Synthesis, Reactions, and Agrochemical Studies of New 4,6-Diaryl-2-hydrazinylnicotinonitriles
Abstract
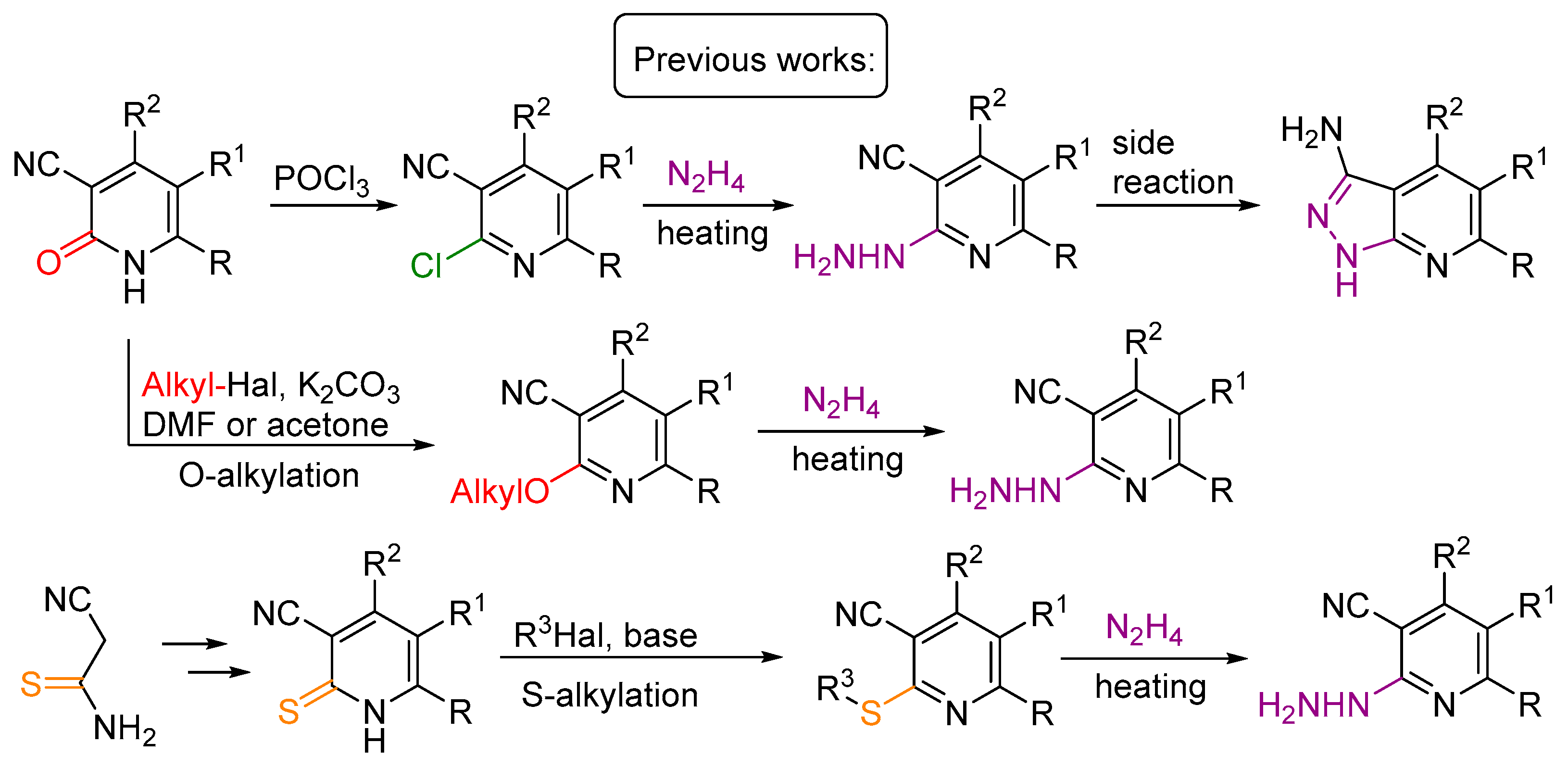
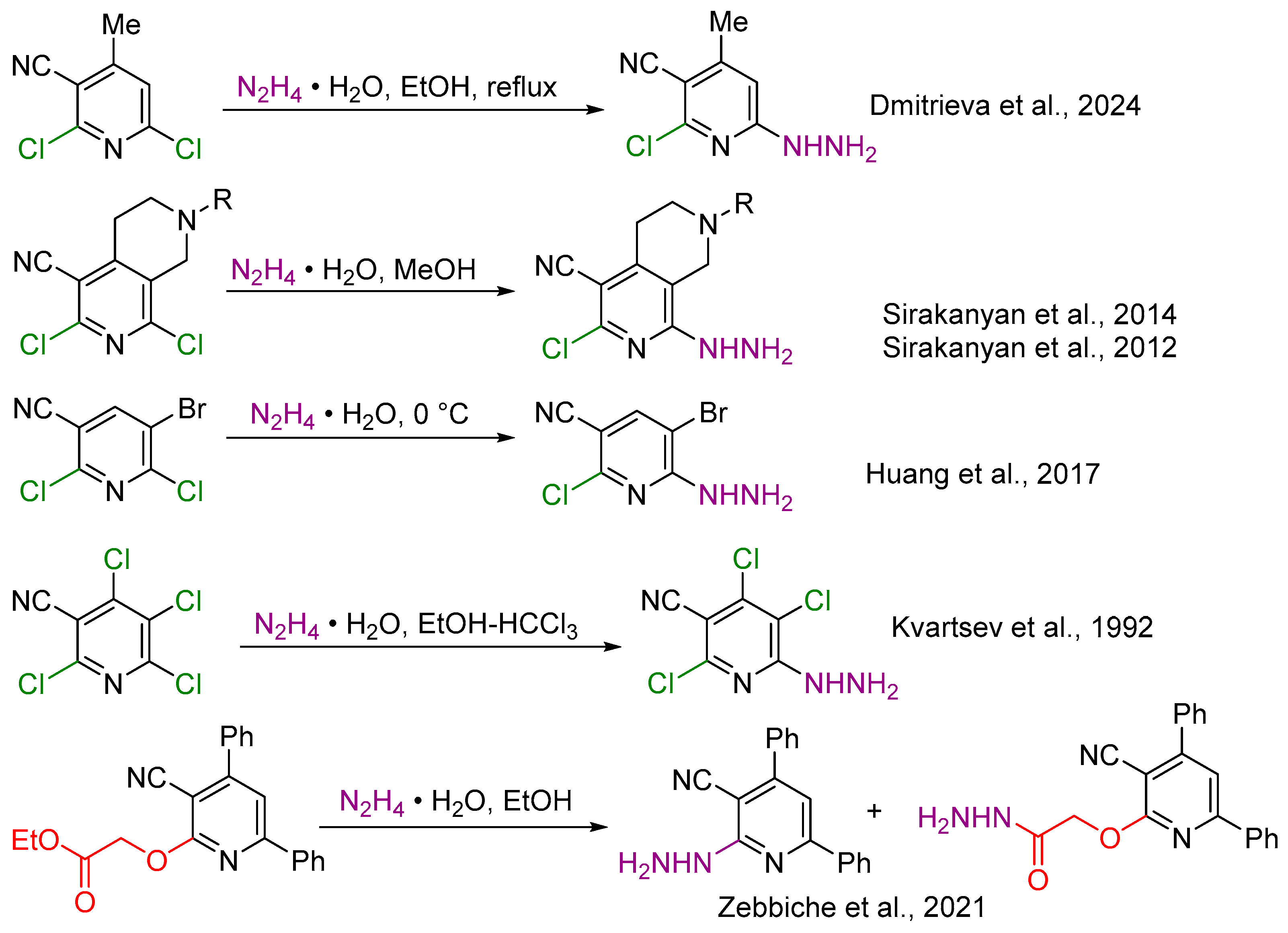
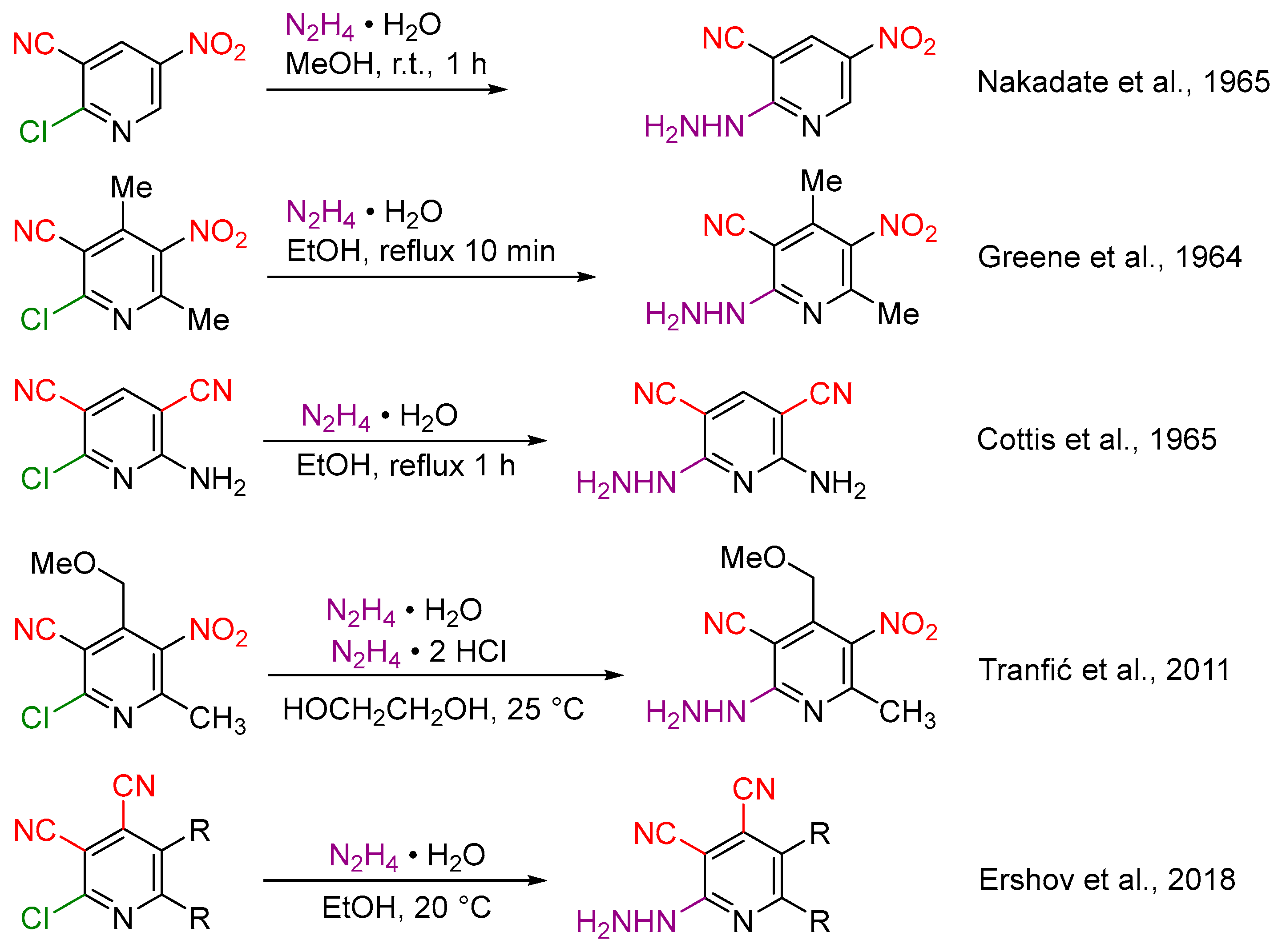
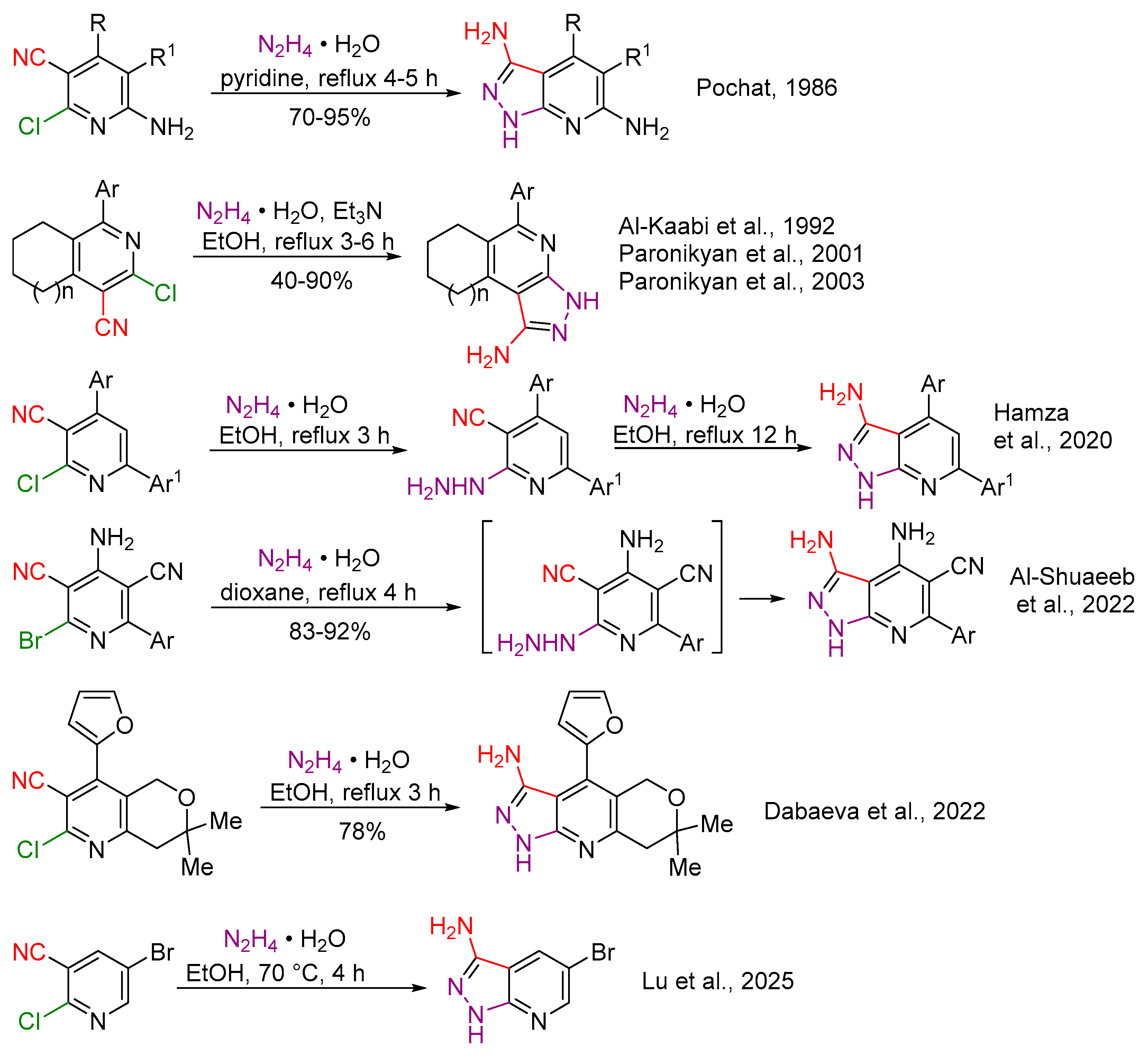
1. Introduction
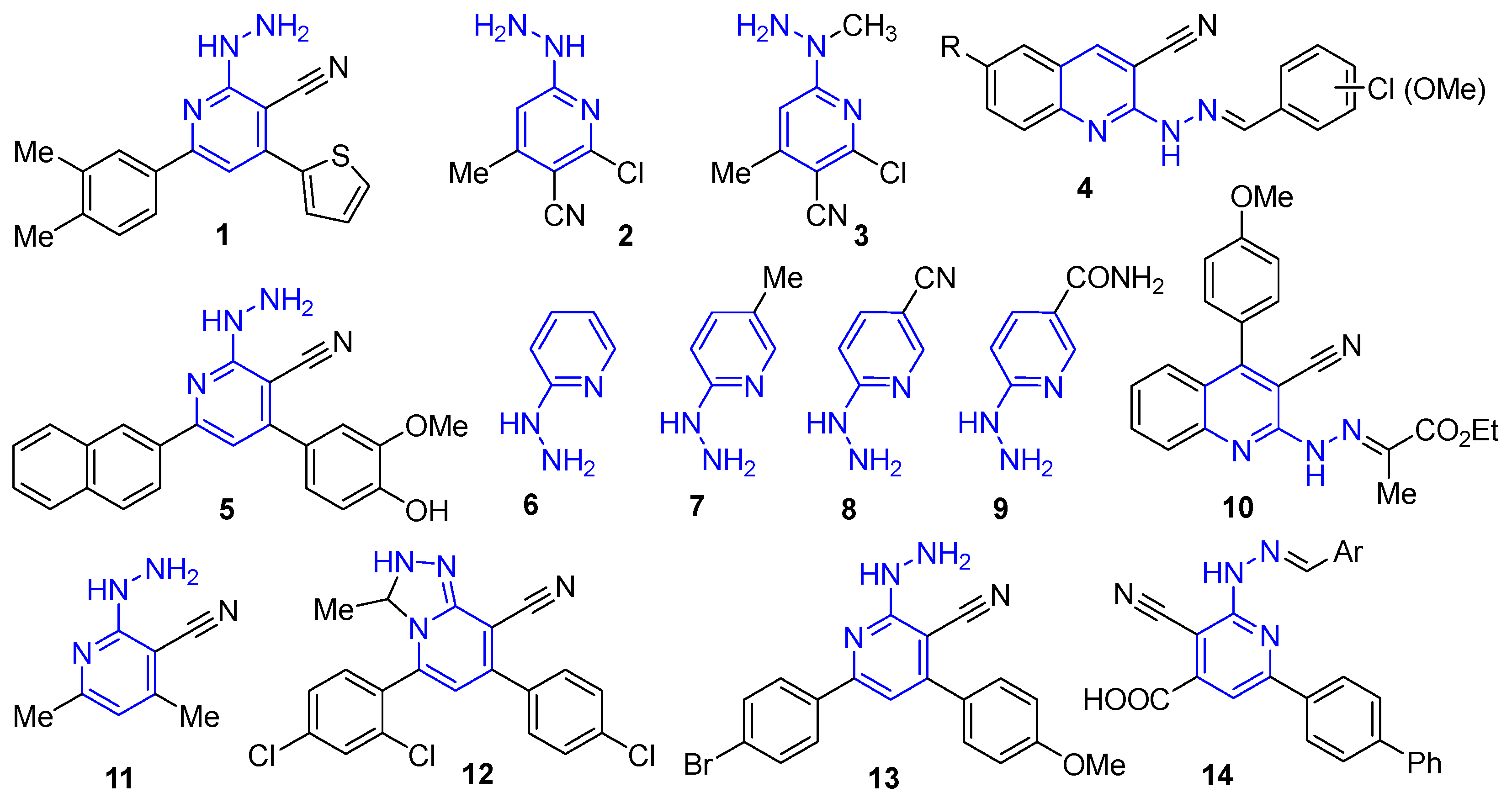
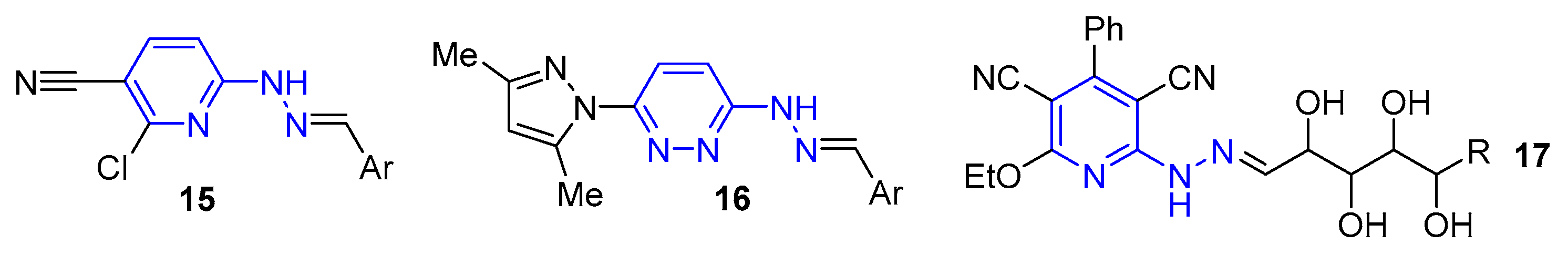
2. Results and Discussion
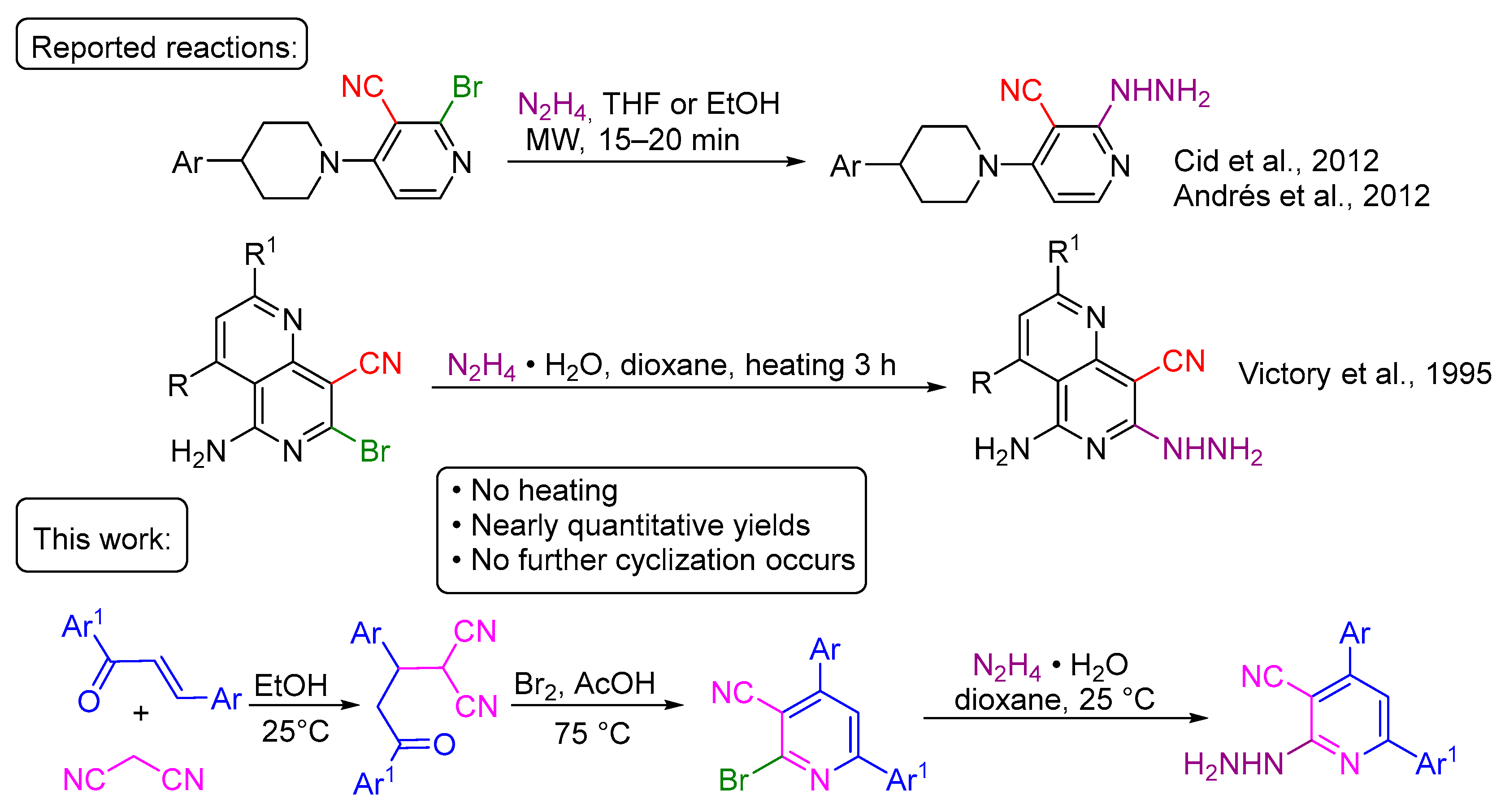
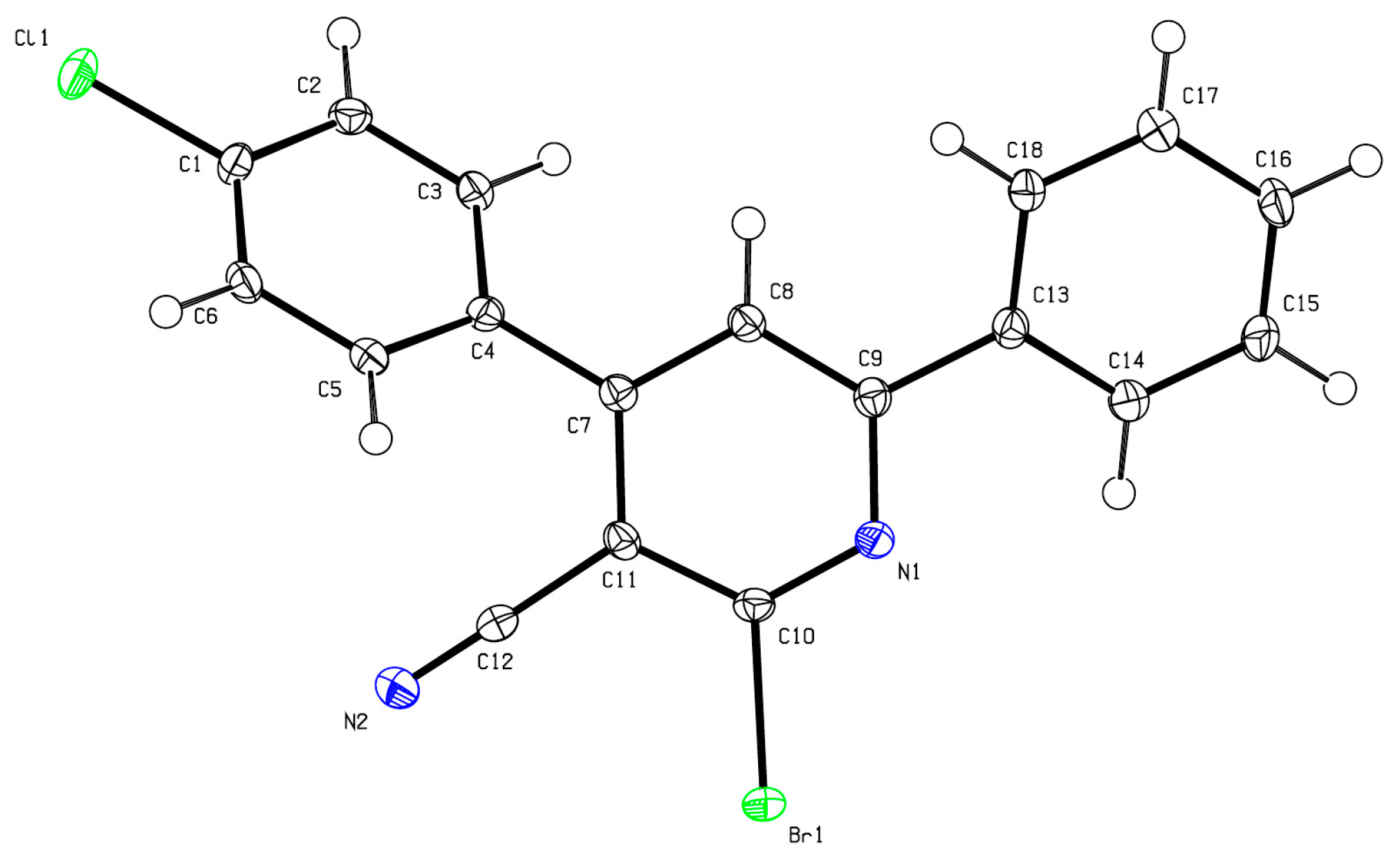
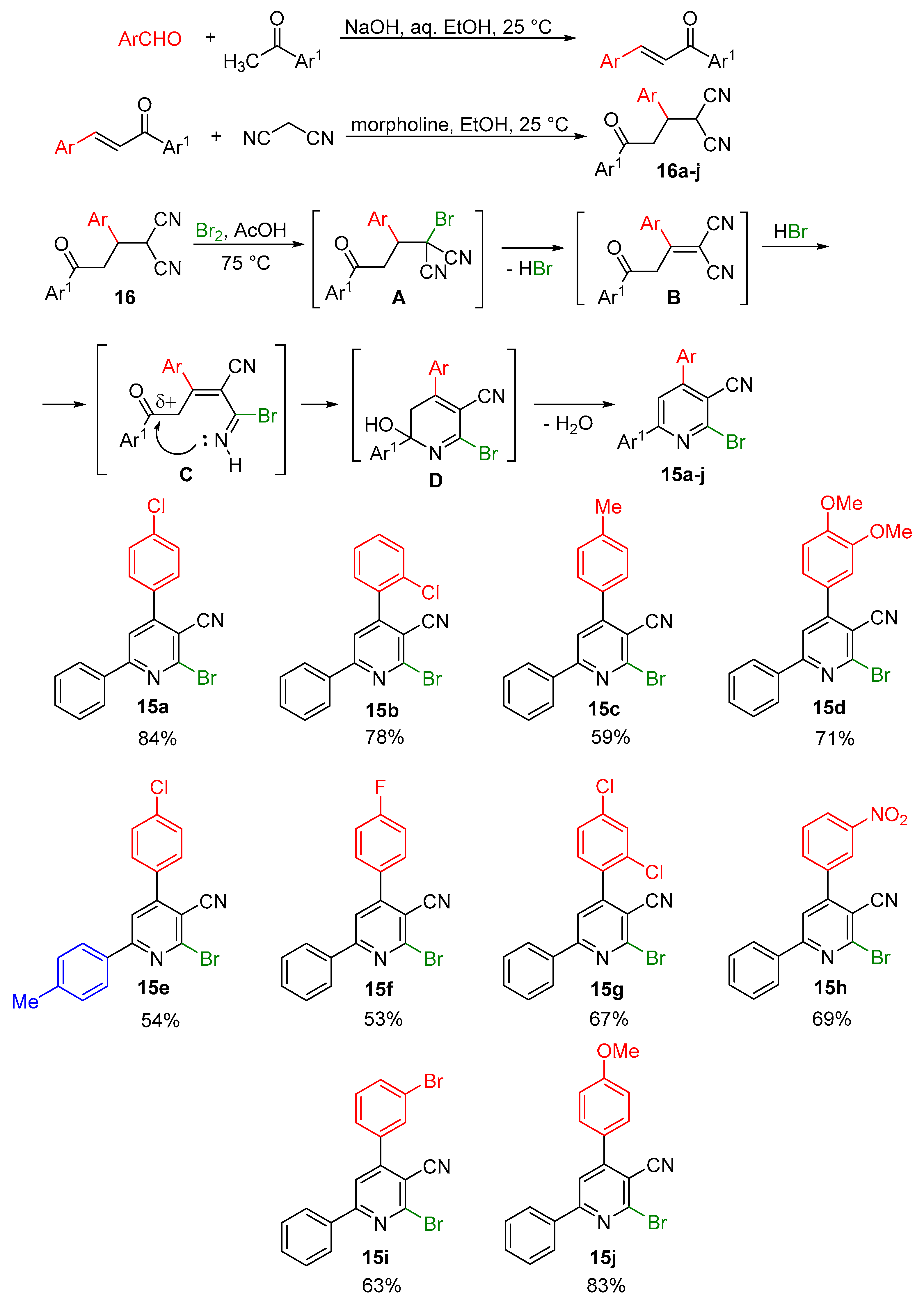
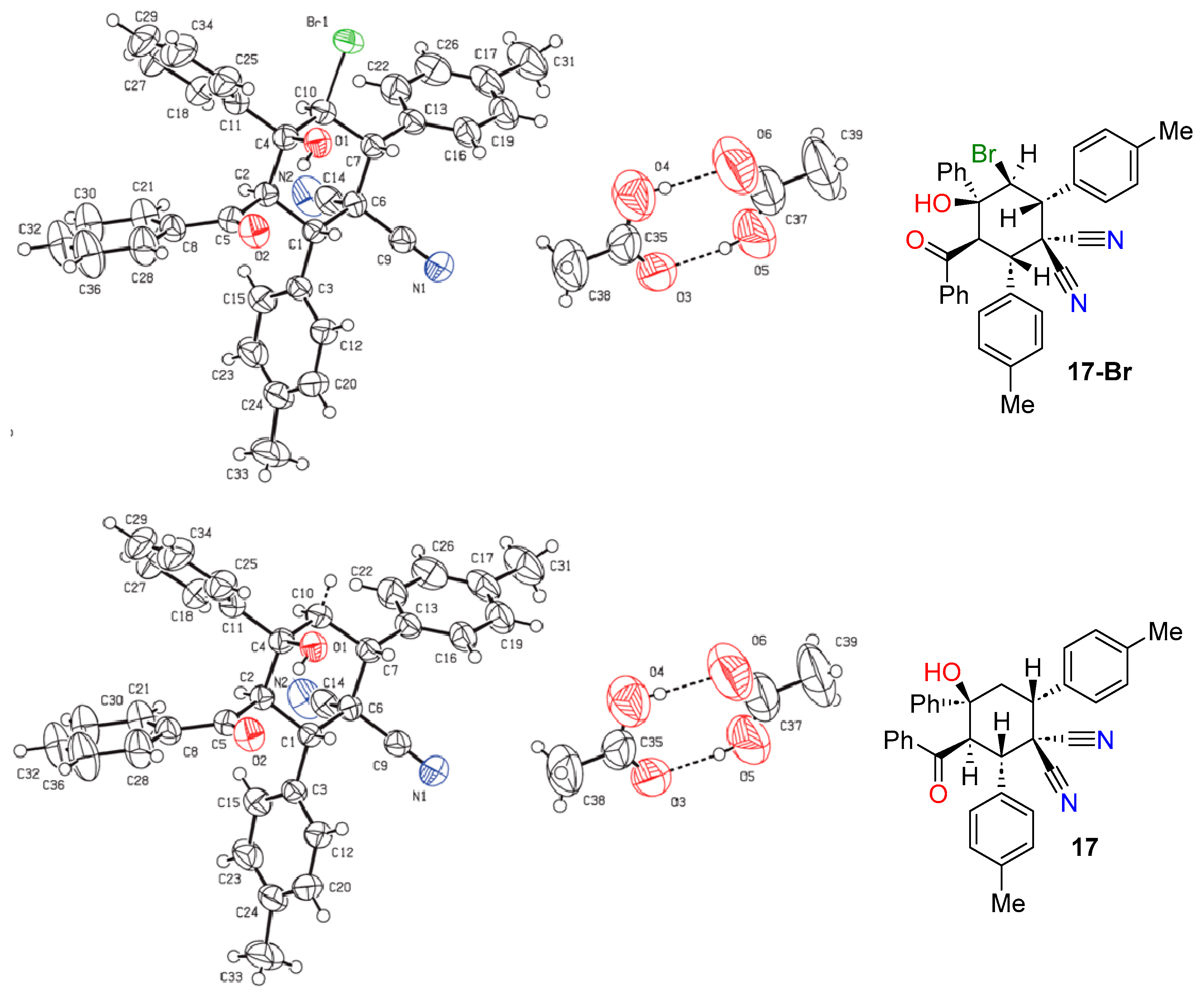
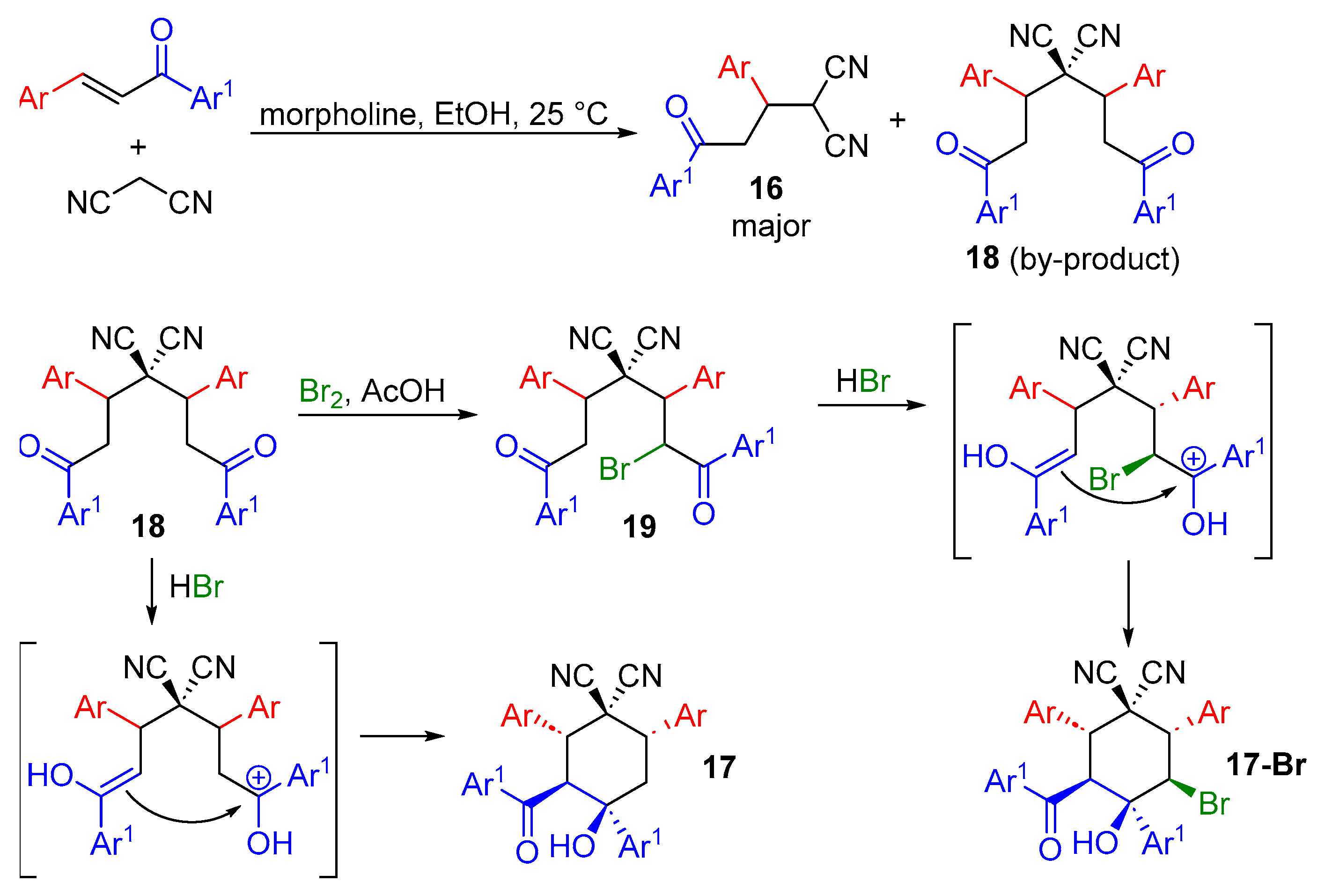
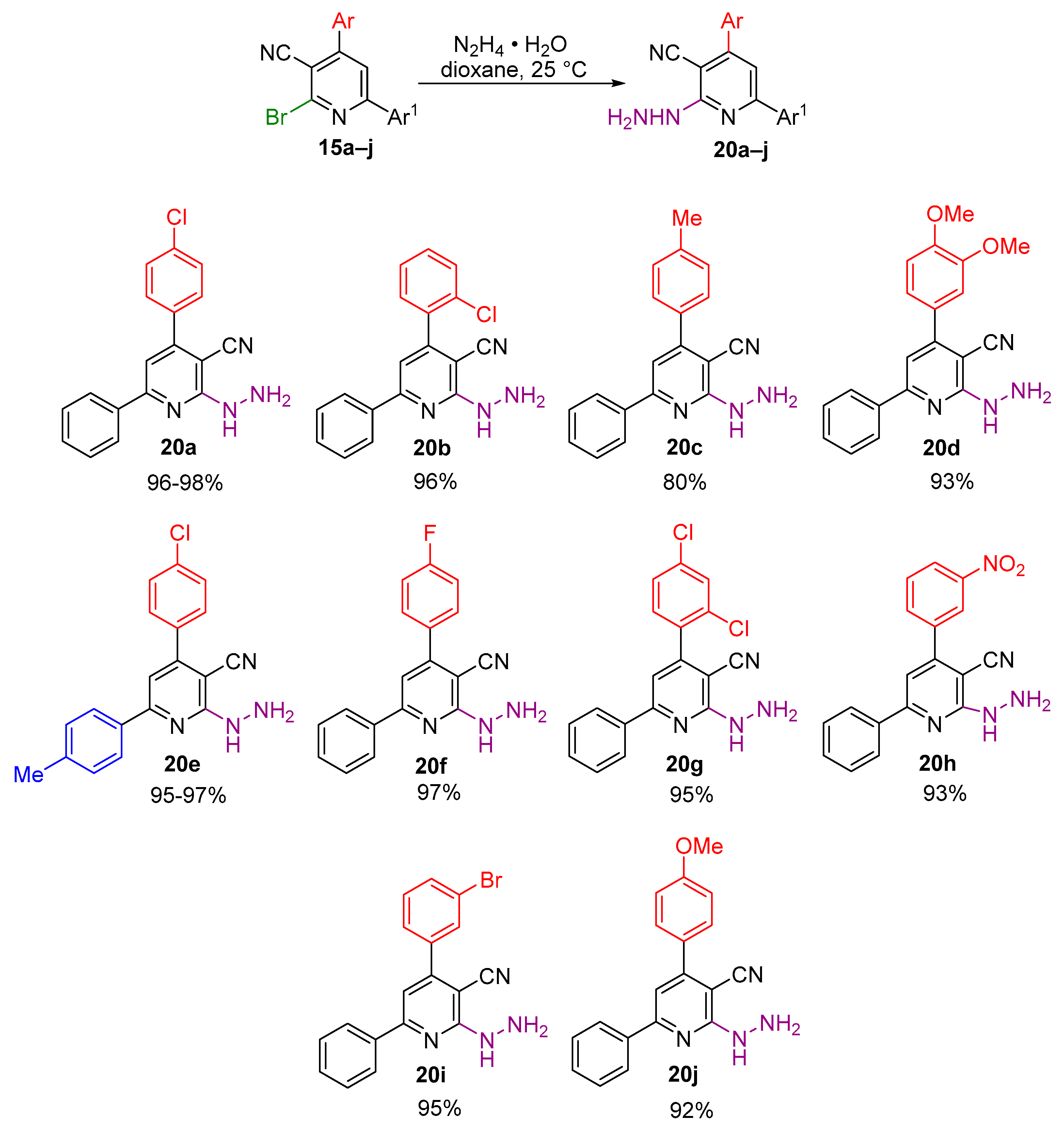
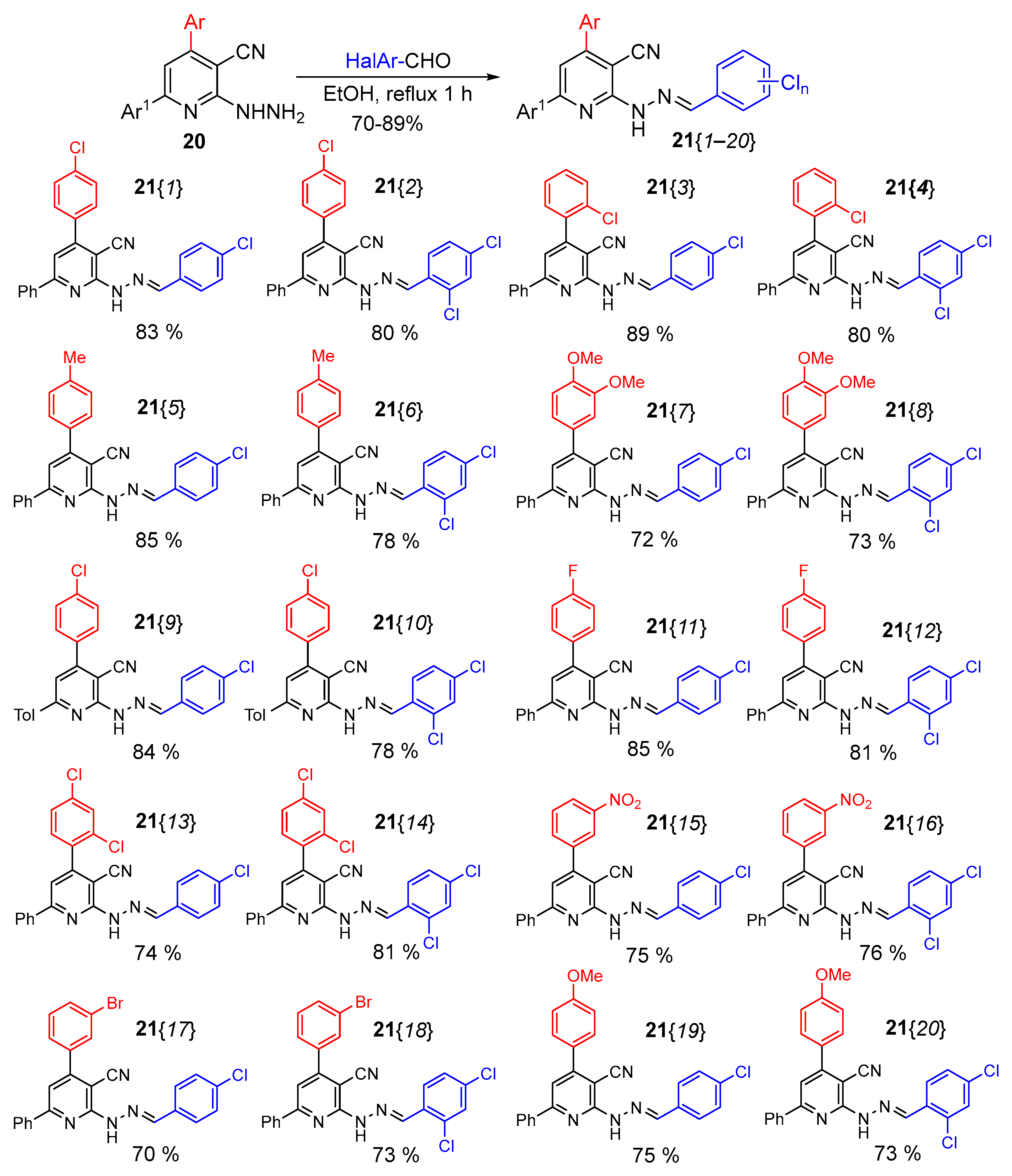
2.1. Synthesis
2.2. Agrochemical Studies
3. Materials and Methods
Herbicide-Safening Effect Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobrakov, K.I.; Ruchkina, A.G.; Rybina, I.I. Methods for the synthesis of hydrazinylpyridines and some of their characteristics. (review). Chem. Heterocycl. Compd. 2003, 39, 283–307. [Google Scholar] [CrossRef]
- Flefel, E.M.; El-Sofany, W.I.; El-Shahat, M.; Naqvi, A.; Assirey, E. Synthesis, molecular docking and in vitro screening of some newly synthesized triazolopyridine, pyridotriazine and pyridine–pyrazole hybrid derivatives. Molecules 2018, 23, 2548. [Google Scholar] [CrossRef]
- Dmitrieva, I.G.; Vasilin, V.K.; Dotsenko, V.V.; Aksenov, N.A. 6-(Pyrazol-1-yl)pyrazolo[3,4-b]pyridines: Synthesis, structure, and wheat growth regulating activity. Russ. J. Gen. Chem. 2024, 94, 2603–2615. [Google Scholar] [CrossRef]
- Dyadyuchenko, L.V.; Balakhov, A.A.; Nazarenko, D.Y.; Morozovskij, V.V.; Tkach, L.N. Method of Increasing Winter Wheat Yield. Patent RU 2623115, 22 June 2017. [Google Scholar]
- El-Gamal, K.M.; El-Morsy, A.M.; Saad, A.M.; Eissa, I.H.; Alswah, M. Synthesis, docking, QSAR, ADMET and antimicrobial evaluation of new quinoline-3-carbonitrile derivatives as potential DNA-gyrase inhibitors. J. Mol. Struct. 2018, 1166, 15–33. [Google Scholar] [CrossRef]
- AbdelHaleem, A.; Mansour, A.O.; AbdelKader, M.; Arafa, R.K. Selective VEGFR-2 inhibitors: Synthesis of pyridine derivatives, cytotoxicity and apoptosis induction profiling. Bioorg. Chem. 2020, 103, 104222. [Google Scholar] [CrossRef]
- Meier, A.A.; Moon, H.J.; Sabuncu, S.; Singh, P.; Ronnebaum, T.A.; Ou, S.; Douglas, J.T.; Jackson, T.A.; Moënne-Loccoz, P.; Mure, M. Insight into the spatial arrangement of the lysine tyrosylquinone and Cu2+ in the active site of lysyl oxidase-like 2. Int. J. Mol. Sci. 2022, 23, 13966. [Google Scholar] [CrossRef]
- Meier, A.A.; Kuczera, K.; Mure, M. A 3D–predicted structure of the amine oxidase domain of lysyl oxidase–like 2. Int. J. Mol. Sci. 2022, 23, 13385. [Google Scholar] [CrossRef]
- Anacona, J.R.; Rincones, M. Tridentate hydrazone metal complexes derived from cephalexin and 2-hydrazinylpyridine: Synthesis, characterization and antibacterial activity. Spectrochim. Acta A 2015, 141, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.A.; Larter, S.J. Complexes of palladium(II) and platinum(II) with 2-hydrazinylpyridine and 8-hydrazinylquinoline. Polyhedron 1986, 5, 1213–1216. [Google Scholar] [CrossRef]
- Dinda, S.; Patra, S.C.; Ganguly, S. Rhodium(III) complex with pyrene-pyridyl-hydrazone: Synthesis, structure, ligand redox, spectral characterization and DFT calculation. J. Chem. Sci. 2019, 131, 24. [Google Scholar] [CrossRef]
- Soliman, A.A.; Attaby, F.A.; Alajrawy, O.I.; Majeed, S.R.; Sahin, C.; Varlikli, C. Soluble cytotoxic Ruthenium(II) complexes with 2-Hydrazinylpyridine. Russ. J. Inorg. Chem. 2019, 64, 742–754. [Google Scholar] [CrossRef]
- Diop, A.; Sarr, M.; Diop, M.; Thiam, I.E.; Barry, A.H.; Coles, S.; Orton, J.; Gaye, M. Metal transition complexes of tridentate Schiff base ligands derived from 2-hydrazinylpyridine: Synthesis, spectroscopic characterization and X-ray structures. Transit. Met. Chem. 2019, 44, 415–423. [Google Scholar] [CrossRef]
- Alajrawy, O.I.; Almhmdi, A.A. Dioxomolybdenum (VI) and oxomolybdenum (IV) complexes with N, O, and S bidentate ligands, syntheses, spectral characterization, and DFT studies. J. Mol. Struct. 2022, 1260, 132813. [Google Scholar] [CrossRef]
- Hirsch-Kuchma, M.; Nicholson, T.; Davison, A.; Davis, W.M.; Jones, A.G. Synthesis and characterization of Rhenium(III) and Technetium(III) organohydrazide Chelate Complexes. reactions of 2-hydrazinylpyridine with complexes of rhenium and Technetium. Inorg. Chem. 1997, 36, 3237–3241. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.L.; Hong, Z.D.; Zhang, T.Y.; Cai, B.D.; Zhang, Y.Z.; Feng, Y.Q. A method for simultaneous determination of 14 carbonyl-steroid hormones in human serum by ultra high performance liquid chromatography–tandem mass spectrometry. J. Anal. Test. 2020, 4, 1–12. [Google Scholar] [CrossRef]
- Nadarajah, N.; Skadberg, Ø.; Adaway, J.; Brede, C. Multiplexed analysis of steroid hormones in saliva by LC-MS/MS with 2-hydrazinylpyridine derivatization. Clin. Mass Spectrom. 2017, 4–5, 1–10, Erratum in J. Mass. Spectrom. Adv. Clin. Lab. 2021, 20, 48. [Google Scholar] [CrossRef]
- Hala, D.; Overturf, M.D.; Petersen, L.H.; Huggett, D.B. Quantification of 2-hydrazinylpyridine derivatized steroid hormones in fathead minnow (Pimephales promelas) blood plasma using LC-ESI+/MS/MS. J. Chromatogr. B 2011, 879, 591–598. [Google Scholar] [CrossRef]
- Im, E.; Lew, B.L.; Lee, M.Y.; Lee, J.; Paeng, K.J.; Chung, B.C. Simultaneous determination of androgens and prostaglandins in human urine using ultra-high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2019, 1109, 45–53. [Google Scholar] [CrossRef]
