Deubiquitinating Enzymes Ubiquitin-Specific Proteases 7 and 10 Regulate TAU Aggregation
Abstract
1. Introduction
2. Results
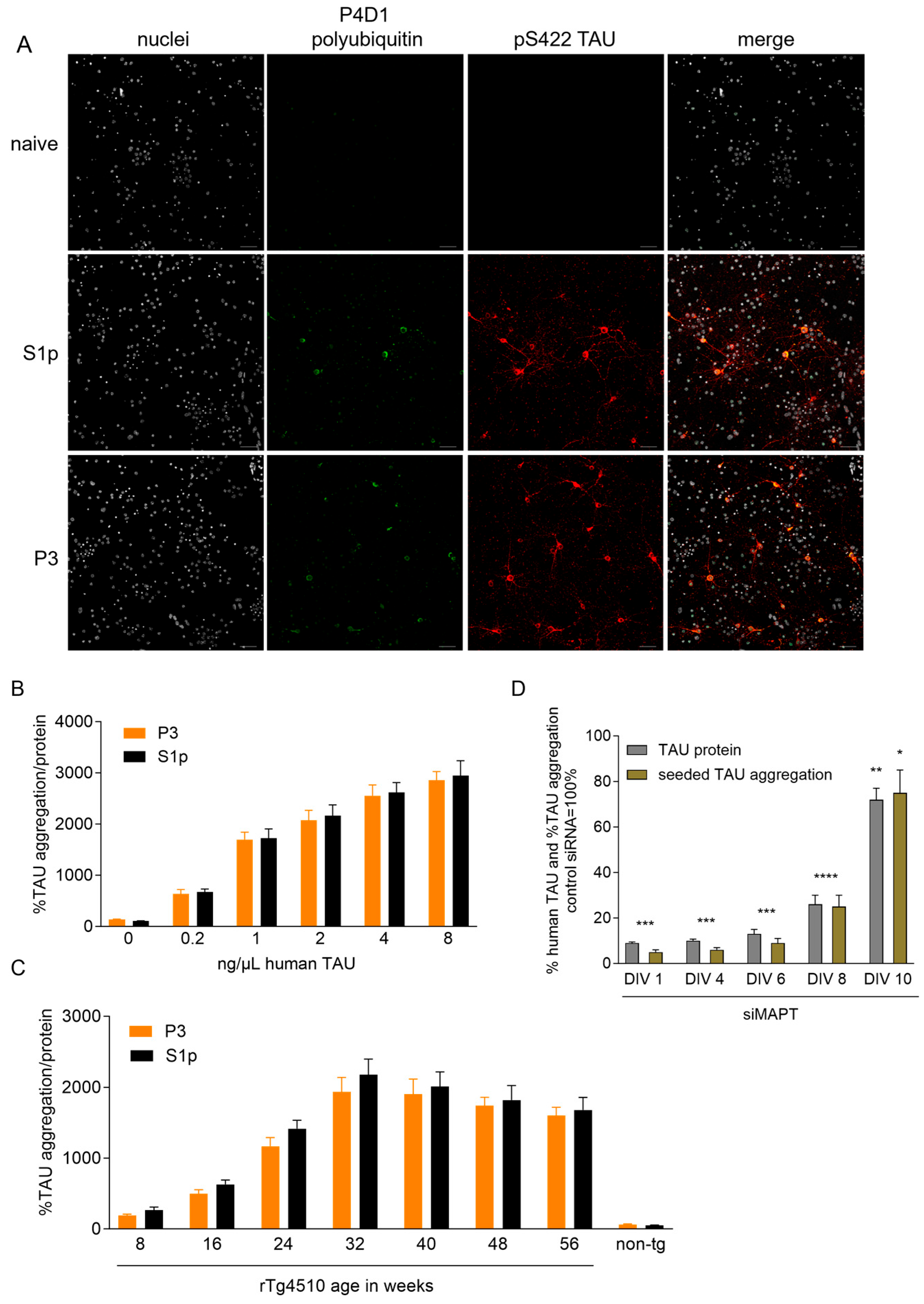
2.1. Defining Optimal Conditions for Seeded TAU Aggregation in Cortical Cultures (CTX) from rTg4510 Mice
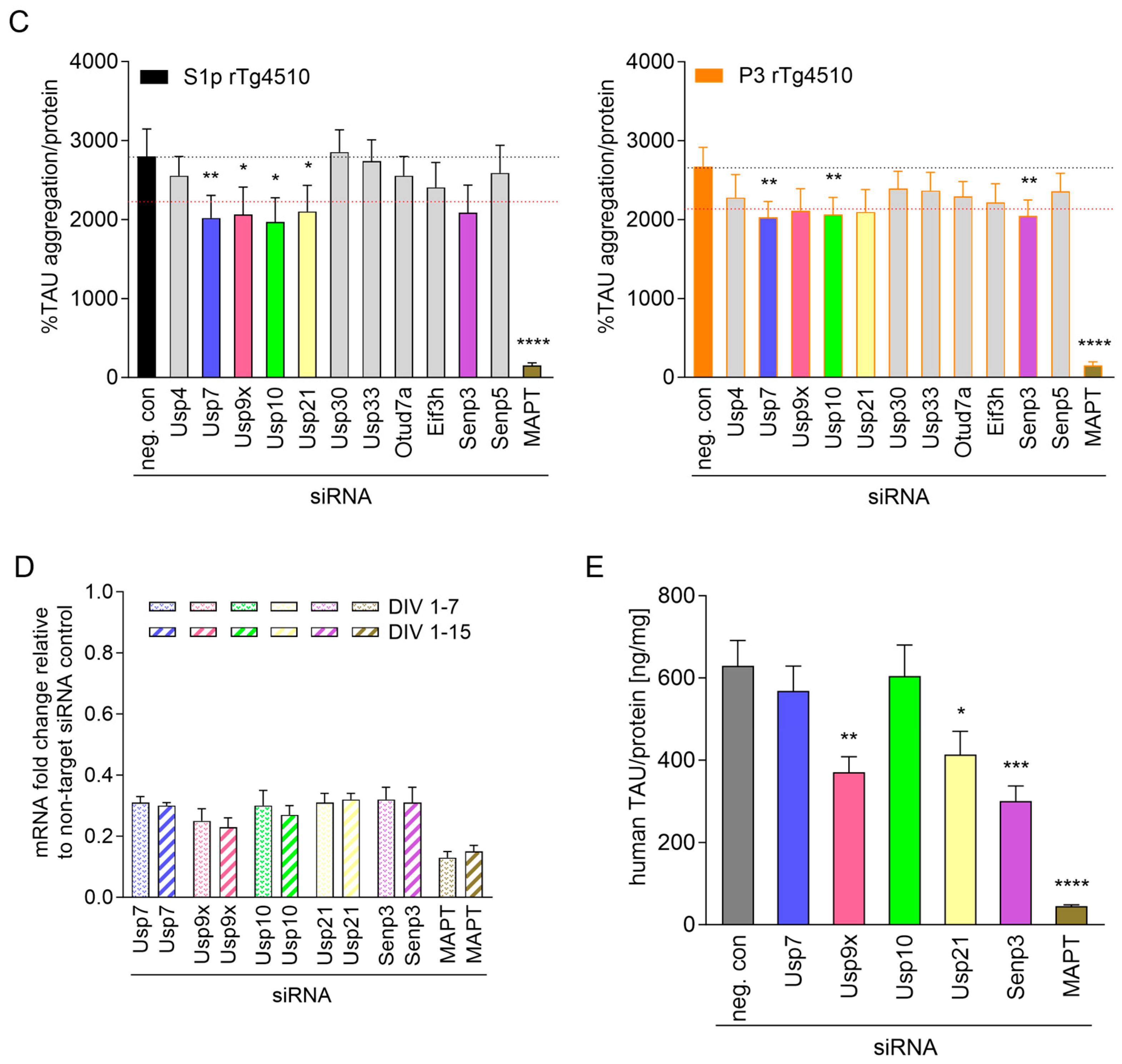
2.2. Focused DUB siRNA Screen on Seeded TAU Aggregation in CTX from rTg4510 Mice
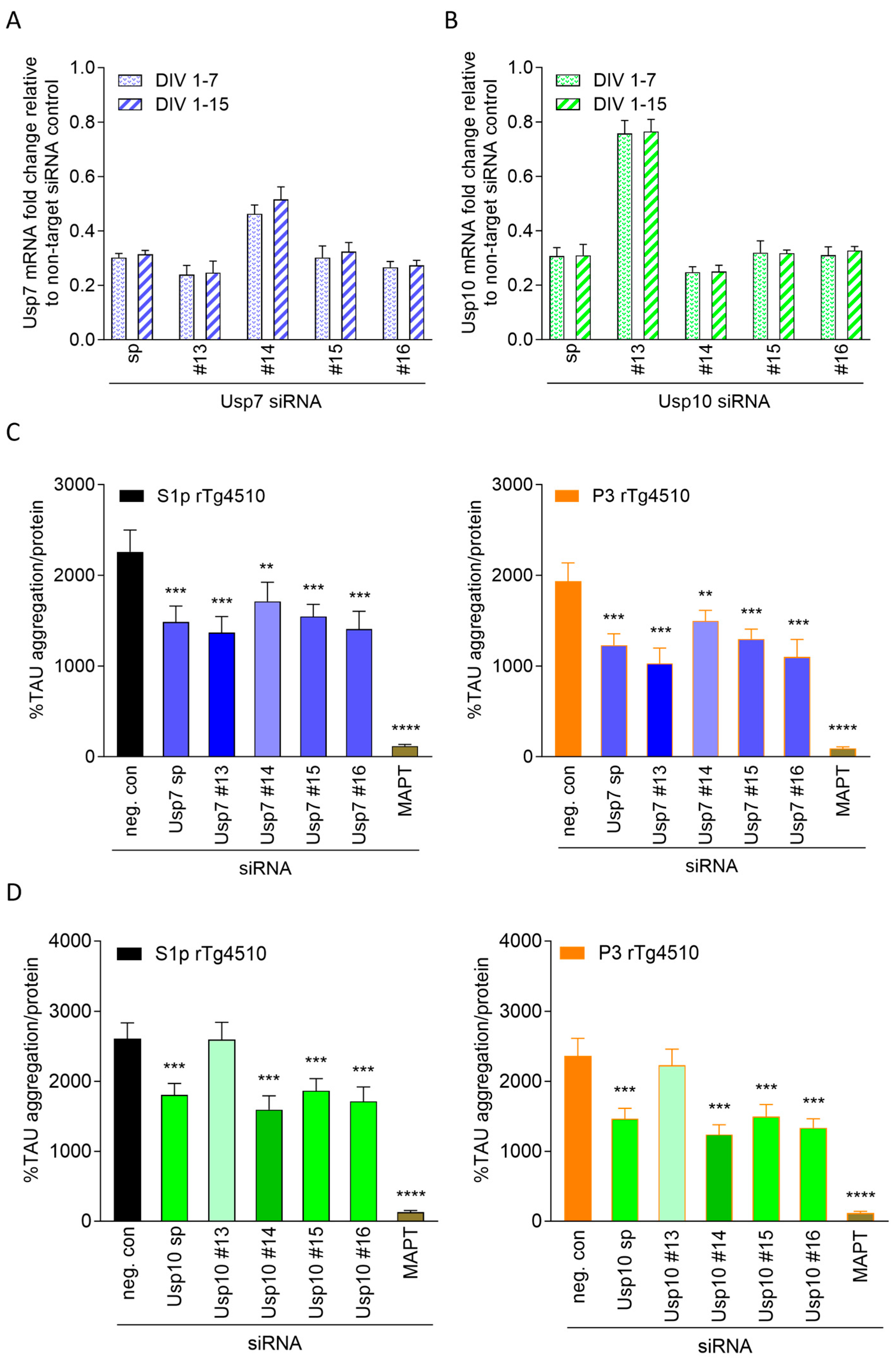
2.2.1. Confirmation of Primary Hits in Seeded TAU Aggregation Induced with S1p TAU Seeds
2.2.2. Validation of Hits in Seeded TAU Aggregation Induced with P3 TAU Seeds
2.2.3. Validated siRNA Hits Lead to Target Knockdown in CTX from rTg4510 Mice
2.2.4. Usp7 and Usp10 Knockdown Does Not Reduce Human TAU Levels in CTX from rTg4510 Mice
2.3. Knockdown Efficiency of Usp7 and Usp10 Correlates with Reduction in Seeded TAU Aggregation in CTX from rTg4510
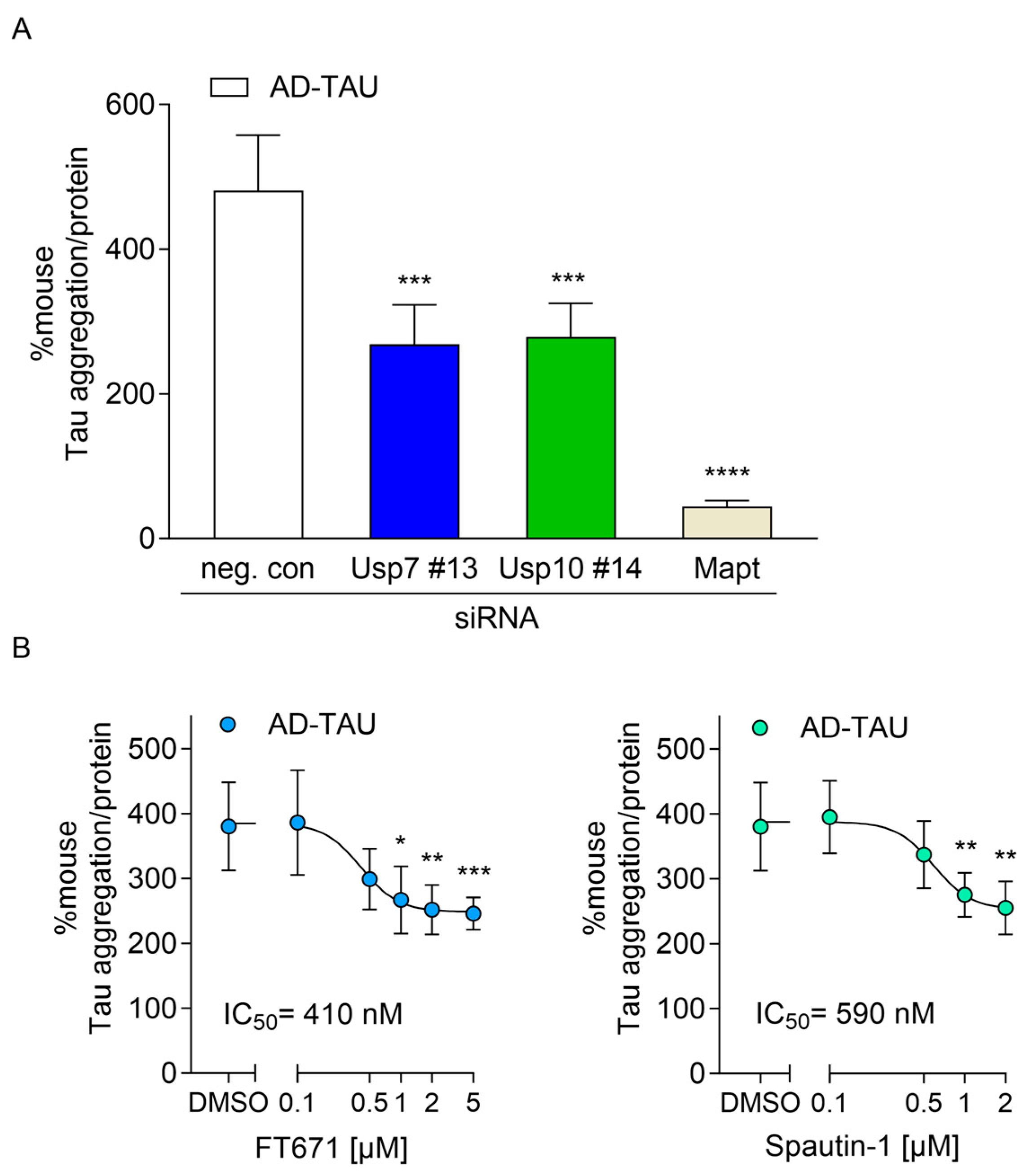
2.4. Usp7 and Usp10 Knockdown Reduced Seeded Tau Aggregation Induced with TAU Seeds Derived from AD Brains in Wildtype CTX
2.5. USP7 and USP10 Inhibitors Reduced Seeded TAU Aggregation in rTg4510 and Wildtype Neurons
2.6. USP7 and USP10 Inhibitors Increase Polyubiquitination Levels of Seeded TAU Aggregates in Neurons from rTg4510 Mice
2.7. Knockdown and Inhibition of Usp7 and Usp10 Reduce Seeded TAU Aggregation in Organotypic Hippocampal Slice Cultures (OHSCs) from rTg4510 Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparations of Pathological TAU Seeds from rTg4510 Mouse and AD Brains
4.3. Seeded TAU Aggregation, siRNA and Inhibitor Treatments in Cortical Cultures (CTX)
4.4. Seeded TAU Aggregation in Cortical Cultures Detected by Sequential TAU Fractionation and Western Blotting
4.5. Measurement of Seeded TAU Aggregation in CTX by HTRF TAU Aggregation Immunoassays
4.6. Measurement of Seeded TAU Aggregation and Polyubiquitination in CTX by Immunocytochemistry
4.7. Measurements of TAU Protein by ELISA
