Prostate Cancer. Molecules 2021, 26, 2812. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Pinon, A.; Gamond, A.; Martin, F.; Laurent, A.; Champavier, Y.; Barette, C.; Liagre, B.; Fagnère, C.; Sol, V.; et al. Synthesis and biological evaluation of chalcone-polyamine conjugates as novel vectorized agents in colorectal and prostate cancer chemotherapy. Eur. J. Med. Chem. 2021, 222, 113586. [Google Scholar] [CrossRef]
- Fu, Y.; Hsieh, T.C.; Guo, J.; Kunicki, J.; Lee, M.Y.; Darzynkiewicz, Z.; Wu, J.M. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem. Biophys. Res. Commun. 2004, 322, 263–270. [Google Scholar] [CrossRef]
- Fu, D.J.; Li, J.H.; Yang, J.J.; Li, P.; Zhang, Y.B.; Liu, S.; Li, Z.R.; Zhang, S.Y. Discovery of novel chalcone-dithiocarbamates as ROS-mediated apoptosis inducers by inhibiting catalase. Bioorganic Chem. 2019, 86, 375–385. [Google Scholar] [CrossRef]
- Sun, Y.W.; Huang, W.J.; Hsiao, C.J.; Chen, Y.C.; Lu, P.H.; Guh, J.H. Methoxychalcone induces cell-cycle arrest and apoptosis in human hormone-resistant prostate cancer cells through PI 3-kinase-independent inhibition of mTOR pathways. Prostate 2010, 70, 1295–1306. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lim, D.Y.; Choi, H.J.; Jung, J.I.; Chung, W.Y.; Park, J.H. Induction of cell cycle arrest in prostate cancer cells by the dietary compound isoliquiritigenin. J. Med. Food 2009, 12, 8–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Srinivasan, B.; Xing, C.; Lü, J. A new chalcone derivative (E)-3-(4-methoxyphenyl)-2-methyl-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one suppresses prostate cancer involving p53-mediated cell cycle arrests and apoptosis. Anticancer Res. 2012, 32, 3689–3698. [Google Scholar] [PubMed]
- Alam, M.J.; Alam, O.; Perwez, A.; Rizvi, M.A.; Naim, M.J.; Naidu, V.G.M.; Imran, M.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Design, Synthesis, Molecular Docking, and Biological Evaluation of Pyrazole Hybrid Chalcone Conjugates as Potential Anticancer Agents and Tubulin Polymerization Inhibitors. Pharmaceuticals 2022, 15, 280. [Google Scholar] [CrossRef]
- Peyrot, V.; Leynadier, D.; Sarrazin, M.; Briand, C.; Rodriquez, A.; Nieto, J.M.; Andreu, J.M. Interaction of tubulin and cellular microtubules with the new antitumor drug MDL 27048. A powerful and reversible microtubule inhibitor. J. Biol. Chem. 1989, 264, 21296–21301. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Mizokami, A.; Tsurimoto, H.; Izumi, K.; Goto, M.; Nakagawa-Goto, K. 5′-Chloro-2,2′-dihydroxychalcone and related flavanoids as treatments for prostate cancer. Eur. J. Med. Chem. 2018, 157, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.M.; Yedla, P.; Babu, K.S.; Shaik, T.B.; Chityal, G.K.; Kamal, A. Synthesis and Biological Evaluation of 1,2,3-triazole tethered Pyrazoline and Chalcone Derivatives. Chem. Biol. Drug Des. 2016, 88, 97–109. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, W.; Wang, Z.; Gao, M.; Wang, X.; Han, W.; Zhang, N.; Xu, X. Flavokawain A inhibits prostate cancer cells by inducing cell cycle arrest and cell apoptosis and regulating the glutamine metabolism pathway. J. Pharm. Biomed. Anal. 2020, 186, 113288. [Google Scholar] [CrossRef]
- Shaffer, C.V.; Cai, S.; Peng, J.; Robles, A.J.; Hartley, R.M.; Powell, D.R.; Du, L.; Cichewicz, R.H.; Mooberry, S.L. Texas native plants yield compounds with cytotoxic activities against prostate cancer cells. J. Nat. Prod. 2016, 79, 531–540. [Google Scholar] [CrossRef]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- Yao, D.; Dai, C.; Peng, S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol. Cancer Res. 2011, 9, 1608–1620. [Google Scholar] [CrossRef]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J. Epithelial-mesenchymal transition (emt) and prostate cancer. In Cell & Molecular Biology of Prostate Cancer: Updates, Insights and New Frontiers; Schatten, H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 101–110. [Google Scholar]
- Montanari, M.; Rossetti, S.; Cavaliere, C.; D’Aniello, C.; Malzone, M.G.; Vanacore, D.; Di Franco, R.; La Mantia, E.; Iovane, G.; Piscitelli, R.; et al. Epithelial-mesenchymal transition in prostate cancer: An overview. Oncotarget 2017, 8, 35376–35389. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Scott, K.F.; Becker, T.M.; Lock, J.; Nimir, M.; Ma, Y.; de Souza, P. The Prospect of Identifying Resistance Mechanisms for Castrate-Resistant Prostate Cancer Using Circulating Tumor Cells: Is Epithelial-to-Mesenchymal Transition a Key Player? Prostate Cancer 2020, 2020, 7938280. [Google Scholar] [CrossRef]
