Abstract
Viral myocarditis (VMC) is the predominant type of myocarditis and currently lacks specific therapies. Salvia miltiorrhiza (Danshen) injection has demonstrated beneficial effects as a supplementary VMC treatment, yet its pharmacological mechanisms are ambiguous, and its efficacy lacks robust evidence. This study aims to preliminarily address these issues through computational approaches and meta-analysis. Using network pharmacology, we identified 257 therapeutic targets, 106 hub genes, and 4 key S. miltiorrhiza ingredients implicated in VMC treatment. Integrating transcriptome data with LASSO and SVM machine learning algorithm yielded six core therapeutic targets from the hub genes—TNF, JUN, PECAM1, KDR, TIMP1, and EPAS1—which are primarily associated with anti-inflammatory activity, vascular remodeling, and fibrosis suppression. GO analysis identified the “inflammatory response” as the most prominent biological process. Concurrently, the PI3K-Akt, TNF, and HIF-1 signaling pathways—each closely associated with inflammation—appeared among the top 20 KEGG pathways. Overall, these results indicate that suppressing excessive inflammation is likely the primary pharmacological mechanism. In molecular docking, four key ingredients—dan-shexinkum D, danshenol A, cryptotanshinone, and methylrosmarinate—exhibited strong binding to the core therapeutic targets, with dan-shexinkum D showing the lowest total binding energy and stable binding confirmed by molecular dynamics simulations. The meta-analysis indicates that S. miltiorrhiza injection improves clinical outcomes and significantly reduces TNF-α, hs-CRP, CK-MB, cTnT, and H-FABP levels. This study used multiple computational approaches to explore the pharmacological mechanisms and identify key active components of S. miltiorrhiza in treating VMC, thereby establishing an evidence-based foundation and providing preliminary groundwork for subsequent clinical application and translational research.
1. Introduction
Myocarditis, characterized by localized or diffuse inflammatory lesions in the myocardium of diverse etiology, is reported to have an approximate 4% all-cause mortality rate among hospitalized acute cases in the UK [1,2]. A retrospective analysis spanning 16 years and involving 684 myocarditis patients across 19 hospitals revealed that about 25% of these patients experienced symptoms like left ventricular systolic dysfunction, ventricular arrhythmias, or acute heart failure [3]. Mammalian cardiomyocytes are essentially nonrenewable after death, so the cell loss caused by myocarditis can precipitate acute heart failure, sudden cardiac death, or chronic dilated cardiomyopathy, posing a major threat to human health [4,5]. Viral infections often result in the abnormal death of cardiomyocytes and are the leading cause of myocarditis, referred to as viral myocarditis (VMC), which has an estimated incidence of 10 to 22 cases per 100,000 individuals [6,7,8]. A variety of viruses can induce VMC, including coxsackievirus, echovirus, adenovirus, human immunodeficiency virus, influenza virus, coronavirus, parvovirus B19, and human herpesvirus 6 [9,10]. Because no specific therapies exist for VMC, clinical management relies on combined symptomatic treatments after assessing acute severity and clinical presentation, so novel therapeutic strategies urgently require investigation and synthesis [11,12].
Traditional Chinese medicine (TCM), notably Chinese medicine injections (CHIs) derived from herbal sources, is widely used as an adjuvant in combination regimens for VMC, and Salvia miltiorrhiza (Danshen) injection is considered one of the most effective CHIs [13,14]. Previous studies indicate that S. miltiorrhiza and its constituents exert diverse pharmacological effects, including modulation of immune function, protection against oxidative DNA damage, regulation of inflammatory responses, antibacterial activity and inhibition of viral proliferation [15,16,17]. Despite the widespread clinical use of S. miltiorrhiza and its injection as an adjunct therapy, its pharmacological mechanisms and key bioactive components in VMC remain insufficiently characterized, and no rigorous systematic review or meta-analysis evaluating efficacy has yet validated its efficacy. These gaps limit the translational application of S. miltiorrhiza and the clinical use of its injection for VMC. The exploration of pharmacological mechanisms and the identification of key components can further inform drug development. For example, a mixed-ingredient injection derived from S. miltiorrhiza (Salvianolic acid injection) has been launched in China and is now widely used clinically to treat cerebral infarction, coronary heart disease, and related conditions, where it has demonstrated favorable efficacy [18,19]. Therefore, this study aims to preliminarily elucidate the molecular mechanisms—identifying core therapeutic targets, principal pharmacological effects, and key active ingredients—by applying computational methods, including network pharmacology, machine learning, in silico docking, and molecular dynamics simulation, to determine the core elements of the treatment process. Meanwhile, relevant literature will be retrieved to establish a meta-analysis on the efficacy of S. miltiorrhiza injection, aiming to provide evidence to guide its adjunctive use in VMC.
2. Results
2.1. Network Pharmacology Analysis of Salvia miltiorrhiza Against Viral Myocarditis
A total of 202 ingredients of S. miltiorrhiza and 1179 targets influenced by it were identified using TCMSP and Swiss Target Prediction. Additionally, 1322 targets linked to VMC were obtained from GeneCards and OMIM. By intersecting the drug and disease targets, 257 therapeutic targets were identified (Figure 1A). The protein–protein interactions (PPI) of these therapeutic targets were analyzed using STRING, where different colored ellipses represent individual targets, and the connecting lines indicate interactions (Figure 1B). Using Cytoscape 3.10.3, 106 hub genes were identified based on three centrality metrics—Degree, Betweenness, and Closeness—retaining those ranked in the top 50% for each metric, with 3615 interactions found among these hub genes (Figure 1C). To elucidate the relationship between S. miltiorrhiza and VMC, we constructed a “Salvia miltiorrhiza–ingredients–therapeutic genes–VMC” network that systematically depicts how the herb components modulate therapeutic genes associated with VMC (Figure 1D). Ranking ingredients by the number of therapeutic genes they affect in the network revealed ten hub components: apigenin (MOL000008), luteolin (MOL000006), ursolic acid (MOL000511), oleic acid (MOL000675), 2-(4-hydroxy-3-methoxyphenyl)-5-(3-hydroxypropyl)-7-methoxy-3-benzofurancarboxaldehyde (MOL007050), dan-shexinkum D (MOL007093), danshenol A (MOL007082), 2-isopropyl-8-methylphenanthrene-3,4-dione (MOL007041), cryptotanshinone (MOL007088), and methylrosmarinate (MOL007116). Dan-shexinkum D, danshenol A, cryptotanshinone, and methylrosmarinate are identified as key ingredients due to their specificity to S. miltiorrhiza or their relatively high concentrations [20,21].