4.8. Seeded TAU Aggregation in Organotypic Hippocampal Slice Cultures (OHSCs)
4.9. Measurement of Seeded TAU Aggregation in OHSCs by TAU Immunocytochemistry
4.10. Measurements of Gene Expression by Quantitative PCR
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, V.M.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef]
- Greenberg, S.G.; Davies, P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc. Natl. Acad. Sci. USA 1990, 87, 5827–5831. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991, 1, 213–216. [Google Scholar] [CrossRef]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Del Tredici, K.; et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef]
- Hutton, M.; Lendon, C.L.; Rizzu, P.; Baker, M.; Froelich, S.; Houlden, H.; Pickering-Brown, S.; Chakraverty, S.; Isaacs, A.; Grover, A.; et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998, 393, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.L.; Kril, J.J.; Stevens, C.H.; Kwok, J.B.; Hallupp, M.; Kim, W.S.; Huang, Y.; McGinley, C.V.; Werka, H.; Kiernan, M.C.; et al. Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies. Brain 2018, 141, 521–534, Correction in Brain 2018, 141, e30. https://doi.org/10.1093/brain/awy012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goedert, M.; Jakes, R.; Crowther, R.A. Effects of frontotemporal dementia FTDP-17 mutations on heparin-induced assembly of tau filaments. FEBS Lett. 1999, 450, 306–311. [Google Scholar] [CrossRef]
- Vogelsberg-Ragaglia, V.; Bruce, J.; Richter-Landsberg, C.; Zhang, B.; Hong, M.; Trojanowski, J.Q.; Lee, V.M. Distinct FTDP-17 missense mutations in tau produce tau aggregates and other pathological phenotypes in transfected CHO cells. Mol. Biol. Cell 2000, 11, 4093–4104. [Google Scholar] [CrossRef] [PubMed]
- Friedhoff, P.; von Bergen, M.; Mandelkow, E.M.; Davies, P.; Mandelkow, E. A nucleated assembly mechanism of Alzheimer paired helical filaments. Proc. Natl. Acad. Sci. USA 1998, 95, 15712–15717. [Google Scholar] [CrossRef]
