Lithocholic Acid Species: Metabolism, Signaling Pathways, and Clinical Significance in Enterohepatic Diseases
Abstract
1. Introduction
2. Metabolic Pathway and Microbial Transformation of LCA Species
3. LCA-Receptor Signaling Pathways in Maintaining Metabolic Homeostasis
4. Distribution and Concentrations of LCA Species in Human Biospecimen
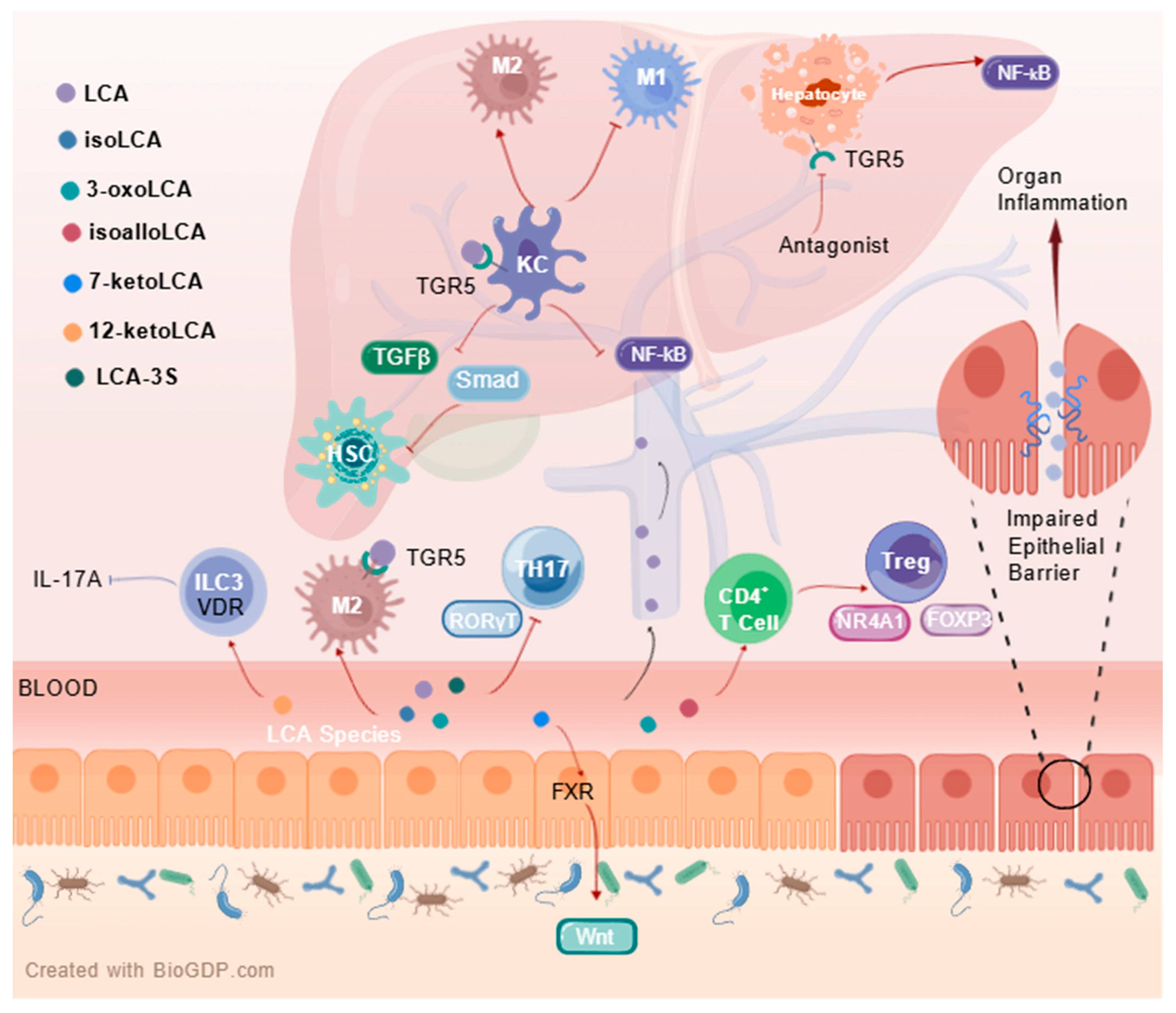
5. Clinical Significance of LCA Species in Enterohepatic Diseases
5.1. Diagnostic Biomarkers
5.2. Therapeutic Targets
5.2.1. Direct Effects of LCA Species on Intestinal Diseases
5.2.2. Direct Effects of LCA Species on Hepatic Diseases
5.2.3. Indirect Effects of LCA Species on Enterohepatic Diseases
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the micrbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, T.; Zhao, A.; Ning, Z.; Kuang, J.; Wang, S.; You, Y.; Bao, Y.; Ma, X.; Yu, H.; et al. Hyocholic acid species as novel biomarkers for metabolic disorders. Nat. Commun. 2021, 12, 1487. [Google Scholar] [CrossRef]
- Liu, A.N.; Xu, C.F.; Liu, Y.R.; Sun, D.Q.; Jiang, L.; Tang, L.J.; Zhu, P.W.; Chen, S.D.; Liu, W.Y.; Wang, X.D.; et al. Secondary bile acids improve risk prediction for non-invasive identification of mild liver fibrosis in nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2023, 57, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Luo, X.; Guo, J.; Xing, B.; Lin, H.; Ma, H.; Wang, Y.; Li, M.; Ye, C.; Yan, S.; et al. Identification of gut microbial bile acid metabolic enzymes via an AI-assisted pipeline. Cell 2025, 188, 6012–6027.e20. [Google Scholar] [CrossRef]
- Lun, W.; Yan, Q.; Guo, X.; Zhou, M.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Mechanism of action of the bile acid receptor TGR5 in obesity. Acta Pharm. Sin. B 2024, 14, 468–491. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.; Yao, L.; Zhang, Y.; Bae, S.; D’Agostino, G.D.; Zhang, M.; Kim, E.; Franzosa, E.A.; Avila-Pacheco, J.; Bisanz, J.E.; et al. Human gut bacteria produce Τ(H)17-modulating bile acid metabolites. Nature 2022, 603, 907–912. [Google Scholar] [CrossRef]
- Jung, Y.; Koo, B.K.; Jang, S.Y.; Kim, D.; Lee, H.; Lee, D.H.; Joo, S.K.; Jung, Y.J.; Park, J.H.; Yoo, T.; et al. Association between circulating bile acid alterations and nonalcoholic steatohepatitis independent of obesity and diabetes mellitus. Liver Int. 2021, 41, 2892–2902. [Google Scholar] [CrossRef]
- Li, W.; Hang, S.; Fang, Y.; Bae, S.; Zhang, Y.; Zhang, M.; Wang, G.; McCurry, M.D.; Bae, M.; Paik, D.; et al. A bacterial bile acid metabolite modulates T(reg) activity through the nuclear hormone receptor NR4A1. Cell Host Microbe 2021, 29, 1366–1377.e9. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, S.; Kovács, P.; Nyerges, P.; Ujlaki, G.; Sipos, A.; Uray, K.; Bai, P.; Mikó, E. The bacterial metabolite, lithocholic acid, has antineoplastic effects in pancreatic adenocarcinoma. Cell Death Discov. 2024, 10, 248. [Google Scholar] [CrossRef]
- Sun, L.; Li, F.; Tan, W.; Zhao, W.; Li, Y.; Zhu, X.; Gao, P.; Shu, G.; Wang, S.; Jiang, Q.; et al. Lithocholic acid promotes skeletal muscle regeneration through the TGR5 receptor. Acta Biochim. Biophys. Sin. 2023, 55, 51–61. [Google Scholar] [CrossRef]
