Differential Gene Expression of Porphyromonas gingivalis in the Presence or Absence of Xanthohumol and Curcumin in a Dynamic In Vitro Biofilm Model
Abstract
1. Introduction
2. Results
2.1. Minimum Inhibitory Concentrations of Xanthohumol and Curcumin Against P. gingivalis
2.2. Scanning Electron Microscopy and Confocal Laser Scanning Microscopy to Monitor P. gingivalis Biofilm Development and the Effect of Treatments
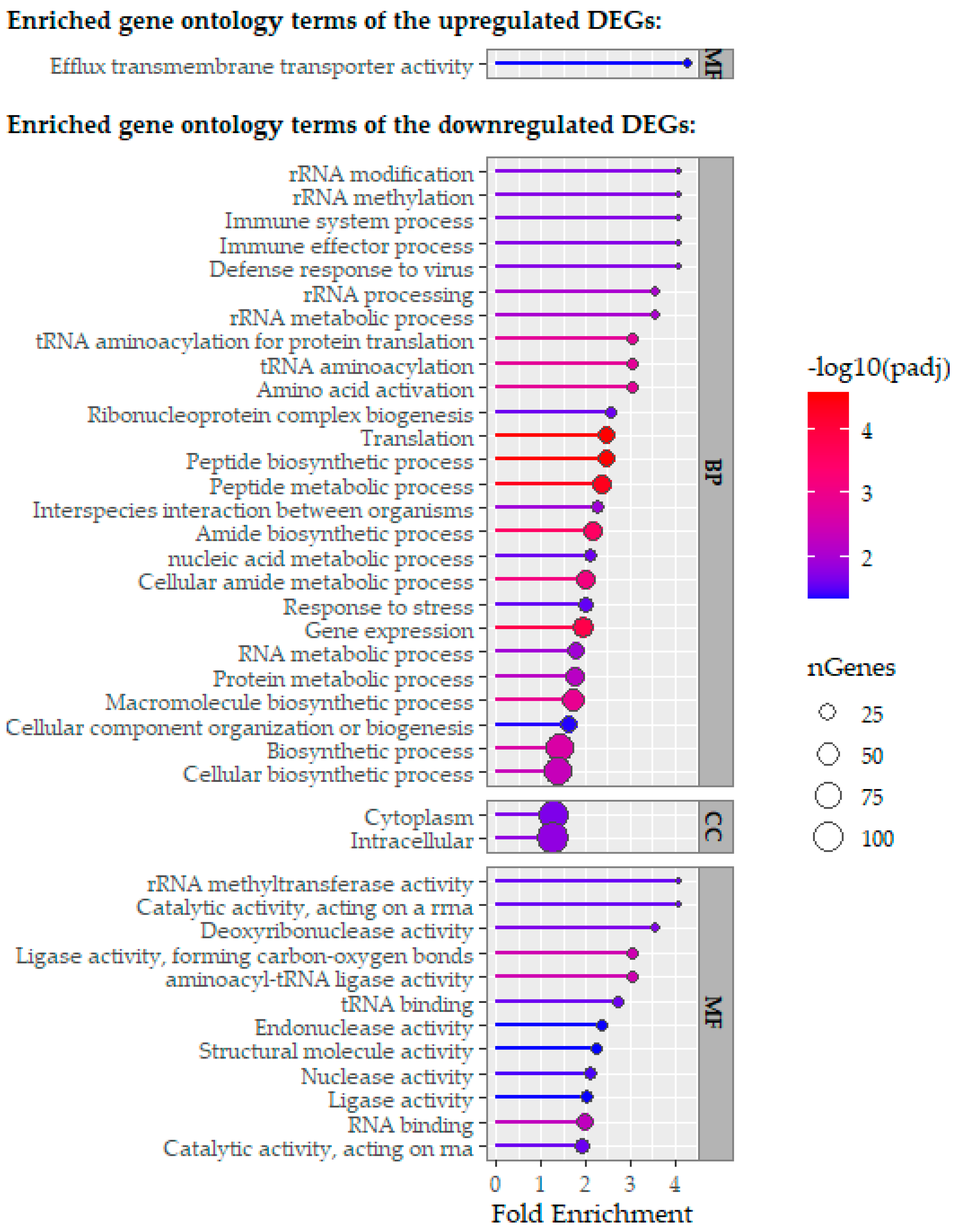
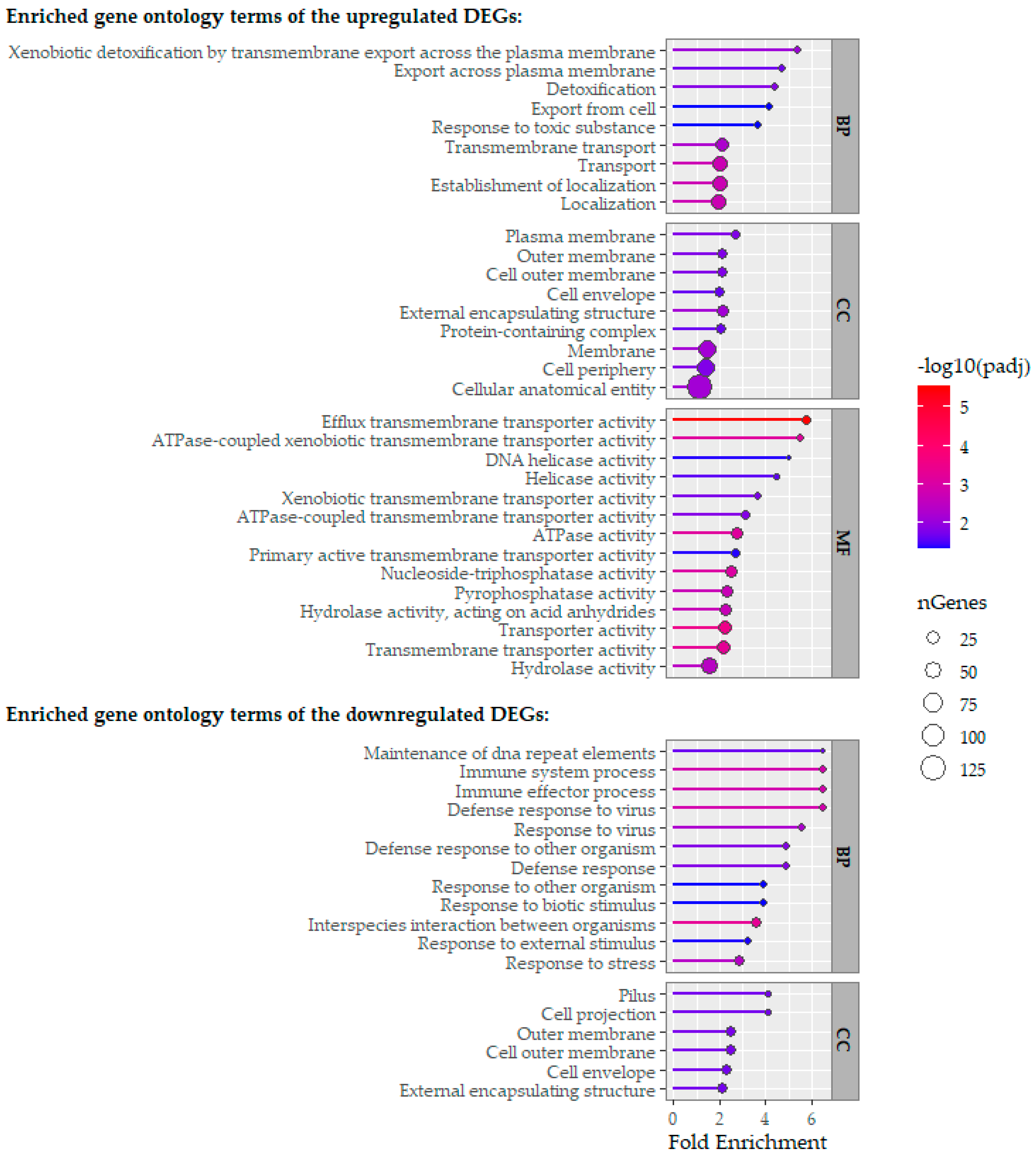
2.3. Comparative Analysis of RNA-Sequencing-Obtained Transcriptomes
3. Discussion
4. Materials and Methods
4.1. Microbial Strains and Culture Conditions
4.2. Minimum Inhibitory Concentrations of Xanthohumol and Curcumin Against P. gingivalis
4.3. In Vitro Dynamic Monospecies Biofilm Model
4.4. Experimental Groups
4.5. Scanning Electron Microscopy
4.6. Confocal Laser Scanning Microscopy
4.7. Total RNA Isolation
4.8. RNA Sequencing
4.9. Reverse Transcriptase Quantitative Polymerase Chain Reaction Validation
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Periodontology 2000 2021, 86, 32–56. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.R. Biofilm architecture and dynamics of the oral ecosystem. BioTechnologia 2024, 105, 395–402. [Google Scholar] [CrossRef]
- Filoche, S.; Wong, L.; Sissons, C.H. Oral biofilms: Emerging concepts in microbial ecology. J. Dent. Res. 2010, 89, 8–18. [Google Scholar] [CrossRef]
- Bloch, S.; Hager-Mair, F.F.; Andrukhov, O.; Schaffer, C. Oral streptococci: Modulators of health and disease. Front. Cell. Infect. Microbiol. 2024, 14, 1357631. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S313–S318. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontol. 