- Chen, C.; Huang, S.; Chen, C.L.; Su, S.B.; Fang, D.D. Isoliquiritigenin Inhibits Ovarian Cancer Metastasis by Reversing Epithelial-to-Mesenchymal Transition. Molecules 2019, 24, 3725. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jang, H.J.; Kwak, S.; Sung, G.J.; Park, S.H.; Song, J.H.; Kim, H.; Na, Y.; Choi, K.C. Novel TGF-β1 inhibitor antagonizes TGF-β1-induced epithelial-mesenchymal transition in human A549 lung cancer cells. J. Cell Biochem. 2019, 120, 977–987. [Google Scholar] [CrossRef]
- Moon, D.O.; Choi, Y.H.; Moon, S.K.; Kim, W.J.; Kim, G.Y. Butein suppresses the expression of nuclear factor-kappa B-mediated matrix metalloproteinase-9 and vascular endothelial growth factor in prostate cancer cells. Toxicol. Vitr. 2010, 24, 1927–1934. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, B.; Yu, J.; Huang, L.; Zeng, X.; Shen, X.; Ren, C.; Ben-David, Y.; Luo, H. Fli-1 activation through targeted promoter activity regulation using a novel 3′, 5′-diprenylated chalcone inhibits growth and metastasis of prostate cancer cells. Int. J. Mol. Sci. 2020, 21, 2216. [Google Scholar] [CrossRef]
- Kwon, G.T.; Cho, H.J.; Chung, W.Y.; Park, K.K.; Moon, A.; Park, J.H. Isoliquiritigenin inhibits migration and invasion of prostate cancer cells: Possible mediation by decreased JNK/AP-1 signaling. J. Nutr. Biochem. 2009, 20, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.B.; Li, J.; Meniel, V.S.; Fennell, C.M.; Waring, P.; Montgomery, K.G.; Rebello, R.J.; Macpherson, A.A.; Koushyar, S.; Furic, L.; et al. Identification of Pik3ca Mutation as a Genetic Driver of Prostate Cancer That Cooperates with Pten Loss to Accelerate Progression and Castration-Resistant Growth. Cancer Discov. 2018, 8, 764–779. [Google Scholar] [CrossRef]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef]
- Grasso, C.S.; Wu, Y.-M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Chung, E.; Seon, M.R.; Shin, H.K.; Kim, E.J.; Lim, S.S.; Chung, W.Y.; Park, K.K.; Park, J.H. Isoliquiritigenin (ISL) inhibits ErbB3 signaling in prostate cancer cells. Biofactors 2006, 28, 159–168. [Google Scholar] [CrossRef]
- Khor, T.O.; Yu, S.; Barve, A.; Hao, X.; Hong, J.L.; Lin, W.; Foster, B.; Huang, M.T.; Newmark, H.L.; Kong, A.N. Dietary feeding of dibenzoylmethane inhibits prostate cancer in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2009, 69, 7096–7102. [Google Scholar] [CrossRef]
- Yo, Y.T.; Shieh, G.S.; Hsu, K.F.; Wu, C.L.; Shiau, A.L. Licorice and licochalcone-A induce autophagy in LNCaP prostate cancer cells by suppression of Bcl-2 expression and the mTOR pathway. J. Agric. Food Chem. 2009, 57, 8266–8273. [Google Scholar] [CrossRef]
- Horta, B.; Freitas-Silva, J.; Silva, J.; Dias, F.; Teixeira, A.L.; Medeiros, R.; Cidade, H.; Pinto, M.; Cerqueira, F. Antitumor Effect of Chalcone Derivatives against Human Prostate (LNCaP and PC-3), Cervix HPV-Positive (HeLa) and Lymphocyte (Jurkat) Cell Lines and Their Effect on Macrophage Functions. Molecules 2023, 28, 2159. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.W.C.; Poon, R.T.P. Clinical implications of angiogenesis in cancers. Vasc. Health Risk Manag. 2006, 2, 97–108. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Green, M.M.L.; Hiley, C.T.; Shanks, J.H.; Bottomley, I.C.; West, C.M.L.; Cowan, R.A.; Stratford, I.J. Expression of vascular endothelial growth factor (VEGF) in locally invasive prostate cancer is prognostic for radiotherapy outcome. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.J.; Mac Gabhann, F. Dysregulation of the vascular endothelial growth factor and semaphorin ligand-receptor families in prostate cancer metastasis. BMC Syst. Biol. 2015, 9, 55. [Google Scholar] [CrossRef]
- Melegh, Z.; Oltean, S. Targeting Angiogenesis in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 2676. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R.; Zurita, A.J.; Werner, L.; Bruce, J.Y.; Carducci, M.A.; Stein, M.N.; Heath, E.I.; Hussain, A.; Tran, H.T.; Sweeney, C.J.; et al. A randomized phase II trial of short-course androgen deprivation therapy with or without Bevacizumab for patients with recurrent prostate cancer after definitive local therapy. J. Clin. Oncol. 2016, 34, 1913–1920. [Google Scholar] [CrossRef]
