Features and In Vitro Assessment of Antiviral Activity of Organic Coatings Doped with Silver-Based Compounds Against Human Coronavirus
Abstract
1. Introduction
2. Results
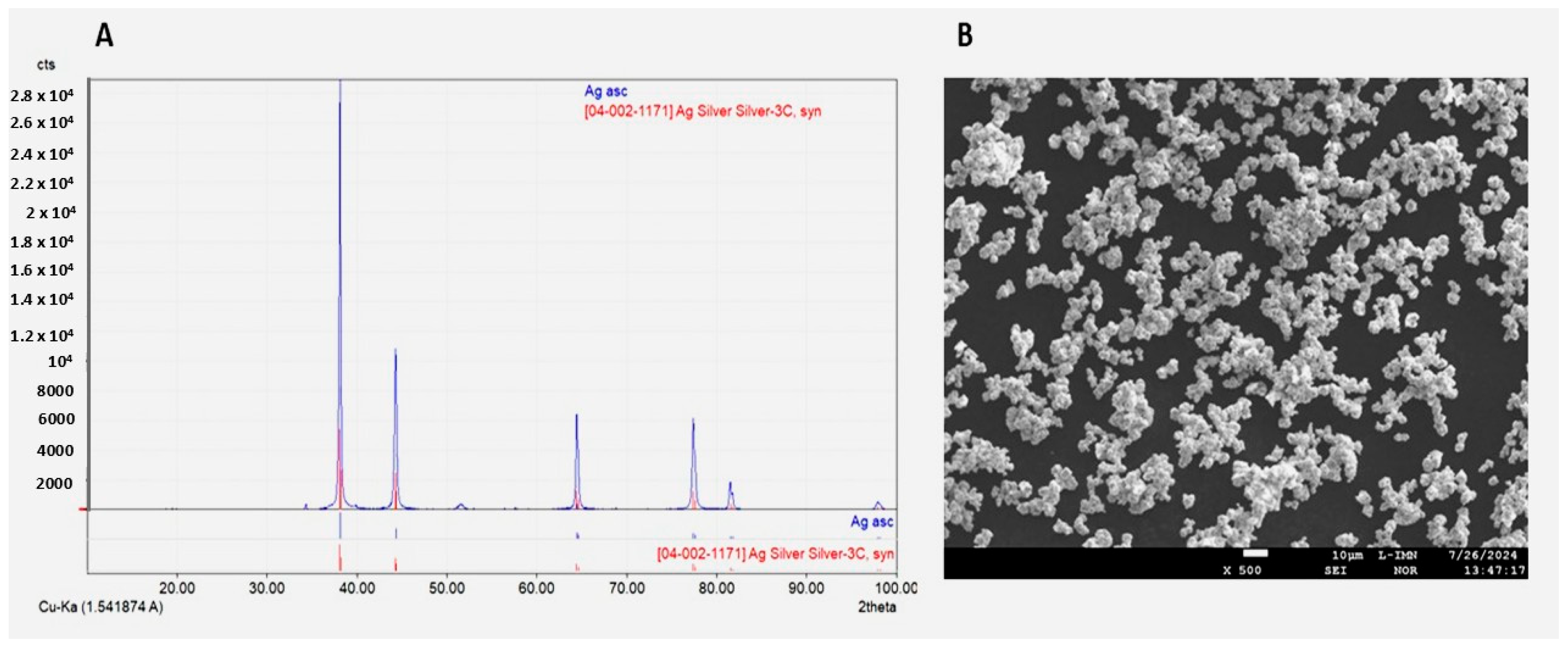
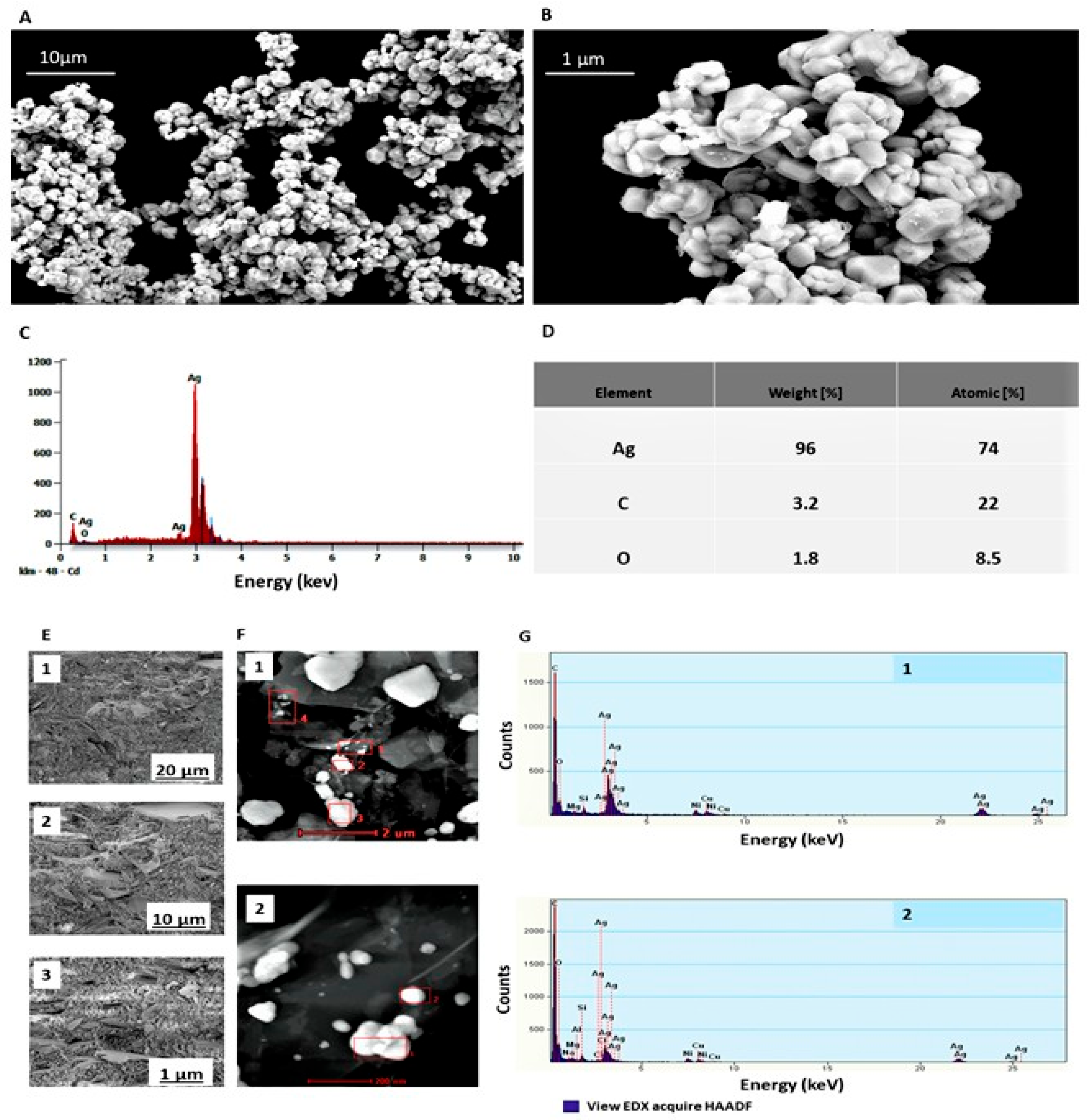
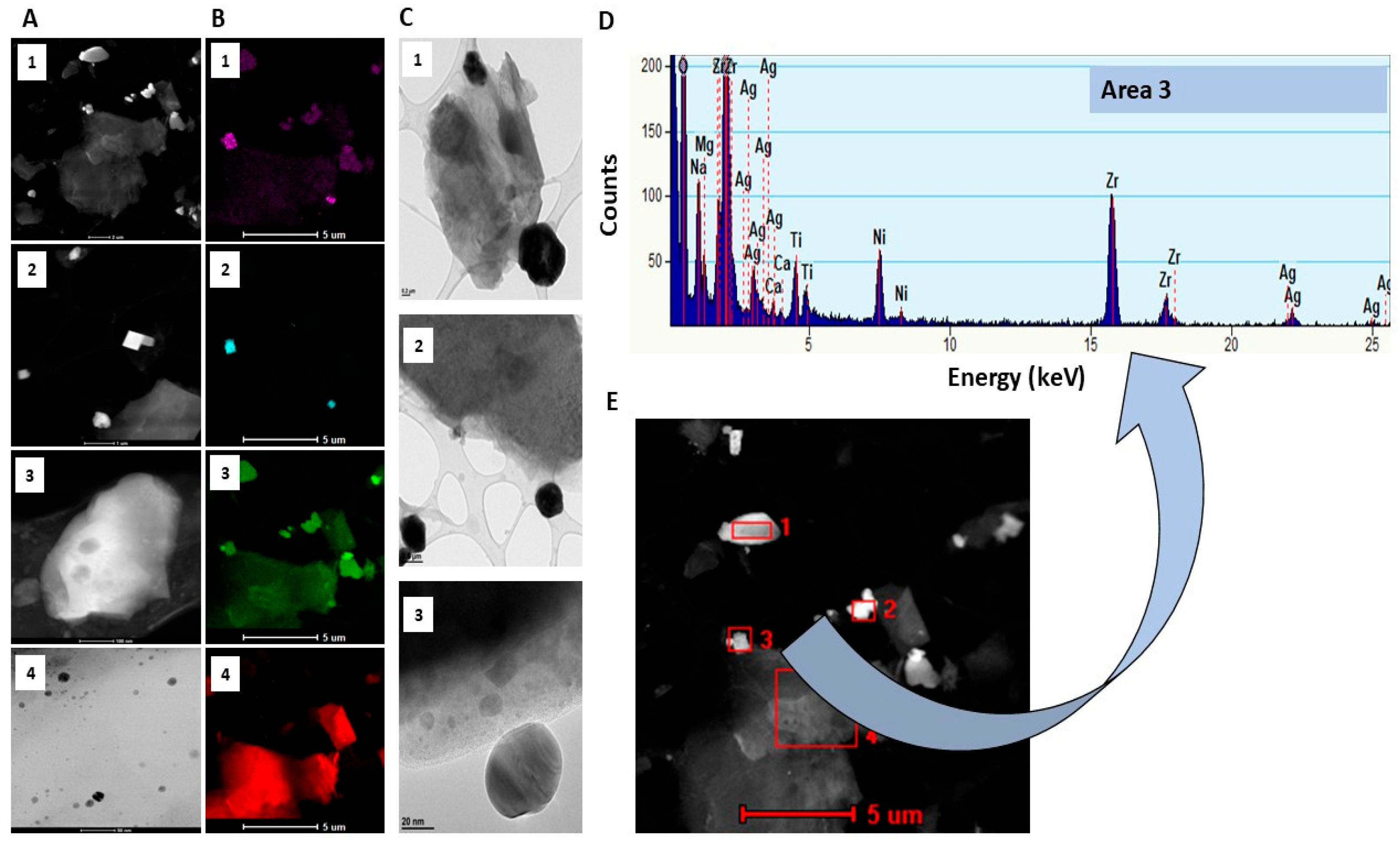
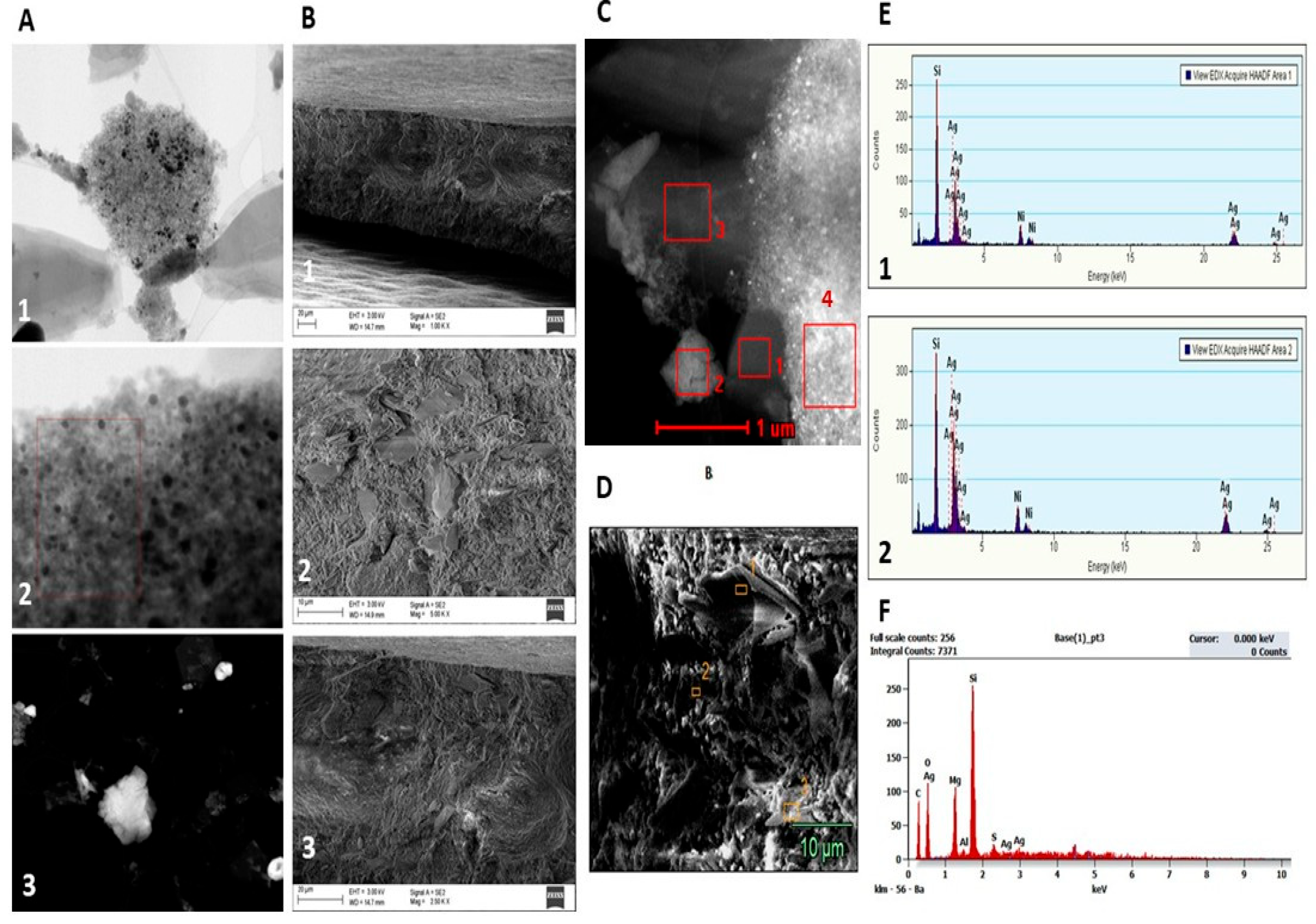