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 223–247. [Google Scholar] [CrossRef]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S5–S11. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, W.; Wang, H.; Liang, S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Struct. Biol. 2020, 120, 45–84. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Kuboniwa, M. The polymicrobial pathogenicity of Porphyromonas gingivalis. Front. Oral Health 2024, 5, 1404917. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, P.; Gao, W. Microbial dysbiosis in periodontitis and peri-implantitis: Pathogenesis, immune responses, and therapeutic. Front. Cell. Infect. Microbiol. 2025, 15, 1517154. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Romero-Lastra, P.; Ribeiro-Vidal, H.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz, M. Comparative gene expression analysis of planktonic Porphyromonas gingivalis ATCC 33277 in the presence of a growing biofilm versus planktonic cells. BMC Microbiol. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Romero-Lastra, P.; Sanchez, M.C.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz, M. Gene expression of Porphyromonas gingivalis ATCC 33277 when growing in an in vitro multispecies biofilm. PLoS ONE 2019, 14, e0221234. [Google Scholar] [CrossRef]
- Romero-Lastra, P.; Sanchez, M.C.; Ribeiro-Vidal, H.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz, M. Comparative gene expression analysis of Porphyromonas gingivalis ATCC 33277 in planktonic and biofilms states. PLoS ONE 2017, 12, e0174669. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Nassani, M.Z.; Alaizari, N.; Kalakonda, B.; Al-Shamiri, H.M.; Alhajj, M.N.; Al-Soneidar, W.A.; Alahmary, A.W. Efficacy of aloe vera mouthwash versus chlorhexidine on plaque and gingivitis: A systematic review. Int. J. Dent. Hyg. 2020, 18, 44–51. [Google Scholar] [CrossRef]
