Targeting Oxidative Stress and Neuroinflammation: Epigallocatechin-3-gallate-Selenium Nanoparticles Mitigate Sleep Deprivation-Induced Cortical Impairment
Abstract
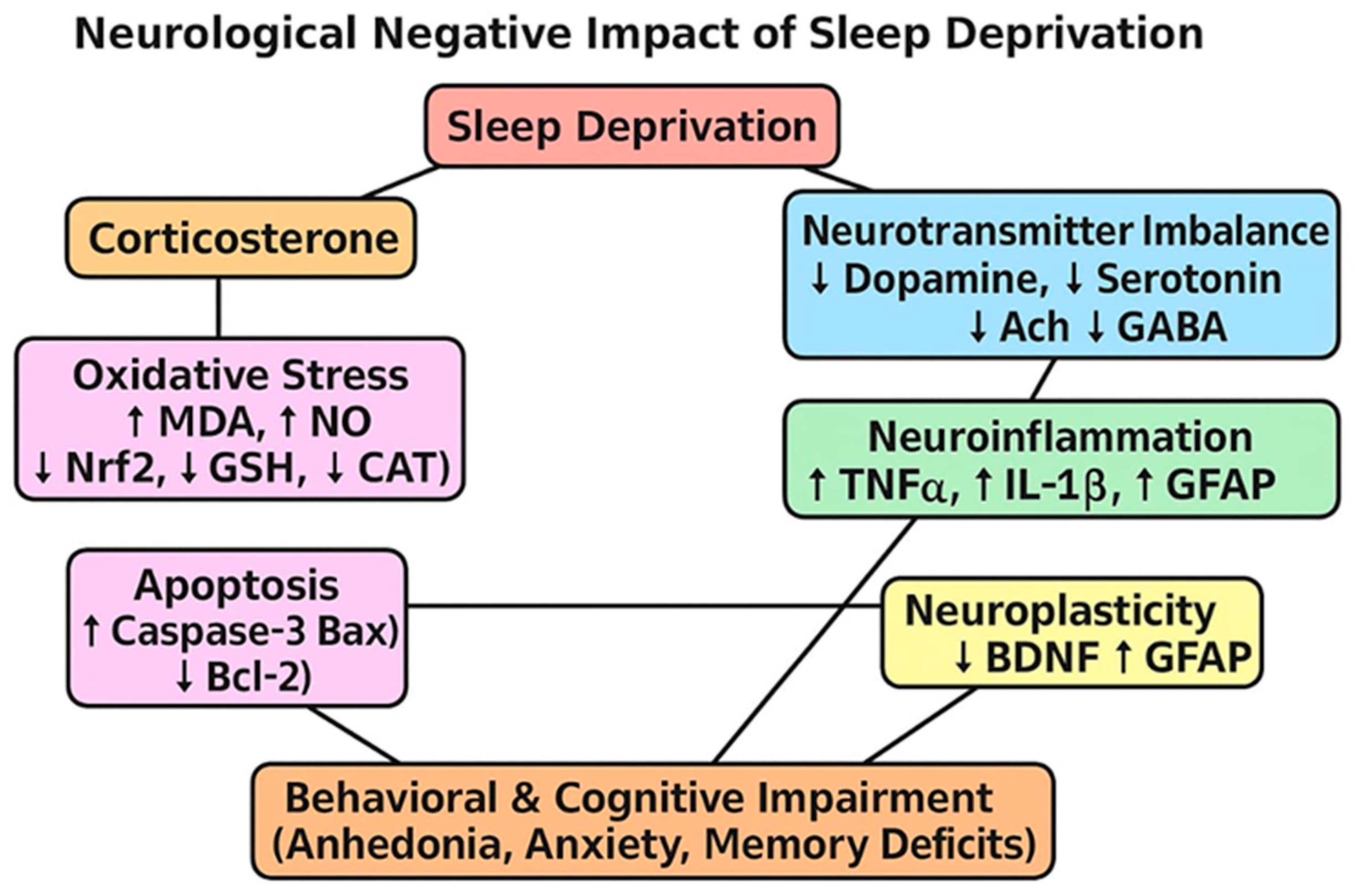
1. Introduction
2. Results
2.1. In Silico Analysis
2.1.1. Docking Score
2.1.2. Absorptivity Measurement of Nanomaterials
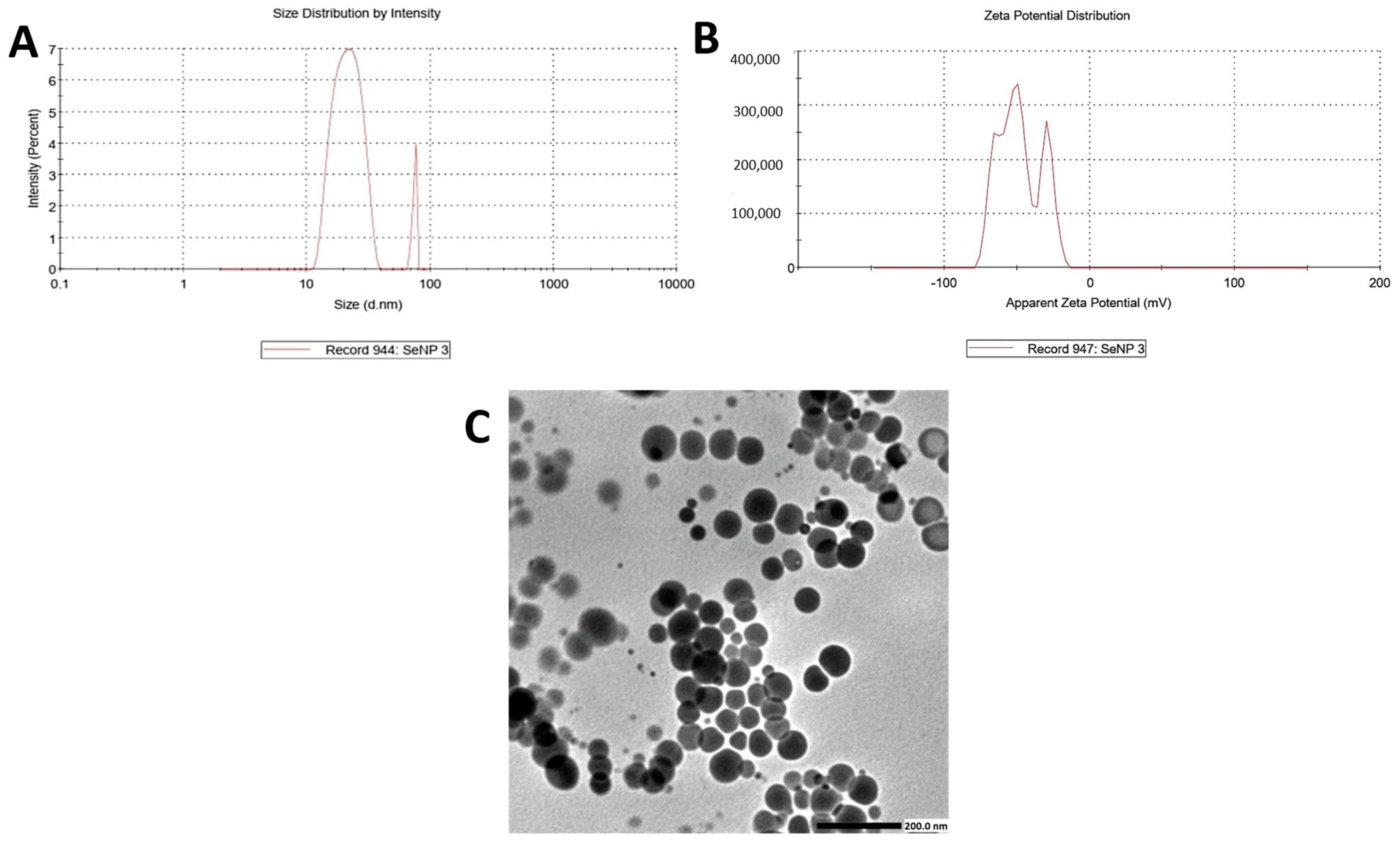
2.2. SeNPs-EGCG Characterization
2.2.1. Transmission Electron Microscope (TEM), Zeta Size, and Zeta Potential
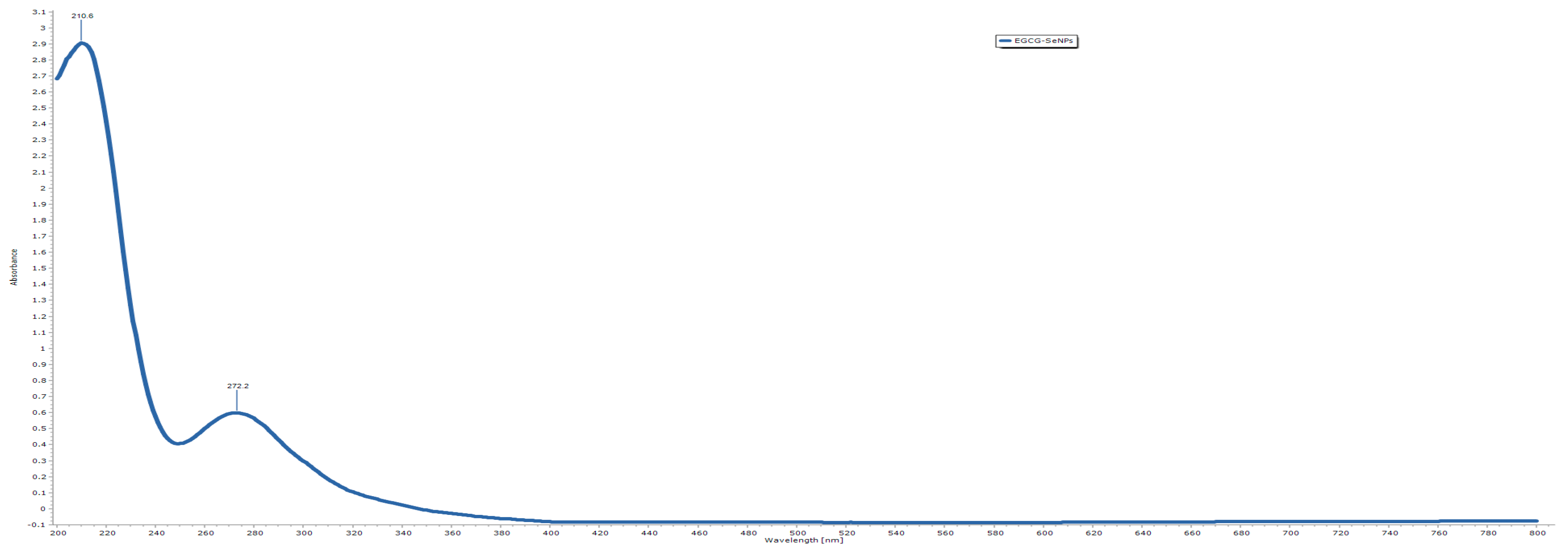
2.2.2. UV–Vis Spectroscopic Characterization
2.2.3. FT-IR Analysis
FTIR Peaks of Pure EGCG
FTIR Peaks and Interaction in the EGCG-SeNPs Complex
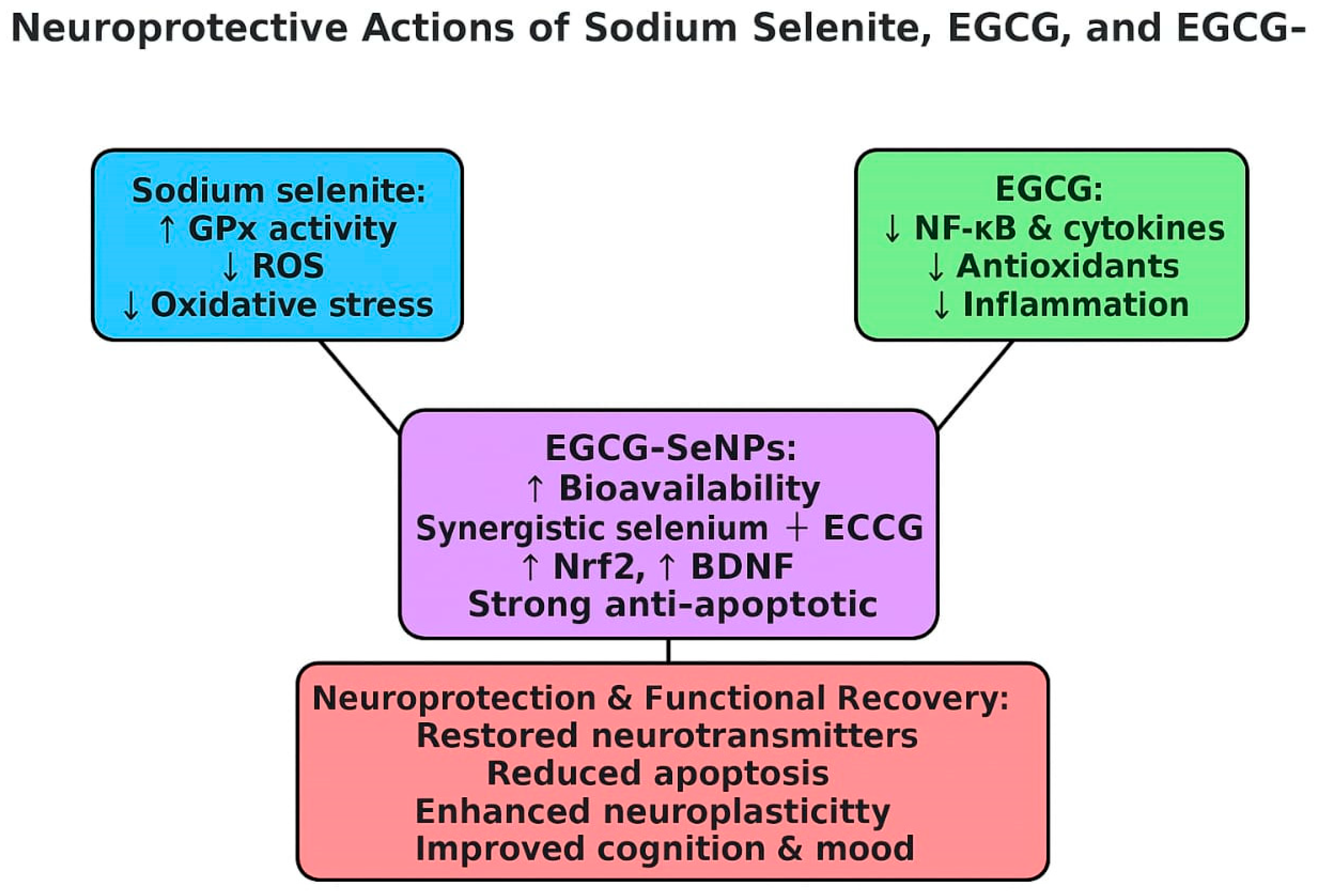
2.3. Sodium Selenite, EGCG, and EGCG-SeNPs Enhance the Performance of Sleep Deprivation-Subjected Rats in the Open-Field Test
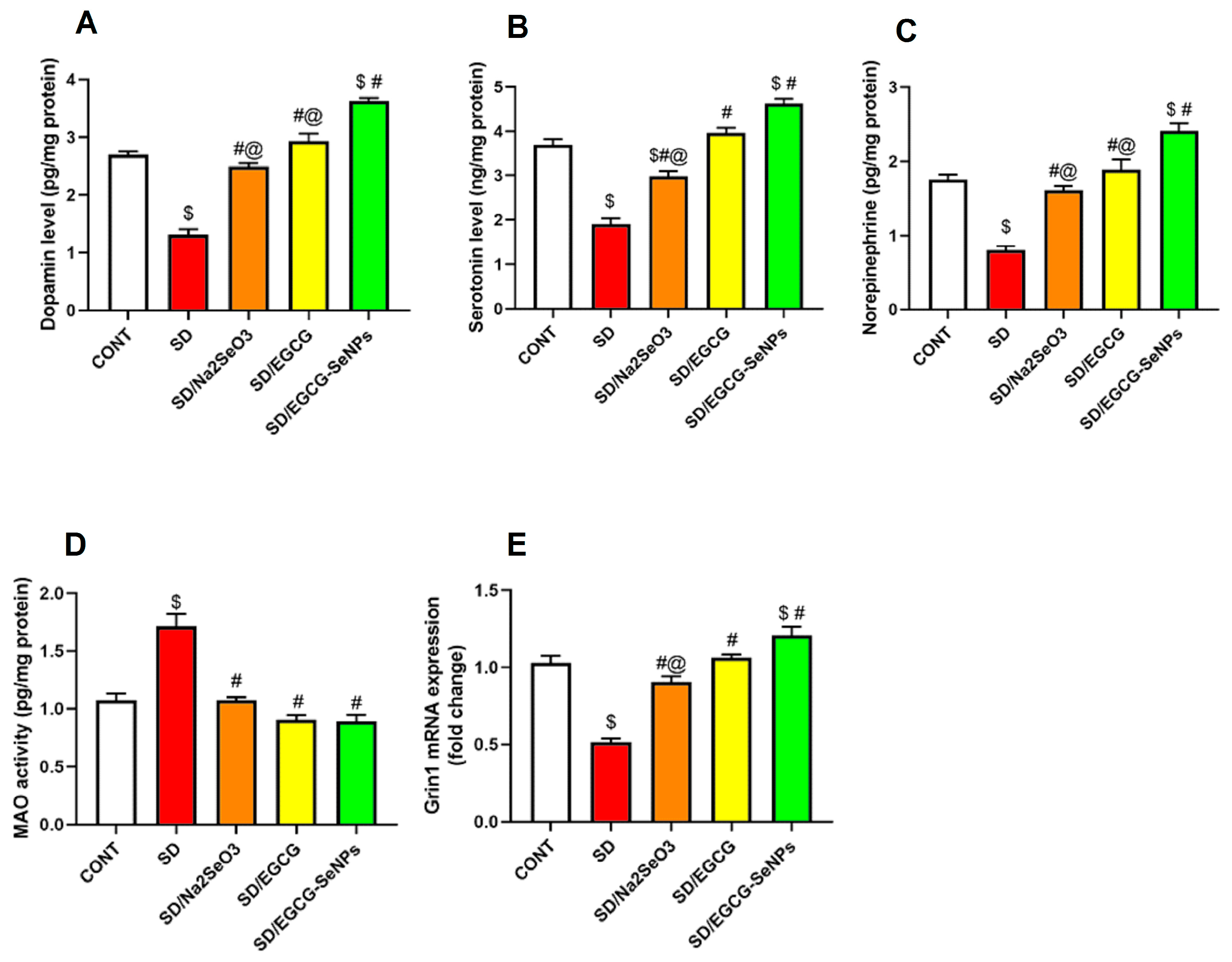
2.4. The Effects of EGCG-SeNPs on Brain Monoamine Contents in the Sleep Deprivation-Subjected Rats
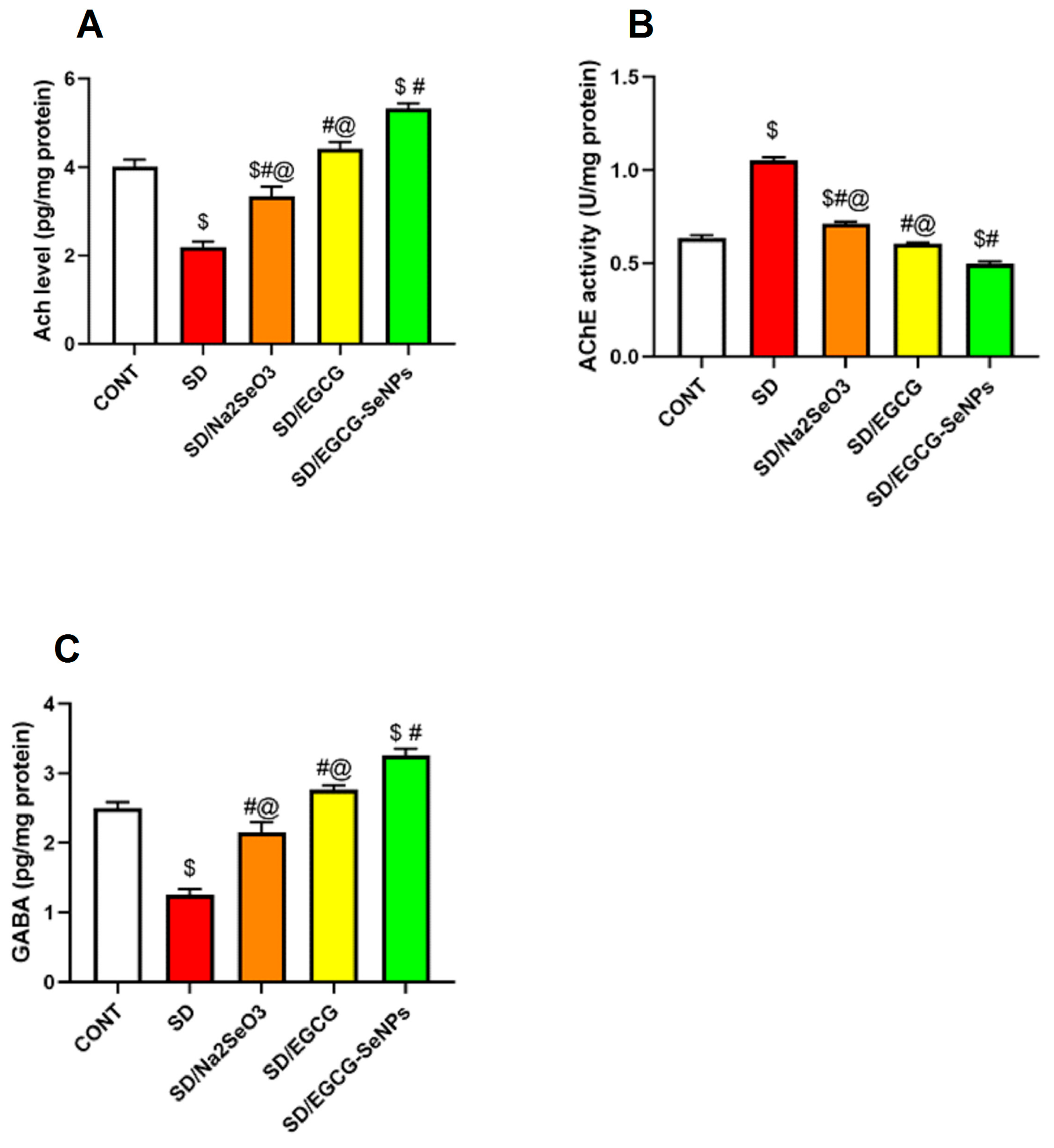
2.5. EGCG-SeNPs Can Restore AChE Activity, ACh and GABA Levels in Sleep Deprivation-Subjected Rats
2.6. Sodium Selenite, EGCG, and EGCG-SeNPs Can Ameliorate the Cortical Oxidative Stress in the Sleep Deprivation-Subjected Rats
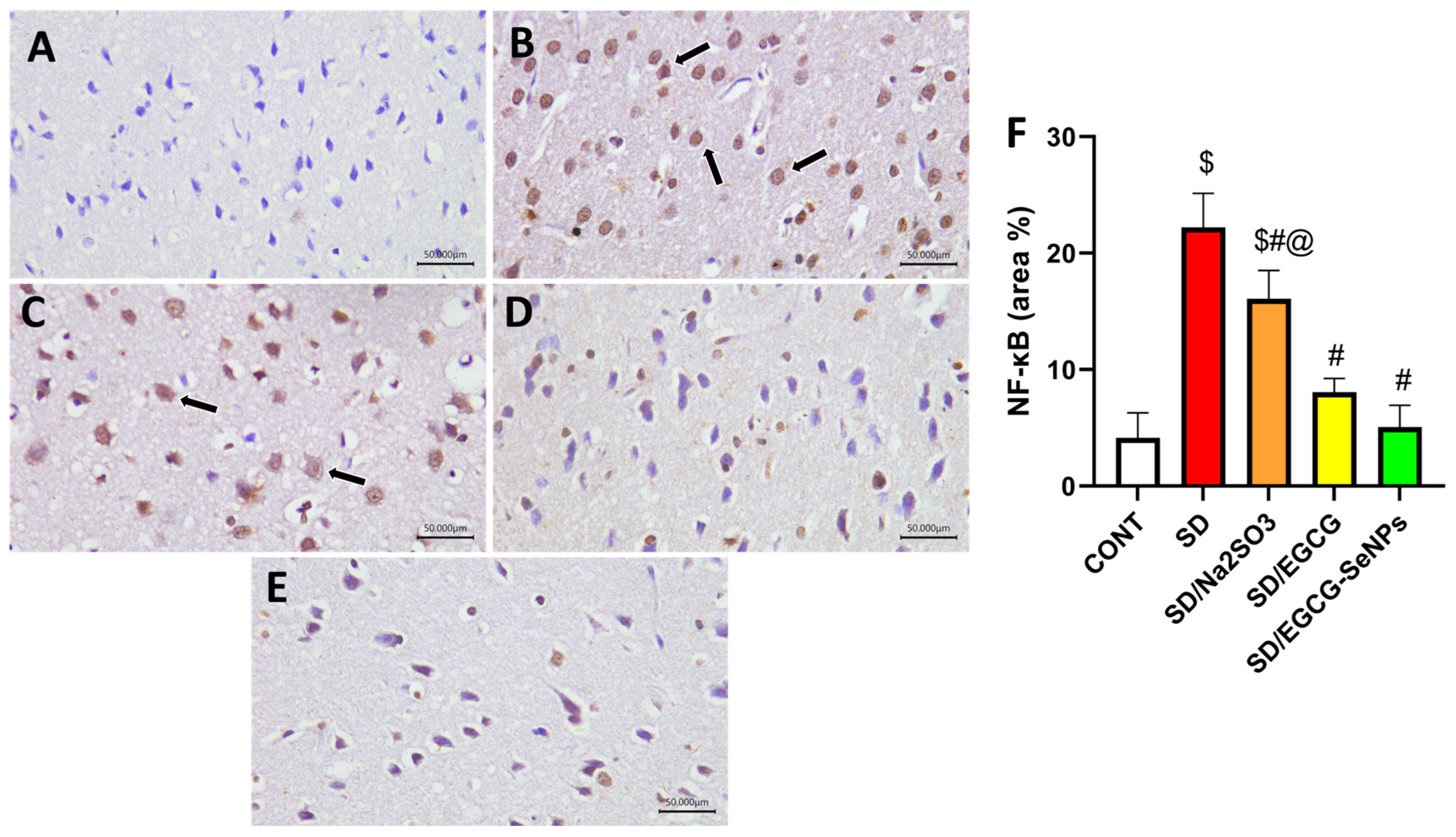
2.7. Sodium Selenite, EGCG, and EGCG-SeNPs Can Abrogate Inflammation in the Sleep Deprivation-Subjected Rats
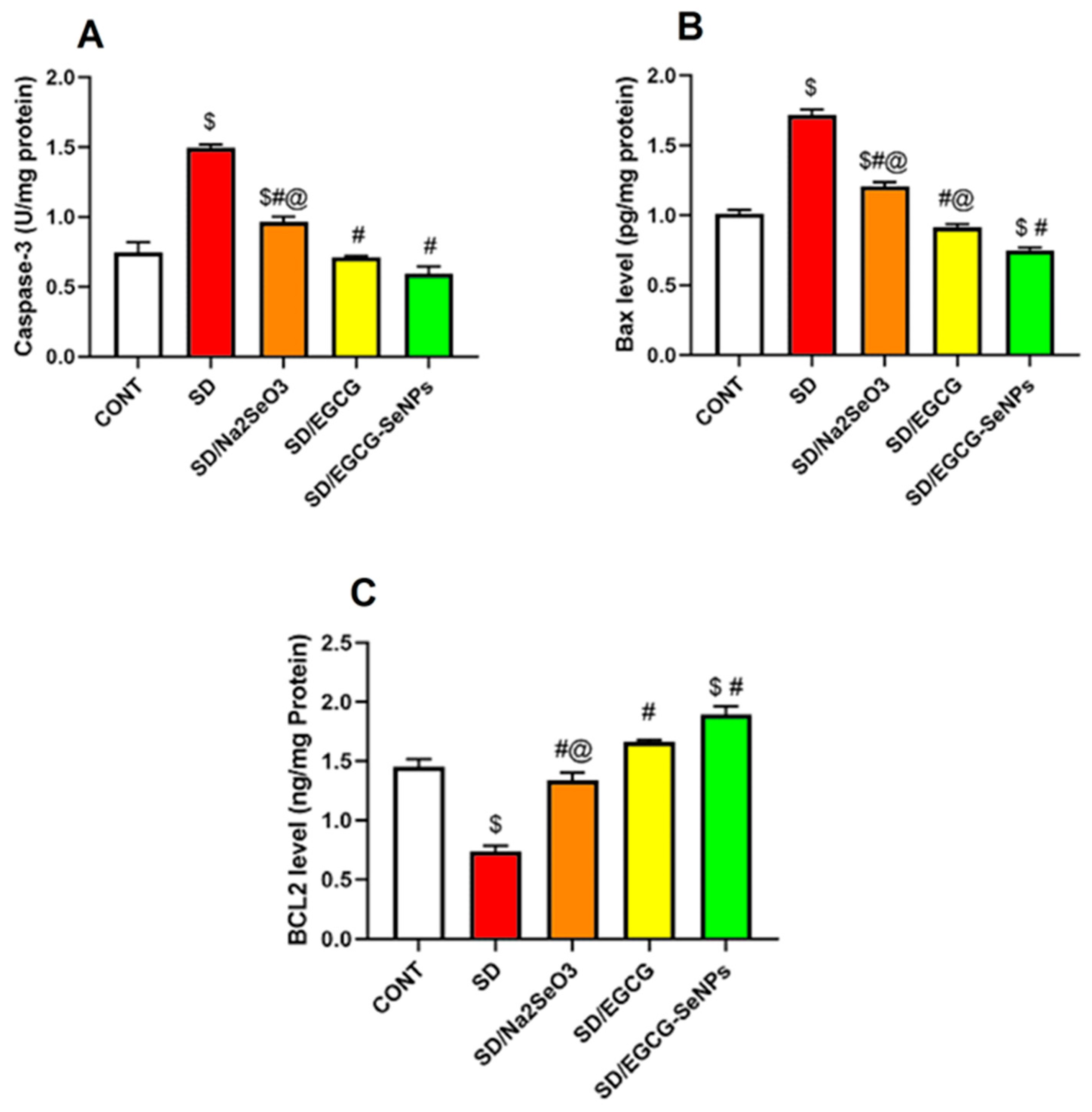
2.8. Selenium Selenite, EGCG, and EGCG-SeNPs Attenuate Apoptosis in the Sleep Deprivation-Subjected Rats
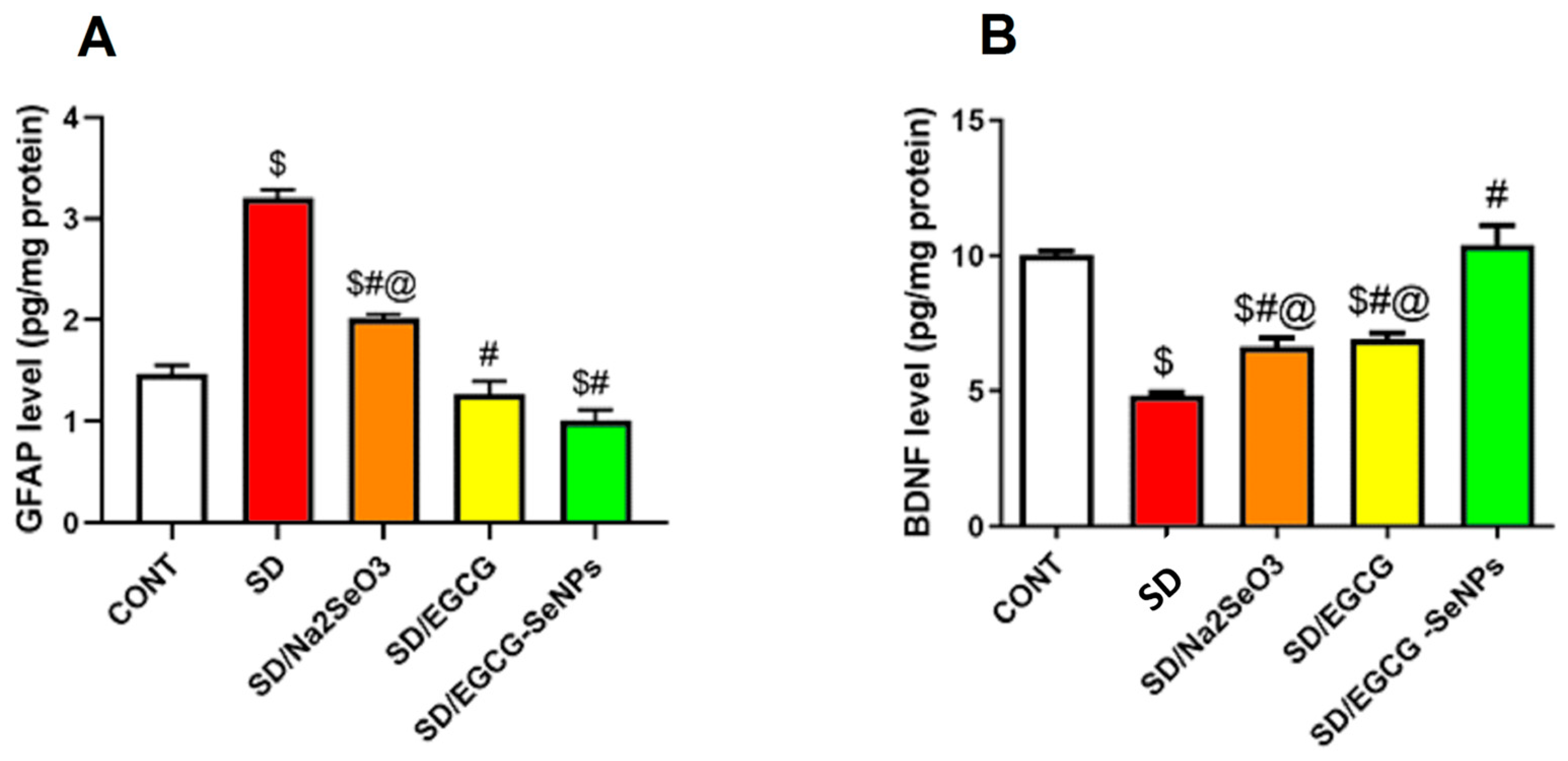
2.9. Selenium Selenite, EGCG, and EGCG-SeNPs Modified the GFAP and BDNF Contents in the Cortical Tissues of the Sleep Deprivation-Subjected Rats
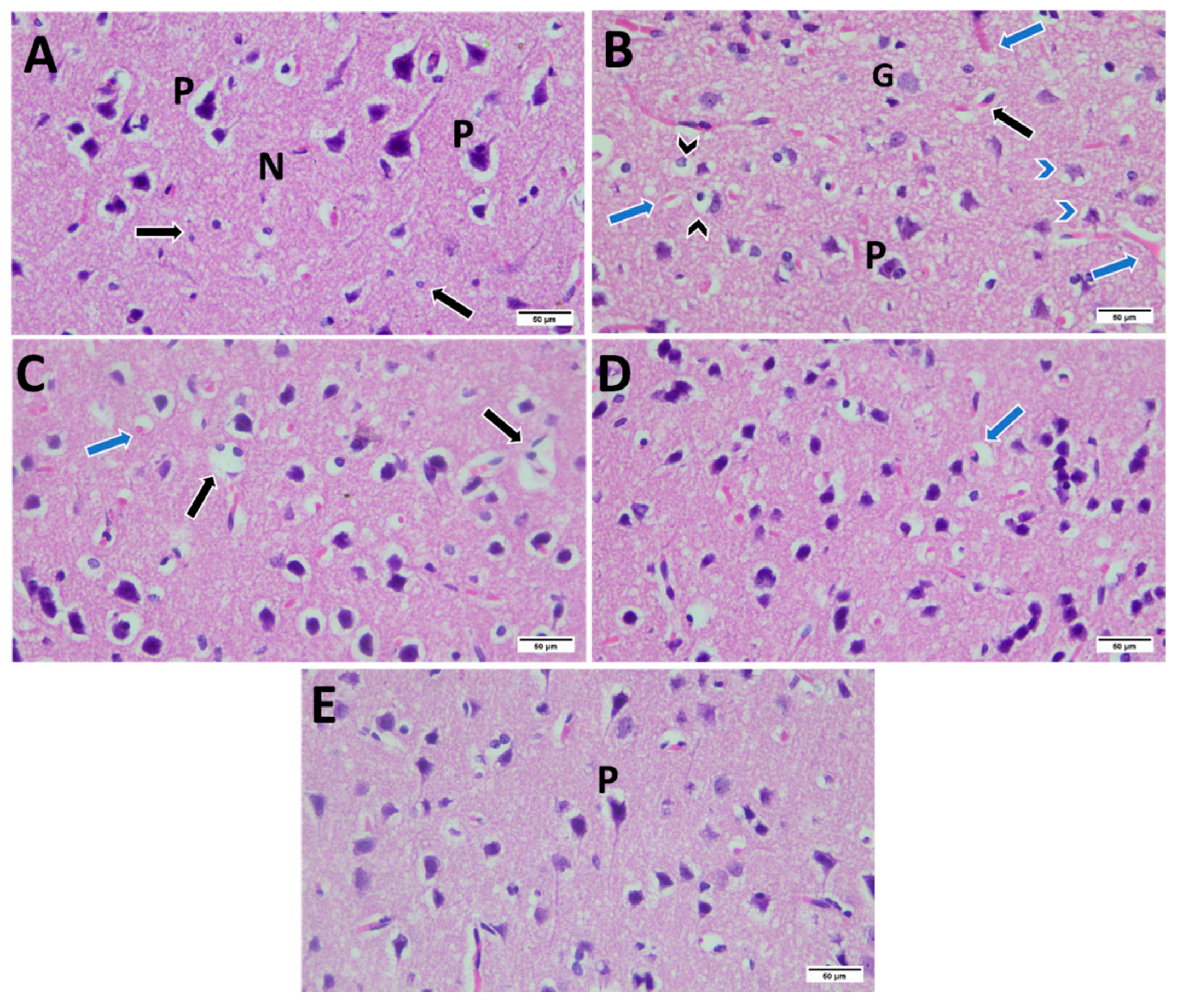
2.10. Histopathological Examination
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.1.1. Molecular Docking
4.1.2. Absorptivity of Nano-Selenium and EGCG–Nano-Selenium with BCL2 and MMP2
4.2. Drugs
4.2.1. Characterization of Nanoparticle
Zeta Potential and Particle Size Distribution
Transmission Electron Microscopy (TEM)
UV–Visible Spectroscopic Analysis
Methods of FTIR Analysis
4.3. Experimental Design
4.3.1. Experimental Animals
4.3.2. Experimental Grouping
4.4. Sleep Deprivation Induced by Rapid Eye Movement (REM) Sleep Interruption
4.5. Behavioral Assessments
4.6. Scarification and Tissue Collection
4.7. Biochemical Assays
4.7.1. Evaluation of Oxidative and Antioxidant Level
4.7.2. Inflammatory Mediators
4.7.3. Apoptotic Markers
4.7.4. Neurotransmitter and Enzymatic Activity
4.7.5. Neurotrophic and Glial Markers
4.7.6. Stress Hormone
4.8. Gene Expression
4.9. Immunohistochemical Analysis
4.10. Quantitative Assessment of IHC Staining
4.11. Histopathological Examination
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SD | Sleep deprivation |
| EGCG | Epigallocatechin-3-gallate |
| Bcl2 | B-cell lymphoma-2 |
| MMP2 | Matrix metallopeptidase 2 |
| SeNPs | Selenium nanoparticles |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NOS2 | Nitric Oxide Synthase 2 |
| MDA | Malondialdehyde |
| LPO | Lipid peroxidation |
| NO | Nitroxide |
| GSH | Glutathione synthetase |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| TNF-α | Tumor necrosis factor alpha |
| IL-6 | Interlukin-6 |
| IL-1β | Interlukin-1β |
| Bax | Bcl-2 Associated X-protein |
| 5-HT | Serotonin |
| DA | Dopamine |
| NE | Norepinephrine |
| Ach | Acetylcholine |
| AChE | Acetylcholinesterase |
| MAO | Monoamine Oxidase |
| BDNF | Brain-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| HPA | Hypothalamic–pituitary–adrenal |
| TEM | Transmission electron microscopy |
| GABA | γ-aminobutyric acid |
| SEM | Standard error of the mean |
| GPx | Glutathione peroxidase |
| Grin1 | Glutamate receptor ionotropic NMDA subunit 1 |
