Abstract
Recent scientific reports have highlighted the physiological role, toxicological effects, and pathophysiological aspects of gasotransmitters, particularly hydrogen sulfide (H2S), which is recognized as a new member of this family. Endogenous generation of H2S in the skin occurs through both enzymatic and non-enzymatic pathways. The main enzymes involved in its endogenous production are cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS), 3-mercaptopyruvate sulfurtransferase (3-MST) and cysteine aminotransferase. 3-MST and CSE are crucial for maintaining the epidermal barrier. H2S may play a role in oncogenesis, acting as a gas signaling molecule that disrupts mitochondrial respiration and influences immune modulation, cell proliferation, apoptosis, tumor cell survival, and metastasis. Interestingly, H2S exhibits dual effects in the biology of skin cancer, promoting tumor growth in some contexts and exerting antitumor activities in others. Data from the European Cancer Information System and Global Cancer Observatory show a significant global increase in skin cancer cases. The most common types of cutaneous malignancies, from both epidemiological and clinical perspectives, are basal cell carcinoma. squamous cell carcinoma, and melanoma. This review aims to evaluate the dysfunctional metabolism of H2S and the specific profiles of the enzymes that synthesize H2S in skin cancer. By comparing the roles of H2S in normal cells with those in cancer cells, we can enhance current understanding of its implications in skin cancer biology. This research paves the way for new clinical strategies, including the development of H2S-modulatory therapies tailored to the dynamics of tumor progression, which could help overcome therapeutic resistance.
1. Introduction
Skin cancer is the most common type of cancer and significantly impacts the lives and health of patients. Evidence from the European Cancer Information System and the Global Cancer Observatory shows a notable global increase in skin cancer cases. Skin cancer can be categorized into two main types: cutaneous melanoma and keratinocyte carcinomas (KCs). Among KCs, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are the most significant from both epidemiological and clinical perspectives []. Cutaneous melanoma, which arises from the malignant transformation of melanocytes, is one of the most aggressive forms of skin cancer, with an incidence that continues to rise worldwide []. Although it constitutes only 4% of all skin cancers, melanoma accounts for 80% of skin cancer-related deaths []. BCC develops from the basal layer of the epidermis and its appendages, while SCC originates from the malignant proliferation of atypical epidermal keratinocytes [,,]. Research on the prevalence of different types of skin cancer across various continents is limited. Skin cancer is among the most prevalent malignancies in Australia and New Zealand, while Europe reports the highest incidence and mortality rates for melanoma []. North America and Asia predominantly report the incidence of non-melanoma skin cancers [].
The potential role of gasotransmitters in regulating many physiological and pathological processes has garnered significant attention. Hydrogen sulfide (H2S) is recognized as a crucial gasotransmitter that may influence the onset and progression of skin tumors. Research conducted over the past decade has provided new insights into the role of H2S in skin tissue damage and repair. Abnormal levels of H2S are linked to various skin conditions. H2S is a gaseous signaling molecule that is essential for maintaining skin homeostasis, influencing keratinocyte and melanocyte biology []. Therefore, recent data indicate its role in skin cancer. Small amounts of naturally produced H2S may enhance or sustain cancer cell growth for a short time, whereas high levels of H2S donors or excessive activity of H2S-producing enzymes can exert antitumor effects when exposure is prolonged. In the skin, there are enzymes responsible for producing H2S, including cystathionine β-synthetase [CBS], cystathionine-γ-lyase [CSE], and 3-mercaptopyruvate sulfurtransferase [3-MST]. However, the expression of these enzymes in different types of skin cells has not been extensively studied or fully understood. These enzymes are present in keratinocytes, melanocytes, and cutaneous fibroblasts. When their expression becomes abnormal, or when there is an accumulation of substrates and intermediates, H2S levels can be disrupted, leading to a breakdown in the metabolic signaling pathways. The exact relationship between H2S production and the metabolism and biology of keratinocytes and melanocytes remains unclear [,,]. To understand the role of H2S in oncogenesis and tumor progression, it is important to examine the complex interactions between the enzymes that synthesize H2S (such as CBS, CSE, 3-MST, cysteine aminotransferase [CAT], D-amino acid oxidase [DAO], and cysteinyl-tRNA synthetase 2 [CARS2]) and those involved in its catabolism (the sulfide oxidation unit [SOU], which includes sulfide–quinone oxidoreductase [SQOR], human ethylmalonic encephalopathy protein 1 [hETHE1], rhodanese, sulfite oxidase [SUOX/SO], and cytochrome c oxidase [CcO]) [,,,].
This review describes recent advances in H2S research concerning dysfunctional H2S metabolism and imbalances in H2S-producing enzymes in skin cancer. The aim of this review is to investigate the role of H2S in the pathogenesis of skin cancer, with a focus on understanding how H2S influences cellular mechanisms involved in tumor development and progression. Understanding the role of H2S in malignant cells compared to normal cells enhances current knowledge about H2S implications in skin cancer biology and may pave the way for new therapeutic concepts that could slow cancer progression and improve patients’ quality of life.
2. Properties and General Characteristics of Hydrogen Sulfide
H2S is a notable member of the expanding family of gasotransmitters, which play important roles in cytoprotection, maintaining homeostasis, and organ development. There is strong evidence supporting the major role of H2S in nearly all mammalian cells and tissues. Research on the physiological, toxicological, and pathophysiological aspects of H2S has significantly increased in recent years. Various endogenous and exogenous H2S donors have been developed, and progress has been made regarding their therapeutic applications [].
Multiple studies have underscored the essential roles of gasotransmitters in the normal physiology and pathogenesis of various malignancies. Research has indicated that these mediators can have both pro-tumorigenic and anti-tumorigenic effects, creating a paradox. Gasotransmitters may stimulate cell proliferation at physiologically relevant concentrations while also exhibiting cytotoxic activity. Additionally, altered expression of gas-producing enzymes in cancer cells across different tissues has been reported, leading to new investigations on the role of these signaling molecules in cancer regulation [,,].
However, the significance of these endogenous gasotransmitters in skin cancer has not been thoroughly analyzed or discussed until now. The current challenge in gasotransmitter research is to explore their physiological and pathological functions, along with clarifying their interactions under pathological conditions [,,].
H2S exists in several forms in extracellular fluids at physiological pH and 37 °C: (1) undissociated gas H2S (approximately 20%), (2) monoanionic ion HS− (approximately 80%), and (3) dianionic ion S2− (less than 1%). In the mitochondrial matrix, at pH 8.0, the main species are (1) HS− (approximately 92%) and (2) H2S (approximately 8%). In lysosomes, at pH 4.7, the non-dissociated form of H2S predominates (over 99%). Of all sulfides in the body, around 40% are in the form of H2S, the rest in the form of HS− and an insignificant amount of S2−. The half-life of H2S in the blood is relatively short, typically ranging from seconds to minutes [,]. H2S is a weak acid, with two ionizable protons. This allows it to dissociate in solution according to the following equilibrium: H2S ↔ H+ + HS− ↔ 2H+ + S2−. In biological systems, the equilibrium between these species (H2S ↔ HS− ↔ S2−) is influenced by the redox environment, as well as by the production, storage, and release of H2S from various sources.
3. H2S and the Skin
Endogenous H2S acts as a signaling molecule that requires a high metabolic flow, a temporally and spatially controlled turnover, tightly regulated low physiological thresholds, and rapid and efficient regulation of H2S kinetics in tissues. There are also flexible methods for storing and mobilizing H2S from cells. Physiological levels of H2S in tissues are determined by the rates of its production and elimination from the body [,,]. The regulated production of H2S ensures the maintenance of an H2S/HS− ratio of 3:1 at physiological pH, positively influencing processes at the molecular, biochemical, and metabolic levels [,,,,]. The widespread distribution of H2S-producing enzymes and the chemical reactivity of H2S account for the altered metabolism of H2S in the pathogenesis of several skin diseases, including inflammatory skin diseases (psoriasis, vitiligo), fibroproliferative disorders, wound healing, vascular disorders, ulcers, pigmentation disorders, and skin cancers (melanoma) [,].
In our analysis, the term H2S refers to the sum of H2S, HS−, and S2− species present at physiological pH. The following sections will outline the pathways of H2S production and inactivation in the skin, the physiological significance of H2S in human skin, and the toxicological and pathophysiological aspects of H2S in skin cancers.
3.1. Production of H2S in the Skin
While H2S is produced and degraded in various cell types, the mechanisms underlying H2S homeostasis in the skin are not fully understood. The regulation of H2S levels depends on several factors: (1) the expression and activity of H2S-producing and degrading enzymes; (2) epigenetic mechanisms; (3) transcriptional regulation; (4) post-transcriptional modifications; (5) post-translational modifications; and (6) cell specificity.
Endogenous generation of H2S in the skin occurs through both enzymatic and non-enzymatic pathways. The production of endogenous H2S is primarily facilitated by enzymatic pathways, with only a small portion generated through non-enzymatic means (Figure 1) [].
