Megakaryocytic Differentiation Regulates the Permissiveness and Antiviral Response of the Megakaryocytic Erythroid Progenitor to Dengue Virus
Abstract
1. Introduction
2. Results
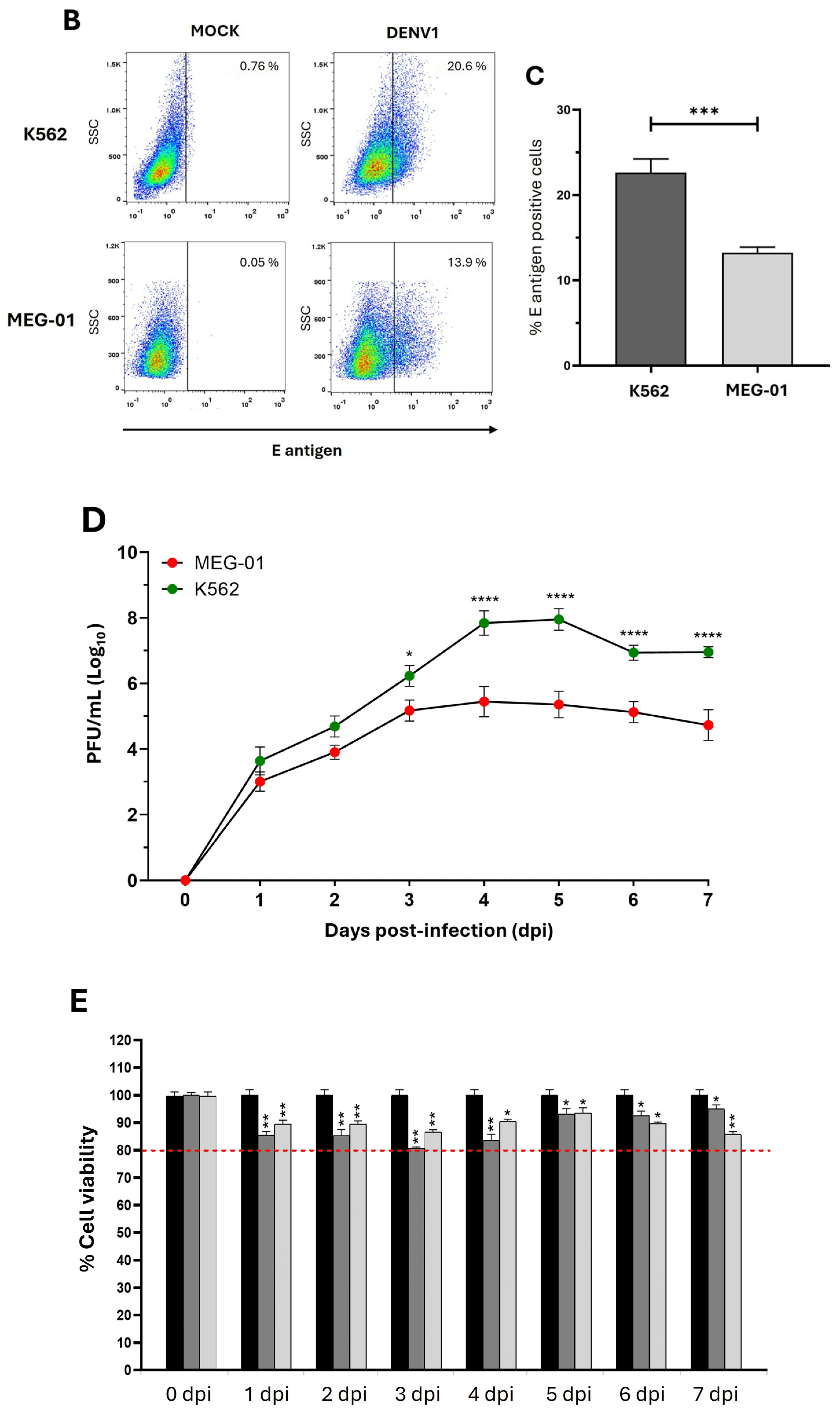
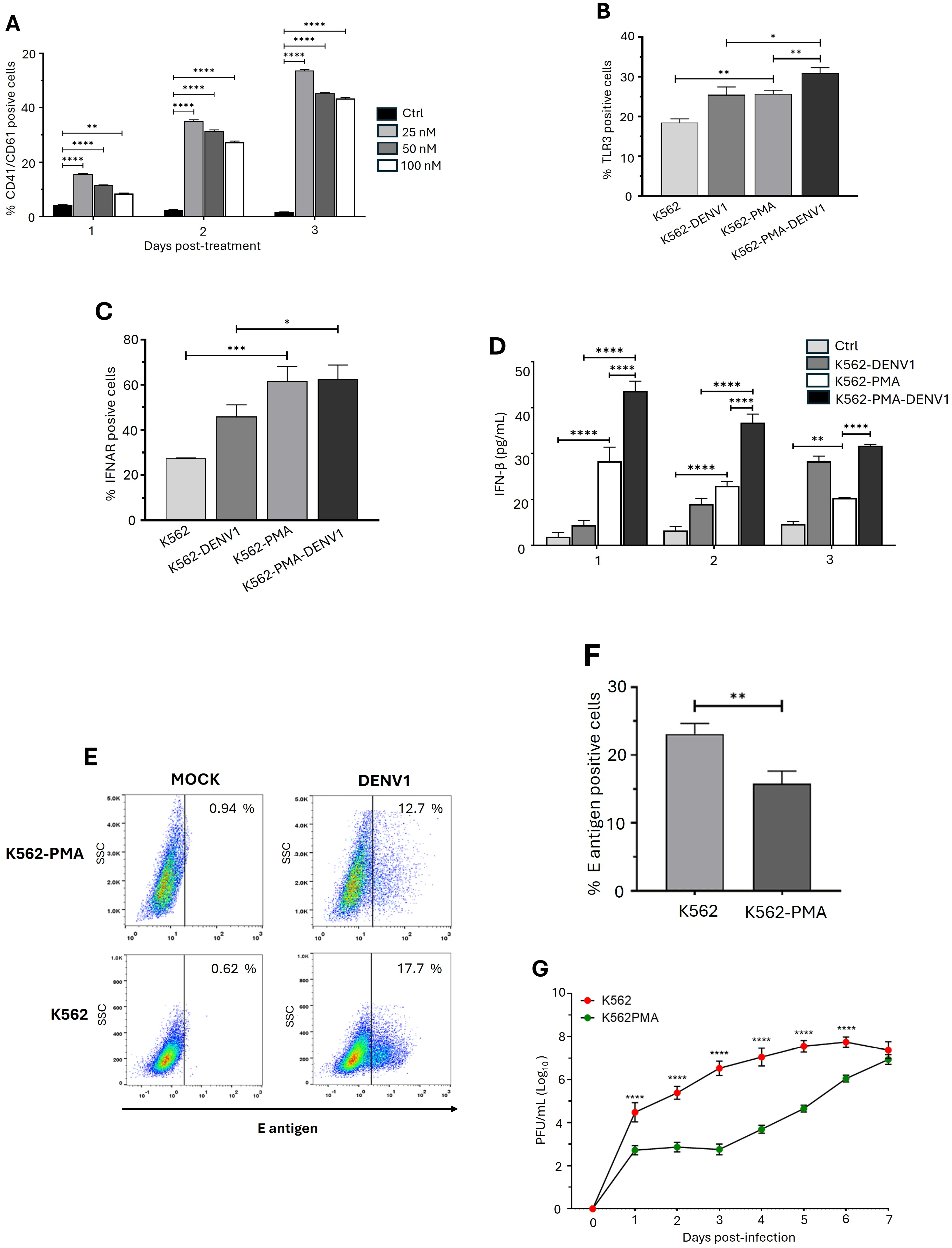
2.1. The Erythroid-Megakaryocytic Precursor Is More Susceptible to DENV1 Infection than Megakaryoblasts
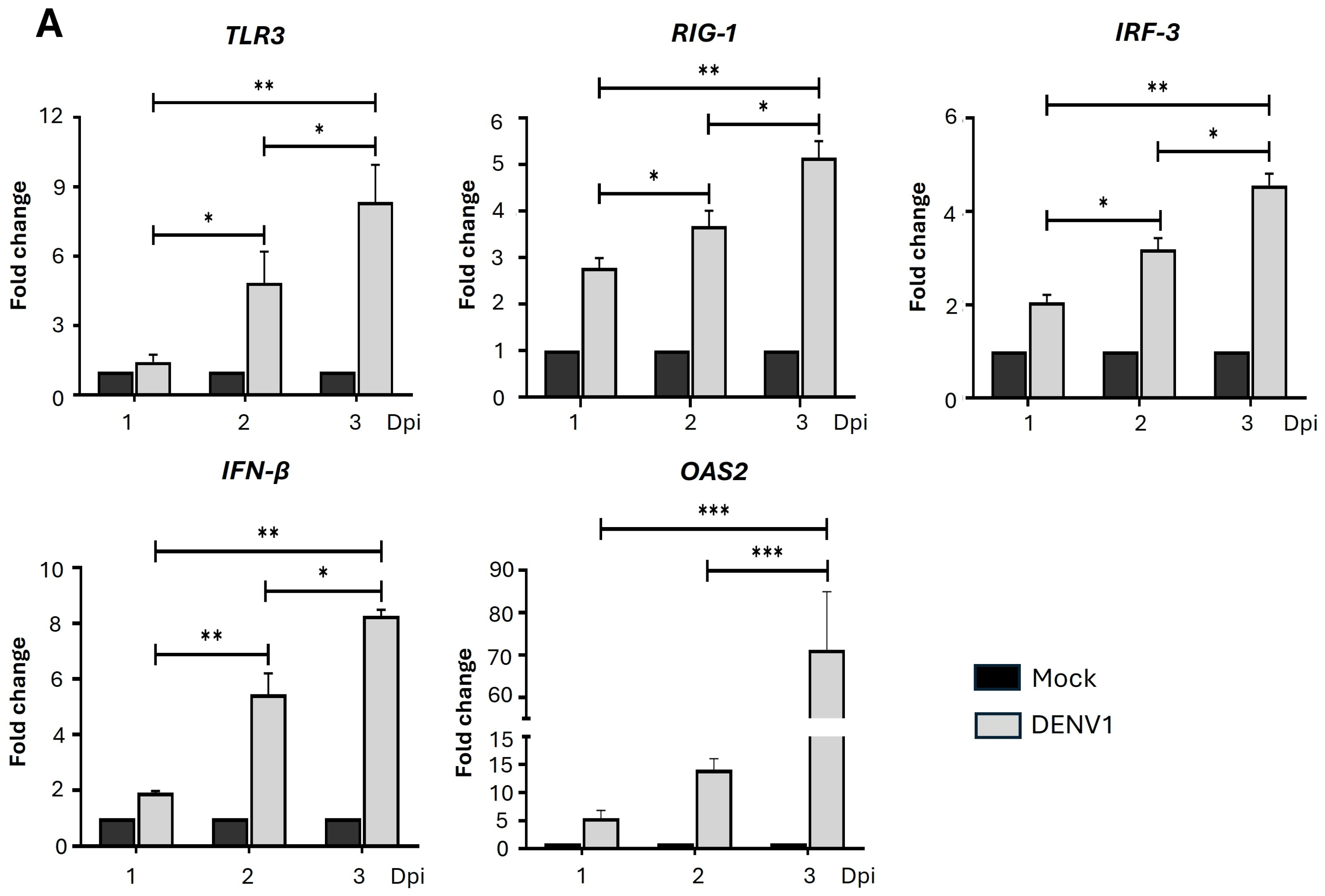
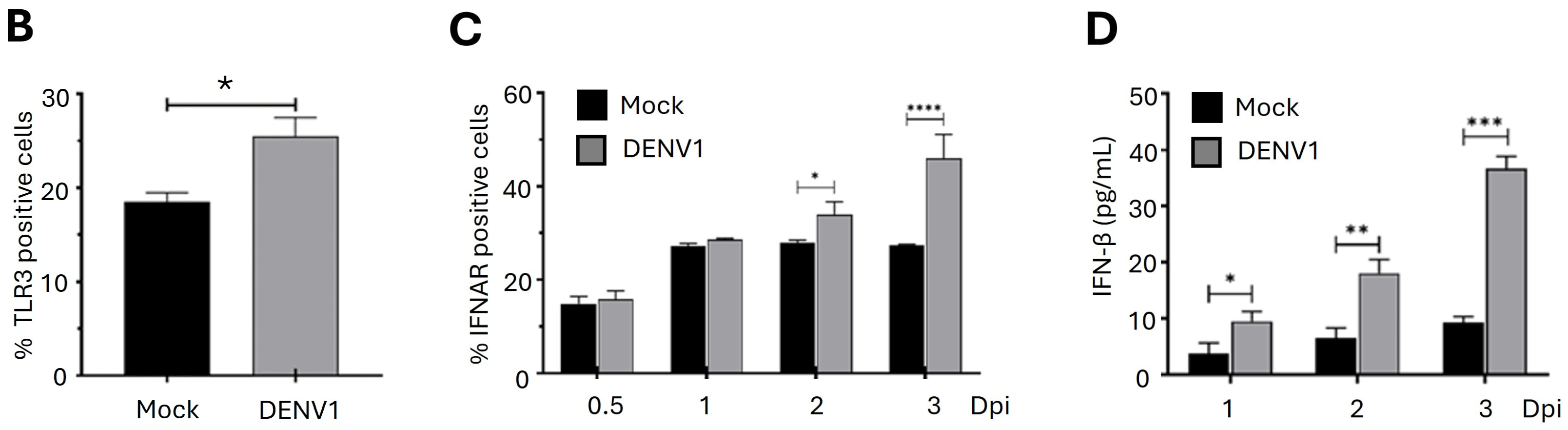