| NMDA | N-methyl-D-aspartate |
| PDB | Protein data bank |
| OFT | Open-Field Test |
| SPT | Sucrose Preference Test |
| cDNA | Complementary DNA |
References
- Mayeli, A.; Sanguineti, C.; Ferrarelli, F. Recent Evidence of Non-Rapid Eye Movement Sleep Oscillation Abnormalities in Psychiatric Disorders. Curr. Psychiatry Rep. 2024, 27, 765–781. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, Sleep, and Circadian Rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, S.-M.; Han, I.-O. Chronic rapid eye movement sleep deprivation aggravates the pathogenesis of Alzheimer’s disease by decreasing brain O-GlcNAc cycling in mice. J. Neuroinflamm. 2024, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Beiyu, Z.; Rong, Z.; Yi, Z.; Shan, W.; Peng, L.; Meng, W.; Wei, P.; Ye, Y.; Qiumin, Q. Oxidative stress is associated with Aβ accumulation in chronic sleep deprivation model. Brain Res. 2024, 1829, 148776. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Varra, F.-N.; Varras, M.; Rekka, E.A. The Effects of Antioxidant Approved Drugs and Under Investigation Compounds with Potential of Improving Sleep Disorders and their Associated Comorbidities Associated with Oxidative Stress and Inflammation. Mini-Rev. Med. Chem. 2025, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Medoro, A.; Savino, R.; Scapagnini, G. Sleep and Oxidative Stress: Current Perspectives on the Role of NRF2. Cell. Mol. Neurobiol. 2024, 44, 52. [Google Scholar] [CrossRef]
- Yuan, X.-L.; Wang, C.-Y. Sleep deprivation-induced cognitive impairment: Unraveling the role of neuroinflammation. Exp. Neurol. 2025, 394, 115419. [Google Scholar] [CrossRef]
- Valencia-Sanchez, S.; Davis, M.; Martensen, J.; Hoeffer, C.; Link, C.; Opp, M.R. Sleep-wake behavior and responses to sleep deprivation and immune challenge of protein kinase RNA-activated knockout mice. Brain. Behav. Immun. 2024, 121, 74–86. [Google Scholar] [CrossRef]
- Gamal, S.M.; Shamseldeen, A.M.; Hosny, S.A.; Fawzy, A. Impact of Sleep Deprivation Combined with Cell Phone Radiation on Behavior, Cognitive Function, Neuroinflammation and Apoptosis in Rat Model: A Functional and Histological Study. Med. J. Cairo Univ. 2024, 91, 1499–1511. [Google Scholar] [CrossRef]
- Weiss, J.T.; Blundell, M.Z.; Singh, P.; Donlea, J.M. Sleep deprivation drives brain-wide changes in cholinergic presynapse abundance in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2024, 121, e2312664121. [Google Scholar] [CrossRef]
- Zhang, R.; Demiral, S.B.; Tomasi, D.; Yan, W.; Manza, P.; Wang, G.-J.; Volkow, N.D. Sleep Deprivation Effects on Brain State Dynamics Are Associated With Dopamine D2 Receptor Availability Via Network Control Theory. Biol. Psychiatry 2025, 97, 89–96. [Google Scholar] [CrossRef]
- Kubota, K.; Hiraide, T.; Nakama, M.; Adachi, M.; Nakashima, M.; Saitsu, H.; Ohnishi, H. GRIN2A-related disorder causes profound developmental delay and a disorder affecting movement. Brain Dev. Case Rep. 2024, 2, 100034. [Google Scholar] [CrossRef]
- Sato, T.; Yamaguchi, A.; Onishi, M.; Abe, Y.; Shiga, T.; Ishikawa, K.; Baba, K.; Akamatsu, W. Comprehensive Gene Expression Analysis Using Human Induced Pluripotent Stem Cells Derived from Patients with Sleep Bruxism: A Preliminary In Vitro Study. Int. J. Mol. Sci. 2024, 25, 13141. [Google Scholar] [CrossRef]
- Azizi, P.; Najafi, A.; Samizadeh, M.; Mohamadian, M.; Jabbari, A.; Rezaie, M.; Vaseghi, S. The Effect of CB1r Hippocampal Activation on Behavioral Changes and BDNF Levels in Rapid-Eye Movement Sleep-Deprived Rats. Eur. J. Neurosci. 2025, 62, e70216. [Google Scholar] [CrossRef]
- Lyckenvik, T.; Olsson, M.; Forsberg, M.; Wasling, P.; Zetterberg, H.; Hedner, J.; Hanse, E. Sleep reduces CSF concentrations of beta-amyloid and tau: A randomized crossover study in healthy adults. Fluids Barriers CNS 2025, 22, 84. [Google Scholar] [CrossRef]
