Abstract
Combined oral contraceptives (COCs) remain one of the most popular reversible contraceptive methods worldwide. Still, regardless of the drug composition and duration of therapy, almost all COCs are associated with the risk of venous thrombosis. This review highlights the main pathogenetic mechanisms of thrombosis development during oral contraceptive use. Increase the production of certain clotting factors; a decrease in antithrombin and protein S levels; acquired resistance to activated protein C; a reduction in tissue factor pathway inhibitor (TFPI); indirect endothelial activation; inhibition of endogenous fibrinolysis; regulation of tissue factor by estradiol-sensitive microRNA; homocysteine imbalance caused by decreased intestinal reabsorption of folates and vitamin B-12; reduced bioavailability of nitric oxide (NO) due to high homocysteine levels; higher blood pressure, water retention, insulin resistance, increased levels of pro-inflammatory C-reactive protein (CRP) and uric acid, and antifibrinolytic (plasminogen activator inhibitor 1 type, PAI-1) biomarkers as consequences of NO deficiency; increased platelet adhesiveness and ADP-induced aggregation, which promote fibrinogen binding; and increased expression of pro-inflammatory cytokines are the main thrombotic effects of COCs use. Clinicians should carefully evaluate each patient’s individual risk factors when prescribing COCs and conduct regular monitoring to reduce the risk of complications.
1. Introduction
Contraceptive pills remain one of the most popular reversible contraceptive methods worldwide, but many factors need to be considered and managed before selecting a method. Clinicians should carefully evaluate each patient’s individual risk factors when prescribing combined oral contraceptives (COCs) and conduct regular monitoring to reduce the risk of complications. Some studies [1] have shown a three- to seven-fold increase in venous [2] and arterial thrombosis [3]. The risk of VTE should be regularly re-evaluated, as risk factors for thrombosis can change over time. Educating women about the potential risks and benefits of hormonal contraception will help them make informed decisions about their health.
This article is a narrative review aimed at summarizing the current evidence on the mechanisms and risk of thrombosis associated with combined oral contraceptive (COC) use, emphasizing differences between formulations and identifying the safest hormonal combinations in terms of thrombotic risk.
1.1. Historical Background
The history of oral contraceptives (OC) dates to the mid-20th century [4]. In the 1950s, American biologists Margaret Sanger and Catherine McCormick funded research on hormonal contraception. Gregory Pincus, along with Ming-Chue Chang, contributed to the basic concept of the pill. In 1956, the first clinical trials of a hormonal contraceptive containing progestin and estrogen were successfully conducted in Puerto Rico. In 1960, the first oral contraceptive, Enovid 10, produced by G.D. Searle & Company, was approved in the United States.
There are four available forms of estrogens: ethinyl estradiol (EE), 17β-estradiol (E2), estradiol valerate (E2V), and mestranol. The estrogen used in the first oral contraceptive was mestranol, which is rarely used today, and the progesterone component was norethynodrel. This medication contained a very high dosage of hormones (9.85 mg of norethynodrel and 150 μg of mestranol), leading to serious side effects, but it marked a revolution in reproductive health.
Nowadays, OC pills contain much lower doses of hormones—ranging from 0.1 to 3.0 mg of progestins and 20 to 50 μg of estrogens [4]. The most common estrogen component in most modern COCs today is EE, and the amount in the tablet determines the estrogenic effects and the risk of thrombosis. One of the goals of reducing the estrogen dose in COCs was to lower the risk of developing thrombotic complications since the risk of thrombosis with estrogen-containing compounds increases as the estrogen dose goes up [5]. Besides reducing the dose of ethinyl estradiol (EE), some combined oral contraceptives (COCs) now include 17β-estradiol, along with various progesterones at different doses. Progestins are the primary component of hormonal contraceptives because they prevent ovulation through their antigonadotropic effects.
1.2. Composition of COCs
There is currently no universal and standardized classification for OCs, but hormonal contraceptive drugs can be grouped based on their progesterone composition (Table 1).