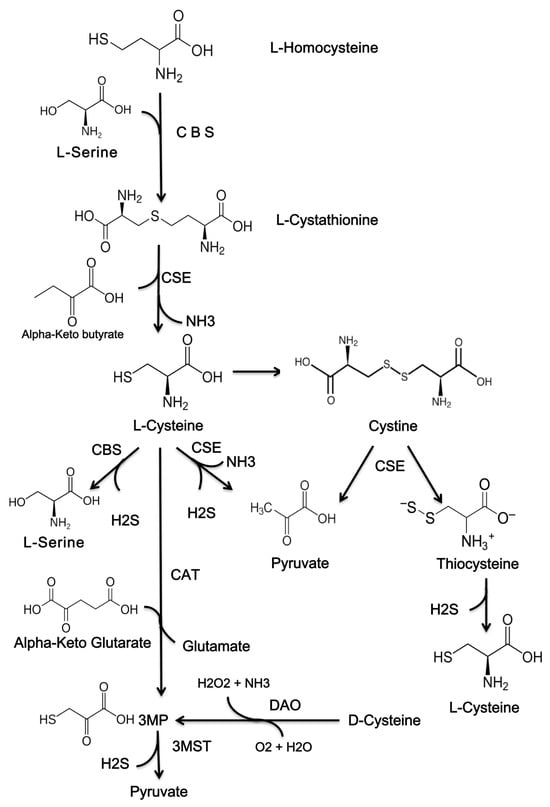
Figure 1.
The chemical structures of compounds that are used to produce H2S. H2S, hydrogen sulfide; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; 3MST, 3-mercaptopyruvate sulfurtransferase; 3MP, 3-methylpyruvate; CAT, cysteine aminotransferase; DAO, D-amino acid oxidase.
In mammals, cysteine is synthesized from methionine through cystathionine via the reverse transsulfuration pathway. This reverse transsulfide pathway contributes significantly to the intracellular reserve of cysteine and aids in the removal of methionine and its toxic intermediates, including homocysteine. Cysteine is essential for the biosynthesis of glutathione and H2S [].
Endogenous H2S is produced by several mechanisms (Figure 2) [,,,].
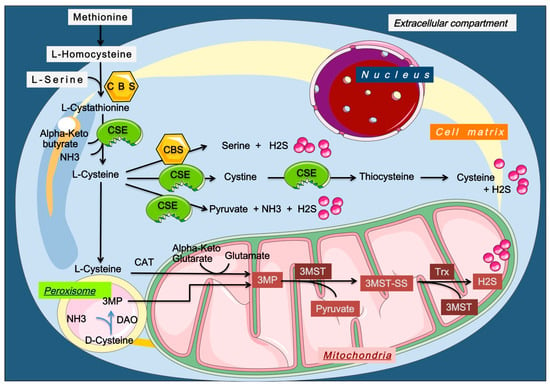
Figure 2.
Enzymatic production of endogenous H2S. H2S, hydrogen sulfide; Trx, Thioredoxin; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; 3MST, 3-mercaptopyruvate sulfurtransferase; 3MP, 3-methylpyruvate; CAT, cysteine aminotransferase; DAO, D-amino acid oxidase. Endogenous H2S is produced through multiple enzymatic pathways, mainly involving cysteine, homocysteine, and methionine metabolism. CBS and CSE generate H2S via transsulfuration, while CAT/3-MST and DAO/3-MST pathways operate in mitochondria and peroxisomes. Additional sources include L-methionine transsulfuration, thiol–disulfide exchange from persulfides, CARS2-mediated cysteine persulfide reduction by thioredoxin, and the methanol-to-olefins (MTO) reaction, which also helps detoxify cells.
Non-enzymatic processes generate small amounts of endogenous H2S, crucial for sulfur recycling, especially in the skin. H2S is produced from L- and D-cysteine via vitamin B6 and metal ions, released from bound sulfane sulfur, Fe-S clusters, and SH-reactive compounds, and formed through the reduction of thiosulfate using pyruvate. Its levels are regulated by removal through methemoglobin and disulfide-containing molecules like GSSG. Under oxidative stress or hyperglycemia, non-enzymatic H2S production markedly increases [,,].
3.2. The Metabolism of H2S in the Skin
The metabolic processes involved in the catabolism of H2S help mitigate the toxicity caused by high concentrations of H2S. To maintain adequate physiological balance after its synthesis: (1) H2S can directly exert biological effects by interacting with various signaling molecules; (2) H2S can be stored in cellular reservoirs and subsequently released in response to physiological signals; and (3) H2S can be catabolized into harmless products [,,].
In vivo, H2S acts as a reducing agent and participates in numerous metabolic pathways. Specifically, (1) H2S can be stored in a bound sulfur pool; (2) it can be oxidized to sulfite in activated neutrophils; (3) it may be captured by various oxidants circulating in the vascular system; (4) H2S can interact with hemoglobin to form sulfhemoglobin and with methemoglobin to create MetHb-Fe(III)-SH2 within the circulatory system; (5) it can mediate interactions with reactive oxygen species (ROS) and reactive nitrogen species (RNS); (6) it can interact with metals through covalent, redox bonds; (7) it is involved in post-translational modifications; and (8) it can chemically reduce protein disulfide bonds [,]. Several forms of existence and storage of H2S in cells have been identified.
In the human body, H2S is eliminated and degraded through several mechanisms: (1) enzymatic oxidation in the mitochondria; (2) enzymatic methylation in the cytosol; (3) oxidation via alternative non-enzymatic ferric heme-dependent pathways; (4) self-oxidation; and (5) exhalation and excretion [,].
In human skin fibroblasts, the oxidation of H2S to thiosulfate and sulfate occurs in the mitochondria. The mitochondrial enzyme system consists of four sulfide enzymes: SQOR, hETHE1 or persulfide dioxygenase (PDO), rhodanese (also known as thiosulfate sulfurtransferase, TST), and sulfite oxidase (SUOX). Thiosulfate is converted to sulfite by TST, followed by its oxidation to sulfate by SUOX [,].
H2S can also be catabolized through methylation in the cytoplasm. In this process, H2S is converted to methanethiol (CH3SH) and dimethyl sulfide (CH3SCH3) by thiol S-methyltransferase (TMT). Dimethyl sulfide serves as a substrate for TST and is subsequently oxidized to thiocyanate and sulfate. Notably, the rate of sulfide methylation in the mucous cells of the mammalian colon is approximately 10,000 times slower than the rate of mitochondrial oxidation of H2S [,]. Intracellular autooxidation of H2S results in the formation of polysulfide and thiosulfate at physiological pH [,,].
The heme-dependent sulfur oxidation pathway is an alternative mechanism that involves metal or sulfur-containing macromolecules, such as methemoglobin and myoglobin. Ferric heme can convert H2S to thiosulfate and polysulfide (HSxS−) through H2S-iron binding [].
3.3. The Dual Role of H2S
Although H2S is commonly associated with toxic effects, it has become clear that when produced endogenously and present at appropriate concentrations, it can play important physiological regulatory roles in the body. The bell-shaped concentration-response dynamics indicate that cells prefer an intermediate concentration of H2S, as notable increases or decreases at this level can be detrimental to cell viability and survival [,].
3.3.1. Physiological Effects of H2S in the Skin
H2S is a versatile gaseous molecule that produces multiple physiological effects. At low concentrations (less than nM), H2S acts as follows [,,]:
- Vasodilation: It regulates NO, cyclic GMP (cGMP), soluble guanylate cyclase (sGC), and protein kinase G (PKG), leading to the opening of ATP-sensitive potassium channels in smooth muscle cell membranes
- Antioxidant Responses: H2S interacts directly with free radicals or oxidants, increases glutathione levels, and suppresses the activity of NADPH oxidase, thereby alleviating oxidative stress in various tissues.
- Cytoprotective Effects: It modulates cytoprotective pathways, including PI3K–Akt, p38–MAPK, and Nrf2.
- Anti-inflammatory Effects: H2S suppresses adhesion and leukocyte infiltration, reduces edema formation, and modulates NF-κB activity.
- Bioenergetic Function: It stimulates bioenergetic functions by donating electrons to the mitochondrial transport chain and cooperating with various signaling systems (cGMP, cAMP).
- Catalytic Activity: H2S mediates the catalytic activity of proteins.
- Detoxification: It binds directly to both endogenous and exogenous toxins.
- Apoptosis Regulation: H2S mediates the apoptosis process through multiple signaling pathways, including PI3K/Akt/mTOR and MAPK.
According to recent studies, H2S exerts specific molecular effects on the growth and adhesion of normal human keratinocytes. For instance, H2S inhibits Raf/MAPK/ERK signaling and the expression of integrins alpha2, alpha6, and beta4. In cultured human keratinocytes treated with NaHS, H2S was found to accumulate in the cytoplasm. Using proteomic, genomic, and biochemical methods, researchers identified that H2S activates superoxide dismutase 2 (SOD2), NAD(P)H quinone dehydrogenase 1 (NQO1), and cullin 3 (CUL3), along with the pro-inflammatory cytokines IL-8, CXCL2, caspase-1, IL-18, and IL-1β. Additionally, H2S inhibited the growth of keratinocyte progenitors and cultured stem cells. Functionally relevant targets of H2S in human keratinocytes include oxidative stress response molecules, the network of pro-inflammatory chemokines, and the inflammasome pathway [].
