Estrogen Attenuates Hypoxia-Induced TRPV1 Activation and Calcium Overload via HIF-1α Suppression in MCF-7 and CHO Cells
Abstract
1. Introduction
2. Results
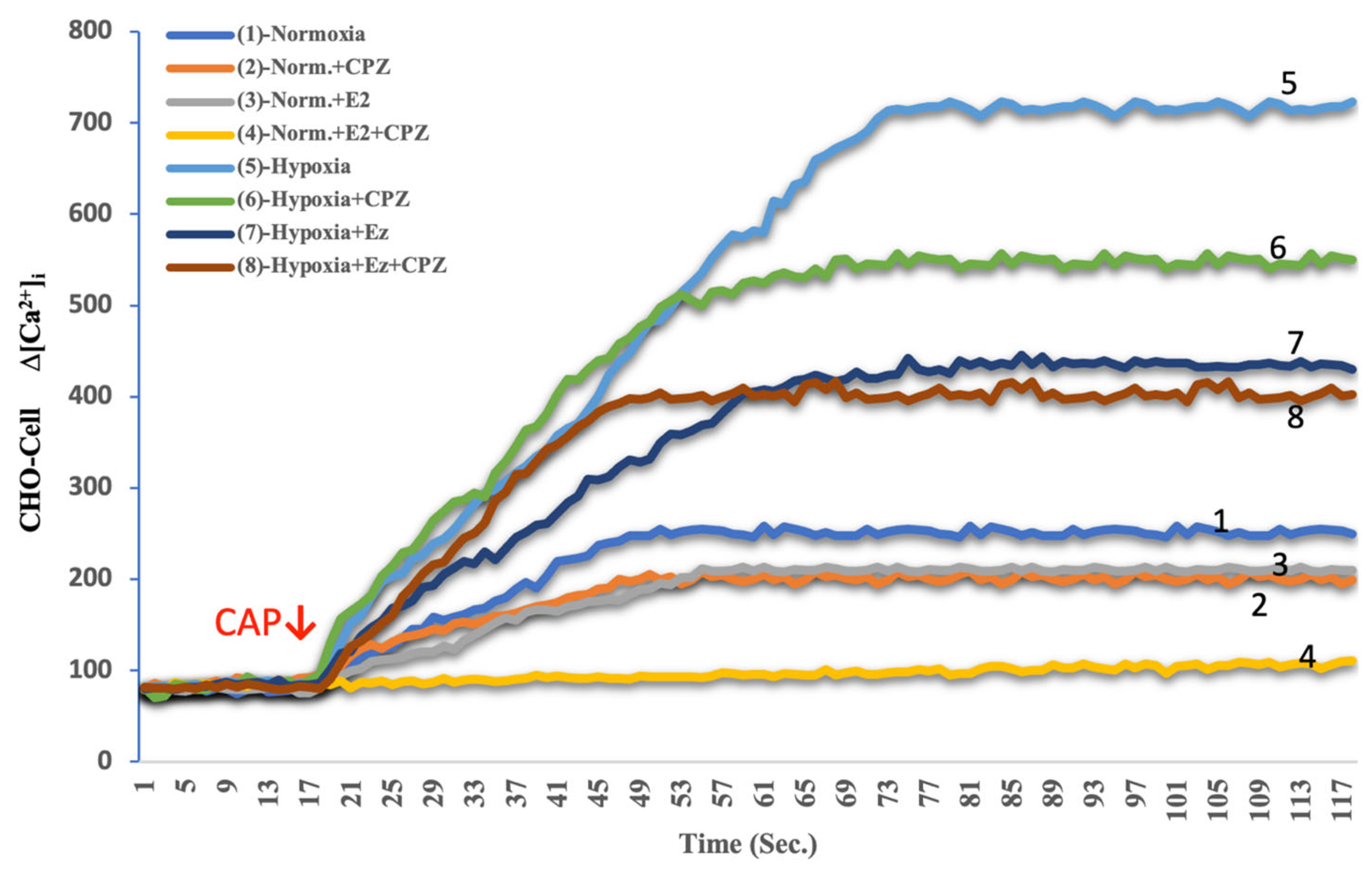
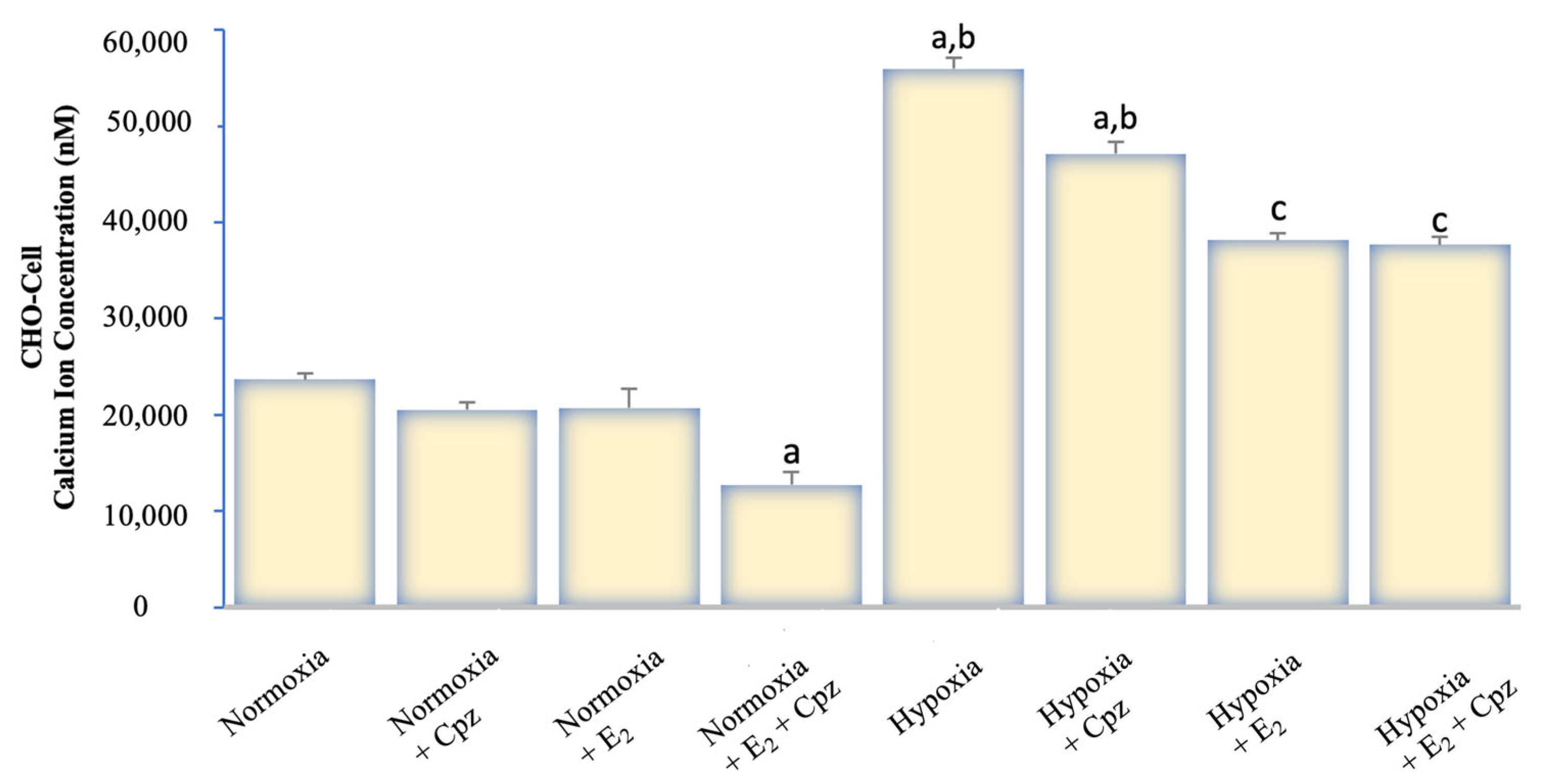
2.1. Effects of Hypoxia and Estrogen on Intracellular Calcium Dynamics in MCF-7 and CHO Cells
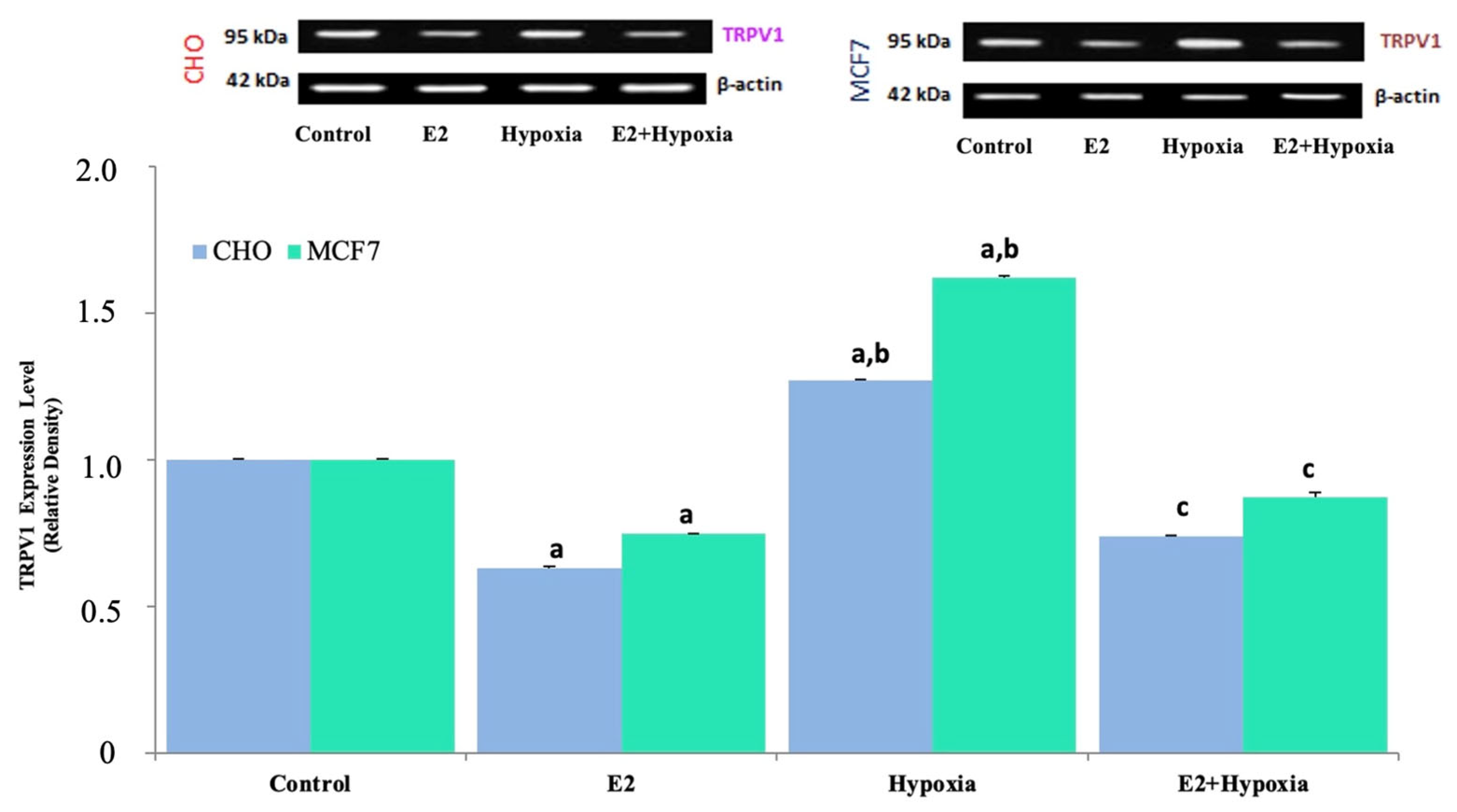
2.2. TRPV1 Expression in Response to Hypoxia and Estradiol
