Abstract
Hypoxia, characterized by insufficient oxygen saturation, triggers a wide array of vascular responses aimed at enhancing cell survival and proliferation. This process is primarily driven by the activation of oxygen-sensing hypoxia-inducible factors (HIFs). HIF-1α, a key mediator in this context, plays a crucial role in vascular restructuring in response to low oxygen tension and oxygen-independent signaling pathways, making it a promising therapeutic target for ischemic cardiovascular diseases such as peripheral artery disease and coronary artery disease. In this review, we explore both oxygen-dependent and oxygen-independent mechanisms of HIF-1α regulation, the role of the HIF protein family in vessel collateralization, and translational efforts to leverage HIF-1α‘s pivotal role in hypoxia signaling for the development of clinical treatments for ischemic cardiovascular disease.
1. Introduction
Oxygen homeostasis, the balance of oxygen supply and demand, is crucial to the normal functioning of molecular and cellular processes involved in cell metabolism, differentiation, proliferation, and survival, as well as organ function and human survival [1,2]. Humans have adapted cellular and biochemical responses to combat hypoxic insult key to many disease processes. Among these, hypoxia-inducible factors (HIFs) are the most extensively studied [3]. HIF-1 was identified in 1992 as a transcription factor that upregulates erythropoietin (EPO) production in response to hypoxia by binding to the EPO enhancer and increases its transcription [4,5,6]. EPO is a glycoprotein hormone produced by specialized interstitial peritubular fibroblast-like cells of the kidney which acts to promote erythropoiesis in the bone marrow in response to hypoxia and/or anemia, thereby increasing the number of circulating red blood cells and increasing oxygen delivery to tissues [7].
Further studies have since uncovered the hypoxic regulatory mechanisms of HIF-1α and the more than 100 genes that it regulates [8]. HIF-1α plays a crucial role in promoting the formation of new blood vessels (angiogenesis) through upregulation of growth factors such as VEGF [9,10,11], facilitating an energy-conserving metabolic switch from aerobic to anaerobic metabolism via the upregulation of key glycolytic enzymes, increase in glucose transporters in the cell membrane, and repression of mitochondrial TCA cycle enzymes—all of which effectively increase the intracellular oxygen tension [12,13]. Additionally, HIF-1α induction has been shown to increase EPO production to increase circulating RBC volume [7], dampen the inflammatory response via extracellular adenosine signaling [14], and promote cell proliferation and survival in hypoxic environments, including solid tumors [15,16].
These HIF-driven cellular mechanisms are central to tissue survival in response to ischemic events, such as the growth of new collateral blood vessels in occluded cerebral, peripheral, and coronary arteries to restore local circulation [17]. The purpose of this review paper is to explore the biological regulation of the HIF proteins, the role of HIFs and their downstream targets in promoting angiogenesis, and the clinical implications of therapeutic angiogenesis in ischemic coronary and peripheral artery disease.
2. Oxygen-Dependent Regulation of the HIFs
HIF-1α and HIF-2α are heterodimeric transcription factors that belong to the basic helix-loop-helix PER-ARNT-SIM family (bHLH-PAS). They consist of an oxygen-sensitive alpha subunit and a constitutively expressed beta subunit (HIF-1β) [18]. The beta subunit is also known as the aryl hydrocarbon receptor nuclear translocator (ARNT) and is encoded by ARNT1 and ARNT2 [19]. HIF-1β forms a heterodimer with both HIF-1α and HIF-2α [18]. There are three isoforms of the alpha subunit, named HIF-1α, HIF-2α, and HIF-3α, respectively [20]. While the alpha subunits of HIF-1 and HIF-2 exhibit stable transcription, they are tightly regulated at the protein level [21]. The alpha subunit contains an oxygen-dependent degradation (ODD) domain with two specific proline residues that undergo hydroxylation by several prolyl hydroxylase domain proteins (PHD1-4) under normal oxygen tension, or “normoxic” conditions [22]. Hydroxylation of the HIF alpha subunits occurs in the cytoplasm, leading to the binding of the alpha subunit to Von Hippel Lindau protein (VHL) [23]. This interaction forms a complex with the E3 ubiquitin ligase, resulting in polyubiquitylation and subsequent degradation via the ubiquitin-proteasome pathway [24]. The half-life of the HIF alpha subunits in the cytosol is approximately 5 min, leading to rapid protein degradation in normoxic conditions [25] (left side of Figure 1).
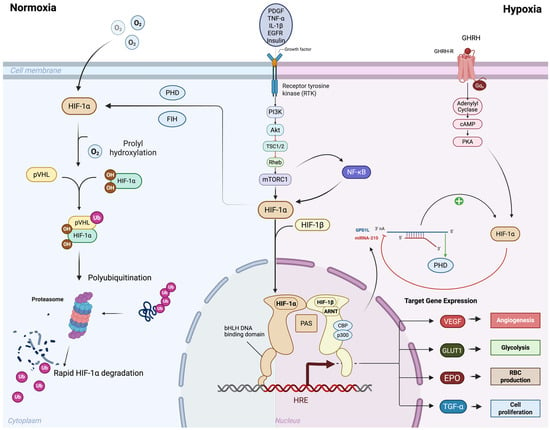
Figure 1.
Oxygen-dependent and oxygen-independent regulation of HIF-1α signaling. Under normoxic conditions (left), HIF-1α undergoes prolyl hydroxylation by prolyl hydroxylase domain proteins (PHDs) and factor inhibiting HIF (FIH), enabling recognition by von Hippel–Lindau protein (pVHL). This leads to ubiquitination and proteasomal degradation of HIF-1α, preventing transcriptional activity. Under hypoxic conditions (right), reduced hydroxylation stabilizes HIF-1α, allowing its accumulation and dimerization with HIF-1β in the nucleus. The HIF-1α/β complex, together with transcriptional co-activators CBP and p300, binds to hypoxia response elements (HREs) to activate transcription of target genes. In addition to this oxygen-dependent regulation, HIF-1α can also be stabilized through oxygen-independent mechanisms, largely mediated by growth factor/receptor signaling pathways. These include PI3K–Akt/mTOR, NF-κB, and EGFR signaling, which enhance HIF-1α synthesis and transcriptional activity even under normoxia. Growth factors such as PDGF, TNF-α, IL-1β, and GHRH further potentiate these effects, amplifying the hypoxic response. Through both oxygen-dependent and oxygen-independent mechanisms, HIF-1α drives the expression of target genes that promote adaptive responses including angiogenesis (VEGF), glycolysis (GLUT1), erythropoiesis (EPO), and cell proliferation (TGF-α). Created in BioRender. Reme, A. (2025) https://BioRender.com/wytkie (accessed on 6 October 2025). Abbreviations: HIF, hypoxia inducible factors; PHD, prolyl hydroxylase domain; FIH, Factor Inhibiting HIF; pVHL, von Hippel–Lindau protein; HRE, hypoxia response elements, EGFR, Epidermal Growth Factor Receptor, PDGF, Platelet-Derived Growth Factor; TNF-α, Tumor Necrosis Factor-alpha; IL-1β, Interleukin-1 beta; GHRH, growth hormone-releasing hormone; VEGF, Vascular Endothelial Growth Factor; GLUT1, Glucose Transporter Type 1; EPO, erythropoietin; TGF-α, Transforming Growth Factor Alpha.
The hydroxylation of the HIF alpha subunit by PHD is contingent upon the presence of molecular oxygen, α-ketoglutarate, ascorbate, as well as iron as a catalyst [26]. Disruption of the iron catalyst, either through iron chelation with deferoxamine (DFO) or by competing with the PHD iron binding site using cobalt chloride (CoCl2), prevents PHD-mediated hydroxylation of HIF alpha subunits, thereby chemically stabilizing them in in vitro experiments [27,28]. Under hypoxic conditions, PHD proteins are unable to hydroxylate the HIF alpha subunits, allowing the HIF alpha subunit to translocate to the nucleus, where it forms a heterodimer with the HIF beta subunit [29]. The HIF heterodimer complex binds to specific core DNA sequences, typically located near the promoters of HIF target genes, known as hypoxic response elements (HREs) [30]. The bHLH sequence is essential for DNA-binding, while the three PAS regions, PAS-A, PAS-B, and PAS-associated C-terminal domain, are involved in heterodimerization [18]. HIF-1α and HIF-2α contain N-terminal and C-terminal transactivation domains (N-TAD and C-TAD, respectively) that are involved in the activation of HIF target genes [31]. These domains interact with additional transcriptional co-activators, most notably CBP and p300, which have lysine acetyl-transferase activity [32]. The CTAD region of the HIF alpha subunit polypeptide is subject to an additional level of oxygen-dependent regulation via the Factor Inhibiting HIF (FIH). FIH hydroxylates an asparagine residue within the CTAD domains at even lower oxygen tensions than PHD proteins, due to its lower Km for oxygen, thus exerting negative regulation on the HIF alpha subunits even under hypoxic conditions [33].
HIF-1α and HIF-2α exhibit strong sequence conservation between their bHLH and PAS regions, indicating their ability to bind identical DNA regions [18]. However, their NTAD regions confer target gene selectivity to the two proteins, likely secondary to distinct interactions with various transcriptional co-activators [34]. Interestingly, the CTAD region shows the least sequence conservation between the two proteins, yet they act to transactivate genes common to both HIF-1α and HIF-2α [35]. Several splice variants of HIF-3α exist, lacking a functional CTAD region and possibly an NTAD region [20]. The most extensively studied variant, HIF-3AF, lacks both transactivation domains, and functions to negatively regulate HIF-1α in an oxygen-independent manner [36]. HIF-3α and its therapeutic potential for neovascularization are less explored in the literature. Therefore, the remainder of this review will focus on HIF-1α and HIF-2α.
HIF-1α and HIF-2α demonstrate temporal differences in their gene expression, with HIF-1α responding to acute hypoxia within minutes and rapidly inducing the expression of its downstream target genes [37,38]. At around 8 h, HIF-1α levels peak and begin to decrease and HIF-2α levels begin to rise. By 24–48 h, HIF-2α levels become the more active responder to chronic hypoxia [34,39]. The fall in HIF-1α protein levels can be partly attributed to hypoxia-associated factor (HAF) mediated ubiquitination which targets HIF-1α for VHL-mediated protein degradation in proliferating cells irrespective of oxygen tension [40]. This oxygen-independent regulation does not apply to HIF-2α [41].
3. Oxygen-Independent Regulation of the HIFs
Although the HIF alpha subunits are traditionally regulated at the post-translational level through oxygen-dependent hydroxylation, they are also influenced by oxygen-independent interactions with other cell signaling pathways [42]. One area of signaling crosstalk comes from the NF-κB pathway [43]. It is unsurprising that crosstalk exists between hypoxia and inflammatory cell signaling. Indeed, several studies have identified an NF-κB binding site within the promoter of HIF-1α [44]. One such study demonstrated that HIF-1α mRNA and protein levels increased in response to exogenous reactive oxygen species (ROS) administration, specifically H2O2, to cultured pulmonary artery smooth muscle cells (PASMCs) under normoxic conditions [44,45]. This finding suggests that NF-κB directly upregulates HIF-1α transcription in a manner that does not depend on oxygen levels [46].
Interestingly, TNF-α, a potent cell surface activator of NF-κB, has been shown to promote HIF-1α protein activity. However, the mechanism remains debated due to conflicting findings regarding increased HIF-1α DNA binding, elevated HIF-1α mRNA levels, and post-translational protein stabilization that may vary based on cell types and experimental conditions [47,48,49]. For example, TNF-α has been shown to upregulate HIF-1α mRNA and protein levels via NF-κB in human pterygium fibroblasts in normoxic conditions [50] while it interferes with the transcription of HIF target genes in cultured smooth muscle cells during hypoxia [51]. IL-1β can also upregulate HIF-1α in an NF-κB-dependent manner [52]. Additionally, NF-κB can enhance HIF-1α expression in hypoxic environments, particularly in the central regions of the solid tumor microenvironment [53]. Hypoxia has been shown to upregulate HIF-1α mRNA via NF-κB through a PI3K/AKT pathway dependent mechanism in PASMCs [54].