- Cai, H.; Chen, J.; Panagodage Perera, N.K.; Liang, X. Effects of Herbal Mouthwashes on Plaque and Inflammation Control for Patients with Gingivitis: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Evid.-Based Complement. Altern. Med. 2020, 2020, 2829854. [Google Scholar] [CrossRef] [PubMed]
- Halboub, E.; Al-Maweri, S.A.; Al-Wesabi, M.; Al-Kamel, A.; Shamala, A.; Al-Sharani, A.; Koppolu, P. Efficacy of propolis-based mouthwashes on dental plaque and gingival inflammation: A systematic review. BMC Oral Health 2020, 20, 198. [Google Scholar] [CrossRef]
- Rozalski, M.; Micota, B.; Sadowska, B.; Stochmal, A.; Jedrejek, D.; Wieckowska-Szakiel, M.; Rozalska, B. Antiadherent and antibiofilm activity of Humulus lupulus L. derived products: New pharmacological properties. Biomed Res. Int. 2013, 2013, 101089. [Google Scholar] [CrossRef]
- Shinada, K.; Tagashira, M.; Watanabe, H.; Sopapornamorn, P.; Kanayama, A.; Kanda, T.; Ikeda, M.; Kawaguchi, Y. Hop bract polyphenols reduced three-day dental plaque regrowth. J. Dent. Res. 2007, 86, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Cermak, P.; Olsovska, J.; Mikyska, A.; Dusek, M.; Kadleckova, Z.; Vanicek, J.; Nyc, O.; Sigler, K.; Bostikova, V.; Bostik, P. Strong antimicrobial activity of xanthohumol and other derivatives from hops (Humulus lupulus L.) on gut anaerobic bacteria. APMIS 2017, 125, 1033–1038. [Google Scholar] [CrossRef]