H2S is known for its antioxidant and cytoprotective activities by directly blocking ROS and RNS, regulating the expression and activity of classical antioxidants (such as glutathione, GSH, and thioredoxin), and increasing the levels of some antioxidant and redox modulators (notably the Nrf2 system). It also enhances endogenous antioxidant systems []. Moreover, H2S protects human skin keratinocytes (HaCaT cells) against hypoxia-induced injury by inhibiting the ROS/NF-kB/COX-2 pathway. This inhibition is associated with the attenuation of inflammatory responses, increased cellular viability by boosting GSH levels, and reduction of ROS, IL-1β, IL-6, IL-8, COX-2, and PGE2 [].
H2S acts as an intercellular physiological messenger that regulates melanocyte proliferation and melanin synthesis in human epidermal melanocytes. H2S influences tyrosinase and associated transcription factors involved in melanogenesis, such as MITF and TRP-1. Additionally, H2S exhibits both pro- and anti-autophagy effects through the PI3K/Akt/mTOR and AMPK/mTOR signaling pathways. It stimulates the multiplication of melanocytes, increases intracellular melanin content, mitigates cell damage, protects and repairs mucous membranes, and regulates the interactions between mucous membranes, bacteria, and viruses via the PI3K/Akt/Nrf2 pathway [,].
CSE is crucial for maintaining the epidermal barrier. Keratinocytes in the hair follicle and basal keratinocytes of the epidermis serve as sources of CSE. In vitro studies have shown that H2S derived from CSE is involved in the expression of early keratinocyte differentiation markers []. Moreover, it is known that all immune cells present in skin tissue synthesize endogenous H2S. This synthesis participates in monocyte apoptosis, cytokine synthesis, polymorphonuclear adhesion, T cell polarization, lymphocyte and neutrophil infiltration and proliferation, Langerhans cell proliferation, mast cell degranulation, and both histaminergic and non-histaminergic adaptive, inflammatory, and pruritic immune responses []. H2S modulates the activity of cutaneous macrophages, impacting migration, phagocytosis, and cytokine production. Cystathionine beta-synthase (CBS) is constitutively expressed in macrophages. It is hypothesized that decreased expression of the CBS protein or its inhibition by NO or carbon monoxide (CO) may be associated with reduced cystathionine levels during macrophage-coordinated inflammatory responses []. In immune cells, the production of H2S has been documented. At the cutaneous level, H2S plays a role in regulating innate immunity by stimulating the activity of neutrophils, macrophages, dendritic cells, natural killer (NK) cells, mast cells, basophils, and eosinophils. The modulation of adaptive immunity involves the interaction of H2S with B and T lymphocytes [,].
H2S plays a crucial role in regulating the proliferation of fibroblasts and the polarization of cutaneous macrophages. In mouse models with CSE deficiency, the impairment of endogenous H2S production accelerated the proliferation of cutaneous fibroblasts through a process known as necroptosis [,].
In experiments with keloid fibroblasts and normal cutaneous fibroblasts stimulated by transforming growth factor-β1, the administration of exogenous H2S reduced the levels of several key markers, including α-smooth muscle actin (α-SMA), proliferating cell nuclear antigen (PCNA), collagen type I, collagen type III and anion superoxide. In summary, H2S regulates the proliferation of cutaneous fibroblasts and the polarization of macrophages by inhibiting oxidative stress and necroptosis. Additionally, research has demonstrated that H2S improves various fibrotic skin diseases. This suggests that impaired H2S production may contribute to the proliferation of human skin fibroblasts [,].
Furthermore, H2S is expressed in the cutaneous circulation, where it regulates vasodilation in humans. Both the endothelium and smooth muscle in blood vessels exhibit a strong expression of the CSE/H2S axis []. H2S promotes vascularization through the peroxisome proliferator-activated receptor-γ (PPAR-γ)/vascular endothelial growth factor (VEGF) axis []. The molecular and cellular mechanisms underlying the physiological effects of H2S in biological systems have been detailed in studies of H2S signaling pathways.
In biological systems, H2S signals through distinct pathways [,,,,,].
3.3.2. Oncogenic Profile of H2S
H2S and its producing enzymes have dual roles in carcinogenesis—low levels promote malignancy, while high levels inhibit it. It also participates in cell invasion and migration, influencing processes such as lymphangiogenesis and the formation of pseudopodia, as well as modulating immune responses []. Cancer cells are particularly vulnerable to oxidative damage, which disrupts cell metabolism, cell homeostasis, macromolecule stability, immune and inflammatory responses, cell signaling, angiogenesis, and apoptosis [,,]. Recent studies demonstrate that gasotransmitters play crucial roles in counteracting these negative processes [,].
H2S has been intensively investigated in terms of its potential to cause cancer. The findings that inhibition of H2S biosynthesis exerts anticancer effects are contradicted by other studies showing that H2S overproduction also exerts antitumor actions [,,]. There is no evidence to suggest that H2S exposure causes cancer in humans []. Currently, there is no data on cancer epigenetics caused by H2S. However, different oncogenic cascades have been attributed to H2S, such as accelerating cell cycle progression, propagating anti-apoptotic signals, and inducing angiogenesis []. Cancer cells upregulate their ambient H2S levels to meet increased metabolic requirements, accelerated proliferation, and tumor angiogenesis [,]. H2S has been reported to be genotoxic, which can lead to chromosomal instability at concentrations of 250 μM [].
H2S has contradictory actions in the context of cancer, including proliferation and apoptosis, invasion and metastasis, angiogenesis and immunomodulation. Exposure of cancer cells to relatively low concentrations of H2S may promote cancer progression by stimulating cancer cell growth, facilitating angiogenesis, and promoting chemotherapy resistance, while high levels inhibit cell proliferation [,].
The mechanism responsible for this progression involves H2S’s ability to regulate multiple signaling pathways, including PI3K/AKT/mTOR, RAS/RAF/MEK/ERK, AKT/GSK-3β/β-catenin, and EGFR/ERK/MMP-2 []. H2S can also alleviate inflammation by inhibiting AMPK and reducing oxidative stress. It functions as a vasoactive agent in an O2-dependent manner, acting as a sensor []. Currently, H2S is recognized as a signaling molecule due to its ability to modify cysteine redox switches. Under certain conditions, H2S can act as either a tumor inhibitor or amplifier []. Consequently, it is accepted that H2S exhibits bimodal characteristics in tumor pathophysiology [].
3.4. Abnormalities in H2S Metabolism in Skin Cancer
Numerous studies have addressed the roles of H2S and its synthesizing enzymes in melanoma and non-melanoma skin cancers. Generally, these studies involve (1) comparing specimens of human skin cancer tissue with adjacent normal skin tissue; (2) analyzing skin cancer-derived cell lines alongside compatible non-malignant cell lines; (3) using animal models with xenografted tumors in vivo; (4) conducting cell fractionation studies to quantify H2S synthesis and assess the localization of producing enzymes; and (5) applying genetic and pharmacological approaches [,,].
3.4.1. Melanoma
In a laborious study, in melanoma cell lines (A375, Sk-Mel-5, Sk-Mel-28, PES-43), versus normal human epidermal melanocytes (NHEM), Panza et al. showed that (1) CBS expression was invariable, CSE expression was increased in melanoma cell lines; (2) in human nevi 100% immunoreaction for CSE, variable staining for 3-MST and lack of reaction for CBS; (3) in human melanoma, the expression of CSE is very high in primary tumors, low in metastases and absent in non-lymphatic metastases, and CBS and 3-MST were downregulated; (4) in the A375 melanoma line, an increased expression of CSE inhibited cell proliferation, while overexpression of CBS and 3-MST had no effect; (5) A-375 cells with inactivated CBS, CSE, 3-MST, did not show changes in cancer cell proliferation []. These data indicate the endogenous regulatory role of H2S-producing enzymes on cell multiplication. CSE exerts inhibitory effects on melanoma growth by inhibiting NF-κB and IκBα, altering apoptosis and cell cycle. In A375 melanoma cells, apoptosis was promoted either by CSE overexpression or by the use of exogenous H2S donors (DATS, GYY4137). Panza et al. claim that the proapoptotic effect is correlated with suppression of NF-Kb, AKT and ERK1/2 and upregulation of c-FLIP, XIAP, and Bcl-2 [].
In a study conducted by Cicco et al., a panel of melanoma cell lines (PES 43, A375) was treated with acetyl deacylasidisulfide (ADA), a natural H2S donor []. The aim was to investigate whether exogenous H2S supplementation affects the malignant phenotype of melanocytes. The treatment with ADA led to several biochemical events: (1) a significant suppression of proliferation in human melanoma cell lines through the induction of apoptosis; (2) a reduction in the nuclear translocation and activation of NF-κB; (3) decreased expression of anti-apoptotic proteins, including c-FLIP, XIAP, and Bcl-2; (4) inhibition of phosphorylation; (5) dysregulation of AKT protein and ERK activity; and (6) a substantial and dose-dependent reduction in the formation of lung metastatic foci in C57BL/6 mice. In conclusion, exogenous H2S suppresses melanoma cell growth and migration both in vivo and in vitro [].