- Qu, Q.; Chen, Y.; Wang, Y.; Wang, W.; Long, S.; Yang, H.Y.; Wu, J.; Li, M.; Tian, X.; Wei, X.; et al. Lithocholic acid binds TULP3 to activate sirtuins and AMPK to slow down ageing. Nature 2024, 643, 201–209. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, M.; Peng, Q.; Sha, K.; Liu, T.; Xia, J.; Xie, H.; Li, J.; Xu, S.; Deng, Z. Lithocholic acid promotes rosacea-like skin inflammation via G protein-coupled bile acid receptor 1. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166563. [Google Scholar] [CrossRef] [PubMed]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Gaskins, H.R. Another renaissance for bile acid gastrointestinal microbiology. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 348–364. [Google Scholar] [CrossRef]
- Wang, D.Q.; Cohen, D.E.; Carey, M.C. Biliary lipids and cholesterol gallstone disease. J. Lipid Res. 2009, 50, S406–S411. [Google Scholar] [CrossRef]
- Deo, A.K.; Bandiera, S.M. 3-ketocholanoic acid is the major in vitro human hepatic microsomal metabolite of lithocholic acid. Drug Metab. Dispos. 2009, 37, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Atarashi, K.; Plichta, D.R.; Arai, Y.; Sasajima, S.; Kearney, S.M.; Suda, W.; Takeshita, K.; Sasaki, T.; Okamoto, S.; et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 2021, 599, 458–464. [Google Scholar] [CrossRef]
- Doden, H.; Sallam, L.A.; Devendran, S.; Ly, L.; Doden, G.; Daniel, S.L.; Alves, J.M.P.; Ridlon, J.M. Metabolism of Oxo-Bile Acids and Characterization of Recombinant 12α-Hydroxysteroid Dehydrogenases from Bile Acid 7α-Dehydroxylating Human Gut Bacteria. Appl. Environ. Microbiol. 2018, 84, e00235-18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhang, H.; Zheng, X.; Zhao, A.; Jia, W. Novel microbial modifications of bile acids and their functional implications. iMeta 2024, 3, e243. [Google Scholar] [CrossRef]
- Rimal, B.; Collins, S.L.; Tanes, C.E.; Rocha, E.R.; Granda, M.A.; Solanki, S.; Hoque, N.J.; Gentry, E.C.; Koo, I.; Reilly, E.R.; et al. Bile salt hydrolase catalyses formation of amine-conjugated bile acids. Nature 2024, 626, 859–863. [Google Scholar] [CrossRef]
- Mohanty, I.; Mannochio-Russo, H.; Schweer, J.V.; El Abiead, Y.; Bittremieux, W.; Xing, S.; Schmid, R.; Zuffa, S.; Vasquez, F.; Muti, V.B.; et al. The underappreciated diversity of bile acid modifications. Cell 2024, 187, 1801–1818.e20. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, Q.; Zhou, J.H.; Xu, G. Enhanced activity and substrate tolerance of 7α-hydroxysteroid dehydrogenase by directed evolution for 7-ketolithocholic acid production. Appl. Microbiol. Biotechnol. 2019, 103, 2665–2674. [Google Scholar] [CrossRef]
- Hylemon, P.B.; Cacciapuoti, A.F.; White, B.A.; Whitehead, T.R.; Fricke, R.J. 7 alpha-Dehydroxylation of cholic acid by cell extracts of Eubacterium species V.P.I. 12708. Am. J. Clin. Nutr. 1980, 33, 2507–2510. [Google Scholar] [CrossRef]
- Mythen, S.M.; Devendran, S.; Méndez-García, C.; Cann, I.; Ridlon, J.M. Targeted Synthesis and Characterization of a Gene Cluster Encoding NAD(P)H-Dependent 3α-, 3β-, and 12α-Hydroxysteroid Dehydrogenases from Eggerthella CAG:298, a Gut Metagenomic Sequence. Appl. Environ. Microbiol. 2018, 84, e02475-17. [Google Scholar] [CrossRef]