- Bastawy, E.M.; Eraslan, I.M.; Voglsanger, L.; Suphioglu, C.; Walker, A.J.; Dean, O.M.; Read, J.L.; Ziemann, M.; Smith, C.M. Novel Insights into Changes in Gene Expression within the Hypothalamus in Two Asthma Mouse Models: A Transcriptomic Lung–Brain Axis Study. Int. J. Mol. Sci. 2024, 25, 7391. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, M.I.; Gerritsen, L. Late-Life Depression, Hippocampal Volumes, and Hypothalamic-Pituitary-Adrenal Axis Regulation: A Systematic Review and Meta-analysis. Biol. Psychiatry 2017, 82, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Kennis, M.; Gerritsen, L.; Van Dalen, M.; Williams, A.; Cuijpers, P.; Bockting, C. Prospective biomarkers of major depressive disorder: A systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Natsubori, A.; Hirai, S.; Kwon, S.; Ono, D.; Deng, F.; Wan, J.; Miyazawa, M.; Kojima, T.; Okado, H.; Karashima, A.; et al. Serotonergic neurons control cortical neuronal intracellular energy dynamics by modulating astrocyte-neuron lactate shuttle. iScience 2023, 26, 105830. [Google Scholar] [CrossRef]
- Mokra, D.; Adamcakova, J.; Mokry, J. Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG): A Time for a New Player in the Treatment of Respiratory Diseases? Antioxidants 2022, 11, 1566. [Google Scholar] [CrossRef]
- Abdelmeguid, N.E.; Hammad, T.M.; Abdel-Moneim, A.M.; Salam, S.A. Effect of Epigallocatechin-3-gallate on Stress-Induced Depression in a Mouse Model: Role of Interleukin-1β and Brain-Derived Neurotrophic Factor. Neurochem. Res. 2022, 47, 3464–3475. [Google Scholar] [CrossRef] [PubMed]
- Babaei, F.G.; Saburi, E.; Forouzanfar, F.; Asgari, M.; Keshavarzi, Z.; Hajali, V. Effect of epigallocatechin-3-gallate (EGCG) on cognitive functioning and the expression of APP and BDNF in the hippocampus of rats with streptozotocin -induced Alzheimer-like disease. Biochem. Biophys. Rep. 2025, 41, 101930. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.A.; Malik, J.A.; Ahmed, S.; Manzoor, M.; Ahemad, N.; Anwar, S. Recent advances in the therapeutic applications of selenium nanoparticles. Mol. Biol. Rep. 2024, 51, 688. [Google Scholar] [CrossRef]
- Hsu, L.-S.; Lin, C.-L.; Pan, M.-H.; Chen, W.-J. Intervention of a Communication Between PI3K/Akt and β-Catenin by (−)-Epigallocatechin-3-Gallate Suppresses TGF-β1-Promoted Epithelial-Mesenchymal Transition and Invasive Phenotype of NSCLC Cells. Environ. Toxicol. 2025, 40, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Al Omairi, N.E.; Albrakati, A.; Alsharif, K.F.; Almalki, A.S.; Alsanie, W.; Abd Elmageed, Z.Y.; Zaafar, D.; Lokman, M.S.; Bauomy, A.A.; Belal, S.K.; et al. Selenium Nanoparticles with Prodigiosin Rescue Hippocampal Damage Associated with Epileptic Seizures Induced by Pentylenetetrazole in Rats. Biology 2022, 11, 354. [Google Scholar] [CrossRef]
- Elfakharany, S.A.; Eskaros, S.S.; Azhary, N.M.E.; Abdelmonsif, D.A.; Zeitoun, T.M.; Ammar, G.A.G.; Hatem, Y.A. Neuroprotective Role of Selenium Nanoparticles Against Behavioral, Neurobiochemical and Histological Alterations in Rats Subjected to Chronic Restraint Stress. Mol. Neurobiol. 2024, 61, 10159–10181. [Google Scholar] [CrossRef]
- Olotu, F.A.; Agoni, C.; Adeniji, E.; Abdullahi, M.; Soliman, M.E. Probing Gallate-Mediated Selectivity and High-Affinity Binding of Epigallocatechin Gallate: A Way-Forward in the Design of Selective Inhibitors for Anti-apoptotic Bcl-2 Proteins. Appl. Biochem. Biotechnol. 2019, 187, 1061–1080. [Google Scholar] [CrossRef]
- Cheng, X.W.; Kuzuya, M.; Kanda, S.; Maeda, K.; Sasaki, T.; Wang, Q.L.; Tamaya-Mori, N.; Shibata, T.; Iguchi, A. Epigallocatechin-3-gallate binding to MMP-2 inhibits gelatinolytic activity without influencing the attachment to extracellular matrix proteins but enhances MMP-2 binding to TIMP-2. Arch. Biochem. Biophys. 2003, 415, 126–132. [Google Scholar] [CrossRef]