- Guo, J.L.; Lee, V.M. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem. 2011, 286, 15317–15331. [Google Scholar] [CrossRef]
- Guo, J.L.; Narasimhan, S.; Changolkar, L.; He, Z.; Stieber, A.; Zhang, B.; Gathagan, R.J.; Iba, M.; McBride, J.D.; Trojanowski, J.Q.; et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med. 2016, 213, 2635–2654. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.; Henderson, M.J.; Anderson, J.; Titus, S.A.; Zakharov, A.; Simeonov, A.; Buist, A.; Delay, C.; Moechars, D.; Trojanowski, J.Q.; et al. Compound screening in cell-based models of tau inclusion formation: Comparison of primary neuron and HEK293 cell assays. J. Biol. Chem. 2020, 295, 4001–4013. [Google Scholar] [CrossRef]
- Ficulle, E.; Kananathan, S.; Airey, D.; Gharbi, S.I.; Humphryes-Kirilov, N.; Scherschel, J.; Dunbar, C.; Eastwood, B.J.; Laing, E.; Collier, D.A.; et al. A human tau seeded neuronal cell model recapitulates molecular responses associated with Alzheimer’s disease. Sci. Rep. 2022, 12, 2673. [Google Scholar] [CrossRef]
- Wang, L.; Sooram, B.; Kumar, R.; Schedin-Weiss, S.; Tjernberg, L.O.; Winblad, B. Tau degradation in Alzheimer’s disease: Mechanisms and therapeutic opportunities. Alzheimers Dement. 2025, 21, e70048. [Google Scholar] [CrossRef]
- Cliffe, R.; Sang, J.C.; Kundel, F.; Finley, D.; Klenerman, D.; Ye, Y. Filamentous Aggregates Are Fragmented by the Proteasome Holoenzyme. Cell Rep. 2019, 26, 2140–2149.e3. [Google Scholar] [CrossRef]
- Ye, Y.; Klenerman, D.; Finley, D. N-Terminal Ubiquitination of Amyloidogenic Proteins Triggers Removal of Their Oligomers by the Proteasome Holoenzyme. J. Mol. Biol. 2020, 432, 585–596. [Google Scholar] [CrossRef]
- Harrigan, J.A.; Jacq, X.; Martin, N.M.; Jackson, S.P. Deubiquitylating enzymes and drug discovery: Emerging opportunities. Nat. Rev. Drug Discov. 2018, 17, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Abreha, M.H.; Dammer, E.B.; Ping, L.; Zhang, T.; Duong, D.M.; Gearing, M.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Quantitative Analysis of the Brain Ubiquitylome in Alzheimer’s Disease. Proteomics 2018, 18, e1800108. [Google Scholar] [CrossRef]
