Abstract
Obesity is linked to metabolic dysfunction-associated steatotic liver disease (MASLD), but the molecular mechanisms and effective treatments remain unclear. This study investigated whether ST32db, an inducer of activating transcription factor 3 (ATF3), affects lipid metabolism in MASLD. An in vitro model was established involving the treatment of HepG2 cells with 1 mM oleic acid (OA) with or without 20 µM ST32db. In an in vivo model, C57BL/6 mice were fed a high-fat diet (HFD) for 18 weeks to induce obesity and treated or not with ST32db (1 mg kg−1). ST32db significantly decreased intracellular lipid accumulation in OA-treated HepG2 cells. In these cells, ST32db remarkably decreased mRNA and protein levels of adipogenesis- and lipogenesis-related genes and increased mRNA levels of adipose triglyceride lipase (ATGL), a lipolytic enzyme. In HFD-fed mice, the ST32db treatment significantly decreased the liver weight, serum triglycerides, and fat vacuole and triglyceride accumulation in the liver. Livers from these mice also showed significantly decreased CCAAT/enhancer-binding protein β mRNA and protein levels, increased ATF3 mRNA and protein and ATGL mRNA levels, and increased levels of phosphorylated AMP-activated protein kinase (AMPK) and protein kinase A (PKA). These findings suggest that ST32db may exert protective effects against MASLD through activating hepatic AMPK and PKA pathways.
1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a common chronic liver disease worldwide with an estimated global prevalence of 30% to 40% [1,2,3]. The condition involves superfluous lipid accumulation in hepatocytes and liver tissues and is tightly associated with various chronic diseases, such as obesity, hyperlipidemia, and diabetes. A considerable proportion of people with MASLD (20–30%) develop nonalcoholic steatohepatitis, which can progress to advanced liver fibrosis, cirrhosis, and hepatocellular carcinoma. Although the pathogenesis of MASLD is not entirely understood, hepatic lipid metabolic disorders are likely crucial in the beginning stages and in its progression [4].
The liver has a central role in the modulation of energy metabolism and lipid transport. Evidence suggests that MASLD is induced by an imbalance of intracellular signaling pathways involved in triglyceride (TG) and fatty acid delivery, export, synthesis, or oxidation [5,6]. Lipogenesis and lipolysis are the main hepatic biological processes modulated by AMP-activated protein kinase (AMPK), a key intracellular energy sensor, and the cyclic AMP–protein kinase A (PKA) signaling pathway. Both signaling pathways represent promising therapeutic targets for the treatment of MASLD [7,8].
Activating transcription factor (ATF) 3 belongs to the ATF/cAMP response element-binding (CREB) family that binds to the cAMP response element in promoters with the consensus sequence TGACGTCA [9]. In adipocytes, ATF3 or ATF3 inducers inhibit adipogenesis and promote adipocyte browning [10]. In addition, hepatic ATF3 is important for the modulation of high-density lipoprotein metabolism, reversal of cholesterol transport, and atherosclerosis [11]. Xu et al. [12] found that the global or hepatocyte-specific loss of ATF3 exacerbates diet-induced steatohepatitis. They further reported that the hepatocyte-specific expression of ATF3 prevents diet-induced steatohepatitis via the induction of hepatic lipolysis and fatty acid oxidation and the suppression of inflammation and apoptosis.
Other ATF/CREB transcription factors, such as ATF4 and ATF6, also modulate hepatocyte function. In mice, ATF4 deficiency protects mice from hepatic steatosis induced by a high-fat diet (HFD) or by high-carbohydrate/high-fructose diets [13,14]. Moreover, a loss of ATF4 decreases TG accumulation in fructose-treated hepatocytes, whereas ATF4 overexpression enhances TG accumulation in HepG2 cells, indicating an effect of ATF4 on lipid metabolism in the liver [15]. ATF6 has been implicated in the regulation of lipogenesis. Whole-body ATF6 knockout mice show decreased mRNA levels of lipogenic genes, such as sterol regulatory element-binding protein 1c (SREBP1c) and peroxisome proliferator-activated receptor (PPAR)γ, and are resistant to HFD-induced MASLD [16]. In addition, Zeng et al. found that ATF6 suppresses lipogenesis and hepatic TG accumulation by recruiting the corepressor histone deacetylase 1 to the ATF6–SREBP2 complex bound to the sterol regulatory element within the promoters of lipogenic genes [17]. ATF6 is reported to alleviate liver lipogenesis and inflammation by stimulating sulfhydrylated sirtuin 1 via a cystathionine β synthetase/hydrogen sulfide synthesis pathway [18]. Conversely, the overexpression of active nuclear ATF6 promotes lipogenesis and hepatic steatosis in alcohol-treated zebrafish [19], even though the majority of evidence indicates that ATF6 suppresses lipid synthesis and alleviates hepatic steatosis.
