Abstract
Plants have the capacity to form adventitious roots (ARs) from detached aerial organs, a process known as de novo root regeneration (DNRR). In Arabidopsis, wounding signals rapidly induce in leaf explants the expression of genes encoding enzymes of auxin biosynthesis, resulting in elevated auxin levels and facilitating AR formation. Here, we report that DEVELOPMENT-RELATED POLYCOMB TARGET IN THE APEX 4 (DPA4/NGAL3), a well-known regulator in seed size and leaf margin development, and a repressor of CUP-SHAPED COTYLEDON 2 (CUC2), inhibits AR formation in detached leaves. Leaf explants of dpa4-2 and cuc2-1D mutants displayed both elevated CUC2 mRNA levels and increased rooting rates. We observed reduced expression of ULTRAPETALA1 (ULT1), a negative regulator of DNRR, while the auxin biosynthesis genes ASA1, YUC4, and YUC9 were upregulated in both mutants. Through pharmacological inhibition of YUCCA-mediated auxin biogenesis, we obtained evidence that the enhanced AR formation in both mutants is at least partially a result of increased auxin production. Genetic analysis of dpa4-2 cuc2-1D double mutants indicates that similar mechanisms promote DNRR in both mutants. In summary, our study suggests that DPA4 suppresses AR formation likely by repression of CUC2 and activation of ULT1, which, in turn, suppresses endogenous auxin biogenesis and DNRR.
1. Introduction
Plant cells exhibit remarkable developmental plasticity, enabling regeneration of new organs and even an entire plant body after suffering wounds [,]. The potential capacity of plants for de novo organogenesis finds wide-ranging applications in agriculture, biotechnology, and biological studies, including tissue culture and vegetative propagation via cuttings and explants [,]. Among various forms of plant regeneration, de novo root regeneration (DNRR) from wounded and/or detached plant tissues and organs is commonly utilized in biotechnological breeding and cultivation research [,]. In contrast to de novo shoot regeneration that requires a proper ratio of exogenous auxin to cytokinin [,], DNRR from Arabidopsis leaf explants can take place on B5 medium without exogenous phytohormones [,].
Upon injury, Arabidopsis leaf explants sense a multitude of signals, including wound-derived signals and other stress-related and environmental stimuli, factors triggering endogenous developmental programs [,,,]. These signals direct the production and transport of auxin towards the future regeneration-competent cells near the wound site, which facilitates adventitious root (AR) formation [,]. Once the leaf is detached, the wounding signal and phytohormone jasmonate (JA) promptly trigger auxin biosynthesis [,]. Therefore, JA signaling activates the expression of ETHYLENE RESPONSE FACTOR 109 (ERF109) and ABSCISIC ACID REPRESSOR 1 (ABR1/ERF111) [,]. The transcriptional activators ERF109 and ABR1 serve as a molecular connection between wounding, JA signaling, and auxin biosynthesis by direct activation of ANTHRANILATE SYNTHASE ALPHA SUBUNIT 1 (ASA1) [,]. ASA1 catalyzes the rate-limiting step in tryptophan (Trp) biosynthesis, which provides the substrate for auxin biosynthesis. Via the conversion of Trp to indole-3-pyruvate (IPA) by the TAA amino transferases, YUCCA (YUC) flavin-containing monooxygenases convert IPA to auxin indole-3-acetic acid (IAA), one of the major natural auxins [,,]. The newly synthesized auxin is polar-transported to the future rooting site adjacent to the wounding site []. The emerging auxin maximum induces the expression of WUSCHEL-RELATED HOMEOBOX 11 (WOX11)/WOX12, which facilitates the cell fate transition from regeneration-competent vasculature-associated pluripotent cells (VPCs) to root founder cells [,,]. Both WOX11/12 and auxin are required to promote the expression of LATERAL ORGAN BOUNDARIES DOMAIN 16 (LBD16) and WOX5, which facilitates the transformation of the root founder cells into root primordia [,,]. Disrupting either auxin biosynthesis or transport prevents the establishment of the auxin maxima in the VPCs and, in turn, AR formation [].
In Arabidopsis, CUP-SHAPED COTYLEDON2 (CUC2) encodes an NAC domain transcription factor that is a key regulator that establishes organ boundaries and participates in multiple developmental pathways []. In leaves, CUC2 is specifically targeted by MIR164A, which triggers the cleavage of the CUC2 mRNA, while cuc2-1D, which carries a point mutation in the MIR164A-targeting site, and mir164a-4 mutants display increased CUC2 expression [,]. During leaf margin patterning, CUC2 establishes auxin activity maxima by directing PIN1 localization at the leaf margin, thereby shaping serrated leaf morphology [,]. Furthermore, CUC2 restricts leaf expansion primarily through modulating cell proliferation rather than influencing cell expansion [].
Notably, CUC2 expression is indispensable for de novo shoot organogenesis from callus tissue [,,] and serves as a molecular indicator of regenerative potential in root-derived explants []. Collectively, these findings establish CUC2 as a key regulator of auxin distribution patterns. Since auxin is essential for AR formation [], it is a reasonable question whether CUC2 has a function in DNRR from leaf explants.
The B3 transcription factor DEVELOPMENT-RELATED POLYCOMB TARGET IN THE APEX4 (DPA4/NGAL3), belonging to the RAV (Related ABI3/VP1) family, has been implicated in multiple developmental processes, including leaf margin development [,], de novo stem cell formation in axillary meristems [], and seed size regulation [,]. DPA4 is expressed in the shoot apex during primordia formation, while also being expressed in the leaf sinuses coinciding with the CUC2 expression domain [,]. Genetic studies demonstrate that DPA4 suppresses CUC2 expression to regulate leaf morphogenesis [,]. Notably, DPA4 expression is induced early by wounding in leaf explants during DNRR []. However, the regulatory role of DPA4 in DNRR remains to be elucidated.
A previous study showed that DPA4 belongs to a group of transcription factor genes that are induced early by wounding during DNRR []. However, it remained unclear whether DPA4 has positive or negative effects on wound-induced DNRR. In this study, we reveal that DPA4, encoding a well-known repressor of CUC2 expression, inhibits DNRR from Arabidopsis leaf explants. Besides the loss-of-function mutant dpa4-2, we employed two other CUC2-overexpressing lines, cuc2-1D and mir164a-4, to test whether increased CUC2 function promotes DNRR in general. All three mutants displayed enhanced AR formation, suggesting that CUC2 promotes DNRR downstream of DPA4. Furthermore, we found similar expression changes in dpa4-2 and cuc2-1D mutant leaf explants, which include increased mRNA levels of CUC2, ERF109, ASA1, and YUC4/9, while ULT1 was lower-expressed compared to the wild-type. The enhanced root regeneration was associated with increased auxin concentrations in both cuc2-1D and dpa4-2 mutant leaf explants, as indicated by intensified DR5::GUS reporter activity, especially in and around the rooting side. Genetic analysis of dpa4-2 cuc2-1D double mutants and pharmacological inhibition of YUC-mediated auxin biogenesis supported the hypothesis that increased auxin production and enhanced DNRR are causally linked. These results indicate a mechanistic link between transcriptional regulation of CUC2 by DPA4 and auxin-dependent developmental reprogramming during DNRR. Our work provides new insights into the molecular control and gene regulatory network of AR formation and offers potential biotechnological applications for improving vegetative propagation of crop plants.