2.1. Characteristics of Bioactive Substances and Coatings
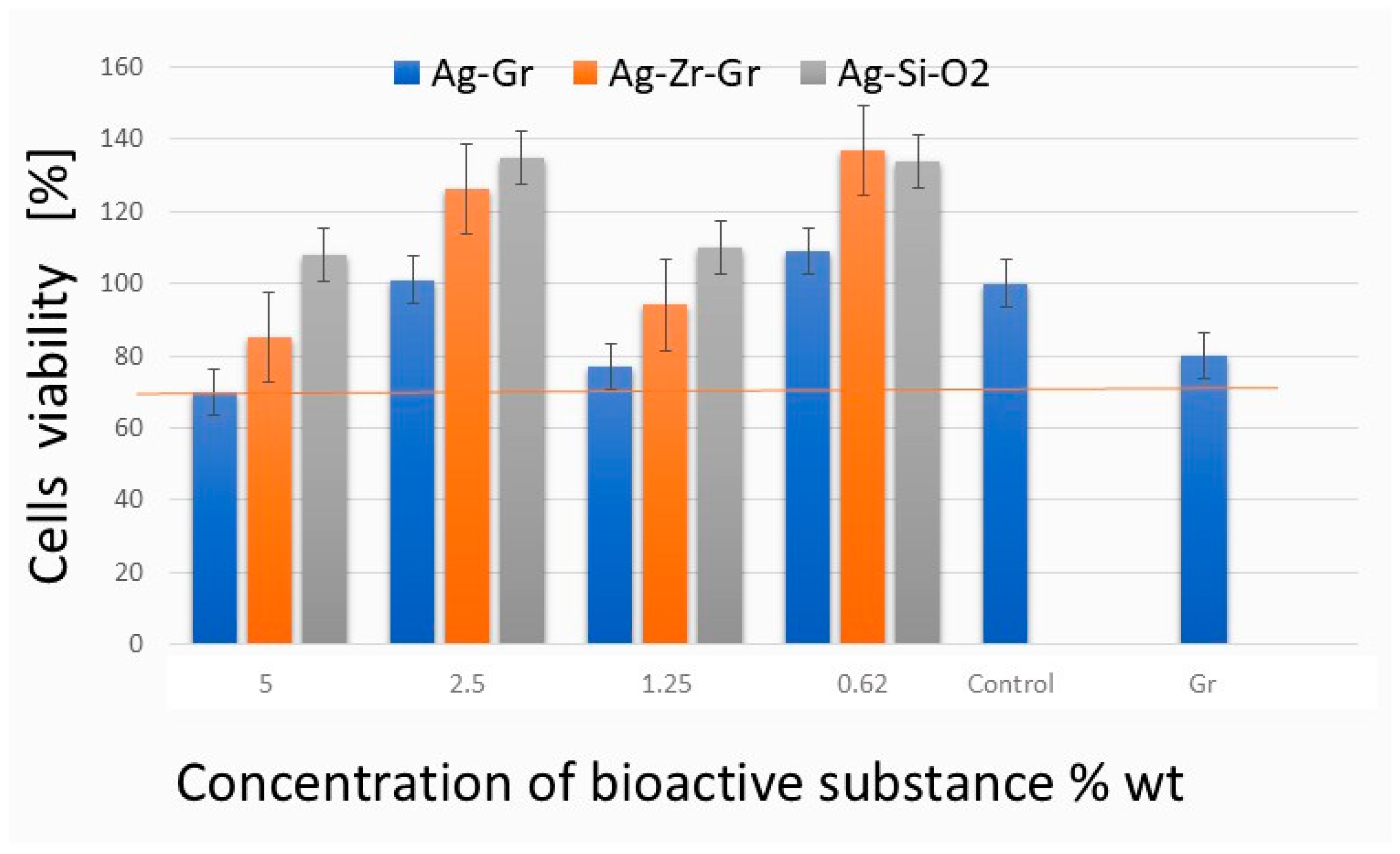
2.2. Cytotoxicity
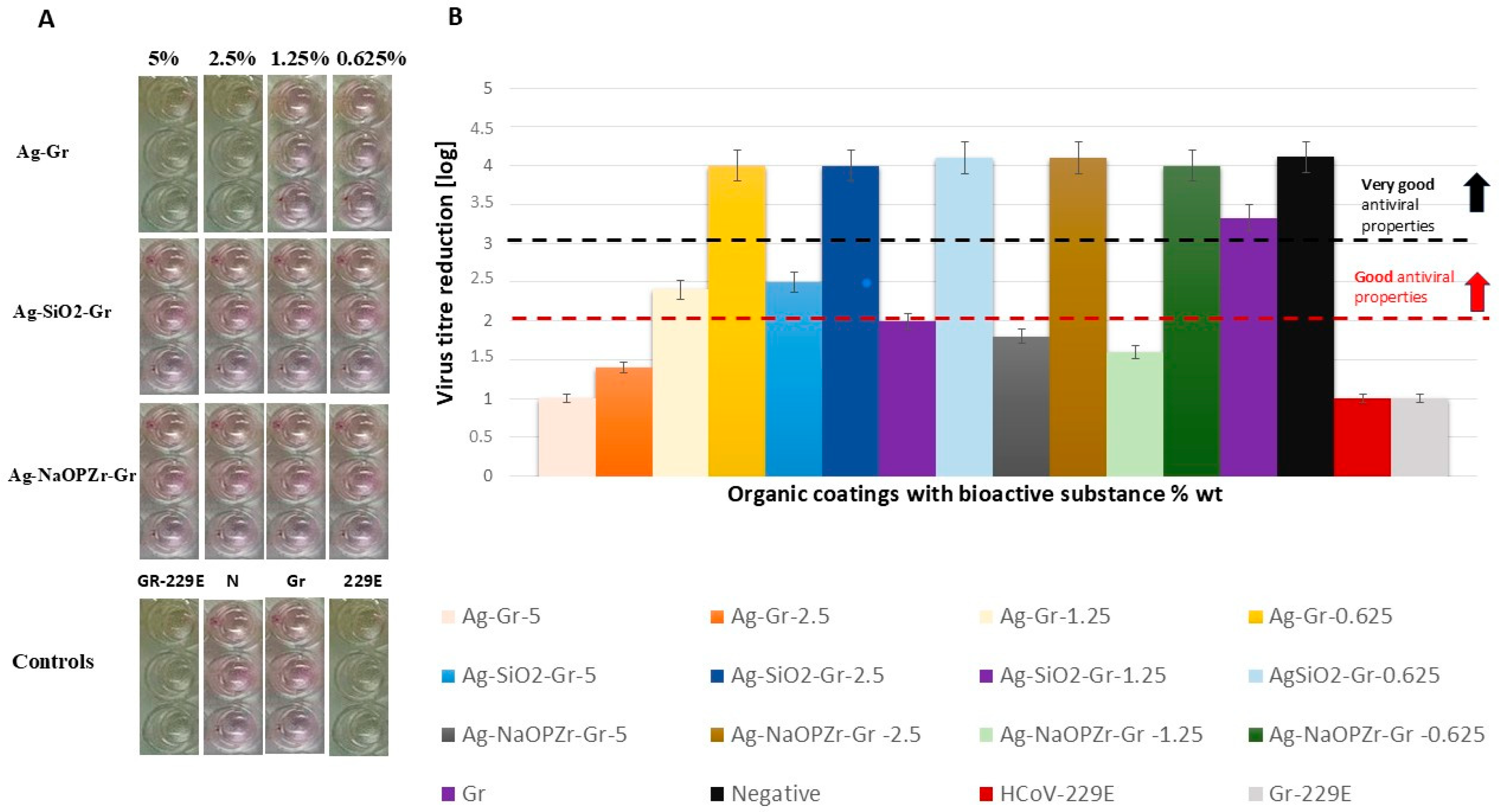
2.3. Antiviral Activity Assessment of Coating Paints
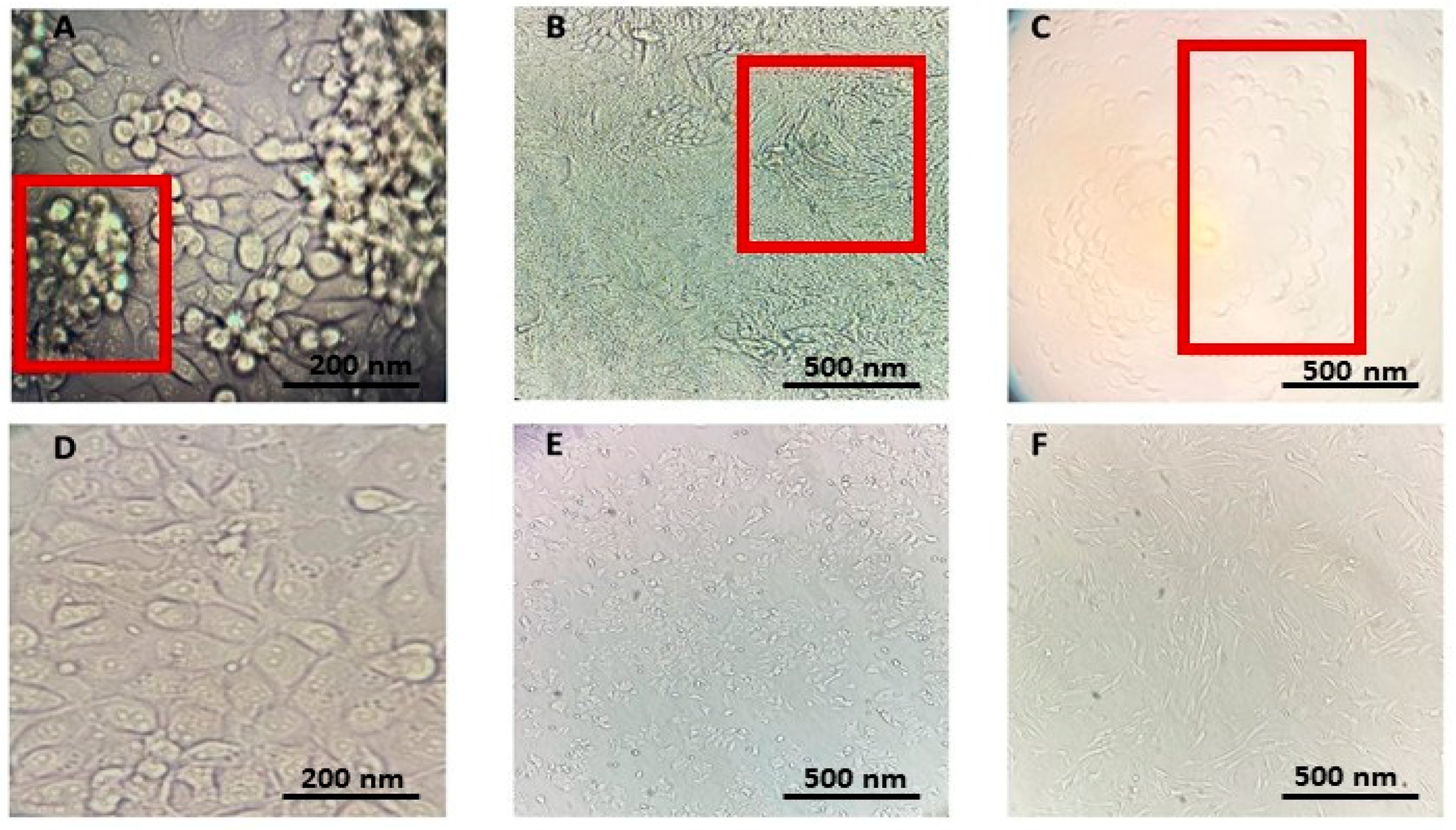
2.3.1. Inverted Fluorescence Microscopic Assessment of Cytopathic Effect Caused by HCoVs
2.3.2. Evaluation of CPE Formation and NRU-CPE Assay
3. Discussion
4. Materials and Methods
4.1. Bioactive Substances
4.2. Coating Paint Preparation and Characterisation
4.2.1. Preparation
4.2.2. Physical and Optical Properties
4.2.3. Characterisation of Synthesised Ag Powder and Gr Organic Coatings Using S/TEM, SEM and EDX
4.2.4. X-Ray Diffraction (XRD)
4.2.5. Electron Probe Microanalysis (EPMA)
4.3. Cell Lines
4.4. Reference HCoV Strains and Replication on Cell Lines
4.5. Testing the Surface Antiviral Activity of Organic Coatings
4.6. Cytotoxicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woo, P.C.Y.; de Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; van der Hoek, L.; Wong, A.C.P.; Yeh, S.-H. ICTV Virus Taxonomy Profile: Coronaviridae 2023. J. Gen. Virol. 2023, 104, 001843. [Google Scholar] [CrossRef]
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1. Ref. Modul. Life Sci. 2020, 2, 428–440. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Cases|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?m49=001&n=o (accessed on 30 October 2025).