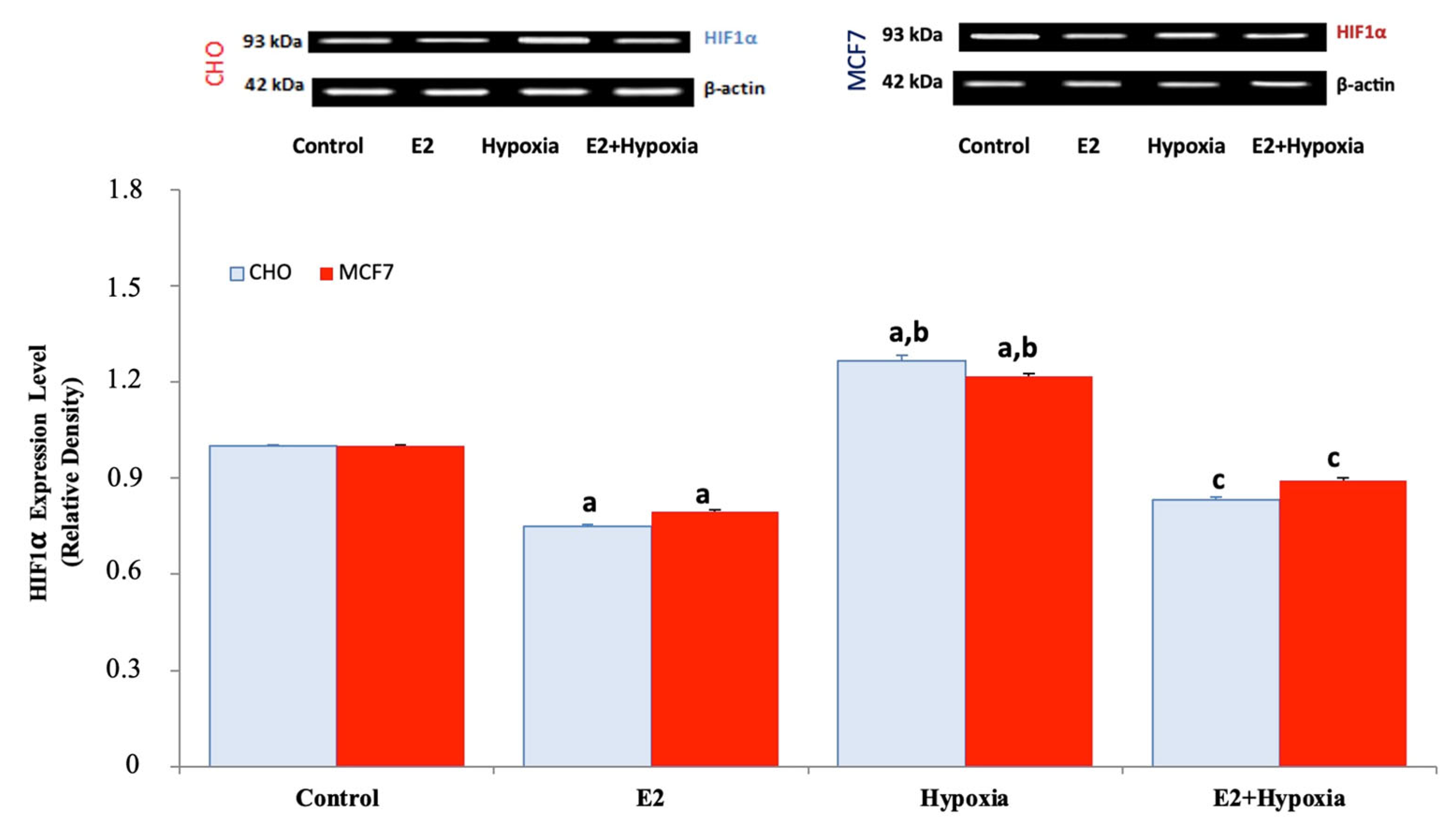
2.3. HIF-1α Expression Under Normoxia, Hypoxia, and Estradiol Treatment
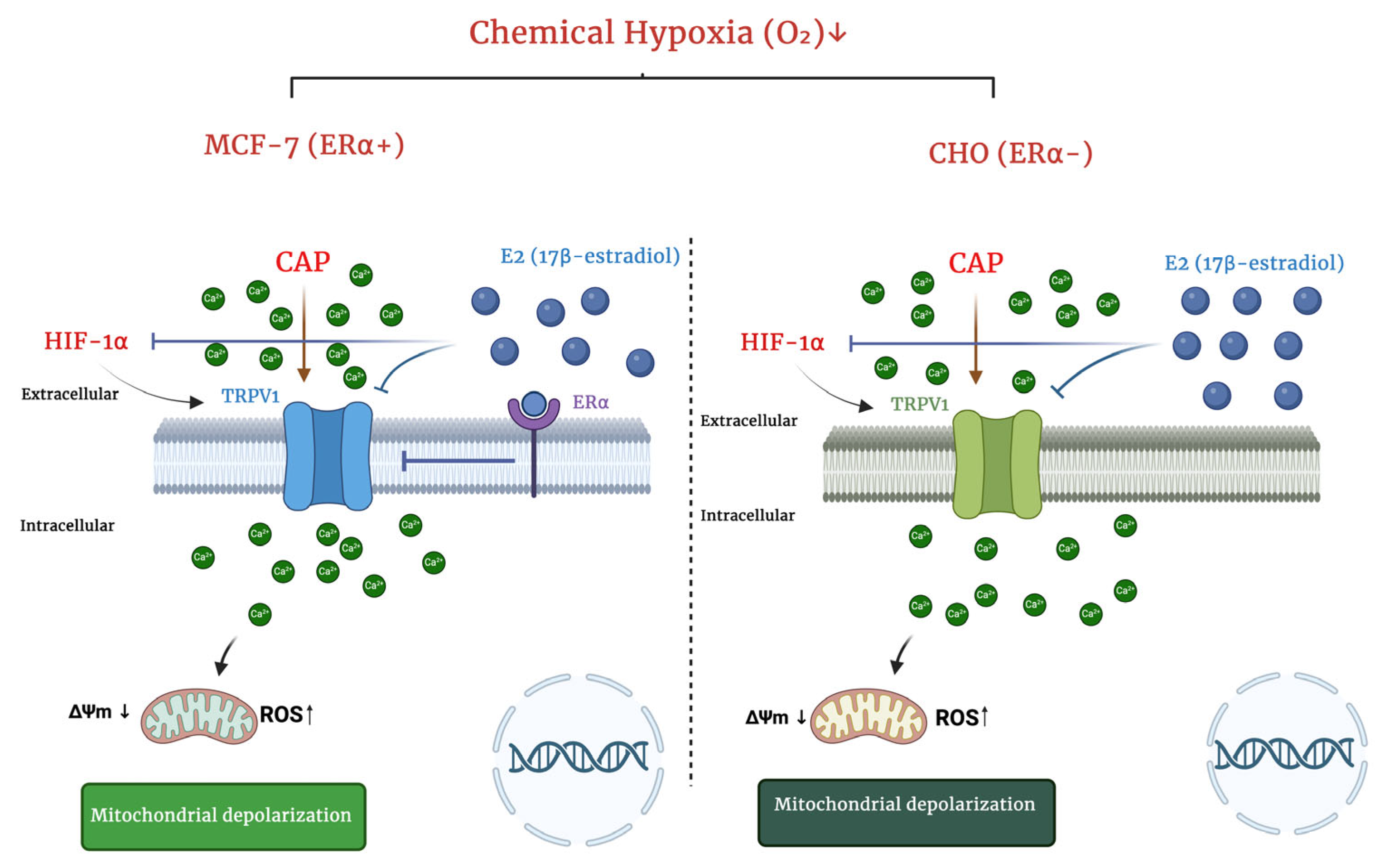
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. TRPV1 Plasmid and Transient Transfection (CHO Cells)
4.3. Reagents and Treatments
4.4. Experimental Design
- (i)
- Group structure and rationale: Four experimental conditions were established—Normoxia, E2, Hypoxia, and Hypoxia + E2 to distinguish ER-dependent and ER-independent effects on HIF-1α and TRPV1. This design allows direct mechanistic comparison between estrogenic modulation and hypoxic induction across both ERα(+) MCF-7 and ERα(–) CHO-TRPV1 cells, ensuring internal control within the same assay framework. Each group was processed under identical culture conditions and time frames to minimize variability arising from incubation duration or media composition. The inclusion of both ERα-positive and ERα-negative backgrounds was deliberate, as it provides a clear internal validation of whether estrogen exerts its effect through receptor-mediated signaling or through indirect modulation of hypoxia-driven pathways. In both cell types, parallel control groups were maintained to capture basal HIF-1α and TRPV1 levels under normoxic conditions, thereby establishing a baseline for relative quantification.