Our previous work has shown that ST32db, a synthetic ATF3 inducer selected from the ATF3 promoter screening platform, upregulates ATF3 expression in association with inhibited adipogenesis and lipogenesis and promoted adipocyte browning [10]. ST32db, which we have previously described in detail, also suppresses lipid droplet accumulation in 3T3-L1 adipocytes [10]. These findings have led to an interest in these pathways in the context of MASLD and other lipid disorders. Given the roles of lipogenesis and lipolysis in modulating lipid accumulation and the potential of ST32db in steatosis prevention, we hypothesized that ST32db would alleviate hepatic steatosis by inhibiting lipogenesis and enhancing lipolysis. Here, we investigated the mechanisms underlying improvements associated with ST32db in hepatic steatosis, using oleic acid (OA)-induced steatosis in HepG2 cells and HFD-induced obese mice as a model of MASLD.
2. Results
2.1. Effects of ST32db, an ATF3 Inducer, on the Viability of HepG2 Cells
To evaluate the cytotoxic effects of ST32db (Figure 1A) on HepG2 cells, we treated cells with various concentrations of ST32db for 24, 48, and 72 h. The cytotoxicity induced by ST32db was determined using a CCK8 assay. The data showed that the ST32db treatment (2–50 μM) for 24 h caused no cytotoxicity in the HepG2 cells (Figure 1B), although one of the higher concentrations of ST32db (100 μM) reduced the cell viability compared with control cells. HepG2 cells treated with ST32db (2–20 μM) for 48 or 72 h also showed no cytotoxic effects (Figure 1C,D). Based on these findings, we selected 20 and 50 μM ST32db for subsequent experiments.
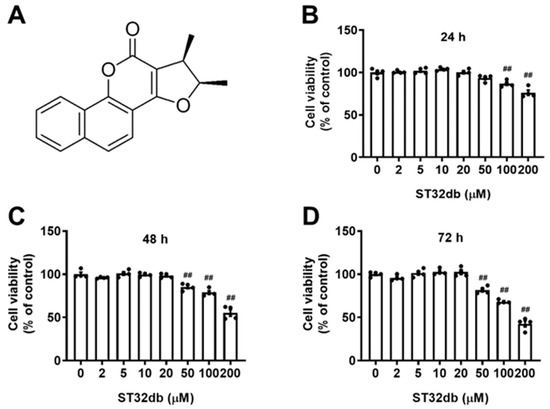
Figure 1.
Effects of the ATF3 inducer, ST32db, on the viability of HepG2 cells. (A) The chemical structure of ST32db. (B–D) Cell viability was measured after the treatment with various concentrations of ST32db for 24 h, 48 h, and 72 h using a CCK8 assay. Data are mean ± SEM (n = 4) and analyzed by one-way ANOVA. ## p < 0.01 compared to the control group.
2.2. ST32db Decreases Lipid Accumulation in OA-Treated HepG2 Cells
To determine the lipid-lowering effects of ST32db, HepG2 cells were treated with 1 mM OA for 24 h, followed by a 20 or 50 µM ST32db treatment for 24 h or 48 h. The intracellular lipid content was measured by Oil Red O staining. A microscopic examination showed that OA-treated HepG2 cells had serious steatosis compared with untreated control cells (Figure 2A). The lipid accumulation in the ST32db-treated cells was significantly decreased in a dose-dependent manner compared with the OA-treated cells (Figure 2B,C).
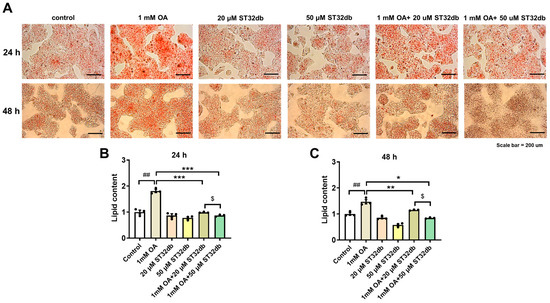
Figure 2.
ST32db decreased lipid accumulation in oleic acid (OA)-treated HepG2 cells. Cells were treated with OA for 24 h, followed by ST32db treatment for 24 h or 48 h. (A) Representative images (200× magnification; scale bar, 200 µm) showing OA-induced lipid accumulation visualized by Oil Red O staining. (B,C) The relative quantification of lipid content. Data are mean ± SEM (n = 3) and analyzed by one-way ANOVA. ## p < 0.01 compared to control group; * p < 0.05, ** p < 0.01, *** p < 0.001 compared to OA group; and $ p < 0.05 compared to 1 mM OA+20 uM ST32db.
2.3. ST32db Regulates mRNA and Protein Levels of Genes Related to Adipogenesis, Lipogenesis, and Lipolysis in OA-Treated HepG2 Cells
To elucidate the mechanism of the ST32db inhibition of hepatic steatosis, we used a qPCR and Western blotting in HepG2 cells to analyze the expression of several key genes that affect adipogenesis (e.g., CCAAT/enhancer-binding protein α (C/EBPα), C/EBPβ, and PPARγ2), lipogenesis (e.g., acetyl-CoA carboxylase (ACC), carbohydrate-responsive element-binding protein (ChREBP), fatty acid synthase (FAS), stearoyl-CoA desaturase 1 (SCD1), SREBP1, diacylglycerol acyltransferase (DGAT)1 and DGAT2), and lipolysis (e.g., adipose triglyceride lipase (ATGL)). As shown in Figure 3A, the downregulation of C/EBPβ, ACC, ChREBP, FAS, SCD1, and SREBP1 mRNA and upregulation of ATGL mRNA were observed in the ST32db-treated HepG2 cells compared with the OA-treated group. Consistent with changes in mRNA levels, protein levels of C/EBPβ and SREBP1 were markedly decreased in ST32db-treated HepG2 cells compared with OA-treated cells (Figure 3B,C). However, ATF3 mRNA and protein levels did not change in OA-exposed HepG2 cells treated with ST32db compared with OA controls (Figure 3). Additionally, a higher dose of ST32db (50 uM) showed similar effects on mRNA levels of these genes (Figure S1). These results suggest that ST32db alleviates hepatic steatosis by modulating the expression of genes involved in lipid synthesis and lipolysis in OA-induced HepG2 cells.