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The Evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Young, M.; Crook, H.; Scott, J.; Edison, P. Covid-19: Virology, Variants, and Vaccines. BMJ Med. 2022, 1, e000040. [Google Scholar] [CrossRef]
- Baranowska-Korczyc, A.; Kowalczyk, D.; Chodkowski, M.; Krzyżowska, M.; Cieślak, M. AgNWs as an Antiviral Agent against Human Coronavirus Infection. Mater. Today Commun. 2025, 48, 113589. [Google Scholar] [CrossRef]
- Nejman, A.; Tkacz-Szczęsna, B.; Chodkowski, M.; Krzyżowska, M.; Cieślak, M. Antiviral and Antibacterial Cotton Woven Fabric Functionalized with CuNPs/ZnONPs- Silane Sols. Int. J. Biol. Macromol. 2025, 310, 143386. [Google Scholar] [CrossRef]
- Asmat-Campos, D.; Rojas-Jaimes, J.; Montes, G.; Nazario-Naveda, R.; Delfín-Narciso, D.; Juárez-Cortijo, L.; Bayona, D.E.; Diringer, B.; Pereira, R.; Menezes, D.M. Biogenic Production of Silver, Zinc Oxide, and Cuprous Oxide Nanoparticles, and Their Impregnation into Textiles with Antiviral Activity against SARS-CoV-2. Sci. Rep. 2023, 13, 9772. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.J.; Gramcianinov, G.B.; Jorge, P.Z.; Malaquias, V.B.; Mori, A.A.; Hirata, M.H.; Lopes, S.A.M.; Bueno, L.A.; Champeau, M.; Carastan, D.J. PVC Containing Silver Nanoparticles with Antimicrobial Properties Effective against SARS-CoV-2. Front. Chem. 2023, 11, 1083399. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent Antiviral Effect of Silver Nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Seyfi, M.; Letafati, A.; Edalat, F.; Malekshahi, S.S.; Pirbonyeh, N.; Moattari, A. Antiviral Activity of Silver Nanoparticles against H1N1 Influenza Virus. BMC Res. Notes 2025, 18, 75. [Google Scholar] [CrossRef]
- Corrêa, J.M.; Mori, M.; Sanches, H.L.; da Cruz, A.D.; Poiate, E.; Poiate, I.A.V.P. Silver Nanoparticles in Dental Biomaterials. Int. J. Biomater. 2015, 2015, 485275. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Reyes, H.; Moreno, S.; Plascencia-López, I.; Alvarado-Vera, M.; Patrón-Romero, L.; Borrego, B.; Reyes-Escamilla, A.; Valencia-Manzo, D.; Brun, A.; Pestryakov, A.; et al. Evaluation of Silver Nanoparticles for the Prevention of SARS-CoV-2 Infection in Health Workers: In Vitro and in Vivo. PLoS ONE 2021, 16, e0256401. [Google Scholar] [CrossRef]
- Kumar, A.; Nath, K.; Parekh, Y.; Ghalib Enayathullah, M.; Bokara, K.K.; Sinhamahapatra, A. Antimicrobial Silver Nanoparticle-Photodeposited Fabrics for SARS-CoV-2 Destruction. Colloid Interface Sci. Commun. 2021, 45, 100542. [Google Scholar] [CrossRef]
- Daoudi, H.; Bouafia, A.; Laouini, S.E.; Meneceur, S.; Fellah, M.; Iqbal, A.; El-Hiti, G.A.; Selmi, B. In Vitro and in Silico Study of Biosynthesized Silver Nanoparticles Using Nigella Sativa Extract against SARS-CoV-2 and Candida Albicans. J. Mol. Liq. 2024, 405, 125059. [Google Scholar] [CrossRef]
- Zatla, I.; Boublenza, L. Battling COVID-19 Leveraging Nanobiotechnology: Gold and Silver Nanoparticle–B-Escin Conjugates as SARS-CoV-2 Inhibitors. Open Life Sci. 2025, 20, 20221047. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2021, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Mosidze, E.; Franci, G.; Dell’Annunziata, F.; Capuano, N.; Colella, M.; Salzano, F.; Galdiero, M.; Bakuridze, A.; Folliero, V. Silver Nanoparticle-Mediated Antiviral Efficacy against Enveloped Viruses: A Comprehensive Review. Glob. Chall. 2025, 9, 2400380. [Google Scholar] [CrossRef]
- He, Q.; Lu, J.; Liu, N.; Lu, W.; Li, Y.; Shang, C.; Li, X.; Hu, L.; Jiang, G. Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomaterials 2022, 12, 990. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.A.; Abdelaziz, A.A.; Abo-Kamar, A.M.; Al-Madboly, L.A.; El-Maradny, Y.A.; El-Fakharany, E.M. Multifunctional Efficacy of the Fabricated Chitosan-Coated Silver Nanoparticles as an Antiviral Agent against SARS-CoV-2: Potent and Safe Mechanistic Insights. Int. J. Biol. Macromol. 2025, 320, 145623. [Google Scholar] [CrossRef]
- Assis, M.; Simoes, L.G.P.; Tremiliosi, G.C.; Coelho, D.; Minozzi, D.T.; Santos, R.I.; Vilela, D.C.B.; Santos, J.R.d.; Ribeiro, L.K.; Rosa, I.L.V.; et al. SiO2-Ag Composite as a Highly Virucidal Material: A Roadmap That Rapidly Eliminates SARS-CoV-2. Nanomaterials 2021, 11, 638. [Google Scholar] [CrossRef]
