Abstract
Severe coronavirus disease 2019 (COVID-19) is characterized by systemic hyperinflammation with cytokine and chemokine release, alongside elevations in conventional laboratory biomarkers such as C-reactive protein (CRP), ferritin, and procalcitonin (PCT). However, the interplay between cytokines, chemokines, growth factors (CCGFs), and standard biomarkers remains incompletely understood. Therefore, we aimed to evaluate associations between CCGFs and conventional biomarkers from a broad aspect, utilizing the prospective PronMed cohort of critically ill COVID-19 patients admitted to the intensive care unit (ICU) at Uppsala University Hospital. Plasma concentrations of 92 CCGFs were analyzed in each patient using the Olink Target 96 Cardiovascular II panel and analyzed in relation to conventional biomarkers and peripheral blood cell counts. Associations were evaluated using Spearman rank correlations with Benjamini–Hochberg correction for multiple testing. A total of 114 patients (median age 61 years (IQR: 19), 75% male, median SAPS-3 52 (IQR: 10) were included. Significant correlations confirmed CRP as a robust surrogate of cytokine-driven inflammation. Ferritin was strongly associated with macrophage-related markers, including IL-18, sCD163-related factors, and PARP1. PCT correlated with a wide range of CCGFs, including ADM, PGF, TRAILR2, and IL-6. Blood cell subsets also showed distinct associations with CCGFs, suggesting functional connections between cytokine signaling and hematological disturbances. Our findings demonstrate that conventional biomarkers of inflammation in COVID-19 reflect complex and distinct interaction patterns with cytokines, chemokines, and growth factors. Mapping these associations improves understanding of COVID-19 immunopathology and may inform biomarker-guided risk stratification in critical illness.
Keywords:
biomarker; nucleated blood cells; COVID-19; critical illness; CRP; cytokine; ferritin; inflammation; procalcitonin 1. Introduction
The pandemic coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, has been characterized not only by viral pneumonia but also by systemic endothelial inflammation, acute kidney injury and myocardial manifestations [1,2,3]. Early in the pandemic, it became clear that severe cases were frequently associated with a state of hyperinflammation, in which multiple inflammatory mediators were elevated and strongly linked to adverse clinical outcomes. This prompted a plethora of questions regarding the relationships between circulating cytokines, growth factors, and chemokines, and more traditional laboratory markers of systemic inflammation such as blood cell counts, C-reactive protein (CRP), ferritin, and procalcitonin (PCT) [4,5,6].
CRP is a frequently used acute-phase reactant and was among the earliest biomarkers reported to be elevated in severe COVID-19 [7]. Multiple studies have consistently demonstrated strong associations between circulating cytokines, especially interleukin-6 (IL6), and CRP levels. The connection between CRP and IL6 is well-established, since IL6 directly induces hepatocyte CRP production. In COVID-19 cohorts, higher CRP concentrations have been tightly linked to elevated IL6 and to worse outcomes, including respiratory failure, intensive care unit (ICU) admission, and mortality [8,9].
Not only other cytokines, e.g., tumor necrosis factor-alpha, but also white blood cells have shown correlations with CRP, underscoring that CRP elevation in COVID-19 reflects a broader pro-inflammatory milieu rather than the action of IL6 alone [10]. Thus, CRP, while non-specific, remains a robust surrogate for the cytokine-driven inflammatory burden in COVID-19 [11].
Hyperferritinemia has emerged as another hallmark of severe COVID-19 [12]. Moreover, elevated ferritin and macrophage activation are mediators of thrombotic complications in COVID-19 [13]. Ferritin levels correlate not only with CRP [14] but also with biomarkers of cell damage [15].
Furthermore, ferritin correlates with sCD163, the soluble form of the CD163 receptor, which is mainly located on the surface of monocytes and macrophages, and also with interleukin-18 (IL18) [16], suggesting a mechanistic link between macrophage activation and cytokine release in COVID-19.
Procalcitonin is commonly used as an infectious biomarker, indicating disease severity, especially in the intensive care unit. Although procalcitonin is relatively specific to bacterial infections, serum procalcitonin levels correlate with disease severity, which decreases its reliability in the setting of critical illness and especially in cases of severe influenza and COVID-19 [17].
Pro-inflammatory cytokines such as IL6 and TNF-α are elevated in patients with increased PCT, and meta-analyses confirm that elevated PCT is associated with severe and fatal outcomes [18,19].