- Chowdhury, A.; Nandy, S.K.; Sarkar, J.; Chakraborti, T.; Chakraborti, S. Inhibition of pro-/active MMP-2 by green tea catechins and prediction of their interaction by molecular docking studies. Mol. Cell. Biochem. 2017, 427, 111–122. [Google Scholar] [CrossRef]
- Sen, T.; Moulik, S.; Dutta, A.; Choudhury, P.R.; Banerji, A.; Das, S.; Roy, M.; Chatterjee, A. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009, 84, 194–204. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, L.; Zhang, D.; Hu, X.; Deng, W. Molecular docking with conformer-dependent charges. Phys. Chem. Chem. Phys. 2024, 26, 22598–22610. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Hasan, S.; Mahato, S.; Celik, I.; Mary, Y.S.; Kumar, A.; Dhamija, P.; Sharma, A.; Choudhary, N.; Chaudhary, P.K.; et al. Molecular docking, DFT analysis, and dynamics simulation of natural bioactive compounds targeting ACE2 and TMPRSS2 dual binding sites of spike protein of SARS-CoV-2. J. Mol. Liq. 2021, 342, 116942. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Dawoud, A.; Helal, M.A. Interaction of nanoparticles with biological macromolecules: A review of molecular docking studies. Nanotoxicology 2021, 15, 66–95. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Maruyama, Y.; Kasahara, K.; Matubayasi, N. Flexible framework of computing binding free energy using the energy representation theory of solution. J. Chem. Phys. 2025, 162, 034103. [Google Scholar] [CrossRef]
- Jang, Y.-H.; Jin, X.; Shankar, P.; Lee, J.H.; Jo, K.; Lim, K.-I. Molecular-Level Interactions between Engineered Materials and Cells. Int. J. Mol. Sci. 2019, 20, 4142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Y.; Hu, Y.; Zhang, D.; Xu, W.; Chen, L.; He, J.; Cheng, S.; Cai, J. Selenium Nanoparticles Synergistically Stabilized by Starch Microgel and EGCG: Synthesis, Characterization, and Bioactivity. Foods 2022, 12, 13. [Google Scholar] [CrossRef]
- Wang, M.; Yu, A.; Han, W.; Chen, J.; Lu, C.; Tu, X. Self-assembled metal-phenolic nanocomplexes comprised of green tea catechin for tumor-specific ferroptosis. Mater. Today Bio 2024, 26, 101040. [Google Scholar] [CrossRef]
- Du, X.; Niu, W.; Xin, Y.; Lou, T. Effect of ultrasound treatment on the structure, physicochemical properties, and functional characteristics of rice-peanut protein/EGCG ternary composite nanoparticles. Int. J. Biol. Macromol. 2025, 312, 144128. [Google Scholar] [CrossRef]
- Zhou, S.-D.; Huang, L.; Meng, L.; Lin, Y.-F.; Xu, X.; Dong, M.-S. Soy protein isolate -(-)-epigallocatechin gallate conjugate: Covalent binding sites identification and IgE binding ability evaluation. Food Chem. 2020, 333, 127400. [Google Scholar] [CrossRef]
- Cao, B.; Wang, C.; Zhou, Z. Insights into the interactions between cellulose and biological molecules. Carbohydr. Res. 2023, 523, 108738. [Google Scholar] [CrossRef]
- Bishir, M.; Bhat, A.; Essa, M.M.; Ekpo, O.; Ihunwo, A.O.; Veeraraghavan, V.P.; Mohan, S.K.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; et al. Sleep Deprivation and Neurological Disorders. BioMed Res. Int. 2020, 2020, 5764017. [Google Scholar] [CrossRef]
- Palagini, L.; Geoffroy, P.A.; Miniati, M.; Perugi, G.; Biggio, G.; Marazziti, D.; Riemann, D. Insomnia, sleep loss, and circadian sleep disturbances in mood disorders: A pathway toward neurodegeneration and neuroprogression? A theoretical review. CNS Spectr. 2022, 27, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Hussain, M.; Ahmed, N.; Ali, A.; Norouziyan, F.; Umar, M.; Moradikor, N. Sleep Deprivation and Cognitive-Behavioral Impairment in Neurodegenerative Disorders. In Integrating Neuroimaging, Computational Neuroscience, and Artificial Intelligence; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-032-71110-2. [Google Scholar]