- Sleha, R.; Radochova, V.; Mikyska, A.; Houska, M.; Bolehovska, R.; Janovska, S.; Pejchal, J.; Muckova, L.; Cermak, P.; Bostik, P. Strong Antimicrobial Effects of Xanthohumol and Beta-Acids from Hops against Clostridioides difficile Infection In Vivo. Antibiotics 2021, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Leonida, M.D.; Belbekhouche, S.; Benzecry, A.; Peddineni, M.; Suria, A.; Carbonnier, B. Antibacterial hop extracts encapsulated in nanochitosan matrices. Int. J. Biol. Macromol. 2018, 120, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
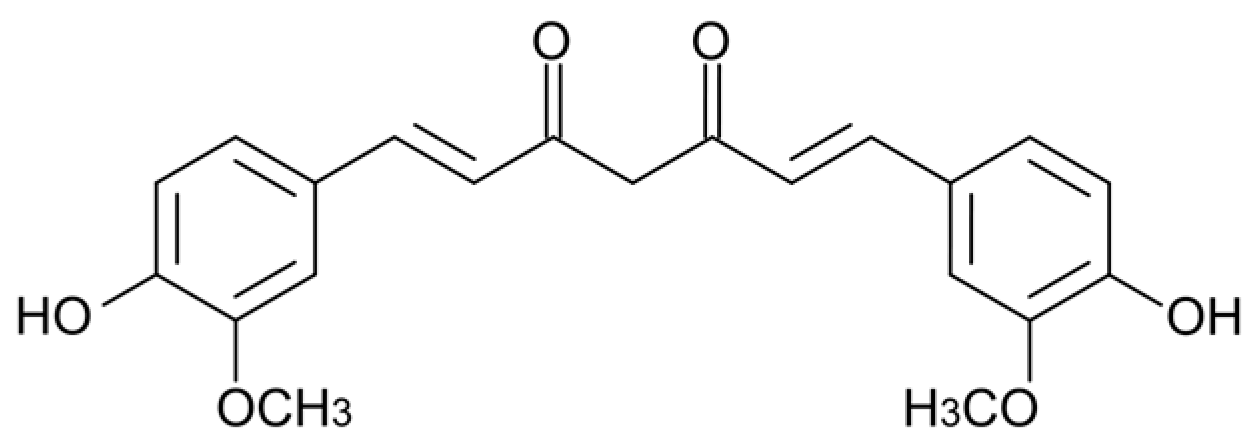
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and curcumin-like molecules: From spice to drugs. Curr. Med. Chem. 2014, 21, 204–222. [Google Scholar] [CrossRef]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef]
- Praditya, D.; Kirchhoff, L.; Bruning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective Properties of the Golden Spice Curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Expert Rev. Anti-Infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef] [PubMed]
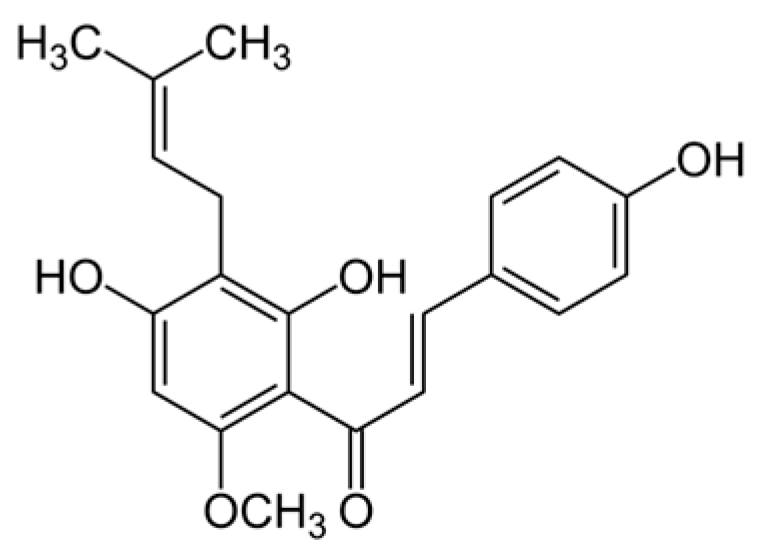
- Alonso-Espanol, A.; Bravo, E.; Ribeiro-Vidal, H.; Virto, L.; Herrera, D.; Alonso, B.; Sanz, M. The Antimicrobial Activity of Curcumin and Xanthohumol on Bacterial Biofilms Developed over Dental Implant Surfaces. Int. J. Mol. Sci. 2023, 24, 2335. [Google Scholar] [CrossRef]
- Alonso-Espanol, A.; Bravo, E.; Carrillo de Albornoz, A.; Martinez, M.; Doll-Nikutta, K.; Winkel, A.; Stiesch, M.; Herrera, D.; Alonso, B.; Sanz, M. Antimicrobial Effect and Cytocompatibility After Using Different Decontamination Methods on Titanium Implant Surfaces: An In Vitro Study. Clin. Oral Implant. Res. 2025, 36, 626–639. [Google Scholar] [CrossRef]