- Sheng, W.; Ji, G.; Zhang, L. The Effect of Lithocholic Acid on the Gut-Liver Axis. Front. Pharmacol. 2022, 13, 910493. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Carazo, A.; Hyrsova, L.; Dusek, J.; Chodounska, H.; Horvatova, A.; Berka, K.; Bazgier, V.; Gan-Schreier, H.; Chamulitrat, W.; Kudova, E.; et al. Acetylated deoxycholic (DCA) and cholic (CA) acids are potent ligands of pregnane X (PXR) receptor. Toxicol. Lett. 2017, 265, 86–96. [Google Scholar] [CrossRef]
- Ðanić, M.; Stanimirov, B.; Pavlović, N.; Goločorbin-Kon, S.; Al-Salami, H.; Stankov, K.; Mikov, M. Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome. Front. Pharmacol. 2018, 9, 1382. [Google Scholar] [CrossRef]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin D receptor as an intestinal bile acid sensor. Science 2002, 296, 1313–1316. [Google Scholar] [CrossRef]
- Fiorucci, S.; Cipriani, S.; Baldelli, F.; Mencarelli, A. Bile acid-activated receptors in the treatment of dyslipidemia and related disorders. Prog. Lipid Res. 2010, 49, 171–185. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhou, J.M.; Ma, Z.C.; Li, H.; Liang, Q.D.; Tan, H.L.; Xiao, C.R.; Zhang, B.L.; Gao, Y. Pregnane X receptor mediated-transcription regulation of CYP3A by glycyrrhizin: A possible mechanism for its hepatoprotective property against lithocholic acid-induced injury. Chem. Biol. Interact. 2012, 200, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, J.; Xie, W.; Rosenfeld, J.M.; Barwick, J.L.; Guzelian, P.S.; Evans, R.M. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR). Proc. Natl. Acad. Sci. USA 2002, 99, 13801–13806. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Repa, J.J.; Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors and lipid physiology: Opening the X-files. Science 2001, 294, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Echchgadda, I.; Song, C.S. Vitamin D receptor regulation of the steroid/bile acid sulfotransferase SULT2A1. Methods Enzymol. 2005, 400, 165–191. [Google Scholar]
- Jahnel, J.; Zöhrer, E.; Scharnagl, H.; Erwa, W.; Fauler, G.; Stojakovic, T. Reference ranges of serum bile acids in children and adolescents. Clin. Chem. Lab. Med. 2015, 53, 1807–1813. [Google Scholar] [CrossRef]
- Tanaka, M.; Sanefuji, M.; Morokuma, S.; Yoden, M.; Momoda, R.; Sonomoto, K.; Ogawa, M.; Kato, K.; Nakayama, J. The association between gut microbiota development and maturation of intestinal bile acid metabolism in the first 3 y of healthy Japanese infants. Gut Microbes 2020, 11, 205–216. [Google Scholar] [CrossRef]
- Setchell, K.D.; Rodrigues, C.M.; Clerici, C.; Solinas, A.; Morelli, A.; Gartung, C.; Boyer, J. Bile acid concentrations in human and rat liver tissue and in hepatocyte nuclei. Gastroenterology 1997, 112, 226–235. [Google Scholar] [CrossRef]
- Mao, F.; Liu, T.; Hou, X.; Zhao, H.; He, W.; Li, C.; Jing, Z.; Sui, J.; Wang, F.; Liu, X.; et al. Increased sulfation of bile acids in mice and human subjects with sodium taurocholate cotransporting polypeptide deficiency. J. Biol. Chem. 2019, 294, 11853–11862. [Google Scholar] [CrossRef]