- Kelly, W.K.; Halabi, S.; Carducci, M.; George, D.; Mahoney, J.F.; Stadler, W.M.; Morris, M.; Kantoff, P.; Monk, J.P.; Kaplan, E.; et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J. Clin. Oncol. 2012, 30, 1534–1540. [Google Scholar] [CrossRef]
- Tannock, I.F.; Fizazi, K.; Ivanov, S.; Karlsson, C.T.; Fléchon, A.; Skoneczna, I.; Orlandi, F.; Gravis, G.; Matveev, V.; Bavbek, S.; et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): A phase 3, double-blind randomised trial. Lancet Oncol. 2013, 14, 760–768. [Google Scholar] [CrossRef]
- Michaelson, M.D.; Oudard, S.; Ou, Y.C.; Sengeløv, L.; Saad, F.; Houede, N.; Ostler, P.; Stenzl, A.; Daugaard, G.; Jones, R.; et al. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. J. Clin. Oncol. 2014, 32, 76–82. [Google Scholar] [CrossRef]
- Keizman, D.; Zahurak, M.; Sinibaldi, V.; Carducci, M.; Denmeade, S.; Drake, C.; Pili, R.; Antonarakis, E.S.; Hudock, S.; Eisenberger, M. Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: Results of a phase I/II double-blinded, randomized study. Clin. Cancer Res. 2010, 16, 5269–5276. [Google Scholar] [CrossRef]
- Mahmoud, A.; Elkhalifa, D.; Alali, F.; Al Moustafa, A.E.; Khalil, A. Novel Polymethoxylated Chalcones as Potential Compounds Against KRAS-Mutant Colorectal Cancers. Curr. Pharm. Des. 2020, 26, 1622–1633. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Wen, Z.; Chen, X.; Yu, J.; Yuan, D.; Xu, B.; Luo, H.; Zhu, J. A novel 3′,5′-diprenylated chalcone induces concurrent apoptosis and GSDME-dependent pyroptosis through activating PKCδ/JNK signal in prostate cancer. Aging 2020, 12, 9103–9124. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Jackson, K.M.; Frazier, M.C.; Harris, W.B. Suppression of androgen receptor expression by dibenzoylmethane as a therapeutic objective in advanced prostate cancer. Anticancer Res. 2007, 27, 1483–1488. [Google Scholar] [PubMed]
- Chen, S.; Gao, J.; Halicka, H.D.; Traganos, F.; Darzynkiewicz, Z. Down-regulation of androgen-receptor and PSA by phytochemicals. Int. J. Oncol. 2008, 32, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Geng, G.; Batist, G.; Wu, J.H. Syntheses and potential anti-prostate cancer activities of ionone-based chalcones. Bioorganic Med. Chem. Lett. 2009, 19, 1183–1186. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.; Bagatini, M.D.; Reetz, L.G.; Chiesa, J.; Battisti, I.E.; Gonçalves, J.F.; Duarte, M.M.; Schetinger, M.R.; Morsch, V.M. Oxidative stress and antioxidant status in prostate cancer patients: Relation to Gleason score, treatment and bone metastasis. Biomed. Pharmacother. 2011, 65, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yamashita, H.; Yu, X.; Wang, J.; Franco, O.E.; Wang, Y.; Hayward, S.W.; Matusik, R.J. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene 2015, 34, 3700–3710. [Google Scholar] [CrossRef]
- Khurana, N.; Sikka, S.C. Targeting Crosstalk between Nrf-2, NF-κB and Androgen Receptor Signaling in Prostate Cancer. Cancers 2018, 10, 352. [Google Scholar] [CrossRef]
- Staal, J.; Beyaert, R. Inflammation and NF-κB Signaling in Prostate Cancer: Mechanisms and Clinical Implications. Cells 2018, 7, 122. [Google Scholar] [CrossRef]
- Ismail, B.; Ghezali, L.; Gueye, R.; Limami, Y.; Pouget, C.; Leger, D.Y.; Martin, F.; Beneytout, J.L.; Duroux, J.L.; Diab-Assaf, M.; et al. Novel methylsulfonyl chalcones as potential antiproliferative drugs for human prostate cancer: Involvement of the intrinsic pathway of apoptosis. Int. J. Oncol. 2013, 43, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.C.; Ehrenfried, C.A.; Lopez, B.G.; de Araujo, T.M.; Pascoal, V.D.; Gilioli, R.; Anhê, G.F.; Ruiz, A.L.; Carvalho, J.E.; Stefanello, M.E.; et al. Antiproliferative activity and induction of apoptosis in PC-3 cells by the chalcone cardamonin from Campomanesia adamantium (Myrtaceae) in a bioactivity-guided study. Molecules 2014, 19, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Z.; Sarkar, F.H.; Wei, W. Targeting prostate cancer stem cells for cancer therapy. Discov. Med. 2012, 13, 135–142. [Google Scholar] [PubMed]
- Maccalli, C.; De Maria, R. Cancer stem cells: Perspectives for therapeutic targeting. Cancer Immunol. Immunother. 2015, 64, 91–97. [Google Scholar] [CrossRef]
