Abstract
S-nitrosoglutathione (GSNO) reductase (GSNOR) is a major and conserved enzyme in prokaryotes and eukaryotes. It reduces a stable nitric oxide (NO) reservoir, GSNO, to balance the organisms’ redox status through S-nitrosylation. Over the last few decades, much of our understanding of GSNOR’s roles in plant biology has been updated. Here, therefore, we review the current knowledge of GSNOR in plant physiology and signaling under abiotic and biotic stresses. We observe that the role of GSNOR in plant abiotic stress is widely studied in both model and crop plants, whereas studies on its role in biotic stress have mainly focused on model plants. Under abiotic stresses, GSNOR plays a pleiotropic role in terms of plant tolerance and sensitivity. The presence or absence of GSNOR activity modulates the endogenous NO pool that balances plant reactive nitrogen species (RNS) and reactive oxygen species (ROS) under stress conditions. Moreover, GSNOR regulates hormonal levels, like ethylene, abscisic acid (ABA), jasmonic acid (JA), and salicylic acid (SA), in response to abiotic and biotic stress conditions. Although GSNOR is important in plant physiology, its regulation of the redox switch is directly influenced by the extent of S-nitrosylation, where S-nitrosylated proteins generally enhance plant tolerance to abiotic stress but simultaneously suppress plant immunity. We further highlight a new perspective on NO-based nanotechnology in agriculture, focusing on GSNO encapsulated in nanocarriers. This technology improves NO stability and opens new avenues by allowing an evaluation of GSNOR’s role for sustainable crop production. Intriguingly, we discuss knowledge gaps, which are crucial to understanding the role of GSNOR in plant stress tolerance. Overall, this review accumulates a comprehensive understanding of the GSNOR enzyme in crop biology, which could aid in harnessing its function to address the impacts of climate change.
1. Introduction
1.1. Nitric Oxide
Nitric oxide (NO) is a short-lived, diatomic gas molecule with a redox-active nature that is produced by one cell, and it penetrates membranes to regulate the functions of other cells [,]. Initially, it gained prominence as a key signaling molecule for muscle relaxation, identified by endothelium-derived relaxing factor (EDRF) [,,]. Even before that, Priestley [] reported that NO consists of a single oxygen and nitrogen. Meanwhile, Klepper and his team observed the possible NO evolution from soybean and winged bean plants in vivo through nitrate reductase (NR) assay [,,]. While the primary source of mammalian NO is nitric oxide synthase (NOS), which converts L-arginine to NO and citrulline [], a homologous gene has not been found in plants []. Instead, plant NO production is mediated by the nitrate reduction pathway, which is catalyzed by NR, which reduces nitrate to nitrite and then to NO (Figure 1A) [,,]. Few studies still hypothesize that plants possess NOS-like activity for NO production [,]. Hence, researchers are still in the race for identifying the additional occurrence and evolution of NO production in plants []. Although its synthesis differs between mammals and plants, NO functions as a paramount signaling molecule in a wide range of biological processes. However, we have little knowledge of how precisely NO signaling regulates crop yield traits to advance agricultural production. Ongoing research continues to reveal additional regulators and mechanisms, expanding our understanding of the NO-mediated complex network. In this review, we highlight how S-nitrosoglutathione (GSNO) reductase (GSNOR) regulates NO response in plants.
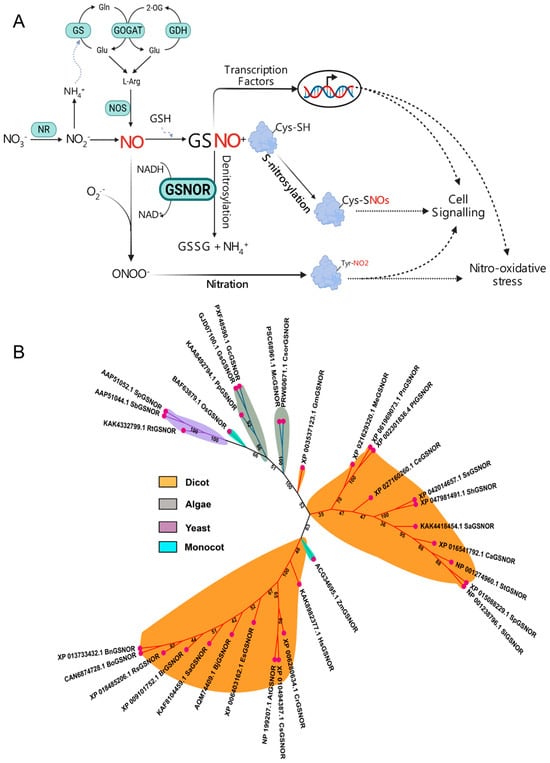
Figure 1.
(A) Schematic of nitric oxide (NO) signaling and associated pathways. NO is produced via different pathways in mammals and plants. In mammals, it is generated through an oxidative pathway by nitric oxide synthase (NOS), while in plants, a reductive pathway involving nitrate reductase (NR) is a major source. NO reacts with glutathione (GSH) in an O2-dependent manner to form S-nitrosoglutathione (GSNO). GSNO functions as a stable NO reservoir and carrier, transferring its NO moiety to the sulfhydryl (-SH) group of a cysteine residue on target proteins. This process is called S-nitrosylation, a unique redox-based post-translational modification that forms S-nitrosothiols (SNOs). In contrast, the conserved enzyme GSNO reductase (GSNOR) facilitates de-nitrosylation by reducing GSNO to glutathione disulfide (GSSG) and ammonium (NH4+) using an electron donor from NADH. The balance between S-nitrosylation and de-nitrosylation is crucial for regulating diverse biological processes. Figure was created with the software BioRender (https://www.biorender.com/). (B) An unrooted phylogenetic tree was prepared using the neighbor-joining method with a bootstrap value of 1000 in the Mega tool and iTOL (https://itol.embl.de/, accessed on 15 September 2025). The protein sequences were downloaded from the NCBI database (https://www.ncbi.nlm.nih.gov/, accessed on 10 September 2025) and used to analyze the evolutionary relationships among the GSNOR orthologs.
1.2. An Introduction to the GSNOR Enzyme and Its Role in GSNO Turnover
NO reacts with glutathione (GSH) through an O2-dependent reaction to form GSNO, which functions as a stable reservoir and carrier of NO (Figure 1A) [,]. Unlike the short-lived NO, GSNO is likely mobile within the phloem, enabling it to travel long distances to redox signaling [,]. GSNO transfers its NO moiety to the sulfhydryl (-SH) group of a cystine residue on target proteins to form low-molecular-mass S-nitrosothiol (SNO) (Figure 1A). This process, called protein S-nitrosylation, is a unique redox-based post-translational modification (PTM) [,,,]. SNO can further transfer the NO moiety to the -SH of other cystine proteins to form high-molecular-mass SNOs, known as thiol-to-thiol SNO formation (S-transnitrosylation) [,]. The stability of GSNO necessitates a mechanism for its controlled turnover. This function is critically managed by the enzyme GSNOR, which acts as the key governor of S-nitrosylation. Over two decades ago, GSNO turnover by GSNOR was first reported in purified Escherichia coli, which is similarly thought to be a reducing agent of GSNO in yeast and mice []. GSNOR uses the electron donor NADH as a cofactor to reduce GSNO into glutathione disulfide (GSSG) and ammonium (NH4+), which ultimately reduces the cellular levels of GSNO and SNO (Figure 1A) []. An ortholog of this single protein (AtGSNOR1; AT5G43940; 379 residues) was also identified in Arabidopsis thaliana, which was originally a class III alcohol dehydrogenase (ADH3, EC 1.1.1.1)/glutathione-dependent formaldehyde dehydrogenase (FALDH, EC 1.2.1.1) [,]. Even a decade before, Koivusalo et al. [] found that ADH3 and FALDH are identical enzymes. Loss-of-function mutation in AtGSNOR1 (Atgsnor1-3) results in higher cellular levels of both GSNO and total SNO. In contrast, gain-of-function (Atgsnor1-1) exhibited elevated GSNOR activity with a marked reduction in GSNO and SNO []. Thus, GSNOR not only turns over cellular GSNO, but, by doing so, also depletes the total SNO pool, which then limits the extent of protein S-nitrosylation (Figure 1A).
To understand the evolutionary map of GSNOR across dicots, monocots, algae, and yeast, we performed a phylogenetic tree analysis. The tree showed that GSNOR maintains an evolutionary relationship across these species (Figure 1B). Additionally, we employed a multiple sequence alignment by Clustal W (SnapGene 8.1.0) that showed a high level of sequence conservation (>95%) across all selected species (Table S1; Figure S1). Notably, a total of nine highly conserved cystine regions were observed among monocots, dicots, and algae, including Cys10, Cys47, Cys99, Cys102, Cys105, Cys113, Cys178, and Cys272 (Figure S1). These results suggest that GSNOR is a conserved protein in plant species, potentially a crucial regulator of S-nitrosylation and de-nitrosylation. The regulatory mechanism of S-nitrosylation is a rapidly updating field of research, as its ubiquitous involvement in biological processes becomes increasingly clear. This review, therefore, racks up a series of studies that have investigated S-nitrosylated proteins in plants under various growth and stress conditions (Table 1). These investigations commonly employed a biotic switch assay coupled with a liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis system [,]. In the following sections, we discuss how the S-nitrosylation of these proteins affects the function of GSNOR in plants or vice versa. Moreover, we noticed that multiple previous discussions were solely focused on S-nitrosylation in diverse proteins [,,] or inadequately addressed the multifaceted role of GSNOR in plants [,,,]. Therefore, this review discusses the role of GSNOR in plant stress responses by integrating up-to-date findings and knowledge gaps from studies that used genetic and exogenous approaches to modify cellular GSNOR activity and GSNO levels.

Table 1.
List of S-nitrosylation sites and detection methods in A. thaliana and crops under abiotic and biotic stress.
Table 1.
List of S-nitrosylation sites and detection methods in A. thaliana and crops under abiotic and biotic stress.
| Species | S-Nitrosylation Sites | Target Protein | Upstream Signal | Detection Method | Reference |
|---|---|---|---|---|---|
| Arabidopsis thaliana | Cys-80 | BIK1 | P/MAMPs | Biotin switch assay | [] |
| Arabidopsis thaliana | Cys-425, Cys-607 | COP1 | Light | Biotin switch assay, nano-LC-MS/MS | [] |
| Arabidopsis thaliana | - | QSOX1 | Heat stress | Biotin switch assay | [] |
| Arabidopsis thaliana | Cys-337 | ERO1 | ER stress | TMT labeling, LC-MS/MS | [] |
| Arabidopsis thaliana | Cys-137 | HDA19 | Oxidative stress | Biotin switch assay, TMT labeling, LC-MS/MS | [] |
| Arabidopsis thaliana | Cys-374 | RGA | Salt stress | Biotin switch assay, LC-MS/MS | [] |
| Arabidopsis thaliana | Cys-164 | HFR1 | High temperature | Biotin switch assay | [] |
| Arabidopsis thaliana | - | AtNRAMP3, AtNRAMP4, AtPIC1 | Iron deficiency | GPS-SNO 1.0 software (in silico and protein stability assay) | [] |
| Arabidopsis thaliana | Cys-10 | GSNOR1 | Hypoxia | Biotin switch assay, DAN Assay, LC-MS/MS | [] |
| Arabidopsis thaliana | Cys-137 | SnRK 2.6 | Drought | Biotin switch assay, LC-MS/MS | [] |
| Arabidopsis thaliana | Cys-32 | APX1 | Oxidative stress | Biotin switch assay, DAN assay, LC-MS/MS | [] |
| Arabidopsis thaliana | Cys-890 | RBOHD | Pathogen | Biotin switch assay, LC-MS | [] |
| Arabidopsis thaliana | Cys-28 | AtSABP3 | Pathogen infection | Biotin switch assay, LC-MS/MS | [] |
| Arabidopsis thaliana | Cys-156 | NPR1 | Pathogen infection | Biotin switch assay | [] |
| Tomato (Solanum lycopersicum) | Cys-316, Cys-258, Cys-316 | SlGABA-TP1, SlGABA-TP2, SlGABA-TP3 | Saline-alkaline stress | Biotin switch assay | [] |
| Tomato (Solanum lycopersicum) | Cys-5 | SlP5CR | Drought and salt stress | Biotin switch assay | [] |
| Tomato (Solanum lycopersicum) | Cys-54 | SlTrxh | Nitrate stress | Biotin switch assay, LC-MS/MS | [] |
| Tomato (Solanum lycopersicum) | Cys-172 | ACOh4 | Salt stress | Biotin switch assay, LC-MS/MS | [] |
| Mini Chinese Cabbage (Brassica rapa ssp. pekinensis) | - | BrGSNOR | Low temperature stress | Biotin switch assay | [] |
| Peach (Prunus persica (L.) Batsch) | Cys-85 | - | Pathogen infection | Iodo-TMT labeling, LC-MS/MS | [] |
Full abbreviations from the table can be found in the main text or Abbreviation section.