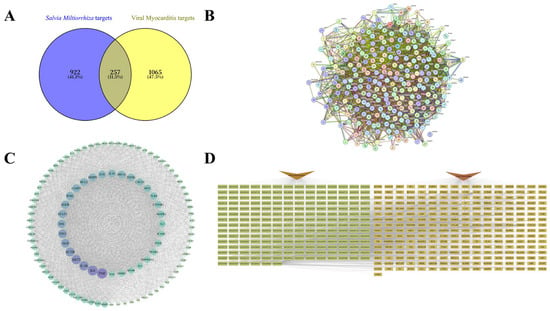
Figure 1.
Network pharmacology analysis of Salvia miltiorrhiza for viral myocarditis. (A) Therapeutic targets were determined by the intersection of targets affected by S. miltiorrhiza and those associated with VMC. (B) Protein–protein Interaction (PPI) network of the therapeutic targets. (C) Network of hub genes identified using Degree, Betweenness, and Closeness metrics in Cytoscape 3.10.3. (D) “Salvia miltiorrhiza–ingredients–therapeutic genes–VMC” network.
2.2. GO and KEGG Analysis of Therapeutic Targets
Gene Ontology (GO) enrichment and KEGG pathway analyses of therapeutic targets were performed using DAVID. Only data with p-value < 0.01 and FDR < 0.01 were retained. According to the p-values ranked from small to large, GO analysis showed that the biological processes through which S. miltiorrhiza acts against VMC mainly involve inflammatory response, negative regulation of apoptotic process, and positive regulation of cell population proliferation. The relevant cellular components include extracellular space, external side of plasma membrane, and plasma membrane. The principal molecular functions comprise histone H3Y41 kinase activity, histone H2AXY 142 kinase activity, and identical protein binding (Figure 2A). KEGG enrichment highlighted Pathways in cancer, Lipid and atherosclerosis, and Kaposi sarcoma-associated herpesvirus infection, ranked by the number of enriched genes (Figure 2B).
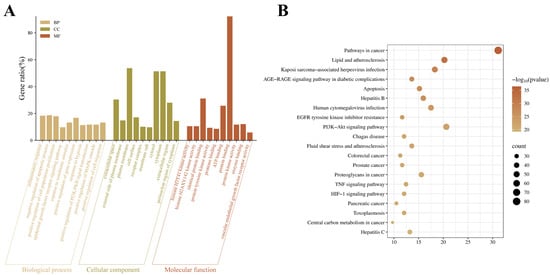
Figure 2.
GO enrichment and KEGG pathway analysis of therapeutic genes. (A) Top 10 enriched terms for biological process (BP), cellular component (CC), and molecular function (MF) among therapeutic targets. (B) Top 20 KEGG pathway entries from the analysis.
2.3. Machine Learning-Guided Identification of Core Therapeutic Targets
Transcriptomic data for VMC (GSE183850) were retrieved from GEO, and differentially expressed genes were identified using thresholds of fold change >2 or <0.5 and p-value < 0.05. Intersecting these genes with the hub genes from Section 2.1 yielded 26 candidate genes. To identify core therapeutic targets among these 26 genes, we applied Least Absolute Shrinkage and Selection Operator (LASSO) regression and support vector machine (SVM) algorithms. LASSO selected 7 core targets; in the coefficient plot, different colored lines represent individual genes, and the “Coefficients” indicate each gene’s contribution to the effect of S. miltiorrhiza in VMC (Figure 3A). The upper abscissa displays the number of model genes for varying λ values, with the optimal λ corresponding to a model of 7 genes (Figure 3B). The SVM approach identified 9 core targets (Figure 3C). The intersection of LASSO and SVM results produced 6 core therapeutic targets: TNF, JUN, PECAM1, KDR, TIMP1, and EPAS1 (Figure 3D).
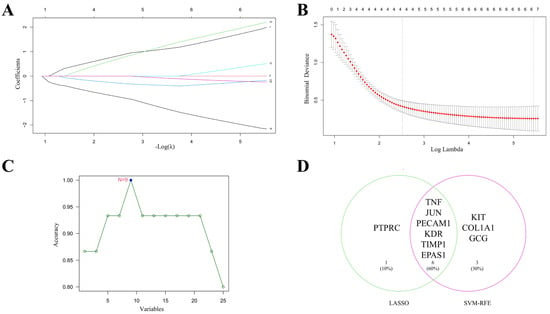
Figure 3.
Machine learning identification of core therapeutic targets. (A,B) Results of Least Absolute Shrinkage and Selection Operator (LASSO) regression. The upper abscissa denotes the number of model genes at different λ values, and the optimal λ corresponds to 7 genes. (C) Support Vector Machine (SVM) results, which reached maximum accuracy with 9 genes. (D) Six genes were identified as core therapeutic targets of S. miltiorrhiza for VMC by intersecting the LASSO and SVM results.
2.4. In Silico Docking of Key Ingredients to Core Therapeutic Targets
Ten hub components of S. miltiorrhiza, as identified in Section 2.1, were docked to six core therapeutic targets: TNF (PDB ID: 5UUI; x center = 45.30, y center = 52.93, z center = 13.77), JUN (PDB ID: 5T01; x center = −16.56, y center = 23.05, z center = 26.23), PECAM1 (PDB ID: 5C14; x center = 20.01, y center = 32.69, z center = −0.87), KDR (PDB ID: 1YWN; x center = 5.03, y center = 38.56, z center = 23.56), TIMP1 (PDB ID: 1UEA; x center = 60.04, y center = 61.00, z center = 38.77), and EPAS1 (PDB ID: 3F1O; x center = 11.33, y center = −52.20, z center = 16.07), utilizing AutoDock Vina 1.1.2. A ligand–receptor pair was considered to have a stable binding state when the binding energy was less than −5 kcal/mol. Except for oleic acid (MOL000675), all nine remaining ingredients showed stable binding to the core therapeutic targets, and dan-shexinkum D (MOL007093) had the lowest total binding energy among them (Figure 4A). Docking poses of dan-shexinkum D with the receptor proteins were visualized in PyMOL 3.0 (Figure 4B).