The PI3K/AKT/mTOR and PI3K/AKT/FRAP pathways can also induce HIF-1α expression independently of NF-κB, in both normoxia and hypoxia [55]. Various cell surface ligands and receptors, such as EGFR, PDGF, TNF-α, IL-1β, and insulin, can activate the PI3K/AKT pathway [9,56]. Interestingly, recent studies have identified growth hormone-releasing hormone (GHRH) as an upstream, oxygen-independent activator of HIF-1α in iPSC-derived cardiomyocytes via GHRH/GHRH-R/cAMP signaling, acting as a mediator of cardiomyocyte proliferation and oxidative phosphorylation [57,58].
Additionally, various post-translational modifications can occur in HIF proteins, such as the phosphorylation and acetylation of the HIF-1α protein [59]. These modifications can either positively or negatively regulate HIF-1α, depending on the location of the modified amino acid within the protein [60]. For instance, the phosphorylation of serine residues by ERK1/2 in the MAPK pathway enhances HIF-1α transcriptional activity and promotes cell survival following hypoxic injury in cardiomyocytes [61]. Conversely, phosphorylation events in the PAS or ODD regions inhibit HIF-1α protein activity [62].
Moreover, the post-transcriptional modification of HIF-1α mRNA by microRNAs (miRNAs) introduces an additional layer of regulation [63]. Active HIF-1α directly upregulates several small ~22 bp miRNAs, which in turn regulate the stability of HIF-1α mRNA or protein, either positively or negatively [64]. Among these, miR-210 is the most extensively studied miRNA involved in regulating HIF-1α activity [65,66,67]. Under hypoxic conditions, HIF-1α directly upregulates miR-210, which then binds to its target protein glycerol-3-phosphate dehydrogenase 1-like (GPD1L) [68]. GPD1L normally enhances the activity of PHD enzymes, promoting HIF-1α protein hydroxylation and degradation [68]. The increase in miR-210 by active HIF-1α during hypoxia creates a positive feedback loop leading to the downregulation of GPD1L, reduced PHD enzyme activity, and stabilized HIF-1α protein [65,69]. Other miRNAs, such as miR-155, can bind to the 3′ UTR region of HIF-1α mRNA transcripts, interfering with translation [70] (right side of Figure 1).
4. HIF Proteins and Neovascularization
The term “neovascularization” encompasses the various processes that lead to the formation of new blood vessels, including vasculogenesis, arteriogenesis, and angiogenesis [71]. Vasculogenesis occurs during embryonic development and involves de novo formation of blood vessels from vascular progenitor cells [72]. Although HIF proteins play a pivotal role in vasculogenesis, this process is confined to embryonic development and is beyond the scope of this review.
Arteriogenesis refers to the formation of collateral vessels from preexisting vessels, triggered by changes in hemodynamic pressure due to distal arterial occlusion [10]. These collaterals can be observed with iodinated contrast beyond the level of arterial occlusion during angiogram procedures. As the arterial lumen narrows due to progressive atherosclerotic stenosis, increased in fluid shear stress remodels the pre-existing artery–arteriolar connections, facilitating blood flow along the path of least resistance [73]. The increase in fluid shear stress enhances the activity of endothelial nitric oxide synthase (eNOS), leading to the release of nitric oxide and promoting the relaxation of smooth muscle cells (SMCs) and vasodilation [74].
Alongside, VEGF is released with monocyte chemotactic protein-1 (MCP-1), which facilitates the upregulation of cell adhesion molecules (CAMs) on the endothelial cell surface and recruitment of monocytes, respectively [75]. Monocytes and platelets localize to the CAMs where they secrete various growth factors and cytokines to stimulate endothelial cell proliferation, induce a switch in SMCs from a contractile to a proliferative phenotype, and ultimately promote the proliferation of collateral arterioles [74]. The process concludes with collateral vessel pruning, where many smaller arterioles occlude in favor of fewer, larger arterioles, optimizing flow and distal perfusion [74,76]. However, arteriogenesis often falls short of restoring adequate distal perfusion, as seen in cases of PAD. Collaterals formed through arteriogenesis are frequently observed in patients undergoing surgical intervention with chronic limb-threatening ischemia (CLTI) [77].
While the initiation of arteriogenesis is triggered by increased fluid shear stress, angiogenesis is initiated by tissue ischemia itself [71,78]. Angiogenesis involves the formation of new capillaries in response to ischemia, enhancing the delivery of oxygen and nutrients to the tissue [72,79]. HIF proteins play a crucial role in angiogenesis, as hypoxia stabilizes the alpha subunits, promoting their translocation to the nucleus, heterodimerization with the beta subunit, and DNA binding, followed by the upregulation of numerous potent pro-angiogenic genes [10,80]. Angiogenesis can occur through two mechanisms: sprouting and non-sprouting, or intussusceptive angiogenesis [79,81].
HIF expression can be upregulated in many cell types in the presence of ischemia, including fibroblasts, cardiomyocytes, skeletal muscle cells, immune cells, and solid tumor cells [80,82]. VEGF, the most extensively studied and potent stimulator of angiogenesis in the ischemic microenvironment, is directly upregulated by HIF [10,83]. In sprouting angiogenesis, VEGF binds its receptor VEGFR-2 on endothelial cells which inducing the formation of endothelial tip cells [76,83]. These tip cells guide the growing vessel towards its chemotactic source through their projections, rather than by elongating the blood vessel.
The close interplay between VEGF and anti-angiogenic Notch signaling facilitates coordinated formation of the new vessel [77,78]. The tip cell exhibits high VEGF/VEGFR-2 and high delta like ligand 4 (Dll4) expression with low Notch signaling [78]. The increased Dll4 increases Notch signaling in neighboring endothelial cells, inhibiting their migration. These endothelial cells, with higher Notch signaling and lower Dll4 expression, form the stalk cells, which exhibit a proliferative phenotype that aids in the elongation of the new vessel [79,80].
HIF signaling in stalk cells maintains a sustained glycolytic metabolism, promoting cellular proliferation in low oxygen tension [84,85]. Additionally, HIF-1α promotes the secretion of matrix metalloproteases (MMPs), urokinase plasminogen activator (uPA), and plasminogen activator inhibitor-1 (PAI-1), which function to degrade the basement membrane and surrounding extracellular matrix (ECM) components, creating space for new blood vessels to form [82]. As the lumen of the new vessel forms through a process known as tubulogenesis, HIF-2α upregulates the expression of VE-cadherin to form new endothelial cell junctions, thereby promoting vascular integrity and preventing luminal collapse [80,86].
Additionally, HIF-1α recruits pericytes to surround the endothelial cells, enhancing the vessel’s structural integrity to the vessel and preventing leakage [87]. Furthermore, the delayed onset of HIF-2α compared to HIF-1α elucidates their complementary roles in angiogenesis. HIF-1α rapidly upregulates VEGF expression to initiate angiogenesis, while HIF-2α sustains the pro-angiogenic response in chronic hypoxia, promoting vascular remodeling and integrity [72,84].
HIF-1α also plays a key role in recruiting hematopoietic and endothelial progenitor cells (EPCs) from the bone marrow to ischemic tissue sites of by directly upregulating its downstream target, stromal-derived factor 1-alpha (SDF-1α) [88,89]. SDF-1α, a cytokine secreted by ischemic tissue cells, enters the peripheral circulation and mobilizes to the bone marrow, where it binds with its receptor, CXCR4, on the cell surface of EPCs [90]. SDF-1α works synergistically with other pro-angiogenic mobilizing factors such as VEGF, hepatocyte growth factor (HGF), and eNOS, to mobilize EPCs from the bone marrow into the peripheral circulation [88,91].
A concentration gradient of SDF-1α is established between the ischemic sites of insult and the peripheral circulation, facilitating the homing of EPCs to ischemic areas [92]. Once there, EPCs proliferate and differentiate into mature endothelial, contributing to the development of new blood vessels [89,90]. Additionally, EPCs secrete various growth factors, including VEGF and SDF-1α, which promote angiogenesis and further recruit EPCs to sites of ischemia [89,91]. Studies have shown that SDF-1 levels rise following ischemic events, and cleavage-resistant gene delivery platforms of SDF-1 show therapeutic potential in rodent models of myocardial infarction [93,94,95].
The HIF proteins upregulate a wide array of known pro-angiogenic genes. A full list can be seen in Table 1.

Table 1.
Pro-angiogenic targets of HIF proteins.
The HIF proteins, while mostly known to upregulate pro-angiogenic genes, also upregulate a limited repertoire of anti-angiogenic genes. This is thought to be a negative feedback system that interacts with pro-angiogenic genes upregulated by HIFs. A full list can be seen in Table 2.

Table 2.
Anti-angiogenic targets of HIF proteins.
HIF-1α vs. HIF-2α
Although HIF-1α and HIF-2α share substantial sequence homology and heterodimerize with HIF-1β to regulate hypoxia-responsive genes, they display distinct temporal kinetics and target gene preferences that define complementary biological functions. HIF-1α acts as an acute responder to hypoxia, rapidly accumulating within minutes of oxygen deprivation and peaking within several hours. It preferentially upregulates glycolytic and angiogenic genes, such as VEGF, GLUT1, LDHA, and PDK1, promoting metabolic adaptation to anaerobic conditions and initiating endothelial sprouting [1,103,104]. In contrast, HIF-2α becomes predominant under chronic or sustained hypoxia, showing slower degradation kinetics due to reduced hypoxia-associated factor (HAF)–mediated ubiquitination and maintaining transcriptional activity for 24–48 h [96,97,98]. HIF-2α selectively regulates genes involved in vascular remodeling and oxygen transport, including VE-cadherin, ANGPTL4, EPO, SOD2, and Tie2, thereby supporting endothelial stability, erythropoiesis, and long-term tissue adaptation [105,106].
The functional distinction between HIF-1α and HIF-2α is reinforced by their different coactivator interactions: HIF-1α primarily associates with CBP/p300, whereas HIF-2α engages PGC-1α and other cofactors that extend transcriptional persistence [107,108]. Together, these differences support a coordinated “handoff” model in which HIF-1α initiates the angiogenic response and metabolic reprogramming, whereas HIF-2α sustains vessel maturation and oxygen homeostasis during prolonged ischemia. Therapeutically, concurrent or sequential activation of both isoforms may produce synergistic benefits: HIF-1α–driven neovascular initiation coupled with HIF-2α–mediated vessel stabilization and erythropoietic support—thereby enhancing the efficacy and durability of angiogenic therapies for ischemic cardiovascular and peripheral arterial diseases.
5. HIFs and Ischemic Cardiovascular Disease
Ischemic cardiovascular disease is the leading cause of death in the United States [109]. Over time, the development of atherosclerotic plaque burden leads to arterial stenosis and subsequent occlusion, resulting in downstream tissue ischemia and hypoxia. This condition is marked by reduced blood flow and an inadequate oxygen supply that fails to meet oxygen demand [110]. Atherosclerotic stenosis and occlusion are the pathological basis for many cardiovascular diseases including coronary artery disease (CAD), cerebral ischemia and stroke, mesenteric and renal ischemia, and PAD of the extremities [3,110]. Prolonged and worsening tissue hypoxia from severe atherosclerotic disease ultimately leads to end organ dysfunction, such as ischemic cardiomyopathy in CAD and tissue loss in CLTI, the most severe form of PAD [3]. Additionally, acute plaque rupture and vessel thrombosis in the coronary, peripheral, or cerebral circulation result in acute severe hypoxia and tissue infarction, manifesting as myocardial infarction, acute limb ischemia, and stroke, respectively [111]. Moreover, myocardial conditions such as atrial fibrillation and left ventricular aneurysm, along with atherosclerotic aortic or carotid artery disease, can predispose patients to embolic events, leading to acute tissue ischemia and infarction [109,110]. The abrupt onset of tissue ischemia is often more catastrophic due to the lack of vessel collateralization that can be seen with chronic stenosis and occlusion [3]. This section will focus on the role of HIF-1α in CAD and PAD, followed by therapeutic implications for promoting angiogenesis and vessel collateralization.