In another pharmacological approach, Cicco et al. utilized cell cultures (B16F10, Sk-Mel-5, Sk-Mel-28, A-375) and a murine melanoma model to show that ATB-346 (the ester of 4-thiocarbamoyl phenyl of propionic acid-2-(6-methoxyphthalene-2-yl)) reduce the proliferation of melanoma cells in both in vivo and in vitro settings. This effect was associated with the stimulation of apoptosis and the repression of NF-κB and Akt [].
In experiments where melanoma cells were injected into mice and treated with diallyl trisulfide (DATS), the results showed: (1) inhibited growth of melanoma; (2) reduced immunosuppressive activity of myeloid-derived suppressor cells (MDSCs) in the spleen, blood, and tumor microenvironment; and (3) restored function of CD8 T cells and dendritic cells. These findings indicate that H2S can modulate tumor growth by influencing the immune system [].
Other studies demonstrated that melanoma cell lines A-375 and SK-MEL-28, treated with Sodium Hydrosulfide (NaSH), showed: (1) a significant reduction of p-PI3K, p-Akt, and mTOR proteins; (2) blocked proliferation, migration, and cell cycle progression; and (3) induction of apoptosis and autophagy. Findings by Xiao et al. confirmed that exogenous H2S could inhibit melanoma growth by suppressing the PI3K/AKT/mTOR pathway [,]. Additionally, Naproxen-HBTA has shown antimetastatic activity in a murine model of cutaneous melanoma, inducing caspase-mediated apoptosis, and inhibiting cell migration and invasiveness [].
Cai et al. demonstrated, using a mouse xenograft model, that 5-(4-hydroxyphenyl)-3H-1,2-dithiol-3-dithione (ADT-OH) induces melanoma cell death both in vitro and in vivo. This effect occurs through the reduction of IkB alpha catabolism, decreased activation of NF-kB, suppression of XIAP and Bcl-2, and upregulation of FADD [].
Furthermore, ADT-OH inhibits the dynamics of epithelial-mesenchymal transition (EMT), reduces cell proliferation, and restricts melanoma invasion in mice (B16F10, A375) by suppressing the CSE/CBS and FAK/Paxillin signaling pathways. Additionally, ADT-OH amplifies FADD-dependent extrinsic apoptosis in melanoma cells. However, overexpression of FAK can reverse the inhibitory effects of ADT-OH on cell migration (B16F10, A375) []. FAK also modulates E-cadherin levels via Src.c/ERK1/2/Stat3 and PPAR/Stat3 signaling in B16F10 cell and human melanoma cell lines [].
In a melanoma cell line A-375, Ma et al. tested the relationship between H2S exogen and promotion of apoptosis by (1) upregulation of gene expression associated with apoptosis; (2) overactivation of the unfolded protein response []; These results highlighted (1) the stimulating effect of reduced exogenous H2S concentrations on human melanoma (increased VEGF expression, decreased intracellular ROS levels, cell cycle acceleration), by regulating the phosphorylation level of protein kinase B/Akt; (2) the inhibitory effect of high H2S concentrations on cancer cells (acceleration of apoptosis and autophagy) by regulating the PI3K/Akt/mTOR signaling pathway [,,].
Based on the data presented above, it is highlighted that MAPK/ERK and PI3K/Akt are the most frequently dysregulated signaling pathways influenced by the H2S levels, associated with the malignant phenotype in melanoma and the mechanism of resistance to targeted therapy [].
3.4.2. Non-Melanoma Skin Cancers
H2S is a potential inducing factor of malignancy in SCC by (1) accelerating cell cycle progression; (2) propagation of anti-apoptotic signals; (3) induction of angiogenesis. Exogenous H2S (NaHS, 200–500 μM) served as a proliferative factor, accelerating cell cycle progression in oral SCC by increased phosphorylation of Akt/ERK and activation of the COX2/AKT/ERK1/2 axis. The propagation of anti-apoptotic signals is maintained via the CSE/H2S pathway. H2S exhibits pro-angiogenic effects mainly by increasing VEGF expression [,]. Several reports indicate that tissue H2S levels are increased in SCC versus nontumor tissue. The comparative analysis of oral SCC and the surrounding mucosa (Western blot, tissue microarray) revealed an increase in H2S concentration by 15%, overexpression of phopho-Stat3, mitoNEET, hTERT, and MAPK protein. These H2S-mediated metabolic alterations identified in oral SCC are associated with overexpression of glucose-6-phosphate dehydrogenase and intracellular NADPH expression. The modest increase in the total level of free cellular H2S (15%) compared to the overexpression of synthesizing enzymes (270%) in oral SCC suggests that H2S is rapidly metabolized in carcinoma, possibly to support malignant cell growth. This finding is supported by previous data showing that inactivation of H2S-generating enzyme activities limits tumor cell growth, while exogenous H2S stimulates tumor cell multiplication [].
Another study using oral SCC cell lines (Cal27, GNM, WSU-HN6) treated with different concentrations of NaHS showed (1) proliferation of CAL-27 and GNM cells in a concentration-dependent manner; (2) acceleration of NaHS-mediated cell cycle progression in all 3 cell lines investigated; (3) the involvement of Akt and Erk1/2 phosphorylation in the proliferation of oral SCC cells []. These results support a detrimental role of H2S in the development of oral SCC []. H2S derived from the metabolism of bacteria in the oral cavity stimulates apoptosis in the cancer cells by inactivating PHLDA1. H2S induces differentiated sensitivity to apoptosis between oral cancer cell lines that overexpress PHLDA1 and oral keratinocytes. The PHDLA1 protein inhibits Akt and acts as a suppressor of apoptosis [,].
Zhang et al. examined the role of the H2S-activated COX2/AKT/ERK1/2 axis in oral cancer. Blockade of AKT, or ERK1/2, or COX2 affected H2S-induced viability of oral cancer cells []. For example, the inactivation of the COX2 pathway by niflumic acid reduced NaHS-induced p-ERK and p-AKT expression. Inactivation of the AKT pathway by GSK690693 upregulated NaHS-induced p-ERK1/2 expression and did not influence COX2 expression, and blocking of the ERK1/2 pathway by U0126 increased NaHS-induced p-AKT expression and did not influence COX2 expression. Consequently, H2S supports the proliferation of oral cancer cells by activating the COX2/AKT/ERK1/2 axis, suggesting new pharmacological modalities for oral cancer [,].
Wang et al. demonstrated that DATS, compared to DADS and DAS, inhibits melanoma and BCC growth by forming ROS, membrane transfer of Ca2+, reduction of mitochondrial membrane potential, DNA disorganization, G2/M arrest, triggering endoplasmic reticulum stress and apoptosis []. The antiproliferative effects of garlic-derived allyl sulfides are associated with their conversion to sulfenic sulfur in tumor cells and/or with the control of proliferative signals (redox-sensitive proteins, cell cycle checkpoint regulators, apoptotic regulatory proteins, transcription factors). This research has shown the role of allyl sulfates in the prevention of skin cancer in cell cultures and in vivo models. The cytotoxicity of organosulfur at similar concentrations decreases in the following order: DATS > DADS > DAS [,].
3.5. Reprogramming of Enzyme Synthesis of H2S in Skin Cancer
To understand the role of H2S in tumor oncogenesis and progression, it is essential to analyze the interaction between the enzymes that synthesize H2S (such as CBS, CSE, 3-MST, and CARS) and those that catabolize H2S (including enzymes involved in sulfide oxidation processes like SQOR, hETHE.1, TST, SUOX/SO, and CcO, as well as enzymes involved in sulfide methylation processes: TMT, TEMT). There are few studies in the literature that identify significant changes in the expression levels of various enzymes involved in H2S turnover in skin cancer. It is estimated that the tissue levels of H2S and the expressions of enzymes involved in the metabolism of this mediator are significantly altered in malignant samples compared to non-malignant tissues. This section focuses on the dysregulation of enzymes involved in H2S synthesis in various forms of skin cancer. In humans, CSE and CBS are two essential metabolic enzymes that synthesize H2S from L-cysteine. Other enzymes that contribute to the endogenous synthesis of H2S include 3-MST (which requires the 3-MP substrate) and CARS (a multifunctional enzymatic system) [,,].
3.5.1. Cystathionine β-Synthetase (CBS, EC 4.2.1.22)
CBS is responsible for the production of H2S in various tissues, including the skin. Many pathophysiological events, including tumorigenesis, have been linked to CBS dysregulation. Cancerous tissues of various types have shown higher CBS expression levels compared to surrounding non-cancerous tissues, although in some malignant tissues, CBS expression was found to be lower than in corresponding non-cancerous tissues []. High intratumoral CBS levels have been influenced by the accumulation of mutations in tumor suppressor genes (such as APC, SMAD4, and TP53) and in oncogenes (such as KRAS), as well as by tumor metabolism, invasion, metastasis, and increased resistance to chemotherapy [].
The role of CBS in melanoma is inconclusive. While CBS overregulation has been observed in melanoma cells, its expression is absent in dysplastic nevi, present in one out of four primary melanoma samples, and found in four out of five melanoma cell lines. Additionally, the biological consequences of modulating CBS expression in melanoma appear to have a minimal functional impact on cell proliferation [,]. CBS may promote tumor immune evasion by destabilizing MHC-I. CBS expression in cutaneous melanoma tissues has been correlated with immune infiltration of CD8+ T cells, response to anti-PD-L1 immunotherapy, tumor microenvironment dynamics, MHC-I antigen presentation pathways, overall survival, tumor immune evasion, redox regulation, and immunotherapy efficacy. CBS-induced metabolic reprogramming in melanoma can activate autophagy regulators, metabolic stress responses, T-cell-mediated cytotoxicity, and lysosomal degradation of MHC-I, thereby establishing an immunosuppressive microenvironment [].