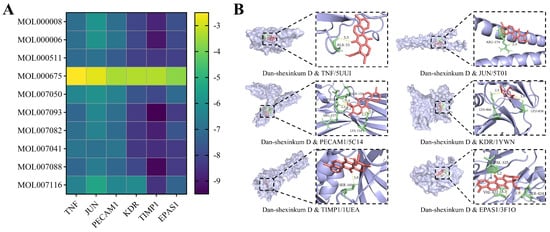
Figure 4.
In silico docking poses and binding interactions of key ingredients with core therapeutic targets. (A) Heatmap of in silico docking between ten hub components of S. miltiorrhiza and core therapeutic targets, showing that dan-shexinkum D (MOL007093) had the lowest total binding energy. (B) Docking poses of dan-shexinkum D with six core therapeutic targets.
2.5. Molecular Dynamics Simulation of Dan-Shexinkum D and Core Therapeutic Targets
To further evaluate the binding affinity of key S. miltiorrhiza components for core therapeutic targets, molecular dynamics simulations of dan-shexinkum D bound to each of six core therapeutic targets were conducted in YASARA 10.3.16. Complex stability was assessed by root-mean-square deviation (RMSD) and binding energy, and root-mean-square fluctuation (RMSF) was used to identify residues that acquired high flexibility upon binding. RMSD measures the deviation in the protein–ligand complex from a reference structure (for example, the initial crystal structure) during the simulation and thus serves as the primary indicator of equilibration and structural stability [22]. The RMSD profiles indicate that the dan-shexinkum D–target complexes remain relatively stable, with the dan-shexinkum D–TNF and dan-shexinkum D–EPAS1 complexes showing smaller RMSD fluctuations, which reflects a more stable interaction state for these two complexes (Figure 5A). System energy stability is another key criterion for assessing binding persistence, since large fluctuations can indicate potential conformational change. The relatively stable binding of dan-shexinkum D to the six target proteins are reflected by the low fluctuations in the complexes’ binding energy (Figure 5B). RMSF quantifies the amplitude of motion of each residue or atom about its mean position, with higher RMSF indicating greater local flexibility that is often associated with activity or binding [23]. Except for the N- and C-termini, residues at the dan-shexinkum D binding sites showed relatively low RMSF, and all residues corresponding to pronounced peaks are labeled (Figure 5C).
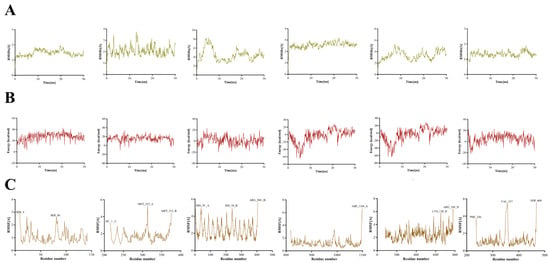
Figure 5.
Molecular dynamics simulation of dan-shexinkum D with six core therapeutic targets. (A) Displays RMSD results for dan-shexinkum D interacting with TNF, JUN, PECAM1, KDR, TIMP1 and EPAS1. (B) Shows Bind Energy outcomes for dan-shexinkum D and TNF, JUN, PECAM1, KDR, TIMP1 and EPAS1. (C) Illustrates RMSF data for dan-shexinkum D TNF, JUN, PECAM1, KDR, TIMP1 and EPAS1.
2.6. Meta-Analysis of Salvia miltiorrhiza Injection for Viral Myocarditis
A total of 467 publications were retrieved from PubMed, Web of Science, Cochrane Library, CNKI, Wanfang, and VIP using the primary search terms “Salvia miltiorrhiza”, “Danshen”, and “Viral Myocarditis”. After screening and eligibility assessment, 15 studies were included in this meta-analysis [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] (Figure 6 and Table 1).
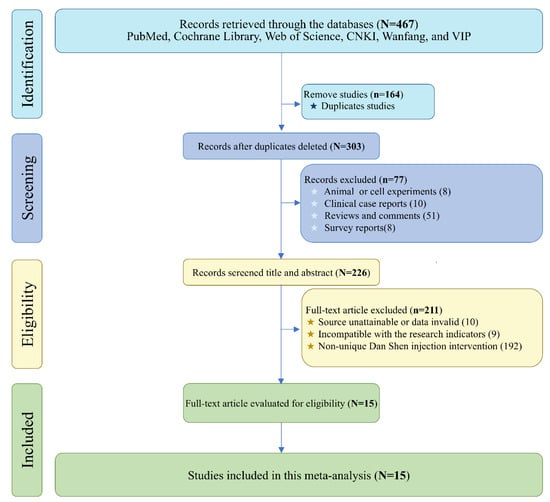
Figure 6.
The relevant literature of Salvia miltiorrhiza Injection against viral myocarditis search flow chart.

Table 1.
Characteristics of included studies.
Fourteen studies documented the effectiveness of S. miltiorrhiza injection for treating VMC, and a meta-analysis of these studies demonstrated its efficacy [OR = 3.68, 95%CI (2.68~5.04)] (Figure 7A). Treatment effects were also reported as changes in VMC clinical indicators in some of the 15 included studies during therapy with S. miltiorrhiza injection. The meta-analysis found that the injection significantly reduced TNF-α [SMD = −3.88, 95%CI (−5.79~−1.97)] (Figure 7B), hypersensitive C-reactive protein [SMD = −4.89, 95%CI (−5.45~−4.33)] (Figure 7C), CK-MB [SMD = −1.78, 95%CI (−3.13~−0.44)] (Figure 7D), cardiac troponin I [SMD = −4.08, 95%CI (−5.36~−2.79)] (Figure 7E), and heart-type fatty acid-binding protein [SMD = −0.77, 95%CI (−1.07~−0.46)] (Figure 7F).

Figure 7.
Meta-analysis results of Salvia miltiorrhiza Injection for viral myocarditis. (A) Forest plot depicting the treatment effectiveness of Salvia miltiorrhiza injection for VMC. (B) Impact on the TNF-α index during the treatment process with S. miltiorrhiza for VMC. (C) Effectiveness of the injection on hypersensitive C-reactive protein (hs-CRP) in VMC. (D) Changes in the CK-MB index. (E) Impact of Cardiac troponin I (cTnT) index. (F) Effect on Heat-fatty acid-binding protein (H-FABP) [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
Risk-of-bias assessment, sensitivity analysis, and publication-bias evaluation were performed to assess the overall quality of the included studies [39]. The risk-of-bias assessment indicates an overall unclear risk, particularly for random sequence generation and blinding (Figure 8A). Sensitivity analysis shows that effect estimates for all efficacy outcomes lie within the 95% CI, indicating consistency across studies and that the pooled results are still robust to exclusion of any single study (Figure 8B). Egger’s test yielded p = 0.363, and the funnel plot shows all points within the 95% confidence interval and roughly symmetric around the central line, indicating minimal publication bias (Figure 8C,D).