As previously discussed, HIF-1α is the major driver of hypoxia-induced angiogenesis and vessel collateralization to ischemic cardiomyocytes due to coronary artery atherosclerosis [3,112]. Many patients with CAD present with vessel collateralization bypassing obstructive plaque, while others lack collaterals. Increased collateralization correlates with reduced infarct size, lower heart failure risk, and decreased mortality [113,114]. In a porcine model of acute myocardial infarction, overexpression of HIF-1α resulted in increased myocardial perfusion post-injury [115]. HIF-1α expression also supports cardioprotection, reduced infarct size, and ischemic preconditioning [116]. In the acute phase of ischemic insult, this HIF-1α–mediated response serves as a protective mechanism to rescue injured tissue and restore perfusion. However, when hypoxic and ischemic insults are prolonged or overwhelming, the compensatory capacity of HIF-1α becomes maladaptive, tipping the balance toward pathological remodeling, chronic inflammation, and disease progression (Figure 2). Hypoxia-inducible factors (HIFs) exhibit a dual role in ischemia–reperfusion injury. While HIF activation promotes angiogenesis and tissue oxygenation, its activation during reperfusion can exacerbate tissue damage by inducing pro-inflammatory signaling pathways and increasing reactive oxygen species (ROS) production [117,118]. These effects contribute to oxidative stress, trigger apoptotic cell death, and amplify inflammatory responses, thereby worsening injury despite HIF’s beneficial functions.
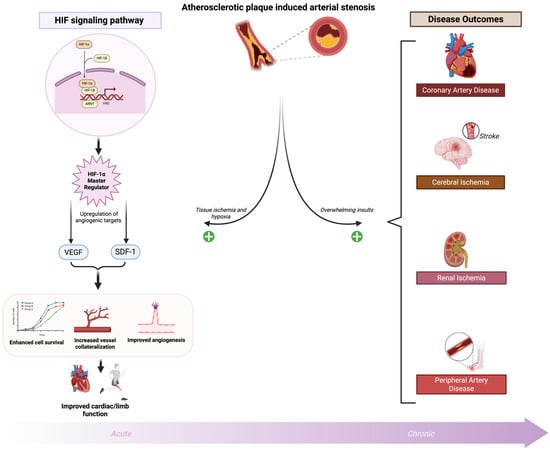
Figure 2.
HIF regulation and ischemic cardiovascular diseases. Atherosclerotic plaque–induced arterial stenosis causes tissue hypoxia from cardiovascular and peripheral ischemia. Hypoxia directly upregulates HIF-1α, which activates transcription of angiogenic targets (e.g., VEGF, SDF-1), promoting cell survival, angiogenesis, vessel collateralization, and improved cardiac/limb function. In the acute phase of ischemic insult, this HIF-1α–mediated response serves as a protective mechanism to rescue injured tissue and restore perfusion. However, when hypoxic and ischemic insults are prolonged or overwhelming, the compensatory capacity of HIF-1α becomes maladaptive, tipping the balance toward pathological remodeling, chronic inflammation, and disease progression. Created in BioRender. Reme, A. (2025) https://BioRender.com/wytkieo (accessed on 5 November 2025). Abbreviations: HIF, hypoxia inducible factor; ARNT, aryl hydrocarbon receptor nuclear translocator; VEGF, Vascular Endothelial Growth Factor; SDF-1, Stromal Cell-Derived Factor-1.
Single nucleotide polymorphisms (SNPs) in the HIF-1 gene, particularly those resulting in a Pro582Ser substitution, are linked to a reduced collateral formation in coronary artery disease (CAD) and are associated with a clinical presentation of stable exertional angina rather than acute myocardial infarction, suggesting a potential role in earlier disease presentation [119]. In a Mexican population, the SNP rs2057482 is correlated with a decreased risk of developing premature CAD [120]. Although beyond the scope of this review, the same SNP is associated with an increased risk of various cancers and is predictive of clinical outcomes, with reduced binding to microRNA-199a, a negative regulator of HIF-1 levels that binds to the 3′-UTR [121,122] This suggests that elevated HIF-1 protein levels may offer protection against coronary ischemic events but could predispose patients to cancer progression, potentially mediated by microRNA-199a. Indeed, the genetic diversity of HIF1A and the varying risks of cancer versus CAD protection are intriguing and warrant further investigation.
Conversely, a recent systemic review and meta-analysis by Chaar and colleagues have found no association between SNPs of HIF-1 and the risk of peripheral artery disease [123]. These risk factors are linked to decreased HIF-1α expression, which reduces VEGF levels and endothelial progenitor cell recruitment. HIF-1α transcriptional activity promotes endothelial cell sprouting, migration, and proliferation under hypoxic conditions [3]. Vascular smooth muscle cells also contribute to vascular integrity during peripheral arterial perfusion [124]. Borton et al. demonstrated that smooth muscle-specific deletion of ARNT (HIF-1β) increased vascular permeability and tissue damage in mice after femoral artery ligation, resembling acute limb ischemia [124]. These findings complicate the development of effective HIF-based therapies for PAD.
Acute limb ischemia, often resulting from emboli, differs from chronic PAD but can manifest in PAD patients as acute on chronic limb ischemia. Tuomisto et al. observed elevated expression of HIF-1α, HIF-2α, VEGF, VEGFR-2, and TNF-α in cases of acute on chronic limb ischemia compared to chronic limb ischemia [125]. The heterogeneity among PAD patient populations, including socioeconomic factors, may affect HIF-1α expression and collateralization [126].
6. Clinical Limitations of Conventional Revascularization: Rationale for HIF-Based Therapeutic Approaches
The standard treatment for ischemic cardiovascular disease involves restoring arterial perfusion to alleviate hypoxia. In cases of CAD and MI, this is typically accomplished through percutaneous coronary intervention (PCI) using balloons and drug-eluting stents [127]. Some patients with multivessel disease or unfavorable anatomy are better candidates for coronary artery bypass grafting (CABG), which traditionally requires sternotomy and cardiopulmonary bypass, although less invasive options are emerging [128]. Ischemic stroke is treated with tissue plasminogen activator (tPA) or mechanical thrombectomy to restore perfusion [129].
Chronic limb-threatening ischemia (CLTI) is characterized by ischemic pain or tissue loss, most often in the distal lower extremities. Without intervention, these patients face a 22% annual risk of major limb amputation [130]. As with CAD, treatment involves endovascular or surgical revascularization to improve distal blood flow, oxygen delivery, pain relief, wound healing, and limb salvage. However, many patients are not candidates for revascularization due to comorbidities, previous failed interventions, or lack of suitable outflow targets.
Diabetes frequently coexists with CLTI and contributes to both macrovascular and microvascular disease [131]. Occlusions often occur in the tibial and foot arteries, making surgical bypass challenging and less durable due to their distal location. Even when large vessels are successfully treated, microvascular disease in the diabetic foot remains a barrier to healing. Patients who cannot undergo revascularization are deemed to have “no-option” CLTI. In this context, targeting molecular pathways that enhance tissue oxygenation, such as upregulating hypoxia-inducible factor 1-alpha (HIF-1α) to promote angiogenesis, emerges as a promising strategy [132]. By addressing microvascular insufficiency and stimulating neovascular growth, HIF-based therapies offer a novel clinical rationale for improving outcomes in patients who cannot benefit from standard perfusion restoration.
7. Prolyl Hydroxylase Domain Inhibition
HIF-1α and HIF-2α are regulated by oxygen-dependent prolyl hydroxylase domain (PHD) enzymes, which target them for degradation. Inhibiting PHD enzymes stabilizes HIF proteins and may promote angiogenesis [133]. Several preclinical studies have shown promise in this approach. In murine hindlimb ischemia models, PHD knockout or knockdown improved perfusion, motor function, and capillary density [134]. Studies using short hairpin RNA (shRNA) targeting PHD2 delivered via a minicircle vector (MC-shPHD2) achieved greater transfection efficiency, higher skeletal muscle HIF-1α levels, and up to 50% blood flow recovery compared to conventional vectors [135,136]. These findings underscore the significance of delivery methods in gene-based therapies.
In myocardial infarction models, Phd2 knockout led to significantly increased HIF-1α and VEGF levels in peri-infarct tissue, leading to enhanced neovascularization, reduced fibrosis, and improved cardiac function [116,137,138]. Dual knockdown of PHD and FIH further augmented angiogenesis, progenitor cell recruitment, and reduced apoptosis, with upregulation of downstream genes such as VEGF, FGF2, and KDR [139]. Similar cardioprotective effects have been observed in various mouse and human tissue models using pharmacologic or genetic silencing of PHD proteins [140]. However, not all findings have been favorable. In vitro treatment of human endothelial cells with dimethyloxalylglycine (DMOG), a chemical PHD inhibitor, reduced endothelial proliferation, migration, and tube formation, despite increased HIF levels [141]. Pharmacologic PHD inhibitors, such as DMOG, increase HIF levels by preventing HIF-α degradation; however, their effects on endothelial proliferation can be paradoxical. Elevated HIF stabilization may induce the expression of genes that inhibit cell cycle progression or promote differentiation rather than proliferation [142,143,144]. This suggests that the method of HIF stabilization, cell type, and experimental context significantly influence angiogenic responses. Additionally, off-target effects or metabolic changes induced by DMOG could impair endothelial cell growth, despite increased HIF signaling. These dual and sometimes opposing effects may contribute to the limited clinical efficacy of PHD inhibitors in PAD. Further mechanistic studies are needed to delineate these complex pathways and optimize therapeutic strategies, including the exploration of alternative delivery methods and the identification of patient subgroups most likely to benefit.
Clinically, translation of treatments for PAD patients has been limited. A randomized trial using an oral PHD inhibitor GSK1278863 in PAD patients failed to improve walking performance or increase expression HIF-1 target genes [145]. The study faced limitations such as a short treatment duration, oral administration, and lack of angiographic assessment. While oral PHD inhibitors like Roxadustat, Daprodustat, and Vadadustat have been approved to stimulate erythropoiesis in chronic kidney disease, their efficacy in promoting angiogenesis for PAD or CLTI remains unproven. Roxadustat has shown some promise in increasing hemoglobin levels in heart failure patients, which could theoretically increase perfusion in PAD, but direct comparisons have yet to be studied [146]. Additionally, safety concerns, including the risks of thromboembolism and pulmonary hypertension, further complicate their use.
8. HIF-1α Gene Overexpression
While inhibiting the inhibitor of HIF-1α is a strategy to promote HIF-1α protein stabilization, inducing HIF-1α overexpression is an alternative to promote neovascularization [132]. Gene therapy for therapeutic angiogenesis employs plasmids or viral vectors to deliver target genes to ischemic tissue, with viral vectors including adenovirus, adeno-associated virus, and retroviruses [146]. Early preclinical studies targeting downstream elements of HIF-1α, such as VEGF, HGF, and FGF showed promise; however, clinical trials involving these growth factors yielded inconsistent results in PAD and CAD [147,148]. A trial in diabetic patients with no-option CLTI using VEGF/HGF bicistronic plasmid therapy reported increased serum VEGF, ABIs, and vessel collateralization, along with improved rest pain [149]. However, the trial was limited by a small cohort. These results suggest that coordinated signaling from multiple factors, as induced by HIF-1α, may be essential for robust angiogenesis.
Xue and colleagues demonstrated in a transgenic diabetes mouse model that cardiomyocyte-specific HIF-1α overexpression increased myocardial capillary density and prevented diabetes-mediated cardiac hypertrophy and glycolytic metabolism remodeling [150]. In a mouse model of myocardial infarction, constitutive expression of HIF-1α attenuated infarct size, increased capillary density, and improved heart function 4 weeks post- myocardial infarction [151]. This supports the rationale for targeting HIF-1α directly rather than its downstream factors. Preclinical studies have reinforced this notion. Intramyocardial injection of HIF-1α/VP16 hybrid increased capillary density and blood flow in rats following LAD occlusion, similar to VEGF treatment [152] Combined HIF-1α and VEGF therapy further increased vessel density but did not reduce infarct size. Remote quadriceps injection of HIF-1α promoted coronary vessel growth, reduced infarct size, and improved ventricular function, suggesting a role in ischemic preconditioning [153]. Sarkar et al. demonstrated in a mouse diabetic model of critical limb ischemia that adenoviral HIF-1α (AdCA5) enhanced arterial remodeling and perfusion, promoting both angiogenesis and arteriogenesis [154]. In diabetic mice, AdCA5 improved perfusion, tissue viability, and motor function and increased circulating angiogenic cells (CACs), which are typically diminished in diabetes [154].