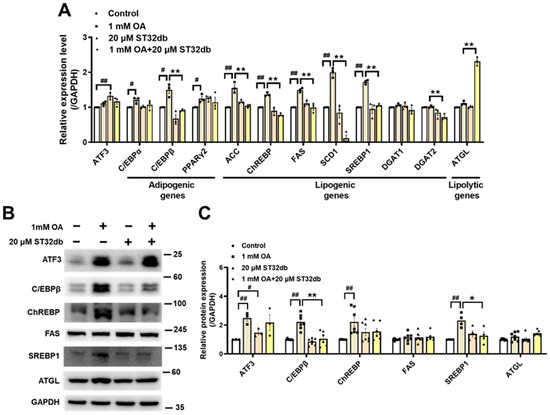
Figure 3.
ST32db regulated adipogenesis-, lipogenesis- and lipolysis-related gene and protein levels in OA-treated HepG2 cells. (A) Real-time PCR analysis of mRNA levels of ATF3, adipogenic (C/EBPα, C/EBPβ, PPARγ2), lipogenic (ACC, ChREBP, FAS, SCD1, SREBP1, DGAT1, and DGAT2), and lipolytic (ATGL) genes after 48 h co-treatment with OA and ST32db normalized to GAPDH and relative to control. (B) The levels of ATF3, C/EBPβ, ChREBP, FAS, SREBP1, and ATGL proteins were measured by Western blot. (C) Densitometry quantification of Western blot. Data are presented as mean ± SEM (n = 3~7) and analyzed by one-way ANOVA. # p < 0.05 and ## p < 0.01 compared to control; * p < 0.05 and ** p < 0.01 compared to OA group.
2.4. ST32db Ameliorates HFD-Induced Hepatic Steatosis
In our previous study [10], a ST32db treatment (1 mg kg−1 twice weekly) for 18 weeks significantly reduced the body weight gain in HFD-induced obese mice compared with controls but did not affect food intake. In the present study, we asked whether the ST32db treatment could alleviate fatty livers in this mouse model of obesity and found that the ST32db treatment substantially reduced the liver weight compared with controls (Figure 4A). In our histopathological assessment of the fat accumulation in livers from these animals, we found that the ST32db treatment substantially decreased the size of the fat droplets in hepatocytes (Figure 4B). A quantification analysis revealed that the ST32db administration led to significantly decreased lipid accumulation (Figure 4B,C) and decreased serum TG levels (Figure 4D). In addition, we found that administering a higher dose of ST32db (10 mg/kg twice weekly) did not further reduce the body weight or total fat mass compared with either the control group or the lower dose group (1 mg/kg twice weekly) (Figure S2).
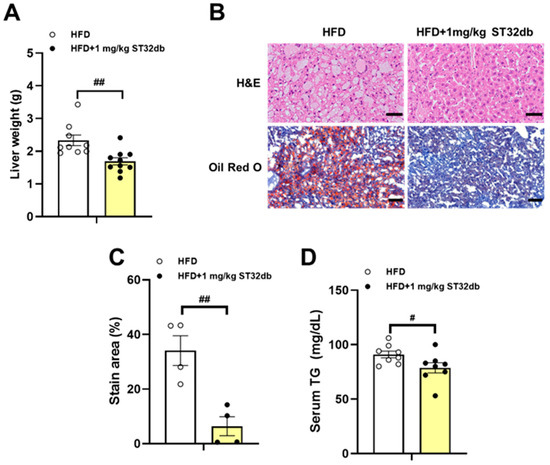
Figure 4.
Inhibitory effect of ST32db on liver weight, hepatic TG accumulation, and serum TG levels in HFD-fed mice. Analysis of wild-type mice fed a HFD for 18 weeks with or without intraperitoneal injection of ST32db at a dose of 1 mg kg−1 twice weekly. (A) Liver weight (n = 9~10). (B) Hematoxylin and eosin (H&E) and Oil Red O staining of liver sections (original magnification × 200, n = 4). (C) Quantification of Oil Red O staining. (D) Serum TG levels (n = 8). Scale bar in H&E and Oil Red O = 50 and 100 µm, respectively. Data are presented as mean ± SEM and analyzed by one-way ANOVA. # p < 0.05 and ## p < 0.01 compared to HFD group.