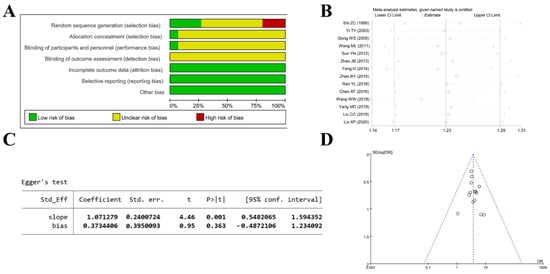
Figure 8.
Quality assessment of included studies. (A) Risk-of-bias evaluation of the included studies using the seven Cochrane domains. (B) Sensitivity analysis of the included studies. (C) Assessment of publication bias using Egger’s test. (D) Funnel plot illustrating publication bias in the included studies [24,25,26,27,28,29,31,32,33,34,35,36,37,38].
3. Discussion
VMC is an inflammatory, nonischemic injury of the myocardium triggered by viral infection, with a lack of pathogenesis-targeted therapies contributing to persistently poor clinical outcomes [40]. Current clinical care relies on multimodal supportive strategies, and S. miltiorrhiza injection is used as an adjunct with reported benefit [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. However, its molecular pharmacology remains unclear, and no systematic review or meta-analysis has yet to be validated. To address this evidence gap, we applied multiple computational approaches to delineate likely molecular mechanisms and conducted a comprehensive meta-analysis to evaluate clinical effectiveness. Our computational mechanistic investigation and evidence-based synthesis provide a clinical and translational rationale for using S. miltiorrhiza and its injection to treat VMC.
Excessive inflammation, triggered by various factors beyond direct viral cytopathic effects, plays a crucial role in myocardial injury in VMC, leading to rapid clinical deterioration [41,42]. Our study provides both computational and clinical evidence that highlights the role of S. miltiorrhiza in reducing hyper-inflammatory responses, which is a key component of its therapeutic potential. We conducted function enrichment analyses (GO and KEGG) on identified 257 therapeutic targets through network pharmacology. The GO analysis identified the “inflammatory response” as the primary biological process. Concurrently, the PI3K-Akt, TNF, and HIF-1 signaling pathways, which are closely linked to the inflammatory response, were among the top 20 pathways in the KEGG results [43,44]. Through multi-computational approaches and transcriptomic data, we identified six core targets (TNF, JUN, PECAM1, KDR (VEGFR2), TIMP1, and EPAS1 (HIF-2α)) closely associated with inflammation, vascular remodeling, and fibrosis suppression [45,46,47,48,49]. This suggests that S. miltiorrhiza likely coordinates an anti-inflammatory (TNF/JUN/PECAM1)—pro-resolving angiogenic (KDR/EPAS1)—anti-fibrotic (TIMP1) axis in VMC treatment. Furthermore, our meta-analysis reinforces the efficacy of the herb in attenuating inflammation in VMC, showing that adjunctive S. miltiorrhiza injection significantly lowers circulating levels of TNF-α and hypersensitive C-reactive protein (hs-CRP) in VMC patients. While the suppression of inflammatory cascades by S. miltiorrhiza has been reported in other conditions [50], our study is the first to elucidate its significance and potential molecular mechanisms in VMC, supported in part by clinical evidence.
In molecular docking, all four key ingredients (dan-shexinkum D, danshenol A, cryptotanshinone, and methylrosmarinate) demonstrated strong binding to the core therapeutic target. Among them, dan-shexinkum D exhibited the lowest total binding energy, and its stable binding was confirmed through molecular dynamics simulations. There are successful precedents for purifying S. miltiorrhiza components and developing them into drugs. Magnesium Lithospermate B, produced by complexing salvianolic acid B with magnesium ions, has been approved in China and is widely used to treat cardiovascular conditions such as coronary heart disease and angina pectoris [51]. Among the four key ingredients, cryptotanshinone demonstrates pharmacological effects in cardiovascular, cancer, and nervous system diseases, while danshenol A has been shown to inhibit inflammation and alleviate hypertension-induced cardiac remodeling [52,53,54]. However, research on dan-shexinkum D and methylrosmarinate remains limited. Our study offers insights into the pharmacological potential of these four components and advocates for further research into their mechanisms and structural modifications to advance their development as novel drugs for VMC and related diseases.
Current clinical practice for treating VMC with S. miltiorrhiza relies mainly on injections prepared from the single herb and on compound CHIs that include it as adjunct [55,56]. Despite multiple randomized controlled trials assessing the efficacy of S. miltiorrhiza injection for VMC, no dedicated systematic review or meta-analysis has synthesized these findings, which has limited its clinical adoption. Consequently, we performed meta-analysis revealing that this injection is efficacious in VMC treatment, leading to notable decreases in myocarditis-associated clinical indicators such as CK-MB, cTnT, and H-FABP [57,58]. However, the risk-of-bias assessment for the included studies was largely unclear, particularly for random sequence generation and blinding, which undermines the overall study quality. Future research should therefore prioritize rigorous randomized, controlled, double-blind clinical trials to strengthen the evidence base for this injection in VMC treatment.
Our study investigated potential mechanisms by which S. miltiorrhiza might combat VMC using various computational methods. However, these in silico predictions lack experimental validation and cannot replace such empirical evidence. Thus, the results should be considered preliminary, serving as a reference for identifying mechanisms and key components of the herb related to VMC, and guiding further translational and clinical research. Experimental validation is necessary, such as assessing mRNA or protein levels of key targets in in vivo or in vitro models treated with and without S. miltiorrhiza. Interactions among the core therapeutic targets TNF, JUN, PECAM1, KDR, TIMP1, and EPAS1, and among the PI3K–Akt, TNF, and HIF–1 signaling pathways, could be investigated by establishing VMC models with cardiotropic viruses such as CVB3 and then treating them with S. miltiorrhiza or its injection [59]. Similarly, further exploration of the pharmacological effects of key components is necessary. For instance, compounds such as dan-shexinkum D should be used in cell or animal models of VMC to examine their pharmacological potential and underlying mechanisms.