Despite promising preclinical data, clinical trials involving intramuscular HIF-1α gene therapy for PAD have yielded disappointing results. A Phase 1 trial showed safety without tumorigenesis or ocular neovascularization, and some patients experiencing pain resolution and ulcer healing [148]. However, a larger double-blinded, randomized control trial in patients with intermittent claudication showed no improvement in walking time, ABIs, or biomarkers [155]. These results may be attributed to low transfection efficiency. Newer vectors like AAV2 and AAV9 may improve outcomes, although they have not yet been tested in humans. Additionally, inadequate preclinical models and variations in patient pathophysiology further complicate the translation [132].
9. Cell-Based HIF-1α Therapies
Stem cell-based therapies hold promise for treating ischemic cardiovascular disease. MSCs, ADSCs, EPCs, and iPSCs can differentiate into various cell types and secrete angiogenic factors [156]. MSCs are particularly appealing due to their ease of harvest and low immunogenicity. Extracellular vesicles (EVs) from stem cells, such as exosomes, deliver pro-angiogenic molecules and influence target cells through paracrine signaling [156]. Stem cells also promote EPC homing via SDF-1α and can differentiate into relevant vascular and cardiac cells [156]. However, the clinical application of unmodified stem cells is limited by poor viability, retention, and homing. Hypoxic/ischemic environments, particularly in diabetics, impair stem cell survival. Strategies to address this include genetic modification, chemical and physical surface modifications, and hydrogel encapsulation. HIF-1α plays a central role in many of these enhancements [132].
Hypoxia preconditioning activates HIF-1α and improves stem cell survival, proliferation, and pro-angiogenic activity [157]. A systematic review of hypoxia-conditioned ADSCs showed consistent upregulation of pro-angiogenic markers and viability [158]. Studies using hypoxia-mimicking agents or reduced oxygen tension confirmed these findings in vitro and in vivo [156]. One murine study showed that hi-MSCs enhanced perfusion, vessel density, and HIF-1α/VEGF expression compared to normoxic MSCs [156]. Direct HIF-1α overexpression in stem cells using plasmids or viral vectors also enhances pro-angiogenic function [159]. CSCs may outperform MSCs in this regard. A study using HIF-1α-transfected CSCs embedded in fibrin gel (HIF-CSC-Gel) improved limb perfusion more than CSCs without the gel [159]. Combined therapy using HIF-1α gene delivery and MSCs in a myocardial infarction model enhanced angiogenesis and cardiac function compared to monotherapies, possibly due to improved MSC engraftment [159]. Future studies should explore combined therapies to optimize outcomes.
Stem cell-derived EVs can also deliver miRNAs like miR-31 and miR-20b to ischemic tissues, promoting angiogenesis and reducing apoptosis in models of myocardial ischemia and reperfusion injury [160]. miR-31 targets FIH, reducing its expression and thereby enhancing HIF-1α activity [161]. Engineering stem cells or EVs with these miRNAs offers another avenue to boost HIF-1α-dependent neovascularization. Interestingly, a recent study shows that overexpressing HIF-1α in MSCs leads to exosomes rich in miRNA-221, capable of shrinking infarct size and cardiac fibrotic scarring in a rat myocardial infarction model [162].
Although HIF-2α’s role in therapeutic angiogenesis has been less explored, it is better understood in cancer, where it aids in vascular remodeling and maintaining integrity during chronic hypoxia [132]. Combining HIF-1α and HIF-2α gene therapies may offer a more durable and functional angiogenic response in atherosclerotic cardiovascular disease [132].
Overall, ischemic cardiovascular diseases, including CAD and PAD, comprise a massive healthcare burden regarding morbidity, mortality, and healthcare economic burden [109]. The mainstay of treatment for both conditions is traditional lifestyle modifications and medical therapy aimed at cardiovascular risk reduction (statins, antiplatelet agents, glycemic control, smoking cessation, etc.) and endovascular or surgical revascularization in cases of AMI and CLTI [127,130]. Surgically or anatomically unfit patients exist in both CAD and PAD, leading to the basis for gene and cell-based therapy to improve neovascularization and improve functional outcomes in cardiac function, maximal walking distance, amputation-free survival, quality of life, morbidity and mortality. Despite the early preclinical promise for both mechanisms of therapy, clinical trials have demonstrated serious limitations of gene- and cell-based therapies, resulting in failed treatment outcomes [148,149]. Hypoxia-inducible factor (HIF) therapies are limited by challenges in drug design, clinical trial execution, safety, and regulatory approval [163]. Additionally, reviews report that HIF inhibitors often lack selectivity and potency, in part because the active sites are elusive and the redundancy between HIF-1α and HIF-2α complicates target validation and drug delivery [164]. A novel drug designed to simultaneously target HIF-1 and HIF-2 could address the redundancy problem that complicates current therapy. Clinical trials have been hampered by poor patient selection, the absence of validated hypoxia biomarkers, and endpoints that fail to capture therapeutic activity in hypoxic environments [163]. Most HIF inhibitors have not progressed beyond early phase trials, resulting in very few approved treatments [165]. Systemic toxicity, off-target effects, and risks such as neoangiogenesis, thrombosis, and cardiovascular events further constrain these approaches. Table 3 summarizes some key human clinical trials evaluating therapeutic strategies for ischemic disease including their delivery challenges and emerging advances.

Table 3.
Clinical trials of therapeutic strategies in ischemic disease.
10. Conclusions
HIF-1α is the master regulator of a myriad of pro-angiogenic growth factors and cell survival responses. Consequently, overexpressing HIF-1 is a crucial strategy for enhancing the outcomes of gene and cell-based therapies in treating ischemic cardiovascular diseases. Future gene therapy-based strategies should focus on optimal vector delivery and transfection efficiency, duration of action, and optimal dosing. Hypoxic preconditioning of stem cells has demonstrated improved pro-angiogenic phenotypes across various stem cell types, primarily through the induction of HIF-1α expression. Future clinical studies on cell-based treatment of ischemic cardiovascular disease should incorporate hypoxic preconditioning to optimize pro-angiogenic and cell retention efficacy. Combining gene and cell-based therapies may yield additive effects in promoting stem cell engraftment, neovascularization, and functional outcomes. Additionally, incorporating stem-cell derived exosomes to deliver miRNAs that promote HIF-1α expression is an active area of ongoing preclinical research and could complement the aforementioned therapeutic strategies. The potential of HIF-2α for therapeutic angiogenesis in ischemic cardiovascular disease is not well studied in the current literature, warranting further investigation, either as a standalone therapy or in combination with HIF-1α gene therapy. Novel strategies to increase neovascularization of ischemic tissues which are not necessarily directly related to HIF-1α are under investigation in our research laboratory and involve the membrane-bound adhesion molecule, E-Selectin [162,166,167,168,169,170,171,172,173]. To date, no mechanistic link has been identified between the angiogenic signaling pathways of HIF-1α and membrane-bound E-Selectin. However, the potential for synergism in combination therapies may warrant further study.
Author Contributions
Conceptualization, E.C., A.-I.S.R., P.J.B., Y.Y.O., D.A.R. and K.G.; writing—original draft preparation, E.C., A.-I.S.R. and P.J.B.; writing—review and editing, A.-I.S.R., B.-N.N., A.B., Z.-J.L. and O.C.V.; visualization, A.-I.S.R. and P.J.B.; supervision, Z.-J.L. and O.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institutes of Health—NIH/NHLBI Catalyze R33 HL156141; NIH T32GM145462 Medical Scientist Training Program; Philanthropy: Eloise & David Kimmelman Foundation.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank all members from the Surgical Vascular Research Labs at DeWitt Daughtry Family Department of Surgery, University of Miami Miller School of Medicine for their helpful discussion and suggestions.
Conflicts of Interest
The authors Ethan Carmichael, Patrick J. Bosco, Anne-Isabelle S. Reme, Yulexi Y. Ortiz, Daniela Alexandra Ramos, Katherine Gomez, Bao-Ngoc Nguyen, and Arash Bornak declare no conflicts of interest. Authors Zhao-Jun Liu (Z.-J.L.) and Omaida C. Velazquez (O.C.V.) declare the following potential conflicts of interest with respect to the research, authorship, and/or presentation and/or publication of some aspects that are indirectly related to this work: E-selectin gene modification technologies aimed as pro neovascularization technologies were developed in our research laboratory and patented/licensed by the University of Miami. These E-Selectin technologies are currently under pre-clinical development by Ambulero Inc., a new start-up company out of the University of Miami that focuses on developing new vascular treatments for ischemic tissue conditions and limb salvage. Z.-J.L. and O.C.V. serve as Ambulero Inc. consultants and chief scientific and medical advisory officers, respectively, and are co-inventors of the E-Selectin technologies and are minority shareholders in Ambulero Inc. Z.-J.L. and O.C.V. are also funded by the NIH/NHLBI and philanthropy in preclinical investigations of E-Selectin technologies and other pro neovascularization technologies.