2. Changes in GSNOR Activity Affect Abiotic Stress Tolerance
Given the multifaceted role of GSNOR in plant growth, it is also vital for balancing endogenous NO levels and regulating signaling in the plant response to abiotic stress [,,]. The multitude of NO responses in plants under abiotic stress poses a major challenge to understanding their precise signaling mechanisms. Additionally, the function of GSNOR in enabling plant stress adaptation is being widely studied, focusing on responses to potential agricultural devastation caused by climate-change-driven catastrophes. In this section, we provide an update on core GSNOR-mediated NO signaling mechanisms connected with signaling transductions under various abiotic stresses.
2.1. High- and Low-Temperature Stress Tolerance
This review explored differences in the thermotolerance of endogenous NO and GSNO levels in A. thaliana, which were then related to GSNOR activity. NO-deficient Arabidopsis mutants, noa1 and nia1nia2, showed an enhanced heat acclimation response []. Conversely, the GSNOR null mutant, hot5-2/4, which accumulates excessive GSNO, displayed high heat sensitivity, suggesting that elevated RNS through nitrosative stress blunts heat acclimation (Figure 2; Table 2) []. A consistent result was observed in tomato plants with suppressed SlGSNOR activity. This compromised heat acclimation was linked to several molecular changes: reduced levels of ABA and salicylic acid (SA) and decreased activation of mitogen-activated protein kinase (MAPK), heat shock protein 90 (HSP90), and respiratory burst oxidase homolog 1 (RBOH1) (Figure 3; Table 3) []. Consequently, the suppressed SlGSNOR lines failed to produce apoplastic H2O2. H2O2 plays an important role in the early activation of heat shock proteins [], and potentiating GSNOR is essential for maintaining redox balance and achieving thermotolerance. Intriguingly, although the broad loss of GSNOR activity compromised thermotolerance, the reduced GSNOR activity of plant quiescin sulfhydryl oxidase homolog (QSOX1) led to enhanced heat tolerance (Figure 2) [,]. The targeted S-nitrosylation of QSOX1 switched from an oxidoreductase to a molecular chaperone, leading to heat resistance (Table 1). Similarly, NO burst in a member of the trihelix transcription factor, GT-1, was S-nitrosylated and bound in the promoter of HsfA2 to activate heat-responsive expression (Figure 2; Table 1) []. While hot5-2/4 or silenced SlGSNOR plants lost heat tolerance, elevated GSNO or S-nitrosylation of other proteins may not inherently be negative (Table 1). Supportively, it is plausible that exogenous NO application increases heat tolerance [].

Figure 2.
This figure illustrates the role of S-nitrosoglutathione (GSNO) reductase (GSNOR) in mediating tolerance in A. thaliana across a wide range of abiotic stresses, including thermo-tolerance, iron homeostasis and tolerance, salt, drought, hypoxia, and cadmium (Cd) and aluminum stress, as well as tolerance to metal and nutritional stress (NH4+ and Zn), and endoplasmic reticulum (ER) stress. A pleiotropic effect of GSNOR has been noticed, where plant tolerance with higher or lower levels of GSNOR activity varies depending on stress conditions and their signaling mechanisms. Black solid or dash arrows indicate direct or indirect activation where red blunt shows inhibition. All the abbreviations are available in the main text.
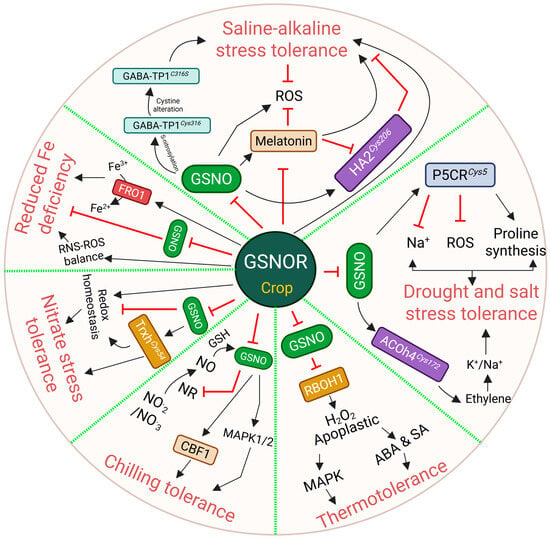
Figure 3.
This figure illustrates the role of S-nitrosoglutathione (GSNO) reductase (GSNOR) in mediating tolerance in crops across a wide range of abiotic stresses, including temperature tolerance, iron homeostasis, and salt and drought stress, as well as tolerance to nitrate stress and saline–alkaline stress. A pleiotropic effect of GSNOR has been noticed, where crop tolerance with higher or lower levels of GSNOR activity varies depending on stress conditions and their signaling mechanisms. GSNOR modulates the reactive oxygen species (ROS)-reactive nitrogen species (RNS) balance and redox switches through S-nitrosylation under abiotic stresses. Black solid arrows indicate direct activation where red blunt shows inhibition. All the abbreviations are available in the main text.
Similar to heat tolerance, exogenous NO application has recently gained research focus in cold stress acclimation [,], as low temperature causes detrimental effects on crop growth and development. The NR null mutant, nia1nia2, failed to undergo cold acclimation, which was restored by the supplementation of SNP []. Conversely, Costa-Broseta et al. [] reported that Arabidopsis nia1nia2noa1-2 were insensitive to cold stress. These discrepancies need to be revisited using both double and triple mutants simultaneously to check whether NOA1-dependent NO regulation has any effect on cold acclimation or whether the observed effects are related to the stress duration, severity, and experimental conditions. However, no studies have been performed using GSNOR null mutants, like gsnor1-3/hot5-2/4, to evaluate cold tolerance. Therefore, null mutants of NR and NOA failed to mimic the role of GSNOR-mediated NO elimination for cold tolerance in A. thaliana. In response to crops, silencing of tomato GSNOR resulted in MAPK1/2 and C-repeat binding factor (CBF1) activation, which helped in cold stress acclimation (Figure 3; Table 3) []. Moreover, brassinosteroid-dependent S-nitrosylation of monodehydroascorbate reductase (MDHAR) also confers low-temperature tolerance by improving ROS detoxification (Table 1; Table 3) []. These findings led us to assume that the loss of GSNOR activity or elevated NO may improve freezing resilience, a notion supported by many studies on exogenous NO application. Moreover, we also hypothesize that the loss-of-GSNOR mutation in gsnor1-3/hot5-2/4 likely exhibits cold tolerance.
2.2. Iron Stress Tolerance and Homeostasis
NO has a high affinity for iron (Fe) that can react with ferric (Fe3+) and/or ferrous (Fe2+) forms of Fe []. Parallelly, NO and Fe can form an iron–nitrosyl complex that is essential for Fe homeostasis []. Excessive Fe is toxic to biological processes, as it reacts with H2O2 to generate highly destructive hydroxyl radicals (OH•) []. NO-mediated oxidative burst is also a reason for nitrosative stress and exerts severe cellular damage []. Examining the previous studies, we observed that the role of GSNOR activity or GSNO levels is highly varied in regulating Fe toxicity and homeostasis. Li et al. [] reported that GSNOR is essential for plant tolerance to high Fe, as evidenced by hot5-2/4 or silenced GSNOR activity of Lotus japonicus and rice (Oryza sativa L.) plants, which were highly sensitive to Fe toxicity (Figure 2 and Figure 3; Table 2 and Table 3). In contrast, overexpression of tomato GSNOR plants mitigated Fe deficiency by upregulating the transcript levels of Fe absorption and transportation, including ferric-reductase oxidase 1 (FRO1) and oligopeptide transporter (OPT), respectively []. Moreover, GSNOR expression and Fe acquisition genes were upregulated under Fe-deficient conditions in A. thaliana []. These results suggest that the presence of GSNOR activity alleviates excessive RNS and ROS, thereby reducing cellular damage and improving Fe homeostasis to better tackle Fe toxicity (Figure 2 and Figure 3; Table 2).
On the other hand, gsnor1-3 plants exhibited a higher relative expression of vacuolar Fe (NRAMP3 and NRAMP4) and chloroplast Fe importer (PIC1) under Fe-deficient conditions (Figure 2; Table 2) []. This result contrasts with the positive regulation of GSNOR activity, but Shee et al. [] discussed that elevated GSNO in gsnor1-3 conferred Fe transportation, mainly dependent on reduced glutathione (GSH). While GSNO is formed from cellular NO and GSH and participates in diverse signaling mechanisms, mutants lacking these precursor molecules—the NO-deficient noa1 and GSH-deficient cad2-1/pad2-1—both exhibited hypersensitivity to Fe deficiency. Hence, both GSH and NO levels are likely crucial for Fe homeostasis in plants. However, it is unclear why the loss of GSNOR simultaneously makes a plant hypersensitive to high Fe but upregulates Fe transport genes during Fe deficiency. Answering with a unifying role of GSNOR under Fe levels will require simultaneous monitoring of different species and conditions.

Table 2.
GSNOR-mediated regulation of abiotic stress tolerance in A. thaliana.
Table 2.
GSNOR-mediated regulation of abiotic stress tolerance in A. thaliana.
| Mode of Genetic Modification | Stress Conditions | GSNOR Activity/Expression | NO/SNO Levels | Crosstalk with Other Proteins | Stress Effects | ROS: Antioxidant | Crosstalk with Hormones | Reference |
|---|---|---|---|---|---|---|---|---|
| hot1 | Heat | – | – | QSOX1 | Tolerance | – | – | [] |
| gsnor1-3 | Cd | – | – | – | Tolerance | ↓:↑ | – | [] |
| GSNOR | Sensitive | ↑:↓ | ||||||
| hot5-4 | ER | – | – | ERO1 | Tolerance | – | – | [] |
| gsnor1 | Oxidative | – | –/Increased | HDA19 | – | – | – | [] |
| hot5-2 | Heat | – | Increased/– | GT-1 | Tolerance | – | – | [] |
| hot5-2 | NH4+ | – | Increased/– | – | Sensitive | – | – | [] |
| gsnor1-3 | Oxidative | – | – | – | Sensitive | – | – | [] |
| gsnor1-3 | Light intensity | – | Increased/Increased | HDA6 | – | – | – | [] |
| gsnor1-3 | Oxidative | Inhibited | –/Increased | ICS1 | – | – | SA | [] |
| gsnor1-3 | Oxidative | – | – | ROG1 | Tolerance | – | – | [] |
| gsnor1-3 | Zn | – | – | APX1 | Tolerance | ↑:↓ | – | [] |
| 35S:FLAG-GSNOR1 | Inhibited | Decreased/Increased | Sensitive | ↑:↓ | ||||
| gsnor1-3 | Cd | – | – | IRT1 and APX | Sensitive | – | – | [] |
| GSNOR | Induced | Tolerance | ||||||
| gsnor1-3 | Fe | – | Increased/– | – | Sensitive | – | – | [] |
| gsnor1-3 | Hypoxia | – | – | ATG8 | Sensitive | – | – | [] |
| gsnor | Salt | Inhibited | Increased/– | CaM | Tolerance | – | – | [] |
| GSNOR | Induced | Decreased/– | Sensitive | |||||
| gsnor1-3 | Oxidative | – | – | APX1 | Tolerance | –:↑ | – | [] |
| gsnor1 | Nitrate | Inhibited | – | – | Sensitive | – | – | [] |
| 35S:FLAG-GSNOR1 | Induced | Tolerance | ||||||
| hot5 | Heat | – | – | – | Sensitive | – | – | [] |
Full abbreviations from the table can be found in the main text or Abbreviation section. ↑, increased; ↓, decreased.