2. Results
2.1. DPA4 Suppresses De Novo Root Regeneration (DNRR) from Leaf Explants
To investigate the potential role of DPA4 in AR formation, we conducted DNRR assays following established protocols [], in which detached leaf explants were cultured on phytohormone-free B5 medium under dark conditions. We quantified the rooting rate and root regeneration capacity in leaf explants of the loss-of-function mutant dpa4-2 and wild-type (Col-0) plants (Figure 1A–D). The time-course analysis revealed that dpa4-2 leaf explants exhibited significantly accelerated root formation rates compared to the wild-type control (Figure 1A–D). Furthermore, the rooting capacity was significantly increased in dpa4-2 mutant leaf explants in comparison with the wild-type (Figure 1D). These findings indicate that DPA4 plays a role in suppressing DNRR in Arabidopsis.
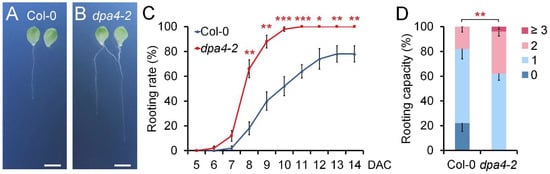
Figure 1.
DPA4 suppresses DNRR. (A,B) Root regeneration assay using wild-type (Col-0) (A) and dpa4-2 (B) mutants on B5 medium at 14 DAC; scale bars indicate 500 µM. (C) Rooting rates in wild-type and dpa4-2 mutant leaf explants on B5 medium under dark conditions. (D) Rooting capacity of leaf explants from wild-type and dpa4-2 mutants, 14 DAC. 0, 1, 2, and ≥3 indicate the number of ARs per leaf explant. (C,D) Average values are shown (N ≥ 40 leaves from 5 individual plates), ±SEM. Asterisks indicate significant differences compared with Col-0 plants (Student’s t-test: * p < 0.05, ** p < 0.01, and *** p < 0.001).
2.2. DPA4 Regulates Genes Known to Be Involved in AR Formation and Auxin Biosynthesis
Since DPA4 encodes a transcription factor, we investigated the expression changes of putative downstream targets of DPA4 after wounding to better understand its function in suppressing DNRR. For the expression analysis of the candidate genes, we harvested leaf explants at 4 h after culturing (HAC) on B5 medium. Since DPA4 is a known repressor of the boundary gene CUC2 in leaf margin development [], we examined CUC2 expression levels in leaf explants. The reverse transcription quantitative PCR (RT-qPCR) analysis revealed significantly increased CUC2 expression in the dpa4-2 mutant compared to the wild-type (Figure 2), confirming that DPA4 functions upstream of CUC2 in leaf explants (4 HAC). In a previous study, we found that ULT1 is a negative regulator of DNRR, likely by repressing ERF109 and, in turn, the auxin biosynthesis gene ASA1 []. Since loss of ULT1 phenocopies the acceleration of AR formation in dpa4-2 mutants, we analyzed the expression of ULT1, ERF109, and ASA1 (Figure 2). Our quantitative analysis revealed significantly reduced levels of ULT1 mRNA, while the expression of ERF109 and ASA1 was increased in dpa4-2 leaf explants compared to the wild-type. Like in ult1 mutants, the expression of ABR1, another wounding-induced activator of ASA1, was not significantly changed in dpa4-2 (Figure 2). These results suggest that DPA4 constrains AR formation at least partially by activation of ULT1 that, in turn, represses ERF109 and thereby limits auxin biosynthesis through the direct repression of ASA1 by ERF109. The expression levels of YUC genes have been reported as the rate-limiting step in auxin biosynthesis and DNRR [,,]. Therefore, we examined the expression levels of YUC genes in dpa4-2 leaf explants, 4 HAC (Figure 2). Notably, YUC4, YUC6, and YUC9 showed significant upregulation in dpa4-2 compared to the wild-type, while YUC1 expression was not significantly changed. Furthermore, the auxin-inducible DNRR-factor WOX11 [,] exhibited significant upregulation in dpa4-2 (Supplementary Figure S3). Collectively, these results indicate that the enhanced rooting rate and rooting capacity observed in dpa4-2 mutant leaf explants are likely caused by an increased auxin biosynthesis.
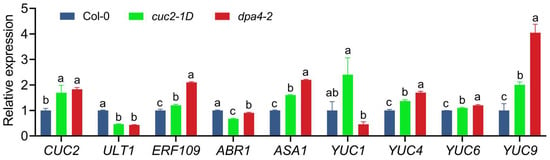
Figure 2.
Gene expression levels in cuc2-1D and dpa4-2 mutant leaf explants. RT-qPCR analysis of mRNA levels in wild-type, cuc2-1D, and dpa4-2, 4 h after culturing (4 HAC) on B5 medium. Average values are shown (N = 4), ±SEM. Statistical significance (p ≤ 0.05) was determined by one-way ANOVA and Duncan’s LSD, and a–c mark groups of significant differences.
2.3. cuc2-1D Phenocopies the Increased AR Formation Phenotype of dpa4-2 Mutant Leaf Explants, While Both Mutants Display Similar Expression Changes in DNRR-Related Genes
Since CUC2 is a known target of DPA4 in leaf margin development [] and plays an essential role in de novo shoot regeneration [,,], we hypothesized that the increased CUC2 expression might promote AR formation in dpa4-2 mutants. To test whether increased CUC2 expression levels can promote DNRR, we employed cuc2-1D mutants that carry a mutation in the CUC2 miRNA target site, resulting in CUC2 overexpression []. We performed DNRR assays to check the rooting rate and capacity in cuc2-1D mutant leaf explants (Figure 3A,E,G). Furthermore, we tested the rooting rate and capacity in mir164a-4 mutant leaf explants (Supplementary Figure S1). Consistent with our hypothesis, cuc2-1D and mir164a-4 mutant leaf explants exhibited similar increased rooting rates and rooting capacity as dpa4-2 mutants, which were significantly higher than those of the wild-type, suggesting that increased CUC2 expression is sufficient in promoting AR formation.