Abbreviations
The following abbreviations are used in this manuscript:
| HIFs | hypoxia inducible factors |
| EPO | erythropoietin |
| RBC | red blood cell |
| VEGF | Vascular Endothelial Growth Factor |
| TCA | tricarboxylic acid cycle |
| ARNT | aryl hydrocarbon receptor nuclear translocator |
| ODD | oxygen-dependent degradation |
| PHD | prolyl hydroxylase domain |
| VHL | Von Hippel Lindau |
| DFO | deferoxamine |
| CoCl2 | cobalt chloride |
| HREs | hypoxic response elements |
| N-TAD | N-terminal activation domains |
| C-TAD | C-terminal activation domains |
| FIH | Factor Inhibiting HIF |
| HAF | hypoxia-associated factor |
| ROS | reactive oxygen species |
| PASMCs | pulmonary artery smooth muscle cells |
| EGFR | Epidermal Growth Factor Receptor |
| PDGF | Platelet-Derived Growth Factor |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-1β | Interleukin-1 beta |
| GHRH | growth hormone-releasing hormone |
| miRNAs | microRNAs |
| GPD1L | glycerol-3-phosphate dehydrogenase 1-like |
| eNOS | endothelial nitric oxide synthase |
| SMC | smooth muscle cell |
| MCP-1 | monocyte chemotactic protein-1 |
| CAMs | cell adhesion molecules |
| PAD | Peripheral Artery Disease |
| CLTI | chronic limb-threatening ischemia |
| Dll4 | delta like ligand 4 |
| MMPs | matrix metalloproteases |
| uPA | urokinase plasminogen activator |
| PAI-1 | plasminogen activator inhibitor-1 |
| ECM | extracellular matrix |
| EPCs | endothelial progenitor cells |
| HGF | hepatocyte growth factor |
| SDF-1α | Stromal Cell-Derived Factor-1 alpha |
| CAD | coronary artery disease |
| SNPs | Single nucleotide polymorphisms |
| MI | Myocardial Infarction |
| PCI | percutaneous coronary intervention |
| CABG | coronary artery bypass grafting |
| tPA | tissue plasminogen activator |
| shRNA | short hairpin RNA |
| MC-shPHD2 | minicircle vector |
| FGF | Fibroblast Growth Factor |
| KDR | Kinase Insert Domain Receptor |
| DMOG | Dimethyloxalylglycine |
| ABI | Ankle-Brachial Index |
| AdCA5 | adenoviral HIF-1α |
| CACs | circulating angiogenic cells |
| AAV | Adeno-Associated Virus |
| MSCs | Mesenchymal Stem Cells |
| ADSCs | Adipose-Derived Stem Cells |
| iPSCs | Induced Pluripotent Stem Cells |
| EVs | Extracellular vesicles |
| CSCs | Cardiac stem cells |
| HIF-CSC-Gel | HIF-1α-transfected CSCs embedded in fibrin gel |
| AMI | acute myocardial infarction |
References
- Semenza, G.L. Regulation of Mammalian O2 Homeostasis by Hypoxia-Inducible Factor 1. Annu. Rev. Cell Dev. Biol. 1999, 15, 551–578. [Google Scholar] [CrossRef]
- Wenger, R.H. Cellular Adaptation to Hypoxia: O2-sensing Protein Hydroxylases, Hypoxia-inducible Transcription Factors, and O2-regulated Gene Expression. FASEB J. 2002, 16, 1151–1162. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor 1 Is a Basic-Helix-Loop-Helix-PAS Heterodimer Regulated by Cellular O2 Tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A Nuclear Factor Induced by Hypoxia via De Novo Protein Synthesis Binds to the Human Erythropoietin Gene Enhancer at a Site Required for Transcriptional Activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-Inducible Nuclear Factors Bind to an Enhancer Element Located 3′ to the Human Erythropoietin Gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef]
- Haase, V.H. Regulation of Erythropoiesis by Hypoxia-Inducible Factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef]
- Liu, W.; Shen, S.-M.; Zhao, X.-Y.; Chen, G.-Q. Targeted Genes and Interacting Proteins of Hypoxia Inducible Factor-1. Int. J. Biochem. Mol. Biol. 2012, 3, 165–178. [Google Scholar]
- Forsythe, J.A.; Jiang, B.-H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of Vascular Endothelial Growth Factor Gene Transcription by Hypoxia-Inducible Factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular Endothelial Growth Factor Induced by Hypoxia May Mediate Hypoxia-Initiated Angiogenesis. Nature 1992, 359, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia Regulates Vascular Endothelial Growth Factor Gene Expression in Endothelial Cells: Identification of a 5′ Enhancer. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 Mediates Adaptation to Hypoxia by Actively Downregulating Mitochondrial Oxygen Consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef]
- Semenza, G.L.; Roth, P.H.; Fang, H.M.; Wang, G.L. Transcriptional Regulation of Genes Encoding Glycolytic Enzymes by Hypoxia-Inducible Factor 1. J. Biol. Chem. 1994, 269, 23757–23763. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Dachs, G.U.; Gleadle, J.M.; Nicholls, L.G.; Harris, A.L.; Stratford, I.J.; Hankinson, O.; Pugh, C.W.; Ratcliffe, P.J. Hypoxia-Inducible Factor-1 Modulates Gene Expression in Solid Tumors and Influences Both Angiogenesis and Tumor Growth. Proc. Natl. Acad. Sci. USA 1997, 94, 8104–8109. [Google Scholar] [CrossRef]
- Hubbi, M.E.; Semenza, G.L. Regulation of Cell Proliferation by Hypoxia-Inducible Factors. Am. J. Physiol. Cell Physiol. 2015, 309, C775–C782. [Google Scholar] [CrossRef] [PubMed]
- Rey, S.; Semenza, G.L. Hypoxia-Inducible Factor-1-Dependent Mechanisms of Vascularization and Vascular Remodelling. Cardiovasc. Res. 2010, 86, 236–242. [Google Scholar] [CrossRef]
- Wu, D.; Rastinejad, F. Structural Characterization of Mammalian bHLH-PAS Transcription Factors. Curr. Opin. Struct. Biol. 2017, 43, 1–9. [Google Scholar] [CrossRef]
- Hirose, K.; Morita, M.; Ema, M.; Mimura, J.; Hamada, H.; Fujii, H.; Saijo, Y.; Gotoh, O.; Sogawa, K.; Fujii-Kuriyama, Y. cDNA Cloning and Tissue-Specific Expression of a Novel Basic Helix-Loop-Helix/PAS Factor (Arnt2) with Close Sequence Similarity to the Aryl Hydrocarbon Receptor Nuclear Translocator (Arnt). Mol. Cell. Biol. 1996, 16, 1706–1713. [Google Scholar] [CrossRef]
- Maynard, M.A.; Evans, A.J.; Hosomi, T.; Hara, S.; Jewett, M.A.S.; Ohh, M. Human HIF-3α4 Is a Dominant-negative Regulator of HIF-1 and Is Down-regulated in Renal Cell Carcinoma. FASEB J. 2005, 19, 1396–1406. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The Tumour Suppressor Protein VHL Targets Hypoxia-Inducible Factors for Oxygen-Dependent Proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Epstein, A.C.R.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A.; et al. C. elegans EGL-9 and Mammalian Homologs Define a Family of Dioxygenases That Regulate HIF by Prolyl Hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef]
- Kaelin, W.G. The von Hippel-Lindau Tumor Suppressor Protein and Clear Cell Renal Carcinoma. Clin. Cancer Res. 2007, 13, 680s–684s. [Google Scholar] [CrossRef]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. HIFα Targeted for VHL-Mediated Destruction by Proline Hydroxylation: Implications for O2 Sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.E.; Arany, Z.; Livingston, D.M.; Bunn, H.F. Activation of Hypoxia-Inducible Transcription Factor Depends Primarily upon Redox-Sensitive Stabilization of Its α Subunit. J. Biol. Chem. 1996, 271, 32253–32259. [Google Scholar] [CrossRef]
- Schofield, C.J.; Ratcliffe, P.J. Oxygen Sensing by HIF Hydroxylases. Nat. Rev. Mol. Cell Biol. 2004, 5, 343–354. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Dunning, S.P.; Bunn, H.F. Regulation of the Erythropoietin Gene: Evidence That the Oxygen Sensor Is a Heme Protein. Science 1988, 242, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hilliard, G.; Ferguson, T.; Millhorn, D.E. Cobalt Inhibits the Interaction between Hypoxia-Inducible Factor-α and von Hippel-Lindau Protein by Direct Binding to Hypoxia-Inducible Factor-α. J. Biol. Chem. 2003, 278, 15911–15916. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; Kriegsheim, A.V.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-α to the von Hippel-Lindau Ubiquitylation Complex by O2 -Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Mediator of Physiological and Pathophysiological Responses to Hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A Novel Protein That Interacts with HIF-1α and VHL to Mediate Repression of HIF-1 Transcriptional Activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef]
- Arany, Z.; Huang, L.E.; Eckner, R.; Bhattacharya, S.; Jiang, C.; Goldberg, M.A.; Bunn, H.F.; Livingston, D.M. An Essential Role for P300/CBP in the Cellular Response to Hypoxia. Proc. Natl. Acad. Sci. USA 1996, 93, 12969–12973. [Google Scholar] [CrossRef]
- Lando, D.; Peet, D.J.; Gorman, J.J.; Whelan, D.A.; Whitelaw, M.L.; Bruick, R.K. FIH-1 Is an Asparaginyl Hydroxylase Enzyme That Regulates the Transcriptional Activity of Hypoxia-Inducible Factor. Genes Dev. 2002, 16, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-J.; Wang, L.-Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential Roles of Hypoxia-Inducible Factor 1α (HIF-1α) and HIF-2α in Hypoxic Gene Regulation. Mol. Cell. Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef]
- Tian, H.; McKnight, S.L.; Russell, D.W. Endothelial PAS Domain Protein 1 (EPAS1), a Transcription Factor Selectively Expressed in Endothelial Cells. Genes Dev. 1997, 11, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Kanopka, A.; Wilson, W.J.; Tanaka, H.; Poellinger, L. Inhibitory PAS Domain Protein (IPAS) Is a Hypoxia-Inducible Splicing Variant of the Hypoxia-Inducible Factor-3α Locus. J. Biol. Chem. 2002, 277, 32405–32408. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R.H.; Gassmann, M. Oxygen(es) and the Hypoxia-Inducible Factor-1. Biol. Chem. 1997, 378, 609–616. [Google Scholar] [PubMed]
- Kietzmann, T.; Görlach, A. Reactive Oxygen Species in the Control of Hypoxia-Inducible Factor-Mediated Gene Expression. Semin. Cell Dev. Biol. 2005, 16, 474–486. [Google Scholar] [CrossRef]
- Koh, M.Y.; Powis, G. Passing the Baton: The HIF Switch. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef]
- Koh, M.Y.; Lemos, R.; Liu, X.; Powis, G. The Hypoxia-Associated Factor Switches Cells from HIF-1α- to HIF-2α-Dependent Signaling Promoting Stem Cell Characteristics, Aggressive Tumor Growth and Invasion. Cancer Res. 2011, 71, 4015–4027. [Google Scholar] [CrossRef]
- Rocha, S. Gene Regulation under Low Oxygen: Holding Your Breath for Transcription. Trends Biochem. Sci. 2007, 32, 389–397. [Google Scholar] [CrossRef]
- Semenza, G.L. Signal Transduction to Hypoxia-Inducible Factor 1. Biochem. Pharmacol. 2002, 64, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-κB Links Innate Immunity to the Hypoxic Response through Transcriptional Regulation of HIF-1α. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef]
