Abstract
The antiarrhythmic drug amiodarone is toxic to yeast cells due to provoking Ca2+ entry into cytosol. Here we show that in Ogataea parapolymorpha, the loss of Cch1 or Mid1, which are the primary components of the high-affinity Ca2+ uptake system (HACS), leads to a delay in the rise of cytosolic Ca2+ concentration ([Ca2+]cyt) in response to amiodarone. This has negligible effect on the ability of the strain with the unaffected Ca2+ sequestration system to grow in the presence of amiodarone. Inactivation of the PMC1 gene encoding the Ca2+ ATPase involved in the cytosolic Ca2+ sequestration in the vacuole dramatically increases sensitivity to amiodarone, while inactivation of CCH1 or MID1 suppresses it. This correlates with a substantially lower [Ca2+]cyt rise in response to amiodarone when the genes encoding the HACS components are inactivated in the mutant lacking Pmc1. Similarly to sodium dodecyl sulfate, which has also been shown to increase [Ca2+]cyt, amiodarone causes activation of the Hog1 protein kinase involved in the cell cycle regulation. The role of HACS in the amiodarone-induced Ca2+ influx is discussed.
1. Introduction
In the eukaryotic cell Ca2+ cations fulfill a number of functions that require their sequestration in certain organelles while their concentration in cytosol ([Ca2+]cyt) is maintained at a very low level. This is achieved due to the action of different ion pumps and channels. In yeast, the main Ca2+ sink/storage organelle is the vacuole, whose Ca2+ ATPase Pmc1 and H+/Ca2+ antiporter Vcx1 pump Ca2+ from the cytosol. Vcx1 is powered by the proton gradient across the vacuolar membrane created by the vacuolar H+ ATPase []. Unlike animal cells, yeast cells do not possess a special Ca2+ ion pump in the endoplasmic reticulum (ER), which receives these cations from the Golgi apparatus [] and possibly from the vacuole []. The Golgi apparatus is supplied with Ca2+ by the Pmr1 Ca2+/Mn2+ ATPase, which is also involved in the control of [Ca2+]cyt [,].
Two Ca2+ uptake systems, namely, low-affinity (LACS) and high-affinity (HACS), have been detected in yeast cells []. No components of LACS have been identified so far except Fig1 [], whose exact function remains unknown. HACS is represented by Cch1, which is a homolog of the mammalian voltage-gated Ca2+ channels [] and by Mid1, which shares some homology with the family of animal proteins associated with the sodium leak channels []. Besides these two proteins, Ecm7 has also been shown to be involved in the functioning of HACS [].
The antiarrhythmic drug amiodarone is toxic to a wide range of fungi []. In S. cerevisiae, it was shown to provoke Ca2+ influx, which causes its toxicity [,,]. The cell death induced by amiodarone in yeast demonstrates hallmarks similar to those of the cell death induced by the alpha-factor pheromone []. Notably, Cch1 and Mid1 are involved in the [Ca2+]cyt rise in response to the alpha factor []. It was reported that in contrast to the loss of Cch1, the loss of Mid1 significantly reduced the [Ca2+]cyt rise in response to amiodarone [], although the other manifestations of these mutations are identical []. In humans, the action of amiodarone as a class III antiarrhythmic drug is achieved through inhibition of potassium channels, and it also blocks cardiac Na+ and Ca2+ channels (for a review see []).
In Ogataea yeasts, the loss of the vacuolar Ca2+ ATPase Pmc1 leads to hypersensitivity to sodium dodecyl sulfate (SDS) since the latter induces Ca2+ influx from the environment [,]. Although the Saccharomyces cerevisiae pmc1-Δ mutant is not hypersensitive to SDS [], this phenotype in some mutants can also be related to Ca2+ homeostasis and signaling. Particularly, SDS sensitivity of the vps13-Δ mutant can be suppressed by inhibition of calcineurin, which is the Ca2+/calmodulin-dependent protein phosphatase regulating Ca2+ homeostasis and adaptation to environmental changes [,], or by an increased PMC1 gene dosage [,]. The loss of Cch1 suppresses SDS hypersensitivity of the pmc1-Δ mutants but does not prevent SDS-induced Ca2+ uptake [,]. This indicates that the main conduit for Ca2+ entry in response to SDS is not the Cch1/Mid1 channel, but its regulation depends on Cch1. At the same time, the role of Mid1 in SDS-induced Ca2+ uptake has not been explored.
The objective of this work was to reveal the role of the Cch1 and Mid1 components of yeast HACS in Ca2+ entry in response to amiodarone using Ogataea parapolymorpha expressing a genetically encoded fluorescent Ca2+ indicator as a model. We have shown that the effects of MID1 and CCH1 inactivation on the [Ca2+]cyt rise in response to amiodarone, as well as to SDS, are very similar and that the loss of the components of HACS inhibits only the initial stage of Ca2+ uptake induced by amiodarone.