- Sosvorova, L.; Vitku, J.; Chlupacova, T.; Mohapl, M.; Hampl, R. Determination of seven selected neuro- and immunomodulatory steroids in human cerebrospinal fluid and plasma using LC-MS/MS. Steroids 2015, 98, 1–8. [Google Scholar] [CrossRef]
- Skodova, T.; Vitku, J.; Bradac, O.; Skalicky, P.; Bubenikova, A.; Kanceva, R.; Kolatorova, L. LC-MS/MS techniques for the analysis of steroid panel in human cerebrospinal fluid. Neurochem. Int. 2025, 191, 106080. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Luo, S.; Tang, S.; Zeng, H.; Zhu, S.; Guo, B.; Chen, B.; Ma, M. Rapid determination of α,β-unsaturated aldehydes in vegetable oils by desorption corona beam ionization-tandem mass spectrometry based on a twin derivatization strategy. Microchem. J. 2025, 208, 112447. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, Y.; Zhang, H.; Chen, S.; Yi, J.; Xie, Q.; Dong, J.; Wu, C. Strategy to empower nontargeted metabolomics by triple-dimensional combinatorial derivatization with MS-TDF Software. Anal. Chem. 2024, 96, 7634–7642. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. 6-Hydrazinylnicotinamide derivatives as bifunctional coupling agents for 99mTc-labeling of small biomolecules. Top. Curr. Chem. 2005, 252, 117–153. [Google Scholar]
- Xu, W.; Yan, J.; Zhong, X.; Pan, D.; Wang, X.; Xu, Y.; Wang, L.; Chen, C.; Yang, M. Development and evaluation of a 99mTc-labeled olaparib analog for PARP imaging. EJNMMI Radiopharm. Chem. 2025, 10, 46. [Google Scholar] [CrossRef]
- Thakur, N.; Rajak, N.; Garg, N.; Pandey, R. Stimuli-responsive and biocompatible pyridyhydrazone-phenol Schiff base and its Zn(II)-complex: Distinct fluorescent detection of SO42−/CO32− in cell lines and real water samples. Anal. Chim. Acta 2025, 1382, 344812. [Google Scholar] [CrossRef]
- Park, J.; Moon, D.I.; Shah, B.; Singh, N.; Jang, D.O. Pyridine-hydrazone-based charge-transfer probe for detecting Cu2+ and Co2+ ions in aqueous solutions. Spectrochim. Acta A 2026, 347, 126979. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, D.B.; Wang, J.Y.; Lu, R.M.; Zheng, C.H.; Pu, S.Z. A novel diarylethene-hydrazinylpyridine-based probe for fluorescent detection of aluminum ion and naked-eye detection of hydroxide ion. Sens. Actuators B Chem. 2017, 245, 263–272. [Google Scholar] [CrossRef]
- Eldem, A.; Ozcan, B.C.; Kalmaz, S.; Ucuncu, M. A Sensitive and Reversible Fluorescent Probe for Selective Detection of Hg2⁺ and Au3⁺ Ions. J. Fluoresc. 2025. in print. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, H.; Fang, X.; He, Y.; Zhang, W.; Yang, Y.; Qian, J. Ratiometic fluorescent detection of Au(III) and fluorescent “turn-off” identification of Cu(II)/Hg(II) with a coumarin-based probe. Tetrahedron 2024, 155, 133934. [Google Scholar] [CrossRef]
- Subasi, N.T. Colorimetric sensing of Au3+ ions in aqueous media: A xanthene-based probe for rapid and visual detection. Sens. Bio-Sens. Res. 2025, 48, 100799. [Google Scholar] [CrossRef]
- Rattanopas, S.; Muangsopa, P.; Khrootkaew, T.; Pinyou, P.; Kamkaew, A.; Chansaenpak, K. Viscosity-sensitive fluorescent probe based on diphenylamino-substituted triazaborolopyridinium. J. Mol. Struct. 2026, 1349, 143936. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Bu, W.; Kang, Z.; Yu, C.; Wu, Q.; Jiao, L.; Hao, E. Boronic acid derived salicylidenehydrazinylpyridine (BOSPY) complexes with aggregation-induced emission for fluorescence imaging of cellular organelles. Dyes Pigments 2023, 210, 111013. [Google Scholar] [CrossRef]
- Madkour, H.M.F.; El-Hashash, M.A.E.A.M.; Salem, M.S.; Mahmoud, A.S.O.A. Synthesis, antileishmanial and cytotoxicity activities of fused and nonfused tetrahydroquinoline derivatives. Res. Chem. Intermed. 2018, 44, 3349–3364. [Google Scholar] [CrossRef]
- Waly, M.A.; EL-Hawary, I.I.; Hamama, W.S.; Zoorob, H.H. Synthesis and antitumor evaluation of some new fused and binary pyridines. J. Heterocycl. Chem. 2013, 50 (Suppl. S1), E12–E17. [Google Scholar] [CrossRef]
- Sayed, H.H.; Flefel, E.M.; Abd El-Fattah, A.M.; El-Sofany, W.I.; Hassan, A.M. Focus on the synthesis and reactions of some new pyridine carbonitrile derivatives as antimicrobial and antioxidant agents. Egypt J. Chem. 2010, 53, 17–35. [Google Scholar]
- EL-Hashash, M.A.; Shaban, S.S.; Ali, R.S. Synthesis of 3-cyano-2-pyridone derivative and its utility in the synthesis of some heterocyclic compounds with expecting antimicrobial activity. J. Heterocycl. Chem. 2021, 58, 329–339. [Google Scholar] [CrossRef]
- Salman, A.S.S. Synthesis and reaction of cyanopyridone derivatives and their potential biological activities. Pharmazie 1999, 54, 178–183. [Google Scholar]
- Poormoradkhan Melal, S.; Mahmoodi, N.O. A review of synthetic methods of 1,2,4-triazolopyridines and their therapeutic properties. Results Chem. 2023, 5, 100782. [Google Scholar] [CrossRef]
- Stoikov, I.I.; Antipin, I.S.; Burilov, V.A.; Kurbangalieva, A.R.; Rostovskii, N.V.; Pankova, A.S.; Balova, I.A.; Remizov, Y.O.; Pevzner, L.M.; Petrov, M.L.; et al. Organic chemistry in russian Universities. achievements of recent Years. Russ. J. Org. Chem. 2024, 60, 1361–1584, Erratum in Russ. J. Org. Chem. 2024, 60, 2052–2053. [Google Scholar] [CrossRef]
- Mohite, P.; Nahar, D.; Pawara, R.; Alqahtani, T.; Eldin, S.M.; Mukherje, N.; Rahman Mohammad Said Al-Tawaha, A.; Iqbal, R.; Bawazeer, S.; Ali, I. Triazolopyridine, a leitmotif of synthetic methods and pharmacological attributes: An extensive review. Arab. J. Chem. 2023, 16, 105181. [Google Scholar] [CrossRef]