- Bonello, S.; Zähringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Görlach, A. Reactive Oxygen Species Activate the HIF-1α Promoter Via a Functional NFκB Site. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Diebold, I.; Petry, A.; Hess, J.; Görlach, A. The NADPH Oxidase Subunit NOX4 Is a New Target Gene of the Hypoxia-Inducible Factor-1. Mol. Biol. Cell 2010, 21, 2087–2096. [Google Scholar] [CrossRef]
- Oliver, K.M.; Taylor, C.T.; Cummins, E.P. Hypoxia. Regulation of NFκB Signalling during Inflammation: The Role of Hydroxylases. Arthritis Res. Ther. 2009, 11, 215. [Google Scholar] [CrossRef]
- Richard, D.E.; Berra, E.; Pouysségur, J. Angiogenesis: How a Tumor Adapts to Hypoxia. Biochem. Biophys. Res. Commun. 1999, 266, 718–722. [Google Scholar] [CrossRef]
- Taylor, C.T.; Cummins, E.P. The Role of NF-κB in Hypoxia-Induced Gene Expression. Ann. N. Y. Acad. Sci. 2009, 1177, 178–184. [Google Scholar] [CrossRef]
- Ghosh, S.; Paul, A.; Sen, E. Tumor Necrosis Factor Alpha-Induced Hypoxia-Inducible Factor 1α–β-Catenin Axis Regulates Major Histocompatibility Complex Class I Gene Activation through Chromatin Remodeling. Mol. Cell. Biol. 2013, 33, 2718–2731. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Lee, S.J.; Kim, J.C. TNF-α Upregulates HIF-1α Expression in Pterygium Fibroblasts and Enhances Their Susceptibility to VEGF Independent of Hypoxia. Exp. Eye Res. 2017, 164, 74–81. [Google Scholar] [CrossRef]
- Tsapournioti, S.; Mylonis, I.; Hatziefthimiou, A.; Ioannou, M.G.; Stamatiou, R.; Koukoulis, G.K.; Simos, G.; Molyvdas, P.; Paraskeva, E. TNFα Induces Expression of HIF-1α mRNA and Protein but Inhibits Hypoxic Stimulation of HIF-1 Transcriptional Activity in Airway Smooth Muscle Cells. J. Cell. Physiol. 2013, 228, 1745–1753. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Isaacs, J.S.; Lee, S.; Trepel, J.; Neckers, L. IL-1β Mediated Up-regulation of HIF-lα via an NFkB/COX-2 Pathway Identifies HIF-1 as a Critical Link between Inflammation and Oncogenesis. FASEB J. 2003, 17, 1–22. [Google Scholar] [CrossRef]
- Zhong, H.; Chiles, K.; Feldser, D.; Laughner, E.; Hanrahan, C.; Georgescu, M.M.; Simons, J.W.; Semenza, G.L. Modulation of Hypoxia-Inducible Factor 1alpha Expression by the Epidermal Growth Factor/Phosphatidylinositol 3-Kinase/PTEN/AKT/FRAP Pathway in Human Prostate Cancer Cells: Implications for Tumor Angiogenesis and Therapeutics. Cancer Res. 2000, 60, 1541–1545. [Google Scholar]
- Görlach, A.; Bonello, S. The Cross-Talk between NF-κB and HIF-1: Further Evidence for a Significant Liaison. Biochem. J. 2008, 412, e17–e19. [Google Scholar] [CrossRef] [PubMed]
- Laughner, E.; Taghavi, P.; Chiles, K.; Mahon, P.C.; Semenza, G.L. HER2 (Neu) Signaling Increases the Rate of Hypoxia-Inducible Factor 1α (HIF-1α) Synthesis: Novel Mechanism for HIF-1-Mediated Vascular Endothelial Growth Factor Expression. Mol. Cell. Biol. 2001, 21, 3995–4004. [Google Scholar] [CrossRef]
- Treins, C.; Giorgetti-Peraldi, S.; Murdaca, J.; Semenza, G.L.; Van Obberghen, E. Insulin Stimulates Hypoxia-Inducible Factor 1 through a Phosphatidylinositol 3-Kinase/Target of Rapamycin-Dependent Signaling Pathway. J. Biol. Chem. 2002, 277, 27975–27981. [Google Scholar] [CrossRef]
- Kanashiro-Takeuchi, R.M.; Takeuchi, L.M.; Balkan, W.; Wanschel, A.C.B.A.; Hatzistergos, K.E.; Kulandavelu, S.; Saltzman, R.G.; Shehadeh, L.A.; Sha, W.; Schally, A.V.; et al. Cardiomyocyte-Specific Expression of HIF-1α Mediates the Cardioprotective Effects of Growth Hormone Releasing Hormone (GHRH). bioRxiv 2025. [Google Scholar] [CrossRef]
- Wanschel, A.; Daiou, A.; Kuznetsoff, J.; Kurtenbach, S.; Petalidou, K.; Mouskeftara, T.; Gika, H.G.; Rodriguez, D.A.; Papadopoulos, E.I.; Siokatas, G.; et al. Oxygen-Independent, Hormonal Control of HIF-1α Regulates the Developmental and Regenerative Growth of Cardiomyocytes. bioRxiv 2022. [Google Scholar] [CrossRef]
- Albanese, A.; Daly, L.A.; Mennerich, D.; Kietzmann, T.; Sée, V. The Role of Hypoxia-Inducible Factor Post-Translational Modifications in Regulating Its Localisation, Stability, and Activity. Int. J. Mol. Sci. 2020, 22, 268. [Google Scholar] [CrossRef] [PubMed]
- Bárdos, J.I.; Ashcroft, M. Negative and Positive Regulation of HIF-1: A Complex Network. Biochim. Biophys. Acta (BBA) Rev. Cancer 2005, 1755, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Iwamatsu, A.; Aki, T.; Kimura, M.; Nakamura, K.; Nao, T.; Okusa, T.; Matsuzaki, M.; Yoshida, K.; Kobayashi, S. ERK1/2 Regulates Intracellular ATP Levels through α-Enolase Expression in Cardiomyocytes Exposed to Ischemic Hypoxia and Reoxygenation. J. Biol. Chem. 2004, 279, 50120–50131. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.; Stiehl, D.P.; Bohensky, J.; Leshchinsky, I.; Srinivas, V.; Caro, J. MAPK Signaling Up-Regulates the Activity of Hypoxia-Inducible Factors by Its Effects on P300. J. Biol. Chem. 2003, 278, 14013–14019. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Loscalzo, J. MicroRNA-210: A Unique and Pleiotropic Hypoxamir. Cell Cycle 2010, 9, 1072–1083. [Google Scholar] [CrossRef]
- Devlin, C.; Greco, S.; Martelli, F.; Ivan, M. miR-210: More than a Silent Player in Hypoxia. IUBMB Life 2011, 63, 94–100. [Google Scholar] [CrossRef]
- Huang, X.; Ding, L.; Bennewith, K.L.; Tong, R.T.; Welford, S.M.; Ang, K.K.; Story, M.; Le, Q.-T.; Giaccia, A.J. Hypoxia-Inducible Mir-210 Regulates Normoxic Gene Expression Involved in Tumor Initiation. Mol. Cell 2009, 35, 856–867. [Google Scholar] [CrossRef]
- Cao, G.; Fan, P.; Ma, R.; Wang, Q.; He, L.; Niu, H.; Luo, Q. MiR-210 Regulates Lung Adenocarcinoma by Targeting HIF-1α. Heliyon 2023, 9, e16079. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Flach, H.; Onizawa, M.; Wei, L.; McManus, M.T.; Weiss, A. Negative Regulation of Hif1a Expression and TH17 Differentiation by the Hypoxia-Regulated microRNA miR-210. Nat. Immunol. 2014, 15, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.J.; Souza, A.L.; Clish, C.B.; Puigserver, P. A Hypoxia-Induced Positive Feedback Loop Promotes Hypoxia-Inducible Factor 1α Stability through miR-210 Suppression of Glycerol-3-Phosphate Dehydrogenase 1-Like. Mol. Cell. Biol. 2011, 31, 2696–2706. [Google Scholar] [CrossRef]
- Fasanaro, P.; D’Alessandra, Y.; Di Stefano, V.; Melchionna, R.; Romani, S.; Pompilio, G.; Capogrossi, M.C.; Martelli, F. MicroRNA-210 Modulates Endothelial Cell Response to Hypoxia and Inhibits the Receptor Tyrosine Kinase Ligand Ephrin-A3. J. Biol. Chem. 2008, 283, 15878–15883. [Google Scholar] [CrossRef]
- Bruning, U.; Cerone, L.; Neufeld, Z.; Fitzpatrick, S.F.; Cheong, A.; Scholz, C.C.; Simpson, D.A.; Leonard, M.O.; Tambuwala, M.M.; Cummins, E.P.; et al. MicroRNA-155 Promotes Resolution of Hypoxia-Inducible Factor 1α Activity during Prolonged Hypoxia. Mol. Cell. Biol. 2011, 31, 4087–4096. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of Angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in Life, Disease and Medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef]
- Pipp, F.; Boehm, S.; Cai, W.-J.; Adili, F.; Ziegler, B.; Karanovic, G.; Ritter, R.; Balzer, J.; Scheler, C.; Schaper, W.; et al. Elevated Fluid Shear Stress Enhances Postocclusive Collateral Artery Growth and Gene Expression in the Pig Hind Limb. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1664–1668. [Google Scholar] [CrossRef]
- Helisch, A.; Schaper, W. Arteriogenesis The Development and Growth of Collateral Arteries. Microcirculation 2003, 10, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Pang, H.; Wu, H.; Peng, X.; Tan, Q.; Lin, S.; Wei, B. CCL2 Promotes Proliferation, Migration and Angiogenesis through the MAPK/ERK1/2/MMP9, PI3K/AKT, Wnt/Β-catenin Signaling Pathways in HUVECs. Exp. Ther. Med. 2022, 25, 77. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF Guides Angiogenic Sprouting Utilizing Endothelial Tip Cell Filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Phng, L.-K.; Gerhardt, H. Angiogenesis: A Team Effort Coordinated by Notch. Dev. Cell 2009, 16, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Lobov, I.B.; Renard, R.A.; Papadopoulos, N.; Gale, N.W.; Thurston, G.; Yancopoulos, G.D.; Wiegand, S.J. Delta-like Ligand 4 (Dll4) Is Induced by VEGF as a Negative Regulator of Angiogenic Sprouting. Proc. Natl. Acad. Sci. USA 2007, 104, 3219–3224. [Google Scholar] [CrossRef]
- Adams, R.H.; Alitalo, K. Molecular Regulation of Angiogenesis and Lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Eilken, H.M.; Adams, R.H. Dynamics of Endothelial Cell Behavior in Sprouting Angiogenesis. Curr. Opin. Cell Biol. 2010, 22, 617–625. [Google Scholar] [CrossRef]
- Mentzer, S.J.; Konerding, M.A. Intussusceptive Angiogenesis: Expansion and Remodeling of Microvascular Networks. Angiogenesis 2014, 17, 499–509. [Google Scholar] [CrossRef]
- Tang, N.; Wang, L.; Esko, J.; Giordano, F.J.; Huang, Y.; Gerber, H.-P.; Ferrara, N.; Johnson, R.S. Loss of HIF-1α in Endothelial Cells Disrupts a Hypoxia-Driven VEGF Autocrine Loop Necessary for Tumorigenesis. Cancer Cell 2004, 6, 485–495. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The Biology of VEGF and Its Receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 Mediates Metabolic Responses to Intratumoral Hypoxia and Oncogenic Mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-Driven Glycolysis in Vessel Sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-Specific Growth Factors and Blood Vessel Formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, R.; Enström, A.; Paul, G. Molecular Regulation of the Response of Brain Pericytes to Hypoxia. Int. J. Mol. Sci. 2023, 24, 5671. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular Mechanisms and Clinical Applications of Angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Dimmeler, S. Endothelial Progenitor Cells: Characterization and Role in Vascular Biology. Circ. Res. 2004, 95, 343–353. [Google Scholar] [CrossRef]
- Ceradini, D.J.; Gurtner, G.C. Homing to Hypoxia: HIF-1 as a Mediator of Progenitor Cell Recruitment to Injured Tissue. Trends Cardiovasc. Med. 2005, 15, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhao, X.; Fang, Y.; Yu, S.; Zhao, J.; Song, M.; Huang, L. SDF-1α Involved in Mobilization and Recruitment of Endothelial Progenitor Cells after Arterial Injury in Mice. Cardiovasc. Pathol. 2010, 19, 218–227. [Google Scholar] [CrossRef]