2.3. Salt, Drought, and Metal Stress Tolerance
NO has a paramount role in regulating plant salt, drought, and heavy metal stress tolerance []. In this section, we discuss how GSNOR modulates plant physiology and signaling under these stress conditions. Zhou et al. [] reported that GSNOR negatively regulates salt tolerance, as the Arabidopsis gsnor mutant showed enhanced survivability to 100 mM NaCl. Moreover, the increased GSNOR activity in Ca2+ sensor protein knockout mutants, cam1 and cam4, effectively abridged NO levels, resulting in salt sensitivity. In contrast, cam4gsnor plants exhibited higher survivability under NaCl stress, pinpointing that NO acts as a downstream regulator of Ca2+ signaling to modulate salt tolerance (Figure 2; Table 2) []. Furthermore, GSNOR RNA interference (RNAi) tomato plants increased ethylene and NO accumulation under salt stress, while GSNOR-overexpressed (OE) plants showed a compromise of both []. Elevated ethylene and NO, together, fight against salt toxicity by upregulating 1-aminocyclopropane-1-carboxylate (ACC) synthase (ACS) and ACC oxidase (ACO) activity and their relative mRNA expression. Later, it was shown that S-nitrosylation of ACOh4Cys172 maintained K+/Na+ homeostasis to promote salt stress tolerance (Figure 3; Table 1 and Table 3). Recently, the same research team reported that the S-nitrosylation of Δ1-pyrroline-5-carboxylate reductase (SlP5CRCys5) enhanced salt and drought tolerance by boosting proline synthesis while limiting the ROS and Na+ accumulation []. Similar to SlP5CRCys5, SlGSNOR RNAi lines exhibited higher proline accumulation. Although proline is known for its role in the mitigation of abiotic stress tolerance [], its intersection with NO remained elusive. Thus, this study first elucidated the crosstalk of proline and NO responses. Overall, these findings suggest that GSNOR plays a negative role in Ca2+, ethylene, and proline accumulation to protect plants from salt stress (Figure 3; Table 1 and Table 3).
During drought stress, ABA signaling is crucial for regulating stomatal movement, which maintains plant transpiration rate and drought acclimation. However, NO acts as a negative regulator of ABA signaling by S-nitrosylating the protein SnRK2.6Cys137, thereby limiting its activation (Table 1) []. Consistent with this, the GSNOR knockout mutant, gsnor1-3, over-accumulates NO in guard cells and consequently impairs stomatal closing (Table 2). This suggests that NO-mediated inhibition of ABA signaling is a critical factor limiting drought tolerance, making the GSNOR gene a key component in plant drought resilience. Despite the contrasting roles of GSNOR in plant salt and drought stress tolerance, GSNOR showed tissue-specific heavy metal tolerance in A. thaliana. Previously, it was reported that GSNOR limits Cd2+ accumulation in roots by restricting iron-regulated transporter 1 (IRT1), which is a Cd transporter (Figure 2; Table 2) []. A few years later, the same group mentioned that GSNOR disfavors Cd tolerance, as gsnor1-3 plants acquired less ROS with higher CAT activity in the shoots (Figure 2; Table 2) []. Similarly, gsnor1-3 also increased APX activity under oxidative stress, indicating its effective ROS detoxification mechanism (Table 2) []. Nevertheless, the conflicting data present a paradox: how can GSNOR limit Cd2+ accumulation in the roots while its absence still results in enhanced metal-dependent ROS production in the leaves? Therefore, this limited knowledge needs to be re-investigated.
2.4. Nutrient Stress Tolerance
Nutrients are available in the soil and are essential for plant growth and development. However, inadequate or excessive fertilization in agricultural lands often causes nutrient pollution or toxicity that restricts crop production. Hence, it is crucial to understand the plant physiology and mechanisms occurring under nutrient stress conditions. Nitrogen assimilation is one of the core metabolic processes in plant growth, which is carried out through the nitrate reduction pathway (Figure 1A). Nitrogen assimilation is largely connected with the formation of NO, which regulates plant development and stress responses. Frungillo et al. [] reported that elevated NO and GSNO differentially modulate nitrogen assimilation by inhibiting nitrate uptake and reduction. Specifically, nitrate-derived NO accumulation represses GSNOR activity, suggesting that NO is the key to the adjustment of nitrogen assimilation. Additionally, S-nitrosylation of tomato SlTrxhCys54 alleviates ROS accumulation under nitrate stress [], indicating a similar NO-induced GSNOR inhibition that likely regulates nitrogen pollution due to over-fertilization. In stark contrast, overexpression of spinach (Spinacia oleracea L.), SoGSNOR, exhibited improved ROS and RNS balance—enhancing the plants’ germination rate under nitrate stress (Figure 2; Table 3) []. Therefore, it would be more informative to be able to justify the role of GSNOR transgenic lines of either tomato or other crops under nitrate stress.
Although NH4+ is less demanded by plants than nitrate, its moderate presence in the soil can cause toxicity directly to plant roots []. Plant hypersensitivity to NH4+ was observed due to disturbed NO signaling in the knockout of the vitamin C1 (VTC1) mutant []. A recent study explored GSNOR’s roles in Arabidopsis and rice under NH4+ stress []. The study exhibited that hot5-2/gsnor and Osgsnor-1/2 plants showed hypersensitivity to NH4+ stress, similar to vtc1-1 plants. This sensitivity reduced the root length and K+ adsorption; however, a double mutant of 35S-GSNOR/vtc1-1 restored the root length, suggesting a GSNOR-dependent positive role of plants’ NH4+ stress tolerance (Figure 2; Table 2). Plants exposed not only to nitrate and NH4+ stress, but also to excess Zn-rich soil experience altered normal growth and development, including inward rolled leaf edges, chlorotic leaves, and retarded and brownish root systems []. In addition, excess Zn accumulation resulted in overaccumulation of RNS, including NO [], which was then responsible for GSNOR inactivation and reduced Zn tolerance []. This inactivation directly accumulated higher H2O2 by reducing glutathione and APX activities. Contrarily, 35S:FLAG-GSNOR1 plants compensated for suboptimal Zn toxicity by reducing H2O2 formation (Figure 2; Table 2) []. Taken together, these findings indicate that the function of GSNOR in Zn toxicity is conditional, varying with the specific Zn levels. This difference underscores the necessity of considering soil characteristics when defining GSNOR’s exact role under Zn stress.
In this review, we examine the pleiotropic role of GSNOR in sensing nutrient toxicity. It is also crucial to understand how GSNOR activity adapts to nutrient deficiency or how plant roots with altered GSNOR activity modulate microbial activity in the rhizosphere in relation to nutrient availability, uptake, and translocation. Moreover, elucidating the role of GSNOR in a phosphorus-deficient medium will be interesting, as phosphorus is the most limiting nutrient in soil owing to its insolubility.
2.5. Saline–Alkaline Stress Tolerance
Saline–alkaline stress is defined by the presence of alkaline salts, primarily NaHCO3 (sodium bicarbonate) and Na2CO3 (sodium carbonate), in the soil. Globally, saline–alkali soils cover 954 million hectares—impacting global agriculture—as shown by Liu et al. []. Plants manage saline–alkaline stress through organic acid metabolism that helps in detoxifying Na+, regulating rhizosphere pH, maintaining osmotic balance, and managing ROS accumulation [,]. Over the last decades, the role of NO signaling in saline–alkaline stress tolerance has been updated through the redox switch of the GSNOR enzyme. Gong et al. [] and Wei et al. [] reported that GSNOR-overexpressed tomato lines were tolerant, while GSNOR-suppressed plants were hypersensitive (Figure 2; Table 3). Although the tolerant lines were found to coordinate ROS and RNS balance, the sensitive GSNOR-suppressed lines presented a paradox, as they displayed a reduced Na+/K+ ratio and increased expression of salt marker genes []. However, the same group recently revealed new findings, where the GSNOR RNAi line accumulated higher Na+, as well as saline–alkaline hypersensitivity (Figure 2; Table 3) []. This conflicting data may be a reason for the differential genetic modification. Despite the higher saline–alkaline sensitivity in GSNOR RNAi lines, these were salt-tolerant, as we discussed in the previous sections. While elevated GSNO levels in GSNOR RNAi lines potentially drive global S-nitrosylation, how this redox switch manages Na+ toxicity but fails to compensate for high pH stress was recently elucidated with malate exudation mechanisms.
Pyruvate-dependent GABA transaminase 1 (SlGABA-TP1) was found to be S-nitrosylated at Cys316/258/316, and increasing γ-aminobutyric acid (GABA) reduces saline–alkaline tolerance through the reduction in malate exudation (Figure 2; Table 3) []. Intriguingly, when S-nitrosylation is blocked by mutating the target cysteine residues to serine, the resulting change in NO levels supports malate movement in both roots and fruits, ultimately increasing overall stress tolerance. Elevated root GABA levels in GSNOR RNAi were also observed, suggesting that excess GABA reduces malate exudation in this line, which is essential for saline–alkaline stress adaptation (Figure 2; Table 3). Similar to the PTM of SlGABA-TP1, S-nitrosylated plasma membrane-localized H+-ATPase 2 (HA2Cys206) limits its interaction with 14-3-3 protein 1 (TFT1)—resulting in impaired HA activity, H+ efflux, and thereby increased saline alkaline sensitivity (Figure 2; Table 3) []. However, this sensitivity is reduced with the help of melatonin, an important phytohormone. GSNOR RNAi/COMT OE lines exhibited saline–alkaline tolerance. COMT (caffeic acid O-methyltransferase) is one of the catalyzing enzymes that facilitates the melatonin biosynthesis from tryptophan []. In contrast, GSNOR RNAi lines were hypersensitive but still accumulated higher melatonin levels, whereas GSNOR OE failed to do so []. Thus, this suggests that the detrimental effects of GSNO/NO dysregulation in the mutant likely overrode the benefits of the elevated melatonin.
2.6. Other Stress Responses
The role of GSNOR has also been studied in plants under hypoxia, aluminum, and endoplasmic reticulum (ER) stress. However, we know little about their crosstalk with GSNOR, where these stress conditions hold importance by their own mode of action on plant physiology and signaling transduction. Hypoxic conditions in plants represent a major concern due to climate change, often resulting from heavy flash flooding. Despite NO’s roles in the regulation of plant metabolism under hypoxic or anoxic conditions [], before the study published by Zhan et al. [], the function of GSNOR in mediating plant response to hypoxic stress was unknown. They reported that S-nitrosylated GSNORCys10 activates the selective autophagy of GSNOR by exposing its autophagy-related protein 8 (ATG8)-interacting motif (AIM) (Figure 2; Table 2). This autophagy was found to be relevant to hypoxia responses. In particular, under 3% O2 levels, despite the marked upregulation of two hypoxia marker genes, alcohol dehydrogenase 1 (ADH1) and pyruvate decarboxylase 1 (PDC1), in gsnor1-3, it showed hypersensitivity during germination. This pleiotropic effect was likely caused by a combination of factors, including inefficient O2 utilization, a mechanism that is independent of GSNOR, and ABI5 downregulation. Conversely, the NO overproducer nox1 mutant exhibited enhanced germination and higher PDC1 expression. This finding implies that NO itself promotes seed germination under low-oxygen conditions, a mechanism distinct from the GSNO-driven effects seen in gsnor1-3. Despite the previously established negative role of NO under low-O2 conditions, the observation that NO promotes germination seems contradictory and is difficult to reconcile—a challenge that Zhan et al. [] addressed by suggesting that this alteration may focus primarily on ABA signaling.
In acidic soils, aluminum is solubilized and available for plants in Al3+ and Al(OH)2+ forms, inhibiting plant growth and development. Peanut (Arachis hypogaea L.) GSNOR1 transcription and protein expression were enhanced under aluminum stress. An introgressed AhGSNOR1-overexpressing transgenic tobacco plant decreased aluminum-induced NO and SNO accumulation, while increasing Thioredoxin H3 (Trxh3) expression and antioxidant activities (Table 3) []. This improvement alleviated plant cellular damage and enhanced aluminum stress resilience. In contrast, Arabidopsis loss-of-function mutation of GSNOR, gsnor1/hot5 mutant, exhibited resistance to oxidative and ER stress []. The ER is a key protein-folding compartment, where one-third of all eukaryotic proteins are translocated from the cytosol to acquire their appropriate conformations. Upon environmental stress, increased protein loading affects ER homeostasis, resulting in unfolded or misfolded proteins. The gsnor1/hot5 mutant exhibits increased expression of ER-stress-responsive genes (BiPs, ERDJ3A, and SHD), suggesting a pre-activated stress state that renders the mutant insensitive to Tm-induced ER stress (Figure 2; Table 2). ER usually induces ROS production, where Qin et al. [] identified that endogenous NO in gsnor1/hot5 reduced oxidative damage by activating S-nitrosylation of ER OXIDOREDUCTASE 1 (ERO1Cys337), which promotes disulfide bond formation and protein folding (Figure 2; Table 1 and Table 2). Collectively, these findings highlight a crucial function for GSNOR-mediated redox switches in regulating ER function in plants.

Table 3.
GSNOR-mediated regulation of abiotic and biotic stress tolerance in crops.
Table 3.