Table 1.
Progestogens characteristics in COCs.
First-generation preparations, which are currently rarely used, include contraceptives containing Lynestrol. The second generation of contraceptives, which were released in the 1970s, had a reduced estrogen dosage, and the introduction of levonorgestrel (LNG) lowered the risk of thrombosis. The second-generation progestins (levonorgestrel and norgestrel) have more profound progestational and androgenic effects in combination with lower EE and strong antiestrogen effects. Nevertheless, the androgenic effects of these combined oral contraceptives (COCs) with a progestin component remained evident [6]. Thus, attempts to reduce the unwanted androgenic impacts of COCs led to the development of third-generation contraceptives, which were introduced in the 1980s and included new progestins such as norgestimate, desogestrel, and gestodene. Despite the modification, most progestins in all three generations are testosterone-derived and, to varying degrees, exhibit undesirable androgenic effects. Finally, the fourth generation of progestins, which contains drospirenone and dienogest (DNG) was designed to be closer in activity to endogenous progesterone than previous generations of progestins and is associated with the lowest risk of side effects.
Oral contraceptives quickly gained popularity despite initial controversies and legal restrictions. Over time, contraceptive formulas improved, with reduced hormone dosages significantly lowering the risk of side effects and increasing the safety of these medications. Today, oral contraceptives are widely used to prevent unwanted pregnancies and to treat various gynecological disorders. Despite the potential risks mentioned earlier, it is essential to recognize the many beneficial effects of hormonal contraceptives. These include a decreased risk of ovarian and endometrial cancer, regulation of the menstrual cycle, and relief from premenstrual syndrome symptoms [7]. However, increasing reports of thromboembolic events since the first use of combined oral contraceptives (COCs) and those associated with COCs have raised concerns about a possible causal relationship between hormonal therapy and the risk of thrombosis [8].
2. Methodology
A structured literature search was performed across PubMed, Scopus, and Web of Science databases to find studies published from January 2010 to June 2024. Various combinations of MeSH terms and keywords were used, including: “combined oral contraceptives,” “venous thromboembolism,” “thrombosis,” “risk factors,” “progestins,” and “estetrol drospirenone.” Preference was given to systematic reviews, meta-analyses, and clinical studies written in English. Reference lists of relevant publications were also screened to find additional sources.
3. Pathogenesis of Thrombotic Complications with Hormonal Contraceptives
Pathogenetic mechanisms of thrombosis development during oral contraceptive use are very diverse but are still not fully understood. The thrombotic effects of COCs are probably mediated through their effects on the blood coagulation system (Figure 1) [9].
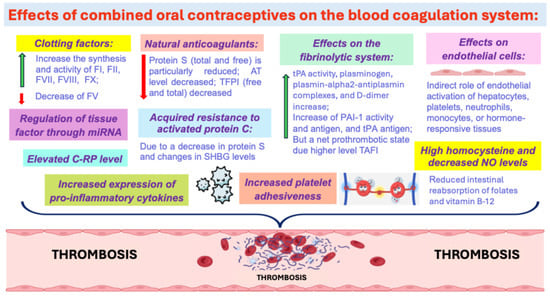
Figure 1.
The thrombotic effects of COCs. Abbreviations: C-RP: C-reactive protein, TAFI—thrombin-activatable fibrinolysis inhibitor, SHBG—sex hormone-binding globulin, TFPI—tissue factor pathway inhibitor.
3.1. Increase the Production of Some Clotting Factors
The rise in coagulation factors related to OCs may be one of the mechanisms that increases the risk of cardiovascular events in contraceptive users. There is a strong link between OC use, especially with higher estrogen doses, and the risk of thromboembolism. Estrogens in OCs increase the synthesis and activity of clotting factors such as fibrinogen (FI), prothrombin (FII), and factors VII, VIII, and X, while decreasing factor V (FV), which promotes hypercoagulability. It is now well recognized that factor V has both pro- and anticoagulant properties [10]. Since FV acts as a cofactor with protein S and effectively inhibits FVIIIa activity, a reduction in FV levels may contribute to the thrombotic effects of OC [11]. The increase in FII and FVII, along with a moderate decrease in FV, is associated with a significantly higher risk of thrombosis. These changes depend on the estrogen dose, the mode of estrogen delivery [12], and the type of progestogen used in COCs [13]. The effect is more pronounced with the desogestrel-containing OC (third generation) compared to the levonorgestrel-containing OC (second generation) [14].