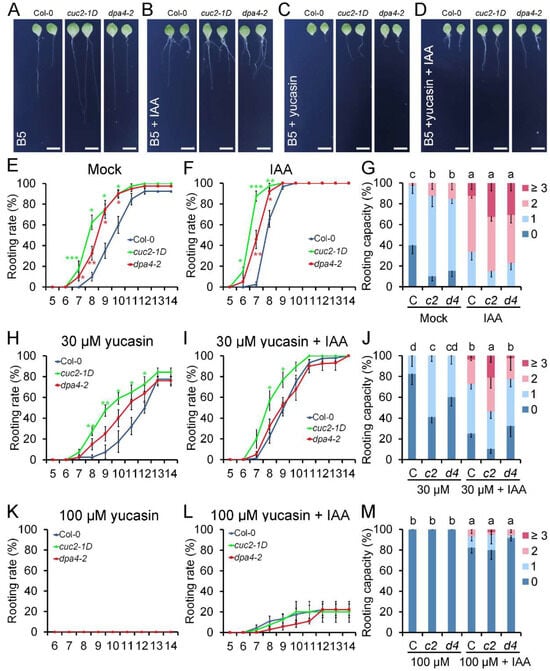
Figure 3.
Auxin (IAA) and yucasin treatment during DNRR. (A–D) Leaf explants of Col-0, cuc2-1D, and dpa4-2 cultured on B5 medium, 14 DAC. (A) mock; (B) 0.1 µM IAA; (C) 30 µM yucasin; (D) 30 µM yucasin + 0.1 µM IAA. Scale bars indicate 500 µm. (E,F) Rooting rate of Col-0, cuc2-1D, and dpa4-2 on B5 medium; (E) mock and (F) 0.1 µM IAA. (G) Rooting capacity of leaf explants from Col-0 (C), cuc2-1D (c2), and dpa4-2 (d4) on B5 medium, mock, and 0.1 µM IAA, 10 DAC. (H,I) Rooting rate of Col-0, cuc2-1D, and dpa4-2 on (H) 30 µM yucasin and (I) 30 µM yucasin + 0.1 µM IAA. (J) Rooting capacity of leaf explants from Col-0 (C), cuc2-1D (c2), and dpa4-2 (d4) on 30 µM yucasin and 30 µM yucasin + 0.1 µM IAA, 10 DAC. (K,L) Rooting rate of leaf explants from Col-0, cuc2-1D, and dpa4-2 on (K) 100 µM yucasin and (L) 100 µM yucasin + 0.1 µM IAA. (M) Rooting capacity of leaf explants from Col-0 (C), cuc2-1D (c2), and dpa4-2 (d4) on 100 µM yucasin and 100 µM yucasin + 0.1 µM IAA, 10 DAC. Average values are shown (N ≥ 40 leaves from 5 individual plates for each single experiment), ± SEM. Statistical significance was determined by Student’s t-test: * p < 0.05, ** p < 0.01, and *** p < 0.001, and a–d mark groups of significant differences (p ≤ 0.05). 0, 1, 2, and ≥3 indicate the number of ARs per leaf explant.
Since cuc2-1D phenocopies the increased AR formation phenotype of dpa4-2 mutants, we analyzed the expression of genes that was misregulated in dpa4-2 in cuc2-1D mutant leaf explants during DNRR (Figure 2). Like in dpa4-2 mutants, ULT1 was downregulated, while CUC2, ERF109, ASA1, YUC4, YUC6, and YUC9 were upregulated in cuc2-1D mutants in comparison to the wild-type, indicating that increased auxin levels could cause the increased AR formation phenotype in both mutants. Notably, also in miR164a-4 mutant leaf explants, CUC2 was upregulated and ULT1 was downregulated (Supplementary Figure S2). Although ASA1 was upregulated in all three CUC2-overexpressing mutants, partially different YUC genes were upregulated in dpa4-2, cuc2-1D, and mir164a-4 (Figure 2 and Supplementary Figure S2), indicating that increased CUC2 is not the only factor that influences YUC expression in these three lines. Nevertheless, the auxin-inducible DNRR-factor WOX11 [,] exhibited significant upregulation in dpa4-2, cuc2-1D, and mir164a-4, indicating increased auxin biosynthesis (Supplementary Figure S3).
To support the hypothesis that increased auxin levels cause the enhanced DNRR in cuc2-1D and dpa4-2 mutants, we conducted DNRR assays that either increased the auxin levels by exogenous application of IAA or decreased endogenous IAA levels in the leaf explant by applying the auxin biosynthesis inhibitor yucasin (Figure 3). Our treatment with 0.1 μM IAA accelerated AR formation in all three genotypes without equalizing the rooting rates [], and cuc2-1D and dpa4-2 leaf explants displayed earlier rooting initiation compared to the wild-type, although the differences in the time-course were reduced relative to mock (Figure 3E,F). Furthermore, the rooting capacity was equalized in wild-type, cuc2-1D, and dpa4-2 leaf explants (Figure 3G), corroborating the idea that accelerated and enhanced AR formation is mainly caused by increased auxin levels in both mutants.
Next, we tested the influence of increased and decreased gibberellin (GA) levels on DNRR in dpa4-2 and cuc2-1D mutant leaf explants. The phytohormone GA is well-known for its negative effects on AR formation, likely through its impact on auxin transport [,]. Although the changes in rooting capacity and the overall effects of the GA synthesis inhibitor PBZ were less distinct, exogenous GA significantly decreased the rooting rates in Col-0, cuc2-1D, and dpa4-2 leaf explants, while the differences in the rooting rate between Col-0 and both mutants with increased CUC2 levels remained (Supplementary Figure S4), indicating that GA signaling is unaffected during DNRR in cuc2-1D and dpa4-2 mutants.
Since the expression of several YUC genes is upregulated in dpa4-2 and cuc2-1D mutant leaf explants, we investigated whether YUC-mediated auxin biosynthesis is required for the enhanced AR formation in both mutants. To test this, we used yucasin, which is a specific inhibitor of the YUC enzymes, reducing endogenous auxin levels during DNRR on B5 medium [,]. At a low concentration of 30 μM yucasin, DNRR in wild-type, cuc2-1D, and dpa4-2 leaf explants was partially reduced, resulting in decelerated rooting rates over the time-course and lower rooting capacity (Figure 3C,H,J). As expected, combined treatment with 30 μM yucasin and 0.1 μM IAA partially rescued the rooting rate and rooting capacity in all three lines (Figure 3D,I,J). Notably, the combined treatment with yucasin and IAA equalized the rooting rate and rooting capacity in wild-type and dpa4-2 leaf explants, strongly suggesting that the enhanced rooting activity in dpa4-2 leaf explants is primarily caused by increased YUC-dependent auxin biosynthesis.
