Oxidative Stress-Mediated Neuroinflammation in the Pathophysiology of Schizophrenia
Abstract
1. Introduction
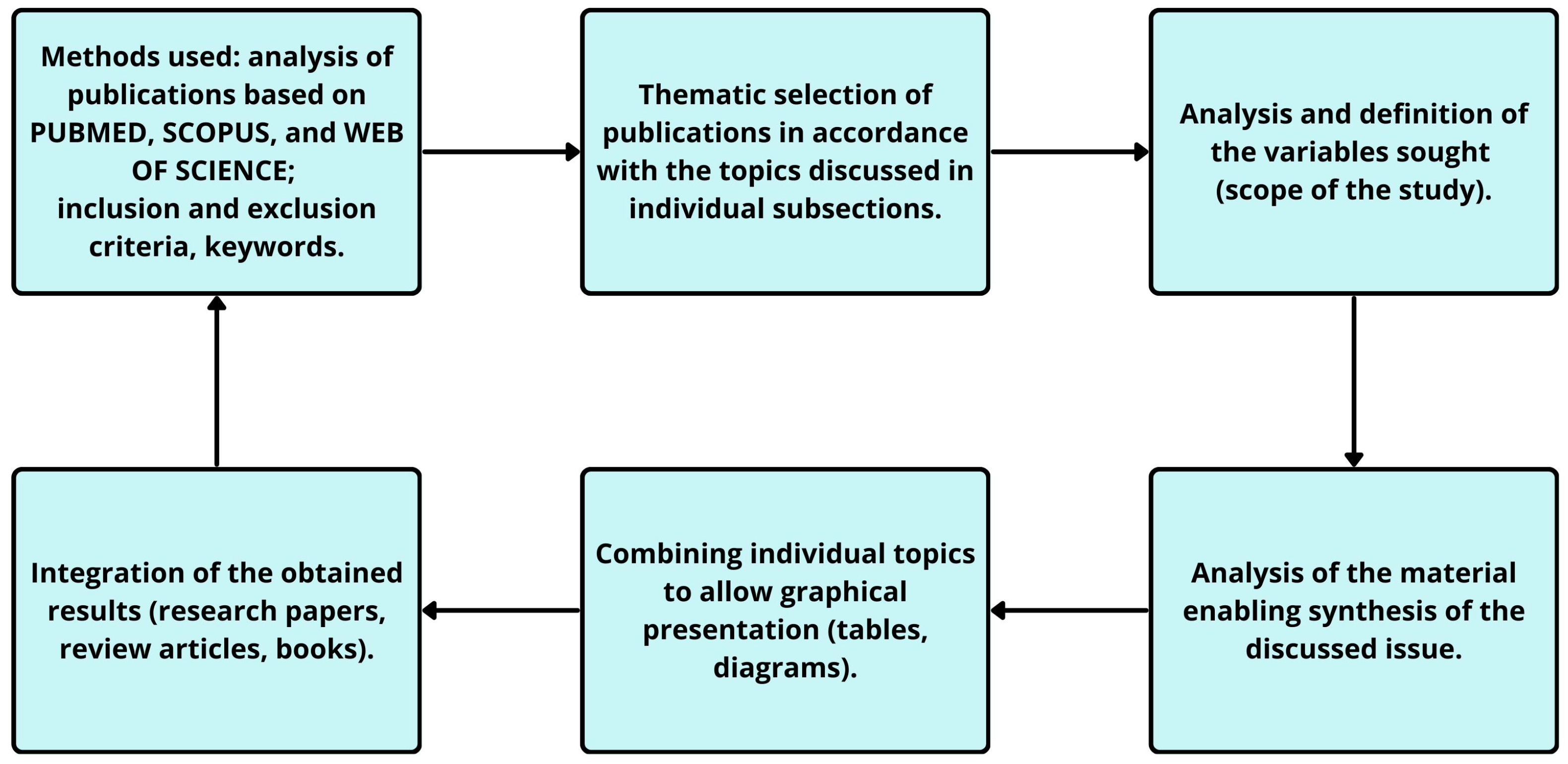
2. Methods

| Cellular Component | Process Characterization |
|---|---|
| Lipids | The oxidative damage to lipids, especially in cell membranes, compromises membrane integrity and function. Lipid peroxidation not only alters the physical properties of membranes but also generates secondary reactive species that propagate damage throughout the cell. This disruption can impair membrane-bound signalling pathways and transport processes, leading to cellular dysfunction [15]. Recent studies in schizophrenia have shown elevated serum levels of oxidized LDL (ox-LDL) and its receptor LOX-1, both of which are products and mediators of lipid oxidation, implicating them as potential biomarkers of oxidative stress–driven neuroinflammation [16]. |
| Proteins | Oxidative modifications of proteins can result in misfolding, aggregation, and loss of function. Enzymes with critical roles in metabolic pathways are particularly vulnerable, as oxidative damage can alter their catalytic efficiency. Moreover, oxidized proteins are often targeted for degradation by the proteasome system, leading to increased protein turnover and stress on the protein synthesis machinery [17]. |
| Nucleic acids | DNA damage caused by ROS includes base modifications, single and double-strand breaks, and cross-linking. These lesions can interfere with transcription and replication processes, leading to genomic instability. The activation of DNA repair mechanisms, while essential for maintaining genomic integrity, consumes cellular resources and can induce further stress if repair processes are overwhelmed or defective [18,19]. |
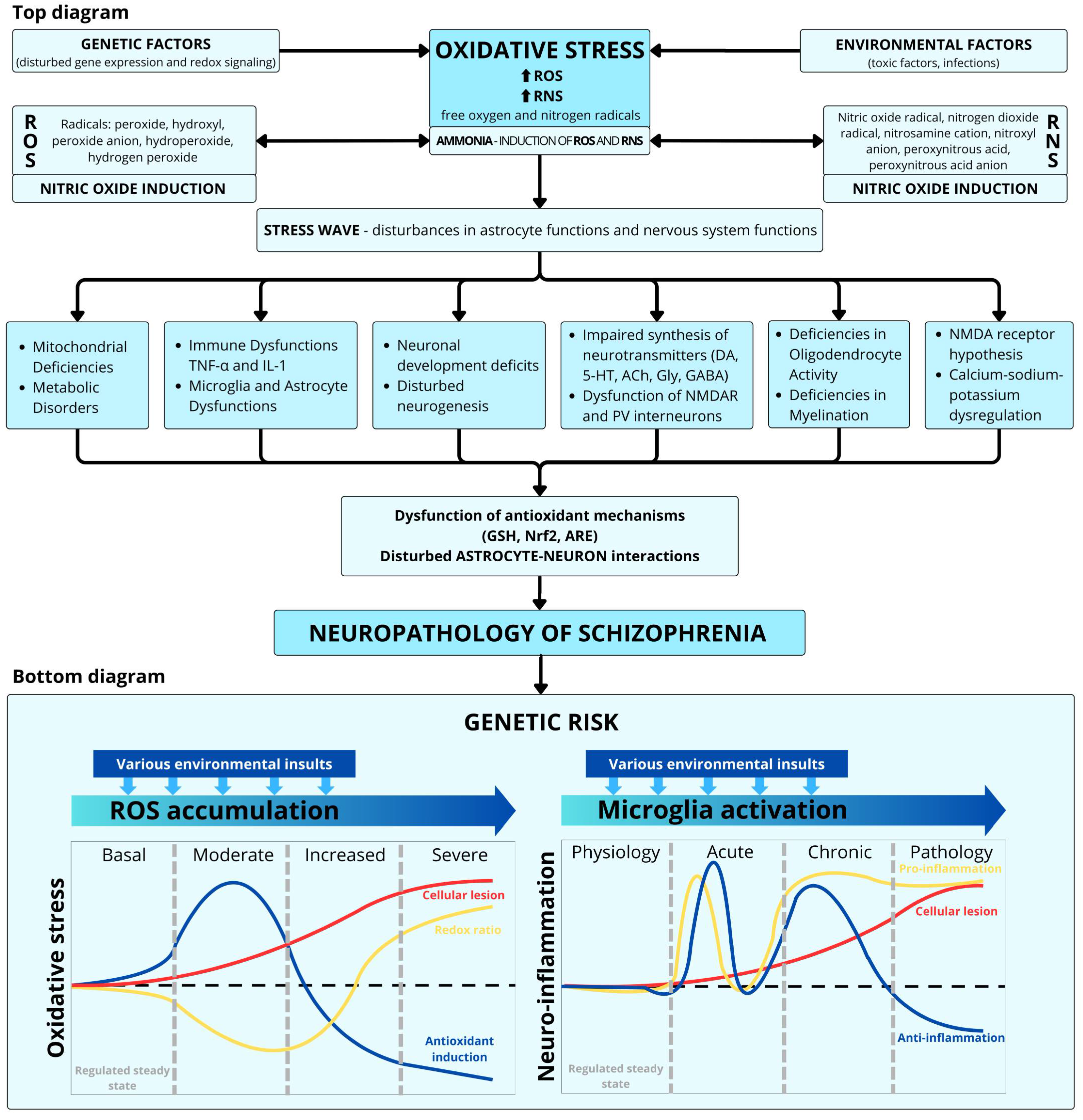
3. Oxidative Stress
4. Impact of Oxidative Stress on Cellular Metabolism
4.1. Disruption of Metabolic Pathways
- (a)
- Glycolysis and the tricarboxylic acid (TCA) Cycle: Oxidative stress significantly affects carbohydrate metabolism, including glycolysis and the TCA cycle. ROS can modify key enzymes such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and aconitase, leading to altered enzyme activities and metabolic fluxes. For example, the oxidation of GAPDH results in decreased glycolytic flow, while aconitase inactivation disrupts the TCA cycle, leading to reduced ATP production and energy imbalance in cells [30,31];