- Cao, Q.; Xiang, H.; Wang, Y.; Liu, F.; Weng, X.; Xu, F. Negative impact of insufficient sleep on the brain. Brain-Appar. Commun. J. Bacomics 2025, 4, 2465538. [Google Scholar] [CrossRef]
- Salih, S.K.; Namiq, H.S.; Othman, H.H. Impact of Sleep Deprivation on the Central Nervous System Neurotransmitters and Immune Function in Male Albino Rats. Al-Rafidain J. Med. Sci. 2025, 9, 28–36. [Google Scholar] [CrossRef]
- Saggu, S.; Pless, A.; Dew, E.; Ware, D.; Jiao, K.; Wang, Q. Monoamine signaling and neuroinflammation: Mechanistic connections and implications for neuropsychiatric disorders. Front. Immunol. 2025, 16, 1543730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.; Sheng, X.; Jiang, Y.; Zhang, S.; Mo, X.; Yang, Y.; Ding, F. Sleep deprivation affects memory function, depression, and anxiety-like behaviours in rats and mice: A systematic review and meta-analysis. Brain Commun. 2025, 7, fcaf309. [Google Scholar] [CrossRef]
- Yasugaki, S.; Okamura, H.; Kaneko, A.; Hayashi, Y. Bidirectional relationship between sleep and depression. Neurosci. Res. 2025, 211, 57–64. [Google Scholar] [CrossRef]
- Kaczmarski, P.; Sochal, M.; Strzelecki, D.; Białasiewicz, P.; Gabryelska, A. Influence of glutamatergic and GABAergic neurotransmission on obstructive sleep apnea. Front. Neurosci. 2023, 17, 1213971. [Google Scholar] [CrossRef]
- Varinthra, P.; Anwar, S.N.M.N.; Shih, S.-C.; Liu, I.Y. The role of the GABAergic system on insomnia. Tzu Chi Med. J. 2024, 36, 103–109. [Google Scholar] [CrossRef]
- Lasisi, T.J.; Shittu, S.-T.T.; Abeje, J.I.; Ogunremi, K.J.; Shittu, S.A. Paradoxical sleep deprivation induces oxidative stress in the submandibular glands of Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2021, 33, 399–408. [Google Scholar] [CrossRef]
- Neculicioiu, V.S.; Colosi, I.A.; Costache, C.; Toc, D.A.; Sevastre-Berghian, A.; Colosi, H.A.; Clichici, S. Sleep Deprivation-Induced Oxidative Stress in Rat Models: A Scoping Systematic Review. Antioxidants 2023, 12, 1600. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Wei, R.-M.; Feng, Y.-Z.; Zhang, K.-X.; Ge, Y.-J.; Kong, X.-Y.; Li, X.-Y.; Chen, G.-H. Sleep deprivation aggravates lipopolysaccharide-induced anxiety, depression and cognitive impairment: The role of pro-inflammatory cytokines and synaptic plasticity-associated proteins. J. Neuroimmunol. 2024, 386, 578252. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Wei, R.-M.; Zhang, J.-Y.; Liu, S.; Zhang, K.-X.; Kong, X.-Y.; Ge, Y.-J.; Li, X.-Y.; Chen, G.-H. Resveratrol prevents cognitive deficits induced by sleep deprivation via modulating sirtuin 1 associated pathways in the hippocampus. J. Biochem. Mol. Toxicol. 2024, 38, e23698. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, M.; Liu, G.; Wu, X.; Feng, X.; Tang, D.; Jiang, H.; Zhang, X. Chronic sleep deprivation induces erectile dysfunction through increased oxidative stress, apoptosis, endothelial dysfunction, and corporal fibrosis in a rat model. J. Sex. Med. 2024, 21, 1098–1110. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.; Liang, Z.; Liu, J.; Wang, J.; Mao, Y.; Xing, F.; Wei, X.; Wang, Z.; Yang, J.; et al. Sleep deprivation affects pain sensitivity by increasing oxidative stress and apoptosis in the medial prefrontal cortex of rats via the HDAC2-NRF2 pathway. Biomed. J. 2025, 100826. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Liu, S.-Z.; Yu, X.; Shi, X.; You, H.; Liu, P.; Wang, F.; Wang, P.; Chen, L.-L. Liuwei Anshen Capsule alleviates cognitive impairment induced by sleep deprivation by reducing neuroapoptosis and inflammation. J. Ethnopharmacol. 2025, 341, 119311. [Google Scholar] [CrossRef]