In contrast, the rooting rate and rooting capacity of cuc2-1D mutant leaf explants remained significantly higher than those of the wild-type during low (30 μM)-yucasin treatments with and without IAA (Figure 3C,D,I,J). This could be caused by higher auxin levels in cuc2-1D than in dpa4-2 leaf explants or an auxin-independent factor that promotes increased DNRR in cuc2-1D, but not in dpa4-2. To rule out the latter one, we repeated the DNRR experiment with a higher concentration of 100 μM yucasin with and without 0.1 μM IAA (Figure 3K–M). The single treatment with 100 μM yucasin annulled the rooting activity in all three lines, confirming that YUC-dependent auxin biosynthesis is, in general, essential for AR formation (Figure 3K,M). The addition of IAA partially rescued the disrupted rooting activity caused by 100 µM yucasin, and the low rooting rates and rooting capacities were not significantly different among all three genotypes (Figure 3L,M). In summary, these findings indicate that the enhanced DNRR ability in cuc2-1D and dpa4-2 mutants results from elevated auxin production. This is in line with the hypothesis that DPA4 regulates DNRR through a CUC2-dependent pathway that controls YUC-mediated auxin biosynthesis.
2.4. DPA4 and CUC2 Promote Endogenous Auxin Levels
To confirm that the enhanced DNRR activity in dpa4-2 and cuc2-1D results from high auxin production in leaf explants during regeneration, we performed a GUS reporter gene assay using the DR5::GUS reporter to show the spatiotemporal pattern of auxin in the leaf explants. We analyzed DR5::GUS staining in wild-type, dpa4-2, and cuc2-1D leaf explants at two time points (0 DAC and 1 DAC) (Figure 4). The DR5::GUS reporter activity reflects free auxin levels []. As expected, the DR5::GUS expression was low and absent in the potential rooting side in all three genotypes at 0 DAC (Figure 4A–C) [,]. After leaf explants were placed on B5 medium (mock) for one day (1 DAC), DR5::GUS staining intensively increased above the wounding sites and was significantly stronger in cuc2-1D and dpa4-2 leaf explants compared to the wild-type, while the staining was stronger in cuc2-1D than dpa4-2 (Figure 4D–F). At 1 DAC, the differences in DR5::GUS expression between dpa4-2 and cuc2-1D remained evident during all treatments with 0.1 μM IAA (Figure 4G–I), 100 μM yucasin (Figure 4J–L), and 100 μM yucasin and 0.1 μM IAA (Figure 4M,N), though the staining was always much weaker in the wild-type. As expected, the IAA treatments increased, while yucasin decreased the DR5::GUS staining intensity, and the yucasin + IAA double treatment rescued the DR5::GUS staining near mock levels. Taken together, the differences in the intensity of the DR5::GUS staining (Figure 4) correlate largely with the previously detected differences in rooting rate and rooting capacity caused by the different treatments and genotypes (Figure 3). This strongly supports the hypothesis that increased auxin levels at the rooting side, likely caused by increased auxin biosynthesis, result in the enhanced DNRR activity in cuc2-1D and dpa4-2 leaf explants.
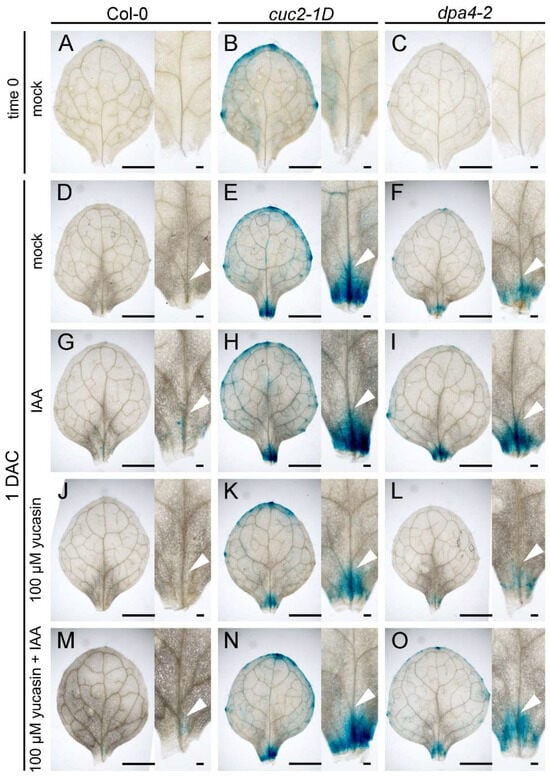
Figure 4.
Auxin distribution in leaf explants affected by yucasin and IAA treatments. (A–O) GUS staining of leaf explants with DR5::GUS reporter in wild-type (Col-0; (A,D,G,J,M)), cuc2-1D (B,E,H,K,N), and dpa4-2 (C,F,I,L,O) at 0 DAC (A–C) and 1 DAC (D–O). (D–F) Mock treatment at 1 DAC. (G–I) 0.1 μM IAA treatment at 1 DAC. (J–L) 100 μM yucasin treatment at 1 DAC. (M–O) 100 μM yucasin + 0.1 μM IAA treatment at 1 DAC. Arrowheads in (D–O) indicate the DR5::GUS signal in vasculature above the wounding side. Scale bars indicate 1000 µm and 100 µm.
2.5. DPA4 and CUC2 Regulate AR Formation Likely Through a Common Genetic Pathway That Controls Auxin Biosynthesis
Given that CUC2 is overexpressed in both cuc2-1D and dpa4-2 (Figure 2), we investigated whether they function in the same genetic pathway by generating cuc2-1D dpa4-2 double mutants. We subsequently examined the rooting rate and capacity in leaf explants from wild-type, cuc2-1D, dpa4-2, and cuc2-1D dpa4-2 double mutants (Figure 5A–C). We found that the dpa4-2 and cuc2-1D single mutants and the cuc2-1D dpa4-2 double mutants exhibited a similar increase in rooting rate and capacity compared to the wild-type; no additive or synergistic effect could be detected. Furthermore, we examined the expression of genes related to DNRR in the cuc2-1D dpa4-2 double mutant leaf explants using RT-qPCR (Figure 5D). We found that ULT1 was reduced to the same level in cuc2-1D, dpa4-2, and cuc2-1D dpa4-2 leaf explants. In contrast, the expression of ERF109, YUC4, and YUC9 was significantly upregulated in cuc2-1D, dpa4-2, and cuc2-1D dpa4-2 compared to the wild-type. Moreover, the expression levels of ULT1, ERF109, YUC4, and YUC9 in the cuc2-1D dpa4-2 double mutants remained similar to those in cuc2-1D and dpa4-2 single mutants, consistent with the observation that the cuc2-1D dpa4-2 double mutants did not show enhanced DNRR compared to the cuc2-1D and dpa4-2 single mutants. Interestingly, ASA1 expression, which was increased in the cuc2-1D and dpa4-2 single mutants in a previous experiment (Figure 2), was only significantly increased in the cuc2-1D dpa4-2 leaf explants (Figure 5D). This might reflect that ASA1 expression levels, which are only slightly increased in ult1-3, cuc2-1D, dpa4-2, and cuc2-1D dpa4-2 double mutants (Figure 2 and Figure 5D) [], are not solitarily dependent on the expression levels of ERF109 but on variable environmental factors. In conclusion, the absence of additive or synergistic effects in cuc2-1D dpa4-2 double mutants on the rooting phenotype and expression patterns strongly supports that DPA4 and CUC2 function in the same genetic pathway that controls the expression of ULT1 and auxin biosynthesis genes regulating DNRR in leaf explants.