- Shen, Z.H.; Wang, Q.; Yang, M.Y.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Wu, H.K.; Han, L.; Liu, X.H. Recent advances of 1,2,4-triazolo[3,4-a]pyridines: Synthesis and Bioactivities. Curr. Org. Chem. 2017, 21, 1626–1650. [Google Scholar]
- Jones, G. The chemistry of the triazolopyridines: An update. Adv. Heterocycl. Chem. 2002, 83, 1–70. [Google Scholar]
- Jones, G.; Sliskovic, D.R. The chemistry of the Triazolopyridines. Adv. Heterocycl. Chem. 1983, 34, 79–143. [Google Scholar]
- Abdel-Aziem, A.; Fouad, S.A. Recent advances in pyrazolo[3,4-b]pyridine chemistry: Synthesis techniques and biological activity. Mol. Divers. 2025. in print. [Google Scholar] [CrossRef]
- Atukuri, D. Pyrazolopyridine: An efficient pharmacophore in recent drug design and development. Chem. Biol. Drug Des. 2022, 100, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Smolobochkin, A.V.; Gazizov, A.S.; Garifzyanov, A.R.; Burilov, A.R.; Pudovik, M.A. Methods for the synthesis of 1H-pyrazolo[3,4-b]pyridine derivatives. Russ. Chem. Bull. 2022, 71, 878–884. [Google Scholar] [CrossRef]
- El-Bana, G.G.; Zoorob, H.H.; Ibrahim, M.E.; Hamama, W.S. Advances in 4,6-dimethyl-3-amino-3H-pyrazolo[3,4-b] pyridine-based and their annulated systems. Synth. Commun. 2020, 50, 2861–2884. [Google Scholar]
- Danel, A.; Gondek, E.; Kucharek, M.; Szlachcic, P.; Gut, A. 1H-Pyrazolo[3,4-b]quinolines: Synthesis and properties over 100 years of Research. Molecules 2022, 27, 2775. [Google Scholar]
- Charushin, V.N.; Verbitskiy, E.V.; Chupakhin, O.N.; Vorobyeva, D.V.; Gribanov, P.S.; Osipov, S.N.; Ivanov, A.V.; Martynovskaya, S.V.; Sagitova, E.F.; Dyachenko, V.D.; et al. The chemistry of heterocycles in the 21st century. Russ. Chem. Rev. 2024, 93, RCR5125. [Google Scholar] [CrossRef]
- Gouda, M.A.; Berghot, M.A.; El Ghani, G.E.A.; Khalil, A.G.M. Recent development in the chemistry of bicyclic 6+5 Systems, part I: Chemistry of Pyrazolo[3,4-b]pyridines. Lett. Org. Chem. 2019, 17, 2–23. [Google Scholar] [CrossRef]
- Dyadyuchenko, L.V.; Dmitrieva, I.G. Microwave-assisted synthesis of pyrazolo[3,4-b]pyridine derivatives (microreview). Chem. Heterocycl. Compd. 2020, 56, 1414–1416. [Google Scholar] [CrossRef]
- Titova, E.M.; Titov, A.A.; Shubina, E.S. Functional pyrazolylpyridine ligands in the design of metal complexes with tunable properties. Russ. Chem. Rev. 2023, 92, RCR5099. [Google Scholar] [CrossRef]
- Komarova, E.S.; Makarov, V.A.; Granik, V.G.; Párkányi, C. Synthesis of pyrazolo[3,4-b]pyridin-6-ones. J. Heterocycl. Chem. 2012, 49, 969–998. [Google Scholar] [CrossRef]
- Wenglowsky, S. Pyrazolo[3,4-b]pyridine kinase inhibitors: A patent review (2008–present). Expert. Opin. Ther. Pat. 2013, 23, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.M.; Makki, M.S.T.; Ali, T.E.; Ibrahim, M.A. 1,2,4-Triazine Chemistry part III: Synthetic strategies to functionalized bridgehead nitrogen heteroannulated 1,2,4-triazine systems and their regiospecific and pharmacological properties. Curr. Org. Synth. 2013, 10, 136–160. [Google Scholar] [PubMed]
- Dotsenko, V.V.; Kindop, V.K.; Kindop, V.K.; Daus, E.S.; Yudaev, I.V.; Daus, Y.V.; Bespalov, A.V.; Buryi, D.S.; Lukina, D.Y.; Aksenov, N.A.; et al. Synthesis of new phenothiazine/3-cyanoquinoline and phenothiazine/3-aminothieno[2,3-b]pyridine(-quinoline) heterodimers. Int. J. Mol. Sci. 2025, 26, 9798. [Google Scholar] [CrossRef]
- Rudenko, S.V.; Lukina, D.Y.; Kuchmas, P.S.; Kindop, V.K.; Bespalov, A.V.; Dotsenko, V.V.; Aksenov, N.A.; Aksenova, I.V. Synthesis of (pyrido[3’,2’:4,5]thieno[3,2-d]pyrimidin-2-ylthio)acetic acids esters by the reaction of N-(thieno[2,3-b]pyridin-3-yl)-α-chloroacetamides with potassium thiocyanate. Russ. J. Gen. Chem. 2025, 95, 2699–2708. [Google Scholar]
- Kindop, V.K.; Bespalov, A.V.; Dotsenko, V.V.; Strelkov, V.D.; Lukina, D.Y.; Baichurin, R.I.; Paronikyan, E.G.; Harutyunyan, A.S.; Ovcharov, S.N.; Aksenov, N.A.; et al. Synthesis and structural characterization of 2-iminothiazoline/quinoline and 2-iminothiazoline/thieno[2,3-b]quinoline molecular hybrids with herbicide safening properties. Tetrahedron 2025, 186, 134889. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Sinotsko, A.E.; Strelkov, V.D.; Varzieva, E.A.; Russkikh, A.A.; Levchenko, A.G.; Temerdashev, A.Z.; Aksenov, N.A.; Aksenova, I.V. Alkyl 4-Aryl-6-amino-7-phenyl-3-(phenylimino)-4,7-dihydro- 3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxylates: Synthesis and agrochemical Studies. Molecules 2023, 28, 609. [Google Scholar] [PubMed]
- Dotsenko, V.V.; Jassim, N.T.; Temerdashev, A.Z.; Abdul-Hussein, Z.R.; Aksenov, N.A.; Aksenova, I.V. New 6′-Amino-5′-cyano-2-oxo-1,2-dihydro-1′H-spiro[indole-3,4′-pyridine]-3′-carboxamides: Synthesis, reactions, molecular docking studies and biological activity. Molecules 2023, 28, 3161. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Muraviev, V.S.; Lukina, D.Y.; Strelkov, V.D.; Aksenov, N.A.; Aksenova, I.V.; Krapivin, G.D.; Dyadyuchenko, L.V. Reaction of 3-Amino-4,6-diarylthieno[2,3-b]pyridine-2-carboxamides with ninhydrin. Russ. J. Gen. Chem. 2020, 90, 948–960. [Google Scholar]