2. Results
2.1. Inactivation of MID1 Suppresses SDS Hypersensitivity Caused by the Lack of the Pmc1 Ca2+ Ion Pump
While the Cch1 subunit of the high-affinity plasma membrane Ca2+ channel in Ogataea yeasts has been characterized previously [,], the Mid1 subunit remained uncharacterized. The only homolog of S. cerevisiae MID1 in the O. parapolymorpha genome (NCBI gene ID: 25772629) is also annotated as MID1 []. Interestingly, this ORF starts only 22 bp downstream of the ORF encoding alanyl-tRNA synthetase. This distance apparently is not sufficient to include the full promoter, which possibly overlaps with the upstream ORF. As in S. cerevisiae Mid1, the O. polymorpha polypeptide encoded by this gene starts with a hydrophobic region (Table 1, Figure S1), whose deletion in S. cerevisiae was shown to affect translocation of this protein to the endoplasmic reticulum []. Unlike the S. cerevisiae protein, the O. parapolymorpha Mid1 possesses a hydrophobic region at the C-terminus, which can potentially serve as a transmembrane domain. Notably, the Mid1 homologs of some other fungi, e.g., Yarrowia lipolytica, Schizosaccharomyces pombe, Candida albicans and Aspergillus fumigatus (Table 1), also possess the C-terminal hydrophobic region. Similar to the S. cerevisiae Mid1, the Cryptococcus neoformans protein contains only one hydrophobic region, which, however, does not start from the N-terminus but from the 58th amino acid residue. The A. fumigatus Mid1 contains both hydrophobic regions, and the N-terminal one starts from the 14th amino acid residue (Table 1, Figure S1). Although the N-terminal hydrophobic region was predicted to be the secretory signal peptide [], its position in the C. neoformans and A. fumigatus proteins contradicts the role as a secretory signal. Moreover, this protein should be membrane-associated, which implies that it should possess a hydrophobic region, which is not cleaved off during maturation. That is why we suppose that this region is not a genuine secretory signal.

Table 1.
Potential transmembrane regions in Mid1 homologs.
Previously we have shown that the loss of Cch1 suppresses SDS hypersensitivity of O. polymorpha and O. parapolymorpha mutants defective in the vacuolar Ca2+ ATPase Pmc1 [,]. To determine whether the loss of Mid1 can cause the same effect, the MID1 gene was inactivated in the O. parapolymorpha pmc1-Δ strain. Indeed, the pmc1-Δ mid1-Δ double mutant was able to grow at higher SDS concentrations than the original pmc1-Δ strain. Notably, as with inactivation of CCH1, inactivation of MID1 suppressed SDS sensitivity not only in the pmc1-Δ mutant but also in the strain with wild-type PMC1 and caused the inability to grow on the Ca2+-deficient medium supplemented with ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetate (EGTA) as a Ca2+ chelator (Figure 1a,b).
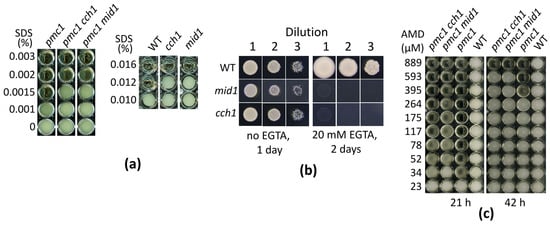
Figure 1.
Ability of O. parapolymorpha mutants to grow in liquid YPD medium in the presence of SDS (a), amiodarone (c), or on the solid Ca2+-deficient medium (b). WT, DL5-LC strain; cch1, DL5-cch1 strain; mid1, DL5-mid1 strain; pmc1, DL5-pmc1-LC strain; pmc1 cch1, DL5-pmc1-cch1 strain, cch1 mid1, DL5-cch1-mid1 strain. Dilutions 1, 2 and 3: 3 μL of 300-, 3000- and 30,000-fold diluted overnight YPD cultures, respectively, were spotted onto plates with the Ca2+-deficient medium with or without EGTA and incubated at 37 °C.
2.2. Effects of Inactivation of MID1 and CCH1 on the SDS-Induced [Ca2+]cyt Rise in the pmc1-Δ Mutant Are the Same
Since we did not observe a noticeable difference between the ability of deletions of MID1 and CCH1 to suppress SDS sensitivity of the pmc1-Δ mutant, we reasoned that the effects of these mutations on the [Ca2+]cyt rise in response to SDS in the pmc1-Δ would also be the same. To study this, we used the genetically encoded Ca2+ indicator GEM-GECO [], which enables monitoring [Ca2+]cyt in individual yeast cells using flow cytometry by measuring the fluorescence ratio at 450 nm and 525 nm (FL450/FL525) []. Indeed, we observed very similar dynamics of the [Ca2+]cyt rise in response to SDS in the pmc1-Δ cch1-Δ and pmc1-Δ mid1-Δ mutants, which was less steep than that in the pmc1-Δ single mutant (Figure 2).
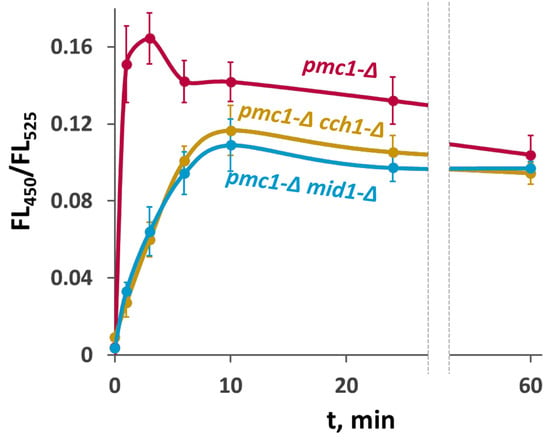
Figure 2.
Comparison of effects of inactivation of CCH1 and MID1 in the strain lacking PMC1 on [Ca2+]cyt dynamics in response to 0.004% SDS. Changes in [Ca2+]cyt were monitored using the GEM-GECO Ca2+ indicator by measuring FL450/FL525 values in individual cells. The median values of 104 cells obtained from 3 replicates were averaged, and standard deviations were calculated.
2.3. Amiodarone Causes a Rapid Increase in [Ca2+]cyt and Hog1 Activation in O. parapolymorpha
According to the previous studies performed in S. cerevisiae using aequorin to monitor [Ca2+]cyt [,], amiodarone causes an increase in [Ca2+]cyt. Here, we studied the effects of this drug on [Ca2+]cyt in O. parapolymorpha using the GEM-GECO Ca2+ indicator. Similar to that in S. cerevisiae, amiodarone induced a [Ca2+]cyt rise in O. parapolymorpha. This was essentially abolished by the presence of EGTA or ethylenediaminetetraacetate (EDTA) as a chelator in culture medium (Figure 3), indicating that Ca2+ is transported from the environment.