Alterations in peripheral blood cell counts, particularly decreased numbers of platelets, lymphopenia and neutrophilia, are some of the striking features of severe COVID-19 infections, and increased neutrophil-to-lymphocyte ratio has been reported to be a strong predictor of severity and mortality [20]. Associations between cytokines (IL6, IL-8, TNF-α) and blood cell abnormalities may connect cytokine signaling directly to hematological disturbances [20,21,22].
Furthermore, several chemokines, e.g., CXCL10 and CCL2, have been found at elevated levels in severe infectious disease, and their correlations with peripheral leukocyte counts point to a functional link between chemokine signaling and observed blood cell dynamics [23,24].
The inflammatory reaction consists of a myriad of interconnected reactions and events, where traditional biomarkers are involved in a complex interplay with circulating cytokines, chemokines, and growth factors (CCGFs). Still, interactions between CCGFs, several biomarkers, and circulating blood cells remain incompletely mapped. Newer technologies, such as proximity extension assays (e.g., Olink panels) enable simultaneous quantification of large numbers of CCGFs with high sensitivity and specificity.
The aim of the present study is to investigate associations between a broad panel of CCGFs and some conventional biomarkers of inflammation in patients with COVID-19 in order to expand current knowledge of COVID-19 pathophysiology and to explore whether yet-undiscovered interactions may be identified. Since severe COVID-19 represents a state of thromboinflammation, endotheliopathy and cardiovascular mortality [25], we decided to focus on a cytokine panel for biomarkers associated with cardiovascular morbidity and mortality.
2. Results
2.1. Patient Characteristics
This cohort consisted of one hundred and fourteen patients aged 24–86 years, of which eighty-six were males. The median age was 61 years and the interquartile range (IQR) was 52–71 years. Median BMI (kg/m2) was 28 IQR (25–33). Fifty-three percent had pre-existing hypertension and twenty-seven percent had diabetes. Median duration of COVID-19 before ICU admission was 10 days (IQR: 8–12) and SAPS-3 was 52 (IQR: 47–57). At ICU admission median C-reactive protein (CRP; mg/L) was 164 (IQR: 113–235), whereas median procalcitonin (microg/L) was 0.46 (IQR: 0.19–1.1) and median ferritin (microg/L) was 1284 (IQR: 543–2395).
2.2. Associations Between Inflammatory Biomarkers (CRP, PCT, IL-6, Ferritin) and Peripheral Blood Nucleated Cells
CRP was significantly correlated with both neutrophil cells and leucocytes, respectively. PCT was significantly correlated with leucocytes and IL6 was significantly correlated with neutrophil cells (Figure 1). The exact correlations and their significance levels are presented in Supplementary Table S2.

Figure 1.
Heatmap displaying Spearman’s rank correlation coefficients. CRP = C-reactive protein; PCT = Procalcitonin; IL6 = Interleukin-6. Increasingly warmer colors (light red to darker red) indicate stronger positive correlations, whereas cooler colors (light blue to blue) indicate more expressed negative correlations. No color denotes absence of correlation.
Significant associations between biomarkers and CCGFs are displayed in Table 1.

Table 1.
Significant associations between biomarkers and cytokines, chemokines, and growth factors (CCGFs). Results were adjusted for multiple testing. Adjusted p-values below 0.10, corresponding to an expected false discovery rate of <10%, were considered statistically significant. Calculations of p-values are explained in Section 4.5.
Interactions between conventional inflammatory biomarkers and CCGFs are displayed as STRING images.
2.3. Associations Between CRP and CCGFs
CRP exhibited 6 significant associations out of the quantified CCGFs (Figure 2). IL6 was the one that was most associated with CRP, followed by SCF and IL1Ra.
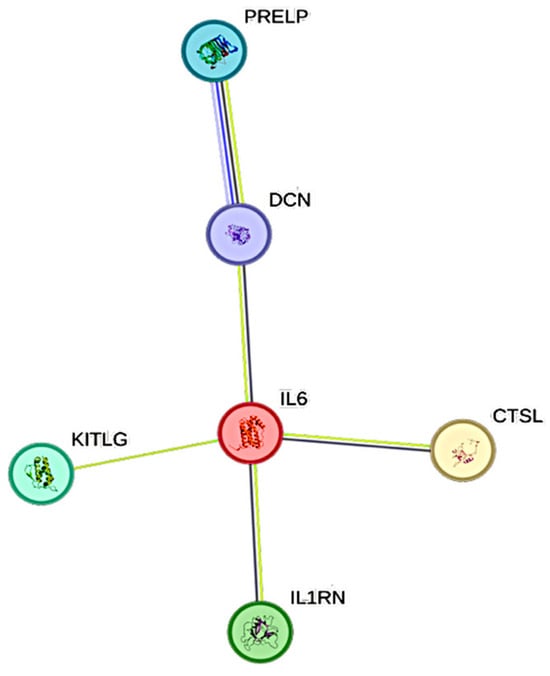
Figure 2.