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Y.; Stolyarova, A.N.; Aksenov, N.A.; Aksenova, I.V.; Strelkov, V.D.; Dyadyuchenko, L.V. Substituted N-(thieno[2,3-b]pyridine-3-yl)acetamides: Synthesis, reactions, and biological activity. Monatsh. Chem. 2019, 150, 1973–1985. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Rudenko, S.V.; Lukina, D.Y.; Smirnova, A.K.; Krivokolysko, S.G.; Temerdashev, A.Z.; Harutyunyan, A.S.; Paronikyan, E.G.; Aksenov, N.A.; Aksenova, I.V. Synthesis of 2,2-dimethyl-2,3-dihydropyrido[3′,2′: 4,5]thieno[3,2-d]pyrimidin-4(1H)-ones by reaction of 3-aminothieno[2,3-b]pyridine- 2-carboxamides with acetone. Russ. J. Gen. Chem. 2025, 95, 1236–1247. [Google Scholar]
- Panaetov, A.O.; Strelkov, V.D.; Dotsenko, V.V.; Aksenov, N.A.; Aksenova, I.V.; Chausov, F.F.; Lomova, N.V.; Kazantseva, I.S.; Isupov, N.Y. Mannich reaction involving 6-amino-4-methyl-2-(thio)oxo-1,2-dihydropyridine-3,5-dicarbonitriles. Russ. J. Gen. Chem. 2023, 93, 1655–1668. [Google Scholar] [CrossRef]
- Temerdashev, A.; Zorina, M.; Feng, Y.Q.; Gashimova, E.; Dotsenko, V.V.; Ioutsi, V.; Atapattu, S.N. Cyanoacetohydrazide as a novel derivatization agent for the determination of UHPLC-HRMS steroids in urine. Molecules 2024, 29, 2433. [Google Scholar] [CrossRef] [PubMed]
- Temerdashev, A.; Nesterenko, P.N.; Atapattu, S.N.; Feng, Y.Q.; Zorina, M.; Zhurkina, K.; Gashimova, E.; Steshin, M.O.; Dotsenko, V.V. Phthalylglycyl chloride as a derivatization reagent for determination of urinary amino acids using ultra high-performance liquid chromatography coupled with high resolution mass spectrometry. J. Chromatogr. Open 2024, 6, 100162. [Google Scholar] [CrossRef]
- Zorina, M.; Feng, Y.Q.; Atapattu, S.N.; Girel, S.; Konshina, D.; Konshin, V.V.; Temerdashev, A. A novel ionic liquid 3-(2-hydrazinyl-2-oxoethyl)-1-methyl-1H-imidazol-3-ium chloride as a derivatization reagent for HPLC-HRMS determination of steroid hormones in urine. J. Chromatogr. B 2025, 1265, 124760. [Google Scholar] [CrossRef]
- Temerdashev, A.; Azaryan, A.; Konshina, D.; Konshin, V.; Steshin, M.; Gashimova, E.; Atapattu, S.N.; Feng, Y.Q.; Zhu, Q.F. Application of alrestatin chloride as a derivatizing reagent for the determination of amino acids, catecholamines and biogenic amines in urine. J. Chromatogr. Open 2025, 8, 100268. [Google Scholar] [CrossRef]
- Dyadyuchenko, L.V.; Dmitrieva, I.G.; Nazarenko, D.Y.; Strelkov, V.D. Antidote and Growth-Regulating Activity of N1-Aryl-N2-(Substituted Nicotinonitrile) Hydrazones. Agrochemistry 2014, N 7, 33–37. (In Russian) [Google Scholar]
- Dyadyuchenko, L.V.; Taranenko, V.V.; Muraviev, V.S. A new growth regulator for rice plants. Agrochemistry 2021, N 4, 57–61. (In Russian) [Google Scholar] [CrossRef]
- Gomktsyan, T.A.; Shainova, R.S.; Karapetyan, A.V.; Yengoyan, A.P. Synthesis and biological Activity of new 3-Pyrazolyl-6-hydrazinylpyridazine Derivatives. Russ. J. Gen. Chem. 2021, 91, 2019–2024. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Moustafa, A.H.; El-Torky, A.E.; Abd El-Salam, E.A. A series of pyridines and pyridine based sulfa-drugs as antimicrobial agents: Design, synthesis and antimicrobial activity. Russ. J. Gen. Chem. 2017, 87, 2401–2408. [Google Scholar] [CrossRef]
- Bomika, Z.A.; Andaburskaya, M.B.; Pelcher, Y.É.; Dubur, G.Y. Some nucleophilic substitution reactions of 2-chloro-3-cyanopyridines. Chem. Heterocycl. Compd. 1976, 12, 896–899. [Google Scholar] [CrossRef]
- Radwan, M.A.A.; Alshubramy, M.A.; Abdel-Motaal, M.; Hemdan, B.A.; El-Kady, D.S. Synthesis, molecular docking and antimicrobial activity of new fused pyrimidine and pyridine derivatives. Bioorg. Chem. 2020, 96, 103516. [Google Scholar]
- Al-Warhi, T.; Al-Karmalawy, A.A.; Elmaaty, A.A.; Alshubramy, M.A.; Abdel-Motaal, M.; Majrashi, T.A.; Asem, M.; Nabil, A.; Eldehna, W.M.; Sharaky, M. Biological evaluation, docking studies, and in silico ADME prediction of some pyrimidine and pyridine derivatives as potential EGFRWT and EGFRT790M inhibitors. J. Enzyme Inhib. Med. Chem. 2023, 38, 176–191. [Google Scholar] [CrossRef]
- Kotb, E.R.; Soliman, H.A.; Morsy, E.M.; Abdelwahed, N.A. New pyridine and triazolopyridine derivatives: Synthesis, antimicrobial and antioxidant evaluation. Acta Pol. Pharm. Drug Res. 2017, 74, 861–872. [Google Scholar]
- Victory, P.; Busquets, N.; Borrell, J.I.; Teixidó, J.; Serra, B.; Matallana, J.L.; Junek, H.; Sterk, H. Cyclization of 2-dicyanomethylene-1,2-dihydro-pyridine-3-carbonitriles with hydrogen halides: A re-examination on the regioselectivity. Heterocycles 1995, 41, 1013–1022. [Google Scholar]
- Kotb, E.R.; Anwar, M.M.; Abbas, H.-A.S.; Abd El-Moez, S.I. A concise synthesis and antimicrobial activity of a novel series of naphthylpyridine-3-carbonitrile compounds. Acta Pol. Pharm. 2013, 70, 667–679. [Google Scholar]
- Bardasov, I.N.; Alekseeva, A.U.; Ievlev, M.Y. Synthesis and spectral–luminescent properties of 2-amino-6-chloro-4-(4-(dimethylamino)phenyl)pyridine-3,5-dicarbonitrile and compounds based on it. Russ. J. Gen. Chem. 2025, 95, 980–989. [Google Scholar]
- Arustamova, I.S.; Piven’, V.T. Investigations on furopyridines. 10. Synthesis of 4-N-substituted 1H-furo[3,4]pyridin-3-ones. Chem. Heterocycl. Compd. 1999, 35, 58–63. [Google Scholar] [CrossRef]
- Sycheva, T.V.; Anisimova, O.S.; Yakhontov, L.N. Azaindole derivatives. 67. Synthesis of N-substituted 1-benzyl-4-methyl-5-cyano-6-amino-7-azaindoles. Chem. Heterocycl. Compd. 1986, 22, 76–80. [Google Scholar] [CrossRef]
- Flefel, E.M.; Abbas, H.A.S.; Abdel Mageid, R.E.; Zaghary, W.A. Synthesis and cytotoxic effect of some novel 1,2-dihydropyridin-3-carbonitrile and nicotinonitrile derivatives. Molecules 2016, 21, 30. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Amr, A.E.G.E.E.; EL-Ziaty, A.K.; Elsayed, E.A. Cytotoxic effects of newly synthesized heterocyclic candidates containing nicotinonitrile and pyrazole moieties on hepatocellular and cervical carcinomas. Molecules 2019, 24, 1965. [Google Scholar] [CrossRef] [PubMed]