- (ii)
- Biological replicates, randomization, and statistical power: All experiments were performed with at least three independent biological replicates, each including two technical repeats per assay to ensure reproducibility. Samples within each replicate were randomized across wells and measurement sessions to minimize operator bias. Each experiment was designed with adequate biological replication and statistical robustness, as detailed in Section 4.7, ensuring that observed differences represent genuine biological effects rather than procedural variability. This approach minimizes both Type I and Type II errors and increases the reliability of the statistical outcomes.
- (iii)
- Validation of TRPV1 dependence: To strengthen mechanistic inference, capsazepine (10 μM, 10 min pre-incubation) was applied in Ca2+ signaling experiments to confirm TRPV1-specific Ca2+ influx. The suppression of Ca2+ elevation by capsazepine under both normoxia and hypoxia demonstrates that the observed effect is TRPV1-mediated. This pharmacological validation step serves as an internal functional control and verifies that any modulation of calcium signaling by hypoxia or estrogen arises from TRPV1 activity rather than from non-specific membrane leakage or unrelated channels. Furthermore, capsaicin was applied acutely (1 μM) to ensure that TRPV1 remained responsive within the physiological activation range, avoiding desensitization or cytotoxic overstimulation.
- (iv)
- Temporal and experimental consistency: All treatments were standardized to a 24 h exposure window to capture early transcriptional and functional responses to hypoxia while preventing secondary adaptive effects related to prolonged stress. Experimental timing, reagent preparation, and imaging parameters were synchronized across replicates to maintain comparability between assays. All reagents were prepared freshly for each experiment, and identical passage ranges (5–15) were used to minimize cell line drift.
- (v)
- Limitations and planned extensions: As now stated in the revised Discussion, the current design establishes a mechanistic relationship between hypoxia, HIF-1α, and TRPV1 activation, but genetic validation (TRPV1 siRNA or CRISPR silencing) and live-cell imaging of TRPV1 localization under hypoxia are planned to be conducted in future studies. Additionally, the inclusion of HIF-1α inhibitors or ERα modulators (e.g., ICI 182,780) is planned to further confirm the directional hierarchy of this signaling pathway. These next-step validations are planned to expand the current pharmacological framework into a fully integrated molecular model.
- Control (Normoxia): vehicle only.
- E2: 10 nM 17β-estradiol (E2), a concentration within the physiological range commonly used in cell culture studies [22].
- Hypoxia + E2: 200 μM CoCl2 + 10 nM E2 (added simultaneously).