- (b)
- Lipid Metabolism: Lipid peroxidation is a primary consequence of oxidative stress, were ROS attack polyunsaturated fatty acids in cell membranes, generating lipid peroxides. This process alters membrane fluidity and permeability, affecting membrane-bound enzymes and receptors. Additionally, lipid peroxidation products, such as malondialdehyde (MDA), can form adducts with proteins and DNA, further impairing cellular functions [32,33]. Additionally, recent evidence indicates that oxidized low-density lipoprotein (ox-LDL) and its receptor LOX-1 play a significant role in oxidative stress–related neuroinflammatory pathways. In a case–sibling–control study, serum ox-LDL and LOX-1 levels were markedly elevated in schizophrenia patients compared to both healthy controls and unaffected first-degree relatives. Interestingly, ox-LDL levels were also higher in relatives than in controls, suggesting a possible endophenotypic marker of vulnerability. Activation of LOX-1 by ox-LDL is known to trigger pro-inflammatory signaling cascades, adhesion molecule expression, and ROS generation via the AMPK/PKC/NADPH oxidase pathway, which may exacerbate neuronal damage and blood–brain barrier dysfunction in schizophrenia [16];
- (c)
- Protein Metabolism: Proteins are susceptible to oxidative modifications by ROS, which can lead to the formation of carbonyl groups, disulfide bonds, and cross-linked aggregates. These modifications often result in loss of enzymatic activity, altered protein structure, and impaired protein-protein interactions. For instance, oxidative damage to enzymes involved in amino acid metabolism can disrupt the synthesis and degradation of proteins, affecting overall cellular homeostasis [34];
- (d)
- Nucleotide Metabolism: ROS can induce oxidative modifications in nucleotides, leading to the formation of 8-oxoguanine and other oxidized bases. These modifications can cause mutations during DNA replication, disrupt gene expression, and activate DNA repair pathways. In severe cases, the accumulation of DNA damage can trigger cell cycle arrest, pathological apoptosis, or carcinogenesis [35].