- Morishima-Kawashima, M.; Hasegawa, M.; Takio, K.; Suzuki, M.; Titani, K.; Ihara, Y. Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron 1993, 10, 1151–1160. [Google Scholar] [CrossRef]
- Petrucelli, L.; Dickson, D.; Kehoe, K.; Taylor, J.; Snyder, H.; Grover, A.; De Lucia, M.; McGowan, E.; Lewis, J.; Prihar, G.; et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 2004, 13, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Schwartz, D.; Gygi, S.P.; Kosik, K.S. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J. Biol. Chem. 2004, 279, 4869–4876. [Google Scholar] [CrossRef] [PubMed]
- Sahara, N.; Murayama, M.; Mizoroki, T.; Urushitani, M.; Imai, Y.; Takahashi, R.; Murata, S.; Tanaka, K.; Takashima, A. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J. Neurochem. 2005, 94, 1254–1263. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xu, Y.F.; Liu, X.H.; Li, D.; Yin, J.; Liu, Y.H.; Chen, X.Q.; Wang, J.Z. Carboxyl terminus of heat-shock cognate 70-interacting protein degrades tau regardless its phosphorylation status without affecting the spatial memory of the rats. J. Neural Transm. 2008, 115, 483–491. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, J.; Zhang, S.; Li, M.; Zuo, C.; Mao, C.; Zhang, Z.; Tang, M.; Shi, C.; Xu, Y. AAV mediated carboxyl terminus of Hsp70 interacting protein overexpression mitigates the cognitive and pathological phenotypes of APP/PS1 mice. Neural Regen. Res. 2025, 20, 253–264. [Google Scholar] [CrossRef]
- Dickey, C.A.; Yue, M.; Lin, W.L.; Dickson, D.W.; Dunmore, J.H.; Lee, W.C.; Zehr, C.; West, G.; Cao, S.; Clark, A.M.; et al. Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J. Neurosci. 2006, 26, 6985–6996. [Google Scholar] [CrossRef]