In oral squamous cell carcinoma (SCC), the expression of three enzymes—CBS, CSE, and 3-MST—that synthesize H2S was significantly increased compared to non-tumoral oral mucosa. CBS levels were found to be approximately three times higher in SCC tissue than in unaffected tissue [,].
3.5.2. Cystathionine γ-Lyase (CSE or CTH, EC 4.4.1.1)
The catalytic activity of CSE depends on pyridoxal phosphate (PLP), L-cysteine (L-Cys), and the metastatic capacity of neoplastic cells. Overexpression of CSE in the cytosol is associated with tumor progression, levels of glutathione, and the presence of certain non-coding RNAs (microRNA-4317, microRNA-939-5p, microRNA-193, microRNA-548) [].
Immunohistochemical analysis of human melanoma tissue showed that CSE was overexpressed in primary melanoma, present at moderate levels in metastases, and occasionally identified in non-lymph node metastases. The reprogramming of CSE expression in melanoma had several biological consequences, including the spontaneous induction of apoptosis, decreased expression of anti-apoptotic proteins, as well as inactivation of NF-kB and AKT pathways. These effects on blocking melanoma progression have been confirmed in vivo using a murine melanoma model and different H2S donors (DATS) and CSE substrates (L-Cys). These results indicate that the L-Cys/CSE/H2S pathway plays a crucial role in the progression and metastasis of melanoma [,].
Gene/protein expression and CSE activity were investigated in human malignant cell lines (specifically melanoma A375 and melanoma WM35) and confirmed through RT-PCR and Western blot analysis. The findings suggested that the investigated neoplastic samples exhibited limited CSE expression and very low enzymatic activity [,]. Furthermore, pharmacological inactivation of CSE in human melanoma cells induced cellular senescence, and gene blocking reduced tumorigenic effects. Consequently, the CSE gene is regarded as a MYC target gene with a significant role in preventing senescence [].
These results imply that, in these cells, the main regulatory pathway governing neoplastic cell proliferation is the reaction catalyzed by CSE/H2S. Meram et al. investigated the tissue levels of CBS, CSE, and 3-MST proteins in paired malignant and normal tissue samples (15 samples) and found approximately a 2.7-fold increase in enzyme proteins in oral SCC compared to intact mucous membranes []. Although the enzymatic activities of the proteins mediating endogenous H2S synthesis have not been determined, it is noted that the catalytic process could be enhanced to ensure sufficient H2S availability. This data suggests that H2S and the enzyme pathways involved in its synthesis may contribute to the oncogenesis of SCC [].
3.5.3. Mercaptopyruvate Sulfurtransferase (MPST, EC 2.8.1.2)
MPST (3MST) plays a significant role in cysteine (Cys) catabolism and cyanide detoxification. It is capable of producing H2S through the CAT/3MST pathway in the presence of dihydrolipoic acid or thioredoxin, alongside Cys and glutathione (GSH) persulfides, H2S2, H2S3, and sulfur oxides [].
The metabolic axis involving 3-MST and H2S in melanoma is not well-explored. In both melanoma A375 and WM35 cell lines, the gene and protein expression levels, as well as 3-MST activity, were higher compared to CSE, indicating that 3-MST is the primary pathway for sulfan formation in these cells. Furthermore, the overregulation of 3-MST in investigated human neoplastic cell lines confers cytoprotective effects and promotes an aggressive metastatic cancer phenotype [,]. In contrast, decreased expression of 3-MST has been observed in human melanoma cell lines and tissue samples (including nevi, primary tumors, and metastases). In A-375 cells, neither overexpression nor inactivation of 3-MST resulted in changes in cancer cell proliferation []. Increased levels of 3-MST have been documented in carcinomas of the oral cavity (such as adenoid cystic carcinomas, mucoepidermoid carcinoma, and squamous cell carcinoma) compared to the surrounding mucosa [,,].
Additionally, the expression and methylation patterns of H2S regulatory genes (CTH, CBS, CAT, DAO, MPST, SQOR) were analyzed in head and neck SCC. The results showed that: (1) CTH, CBS, and SQOR were significantly overregulated; (2) DAO remained unchanged in malignant tissues; (3) hypomethylated CTH and SQOR are associated with stimulation processes; and (4) hypermethylated CBS, MPST, and DAO indicate repression. Therefore, the regulatory genes of enzymes involved in H2S metabolism could play an important role in the progression of SCC by influencing enzyme expression and epigenetic regulation [].
3.5.4. Cysteinyl-tRNA Synthetase (CARS2, E.C 6.1.1.16)
CARS2 is responsible for the aminoacylation of tRNA molecules, catalyzing the binding of L-cysteine (L-Cys) and ATP, which leads to the formation of cysteinyl-tRNA. CARS2 contributes to various aspects of cancer biology, including cell proliferation and migration, mitochondrial biogenesis, stress response, and the regulation of apoptosis []. In human cells, these enzymes exist in free form or are organized into a multi-tRNA synthesis complex, consisting of nine ARS cytoplasmic entities and three multifunctional proteins (AIMP). Generally, ARS have antitumorigenic functions, whereas AIMP has protumorigenic roles [].
In melanoma, ARS may regulate the proliferation, migration, and invasion of uveal melanoma cells by activating the PI3K/AKT/mTOR pathway, suggesting that ARS could be a novel tumor factor []. ARS has been reported to participate in pathways that either promote or suppress tumorigenesis []. AIMP promotes Th1 polarization through the p38/MAPK signaling pathway, exerting an antitumor effect []. In head and neck SCCs, AIMP were overregulated compared to normal tissues, and AIMP levels correlated with reduced patient survival []. CARS levels were found to be increased in esophageal SCC tissues compared to normal esophageal tissue. CARS1 may function in an antitumor capacity by promoting the ferroptosis process, regulating GPX4, reducing cell proliferation, migration, and tumor invasion. CARS also serves as a potential clinical prognosis predictor in esophageal SCC [].
4. Future Directions and Clinical Significance
Research on the potential of gasotransmitters in biology and medicine is rapidly expanding. In addition to the considerable volume of studies demonstrating the roles of NO, CO and H2S in mammalian systems, the authors propose that an increasing number of gaseous messengers, essential for cellular communication and homeostasis, could serve as signaling entities. The authors anticipate that the development of agonists and antagonists for CBS, CSE, and 3-MST, along with compounds that release H2S, will progress quickly. Consequently, further research will be crucial for advancing fundamental science, clinical applications, and therapeutic approaches, as well as for exploring commercial applications.
The management of gaseous signaling molecules in cancer therapy has been extensively investigated in recent years. Different endogenous and exogenous H2S donors have been developed and progress has been made in their therapeutic applications [,,]. Numerous studies have revealed the particularities of H2S-anticancer therapy. The use of H2S as a therapeutic agent is still in its early stages and faces several challenges. The therapeutic potential of H2S may be influenced by (1) the properties of the gas mediator, including: bimodal role of H2S in cancer, effective biocompatibility and solubility, negligible side effects, small size, accelerated metabolism, rapid diffusion at the site of injury, (2) concentration/dose, time, cell type, and drug used []. The effects of natural and synthetic H2S donors range from cancer suppressants to promoters [].
The exogenous H2S donor, NaHS, amplifies the apoptotic process in melanoma A375 and SK-MEL-282 cells, demonstrating the antitumor effect of H2S treatment at high concentrations (via suppression of the PI3K/AKT/mTOR, NF-KB, AKT, ERK1/2, MAPK/ERK, PI3K/Akt pathways, FLIP, XIAP, Bcl-2, PHLDA genes) [,,,]. Exogenous administration of H2S at low levels amplifies the growth/proliferation of melanoma by modulating angiogenesis, energy metabolism, antioxidant profile. Increased levels of exogenous H2S have an antitumor effect by disrupting apoptosis, cell cycle and genomic stability. In summary, it is documented that several types of cancer cells overexpress CBS, CSE or 3MST or synthesize larger amounts of H2S compared to adjacent non-tumor tissue. Overexpression or reduction of expression of genes encoding H2S-generating enzymes has been tested in tumors of various tissue origins and in animal models []. H2S donors orchestrate intracellular signaling pathways and tumor microenvironment dynamics in skin cancer [,,]. The maximum concentration of H2S that provides safety should not exceed 10 ppm by inhalation; effective therapeutic concentrations should be in the nM–mM range. The effects of H2S on tumor cells are dose-dependent. At physiological concentrations, H2S mediates positive bioenergetic mechanisms, antioxidant signaling, cytoprotective responses. At high concentrations, it exhibits remarkable toxicity, inhibits mitochondrial respiratory metabolism, and induces reactive oxidative stress and apoptosis []
H2S donors play a crucial role in either promoting or inhibiting skin cancer. For instance, NaHS has been shown to promote the growth of NCI-H929 melanoma cell lines, while another compound, GYY4137, has exhibited similar promotional effects on SKMel5 and SKMel28 lines. On the other hand, BITC has demonstrated inhibitory effects on the promotion of B16F10 melanoma cells. Additionally, NaHS has positively influenced the development of squamous cell carcinoma (SCC) lines, such as Cal-27 and WSU-HN612, while BITC has negatively affected the progression of other SCC lines (OG2, SCC9) [].