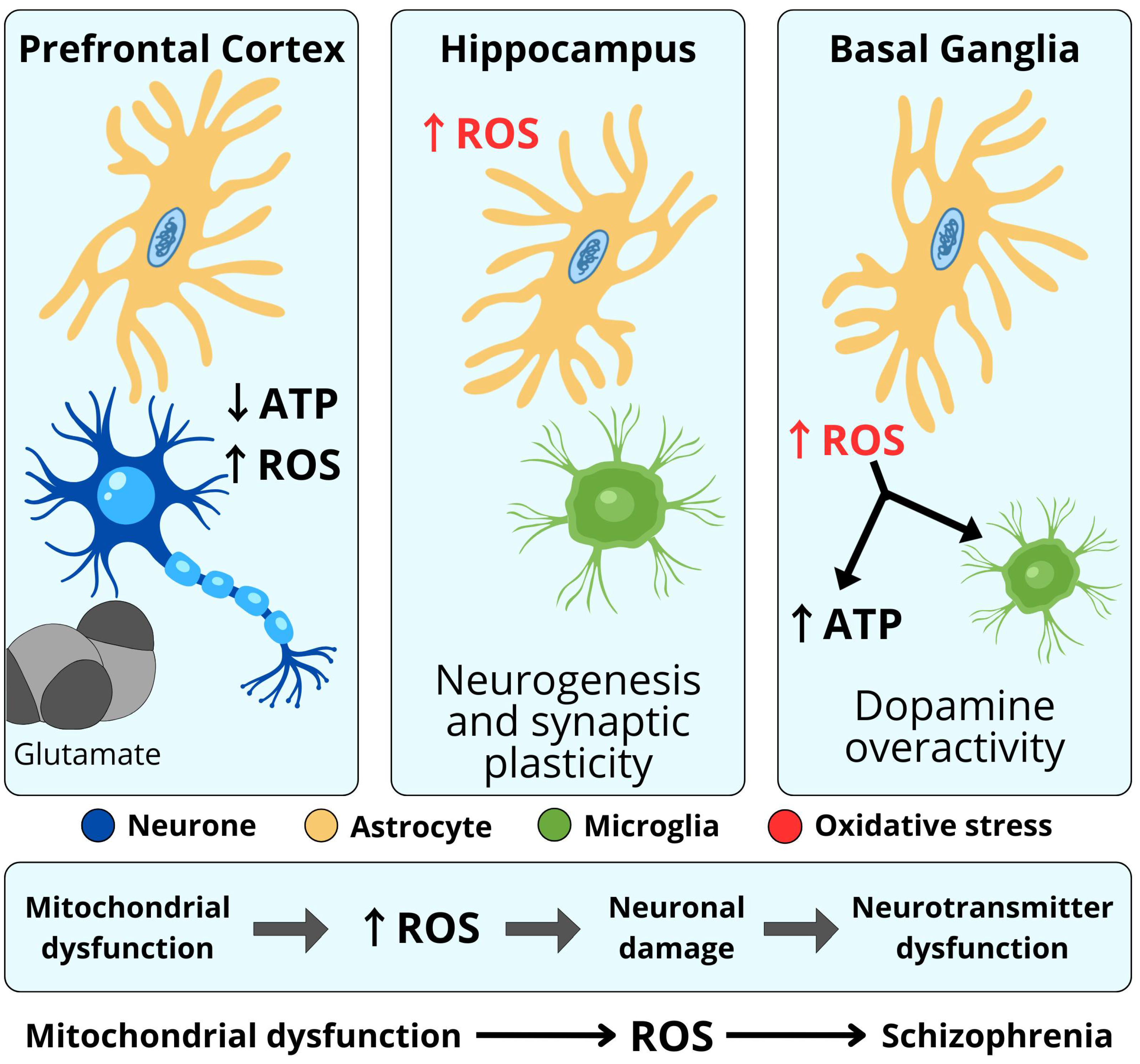
4.2. Mitochondrial Dysfunction
4.3. Altered Redox Balance
4.4. Dysregulated Metabolic Pathways
4.5. Consequences for Cellular Health
5. Disturbance in Neurotransmission
5.1. Dopamine
5.2. Serotonin
5.3. Acetylcholine
5.4. Gamma-Aminobutyric Acid
5.5. Glutamate
5.6. Other Neurotransmitters
6. Neuroinflammation Hypothesis of Schizophrenia
7. Neurodevelopmental Hypotheses
8. Antioxidant Therapy
9. Oxidative Stress and Neuroinflammation in General Models of Schizophrenia
- Neurodevelopmental models. Regardless of whether these models approach schizophrenia as an inevitable cost to the emergence of the human species or as a variable sum of errors at key stages of an individual’s development, they address the interplay of unimpaired development and pathological processes such as oxidative stress and neuroinflammation [164,165].
- The vulnerability-stress models. The phenomena of oxidative stress and neuroinflammation may be particularly useful for explaining not only the initial or final psychotic pathogeneses, but mostly the “silent”, long-term accumulating dysfunctions of the prodromal and latent psychotic phases [120,141].
- Neuroplasticity models. Oxidative stress and neuroinflammation concepts allow us to avoid understanding schizophrenia through a single trigger or disruption of a key stage of development and to see the pathogenesis of psychosis as an imbalance of continuous pro-health and disruptive neuroplastic processes, such as apoptosis induced by oxidative stress.
- Neurotransmitter models. All these concepts, such as early hyperdopaminergic or late hypodopaminergic hypotheses, refer to quantitative making qualitative changes in neurotransmitter functioning and are finally related to cumulative damage to the CNS [29,60,100,108]. While the list of primary causes damaging the CNS is quite long or in fact endless, the secondary reasons such as oxidative stress and neuroinflammation are limited.
- Microbiome-brain axis models. Oxidative stress and inflammation may contribute to the disruption of the intestinal and blood–brain barriers, and ultimately brain inflammation [60].
- Psychological stress models. All concepts of psychological stress use oxidative and neuroinflammatory dysfunctions as a common link between non-compensated stress and neuropathological consequences [137,138,139]. When stressful life events exceed an individual’s neuroplasticity buffering threshold, a psychotic episode is likely to develop [134].
- Factor models. Because there is a relative independence of factors creating clinical symptoms of schizophrenia (positive, negative, cognitive, disorganized, emotional, others), non-specific oxidative stress and neuroinflammation can explain the individual diversity of clinical symptoms of schizophrenia and varying susceptibility to antipsychotic treatment [5,140,162].
- To provide a broader perspective on the mechanisms discussed, Table 2 compiles and synthesizes key clinical and preclinical findings that demonstrate a consistent association between redox dysfunction and schizophrenia.
10. Conclusions
- (1)
- mitochondrial dysfunction, abnormal neuron formation, abnormal myelination, impaired neurotransmitter production, and impaired reorganization of synaptic connections,
- (2)
- impaired release of neurotransmitters, abnormal processes of neurogenesis,
- (3)
- a deficit of ATP and a disturbed energy balance of cells and their enzymatic activity,
- (4)
- lipid peroxidation, altered protein structure, mutations in protein coding, disturbed protein–protein interaction,
- (5)
- DNA mutations, impaired gene expression, and impaired activation of DNA repair pathways,
- (6)
- disrupted cell homeostasis as a toxic effect of high H2S concentration,
- (7)
- destruction of metabolic pathways and changes in substrate utilization,
- (8)
- increased apoptosis and increased inflammatory responses,
- (9)
- microdamage of dopaminergic endings and loss of neuronal connections,
- (10)
- impaired synthesis and transmission of neurotransmitters,
- (11)
- impaired synaptic signaling,
- (12)
- non-enzymatic oxidation of dopamine—an increase in hydrogen peroxide,
- (13)
- increased levels of nitric oxide (peroxynitrite)—neurotoxic effects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girdler, S.; Confino, J.; Woesner, M. Exercise as a Treatment for Schizophrenia: A Review. Psychopharmacol. Bull. 2019, 49, 56–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fonseka, L.N.; Woo, B.K.P. Wearables in Schizophrenia: Update on Current and Future Clinical Applications. JMIR Mhealth uHealth 2022, 10, e35600. [Google Scholar] [CrossRef]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef]
- Khavari, B.; Cairns, M. Epigenomic Dysregulation in Schizophrenia: In Search of Disease Etiology and Biomarkers. Cells 2020, 9, 1837. [Google Scholar] [CrossRef]
- Stilo, S.; Murray, R. Non-Genetic Factors in Schizophrenia. Curr. Psychiatry Rep. 2019, 21, 100. [Google Scholar] [CrossRef]
- Hartwig, F.; Borges, M.; Horta, B.; Bowden, J.; Smith, G.D. Inflammatory Biomarkers and Risk of Schizophrenia: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry 2017, 74, 1226–1233. [Google Scholar] [CrossRef]
- Williams, J.; Burgess, S.; Suckling, J.; Lalousis, P.A.; Batool, F.; Griffiths, S.L.; Palmer, E.; Karwath, A.; Barsky, A.; Gkoutos, G.V.; et al. PIMS Collaboration. Inflammation and Brain Structure in Schizophrenia and Other Neuropsychiatric Disorders: A Mendelian Randomization Study. JAMA Psychiatry 2022, 79, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Rogers, J.; Katshu, M.; Liddle, P.; Upthegrove, R. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front. Psychiatry 2021, 12, 703452. [Google Scholar] [CrossRef]
- Bošković, M.; Vovk, T.; Kores Plesničar, B.; Grabnar, I. Oxidative stress in schizophrenia. Curr. Neuropharmacol. 2011, 9, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, S.I.; Rosse, R.B.; Schwartz, B.L.; Mastropaolo, J. A revised excitotoxic hypothesis of schizophrenia: Therapeutic implications. Clin. Neuropharmacol. 2001, 24, 43–49. [Google Scholar] [CrossRef]