- Wei, Z.; Zeng, K.; Hu, J.; Li, X.; Huang, F.; Zhang, B.; Wang, J.Z.; Liu, R.; Li, H.L.; Wang, X. USP10 deubiquitinates Tau, mediating its aggregation. Cell Death Dis. 2022, 13, 726. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Chaput, D.; Shin, M.K.; Koh, Y.; Gan, L.; Pieper, A.A.; Woo, J.A.; Kang, D.E. X-linked ubiquitin-specific peptidase 11 increases tauopathy vulnerability in women. Cell 2022, 185, 3913–3930.e19. [Google Scholar] [CrossRef]
- Liu, X.; Hebron, M.L.; Mulki, S.; Wang, C.; Lekah, E.; Ferrante, D.; Shi, W.; Kurd-Misto, B.; Moussa, C. Ubiquitin Specific Protease 13 Regulates Tau Accumulation and Clearance in Models of Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 425–441, Correction in J. Alzheimers Dis. 2024, 98, 1543–1546. https://doi.org/10.3233/JAD-249007. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Boyd-Kimball, D.; Cai, J.; Pierce, W.M.; Klein, J.B.; Merchant, M.; Butterfield, D.A. Proteomics analysis of the Alzheimer’s disease hippocampal proteome. J. Alzheimers Dis. 2007, 11, 153–164. [Google Scholar] [CrossRef]
- Piatnitskaia, S.; Takahashi, M.; Kitaura, H.; Katsuragi, Y.; Kakihana, T.; Zhang, L.; Kakita, A.; Iwakura, Y.; Nawa, H.; Miura, T.; et al. USP10 is a critical factor for Tau-positive stress granule formation in neuronal cells. Sci. Rep. 2019, 9, 10591. [Google Scholar] [CrossRef] [PubMed]
- Minjarez, B.; Valero Rustarazo, M.L.; Sanchez del Pino, M.M.; Gonzalez-Robles, A.; Sosa-Melgarejo, J.A.; Luna-Munoz, J.; Mena, R.; Luna-Arias, J.P. Identification of polypeptides in neurofibrillary tangles and total homogenates of brains with Alzheimer’s disease by tandem mass spectrometry. J. Alzheimers Dis. 2013, 34, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Joberty, G.; Buist, A.; Vanoosthuyse, A.; Stancu, I.C.; Vasconcelos, B.; Pierrot, N.; Faelth-Savitski, M.; Kienlen-Campard, P.; Octave, J.N.; et al. Tau interactome mapping based identification of Otub1 as Tau deubiquitinase involved in accumulation of pathological Tau forms in vitro and in vivo. Acta Neuropathol. 2017, 133, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Han, Y.; Yu, Q.; Wang, X.; Wang, S.; Liao, X. UCH-L1 Inhibition Decreases the Microtubule-Binding Function of Tau Protein. J. Alzheimers Dis. 2016, 49, 353–363. [Google Scholar] [CrossRef]