4.5. Determination of Intracellular Free Ca2+ Concentration ([Ca2+]ᵢ)
4.6. Western Blotting
4.7. Statistics
4.8. Reproducibility and Reporting
4.9. Ethics Statement
5. Conclusions
- Limitations:
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaltel-Lima, L.; Domínguez, F.; Domínguez-Ramírez, L.; Cortes-Hernandez, P. The Role of the Estrogen-Related Receptor Alpha (ERRa) in Hypoxia and Its Implications for Cancer Metabolism. Int. J. Mol. Sci. 2023, 24, 7983. [Google Scholar] [CrossRef]
- Feng, D.; Gao, J.; Liu, R.; Liu, W.; Gao, T.; Yang, Y.; Zhang, D.; Yang, T.; Yin, X.; Yu, H.; et al. CARM1 drives triple-negative breast cancer progression by coordinating with Hif1α. Protein Cell 2024, 15, 744–765. [Google Scholar] [CrossRef] [PubMed]
- Tessier, N.; Ducrozet, M.; Dia, M.; Badawi, S.; Chouabe, C.; Crola Da Silva, C.; Ovize, M.; Bidaux, G.; Van Coppenolle, F.; Ducreux, S. TRPV1 Channels Are New Players in the Reticulum-Mitochondria Ca2+ Coupling in a Rat Cardiomyoblast Cell Line. Cells 2023, 12, 2322. [Google Scholar] [CrossRef] [PubMed]
- Holmes, T.; Brown, A.W.; Suggitt, M.; Shaw, L.A.; Simpson, L.; Harrity, J.P.A.; Tozer, G.M.; Kanthou, C. The influence of hypoxia and energy depletion on the response of endothelial cells to the vascular disrupting agent combretastatin A-4-phosphate. Sci. Rep. 2020, 10, 9926. [Google Scholar] [CrossRef] [PubMed]
- Devi, U.; Singh, M.; Roy, S.; Tripathi, A.C.; Gupta, P.S.; Saraf, S.K.; Ansari, M.N.; Saeedan, A.S.; Kaithwas, G. PHD-2 activation: A novel strategy to control HIF-1α and mitochondrial stress to modulate mammary gland pathophysiology in ER+ subtype. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1239–1256. [Google Scholar] [CrossRef]
- Simińska, D.; Kojder, K.; Jeżewski, D.; Tarnowski, M.; Tomasiak, P.; Piotrowska, K.; Kolasa, A.; Patrycja, K.; Chlubek, D.; Baranowska-Bosiacka, I. Estrogen α and β Receptor Expression in the Various Regions of Resected Glioblastoma Multiforme Tumors and in an In Vitro Model. Int. J. Mol. Sci. 2024, 25, 4130. [Google Scholar] [CrossRef]
- Jehanno, C.; Le Goff, P.; Habauzit, D.; Le Page, Y.; Lecomte, S.; Lecluze, E.; Percevault, F.; Avner, S.; Métivier, R.; Michel, D.; et al. Hypoxia and ERα Transcriptional Crosstalk Is Associated with Endocrine Resistance in Breast Cancer. Cancers 2022, 14, 4934. [Google Scholar] [CrossRef]
- Rasmussen, M.; Tan, S.; Somisetty, V.S.; Hutin, D.; Olafsen, N.E.; Moen, A.; Anonsen, J.H.; Grant, D.M.; Matthews, J. PARP7 and Mono-ADP-Ribosylation Negatively Regulate Estrogen Receptor α Signaling in Human Breast Cancer Cells. Cells 2021, 10, 623. [Google Scholar] [CrossRef]
- Scherbakov, A.M.; Shestakova, E.A.; Galeeva, K.E.; Bogush, T.A. BRCA1 and Estrogen Receptor α Expression Regulation in Breast Cancer Cells. Mol. Biol. 2019, 53, 502–512. (In Russian) [Google Scholar] [CrossRef]
- Brizzi, A.; Maramai, S.; Aiello, F.; Baratto, M.C.; Corelli, F.; Mugnaini, C.; Paolino, M.; Scorzelli, F.; Aldinucci, C.; De Petrocellis, L.; et al. Lipoic/Capsaicin-Related Amides: Synthesis and Biological Characterization of New TRPV1 Agonists Endowed with Protective Properties against Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 13580. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Q.; Hu, H.; Yang, C.; Zhao, Q. Esketamine alleviates hypoxia/reoxygenation injury of cardiomyocytes by regulating TRPV1 expression and inhibiting intracellular Ca2+ concentration. Clinics 2024, 79, 100363. [Google Scholar] [CrossRef]
- Lozano, C.; Córdova, C.; Marchant, I.; Zúñiga, R.; Ochova, P.; Ramírez-Barrantes, R.; González-Arriagada, W.A.; Rodriguez, B.; Olivero, P. Intracellular aggregated TRPV1 is associated with lower survival in breast cancer patients. Breast Cancer Targets Ther. 2018, 10, 161–168. [Google Scholar] [CrossRef]
- Calahorra, J.; Martínez-Lara, E.; De Dios, C.; Siles, E. Hypoxia modulates the antioxidant effect of hydroxytyrosol in MCF-7 breast cancer cells. PLoS ONE 2018, 13, e0203892. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Wang, X.; Shao, L.; Jiang, G.T.; Min, J.W.; Mei, X.Y.; He, X.H.; Liu, W.H.; Huang, W.X.; Peng, B.W. TRPV1 mediates astrocyte activation and interleukin-1β release induced by hypoxic ischemia (HI). J. Neuroinflammation 2019, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.S.; Harb, A.A.; Almasri, I.M.; Bustanji, Y.K. The interaction of TRPV1 and lipids: Insights into lipid metabolism. Front. Physiol. 2022, 13, 1066023. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.Y.; Zhu, T.T.; Lian, S.; Wang, J.F.; Wu, R.; Zheng, J.S. Estrogen-related Receptor α (ERRα) Functions in The Hypoxic Injury of Microglial Cells. J. Vet. Res. 2022, 66, 131–140. [Google Scholar] [CrossRef]