3.2. Decrease in the Activity of Natural Anticoagulants
At the same time, there is a decrease in the activity of natural anticoagulants, such as protein S and antithrombin (AT), along with reduced sensitivity to activated protein C (APC), which also enhances the blood’s clotting properties.
3.2.1. Decreased Antithrombin Levels
Antithrombin (SERPINC 1) is a liver-produced glycoprotein that belongs to a family of serine protease inhibitors and is actively involved in the coagulation cascade. AT binds to serine factor II (thrombin), factor IXa, and factor Xa, which inhibit the blood clotting process. Several studies have established a relationship between oral contraceptive use and changes in AT level [15,16]. Burkman and colleagues found that antithrombin levels in different evaluated oral contraceptive groups decreased from baseline by 19.7% and 28.8% for the immunologic and activity methods, respectively. Initially, they suggested that the estrogen component in OCs is responsible for changes in antithrombin. However, it was later revealed that the highest decline in antithrombin level was observed in type O blood group COC users (31.6%) taking high doses (50 μg) of ethinyl estradiol, and in non-type O women (38.9%) taking the lowest progestin dose (0.5 mg norethindrone) [15,16].
3.2.2. Acquired Resistance to Activated Protein C (APC-R)
The protein C system is the natural anticoagulant system, regulating coagulation, maintaining blood fluidity, preventing thrombosis, and thereby preventing vascular injury and stress. The key protease of the protein C system is activated protein C (APC). Normally, APC inhibits the coagulation cascade by cleaving peptide bonds in FV/Va and VIII/VIIIa. Circulating inactive factor V can exhibit either procoagulant or anticoagulant activity, depending on modification by pro- or anticoagulant enzymes. Under the influence of thrombin, the active form of factor V is produced, which has procoagulant activity. After proteolytic inactivation by activated protein C, FVa is degraded into inactive factor FVi, which effectively downregulates the coagulation and limits blood clot formation. In addition to its anticoagulant activity, APC exhibits cytoprotective and anti-inflammatory effects on vascular endothelial cells, neuronal cells, and various immune system cells. The most common cause of the inability of APC to cleave factor Va and/or factor VIIIa (APC-resistance) is the factor V Leiden mutation.
The use of COCs may result in the development of resistance to the anticoagulant action of APC, regardless of the presence of a genetic mutation of factor V Leiden, especially in women using third-generation (desogestrel-containing) COCs compared with users of second-generation (levonorgestrel-containing) oral contraceptives [10].
However, some studies indicate that COC-induced APC resistance (APC-R) has a low prothrombotic potential and that COC-associated thrombosis occurs rarely, particularly in comparison to high-risk situations, such as major surgery [17]. At the same time, the authors of this study suggest that thrombin generation may increase during COC use when additional risk factors are present.
Protein S is a natural anticoagulant that acts as a cofactor for activated protein C and, in addition, exhibits anticoagulant properties by directly inhibiting thrombin formation (APC-independent action). Protein S (total and free) is remarkably reduced when taking OCs containing 3rd generation progesterone and in postmenopausal women [18].