- Murai, H.; Kuboniwa, M.; Kakiuchi, M.; Matsumura, R.; Hirata, Y.; Amano, A. Curcumin inhibits growth of Porphyromonas gingivalis by arrest of bacterial dipeptidyl peptidase activity. J. Oral Microbiol. 2024, 16, 2373040. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, V.M.; Peram, M.R.; Kugaji, M.S.; Shah, T.; Patil, S.P.; Muddapur, U.M.; Bhat, K.G. Effect of curcumin on growth, biofilm formation and virulence factor gene expression of Porphyromonas gingivalis. Odontology 2021, 109, 18–28. [Google Scholar] [CrossRef]
- Izui, S.; Sekine, S.; Murai, H.; Takeuchi, H.; Amano, A. Inhibitory effects of curcumin against cytotoxicity of Porphyromonas gingivalis outer membrane vesicles. Arch. Oral Biol. 2021, 124, 105058. [Google Scholar] [CrossRef]
- Naito, M.; Hirakawa, H.; Yamashita, A.; Ohara, N.; Shoji, M.; Yukitake, H.; Nakayama, K.; Toh, H.; Yoshimura, F.; Kuhara, S.; et al. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res. 2008, 15, 215–225. [Google Scholar] [CrossRef]
- Cheng, W.; Xu, T.; Cui, L.; Xue, Z.; Liu, J.; Yang, R.; Qin, S.; Guo, Y. Discovery of Amphiphilic Xanthohumol Derivatives as Membrane-Targeting Antimicrobials against Methicillin-Resistant Staphylococcus aureus. J. Med. Chem. 2023, 66, 962–975. [Google Scholar] [CrossRef]
- Betancur, M.; López, J.; Salazar, F. Antimicrobial activity of compounds from hop (Humulus lupulus L.) following supercritical fluid extraction: An overview. Chil. J. Agric. Res. 2023, 83, 499–509. [Google Scholar] [CrossRef]
- Kolodziejczak, A.; Dziedzic, M.; Algiert-Zielinska, B.; Mucha, P.; Rotsztejn, H. A Novel Look at Mechanisms and Applications of Xanthohumol (XN) in Dermatology and Cosmetology. Int. J. Mol. Sci. 2024, 25, 11938. [Google Scholar] [CrossRef]