2.5. ST32db Increases ATF3 mRNA Levels but Decreases Adipogenesis- and Lipogenesis-Related mRNA in Mouse Liver
To elucidate the molecular mechanism of the effects of ST32db on MASLD, we examined mRNA levels associated with ATF3 and genes involved in hepatic lipogenesis. We found that compared with HFD-only controls, ST32db upregulated ATF3 mRNA levels in the liver (Figure 5A) and that mRNA levels of C/EBPβ and DGAT2 were downregulated in ST32db-treated livers (Figure 5B), similar to the effect of ST32db on HepG2 cells. However, ST32db exerted a weaker suppressive effect on the SREBP1 mRNA expression compared with HFD-only controls (p = 0.31). These results suggest that ST32db can alleviate hepatic steatosis in MASLD by upregulating ATF3 expression and suppressing de novo lipogenesis in the liver.
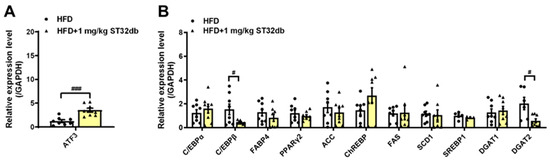
Figure 5.
ST32db increased ATF3 mRNA levels but decreased the mRNA levels of adipogenesis- and lipogenesis-related genes in the liver of HFD-fed mice. (A) The mRNA levels of ATF3 and (B) adipogenesis- and lipogenesis-related gene expression were measured by real-time PCR analysis. Data are presented as mean ± SEM (n = 8) and analyzed by one-way ANOVA. # p < 0.05 and ### p < 0.001 compared to HFD group.
2.6. ST32db Promotes Lipolysis in the Mouse Liver
We further examined the lipolytic activity of ST32db by analyzing the expression of key enzymes in the hepatic TG metabolism in mice, including ATGL, monoacylglycerol lipase, and hormone-sensitive lipase, and the β-oxidation enzymes carnitine palmitoyl transferase 1, PPARα, and PPARγ coactivator 1α. Compared with controls, ATGL mRNA levels in the liver increased after the treatment with ST32db (Figure 6A), but mRNA levels of β-oxidation enzymes seemed to be unaffected (Figure 6B). Our data indicate that the increased lipolytic ability of ST32db depends primarily on the upregulated expression of ATGL, similar to the effect of ST32db on HepG2 cells.
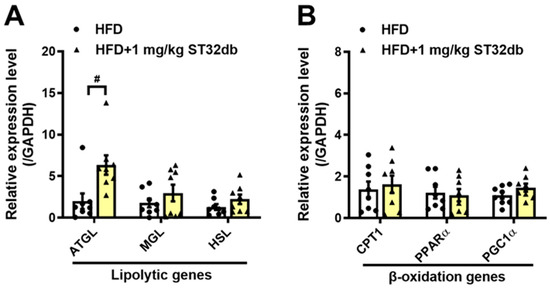
Figure 6.
ST32db upregulated the mRNA levels of lipolytic genes in the liver of HFD-fed mice. (A) The mRNA levels of lipolytic genes, including ATGL, MGL, and HSL and (B) β-oxidation genes, including Cpt1, PPARα, and PGC1α, were measured by a real-time PCR analysis. Data are presented as the mean ± SEM (n = 8) and analyzed by one-way ANOVA. # p < 0.05 compared to the HFD group.
2.7. ST32db Increases Levels of ATF3, Phospho-PKA, and Phospho-AMPK Proteins but Decreases C/EBPβ Protein Levels in Mouse Liver
To clarify the molecular mechanism underlying the effects of ST32db on MASLD, including suppressed lipid accumulation in the liver and improved TG levels, we examined the expression of proteins involved in hepatic lipogenesis (C/EBPβ and SREBP1) and lipolysis (phosphorylation [p] of AMPK, PKA, p38, and ERK) (Figure 7). Consistent with the in vitro results, levels of C/EBPβ protein were downregulated in ST32db-treated livers compared with the control mouse liver. ST32db showed a trend toward decreasing SREBP1 protein levels relative to the control group (p = 0.052). In addition, ST32db significantly increased ATF3, p-AMPK, and p-PKA protein levels compared with controls. These results suggest that ST32db can ameliorate hepatic steatosis in MASLD by enhancing lipolysis and suppressing de novo lipogenesis through the activation of the AMPK and PKA pathways in the liver.
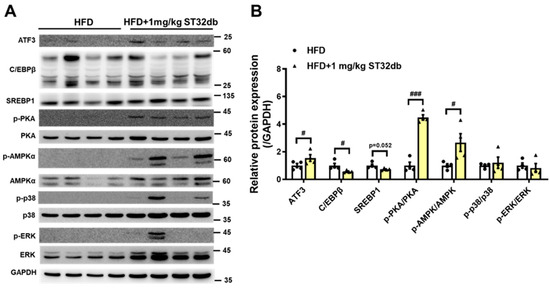
Figure 7.
(A) Western blot and (B) densitometry quantification showing effects of ST32db on protein expression of ATF3; C/EBPβ; and phosphorylated form of AMPK, PKA, p38, and ERK in the liver of HFD-fed mice. The protein levels were measured by Western blot. Data are presented as mean ± SEM (n = 4) and analyzed by one-way ANOVA. # p < 0.05 and ### p < 0.001 compared to HFD group.