In conclusion, this study identified the key constituents and potential pharmacological mechanisms of S. miltiorrhiza against VMC using multiple computational approaches, established an evidence-based rationale for its injection, and provided a preliminary foundation for future clinical translation and application.
4. Materials and Methods
4.1. Acquisition of Drug, Disease and Therapeutic Targets Data
The components of S. miltiorrhiza and their corresponding targets were identified using TCMSP (https://go.drugbank.com/, accessed on 31 October 2025) with the search term “Danshen”, the Chinese name of Salvia miltiorrhiza. After downloading the SDF format of these ingredients from TCMSP, they were uploaded to Swiss Target Prediction (https://go.drugbank.com/, accessed on 31 October 2025) to determine the targets recorded in this database. The targets obtained from TCMSP were converted to standard gene names using Uniprot IDs from the Uniprot database (https://www.uniprot.org/, accessed on 31 October 2025). Targets related to VMC were sourced from GeneCards (https://www.genecards.org/, accessed on 31 October 2025) and Online Mendelian Inheritance in Man (https://www.omim.org/, accessed on 31 October 2025). The therapeutic targets of S. miltiorrhiza for VMC were determined by intersecting the drug and disease targets using Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/, accessed on 31 October 2025).
4.2. PPI Network Construction and Hub Targets Validation
Using STRING (https://cn.string-db.org/, accessed on 31 October 2025), we generated a protein–protein interaction (PPI) network by uploading the therapeutic targets identified in Section 4.1. From the PPI dataset, we selected hub targets using three centrality metrics—Degree, Betweenness, and Closeness—and retained genes ranked in the top 50% for each metric using Cytoscape 3.10.3 (Institute for Systems Biology, Seattle, WA, USA) [60]. The 50% threshold was selected to ensure a substantial pool of candidates for reliable machine-learning modeling without excessively strict filtering that could eliminate valuable features. To visualize potential links between S. miltiorrhiza and VMC, we then built a “Drug–Ingredients–Therapeutic genes–Disease” network in Cytoscape 3.10.3. Hub components were defined by the number of therapeutic genes they affected within the network. Key ingredients were then selected from those hub components based on their specificity or high content in S. miltiorrhiza, as reported in the previous literature.
4.3. Functional Enrichment Analysis of Therapeutic Genes
Functional enrichment analysis (GO and KEGG) was performed on the therapeutic targets dataset using the Database for Annotation, Visualization, and Integrated Discovery (DAVID; https://davidbioinformatics.nih.gov/, accessed on 31 October 2025). GO analysis encompassed biological process (BP), cellular component (CC), and molecular function (MF). The top 10 entries from each GO category and the top 20 KEGG pathways were exported for visualization with Bioinformatics (https://www.bioinformatics.com.cn/, accessed on 31 October 2025).
4.4. Machine Learning for Identification of Core Therapeutic Targets
Transcriptomic data for VMC were obtained from GEO (https://www.ncbi.nlm.nih.gov/geo/, accessed on 31 October 2025). Significant differential genes were defined as those with fold change >2 or <0.5 and p-value < 0.05. Reliable therapeutic targets were identified as the intersection of hub genes and these significant differential genes. From these targets, two machine-learning algorithms—Least Absolute Shrinkage and Selection Operator (LASSO) regression and Support Vector Machine (SVM)—were applied to identify core therapeutic targets. LASSO regression was run with the R 4.4.3 package glmnet using standardize = TRUE, alpha = 1, family = “binomial”, and ten-fold cross-validation was used and that the final penalty corresponds to the “lambda.min” criterion. SVM was implemented with the linear kernel (svmLinear), the default cost parameter exactly as implemented by caret’s svmLinear method (i.e., no user tuning), Z-score standardization (centering and scaling via preProcess) performed before training, and the stopping rule defined by sizes = c(seq(1, 23, by = 2)).
4.5. In Silico Docking of Key Components to Core Therapeutic Targets
Hub components of S. miltiorrhiza were identified by the “Drug–Ingredients–Therapeutic targets–Disease” network in Section 4.2 according to the number of influenced targets (quasi “Degree”). SDF files of the hub components were retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov, accessed on 31 October 2025), energy-minimized to their lowest-energy conformers with Chem3D 22.0.0 (CambridgeSoft, Cambridge, MA, USA), and exported in MOL2 format. PDB files of the core therapeutic targets were downloaded from RCSB (https://www.rcsb.org/, accessed on 31 October 2025) and had water molecules and organic ligands removed using PyMOL 3.0 (Schrödinger, South San Francisco, CA, USA). In silico docking between the hub components and core therapeutic targets was performed with AutoDock Vina 1.1.2 (Scripps Research, La Jolla, CA, USA) using AutoDockTools 1.5.7 (Scripps Research, La Jolla, CA, USA), with the docking grid box spacing set to 1 Å. Docking results were visualized in PyMOL 3.0.
4.6. Molecular Dynamics Simulation of Key Components with Core Therapeutic Targets
The receptor protein and the ligand (dan-shexinkum D) were prepared using YASARA 10.3.16 (YASARA Biosciences GmbH, Vienna, Austria). This involved removing water molecules, ions, and any co-crystallized ligands, adding hydrogen atoms, and assigning protonation states at pH 7.4. Molecular docking was subsequently performed, and the complex exhibiting the most favorable binding energy and stereo-chemically reasonable geometry was selected for molecular dynamics simulation. The selected complex was parameterized using the AMBER force field and solvated in a cubic box of explicit solvent environment. A cubic simulation box was built using periodic boundary conditions in “around-all-atoms” mode with a 5 Å buffer (α = β = γ = 90°), and water molecules and ions were added using the software defaults. The root-mean-square deviation (RMSD), binding energy, and root-mean-square fluctuation (RMSF) were recorded throughout the 30 ns (30,000 ps) simulation. The resulting trajectories were analyzed, and the data were visualized as line plots using GraphPad Prism 10.4.2 (GraphPad Software, San Diego, CA, USA).
4.7. Meta-Analysis of Salvia miltiorrhiza Injection for Viral Myocarditis
We searched PubMed, Web of Science, the Cochrane Library, and three Chinese databases (CNKI: https://www.cnki.net/, accessed on 31 October 2025; Wanfang: https://www.wanfangdata.com.cn/, accessed on 31 October 2025; VIP: https://qikan.cqvip.com/, accessed on 31 October 2025) for studies of S. miltiorrhiza injection for VMC from inception through November 2025. Drug search terms included “Salvia miltiorrhiza” and “Danshen,” and disease terms included “myocarditis” and “viral myocarditis”. We used a combined subject heading and keyword strategy with no language or geographic restrictions. For example, PubMed searches combined the MeSH terms “Salvia miltiorrhiza”[Mesh] and “Myocarditis”[Mesh] with free text words such as “Danshen”, “Dan Shen”, “Chinese Salvia”, “Salvias, Chinese”, “Tan Seng”, and “viral myocarditis”.