- Hussein, O.J.; Rayan, M.; Matarid, T.R.; Elkhalifa, D.; Abunada, H.H.; Therachiyil, L.; Khalil, A.; Uddin, S.; Maccalli, C.; Korashy, H.M. The role of immune checkpoints in modulating cancer stem cells anti-tumor immune responses: Implications and perspectives in cancer therapy. J. Exp. Clin. Cancer Res. 2025, 44, 305. [Google Scholar] [CrossRef]
- Tout, I.; Bougarn, S.; Toufiq, M.; Gopinath, N.; Hussein, O.; Sathappan, A.; Chin-Smith, E.; Rehaman, F.; Mathew, R.; Mathew, L.; et al. The integrative genomic and functional immunological analyses of colorectal cancer initiating cells to modulate stemness properties and the susceptibility to immune responses. J. Transl. Med. 2025, 23, 193. [Google Scholar] [CrossRef]
- Rybak, A.P.; Bristow, R.G.; Kapoor, A. Prostate cancer stem cells: Deciphering the origins and pathways involved in prostate tumorigenesis and aggression. Oncotarget 2015, 6, 1900–1919. [Google Scholar] [CrossRef]
- Wen, D.; Peng, Y.; Lin, F.; Singh, R.K.; Mahato, R.I. Micellar Delivery of miR-34a Modulator Rubone and Paclitaxel in Resistant Prostate Cancer. Cancer Res. 2017, 77, 3244–3254. [Google Scholar] [CrossRef]
- Lin, F.; Wen, D.; Wang, X.; Mahato, R.I. Dual responsive micelles capable of modulating miRNA-34a to combat taxane resistance in prostate cancer. Biomaterials 2019, 192, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Bartmańska, A.; Tronina, T.; Popłoński, J.; Milczarek, M.; Filip-Psurska, B.; Wietrzyk, J. Highly Cancer Selective Antiproliferative Activity of Natural Prenylated Flavonoids. Molecules 2018, 23, 2922. [Google Scholar] [CrossRef]
- Burcu Gürdere, M.; Aydin, A.; Yencilek, B.; Ertürk, F.; Özbek, O.; Erkan, S.; Budak, Y.; Ceylan, M. Synthesis, Antiproliferative and Cytotoxic Activities, DNA Binding Features and Molecular Docking Study of Novel Enamine Derivatives. Chem. Biodivers. 2020, 17, e2000139. [Google Scholar] [CrossRef] [PubMed]
- Ocasio-Malavé, C.; Donate, M.J.; Sánchez, M.M.; Sosa-Rivera, J.M.; Mooney, J.W.; Pereles-De León, T.A.; Carballeira, N.M.; Zayas, B.; Vélez-Gerena, C.E.; Martínez-Ferrer, M.; et al. Synthesis of novel 4-Boc-piperidone chalcones and evaluation of their cytotoxic activity against highly-metastatic cancer cells. Bioorganic Med. Chem. Lett. 2020, 30, 126760. [Google Scholar] [CrossRef]
- Cancino, K.; Castro, I.; Yauri, C.; Jullian, V.; Arévalo, J.; Sauvain, M.; Adaui, V.; Castillo, D. Toxicity assessment of synthetic chalcones with antileishmanial potential in BALB/c mice. Rev. Peru. Med. Exp. Salud Publica 2021, 38, 424–433. [Google Scholar] [CrossRef]
- Li, W.; Xu, F.; Shuai, W.; Sun, H.; Yao, H.; Ma, C.; Xu, S.; Yao, H.; Zhu, Z.; Yang, D.-H.; et al. Discovery of Novel Quinoline–Chalcone Derivatives as Potent Antitumor Agents with Microtubule Polymerization Inhibitory Activity. J. Med. Chem. 2019, 62, 993–1013. [Google Scholar] [CrossRef]
- WalyEldeen, A.A.; El-Shorbagy, H.M.; Hassaneen, H.M.; Abdelhamid, I.A.; Sabet, S.; Ibrahim, S.A. [1,2,4] Triazolo [3,4-a]isoquinoline chalcone derivative exhibits anticancer activity via induction of oxidative stress, DNA damage, and apoptosis in Ehrlich solid carcinoma-bearing mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1225–1238. [Google Scholar] [CrossRef]
- Zhou, P.; Gross, S.; Liu, J.H.; Yu, B.Y.; Feng, L.L.; Nolta, J.; Sharma, V.; Piwnica-Worms, D.; Qiu, S.X. Flavokawain B, the hepatotoxic constituent from kava root, induces GSH-sensitive oxidative stress through modulation of IKK/NF-kappaB and MAPK signaling pathways. FASEB J. 2010, 24, 4722–4732. [Google Scholar]
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Rychlicka, M.; Sordon, S.; Filip-Psurska, B.; Milczarek, M.; Wietrzyk, J.; Huszcza, E. Prenylated Flavonoids with Selective Toxicity against Human Cancers. Int. J. Mol. Sci. 2023, 24, 7408. [Google Scholar] [CrossRef] [PubMed]
- d’Oliveira, G.D.C.; Custodio, J.M.F.; Moura, A.F.; Napolitano, H.B.; Pérez, C.N.; Moraes, M.O.; Prókai, L.; Perjési, P. Different reactivity to glutathione but similar tumor celltoxicity of chalcones and their quinolinone analogues. Med. Chem. Res. 2019, 28, 1448–1460. [Google Scholar] [CrossRef]