- Santos Silva, E.; Rocha, S.; Candeias Ramos, R.; Coutinho, H.; Catarino, C.; Teixeira, F.; Henriques, G.; Lopes, A.I.; Santos-Silva, A.; Brites, D. Bile acids profile and redox status in healthy infants. Pediatr. Res. 2023, 93, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Bathena, S.P.R.; Mukherjee, S.; Olivera, M.; Alnouti, Y. The profile of bile acids and their sulfate metabolites in human urine and serum. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 942–943, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Choucair, I.; Mallela, D.P.; Hilser, J.R.; Hartiala, J.A.; Nemet, I.; Gogonea, V.; Li, L.; Lusis, A.J.; Fischbach, M.A.; Tang, W.H.W.; et al. Comprehensive Clinical and Genetic Analyses of Circulating Bile Acids and Their Associations with Diabetes and Its Indices. Diabetes 2024, 73, 1215–1228. [Google Scholar] [CrossRef]
- Zheng, D.; Ge, K.; Qu, C.; Sun, T.; Wang, J.; Jia, W.; Zhao, A. Comparative profiling of serum, urine, and feces bile acids in humans, rats, and mice. Commun. Biol. 2024, 7, 641. [Google Scholar] [CrossRef]
- Humbert, L.; Maubert, M.A.; Wolf, C.; Duboc, H.; Mahe, M.; Farabos, D.; Seksik, P.; Mallet, J.M.; Trugnan, G.; Masliah, J.; et al. Bile acid profiling in human biological samples: Comparison of extraction procedures and application to normal and cholestatic patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 899, 135–145. [Google Scholar] [CrossRef]
- Schauermann, M.; Wang, R.; Pons-Kuehnemann, J.; Hartmann, M.F.; Remer, T.; Hua, Y.; Bereket, A.; Wasniewska, M.; Shmoish, M.; Hochberg, Z.; et al. Targeted quantitative analysis of urinary bile acids by liquid chromatography-tandem mass spectrometry: Method development and application to healthy and obese children. J. Steroid Biochem. Mol. Biol. 2025, 249, 106712. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Myint, K.T.; Sato, K.; Wada, O.; Kakiyama, G.; Iida, T.; Hishinuma, T.; Mano, N.; Goto, J. LC/ESI-tandem mass spectrometric determination of bile acid 3-sulfates in human urine 3beta-Sulfooxy-12alpha-hydroxy-5beta-cholanoic acid is an abundant nonamidated sulfate. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 846, 69–77. [Google Scholar] [CrossRef]
- Sommersberger, S.; Gunawan, S.; Elger, T.; Fererberger, T.; Loibl, J.; Huss, M.; Kandulski, A.; Krautbauer, S.; Muller, M.; Liebisch, G.; et al. Altered fecal bile acid composition in active ulcerative colitis. Lipids Health Dis. 2023, 22, 199. [Google Scholar] [CrossRef]
- Farhat, Z.; Sampson, J.N.; Hildesheim, A.; Safaeian, M.; Porras, C.; Cortes, B.; Herrero, R.; Romero, B.; Vogtmann, E.; Sinha, R.; et al. Reproducibility, Temporal Variability, and Concordance of Serum and Fecal Bile Acids and Short Chain Fatty Acids in a Population-Based Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1875–1883. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef]
- Adams, L.A.; Wang, Z.; Liddle, C.; Melton, P.E.; Ariff, A.; Chandraratna, H.; Tan, J.; Ching, H.; Coulter, S.; de Boer, B.; et al. Bile acids associate with specific gut microbiota, low-level alcohol consumption and liver fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2020, 40, 1356–1365. [Google Scholar] [CrossRef]