- Manna, S.; Naskar, M.K.; Saha, D.; Pal, P.; Medda, S.K. Long-Term Stability of Ag NPs in a Room Temperature Cured SiO2–ZrO2 Crosslinking Hybrid Robust Coating towards Enhanced Weather Resistance Properties and Its Antibacterial Applications. New J. Chem. 2025, 49, 6914–6929. [Google Scholar] [CrossRef]
- Ferrari, I.V.; Giuntoli, G.; Pisani, A.; Cavallo, A.; Mazzetti, P.; Fonnesu, R.; Rosellini, A.; Pistello, M.; Al Kayal, T.; Cataldo, A.; et al. One-Step Silver Coating of Polypropylene Surgical Mask with Antibacterial and Antiviral Properties. Heliyon 2024, 10, e23196. [Google Scholar] [CrossRef]
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal Effect against Coronavirus SARS-CoV-2 of a Silver Nanocluster/Silica Composite Sputtered Coating. Open Ceram. 2020, 1, 100006. [Google Scholar] [CrossRef]
- He, J.; Ma, Y.; Niu, X.; Pei, J.; Yan, R.; Xu, F.; Ma, J.; Ma, X.; Jia, S.; Ma, W. Silver Nanoparticles Induce Endothelial Cytotoxicity through ROS-Mediated Mitochondria-Lysosome Damage and Autophagy Perturbation: The Protective Role of N-Acetylcysteine. Toxicology 2024, 502, 153734. [Google Scholar] [CrossRef]
- Rohde, M.M.; Snyder, C.M.; Sloop, J.; Solst, S.R.; Donati, G.L.; Spitz, D.R.; Furdui, C.M.; Singh, R. The Mechanism of Cell Death Induced by Silver Nanoparticles Is Distinct from Silver Cations. Part. Fibre Toxicol. 2021, 18, 37. [Google Scholar] [CrossRef]
- Byrd, G.; Goldstein-Plesser, A.; Nyffeler, J.; Willis, C.M.; Fisher, A.; Boyes, W.K.; Harrill, J.A. Assessing the Effects of Silver Nanoparticles on ARPE-19 Cells via High-Throughput Phenotypic Profiling with the Cell Painting Assay. Toxicol. Appl. Pharmacol. 2025, 502, 117444. [Google Scholar] [CrossRef]
- Perde-Schrepler, M.; Florea, A.; Brie, I.; Virag, P.; Fischer-Fodor, E.; Vâlcan, A.; Gurzău, E.; Lisencu, C.; Maniu, A. Size-Dependent Cytotoxicity and Genotoxicity of Silver Nanoparticles in Cochlear Cells in Vitro. J. Nanomater. 2019, 2019, e6090259. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 30 October 2025).
- ISO 21702:2019; Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/71365.html (accessed on 30 October 2025).
- Naumenko, K.; Zahorodnia, S.; Pop, C.V.; Rizun, N. Antiviral Activity of Silver Nanoparticles against the Influenza a Virus. J. Virus Erad. 2023, 9, 100330. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Das, S.; Aich, S.; Bhattacharyya, P.; Acharya, K. Antiviral potential of nanoparticles for the treatment of Coronavirus infections. J. Trace Elem. Med. Biol. 2022, 72, 126977, Erratum in J. Trace Elem. Med. Biol. 2024, 83, 127400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kokkarachedu, V.; Chandrasekaran, K.; Sisubalan, N.; Jayaramudu, T.; Vijayan, A.; Sadiku, R. SiO2-Based Nanomaterials as Antibacterial and Antiviral Agents: Potential Applications. In Nanoparticles in Modern Antimicrobial and Antiviral Applications; Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2024; pp. 65–95. [Google Scholar] [CrossRef]
- Marques, G.N.; Reis, R.Y.N.; Ribeiro, L.K.; Simões, L.G.P.; Minozzi, D.T.; Andrés, J.; Assis, M.; Mascaro, L.H.; Longo, E. Antiviral Leather: A Functional Coating Based on SiO2-AgNPs to Eliminate Pathogens. J. Environ. Chem. Eng. 2023, 11, 110919. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Perero, S.; Lembo, D.; Ferraris, M.; Balagna, C. Antibacterial and Antiviral Activities of Silver Nanocluster/Silica Composite Coatings Deposited onto Air Filters. ACS Appl. Mater. Interfaces 2024, 16, 3955–3965. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Sefrji, F.O.; Obaid, R.; Alsharari, A.M.; Mojally, M.; Alisaac, A.; Alsahag, M.; El-Metwaly, N.M. Rosmarinus Officinalis-Based Ag/SiO2 and CeO2-Ag/SiO2 Core-Shell Nanocomposites: A Green Approach to Phytochemical Analyses, Molecular Docking, Antioxidant and Antimicrobial Applications with Enhanced Biocompatibility. Results Eng. 2024, 24, 103478. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic Potential of Silver Nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Sharma, A.K.; Loeschner, K.; Jacobsen, N.R. Pulmonary Toxicity of Silver Vapours, Nanoparticles and Fine Dusts: A Review. Regul. Toxicol. Pharmacol. 2020, 115, 104690. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical Aspects of Silver Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017, 46, 115–126. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, B.; Ryu, H.; Sung, J.; Chung, K.; Yu, I. Effects of Repeated Silver Nanoparticles Exposure on the Histological Structure and Mucins of Nasal Respiratory Mucosa in Rats. Toxicol. Lett. 2008, 182, 24–28. [Google Scholar] [CrossRef]