Most users of 3rd generation contraceptive pills have acquired resistance to APC, which is mediated not only by a decrease in PS but is also associated with changes in the level of sex hormone-binding globulin (SHBG), a liver protein, whose synthesis is highly estrogen dose-dependent [19]. Oral administration of EE alone causes a significant dose-dependent increase in SHBG. In contrast, administration of progestogens results in a decrease in SHBG, the extent of which depends on the androgenic activity of the progestogen [20]. Additionally, HSPG can serve as a measure of total estrogenicity and an indirect marker of thrombosis in OC users [21]. When taking OC with high concentrations of estrogens, there is an increase in the level of SHBG. At the same time, it decreases depending on the antiestrogenic properties of the progesterone component of pills [22]. For example, there is a 50% SHBG increase with LNG-containing contraceptives, 150% with norgestimate, 250–300% with drospirenone and dienogest, 200–300% with desogestrel and gestodene, and 300–400% with cyproterone acetate, carrying a higher risk of VTE [20,21,22]. OCs containing drospirenone, dienogest, cyproterone acetate, and norgestimate may cause more pronounced alterations to APC and, therefore, lead to a higher risk of VTE compared to users of 2nd-generation OCs.
3.2.3. Decrease in TFPI Levels
The anticoagulant properties of endothelial cells (ECs) predominate over procoagulant properties due to the expression of proteins such as tissue factor pathway inhibitor (TFPI), which is the primary natural inhibitor of TF-induced coagulation. TFPI inhibits the FVIIa/tissue factor complex, thereby blocking the initiation of the coagulation process. In 2003, Dahm and colleagues assessed the TFPI level (total and free) and its activity in COC users compared to non-using women [23]. Additionally, they investigated whether a decreased level of TFPI is a risk factor for the development of deep vein thrombosis (DVT). The results showed that forty-one percent of oral contraceptive users had a more profound decrease in free and total TFPI compared with premenopausal nonusers, who had lower levels than men and women in post menopause [23]. They revealed that plasma TFPI level below the 10th percentile is a weak risk factor for DVT (10th percentile = 9 ng/mL), and further analyses found that lower TFPI levels (e.g., cut-off at the 5th and 2nd percentiles) were associated with a higher odds ratio for DVT. The authors concluded that this relationship between higher risk of VTE and lower TFPI levels suggests a threshold level for the protective effect of TFPI against DVT.
3.3. Effects on Endothelial Cells
Both estrogen and progesterone receptors are present on vascular endothelial cells and smooth muscle cells, suggesting that these compounds may influence vascular function [24]. It has been well established that 17β-estradiol, the naturally produced estrogen, exerts protective cardiovascular effects through interaction with multiple cell types via estrogen receptors (ER), including nuclear estrogen receptors ERα and ERβ, membrane ER, and G protein-coupled estrogen receptor. ECs are the primary target of E2’s beneficial effects, such as neovascularization, which helps reduce ischemic injury.
To identify whether COC hormones provoke endothelial prothrombotic activity, Bouck et al. conducted a study using an in vitro model of the plasma-endothelial interface [25]. They treated the human umbilical vein and microvascular endothelium with ethinylestradiol and/or drospirenone. However, neither of these drugs alone nor their combination resulted in changes in the gene expression encoding anti- or procoagulant proteins (TFPI, THBD, F3), integrins (ITGAV, ITGB3), or fibrinolytic mediators (SERPINE1, PLAT). Also, there was no increase in thrombin or fibrin generation. Thus, the authors claim that there is no direct procoagulant effect of COCs (EE and drospirenone) on endothelial cells. However, it is generally appreciated that EE carries a dose-dependent risk of VTE, and drospirenone increases VTE risk 6.3-fold compared with no OC users and 2–3-fold compared with levonorgestrel [25]. The authors provide possible explanations for the contradictory results obtained. First, women with VTE may carry undetected risk factors or polymorphisms that interact with OCs, promoting endothelial dysfunction and thrombus formation. Secondly, the results do not exclude an indirect role of endothelial activation in OC-associated VTE (activation of hepatocytes, platelets, neutrophils, monocytes, or hormone-responsive tissues), which secondarily release factors that activate ECs and trigger procoagulant activity on the vessel wall. Therefore, it is necessary to continue the search for possible mechanisms of OC effects on the endothelium [25].