This figure represents a network of interactions among cytokines, demonstrating significant associations between circulating cytokine levels and CRP. The nodes (CCGFs) are connected by edges of varying thickness, indicating confidence of interactions.
2.4. Associations Between PCT and CCGFs
Procalcitonin was significantly associated with 19 CCGFs, out of which the most expressed association was noted with ADM, followed by TNFRSF10A and in turn by CTSL1 (Figure 3). CRP was significantly more associated with procalcitonin than IL6 was.

Figure 3.
This figure represents a network of interactions among cytokines, demonstrating significant associations between circulating cytokine levels and procalcitonin. The nodes (CCGFs) are connected by edges of varying thickness, indicating confidence of interactions.
2.5. Associations Between IL6 and CCGFs
IL6 was most significantly associated with ADM, CRP, and CCL3 (Figure 4). PCT exhibited the second weakest association with IL6.
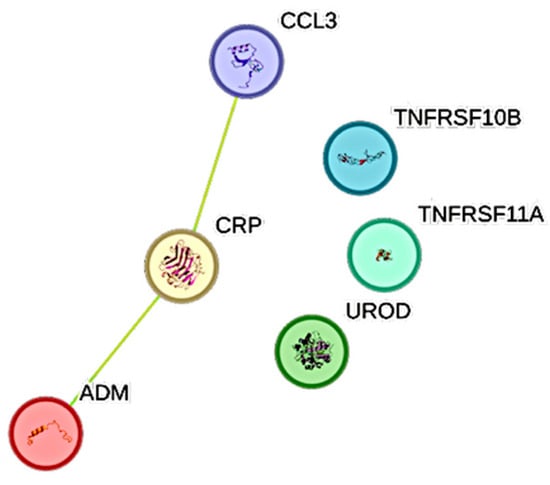
Figure 4.
This figure represents a network of interactions among cytokines, demonstrating significant associations between circulating cytokine levels and IL6. The nodes (CCGFs) are connected by edges of varying thickness, indicating confidence of interactions.
2.6. Associations Between Ferritin and CCGFs
Ferritin was significantly associated with ten CCGFs. The three most expressed associations were between ferritin and, in the following order, CTSL1, PARP1, and IL18 (Figure 5).

Figure 5.
This figure represents a network of interactions among cytokines, demonstrating significant associations between circulating cytokine levels and ferritin. The nodes (CCGFs) are connected by edges of varying thickness, indicating confidence of interactions.
3. Discussion
In this prospective cohort of critically ill COVID-19 patients, we investigated the associations between conventional inflammatory biomarkers (CRP, PCT, ferritin, and blood cell counts) and a broad panel of circulating cytokines, chemokines, and growth factors (CCGFs) using proximity extension assays. Our data provide new insights into the complex inflammatory milieu of severe COVID-19 and highlight several previously unrecognized links between routine clinical biomarkers and mediators of cytokine signaling, macrophage activation, and endothelial dysfunction.
As expected, CRP showed a strong and highly significant association with IL6, confirming prior studies that have consistently demonstrated the IL6–CRP axis as a central pathway in COVID-19-related inflammation [26]. Beyond IL6, we identified significant associations between CRP and IL1 receptor antagonist (IL1Ra), stem cell factor (SCF), and extracellular matrix components such as decorin (DCN) and prolargin (PRELP). These findings suggest that CRP may not only reflect hepatocyte stimulation by IL6 but may also serve as a surrogate for broader cytokine-driven tissue and extracellular matrix remodeling processes during severe infection.