- Reveron, I.; Plaza-Vinuesa, L.; Santamaria, L.; Oliveros, J.C.; Rivas, B.L.; Munoz, R.; Lopez de Felipe, F. Transcriptomic Evidence of Molecular Mechanisms Underlying the Response of Lactobacillus Plantarum WCFS1 to Hydroxytyrosol. Antioxidants 2020, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Nakao, R.; Takatsuka, A.; Mandokoro, K.; Narisawa, N.; Ikeda, T.; Takai, H.; Ogata, Y. Multimodal inhibitory effect of matcha on Porphyromonas gingivalis. Microbiol. Spectr. 2024, 12, e0342623. [Google Scholar] [CrossRef]
- Ding, J.; Tan, L.; Wu, L.; Li, J.; Zhang, Y.; Shen, Z.; Zhang, C.; Zhao, C.; Gao, L. Regulation of tryptophan-indole metabolic pathway in Porphyromonas gingivalis virulence and microbiota dysbiosis in periodontitis. NPJ Biofilms Microbiomes 2025, 11, 37. [Google Scholar] [CrossRef]
- Confessor, M.V.A.; Agreles, M.A.A.; Campos, L.A.D.; Neto, A.F.S.; Borges, J.C.; Martins, R.M.; Scavuzzi, A.M.L.; Lopes, A.C.S.; Kretzschmar, E.A.D.; Cavalcanti, I.M.F. Olive oil nanoemulsion containing curcumin: Antimicrobial agent against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 2024, 108, 241. [Google Scholar] [CrossRef]
- Janesomboon, S.; Sawaengwong, T.; Muangsombut, V.; Vanaporn, M.; Santanirand, P.; Kritsiriwuthinan, K.; Gundogdu, O.; Chantratita, N.; Nale, J.Y.; Korbsrisate, S.; et al. Synergistic antibacterial activity of curcumin and phage against multidrug-resistant acinetobacter baumannii. Sci. Rep. 2025, 15, 8959. [Google Scholar] [CrossRef] [PubMed]