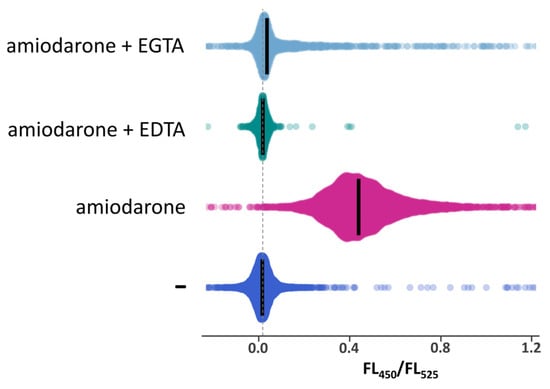
Figure 3.
Distribution of cells according to FL450/FL525 in exponentially grown cultures of the DL5-LC strain, which has the wild-type Ca2+ transport system, before (−) or after 10′-incubation with 30 μM amiodarone in the absence or the presence of 10 mM EDTA or 20 mM EGTA. Median values are indicated by bars.
Similarly to that observed for the SDS treatment [], the [Ca2+]cyt rise in response to amiodarone treatment was accompanied by activation of the Hog1 protein kinase. Notably, the effect of amiodarone lasted longer than that of SDS but still was not as intensive and prolonged as in case of Hog1 activation in response to high osmolality of NaCl (Figure 4). This indicates that, similar to sensitivity to SDS [], sensitivity to amiodarone can be mediated by the Hog1 protein kinase.
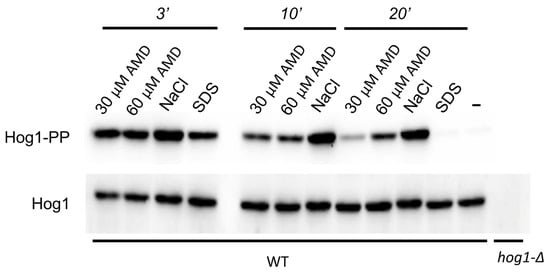
Figure 4.
Immunoblotting of phosphorylated (Hog1-PP) and total Hog1 (Hog1) in cell lysates. Exponentially growing culture of the O. parapolymorpha DL5-LC strain (WT) was supplemented either with 30 μM amiodarone (30 μM AMD), 60 μM amiodarone (60 μM AMD), 0.6 M NaCl (NaCl), or 0.004% SDS (SDS) and incubated for 3, 10 and 20 min except for samples with SDS, which were incubated for 3 and 20 min. Untreated cells (−) represent the basal level of Hog1 phosphorylation. Cells of the untreated hog1-Δ mutant (hog1-Δ) were used as a negative control.
2.4. Effects of the Loss of HACS in the Strain with Wild-Type PMC1 and in the pmc1-Δ Mutant on Survival Rate on Amiodarone-Containing Medium Are Opposite
It was reasonable to expect that, similar to that observed in S. cerevisiae [], the amiodarone-induced [Ca2+]cyt rise in O. parapolymorpha cells may affect growth rate and cause cell death. Indeed, when dilutions of cell suspensions were spotted onto YPD supplemented with amiodarone concentrations ranging from 0.1 to 1 mM, some growth attenuation was observed at the 0.1 mM concentration of this drug, but the number of colonies on the spots was similar to that on the control plate (Figure S2). At higher amiodarone concentrations, a successive decrease in growth rate as well as some reduction in the number of colonies was observed; however, colonies emerged even with 1 mM amiodarone (Figure S2). This means that only some fractions of cells were unable to form colonies on the amiodarone-containing medium. To determine whether the proportion of cells able to survive in the presence of amiodarone depends on the HACS, exponentially growing cultures of the mid1-Δ and cch1-Δ mutants, as well as the wild-type control strain, were spread on YPD plated with and without amiodarone to count the numbers of colony-forming units as described in Materials and Methods. Inactivation of MID1 or CCH1 decreased the survival rate (Figure 5a); however, the attenuation of the colony growth on the medium supplemented with amiodarone was very similar in the mutants and wild-type control strain (Figure S3).
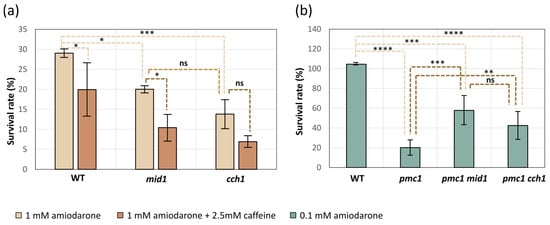
Figure 5.
Effects of the mid1-Δ and cch1-Δ mutations on survival rate on medium containing 1 mM amiodarone or 1 mM amiodarone and 2.5 mM caffeine in strains with wild-type PMC1 (a) and on medium containing 0.1 mM amiodarone in strains with the inactivated PMC1 (b). WT, cch1, mid1, pmc1, pmc1 mid1 and pmc1 cch1: DL5-LC, DL5-mid1, DL5-cch1, DL5-pmc1-LC, DL5-pmc1-mid1 and DL5-pmc1-cch1 strains, respectively. Data represent mean ± SD from at least 3 independent experiments. Significance was determined by two-way ANOVA with Tukey’s multiple comparisons test (* p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001; ns, not significant).