- Suga, T.; Yamaguchi, H.; Ogura, J.; Shoji, S.; Maekawa, M.; Mano, N. Altered bile acid composition and disposition in a mouse model of non-alcoholic steatohepatitis. Toxicol. Appl. Pharmacol. 2019, 379, 114664. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Jiang, R.; Wang, X.; Liu, P.; Zhao, A.; Wu, Y.; Huang, F.; Liu, Z.; Rajani, C.; Zheng, X.; et al. Conjugated secondary 12α-hydroxylated bile acids promote liver fibrogenesis. EBioMedicine 2021, 66, 103290. [Google Scholar] [CrossRef] [PubMed]
- Nimer, N.; Choucair, I.; Wang, Z.; Nemet, I.; Li, L.; Gukasyan, J.; Weeks, T.L.; Alkhouri, N.; Zein, N.; Tang, W.H.W.; et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism 2021, 116, 154457. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, D.; Mai, M.; Song, W.; Yuan, Q.; Xie, Y.; Mo, B.; Guo, H. Biological gender difference of bile acid metabolism in susceptibility to cholelithiasis in patients with nonalcoholic fatty liver disease. J. Steroid Biochem. Mol. Biol. 2025, 253, 106812. [Google Scholar] [CrossRef] [PubMed]
- Aliwa, B.; Horvath, A.; Traub, J.; Feldbacher, N.; Habisch, H.; Fauler, G.; Madl, T.; Stadlbauer, V. Altered gut microbiome, bile acid composition and metabolome in sarcopenia in liver cirrhosis. J. Cachexia Sarcopenia Muscle 2023, 14, 2676–2691. [Google Scholar] [CrossRef]
- Sanchez, J.I.; Fontillas, A.C.; Kwan, S.Y.; Sanchez, C.I.; Calderone, T.L.; Lee, J.L.; Elsaiey, A.; Cleere, D.W.; Wei, P.; Vierling, J.M.; et al. Metabolomics biomarkers of hepatocellular carcinoma in a prospective cohort of patients with cirrhosis. JHEP Rep. 2024, 6, 101119. [Google Scholar] [CrossRef]
- Wei, H.; Suo, C.; Gu, X.; Shen, S.; Lin, K.; Zhu, C.; Yan, K.; Bian, Z.; Chen, L.; Zhang, T.; et al. AKR1D1 suppresses liver cancer progression by promoting bile acid metabolism-mediated NK cell cytotoxicity. Cell Metab. 2025, 37, 1103–1118.e7. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, C.; Yao, J.; Zhu, C.; Li, Z.; Liu, H.Y.; Zhu, M.; Li, K.; Ahmed, A.A.; Li, S.; et al. Lithocholic Acid Alleviates Deoxynivalenol-Induced Inflammation and Oxidative Stress via PPARγ-Mediated Epigenetically Transcriptional Reprogramming in Porcine Intestinal Epithelial Cells. J. Agric. Food Chem. 2024, 72, 5452–5462. [Google Scholar] [CrossRef]
- Li, Y.; Gu, F.; Gu, H.; Hu, P.; Liu, H.X.; Cai, D. Lithocholic Acid Alleviates Deoxynivalenol-Induced Lethal Cholesterol Metabolic Abnormalities in IPI-2I Cells. Metabolites 2022, 12, 659. [Google Scholar] [CrossRef]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670.e5. [Google Scholar] [CrossRef]
- Li, T.; Ding, N.; Guo, H.; Hua, R.; Lin, Z.; Tian, H.; Yu, Y.; Fan, D.; Yuan, Z.; Gonzalez, F.J.; et al. A gut microbiota-bile acid axis promotes intestinal homeostasis upon aspirin-mediated damage. Cell Host Microbe 2024, 32, 191–208.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, P.; Li, Y.; Shang, X.; Nan, X.; Shi, L.; Han, X.; Liu, J.; Hong, Y.; Li, Q.; et al. Gut microbiota-derived 12-ketolithocholic acid suppresses the IL-17A secretion from colonic group 3 innate lymphoid cells to prevent the acute exacerbation of ulcerative colitis. Gut Microbes 2023, 15, 2290315. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yue, C.; Zhang, H.; Chen, H.; Liu, Y.; Li, J. Deoxycholic Acid and Lithocholic Acid Alleviate Liver Injury and Inflammation in Mice with Klebsiella pneumoniae-Induced Liver Abscess and Bacteremia. J. Inflamm. Res. 2021, 14, 777–789. [Google Scholar] [CrossRef]