- Kim, J.; de Haro, M.; Al-Ramahi, I.; Garaicoechea, L.L.; Jeong, H.H.; Sonn, J.Y.; Tadros, B.; Liu, Z.; Botas, J.; Zoghbi, H.Y. Evolutionarily conserved regulators of tau identify targets for new therapies. Neuron 2023, 111, 824–838.e7. [Google Scholar] [CrossRef]
- Koglsberger, S.; Cordero-Maldonado, M.L.; Antony, P.; Forster, J.I.; Garcia, P.; Buttini, M.; Crawford, A.; Glaab, E. Gender-Specific Expression of Ubiquitin-Specific Peptidase 9 Modulates Tau Expression and Phosphorylation: Possible Implications for Tauopathies. Mol. Neurobiol. 2017, 54, 7979–7993. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, S.K.; Jiang, Y.; Choi, W.H.; Hong, C.; Kim, D.E.; Lee, M.J. Facilitated Tau Degradation by USP14 Aptamers via Enhanced Proteasome Activity. Sci. Rep. 2015, 5, 10757. [Google Scholar] [CrossRef]
- Boselli, M.; Lee, B.H.; Robert, J.; Prado, M.A.; Min, S.W.; Cheng, C.; Silva, M.C.; Seong, C.; Elsasser, S.; Hatle, K.M.; et al. An inhibitor of the proteasomal deubiquitinating enzyme USP14 induces tau elimination in cultured neurons. J. Biol. Chem. 2017, 292, 19209–19225. [Google Scholar] [CrossRef]
- Qin, B.; Chen, X.; Wang, F.; Wang, Y. DUBs in Alzheimer’s disease: Mechanisms and therapeutic implications. Cell Death Discov. 2024, 10, 475. [Google Scholar] [CrossRef]
- Sahara, N.; DeTure, M.; Ren, Y.; Ebrahim, A.S.; Kang, D.; Knight, J.; Volbracht, C.; Pedersen, J.T.; Dickson, D.W.; Yen, S.H.; et al. Characteristics of TBS-extractable hyperphosphorylated tau species: Aggregation intermediates in rTg4510 mouse brain. J. Alzheimers Dis. 2013, 33, 249–263. [Google Scholar] [CrossRef]
- Rostgaard, N.; Jul, P.H.; Garmer, M.; Volbracht, C. Increasing O-GlcNAcylation Attenuates tau Hyperphosphorylation and Behavioral Impairment in rTg4510 Tauopathy Mice. J. Integr. Neurosci. 2023, 22, 135. [Google Scholar] [CrossRef] [PubMed]
