Abstract
Secondary bile acids are generated from the metabolism of primary bile acids by intestinal flora and play important roles in lipid digestion, regulation of metabolic homeostasis, and intestinal-hepatic axis signaling. Recent studies indicate that lithocholic acid (LCA) and its derivatives (e.g., 3-oxoLCA and isoLCA) are significantly dysregulated in inflammatory bowel disease, nonalcoholic fatty liver disease, and hepatocellular carcinoma. Consequently, LCA species are emerging as promising biomarkers and potential targets for early diagnosis. This review systematically summarizes the metabolic pathways of LCA species, their distribution and concentrations in human blood, urine, and fecal samples, as well as the progress of recent research studies on enterohepatic disorders, which will serve as a reference for the development of new diagnostic and therapeutic methods in the future.
1. Introduction
Bile acids (BAs) are synthesized from cholesterol in hepatocytes and regulate glucose, lipid, steroid and xenobiotic metabolic homeostasis in humans and animals []. As signalling molecules, bile acids exhibit metabolic dysregulation under pathological conditions, rendering them potential biomarkers for diagnosing various diseases. Recent studies have integrated biochemical indicators with serum bile acid biomarkers to construct disease prediction models, offering non-invasive detection methods that facilitate early disease detection and intervention [,]. Bile acids represent a focal point in metabolomics research. Current studies, through the refinement of mass spectrometry techniques, continue to identify novel bile acids. This is complemented by the application of artificial intelligence to assist in the identification of bile acid-related metabolic enzymes, thereby advancing developments within the bile acid field []. LCA is a secondary bile acid synthesised via an alternative pathway. It is formed through the 7α-dehydroxylation of chenodeoxycholic acid (CDCA) under the influence of the gut microbiota and has garnered attention due to its role in intestinal and hepatic diseases []. Advances in microbiome and metabolome technologies have revealed LCA and its novel derivatives in a variety of diseases, especially those related to the enterohepatic circulatory system, which are closely related to BA synthesis and metabolism. Further studies have demonstrated the immunomodulatory functions of novel LCA derivatives, including 3-oxoLCA, iso-LCA, and isoallo-LCA, in inflammatory bowel disease (IBD) and metabolic dysfunction-associated steatotic liver disease (MASLD) [,,]. Recent studies also report anti-tumor, skeletal muscle regenerative, and anti-aging properties of LCA [,,]. However, LCA may exacerbate skin inflammation [] or steatohepatitis through distinct mechanisms. Given these multifaceted roles, LCA species are increasingly recognized as critical regulators of human health and diseases. This review comprehensively examines the metabolic pathways, tissue distribution, and clinical relevance of LCA species, providing a foundation for future diagnostic and therapeutic innovations.
2. Metabolic Pathway and Microbial Transformation of LCA Species
Advances in analytical techniques have expanded the characterization of LCA derivatives. We summarize their interconversion pathways and key metabolic enzymes in Figure 1. In hepatocytes, cholesterol is converted to primary BAs by the enzymes cholesterol 7α-hydroxylase (CYP7A1) and sterol 27 hydroxylase (CYP27A1). These Primary BAs are then conjugated to taurine or glycine in the liver, stored in the gallbladder and released into the duodenum during food intake. There they undergo microbial transformations in the intestine []. Conjugated BAs undergo deconjugation via intestinal bacterial bile salt hydrolases (BSH) enzymes, subsequently being converted into secondary BAs by 7α-dehydroxylation []. Both conjugated and unconjugated LCA are actively transported with high affinity by intestinal BA transporter protein (IBAT) from the terminal ileum into the enterocytes. There, they bind to Fatty Acid Binding Protein 6 (FABP6) and are transported via organic solute transporter α (OSTα) and organic solute transporter β (OSTβ), expressed on the intestinal cell basement membrane, ultimately return to the liver via the portal venous circulation []. LCA is present in the human body at low concentrations, but it can be toxic to the liver if present in high concentrations. Around five percent of bile acids that are not absorbed will enter the large intestine and are excreted in faeces, while a small quantity of LCA that returning to the liver is secreted into bile after sulphation, then excreted in urine via the kidneys through the bloodstream [].
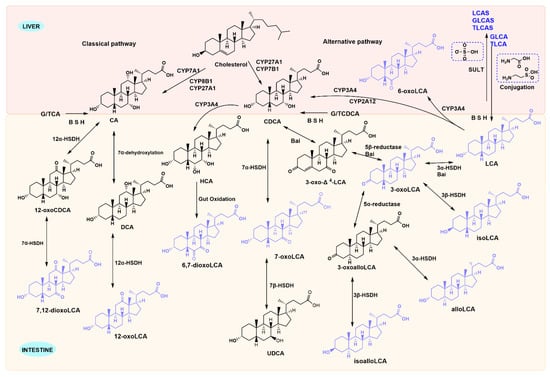
Figure 1.
Metabolic pathways of LCA species. Primary bile acids generated in the liver are converted into LCA species by gut microbiota and host enzymes. However, 6-oxo-LCA is formed when LCA is reabsorbed into the liver via enterohepatic circulation and subsequently metabolised by CYP3A4 enzymes. LCA reabsorbed into the liver conjugates with glycine and taurine to form TLCA and GLCA, respectively. All three undergo sulphonation metabolism in the liver via the action of hepatic sulphotransferase enzymes.