- Shao, J.; Ge, T.; Tang, C.; Wang, G.; Pang, L.; Chen, Z. Synergistic anti-inflammatory effect of gut microbiota and lithocholic acid on liver fibrosis. Inflamm. Res. 2022, 71, 1389–1401. [Google Scholar] [CrossRef]
- Keitel, V.; Donner, M.; Winandy, S.; Kubitz, R.; Häussinger, D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem. Biophys. Res. Commun. 2008, 372, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, Y.; Wang, H.; Yin, A.; Hu, J.; Yuan, T.; Zhou, S.; Xu, W.; Wei, P.; Yin, S.; et al. Gut commensal Parabacteroides distasonis alleviates inflammatory arthritis. Gut 2023, 72, 1664–1677. [Google Scholar] [CrossRef]
- Xiao, R.; Lei, K.; Kuok, H.; Deng, W.; Zhuang, Y.; Tang, Y.; Guo, Z.; Qin, H.; Bai, L.P.; Li, T. Synthesis and identification of lithocholic acid 3-sulfate as RORγt ligand to inhibit Th17 cell differentiation. J. Leukoc. Biol. 2022, 112, 835–843. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Huws, S.A.; Xu, G.; Li, J.; Ren, J.; Xu, J.; Guan, L.L.; Yao, J.; Wu, S. Ileal microbial microbiome and its secondary bile acids modulate susceptibility to nonalcoholic steatohepatitis in dairy goats. Microbiome 2024, 12, 247. [Google Scholar] [CrossRef]
- Marchianò, S.; Biagioli, M.; Giorgio, C.D.; Massa, C.; Bellini, R.; Bordoni, M.; Urbani, G.; Lachi, G.; Sepe, V.; Morretta, E.; et al. Allo-lithocholic acid, a microbiome derived secondary bile acid, attenuates liver fibrosis. Biochem. Pharmacol. 2025, 236, 116883. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, 53, D1670–D1676. [Google Scholar]
- Lian, S.; Lu, M.; Jiajing, L.; Zhang, B.; Fang, Y.; Wang, X.; Zheng, M.; Ni, Y.; Xu, G.; Yang, Y.; et al. Conjugated Lithocholic Acid Activates Hepatic TGR5 to Promote Lipotoxicity and MASLD-MASH Transition by Disrupting Carnitine Biosynthesis. Adv. Sci. 2025, 12, e2410602. [Google Scholar] [CrossRef]
- Varanasi, S.K.; Chen, D.; Liu, Y.; Johnson, M.A.; Miller, C.M.; Ganguly, S.; Lande, K.; LaPorta, M.A.; Hoffmann, F.A.; Mann, T.H.; et al. Bile acid synthesis impedes tumor-specific T cell responses during liver cancer. Science 2025, 387, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tang, L.; Chen, Q.; Wu, L.; He, W.; Tu, D.; Wang, S.; Chen, Y.; Liu, S.; Xie, Z.; et al. Disulfiram ameliorates nonalcoholic steatohepatitis by modulating the gut microbiota and bile acid metabolism. Nat. Commun. 2022, 13, 6862. [Google Scholar] [CrossRef] [PubMed]
- Marchianò, S.; Biagioli, M.; Roselli, R.; Zampella, A.; Di Giorgio, C.; Bordoni, M.; Bellini, R.; Morretta, E.; Monti, M.C.; Distrutti, E.; et al. Atorvastatin protects against liver and vascular damage in a model of diet induced steatohepatitis by resetting FXR and GPBAR1 signaling. FASEB J. 2022, 36, e22060. [Google Scholar]
- Juárez-Fernández, M.; Porras, D.; Petrov, P.; Román-Sagüillo, S.; García-Mediavilla, M.V.; Soluyanova, P.; Martínez-Flórez, S.; González-Gallego, J.; Nistal, E.; Jover, R.; et al. The Synbiotic Combination of Akkermansia muciniphila and Quercetin Ameliorates Early Obesity and NAFLD through Gut Microbiota Reshaping and Bile Acid Metabolism Modulation. Antioxidants 2021, 10, 2001. [Google Scholar] [CrossRef]