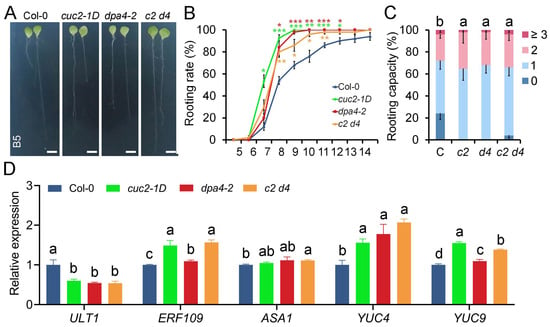
Figure 5.
DPA4 and CUC2 regulate DNRR in a common genetic pathway. (A) Leaf explants cultured on B5 medium at 14 DAC from Col-0, cuc2-1D, dpa4-2, and cuc2-1D dpa4-2; scale bars indicate 500 µm. (B) Rooting rate of leaf explants from Col-0, cuc2-1D, dpa4-2, and cuc2-1D dpa4-2 (c2 d4) on B5 medium. (C) Rooting capacity of leaf explants from Col-0 (C), cuc2-1D (c2), dpa4-2 (d4), and cuc2-1D dpa4-2 (c2 d4) on B5 medium at 10 DAC. (D) RT-qPCR analysis of gene expression levels in Col-0, cuc2-1D, dpa4-2, and cuc2-1D dpa4-2 (c2 d4) leaf explants, 4 HAC on B5 medium, N = 4. (B,C) Average values are shown (N ≥ 40 leaves from 5 individual plates for each single experiment), ±SEM. Statistical significance was determined by Student’s t-test: * p < 0.05, ** p < 0.01, and *** p < 0.001 and a–d mark groups of significant differences (p ≤ 0.05). 0, 1, 2, and ≥3 represent AR numbers per leaf explant.
3. Discussion
DNRR is a developmental process that enables plants to regenerate new roots post injury, after detachment, and under other stress conditions, representing a crucial survival strategy of higher plants [,]. Mechanical wounding primarily drives this process of AR formation by creating high auxin concentrations near the wounding site []. Despite its importance, the molecular pathways coordinating DNRR, which include hormone responses and the control of intrinsic developmental programs by transcription and epigenetic factors [,], are poorly characterized.
A previous study showed that DPA4 belongs to a group of transcription factor genes that are induced early by wounding during DNRR, but it remained unclear whether DPA4, which belongs to the B3 transcription factor gene subfamily, has positive or negative effects on wound-induced DNRR []. In this study, we investigated the role of DPA4 in AR formation in a series of DNRR assays and found that DPA4 is a suppressor of root regeneration from leaf explants via reduced auxin content. Therefore, DPA4 likely controls auxin biosynthesis through the indirect activation of ULT1, a previously known suppressor of DNRR [], and the repression of CUC2, a newly identified promoter of DNRR (Figure 6).
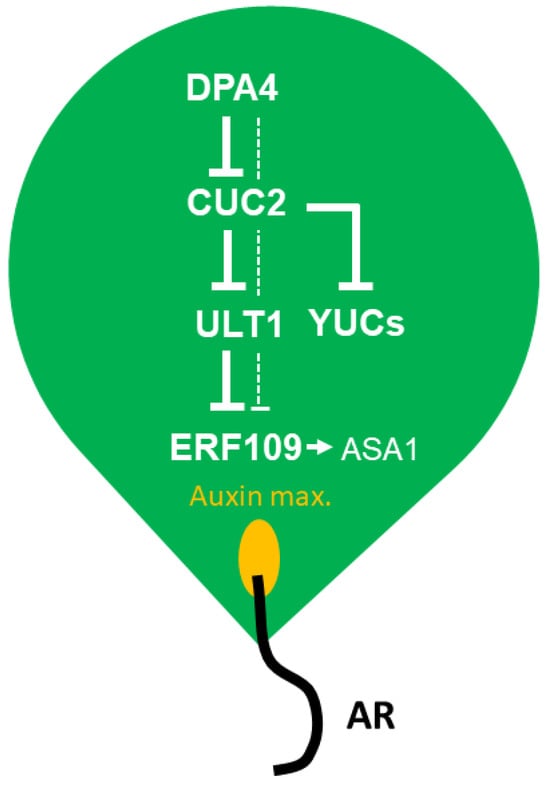
Figure 6.
Conceptual model of the DPA4-CUC2-ULT1 module limiting auxin biosynthesis and adventitious root (AR) formation in Arabidopsis leaf explants. See the discussion for more details.
During screening of candidate genes in DNRR assays, we observed significantly enhanced AR formation in dpa4-2 mutant leaf explants compared to wild-type (Figure 1). This finding suggests that DPA4 functions as a key regulator of root regeneration, supporting the emerging view that plant B3 transcription factors play important roles in regulating DNRR [,,]. Our RT-qPCR analysis confirmed increased CUC2 expression in dpa4-2 leaf explants, consistent with previous reports showing that CUC2 is a downstream target of DPA4 in various developmental processes [,,]. Furthermore, we detected enhanced expression of JA-induced ERF109, a member of the AP2/ERF transcription factor subfamily that mediates crosstalk between JA signaling and auxin biosynthesis [,]. These observations suggest that DPA4 may regulate DNRR through modulation of auxin biosynthesis.
Furthermore, we found that several YUC genes were significantly upregulated in dpa4-2, cuc2-1D, and miR164a-4 mutants that all displayed increased CUC2 expression (Figure 2 and Supplementary Figure S1). This result strongly suggests that increased CUC2 levels are sufficient in promoting DNRR by activating the expression of various YUC genes, corroborating previous findings that YUC1 and YUC4 expression depend on CUC2 during embryogenesis []. We found that ULT1, which encodes a known negative regulator of DNRR [], was equally downregulated in all three CUC2 overexpression mutants, dpa4-2, cuc2-1D, and miR164a-4 (Figure 2 and Supplementary Figure S1), suggesting that CUC2 is a direct or indirect repressor of ULT1 expression (Figure 6). Since ULT1 is a general repressor of ERF109 [] that encodes an activator of ASA1 during DNRR [,], the low ULT1 expression levels may contribute to the high auxin levels via ASA1-dependent auxin biosynthesis (Figure 6). However, since the increase in ASA1 expression is weak and variable, ULT1 may suppress DNRR also through ASA1-independent pathways. Notably, the enhanced root regeneration phenotype of cuc2-1D dpa4-2 double mutants was very similar to that of the single mutants, and no additive or synergistic effect could be detected (Figure 5), indicating that DPA4 and CUC2 function mainly in the same DNRR pathway (Figure 6).