2.2. DENV Progressively Induces the Expression of Genes in the IFN-I Pathway in Erythroid-Megakaryocytic Progenitor Cells
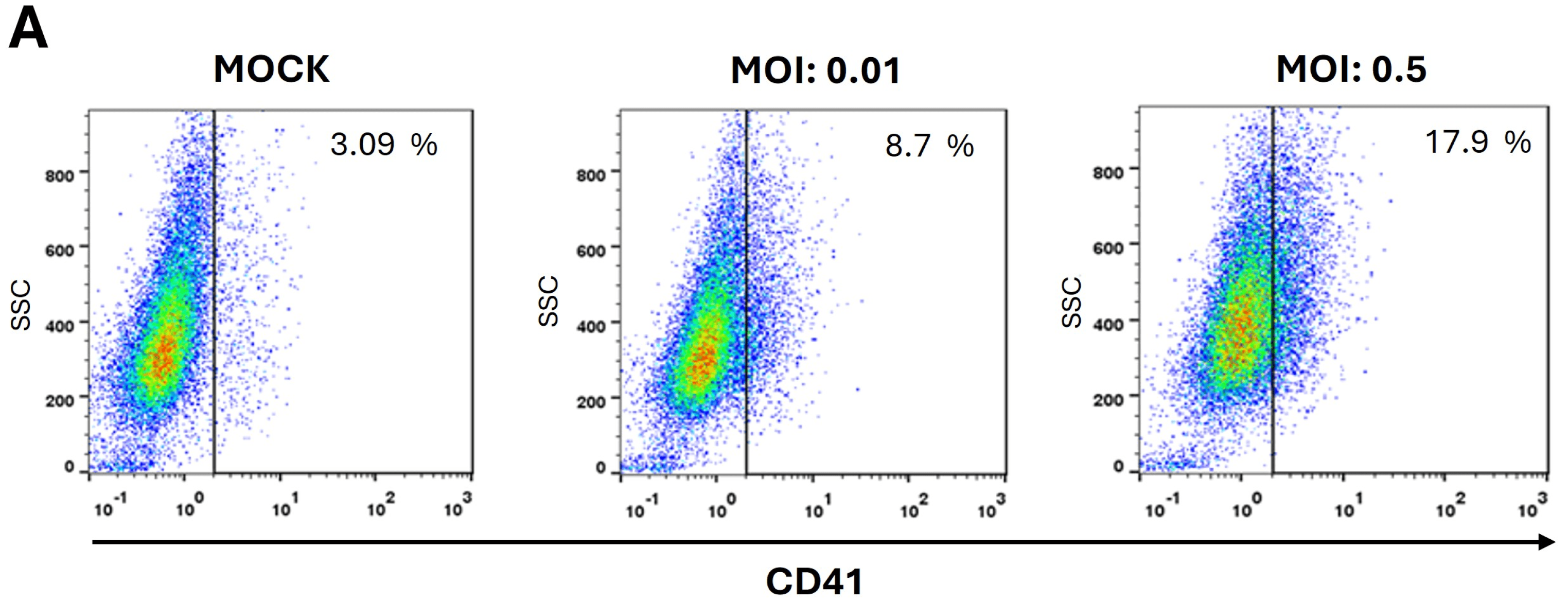
2.3. DENV Promotes the Differentiation of Erythroid-Megakaryocytic Progenitors
2.4. Differentiation of Erythroid-Megakaryocytic Progenitor Cells Regulates the Antiviral Response to DENV
2.5. Differentiation of K562 Cells Increases the Expression of IRF3 and OAS2