The retrieved literature was screened based on the inclusion and exclusion criteria of this study. Studies were included if they met all of the following: (1) the population comprised patients diagnosed with VMC according to established guidelines; (2) the design was a randomized controlled trial (RCT); (3) the only difference in intervention between comparison groups was administration of S. miltiorrhiza injection from a single-herb source; and (4) the study reported treatment outcomes or relevant clinical indicators of VMC. Studies were excluded for any of the following: (1) noncontrolled designs, including reviews, retrospective analyses, and animal studies; (2) sources that could not be obtained; or (3) invalid original data or duplicate publications.
The risk-of-bias evaluation of the included studies was assessed using seven domains recommended by Cochrane. Key information, including group sizes (experiment and control) and outcome values, were extracted from the studies selected for the meta-analysis. The meta-analysis was performed using RevMan 5.4.1 (Epic Gecko Ltd., Bergen, Norway). Heterogeneity was determined using Cochrane’s Q test and the I2 statistic. A random-effects model was applied when there was significant heterogeneity in the data (I2 > 50% or p < 0.1), while a fixed-effects model was used for low heterogeneity (I2 ≤ 50% and p ≥ 0.1). Sensitivity analyses and evaluation of publication bias were conducted using Stata 18 (StataCorp LLC, College Station, TX, USA). Publication bias was also assessed visually with funnel plots in RevMan 5.4.1.
5. Conclusions
The core therapeutic targets of S. miltiorrhiza for VMC include TNF, JUN, PECAM1, KDR, TIMP1, and EPAS1, focusing on anti-inflammatory effects, vascular remodeling, and fibrosis suppression. Dan–shexinkum D, danshenol A, cryptotanshinone and methylrosmarinate were identified as key ingredients, with dan–shexinkum D demonstrating particularly promising results. When used as an adjunct therapy, S. miltiorrhiza injection effectively improves outcome and significantly reduces levels of TNF-α, hs-CRP, CK-MB, cTnT, and H-FABP.
Author Contributions
Conceptualization, X.C. and Z.H.; methodology, X.C., M.L. and X.X.; software, X.C.; Validation, X.C. and M.L.; formal analysis, X.C. and Z.F.; investigation, X.C., S.D. and W.J.; resources, S.D.; data curation, X.C., Y.L., F.Y. and Z.H.; writing—original draft preparation, X.C.; writing—review and editing, X.C. and Z.H.; visualization, X.C. and S.D.; supervision, Z.H.; project administration, X.C., S.D. and Z.H.; funding acquisition, S.D. and Z.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China, under grant no: 2024YFF0728801; Fundamental Research Funds for the Central Universities, Peking Union Medical College, under grant no: 3332025199; Yunnan Province Applied Basic Research Special Project-General Project under grant no: 202401CF070048.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brociek, E.; Tymińska, A.; Giordani, A.S.; Caforio, A.L.P.; Wojnicz, R.; Grabowski, M.; Ozierański, K. Myocarditis: Etiology, Pathogenesis, and Their Implications in Clinical Practice. Biology 2023, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Lampejo, T.; Durkin, S.M.; Bhatt, N.; Guttmann, O. Acute myocarditis: Aetiology, diagnosis and management. Clin. Med. 2021, 21, e505–e510. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: Multicenter lombardy registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Sagar, S.; Liu, P.P.; Cooper, L.T.J. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef]
- Olejniczak, M.; Schwartz, M.; Webber, E.; Shaffer, A.; Perry, T.E. Viral Myocarditis-Incidence, Diagnosis and Management. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1591–1601. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; You, B.; Zhou, C. Advances in cell death mechanisms involved in viral myocarditis. Front. Cardiovasc. Med. 2022, 9, 968752. [Google Scholar] [CrossRef]
- Mahrholdt, H.; Wagner, A.; Deluigi, C.C.; Kispert, E.; Hager, S.; Meinhardt, G.; Vogelsberg, H.; Fritz, P.; Dippon, J.; Bock, C.-T.; et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006, 114, 1581–1590. [Google Scholar] [CrossRef]
- Rezkalla, S.H.; Kloner, R.A. Viral myocarditis: 1917–2020: From the Influenza A to the COVID-19 pandemics. Trends Cardiovasc. Med. 2021, 31, 163–169. [Google Scholar] [CrossRef]
- Ammirati, E.; Moslehi, J.J. Diagnosis and Treatment of Acute Myocarditis: A Review. JAMA 2023, 329, 1098–1113. [Google Scholar] [CrossRef]
- Tschöpe, C.; Cooper, L.T.; Torre-Amione, G.; Van Linthout, S. Management of Myocarditis-Related Cardiomyopathy in Adults. Circ. Res. 2019, 124, 1568–1583. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.S.; Wang, S.Q.; Lu, S.C.; Liu, Y.M.; Zhang, Z.P.; Lian, W.J.; Zhou, S.Y.; Zhang, H.; Zhang, J.S.; Wang, J. Chinese Herbal Medicine for the Treatment of Adult Viral Myocarditis: An Overview of Systematic Reviews and Meta-analyses of Randomized Controlled Trials. Clin. Ther. 2023, 45, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Deng, D.; Yu, B.; Han, Z.; Huang, L.; He, Y.; Yan, X.; Wang, D. Evaluation of the Efficacy and Safety of Chinese Herbal Injection Combined with Trimetazidine for Viral Myocarditis: A Network Meta-Analysis. Front. Pharmacol. 2021, 12, 630896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, H.; Qiu, T.; Fu, H.; Dai, J.; Lian, Q.; Yan, F.; Guo, D.; Lin, J.; Xu, S.; et al. Tanshinone IIA alleviates myocarditis in Trex1-D18N lupus-like mice by inhibiting the interaction between STING and SEC24C. Int. Immunopharmacol. 2025, 156, 114659. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.; Jung, Y.L.; Hong, A.; Nam, S.J.; Lim, B.K. Salvianolic Acid B Inhibits Hand-Foot-Mouth Disease Enterovirus 71 Replication through Enhancement of AKT Signaling Pathway. J. Microbiol. Biotechnol. 2020, 30, 38–43. [Google Scholar]