GSNOR-mediated regulation of abiotic and biotic stress tolerance in crops.
| Species | Mode of Genetic Modification | Stress Conditions | GSNOR Activity/Expression | NO/SNO Levels | Crosstalk with Other Proteins | Stress Effects | ROS: Antioxidant | Crosstalk with Hormones | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Solanum lycopersicum L. | GSNOR | Saline–Alkali Stress | – | Decreased/Decreased | HA2 | Tolerance | ↓:– | Melatonin | [] |
| GSNOR-RNAi | Increased/Increased | Sensitive | ↑:– | ||||||
| Nicotiana tabacum | GSNOR1 | Aluminum Stress | Induced | Decreased/Decreased | Trxh3 | Tolerance | ↓:↑ | – | [] |
| Solanum lycopersicum L. | GSNOR-RNAi | Salt Stress | – | – | P5CR | Sensitive | – | – | [] |
| Solanum lycopersicum cv. Ailsa | GSNOR-RNAi | Salt Stress | – | – | ACOh4 | Sensitive | – | Ethylene | [] |
| Ganoderma lucidum | GSNOR-RNAi | Heat Stress | Inhibited | – | CAT | Tolerance | ↓:↑ | – | [] |
| Solanum lycopersicum L. | GSNOR-RNAi | Salt Stress | – | Increased/– | MAPK3, ACO1 | Sensitive | ↑:– | – | [] |
| Solanum lycopersicum L. cv. Ailsa | GSNOR-Silenced | High-Temperature Stress | Inhibited | –/Increased | RBOH1 | Sensitive | ↑:↓ | ABA and SA | [] |
| Nicotiana tabacum | GSNOR | Nitrate Stress | Induced | Decreased/Decreased | – | Tolerance | ↓:↑ | – | [] |
| Solanum lycopersicum L. | GSNOR | Fe Deficiency Stress | Induced | Decreased/Decreased | – | Tolerance | ↓:↑ | – | [] |
| Solanum lycopersicum L. cv. Condine Red | GSNOR-Silenced | Cold Acclimation | Inhibited | Increased/ | NR, MPK1/2 | Tolerance | – | – | [] |
| Solanum lycopersicum L. | GSNOR | Alkaline Stress | Induced | Decreased/Decreased | – | Tolerance | ↓:↑ | – | [] |
| GSNOR-Suppressed | Inhibited | Increased/Increased | Sensitive | ↑:↓ | |||||
| Oryza sativa | GSNOR | Oxidative Stress | Induced | –/Decreased | – | Tolerance | ↑:↓ | – | [] |
| GSNOR-RNAi | Inhibited | –/Increased | Sensitive | – | |||||
| Solanum lycopersicum L. | GSNOR | Botrytis cinerea | – | Decreased/Decreased | COMT2 | Sensitive | – | JA, Melatonin | [] |
| GSNOR-RNAi | Increased/Increased | Tolerance | |||||||
| GSNOR-Silenced | |||||||||
| Solanum lycopersicum L. | gsnor | P. capsici, flg22 | Inhibited | – | PcRD18, ATG8c | Sensitive | ↓:– | SA | [] |
| GSNOR-silenced | |||||||||
| Solanum lycopersicum L. | GSNOR | Botrytis cinerea | Induced | Decreased/– | – | Tolerance | – | – | [] |
| GSNOR-RNAi | Inhibited | Increased/– | Sensitive | ||||||
| Solanum lycopersicum L. | GSNOR | Pst DC3000 | Induced | – | – | Tolerance | – | – | [] |
| Solanum lycopersicum L. | GSNOR | Pst DC3000 | Induced | – | PR1 | Tolerance | – | SA | [] |
| GSNOR-RNAi | Inhibited | Sensitive | |||||||
| Medicago truncatula L. | 35S:GSNOR | Aphanomyces euteiches | Induced | –/Increased | NR | Tolerance | – | – | [] |
Full abbreviations from the table can be found in the main text or Abbreviation section. ↑, increased; ↓, decreased.
3. Changes in GSNOR Activity Affect Biotic Stress Tolerance
A defining feature of plant immunity is the dynamic reprogramming of cellular redox homeostasis, shaped by the interplay between ROS and RNS. Within this mechanism, NO has emerged as a central signaling molecule that orchestrates defense responses against diverse biotic stresses. As previously discussed, the NO moiety transferred to a reactive cysteine residue is central to a redox-based PTM, S-nitrosylation, which regulates protein function and immune defense. This entire process is controlled by GSNOR, which acts as a critical checkpoint in NO signaling for plant immunity (Figure 4; Table 3 and Table 4).

Figure 4.
This figure illustrates the integration of NO and GSNOR signaling into plant immunity. Recognition of pathogen-associated molecular patterns (PAMPs) by receptor-like kinases such as FLS2 and BAK1 activates the cytoplasmic kinase BIK1, triggering Ca2+ influx, MAPK cascades, and NADPH oxidase (RBOHD)-dependent ROS bursts. Nitric oxide (NO) interacts with ROS to regulate hypersensitive response (HR) and programmed cell death (PCD), and reacts with glutathione (GSH) to form S-nitrosoglutathione (GSNO), a mobile reservoir of NO bioactivity and the main donor of S-nitrosylation (SNO). GSNO levels are tightly controlled by S-nitrosoglutathione reductase (GSNOR), while regulators such as QSOX1 and PcRD18 modulate its activity. Protein S-nitrosylation modifies immune regulators, including SRG1, SABP3, and NPR1, thereby controlling their stability, activity, and redox-dependent transitions. NPR1, in particular, undergoes S-nitrosylation to regulate its oligomer–monomer transition and SA-dependent transcriptional activity (Figure 4; Table 1 and Table 4). Denitrosylation is mediated by thioredoxin h5 (Trx5), linking NO/SNO homeostasis to SA signaling. Pathogen effectors can suppress immunity by targeting GSNOR, whereas R proteins and the EDS1–PAD4 complex activate effector-triggered immunity (ETI). Through the GSNO/GSNOR balance, NO signaling intersects with salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) pathways, coordinating defense gene expression, HR/PCD, and systemic acquired resistance (SAR). Black solid arrows indicate direct activation where blunt shows inhibition.
S-nitrosylation modifies a suite of immune-related proteins, including RBOHD, NPR1, TGA1, SABP3, and peroxiredoxins, thereby wiring NO signaling into both pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) [,,,,]. Importantly, this modification can act as a double-edged sword. While host cells employ it to fine-tune defense, pathogens may exploit S-nitrosylation machinery to suppress immunity and promote virulence. NO mediates PTI through stage-specific S-nitrosylation of immune regulators. Recognition of pathogen-associated molecular patterns (PAMPs) by receptor-like kinases such as FLS2 activates BIK1, which phosphorylates the NADPH oxidase RBOHD to induce ROS bursts (Figure 4; Table 4) []. Early S-nitrosylation of BIK1Cys80 enhances its stability and phosphorylation, thereby amplifying ROS production, whereas later modification of RBOHDCys890 suppresses ROS output to prevent excessive oxidative stress (Figure 4; Table 1 and Table 4) []. This temporal regulation demonstrates how NO balances immune activation with cellular homeostasis. In atgsnor1 mutants, excessive SNO accumulation disrupts SA-dependent transcriptional reprogramming, impairs NPR1 monomerization, and compromises basal and nonhost resistance (Figure 4; Table 4). Conversely, GSNOR overexpression restores redox balance and enhances immune responses. These results underscore GSNOR as a critical metabolic checkpoint.
Additionally, HMAD1 negatively regulates immunity by modulating GSNOR activity [], whereas QSOX1, a redox-sensitive enzyme, interacts with both GSNOR and RBOHD to adjust ROS/RNS balance, limiting excessive cell death while sustaining basal defense (Figure 4; Table 4) []. At the nuclear level, SRG1, a zinc-finger transcription factor, is activated by pathogen-induced NO bursts to repress negative regulators of defense []. Subsequent S-nitrosylation of conserved cysteines (e.g., Cys87) disrupts Zn2+ coordination and DNA binding, releasing SRG1 repression in a redox-controlled feedback loop. GSNOR-mediated SNO homeostasis is pivotal in fine-tuning this regulatory switch (Figure 4; Table 1 and Table 4). NO also modulates other immune regulators in Arabidopsis. S-nitrosylation of NPR1Cys156 promotes oligomerization and protein stability, whereas modification of SA-binding protein 3 (AtSABP3Cys280) reduces SA binding and carbonic anhydrase activity, thereby providing negative feedback to SA-mediated immunity (Figure 4; Table 1 and Table 4) []. Moreover, NO/GSNOR signaling intersects with abiotic and biotic stress pathways. The aldehyde oxidase gene AtAO3, involved in ABA biosynthesis, antagonizes SA signaling while promoting drought tolerance through ABA-dependent stomatal closure []. Accordingly, atao3 mutants show elevated PR1 expression and hypersensitive response (HR), illustrating the antagonistic interplay between ABA and SA, with NO functioning as a molecular integrator of biotic and abiotic stress signaling.
Pathogens exploit this hub to suppress host immunity. For example, Phytophthora effectors directly inhibit GSNOR, disrupting SA-dependent transcriptional activation and ROS bursts (Figure 4; Table 4) []. The RxLR effector PcRD18 further promotes autophagic degradation of GSNOR via ATG8c, elevating SNO accumulation, suppressing ROS production, and ultimately enhancing pathogen virulence. These regulatory mechanisms are conserved in crops. In wheat, pathogen-induced expression of TaNIA and TaGSNOR correlates with increased NO production and dynamic GSNO turnover, suggesting roles in both PTI and ETI []. In tomato, SlGSNOR overexpression enhances resistance to Pseudomonas syringae pv. tomato and promotes fruit development [], while silencing SlGSNOR compromises SA-dependent defense []. In rice, the noe1 mutant, defective in catalase, shows uncontrolled NO/ROS accumulation, whereas OsGSNOR overexpression restores redox balance and prevents runaway cell death []. Even fungal pathogens such as Magnaporthe oryzae rely on GSNOR-mediated denitrosylation for infection, as loss of GSNOR impairs appressorium formation, turgor generation, and virulence []. Collectively, these studies highlight the role of GSNOR as a conserved regulatory hub across plant species. By controlling NO bioactivity and S-nitrosylation, GSNOR can coordinate immune responses, prevent nitrosative stress, and balance defense with plant growth.

Table 4.
GSNOR-mediated regulation of biotic stress tolerance in A. thaliana.
Table 4.
GSNOR-mediated regulation of biotic stress tolerance in A. thaliana.
| Mode of Genetic Modification | Stress Conditions | GSNOR Activity/Expression | NO/SNO Levels | Crosstalk with Other Proteins | Stress Effects | ROS: Antioxidant | Crosstalk with Hormones | Reference |
|---|---|---|---|---|---|---|---|---|
| gsnor1-3 | Pst DC3000 hrcC−, flg22 | – | –/Increased | BIK1, RBOHD, FLS2, BAK1 | Sensitive | ↑:– | – | [] |
| par2-1 | ||||||||
| gsnor1-1 | Phytophthora parasitica | – | – | – | – | – | – | [] |
| gsnor1-3 | Sensitive | ↓:– | SA | |||||
| par2-1 | – | – | ||||||
| gsnor1-3 | Pst DC3000 (avrRpt2), Pst DC3000 (avrRpm1), Pst DC3000 (avrRps4) | Inhibited | –/Increased | RBOHD | Sensitive | – | – | [] |
| gsnor1-3 | Pst DC3000 (avrB) | – | –/Increased | – | Sensitive | – | SA | [] |
| gsnor1-3 | Pst DC3000, Pst DC3000 (avrRpm1) | – | –/Increased | SRG1 | Sensitive | ↓:– | SA | [] |
| gsnor1-3 | Pst DC3000, Pst DC 3000 (avrB) | – | –/Increased | – | Sensitive | – | SA | [] |
| gsnor1-1 | Pst DC3000 (avrB), Pst DC3000 (avrRps4), Pst DC3000 (virulent), Psp | – | Decreased/Decreased | – | Tolerance | – | – | [] |
| 35S:FLAG-GSNOR1 | – | |||||||
| gnsor1-3 | Inhibited | Increased/Increased | Sensitive | SA | ||||
| par2-1 | – | – | – | |||||
| gsnor1-1 | Pst DC3000 (avrB), Pst DC3000 (avrRps4), Pst DC3000, H. arabidopsidis Emwa1 | Induced | Decreased/Decreased | RBOHD | Sensitive | ↑:– | SA | [] |
| gnsor1-3 | Inhibited | Increased/Increased | Tolerance | ↓:– | ||||
| gsnor | Pst DC3000 (avrRpt2), Pst DC3000 | Inhibited | – | – | – | –:↑ | – | [] |
| gsnor1-1 | Pst DC3000 (avrB) | – | –/Decreased | SABP3 | Tolerance | – | SA | [] |
| gsnor1-3 | –/Increased | Sensitive | ||||||
| gsnor1-1 | Pst DC3000 (avrB), Pst DC3000 | Induced | –/Decreased | – | Tolerance | – | SA | [] |
| gsnor1-2 | ||||||||
| gsnor1-3 | Inhibited | –/Increased | Sensitive |
Full abbreviations from the table can be found in the main text or Abbreviation section. ↑, increased; ↓, decreased.