- Amslinger, S. The tunable functionality of α,β-unsaturated carbonyl compounds enables their differential application in biological systems. ChemMedChem 2010, 5, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Suliman, A.; Hassan, A.F.; Saeed, S.; Al Moustafa, A.-E.; Khalil, A.; Elhissi, A. Liposomal delivery of DK14 chalcone analogue: A promising therapeutic strategy against triple-negative breast cancer. J. Drug Deliv. Sci. Technol. 2026, 115, 107601. [Google Scholar] [CrossRef]
- Souza, J.M.T.; de Negreiros Matos Silva, S.A.; de Sá, R.E.; Ribeiro, R.; de Souza Castro, A.J.; Eaton, P.; de Castro, M.R.C.; Pérez, C.N.; Machado-Neto, J.A.; da Silva, D.A.; et al. ZnO nanoparticles enhance the cytotoxic effects of a chalcone-sulfonamide hybrid by impairing the autophagic flux and upregulating HIF-1α expression. Bioorganic Chem. 2025, 166, 109127. [Google Scholar] [CrossRef]
- Nakao, I.A.; Hermenegildo, A.M.; Vaz, L.B.A.; Reis, A.C.C.; Coutinho, G.G.; Campbell, G.S.G.; Almeida, T.C.; Amparo, T.R.; de Fatima Anunciação, K.; da Silva, G.N.; et al. Development of new glycosyl-chalcones targeting cancer cells through recognition of cellular carbohydrate receptors. Future Med. Chem. 2025, 17, 2999–3012. [Google Scholar] [CrossRef] [PubMed]
- Manna, T.; Pal, K.; Jana, K.; Misra, A.K. Anti-cancer potential of novel glycosylated 1,4-substituted triazolylchalcone derivatives. Bioorganic Med. Chem. Lett. 2019, 29, 126615. [Google Scholar] [CrossRef]
- Toublet, F.-X.; Laurent, A.; Pouget, C. A Review of Natural and Synthetic Chalcones as Anticancer Agents Targeting Topoisomerase Enzymes. Molecules 2025, 30, 2498. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Nath, P.; Deb, V.K.; Das, N.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Pharmacological potential of natural chalcones: A recent studies and future perspective. Front. Pharmacol. 2025, 16, 1570385. [Google Scholar] [CrossRef]
- Laiche, M.H.; Barlow, J.W. Recent Advances in the Synthesis and Biological Applications of Prenylated Chalcones. Int. J. Mol. Sci. 2025, 26, 9845. [Google Scholar] [CrossRef]
- Buckett, L.; Sus, N.; Spindler, V.; Rychlik, M.; Schoergenhofer, C.; Frank, J. The Pharmacokinetics of Individual Conjugated Xanthohumol Metabolites Show Efficient Glucuronidation and Higher Bioavailability of Micellar than Native Xanthohumol in a Randomized, Double-Blind, Crossover Trial in Healthy Humans. Mol. Nutr. Food Res. 2023, 67, 2200684. [Google Scholar] [CrossRef]
- Nikolic, D.; Li, Y.; Chadwick, L.R.; Pauli, G.F.; van Breemen, R.B. Metabolism of xanthohumol and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J. Mass Spectrom. JMS 2005, 40, 289–299. [Google Scholar] [CrossRef]
- Krajnović, T.; Kaluđerović, G.N.; Wessjohann, L.A.; Mijatović, S.; Maksimović-Ivanić, D. Versatile antitumor potential of isoxanthohumol: Enhancement of paclitaxel activity in vivo. Pharmacol. Res. 2016, 105, 62–73. [Google Scholar] [CrossRef]
- Sung, B.; Cho, S.G.; Liu, M.; Aggarwal, B.B. Butein, a tetrahydroxychalcone, suppresses cancer-induced osteoclastogenesis through inhibition of receptor activator of nuclear factor-kappaB ligand signaling. Int. J. Cancer 2011, 129, 2062–2072. [Google Scholar] [CrossRef]
- Khan, N.; Adhami, V.M.; Afaq, F.; Mukhtar, H. Butein induces apoptosis and inhibits prostate tumor growth in vitro and in vivo. Antioxid. Redox Signal. 2012, 16, 1195–1204. [Google Scholar] [CrossRef]
- Kanazawa, M.; Satomi, Y.; Mizutani, Y.; Ukimura, O.; Kawauchi, A.; Sakai, T.; Baba, M.; Okuyama, T.; Nishino, H.; Miki, T. Isoliquiritigenin inhibits the growth of prostate cancer. Eur. Urol. 2003, 43, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lai, Y.; Li, Y.; Shu, N.; Wang, Z.; Wang, Y.; Li, Y.; Chen, Z. Antineoplastic activity of isoliquiritigenin, a chalcone compound, in androgen-independent human prostate cancer cells linked to G2/M cell cycle arrest and cell apoptosis. Eur. J. Pharmacol. 2018, 821, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, E.D.; Wang, J.; Panzhinskiy, E.E.; Tong, C.; Li, W.; Li, J. Isoliquiritigenin, a natural anti-oxidant, selectively inhibits the proliferation of prostate cancer cells. Clin. Exp. Pharmacol. Physiol. 2010, 37, 841–847. [Google Scholar] [CrossRef]