In conclusion, there are two main strategies for utilizing H2S in cancer treatment: inhibiting endogenous H2S synthesis or supplementing with exogenous H2S donors. H2S donors have been shown to induce persulfidation modifications of cysteine residues, leading to alterations in various signaling pathways []. Specifically, H2S donors modulate key signal transduction pathways involved in malignancy progression, including PI3K/AKT/mTOR, RAS/RAF/MEK/ERK, AKT/GSK-3β/β-catenin, JAK2/STAT3, and EGFR/ERK/MMP-2 [,]. The ability of H2S to treat various malignancies stems from its capacity to modulate immune responses, reduce oxidative stress, promote tissue repair, maintain redox status, stimulate the phagocytic activity of immune cells, alleviate vascular inflammation, induce vasodilation, and reduce apoptosis [,]. Future multidisciplinary collaborations involving nanomaterials, chemistry, pharmaceuticals, and biological sciences could enhance the potential of H2S therapy.
This paper investigates recent developments and emerging trends of H2S in skin cancer pathology. It is a relevant and timely study because there is a real need for non-invasive biomarkers for cancer management. This analysis identifies opportunities and challenges to guide future work. The discussion could be enriched by considering the interaction of H2S with other gasotransmitters, such as NO and CO.
5. Conclusions
The presence of H2S is vital for maintaining cellular homeostasis in both normal and malignant cells. Studies have reported significant differences in endogenous H2S levels between cancerous and non-cancerous cells, with higher levels detected in patients with skin cancer. Additionally, upregulation of various H2S-producing enzymes (such as CSE, CBS, and 3-MST) has been observed in human skin cell lines and in malignancies like melanoma and non-melanoma skin cancers. These enzymes may serve as potential biomarkers for cancer diagnosis and treatment. The role of H2S donors in promoting or inhibiting skin cancer presents a complex approach that merits further exploration in the development of possible antitumor therapeutic strategies.
Author Contributions
Conceptualization, I.N., M.T., C.C., S.R.G. and C.M.; writing—original draft preparation, I.N., C.I.M., M.I.M. and C.D.E.; writing—review and editing, M.T., C.M. and C.D.E.; supervision, C.C., S.R.G. and I.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mallardo, D.; Basile, D.; Vitale, M.G. Advances in Melanoma and Skin Cancers. Int. J. Mol. Sci. 2025, 26, 1849. [Google Scholar] [CrossRef]
- Caraviello, C.; Nazzaro, G.; Tavoletti, G.; Boggio, F.; Denaro, N.; Murgia, G.; Passoni, E.; Benzecry Mancin, V.; Marzano, A.V. Melanoma Skin Cancer: A Comprehensive Review of Current Knowledge. Cancers 2025, 17, 2920. [Google Scholar] [CrossRef]
- Pan, Y.; Tang, B.; Guo, Y.; Cai, Y.; Li, Y.-Y. Global Burden of Non-Melanoma Skin Cancers among Older Adults: A Comprehensive Analysis Using Machine Learning Approaches. Sci. Rep. 2025, 15, 15266. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, Y.; Shao, X.; Chen, T.; Zhong, J.; Ou, Y.; Chen, J. Burden of Skin Cancer in Older Adults From 1990 to 2021 and Modelled Projection to 2050. JAMA Dermatol. 2025, 161, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Georgescu, S.R.; Mitran, M.I.; Mitran, C.I.; Matei, C.; Caruntu, A.; Scheau, C.; Nicolae, I.; Matei, A.; Caruntu, C.; et al. Current Perspectives on the Role of Matrix Metalloproteinases in the Pathogenesis of Basal Cell Carcinoma. Biomolecules 2021, 11, 903. [Google Scholar] [CrossRef]
- Xiao, Q.; Xiong, L.; Tang, J.; Li, L.; Li, L. Hydrogen Sulfide in Skin Diseases: A Novel Mediator and Therapeutic Target. Oxid. Med. Cell. Longev. 2021, 2021, 6652086. [Google Scholar] [CrossRef]
- Coavoy-Sánchez, S.A.; Costa, S.K.P.; Muscará, M.N. Hydrogen Sulfide and Dermatological Diseases. Br. J. Pharmacol. 2020, 177, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-Y.; Wang, Y.; Zhu, Y.-W.; Zhang, Y.-X.; Yuan, H.; Liu, Y.-F.; Jin, Y.-Q.; Gao, W.; Ren, Z.-G.; Ji, X.-Y.; et al. Role of Hydrogen Sulfide in Dermatological Diseases. Nitric Oxide Biol. Chem. 2024, 150, 18–26. [Google Scholar] [CrossRef]
- Liu, J.; Mesfin, F.M.; Hunter, C.E.; Olson, K.R.; Shelley, W.C.; Brokaw, J.P.; Manohar, K.; Markel, T.A. Recent Development of the Molecular and Cellular Mechanisms of Hydrogen Sulfide Gasotransmitter. Antioxidants 2022, 11, 1788. [Google Scholar] [CrossRef]
- Dawoud, A.; Youness, R.A.; Elsayed, K.; Nafae, H.; Allam, H.; Saad, H.A.; Bourquin, C.; Szabo, C.; Abdel-Kader, R.; Gad, M.Z. Emerging Roles of Hydrogen Sulfide-Metabolizing Enzymes in Cancer. Redox Rep. Commun. Free Radic. Res. 2024, 29, 2437338. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Y.-F.; Zhang, Y.-X.; Wang, Y.; Jin, Y.-Q.; Yuan, H.; Liang, X.-Y.; Ji, X.-Y.; Jiang, Q.-Y.; Wu, D.-D. The Potential Role of Hydrogen Sulfide in Cancer Cell Apoptosis. Cell Death Discov. 2024, 10, 114. [Google Scholar] [CrossRef]
- Ghouse, M.R.; Egambaram, S.; Ramachandran, A.; Parsanathan, R. The Impact of Hydrogen Sulfide Regulatory Gene Alterations on Head and Neck Squamous Cell Carcinoma Prognosis and Tumor Microenvironment. Egypt. J. Med. Hum. Genet. 2025, 26, 78. [Google Scholar] [CrossRef]
- Szabo, C. Novel Regulatory Roles of Hydrogen Sulfide in Health and Disease. Biomolecules 2022, 12, 1372. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y. Hydrogen Sulfide: A Rising Star for Cancer Treatment. Med. Gas Res. 2025, 15, 114–116. [Google Scholar] [CrossRef]
- Ene, C.D.; Tampa, M.; Georgescu, S.R.; Matei, C.; Leulescu, I.M.T.; Dogaru, C.I.; Penescu, M.N.; Nicolae, I. Disturbances in Nitric Oxide Cycle and Related Molecular Pathways in Clear Cell Renal Cell Carcinoma. Cancers 2023, 15, 5797. [Google Scholar] [CrossRef]
- Rodkin, S.; Nwosu, C.; Sannikov, A.; Tyurin, A.; Chulkov, V.S.; Raevskaya, M.; Ermakov, A.; Kirichenko, E.; Gasanov, M. The Role of Gasotransmitter-Dependent Signaling Mechanisms in Apoptotic Cell Death in Cardiovascular, Rheumatic, Kidney, and Neurodegenerative Diseases and Mental Disorders. Int. J. Mol. Sci. 2023, 24, 6014. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, L.; Xie, Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.-S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, H.; Shen, P.; Zhou, Y.; Li, Q.; Zhang, J.; Chen, Y. Gasotransmitter Research Advances in Respiratory Diseases. Antioxid. Redox Signal. 2024, 40, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Coavoy-Sanchez, S.A.; da Costa Marques, L.A.; Costa, S.K.P.; Muscara, M.N. Role of Gasotransmitters in Inflammatory Edema. Antioxid. Redox Signal. 2024, 40, 272–291. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, R.; Matson, J.B. Hydrogels for Gasotransmitter Delivery: Nitric Oxide, Carbon Monoxide, and Hydrogen Sulfide. Macromol. Biosci. 2024, 24, e2300138. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Sulfide-Pathological and Physiological Functions in Mammalian Cells. Cells 2023, 12, 2684. [Google Scholar] [CrossRef]
- Tan, B.; Jin, S.; Sun, J.; Gu, Z.; Sun, X.; Zhu, Y.; Huo, K.; Cao, Z.; Yang, P.; Xin, X.; et al. New Method for Quantification of Gasotransmitter Hydrogen Sulfide in Biological Matrices by LC-MS/MS. Sci. Rep. 2017, 7, 46278. [Google Scholar] [CrossRef]