2.6. Exogenous IFN-β Enhances the Antiviral Response of Erythroid-Megakaryocytic Progenitors Against DENV in the Early Days of Infection
3. Discussion
4. Materials and Methods
4.1. Cultivation of K562 Cells and Differentiation with PMA
4.2. Virus
4.3. Viral Infection and Supernatant Collection
4.4. Flow Cytometry Analysis
4.5. Detection of E Antigen, IRF3 and OAS2 by Immunofluorescence
4.6. Gene Expression Análisis
4.7. Quantification of IFN-β
4.8. Pretreatment with Recombinant IFN-β
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Postler, T.S.; Beer, M.; Blitvich, B.J.; Bukh, J.; de Lamballerie, X.; Drexler, J.F.; Imrie, A.; Kapoor, A.; Karganova, G.G.; Lemey, P.; et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol. 2023, 168, 224. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef]
- Yang, X.; Quam, M.B.M.; Zhang, T.; Sang, S. Global burden for dengue and the evolving pattern in the past 30 years. J. Travel Med. 2021, 28, taab146. [Google Scholar] [CrossRef]
- Nakase, T.; Giovanetti, M.; Obolski, U.; Lourenço, J. Population at risk of dengue virus transmission has increased due to coupled climate factors and population growth. Commun. Earth Environ. 2024, 5, 475. [Google Scholar] [CrossRef]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Available online: https://www.who.int/publications/i/item/9789241547871 (accessed on 22 July 2025).