In addition, LCA species are mainly generated through four pathways under the action of gut microbiota: deconjugation (removal of glycine or taurine), 7α-dehydroxylation, oxidation, and isomerization []. BSH enzymes can hydrolyze conjugated bile acids that are bound to glycine and taurine, while hydroxysteroid dehydrogenase is responsible for the biotransformation of primary BAs into secondary BAs. CYP3A4 is the enzyme involved in the biotransformation of LCA in liver microsomes to form metabolites with LCA as the parent structure (such as 3-keto-LCA and 6-keto-LCA). The formation of these LCA derivatives enhances hepatic detoxification and elimination functions []. Enzymes involved in LCA metabolism are gradually being characterized in microorganisms (Table 1). BSH enzyme activity have been expressed in gut microbiota, including Clostridium, Bacteroides, Lactobacillus, Bifidobacterium, Enterococcus, and methanogenic bacteria. Gut microbiota members with 3α-hydroxysteroid dehydrogenase (3α-HSDH), bile acid 5β-reductase, and bile acid 5α-reductase produce allo-secondary bile acids via 3-oxo-∆4-LCA intermediate that resets the ring stereochemistry []. 7α-HSDH has been primarily characterized in human gut microbiota species such as C. absonum, C. hiranonis, and C. hylemonae. Phylogenetic analysis indicates that 12α-HSDH is widely distributed across the Actinobacteria phylum, the Coriobacteriaceae family, and human gut archaea []. Recent studies have proposed a fifth microbial modification, recombination, which includes the formation of BA-24-amides with amino acids or polyamines at the C-24 position and BA-3-O-acyl esters with fatty acids or organic acids at the C-3 position []. Research has shown that BAs decorated by the gut microbiota do not only bind only to glycine, taurine at the C-24 position, but also produce more than 200 amino acid-BA conjugates [,,]. Sato et al. found a series of LCA derivatives produced by enriched gut microorganisms in feces of Japanese centenarians, including 3-iso-, 3-oxo-, 3-allo-, 3-isoallo-, and 3-oxoallo-LCA [].

Table 1.
Bacterial taxa involved in the metabolism of LCA species.
3. LCA-Receptor Signaling Pathways in Maintaining Metabolic Homeostasis
BAs function as signaling molecules that attach to specific receptors, such as the farnesoid X receptor (FXR), G protein-coupled receptor 5 (TGR5), vitamin D receptor (VDR), pregnane X receptor (PXR), liver X receptor (LXR) and constitutive androstane receptor (CAR). Previous studies have summarized the roles of LCA in anti-inflammation, immune regulation, and glycolipid metabolism modulation, which are mediated through the activation of signaling pathways involving the receptors mentioned above []. The potency of unconjugated BAs in activating FXR is as follows: CDCA > DCA > LCA ≫ CA, while the most effective endogenous ligands for TGR5 are LCA and its derivatives [,,]. Upon activation by BAs, the intestinal FXR regulates BA levels within the enterohepatic circulation. TGR5 mainly participates in the regulation of glucose, lipid, and energy metabolism after BAs activation [,]. Beyond acetylated DCA and CA, LCA and its oxidative metabolite 3-oxo-LCA are also primary bile acid ligands for PXR, whereas conjugated bile acids fail to activate PXR [,]. VDR exhibits heightened sensitivity to bile acids compared to FXR and PXR, demonstrating particularly pronounced responses to ketone LCAs and glycine-LCA within the LCA species []. The ketone derivatives of LCA, 6-keto and 7-keto-LCA, have been suggested to exert a trans-inhibitory effect on CAR activity [,]. Up-regulation of PXR expression, on the one hand, inhibits BA synthesis by blocking transcriptional activation of the promoter region of the CYP7A1 gene via the SHP-LRH-1 pathway and suppressing its gene expression. On the other hand, PXR serves as key transcription factor to govern the inducible expression of the CYP3A gene, accelerating the metabolism of drugs in vivo and potentially reducing the efficacy of these drugs or generating adverse effects []. It has been shown that PXR has the capacity to counteract the adverse impacts of toxic hydrophobic bile acids (e.g., LCA) by activating cytochromes. These enzymes hydroxylate BAs to less toxic and more hydrophilic BA species. Furthermore, PXR induces sulphatase activity, thereby facilitating the second phase of BA metabolism and the detoxification process []. The regulatory mechanisms governing the metabolic cascade of the vitamin D receptor (VDR) bear a strong resemblance to those mediated by other nuclear receptors. These receptors function as lipid sensors to mediate the detoxification of their ligands. LCA may activate feedforward catabolic pathways by binding to VDR, thereby increasing CYP3A expression and ultimately achieving LCA detoxification []. Concurrently, inducing sulfotransferase expression leads to LCA sulphation, which inhibits its passive uptake by enteric pericytes and promotes its excretion [].
4. Distribution and Concentrations of LCA Species in Human Biospecimen
Next, we systematically summarized the distribution and concentrations of LCA species in the blood, urine, and fecal samples of healthy subjects that have been reported in previous studies. First, more studies prefer the identification of BAs in the blood samples than urine or fecal samples, and there are differences in the distribution and concentration of LCA species in different sample types. We noticed that most of the LCA species can be detected in blood samples, mostly glycine-conjugating LCA analogs. Interestingly, LCA concentrations increase during infancy and early childhood (0–3 years) but stabilise with advancing age [] (Table 2). This phenomenon confirms that intestinal bile acid metabolism undergoes phased maturation alongside microbiota development, by which stage the microbiota has matured into an adult-type composition []. In addition, the potential biological significance of the evolution of LCA levels in the human body at different ages for growth and development remains to be further studied. In urine samples, the LCA series are dominated by the II phase metabolite 3-sulfated-LCA in high concentrations (Table 3). Among the secondary bile acid LCA derivatives produced by the conversion of primary bile acids by gut microbiota, LCA remains the most abundant in fecal samples (Table 4). Due to the difficulty in obtaining liver samples, only one study has reported the concentration of LCA in normal liver tissue. This research selected liver parenchymal tissue from the perilesional area of patients with focal liver disease (n = 6, aged 40–60 years) as samples. Using gas chromatography-mass spectrometry, the LCA concentration was detected as 1.5 ± 0.2 nmol/g (mean ± SEM) [].

Table 2.
Concentrations of LCA species in the blood samples of healthy individuals.

Table 3.
Concentrations of LCA species in the urine samples of healthy individuals.

Table 4.
Concentration of LCA species in the fecal samples of healthy individuals.