- Khodade, V.S.; Toscano, J.P. Reactive Sulfur Species in Biology and Medicine. Antioxidants 2023, 12, 1759. [Google Scholar] [CrossRef]
- Iciek, M.; Bilska-Wilkosz, A.; Kozdrowicki, M.; Górny, M. Reactive Sulfur Species in Human Diseases. Antioxid. Redox Signal. 2023, 39, 1000–1023. [Google Scholar] [CrossRef]
- Wang, H.C.; Yang, J.-H.; Hsieh, S.-C.; Sheen, L.-Y. Allyl Sulfides Inhibit Cell Growth of Skin Cancer Cells through Induction of DNA Damage Mediated G2/M Arrest and Apoptosis. J. Agric. Food Chem. 2010, 58, 7096–7103. [Google Scholar] [CrossRef]
- Gross-Amat, O.; Guillen, M.; Gimeno, J.-P.; Salzet, M.; Lebonvallet, N.; Misery, L.; Auxenfans, C.; Nataf, S. Molecular Mapping of Hydrogen Sulfide Targets in Normal Human Keratinocytes. Int. J. Mol. Sci. 2020, 21, 4648. [Google Scholar] [CrossRef]
- Birg, A.; Lin, H.C. The Role of Bacteria-Derived Hydrogen Sulfide in Multiple Axes of Disease. Int. J. Mol. Sci. 2025, 26, 3340. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological Roles of Hydrogen Sulfide in Mammalian Cells, Tissues, and Organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-J.; Wang, Y.; Jin, Y.-Q.; Zhu, Y.-W.; Zhu, S.-G.; Wang, Q.-M.; Jing, M.-R.; Zhang, Y.-X.; Cai, C.-B.; Feng, Z.-F.; et al. Recent Advances in the Role of Hydrogen Sulfide in Age-Related Diseases. Exp. Cell Res. 2024, 441, 114172. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, X.; Zhuang, L. Exogenous Hydrogen Sulfide Induces A375 Melanoma Cell Apoptosis Through Overactivation of the Unfolded Protein Response. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-Q.; Yuan, H.; Liu, Y.-F.; Zhu, Y.-W.; Wang, Y.; Liang, X.-Y.; Gao, W.; Ren, Z.-G.; Ji, X.-Y.; Wu, D.-D. Role of Hydrogen Sulfide in Health and Disease. MedComm 2024, 5, e661. [Google Scholar] [CrossRef]
- Youness, R.A.; Habashy, D.A.; Khater, N.; Elsayed, K.; Dawoud, A.; Hakim, S.; Nafea, H.; Bourquin, C.; Abdel-Kader, R.M.; Gad, M.Z. Role of Hydrogen Sulfide in Oncological and Non-Oncological Disorders and Its Regulation by Non-Coding RNAs: A Comprehensive Review. Non-Coding RNA 2024, 10, 7. [Google Scholar] [CrossRef]
- Marutani, E.; Ichinose, F. Emerging Pharmacological Tools to Control Hydrogen Sulfide Signaling in Critical Illness. Intensive Care Med. Exp. 2020, 8, 5. [Google Scholar] [CrossRef]
- Santana Maldonado, C.; Weir, A.; Rumbeiha, W.K. A Comprehensive Review of Treatments for Hydrogen Sulfide Poisoning: Past, Present, and Future. Toxicol. Mech. Methods 2023, 33, 183–196. [Google Scholar] [CrossRef]
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.-Q.; Chen, H.-J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.-Y.; et al. Hydrogen Sulfide Biology and Its Role in Cancer. Molecules 2022, 27, 3389. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Skalny, A.V.; Ke, T.; da Rocha, J.B.; Paoliello, M.M.; Santamaria, A.; Bornhorst, J.; Rongzhu, L.; Svistunov, A.A.; Djordevic, A.B.; et al. Hydrogen Sulfide (H2S) Signaling as a Protective Mechanism against Endogenous and Exogenous Neurotoxicants. Curr. Neuropharmacol. 2022, 20, 1908–1924. [Google Scholar] [CrossRef]
- Munteanu, C.; Turnea, M.A.; Rotariu, M. Hydrogen Sulfide: An Emerging Regulator of Oxidative Stress and Cellular Homeostasis-A Comprehensive One-Year Review. Antioxidants 2023, 12, 1737. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y.; Mao, S.; Dong, C.; Yu, Z.; Yao, X.; Shen, B. CBS Promotes Tumor Immune Evasion by Reducing MHC-I Stability. Genes Dis. 2025; in press. [Google Scholar] [CrossRef]
- Bege, M.; Lovas, M.; Priksz, D.; Bernát, B.; Bereczki, I.; Kattoub, R.G.; Kajtár, R.; Eskeif, S.; Novák, L.; Hodek, J.; et al. Synthesis, H2S Releasing Properties, Antiviral and Antioxidant Activities and Acute Cardiac Effects of Nucleoside 5′-Dithioacetates. Sci. Rep. 2025, 15, 2876. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Wang, Q.; Jiang, M.; Wang, X.; Liu, W.; Wang, X.; Zhang, C.; Xiang, L. Hydrogen Sulfide Promotes Cell Proliferation and Melanin Synthesis in Primary Human Epidermal Melanocytes. Ski. Pharmacol. Physiol. 2020, 33, 61–68. [Google Scholar] [CrossRef]
- Goren, I.; Köhler, Y.; Aglan, A.; Pfeilschifter, J.; Beck, K.-F.; Frank, S. Increase of Cystathionine-γ-Lyase (CSE) during Late Wound Repair: Hydrogen Sulfide Triggers Cytokeratin 10 Expression in Keratinocytes. Nitric Oxide Biol. Chem. 2019, 87, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ozlen, N.; Ercetin, D.; Sapmaz-Metin, M.; Gunduz, O. Anti-Inflammatory Effect of Hydrogen Sulfide Donor Sodium-Sulfide in an Experimental Mouse Model of Contact Hypersensitivity. Dermatol. Sin. 2023, 41, 94. [Google Scholar] [CrossRef]
- Cornwell, A.; Badiei, A. From Gasotransmitter to Immunomodulator: The Emerging Role of Hydrogen Sulfide in Macrophage Biology. Antioxidants 2023, 12, 935. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen Sulfide: An Endogenous Regulator of the Immune System. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Sawaya, A.; Regina, A.C.; Menezes, R.G. Hydrogen Sulfide Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Li, Y.; Liu, H. Clinical Powers of Aminoacyl tRNA Synthetase Complex Interacting Multifunctional Protein 1 (AIMP1) for Head-Neck Squamous Cell Carcinoma. Cancer Biomark. Sect. Dis. Markers 2022, 34, 359–374. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Liu, C.; He, Z.; Shen, Q.; Zhu, Y.; Wang, X.; Cao, S.; Yang, S. Endogenous Hydrogen Sulphide Deficiency and Exogenous Hydrogen Sulphide Supplement Regulate Skin Fibroblasts Proliferation via Necroptosis. Exp. Dermatol. 2024, 33, e14972. [Google Scholar] [CrossRef]
- Lei, Y.-Y.; Feng, Y.-F.; Zeng, B.; Zhang, W.; Xu, Q.; Cheng, F.; Lan, J.; Luo, H.-H.; Zou, J.-Y.; Chen, Z.-G.; et al. Exogenous H2S Promotes Cancer Progression by Activating JAK2/STAT3 Signaling Pathway in Esophageal EC109 Cells. Int. J. Clin. Exp. Pathol. 2018, 11, 3247–3256. [Google Scholar]
- Xiao, Q.; Ying, J.; Qiao, Z.; Yang, Y.; Dai, X.; Xu, Z.; Zhang, C.; Xiang, L. Exogenous Hydrogen Sulfide Inhibits Human Melanoma Cell Development via Suppression of the PI3K/AKT/mTOR Pathway. J. Dermatol. Sci. 2020, 98, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, L.; Song, S.; Pan, L.; Muhammad Arslan, I.; Chen, Y.; Yang, S. Hydrogen Sulfide: Recent Progress and Perspectives for the Treatment of Dermatological Diseases. J. Adv. Res. 2021, 27, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, R.E.; Mohammad, I.Z.; Meram, A.T.; Kim, D.; Alotaibi, F.; Patel, S.; Ghali, G.E.; Kevil, C.G. Molecular Functions of Hydrogen Sulfide in Cancer. Pathophysiology 2021, 28, 437–456. [Google Scholar] [CrossRef]
- Shi, Y.-B.; Cheng, L.; Lyu, Y.; Shi, Z.-J. The New Perspective of Gasotransmitters in Cancer Metastasis. Nitric Oxide Biol. Chem. 2025, 156, 1–8. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Tampa, M.; Matei, C.; Lupu, A.; Manole, E.; Ion, R.-M.; Fenga, C.; Tsatsakis, A.M. Toxicological and Efficacy Assessment of Post-Transition Metal (Indium) Phthalocyanine for Photodynamic Therapy in Neuroblastoma. Oncotarget 2016, 7, 69718–69732. [Google Scholar] [CrossRef]
- Salihi, A.; Al-Naqshabandi, M.A.; Khudhur, Z.O.; Housein, Z.; Hama, H.A.; Abdullah, R.M.; Hussen, B.M.; Alkasalias, T. Gasotransmitters in the Tumor Microenvironment: Impacts on Cancer Chemotherapy (Review). Mol. Med. Rep. 2022, 26, 233. [Google Scholar] [CrossRef] [PubMed]