- Aboukhatwa, S.M.; Ibrahim, A.O.; Aoyama, H.; Al-Behery, A.S.; Shaldam, M.A.; El-Ashmawy, G.; Tawfik, H.O. Nicotinonitrile-derived apoptotic inducers: Design, synthesis, X-ray crystal structure and pim kinase inhibition. Bioorg. Chem. 2022, 129, 106126. [Google Scholar] [CrossRef]
- Mohamed, S.F.; Kotb, E.R.; Abd El-Meguid, E.A.; Awad, H.M. Synthesis and anticancer activity of novel 2-substituted pyranopyridine derivatives. Res. Chem. Intermed. 2017, 43, 437–456. [Google Scholar] [CrossRef]
- Cheney, I.W.; Yan, S.; Appleby, T.; Walker, H.; Vo, T.; Yao, N.; Hamatake, R.; Hong, Z.; Wu, J.Z. Identification and structure–activity relationships of substituted pyridones as inhibitors of Pim-1 kinase. Bioorg. Med. Chem. Lett. 2007, 17, 1679–1683. [Google Scholar] [CrossRef]
- Nikam, P.S.; Patil, S.P.; Kanawade, S.B.; Gangurde, S.A.; Rathi, M.; Toche, R.B. The use of Pinner type reaction for the synthesis of new indeno[2,1-c]pyridine-4-carbonitrile derivatives. J. Heterocycl. Chem. 2015, 52, 150–155. [Google Scholar] [CrossRef]
- Salem, M.S.; Sakr, S.I.; El-Senousy, W.M.; Madkour, H.M.F. Synthesis, Antibacterial, and antiviral Evaluation of new Heterocycles containing the pyridine Moiety. Arch. Pharm. 2013, 346, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Hamza, E.K.; Hamdy, N.A.; Zarie, E.S.; Fakhr, I.M.I.; Elwahy, A.H.M.; Awad, H.M. Synthesis and in vitro evaluation of novel tetralin-pyrazolo[3,4-b]pyridine hybrids as potential anticancer agents. J. Heterocycl. Chem. 2020, 57, 182–196. [Google Scholar] [CrossRef]
- Al-Issa, S.A.R. Synthesis of a new series of pyridine and fused pyridine derivatives. Molecules 2012, 17, 10902–10915. [Google Scholar] [CrossRef]
- Alsafi, M.A.; Al-Dhuwayin, B.H.A.; El-Sofany, W.I.; Rateb, H.S.; Flefel, E.M. Functionalization of novel anti-microbial drug based on molecular docking study for nicotinonitrile analogs prepared by microwave irradiation. J. Mol. Struct. 2022, 1264, 133261. [Google Scholar] [CrossRef]
- Elewa, S.I.; Abdel-Haleem, D.R.; Tantawy, A.H.; Mohamed, H.I.; Lashin, W.H. Exploring the larvicidal and pupicidal activities of new functionalized pyridines against Culex pipiens L. referring to molecular docking and SAR studies. Bioorg. Chem. 2025, 157, 108283. [Google Scholar]
- Keshk, R.M. Design and synthesis of new series of 3-cyanopyridine and pyrazolopyridine derivatives. J. Heterocycl. Chem. 2020, 57, 3384–3393. [Google Scholar]
- Abbas, H.A.S.; Abo Zeina, E.A.; Radwan, H.A.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I. Efficient synthesis and biological evaluation of some new series of pyridine derivatives: Promising and potent new class of anticancer agents. J. Heterocycl. Chem. 2022, 59, 1971–1989. [Google Scholar] [CrossRef]
- Rashad, A.E.; Shamroukh, A.H.; El-Hashash, M.A.; El-Farargy, A.F.; Yousif, N.M.; Salama, M.A.; Mostafa, A.; El-Shahat, M. Synthesis and Anti-avian Influenza virus (H5N1) evaluation of some novel nicotinonitriles and their N-acylic nucleosides. J. Heterocycl. Chem. 2012, 49, 1130–1135. [Google Scholar] [CrossRef]
- Moustafa, O.S.; Badr, M.Z.A.; Kamel, E.M. Synthesis of new pyridoquinoxalines, thienopyridoquinoxalines and pyrimidothienopyridoquinoxalines. Pharmazie 2000, 55, 896–899. [Google Scholar]
- Sanad, S.M.H.; Hawass, M.A.E.; Ahmed, A.A.M.; Elneairy, M.A.A. Facile synthesis and characterization of novel pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-4(3H)-one and pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine incorporating 1,3-diarylpyrazole moiety. Synth. Commun. 2018, 48, 1847–1856. [Google Scholar] [CrossRef]
- Abdel-Megid, M. A convenient route for the synthesis of some new bi- and triheterocondensed uracils. Chem. Heterocycl. Compd. 2010, 46, 316–324. [Google Scholar] [CrossRef]
- Rateb, N.M.; Abdelaziz, S.H.; Zohdi, H.F. Synthesis and antimicrobial evaluation of some new thienopyridine, pyrazolopyridine and pyridothienopyrimidine derivatives. J. Sulfur. Chem. 2011, 32, 345–354. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Hovakimyan, A.A.; Noravyan, A.S. New heterocyclic systems derived from pyridine: New substrates for the investigation of the azide/tetrazole equilibrium. Tetrahedron 2014, 70, 8648–8656. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Avetisyan, N.G.; Noravyan, A.S. New heterocyclic systems based on 1-hydrazinyl-5,6,7,8- tetrahydro[2,7]naphthyridine: 7,8,9,10-tetrahydro[1,2,4]triazolo[3,4-a]- and 7,8,9,10-tetrahydro[1,2,4]triazolo[5,1-a][2,7]- naphthyridines. Chem. Heterocycl. Compd. 2012, 48, 470–475. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Yu, Z.; Zhang, H.; Wang, Y.; Lingel, A.; Qi, W.; Gu, J.; Zhao, K.; Shultz, M.D.; et al. Discovery of first-in-class, potent, and orally bioavailable embryonic ectoderm development (EED) inhibitor with robust anticancer efficacy. J. Med. Chem. 2017, 60, 2215–2226. [Google Scholar] [CrossRef]
- Kvartsev, V.G.; Gizatullina, M.; Aliev, Z.G. Synthesis and structure of 6-hydrazinyl-2,4,5-trichloronicotinonitrile. Chem. Heterocycl. Compd. 1992, 28, 309–313. [Google Scholar] [CrossRef]
- Zebbiche, Z.; Tekin, S.; Küçükbay, H.; Yüksel, F.; Boumoud, B. Synthesis and anticancer properties of novel hydrazone derivatives incorporating pyridine and isatin moieties. Arch. Pharm. 2021, 354, 2000377. [Google Scholar] [CrossRef]