- Kaygısız, M.; Sönmez, G.E. Determination of the antimicrobial, antioxidant activities and effects on oxidative DNA damage of extracts from three different Salvia species grown in Turkey. Prospect. Pharm. Sci. 2025, 23, 1–8. [Google Scholar] [CrossRef]
- Neurology Specialty Committee of the Chinese Association of Integrative Medicine. Chinese Expert Consensus on the Clinical Application of Salvianolic Acid for Injection in the Treatment of Cerebral Infarction. Chin. J. Stroke 2025, 20, 878–898. [Google Scholar]
- Xin, L.; Bing, Z.; Jiahu, Y.; Yan, L.; Fang, W. Analysis of the therapeutic effect and influencing factors of salvianolic acid combined with trimetazidine in the treatment of angina pectoris in coronary heart disease. Med. Sci. J. Cent. South China 2024, 52, 853–856. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Luo, D.; He, L.; Wang, H.; Peng, B. Mechanism of action of Salvia miltiorrhiza on avascular necrosis of the femoral head determined by integrated network pharmacology and molecular dynamics simulation. Sci. Rep. 2024, 14, 28479. [Google Scholar] [CrossRef]
- Shan, X.X.; Hong, B.Z.; Liu, J.; Wang, G.K.; Chen, W.D.; Yu, N.J.; Peng, D.Y.; Wang, L.; Zhang, C.Y. Review of chemical composition, pharmacological effects, and clinical application of Salviae Miltiorrhizae Radix et Rhizoma and prediction of its Q-markers. China J. Chin. Mater. Medica 2021, 46, 5496–5511. [Google Scholar]
- Coutsias, E.A.; Wester, M.J. RMSD and Symmetry. J. Comput. Chem. 2019, 40, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Sharanya, C.S.; Wilbee, D.S.; Sathi, S.N.; Natarajan, K. Computational screening combined with well-tempered metadynamics simulations identifies potential TMPRSS2 inhibitors. Sci. Rep. 2024, 14, 16197. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P. Therapeutic Efficacy of Levocarnitine plus Danshen Injection in Pediatric Viral Myocarditis. Henan Med. Res. 2020, 29, 3782–3784. [Google Scholar]
- Liu, C.C. Efficacy of Danshen Injection in the Treatment of Viral Myocarditis. China Sci. Technol. J. Database (Abstr. Ed.) Med. Health 2019, 3, 00073-0007378. [Google Scholar]
- Wang, W.W.; Zhang, X.F. Anti-Inflammatory and Antioxidant Effects of Adjuvant Danshen Injection in Viral Myocarditis. World Latest Med. Inf. 2018, 18, 165+167. [Google Scholar] [CrossRef]
- Yang, M.D. Anti-Inflammatory and Antioxidant Effects of Adjuvant Danshen Injection in Viral Myocarditis. Cardiovasc. Dis. J. Integr. Tradit. Chin. West. Med. 2018, 6, 46–47. [Google Scholar] [CrossRef]
- Ren, Y.L. Efficacy of Salvia miltiorrhiza Injection in Viral Myocarditis and Its Antioxidant Mechanism Targeting Oxygen Free Radicals. Guide China Med. 2016, 14, 193–194. [Google Scholar] [CrossRef]
- Chen, X.F.; Meng, R.; Lin, J.R. Therapeutic Effect of Salvia miltiorrhiza Injection on Pediatric Viral Myocarditis and Its Underlying Mechanism. Chin. J. Woman Child Health Res. 2016, 27, 389–390. [Google Scholar]
- Liang, W.B.; Chen, J. Anti-inflammation and anti-oxygen free radical effect of adjuvant Danshen injection treatment on viral myocarditis. J. Hainan Med. Univ. 2016, 22, 1630–1632. [Google Scholar] [CrossRef]
- Zhao, B.Y. Clinical effect observation of Danshen injection combined with sodium fructose diphosphate on treatment of viral myocarditis. J. Clin. Med. Lit. 2015, 2, 2211+2213. [Google Scholar] [CrossRef]
- Feng, H.; Chen, X.J. Efficacy of salvia miltiorrhiza injection on viral myocarditis and its effect of antioxygen free radicals. Chin. J. Biochem. Pharm. 2014, 34, 81–83. [Google Scholar]
- Sun, Y.N.; Lu, S.; Lu, M.R. Clinical Observation of Effect of Salvia injection on Viral myocarditis. Chin. Arch. Tradit. Chin. Med. 2013, 31, 1617–1619. [Google Scholar] [CrossRef]
- Zhao, J.B.; Ma, L.; Jin, Y.C.; Zhu, W. Observation on the effect of Danshen injection combined with western medicine in the treatment of children with viral myocarditis. China Mod. Med. 2013, 20, 72–73. [Google Scholar]
- Wang, M.L.; Cao, X.R. Efficacy Analysis of Salvia miltiorrhiza Injection Combined with Taurine in the Treatment of Viral Myocarditis. Chin. Manip. Rehabil. Med. 2011, 2, 32. [Google Scholar]
- Gong, W.S. Pharmacological Mechanisms of Salvia miltiorrhiza in Pediatric Viral Myocarditis: A Mechanistic Exploration. Chin. Prim. Health Care 2005, 12, 82–83. [Google Scholar]
- Yi, T.Y.; Liu, D.X.; Sun, L.; Yang, F.; Chen, J.J.; Jiang, Z.Y.; Huang, X.Y. Observations on the Efficacy of Salvia miltiorrhiza Injection in Treating Pediatric Viral Myocarditis. J. Xianning Coll. (Med. Sci.) 2003, 17, 117–118. [Google Scholar] [CrossRef]
- Shi, Z.C.; Li, Q.W.; Zhang, M. High-Dose Salvia miltiorrhiza Injection in the Treatment of Acute Viral Myocarditis: A Study of 64 Cases. J. Shandong Univ. Tradit. Chin. Med. 1995, 3, 167–168. [Google Scholar] [CrossRef]