- Lackova, Z.; Buchtelova, H.; Buchtova, Z.; Klejdus, B.; Heger, Z.; Brtnicky, M.; Kynicky, J.; Zitka, O.; Adam, V. Anticarcinogenic Effect of Spices Due to Phenolic and Flavonoid Compounds-In Vitro Evaluation on Prostate Cells. Molecules 2017, 22, 1626. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.I.; Yerdelen, K.O.; Das, U.; Gul, M.; Pandit, B.; Li, P.K.; Dimmock, J.R. Synthesis and cytotoxicity of novel 3-aryl-1-(3′-dibenzylaminomethyl-4′-hydroxyphenyl)-propenones and related compounds. Chem. Pharm. Bull. 2008, 56, 1675–1681. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Ji, T.; Liu, Z.; Gu, M.; Hoang, B.H.; Zi, X. Dietary feeding of Flavokawain A, a Kava chalcone, exhibits a satisfactory safety profile and its association with enhancement of phase II enzymes in mice. Toxicol. Rep. 2014, 1, 2–11. [Google Scholar] [CrossRef]
- Li, X.; Yokoyama, N.N.; Zhang, S.; Ding, L.; Liu, H.M.; Lilly, M.B.; Mercola, D.; Zi, X. Flavokawain A induces deNEDDylation and Skp2 degradation leading to inhibition of tumorigenesis and cancer progression in the TRAMP transgenic mouse model. Oncotarget 2015, 6, 41809–41824. [Google Scholar] [CrossRef]
- Song, L.; Mino, M.; Yamak, J.; Nguyen, V.; Lopez, D.; Pham, V.; Fazelpour, A.; Le, V.; Fu, D.; Tippin, M.; et al. Flavokawain A Reduces Tumor-Initiating Properties and Stemness of Prostate Cancer. Front. Oncol. 2022, 12, 943846. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Liu, Z.; Simoneau, A.R.; Xie, J.; Zi, X. Flavokawain B, a kava chalcone, induces apoptosis via up-regulation of death-receptor 5 and Bim expression in androgen receptor negative, hormonal refractory prostate cancer cell lines and reduces tumor growth. Int. J. Cancer 2010, 127, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- El-Naga, R.N. Pre-treatment with cardamonin protects against cisplatin-induced nephrotoxicity in rats: Impact on NOX-1, inflammation and apoptosis. Toxicol. Appl. Pharmacol. 2014, 274, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sikka, S.; Siveen, K.S.; Lee, J.H.; Um, J.Y.; Kumar, A.P.; Chinnathambi, A.; Alharbi, S.A.; Basappa; Rangappa, K.S.; et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis 2017, 22, 158–168. [Google Scholar] [CrossRef]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of chalcones with anticancer activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kumar, V.; Lee, S.; Iwai, A.; Neckers, L.; Malhotra, S.V.; Trepel, J.B. Methoxychalcone inhibitors of androgen receptor translocation and function. Bioorganic Med. Chem. Lett. 2012, 22, 2105–2109. [Google Scholar] [CrossRef]
- Moses, M.A.; Kim, Y.S.; Rivera-Marquez, G.M.; Oshima, N.; Watson, M.J.; Beebe, K.E.; Wells, C.; Lee, S.; Zuehlke, A.D.; Shao, H.; et al. Targeting the Hsp40/Hsp70 Chaperone Axis as a Novel Strategy to Treat Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 4022–4035. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, H.Y.; Riaz, T.A.; Bhattarai, K.R.; Chaudhary, M.; Ahn, J.H.; Jeong, J.; Kim, H.R.; Chae, H.J. Chalcone suppresses tumor growth through NOX4-IRE1α sulfonation-RIDD-miR-23b axis. Redox Biol. 2021, 40, 101853. [Google Scholar] [CrossRef]
- Delmulle, L.; Bellahcène, A.; Dhooge, W.; Comhaire, F.; Roelens, F.; Huvaere, K.; Heyerick, A.; Castronovo, V.; De Keukeleire, D. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine 2006, 13, 732–734. [Google Scholar] [CrossRef]
- Li, K.; Zheng, Q.; Chen, X.; Wang, Y.; Wang, D.; Wang, J. Isobavachalcone Induces ROS-Mediated Apoptosis via Targeting Thioredoxin Reductase 1 in Human Prostate Cancer PC-3 Cells. Oxid. Med. Cell. Longev. 2018, 2018, 1915828. [Google Scholar] [CrossRef]
- Borges-Argáez, R.; Balnbury, L.; Flowers, A.; Giménez-Turba, A.; Ruiz, G.; Waterman, P.G.; Peña-Rodríguez, L.M. Cytotoxic and antiprotozoal activity of flavonoids from Lonchocarpus spp. Phytomedicine 2007, 14, 530–533. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Wen, R.; Tu, G.Z.; Chen, H.B.; Liang, H.; Zhao, Y.Y. Anti-inflammatory and antiproliferative prenylated chalcones from Hedysarum gmelinii. J. Asian Nat. Prod. Res. 2018, 20, 1009–1018. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, Y.; Wang, X.; Zeng, X.; Hu, Z.; Liu, Y.; Xie, Y.; Liang, G.; Zhu, J.; Luo, H.; et al. Novel 3′,5′-diprenylated chalcones inhibited the proliferation of cancer cells in vitro by inducing cell apoptosis and arresting cell cycle phase. Eur. J. Med. Chem. 2017, 133, 227–239. [Google Scholar] [CrossRef]
- Su, S.Y.; Xue, J.J.; Yang, G.Y.; Lei, C.; Hou, A.J. New cytotoxic alkylated chalcones from fatoua villosa. Chem. Biodivers. 2017, 14, e1700076. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, Y.; Wang, X.; Han, Y.; Peng, X.; Efferth, T.; Fu, Y. Synthesis and anti-tumor activity of novel aminomethylated derivatives of isoliquiritigenin. Molecules 2014, 19, 17715–17726. [Google Scholar] [CrossRef]