Notably, YPD supplemented with more than 0.5 mM amiodarone becomes visibly turbid, indicating a colloid state of amiodarone, which tended to precipitate onto the well surface after a while if the experiment was performed in a 96-well plate in liquid medium. That was why we could not reach the amiodarone concentration, which prevented growth of cells with the wild-type Ca2+ transport system since those cells were able to grow in liquid YPD containing more than 0.5 mM amiodarone. The lack of the Pmc1 vacuolar Ca2+ ATPase significantly decreased resistance to amiodarone, while the loss of Cch1 or Mid1 suppressed this amiodarone hypersensitivity (Figure 1c and Figure 5b).
2.5. Caffeine Does Not Alleviate Effects of Amiodarone on Cell Growth and Has a Negligible Effect on the Amiodarone-Induced [Ca2+]cyt Rise
It was observed in a previous study [], which employed aequorin to monitor Ca2+ concentration in S. cerevisiae, that the [Ca2+]cyt rise in response to amiodarone is strongly inhibited in the presence of caffeine. In contrast to those data, the use of the GEM-GECO Ca2+ indicator revealed only a slight decrease in the FL450/FL525 ratio when cells were treated with amiodarone in the presence of caffeine (Figure 6), which could be due to some unrelated effect of caffeine on cell physiology. It was reasonable to expect that caffeine should rescue cells from amiodarone action if it inhibits the amiodarone-induced Ca2+ uptake. However, the presence of caffeine did not improve the survival or colony growth rate on amiodarone-containing medium in either O. polymorpha or S. cerevisiae (Figure 5a, Figures S3 and S4). This indicates that the amiodarone action is not inhibited by caffeine.
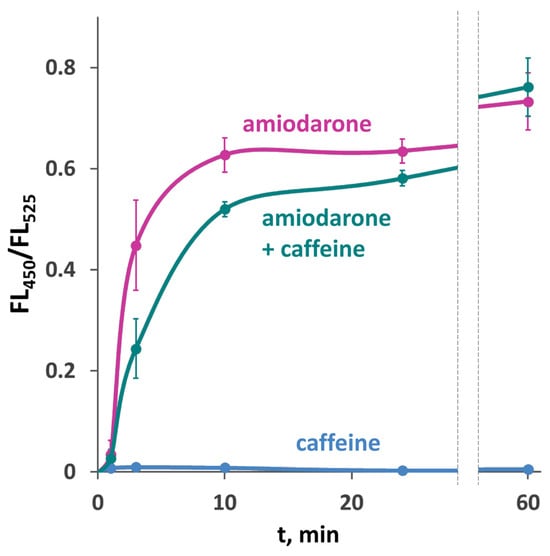
Figure 6.
Effect of 10 mM caffeine on [Ca2+]cyt rise in response to 30 μM amiodarone in the DL5-LC strain with the wild-type Ca2+ transport system. Changes in [Ca2+]cyt were monitored using the GEM-GECO Ca2+ indicator by measuring FL450/FL525 values in individual cells. The median values of 104 cells obtained from 3 replicates were averaged, and standard deviations were calculated.
2.6. Deletion Mutations of MID1 and CCH1 Delay the Amiodarone-Induced Ca2+ Influx
To explore whether HACS is involved in the amiodarone-induced Ca2+ entry, the FL450/FL525 ratio was determined in strains expressing the GEM-GECO indicator. Notably, the rapid [Ca2+]cyt rise in response to amiodarone was not immediate. In the strain with the wild-type Ca2+ metabolism, it was observed after 1–2 min of incubation with amiodarone and continued for more than 10 min. Inactivation of MID1 or CCH1 led to an additional delay in the amiodarone-induced [Ca2+]cyt rise lasting approximately 3–5 min, but then [Ca2+]cyt started rising, reaching similar levels to the wild-type strain (Figure 7a).
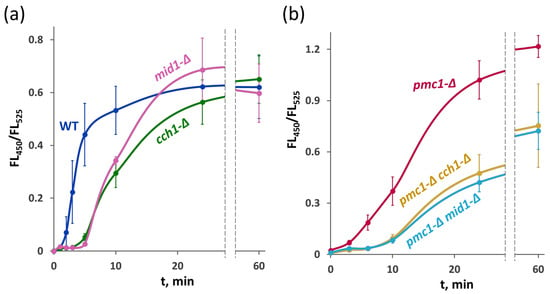
Figure 7.
[Ca2+]cyt rise in response to 30 μM amiodarone in the strains with the wild-type cytosolic Ca2+ sequestration system (a) and in the strains lacking the Pmc1 Ca2+ ATPase (b). Changes in [Ca2+]cyt were monitored using the GEM-GECO Ca2+ indicator by measuring FL450/FL525 values in individual cells. The median values of 104 cells obtained from 3 replicates were averaged, and standard deviations were calculated.
In the strain lacking the Pmc1 vacuolar Ca2+ ATPase, the initial [Ca2+]cyt rise was not as steep as in the wild-type strain, but then it sped up and after prolonged incubation reached higher levels than in the wild-type strain (Figure 7b), apparently due to the inability to efficiently sequester cytosolic Ca2+ in the vacuole. Inactivation of either MID1 or CCH1 in the pmc1-Δ mutant increased the delay in the [Ca2+]cyt rise, and [Ca2+]cyt reached a noticeably lower level after prolonged incubation (Figure 7b). This is in agreement with the observation that the loss of HACS components suppresses pmc1-Δ hypersensitivity to amiodarone (Figure 1c and Figure 5b).