3. Discussion
ATF3 is a crucial metabolic modulator with important roles in regulating metabolism, immunity, and oncogenesis [20]. We previously demonstrated that the ATF3 inducer ST32db may have beneficial effects in obesity and metabolic dyshomeostasis [10]. Because MASLD is a related condition, here we sought to investigate the potential therapeutic effects of ST32db on HFD-induced MASLD and to explore the molecular mechanisms involved. MASLD is characterized by an enormous aggregation of fat in the liver, resulting from the conversion of superfluous free fatty acids into TGs and their accumulation in hepatocytes [21]. To assess the protective impacts and molecular mechanisms of action of ST32db in MASLD, we used in vitro (OA-treated HepG2 cells) and in vivo (HFD-induced obese mice) models.
Although OA is an unsaturated fatty acid with reported antioxidative and anti-inflammatory properties, it is widely used to establish in vitro models of hepatic steatosis because it effectively promotes neutral lipid accumulation while causing minimal cytotoxicity. The biological actions of OA may vary depending on its concentration and the cellular context. In this study, 1 mM OA was applied to HepG2 cells to induce steatosis [22], leading to a significantly increased accumulation of lipid droplets. In these cells, the ST32db treatment exerted a suppressive effect on this OA-induced hepatic steatosis. In addition, ST32db significantly decreased the OA-induced mRNA levels of C/EBPβ, ACC, ChREBP, FAS, SCD1, SREBP1, and DGAT2. At the protein level, the ST32db treatment in these cells led to a significantly dampened production of OA-induced C/EBPβ and SREBP1, transcriptional factors that modulate downstream genes involved in de novo lipogenesis, such as those encoding ACC, FAS, and SCD1 [23]. Furthermore, we found that ST32db reduced hepatic steatosis by decreasing the expression of de novo lipogenesis genes and by upregulating lipolysis-related ATGL mRNA. The physiological function of ATGL may not be limited to adipose tissue and could involve many other tissues, such as the liver [24,25], although it is expressed at low levels in hepatocytes. Liver-specific ATGL overexpression decreases hepatic steatosis and slightly improves liver insulin sensitivity [26], and hepatic ATGL expression is positively associated with fatty acid β-oxidation [27]. In addition, patients with MASLD have decreased hepatic ATGL levels [28]. Although ST32db is an ATF3 inducer, it did not increase ATF3 mRNA or protein levels in OA-treated cells. This lack of upregulation may reflect the influence of OA-induced lipogenic signaling, which could dampen ST32db-mediated ATF3 induction through feedback regulation or altered cellular stress response pathways.
In the in vivo experiment, we investigated whether ST32db improves hepatic steatosis in HFD-induced MASLD. We found that ST32db significantly decreased the liver weight in the HFD-fed obese mice. Consistent with the in vitro results, ST32db significantly slowed the progression of HFD-induced MASLD, as evidenced by fewer and smaller lipid vacuoles on histological examination. In addition, serum TG levels declined significantly with the ST32db treatment.
Also consistent with the in vitro findings, ST32db decreased C/EBPβ mRNA and protein levels in the mouse liver in our in vivo model. We further observed the downregulation of DGAT2 mRNA and the upregulation of ATGL mRNA in ST32db-treated mouse livers compared to controls, along with an increased expression of ATF3, phospho-PKA, and phospho-AMPK proteins in ST32db-treated livers. A potential limitation of this study is the absence of a normal diet control group, which would have provided an additional baseline for the comparison of metabolic and histological parameters.
The results of the animal and cell experiments in the present study together indicate that ST32db modulates the expression of genes involved in lipogenesis and lipolysis, resulting in the amelioration of hepatic steatosis. PKA plays an important role in the modulation of lipid metabolism, and the dysmodulation of its signaling is linked to pathogenic mechanisms underlying several metabolic diseases, such as MASLD [29]. PKA is an upstream signaling molecule of AMPK, which it can activate by phosphorylating Thr 172 [30]. To date, no available drugs target PKA or AMPK to treat lipid-related metabolic syndromes in hepatocytes.
The activation of AMPK, an important modulator of numerous metabolic pathways, inhibits hepatic lipogenesis and increases fatty acid oxidation [31]. AMPK also inhibits adipogenesis-related proteins such as C/EBPβ and PPARγ [32]. C/EBPβ can induce the expression of PPARγ and C/EBPα, which subsequently promote the expression of adipocyte-specific genes. Our qPCR and Western blot results indicate that the OA treatment increased the C/EBPβ expression in HepG2 cells and that ST32db dampened this effect. These results suggest that the suppression of the adipogenic protein expression may underlie the decline in lipid accumulation following ST32db exposure. Our in vivo and in vitro experiments showed that ST32db modulates the expression of genes involved in adipogenesis, lipogenesis, and lipolysis, leading to the amelioration of hepatic steatosis (Figure 8).
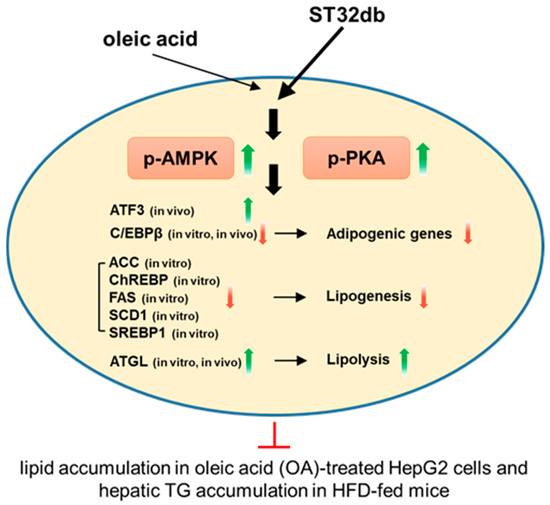
Figure 8.