5. Clinical Significance of LCA Species in Enterohepatic Diseases
5.1. Diagnostic Biomarkers
Numerous studies have shown that MASLD patients have increased levels of total BAs, increased hepatic BA synthesis, and intestinal flora disorders leading to an imbalance in secondary BA metabolism, which promotes disease progression []. Higher levels of BAs in both serum and feces have been strongly associated with the severity of liver fibrosis in NAFLD patients [,]. LCA, GLCA, and TLCA were significantly elevated in both liver fibrosis patients and in three distinct C57BL/6J mouse models of liver fibrosis induced by high-fat diet (HFD), streptozotocin-high-fat diet (STZ-HFD) and carbon tetrachloride (CCl4), respectively. Notably, the Pseudomonas species significantly increased in the HFD-induced model showed a significant positive correlation with LCA and GLCA []. In our large cohort study using biopsy-confirmed NAFLD, patients with mild fibrosis (F1) had a significant increase in secondary BAs, including DehydroLCA, 6-ketoLCA, and TLCA. These were associated with clinical indicators to construct and validate a liver fibrosis prediction model. In the early stage (F1 vs. F0) non-obese patient cohort (n = 119), the training set AUROC value was 0.78 and the testing set AUROC value was 0.69. This outperformed the traditional non-invasive Hepamet fibrosis score, offering a novel strategy for non-invasive detection of early-stage liver fibrosis []. The above studies demonstrate the LCA species could serve as early MASLD markers and key measures of disease advancement.
Furthermore, when examining the changes in BA profiles during NAFLD progression, plasma 7-ketoLCA levels were found to be significantly elevated in patients with advanced steatosis and were associated with the progression of NASH, hepatocellular edema, and steatosis []. Interestingly, the BA profile characteristics of NAFLD patients at risk for gallstone disease exhibit gender specificity. Compared to males, females demonstrate significantly elevated serum concentrations of GLCA, isoLCA, and 12-ketoLCA, alongside markedly increased fecal LCA levels. Conversely, fecal 7-ketoLCA levels are significantly reduced []. In patients with cirrhosis with sarcopenia, serum secondary BAs (total DCA, total LCA, unconjugated DCA, and unconjugated LCA) and fecal total LCA were markedly higher than in cirrhotic patients without sarcopenia []. Recent research has indicated that microbiota-generated metabolites contribute to hepatocellular carcinoma (HCC) development, and BAs may serve as biomarkers of HCC risk []. Serum iso-LCA levels and fecal abundance of B. ovatus were significantly increased in patients with HCC, both of which may serve as biomarkers for HCC diagnosis [].
5.2. Therapeutic Targets
5.2.1. Direct Effects of LCA Species on Intestinal Diseases
In vitro cellular experiments demonstrated that LCA inhibited deoxynivalenol (DON)-induced intestinal inflammatory responses and oxidative stress in intestinal epithelial cells (IPI-2I) by modulating PPARγ-mediated core inflammatory and antioxidant genes []. Furthermore, LCA reverses DON-induced apoptosis in IPI-2I cells by reducing the expression of cleaved caspase-3 and PARP-1. Further investigations revealed that LCA reduces elevated bile acid levels in IPI-2I caused by DON-promoted expression of the rate-limiting bile acid synthase CYP7A1. It significantly downregulates CYP7A1, CYP7B1, CYP8B1, and CYP27A1 expression, a regulatory process likely closely associated with the nuclear receptor RORγ []. In vivo animal experiments verified that LCA treatment significantly reduced a variety of chemokines and cytokines associated with intestinal inflammation, such as chemokine ligands CCL5, CXCL10, IL-17A and TNF-α []. 7-Keto-LCA was shown to be an FXR antagonist that promotes Wnt signaling in an aspirin-induced C57BL/6J mouse model of intestinal injury, thereby facilitating self-renewal of intestinal stem cells []. 12-ketoLCA inhibits the secretion of pro-inflammatory cytokine IL-17A by promoting the expression of the nuclear receptor VDR in ILC3s, which may be important for its efficacy against colitis []. Similarly, isoalloLCA attenuates intestinal inflammation by increasing the binding of the orphan nuclear receptor NR4A1 at the Foxp3 locus and enhancing the transcription of the Foxp3 gene to induce the differentiation of naïve T cells into Treg cells []. The above studies suggest that LCA species play a beneficial role in the gut, especially through immune modulation. However, when the intestinal barrier is damaged, tight junctions break down, allowing more LCA species to diffuse from this site to various tissues and organs throughout the body. When the concentration reaches a certain level, it might trigger various inflammatory responses.
5.2.2. Direct Effects of LCA Species on Hepatic Diseases
The enterohepatic axis pathway is increasingly recognized internationally, and the role of LCA in the gut can further influence liver function. It was noted that LCA downregulates the expression of proteins critical to the NF-κB inflammatory signaling pathway, such as IκBα and NF-κB p50 phosphorylation, and nuclear translocation of NF-κB p65. It exerts anti-inflammatory and hepatoprotective effects on Klebsiella pneumoniae-infected mice via TGR5 []. LCA has been demonstrated to inhibit the activation of HSCs by reducing the activation of transforming growth factor β (TGF-β) Smad-dependent and Smad-independent pathways and inducing apoptosis. Meanwhile, LCA suppresses glycolysis and enhances oxidative phosphorylation, driving hepatic macrophage polarization toward M2 phenotype while preventing their transformation into M1 type []. In macrophages, the NF-κB pathway activation is primarily linked to the cell’s capacity to enhance inflammation. TGR5 reduces inflammation in liver macrophages by suppressing the activation of the NF-κB pathway, which is in contrast to its role in hepatocytes []. Another study reported that LCA, 3-oxoLCA and isoLCA depend on and up-regulate TGR5 to promote M2 macrophage polarization and inhibit TH17 cell differentiation, but also directly inhibit the expression of RORγt, which drives the differentiation of TH17 cells, to exert anti-inflammatory effects []. The reduction of LCA-3-S due to BA metabolism disorders may be one of the pathogenic mechanisms of cholestatic liver disease. It has been suggested that LCA-3-S binds better to RORγt than 3-oxo-LCA. It specifically inhibits TH17 cell differentiation by targeting RORγt without affecting TH1, TH2 and Treg cells []. Recently, it was found that isoLCA administration to HFD-induced C57BL/6J mice NASH model prevented the progression of NAFLD to NASH by regulating the expression of genes related to TH17 cell differentiation/IL-17/PPAR signaling pathway to inhibit TH17 cell differentiation and promote Treg cell proliferation []. Administration of alloLCA in the HFD and CCl4-induced C57BL/6J mouse model of MASH corrected the disruption of various pathways linked to immune, inflammatory, and metabolic signaling, indicating that alloLCA might play a role in treating chronic inflammation and fibrosis-associated liver diseases (Figure 2) [].