3. Discussion
As mentioned in the Introduction, the yeast plasma membrane high-affinity Ca2+ ion channel consists of two subunits, namely, Cch1 and Mid1. While the Cch1 predicted topology [] and its homology to mammalian voltage-gated Ca2+ channels [] indicate that it is the channel-forming subunit, the exact role of Mid1 in the complex remains unclear. Some published data indicate that the Mid1 subunit of the Cch1/Mid1 Ca2+ channel may have a function outside of this complex. Particularly, production of S. cerevisiae Mid1 alone in animal cells conferred sensitivity to mechanical stress []. The lack of Mid1, but not Cch1, affected the [Ca2+]cyt rise in response to amiodarone in S. cerevisiae, while cell growth in the presence of amiodarone was improved by the loss of Cch1, but not Mid1 []. The effects of amiodarone on [Ca2+]cyt in S. cerevisiae were studied using the Ca2+-dependent luciferase aequorin [,]. However, this approach does not allow [Ca2+]cyt to be monitored in single cells. Previously [], we have shown that the GEM-GECO Ca2+ indicator [] can be used to monitor [Ca2+]cyt in single cells of the O. parapolymorpha yeast, and this was applied here to study the role of these HACS components in reaction of yeast cells to amiodarone. Similar to previous observations made in S. cerevisiae, we have observed a substantial [Ca2+]cyt rise in response to amiodarone in O. parapolymorpha; however, in contrast to that observed in S. cerevisiae [], it did not depend on caffeine. There is strong evidence that the direct target of caffeine in yeast is Tor1 protein kinase [,], and how this compound can affect the Ca2+ transport system is unclear. We do not think that HACS reacts differently to caffeine in these two yeast species. Indeed, caffeine had the same effect on cell growth in both yeast species in our experiments. Caffeine interfering with Ca2+ detection by aequorin may have caused this discrepancy; however, this needs to be verified.
The effects of the loss of Cch1 and Mid1 in O. parapolymorpha were very similar: these mutations similarly affected the dynamics of the [Ca2+]cyt rise in the response to amiodarone and SDS, improved SDS resistance and slightly decreased survival in the presence of amiodarone. This indicates that these effects are related to the function of the Cch1/Mid1 complex but not to hypothetical functions of Cch1 or Mid1 outside the complex even if such functions exist.
In the strain with the wild-type Ca2+ transport system, amiodarone induced a rapid [Ca2+]cyt rise, which started within the first two minutes of incubation and continued for more than 10 min until it was probably compensated by the action of ion transporters sequestering Ca2+ in the vacuole. The loss of the HACS components caused some delay in the [Ca2+]cyt rise that might lead to suggestion that amiodarone induces Ca2+ entry via the Cch1/Mid1 channel, while the following [Ca2+]cyt increase is mediated by an alternative pathway. However this raises a question: why does this alternative pathway not work at the beginning? A more consistent explanation is that the pathway of Ca2+ entry in response to amiodarone can be recruited by Cch1/Mid1 and by amiodarone itself, but this recruitment takes some time, causing the delay observed in the mutants lacking Cch1 and Mid1. Notably, the loss of the HACS components did not improve survival rate on amiodarone-containing medium, indicating that the survival does not depend on the delay in the amiodarone-induced [Ca2+]cyt rise.
The survival in the presence of amiodarone may involve Hog1-dependent cell cycle regulation as described for SDS hypersensitivity in the pmc1-Δ mutant [] since both SDS and amiodarone induced a [Ca2+]cyt rise accompanied with Hog1 activation. At the same time the primary cellular target of amiodarone can be different from that of SDS since SDS induces the immediate [Ca2+]cyt rise, while the [Ca2+]cyt rise in response to amiodarone starts after some delay.
In yeast, the homolog of mammalian p38 protein kinase Hog1 is implicated into adaptation to high osmolarity conditions, being activated in response to high osmolarity shock (for a review see []). It has also been shown that Hog1 is activated in response to caffeine []. One of the consequences of its activation is stabilization of the Wee1 protein kinase, which inhibits the G2/M cell-cycle transition [,]. Thus, both amiodarone and caffeine can cause Hog1 activation but via different pathways. This is probably why caffeine aggravates the inhibitory effect of amiodarone on cell growth (Figures S3 and S4).
The delay in the [Ca2+]cyt rise lasted longer in the mutant lacking the Pmc1 Ca2+ ATPase than in the strain with the wild-type Ca2+ transport system, while the loss of Cch1 or Mid1 additionally extended it. As we observed previously, the basal [Ca2+]cyt level is increased in the pmc1-Δ mutant [] that hypothetically may suppress Ca2+ uptake systems, thus resulting in the delayed response to amiodarone. After prolonged incubation with amiodarone, the [Ca2+]cyt level in the pmc1-Δ cch1-Δ and pmc1-Δ mid1-Δ mutants was substantially lower than in the pmc1-Δ mutant. This correlated with suppression of the pmc1-Δ amiodarone hypersensitivity. This might indicate that HACS is directly involved in the amiodarone-induced Ca2+ entry; however, in terms of the “indirect involvement” hypothesis expressed above, this occurs via an alternative pathway, whose activity depends on the HACS components. The less steep [Ca2+]cyt rise in the strains lacking Pmc1 Ca2+ ATPase can be explained by repression of this alternative pathway due to higher basal [Ca2+]cyt. This suggestion is consistent with the previous observation that increased external Ca2+ concentration reduces the amiodarone-induced [Ca2+]cyt burst and rescues cells from amiodarone toxicity in S. cerevisiae [].
Thus, here we have characterized the O. parapolymorpha MID1 gene and shown that its inactivation leads to the same phenotypes as inactivation of CCH1 encoding the channel-forming subunit of the Mid1/Cch1 complex. The effects of inactivation of these genes on the amiodarone-induced [Ca2+]cyt rise were also very similar, which contradicts the previously published results obtained in S. cerevisiae []. Our data suggest that the Mid1/Cch1 complex is not the conduit for Ca2+ entry in response to amiodarone but is involved in the regulation of this process.