- Qu, Y.; Li, N.; Xu, M.; Zhang, D.; Xie, J.; Wang, J. Estrogen Up-Regulates Iron Transporters and Iron Storage Protein Through Hypoxia Inducible Factor 1 Alpha Activation Mediated by Estrogen Receptor β and G Protein Estrogen Receptor in BV2 Microglia Cells. Neurochem. Res. 2022, 47, 3659–3669. [Google Scholar] [CrossRef]
- Barrak, N.H.; Khajah, M.A.; Luqmani, Y.A. Hypoxic environment may enhance migration/penetration of endocrine resistant MCF7- derived breast cancer cells through monolayers of other non-invasive cancer cells in vitro. Sci. Rep. 2020, 10, 1127. [Google Scholar] [CrossRef]
- Wang, L.; Fan, J.; Yan, C.Y.; Ling, R.; Yun, J. Activation of hypoxia-inducible factor-1α by prolonged in vivo hyperinsulinemia treatment potentiates cancerous progression in estrogen receptor-positive breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 491, 545–551. [Google Scholar] [CrossRef]
- Jia, X.; Cheng, J.; Shen, Z.; Shao, Z.; Liu, G. Zoledronic acid sensitizes breast cancer cells to fulvestrant via ERK/HIF-1 pathway inhibition in vivo. Mol. Med. Rep. 2018, 17, 5470–5476. [Google Scholar] [CrossRef]
- Patil, M.J.; Belugin, S.; Akopian, A.N. Chronic alteration in phosphatidylinositol 4,5-biphosphate levels regulates capsaicin and mustard oil responses. J. Neurosci. Res. 2011, 89, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Ab-Rahim, S.; Selvaratnam, L.; Kamarul, T. The effect of TGF-beta1 and beta-estradiol on glycosaminoglycan and type II collagen distribution in articular chondrocyte cultures. Cell Biol. Int. 2008, 32, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, J.; Kong, B.; Park, J.; Song, H.; Choi, K.; Guon, T.; Lee, Y. The effects of bisphenol A, benzyl butyl phthalate, and di(2-ethylhexyl) phthalate on estrogen receptor alpha in estrogen receptor-positive cells under hypoxia. Env. Environ. Pollut. 2019, 248, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.K.; Singh, P.; Koch, B. CoCl2 simulated hypoxia induce cell proliferation and alter the expression pattern of hypoxia associated genes involved in angiogenesis and apoptosis. Biol. Res. 2019, 52, 12. [Google Scholar] [CrossRef]
- Isaja, L.; Mucci, S.; Vera, J.; Rodríguez-Varela, M.S.; Marazita, M.; Morris-Hanon, O.; Videla-Richardson, G.A.; Sevlever, G.E.; Scassa, M.E.; Romorini, L. Chemical hypoxia induces apoptosis of human pluripotent stem cells by a NOXA-mediated HIF-1α and HIF-2α independent mechanism. Sci. Rep. 2020, 10, 20653. [Google Scholar] [CrossRef]
- Ruan, Y.; Ling, J.; Ye, F.; Cheng, N.; Wu, F.; Tang, Z.; Cheng, X.; Liu, H. Paeoniflorin alleviates CFA-induced inflammatory pain by inhibiting TRPV1 and succinate/SUCNR1-HIF-1α/NLPR3 pathway. Int. Immunopharmacol. 2021, 101 Part B, 108364. [Google Scholar] [CrossRef]
- Tang, R.F.; Li, W.J.; Lu, Y.; Wang, X.X.; Gao, S.Y. LncRNA SNHG1 alleviates myocardial ischaemia-reperfusion injury by regulating the miR-137-3p/KLF4/TRPV1 axis. ESC Heart Fail. 2024, 11, 1009–1021. [Google Scholar] [CrossRef]
- Luo, X.; Chen, O.; Wang, Z.; Bang, S.; Ji, J.; Lee, S.H.; Huh, Y.; Furutani, K.; He, Q.; Tao, X.; et al. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 2021, 109, 2691–2706.e5. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Cipolletti, M.; Ascenzi, P.; Marino, M. Dissecting the 17β-estradiol pathways necessary for neuroglobin anti-apoptotic activity in breast cancer. J. Cell Physiol. 2018, 233, 5087–5103. [Google Scholar] [CrossRef]
- Melone, V.; Palumbo, D.; Palo, L.; Brusco, N.; Salvati, A.; Tarallo, A.; Giurato, G.; Rizzo, F.; Nassa, G.; Weisz, A.; et al. LncRNA PVT1 links estrogen receptor alpha and the polycomb repressive complex 2 in suppression of pro-apoptotic genes in hormone-responsive breast cancer. Cell Death Dis. 2025, 16, 80. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, J.R.; Kim, K.J.; Jun, J.H.; Hwang, H.J.; Lee, W.; Nam, S.H.; Oh, J.E.; Yoo, Y.C. Identification for antitumor effects of tramadol in a xenograft mouse model using orthotopic breast cancer cells. Sci. Rep. 2021, 11, 22113. [Google Scholar] [CrossRef]