Procalcitonin, typically regarded as a biomarker of bacterial superinfection [27], demonstrated extensive associations with multiple CCGFs, including adrenomedullin (ADM), placental growth factor (PGF), programmed death-ligand 2 (PD-L2), and TRAIL receptor 2 (TRAILR2). Interestingly, PCT was also tightly linked to IL6 and TNF superfamily members, underscoring that elevated PCT in COVID-19 is not solely attributable to bacterial coinfection but may instead reflect a state of profound systemic inflammation. This aligns with prior meta-analyses showing that PCT is associated with adverse outcomes in COVID-19, irrespective of concurrent bacterial infection. The broad CCGF interaction profile of PCT in our study reinforces the notion that this biomarker reflects inflammatory severity rather than etiology in critical illness [28,29].
Hyperferritinemia, a hallmark of severe COVID-19 [12], also displayed strong associations with cytokines and enzymes linked to macrophage activation and oxidative stress. In particular, ferritin correlated with IL18, heme oxygenase-1 (HO-1), soluble transglutaminase 2 (TGM2), and the protease cathepsin L1 (CTSL1). These findings reinforce the role of ferritin as more than a passive bystander, instead reflecting macrophage activation and cellular stress responses that contribute to endothelial injury and thromboinflammation. The link between ferritin and PARP1 further implicates DNA damage and repair pathways in the hyperinflammatory response of COVID-19 [30].
Peripheral blood cell counts, while widely used in clinical monitoring, showed meaningful correlations with several immune mediators. For example, basophil and eosinophil counts were linked to galectin-9 (Gal-9) and receptor for advanced glycation end-products (RAGE), both molecules implicated in immune checkpoint regulation and tissue damage responses. Neutrophils and total leukocyte counts associated with ADAMTS13, GIF, and CEACAM8, highlight a functional connection between hematological changes and endothelial as well as granulocyte activity. Such findings may help explain why hematological parameters, particularly the neutrophil-to-lymphocyte ratio, consistently predict outcome in COVID-19 [20].
Our findings extend current knowledge by integrating cytokines, chemokines, growth factors, and standard biomarkers by revealing distinct and biologically coherent correlation patterns. This comprehensive mapping clarifies how conventional biomarkers reflect underlying immune pathways, which may contribute to more refined biomarker-guided risk stratification.
In this exploratory trial, we used a cardiovascular Olink panel to evaluate a broad spectrum of CCGFs reflecting cardiac injury in patients infected with SARS-CoV-2. It is a drawback that some important clinical covariates that influence inflammatory markers (e.g., immunomodulatory treatments, bacterial coinfection, comorbidities, organ dysfunction) are not evaluated regarding their potential impact on the CCGFs measured. As expected, elevated levels of IL6, CRP, ferritin, and PCT were observed, all of which have been associated with cardiovascular events [6,31,32]. Treatment with dexamethasone in severe COVID-19 may have attenuated some of the CCGFs analyzed. In a previous study, we demonstrated that IL6 was significantly associated with body mass index, high-density lipoproteins, estimated glomerular filtration rate, and triglycerides in presumed healthy individuals [33]. Thus, it cannot be excluded that certain comorbidities may influence the interpretation of IL6 levels, and possibly those of other CCGFs associated with conventional biomarkers and nucleated blood cells. Viral pneumonia has been predicted by IL6, IL27, and CRP, with distinct cytokine expression profiles differentiating viral from bacterial community-acquired pneumonia [34]. In SARS-CoV-2 infection, IL6 is one of the cytokines most clearly associated with poor clinical outcomes [35].
Thus, prospective validation studies—such as cluster analyses using artificial intelligence—may help determine whether specific cytokine patterns can guide therapeutic decision-making. If so, the Olink panel (or similar platforms) may be cost-effective, particularly when evaluated in relation to ICU care costs.
4. Materials and Methods
4.1. Study Population
This investigation was conducted as a sub-study of the single-center, prospective observational PronMed cohort, carried out at the Intensive Care Units of Uppsala University Hospital, Uppsala, Sweden, which treated critically ill COVID-19 patients during the pandemic. Adult patients with severe COVID-19 treated in intensive care were screened for eligibility. COVID-19 was confirmed by a positive polymerase chain reaction (PCR) test of a nasopharyngeal sample. Baseline characteristics, including age, sex, and BMI, were recorded at ICU admission, and comorbidity data were extracted from medical records. Data, including the Simplified Physiology Score (SAPS-3) [36,37], were collected between 14 March 2020 and 10 March 2021. Blood samples for biochemical analyses were obtained as part of routine clinical care. Plasma samples were stored at −80 °C until analyzed. The median time (IQR) from symptom onset to ICU admission was 10 days (8–12). From 22 June 2020 onward, patients with COVID-19 requiring supplemental oxygen received dexamethasone 6 mg daily. Demographic data at ICU admission have been reported previously [38].