Auxin levels play a pivotal role in DNRR. Previous studies have demonstrated that both exogenous IAA application and inhibition of auxin production by yucasin significantly affect the root regeneration process []. Our findings confirmed that increased auxin biosynthesis is essential for the enhanced DNRR in dpa4-2 and cuc2-1D mutants. This conclusion is further supported by our analysis of the DR5::GUS expression patterns in leaf explants in DNRR assays with and without IAA and/or yucasin treatment (Figure 4). The intensity of the DR5::GUS staining correlates largely with the differences in rooting rate and rooting capacity caused by the different treatments and genotypes (Figure 3). Furthermore, the auxin-inducible DNRR factor WOX11 exhibited significant upregulation in dpa4-2, cuc2-1D, and miR164a-4 (Supplementary Figure S3), indicating a link between increased CUC2 expression and auxin biosynthesis, but more importantly, an immediate increase in a key regulator of cell fate reprogramming during DNRR, WOX11 [,]. Nevertheless, we cannot exclude the possibility that CUC2 and DPA4 regulate root regeneration also through other pathways [,,].
In summary, our findings suggest that DPA4 plays a crucial role in DNRR in Arabidopsis leaf explants by serving as a key player in inhibiting ASA1- and YUC-mediated auxin biosynthesis. Furthermore, DPA4 and CUC2 regulate DNRR through a common auxin-dependent pathway. In our conceptual model (Figure 6), DPA4 suppresses DNRR by downregulating CUC2, which, in turn, upregulates ULT1 that represses ERF109 and, ultimately, ASA1. Likely independently of ULT1, CUC2 represses YUC-mediated auxin biosynthesis. Overproliferation of ARs would decrease the survival rate of detached aerial organs []. Therefore, the DPA4-CUC2-ULT1 module likely evolved to limit DNRR, thereby increasing the persistence of detached Arabidopsis leaves.
4. Materials and Methods
4.1. Plant Materials and Culturing Conditions
All plant materials were in the Col-0 background. Seeds of the T-DNA insertion mutant dpa4-2 [] (N650707) and the miRNA-resistant cuc2-1D allele [] (N16485) were obtained from the Nottingham Arabidopsis Stock Center. DR5::GUS were kindly provided by Rüdiger Simon. The seeds were surfaced-sterilized by three sequential treatments with 70% ethanol (3 min each), and then the seeds were sown on half-strength Murashige and Skoog (1/2 MS) plates containing 1% sucrose and 1% agar with pH adjusted to 5.8. After sowing, the plates were first kept at 4 °C for 3 days in darkness, and then transferred to growth chambers maintained at 22 °C under long-day photoperiod conditions (16 h light/8 h dark cycle).
4.2. De Novo Root Regeneration (DNRR) Assays with and Without Hormone Treatment
The first pair of primary leaves was cut from 12-day-old Arabidopsis seedlings for the DNRR assay. Leaf explants were cultured following previously described methods [] on B5 medium (Gamborg B5 medium containing 3% sucrose, 1% agar, and 0.5 g/L MES, with pH adjusted to 5.7) under dark conditions. The rooting rate was calculated as the percentage of leaf explants developing roots at a given time point [], while regeneration capacity was assessed based on the percentage of leaf explants showing varying numbers of regenerated roots []. For IAA and yucasin treatments, we used 0.1 µM IAA (Sigma-Aldrich, Inc., St. Louis, MO, USA) and 30 µM or 100 µM yucasin (MACKLIN, Beijing, China) in B5 medium (applied continuously after detachment). Each experiment included more than five plates per treatment, with at least eight explants per genotype per plate. A one-tailed Student’s t test was employed to assess the statistical significance between value pairs of different genotypes, resulting in p values. In this study, all error bars represent the mean ± standard error.
4.3. Quantitative RT-PCR and GUS Staining
The leaf explants after 4 HAC (hours after culture) on B5 medium were collected and snap-frozen in liquid nitrogen. Total RNA was isolated using the TRIZOL reagent (Invitrogen, Waltham, MA, USA), and cDNA was synthesized using a commercial kit (Thermo Scientific, Waltham, MA, USA). RT-qPCR was performed using the SYBR green supermix (Vazyme, Nanjing, China) on a Biorad CFX96 system (Biorad, Hercules, CA, USA). The RT-qPCR procedure and the primers for CUC2, ABR1, ERF109, ASA1, ULT1, YUC1, YUC4, YUC6, YUC9, and eIF4A (Supplementary Table S1) have been previously described [,,,]. All expression data represent the average of four biological replicates.
The detection of β-glucuronidase (GUS) activity in leaf explants was performed according to a published protocol with minor modifications [,]. In brief, leaf explants were harvested at specific time points after detachment (time 0 DAC and 1 DAC (day after culturing on B5 medium)) and immersed in the GUS assay solution (0.5 mM ferrocyanide, 0.5 mM ferricyanide, 50 mM NaHPO4, 50 mM Na2HPO4, and 1% Triton X-100) containing 1 mM X-Gluc (Solarbio, LIFE SCIENCE, Beijing, China). Then, the leaf explants in the GUS staining solution were vacuum-infiltrated for 30 min and subsequently incubated overnight at 37 °C. To remove the chlorophyll, the stained leaves were passed through an ethanol series and then photographed using a stereomicroscope (Olympus, Tokyo, Japan). Finally, the digital photographs were collated with Adobe Photoshop.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms262311336/s1.