4. Nitric Oxide for Innovative Agricultural Application: Potential Nanotechnology and Its Limitations
Recent advances in nanotechnology have expanded the potential of smart fertilizers by providing novel platforms for precise nutrient delivery [,]. In the previous sections, we discussed the various roles of NO in plant physiology and its involvement in developmental processes. Despite its considerable applications, its utility is limited by a short shelf life due to its rapid redox nature in the presence of light, high temperatures, and catalytic metal ions [,,]. Therefore, by believing in its importance, studies focusing on the creation of innovative NO-based nanomaterials could be applied to basic and applied plant research (Figure 5) []. This review, while focused on GSNOR’s role in NO signaling, also explores NO-based nanoparticles (NPs), especially those using GSNO as a donor. Ultimately, we examine the significance of GSNOR in the context of nano-based agricultural solutions.
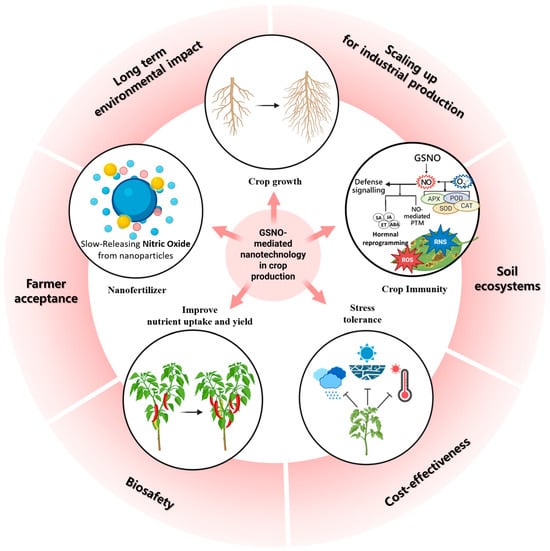
Figure 5.
The figure illustrates the potential of nitric-oxide-based nanotechnology in agriculture, including (i) controlled and slow release of nitric oxide (NO) from nanoparticles, (ii) enhancement of plant defense signaling and stress tolerance through modulation of reactive oxygen/nitrogen species (ROS/RNS) and hormone crosstalk, and (iii) improvements in growth and productivity under abiotic and biotic stresses. Moreover, this figure highlights key limitations and challenges that must be addressed for field application: (i) scaling up nanoparticle production for industrial use, (ii) understanding impacts on soil ecosystems and microbial interactions, (iii) ensuring cost-effectiveness for farmers, (iv) biosafety and risk assessment, (v) farmer acceptance and regulatory approval, and (vi) evaluation of long-term environmental impacts.
NO-based NPs have been shown to improve NO’s shelf-life and provide a slow release mechanism (Figure 5) [,,,]. To date, most NO-based NPs have been encapsulated with chitosan-based NPs, which increases NO stability []. NO release from these encapsulated nanocarriers improves plant resilience to salt and drought stress. These tolerance mechanisms are involved in increasing plant biomass, photosynthesis, ROS detoxification, antioxidant properties, and crop yield compared to NO donors alone (Figure 5) [,,,]. Together with these findings, a recent study showed that encapsulation of GSNO into chitosan nanocapsules applied in Brassica napus promoted stable SNO accumulation and redox balance while minimizing nitrosative stress []. Intriguingly, this NP exhibited NO release for more than 4 h, demonstrating the potential of nano-formulated NO availability. In line with the NO release, GSNO–chitosan NPs inactivated GSNOR activity in the seedling of B. napus, where Methela et al. [] reported that the relative expression of GSNOR1 in soybean shoots was increased upon drought stress. These results suggest that slow-release GSNO–chitosan NPs do not trigger GSNOR activity, while drought stress forces GSNOR to break down the excess NO to balance ROS and RNS for plant tolerance under stress. Hence, we speculate that such improvised NO-based nanocarriers are building a strong field in NO-based nanotechnology for future agricultural applications.
Despite the promising potential of nanotechnology-based smart fertilizers and NO-delivery platforms such as HAs and chitosan NPs, several limitations remain before their widespread application in agriculture can be realized (Figure 5). First, the large-scale synthesis, stability, and cost-effectiveness of NP-based formulations are still major challenges, as production methods often require complex processes that may not be easily scalable for field use. Second, the long-term environmental and ecological impacts of nanomaterials in soil ecosystems are not fully understood [,]. Potential concerns include the persistence and accumulation of NPs, as well as their unintended interactions with soil microbiota [,]. These interactions may affect crucial soil processes such as nutrient cycling, the structure of microbial communities, and beneficial functions like nitrogen fixation or phosphate solubilization [,]. For example, studies have shown that low concentrations of silver NPs can adversely affect plant species and significantly alter bacterial community composition and enzyme activities in soil [].
Nanomaterials can enter soil and aquatic ecosystems through various routes, including their presence in industrial products, environmental cleanup technologies, and unintentional releases from air, water, and sewage sludge applications (Figure 5) [,]. Engineered nanomaterials (ENMs) are expected to accumulate significantly in terrestrial environments, with biosolids from wastewater treatment serving as a major pathway for their introduction into the environment, exposing important soil microorganisms []. The unique properties of NPs, such as high surface area and reactivity, can lead to environmental hazards and potentially harm soil health (Figure 5) [,]. Another limitation lies in the complexity of NO/GSNOR signaling itself. While controlled NO release provides multiple physiological benefits, excessive or misregulated NO levels can lead to nitrosative stress, disrupting redox balance and impairing plant growth []. Therefore, fine-tuning NO dosage, release kinetics, and interactions with GSNOR pathways remains a critical challenge for practical implementation. In addition, crop-specific responses to NO and GSNO delivery need to be carefully evaluated, as species-dependent differences may influence the efficiency and outcomes of such interventions []. Finally, regulatory frameworks, biosafety assessments, and farmer acceptance of nanotechnology-based fertilizers are still in the early stages of development [,]. To achieve successful implementation in the field, interdisciplinary efforts are required, combining expertise from plant physiology, soil microbiology, materials science, systems biology, and agricultural policy [,]. Addressing these multifaceted challenges is crucial to fully harness the potential of smart NO-delivery systems for sustainable and climate-smart agriculture, and will likely improve our understanding of the GSNOR response [,].
5. Conclusions and Future Perspectives
Understanding the role of GSNOR in plant biology is crucial for stress tolerance under changing climatic conditions. A wide range of studies on NO signaling have been carried out with the endogenous modification of GSNOR activity in model plants and crops. This review aims to consolidate current knowledge on GSNOR’s involvement in plant tolerance to both abiotic and biotic stresses while highlighting key knowledge gaps. While stress tolerance depends on multiple variables, GSNOR shows pleiotropic effects on it. It is established that GSNOR controls global S-nitrosylation, which primarily contributes to resilience against abiotic stress but shows negligible efficacy against plant pathogenicity. However, the precise mechanism by which GSNOR confers stress tolerance needs to be revisited. For instance, (i) why cellular GSNO inhibits heat tolerance but S-nitrosylated proteins exhibit enhanced tolerance needs to be explored; (ii) the regulatory negative effect between NO/GSNOR and ABA under drought conditions is insufficiently studied; (iii) when elevated GSNO is present in the loss-of-function mutation of GSNOR, how it modulates plant nutrient availability and uptake is poorly investigated; (iv) except for seed germination status in the loss-of-function mutation of GSNOR under low-O2 conditions, its survivability and signaling are completely overlooked; (v) despite the knowledge of auxin-dependent growth and development of GSNOR-mutated plants, their crosstalk in response to stress tolerance is still enigmatic, and (vi) there is insufficient knowledge on NO/GSNOR-mediated PAMP recognition for plant immunity. Addressing these critical gaps will be crucial for improving GSNOR-dependent crop growth, stress resilience, and productivity, particularly under future climate challenges, and may also be informative in the development of NO-based agricultural technologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262311486/s1.
Author Contributions
A.K.D., D.-S.L., and B.-W.Y. conceptualization. G.-J.L., Y.-S.K., and S.H. table preparation. A.K.D. and D.-S.L. writing—original draft preparation. A.K.D. and B.-W.Y. writing—review and editing. M.-S.L. and B.-G.M. resources and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Chungbuk National University NUDP program (2025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| ADH3 | Class III alcohol dehydrogenase |
| APX1 | Ascorbate Peroxidase 1 |
| BIK1 | Botrytis-induced kinase 1 |
| COP1 | Constitutive Photomorphogenesis Protein 1 |
| Cys | Cystein |
| ER | Endoplasmic Reticulum |
| ERO1 | Endoplasmic Reticulum Oxidoreductin 1 |
| ET | Ethylene |
| ETI | Effector-Triggered Immunity |
| FRO1 | Ferric-Reductase Oxidase1 |
| GABA-TP1/2 | Gamma-aminobutyrate Transaminase 1/2 |
| GSH | Reduced Glutathione |
| GSNO | S-nitrosoglutathione |
| GSNOR | S-nitrosoglutathione Reductase |
| GSSG | Glutathione disulfide |
| HDA19 | Histone Deacetylase 19 |
| HFR1 | Far-red elongated hypocotyl 1 |
| JA | Jasmonic Acid |
| MDHAR | Monodehydroascorbate Reductase |
| NADH | Nicotinamide adenine dinucleotide |
| NIA1/2 | Nitrate Reductase 1/2 |
| NO | Nitric Oxide |
| NOA1 | Nitric Oxide-Associated 1 |
| NOS | Nitric Oxide Synthase |
| NOX1 | NADPH oxidase 1 |
| NPR1 | Nonexpressor of Pathogenesis-Related genes 1 |
| NRAMP3/4 | Natural Resistance-Associated Macrophage Protein 3/4 |
| OPT | Oligopeptide transporter |
| P5CR | Pyrroline-5-Carboxylate Reductase |
| PDC1 | Pyruvate Decarboxylase 1 |
| PIC1 | Permease in Chloroplasts 1 |
| QSOX1 | Quiescin Sulfhydryl Oxidase 1 |
| RBOHD | Respiratory Burst Oxidase Homologue D |
| RGA | Repressor of Gibberellin 1-3 |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SA | Salicylic Acid |
| SABP3 | Salicylic Acid-Binding Protein 3 |
| SAR | Systemic Acquired Resistance |
| SNO | S-nitrosothiol |
| SNP | Single-Nucleotide Polymorphism |
| SnRK | Sucrose Non-Fermenting 1-related Protein Kinase 2.6 |
| Trxh3 | Thioredoxin H3 |
References
- Tennyson, A.G.; Lippard, S.J. Generation, Translocation, and Action of Nitric Oxide in Living Systems. Chem. Biol. 2011, 18, 1211–1220. [Google Scholar] [CrossRef]
- Dixit, V.D.; Parvizi, N. Nitric oxide and the control of reproduction. Anim. Reprod. Sci. 2001, 65, 1–16. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Priestley, J. Experiments and Observations on Different Kinds of Air; J. Johnson: Chicago, IL, USA, 1776; Volume 2. [Google Scholar]
- Klepper, L.A. Nitric Oxide Emissions from Soybean Leaves during in Vivo Nitrate Reductase Assays. Plant Physiol. 1987, 85, 96–99. [Google Scholar] [CrossRef]