- Beganovic, S.; Wittmann, C. Medical properties, market potential, and microbial production of golden polyketide curcumin for food, biomedical, and cosmetic applications. Curr. Opin. Biotechnol. 2024, 87, 103112. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, S.; Sharma, K.; Firdaus, Z.; Aditi, P.; Neogi, K.; Bansal, M.; Gupta, M.K.; Shanker, A.; Singh, R.K.; et al. Correction: Quantum curcumin mediated inhibition of gingipains and mixed-biofilm of Porphyromonas gingivalis causing chronic periodontitis. RSC Adv. 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Vidal, H.; Sanchez, M.C.; Alonso-Espanol, A.; Figuero, E.; Ciudad, M.J.; Collado, L.; Herrera, D.; Sanz, M. Antimicrobial Activity of EPA and DHA against Oral Pathogenic Bacteria Using an In Vitro Multi-Species Subgingival Biofilm Model. Nutrients 2020, 12, 2812. [Google Scholar] [CrossRef] [PubMed]
- Blanc, V.; Isabal, S.; Sanchez, M.C.; Llama-Palacios, A.; Herrera, D.; Sanz, M.; Leon, R. Characterization and application of a flow system for in vitro multispecies oral biofilm formation. J. Periodontal Res. 2014, 49, 323–332. [Google Scholar] [CrossRef]
- Bravo, E.; Arce, M.; Ribeiro-Vidal, H.; Herrera, D.; Sanz, M. The Impact of Candida albicans in the Development, Kinetics, Structure, and Cell Viability of Biofilms on Implant Surfaces-An In Vitro Study with a Validated Multispecies Biofilm Model. Int. J. Mol. Sci. 2024, 25, 3277. [Google Scholar] [CrossRef]
- Chen, S.; Huang, T.; Zhou, Y.; Han, Y.; Xu, M.; Gu, J. AfterQC: Automatic filtering, trimming, error removing and quality control for fastq data. BMC Bioinform. 2017, 18, 80. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Thorvaldsdottir, H.; Turner, D.; Mesirov, J.P. igv.js: An embeddable JavaScript implementation of the Integrative Genomics Viewer (IGV). Bioinformatics 2023, 39, btac830. [Google Scholar] [CrossRef] [PubMed]
- Kopylova, E.; Noe, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