- Rosenqvist, N.; Asuni, A.A.; Andersson, C.R.; Christensen, S.; Daechsel, J.A.; Egebjerg, J.; Falsig, J.; Helboe, L.; Jul, P.; Kartberg, F.; et al. Highly specific and selective anti-pS396-tau antibody C10.2 targets seeding-competent tau. Alzheimers Dement. 2018, 4, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Helboe, L.; Egebjerg, J.; Barkholt, P.; Volbracht, C. Early depletion of CA1 neurons and late neurodegeneration in a mouse tauopathy model. Brain Res. 2017, 1665, 22–35. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Turnbull, A.P.; Ioannidis, S.; Krajewski, W.W.; Pinto-Fernandez, A.; Heride, C.; Martin, A.C.L.; Tonkin, L.M.; Townsend, E.C.; Buker, S.M.; Lancia, D.R.; et al. Molecular basis of USP7 inhibition by selective small-molecule inhibitors. Nature 2017, 550, 481–486. [Google Scholar] [CrossRef]
- Kategaya, L.; Di Lello, P.; Rouge, L.; Pastor, R.; Clark, K.R.; Drummond, J.; Kleinheinz, T.; Lin, E.; Upton, J.P.; Prakash, S.; et al. USP7 small-molecule inhibitors interfere with ubiquitin binding. Nature 2017, 550, 534–538. [Google Scholar] [CrossRef]
- Liu, J.; Xia, H.; Kim, M.; Xu, L.; Li, Y.; Zhang, L.; Cai, Y.; Norberg, H.V.; Zhang, T.; Furuya, T.; et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 2011, 147, 223–234. [Google Scholar] [CrossRef]
- Kedersha, N.; Panas, M.D.; Achorn, C.A.; Lyons, S.; Tisdale, S.; Hickman, T.; Thomas, M.; Lieberman, J.; McInerney, G.M.; Ivanov, P.; et al. G3BP-Caprin1-USP10 complexes me-diate stress granule condensation and associate with 40S subunits. J. Cell Biol. 2016, 212, 845–860, Correction in J. Cell Biol. 2020, 219, e20150802809202019c. https://doi.org/10.1083/jcb.20150802809202019c. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santacruz, K.; Lewis, J.; Spires, T.; Paulson, J.; Kotilinek, L.; Ingelsson, M.; Guimaraes, A.; DeTure, M.; Ramsden, M.; McGowan, E.; et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef]