- Oza, P.P.; Kashfi, K. The Triple Crown: NO, CO, and H2S in Cancer Cell Biology. Pharmacol. Ther. 2023, 249, 108502. [Google Scholar] [CrossRef]
- Zhu, J.; Ligi, S.; Yang, G. An Evolutionary Perspective on the Interplays between Hydrogen Sulfide and Oxygen in Cellular Functions. Arch. Biochem. Biophys. 2021, 707, 108920. [Google Scholar] [CrossRef]
- Zou, Y.; Ge, M.; Wang, X. Targeting PI3K-AKT-mTOR by LY3023414 Inhibits Human Skin Squamous Cell Carcinoma Cell Growth in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 2017, 490, 385–392. [Google Scholar] [CrossRef]
- Wang, H.-C.; Chu, Y.-L.; Hsieh, S.-C.; Sheen, L.-Y. Diallyl Trisulfide Inhibits Cell Migration and Invasion of Human Melanoma A375 Cells via Inhibiting Integrin/Facal Adhesion Kinase Pathway. Environ. Toxicol. 2017, 32, 2352–2359. [Google Scholar] [CrossRef]
- Panza, E.; De Cicco, P.; Armogida, C.; Scognamiglio, G.; Gigantino, V.; Botti, G.; Germano, D.; Napolitano, M.; Papapetropoulos, A.; Bucci, M.; et al. Role of the Cystathionine γ Lyase/Hydrogen Sulfide Pathway in Human Melanoma Progression. Pigment Cell Melanoma Res. 2015, 28, 61–72. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Panza, E.; Armogida, C.; Ercolano, G.; Taglialatela-Scafati, O.; Shokoohinia, Y.; Camerlingo, R.; Pirozzi, G.; Calderone, V.; Cirino, G.; et al. The Hydrogen Sulfide Releasing Molecule Acetyl Deacylasadisulfide Inhibits Metastatic Melanoma. Front. Pharmacol. 2017, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; De Cicco, P.; Frecentese, F.; Saccone, I.; Corvino, A.; Giordano, F.; Magli, E.; Fiorino, F.; Severino, B.; Calderone, V.; et al. Anti-Metastatic Properties of Naproxen-HBTA in a Murine Model of Cutaneous Melanoma. Front. Pharmacol. 2019, 10, 66. [Google Scholar] [CrossRef]
- Cai, F.; Xu, H.; Cao, N.; Zhang, X.; Liu, J.; Lu, Y.; Chen, J.; Yang, Y.; Cheng, J.; Hua, Z.-C.; et al. ADT-OH, a Hydrogen Sulfide-Releasing Donor, Induces Apoptosis and Inhibits the Development of Melanoma in Vivo by Upregulating FADD. Cell Death Dis. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Lan, Y.; Chen, D.; Ji, L.; Hua, Z.-C. FAK Regulates E-Cadherin Expression via p-SrcY416/p-ERK1/2/p-Stat3Y705 and PPARγ/miR-125b/Stat3 Signaling Pathway in B16F10 Melanoma Cells. Oncotarget 2017, 8, 13898–13908. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.; Han, R.; Wang, S. Benzyl Isothiocyanate Inhibits Invasion and Induces Apoptosis via Reducing S100A4 Expression and Increases PUMA Expression in Oral Squamous Cell Carcinoma Cells. Braz. J. Med. Biol. Res. 2019, 52, e8409. [Google Scholar] [CrossRef]
- Caruntu, C.; Mirica, A.; Roşca, A.E.; Mirica, R.; Caruntu, A.; Tampa, M.; Matei, C.; Constantin, C.; Neagu, M.; Badarau, A.I.; et al. The Role of Estrogens and Estrogen Receptors in Melanoma Development and Progression. Acta Endocrinol. 2016, 12, 234–241. [Google Scholar] [CrossRef]
- Meram, A.T.; Chen, J.; Patel, S.; Kim, D.D.; Shirley, B.; Covello, P.; Coppola, D.; Wei, E.X.; Ghali, G.; Kevil, C.G.; et al. Hydrogen Sulfide Is Increased in Oral Squamous Cell Carcinoma Compared to Adjacent Benign Oral Mucosae. Anticancer Res. 2018, 38, 3843–3852. [Google Scholar] [CrossRef]
- Zhang, S.; Bian, H.; Li, X.; Wu, H.; Bi, Q.; Yan, Y.; Wang, Y. Hydrogen Sulfide Promotes Cell Proliferation of Oral Cancer through Activation of the COX2/AKT/ERK1/2 Axis. Oncol. Rep. 2016, 35, 2825–2832. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Nie, A.; Yu, D.; Bian, M. Roles of Aminoacyl-tRNA Synthetases in Cancer. Front. Cell Dev. Biol. 2020, 8, 599765. [Google Scholar] [CrossRef]
- Wang, H.-C.; Pao, J.; Lin, S.-Y.; Sheen, L.-Y. Molecular Mechanisms of Garlic-Derived Allyl Sulfides in the Inhibition of Skin Cancer Progression. Ann. N. Y. Acad. Sci. 2012, 1271, 44–52. [Google Scholar] [CrossRef]
- Zuhra, K.; Augsburger, F.; Majtan, T.; Szabo, C. Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules 2020, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiong, Y.; Zhou, L.; Huang, Y.; Chen, W.; Wang, B. Soluble E-cadherin Is Associated with Oxidative Stress in Patients with Chronic HBV Infection. J. Med. Virol. 2020, 92, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Ianoși, S.L.; Batani, A.; Ilie, M.A.; Tampa, M.; Georgescu, S.-R.; Zurac, S.; Boda, D.; Ianosi, N.G.; Neagoe, D.; Calina, D.; et al. Non-Invasive Imaging Techniques for the in Vivo Diagnosis of Bowen’s Disease: Three Case Reports. Oncol. Lett. 2019, 17, 4094–4101. [Google Scholar] [CrossRef] [PubMed]
- Jurkowska, H.; Placha, W.; Nagahara, N.; Wróbel, M. The Expression and Activity of Cystathionine-γ-Lyase and 3-Mercaptopyruvate Sulfurtransferase in Human Neoplastic Cell Lines. Amino Acids 2011, 41, 151–158. [Google Scholar] [CrossRef]
- Rao, S.P.; Dobariya, P.; Bellamkonda, H.; More, S.S. Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease. Antioxidants 2023, 12, 603. [Google Scholar] [CrossRef]
- Leikam, C.; Hufnagel, A.; Walz, S.; Kneitz, S.; Fekete, A.; Müller, M.J.; Eilers, M.; Schartl, M.; Meierjohann, S. Cystathionase Mediates Senescence Evasion in Melanocytes and Melanoma Cells. Oncogene 2014, 33, 771–782. [Google Scholar] [CrossRef]
- Dongsoo, K.; Chen, J.; Wei, E.; Ansari, J.; Meram, A.; Patel, S.; Ghali, G.; Kevil, C.; Shackelford, R.E. Hydrogen Sulfide and Hydrogen Sulfide-Synthesizing Enzymes Are Altered in a Case of Oral Adenoid Cystic Carcinoma. Case Rep. Oncol. 2018, 11, 585–590. [Google Scholar] [CrossRef]
- Kim, D.; Chen, J.; Meram, A.; Patel, S.; Wei, E.; Ansari, J.; Ghali, G.; Kevil, C.; Shackelford, R.E. Hydrogen Sulfide-Synthesizing Enzymes Are Altered in a Case of Oral Cavity Mucoepidermoid Carcinoma. Case Rep. Oncol. 2018, 11, 682–687. [Google Scholar] [CrossRef]
- Khan, K.; Gogonea, V.; Fox, P.L. Aminoacyl-tRNA Synthetases of the Multi-tRNA Synthetase Complex and Their Role in Tumorigenesis. Transl. Oncol. 2022, 19, 101392. [Google Scholar] [CrossRef] [PubMed]
- Wusiman, W.; Zhang, Z.; Ding, Q.; Liu, M. The Pathophyiological Role of Aminoacyl-tRNA Synthetases in Digestive System Diseases. Front. Physiol. 2022, 13, 935576. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, X.; Chen, S. Cysteinyl-tRNA Synthetase 1 Promotes Ferroptosis-Induced Cell Death via Regulating GPX4 Expression. J. Oncol. 2022, 2022, 4849174. [Google Scholar] [CrossRef]
- Pu, Y.; Lin, W.; Ren, S.; Gao, Y.; Wang, G. The Therapeutic Potential of Hydrogen Sulfide and Its Donors, a New Discovery in Vascular Diseases. J. Cardiovasc. Pharmacol. 2025, 86, 128–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X.; Ke, B.; Huang, W.; Jiang, X.; He, G. Recent Advances on Endogenous Gasotransmitters in Inflammatory Dermatological Disorders. J. Adv. Res. 2021, 38, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.V.; Pinto, M.L.; Serre, C. Gasotransmitters in Modern Medicine: Promises and Challenges in the Use of Porous Crystalline Carriers. Adv. Healthc. Mater. 2025, 14, e2404553. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.D.; Georgescu, S.R.; Tampa, M.; Matei, C.; Mitran, C.I.; Mitran, M.I.; Penescu, M.N.; Nicolae, I. Cellular Response against Oxidative Stress, a Novel Insight into Lupus Nephritis Pathogenesis. J. Pers. Med. 2021, 11, 693. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).