- Pathak, P.; Liu, H.; Boehme, S.; Xie, C.; Krausz, K.W.; Gonzalez, F.; Chiang, J.Y.L. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem. 2017, 292, 11055–11069. [Google Scholar] [CrossRef]
- McMahan, R.H.; Wang, X.X.; Cheng, L.L.; Krisko, T.; Smith, M.; El Kasmi, K.; Pruzanski, M.; Adorini, L.; Golden-Mason, L.; Levi, M.; et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J. Biol. Chem. 2013, 288, 11761–11770. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Wang, H.; Luo, W.; Clayton, Y.D.; Gu, L.; Du, Y.; Palle, S.K.; Chen, J.; Li, T. Gly-β-MCA is a potent anti-cholestasis agent against “human-like” hydrophobic bile acid-induced biliary injury in mice. J. Lipid Res. 2024, 65, 100649. [Google Scholar] [CrossRef] [PubMed]

| Enzymes | Bacterial Taxa | Substrate | Product | Refs. |
|---|---|---|---|---|
| BSH | Clostridia Bacteroides Enterococcus Bifidobacteria Lactobacilli methanogenic archaea | Conjugated bile acids or free bile acids | Unconjugated bile acids | [15] |
| 5α-reductase | P. goldsteinii B. theta. A. onderdonkii | 3-oxo-∆4-LCA | 3-oxoalloLCA | [18] |
| 5β-reductase | H. hathewayi Lachnospiraceae | 3-oxo-∆4-LCA | 3-oxoLCA | [18] |
| 3α-HSDH | R. timonensis Lachnospiraceae P. distasonis | LCA 3-oxoalloLCA | 3-oxoLCA alloLCA | [18] |
| 3β-HSDH | O. laneus Odoribacteraceae P. distasonis P. merdae | 3-oxoLCA 3-oxoalloLCA | isoLCA isoalloLCA | [18] |
| 7α-HSDH | C. absonum C. hiranonis C. hylemonae Eubacterium sp. | CDCA | 7-oxoLCA | [19,23,24] |
| 12α-HSDH | C. scindens C. hiranonis C. hylemonae Eggerthella sp. | 12-oxoLCA | DCA | [19,25] |
| Sample Type | Plasma | Plasma | Serum | Serum | Serum | Serum |
|---|---|---|---|---|---|---|
| Age | / | 2 months | 6~24 months | 19~65 years | 54.5~71.3 years | 21~31 years |
| Instrument type | UPLC–MS/MS | HPLC | UPLC–HRMS | LC–MS/MS | LC–MS/MS | UPLC–TQMS |
| sample size | n = 43 Mean ± SD | n = 32 Median (IQR) | n = 15 Mean ± SD | n = 90 Mean ± SEM | n = 1670 Median (IQR) | n = 50 Mean ± SEM |
| LCA | 3.98 ± 4.07 | / | 10 ± 20 | 7.50 ± 0.38 | 28.2 (19. 5–39.2) | 20.06 ± 13.97 |
| isoLCA | 1.48 ± 2.73 | / | / | 4.93 ± 0.28 | 16.9 (8. 8–30.3) | 37.22 ± 39.41 |
| alloLCA | / | / | / | / | / | 4.46 ± 4.20 |
| isoalloLCA | 1.08 ± 0.71 | / | / | / | / | / |
| GLCA | 14.42 ± 27.68 | 300 (200–480) | 40 ± 60 | 10.7 ± 0.8 | 7.9 (4.2–17.3) | 11.15 ± 8.34 |
| TLCA | 1.21 ± 1.99 | 250 (130–400) | 80 ± 90 | 1.64 ± 0.15 | 4.0 (3.0–6.1) | 2.05 ± 1.82 |
| 3-oxoLCA | 0.8 ± 1.31 | / | / | / | 1.4 (0.1–3.3) | / |
| 6-oxoLCA | / | / | / | / | 0.7 (0.1–1.5) | / |
| 7-oxoLCA | 14.61 ± 23.82 | / | / | 5.90 ± 0.57 | 12.7 (8.2–22.4) | 7.92 ± 12.54 |
| 12-oxoLCA | 2.36 ± 4.02 | / | / | 8.58 ± 0.97 | 4.4 (1.7–9.6) | 14.44 ± 11.4 |
| 6,7-dioxoLCA | 3.06 ± 5.55 | / | / | / | / | ND |
| 7,12-dioxoLCA | 7.12 ± 9.05 | / | / | / | / | / |
| LCA-3-S | / | / | / | 5.36 ± 0.54 | 7.5 (3.4–15.3) | 20.87 ± 28.21 |
| GLCA-3-S | 130.94 ± 261.36 | / | / | 275.5 ± 15.0 | / | 509.08 ± 468.34 |
| TLCA-3-S | 0.68 ± 1.28 | / | / | 83.0 ± 5.1 | / | 90.32 ± 84.24 |
| Refs. | [42] | [43] | [39] | [44] | [45] | [46] |
| Sample Type | Random Urine | Random Urine | Random Urine | 24 h Urine | Random Urine |
|---|---|---|---|---|---|
| Age | 19~65 years | 21~31 years | / | 2.98~18.37 years | 22~23 years |
| Instrument type | LC–MS/MS | UPLC–TQMS | HPLC–MS/MS | LC–MS/MS | LC/ESI–MS/MS |