- Klepper, L. Comparison between NOx Evolution Mechanisms of Wild-Type and nr1 Mutant Soybean Leaves. Plant Physiol. 1990, 93, 26–32. [Google Scholar] [CrossRef]
- Dean, J.V.; Harper, J.E. Nitric Oxide and Nitrous Oxide Production by Soybean and Winged Bean during the in Vivo Nitrate Reductase Assay. Plant Physiol. 1986, 82, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2011, 33, 829–837. [Google Scholar] [CrossRef]
- Wilson, I.D.; Neill, S.J.; Hancock, J.T. Nitric oxide synthesis and signalling in plants. Plant Cell Environ. 2008, 31, 622–631. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In Vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000, 468, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Rockel, P.; Strube, F.; Rockel, A.; Wildt, J.; Kaiser, W.M. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and In Vitro. J. Exp. Bot. 2002, 53, 103–110. [Google Scholar] [CrossRef]
- Jahnová, J.; Luhová, L.; Petřivalský, M. S-Nitrosoglutathione Reductase—The Master Regulator of Protein S-Nitrosation in Plant NO Signaling. Plants 2019, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.; Ramchiary, C.; Sen, B.; Daimary, M.; Prasad, R. Integration of nitric oxide signaling in plant stress responses: Unveiling its role in enhancing crop tolerance to abiotic stress. Discov. Plants 2025, 2, 32. [Google Scholar] [CrossRef]
- Stuehr, D.J. Arginine metabolism: Enzymology, nutrition, and clinical significance. J. Nutr. 2004, 134, 2748S–2751S. [Google Scholar] [CrossRef] [PubMed]
- Jeandroz, S.; Wipf, D.; Stuehr, D.J.; Lamattina, L.; Melkonian, M.; Tian, Z.; Zhu, Y.; Carpenter, E.J.; Wong, G.K.-S.; Wendehenne, D. Occurrence, structure, and evolution of nitric oxide synthase–like proteins in the plant kingdom. Sci. Signal. 2016, 9, re2. [Google Scholar] [CrossRef]
- Gaston, B.; Reilly, J.; Drazen, J.M.; Fackler, J.; Ramdev, P.; Arnelle, D.; Mullins, M.E.; Sugarbaker, D.J.; Chee, C.; Singel, D.J.; et al. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc. Natl. Acad. Sci. USA 1993, 90, 10957–10961. [Google Scholar] [CrossRef]
- Spadaro, D.; Yun, B.-W.; Spoel, S.H.; Chu, C.; Wang, Y.-Q.; Loake, G.J. The redox switch: Dynamic regulation of protein function by cysteine modifications. Physiol. Plant. 2010, 138, 360–371. [Google Scholar] [CrossRef]
- Durner, J.; Klessig, D.F. Nitric oxide as a signal in plants. Curr. Opin. Plant Biol. 1999, 2, 369–374. [Google Scholar] [CrossRef]
- Espunya, M.C.; Díaz, M.; Moreno-Romero, J.; Martínez, M.C. Modification of intracellular levels of glutathione-dependent formaldehyde dehydrogenase alters glutathione homeostasis and root development. Plant Cell Environ. 2006, 29, 1002–1011. [Google Scholar] [CrossRef]
- Hogg, N. The Biochemistry and Physiology of S-Nitrosothiols. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, A.; Lamas, S. Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: Convergences and divergences. Cardiovasc. Res. 2007, 75, 220–228. [Google Scholar] [CrossRef]
- Stamler, J.S.; Lamas, S.; Fang, F.C. Nitrosylation: The Prototypic Redox-Based Signaling Mechanism. Cell 2001, 106, 675–683. [Google Scholar] [CrossRef]
- Wang, Y.; Yun, B.-W.; Kwon, E.; Hong, J.K.; Yoon, J.; Loake, G.J. S-Nitrosylation: An emerging redox-based post-translational modification in plants. J. Exp. Bot. 2006, 57, 1777–1784. [Google Scholar] [CrossRef]
- Corpas, F.J.; Alché, J.d.; Barroso, J.B. Current overview of S-nitrosoglutathione (GSNO) in higher plants. Front. Plant Sci. 2013, 4, 126. [Google Scholar] [CrossRef]
- Liu, L.; Hausladen, A.; Zeng, M.; Que, L.; Heitman, J.; Stamler, J.S. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 2001, 410, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.E.; Belka, G.K.; Bois, G.C.D. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem. J. 1998, 331, 659–668. [Google Scholar] [CrossRef]
- Sakamoto, A.; Ueda, M.; Morikawa, H. Arabidopsis glutathione-dependent formaldehyde dehydrogenase is an S-nitrosoglutathione reductase. FEBS Lett. 2002, 515, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Feechan, A.; Kwon, E.; Yun, B.-W.; Wang, Y.; Pallas, J.A.; Loake, G.J. A central role for S-nitrosothiols in plant disease resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 8054–8059. [Google Scholar] [CrossRef]
- Koivusalo, M.; Baumann, M.; Uotila, L. Evidence for the identity of glutathione-dependent formaldehyde dehydrogenase and class III alcohol dehydrogenase. FEBS Lett. 1989, 257, 105–109. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Erdjument-Bromage, H.; Ferris, C.D.; Tempst, P.; Snyder, S.H. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001, 3, 193–197. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Ching, W.-C.; Lin, Y.-P.; Chen, Y.-J. Methods for detection and characterization of protein S-nitrosylation. Methods 2013, 62, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, L.; Zuo, J. Protein S-Nitrosylation in plants: Current progresses and challenges. J. Integr. Plant Biol. 2019, 61, 1206–1223. [Google Scholar] [CrossRef]
- Machchhu, F.; Wany, A. Protein S-nitrosylation in plants under biotic stress. Theor. Exp. Plant Physiol. 2023, 35, 331–339. [Google Scholar] [CrossRef]
- Li, B.; Sun, C.; Lin, X.; Busch, W. The Emerging Role of GSNOR in Oxidative Stress Regulation. Trends Plant Sci. 2021, 26, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Wu, X.; Fang, H.; Huang, D.; Pan, X.; Liao, W. Role of protein S-nitrosylation in plant growth and development. Plant Cell Rep. 2024, 43, 204. [Google Scholar] [CrossRef]
- Huang, D.; Huo, J.; Zhang, J.; Wang, C.; Wang, B.; Fang, H.; Liao, W. Protein S-nitrosylation in programmed cell death in plants. Cell. Mol. Life Sci. 2019, 76, 1877–1887. [Google Scholar] [CrossRef]
- Cui, B.; Pan, Q.; Cui, W.; Wang, Y.; Loake, V.I.P.; Yuan, S.; Liu, F.; Loake, G.J. S-nitrosylation of a receptor-like cytoplasmic kinase regulates plant immunity. Sci. Adv. 2024, 10, eadk3126. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, X.; Wu, B.; Tong, B.; Xu, D.; Wang, J.; Cui, B.; Yin, R.; Lin, L. S-nitrosylation may inhibit the activity of COP1 in plant photomorphogenesis. Biochem. Biophys. Res. Commun. 2024, 719, 150096. [Google Scholar] [CrossRef]
- Chae, H.B.; Bae, S.B.; Paeng, S.K.; Wi, S.D.; Thi Phan, K.A.; Lee, S.Y. S-nitrosylation switches the Arabidopsis redox sensor protein, QSOX1, from an oxidoreductase to a molecular chaperone under heat stress. Plant Physiol. Biochem. 2024, 206, 108219. [Google Scholar] [CrossRef]
- Qin, G.; Qu, M.; Jia, B.; Wang, W.; Luo, Z.; Song, C.-P.; Tao, W.A.; Wang, P. FAT-switch-based quantitative S-nitrosoproteomics reveals a key role of GSNOR1 in regulating ER functions. Nat. Commun. 2023, 14, 3268. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Cui, X.; Yang, Z.; Bao, C.; Pan, L.; Liu, X.; Chatel-Innocenti, G.; Vanacker, H.; Noctor, G.; et al. S-Nitrosylation of the histone deacetylase HDA19 stimulates its activity to enhance plant stress tolerance in Arabidopsis. Plant J. 2023, 114, 836–854. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; Song, C.-P.; Zhou, J.-M.; Li, J.; Zuo, J. Nitric oxide negatively regulates gibberellin signaling to coordinate growth and salt tolerance in Arabidopsis. J. Genet. Genom. 2022, 49, 756–765. [Google Scholar] [CrossRef]
- Ying, S.; Yang, W.; Li, P.; Hu, Y.; Lu, S.; Zhou, Y.; Huang, J.; Hancock, J.T.; Hu, X. Phytochrome B enhances seed germination tolerance to high temperature by reducing S-nitrosylation of HFR1. EMBO Rep. 2022, 23, e54371. [Google Scholar] [CrossRef]
- Shee, R.; Ghosh, S.; Khan, P.; Sahid, S.; Roy, C.; Shee, D.; Paul, S.; Datta, R. Glutathione regulates transcriptional activation of iron transporters via S-nitrosylation of bHLH factors to modulate subcellular iron homoeostasis. Plant Cell Environ. 2022, 45, 2176–2190. [Google Scholar] [CrossRef] [PubMed]
- Zhan, N.; Wang, C.; Chen, L.; Yang, H.; Feng, J.; Gong, X.; Ren, B.; Wu, R.; Mu, J.; Li, Y.; et al. S-Nitrosylation Targets GSNO Reductase for Selective Autophagy during Hypoxia Responses in Plants. Mol. Cell 2018, 71, 142–154.e146. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.; Hou, Y.-J.; Zhao, Y.; Hsu, C.-C.; Yuan, F.; Zhu, X.; Tao, W.A.; Song, C.-P.; Zhu, J.-K. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc. Natl. Acad. Sci. USA 2015, 112, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mu, J.; Chen, L.; Feng, J.; Hu, J.; Li, L.; Zhou, J.-M.; Zuo, J. S-Nitrosylation Positively Regulates Ascorbate Peroxidase Activity during Plant Stress Responses. Plant Physiol. 2015, 167, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.-W.; Feechan, A.; Yin, M.; Saidi, N.B.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.-G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Feechan, A.; Yun, B.-W.; Shafiei, R.; Hofmann, A.; Taylor, P.; Xue, P.; Yang, F.-Q.; Xie, Z.-S.; Pallas, J.A.; et al. S-Nitrosylation of AtSABP3 Antagonizes the Expression of Plant Immunity. J. Biol. Chem. 2009, 284, 2131–2137. [Google Scholar] [CrossRef]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant Immunity Requires Conformational Charges of NPR1 via S-Nitrosylation and Thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef]
- Liu, M.; Cao, B.; Wei, J.-W.; Gong, B. Redesigning a S-nitrosylated pyruvate-dependent GABA transaminase 1 to generate high-malate and saline–alkali-tolerant tomato. New Phytol. 2024, 242, 2148–2162. [Google Scholar] [CrossRef]
- Liu, W.; Wei, J.-W.; Shan, Q.; Liu, M.; Xu, J.; Gong, B. Genetic engineering of drought- and salt-tolerant tomato via Δ1-pyrroline-5-carboxylate reductase S-nitrosylation. Plant Physiol. 2024, 195, 1038–1052. [Google Scholar] [CrossRef]
- Zeng, S.; Sun, X.; Zhai, J.; Li, X.; Pedro, G.-C.; Nian, H.; Li, K.; Xu, H. SlTrxh functions downstream of SlMYB86 and positively regulates nitrate stress tolerance via S-nitrosation in tomato seedling. Hortic. Res. 2024, 11, uhae184. [Google Scholar] [CrossRef]
- Liu, M.; Wei, J.-W.; Liu, W.; Gong, B. S-nitrosylation of ACO homolog 4 improves ethylene synthesis and salt tolerance in tomato. New Phytol. 2023, 239, 159–173. [Google Scholar] [CrossRef]
- Gao, X.; Ma, J.; Tie, J.; Li, Y.; Hu, L.; Yu, J. BR-Mediated Protein S-Nitrosylation Alleviated Low-Temperature Stress in Mini Chinese Cabbage (Brassica rapa ssp. pekinensis). Int. J. Mol. Sci. 2022, 23, 10964. [Google Scholar] [CrossRef]
- Yu, Z.; Cao, J.; Zhu, S.; Zhang, L.; Peng, Y.; Shi, J. Exogenous Nitric Oxide Enhances Disease Resistance by Nitrosylation and Inhibition of S-Nitrosoglutathione Reductase in Peach Fruit. Front. Plant Sci. 2020, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, M.; Chaki, M.; Airaki, M.; Valderrama, R.; Palma, J.M.; Barroso, J.B.; Corpas, F.J. Function of S-nitrosoglutathione reductase (GSNOR) in plant development and under biotic/abiotic stress. Plant Signal. Behav. 2011, 6, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hou, X.; Wei, L.; Deng, Y.; Zhao, Z.; Liang, C.; Liao, W. Protein S-nitrosylation under abiotic stress: Role and mechanism. Plant Physiol. Biochem. 2024, 207, 108329. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Zhou, S.; Wang, L.; Cheng, Y.; Zhao, L. Nitric Oxide Functions as a Signal and Acts Upstream of AtCaM3 in Thermotolerance in Arabidopsis Seedlings. Plant Physiol. 2010, 153, 1895–1906. [Google Scholar] [CrossRef]