- Basu, A.; Jain, P.; Gangodkar, S.V.; Shetty, S.; Ghosh, K. Dengue 2 virus inhibits in vitro megakaryocytic colony formation and induces apoptosis in thrombopoietin-inducible megakaryocytic differentiation from cord blood CD34+ cells. FEMS Immunol. Med. Microbiol. 2008, 53, 46–51. [Google Scholar] [CrossRef]
- Hottz, E.D.; Oliveira, M.F.; Nunes, P.C.G.; Nogueira, R.M.R.; Valls-de-Souza, R.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, P.T.; Bozza, F.A. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC-SIGN and caspases. J. Thromb. Haemost. 2013, 11, 951–962. [Google Scholar] [CrossRef]
- Nelson, E.R.; Bierman, H.R. Dengue Fever: A Thrombocytopenic Disease? JAMA 1964, 190, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Tripathi, A.; Duggal, S.; Banerjee, A.; Vrati, S. Dengue virus infection impedes megakaryopoiesis in MEG-01 cells where the virus envelope protein interacts with the transcription factor TAL-1. Sci. Rep. 2020, 10, 19772. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B.; Noisakran, S.; Onlamoon, N.; Hsiao, H.-M.; Roback, J.; Villinger, F.; Ansari, A.A.; Perng, G.C. Multiploid CD61+ cells are the pre-dominant cell lineage infected during acute dengue virus infection in bone marrow. PLoS ONE 2012, 7, e52902. [Google Scholar] [CrossRef]
- Hsu, A.Y.; Ho, T.-Z.; Lai, M.-L.; Tan, S.S.; Chen, T.-Y.; Lee, M.; Chien, Y.-W.; Chen, Y.-P.; Perng, G.C. Identification and characterization of permissive cells to dengue virus infection in human hematopoietic stem and progenitor cells. Transfusion 2019, 59, 2938–2951. [Google Scholar] [CrossRef]
- Nakao, S.; Lai, C.-J.; Young, N.S. Dengue virus, a flavivirus, propagates in human bone marrow progenitors and hematopoietic cell lines. Blood 1989, 74, 1235–1240. [Google Scholar] [CrossRef]
- Tsai, J.-J.; Liu, L.-T.; Chang, K.; Wang, S.-H.; Hsiao, H.-M.; Clark, K.B.; Perng, G.C. The importance of hematopoietic progenitor cells in dengue. Ther. Adv. Hematol. 2012, 3, 59–71. [Google Scholar] [CrossRef]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Clinton, J.L.S.; Rico-Hesse, R. Dengue viruses infect human megakaryocytes, with probable clinical consequences. PLoS Neglected Trop. Dis. 2019, 13, e0007837. [Google Scholar] [CrossRef]
- Geddis, A.E. Megakaryopoiesis. Semin. Hematol. 2010, 47, 212–219. [Google Scholar] [CrossRef]
- Kurane, I.; Kontny, U.; Janus, J.; Ennis, F.A. Dengue-2 virus infection of human mononuclear cell lines and establishment of persistent infections. Arch. Virol. 1990, 110, 91–101. [Google Scholar] [CrossRef]
- Diamond, M.S.; Edgil, D.; Roberts, T.G.; Lu, B.; Harris, E. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 2000, 74, 7814–7823. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B.; Hsiao, H.-M.; Bassit, L.; Crowe, J.E., Jr.; Schinazi, R.F.; Perng, G.C.; Villinger, F. Characterization of dengue virus 2 growth in megakaryocyte–erythrocyte progenitor cells. Virology 2016, 493, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Rawat, Y.; Sood, V.; Periwal, N.; Rathore, D.K.; Kumar, S.; Kumar, N.; Bhattacharyya, S. Replication of Dengue Virus in K562-Megakaryocytes Induces Suppression in the Accumulation of Reactive Oxygen Species. Front. Microbiol. 2022, 12, 784070. [Google Scholar] [CrossRef]