- Liu, G.; Ge, Z.; Zhao, M.; Zhou, Y. Design, synthesis and cytotoxic activities of novel aliphatic amino-substituted flavonoids. Molecules 2013, 18, 14070–14084. [Google Scholar] [CrossRef]
- Lorenzo, P.; Alvarez, R.; Ortiz, M.A.; Alvarez, S.; Piedrafita, F.J.; de Lera, A.R. Inhibition of IkappaB kinase-beta and anticancer activities of novel chalcone adamantyl arotinoids. J. Med. Chem. 2008, 51, 5431–5440. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.; Ortiz, M.A.; Alvarez, R.; Piedrafita, F.J.; de Lera, A.R. Adamantyl arotinoids that inhibit IκB kinase α and IκB kinase β. ChemMedChem 2013, 8, 1184–1198. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Abramjuk, C.; Vásquez, L.; Gamboa, N.; Domínguez, J.; Nitzsche, B.; Höpfner, M.; Georgieva, R.; Bäumler, H.; Stephan, C.; et al. New 4-maleamic acid and 4-maleamide peptidyl chalcones as potential multitarget drugs for human prostate cancer. Pharm. Res. 2011, 28, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Pouget, C.; Fidanzi-Dugas, C.; Gamond, A.; Laurent, A.; Semaan, J.; Pinon, A.; Champavier, Y.; Léger, D.Y.; Liagre, B.; et al. Design and multi-step synthesis of chalcone-polyamine conjugates as potent antiproliferative agents. Bioorganic Med. Chem. Lett. 2017, 27, 4354–4357. [Google Scholar] [CrossRef]
- Gul, H.I.; Yerdelen, K.O.; Gul, M.; Das, U.; Pandit, B.; Li, P.K.; Secen, H.; Sahin, F. Synthesis of 4′-hydroxy-3′-piperidinomethylchalcone derivatives and their cytotoxicity against PC-3 cell lines. Arch. Pharm. 2007, 340, 195–201. [Google Scholar] [CrossRef]
- Reddy, M.V.; Su, C.R.; Chiou, W.F.; Liu, Y.N.; Chen, R.Y.; Bastow, K.F.; Lee, K.H.; Wu, T.S. Design, synthesis, and biological evaluation of Mannich bases of heterocyclic chalcone analogs as cytotoxic agents. Bioorganic Med. Chem. 2008, 16, 7358–7370. [Google Scholar] [CrossRef]
- Shankaraiah, N.; Nekkanti, S.; Brahma, U.R.; Praveen Kumar, N.; Deshpande, N.; Prasanna, D.; Senwar, K.R.; Jaya Lakshmi, U. Synthesis of different heterocycles-linked chalcone conjugates as cytotoxic agents and tubulin polymerization inhibitors. Bioorganic Med. Chem. 2017, 25, 4805–4816. [Google Scholar] [CrossRef]
- Tu, H.Y.; Huang, A.M.; Hour, T.C.; Yang, S.C.; Pu, Y.S.; Lin, C.N. Synthesis and biological evaluation of 2′,5′-dimethoxychalcone derivatives as microtubule-targeted anticancer agents. Bioorganic Med. Chem. 2010, 18, 2089–2098. [Google Scholar] [CrossRef]
- Lei, Q.; Zhang, S.; Liu, M.; Li, J.; Zhang, X.; Long, Y. Synthesis and biological evaluation of glycosides containing triazene-chalcones. Mol. Divers. 2017, 21, 957–966. [Google Scholar] [CrossRef]
- Wang, M.; Xu, S.; Wu, C.; Liu, X.; Tao, H.; Huang, Y.; Liu, Y.; Zheng, P.; Zhu, W. Design, synthesis and activity of novel sorafenib analogues bearing chalcone unit. Bioorganic Med. Chem. Lett. 2016, 26, 5450–5454. [Google Scholar] [CrossRef] [PubMed]
- Wani, Z.A.; Guru, S.K.; Rao, A.V.; Sharma, S.; Mahajan, G.; Behl, A.; Kumar, A.; Sharma, P.R.; Kamal, A.; Bhushan, S.; et al. A novel quinazolinone chalcone derivative induces mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR signaling pathway in human colon cancer HCT-116 cells. Food Chem. Toxicol. 2016, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Ramakrishna, G.; Raju, P.; Viswanath, A.; Ramaiah, M.J.; Balakishan, G.; Pal-Bhadra, M. Synthesis and anti-cancer activity of chalcone linked imidazolones. Bioorganic Med. Chem. Lett. 2010, 20, 4865–4869. [Google Scholar] [CrossRef]
- Singh, P.; Raj, R.; Kumar, V.; Mahajan, M.P.; Bedi, P.M.; Kaur, T.; Saxena, A.K. 1,2,3-Triazole tethered β-lactam-chalcone bifunctional hybrids: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2012, 47, 594–600. [Google Scholar] [CrossRef]
- Pinheiro, S.; Pessôa, J.C.; Pinheiro, E.M.C.; Muri, E.M.F.; Filho, E.V.; Loureiro, L.B.; Freitas, M.C.R.; Silva Junior, C.M.D.; Fiorot, R.G.; Carneiro, J.W.M.; et al. 2H-1,2,3-Triazole-chalcones as novel cytotoxic agents against prostate cancer. Bioorganic Med. Chem. Lett. 2020, 30, 127454. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, B.; Wang, Y.; Li, M.; Jernigan, F.; Sun, L. Novel indolyl-chalcones target stathmin to induce cancer cell death. Cell Cycle 2016, 15, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hedblom, A.; Koerner, S.K.; Li, M.; Jernigan, F.E.; Wegiel, B.; Sun, L. Novel synthetic chalcones induce apoptosis in the A549 non-small cell lung cancer cells harboring a KRAS mutation. Bioorganic Med. Chem. Lett. 2016, 26, 5703–5706. [Google Scholar] [CrossRef] [PubMed]