A schematic diagram of the molecular mechanisms of the anti-MASLD effect of the ATF3 inducer ST32db. ST32db protects against diet-induced MASLD by inhibiting lipogenesis and enhancing lipolysis via the activation of AMPK and PKA pathways. Green upward arrows indicate increases; red downward arrows indicate decreases; red T-shaped lines indicate inhibition.
4. Materials and Methods
4.1. Materials and Reagents
Fetal bovine serum and Dulbecco’s modified eagle medium (DMEM) were purchased from Gibco Life Technology (Grand Island, NY, USA). Penicillin, streptomycin, oleic acid, and Oil Red O staining powder were purchased from Sigma-Aldrich (St. Louis, MO, USA). A cell counting kit (CCK-8) was purchased from Abcam (Cambridge, UK).
4.2. Cell Culture
Human hepatoma HepG2 cells were obtained from ATCC (HB-8065, ATCC, Manassas, VA, USA). HepG2 cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin in a humidified incubator with 5% CO2 at 37 °C.
4.3. Analysis of Cell Viability
HepG2 cells were cultured in 96-well plates at 5 × 103 per well and treated with ST32db (at a concentration of 2, 5, 10, 20, 50, 100, and 200 μM) for 24, 48, and 72 h. Then, cell viability was analyzed by CCK-8 assay.
4.4. Oil Red O Staining
Lipid droplet accumulation in HepG2 cells was measured using Oil Red O staining. Cells were seeded in 6-well plates (4 × 105 cells/well) and incubated with 1 mM oleic acid (OA) for 24 h. After washing with PBS, cells were treated with ST32db (20 or 50 μM) for 24 or 48 h. Cells were then fixed with 10% formalin for 1 h, stained with Oil Red O for 1 h, and washed with distilled water. The dye was eluted with 100% isopropanol, and absorbance was measured at 500 nm.
4.5. Real-Time Quantitative PCR
Total RNA from cultured cells or liver tissues was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Real-time quantitative PCR (qPCR) was performed on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using SYBR Green (Bio-Rad). Primer sequences are listed in Table 1. Relative gene expression levels were normalized to GAPDH, and fold changes were calculated using the 2−ΔΔCT method.

Table 1.
Primer sequences for real-time PCR.
4.6. Western Blot Analysis
Cells were lysed in RIPA buffer, and liver tissues (200 mg) were homogenized using a tissue ruptor (Qiagen, Hilden, Germany). Lysates were centrifuged at 14,000 rpm for 15 min at 4 °C, and protein concentrations were measured by the Bradford assay. Proteins were separated by SDS-PAGE, transferred to PVDF membranes (Merck Millipore, Burlington, MA, USA), and probed with antibodies, including AMPKα (#2603, Cell Signaling Technology), Phospho-AMPKα (Thr172) (#2535, Cell Signaling Technology, Danvers, MA, USA), p44/42 MAPK (ERK1/2) (#4695, Cell Signaling Technology), Phospho-p44/42 MAPK (ERK1/2) (#9101, Cell Signaling Technology), PKA C-α (#4782, Cell Signaling Technology), Phospho-PKA C (Thr197) (#4781, Cell Signaling Technology), p38 MAPK (#9212, Cell Signaling Technology), Phospho-p38 MAPK (Thr180/Tyr182) (#9211, Cell Signaling Technology), ATF3 (#ab207434, Abcam, Cambridge, UK), ChREBP (#81958, Abcam), C/EBPβ (sc-7962, Santa Cruz, Dallas, TX, USA), fatty acid synthase (#3180, Cell Signaling Technology), SREBP1 (14088-1-AP, Proteintech, Rosemont, IL, USA), ATGL (#2138, Cell Signaling Technology), and GAPDH (#2118, Cell Signaling Technology). Secondary antibodies (1:1000 in 5% milk) included anti-rabbit IgG-HRP (#7074) and anti-mouse IgG-HRP (#7076, Cell Signaling Technology). Protein bands were detected using a chemiluminescence reagent (PerkinElmer, Shelton, CT, USA) and quantified with ImageJ software version 1.53t (National Institutes of Health, Bethesda, MD, USA).
4.7. Animal Studies
Six-week-old male C57BL/6 mice (National Center for Biomodels, Taipei, Taiwan) were fed a high-fat diet (HFD, 45% kcal from fat) with or without intraperitoneal ST32db for 18 weeks to assess its effects on fatty liver. Body weight and food intake were recorded weekly. All procedures were performed according to the protocols approved by the Institutional Animal Care and Utilization Committee, Academia Sinica, Taipei, Taiwan. The HFD alone was set as control group. HFD: n = 9; HFD + 1 mg·kg−1 ST32db: n = 10; HFD + 10 mg·kg−1 ST32db: n = 7.
4.8. Histopathological Assessments of Livers
The liver samples of mice were fixed in 4% paraformaldehyde at room temperature for 24 h and embedded in paraffin and sectioned into 5 μm thick sections. Liver slices were stained with hematoxylin–eosin (H&E) and Oil Red O for histological examinations.