- Lin, C.H.; Lane, H.Y. Early Identification and Intervention of Schizophrenia: Insight from Hypotheses of Glutamate Dysfunction and Oxidative Stress. Front. Psychiatry 2019, 10, 93. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kozubski, W.; Dorszewska, J. Apoptosis in Diseases of the Central Nervous System; Wydawnictwo Czelej: Lublin, Poland, 2000. [Google Scholar]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Akkuş, M.; Solak, H. Elevated levels of oxLDL and LOX-1: Implications for schizophrenia pathophysiology. J. Psychiatr. Res. 2024, 177, 140–146. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.; Medeiros, M.; Di Mascio, P.; Wagner, J. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef]
- Murphy, M. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Fendri, C.; Mechri, A.; Khiari, G.; Othman, A.; Kerkeni, A.; Gaha, L. Oxidative stress involvement in schizophrenia pathophysiology: A review. Encephale 2006, 32, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Kosten, T.; Zhang, X. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Flatow, J.; Buckley, P.; Miller, B.J. Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatry 2013, 74, 400–409. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Bandodkar, V.V.; Moger, V.S.; Baliga, P. Antioxidant Defense Mechanisms: Enzymatic and Non-Enzymatic. In The Role of Reactive Oxygen Species in Human Health and Disease; Prabhakar, P.K., Ed.; IGI Global Scientific Publishing: Hershey, PA, USA, 2025; pp. 43–80. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Rupniewska, Z.; Bojarska-Junak, A. Apoptosis: Mitochondrial membrane permeability and the role of BCL-2 family proteins. Postępy Hig. Med. Dosw. 2004, 58, 538–547. [Google Scholar] [PubMed]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W., 2nd; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N.; et al. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement from the American Heart Association. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef]
- Pham, L.; Arroum, T.; Wan, J.; Pavelich, L.; Bell, J.; Morse, P.T.; Lee, I.; Grossman, L.I.; Sanderson, T.H.; Malek, M.H.; et al. Regulation of mitochondrial oxidative phosphorylation through tight control of cytochrome c oxidase in health and disease—Implications for ischemia/reperfusion injury, inflammatory diseases, diabetes, and cancer. Redox Biol. 2024, 78, 103426. [Google Scholar] [CrossRef]
- Yoo, J.; Han, J.; Lim, H. Transition metal ions and neurotransmitters: Coordination chemistry and implications for neurodegeneration. RSC Chem. Biol. 2023, 4, 548–563. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Avery, S. Molecular targets of oxidative stress. Biochem. J. 2011, 434, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signalling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Davies, M. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Wallace, D. A mitochondrial bioenergetic etiology of disease. J. Clin. Investig. 2013, 123, 1405–1412. [Google Scholar] [CrossRef]
- Quinlan, C.; Perevoshchikova, I.; Hey-Mogensen, M.; Orr, A.; Brand, M. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013, 1, 304–312. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.; Petrosillo, G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 14205–14218. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H. Redox metabolism: ROS as specific molecular regulators of cell signalling and function. Mol. Cell. 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defence. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Liemburg-Apers, D.; Willems, P.; Koopman, W.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Górny, M.; Bilska-Wilkosz, A.; Iciek, M.; Rogóż, Z.; Lorenc-Koci, E. Treatment with aripiprazole and N-acetylcysteine affects anaerobic cysteine metabolism in the hippocampus and reverses schizophrenia-like behavior in the neurodevelopmental rat model of schizophrenia. FEBS J. 2023, 290, 5773–5793. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.J.; Chakraborty, S.; Miller, E.; Pieper, A.A.; Paul, B.D. Hydrogen sulfide signaling in neurodegenerative diseases. Br. J. Parmacol. 2023. [Google Scholar] [CrossRef]
- Yao, J.K.; Leonard, S.; Reddy, R. Altered glutathione redox state in schizophrenia. Dis. Markers 2006, 22, 83–93. [Google Scholar] [CrossRef]
- Hassan, M.I.; Boosen, M.; Schaefer, L.; Kozlowska, J.; Eisel, F.; von Knethen, A.; Beck, M.; Hemeida, R.A.M.; El-Moselhy, M.A.M.; Hamada, F.M.A.; et al. Platelet-derived growth factor-BB induces cystathionine γ-lyase expression in rat mesangial cells via a redox-dependent mechanism. Br. J. Pharmacol. 2012, 166, 2231–2242. [Google Scholar] [CrossRef]
- Guo, C.; Liang, F.; Shah Masood, W.; Yan, X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-kappaB dependent anti-inflammation pathway. Eur. J. Pharmacol. 2014, 725, 70–78. [Google Scholar] [CrossRef]
- Munteanu, C.; Galaction, A.I.; Onose, G.; Turnea, M.; Rotariu, M. Hydrogen Sulfide (H2S- or H2Sn-Polysulfides) in Synaptic Plasticity: Modulation of NMDA Receptors and Neurotransmitter Release in Learning and Memory. Int. J. Mol. Sci. 2025, 26, 3131. [Google Scholar] [CrossRef]
- Kimura, H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013, 63, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Jones, D. Redox theory of aging. Redox Biol. 2015, 5, 71–79. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Morgan, M.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signalling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.; Chaturvedi, M.; Aggarwal, B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Serasanambati, M.; Shanmuga, R.C. Function of Nuclear Factor Kappa B (NF-kB) in Human Diseases-A Review. South Indian J. Biol. Sci. 2016, 2, 368–387. [Google Scholar] [CrossRef]
- Madamanchi, N.; Runge, M. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Pino, O.; Guilera, G.; Gómez-Benito, J.; Najas-García, A.; Rufián, S.; Rojo, E. Neurodevelopment or neurodegeneration: Review of theories of schizophrenia. Actas Esp. Psiquiatr. 2014, 42, 185–195. [Google Scholar] [PubMed]