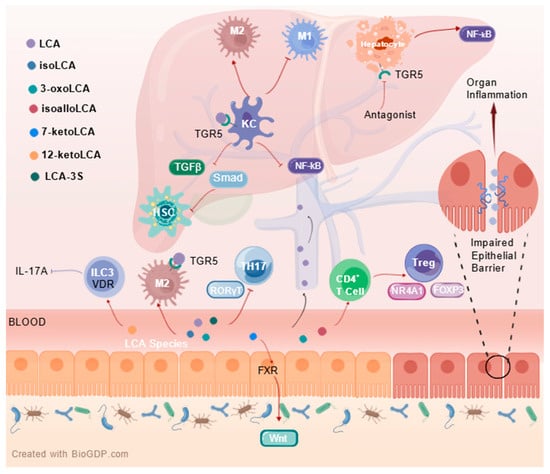
Figure 2.
Immune signalling pathways and mechanisms of LCA species in the enterohepatic axis. Created with http://BioGDP.com [].
Our studies have reported that conjugated LCA activates hepatic TGR5 to promote lipotoxicity and the transition of MASLD to MASH by disrupting carnosine biosynthesis []. Notably, LCA plays a negative role in treating the progression of hepatocellular carcinoma. AKR1D1, an enzyme involved in BA metabolism, plays an important tumor suppressor role in hepatocellular carcinoma, and its deficiency significantly accelerated the progression of hepatocellular carcinoma by a mechanism that may be a significant increase in secondary BA iso-LCA impairs the cytotoxic function of CREB1 by inhibiting its phosphorylation in NK cells, dose-dependently decreasing the ability of NK cells to kill tumor cells []. LCA, a secondary BA accumulated in the microenvironment of hepatocellular carcinoma, can promote tumorigenesis and T cell dysfunction in vivo by inducing endoplasmic reticulum (ER) stress in tumor-specific T cells []. In summary, the application of LCA and other derivatives in the field of liver disease needs to be further explored.
5.2.3. Indirect Effects of LCA Species on Enterohepatic Diseases
In addition to direct intervention with LCA species, many studies have attempted to target BA receptors, BA synthases, and gut flora to regulate secondary BA metabolic homeostasis in vivo. Secondary BA synthesis is regulated by remodeling the gut microbiota including Parabacteroides, Clostridium, and Akkermansia muciniphila, which encode 7α- HSDH [,,]. FXR and TGR5 are two of the most extensively researched BA receptors. The agonists that have been developed for these related receptors, which inhibit NF-κB pathway-mediated pro-inflammatory cytokine releases, have demonstrated their efficacy in ameliorating hepatic steatosis and inflammation in the clinic [,]. The activation of the NF-κB pathway in hepatocytes is generally seen as protective, particularly during inflammatory damage, as it enhances the expression of genes that prevent apoptosis and supports liver function []. The TGR5 receptor, which is highly expressed on hepatocytes following disease onset, inhibits NF-κB signalling when activated by LCA, thereby exacerbating disease progression. This suggests that specific inhibitors targeting hepatocyte TGR5 may represent a viable therapeutic strategy. Atorvastatin increases BA synthesis and regulates the gut microbiota by promoting the abundance of 7-dehydroxylase-expressing Clostridium, increasing LCA level and activating TGR5, and attenuating liver injury in a diet-induced steatohepatitis model []. By binding to PXR and VDR, LCA derivatives increase the excretion of cytotoxic LCA and prevents cholestatic liver injury and steatohepatitis due to liver accumulation []. Gly-β-MCA prevents cholestatic liver injury by promoting fecal BAs excretion and reducing colonic LCA exposure [].
6. Conclusions
A total of 15 LCA species have been quantitatively identified and reported in the blood, urine and fecal samples. Yet, more novel compounds are to be unearthed with the development of mass spectrometry. LCA derivatives have been reported to play roles in anti-inflammatory, reducing lipid accumulation, antiviral and antibacterial activities. Although LCA and its derivatives have shown promising therapeutic targets in the field of enterohepatic diseases, the dual effects of LCA (protection and toxicity) that dependent on the concentrations, tissue microenvironment and species differences in bile acid metabolism, and the precise regulatory mechanisms have not been fully elucidated. Thus, more systematic work is required to explore the specific roles of LCA and its derivatives in the gut and liver. Furthermore, existing research is largely confined to cellular or animal models, necessitating further validation to determine whether the mechanisms observed in animals are applicable to humans. Individual differences in gut microbiota composition may significantly influence LCA metabolism and therapeutic efficacy, and future research should integrate multi-omics technologies to explore personalised intervention strategies; The dynamic changes of LCA across different disease stages (e.g., progression from MASLD to MASH) and its therapeutic window require systematic investigation.
In summary, how LCA interacts with the gut microbiota to exert its effects in the gut and avoid enterohepatic circulation-induced damage to other organs warrants further investigation. Future efforts should also focus on technological advancements, the discovery of new compounds, and in-depth mechanistic studies of the association between LCA metabolic networks and disease, with the aim of developing multi-target therapies based on the microbiota-BA axis. This approach holds the promise of providing novel diagnostic tools and treatment strategies for enterohepatic diseases.