- Vaseghi, S.; Mostafavijabbari, A.; Alizadeh, M.-S.; Ghaffarzadegan, R.; Kholghi, G.; Zarrindast, M.-R. Intricate role of sleep deprivation in modulating depression: Focusing on BDNF, VEGF, serotonin, cortisol, and TNF-α. Metab. Brain Dis. 2023, 38, 195–219. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Zheng, Y.; Zhu, S.; Sun, J.; Deng, Y.; Wang, Q.; Zhai, Q. Dexmedetomidine attenuates sleep deprivation-induced inhibition of hippocampal neurogenesis via VEGF-VEGFR2 signaling and inhibits neuroinflammation. Biomed. Pharmacother. 2023, 165, 115085. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Mirabzadeh, Y.; Khesali, G.; Ebrahimkhani, Z.; Karimi, H.; Vaseghi, S. Chronic REM sleep deprivation leads to manic- and OCD-related behaviors, and decreases hippocampal BDNF expression in female rats. Psychopharmacology 2024, 241, 1345–1363. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, S.; Mehan, S.; Khan, Z.; Das Gupta, G.; Narula, A.S. Exploring the Connection Between BDNF/TrkB and AC/cAMP/PKA/CREB Signaling Pathways: Potential for Neuroprotection and Therapeutic Targets for Neurological Disorders. Mol. Neurobiol. 2025, 62, 14627–14659. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. ISBN 978-1-60327-198-1. [Google Scholar]
- Dougherty, G.M.; Rose, K.A.; Tok, J.B.-H.; Pannu, S.S.; Chuang, F.Y.S.; Sha, M.Y.; Chakarova, G.; Penn, S.G. The zeta potential of surface-functionalized metallic nanorod particles in aqueous solution. Electrophoresis 2008, 29, 1131–1139. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Kheirollahi-Kouhestani, M.; Razavi, S.; Tavalaee, M.; Deemeh, M.R.; Mardani, M.; Moshtaghian, J.; Nasr-Esfahani, M.H. Selection of sperm based on combined density gradient and Zeta method may improve ICSI outcome. Hum. Reprod. 2009, 24, 2409–2416. [Google Scholar] [CrossRef]
- Van Erum, J.; Van Dam, D.; De Deyn, P.P. PTZ-induced seizures in mice require a revised Racine scale. Epilepsy Behav. 2019, 95, 51–55. [Google Scholar] [CrossRef]
- Abdel-Wahhab, K.G.; Ashry, M.; Hassan, L.K.; Gadelmawla, M.H.A.; Elqattan, G.M.; El-Fakharany, E.M.; Mannaaa, F.A. Nano-chitosan/bovine lactoperoxidase and lactoferrin formulation modulates the hepatic deterioration induced by 7,12-dimethylbenz[a]anthracene. Comp. Clin. Pathol. 2023, 32, 981–991. [Google Scholar] [CrossRef]
- Saad, S.S.; El-Sakka, S.S.A.; Soliman, M.H.A.; Gadelmawla, M.H.A.; Mahmoud, N.M. Thiazolidin-4-one derivatives as antitumor agents against (Caco-2) cell line: Synthesis, characterization, in silico and in vitro studies. Phosphorus Sulfur Silicon Relat. Elem. 2024, 199, 732–745. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Mohamed, E.-B.; AL Badawi, M.H.; Abdelhady, A.A. The Protective Role of Selenium Against Acrylamide-Induced Cerebellar Neurotoxicity in Adult Male Albino Rats. Egypt. J. Histol. 2024, 47, 13. [Google Scholar] [CrossRef]
- Dahiya, S.; Rani, R.; Dilbaghi, N.; Dhingra, D.; Lal, S.; Verma, J. Evaluation of the anti-depressant potential of EGCG-loaded nanoparticles in unstressed and stressed mice. RSC Pharm. 2024, 1, 344–356. [Google Scholar] [CrossRef]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef]
- Alrashdi, B.M.; Fehaid, A.; Kassab, R.B.; Rizk, S.; Habotta, O.A.; Abdel Moneim, A.E. Biosynthesized Selenium Nanoparticles Using Epigallocatechin Gallate Protect against Pentylenetetrazole-Induced Acute Epileptic Seizures in Mice via Antioxidative, Anti-Inflammatory, and Anti-Apoptotic Activities. Biomedicines 2023, 11, 1955. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-C.; Lai, C.-L.; Wu, M.-N.; Lin, H.-C.; Lee, K.-W.; Li, Y.-S.; Hsu, C.-Y. Rapid eye movement sleep disturbance in patients with refractory epilepsy: A polysomnographic study. Sleep Med. 2021, 81, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, P.; Farmer, C.A.; Medeiros, G.C.; Yuan, P.; Johnston, J.; Kadriu, B.; Gould, T.D.; Zarate, C.A. Associations between hypothalamic-pituitary-adrenal (HPA) axis hormone levels, major depression features and antidepressant effects of ketamine. J. Affect. Disord. 2025, 373, 126–132. [Google Scholar] [CrossRef]