- Nakadate, M.; Takano, Y.; Hirayama, T.; Sakaizawa, S.; Hirano, T.; Okamoto, K.; Hirao, K.; Kawamura, T.; Kimura, M. Janovsky reaction of Nitropyridines. II. Preparation of 5-nitronicotinic acid and its related compounds. Chem. Pharm. Bull. 1965, 13, 113–118. [Google Scholar] [CrossRef]
- Greene, J.L.; Montgomery, J.A. Vitamin B6 Analogs. II.1,2 synthesis of 4,6-dimethyl-5-mercapto-3-pyridinemethanol and of 5-mercapto-6-methyl-3,4-pyridinedimethanol hydrochlorides. J. Med. Chem. 1964, 7, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Cottis, S.G.; Clarke, P.B.; Tieckelmann, H. Pyrazolo[3,4-b]pyridines and pyrazolo[3′,4′:6,5]pyrido[2,3-d]pyrimidines. J. Heterocycl. Chem. 1965, 2, 192–195. [Google Scholar] [CrossRef]
- Tranfić, M.; Halambek, J.; Cetina, M.; Jukić, M. Synthesis, X-ray and spectroscopic analysis of 2-hydrazinyl-6-methyl-4-(methoxymethyl)-5-nitropyridine-3-carbonitrile. J. Mol. Struct. 2011, 1001, 145–151. [Google Scholar] [CrossRef]
- Ershov, O.V.; Ievlev, M.Y.; Belikov, M.Y.; Maksimova, V.N. Synthesis of 2-Hydrazinylpyridine-3,4-dicarbonitriles and their Reaction with salicylaldehyde Derivatives. Russ. J. Org. Chem. 2018, 54, 873–877. [Google Scholar] [CrossRef]
- Pochat, F. Acces aux alkyl (ou aryl)-4 diamino-3,6 pyrazolo[3,4-b]pyridines substituees en 5 par un groupe SR ou Cl. Tetrahedron 1986, 42, 4461–4469. [Google Scholar] [CrossRef]
- Al-Kaabi, S.S.; Elgemeie, G.E.H. Studies on fused 2(1H)-pyridenethiones: New routes for the synthesis of fused 1H-pyrazolo[3,4-b]pyridines and fused thieno[2,3-b]pyridines. Bull. Chem. Soc. Jpn. 1992, 65, 2241–2245. [Google Scholar]
- Paronikyan, E.G.; Sirakanyan, S.N.; Noravyan, A.S.; Paronikyan, R.G.; Dzhagatspanyan, I.A. Synthesis and anticonvulsant activity of pyrazolo[3,4-b]pyrano(thiopyrano)[4,3-d]pyridine and pyrazolo[3,4-c]isoquinoline derivatives. Pharm. Chem. J. 2001, 35, 8–10. [Google Scholar] [CrossRef]
- Paronikyan, E.G.; Sirakanyan, S.N.; Noravyan, A.S. Synthesis ad some conversions of partially hydrogenated 1-aminopyrano(thiopyrano)[4,3-d]pyrazolo[3,4-b]pyridines and pyrazolo[3,4-c]isoquinolines. Chem. Heterocycl. Compd. 2003, 39, 374–378. [Google Scholar] [CrossRef]
- Al-Shuaeeb, R.A.A.; Alekseeva, A.Y.; Yashchenko, N.N.; Zhitar, S.V.; Mel’nik, E.A.; Bardasov, I.N. Synthesis and optical Properties of 3,4-diamino-6-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles. Russ. J. Org. Chem. 2022, 58, 997–1001. [Google Scholar] [CrossRef]
- Dabaeva, V.V.; Baghdasaryan, M.R.; Barkhudaryants, I.M.; Paronikyan, E.G.; Panosyan, H.A.; Dashyan, S.S. Synthesis of new fused derivatives of 4-(fur-2-yl)pyrano[4,3-b]pyridines. Russ. J. Gen. Chem. 2022, 92, 1686–1691. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Ghazaryan, S.G.; Hakobyan, E.K.; Hovakimyan, A.A. Synthesis of novel 1-pyrazolyl-2,7-naphthyridine derivatives. Russ. J. Org. Chem. 2020, 56, 840–844. [Google Scholar] [CrossRef]
- Lu, J.; Yin, A.; Tan, S.; Zhuge, R.; Liu, Y.; Zhang, P.; Liu, L.; Xuan, X.; Li, H.; Wang, W.; et al. Design, synthesis, and evaluation of pyrazolopyridine derivatives as novel calreticulin (CALR) ligands that inhibit triple-negative breast cancer (TNBC) via inducing calcium overloading. J. Med. Chem. 2025, 68, 11419–11436. [Google Scholar] [CrossRef]
- Cid, J.M.; Tresadern, G.; Vega, J.A.; de Lucas, A.I.; Matesanz, E.; Iturrino, L.; Linares, M.L.; Garcia, A.; Andrés, J.I.; Macdonald, G.J.; et al. Discovery of 3-cyclopropylmethyl-7-(4-phenylpiperidin-1-yl)-8-trifluoromethyl [1,2,4]triazolo[4,3-a]pyridine (JNJ-42153605): A positive allosteric modulator of the metabotropic glutamate 2 receptor. J. Med. Chem. 2012, 55, 8770–8789. [Google Scholar] [CrossRef]
- Andrés, J.I.; Alcázar, J.; Cid, J.M.; De Angelis, M.; Iturrino, L.; Langlois, X.; Lavreysen, H.; Trabanco, A.A.; Celen, S.; Bormans, G. Synthesis, Evaluation, and radiolabeling of new potent positive allosteric modulators of the metabotropic glutamate receptor 2 as potential tracers for positron emission tomography imaging. J. Med. Chem. 2012, 55, 8685–8699. [Google Scholar] [CrossRef]
- Sharanin, Y.A.; Promonenkov, V.K.; Shestopalov, A.M. Cyclization reactions of nitriles. V. 2-Aryl-3-(2-thenoyl)-1,1-dicyanopropanes and pyridine derivatives based on them. J. Org. Chem. USSR 1982, 18, 548–556. [Google Scholar]
- Shestopalov, A.M.; Promonenkov, V.K.; Sharanin, Y.A.; Rodinovskaya, L.A.; Sharanin, S.Y. Cyclization of nitriles. IX. Syntheses based on 2-aryl-3-aroyl-1,1-dicyanopropanes. J. Org. Chem. USSR 1984, 20, 1382–1401. [Google Scholar]
- Mishriky, N.; Asaad, F.M.; Girgis, A.S.; Ibrahim, Y.A. New pyridinecarbonitriles from fluoro arylpropenones. Recl. Trav. Chim. Pays-Bas 1994, 113, 35–39. [Google Scholar] [CrossRef]
- Victory, P.; Borrell, J.I.; Vidal-Ferran, A.; Seoane, C.; Soto, J.L. The reaction of malononitrile with chalcone: A controversial chemical process. Tetrahedron Lett. 1991, 32, 5375–5378. [Google Scholar] [CrossRef]
- Igushkina, A.V.; Golovanov, A.A.; Boyarskaya, I.A.; Kolesnikov, I.E.; Vasilyev, A.V. Stereoselective synthesis of multisubstituted cyclohexanes by reaction of conjugated enynones with malononitrile in the presence of LDA. Molecules 2020, 25, 5920. [Google Scholar] [CrossRef]
- Al-Arab, M.M.; Tabba, H.D.; Ghanem, B.S.; Olmstead, M.M. Michael-addition of malononitrile to 1,3-diaryl-2-propen-1-ones. Synthesis 1990, 1990, 1157–1159. [Google Scholar] [CrossRef]
- El-Sadany, S.K.; Sharaf, S.M.; Darwish, A.I.; Youssef, A.A. New aspects of Michael reaction: Reaction of 1,3-diarylpropenones with active cyano compounds. Ind. J. Chem. Sect. B 1991, 30, 567–573. [Google Scholar]