4.9. Statistical Analyses
Values are expressed as means ± SEM from at least three experiments. The data were analyzed using one-way ANOVA, followed by Tukey’s test. A value of p < 0.05 was considered statistically significant.
5. Conclusions
The ST32db treatment suppressed lipid accumulation and steatosis in vitro and in an in vivo MASLD model. It also significantly decreased the liver weight and liver steatosis in HFD-fed obese mice. Results from both models implicated the modulation of lipid metabolism, including the inhibition of C/EBPβ, ACC, ChREBP, FAS, SCD1, SREBP1, and DGAT2 expression and the stimulation of ATGL, p-PKA, and p-AMPK production, in the effects of the ST32db treatment. These pathways may thus underlie the protective effects of ST32db against MASLD.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms262411877/s1.
Author Contributions
Conceptualization, C.-F.C. and H.-C.K.; methodology, W.-T.C. and J.-F.C.; validation, W.-T.C. and J.-F.C.; formal analysis, R.-B.Y.; investigation, C.-F.C. and H.-C.K.; data curation, W.-T.C. and J.-F.C.; writing—original draft preparation, H.-C.K.; writing—review and editing, H.-C.K. and C.-F.C.; visualization, C.-F.C. and H.-C.K.; supervision, C.-F.C. and H.-C.K.; funding acquisition, C.-F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Science and Technology Council, Taiwan (grant number NSTC 113-2314-B-303-025-MY3 and NSTC 114-2314-B-303-005); Taipei Tzu Chi Hospital; Buddhist Tzu Chi Medical Foundation, Taiwan (grant numbers: TCRD-TPE-NCU-113-01, TCRD-TPE-NCU-114-01, and TCMMP114-01-02); and collaborative grants from the Tzu Chi Medical Foundation, the Tzu Chi University, and Academia Sinica (TCAS-112-02).
Institutional Review Board Statement
All procedures were performed according to the protocols approved by the Institutional Animal Care and Utilization Committee, Academia Sinica, Taipei, Taiwan (AS_IACUC 23-12-2087; approval date: 28 February 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| AMPK | AMP-activated protein kinase |
| ATF | Activating transcription factor |
| ATGL | Adipose triglyceride lipase |
| C/EBPα | CCAAT/enhancer-binding protein α |
| ChREBP | Carbohydrate-responsive element-binding protein |
| CREB | cAMP response element-binding protein |
| DGAT1 | Diacylglycerol acyltransferase 1 |
| DMEM | Dulbecco’s modified eagle medium |
| FAS | Fatty acid synthase |
| H&E | Hematoxylin–eosin |
| HFD | High-fat diet |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| OA | Oleic acid |
| PKA | Protein kinase A |
| PPAR | Peroxisome proliferator-activated receptor |
| SCD1 | Stearoyl-CoA desaturase 1 |
| SREBP1 | Sterol regulatory element binding protein 1 |
| TG | Triglyceride |
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Tilg, H.; Petta, S.; Stefan, N.; Targher, G. Metabolic Dysfunction–Associated Steatotic Liver Disease in Adults: A Review. JAMA, 2025; epub ahead of print. [Google Scholar] [CrossRef]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. CMLS 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Stefan, N.; Kantartzis, K.; Häring, H.-U. Causes and Metabolic Consequences of Fatty Liver. Endocr. Rev. 2008, 29, 939–960. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, Y.; Bai, M.; Huang, Y.; Yang, H.; Liu, L.; Wang, S.; Yu, C.; Song, Z.; Bao, Y.; et al. Hypericin attenuates nonalcoholic fatty liver disease and abnormal lipid metabolism via the PKA-mediated AMPK signaling pathway in vitro and in vivo. Pharmacol. Res. 2020, 153, 104657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Saltiel, A.R. From overnutrition to liver injury: AMP-activated protein kinase in nonalcoholic fatty liver diseases. J. Biol. Chem. 2020, 295, 12279–12289. [Google Scholar] [CrossRef]
- Montminy, M.R.; Bilezikjian, L.M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature 1987, 328, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.C.; Chan, T.Y.; Chung, J.F.; Kao, Y.H.; Cheng, C.F. The ATF3 inducer protects against diet-induced obesity via suppressing adipocyte adipogenesis and promoting lipolysis and browning. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 145, 112440. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Jadhav, K.; Pan, X.; Zhu, Y.; Hu, S.; Chen, S.; Chen, L.; Tang, Y.; Wang, H.H.; et al. Hepatocyte ATF3 protects against atherosclerosis by regulating HDL and bile acid metabolism. Nat. Metab. 2021, 3, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, S.; Jadhav, K.; Zhu, Y.; Pan, X.; Bawa, F.C.; Yin, L.; Zhang, Y. Hepatocytic Activating Transcription Factor 3 Protects Against Steatohepatitis via Hepatocyte Nuclear Factor 4α. Diabetes 2021, 70, 2506–2517. [Google Scholar] [CrossRef]