Author Contributions
Y.Z. performed all experiments; R.M.-X. created the concept and designed the research; X.L., Q.X. and R.M.-X. co-supervised; Y.Z. and R.M.-X. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was kindly funded by the National Natural Science Foundation of China (project nos. 32460150, 31640054, and 31771602) and the Jiangxi Province Recruitment Program of Foreign Experts (project no. jxsq2023104003), to R.M.-X.; two Starting Grants of the Lushan Botanical Garden, Chinese Academy of Sciences, Jiujiang (Grant Nos. 2021ZWZX24 and 2021ZWZX25) to Q.X. and R.M.-X.; and the High-Level Foreign Experts Project (G2021022004) from the Chinese Ministry of Science and Technology and the National Demonstration Platform for Attracting Talents and Intelligence (project no. YZJD2023020) to R.M.-X.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank all members of our research groups for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.L.; Cheng, Z.J.; Zhang, X.S. iPSCs: A Comparison between Animals and Plants. Trends Plant Sci. 2018, 23, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Tazeb, A. Plant tissue culture technique as a novel tool in plant breeding: A review article. Am. Eurasian J. Agric. Environ. Sci. 2017, 17, 111–118. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Fang, X.; Tran, S.; Zhai, N.; Yang, Z.; Guo, F.; Chen, L.; Yu, J.; Ison, M.S.; et al. Transcriptional landscapes of de novo root regeneration from detached Arabidopsis leaves revealed by time-lapse and single-cell RNA sequencing analyses. Plant Commun. 2022, 3, 100306. [Google Scholar] [CrossRef]
- Duclercq, J.; Sangwan-Norreel, B.; Catterou, M.; Sangwan, R.S. De novo shoot organogenesis: From art to science. Trends Plant Sci. 2011, 16, 597–606. [Google Scholar] [CrossRef]
- Long, Y.; Yang, Y.; Pan, G.; Shen, Y. New Insights Into Tissue Culture Plant-Regeneration Mechanisms. Front. Plant Sci. 2022, 13, 926752. [Google Scholar] [CrossRef]
- Doll, Y.; Ikeuchi, M. All roads lead to dome: Multicellular dynamics during de novo meristem establishment in shoot regeneration. Curr. Opin. Plant Biol. 2025, 85, 102733. [Google Scholar] [CrossRef]
- Šmeringai, J.; Schrumpfová, P.P.; Pernisová, M. Cytokinins—Regulators of de novo shoot organogenesis. Front. Plant Sci. 2023, 14, 1239133. [Google Scholar] [CrossRef]
- Chen, X.; Qu, Y.; Sheng, L.; Liu, J.; Huang, H.; Xu, L. A simple method suitable to study de novo root organogenesis. Front. Plant Sci. 2014, 5, 208. [Google Scholar] [CrossRef]
- Garg, T.; Yadav, M.; Mushahary, K.K.K.; Kumar, A.; Pal, V.; Singh, H.; Jain, M.; Yadav, S.R. Spatially activated conserved auxin-transcription factor regulatory module controls de novo root organogenesis in rice. Planta 2023, 258, 52. [Google Scholar] [CrossRef]
- Ye, B.-B.; Shang, G.-D.; Pan, Y.; Xu, Z.-G.; Zhou, C.-M.; Mao, Y.-B.; Bao, N.; Sun, L.; Xu, T.; Wang, J.-W. AP2/ERF Transcription Factors Integrate Age and Wound Signals for Root Regeneration. Plant Cell 2020, 32, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, F.; Chen, L.; Pan, Y.; Sun, L.; Bao, N.; Zhang, T.; Cui, C.-X.; Qiu, Z.; Zhang, Y.; et al. Jasmonate-mediated wound signalling promotes plant regeneration. Nat. Plants 2019, 5, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Song, Y.; Li, M.; Hu, T.; Hsu, Y.-F.; Zheng, M. IRR1 contributes to de novo root regeneration from Arabidopsis thaliana leaf explants. Physiol. Plant 2023, 175, e14047. [Google Scholar] [CrossRef] [PubMed]
- Bustillo-Avendaño, E.; Ibáñez, S.; Sanz, O.; Sousa Barros, J.A.; Gude, I.; Perianez-Rodriguez, J.; Micol, J.L.; Del Pozo, J.C.; Moreno-Risueno, M.A.; Pérez-Pérez, J.M. Regulation of Hormonal Control, Cell Reprogramming, and Patterning during De Novo Root Organogenesis. Plant Physiol. 2018, 176, 1709–1727. [Google Scholar] [CrossRef]
- Jing, T.; Ardiansyah, R.; Xu, Q.; Xing, Q.; Müller-Xing, R. Reprogramming of Cell Fate During Root Regeneration by Transcriptional and Epigenetic Networks. Front. Plant Sci. 2020, 11, 317, Correction in Front. Plant Sci. 2021, 12, 698412. [Google Scholar] [CrossRef]
- Xu, L. De novo root regeneration from leaf explants: Wounding, auxin, and cell fate transition. Curr. Opin. Plant Biol. 2018, 41, 39–45. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, H.; Park, O.-S.; Seo, P.J. ENHANCER OF SHOOT REGENERATION1 promotes de novo root organogenesis after wounding in Arabidopsis leaf explants. Plant Cell 2024, 36, 2359–2374. [Google Scholar] [CrossRef]
- Cai, X.-T.; Xu, P.; Zhao, P.-X.; Liu, R.; Yu, L.-H.; Xiang, C.-B. Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat. Commun. 2014, 5, 5833. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Zhao, Y.; Hull, A.K.; Gupta, N.R.; Goss, K.A.; Alonso, J.; Ecker, J.R.; Normanly, J.; Chory, J.; Celenza, J.L. Trp-dependent auxin biosynthesis in Arabidopsis: Involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002, 16, 3100–3112. [Google Scholar] [CrossRef]
- Luo, P.; Di, D.-W. Precise Regulation of the TAA1/TAR-YUCCA Auxin Biosynthesis Pathway in Plants. Int. J. Mol. Sci. 2023, 24, 8514. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Chen, L.; Liu, J.; Zhang, X.; Yang, Z.; Liu, W.; Xu, L. TAA family contributes to auxin production during de novo regeneration of adventitious roots from Arabidopsis leaf explants. Sci. Bull. 2016, 61, 1728–1731. [Google Scholar] [CrossRef]
- Hu, X.; Xu, L. Transcription Factors WOX11/12 Directly Activate WOX5/7 to Promote Root Primordia Initiation and Organogenesis. Plant Physiol. 2016, 172, 2363–2373. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef]
- Müller-Xing, R.; Xing, Q. The plant stem-cell niche and pluripotency: 15 years of an epigenetic perspective. Front. Plant Sci. 2022, 13, 1047. [Google Scholar] [CrossRef]
- Sang, Y.L.; Cheng, Z.J.; Zhang, X.S. Plant stem cells and de novo organogenesis. N. Phytol. 2018, 218, 1334–1339. [Google Scholar] [CrossRef]
- Jing, T.; Xing, Q.; Shi, Y.; Liu, X.; Müller-Xing, R. Depletion of Gibberellin Signaling Up-Regulates LBD16 Transcription and Promotes Adventitious Root Formation in Arabidopsis Leaf Explants. Int. J. Mol. Sci. 2024, 25, 13340. [Google Scholar] [CrossRef]