4. Materials and Methods
4.1. Culture Media and Yeast Transformation
Complex media contained 2% Peptone, 1% yeast extract and 2% glucose (YPD) or 1% sucrose (YP-Suc) as a carbon source. The synthetic medium SC-D (2% glucose, 0.67% Yeast Nitrogen Base with ammonium sulfate) was used for the selection of transformants. The Ca2+-deficient medium was prepared as described previously []. Yeast cells were transformed using the Li-acetate method [] with some modifications [].
4.2. Yeast Strains
The O. parapolymorpha strains used in this study are listed in Table 2. The process of obtaining these strains except DL5-mid1 bearing the MID1 deletion allele was described previously []. The MID1 deletion was performed as follows. The DNA fragment with inverted recombination arms was obtained by PCR with OpMID1U1 and OpMID1L1 primers using HindIII-digested and self-ligated O. parapolymorpha genomic DNA as a template. This fragment was digested with Bsp1407 and inserted between Bsp1407 and PvuII sites of the pAM576 vector. This vector was obtained by replacement of the wild-type S. cerevisiae LEU2 selectable marker in the AMIpSL1 vector [] for the modified one described previously []. The obtained plasmid pMK4 was digested with HindIII that resulted in a linear construct possessing vector sequence flanked by the recombination arms according to the scheme described previously []. Integration of this construct into the MID1 locus was confirmed by PCR with primer pairs OpMID1AU1-oriA and OpMID1AL1-SL3. Sequences of PCR primers are presented in Table S1.

Table 2.
O. parapolymorpha strains used in this study.
Besides O. parapolymorpha, S. cerevisiae strain BY4742 and its cch1-Δ and mid1-Δ derivatives [] were used in this study.
4.3. Monitoring of the Cytosolic Ca2+ Concentration
The GEM-GECO genetically encoded Ca2+ indicator [] was used to monitor [Ca2+]cyt in individual O. parapolymorpha cells using flow cytometry as described previously [,].
4.4. Growth Inhibition and Cell Survival Assays
To determine minimum inhibitory concentrations of SDS and amiodarone, logarithmic-phase cultures were diluted to an OD600 of 0.05 in medium containing serial dilutions of each compound in 96-well plates. The plates were incubated at 37 °C in a shaker incubator, and yeast growth was assessed visually by culture turbidity after 20–24 h incubation for SDS or 20–24 and 44–48 h incubation for amiodarone.
To assess the survival rates on amiodarone-containing medium, logarithmic cultures at OD600 0.8–1.0 were spread onto YPD plates with or without amiodarone. Prior to the plating, the cultures were properly diluted to obtain 500–1000 colonies per plate. Plates were incubated at 37 °C, and yeast colonies were counted after 24 h on the control plates or after 48 h on the amiodarone-containing plates. The survival rate was calculated as a ratio of colony numbers on plates with and without amiodarone with respect to the dilution factor.
4.5. Analysis of Hog1 Phosphorylation
The alterations in Hog1 phosphorylation were assayed by immunoblotting using anti Phospho-p38 monoclonal antibody (Cell Signaling Technology, Inc., Danvers, MA, USA, cat. #9215). Total Hog1 content was assayed by immunoblotting using polyclonal rabbit antisera against Escherichia coli-expressed O. polymorpha Hog1. A more detailed protocol of the analysis of Hog1 phosphorylation was described previously [].
4.6. Statistical Analysis
Statistical analysis was conducted using GraphPad Prism 8.0.1. Data are presented as mean ± SD from at least three independent replicates. Two-way analysis of variance (ANOVA) followed by Tukey’s Honestly Significant Difference (HSD) post hoc test was used for multiple comparisons.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262311386/s1.
Author Contributions
Conceptualization, M.A. and M.K.; methodology, M.K. and V.B.; formal analysis, M.A., M.K. and V.B.; investigation, M.K., V.B. and M.P.; writing—original draft preparation, M.A.; writing—review and editing, M.A. and V.B.; supervision, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Ministry of Science and Higher Education of the Russian Federation (Federal Scientific and Technical Program for the Development of Genetic Technologies for 2019–2030, agreement #075-15-2025-470 dated 29 May 2025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pittman, J.K. Vacuolar Ca2+ Uptake. Cell Calcium 2011, 50, 139–146. [Google Scholar] [CrossRef]
- Dürr, G.; Strayle, J.; Plemper, R.; Elbs, S.; Klee, S.K.; Catty, P.; Wolf, D.H.; Rudolph, H.K. The Medial-Golgi Ion Pump Pmr1 Supplies the Yeast Secretory Pathway with Ca2+ and Mn2+ Required for Glycosylation, Sorting, and Endoplasmic Reticulum-Associated Protein Degradation. Mol. Biol. Cell 1998, 9, 1149–1162. [Google Scholar] [CrossRef]