- Stone, W.S.; Phillips, M.R.; Yang, L.H.; Kegeles, L.S.; Susser, E.S.; Lieberman, J.A. Neurodegenerative model of schizophrenia: Growing evidence to support a revisit. Schizophr. Res. 2022, 243, 154–162. [Google Scholar] [CrossRef]
- Parellada, E.; Gassó, P. Glutamate and microglia activation as a driver of dendritic apoptosis: A core pathophysiological mechanism to understand schizophrenia. Transl. Psychiatry 2021, 11, 271. [Google Scholar] [CrossRef]
- Catts, V.S.; Catts, S.V.; McGrath, J.J.; Féron, F.; McLean, D.; Coulson, E.J.; Lutze-Mann, L.H. Apoptosis and schizophrenia: A pilot study based on dermal fibroblast cell lines. Schizophr. Res. 2006, 84, 20–28. [Google Scholar] [CrossRef][Green Version]
- Beyazyüz, M.; Küfeciler, T.; Bulut, L.; Ünsal, C.; Albayrak, Y.; Akyol, E.S.; Baykal, S.; Kuloglu, M.; Hashimoto, K. Increased serum levels of apoptosis in deficit syndrome schizophrenia patients: A preliminary study. Neuropsychiatr. Dis. Treat. 2016, 23, 1261–1268. [Google Scholar] [CrossRef]
- Dirican, E.; Özcan, H.; Uzunçakmak, S.K.; Takım, U. Evaluation Expression of the Caspase-3 and Caspase-9 Apoptotic Genes in Schizophrenia Patients. Clin. Psychopharmacol. Neurosci. 2023, 21, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Dwir, D.; Khadimallah, I.; Xin, L.; Rahman, M.; Du, F.; Öngür, D.; Do, K.Q. Redox and Immune Signaling in Schizophrenia: New Therapeutic Potential. Int. J. Neuropsychopharmacol. 2023, 26, 309–321. [Google Scholar] [CrossRef]
- de Vries, L.P.; van de Weijer, M.P.; Bartels, M. The human physiology of well-being: A systematic review on the association between neurotransmitters, hormones, inflammatory markers, the microbiome and well-being. Neurosci. Biobehav. Rev. 2022, 139, 104733. [Google Scholar] [CrossRef]
- Miller, M. Dopamine as a Multifunctional Neurotransmitter in Gastropod Molluscs: An Evolutionary Hypothesis. Biol. Bull. 2020, 239, 189–208. [Google Scholar] [CrossRef]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Howes, O.; Kapur, S. The dopamine hypothesis of schizophrenia: Version III--the final common pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, F. The interplay of dopamine metabolism abnormalities and mitochondrial defects in the pathogenesis of schizophrenia. Transl. Psychiatry 2022, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, J511, 421–427. [Google Scholar] [CrossRef]
- Yang, A.; Tsai, S. New Targets for Schizophrenia Treatment beyond the Dopamine Hypothesis. Int. J. Mol. Sci. 2017, 18, 1689. [Google Scholar] [CrossRef]
- Carlsson, A.; Carlsson, M.L. A dopaminergic deficit hypothesis of schizophrenia: The path to discovery. Dialogues Clin. Neurosci. 2006, 8, 137–142. [Google Scholar] [CrossRef]
- Howes, O.; McCutcheon, R.; Stone, J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 2015, 29, 97–115. [Google Scholar] [CrossRef]
- McCutcheon, R.; Krysta, J.; Howes, O. Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry 2020, 19, 15–33. [Google Scholar] [CrossRef]
- Luvsannyam, E.; Jain, M.; Pormento, M.; Siddiqui, H.; Balagtas, A.; Emuze, B.; Poprawski, T. Neurobiology of schizophrenia: A comprehensive review. Cureus 2022, 14, e23959. [Google Scholar] [CrossRef] [PubMed]
- Kokkinou, M.; Irvine, E.; Bonsall, D. Reproducing the dopamine pathophysiology of schizophrenia and approaches to ameliorate it: A translational imaging study with ketamine. Mol. Psychiatry 2021, 26, 2562–2576. [Google Scholar] [CrossRef]
- Brugger, S.; Angelescu, I.; Abi-Dargham, A.; Mizrahi, R.; Shahrezaei, V.; Howes, O. Heterogeneity of striatal dopamine function in schizophrenia: Meta-analysis of Variance. Biol. Psychiatry 2020, 87, 215–224, Erratum in Biol. Psychiatry 2020, 87, 305. [Google Scholar] [CrossRef]
- Chowardi, K.V.; Bamne, M.N.; Nimgaonkar, V.L. Generic association studies of antioxidant pathway genes and schizophrenia. Antioxid. Redox Signal. 2011, 15, 2037–2045. [Google Scholar] [CrossRef]
- Hermida-Ameijeiras, A.; Méndez-Alvarez, E.; Sánchez-Iglesias, S.; Sanmartín-Suárez, C.; Soto-Otero, R. Autoxidation and MAO-mediated metabolism of dopamine as a potential cause of oxidative stress: Role of ferrous and ferric ions. Neurochem. Int. 2004, 45, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Arnone, D.; Cappai, A.; Howes, O. Alterations in the serotonin system in schizophrenia: A systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci. Biobehav. Rev. 2014, 45, 233–245. [Google Scholar] [CrossRef]
- Wang, D.; Chand, G.; Caito, N.; Eramo, A.; Gratten, V.; Hixon, M.; Nicol, G.; Lessie, E.; Prensky, Z.; Kuwabara, H.; et al. PET clinical study of novel antipsychotic LB-102 demonstrates unexpectedly prolonged dopamine receptor target engagement. Neuropsychopharmocology 2024, 50, 372–377, Correction in Neuropsychopharmocology 2024, 50, 856. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kanazawa, M.; Iwaki, M.; Jokanovic, A.; Minakuchi, S. Effects of offset values for artificial teeth positions in CAD/CAM complete denture. Comput. Biol. Med. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Grima, G.; Benz, B.; Parpura, V.; Cuénod, M.; Kim, Q. Dopamine-induced oxidative stress in neurons with glutathione deficit: Implication for schizophrenia. Schizophr. Res. 2003, 62, 213–224. [Google Scholar] [CrossRef]
- Karmakar, S.; Lal, G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics 2021, 11, 5296–5312. [Google Scholar] [CrossRef]
- Yabut, J.; Crane, J.; Green, A.; Keating, D.; Khan, W.; Steinberg, G. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Jenkins, T.; Nguyen, J.; Polglaze, K.; Bertrand, P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 28, 56. [Google Scholar] [CrossRef] [PubMed]
- Hrovatin, K.; Kunej, T.; Dolžan, V. Genetic variability of serotonin pathway associated with schizophrenia onset, progression, and treatment. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2020, 183, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Imamdin, A.; van der Vorst, E.P.C. Exploring the Role of Serotonin as an Immune Modulatory Component in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 1549. [Google Scholar] [CrossRef]
- Pytliak, M.; Vargová, V.; Mechírová, V.; Felšöci, M. Serotonin receptors—From molecular biology to clinical applications. Physiol. Res. 2011, 60, 15–25. [Google Scholar] [CrossRef]