Author Contributions
Conceptualization, Y.N.; investigation, L.L. and G.Z.; data curation, G.Z. and L.L.; writing—original draft preparation, L.L.; writing—review and editing, L.L., G.Z., A.L. and H.W.; visualization, L.L.; funding acquisition, Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (82570672).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The author acknowledges the contributions of the research team members, especially Yan Ni from the Children’s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China, for her invaluable insights to this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
BA, bile acid; BAs, Bile acids; bai, manipulator; BSH, bile salt hydrolases; CA, cholic acid; CAR, constitutive androstane receptor; CDCA, chenodeoxycholic acid; CYP7A1, cholesterol 7α-hydroxylase; CYP27A1, sterol 27 hydroxylase; DON, deoxynivalenol; FABP6, Fatty Acid Binding Protein 6; FXR, farnesoid X receptor; HCC, hepatocellular carcinoma; IBAT, intestinal BAs transporter; IBD, inflammatory bowel disease; IPI-2I, intestinal epithelial cells; LCA, lithocholic acid; LXR, liver X receptor; MASLD, metabolic dysfunction-associated steatotic liver disease; NAFLD, nonalcoholic fatty liver disease; OSTα, organic solute transporter α; OSTβ, organic solute transporter β; PXR, pregnane X receptor; SULT, sulfotransferase; TGF-β, transforming growth factor β; TGR5, G protein-coupled receptor 5; VDR, vitamin D receptor; 5AR, 5α-reductase; 5BR, 5β-reductase; 3α-HSDH, 3α-hydroxysteroid dehydrogenase; 3β-HSDH, 3β-hydroxysteroid dehydrogenase; 7α-HSDH, 7α-hydroxysteroid dehydrogenase; 7β-HSDH, 7β-hydroxysteroid dehydrogenase; 12α-HSDH, 12α-hydroxysteroid dehydrogenase.
References
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the micrbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, T.; Zhao, A.; Ning, Z.; Kuang, J.; Wang, S.; You, Y.; Bao, Y.; Ma, X.; Yu, H.; et al. Hyocholic acid species as novel biomarkers for metabolic disorders. Nat. Commun. 2021, 12, 1487. [Google Scholar] [CrossRef]
- Liu, A.N.; Xu, C.F.; Liu, Y.R.; Sun, D.Q.; Jiang, L.; Tang, L.J.; Zhu, P.W.; Chen, S.D.; Liu, W.Y.; Wang, X.D.; et al. Secondary bile acids improve risk prediction for non-invasive identification of mild liver fibrosis in nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2023, 57, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Luo, X.; Guo, J.; Xing, B.; Lin, H.; Ma, H.; Wang, Y.; Li, M.; Ye, C.; Yan, S.; et al. Identification of gut microbial bile acid metabolic enzymes via an AI-assisted pipeline. Cell 2025, 188, 6012–6027.e20. [Google Scholar] [CrossRef]
- Lun, W.; Yan, Q.; Guo, X.; Zhou, M.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Mechanism of action of the bile acid receptor TGR5 in obesity. Acta Pharm. Sin. B 2024, 14, 468–491. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.; Yao, L.; Zhang, Y.; Bae, S.; D’Agostino, G.D.; Zhang, M.; Kim, E.; Franzosa, E.A.; Avila-Pacheco, J.; Bisanz, J.E.; et al. Human gut bacteria produce Τ(H)17-modulating bile acid metabolites. Nature 2022, 603, 907–912. [Google Scholar] [CrossRef]
- Jung, Y.; Koo, B.K.; Jang, S.Y.; Kim, D.; Lee, H.; Lee, D.H.; Joo, S.K.; Jung, Y.J.; Park, J.H.; Yoo, T.; et al. Association between circulating bile acid alterations and nonalcoholic steatohepatitis independent of obesity and diabetes mellitus. Liver Int. 2021, 41, 2892–2902. [Google Scholar] [CrossRef]
- Li, W.; Hang, S.; Fang, Y.; Bae, S.; Zhang, Y.; Zhang, M.; Wang, G.; McCurry, M.D.; Bae, M.; Paik, D.; et al. A bacterial bile acid metabolite modulates T(reg) activity through the nuclear hormone receptor NR4A1. Cell Host Microbe 2021, 29, 1366–1377.e9. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, S.; Kovács, P.; Nyerges, P.; Ujlaki, G.; Sipos, A.; Uray, K.; Bai, P.; Mikó, E. The bacterial metabolite, lithocholic acid, has antineoplastic effects in pancreatic adenocarcinoma. Cell Death Discov. 2024, 10, 248. [Google Scholar] [CrossRef]
- Sun, L.; Li, F.; Tan, W.; Zhao, W.; Li, Y.; Zhu, X.; Gao, P.; Shu, G.; Wang, S.; Jiang, Q.; et al. Lithocholic acid promotes skeletal muscle regeneration through the TGR5 receptor. Acta Biochim. Biophys. Sin. 2023, 55, 51–61. [Google Scholar] [CrossRef]
- Qu, Q.; Chen, Y.; Wang, Y.; Wang, W.; Long, S.; Yang, H.Y.; Wu, J.; Li, M.; Tian, X.; Wei, X.; et al. Lithocholic acid binds TULP3 to activate sirtuins and AMPK to slow down ageing. Nature 2024, 643, 201–209. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, M.; Peng, Q.; Sha, K.; Liu, T.; Xia, J.; Xie, H.; Li, J.; Xu, S.; Deng, Z. Lithocholic acid promotes rosacea-like skin inflammation via G protein-coupled bile acid receptor 1. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166563. [Google Scholar] [CrossRef] [PubMed]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Gaskins, H.R. Another renaissance for bile acid gastrointestinal microbiology. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 348–364. [Google Scholar] [CrossRef]
- Wang, D.Q.; Cohen, D.E.; Carey, M.C. Biliary lipids and cholesterol gallstone disease. J. Lipid Res. 2009, 50, S406–S411. [Google Scholar] [CrossRef]