4.2. Ethical Considerations
The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki [39] and was consistent with ICH/GCP E6 (R2), the EU Clinical Trials Directive, GDPR (EU 2016/679), and applicable local regulations. Approval was obtained from the Swedish National Ethical Review Agency (Dnr 2017-043 approved 23 August 2017 by Per-Erik Nistér; amendments 2019-00169, 2020-01623, 2020-02719, 2020-05730, 2021-01469, and 2022-00526-01). Informed consent was obtained from all patients or from their next of kin if the patient was unable to provide consent. The study protocol was prospectively registered (Clinical Trials ID: NCT04316884) and conducted in accordance with relevant directives.
4.3. Proximity Extension Assay
The proximity extension assay (PEA) using the Olink Target 96 Cardiovascular II panel was conducted on citrate plasma samples at SciLifeLab Affinity Proteomics, Uppsala University (Olink Proteomics, Uppsala, Sweden) [40]. The PEA has been reported to provide protein plasma levels in good agreement with conventional immunoassays [41]. In brief, 1 µL of each plasma sample was incubated with pairs of oligonucleotide-labeled antibodies targeting 92 inflammation-related proteins. When both antibodies bound to the same target protein, their attached oligonucleotides hybridized and were extended by DNA polymerase to form a unique reporter sequence. These reporter molecules were then amplified by universal PCR and quantified using high-throughput real-time PCR (Fluidigm BioMark HD real-time PCR, Standard BioTools, San Francisico, CA, USA), generating Normalized Protein eXpression (NPX) values on a log2 scale. Assay performance was monitored using Olink’s internal controls (incubation, extension, and detection controls). Data processing, QC, and normalization were performed using Olink NPX Manager software v2.2.1. 311.
Analyzed cytokines, chemokines, and growth factors (CCGFs) are seen in Supplementary Table S1, where their genes, full names, and Uniprot IDs are also displayed.
4.4. STRING Images
Cytokine, chemokine, and growth factor interactions, together with concentration patterns, were visualized using STRING database–generated images [42]. Edge weights were calculated in STRING based on the associations identified in this study. Protein names were displayed to create interaction networks, and edge thickness reflected confidence scores, indicating the likelihood of a true interaction according to STRING’s integrated evidence. Images were exported in high resolution for figure preparation, enabling simultaneous visualization of quantitative concentration data and qualitative interaction patterns.
4.5. Statistical Analysis
Coefficients of variation were examined using Spearman rank correlations in Statistica (StatSoft, v14; Tulsa, OK, USA). Cytokine values below the lowest standard point were included in analyses in agreement with Olink recommendations, and no values exceeded the assay’s upper or lower standard curve limits. To account for the increased risk of false positives, due to multiple comparisons, p-values were adjusted using the Benjamini–Hochberg false discovery rate (FDR) method [40]. Adjusted p-values < 0.10, corresponding to an expected FDR ≤ 10%, were considered statistically significant. As this is an exploratory study, we used somewhat higher levels of significance to ensure the avoidance of a statistical type II (beta) error. Continuous variables are denoted as median and interquartile range [IQR (75th percentile–25th percentile)].
5. Conclusions
In conclusion, our findings demonstrate that conventional inflammatory biomarkers such as CRP, PCT, and ferritin reflect distinct yet overlapping aspects of the cytokine, chemokine, and growth factor network in severe COVID-19. CRP closely mirrored the IL6–driven acute-phase response, PCT revealed broader links to pro-inflammatory and endothelial mediators, and ferritin highlighted macrophage activation and oxidative stress pathways. Hematological parameters also proved to be integrated within this network. These results may turn out to have clinical implications by clarifying which routinely measured biomarkers best reflect specific immune pathways, improve risk stratification, and guide monitoring strategies in critical care. Together, these results emphasize that routine laboratory markers remain valuable as accessible surrogates of complex immune responses, and they provide new biological insights that may inform biomarker-guided monitoring and targeted therapeutic strategies in COVID-19.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262311419/s1.