3.4. Effects on the Fibrinolytic System
Although the procoagulant effects of estrogens are well documented, their impact on the fibrinolytic system has only recently emerged as a topic of interest. OCs significantly affect the fibrinolytic process, which may also lead to thrombosis formation. Fibrinolysis plays a key role in maintaining the balance between clot formation and destruction by preventing excessive fibrin accumulation in blood vessels. The main components of this system include plasminogen, plasminogen activators, and inhibitors, such as tissue plasminogen activator (tPA), and plasminogen activator inhibitor-1 (PAI-1) [26]. During OC use, the levels of tPA activity, plasminogen, plasmin-alpha2-antiplasmin complexes, and D-dimer increase significantly (by 30–80%), while the levels of PAI-1 antigen, PAI-1 activity, and tPA antigen decrease (by 25–50%), suggesting the profibrinolytic effect of OC [27]. However, these changes were counteracted by higher levels of the thrombin-activatable fibrinolysis inhibitor (TAFI), resulting in a net prothrombotic state. Inhibition of endogenous fibrinolysis occurs independently of factor XI and is more pronounced with desogestrel-containing OC, rather than levonorgestrel-containing OC [27].
3.5. Regulator of Tissue Factor Through miRNA
miRNAs are small, single-stranded, non-coding RNAs that target mRNAs via the 3′-UTR and regulate target gene expression at a post-transcriptional level by RNA silencing, leading to translation inhibition and potential mRNA degradation [28]. Almost every biological process in the body is mediated by microRNA. Several epidemiological studies have identified microRNAs that can modulate the expression of target mRNAs associated with thrombosis and hemostasis and may serve as potential biomarkers for VTE in the general population. Through interaction with the 3′-UTR, miRNAs directly regulate multiple hemostatic functions and are involved in the relationship between the hemostatic system and inflammation, controlling the expression of fibrinogen, coagulation factors III, VII, VIII, IX, XI, von Willebrand Factor (vWF), PAI-1, antithrombin, proteins C, and S.
Tian et al. identified an estradiol-sensitive microRNA (miR), miR-494-3p, which suppresses protein S expression and hypothesized that miRs may similarly regulate other coagulation factors. They found a direct interaction between miR-365a-3p and the 3′-untranslated region of tissue factor mRNA. Tissue factor (TF), as the primary trigger of blood coagulation, was upregulated by estradiol treatment in plasma samples obtained from pregnant women and women on the contraceptive pill compared with sex-matched controls. Thus, miR-365a-3p can be considered a new regulator of tissue factor that initiates thrombin formation [28].
3.6. Effects on Nitric Oxide (NO) and Homocysteine Levels
Possible blood clot formation pathways during OC use include effects on nitric oxide (NO) and homocysteine levels. NO is an important biomolecule that plays a key role in the regulation of hemostasis by influencing various components of the blood coagulation system. It is synthesized by endothelial cells via the enzyme nitric oxide synthase (eNOS) and acts as a vasodilator, antiaggregant, and anticoagulant [29]. Many of the NO effects are cardioprotective. Endogenous 17beta-estradiol has a direct vasodilator effect, increasing NO synthesis in endothelial cells and platelets [30]. Numerous studies were conducted to assess the impact of exogenous female hormones on NO levels. In the study by Olatunji, it has been shown that COC-treated female rats (1.0 μg ethinylestradiol + 5.0 μg levonorgestrel) with NO deficiency had significantly higher blood pressure, water retention, insulin resistance, increased levels of pro-inflammatory (C-reactive protein; CRP and uric acid) and antifibrinolytic (PAI-1) biomarkers [31].