- Deo, A.K.; Bandiera, S.M. 3-ketocholanoic acid is the major in vitro human hepatic microsomal metabolite of lithocholic acid. Drug Metab. Dispos. 2009, 37, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Atarashi, K.; Plichta, D.R.; Arai, Y.; Sasajima, S.; Kearney, S.M.; Suda, W.; Takeshita, K.; Sasaki, T.; Okamoto, S.; et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 2021, 599, 458–464. [Google Scholar] [CrossRef]
- Doden, H.; Sallam, L.A.; Devendran, S.; Ly, L.; Doden, G.; Daniel, S.L.; Alves, J.M.P.; Ridlon, J.M. Metabolism of Oxo-Bile Acids and Characterization of Recombinant 12α-Hydroxysteroid Dehydrogenases from Bile Acid 7α-Dehydroxylating Human Gut Bacteria. Appl. Environ. Microbiol. 2018, 84, e00235-18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhang, H.; Zheng, X.; Zhao, A.; Jia, W. Novel microbial modifications of bile acids and their functional implications. iMeta 2024, 3, e243. [Google Scholar] [CrossRef]
- Rimal, B.; Collins, S.L.; Tanes, C.E.; Rocha, E.R.; Granda, M.A.; Solanki, S.; Hoque, N.J.; Gentry, E.C.; Koo, I.; Reilly, E.R.; et al. Bile salt hydrolase catalyses formation of amine-conjugated bile acids. Nature 2024, 626, 859–863. [Google Scholar] [CrossRef]
- Mohanty, I.; Mannochio-Russo, H.; Schweer, J.V.; El Abiead, Y.; Bittremieux, W.; Xing, S.; Schmid, R.; Zuffa, S.; Vasquez, F.; Muti, V.B.; et al. The underappreciated diversity of bile acid modifications. Cell 2024, 187, 1801–1818.e20. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, Q.; Zhou, J.H.; Xu, G. Enhanced activity and substrate tolerance of 7α-hydroxysteroid dehydrogenase by directed evolution for 7-ketolithocholic acid production. Appl. Microbiol. Biotechnol. 2019, 103, 2665–2674. [Google Scholar] [CrossRef]
- Hylemon, P.B.; Cacciapuoti, A.F.; White, B.A.; Whitehead, T.R.; Fricke, R.J. 7 alpha-Dehydroxylation of cholic acid by cell extracts of Eubacterium species V.P.I. 12708. Am. J. Clin. Nutr. 1980, 33, 2507–2510. [Google Scholar] [CrossRef]
- Mythen, S.M.; Devendran, S.; Méndez-García, C.; Cann, I.; Ridlon, J.M. Targeted Synthesis and Characterization of a Gene Cluster Encoding NAD(P)H-Dependent 3α-, 3β-, and 12α-Hydroxysteroid Dehydrogenases from Eggerthella CAG:298, a Gut Metagenomic Sequence. Appl. Environ. Microbiol. 2018, 84, e02475-17. [Google Scholar] [CrossRef]
- Sheng, W.; Ji, G.; Zhang, L. The Effect of Lithocholic Acid on the Gut-Liver Axis. Front. Pharmacol. 2022, 13, 910493. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Carazo, A.; Hyrsova, L.; Dusek, J.; Chodounska, H.; Horvatova, A.; Berka, K.; Bazgier, V.; Gan-Schreier, H.; Chamulitrat, W.; Kudova, E.; et al. Acetylated deoxycholic (DCA) and cholic (CA) acids are potent ligands of pregnane X (PXR) receptor. Toxicol. Lett. 2017, 265, 86–96. [Google Scholar] [CrossRef]
- Ðanić, M.; Stanimirov, B.; Pavlović, N.; Goločorbin-Kon, S.; Al-Salami, H.; Stankov, K.; Mikov, M. Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome. Front. Pharmacol. 2018, 9, 1382. [Google Scholar] [CrossRef]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin D receptor as an intestinal bile acid sensor. Science 2002, 296, 1313–1316. [Google Scholar] [CrossRef]
- Fiorucci, S.; Cipriani, S.; Baldelli, F.; Mencarelli, A. Bile acid-activated receptors in the treatment of dyslipidemia and related disorders. Prog. Lipid Res. 2010, 49, 171–185. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhou, J.M.; Ma, Z.C.; Li, H.; Liang, Q.D.; Tan, H.L.; Xiao, C.R.; Zhang, B.L.; Gao, Y. Pregnane X receptor mediated-transcription regulation of CYP3A by glycyrrhizin: A possible mechanism for its hepatoprotective property against lithocholic acid-induced injury. Chem. Biol. Interact. 2012, 200, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, J.; Xie, W.; Rosenfeld, J.M.; Barwick, J.L.; Guzelian, P.S.; Evans, R.M. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR). Proc. Natl. Acad. Sci. USA 2002, 99, 13801–13806. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Repa, J.J.; Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors and lipid physiology: Opening the X-files. Science 2001, 294, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Echchgadda, I.; Song, C.S. Vitamin D receptor regulation of the steroid/bile acid sulfotransferase SULT2A1. Methods Enzymol. 2005, 400, 165–191. [Google Scholar]
- Jahnel, J.; Zöhrer, E.; Scharnagl, H.; Erwa, W.; Fauler, G.; Stojakovic, T. Reference ranges of serum bile acids in children and adolescents. Clin. Chem. Lab. Med. 2015, 53, 1807–1813. [Google Scholar] [CrossRef]
- Tanaka, M.; Sanefuji, M.; Morokuma, S.; Yoden, M.; Momoda, R.; Sonomoto, K.; Ogawa, M.; Kato, K.; Nakayama, J. The association between gut microbiota development and maturation of intestinal bile acid metabolism in the first 3 y of healthy Japanese infants. Gut Microbes 2020, 11, 205–216. [Google Scholar] [CrossRef]
- Setchell, K.D.; Rodrigues, C.M.; Clerici, C.; Solinas, A.; Morelli, A.; Gartung, C.; Boyer, J. Bile acid concentrations in human and rat liver tissue and in hepatocyte nuclei. Gastroenterology 1997, 112, 226–235. [Google Scholar] [CrossRef]
- Mao, F.; Liu, T.; Hou, X.; Zhao, H.; He, W.; Li, C.; Jing, Z.; Sui, J.; Wang, F.; Liu, X.; et al. Increased sulfation of bile acids in mice and human subjects with sodium taurocholate cotransporting polypeptide deficiency. J. Biol. Chem. 2019, 294, 11853–11862. [Google Scholar] [CrossRef]