- Darwish, A.I. Reactions of vinylketones and β-chloroketones with malononitrile: A novel synthesis of cyclohexanol derivatives. Egypt. J. Chem. 2001, 44, 373–385. [Google Scholar]
- Laskar, D.D.; Prajapati, D.; Sandhu, J.S. A rapid one-pot synthesis of 2,4,6-triaryl-3-aroyl-4-hydroxy-1,1-cyclohexane- dicarbonitriles and 2-aminoisophthalo nitriles under microwave activation. Ind. J. Chem. Sect. B 2003, 42, 135–139. [Google Scholar]
- Sanin, A.V.; Nenaidenko, V.G.; Krasovskii, A.L.; Churakov, A.V.; Howard, J.A.K.; Balenkova, E.S. Synthesis of trifluoromethyl-containing carbo- and heterocycles based on reactions of α,β-unsaturated ketones with malonodinitrile and cyanoacetamides. Russ. J. Nondestruct. Test. 1997, 33, 205–212. [Google Scholar]
- Greiner-Bechert, L.; Sprang, T.; Otto, H.H. Reactions of heteroaryl substituted propenones. Monatsh. Chem. 2005, 136, 635–653. [Google Scholar] [CrossRef]
- Rong, L.C.; Li, X.Y.; Yang, F.; Wang, H.Y.; Shi, D.Q. 3-Benzoyl-4-hydroxy-2,4,6-triphenylcyclohexane-1,1-dicarbonitrile. Acta Crystallogr. E 2006, 62, o1766–o1767. [Google Scholar] [CrossRef]
- Wang, A.-Q.; Jin, T.-S.; Liu, L.-B.; Cheng, Z.-L.; Li, T.-S. A clean and efficient method for the synthesis of 3-aroyl-2,4,6-triaryl-4-hydroxy-1,1-cyclohexanedicarbonitriles in water. Asian J. Chem. 2010, 22, 1977–1981. [Google Scholar]
- Lu, G.; Cai, C. Synthesis of a series of highly substituted cyclohexanols via Michael addition in an aqueous medium. J. Chem. Res. 2011, 35, 147–150. [Google Scholar] [CrossRef]
- Gein, V.L.; Nosova, N.V.; Vagapov, A.V.; Gein, L.F. Synthesis of 2,6-diaryl-3-benzoyl-4-hydroxy-4-phenyl- 1,1-cyclohexanedinitriles. Russ. J. Org. Chem. 2011, 47, 1247–1248. [Google Scholar] [CrossRef]
- Diao, X.-J.; Ji, H.-L.; Yin, S.; Rong, L.-C.; Tu, S.-J. Synthesis of 1,3,5-triaryl-2-aroylcyclohexanol derivatives and crystal structure under solvent-free conditions. Chin. J. Org. Chem. 2011, 31, 1064–1068. [Google Scholar]
- Castro-Osma, J.A.; Comerford, J.W.; Heath, S.; Jones, O.; Morcillo, M.; North, M. Quinine catalysed asymmetric Michael additions in a sustainable solvent. RSC Adv. 2014, 5, 3678–3685. [Google Scholar] [CrossRef]
- Kuznetcova, A.V.; Odin, I.S.; Golovanov, A.A.; Grigorev, I.M.; Vasilyev, A.V. Multicomponent reaction of conjugated enynones with malononitrile and sodium alkoxides: Complex reaction mechanism of the formation of pyridine derivatives. Tetrahedron 2019, 75, 4516–4530. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, Q.; Lu, C.; Zhao, B. Enantioselective Michael addition of malononitrile to unsaturated ketones catalyzed by rare-earth metal amides RE[N(SiMe3)2]3 with phenoxy-functionalized TsDPEN ligands. J. Org. Chem. 2023, 88, 13205–13213. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhou, R.; Liu, B.; Wang, R.; Kong, X.; He, S.; Wang, J.; Fan, L.; Fu, X.; Tang, L. Unanticipated three-component cascade and sequential cyclization: Highly diastereoselective synthesis of polyfunctionalized cyclohexanes. J. Org. Chem. 2025, 90, 5349–5358. [Google Scholar] [CrossRef] [PubMed]
- Benko, Z.L.; Crouse, G.D.; Erickson, W.R.; Gifford, J.M.; Gustafson, G.D.; Orr, N.; Watson, G.B. Pesticidal Compositions. U.S. Patent 20090093500, 9 April 2009. [Google Scholar]
- Jeschke, P. The unique role of halogen substituents in the design of modern agrochemicals. Pest. Manag. Sci. 2010, 66, 10–27. [Google Scholar] [CrossRef]
- Jeschke, P. Latest generation of halogen-containing pesticides. Pest. Manag. Sci. 2017, 73, 1053–1066. [Google Scholar] [CrossRef]
- Jeschke, P. The unique role of halogen substituents in the design of modern crop protection compounds (Chapter 4). In Modern Methods in Crop Protection Research; Jeschke, P., Krämer, W., Schirmer, U., Witschel, M., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 73–128. [Google Scholar]
- Jeschke, P. Manufacturing approaches of new Halogenated Agrochemicals. Eur. J. Org. Chem. 2022, 2022, e202101513. [Google Scholar] [CrossRef]
- Naumann, K. Influence of chlorine substituents on biological activity of chemicals. J. Prakt. Chem. 1999, 341, 417–435. [Google Scholar] [CrossRef]
- Geilfus, C.M. Review on the significance of chlorine for crop yield and quality. Plant Sci. 2018, 270, 114–122. [Google Scholar] [CrossRef]
- Jeschke, P. New active ingredients for sustainable modern chemical crop protection in agriculture. ChemSusChem 2025, 18, e202401042. [Google Scholar] [CrossRef]
- Parisotto, S.; Azzi, E.; Lanfranco, A.; Renzi, P.; Deagostino, A. Recent progresses in the preparation of chlorinated molecules: Electrocatalysis and photoredox catalysis in the spotlight. Reactions 2022, 3, 233–253. [Google Scholar] [CrossRef]
- Zakharychev, V.V.; Kuzenkov, A.V.; Martsynkevich, A.M. Good pyridine hunting: A biomimic compound, a modifier and a unique pharmacophore in agrochemicals. Chem. Heterocycl. Compd. 2020, 56, 1491–1516. [Google Scholar] [CrossRef]
- Guan, A.Y.; Liu, C.L.; Sun, X.F.; Xie, Y.; Wang, M.A. Discovery of pyridine-based agrochemicals by using intermediate derivatization methods. Bioorg. Med. Chem. 2016, 24, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Zakharychev, V.V.; Martsynkevich, A.M. Development of novel pyridine-based agrochemicals: A review. Adv. Agrochem. 2025, 4, 30–48. [Google Scholar] [CrossRef]