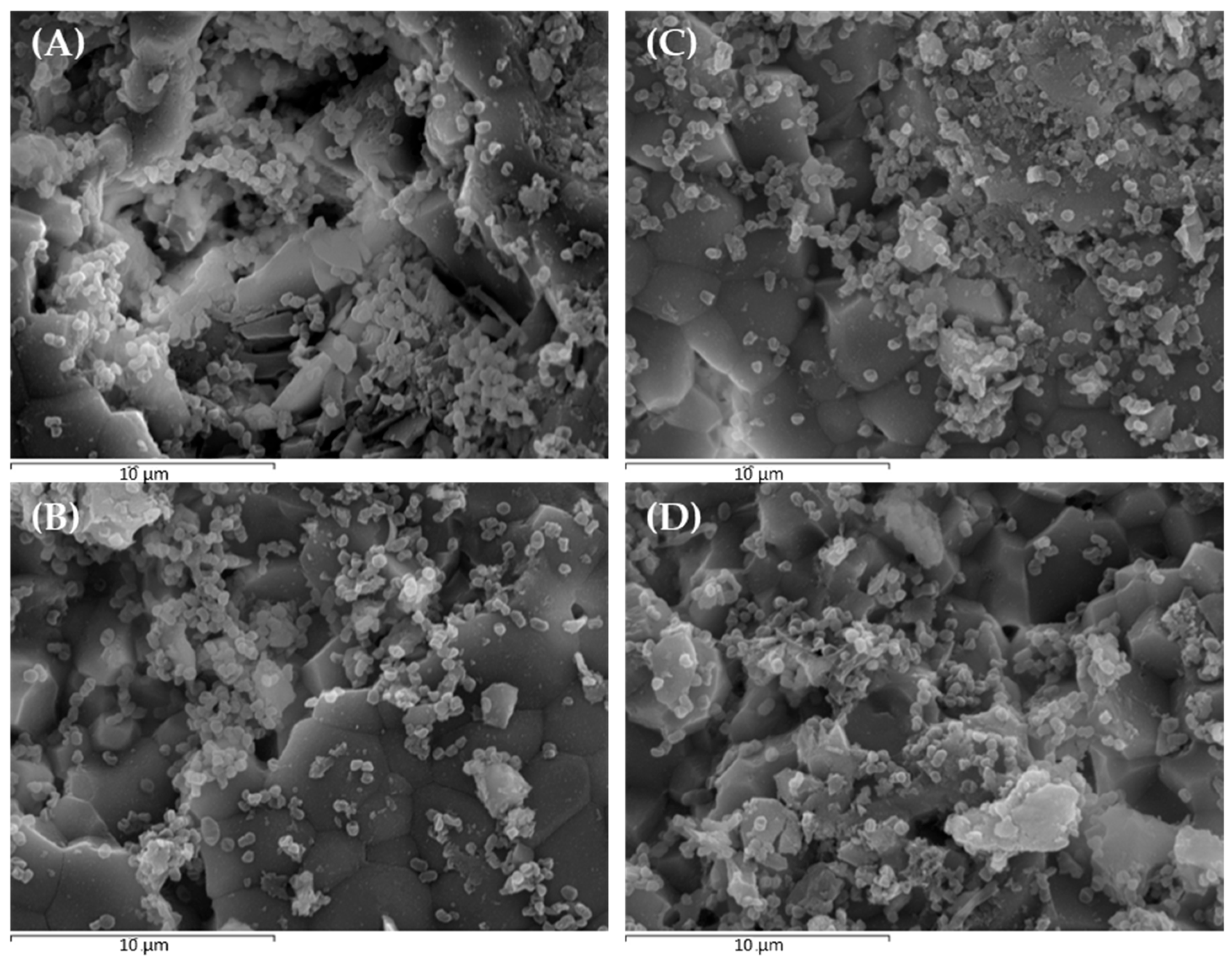
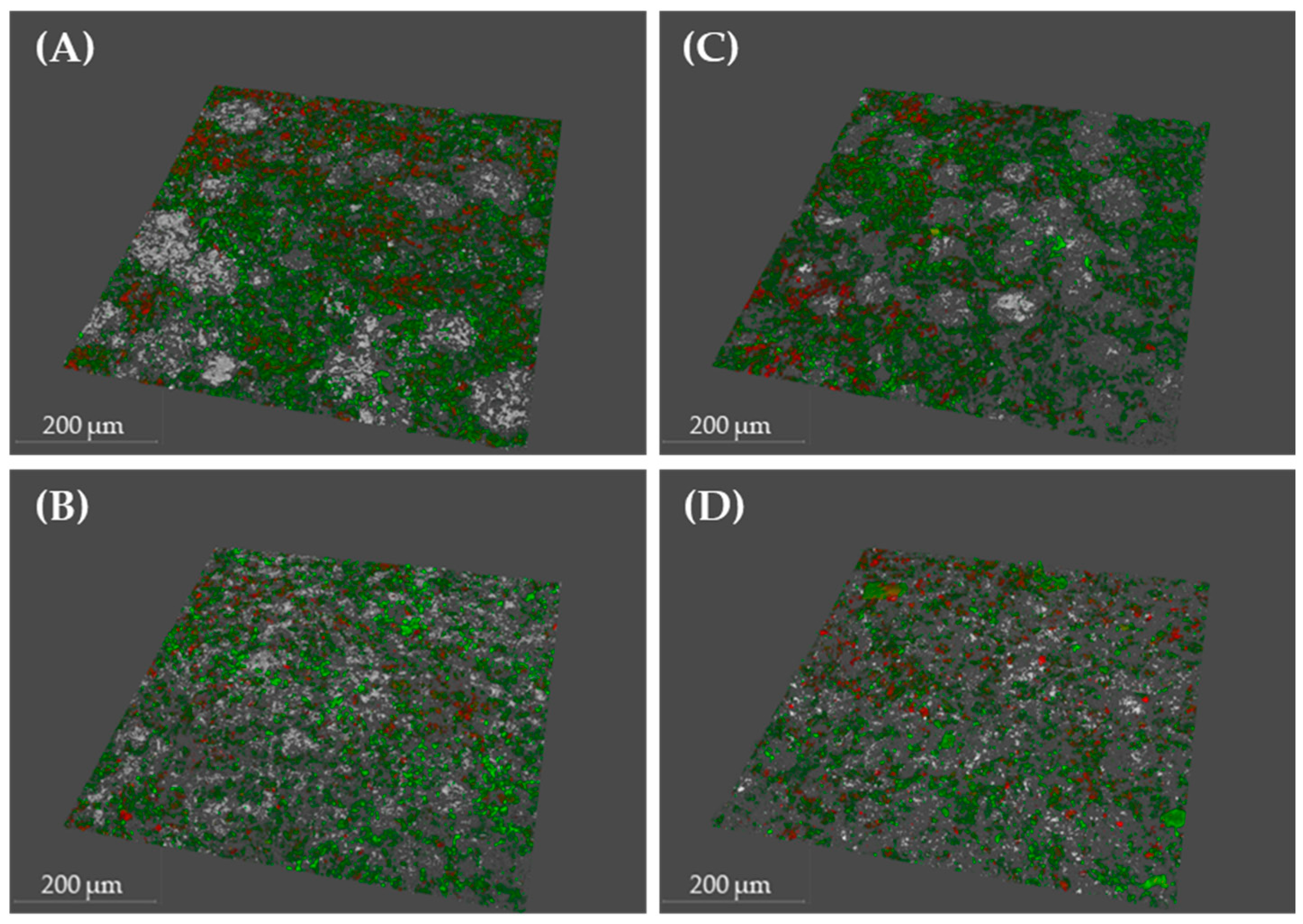

| Condition | Bacterial Density (µm3/µm2) | % Viability | Roughness Coefficient (Ra*) | |||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| PBS | 8.4 | 1.64 | 79.29 | 4.79 | 0.61 | 0.15 |
| DMSO | 10.33 | 1.24 | 78.57 | 3.1 | 0.51 | 0.06 |
| XN | 10.61 | 2.13 | 72.59 | 12.32 | 0.49 | 0.15 |
| Cur | 8.23 | 0.57 | 78.24 | 5.95 | 0.63 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo, E.; Chamorro, C.; Herrera, D.; Sanz, M. Differential Gene Expression of Porphyromonas gingivalis in the Presence or Absence of Xanthohumol and Curcumin in a Dynamic In Vitro Biofilm Model. Int. J. Mol. Sci. 2025, 26, 11315. https://doi.org/10.3390/ijms262311315
Bravo E, Chamorro C, Herrera D, Sanz M. Differential Gene Expression of Porphyromonas gingivalis in the Presence or Absence of Xanthohumol and Curcumin in a Dynamic In Vitro Biofilm Model. International Journal of Molecular Sciences. 2025; 26(23):11315. https://doi.org/10.3390/ijms262311315
Chicago/Turabian StyleBravo, Enrique, Cristina Chamorro, David Herrera, and Mariano Sanz. 2025. "Differential Gene Expression of Porphyromonas gingivalis in the Presence or Absence of Xanthohumol and Curcumin in a Dynamic In Vitro Biofilm Model" International Journal of Molecular Sciences 26, no. 23: 11315. https://doi.org/10.3390/ijms262311315
APA StyleBravo, E., Chamorro, C., Herrera, D., & Sanz, M. (2025). Differential Gene Expression of Porphyromonas gingivalis in the Presence or Absence of Xanthohumol and Curcumin in a Dynamic In Vitro Biofilm Model. International Journal of Molecular Sciences, 26(23), 11315. https://doi.org/10.3390/ijms262311315