- Askari, A.T.; Unzek, S.; Popovic, Z.B.; Goldman, C.K.; Forudi, F.; Kiedrowski, M.; Rovner, A.; Ellis, S.G.; Thomas, J.D.; DiCorleto, P.E.; et al. Effect of Stromal-Cell-Derived Factor 1 on Stem-Cell Homing and Tissue Regeneration in Ischaemic Cardiomyopathy. Lancet 2003, 362, 697–703. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, G.; Jin, Y.M.; Lee, J.Y.; Shin, J.W.; Jo, I. Expression of Angiopoietin-1 in Hypoxic Pericytes: Regulation by Hypoxia-Inducible Factor-2α and Participation in Endothelial Cell Migration and Tube Formation. Biochem. Biophys. Res. Commun. 2016, 469, 263–269. [Google Scholar] [CrossRef]
- Le Jan, S.; Amy, C.; Cazes, A.; Monnot, C.; Lamandé, N.; Favier, J.; Philippe, J.; Sibony, M.; Gasc, J.-M.; Corvol, P.; et al. Angiopoietin-Like 4 Is a Proangiogenic Factor Produced during Ischemia and in Conventional Renal Cell Carcinoma. Am. J. Pathol. 2003, 162, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, A.; Hendee, K.; Muyleart, M.; Mammoto, T. Endothelial Twist1-PDGFB Signaling Mediates Hypoxia-Induced Proliferation and Migration of αSMA-Positive Cells. Sci. Rep. 2020, 10, 7563. [Google Scholar] [CrossRef] [PubMed]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast Growth Factor-2 (FGF-2) Induces Vascular Endothelial Growth Factor (VEGF) Expression in the Endothelial Cells of Forming Capillaries: An Autocrine Mechanism Contributing to Angiogenesis. J. Cell Biol. 1998, 141, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Luttun, A.; Tjwa, M.; Moons, L.; Wu, Y.; Angelillo-Scherrer, A.; Liao, F.; Nagy, J.A.; Hooper, A.; Priller, J.; De Klerck, B.; et al. Revascularization of Ischemic Tissues by PlGF Treatment, and Inhibition of Tumor Angiogenesis, Arthritis and Atherosclerosis by Anti-Flt1. Nat. Med. 2002, 8, 831–840. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yan, L.; Du, W.; Zhang, M.; Chen, H.; Zhang, L.; Li, G.; Li, J.; Dong, Y.; et al. MMP-2 and MMP-9 Contribute to the Angiogenic Effect Produced by Hypoxia/15-HETE in Pulmonary Endothelial Cells. J. Mol. Cell. Cardiol. 2018, 121, 36–50. [Google Scholar] [CrossRef]
- Revuelta-López, E.; Castellano, J.; Roura, S.; Gálvez-Montón, C.; Nasarre, L.; Benitez, S.; Bayes-Genis, A.; Badimon, L.; Llorente-Cortés, V. Hypoxia Induces Metalloproteinase-9 Activation and Human Vascular Smooth Muscle Cell Migration Through Low-Density Lipoprotein Receptor–Related Protein 1–Mediated Pyk2 Phosphorylation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2877–2887. [Google Scholar] [CrossRef]
- Jin, Y.; An, X.; Ye, Z.; Cully, B.; Wu, J.; Li, J. RGS5, a Hypoxia-Inducible Apoptotic Stimulator in Endothelial Cells. J. Biol. Chem. 2009, 284, 23436–23443. [Google Scholar] [CrossRef]
- Norman, J.T.; Clark, I.M.; Garcia, P.L. Hypoxia Promotes Fibrogenesis in Human Renal Fibroblasts. Kidney Int. 2000, 58, 2351–2366. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, A.H.; Tracy, K.; Frankenberger, C.; Boland, M.L.; Sharifi, M.N.; Drake, L.E.; Sachleben, J.R.; Asara, J.M.; Locasale, J.W.; Karczmar, G.S.; et al. Mitophagy Defects Arising from BNip3 Loss Promote Mammary Tumor Progression to Metastasis. EMBO Rep. 2015, 16, 1145–1163. [Google Scholar] [CrossRef]
- Basheeruddin, M.; Qausain, S. Hypoxia-Inducible Factor 1-Alpha (HIF-1α): An Essential Regulator in Cellular Metabolic Control. Cureus 2024, 16, e63852. [Google Scholar] [CrossRef]
- Lindholm, M.E.; Rundqvist, H. Skeletal Muscle Hypoxia-inducible Factor-1 and Exercise. Exp. Physiol. 2016, 101, 28–32. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, W.; Granucci, E.J.; Tu, A.B.; Kim, D.; Dahms, P.; Pasupneti, S.; Peng, G.; Kim, Y.; Lim, A.H.; et al. Decreased Lymphatic HIF-2α Accentuates Lymphatic Remodeling in Lymphedema. J. Clin. Investig. 2020, 130, 5562–5575. [Google Scholar] [CrossRef]
- Skuli, N.; Majmundar, A.J.; Krock, B.L.; Mesquita, R.C.; Mathew, L.K.; Quinn, Z.L.; Runge, A.; Liu, L.; Kim, M.N.; Liang, J.; et al. Endothelial HIF-2α Regulates Murine Pathological Angiogenesis and Revascularization Processes. J. Clin. Investig. 2012, 122, 1427–1443. [Google Scholar] [CrossRef]
- Tavares, C.D.J.; Aigner, S.; Sharabi, K.; Sathe, S.; Mutlu, B.; Yeo, G.W.; Puigserver, P. Transcriptome-Wide Analysis of PGC-1α–Binding RNAs Identifies Genes Linked to Glucagon Metabolic Action. Proc. Natl. Acad. Sci. USA 2020, 117, 22204–22213. [Google Scholar] [CrossRef]
- Wu, H.; Deng, X.; Shi, Y.; Su, Y.; Wei, J.; Duan, H. PGC-1α, Glucose Metabolism and Type 2 Diabetes Mellitus. J. Endocrinol. 2016, 229, R99–R115. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Circulation 2019, 139, 107280. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in Atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; De Zeeuw, P.; Simons, M.; Carmeliet, P. Endothelial Cell Metabolism in Normal and Diseased Vasculature. Circ. Res. 2015, 116, 1231–1244. [Google Scholar] [CrossRef]
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early Expression of Angiogenesis Factors in Acute Myocardial Ischemia and Infarction. N. Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef]
- Habib, G.B.; Heibig, J.; Forman, S.A.; Brown, B.G.; Roberts, R.; Terrin, M.L.; Bolli, R. Influence of Coronary Collateral Vessels on Myocardial Infarct Size in Humans. Results of Phase I Thrombolysis in Myocardial Infarction (TIMI) Trial. The TIMI Investigators. Circulation 1991, 83, 739–746. [Google Scholar] [CrossRef]
- Elias, J.; Hoebers, L.P.C.; Van Dongen, I.M.; Claessen, B.E.P.M.; Henriques, J.P.S. Impact of Collateral Circulation on Survival in ST-Segment Elevation Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention With a Concomitant Chronic Total Occlusion. JACC Cardiovasc. Interv. 2017, 10, 906–914. [Google Scholar] [CrossRef]
- Heinl-Green, A.; Radke, P.W.; Munkonge, F.M.; Frass, O.; Zhu, J.; Vincent, K.; Geddes, D.M.; Alton, E.W.F.W. The Efficacy of a ‘Master Switch Gene’ HIF-1α in a Porcine Model of Chronic Myocardial Ischaemia. Eur. Heart J. 2005, 26, 1327–1332. [Google Scholar] [CrossRef]
- Milkiewicz, M.; Pugh, C.W.; Egginton, S. Inhibition of Endogenous HIF Inactivation Induces Angiogenesis in Ischaemic Skeletal Muscles of Mice. J. Physiol. 2004, 560, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Minhas, G.; Sharma, J.; Khan, N. Cellular Stress Response and Immune Signaling in Retinal Ischemia–Reperfusion Injury. Front. Immunol. 2016, 7, 444. [Google Scholar] [CrossRef]
- Hlatky, M.A.; Quertermous, T.; Boothroyd, D.B.; Priest, J.R.; Glassford, A.J.; Myers, R.M.; Fortmann, S.P.; Iribarren, C.; Tabor, H.K.; Assimes, T.L.; et al. Polymorphisms in Hypoxia Inducible Factor 1 and the Initial Clinical Presentation of Coronary Disease. Am. Heart J. 2007, 154, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- López-Reyes, A.; Rodríguez-Pérez, J.M.; Fernández-Torres, J.; Martínez-Rodríguez, N.; Pérez-Hernández, N.; Fuentes-Gómez, A.J.; Aguilar-González, C.A.; Álvarez-León, E.; Posadas-Romero, C.; Villarreal-Molina, T.; et al. The HIF1A Rs2057482 Polymorphism Is Associated with Risk of Developing Premature Coronary Artery Disease and with Some Metabolic and Cardiovascular Risk Factors. The Genetics of Atherosclerotic Disease (GEA) Mexican Study. Exp. Mol. Pathol. 2014, 96, 405–410. [Google Scholar] [CrossRef]
- Wu, L.-F.; Xu, G.-P.; Zhao, Q.; Zhou, L.-J.; Wang, D.; Chen, W.-X. The Association between Hypoxia Inducible Factor 1 Subunit Alpha Gene Rs2057482 Polymorphism and Cancer Risk: A Meta-Analysis. BMC Cancer 2019, 19, 1123. [Google Scholar] [CrossRef]
- Guo, X.; Li, D.; Chen, Y.; An, J.; Wang, K.; Xu, Z.; Chen, Z.; Xing, J. SNP Rs2057482 in HIF1A Gene Predicts Clinical Outcome of Aggressive Hepatocellular Carcinoma Patients after Surgery. Sci. Rep. 2015, 5, 11846. [Google Scholar] [CrossRef]
- Ochoa Chaar, C.I.; Kim, T.; Alameddine, D.; DeWan, A.; Guzman, R.; Dardik, A.; Grossetta Nardini, H.K.; Wallach, J.D.; Kullo, I.; Murray, M. Systematic Review and Meta-Analysis of the Genetics of Peripheral Arterial Disease. JVS Vasc. Sci. 2024, 5, 100133. [Google Scholar] [CrossRef]
- Borton, A.H.; Benson, B.L.; Neilson, L.E.; Saunders, A.; Alaiti, M.A.; Huang, A.Y.; Jain, M.K.; Proweller, A.; Ramirez-Bergeron, D.L. Aryl Hydrocarbon Receptor Nuclear Translocator in Vascular Smooth Muscle Cells Is Required for Optimal Peripheral Perfusion Recovery. J. Am. Heart Assoc. 2018, 7, e009205. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, T.T.; Rissanen, T.T.; Vajanto, I.; Korkeela, A.; Rutanen, J.; Ylä-Herttuala, S. HIF-VEGF-VEGFR-2, TNF-α and IGF Pathways Are Upregulated in Critical Human Skeletal Muscle Ischemia as Studied with DNA Array. Atherosclerosis 2004, 174, 111–120. [Google Scholar] [CrossRef]
- Annex, B.H. Therapeutic Angiogenesis for Critical Limb Ischaemia. Nat. Rev. Cardiol. 2013, 10, 387–396. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; De Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e99. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.-B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109.e33. [Google Scholar] [CrossRef] [PubMed]
- Jude, E.B.; Eleftheriadou, I.; Tentolouris, N. Peripheral Arterial Disease in Diabetes—A Review. Diabet. Med. 2010, 27, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Targeting HIF-1 for Cancer Therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Takeda, K.; Cowan, A.; Fong, G.-H. Essential Role for Prolyl Hydroxylase Domain Protein 2 in Oxygen Homeostasis of the Adult Vascular System. Circulation 2007, 116, 774–781. [Google Scholar] [CrossRef]
- Rishi, M.T.; Selvaraju, V.; Thirunavukkarasu, M.; Shaikh, I.A.; Takeda, K.; Fong, G.-H.; Palesty, J.A.; Sanchez, J.A.; Maulik, N. Deletion of Prolyl Hydroxylase Domain Proteins (PHD1, PHD3) Stabilizes Hypoxia Inducible Factor-1 Alpha, Promotes Neovascularization, and Improves Perfusion in a Murine Model of Hind-Limb Ischemia. Microvasc. Res. 2015, 97, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, Z.; Ren, P.; You, M.; Zhang, J.; Fang, L.; Wang, J.; Chen, Y.; Yan, F.; Zheng, H.; et al. Localized Delivery of shRNA against PHD2 Protects the Heart from Acute Myocardial Infarction through Ultrasound-Targeted Cationic Microbubble Destruction. Theranostics 2017, 7, 51–66. [Google Scholar] [CrossRef]
- Huang, M.; Chan, D.A.; Jia, F.; Xie, X.; Li, Z.; Hoyt, G.; Robbins, R.C.; Chen, X.; Giaccia, A.J.; Wu, J.C. Short Hairpin RNA Interference Therapy for Ischemic Heart Disease. Circulation 2008, 118, S226–S233. [Google Scholar] [CrossRef]
- Pradeep, S.R.; Lim, S.T.; Thirunavukkarasu, M.; Joshi, M.; Cernuda, B.; Palesty, J.A.; Maulik, N. Protective Effect of Cardiomyocyte-Specific Prolyl-4-Hydroxylase 2 Inhibition on Ischemic Injury in a Mouse MI Model. J. Am. Coll. Surg. 2022, 235, 240–254. [Google Scholar] [CrossRef]