- Kazi, A.A.; Gilani, R.A.; Schech, A.J.; Chumsri, S.; Sabnis, G.; Shah, P.; Goloubeva, O.; Kronsberg, S.; Brodie, A.H. Nonhypoxic regulation and role of hypoxia-inducible factor 1 in aromatase inhibitor resistant breast cancer. Breast Cancer Res. 2014, 16, R15. [Google Scholar] [CrossRef]
- Miyara, S.J.; Shinozaki, K.; Hayashida, K.; Shoaib, M.; Choudhary, R.C.; Zafeiropoulos, S.; Guevara, S.; Kim, J.; Molmenti, E.P.; Volpe, B.T.; et al. Differential Mitochondrial Bioenergetics in Neurons and Astrocytes Following Ischemia-Reperfusion Injury and Hypothermia. Biomedicines 2024, 12, 1705. [Google Scholar] [CrossRef]
- Silva, D.; Rocha, R.; Correia, A.S.; Mota, B.; Madeira, M.D.; Vale, N.; Cardoso, A. Repurposed Edaravone, Metformin, and Perampanel as a Potential Treatment for Hypoxia-Ischemia Encephalopathy: An In Vitro Study. Biomedicines 2022, 10, 3043. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Yao, L.; Hu, Q.; Liu, H.; Zhao, G.; Wang, K.; Zeng, J.; Sun, M.; Lv, C. Capsaicin Enhanced the Efficacy of Photodynamic Therapy Against Osteosarcoma via a Pro-Death Strategy by Inducing Ferroptosis and Alleviating Hypoxia. Small 2024, 20, e2306916. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhao, B.C.; Yang, X.; Lin, Z.B.; Sun, Q.S.; Wang, Y.F.; Yan, Z.Z.; Liu, W.F.; Li, C.; Hu, J.J.; et al. The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes. 2021, 13, 1902719. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, M.; Mauro, A.; Delle Monache, S.; Pulcini, F.; Russo, V.; Berardinelli, P.; Citeroni, M.R.; Turriani, M.; Peserico, A.; Barboni, B. Hypoxia-Mimetic CoCl2 Agent Enhances Pro-Angiogenic Activities in Ovine Amniotic Epithelial Cells-Derived Conditioned Medium. Cells 2022, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Schoch, H.J.; Fischer, S.; Marti, H.H. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain 2002, 125 Part 11, 2549–2557. [Google Scholar] [CrossRef]
- Kang, K.; Wang, D.P.; Lv, Q.L.; Chen, F. VEGF-A ameliorates ischemia hippocampal neural injury via regulating autophagy and Akt/CREB signaling in a rat model of chronic cerebral hypoperfusion. J. Stroke Cerebrovasc. Dis. 2023, 32, 107367. [Google Scholar] [CrossRef]
- Çiğ, B. Selenium reduces oxaliplatin induced neuropathic pain: Focus on TRPV1. Front Pharmacol. 2025, 16, 1549190. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Öz, A.; Yılmaz, B.; Altunbaş, S.; Çelik, Ö. Melatonin attenuates apoptosis and mitochondrial depolarization levels in hypoxic conditions of SH-SY5Y neuronal cells induced by cobalt chloride (CoCl2). Turk. J. Biol. 2015, 39, 896–903. [Google Scholar] [CrossRef]
- Tan, R.; Tian, H.; Yang, B.; Zhang, B.; Dai, C.; Han, Z.; Wang, M.; Li, Y.; Wei, L.; Chen, D.; et al. Autophagy and Akt in the protective effect of erythropoietin helix B surface peptide against hepatic ischaemia/reperfusion injury in mice. Sci. Rep. 2018, 8, 14703. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1958, 260, 3440–3450. [Google Scholar] [CrossRef]
- Kahya, M.C.; Nazıroğlu, M.; Övey, İ.S. Modulation of diabetes-induced oxidative stress, apoptosis, and Ca2+ Entry Through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol. Neurobiol. 2017, 54, 2345–2360. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çiğ, B. Estrogen Attenuates Hypoxia-Induced TRPV1 Activation and Calcium Overload via HIF-1α Suppression in MCF-7 and CHO Cells. Int. J. Mol. Sci. 2025, 26, 11110. https://doi.org/10.3390/ijms262211110
Çiğ B. Estrogen Attenuates Hypoxia-Induced TRPV1 Activation and Calcium Overload via HIF-1α Suppression in MCF-7 and CHO Cells. International Journal of Molecular Sciences. 2025; 26(22):11110. https://doi.org/10.3390/ijms262211110
Chicago/Turabian StyleÇiğ, Bilal. 2025. "Estrogen Attenuates Hypoxia-Induced TRPV1 Activation and Calcium Overload via HIF-1α Suppression in MCF-7 and CHO Cells" International Journal of Molecular Sciences 26, no. 22: 11110. https://doi.org/10.3390/ijms262211110
APA StyleÇiğ, B. (2025). Estrogen Attenuates Hypoxia-Induced TRPV1 Activation and Calcium Overload via HIF-1α Suppression in MCF-7 and CHO Cells. International Journal of Molecular Sciences, 26(22), 11110. https://doi.org/10.3390/ijms262211110