- Volbracht, C.; Penzkofer, S.; Mansson, D.; Christensen, K.V.; Fog, K.; Schildknecht, S.; Leist, M.; Nielsen, J. Measurement of cellular beta-site of APP cleaving enzyme 1 activity and its modulation in neuronal assay systems. Anal. Biochem. 2009, 387, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Buist, A.; Soares, A.; Callaerts, K.; Calafate, S.; Stevenaert, F.; Daniels, J.P.; Zoll, B.E.; Crowe, A.; Brunden, K.R.; et al. The Dynamics and Turnover of Tau Aggregates in Cultured Cells: Insights into therapies for tauopathies. J. Biol. Chem. 2016, 291, 13175–13193. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.; Ksiezak-Reding, H.; Liu, W.K.; Dickson, D.W.; Yen, S.H. The N terminal region of human tau is present in Alzheimer’s disease protein A68 and is incorporated into paired helical filaments. Am. J. Pathol. 1991, 139, 1463–1470. [Google Scholar] [PubMed]
- Opitz-Araya, X.; Barria, A. Organotypic hippocampal slice cultures. J. Vis. Exp. 2011, 48, 2462. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volbracht, C.; Fog, K. Deubiquitinating Enzymes Ubiquitin-Specific Proteases 7 and 10 Regulate TAU Aggregation. Int. J. Mol. Sci. 2025, 26, 11062. https://doi.org/10.3390/ijms262211062
Volbracht C, Fog K. Deubiquitinating Enzymes Ubiquitin-Specific Proteases 7 and 10 Regulate TAU Aggregation. International Journal of Molecular Sciences. 2025; 26(22):11062. https://doi.org/10.3390/ijms262211062
Chicago/Turabian StyleVolbracht, Christiane, and Karina Fog. 2025. "Deubiquitinating Enzymes Ubiquitin-Specific Proteases 7 and 10 Regulate TAU Aggregation" International Journal of Molecular Sciences 26, no. 22: 11062. https://doi.org/10.3390/ijms262211062
APA StyleVolbracht, C., & Fog, K. (2025). Deubiquitinating Enzymes Ubiquitin-Specific Proteases 7 and 10 Regulate TAU Aggregation. International Journal of Molecular Sciences, 26(22), 11062. https://doi.org/10.3390/ijms262211062