- Thirunavukkarasu, M.; Pradeep, S.R.; Oriowo, B.; Lim, S.T.; Maloney, M.; Ahmed, S.; Taylor, N.; Russell, D.M.; Socrates, P.; Batko, E.; et al. Stabilization of Transcription Factor, HIF-1α by Prolylhydroxylase 1 Knockout Reduces Cardiac Injury After Myocardial Infarction in Mice. Cells 2025, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Nguyen, P.; Jia, F.; Hu, S.; Gong, Y.; De Almeida, P.E.; Wang, L.; Nag, D.; Kay, M.A.; Giaccia, A.J.; et al. Double Knockdown of Prolyl Hydroxylase and Factor-Inhibiting Hypoxia-Inducible Factor With Nonviral Minicircle Gene Therapy Enhances Stem Cell Mobilization and Angiogenesis After Myocardial Infarction. Circulation 2011, 124, S46–S54. [Google Scholar] [CrossRef]
- Xie, L.; Pi, X.; Wang, Z.; He, J.; Willis, M.S.; Patterson, C. Depletion of PHD3 Protects Heart from Ischemia/Reperfusion Injury by Inhibiting Cardiomyocyte Apoptosis. J. Mol. Cell. Cardiol. 2015, 80, 156–165. [Google Scholar] [CrossRef]
- Tiwari, R.; Bommi, P.V.; Gao, P.; Schipma, M.J.; Zhou, Y.; Quaggin, S.E.; Chandel, N.S.; Kapitsinou, P.P. Chemical Inhibition of Oxygen-Sensing Prolyl Hydroxylases Impairs Angiogenic Competence of Human Vascular Endothelium through Metabolic Reprogramming. iScience 2022, 25, 105086. [Google Scholar] [CrossRef]
- Iida, T.; Mine, S.; Fujimoto, H.; Suzuki, K.; Minami, Y.; Tanaka, Y. Hypoxia-inducible factor-1alpha induces cell cycle arrest of endothelial cells. Genes Cells 2002, 7, 143. [Google Scholar] [CrossRef]
- Chamboredon, S.; Ciais, D.; Desroches-Castan, A.; Savi, P.; Bono, F.; Feige, J.-J.; Cherradi, N. Hypoxia-Inducible Factor-1α mRNA: A New Target for Destabilization by Tristetraprolin in Endothelial Cells. Mol. Biol. Cell 2011, 22, 3366–3378. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhou, H.; Binmadi, N.O.; Basile, J.R. Hypoxia-Inducible Factor-1-Mediated Regulation of Semaphorin 4D Affects Tumor Growth and Vascularity. J. Biol. Chem. 2009, 284, 32066–32074. [Google Scholar] [CrossRef]
- Olson, E.; Demopoulos, L.; Haws, T.F.; Hu, E.; Fang, Z.; Mahar, K.M.; Qin, P.; Lepore, J.; Bauer, T.A.; Hiatt, W.R. Short-Term Treatment with a Novel HIF-Prolyl Hydroxylase Inhibitor (GSK1278863) Failed to Improve Measures of Performance in Subjects with Claudication-Limited Peripheral Artery Disease. Vasc. Med. 2014, 19, 473–482. [Google Scholar] [CrossRef]
- Liu, J.; Meng, Z.; Feng, L.; Zhuo, L.; Liang, Y.; Yang, M.; Su, L.; Zheng, Z.; Liu, B.; Ren, J. Efficacy and Safety of Roxadustat for Treating Anaemia in Patients with Chronic Kidney Disease and Heart Failure: A Retrospective Cohort Study. Clin. Kidney J. 2025, 18, sfaf061. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Yu, Y.; Li, C.; Zhang, C. Advances in the Study of Exosomes Derived from Mesenchymal Stem Cells and Cardiac Cells for the Treatment of Myocardial Infarction. Cell Commun. Signal. 2023, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Yasuda, K.; Iwai, T.; Sasajima, T.; Ishimaru, S.; Ohashi, Y.; Yamaguchi, T.; Ogihara, T.; Morishita, R. Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Hepatocyte Growth Factor Plasmid for Critical Limb Ischemia. Gene Ther. 2010, 17, 1152–1161. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Mohler, E.R.; Lederman, R.J.; Mendelsohn, F.O.; Saucedo, J.F.; Goldman, C.K.; Blebea, J.; Macko, J.; Kessler, P.D.; Rasmussen, H.S.; et al. Regional Angiogenesis With Vascular Endothelial Growth Factor in Peripheral Arterial Disease: A Phase II Randomized, Double-Blind, Controlled Study of Adenoviral Delivery of Vascular Endothelial Growth Factor 121 in Patients With Disabling Intermittent Claudication. Circulation 2003, 108, 1933–1938. [Google Scholar] [CrossRef]
- Barć, P.; Antkiewicz, M.; Śliwa, B.; Frączkowska, K.; Guziński, M.; Dawiskiba, T.; Małodobra-Mazur, M.; Witkiewicz, W.; Kupczyńska, D.; Strzelec, B.; et al. Double VEGF/HGF Gene Therapy in Critical Limb Ischemia Complicated by Diabetes Mellitus. J. Cardiovasc. Transl. Res. 2021, 14, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Cai, L.; Tan, Y.; Thistlethwaite, P.; Kang, Y.J.; Li, X.; Feng, W. Cardiac-Specific Overexpression of HIF-1α Prevents Deterioration of Glycolytic Pathway and Cardiac Remodeling in Streptozotocin-Induced Diabetic Mice. Am. J. Pathol. 2010, 177, 97–105. [Google Scholar] [CrossRef]
- Kido, M.; Du, L.; Sullivan, C.C.; Li, X.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Hypoxia-Inducible Factor 1-Alpha Reduces Infarction and Attenuates Progression of Cardiac Dysfunction After Myocardial Infarction in the Mouse. J. Am. Coll. Cardiol. 2005, 46, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Shyu, K.-G.; Wang, M.-T.; Wang, B.-W.; Chang, C.-C.; Leu, J.-G.; Kuan, P.; Chang, H. Intramyocardial Injection of Naked DNA Encoding HIF-1/VP16 Hybrid to Enhance Angiogenesis in an Acute Myocardial Infarction Model in the Rat. Cardiovasc. Res. 2002, 54, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Czibik, G.; Martinov, V.; Ruusalepp, A.; Sagave, J.; Skare, Ø.; Valen, G. In Vivo Remote Delivery of DNA Encoding for Hypoxia-inducible Factor 1 Alpha Reduces Myocardial Infarct Size. Clin. Transl. Sci. 2009, 2, 33–40. [Google Scholar] [CrossRef]
- Sarkar, K.; Fox-Talbot, K.; Steenbergen, C.; Bosch-Marcé, M.; Semenza, G.L. Adenoviral Transfer of HIF-1α Enhances Vascular Responses to Critical Limb Ischemia in Diabetic Mice. Proc. Natl. Acad. Sci. USA 2009, 106, 18769–18774. [Google Scholar] [CrossRef]
- Creager, M.A.; Olin, J.W.; Belch, J.J.F.; Moneta, G.L.; Henry, T.D.; Rajagopalan, S.; Annex, B.H.; Hiatt, W.R. Effect of Hypoxia-Inducible Factor-1α Gene Therapy on Walking Performance in Patients with Intermittent Claudication. Circulation 2011, 124, 1765–1773. [Google Scholar] [CrossRef]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.-A.; Wei, L. Transplantation of Hypoxia-Preconditioned Mesenchymal Stem Cells Improves Infarcted Heart Function via Enhanced Survival of Implanted Cells and Angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef]
- Rosová, I.; Dao, M.; Capoccia, B.; Link, D.; Nolta, J.A. Hypoxic Preconditioning Results in Increased Motility and Improved Therapeutic Potential of Human Mesenchymal Stem Cells. Stem Cells 2008, 26, 2173–2182. [Google Scholar] [CrossRef]
- Garcia, J.P.; Avila, F.R.; Torres, R.A.; Maita, K.C.; Eldaly, A.S.; Rinker, B.D.; Zubair, A.C.; Forte, A.J.; Sarabia-Estrada, R. Hypoxia-Preconditioning of Human Adipose-Derived Stem Cells Enhances Cellular Proliferation and Angiogenesis: A Systematic Review. J. Clin. Transl. Res. 2022, 8, 61–70. [Google Scholar]
- Pei, X.; Kim, H.; Lee, M.; Wang, N.; Shin, J.; Lee, S.; Yoon, M.; Yang, V.C.; He, H. Local Delivery of Cardiac Stem Cells Overexpressing HIF-1α Promotes Angiogenesis and Muscular Tissue Repair in a Hind Limb Ischemia Model. J. Control. Release 2020, 322, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome Secreted by MSC Reduces Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; Sun, Z.; Chi, B.; Zou, A.; Mao, L.; Xiong, X.; Jiang, J.; Sun, L.; Zhu, W.; et al. HIF-1α Overexpression in Mesenchymal Stem Cell-Derived Exosome-Encapsulated Arginine-Glycine-Aspartate (RGD) Hydrogels Boost Therapeutic Efficacy of Cardiac Repair after Myocardial Infarction. Mater. Today Bio 2021, 12, 100171. [Google Scholar] [CrossRef]
- Bich Phuong Bui T, Phuong Linh Nguyen T, Kyeong Lee and Jungsook Cho Hypoxia-Inducible Factor-1: A Novel Therapeutic Target for the Management of Cancer, Drug Resistance, and Cancer-Related Pain. Cancers 2022, 14, 6054. [CrossRef]
- Ortmann, B.M. Hypoxia-Inducible Factor in Cancer: From Pathway Regulation to Therapeutic Opportunity. BMJ Oncol. 2024, 3, e000154. [Google Scholar] [CrossRef]
- Tianchi Yu, Bo Tang, and Xueying Sun Development of Inhibitors Targeting Hypoxia-Inducible Factor 1 and 2 for Cancer Therapy. Yonsei Med. J. 2017, 58, 489–496. [CrossRef] [PubMed]
- Chen, T.; Yao, L.-Q.; Shi, Q.; Ren, Z.; Ye, L.-C.; Xu, J.-M.; Zhou, P.-H.; Zhong, Y.-S. MicroRNA-31 Contributes to Colorectal Cancer Development by Targeting Factor Inhibiting HIF-1α (FIH-1). Cancer Biol. Ther. 2014, 15, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.P.; Lassance-Soares, R.M.; Shao, H.; Regueiro, M.M.; Li, Y.; Liu, Z.-J.; Velazquez, O.C. Intramuscular E-Selectin/Adeno-Associated Virus Gene Therapy Promotes Wound Healing in an Ischemic Mouse Model. J. Surg. Res. 2018, 228, 68–76. [Google Scholar] [CrossRef]
- Quiroz, H.J.; Parikh, P.P.; Lassance-Soares, R.M.; Regueiro, M.M.; Li, Y.; Shao, H.; Vazquez-Padron, R.; Percival, J.; Liu, Z.-J.; Velazquez, O.C. Gangrene, Revascularization, and Limb Function Improved with E-Selectin/Adeno-Associated Virus Gene Therapy. JVS Vasc. Sci. 2021, 2, 20–32. [Google Scholar] [CrossRef]
- Quiroz, H.J.; Valencia, S.F.; Shao, H.; Li, Y.; Ortiz, Y.Y.; Parikh, P.P.; Lassance-Soares, R.M.; Vazquez-Padron, R.I.; Liu, Z.-J.; Velazquez, O.C. E-Selectin-Overexpressing Mesenchymal Stem Cell Therapy Confers Improved Reperfusion, Repair, and Regeneration in a Murine Critical Limb Ischemia Model. Front. Cardiovasc. Med. 2022, 8, 826687. [Google Scholar] [CrossRef]
- Ribieras, A.J.; Ortiz, Y.Y.; Li, Y.; Huerta, C.T.; Le, N.; Shao, H.; Vazquez-Padron, R.I.; Liu, Z.-J.; Velazquez, O.C. E-Selectin/AAV2/2 Gene Therapy Alters Angiogenesis and Inflammatory Gene Profiles in Mouse Gangrene Model. Front. Cardiovasc. Med. 2022, 9, 929466. [Google Scholar] [CrossRef]
- Ribieras, A.J.; Ortiz, Y.Y.; Li, Y.; Le, N.T.; Huerta, C.T.; Voza, F.A.; Shao, H.; Vazquez-Padron, R.I.; Liu, Z.-J.; Velazquez, O.C. E-Selectin/AAV Gene Therapy Promotes Myogenesis and Skeletal Muscle Recovery in a Mouse Hindlimb Ischemia Model. Cardiovasc. Ther. 2023, 2023, 679390. [Google Scholar] [CrossRef] [PubMed]
- Huerta, C.T.; Ortiz, Y.Y.; Li, Y.; Ribieras, A.J.; Voza, F.; Le, N.; Dodson, C.; Wang, G.; Vazquez-Padron, R.I.; Liu, Z.-J.; et al. Novel Gene-Modified Mesenchymal Stem Cell Therapy Reverses Impaired Wound Healing in Ischemic Limbs. Ann. Surg. 2023, 278, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Voza, F.A.; Byrne, B.J.; Ortiz, Y.Y.; Li, Y.; Le, N.; Osafo, L.; Ribieras, A.C.; Shao, H.; Huerta, C.T.; Wei, Y.; et al. Codon-Optimized and de Novo–Synthesized E-Selectin/AAV2 Dose–Response Study for Vascular Regeneration Gene Therapy. Ann. Surg. 2024, 280, 570–583. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).