- Fokina, A.V.; Chechenova, M.B.; Karginov, A.V.; Ter-Avanesyan, M.D.; Agaphonov, M.O. Genetic Evidence for the Role of the Vacuole in Supplying Secretory Organelles with Ca2+ in Hansenula Polymorpha. PLoS ONE 2015, 10, e0145915. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.; Frank, C.G.; Jakob, C.A.; Ng, D.T.W. Two Distinctly Localized P-Type ATPases Collaborate to Maintain Organelle Homeostasis Required for Glycoprotein Processing and Quality Control. Mol. Biol. Cell 2002, 13, 3955–3966. [Google Scholar] [CrossRef] [PubMed]
- Strayle, J.; Pozzan, T.; Rudolph, H.K. Steady-State Free Ca2+ in the Yeast Endoplasmic Reticulum Reaches Only 10 MicroM and Is Mainly Controlled by the Secretory Pathway Pump Pmr1. EMBO J. 1999, 18, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.M.; Locke, E.G.; Cunningham, K.W.; Muller, E.M.; Locke, E.G.; Cunningham, K.W. Differential Regulation of Two Ca2+ Influx Systems by Pheromone Signaling in Saccharomyces Cerevisiae. Genetics 2001, 159, 1527–1538. [Google Scholar] [CrossRef]
- Muller, E.M.; Mackin, N.A.; Erdman, S.E.; Cunningham, K.W. Fig1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 38461–38469. [Google Scholar] [CrossRef]
- Paidhungat, M.; Garrett, S. A Homolog of Mammalian, Voltage-Gated Calcium Channels Mediates Yeast Pheromone-Stimulated Ca2+ Uptake and Exacerbates the Cdc1(Ts) Growth Defect. Mol. Cell. Biol. 1997, 17, 6339–6347. [Google Scholar] [CrossRef]
- Ghezzi, A.; Liebeskind, B.J.; Thompson, A.; Atkinson, N.S.; Zakon, H.H. Ancient Association between Cation Leak Channels and Mid1 Proteins Is Conserved in Fungi and Animals. Front. Mol. Neurosci. 2014, 7, 15. [Google Scholar] [CrossRef]
- Martin, D.C.; Kim, H.; Mackin, N.A.; Maldonado-Báez, L.; Evangelista, C.C.; Beaudry, V.G.; Dudgeon, D.D.; Naiman, D.Q.; Erdman, S.E.; Cunningham, K.W. New Regulators of a High Affinity Ca2+ Influx System Revealed through a Genome-Wide Screen in Yeast. J. Biol. Chem. 2011, 286, 10744–10754. [Google Scholar] [CrossRef]
- Courchesne, W.E. Characterization of a Novel, Broad-Based Fungicidal Activity for the Antiarrhythmic Drug Amiodarone. J. Pharmacol. Exp. Ther. 2002, 300, 195–199. [Google Scholar] [CrossRef]
- Muend, S.; Rao, R. Fungicidal Activity of Amiodarone Is Tightly Coupled to Calcium Influx. FEMS Yeast Res. 2008, 8, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, W.E.; Ozturk, S. Amiodarone Induces a Caffeine-inhibited, MID1 -depedent Rise in Free Cytoplasmic Calcium in Saccharomyces Cerevisiae. Mol. Microbiol. 2003, 47, 223–234. [Google Scholar] [CrossRef]
- Gupta, S.S.; Ton, V.-K.; Beaudry, V.; Rulli, S.; Cunningham, K.; Rao, R. Antifungal Activity of Amiodarone Is Mediated by Disruption of Calcium Homeostasis. J. Biol. Chem. 2003, 278, 28831–28839. [Google Scholar] [CrossRef]
- Pozniakovsky, A.I.; Knorre, D.A.; Markova, O.V.; Hyman, A.A.; Skulachev, V.P.; Severin, F.F. Role of Mitochondria in the Pheromone- and Amiodarone-Induced Programmed Death of Yeast. J. Cell Biol. 2005, 168, 257–269. [Google Scholar] [CrossRef]
- Peiter, E.; Fischer, M.; Sidaway, K.; Roberts, S.K.; Sanders, D. The Saccharomyces Cerevisiae Ca2+ Channel Cch1pMid1p Is Essential for Tolerance to Cold Stress and Iron Toxicity. FEBS Lett. 2005, 579, 5697–5703. [Google Scholar] [CrossRef]
- Gelman, I.; Sharma, N.; Mckeeman, O.; Lee, P.; Campagna, N.; Tomei, N.; Baranchuk, A.; Zhang, S.; El-Diasty, M. The Ion Channel Basis of Pharmacological Effects of Amiodarone on Myocardial Electrophysiological Properties, a Comprehensive Review. Biomed. Pharmacother. 2024, 174, 116513. [Google Scholar] [CrossRef] [PubMed]
- Fokina, A.V.; Sokolov, S.S.; Kang, H.A.; Kalebina, T.S.; Ter-Avanesyan, M.D.; Agaphonov, M.O. Inactivation of Pmc1 Vacuolar Ca2+ ATPase Causes G2 Cell Cycle Delay in Hansenula Polymorpha. Cell Cycle 2012, 11, 778–784. [Google Scholar] [CrossRef]
- Kulakova, M.; Pakhomova, M.; Bidiuk, V.; Ershov, A.; Alexandrov, A.; Agaphonov, M. High-Affinity Plasma Membrane Ca2+ Channel Cch1 Modulates Adaptation to Sodium Dodecyl Sulfate-Triggered Rise in Cytosolic Ca2+ Concentration in Ogataea Parapolymorpha. Int. J. Mol. Sci. 2024, 25, 11450. [Google Scholar] [CrossRef] [PubMed]
- Cyert, M.S. Calcineurin Signaling in Saccharomyces Cerevisiae: How Yeast Go Crazy in Response to Stress. Biochem. Biophys. Res. Commun. 2003, 311, 1143–1150. [Google Scholar] [CrossRef]
- Goldman, A.; Roy, J.; Bodenmiller, B.; Wanka, S.; Landry, C.R.; Aebersold, R.; Cyert, M.S. The Calcineurin Signaling Network Evolves via Conserved Kinase-Phosphatase Modules That Transcend Substrate Identity. Mol. Cell 2014, 55, 422–435. [Google Scholar] [CrossRef]