Author Contributions
M.B.E., A.O.L., M.L., R.F., M.Å. and M.M.-H. conceived the present study. M.L., R.F., M.M.-H. and the Intensive Care COVID-19 research group collected patient data. A.O.L. and M.Å., respectively, analyzed the blood samples. M.B.E. and A.O.L. performed the initial data analysis. M.B.E. and A.O.L. drafted the original manuscript. A.O.L. performed statistical analyses. All authors participated in the revisions of the manuscript. All authors read, commented on and approved the final manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by funds from the Swedish Research Council (R.F. grant no 2014-02569 and 2014-07606), by grants from the Swedish state under the ALF agreement between the Swedish government and the county councils, the SciLifeLab/Knut and Alice Wallenberg national COVID-19 research program (M.M-H.: KAW 2020.0182, KAW 2020.0241), the Swedish Heart-Lung Foundation (M.M-H.: 20210089, 20190639, 20190637, 20230732, 20230627), and Swedish Society of Medicine (M.M-H.: SLS-938101), and by grants from the Swedish state under the ALF agreement between the Swedish government and the county councils (A.O.L.).
Institutional Review Board Statement
This study was performed in accordance with ethical principles that have their origin in the Declaration of Helsinki [39] and consistent with ICH/GCP E6 (R2). The study was approved by the National Ethical Review Agency (protocol code Dnr 2017-043 and 23 August 2017 of approval).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. The protocol of the study was registered a priori at Clinical Trials ID: NCT04316884. The study was performed according to relevant directives.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ma, Z.; Yang, K.Y.; Huang, Y.; Lui, K.O. Endothelial contribution to COVID-19: An update on mechanisms and therapeutic implications. J. Mol. Cell. Cardiol. 2022, 164, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, A.; Gheibi Hayat, S.M.; Taghizadeh, H.; Akbari, A.; Inabadi, M.; Savardashtaki, A.; Johnston, T.P.; Sahebkar, A. COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti Infect. Ther. 2021, 19, 345–357. [Google Scholar] [CrossRef]
- Hultström, M.; Lipcsey, M.; Wallin, E.; Larsson, I.M.; Larsson, A.; Frithiof, R. Severe acute kidney injury associated with progression of chronic kidney disease after critical COVID-19. Crit. Care 2021, 25, 37. [Google Scholar] [CrossRef]
- Fouladseresht, H.; Doroudchi, M.; Rokhtabnak, N.; Abdolrahimzadehfard, H.; Roudgari, A.; Sabetian, G.; Paydar, S. Predictive monitoring and therapeutic immune biomarkers in the management of clinical complications of COVID-19. Cytokine Growth Factor Rev. 2021, 58, 32–48. [Google Scholar] [CrossRef]
- Goudouris, E.S. Laboratory diagnosis of COVID-19. J. Pediatr. 2021, 97, 7–12. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Hou, Y.L.; Li, D.T.; Li, F.Z. Laboratory findings of COVID-19: A systematic review and meta-analysis. Scand. J. Clin. Lab. Investig. 2020, 80, 441–447. [Google Scholar] [CrossRef]
- Quartuccio, L.; Fabris, M.; Sonaglia, A.; Peghin, M.; Domenis, R.; Cifù, A.; Curcio, F.; Tascini, C. Interleukin 6, soluble interleukin 2 receptor alpha (CD25), monocyte colony-stimulating factor, and hepatocyte growth factor linked with systemic hyperinflammation, innate immunity hyperactivation, and organ damage in COVID-19 pneumonia. Cytokine 2021, 140, 155438. [Google Scholar] [CrossRef]
- Bivona, G.; Agnello, L.; Ciaccio, M. Biomarkers for Prognosis and Treatment Response in COVID-19 Patients. Ann. Lab. Med. 2021, 41, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin. Chem. Lab. Med. 2023, 61, 1540–1545. [Google Scholar] [CrossRef]