- Shah, U.H.; González-Maeso, J. Serotonin and Glutamate Interactions in Preclinical Schizophrenia Models. ACS Chem. Neurosci. 2019, 10, 3068–3077. [Google Scholar] [CrossRef]
- Świt, P.; Pollap, A.; Orzeł, J. Spectroscopic Determination of Acetylcholine (ACh): A Representative Review. Top. Curr. Chem. 2023, 381, 16. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.; Bassi, C.; Saunders, M.; Nechanitzky, R.; Morgado-Palacin, I.; Zheng, C.; Mak, T. Beyond neurotransmission: Acetylcholine in immunity and inflammation. J. Intern. Med. 2020, 287, 120–133. [Google Scholar] [CrossRef]
- Higley, M.; Picciotto, M. Neuromodulation by acetylcholine: Examples from schizophrenia and depression. Curr. Opin. Neurobiol. 2014, 29, 88–95. [Google Scholar] [CrossRef]
- Picciotto, M.; Higley, M.; Mineur, Y. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Chen, J.; Cheuk, I.; Shin, V.Y.; Kwong, A. Acetylcholine receptors: Key players in cancer development. Surg. Oncol. 2019, 31, 46–53. [Google Scholar] [CrossRef]
- Ryan, A.; Mowry, B.; Kesby, J.; Scott, J.; Greer, J. Is there a role for antibodies targeting muscarinic acetylcholine receptors in the pathogenesis of schizophrenia? Aust. N. Z. J. Psychiatry 2019, 53, 1059–1069. [Google Scholar] [CrossRef]
- Shin, S.; Dixon, C. Alterations in Cholinergic Pathways and Therapeutic Strategies Targeting Cholinergic System after Traumatic Brain Injury. J. Neurotrauma 2015, 32, 1429–1440. [Google Scholar] [CrossRef]
- Terry, A.; Callahan, P. α7 nicotinic acetylcholine receptors as therapeutic targets in schizophrenia: Update on animal and clinical studies and strategies for the future. Neuropharmacology 2020, 170, 108053. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.; Ashok, A.; Angelescu, I.; Borgan, F.; Myers, J.; Lingford-Hughes, A.; Nutt, D.J.; Veronese, M.; Turkheimer, F.E.; Howes, O.D. GABA-A receptor differences in schizophrenia: A positron emission tomography study using [11C] Ro154513. Mol. Psychiatry 2021, 26, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Miller, M. GABA as a Neurotransmitter in Gastropod Molluscs. Biol. Bull. 2019, 236, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Buddhala, C.; Hsu, C.; Wu, J. A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem. Int. 2009, 55, 9–12. [Google Scholar] [CrossRef]
- Sarasa, S.; Mahendran, R.; Muthusamy, G.; Thankappan, B.; Selta, D.; Angayarkanni, J. A Brief Review on the Non-protein Amino Acid, Gamma-amino Butyric Acid (GABA): Its Production and Role in Microbes. Curr. Microbiol. 2020, 77, 534–544. [Google Scholar] [CrossRef]
- Reddy-Thootkur, M.; Kraguljac, N.; Lahti, A. The role of glutamate and GABA in cognitive dysfunction in schizophrenia and mood disorders–A systematic review of magnetic resonance spectroscopy studies. Schizophr. Res. 2022, 249, 74–84. [Google Scholar] [CrossRef]
- Merritt, K.; Egerton, A.; Kempton, M.; Taylor, M.; McGuire, P. Nature of glutamate alterations in schizophrenia a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry 2016, 73, 665–674. [Google Scholar] [CrossRef]
- Rasool, M.; Malik, A.; Saleem, S.; Ashraf, M.; Khan, A.; Waquar, S.; Pushparaj, P. Role of oxidative stress and the identification of biomarkers associated with thyroid dysfunction in schizophrenics. Front. Pharmacol. 2021, 12, 646287. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, G.; Fish, K.; Lewis, D. GABA Neuron Alterations, Cortical Circuit Dysfunction and Cognitive Deficits in Schizophrenia. Neural Plast. 2011, 2011, 723184. [Google Scholar] [CrossRef]
- Lodge, D.; Behrens, M.; Grace, A. A Loss of parvalbumin-containing interneurons is associated with reduced oscillatory activity in an animal model of schizophrenia. J. Neurosci. 2009, 29, 2344–2354. [Google Scholar] [CrossRef]
- Yuan, H.; Low, C.; Moody, O.; Jenkins, A.; Traynelis, S. Ionotropic GABA and Glutamate Receptor Mutations and Human Neurologic Diseases. Mol. Pharmacol. 2015, 88, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Charych, E.; Liu, F.; Moss, S.; Brandon, N. GABA(A) receptors and their associated proteins: Implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology 2009, 57, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef]
- Moghaddam, B.; Javitt, D. From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012, 37, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Pilowsky, L.S.; ABressan, R.; Stone, J.M.; Erlandsson, K.; Mulligan, R.S.; Krystal, J.H.; Ell, P.J. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol. Psychiatry 2006, 11, 118–119. [Google Scholar] [CrossRef]
- Kruse, A.; Bustillo, J. Glutamatergic dysfunction in Schizophrenia. Transl. Psychiatry 2022, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Liddle, E.B.; Fernandes, C.C.; Palaniyappan, L.; Hall, E.L.; Robson, S.E.; Simmonite, M.; Fiesal, J.; Katshu, M.Z.; Qureshi, A.; et al. Glutathione and glutamate in schizophrenia: A 7T MRS study. Mol. Psychiatry 2020, 25, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.M. Glutamatergic antipsychotic drugs: A new dawn in the treatment of schizophrenia? Ther. Adv. Psychopharmacol. 2011, 1, 5–18. [Google Scholar] [CrossRef]
- Moghaddam, B.; Jackson, M.E. Glutamatergic animal models of schizophrenia. Ann. N. Y. Acad. Sci. 2003, 1003, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. Neuroinflammation in Schizophrenia: The Key Role of the WNT/β-Catenin Pathway. Int. J. Mol. Sci. 2022, 23, 2810. [Google Scholar] [CrossRef]
- Kumari, V.; Postma, P. Nicotine use in schizophrenia: The self-medication hypotheses. Neurosci. Biobehav. Rev. 2005, 29, 1021–1034. [Google Scholar] [CrossRef]
- Rantala, M.; Luoto, S.; Borráz-León, J.; Krams, I. Schizophrenia: The new etiological synthesis. Neurosci. Biobehav. Rev. 2022, 142, 104894. [Google Scholar] [CrossRef]
- Ermakov, E.; Mednova, I.; Boiko, A.; Buneva, V.; Ivanova, S. Chemokine Dysregulation and Neuroinflammation in Schizophrenia: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2215. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like receptors in the pathogenesis o neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef]