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D past, present, and future: A review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Chkanikov, N.D.; Spiridonov, Y.Y.; Khalikov, S.S.; Muzafarova, A.M. Antidotes for reduction of phytotoxicity of the residues of sulfonylurea herbicides. INEOS OPEN 2019, 2, 145–152. [Google Scholar] [CrossRef]
- Deng, X. Current advances in the action mechanisms of safeners. Agronomy 2022, 12, 2824. [Google Scholar] [CrossRef]
- Jia, L.; Jin, X.Y.; Zhao, L.X.; Fu, Y.; Ye, F. Research progress in the design and synthesis of herbicide safeners: A review. J. Agric. Food Chem. 2022, 70, 5499–5515. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.Y.; Zhao, L.X.; Gao, S.; Ye, F.; Fu, Y. Review on the discovery of novel natural herbicide safeners. J. Agric. Food Chem. 2023, 71, 11320–11331. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, F.; Fu, Y. Herbicide safeners: From molecular structure design to safener activity. J. Agric. Food Chem. 2024, 72, 2451–2466. [Google Scholar] [CrossRef]
- Dahno, P.G.; Zhilyaev, D.M.; Dotsenko, V.V.; Strelkov, V.D.; Krapivin, G.D.; Aksenov, N.A.; Aksenova, I.V.; Likhovid, N.G. Oxidation of 2-cyanothioacrylamides with sodium nitrite in acidic medium. Russ. J. Gen. Chem. 2022, 92, 1667–1676. [Google Scholar] [CrossRef]
- Krivokolysko, B.S.; Dotsenko, V.V.; Pakholka, N.A.; Dakhno, P.G.; Strelkov, V.D.; Aksenov, N.A.; Aksenova, I.V.; Krivokolysko, S.G. Bromine- and iodine-mediated oxidative dimerization of cyanothioacetamide derivatives: Synthesis of new functionalized 1,2,4-thiadiazoles. J. Iran. Chem. Soc. 2023, 20, 609–628. [Google Scholar] [CrossRef]
- Stepanova, S.F.; Semenova, A.M.; Dotsenko, V.V.; Strelkov, V.D.; Temerdashev, A.Z.; Gasyuk, O.A.; Volchenko, N.N.; Aksenov, N.A.; Aksenova, I.V. 7-(2-Aryl-1-cyanovinyl)-1,2,3,4-tetrahydropyrazolo[1,5-a][1,3,5]triazine-8-carbonitriles: Synthesis and biological Activity. Russ. J. Gen. Chem. 2023, 93, 1360–1373. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Bespalov, A.V.; Sinotsko, A.E.; Temerdashev, A.Z.; Vasilin, V.K.; Varzieva, E.A.; Strelkov, V.D.; Aksenov, N.A.; Aksenova, I.V. 6-Amino-4-aryl-7-phenyl-3-(phenylimino)-4,7-dihydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxamides: Synthesis, biological activity, quantum chemical studies and in silico docking studies. Int. J. Mol. Sci. 2024, 25, 769. [Google Scholar] [CrossRef]
- Shestopalov, A.M.; Rodinovskaya, L.A.; Fedorov, A.E.; Kalugin, V.E.; Nikishin, K.G.; Shestopalov, A.A.; Gakh, A.A. Synthesis of 3-cyano-2-fluoropyridines. J. Fluor. Chem. 2009, 130, 236–240. [Google Scholar] [CrossRef]
- Bou-Petit, E.; Hümmer, S.; Alarcon, H.; Slobodnyuk, K.; Cano-Galietero, M.; Fuentes, P.; Guijarro, P.J.; Muñoz, M.J.; Suarez-Cabrera, L.; Santamaria, A.; et al. Overcoming paradoxical Kinase priming by a novel MNK1 Inhibitor. J. Med. Chem. 2022, 65, 6070–6087. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
| N | Pyridine | Plant Organ | Antidote Effect (A) at Different Concentrations, % 1 | |||
|---|---|---|---|---|---|---|
| 10−2 | 10−3 | 10−4 | 10−5 | |||
| 1 | 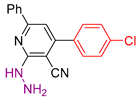 20a | roots | 141 | 134 | 178 | 186 |
| hypocotyls | 135 | 134 | 146 | 130 | ||
| 2 | 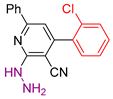 20b | roots | 132 | 113 | 116 | 136 |
| hypocotyls | 140 | 97 | 93 | 96 | ||
| 3 | 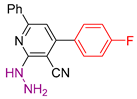 20f | roots | 130 | 93 | 140 | 134 |
| hypocotyls | 111 | 73 | 102 | 98 | ||
| 4 | 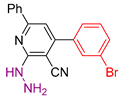 20i | roots | 170 | 130 | 180 | 138 |
| hypocotyls | 104 | 117 | 92 | 88 | ||
| 5 | 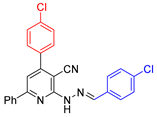 21{1} | roots | 154 | 154 | 150 | 184 |
| hypocotyls | 123 | 112 | 101 | 132 | ||
| 6 | 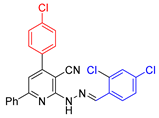 21{2} | roots | 163 | 173 | 167 | 152 |
| hypocotyls | 156 | 119 | 119 | 122 | ||
| 7 | 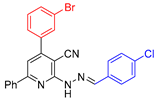 21{17} | roots | 186 | 171 | 168 | 161 |
| hypocotyls | 148 | 152 | 138 | 145 | ||
| 8 | 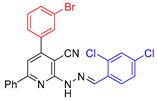 21{18} | roots | 188 | 197 | 180 | 159 |
| hypocotyls | 146 | 152 | 127 | 137 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsenko, V.V.; Kindop, V.K.; Kindop, V.K.; Achmiz, R.G.; Levchenko, A.G.; Dakhno, P.G.; Temerdashev, A.Z.; Feng, Y.-Q.; Zhu, Q.-F.; Daus, E.S.; et al. Synthesis, Reactions, and Agrochemical Studies of New 4,6-Diaryl-2-hydrazinylnicotinonitriles. Int. J. Mol. Sci. 2025, 26, 11874. https://doi.org/10.3390/ijms262411874
Dotsenko VV, Kindop VK, Kindop VK, Achmiz RG, Levchenko AG, Dakhno PG, Temerdashev AZ, Feng Y-Q, Zhu Q-F, Daus ES, et al. Synthesis, Reactions, and Agrochemical Studies of New 4,6-Diaryl-2-hydrazinylnicotinonitriles. International Journal of Molecular Sciences. 2025; 26(24):11874. https://doi.org/10.3390/ijms262411874
Chicago/Turabian StyleDotsenko, Victor V., Vladislav K. Kindop, Vyacheslav K. Kindop, Renat G. Achmiz, Arina G. Levchenko, Polina G. Dakhno, Azamat Z. Temerdashev, Yu-Qi Feng, Quan-Fei Zhu, Eva S. Daus, and et al. 2025. "Synthesis, Reactions, and Agrochemical Studies of New 4,6-Diaryl-2-hydrazinylnicotinonitriles" International Journal of Molecular Sciences 26, no. 24: 11874. https://doi.org/10.3390/ijms262411874
APA StyleDotsenko, V. V., Kindop, V. K., Kindop, V. K., Achmiz, R. G., Levchenko, A. G., Dakhno, P. G., Temerdashev, A. Z., Feng, Y.-Q., Zhu, Q.-F., Daus, E. S., Yudaev, I. V., Daus, Y. V., Aksenov, A. V., Aksenov, N. A., & Aksenova, I. V. (2025). Synthesis, Reactions, and Agrochemical Studies of New 4,6-Diaryl-2-hydrazinylnicotinonitriles. International Journal of Molecular Sciences, 26(24), 11874. https://doi.org/10.3390/ijms262411874