- Ji, J.; Chao, H.; Chen, H.; Liao, J.; Shi, W.; Ye, Y.; Wang, T.; You, Y.; Liu, N.; Ji, J.; et al. Decoding frontotemporal and cell-type-specific vulnerabilities to neuropsychiatric disorders and psychoactive drugs. Open Biol. 2024, 14, 240063. [Google Scholar] [CrossRef] [PubMed]
- Markov, D.D. Sucrose Preference Test as a Measure of Anhedonic Behavior in a Chronic Unpredictable Mild Stress Model of Depression: Outstanding Issues. Brain Sci. 2022, 12, 1287. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Elhemiely, A.A.; El-Fayoumi, S.H.; Gadelmawla, M.H.A.; Mahran, N.A.; Gad, A.M. Hesperidin Reduces Hepatic Injury Induced by Doxorubicin in Rat Model Through Its Antioxidative and Anti-Inflammatory Effects, Focusing on SIRT-1/NRF-2 Pathways. J. Biochem. Mol. Toxicol. 2025, 39, e70465. [Google Scholar] [CrossRef]
- Khames, A.; Gad, A.M.; Abd El-raouf, O.M.; Kandeil, M.A.; Khalaf, M.M. Sodium thiosulphate shows promising anti-inflammatory role against doxorubicin-induced renal injury depending on tlr4 pathway inhibition. Plant Arch. 2020, 20, 2948–2958. [Google Scholar]
- Alrashdi, B.M.; Ashry, M.; Germoush, M.O.; Fouda, M.; Abdel-Farid, I.; Massoud, D.; Shaldoum, F.; Abdel Moneim, A.E.; Gadel-Rab, A.G.; Mahrous, M.; et al. Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in rats. Open Vet. J. 2025, 15, 402–415. [Google Scholar] [CrossRef]
- Pagel, P.; Blome, J.; Wolf, H.U. High-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson’s disease. J. Chromatogr. B Biomed. Sci. App. 2000, 746, 297–304. [Google Scholar] [CrossRef]
- Towers, P.R. The Role of Glial Cell Line-Derived Neurotrophic Factor Signalling Pathway in Mammalian Organogenesis; University of London: London, UK, 1998; ISBN 1-339-30875-4. [Google Scholar]
- Gadelmawla, M.H.A.; Alazzouni, A.S.; Farag, A.H.; Gabri, M.S.; Hassan, B.N. Enhanced effects of ferulic acid against the harmful side effects of chemotherapy in colon cancer: Docking and in vivo study. J. Basic Appl. Zool. 2022, 83, 28. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Alsufyani, S.E.; Ashour, A.M.; Gad, A.M.; Elhemiely, A.A.; Gadelmawla, M.H.A.; Mahmoud, M.A.; Khames, A. Targeting JAK2/STAT3, NLRP3/Caspase-1, and PK2/PKR2 Pathways with Arbutin Ameliorates Lead Acetate-Induced Testicular Injury in Rats. Pharmaceuticals 2024, 17, 909. [Google Scholar] [CrossRef]
- Abdel-Wahhab, K.G.; Ashry, M.; Hassan, L.K.; El-Azma, M.H.; Elqattan, G.M.; Gadelmawla, M.H.A.; Mannaa, F.A. Hepatic and immune modulatory effectiveness of lactoferrin loaded Selenium nanoparticles on bleomycin induced hepatic injury. Sci. Rep. 2024, 14, 21066. [Google Scholar] [CrossRef]
- Alrashdi, B.; Askar, H.; Germoush, M.; Fouda, M.; Abdel-Farid, I.; Massoud, D.; Alzwain, S.; Gadelmawla, M.; Ashry, M. Evaluation of the anti-diabetic and anti-inflammatory potentials of curcumin nanoparticle in diabetic rat induced by streptozotocin. Open Vet. J. 2024, 14, 3375. [Google Scholar] [CrossRef]
- Alrashdi, B.M.; Askar, H.; Germoush, M.O.; Fouda, M.; Massoud, D.; Alzwain, S.; Abdelsater, N.; Salim, L.M.S.; Gadelmawla, M.H.A.; Ashry, M. Cardioprotective, anti-inflammatory, and antioxidative outcome of costus against bleomycin-induced cardiotoxicity in rat model. J. Genet. Eng. Biotechnol. 2025, 23, 100466. [Google Scholar] [CrossRef] [PubMed]







| Target Protein | Glide XP G-Score (kcal mol−1) | Binding Affinity Classification | Hydrogen-Bonding Residues |
|---|---|---|---|
| Anti-apoptotic BCL-2 | −4.033 | Good | Leu 121, Glu 160, Asn 163, Arg 164 |