- Cao, X.; Duan, S.; Li, A.; He, Z. Astragalus in Acute Pancreatitis: Insights from Network Pharmacology, Molecular Docking, and Meta-Analysis Validation. Curr. Issues Mol. Biol. 2025, 47, 379. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C.; Zhang, W.; Zhang, M.; Li, F.; Shang, H.; Zheng, W.; Li, Y.; Quan, L.; Li, X.; et al. Danhong Injection targets CaMKII through Dihydrotanshinone I to alleviate cardiomyocyte death and inflammation in viral myocarditis. Sci. China Life Sci. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Wang, J.J.; Shen, J.; Abuduwufuer, K.; Zhang, Z.; Dong, Z.; Wen, Z.; He, J.; Chen, S.; et al. Spatiotemporal transcriptomics elucidates the pathogenesis of fulminant viral myocarditis. Signal Transduct. Target. Ther. 2025, 10, 59. [Google Scholar] [CrossRef]
- Corsten, M.F.; Schroen, B.; Heymans, S. Inflammation in viral myocarditis: Friend or foe? Trends Mol. Med. 2012, 18, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative Stress: Signaling Pathways, Biological Functions, and Disease. MedComm 2020 2025, 6, e70268. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Wang, D.C.; Wang, Y.Q.; Huang, A.F.; Xu, W.D. Emerging role of hypoxia-inducible factor-1α in inflammatory autoimmune diseases: A comprehensive review. Front. Immunol. 2023, 13, 1073971. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Sullivan, D.P.; Gonzalez, A.M.; Haynes, M.E.; Dalal, P.J.; Rutledge, N.S.; Tierney, A.L.; Yescas, J.A.; Weber, E.W.; Muller, W.A. Mechanotransduction via endothelial adhesion molecule CD31 initiates transmigration and reveals a role for VEGFR2 in diapedesis. Immunity 2023, 56, 2311–2324.e6. [Google Scholar] [CrossRef]
- Lee, S.Y. Anti-Metastatic and Anti-Inflammatory Effects of Matrix Metalloproteinase Inhibition by Ginsenosides. Biomedicines 2021, 9, 198. [Google Scholar] [CrossRef]
- Das, A.; Ash, D.; Fouda, A.Y.; Sudhahar, V.; Kim, Y.-M.; Hou, Y.; Hudson, F.Z.; Stansfield, B.K.; Caldwell, R.B.; McMenamin, M.; et al. Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat. Cell Biol. 2022, 24, 35–50. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, J.; Babapoor-Farrokhran, S.; Applewhite, B.; Deshpande, M.; Megarity, H.; Flores-Bellver, M.; Aparicio-Domingo, S.; Ma, T.; Rui, Y.; et al. PAI-1 is a vascular cell-specific HIF-2-dependent angiogenic factor that promotes retinal neovascularization in diabetic patients. Sci. Adv. 2022, 8, eabm1896. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Ramis, J.M.; Monjo, M. Anti-fibrotic and anti-inflammatory properties of melatonin on human gingival fibroblasts in vitro. Biochem. Pharmacol. 2013, 86, 1784–1790. [Google Scholar] [CrossRef]
- Lan, T.; Yu, D.; Zhao, Q.; Qu, C.; Wu, Q. Ethnomedicine, phytochemistry, pharmacology, pharmacokinetics, and clinical application of Salvia miltiorrhiza Bunge (Lamiaceae): A comprehensive review. J. Ethnopharmacol. 2025, 350, 120032. [Google Scholar] [CrossRef]
- Chenfeng, J.M.; Wang, L.X.; Xie, Y.M. Four-dimensional Design of Clinical Localization of Salvianolate Injection Combined with Aspirin in the Treatment of Coronary Heart Disease Angina Pectoris. World Chinses Med. 2020, 15, 1–6+12. [Google Scholar]
- Zheng, Z.; Ke, L.; Ye, S.; Shi, P.; Yao, H. Pharmacological Mechanisms of Cryptotanshinone: Recent Advances in Cardiovascular, Cancer, and Neurological Disease Applications. Drug Des. Dev. Ther. 2024, 18, 6031–6060. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, D.; Lou, H.; Sun, L.; Ji, J. Evaluation of the anti-inflammatory activities of tanshinones isolated from Salvia miltiorrhiza var. alba roots in THP-1 macrophages. J. Ethnopharmacol. 2016, 188, 193–999. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Guan, Y.; Ma, Y.; Quan, D.; Zhang, J.; Wu, S.; Liu, X.; Lv, L.; Zhang, G. Danshenol A Alleviates Hypertension-Induced Cardiac Remodeling by Ameliorating Mitochondrial Dysfunction and Suppressing Reactive Oxygen Species Production. Oxid. Med. Cell. Longev. 2019, 2019, 2580409. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.R.; Chen, Y.M.; Li, K.J.; Zhang, R.Z.; Niu, H.L.; Liu, W.H.; Wang, C. Research progress on pharmacological effects and clinical application of Danshen Injection. Drugs Clin. 2024, 39, 2977–2982. [Google Scholar]
- Zhu, Q.B.; Huang, H.F.; Xu, Y.; Pan, Z.H.; Xiang, J.H. Effects of Salvia miltiorrhiza and Ligustrazine on Myocardial Enzymes, Oxidative Stress, and Inflammatory Factors in Children with Viral Myocarditis. Matern. Child Health Care China 2020, 35, 259–262. [Google Scholar] [CrossRef]
- Marino, Y.; Arangia, A.; D’amico, R.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Gugliandolo, E.; Fusco, R.; Cuzzocrea, S.; Di Paola, R. Aggravation of TGFβ1-Smad Pathway and Autoimmune Myocarditis by Fungicide (Tebuconazole) Exposure. Int. J. Mol. Sci. 2023, 24, 11510. [Google Scholar] [CrossRef]
- Cui, X.; Song, J.; Liu, P.; Chen, Z. Analysis of the Clinical Value of Galectin-3 and Heart-Type Fatty Acid-Binding Protein Levels in the Diagnosis and Prognosis of Viral Myocarditis in Children. Int. Heart J. 2025, 66, 570–576. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Li, H.; Wang, M.; Qiu, Y.; Lu, L. miR-29b-3p regulates cardiomyocytes pyroptosis in CVB3-induced myocarditis through targeting DNMT3A. Cell. Mol. Biol. Lett. 2024, 29, 55. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.; Chen, X.; Liu, Y.; Qin, Z.; Lin, P.; Shang, H.; Ye, M.; He, L.; Yao, Z. Development of a three-step-based novel strategy integrating DMPK with network pharmacology and bioactivity evaluation for the discovery of Q-markers of traditional Chinese medicine prescriptions: Danlou tablet as an example. Phytomedicine 2023, 108, 154511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).