- Muhammad, J.S.; ElGhazali, G.; Shafarin, J.; Mohammad, M.G.; Abu-Qiyas, A.; Hamad, M. SARS-CoV-2-induced hypomethylation of the ferritin heavy chain (FTH1) gene underlies serum hyperferritinemia in severe COVID-19 patients. Biochem. Biophys. Res. Commun. 2022, 631, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hanff, T.C.; Mohareb, A.M.; Giri, J.; Cohen, J.B.; Chirinos, J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020, 95, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Meroni, P.L.; Croci, S.; Lonati, P.A.; Pregnolato, F.; Spaggiari, L.; Besutti, G.; Bonacini, M.; Ferrigno, I.; Rossi, A.; Hetland, G.; et al. Complement activation predicts negative outcomes in COVID-19: The experience from Northen Italian patients. Autoimmun. Rev. 2023, 22, 103232. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Volfovitch, Y.; Tsur, A.M.; Gurevitch, M.; Novick, D.; Rabinowitz, R.; Mandel, M.; Achiron, A.; Rubinstein, M.; Shoenfeld, Y.; Amital, H. The intercorrelations between blood levels of ferritin, sCD163, and IL-18 in COVID-19 patients and their association to prognosis. Immunol. Res. 2022, 70, 817–828. [Google Scholar] [CrossRef]
- Maves, R.C.; Enwezor, C.H. Uses of Procalcitonin as a Biomarker in Critical Care Medicine. Infect. Dis. Clin. N. Am. 2022, 36, 897–909. [Google Scholar] [CrossRef]
- Mazaheri, T.; Ranasinghe, R.; Al-Hasani, W.; Luxton, J.; Kearney, J.; Manning, A.; Dimitriadis, G.K.; Mare, T.; Vincent, R.P. A cytokine panel and procalcitonin in COVID-19, a comparison between intensive care and non-intensive care patients. PLoS ONE 2022, 17, e0266652. [Google Scholar] [CrossRef]
- Oberhoffer, M.; Karzai, W.; Meier-Hellmann, A.; Reinhart, K. Procalcitonin. A new diagnostic parameter for severe infections and sepsis. Anaesthesist 1998, 47, 581–587. [Google Scholar] [CrossRef]
- Palladino, M. Complete blood count alterations in COVID-19 patients: A narrative review. Biochem. Med. 2021, 31, 030501. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, P.; Zhao, Y.; Zhuang, Z.; Wang, Z.; Song, R.; Zhang, J.; Liu, C.; Gao, Q.; Xu, Q.; et al. Single-Cell Sequencing of Peripheral Mononuclear Cells Reveals Distinct Immune Response Landscapes of COVID-19 and Influenza Patients. Immunity 2020, 53, 685–696.e683. [Google Scholar] [CrossRef]
- Carreto-Binaghi, L.E.; Herrera, M.T.; Guzmán-Beltrán, S.; Juárez, E.; Sarabia, C.; Salgado-Cantú, M.G.; Juarez-Carmona, D.; Guadarrama-Pérez, C.; González, Y. Reduced IL-8 Secretion by NOD-like and Toll-like Receptors in Blood Cells from COVID-19 Patients. Biomedicines 2023, 11, 1078. [Google Scholar] [CrossRef] [PubMed]
- Méndez Rodríguez, M.L.; Ponciano-Gómez, A.; Campos-Aguilar, M.; Tapia-Sánchez, W.D.; Duarte-Martínez, C.L.; Romero-Herrera, J.S.; Olivas-Quintero, S.; Saucedo-Campos, A.D.; Méndez-Cruz, A.R.; Jimenez-Flores, R.; et al. Neutrophil-to-Lymphocyte Ratio and Cytokine Profiling as Predictors of Disease Severity and Survival in Unvaccinated COVID-19 Patients. Vaccines 2024, 12, 861. [Google Scholar] [CrossRef] [PubMed]
- Naylor, D.; Sharma, A.; Li, Z.; Monteith, G.; Mallard, B.A.; Bergeron, R.; Baes, C.; Karrow, N.A. Endotoxin-induced cytokine, chemokine and white blood cell profiles of variable stress-responding sheep. Stress 2021, 24, 888–897. [Google Scholar] [CrossRef]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021, 18, 194–209. [Google Scholar] [CrossRef]
- Pushkala, S.; Seshayyan, S.; Theranirajan, E.; Sudhakar, D.; Raghavan, K.; Dedeepiya, V.D.; Ikewaki, N.; Iwasaki, M.; Preethy, S.; Abraham, S.J. Efficient Control of IL-6, CRP and Ferritin in COVID-19 Patients With Two Variants of Beta-1,3-1,6 Glucans in Combination: An Open-Label, Prospective, Randomised Clinical Trial. Glob. Adv. Integr. Med. Health 2025, 14, 27536130251327134. [Google Scholar] [CrossRef]