- Taniuchi, I. CD4 helper and CD8 cytotoxic T cell differentiation. Annu. Rev. Immunol. 2018, 36, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Nedjai, B.; Hurst, T.; Pennington, D. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Murray, P.; Wynn, T. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: New approaches to hippocampal neurogenesis. J. Neuroinflamm. 2023, 20, 283. [Google Scholar] [CrossRef]
- Gu, M.; Mei, X.L.; Zhao, Y.N. Sepsis and Cerebral Dysfunction: BBB Damage, Neuroinflammation, Oxidative Stress, Apoptosis and Autophagy as Key Mediators and the Potential Therapeutic Approaches. Neurotox. Res. 2021, 39, 489–503. [Google Scholar] [CrossRef]
- Stanca, S.; Rossetti, M.; Bokulic Panichi, L.; Bongioanni, P. The Cellular Dysfunction of the Brain-Blood Barrier from Endothelial Cells to Astrocytes: The Pathway towards Neurotransmitter Impairment in Schizophrenia. Int. J. Mol. Sci. 2024, 25, 1250. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Gaglione, A.; Busceti, C.; Fornai, F. A sentinel in the crosstalk between the nervous and immune system: The (immuno)-proteasome. Front. Immunol. 2019, 10, 628. [Google Scholar] [CrossRef]
- Mosser, D.; Edwards, J. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Wicker, A.; Denovan-Wright, E.; Uher, R. Gene-environment interplay in the etiology of psychosis. Psychol. Med. 2018, 48, 1925–1936. [Google Scholar] [CrossRef]
- Bergdolt, L.; Dunaevsky, A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog. Neurobiol. 2019, 175, 1–19. [Google Scholar] [CrossRef]
- Mongan, D.; Ramesar, M.; Föcking, M.; Cannon, M.; Cotter, D. Role of inflammation in the pathogenesis of schizophrenia: A review of the evidence, proposed mechanisms and implications for treatment. Early Interv. Psychiatry 2019, 14, 385–397. [Google Scholar] [CrossRef]
- Purves-Tyson, T.; Weber-Stadlbauer, U.; Richetto, J.; Rothmond, D.; Labouesse, M.; Polesel, M.; Robinson, K.; Weickert, C.S.; Meyer, U. Increased levels of midbrain immune-related transcripts in schizophrenia and in murine offspring after maternal immune activation. Mol. Psychiatry 2019, 26, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.; Beggs, S. Sublime microglia: Expanding roles for the guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Boddeke, H.; Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Zhu, Y.; Klomparens, E.; Guo, S.; Geng, X. Neuroinflammation caused by mental stress: The effect of chronic restraint stress and acute repeated social defeat stress in mice. Neurol. Res. 2019, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhao, Z.; Feng, J.; Bo, J.; Rong, H.; Lei, Y.; Lu, C.; Zhang, X.; Hou, B.; Sun, Y.; et al. Glucocorticoid-potentiated spinal microglia activation contributes to preoperative anxiety-induced postoperative hyperalgesia. Mol. Neurobiol. 2017, 54, 4316–4328. [Google Scholar] [CrossRef]
- Niu, Z.; Yang, L.; Wu, X.; Zhu, Y.; Chen, J.; Fang, Y. The relationship between neuroimmunity and bipolar disorder: Mechanism and translational application. Neurosci. Bull. 2019, 35, 595–607. [Google Scholar] [CrossRef]
- Lurie, D. An integrative approach to neuroinflammation in psychiatric disorders and neuropathicpain. J. Exp. Neurosci. 2018, 12, 1179069518793639. [Google Scholar] [CrossRef]
- Haroon, E.; Chen, X.; Li, Z.; Patel, T.; Woolwine, B.; Hu, X.; Felger, J.C.; Miller, A.H. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl. Psychiatry 2018, 8, 189. [Google Scholar] [CrossRef]
- Lanz, T.A.; Reinhart, V.; Sheehan, M.J.; Rizzo, S.J.S.; Bove, S.E.; James, L.C.; Volfson, D.; Lewis, D.A.; Kleiman, R.J. Postmortem transcriptional profiling reveals widespread increase in inflammation in schizophrenia: A comparison of prefrontal cortex, striatum, and hippocampus among matched tetrads of controls with subjects diagnosed with schizophrenia, bipolar or major depressive disorder. Transl. Psychiatry 2019, 9, 151. [Google Scholar] [CrossRef]
- Zamanpoor, M. Schizophrenia in a genomic era: A review from the pathogenesis, genetic and environmental etiology to diagnosis and treatment insights. Psychiatr. Genet. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Davis, J.; Eyre, H.; Jacka, F.N.; Dodd, S.; Dean, O.; McEwen, S.; Debnath, M.; McGrath, J.; Maes, M.; Amminger, P.; et al. A review of vulnerability and risks for schizophrenia: Beyond the twohit hypothesis. Neurosci. Biobehav. Rev. 2016, 65, 185–194. [Google Scholar] [CrossRef]
- Feigenson, K.; Kusnecov, A.; Silverstein, S. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci. Biobehav. Rev. 2014, 38, 72–93. [Google Scholar] [CrossRef]
- Marenco, S.; Weinberger, D.R. The neurodevelopmental hypothesis of schizophrenia: Following a trail of evidence from cradle to grave. Dev. Psychopathol. 2000, 12, 501–527. [Google Scholar] [CrossRef]
- Maynard, T.; Sikich, L.; Lieberman, J.; LaMantia, A. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr. Bull. 2001, 27, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Chen, C.; Li, Z.; Martin, A.; Bryois, J.; Ma, X.; Gaspar, H.; Ikeda, M.; Benyamin, B.; Brown, B.; et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat. Genet. 2019, 51, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.; McCutcheon, R.; Owen, M.; Murray, R. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol. Psychiatry 2017, 81, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Tovar, M.; Rodríguez-Ramírez, A.M.; Rodríguez-Cárdenas, L.; Sotelo-Ramírez, C.E.; Camarena, B.; Sanabrais-Jiménez, M.A.; López-Riquelme, G. Insights into myelin dysfunction in schizophrenia and bipolar disorder. World J. Psychiatry 2022, 12, 264–285. [Google Scholar] [CrossRef]
- Schmitt, A.; Simons, M.; Cantuti-Castelvetri, L. A new role for oligodendrocytes and myelination in schizophrenia and affective disorders? Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 371–372. [Google Scholar] [CrossRef]
- Imosemi, Ł.I. A review of the importance of glutathione in neurodegenerative diseases. Ejpmr 2021, 8, 61–71. [Google Scholar]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.M.; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell. Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef]