| sample size | n = 90 Mean ± SEM | n = 50 Mean ± SEM [nmol/mmol creatinine] | n = 39 Mean ± SD | n = 80 Mean ± SD | n = 12 Mean ± SD [nmol/mmol creatinine] |
| LCA | 0.15 ± 0.02 | 0.28 ± 0.26 | ND | ND | / |
| isoLCA | ND | ND | / | / | / |
| alloLCA | / | ND | / | / | / |
| isoalloLCA | / | / | / | / | / |
| GLCA | 0.36 ± 0.03 | ND | ND | ND | / |
| TLCA | 0.32 ± 0.03 | ND | ND | ND | / |
| 3-oxoLCA | / | ND | / | / | / |
| 6-oxoLCA | / | ND | / | / | / |
| 7-oxoLCA | 2.27 ± 0.55 | 0.16 ± 0.26 | / | / | / |
| 12-oxoLCA | 2.17 ± 0.48 | 0.18 ± 0.34 | / | / | / |
| 6,7-dioxoLCA | / | 0.03 ± 0.14 | / | / | / |
| 7,12-dioxoLCA | / | 0.1 ± 0.23 | / | / | / |
| LCA-3-S | 7.19 ± 0.63 | 2.76 ± 5.36 | ND | 4. 2 ± 13.5 | ND |
| GLCA-3-S | 8082 ± 46.1 | 109.6 ± 110.79 | 185.73 ± 151.63 | 580.6 ± 637.6 | 73.99 ± 68.71 |
| TLCA-3-S | 222.9 ± 12.5 | 17.96 ± 19.44 | 108.6 ± 95.39 | 234.3 ± 232.5 | 17.10 ± 18.06 |
| Refs. | [44] | [46] | [47] | [48] | [49] |
| Sample Type | Freeze-Dried Fecal Sample | Freeze-Dried Fecal Sample | Freeze-Dried Fecal Sample | Fresh Fecal Sample |
|---|---|---|---|---|
| Age | 21~31 years | / | 23~78 years | ≥18 years |
| Instrument type | UPLC–TQMS | UPLC/MRM–MS | LC–MS/MS | LC–MS/MS |
| sample size | n = 50 Mean ± SEM | n = 43 Mean ± SD | n = 17 Median (Min, Max) | n = 136 Median (IQR) |
| LCA | 3643.61 ± 2385.74 | 1016.60 ± 647.31 | 8401.19 (2112.54, 23,499.31) | 549.79 (375.44–764.44) |
| isoLCA | 480.8 ± 455.9 | / | / | / |
| alloLCA | 37.6 ± 62.36 | / | / | / |
| isoalloLCA | / | / | / | / |
| GLCA | 0.64 ± 0.73 | 6.68 ± 18.49 | 5.06 (0.00, 8.59) | 0.13 (0.08–0.23) |
| TLCA | 2.4 ± 2.05 | 0.51 ± 0.40 | 0.32 (0.00, 8.90) | 0.27 (0.10–0.75) |
| 3-oxoLCA | 402.77 ± 600.14 | / | / | / |
| 6-oxoLCA | 18.93 ± 16.57 | / | / | / |
| 7-oxoLCA | / | / | / | / |
| 12-oxoLCA | / | / | / | / |
| 6,7-dioxoLCA | ND | / | / | / |
| 7,12-dioxoLCA | 4.52 ± 6.37 | / | / | / |
| LCA-3-S | 174.03 ± 282.05 | 7.76 ± 9.24 | / | / |
| GLCA-3-S | 7.71 ± 10.4 | 1.26 ± 1.24 | / | / |
| TLCA-3-S | 4.16 ± 4.97 | 0.77 ± 0.63 | / | / |
| Refs. | [46] | [47] | [50] | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, L.; Zhou, G.; Liu, A.; Wang, H.; Ni, Y. Lithocholic Acid Species: Metabolism, Signaling Pathways, and Clinical Significance in Enterohepatic Diseases. Int. J. Mol. Sci. 2025, 26, 11530. https://doi.org/10.3390/ijms262311530
Leng L, Zhou G, Liu A, Wang H, Ni Y. Lithocholic Acid Species: Metabolism, Signaling Pathways, and Clinical Significance in Enterohepatic Diseases. International Journal of Molecular Sciences. 2025; 26(23):11530. https://doi.org/10.3390/ijms262311530
Chicago/Turabian StyleLeng, Lianggui, Guangzeng Zhou, Ana Liu, Huiying Wang, and Yan Ni. 2025. "Lithocholic Acid Species: Metabolism, Signaling Pathways, and Clinical Significance in Enterohepatic Diseases" International Journal of Molecular Sciences 26, no. 23: 11530. https://doi.org/10.3390/ijms262311530
APA StyleLeng, L., Zhou, G., Liu, A., Wang, H., & Ni, Y. (2025). Lithocholic Acid Species: Metabolism, Signaling Pathways, and Clinical Significance in Enterohepatic Diseases. International Journal of Molecular Sciences, 26(23), 11530. https://doi.org/10.3390/ijms262311530