- Santos Silva, E.; Rocha, S.; Candeias Ramos, R.; Coutinho, H.; Catarino, C.; Teixeira, F.; Henriques, G.; Lopes, A.I.; Santos-Silva, A.; Brites, D. Bile acids profile and redox status in healthy infants. Pediatr. Res. 2023, 93, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Bathena, S.P.R.; Mukherjee, S.; Olivera, M.; Alnouti, Y. The profile of bile acids and their sulfate metabolites in human urine and serum. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 942–943, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Choucair, I.; Mallela, D.P.; Hilser, J.R.; Hartiala, J.A.; Nemet, I.; Gogonea, V.; Li, L.; Lusis, A.J.; Fischbach, M.A.; Tang, W.H.W.; et al. Comprehensive Clinical and Genetic Analyses of Circulating Bile Acids and Their Associations with Diabetes and Its Indices. Diabetes 2024, 73, 1215–1228. [Google Scholar] [CrossRef]
- Zheng, D.; Ge, K.; Qu, C.; Sun, T.; Wang, J.; Jia, W.; Zhao, A. Comparative profiling of serum, urine, and feces bile acids in humans, rats, and mice. Commun. Biol. 2024, 7, 641. [Google Scholar] [CrossRef]
- Humbert, L.; Maubert, M.A.; Wolf, C.; Duboc, H.; Mahe, M.; Farabos, D.; Seksik, P.; Mallet, J.M.; Trugnan, G.; Masliah, J.; et al. Bile acid profiling in human biological samples: Comparison of extraction procedures and application to normal and cholestatic patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 899, 135–145. [Google Scholar] [CrossRef]
- Schauermann, M.; Wang, R.; Pons-Kuehnemann, J.; Hartmann, M.F.; Remer, T.; Hua, Y.; Bereket, A.; Wasniewska, M.; Shmoish, M.; Hochberg, Z.; et al. Targeted quantitative analysis of urinary bile acids by liquid chromatography-tandem mass spectrometry: Method development and application to healthy and obese children. J. Steroid Biochem. Mol. Biol. 2025, 249, 106712. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Myint, K.T.; Sato, K.; Wada, O.; Kakiyama, G.; Iida, T.; Hishinuma, T.; Mano, N.; Goto, J. LC/ESI-tandem mass spectrometric determination of bile acid 3-sulfates in human urine 3beta-Sulfooxy-12alpha-hydroxy-5beta-cholanoic acid is an abundant nonamidated sulfate. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 846, 69–77. [Google Scholar] [CrossRef]
- Sommersberger, S.; Gunawan, S.; Elger, T.; Fererberger, T.; Loibl, J.; Huss, M.; Kandulski, A.; Krautbauer, S.; Muller, M.; Liebisch, G.; et al. Altered fecal bile acid composition in active ulcerative colitis. Lipids Health Dis. 2023, 22, 199. [Google Scholar] [CrossRef]
- Farhat, Z.; Sampson, J.N.; Hildesheim, A.; Safaeian, M.; Porras, C.; Cortes, B.; Herrero, R.; Romero, B.; Vogtmann, E.; Sinha, R.; et al. Reproducibility, Temporal Variability, and Concordance of Serum and Fecal Bile Acids and Short Chain Fatty Acids in a Population-Based Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1875–1883. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef]
- Adams, L.A.; Wang, Z.; Liddle, C.; Melton, P.E.; Ariff, A.; Chandraratna, H.; Tan, J.; Ching, H.; Coulter, S.; de Boer, B.; et al. Bile acids associate with specific gut microbiota, low-level alcohol consumption and liver fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2020, 40, 1356–1365. [Google Scholar] [CrossRef]
- Suga, T.; Yamaguchi, H.; Ogura, J.; Shoji, S.; Maekawa, M.; Mano, N. Altered bile acid composition and disposition in a mouse model of non-alcoholic steatohepatitis. Toxicol. Appl. Pharmacol. 2019, 379, 114664. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Jiang, R.; Wang, X.; Liu, P.; Zhao, A.; Wu, Y.; Huang, F.; Liu, Z.; Rajani, C.; Zheng, X.; et al. Conjugated secondary 12α-hydroxylated bile acids promote liver fibrogenesis. EBioMedicine 2021, 66, 103290. [Google Scholar] [CrossRef] [PubMed]
- Nimer, N.; Choucair, I.; Wang, Z.; Nemet, I.; Li, L.; Gukasyan, J.; Weeks, T.L.; Alkhouri, N.; Zein, N.; Tang, W.H.W.; et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism 2021, 116, 154457. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, D.; Mai, M.; Song, W.; Yuan, Q.; Xie, Y.; Mo, B.; Guo, H. Biological gender difference of bile acid metabolism in susceptibility to cholelithiasis in patients with nonalcoholic fatty liver disease. J. Steroid Biochem. Mol. Biol. 2025, 253, 106812. [Google Scholar] [CrossRef] [PubMed]
- Aliwa, B.; Horvath, A.; Traub, J.; Feldbacher, N.; Habisch, H.; Fauler, G.; Madl, T.; Stadlbauer, V. Altered gut microbiome, bile acid composition and metabolome in sarcopenia in liver cirrhosis. J. Cachexia Sarcopenia Muscle 2023, 14, 2676–2691. [Google Scholar] [CrossRef]
- Sanchez, J.I.; Fontillas, A.C.; Kwan, S.Y.; Sanchez, C.I.; Calderone, T.L.; Lee, J.L.; Elsaiey, A.; Cleere, D.W.; Wei, P.; Vierling, J.M.; et al. Metabolomics biomarkers of hepatocellular carcinoma in a prospective cohort of patients with cirrhosis. JHEP Rep. 2024, 6, 101119. [Google Scholar] [CrossRef]
- Wei, H.; Suo, C.; Gu, X.; Shen, S.; Lin, K.; Zhu, C.; Yan, K.; Bian, Z.; Chen, L.; Zhang, T.; et al. AKR1D1 suppresses liver cancer progression by promoting bile acid metabolism-mediated NK cell cytotoxicity. Cell Metab. 2025, 37, 1103–1118.e7. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, C.; Yao, J.; Zhu, C.; Li, Z.; Liu, H.Y.; Zhu, M.; Li, K.; Ahmed, A.A.; Li, S.; et al. Lithocholic Acid Alleviates Deoxynivalenol-Induced Inflammation and Oxidative Stress via PPARγ-Mediated Epigenetically Transcriptional Reprogramming in Porcine Intestinal Epithelial Cells. J. Agric. Food Chem. 2024, 72, 5452–5462. [Google Scholar] [CrossRef]