- Losada, P.X.; Bosch, I.; Frydman, G.H.; Gehrke, L.; Narváez, C.F. Dengue and Zika virus differential infection of human megakaryoblast MEG-01 reveals unique cellular markers. Virology 2022, 577, 16–23. [Google Scholar] [CrossRef]
- Arya, R.P.; Lahon, A.; Patel, A.K. Dengue virus induces interferon-β by activating RNA sensing pathways in megakaryocytes. Immunol. Lett. 2021, 236, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yue, Y.; Li, D.; Zhao, Y.; Qiu, L.; Chen, J.; Pan, Y.; Xi, J.; Wang, X.; Sun, Q.; et al. Antibody-dependent enhancement of dengue virus infection inhibits RLR-mediated Type-I IFN-independent signalling through upregulation of cellular autophagy. Sci. Rep. 2016, 6, 22303. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, L.P.; Etulain, J.; Rivadeneyra, L.; Lapponi, M.J.; Centurión, M.; Cheng, K.; Yin, H.; Schattner, M. Expression and functionality of Toll-like receptor 3 in the megakaryocytic lineage. J. Thromb. Haemost. 2015, 13, 839–850. [Google Scholar] [CrossRef]
- Negrotto, S.; De Giusti, C.J.; Lapponi, M.J.; Etulain, J.; Rivadeneyra, L.; Pozner, R.G.; Gomez, R.M.; Schattner, M. Expression and functionality of type I interferon receptor in the megakaryocytic lineage. J. Thromb. Haemost. 2011, 9, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Schwertz, H.; Hottz, E.D.; Rowley, J.W.; Manne, B.K.; Washington, A.V.; Hunter-Mellado, R.; Tolley, N.D.; Christensen, M.; Eustes, A.S.; et al. Human megakaryocytes possess intrinsic antiviral immunity through regulated induction of IFITM3. Blood 2019, 133, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Rommel, M.G.E.; Walz, L.; Fotopoulou, F.; Kohlscheen, S.; Schenk, F.; Miskey, C.; Botezatu, L.; Krebs, Y.; Voelker, I.M.; Wittwer, K.; et al. Influenza A virus infection instructs hematopoiesis to megakaryocyte-lineage output. Cell Rep. 2022, 41, 111447. [Google Scholar] [CrossRef]
- Honda, K.; Taniguchi, T. IRFs: Master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef]
- Kristiansen, H.; Gad, H.H.; Eskildsen-Larsen, S.; Despres, P.; Hartmann, R. The oligoadenylate synthetase family: An ancient protein family with multiple antiviral activities. J. Interferon Cytokine Res. 2011, 31, 41–47. [Google Scholar] [CrossRef]
- Loo, Y.-M.; Fornek, J.; Crochet, N.; Bajwa, G.; Perwitasari, O.; Martinez-Sobrido, L.; Akira, S.; Gill, M.A.; García-Sastre, A.; Katze, M.G.; et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 2008, 82, 335–345. [Google Scholar] [CrossRef]
- Nasirudeen, A.M.A.; Wong, H.H.; Thien, P.; Xu, S.; Lam, K.-P.; Liu, D.X. RIG-I, MDA5 and TLR3 synergistically play an important role in restriction of dengue virus infection. PLOS Neglected Trop. Dis. 2011, 5, e926. [Google Scholar] [CrossRef]
- Lion, E.; Anguille, S.; Berneman, Z.N.; Smits, E.L.J.M.; Van Tendeloo, V.F.I. Poly(I:C) enhances the susceptibility of leukemic cells to NK cell cytotoxicity and phagocytosis by DC. PLoS ONE 2011, 6, e20952. [Google Scholar] [CrossRef]