- Chen, L.; Tong, J.; Xiao, L.; Ruan, Y.; Liu, J.; Zeng, M.; Huang, H.; Wang, J.-W.; Xu, L. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J. Exp. Bot. 2016, 67, 4273–4284. [Google Scholar] [CrossRef]
- Aida, M.; Ishida, T.; Fukaki, H.; Fujisawa, H.; Tasaka, M. Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell 1997, 9, 841–857. [Google Scholar] [CrossRef]
- Nikovics, K.; Blein, T.; Peaucelle, A.; Ishida, T.; Morin, H.; Aida, M.; Laufs, P. The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 2006, 18, 2929–2945. [Google Scholar] [CrossRef]
- Larue, C.T.; Wen, J.; Walker, J.C. A microRNA-transcription factor module regulates lateral organ size and patterning in Arabidopsis. Plant J. 2009, 58, 450–463. [Google Scholar] [CrossRef]
- Nicolas, A.; Maugarny-Calès, A.; Adroher, B.; Chelysheva, L.; Li, Y.; Burguet, J.; Bågman, A.-M.; Smit, M.E.; Brady, S.M.; Li, Y.; et al. De novo stem cell establishment in meristems requires repression of organ boundary cell fate. Plant Cell 2022, 34, 4738–4759. [Google Scholar] [CrossRef]
- Bilsborough, G.D.; Runions, A.; Barkoulas, M.; Jenkins, H.W.; Hasson, A.; Galinha, C.; Laufs, P.; Hay, A.; Prusinkiewicz, P.; Tsiantis, M. Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Natl. Acad. Sci. USA 2011, 108, 3424–3429. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Xing, Q.; Ardiansyah, R.; Zhou, H.; Ali, S.; Jing, T.; Tian, J.; Song, X.S.; Li, Y.; et al. Ectopic expression of the transcription factor CUC2 restricts growth by cell cycle inhibition in Arabidopsis leaves. Plant Signal. Behav. 2020, 15, 1706024. [Google Scholar] [CrossRef]
- Daimon, Y.; Takabe, K.; Tasaka, M. The CUP-SHAPED COTYLEDON genes promote adventitious shoot formation on calli. Plant Cell Physiol. 2003, 44, 113–121. [Google Scholar] [CrossRef]
- Gordon, S.P.; Heisler, M.G.; Reddy, G.V.; Ohno, C.; Das, P.; Meyerowitz, E.M. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development 2007, 134, 3539–3548. [Google Scholar] [CrossRef] [PubMed]
- Kareem, A.; Durgaprasad, K.; Sugimoto, K.; Du, Y.; Pulianmackal, A.J.; Trivedi, Z.B.; Abhayadev, P.V.; Pinon, V.; Meyerowitz, E.M.; Scheres, B.; et al. PLETHORA Genes Control Regeneration by a Two-Step Mechanism. Curr. Biol. 2015, 25, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Motte, H.; Verstraeten, I.; Werbrouck, S.; Geelen, D. CUC2 as an early marker for regeneration competence in Arabidopsis root explants. J. Plant Physiol. 2011, 168, 1598–1601. [Google Scholar] [CrossRef] [PubMed]
- Engelhorn, J.; Reimer, J.J.; Leuz, I.; Göbel, U.; Huettel, B.; Farrona, S.; Turck, F. Development-related PcG target in the apex 4 controls leaf margin architecture in Arabidopsis thaliana. Development 2012, 139, 2566–2575. [Google Scholar] [CrossRef]
- Shao, J.; Meng, J.; Wang, F.; Shou, B.; Chen, Y.; Xue, H.; Zhao, J.; Qi, Y.; An, L.; Yu, F.; et al. NGATHA-LIKEs Control Leaf Margin Development by Repressing CUP-SHAPED COTYLEDON2 Transcription. Plant Physiol. 2020, 184, 345–358. [Google Scholar] [CrossRef]
- Swaminathan, K.; Peterson, K.; Jack, T. The plant B3 superfamily. Trends Plant Sci. 2008, 13, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, L.; Xu, R.; Cui, R.; Hao, J.; Sun, C.; Li, Y. Transcription factors SOD7/NGAL2 and DPA4/NGAL3 act redundantly to regulate seed size by directly repressing KLU expression in Arabidopsis thaliana. Plant Cell 2015, 27, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Xing, Q.; Jing, T.; Fan, X.; Zhang, Q.; Müller-Xing, R. The epigenetic regulator ULTRAPETALA1 suppresses de novo root regeneration from Arabidopsis leaf explants. Plant Signal. Behav. 2022, 17, 2031784. [Google Scholar] [CrossRef]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Hanada, A.; Yaeno, T.; Shirasu, K.; Yao, H.; et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014, 78, 372–384. [Google Scholar] [CrossRef]
- Nishimura, T.; Hayashi, K.-I.; Suzuki, H.; Gyohda, A.; Takaoka, C.; Sakaguchi, Y.; Matsumoto, S.; Kasahara, H.; Sakai, T.; Kato, J.-I.; et al. Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis. Plant J. 2014, 77, 352–366, Erratum in Plant. J. 2015, 81, 649. [Google Scholar] [CrossRef]
- Ulmasov, T.; Murfett, J.; Hagen, G.; Guilfoyle, T.J. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 1997, 9, 1963–1971. [Google Scholar] [CrossRef]
- Sengupta, S.; Ray, A.; Mandal, D.; Nag Chaudhuri, R. ABI3 mediated repression of RAV1 gene expression promotes efficient dehydration stress response in Arabidopsis thaliana. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194582. [Google Scholar] [CrossRef]
- Yamada, M.; Tanaka, S.; Miyazaki, T.; Aida, M. Expression of the auxin biosynthetic genes YUCCA1 and YUCCA4 is dependent on the boundary regulators CUP-SHAPED COTYLEDON genes in the Arabidopsis thaliana embryo. Plant Biotechnol. 2022, 39, 37–42. [Google Scholar] [CrossRef]
- Tyler, L.; Miller, M.J.; Fletcher, J.C. The Trithorax Group Factor ULTRAPETALA1 Regulates Developmental as Well as Biotic and Abiotic Stress Response Genes in Arabidopsis. G3 Genes Genomes Genet. 2019, 9, 4029–4043. [Google Scholar] [CrossRef]
- Wan, Q.; Yao, R.; Zhao, Y.; Xu, L. JA and ABA signaling pathways converge to protect plant regeneration in stress conditions. Cell Rep. 2025, 44, 115423. [Google Scholar] [CrossRef]
- Tu, T.; Zheng, S.; Ren, P.; Meng, X.; Zhao, J.; Chen, Q.; Li, C. Coordinated cytokinin signaling and auxin biosynthesis mediates arsenate-induced root growth inhibition. Plant Physiol. 2021, 185, 1166–1181. [Google Scholar] [CrossRef]
- Xiong, Y.; Xing, Q.; Müller-Xing, R. A novel UV-B priming system reveals an UVR8-depedent memory, which provides resistance against UV-B stress in Arabidopsis leaves. Plant Signal. Behav. 2021, 16, 1879533. [Google Scholar] [CrossRef]
- Müller-Xing, R.; Ardiansyah, R.; Xing, Q.; Faivre, L.; Tian, J.; Wang, G.; Zheng, Y.; Wang, X.; Jing, T.; de Leau, E.; et al. Polycomb proteins control floral determinacy by H3K27me3-mediated repression of pluripotency genes in Arabidopsis thaliana. J. Exp. Bot. 2022, 73, 2385–2402. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).