- Li, Y.; Gu, F.; Gu, H.; Hu, P.; Liu, H.X.; Cai, D. Lithocholic Acid Alleviates Deoxynivalenol-Induced Lethal Cholesterol Metabolic Abnormalities in IPI-2I Cells. Metabolites 2022, 12, 659. [Google Scholar] [CrossRef]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670.e5. [Google Scholar] [CrossRef]
- Li, T.; Ding, N.; Guo, H.; Hua, R.; Lin, Z.; Tian, H.; Yu, Y.; Fan, D.; Yuan, Z.; Gonzalez, F.J.; et al. A gut microbiota-bile acid axis promotes intestinal homeostasis upon aspirin-mediated damage. Cell Host Microbe 2024, 32, 191–208.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, P.; Li, Y.; Shang, X.; Nan, X.; Shi, L.; Han, X.; Liu, J.; Hong, Y.; Li, Q.; et al. Gut microbiota-derived 12-ketolithocholic acid suppresses the IL-17A secretion from colonic group 3 innate lymphoid cells to prevent the acute exacerbation of ulcerative colitis. Gut Microbes 2023, 15, 2290315. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yue, C.; Zhang, H.; Chen, H.; Liu, Y.; Li, J. Deoxycholic Acid and Lithocholic Acid Alleviate Liver Injury and Inflammation in Mice with Klebsiella pneumoniae-Induced Liver Abscess and Bacteremia. J. Inflamm. Res. 2021, 14, 777–789. [Google Scholar] [CrossRef]
- Shao, J.; Ge, T.; Tang, C.; Wang, G.; Pang, L.; Chen, Z. Synergistic anti-inflammatory effect of gut microbiota and lithocholic acid on liver fibrosis. Inflamm. Res. 2022, 71, 1389–1401. [Google Scholar] [CrossRef]
- Keitel, V.; Donner, M.; Winandy, S.; Kubitz, R.; Häussinger, D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem. Biophys. Res. Commun. 2008, 372, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, Y.; Wang, H.; Yin, A.; Hu, J.; Yuan, T.; Zhou, S.; Xu, W.; Wei, P.; Yin, S.; et al. Gut commensal Parabacteroides distasonis alleviates inflammatory arthritis. Gut 2023, 72, 1664–1677. [Google Scholar] [CrossRef]
- Xiao, R.; Lei, K.; Kuok, H.; Deng, W.; Zhuang, Y.; Tang, Y.; Guo, Z.; Qin, H.; Bai, L.P.; Li, T. Synthesis and identification of lithocholic acid 3-sulfate as RORγt ligand to inhibit Th17 cell differentiation. J. Leukoc. Biol. 2022, 112, 835–843. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Huws, S.A.; Xu, G.; Li, J.; Ren, J.; Xu, J.; Guan, L.L.; Yao, J.; Wu, S. Ileal microbial microbiome and its secondary bile acids modulate susceptibility to nonalcoholic steatohepatitis in dairy goats. Microbiome 2024, 12, 247. [Google Scholar] [CrossRef]
- Marchianò, S.; Biagioli, M.; Giorgio, C.D.; Massa, C.; Bellini, R.; Bordoni, M.; Urbani, G.; Lachi, G.; Sepe, V.; Morretta, E.; et al. Allo-lithocholic acid, a microbiome derived secondary bile acid, attenuates liver fibrosis. Biochem. Pharmacol. 2025, 236, 116883. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, 53, D1670–D1676. [Google Scholar]
- Lian, S.; Lu, M.; Jiajing, L.; Zhang, B.; Fang, Y.; Wang, X.; Zheng, M.; Ni, Y.; Xu, G.; Yang, Y.; et al. Conjugated Lithocholic Acid Activates Hepatic TGR5 to Promote Lipotoxicity and MASLD-MASH Transition by Disrupting Carnitine Biosynthesis. Adv. Sci. 2025, 12, e2410602. [Google Scholar] [CrossRef]
- Varanasi, S.K.; Chen, D.; Liu, Y.; Johnson, M.A.; Miller, C.M.; Ganguly, S.; Lande, K.; LaPorta, M.A.; Hoffmann, F.A.; Mann, T.H.; et al. Bile acid synthesis impedes tumor-specific T cell responses during liver cancer. Science 2025, 387, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tang, L.; Chen, Q.; Wu, L.; He, W.; Tu, D.; Wang, S.; Chen, Y.; Liu, S.; Xie, Z.; et al. Disulfiram ameliorates nonalcoholic steatohepatitis by modulating the gut microbiota and bile acid metabolism. Nat. Commun. 2022, 13, 6862. [Google Scholar] [CrossRef] [PubMed]
- Marchianò, S.; Biagioli, M.; Roselli, R.; Zampella, A.; Di Giorgio, C.; Bordoni, M.; Bellini, R.; Morretta, E.; Monti, M.C.; Distrutti, E.; et al. Atorvastatin protects against liver and vascular damage in a model of diet induced steatohepatitis by resetting FXR and GPBAR1 signaling. FASEB J. 2022, 36, e22060. [Google Scholar]
- Juárez-Fernández, M.; Porras, D.; Petrov, P.; Román-Sagüillo, S.; García-Mediavilla, M.V.; Soluyanova, P.; Martínez-Flórez, S.; González-Gallego, J.; Nistal, E.; Jover, R.; et al. The Synbiotic Combination of Akkermansia muciniphila and Quercetin Ameliorates Early Obesity and NAFLD through Gut Microbiota Reshaping and Bile Acid Metabolism Modulation. Antioxidants 2021, 10, 2001. [Google Scholar] [CrossRef]
- Pathak, P.; Liu, H.; Boehme, S.; Xie, C.; Krausz, K.W.; Gonzalez, F.; Chiang, J.Y.L. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem. 2017, 292, 11055–11069. [Google Scholar] [CrossRef]
- McMahan, R.H.; Wang, X.X.; Cheng, L.L.; Krisko, T.; Smith, M.; El Kasmi, K.; Pruzanski, M.; Adorini, L.; Golden-Mason, L.; Levi, M.; et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J. Biol. Chem. 2013, 288, 11761–11770. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Wang, H.; Luo, W.; Clayton, Y.D.; Gu, L.; Du, Y.; Palle, S.K.; Chen, J.; Li, T. Gly-β-MCA is a potent anti-cholestasis agent against “human-like” hydrophobic bile acid-induced biliary injury in mice. J. Lipid Res. 2024, 65, 100649. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).