- Lee, U.; Wie, C.; Fernandez, B.O.; Feelisch, M.; Vierling, E. Modulation of Nitrosative Stress by S-Nitrosoglutathione Reductase Is Critical for Thermotolerance and Plant Growth in Arabidopsis. Plant Cell 2008, 20, 786–802. [Google Scholar] [CrossRef]
- Song, X.; Wang, T.; Zhang, Y.; Yu, J.-Q.; Xia, X.-J. S-Nitrosoglutathione Reductase Contributes to Thermotolerance by Modulating High Temperature-Induced Apoplastic H2O2 in Solanum lycopersicum. Front. Plant Sci. 2022, 13, 862649. [Google Scholar] [CrossRef] [PubMed]
- Volkov, R.A.; Panchuk, I.I.; Mullineaux, P.M.; Schöffl, F. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 2006, 61, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.B.; Kim, M.G.; Kang, C.H.; Park, J.H.; Lee, E.S.; Lee, S.-U.; Chi, Y.H.; Paeng, S.K.; Bae, S.B.; Wi, S.D.; et al. Redox sensor QSOX1 regulates plant immunity by targeting GSNOR to modulate ROS generation. Mol. Plant 2021, 14, 1312–1327. [Google Scholar] [CrossRef] [PubMed]
- He, N.-Y.; Chen, L.-S.; Sun, A.-Z.; Zhao, Y.; Yin, S.-N.; Guo, F.-Q. A nitric oxide burst at the shoot apex triggers a heat-responsive pathway in Arabidopsis. Nat. Plants 2022, 8, 434–450. [Google Scholar] [CrossRef]
- Parankusam, S.; Adimulam, S.S.; Bhatnagar-Mathur, P.; Sharma, K.K. Nitric Oxide (NO) in Plant Heat Stress Tolerance: Current Knowledge and Perspectives. Front. Plant Sci. 2017, 8, 1582. [Google Scholar] [CrossRef]
- Cui, J.; Huang, M.; Qi, J.; Yu, W.; Li, C. Nitric Oxide in Plant Cold Stress: Functions, Mechanisms and Challenges. Agronomy 2025, 15, 1072. [Google Scholar] [CrossRef]
- Babuta, P.; Sougrakpam, Y.; Deswal, R. Nitric oxide cross-talks during low-temperature stress in plants. Plant Sci. 2025, 360, 112708. [Google Scholar] [CrossRef]
- Zhao, M.-G.; Chen, L.; Zhang, L.-L.; Zhang, W.-H. Nitric Reductase-Dependent Nitric Oxide Production Is Involved in Cold Acclimation and Freezing Tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef]
- Costa-Broseta, Á.; Perea-Resa, C.; Castillo, M.-C.; Ruíz, M.F.; Salinas, J.; León, J. Nitric Oxide Controls Constitutive Freezing Tolerance in Arabidopsis by Attenuating the Levels of Osmoprotectants, Stress-Related Hormones and Anthocyanins. Sci. Rep. 2018, 8, 9268. [Google Scholar] [CrossRef]
- Lv, X.; Ge, S.; Jalal Ahammed, G.; Xiang, X.; Guo, Z.; Yu, J.; Zhou, Y. Crosstalk between Nitric Oxide and MPK1/2 Mediates Cold Acclimation-induced Chilling Tolerance in Tomato. Plant Cell Physiol. 2017, 58, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ma, J.; Wang, G.; Huang, S.; Wu, X.; Hu, L.; Yu, J. The S-nitrosylation of monodehydroascorbate reductase positively regulated the low temperature tolerance of mini Chinese cabbage. Int. J. Biol. Macromol. 2024, 281, 136047. [Google Scholar] [CrossRef]
- Stamler, J.S.; Singel, D.J.; Loscalzo, J. Biochemistry of Nitric Oxide and Its Redox-Activated Forms. Science 1992, 258, 1898–1902. [Google Scholar] [CrossRef]
- Wink, D.A.; Mitchell, J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Li, B.; Sun, L.; Huang, J.; Göschl, C.; Shi, W.; Chory, J.; Busch, W. GSNOR provides plant tolerance to iron toxicity via preventing iron-dependent nitrosative and oxidative cytotoxicity. Nat. Commun. 2019, 10, 3896. [Google Scholar] [CrossRef]
- Wen, D.; Sun, S.; Yang, W.; Zhang, L.; Liu, S.; Gong, B.; Shi, Q. Overexpression of S-nitrosoglutathione reductase alleviated iron-deficiency stress by regulating iron distribution and redox homeostasis. J. Plant Physiol. 2019, 237, 1–11. [Google Scholar] [CrossRef]
- García, M.J.; Corpas, F.J.; Lucena, C.; Alcántara, E.; Pérez-Vicente, R.; Zamarreño, Á.M.; Bacaicoa, E.; García-Mina, J.M.; Bauer, P.; Romera, F.J. A Shoot Fe Signaling Pathway Requiring the OPT3 Transporter Controls GSNO Reductase and Ethylene in Arabidopsis thaliana Roots. Front. Plant Sci. 2018, 9, 1325. [Google Scholar] [CrossRef]
- Guan, M.; Zheng, X.; Zhu, Y. S-nitrosoglutathione reductase disfavors cadmium tolerance in shoots of Arabidopsis. Sci. Rep. 2024, 14, 26401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, H.; Li, B.; Wang, M.; Di, D.; Lin, X.; Kronzucker, H.J.; Shi, W.; Li, G. Induction of S-nitrosoglutathione reductase protects root growth from ammonium toxicity by regulating potassium homeostasis in Arabidopsis and rice. J. Exp. Bot. 2021, 72, 4548–4564. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Al Azawi, T.N.I.; Pande, A.; Mun, B.-G.; Lee, D.-S.; Hussain, A.; Lee, B.-H.; Yun, B.-W. The Role of Nitric Oxide-Induced ATILL6 in Growth and Disease Resistance in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 685156. [Google Scholar] [CrossRef]
- Ageeva-Kieferle, A.; Georgii, E.; Winkler, B.; Ghirardo, A.; Albert, A.; Hüther, P.; Mengel, A.; Becker, C.; Schnitzler, J.-P.; Durner, J.; et al. Nitric oxide coordinates growth, development, and stress response via histone modification and gene expression. Plant Physiol. 2021, 187, 336–360. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, M.; Chen, T.; Zhang, L.; Fan, L.; Zhang, W.; Wei, B.; Li, S.; Xuan, W.; Noctor, G.; et al. Glutathione-dependent denitrosation of GSNOR1 promotes oxidative signalling downstream of H2O2. Plant Cell Environ. 2020, 43, 1175–1191. [Google Scholar] [CrossRef]
- Chen, L.; Wu, R.; Feng, J.; Feng, T.; Wang, C.; Hu, J.; Zhan, N.; Li, Y.; Ma, X.; Ren, B.; et al. Transnitrosylation Mediated by the Non-canonical Catalase ROG1 Regulates Nitric Oxide Signaling in Plants. Dev. Cell 2020, 53, 444–457.e445. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z.; Molnár, Á.; Oláh, D.; Feigl, G.; Horváth, E.; Erdei, L.; Ördög, A.; Rudolf, E.; Barth, T.; Lindermayr, C. S-Nitrosothiol Signaling Is involved in Regulating Hydrogen Peroxide Metabolism of Zinc-Stressed Arabidopsis. Plant Cell Physiol. 2019, 60, 2449–2463. [Google Scholar] [CrossRef]
- Guan, M.Y.; Zhu, Y.X.; Liu, X.X.; Jin, C.W. Induction of S-nitrosoglutathione reductase reduces root cadmium uptake by inhibiting Iron-regulated transporter 1. Plant Soil 2019, 438, 251–262. [Google Scholar] [CrossRef]
- Zhou, S.; Jia, L.; Chu, H.; Wu, D.; Peng, X.; Liu, X.; Zhang, J.; Zhao, J.; Chen, K.; Zhao, L. Arabidopsis CaM1 and CaM4 Promote Nitric Oxide Production and Salt Resistance by Inhibiting S-Nitrosoglutathione Reductase via Direct Binding. PLoS Genet. 2016, 12, e1006255. [Google Scholar] [CrossRef] [PubMed]
- Frungillo, L.; Skelly, M.J.; Loake, G.J.; Spoel, S.H.; Salgado, I. S-nitrosothiols regulate nitric oxide production and storage in plants through the nitrogen assimilation pathway. Nat. Commun. 2014, 5, 5401. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Proline Alleviates Abiotic Stress Induced Oxidative Stress in Plants. J. Plant Growth Regul. 2023, 42, 4629–4651. [Google Scholar] [CrossRef]
- Wang, M.; Dong, Y.; Yan, J.; Han, Q.; Li, K.; Xu, H. Overexpression of the spinach S-nitrosoglutathione reductase (SoGSNOR) in tobacco resulted in enhanced nitrate stress tolerance. Plant Cell Tissue Organ Cult. 2020, 143, 173–187. [Google Scholar] [CrossRef]
- Lay-Pruitt, K.S.; Takahashi, H. Integrating N signals and root growth: The role of nitrate transceptor NRT1.1 in auxin-mediated lateral root development. J. Exp. Bot. 2020, 71, 4365–4368. [Google Scholar] [CrossRef] [PubMed]
- Barth, C.; Gouzd, Z.A.; Steele, H.P.; Imperio, R.M. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J. Exp. Bot. 2009, 61, 379–394. [Google Scholar] [CrossRef]
- Sagardoy, R.; Morales, F.; López-Millán, A.-F.; Abadía, A.; Abadía, J. Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics. Plant Biol. 2009, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Feigl, G.; Lehotai, N.; Molnár, Á.; Ördög, A.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J.; Erdei, L.; Kolbert, Z. Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 2014, 116, 613–625. [Google Scholar] [CrossRef]
- Oláh, D.; Kondak, S.; Molnár, Á.; Adedokun, O.P.; Czékus, Z.; Gémes, K.; Galbács, G.; Kolbert, Z. Suboptimal zinc supply affects the S-nitrosoglutathione reductase enzyme and nitric oxide signaling in Arabidopsis. Plant Stress 2023, 10, 100250. [Google Scholar] [CrossRef]
- Gong, B.; Wen, D.; Bloszies, S.; Li, X.; Wei, M.; Yang, F.; Shi, Q.; Wang, X. Comparative effects of NaCl and NaHCO3 stresses on respiratory metabolism, antioxidant system, nutritional status, and organic acid metabolism in tomato roots. Acta Physiol. Plant. 2014, 36, 2167–2181. [Google Scholar] [CrossRef]
- Sharma, T.; Dreyer, I.; Kochian, L.; Piñeros, M.A. The ALMT Family of Organic Acid Transporters in Plants and Their Involvement in Detoxification and Nutrient Security. Front. Plant Sci. 2016, 7, 1488. [Google Scholar] [CrossRef]
- Gong, B.; Wen, D.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. S-Nitrosoglutathione Reductase-Modulated Redox Signaling Controls Sodic Alkaline Stress Responses in Solanum lycopersicum L. Plant Cell Physiol. 2014, 56, 790–802. [Google Scholar] [CrossRef]
- Wei, J.-W.; Liu, M.; Zhao, D.; Du, P.; Yan, L.; Liu, D.; Shi, Q.; Yang, C.; Qin, G.; Gong, B. Melatonin confers saline-alkali tolerance in tomato by alleviating nitrosative damage and S-nitrosylation of H+-ATPase 2. Plant Cell 2025, 37, koaf035. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.-X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.; Sasidharan, R.; Visser, E.J.; Bailey, J. Flooding stress signaling through perturbations in oxygen, ethylene, nitric oxide and light. New Phytol. 2016, 209, 39–43. [Google Scholar] [CrossRef]
- Pan, C.; Li, X.; Jian, C.; Zhou, Y.; Wang, A.; Xiao, D.; Zhan, J.; He, L. AhGSNOR1 negatively regulates Al-induced programmed cell death by regulating intracellular NO and redox levels. Plant Sci. 2024, 349, 112275. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, T.; Chen, X.; Wang, Z.; Yang, Z.; Ren, A.; Shi, L.; Yu, H.; Zhao, M. GSNOR regulates ganoderic acid content in Ganoderma lucidum under heat stress through S-nitrosylation of catalase. Commun. Biol. 2022, 5, 32. [Google Scholar] [CrossRef]
- Zhang, X.; Du, H.; Shi, Q.; Gong, B. Loss of GSNOR increases abiotic stress sensitivity via regulating MAPK-ethylene cascade signaling in Solanum lycopersicum L. Environ. Exp. Bot. 2022, 199, 104872. [Google Scholar] [CrossRef]
- Lin, A.; Wang, Y.; Tang, J.; Xue, P.; Li, C.; Liu, L.; Hu, B.; Yang, F.; Loake, G.J.; Chu, C. Nitric Oxide and Protein S-Nitrosylation Are Integral to Hydrogen Peroxide-Induced Leaf Cell Death in Rice. Plant Physiol. 2011, 158, 451–464. [Google Scholar] [CrossRef]