- Rahman, M.F.; Wang, J.; Patterson, T.A.; Saini, U.T.; Robinson, B.L.; Newport, G.D.; Murdock, R.C.; Schlager, J.J.; Hussain, S.M.; Ali, S.F. Expression of Genes Related to Oxidative Stress in the Mouse Brain after Exposure to Silver-25 Nanoparticles. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of Silver Nanoparticles on Human Cells: Effect of Particle Size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef]
- Stoehr, L.C.; Gonzalez, E.; Stampfl, A.; Casals, E.; Duschl, A.; Puntes, V.; Oostingh, G.J. Shape Matters: Effects of Silver Nanospheres and Wires on Human Alveolar Epithelial Cells. Part. Fibre Toxicol. 2011, 8, 36. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Roto, R.; Rasydta, H.P.; Suratman, A.; Aprilita, N.H. Effect of Reducing Agents on Physical and Chemical Properties of Silver Nanoparticles. Indones. J. Chem. 2018, 18, 614. [Google Scholar] [CrossRef]
- Kopyciński, B.; Langer, E.; Kowalczyk, D.; Baranowska-Korczyc, A.; Bodzek-Kochel, M.; Cieślak, M. Nonwoven Fabrics with Camouflage and Antibacterial Properties. Appl. Surf. Sci. 2025, 709, 163856. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, C.; Wang, J. Cytopathic Effect Assay and Plaque Assay to Evaluate in Vitro Activity of Antiviral Compounds against Human Coronaviruses 229E, OC43, and NL63. Bio-Protocol 2022, 12, e4314. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Liu, Z.; Zhao, L.; Bao, M.; Mei, X.; Li, D. Promising Instrument-Free Detections of Various Analytes Using Smartphones with Spotxel® Reader. Anal. Sci. 2022, 39, 139–148. [Google Scholar] [CrossRef] [PubMed]
- 3T3 Neutral Red Uptake Cytotoxicity Assay|EURL ECVAM—TSAR. Available online: https://tsar.jrc.ec.europa.eu/test-method/tm2007-03 (accessed on 26 August 2025).
| Compound/Purity | Particle Size [nm] | Content of Ag [%] | Carrier/Concentration |
|---|---|---|---|
| Ag-NaOPZr/99% | up to 1300 | 10 | N/A |
| Ag-SiO2 | 5–15 | 5 | silicate/70% |
| Ag | up to 3000 | 96–99 | N/A |
| Name | ca. [%] 1 | CPE 9 | RF [log] |
|---|---|---|---|
| Ag-Gr 2 | 5.0 | (3) | 1.0 |
| 2.5 | (3) | 1.4 | |
| 1.3 | (0) | 2.4 | |
| 0.6 | (0) | 4.0 | |
| Ag-SiO2-Gr 3 | 5.0 | (0) | 2.5 |
| 2.5 | (0) | 4.0 | |
| 1.3 | (0) | 2.0 | |
| 0.6 | (0) | 4.1 | |
| Ag-NaOPZr-Gr 4 | 5.0 | (2) | 1.8 |
| 2.5 | (0) | 4.1 | |
| 1.3 | (2) | 1.6 | |
| 0.6 | (0) | 4.0 | |
| Control-Gr 5 | 0.0 | (0) | 3.3 |
| Control-Gr-229E 6 | 0.0 | (3) | 1.1 |
| Control-N 7 | 0.0 | (0) | 4.1 |
| Control-229E 8 | 0.0 | (3) | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaczek-Moczydłowska, M.A.; Kopyciński, B.; Hryniszyn, A.; Osadnik, M.; Czech, A.; Pęcak, K.; Markowska, A.; Ghavami, S.; Matus, K.; Langer, E.; et al. Features and In Vitro Assessment of Antiviral Activity of Organic Coatings Doped with Silver-Based Compounds Against Human Coronavirus. Int. J. Mol. Sci. 2025, 26, 11068. https://doi.org/10.3390/ijms262211068
Zaczek-Moczydłowska MA, Kopyciński B, Hryniszyn A, Osadnik M, Czech A, Pęcak K, Markowska A, Ghavami S, Matus K, Langer E, et al. Features and In Vitro Assessment of Antiviral Activity of Organic Coatings Doped with Silver-Based Compounds Against Human Coronavirus. International Journal of Molecular Sciences. 2025; 26(22):11068. https://doi.org/10.3390/ijms262211068
Chicago/Turabian StyleZaczek-Moczydłowska, Maja A., Bartosz Kopyciński, Alicja Hryniszyn, Małgorzata Osadnik, Anna Czech, Krzysztof Pęcak, Aleksandra Markowska, Saeid Ghavami, Krzysztof Matus, Ewa Langer, and et al. 2025. "Features and In Vitro Assessment of Antiviral Activity of Organic Coatings Doped with Silver-Based Compounds Against Human Coronavirus" International Journal of Molecular Sciences 26, no. 22: 11068. https://doi.org/10.3390/ijms262211068
APA StyleZaczek-Moczydłowska, M. A., Kopyciński, B., Hryniszyn, A., Osadnik, M., Czech, A., Pęcak, K., Markowska, A., Ghavami, S., Matus, K., Langer, E., & Łos, M. J. (2025). Features and In Vitro Assessment of Antiviral Activity of Organic Coatings Doped with Silver-Based Compounds Against Human Coronavirus. International Journal of Molecular Sciences, 26(22), 11068. https://doi.org/10.3390/ijms262211068