| Metalloproteinase MMP-2 | −6.749 | High | Phe 80, Pro 466, Gly 505, Pro 506 |
| Structures | Ligand | Total Energy | Adsorption Energy | Rigid Adsorption Energy | Deformation Energy | MMP2 (2): dEad/dNi | dEad/dNi |
|---|---|---|---|---|---|---|---|
| BCL2 | SeNPs | 2.49 × 104 | −5.44 × 104 | 2.467 | −5.44 × 104 | −1.19 × 104 | −4.25 × 104 |
| EGCG-SeNPs | 1.21 × 105 | −8.16 × 104 | 6.015 | −8.16 × 104 | −1.026 × 104 | −7.14 × 104 | |
| MMP2-1 | SeNPs | 3.64 × 104 | −7.69 × 104 | −22.15 | −7.68 × 104 | −3.62 × 104 | −4.07 × 104 |
| EGCG-SeNPs | 1.36 × 105 | −1.01 × 105 | −8.16 | −1.01 × 105 | −3.21 × 104 | −6.91 × 104 |
| Wavenumber (cm−1) | Assignment (Functional Group) |
|---|---|
| 3241 | O-H stretching (Phenolic, H-bonded) |
| 2918, 2849 | C-H stretching (Aliphatic-CH2, -CH3) |
| 1704 | C=O stretching (Ester carbonyl) |
| 1610, 1448 | C=C stretching (Aromatic ring) |
| 1242, 1053 | C-O stretching (Phenolic and ester) |
| Wavenumber (cm−1) (EGCG) | Wavenumber (cm−1) (EGCG-SeNPs) | Change Observed | Interpretation |
|---|---|---|---|
| 3241 | ~3225 | Shift to lower wavenumber, broadening | Involvement of phenolic-OH in coordination/interaction with nano-Se. |
| 2918, 2849 | 2916, 2847 | Minor shift | Aliphatic C-H groups are not directly involved in the interaction. |
| 1704 | 1698 | Shift to lower wavenumber | Coordination of ester carbonyl oxygen with nano-Se surface. |
| 1610, 1448 | 1608, 1450 | Minor shift | Slight change in the electronic environment of the aromatic rings. |
| 1242, 1053 | 1235, 1048 | Shift to lower wavenumber | Involvement of C-O groups (phenolic/ester) in binding to nano-Se. |
| Forward Sequence | Reverse Sequence | Accession Number | |
|---|---|---|---|
| Nrf2 | CCTCAGCATGATGGACTTGGA | GCGACTGAAATGTAGGTGAAGA | NM_001399173.1 |
| Nos2 | GGTGAGGGGACTGGACTTTTAG | TTGTTGGGCTGGGAATAGCA | NM_012611.3 |
| Grin1 | GGCAACTTGTATGGGAGCCT | GGCAACTTGTATGGGAGCCT | NM_012573.4 |
| Β-actin | GTCCACCCGCGAGTACAAC | GGATGCCTCTCTTGCTCTGG | NM_031144.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutfy, R.H.; Ashour, A.M.; Khames, A.; Elhemiely, A.A.; Alam-ElDein, K.M.; Faraag, A.H.I.; Hamed, M.O.A.; Abdel Daim, Z.J.; Attia, N.I.; Gadelmawla, M.H.A. Targeting Oxidative Stress and Neuroinflammation: Epigallocatechin-3-gallate-Selenium Nanoparticles Mitigate Sleep Deprivation-Induced Cortical Impairment. Int. J. Mol. Sci. 2025, 26, 11173. https://doi.org/10.3390/ijms262211173
Lutfy RH, Ashour AM, Khames A, Elhemiely AA, Alam-ElDein KM, Faraag AHI, Hamed MOA, Abdel Daim ZJ, Attia NI, Gadelmawla MHA. Targeting Oxidative Stress and Neuroinflammation: Epigallocatechin-3-gallate-Selenium Nanoparticles Mitigate Sleep Deprivation-Induced Cortical Impairment. International Journal of Molecular Sciences. 2025; 26(22):11173. https://doi.org/10.3390/ijms262211173
Chicago/Turabian StyleLutfy, Radwa Hussein, Ahmed M. Ashour, Ali Khames, Alzahraa A. Elhemiely, Khaled M. Alam-ElDein, Ahmed Hassan Ibrahim Faraag, Mariam O. A. Hamed, Zainab J. Abdel Daim, Nagwa Ibrahim Attia, and Mohamed H. A. Gadelmawla. 2025. "Targeting Oxidative Stress and Neuroinflammation: Epigallocatechin-3-gallate-Selenium Nanoparticles Mitigate Sleep Deprivation-Induced Cortical Impairment" International Journal of Molecular Sciences 26, no. 22: 11173. https://doi.org/10.3390/ijms262211173
APA StyleLutfy, R. H., Ashour, A. M., Khames, A., Elhemiely, A. A., Alam-ElDein, K. M., Faraag, A. H. I., Hamed, M. O. A., Abdel Daim, Z. J., Attia, N. I., & Gadelmawla, M. H. A. (2025). Targeting Oxidative Stress and Neuroinflammation: Epigallocatechin-3-gallate-Selenium Nanoparticles Mitigate Sleep Deprivation-Induced Cortical Impairment. International Journal of Molecular Sciences, 26(22), 11173. https://doi.org/10.3390/ijms262211173