- Shan, Q.; Zhao, D.; Cao, B.; Zhu, X.; Wang, C.; Deng, L.; Li, C.; Zhang, Y.; Shi, Q.; Gong, B. Jasmonic acid and nitric oxide orchestrate a hierarchical melatonin cascade for Botrytis cinerea resistance in tomato. Plant Physiol. 2025, 197, kiaf078. [Google Scholar] [CrossRef]
- Li, T.; Kang, J.; Zhang, H.; Wang, L.; Lu, M.; Cai, L.; Li, J.; Joosten, M.H.A.J.; Du, Y. Phytophthora Disrupts Plant Immunity by Manipulating Nitric Oxide Homeostasis Through GSNOR Inhibition. Adv. Sci. 2025, 12, e03633. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, J.; Qiao, J.; Pan, J.; Zhang, S.; Li, Q.; Wang, Q.; Gong, B.; Shi, J. GABA keeps nitric oxide in balance by regulating GSNOR to enhance disease resistance of harvested tomato against Botrytis cinerea. Food Chem. 2022, 392, 133299. [Google Scholar] [CrossRef]
- Rasool, G.; Buchholz, G.; Yasmin, T.; Shabbir, G.; Abbasi, N.A.; Malik, S.I. Overexpression of SlGSNOR impairs In Vitro shoot proliferation and developmental architecture in tomato but confers enhanced disease resistance. J. Plant Physiol. 2021, 261, 153433. [Google Scholar] [CrossRef]
- Hussain, A.; Yun, B.W.; Kim, J.H.; Gupta, K.J.; Hyung, N.I.; Loake, G.J. Novel and conserved functions of S-nitrosoglutathione reductase in tomato. J. Exp. Bot. 2019, 70, 4877–4886. [Google Scholar] [CrossRef]
- Thalineau, E.; Truong, H.-N.; Berger, A.; Fournier, C.; Boscari, A.; Wendehenne, D.; Jeandroz, S. Cross-Regulation between N Metabolism and Nitric Oxide (NO) Signaling during Plant Immunity. Front. Plant Sci. 2016, 7, 472. [Google Scholar] [CrossRef]
- Lindermayr, C.; Saalbach, G.; Durner, J.r. Proteomic Identification of S-Nitrosylated Proteins in Arabidopsis. Plant Physiol. 2005, 137, 921–930. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Campostrini, N.; Mattè, A.; Righetti, P.G.; Perazzolli, M.; Zolla, L.; Roepstorff, P.; Delledonne, M. Proteomic analysis of S-nitrosylated proteins in Arabidopsis thaliana undergoing hypersensitive response. Proteomics 2008, 8, 1459–1469. [Google Scholar] [CrossRef]
- Imran, Q.M.; Falak, N.; Hussain, A.; Mun, B.-G.; Sharma, A.; Lee, S.-U.; Kim, K.-M.; Yun, B.-W. Nitric Oxide Responsive Heavy Metal-Associated Gene AtHMAD1 Contributes to Development and Disease Resistance in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1712. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Pan, Q.; Clarke, D.; Villarreal, M.O.; Umbreen, S.; Yuan, B.; Shan, W.; Jiang, J.; Loake, G.J. S-nitrosylation of the zinc finger protein SRG1 regulates plant immunity. Nat. Commun. 2018, 9, 4226. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Imran, Q.M.; Shahid, M.; Mun, B.-G.; Lee, S.-U.; Khan, M.A.; Hussain, A.; Lee, I.-J.; Yun, B.-W. Nitric oxide- induced AtAO3 differentially regulates plant defense and drought tolerance in Arabidopsis thaliana. BMC Plant Biol. 2019, 19, 602. [Google Scholar] [CrossRef]
- Cui, B.; Ma, X.; Li, Y.; Zhou, Y.; Ju, X.; Hussain, A.; Umbreen, S.; Yuan, B.; Tabassum, A.; Lubega, J.; et al. Perturbations in nitric oxide homeostasis promote Arabidopsis disease susceptibility towards Phytophthora parasitica. Mol. Plant Pathol. 2021, 22, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Hurali, D.T.; Bhurta, R.; Tyagi, S.; Sathee, L.; Sandeep, A.B.; Singh, D.; Mallick, N.; Vinod; Jha, S.K. Analysis of NIA and GSNOR family genes and nitric oxide homeostasis in response to wheat-leaf rust interaction. Sci. Rep. 2022, 12, 803. [Google Scholar] [CrossRef]
- Wünsche, H.; Baldwin, I.T.; Wu, J. S-Nitrosoglutathione reductase (GSNOR) mediates the biosynthesis of jasmonic acid and ethylene induced by feeding of the insect herbivore Manduca sexta and is important for jasmonate-elicited responses in Nicotiana attenuata. J. Exp. Bot. 2011, 62, 4605–4616. [Google Scholar] [CrossRef]
- Yun, B.-W.; Skelly, M.J.; Yin, M.; Yu, M.; Mun, B.-G.; Lee, S.-U.; Hussain, A.; Spoel, S.H.; Loake, G.J. Nitric oxide and S-nitrosoglutathione function additively during plant immunity. New Phytol. 2016, 211, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Holzmeister, C.; Fröhlich, A.; Sarioglu, H.; Bauer, N.; Durner, J.; Lindermayr, C. Proteomic analysis of defense response of wildtype Arabidopsis thaliana and plants with impaired NO-homeostasis. Proteomics 2011, 11, 1664–1683. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.; Waseem, M.; Felix, A.; Shaheen, I. Nitrogen Uptake and Utilization Toward Sustainable Agriculture Development. In Nitric Oxide in Plants: A Molecule with Dual Roles; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 78–94. [Google Scholar]
- Pramanik, P.; Krishnan, P.; Maity, A.; Mridha, N.; Mukherjee, A.; Rai, V. Application of Nanotechnology in Agriculture. In Environmental Nanotechnology Volume 4; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 317–348. [Google Scholar]
- de Souza, G.F.P.; Denadai, J.P.; Picheth, G.F.; de Oliveira, M.G. Long-term decomposition of aqueous S-nitrosoglutathione and S-nitroso-N-acetylcysteine: Influence of concentration, temperature, pH and light. Nitric Oxide 2019, 84, 30–37. [Google Scholar] [CrossRef]
- Manoj, V.M.; Aravindakumar, C.T. Hydroxyl radical induced decomposition of S-nitrosoglutathione. Chem. Commun. 2000, 2361–2362. [Google Scholar] [CrossRef]
- Trujillo, M.; Alvarez, M.a.N.; Peluffo, G.; Freeman, B.A.; Radi, R. Xanthine Oxidase-mediated Decomposition of S-Nitrosothiols. J. Biol. Chem. 1998, 273, 7828–7834. [Google Scholar] [CrossRef]
- Seabra, A.B.; Silveira, N.M.; Ribeiro, R.V.; Pieretti, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M.; Hancock, J.T.; Petřivalský, M.; Gupta, K.J.; et al. Nitric oxide-releasing nanomaterials: From basic research to potential biotechnological applications in agriculture. New Phytol. 2022, 234, 1119–1125. [Google Scholar] [CrossRef]
- Pereira, A.; Narciso, A.; Seabra, A.; Fraceto, L. Evaluation of the effects of nitric oxide-releasing nanoparticles on plants. J. Phys. Conf. Ser. 2015, 617, 012025. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Gomes, B.C.R.; Pelegrino, M.T.; Seabra, A.B. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide 2016, 61, 10–19. [Google Scholar] [CrossRef]
- Methela, N.J.; Pande, A.; Islam, M.S.; Rahim, W.; Hussain, A.; Lee, D.-S.; Mun, B.-G.; Maria Joseph Raj, N.P.; Kim, S.-J.; Kim, Y.; et al. Chitosan-GSNO nanoparticles: A positive modulator of drought stress tolerance in soybean. BMC Plant Biol. 2023, 23, 639. [Google Scholar] [CrossRef] [PubMed]
- Kondak, D.; Deák, Á.; Rónavári, A.; Bodor, T.; Kondak, S.; Adedokun, O.P.; Benkő, P.; Szőllősi, R.; Szalai, G.; Janda, T.; et al. Chitosan encapsulated S-nitrosoglutathione is an efficient nanodonor in Brassica napus seedlings. Plant Physiol. Biochem. 2025, 227, 110184. [Google Scholar] [CrossRef]
- Cardozo, V.F.; Lancheros, C.A.C.; Narciso, A.M.; Valereto, E.C.S.; Kobayashi, R.K.T.; Seabra, A.B.; Nakazato, G. Evaluation of antibacterial activity of nitric oxide-releasing polymeric particles against Staphylococcus aureus and Escherichia coli from bovine mastitis. Int. J. Pharm. 2014, 473, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Silveira, N.M.; Seabra, A.B.; Marcos, F.C.C.; Pelegrino, M.T.; Machado, E.C.; Ribeiro, R.V. Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide 2019, 84, 38–44. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, L.; Hussain, Z.; Huang, D.; Zhu, S. Enhancement of storability and antioxidant systems of sweet cherry fruit by nitric oxide-releasing chitosan nanoparticles (GSNO-CS NPs). Food Chem. 2019, 285, 10–21. [Google Scholar] [CrossRef]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and In Situ Remediation: A Review of the Benefits and Potential Risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef]
- Santos, P.A.; Biraku, X.; Nielsen, E.; Ozketen, A.C.; Ozketen, A.A.; Hakki, E.E. Agricultural nanotechnology for a safe and sustainable future: Current status, challenges, and beyond. J. Sci. Food Agric. 2025, 105, 3159–3169. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K. Exploring the Effect of Engineered Nanomaterials on Soil Microbial Diversity and Functions: A Review. J. Environ. Nanotechnol. 2024, 13, 48–62. [Google Scholar] [CrossRef]
- Jafar, G.; Hamzeh, G. Ecotoxicity of nanomaterials in soil. Ann. Biol. Res. 2013, 4, 86–92. [Google Scholar]
- Colman, B.P.; Arnaout, C.L.; Anciaux, S.; Gunsch, C.K.; Hochella, M.F., Jr.; Kim, B.; Lowry, G.V.; McGill, B.M.; Reinsch, B.C.; Richardson, C.J.; et al. Low Concentrations of Silver Nanoparticles in Biosolids Cause Adverse Ecosystem Responses under Realistic Field Scenario. PLoS ONE 2013, 8, e57189. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhang, L.; Huang, Y.; Ouyang, Z. Research status on soil and water pollution remediation and environmental impact of nanomaterials. IOP Conf. Ser. Earth Environ. Sci. 2019, 371, 032015. [Google Scholar] [CrossRef]
- Kumar, R.; Kishan Singh, R.; Mishra, A.K. Nanoparticles in the soil environment and their behaviour: An overview. J. Appl. Nat. Sci. 2012, 4, 310–324. [Google Scholar] [CrossRef]
- Lewis, R.W.; Bertsch, P.M.; McNear, D.H. Nanotoxicity of engineered nanomaterials (ENMs) to environmentally relevant beneficial soil bacteria—A critical review. Nanotoxicology 2019, 13, 392–428. [Google Scholar] [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in daily life: Applications, toxicity and regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Kaya, C.; Uğurlar, F.; Seth, C.S. Sodium nitroprusside modulates oxidative and nitrosative processes in Lycopersicum esculentum L. under drought stress. Plant Cell Rep. 2024, 43, 152, Correction in Plant Cell Rep. 2025, 44, 145. https://doi.org/10.1007/s00299-025-03529-3. [Google Scholar] [CrossRef] [PubMed]
- Ghita, R.V.; Predoi, D.; Iconaru, S.L.; Cimpeanu, C.L.; Raita, S.M. Application of Nanotechnology Solutions in Plants Fertilization. In Urban Horticulture-Necessity of the Future; Solankey, S.S., Akhtar, S., Luna Maldonado, A.I., Rodriguez-Fuentes, H., Vidales Contreras, J.A., Reyes, J.M.M., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Yadav, A.; Yadav, K.; Ahmad, R.; Abd-Elsalam, K.A. Emerging Frontiers in Nanotechnology for Precision Agriculture: Advancements, Hurdles and Prospects. Agrochemicals 2023, 2, 220–256. [Google Scholar] [CrossRef]
- Klaine, S.J.; Koelmans, A.A.; Horne, N.; Carley, S.; Handy, R.D.; Kapustka, L.; Nowack, B.; von der Kammer, F. Paradigms to assess the environmental impact of manufactured nanomaterials. Environ. Toxicol. Chem. 2012, 31, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Tripathi, S.; Bajpai, S.; Kamboj, M. Approaches, Challenges, and Prospects of Nanotechnology for Sustainable Agriculture. In Agricultural and Environmental Nanotechnology: Novel Technologies and Their Ecological Impact; Fernandez-Luqueno, F., Patra, J.K., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 83–103. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).