In turn, homocysteine reduces the bioavailability of NO. Homocysteine imbalance leads to redox imbalance, increased protein/nucleic acid/carbohydrate oxidation, lipid peroxidation, and ultimately cytotoxicity [32]. One mechanism for triggering the coagulation cascade is the denudation of the subendothelial matrix and endothelial smooth muscle cells by homocysteine metabolism products. It is widely accepted that hyperhomocysteinemia is an independent risk factor for cardiovascular diseases [32]. Detoxification of homocysteine occurs through remethylation to methionine (B vitamins and folate-dependent and independent routes) and transsulfuration to cystathionine, which requires the activity of essential transsulfuration enzymes (cystathionine β-synthase, cystathionine γ-lyase). Homocysteine is expressed in all tissues, but the brain lacks cystathionine γ-lyase and some other enzymes, being dependent on folic acid/B12 pathways of homocysteine disposal and making the brain parenchyma vulnerable to excess homocysteine.
Moreover, hormonal contraceptives reduce intestinal reabsorption of folates and vitamin B-12, which leads to a significant decrease in their levels in women taking OCs, even when their intake with food is controlled, and can lead to hyperhomocysteinemia and megaloblastic anemia [33].
An additional pathway is the induction of inflammatory responses by homocysteine, which increases proinflammatory cytokines and subsequently induces cellular apoptosis [34]. Fallah et al. confirmed the effect of OCs on homocysteine and NO concentrations, as well as their role in the development of a prothrombotic state [33]. They found that COCs increase homocysteine levels and decrease NO levels, which may result in increased cardiovascular risk [33]. However, the studies of Merki-Feld et al. [35] and Momeni et al. [36] demonstrated no changes in NO and homocysteine levels in women taking OCs, which requires further studies of this mechanism in the development of thrombosis.
Anyway, the alteration in homocysteine levels and inhibition of NO induced by COCs could suggest that the use of oral contraceptives should be reviewed in women with increased cardiovascular risk factors.
3.7. COCs and Platelets
Platelets are actively involved in the coagulation processes and play a central role in the development of arterial and venous thrombosis. The effect of OCs on platelet counts and function remains unclear. Estimates of the estrogen effect on platelet counts vary widely in the literature. For example, Fox et al. demonstrated that high doses of estrogen (500 µg/kg) resulted in an increased level of mature CD41+ megakaryocytes and circulating platelets in mice [37]. However, administration of 10–100 μg/kg/day of 17β-estradiol (17β-E2) for 10 days resulted in a decrease in platelet counts.
There are many studies with contradictory results regarding the effects of COCs on platelet function. For example, a survey by Saleh et al. showed no evidence of altered platelet function [38]. They found no significant differences (p = 0.05) in platelet aggregation using adenosine diphosphate (ADP) and collagen as inducing agents in women using monophasic COCs (n = 12), triphasic COCs (n = 13), Norplant (n = 19) after 3 months of hormone intake compared to 29 control healthy women. The research group led by Bulur et al. found that the use of oral contraceptives did not cause changes in the mean platelet volume (MPV) value in young women [39]. Beltran et al. performed a comparative study of four different hormonal combinations (a transdermal release system that contains 6 mg of norelgestromin (NGMN)/0.600 mg of EE; a vaginal release system containing 11.7 mg of Etonogestrel/2.7 mg of EE; oral COC containing 2 mg of chlormadinone acetate/0.030 mg of EE; COC with 3 mg of drospirenone/0.030 mg of EE) on platelet count and coagulation parameters after 6 months of intake [40]. A statistically significant increase was seen in platelet count and prothrombin activity in the group treated with NGMN/0.600 mg of EE.
Oral contraceptives increase platelet adhesiveness and ADP-induced aggregation, which causes fibrinogen binding [41]. Moreover, in women on OCs, the platelet lipid biosynthesis is increased dramatically, which implies a higher response of platelets to thrombin-induced aggregation [42]. The lipid membrane is crucial for initiating the coagulation cascade. The procoagulant platelet acts as a scaffold that supports the assembly of the prothrombinase complex, which leads to the explosion of thrombin (so-called thrombin burst), the formation of fibrin, resulting in the formation of a fragile fibrin network [43]. Moreover, procoagulant platelets can be considered a new player in the process of thromboinflammation, performing a proinflammatory role by releasing platelet microparticles and inorganic polyphosphate. Once platelets become activated, their aggregation begins, and von Willebrand factor (vWF) is released from alpha granules of platelets.