- Khattar, N.; Triebswetter, C.; Kiely, M.; Ferrucci, L.; Resnick, S.; Spencer, R.; Bouhrara, M. Investigation of the association between cerebral iron content and myelin content in normative aging using quantitative magnetic resonance neuroimaging. Neuroimage 2021, 239, 118267. [Google Scholar] [CrossRef]
- Cheli, V.; Correale, J.; Paez, P.; Pasquini, J. Iron metabolism in oligodendrocytes and astrocytes, implications for myelination and remyelination. ASN Neuro. 2020, 12, 1759091420962681. [Google Scholar] [CrossRef]
- Müller, N. Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations. Schizophr. Bull. 2018, 44, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Byron, K.Y.; Bitanihirwe, B.K.Y.; Woo, T.-U.W. Oxidative stress in schizophrenia: An integrated approach. Neurosci. Biobehav. Rev. 2011, 35, 878–893. [Google Scholar] [CrossRef]
- Magalhães, P.V.; Dean, O.; Andreazza, A.C.; Berk, M.; Kapczinski, F. Antioxidant treatments for schizophrenia. Cochrane Database Syst. Rev. 2016, 2, CD008919. [Google Scholar] [CrossRef]
- Schmidt, L.; Phelps, E.; Friedel, J.; Shokraneh, F. Acetylsalicylic acid (aspirin) for schizophrenia. Cochrane Database Syst. Rev. 2019, 8, CD012116. [Google Scholar] [CrossRef]
- Isom, A.M.; Gudelsky, G.A.; Benoit, S.C.; Richtand, N.M. Antipsychotic medications, glutamate, and cell death: A hidden, but common medication side effect? Med. Hypotheses 2013, 80, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, V.L.; Millen, B.A.; Andersen, S.; Kinon, B.J.; Lagrandeur, L.; Lindenmayer, J.P.; Gomez, J.C. Pomaglumetad methionil: No significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr. Res. 2013, 150, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, R.; Markiewicz-Gospodarek, A.A.; Dobrowolska, B.; Bojar, I.; Łoza, B. Brain-derived neurotrophic factor and matrix metalloproteinase-9 activity during rehabilitation therapy of schizophrenic patients—Environmental pilot study. Ann. Agric. Environ. Med. 2023, 30, 315–321. [Google Scholar] [CrossRef]
- Ľupták, M.; Michaličková, D.; Fišar, Z.; Kitzlerová, E.; Hroudová, J. Novel approaches in schizophrenia-from risk factors and hypotheses to novel drug targets. World J. Psychiatry 2021, 11, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Crow, T.J. Schizophrenia as the price that homo sapiens pays for language: A resolution of the central paradox in the origin of the species. Brain Res. Rev. 2000, 31, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Voineskos, D.; Daskalakis, Z.J.; Rajji, T.K.; Blumberger, D.M.A. Review of Impaired Neuroplasticity in Schizophrenia Investigated with Non-invasive Brain Stimulation. Front. Psychiatry 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed]
| Type of Evidence | Brief Summary/Significance | Ref. No. |
|---|---|---|
| Humans—clinical (biochemistry) | Demonstrated disturbed redox balance (reduced GSH) in patients with schizophrenia—one of the most frequently cited studies linking antioxidant deficit with schizophrenia. | Yao et al., 2006 [47] |
| Humans—7T MRS (imaging) | 7-Tesla MRS revealed alterations in glutathione/glutamate levels in patients’ brains, providing direct evidence of redox dysfunction in brain tissue. | Kumar et al., 2020 [115] |
| In vitro—neurons | Experiments showing that dopamine, under glutathione deficiency, induces oxidative stress in neurons—a mechanistic explanation for the DA-ROS relationship. | Grima et al., 2003 [84] |
| Mixed—post-mortem humans + MIA mice | Increased expression of immune transcripts in the midbrain of patients and in the MIA model, linking neuroinflammation with developmental disorder models. | Purves-Tyson et al., 2019 [134] |
| Humans—post-mortem transcriptomics | Broad upregulation of inflammatory markers in the PFC/striatum/hippocampus in post-mortem samples from schizophrenia patients. | Lanz et al., 2019 [142] |
| Animals—neurodevelopmental rat model | Aripiprazole + NAC intervention normalizes cysteine-related disturbances and “schizophrenia-like” behaviors—preclinical evidence that redox modulation has behavioral effects. | Górny et al., 2023 [45] |
| In vitro/ex vivo—human fibroblasts | Pilot study on fibroblasts showing apoptotic shifts in schizophrenia patients—cellular evidence of mitochondrial dysfunction/apoptosis. | Catts et al., 2006 [62] |
| In vitro/biochemistry | Identification of mitochondrial ROS generation sites for different substrates—mechanistic basis linking mitochondrial dysfunction with increased ROS production. | Quinlan et al., 2013 [38] |
| In vitro/OPC | Oxidative stress disrupts differentiation of oligodendrocyte precursor cells—a link between ROS and abnormal myelination, relevant to schizophrenia pathology. | Spaas et al., 2021 [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trubalski, M.; Markiewicz-Gospodarek, A.; Żerebiec, M.; Poleszak, J.; Szczotka, M.; Markiewicz, R.; Łoza, B.; Szymańczyk, S. Oxidative Stress-Mediated Neuroinflammation in the Pathophysiology of Schizophrenia. Int. J. Mol. Sci. 2025, 26, 11139. https://doi.org/10.3390/ijms262211139
Trubalski M, Markiewicz-Gospodarek A, Żerebiec M, Poleszak J, Szczotka M, Markiewicz R, Łoza B, Szymańczyk S. Oxidative Stress-Mediated Neuroinflammation in the Pathophysiology of Schizophrenia. International Journal of Molecular Sciences. 2025; 26(22):11139. https://doi.org/10.3390/ijms262211139
Chicago/Turabian StyleTrubalski, Mateusz, Agnieszka Markiewicz-Gospodarek, Marta Żerebiec, Julia Poleszak, Miłosz Szczotka, Renata Markiewicz, Bartosz Łoza, and Sylwia Szymańczyk. 2025. "Oxidative Stress-Mediated Neuroinflammation in the Pathophysiology of Schizophrenia" International Journal of Molecular Sciences 26, no. 22: 11139. https://doi.org/10.3390/ijms262211139
APA StyleTrubalski, M., Markiewicz-Gospodarek, A., Żerebiec, M., Poleszak, J., Szczotka, M., Markiewicz, R., Łoza, B., & Szymańczyk, S. (2025). Oxidative Stress-Mediated Neuroinflammation in the Pathophysiology of Schizophrenia. International Journal of Molecular Sciences, 26(22), 11139. https://doi.org/10.3390/ijms262211139