- Kaminska, J.; Soczewka, P.; Rzepnikowska, W.; Zoladek, T. Yeast as a Model to Find New Drugs and Drug Targets for VPS13-Dependent Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5106. [Google Scholar] [CrossRef] [PubMed]
- Soczewka, P.; Kolakowski, D.; Smaczynska-de Rooij, I.; Rzepnikowska, W.; Ayscough, K.R.; Kaminska, J.; Zoladek, T. Yeast-Model-Based Study Identified Myosin- and Calcium-Dependent Calmodulin Signalling as a Potential Target for Drug Intervention in Chorea-Acanthocytosis. Dis. Model. Mech. 2019, 12, dmm036830. [Google Scholar] [CrossRef]
- Ravin, N.V.; Eldarov, M.A.; Kadnikov, V.V.; Beletsky, A.V.; Schneider, J.; Mardanova, E.S.; Smekalova, E.M.; Zvereva, M.I.; Dontsova, O.A.; Mardanov, A.V.; et al. Genome Sequence and Analysis of Methylotrophic Yeast Hansenula Polymorpha DL1. BMC Genom. 2013, 14, 837. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Teng, J.; Cho, T.; Yoshikawa-Kimura, S.; Iida, H. Post-Translational Processing and Membrane Translocation of the Yeast Regulatory Mid1 Subunit of the Cch1/VGCC/NALCN Cation Channel Family. J. Biol. Chem. 2017, 292, 20570–20582. [Google Scholar] [CrossRef]
- Kulakova, M.V.; Karginov, A.V.; Alexandrov, A.I.; Agaphonov, M.O. The GEM-GECO Calcium Indicator Is Useable in Ogataea Parapolymorpha Yeast, but Aggravates Effects of Increased Cytosolic Calcium Levels. Int. J. Mol. Sci. 2022, 23, 10004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.-F.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An Expanded Palette of Genetically Encoded Ca2+ Indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef]
- Volkova, M.; Atamas, A.; Tsarenko, A.; Rogachev, A.; Guskov, A. Cation Transporters of Candida Albicans—New Targets to Fight Candidiasis? Biomolecules 2021, 11, 584. [Google Scholar] [CrossRef]
- Kanzaki, M.; Nagasawa, M.; Kojima, I.; Sato, C.; Naruse, K.; Sokabe, M.; Iida, H. Molecular Identification of a Eukaryotic, Stretch-Activated Nonselective Cation Channel. Science 1999, 285, 882–886, Erratum in Science 1999, 285, 1493. https://doi.org/10.1126/science.288.5470.1347. [Google Scholar] [CrossRef]
- Kuranda, K.; Leberre, V.; Sokol, S.; Palamarczyk, G.; François, J. Investigating the Caffeine Effects in the Yeast Saccharomyces Cerevisiae Brings New Insights into the Connection between TOR, PKC and Ras/CAMP Signalling Pathways. Mol. Microbiol. 2006, 61, 1147–1166. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; Li, G.; Wang, X. TORC1 Signaling in Fungi: From Yeasts to Filamentous Fungi. Microorganisms 2023, 11, 218. [Google Scholar] [CrossRef]
- Hohmann, S.; Krantz, M.; Nordlander, B. Yeast Osmoregulation. Methods Enzymol. 2007, 428, 29–45. [Google Scholar] [CrossRef]
- Elhasi, T.; Blomberg, A. Caffeine Activates HOG-Signalling and Inhibits Pseudohyphal Growth in Saccharomyces Cerevisiae. BMC Res. Notes 2023, 16, 52. [Google Scholar] [CrossRef]
- Clotet, J.; Escoté, X.; Adrover, M.A.; Yaakov, G.; Garí, E.; Aldea, M.; de Nadal, E.; Posas, F. Phosphorylation of Hsl1 by Hog1 Leads to a G2 Arrest Essential for Cell Survival at High Osmolarity. EMBO J. 2006, 25, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.; Park, J.; Sakchaisri, K.; Yu, L.; Song, S.; Supavilai, P.; Veenstra, T.D.; Lee, K.S. Concerted Mechanism of Swe1/Wee1 Regulation by Multiple Kinases in Budding Yeast. EMBO J. 2005, 24, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Schiestl, R.H. Quick and Easy Yeast Transformation Using the LiAc/SS Carrier DNA/PEG Method. Nat. Protoc. 2007, 2, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Karginov, A.V.; Alexandrov, A.I.; Kushnirov, V.V.; Agaphonov, M.O. Perturbations in the Heme and Siroheme Biosynthesis Pathways Causing Accumulation of Fluorescent Free Base Porphyrins and Auxotrophy in Ogataea Yeasts. J. Fungi 2021, 7, 884. [Google Scholar] [CrossRef]
- Agaphonov, M.O.; Trushkina, P.M.; Sohn, J.H.; Choi, E.S.; Rhee, S.K.; Ter-Avanesyan, M.D. Vectors for Rapid Selection of Integrants with Different Plasmid Copy Numbers in the Yeast Hansenula Polymorpha DL1. Yeast 1999, 15, 541–551. [Google Scholar] [CrossRef]
- Agaphonov, M.; Alexandrov, A. Self-Excising Integrative Yeast Plasmid Vectors Containing an Intronated Recombinase Gene. FEMS Yeast Res. 2014, 14, 1048–1054. [Google Scholar] [CrossRef]
- Agaphonov, M.; Romanova, N.; Choi, E.-S.; Ter-Avanesyan, M. A Novel Kanamycin/G418 Resistance Marker for Direct Selection of Transformants in Escherichia Coli and Different Yeast Species. Yeast 2010, 27, 189–195. [Google Scholar] [CrossRef]
- Giaever, G.; Chu, A.M.; Ni, L.; Connelly, C.; Riles, L.; Véronneau, S.; Dow, S.; Lucau-Danila, A.; Anderson, K.; André, B.; et al. Functional Profiling of the Saccharomyces Cerevisiae Genome. Nature 2002, 418, 387–391. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).