- Gao, D.; Ciancanelli, M.J.; Zhang, P.; Harschnitz, O.; Bondet, V.; Hasek, M.; Chen, J.; Mu, X.; Itan, Y.; Cobat, A.; et al. TLR3 controls constitutive IFN-β antiviral immunity in human fibroblasts and cortical neurons. J. Clin. Investig. 2021, 131, e134529. [Google Scholar] [CrossRef] [PubMed]
- Castillo Ramirez, J.A.; Urcuqui-Inchima, S. Dengue Virus Control of Type I IFN Responses: A History of Manipulation and Control. J Interferon Cytokine Res. 2015, 35, 421–430. [Google Scholar] [CrossRef]
- Dalrymple, N.A.; Cimica, V.; Mackow, E. Dengue Virus NS Proteins Inhibit RIG-I/MAVS Signaling by Blocking TBK1/IRF3 Phosphorylation: Dengue Virus Serotype 1 NS4A Is a Unique Interferon-Regulating Virulence Determinant. MBio 2015, 6, 1028–1128. [Google Scholar] [CrossRef]
- François-Newton, V.; Magno de Freitas, G.A.; Payelle-Brogard, B.; Monneron, D.; Pichard-Garcia, L.; Piehler, J.; Pellegrini, S.; Uzé, G. USP18-Based Negative Feedback Control Is Induced by Type I and Type III Interferons and Specifically Inactivates Interferon α Response. PLoS ONE 2011, 6, e22200. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Roberts, T.G.; Edgil, D.; Lu, B.; Ernst, J.; Harris, E. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 2000, 74, 4957–4966. [Google Scholar] [CrossRef] [PubMed][Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Hernández, D.S.; Sánchez-Peña, F.J.; Cruz-Cruz, M.; Guillén-Morales, D.d.J.; Castillo-Soriano, M.C.; Cruz-Altamirano, E.; Alpuche, J.; Bustos-Arriaga, J.; Romero-Tlalolini, M.d.L.Á.; Torres-Aguilar, H.; et al. Megakaryocytic Differentiation Regulates the Permissiveness and Antiviral Response of the Megakaryocytic Erythroid Progenitor to Dengue Virus. Int. J. Mol. Sci. 2025, 26, 11081. https://doi.org/10.3390/ijms262211081
Cruz-Hernández DS, Sánchez-Peña FJ, Cruz-Cruz M, Guillén-Morales DdJ, Castillo-Soriano MC, Cruz-Altamirano E, Alpuche J, Bustos-Arriaga J, Romero-Tlalolini MdLÁ, Torres-Aguilar H, et al. Megakaryocytic Differentiation Regulates the Permissiveness and Antiviral Response of the Megakaryocytic Erythroid Progenitor to Dengue Virus. International Journal of Molecular Sciences. 2025; 26(22):11081. https://doi.org/10.3390/ijms262211081
Chicago/Turabian StyleCruz-Hernández, Diego Sait, Francisco Javier Sánchez-Peña, Marymar Cruz-Cruz, Darío de Jesús Guillén-Morales, Martha Cristina Castillo-Soriano, Elizabeth Cruz-Altamirano, Juan Alpuche, José Bustos-Arriaga, María de Los Ángeles Romero-Tlalolini, Honorio Torres-Aguilar, and et al. 2025. "Megakaryocytic Differentiation Regulates the Permissiveness and Antiviral Response of the Megakaryocytic Erythroid Progenitor to Dengue Virus" International Journal of Molecular Sciences 26, no. 22: 11081. https://doi.org/10.3390/ijms262211081
APA StyleCruz-Hernández, D. S., Sánchez-Peña, F. J., Cruz-Cruz, M., Guillén-Morales, D. d. J., Castillo-Soriano, M. C., Cruz-Altamirano, E., Alpuche, J., Bustos-Arriaga, J., Romero-Tlalolini, M. d. L. Á., Torres-Aguilar, H., Rodríguez-Alba, J. C., & Aguilar-Ruíz, S. R. (2025). Megakaryocytic Differentiation Regulates the Permissiveness and Antiviral Response of the Megakaryocytic Erythroid Progenitor to Dengue Virus. International Journal of Molecular Sciences, 26(22), 11081. https://doi.org/10.3390/ijms262211081