- Bagul, C.; Rao, G.K.; Makani, V.K.K.; Tamboli, J.R.; Pal-Bhadra, M.; Kamal, A. Synthesis and biological evaluation of chalcone-linked pyrazolo [1,5-a]pyrimidines as potential anticancer agents. Medchemcomm 2017, 8, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.B.; Zhang, S.J.; Ge, Q.F.; Guo, D.W.; Cai, C.M.; Hu, W.X. Synthesis and anticancer evaluation of thiazolyl-chalcones. Bioorganic Med. Chem. Lett. 2010, 20, 6555–6559. [Google Scholar] [CrossRef]
- Kamal, A.; Balakrishna, M.; Nayak, V.L.; Shaik, T.B.; Faazil, S.; Nimbarte, V.D. Design and synthesis of imidazo[2,1-b]thiazole-chalcone conjugates: Microtubule-destabilizing agents. ChemMedChem 2014, 9, 2766–2780. [Google Scholar] [CrossRef]
- Wang, Q.; Arnst, K.E.; Wang, Y.; Kumar, G.; Ma, D.; Chen, H.; Wu, Z.; Yang, J.; White, S.W.; Miller, D.D.; et al. Structural modification of the 3,4,5-trimethoxyphenyl moiety in the tubulin inhibitor VERU-111 leads to improved antiproliferative activities. J. Med. Chem. 2018, 61, 7877–7891. [Google Scholar] [CrossRef]
- Nagaraju, M.; Gnana Deepthi, E.; Ashwini, C.; Vishnuvardhan, M.V.; Lakshma Nayak, V.; Chandra, R.; Ramakrishna, S.; Gawali, B.B. Synthesis and selective cytotoxic activity of novel hybrid chalcones against prostate cancer cells. Bioorganic Med. Chem. Lett. 2012, 22, 4314–4317. [Google Scholar] [CrossRef]
- Coskun, D.; Erkisa, M.; Ulukaya, E.; Coskun, M.F.; Ari, F. Novel 1-(7-ethoxy-1-benzofuran-2-yl) substituted chalcone derivatives: Synthesis, characterization and anticancer activity. Eur. J. Med. Chem. 2017, 136, 212–222. [Google Scholar] [CrossRef]
- Isa, N.M.; Abdelwahab, S.I.; Mohan, S.; Abdul, A.B.; Sukari, M.A.; Taha, M.M.; Syam, S.; Narrima, P.; Cheah, S.; Ahmad, S.; et al. In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.) (fingerroot). Braz. J. Med. Biol. Res. 2012, 45, 524–530. [Google Scholar] [CrossRef]
- Chinthala, Y.; Thakur, S.; Tirunagari, S.; Chinde, S.; Domatti, A.K.; Arigari, N.K.; Srinivas, K.V.N.S.; Alam, S.; Jonnala, K.K.; Khan, F.; et al. Synthesis, docking and ADMET studies of novel chalcone triazoles for anti-cancer and anti-diabetic activity. Eur. J. Med. Chem. 2015, 93, 564–573. [Google Scholar] [CrossRef]
- Popłoński, J.; Turlej, E.; Sordon, S.; Tronina, T.; Bartmańska, A.; Wietrzyk, J.; Huszcza, E. Synthesis and Antiproliferative Activity of Minor Hops Prenylflavonoids and New Insights on Prenyl Group Cyclization. Molecules 2018, 23, 776. [Google Scholar] [CrossRef]
- Vijaya Bhaskar Reddy, M.; Shen, Y.C.; Ohkoshi, E.; Bastow, K.F.; Qian, K.; Lee, K.H.; Wu, T.S. Bis-chalcone analogues as potent NO production inhibitors and as cytotoxic agents. Eur. J. Med. Chem. 2012, 47, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Kelsang, N.; Tu, G.; Kong, D.; Lu, J.; Zhang, Y.; Liang, H.; Tu, P.; Zhang, Q. Structurally diverse cytotoxic dimeric chalcones from Oxytropis chiliophylla. J. Nat. Prod. 2018, 81, 307–315. [Google Scholar] [CrossRef]
- El Sayed Aly, M.R.; Abd El Razek Fodah, H.H.; Saleh, S.Y. Antiobesity, antioxidant and cytotoxicity activities of newly synthesized chalcone derivatives and their metal complexes. Eur. J. Med. Chem. 2014, 76, 517–530. [Google Scholar] [CrossRef]
- Gibson, M.Z.; Nguyen, M.A.; Zingales, S.K. Design, Synthesis, and Evaluation of (2-(Pyridinyl)methylene)-1-tetralone Chalcones for Anticancer and Antimicrobial Activity. Med. Chem. 2018, 14, 333–343. [Google Scholar] [CrossRef]
- Maguire, C.J.; Carlson, G.J.; Ford, J.W.; Strecker, T.E.; Hamel, E.; Trawick, M.L.; Pinney, K.G. Synthesis and biological evaluation of structurally diverse α-conformationally restricted chalcones and related analogues. Medchemcomm 2019, 10, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, B.V.S.; Prasad, Y.R.; Shaik, A.B. Synthesis, Biological Evaluation and Molecular Docking Studies of New Pyrazolines as an Antitubercular and Cytotoxic Agents. Infect. Disord. Drug Targets 2019, 19, 310–321. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).