- Atallah, N.J.; Warren, H.M.; Roberts, M.B.; Elshaboury, R.H.; Bidell, M.R.; Gandhi, R.G.; Adamsick, M.; Ibrahim, M.K.; Sood, R.; Bou Zein Eddine, S.; et al. Baseline procalcitonin as a predictor of bacterial infection and clinical outcomes in COVID-19: A case-control study. PLoS ONE 2022, 17, e0262342. [Google Scholar] [CrossRef]
- Cohen, A.J.; Glick, L.R.; Lee, S.; Kunitomo, Y.; Tsang, D.A.; Pitafi, S.; Valda Toro, P.; Ristic, N.R.; Zhang, E.; Carey, G.B.; et al. Nonutility of procalcitonin for diagnosing bacterial pneumonia in patients with severe COVID-19. Eur. Clin. Respir. J. 2023, 10, 2174640. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Alessandri, F.; Migliara, G.; Baccolini, V.; Giordano, G.; Galardo, G.; Marzuillo, C.; De Vito, C.; Russo, A.; Ciccozzi, M.; et al. Reduced Reliability of Procalcitonin (PCT) as a Biomarker of Bacterial Superinfection: Concerns about PCT-Driven Antibiotic Stewardship in Critically Ill COVID-19 Patients-Results from a Retrospective Observational Study in Intensive Care Units. J. Clin. Med. 2023, 12, 6171. [Google Scholar] [CrossRef]
- Adachi, T.; Nonomura, S.; Horiba, M.; Hirayama, T.; Kamiya, T.; Nagasawa, H.; Hara, H. Iron stimulates plasma-activated medium-induced A549 cell injury. Sci. Rep. 2016, 6, 20928. [Google Scholar] [CrossRef]
- Azevedo, R.B.; Botelho, B.G.; Hollanda, J.V.G.; Ferreira, L.V.L.; Junqueira de Andrade, L.Z.; Oei, S.; Mello, T.S.; Muxfeldt, E.S. COVID-19 and the cardiovascular system: A comprehensive review. J. Hum. Hypertens. 2021, 35, 4–11. [Google Scholar] [CrossRef]
- Magadum, A.; Kishore, R. Cardiovascular Manifestations of COVID-19 Infection. Cells 2020, 9, 2508. [Google Scholar] [CrossRef]
- Eriksson, M.B.; Eriksson, L.B.; Larsson, A.O. Significant Interplay Between Lipids, Cytokines, Chemokines, Growth Factors, and Blood Cells in an Outpatient Cohort. Int. J. Mol. Sci. 2025, 26, 7746. [Google Scholar] [CrossRef] [PubMed]
- Burgmeijer, E.H.; Duijkers, R.; Lutter, R.; Bonten, M.J.M.; Schweitzer, V.A.; Boersma, W.G. Plasma cytokine profile on admission related to aetiology in community-acquired pneumonia. Clin. Respir. J. 2019, 13, 605–613. [Google Scholar] [CrossRef]
- Lee, S.; Channappanavar, R.; Kanneganti, T.D. Coronaviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Trends Immunol. 2020, 41, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Metnitz, B.; Sprung, C.L.; Timsit, J.F.; Lemaire, F.; Bauer, P.; Schlemmer, B.; Moreno, R.; Metnitz, P. End-of-life practices in 282 intensive care units: Data from the SAPS 3 database. Intensive Care Med. 2009, 35, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.P.; Metnitz, P.G.; Almeida, E.; Jordan, B.; Bauer, P.; Campos, R.A.; Iapichino, G.; Edbrooke, D.; Capuzzo, M.; Le Gall, J.R. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005, 31, 1345–1355. [Google Scholar] [CrossRef]
- Larsson, A.O.; Hultström, M.; Frithiof, R.; Lipcsey, M.; Eriksson, M.B. Shrunken Pore Syndrome Is Frequently Occurring in Severe COVID-19. Int. J. Mol. Sci. 2022, 23, 15687. [Google Scholar] [CrossRef]
- WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects (accessed on 23 March 2022).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 2018, 57, 289–300. [Google Scholar] [CrossRef]
- Skau, E.; Wagner, P.; Leppert, J.; Ärnlöv, J.; Hedberg, P. Are the results from a multiplex proteomic assay and a conventional immunoassay for NT-proBNP and GDF-15 comparable? Clin. Proteom. 2023, 20, 5. [Google Scholar] [CrossRef]
- STRING: Functional Protein Association Networks. Available online: https://string-db.org (accessed on 1 February 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).