- Li, H.; Meng, Q.; Xiao, F.; Chen, S.; Du, Y.; Yu, J.; Wang, C.; Guo, F. ATF4 deficiency protects mice from high-carbohydrate-diet-induced liver steatosis. Biochem. J. 2011, 438, 283–289. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, T.; Yu, S.; Lee, S.; Calabuig-Navarro, V.; Yamauchi, J.; Ringquist, S.; Dong, H.H. ATF4 protein deficiency protects against high fructose-induced hypertriglyceridemia in mice. J. Biol. Chem. 2013, 288, 25350–25361. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.P.; Yu, X.; Song, G.Y.; Zhang, P.; Sun, L.N.; Chen, S.C.; Hu, Z.J.; Zhang, X.M. Impact of activating transcription factor 4 signaling on lipogenesis in HepG2 cells. Mol. Med. Rep. 2016, 14, 1649–1658. [Google Scholar] [CrossRef]
- Usui, M.; Yamaguchi, S.; Tanji, Y.; Tominaga, R.; Ishigaki, Y.; Fukumoto, M.; Katagiri, H.; Mori, K.; Oka, Y.; Ishihara, H. Atf6α-null mice are glucose intolerant due to pancreatic β-cell failure on a high-fat diet but partially resistant to diet-induced insulin resistance. Metab. Clin. Exp. 2012, 61, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lu, M.; Mori, K.; Luo, S.; Lee, A.S.; Zhu, Y.; Shyy, J.Y. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J. 2004, 23, 950–958, Erratum in EMBO J. 2008, 27, 2941. [Google Scholar] [CrossRef]
- Dong, B.; Sun, Y.; Cheng, B.; Xue, Y.; Li, W.; Sun, X. Activating transcription factor (ATF) 6 upregulates cystathionine β synthetase (CBS) expression and hydrogen sulfide (H2S) synthesis to ameliorate liver metabolic damage. Eur. J. Med. Res. 2023, 28, 540. [Google Scholar] [CrossRef]
- Howarth, D.L.; Lindtner, C.; Vacaru, A.M.; Sachidanandam, R.; Tsedensodnom, O.; Vasilkova, T.; Buettner, C.; Sadler, K.C. Activating transcription factor 6 is necessary and sufficient for alcoholic fatty liver disease in zebrafish. PLoS Genet. 2014, 10, e1004335. [Google Scholar] [CrossRef]
- Ku, H.C.; Cheng, C.F. Master Regulator Activating Transcription Factor 3 (ATF3) in Metabolic Homeostasis and Cancer. Front. Endocrinol. 2020, 11, 556. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Cominguez, D.C.; Park, Y.J.; Kang, Y.M.; Nugroho, A.; Kim, S.; An, H.J. Clitorin ameliorates western diet-induced hepatic steatosis by regulating lipogenesis and fatty acid oxidation in vivo and in vitro. Sci. Rep. 2022, 12, 4154. [Google Scholar] [CrossRef]
- Osborne, T.F. Sterol regulatory element-binding proteins (SREBPs): Key regulators of nutritional homeostasis and insulin action. J. Biol. Chem. 2000, 275, 32379–32382. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, T.O.; Grumet, L.; Taschler, U.; Hartler, J.; Heier, C.; Woblistin, A.; Pajed, L.; Kollroser, M.; Rechberger, G.; Thallinger, G.G.; et al. ATGL and CGI-58 are lipid droplet proteins of the hepatic stellate cell line HSC-T6. J. Lipid Res. 2015, 56, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.; Nakatsuka, A.; Fears, S.; Davis, W.; Tsukamoto, H.; Bosron, W.F.; Sanghani, S.P. Expression of carboxylesterase and lipase genes in rat liver cell-types. Biochem. Biophys. Res. Commun. 2008, 374, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Turpin, S.M.; Hoy, A.J.; Brown, R.D.; Rudaz, C.G.; Honeyman, J.; Matzaris, M.; Watt, M.J. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia 2011, 54, 146–156. [Google Scholar] [CrossRef]
- Reid, B.N.; Ables, G.P.; Otlivanchik, O.A.; Schoiswohl, G.; Zechner, R.; Blaner, W.S.; Goldberg, I.J.; Schwabe, R.F.; Chua, S.C., Jr.; Huang, L.S. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 2008, 283, 13087–13099. [Google Scholar] [CrossRef]
- Kato, M.; Higuchi, N.; Enjoji, M. Reduced hepatic expression of adipose tissue triglyceride lipase and CGI-58 may contribute to the development of non-alcoholic fatty liver disease in patients with insulin resistance. Scand. J. Gastroenterol. 2008, 43, 1018–1019. [Google Scholar] [CrossRef]
- Ladiges, W.; Enns, L. Targeting PKA Signaling to Prevent Metabolic Syndrome and Delay Aging. In Medical Complications of Type 2 Diabetes; Croniger, C.M., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Yan, F.; Wang, Q.; Lu, M.; Chen, W.; Song, Y.; Jing, F.; Guan, Y.; Wang, L.; Lin, Y.; Bo, T.; et al. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. J. Hepatol. 2014, 61, 1358–1364. [Google Scholar] [CrossRef]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; Enjoji, M.; et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef]
- Feng, S.; Reuss, L.; Wang, Y. Potential of Natural Products in the Inhibition of Adipogenesis through Regulation of PPARγ Expression and/or Its Transcriptional Activity. Molecules 2016, 21, 1278. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).