Willebrand factor binds platelets when the integrity of the vascular wall is compromised (thereby participating in primary hemostasis) and when it binds to factor VIII. VWF protects FVIII from the proteolytic activity of protein C and delivers it to the site of vascular injury, playing an important role in secondary hemostasis. vWF facilitates the attachment of platelets to the damaged endothelium due to the formation of an adhesive bridge [44]. There have been reports that estrogen directly stimulates the synthesis of vWF by endothelial cells, resulting in a slight increase in endothelial replication [44].
3.8. COCs and Inflammation
OCs also play a significant role in inflammatory processes and the immune system. In vitro and in vivo models have demonstrated that low-dose 17β-estradiol (17β-E2) administration is associated with increased expression of pro-inflammatory cytokines [45]. The regulation of inflammatory mechanisms likely occurs through nuclear/membrane estrogen receptor-mediated stimulation of Toll-like receptor 4 ligands, leading to increased IL-1β and IL-6, inhibition of phosphatidylinositol 3-kinase (PI3K) pathway activity, and regulation of NF-κB-mediated transcriptional activity [46,47]. Results of a new UCLA Health study, published in the journal Brain, Behavior, and Immunity [48], found that OC users exhibit numerous signs of chronic inflammation, including elevated C-reactive protein levels and a greater risk of developing mood and autoimmune disorders.
For comparison, it is worth mentioning that endogenous estrogen is negatively associated with C-reactive protein [49]. However, the opposite relationship has been found between exogenously administered female hormones and CRP levels, suggesting that the effect of sex steroid hormones on inflammatory activity in the body may depend, among other factors, on their source. Cauci et al. showed an association between the presence of high-sensitivity C-reactive protein (hsCRP), indicating an ongoing inflammatory process in women of reproductive age taking OCs [50]. Elevated hsCRP levels ≥ 2.0 mg/L are considered a risk factor for cardiovascular disease. These hsCRP concentrations were found in 41.0% of women taking OCs compared to controls [OR = 6.6, 95%CI 3.5–12.4, p < 0.001] [50].
3.9. Role of Lipoprotein(a) in Thrombosis
Recent studies have also highlighted the potential role of lipoprotein(a) (Lp(a)) as a prothrombotic factor in the context of COC use [51]. Elevated plasma levels of Lp(a) may promote both venous and arterial thrombosis through different pathways, including antifibrinolytic and proinflammatory mechanisms, such as inhibiting plasminogen activation and increasing oxidative stress. Oxidized phospholipids carried by Lp(a), along with vascular endothelial dysfunction, may further raise the thrombotic risk associated with COC use [52]. Although the clinical significance of Lp(a) in women using COCs remains limited, measuring it could provide additional insights for women with hereditary or acquired thrombophilia, supporting a more personalized assessment of thrombotic risk.
Table 2 presents a comprehensive overview of the key pathogenetic mechanisms that link COC use to a prothrombotic state, including both classical and recently discovered molecular pathways such as NET formation and elevated Lp(a) levels.

Table 2.
Pathogenetic mechanisms involved in COC-related thrombosis.
4. Hormonal Contraception and the Risk of Thrombosis
A large meta-analysis and systematic review of 3110 publications (25 publications reporting on 26 studies) was conducted to comprehensively assess the risk of venous thromboembolism in women using different combined oral contraceptives [53]. The results of the study reported that the use of COCs leads to almost a four-fold increase in the risk of venous thrombosis (OR 3.5, 95% CI 2.9–4.3 In comparison, the incidence of VTE in individuals not using contraception was 1.9 and 3.7 per 10,000 women per year [53].
4.1. Risk of Venous Thrombosis
Mohammed Al Sheef et al., in their five-year retrospective review, analyzed the risk of various thrombotic complications in 100 female patients who were taking OCs [54]. In this, several types of complications, such as DVT, pulmonary embolism (PE), and other various foci of thrombosis and embolism, were identified [54].
Venous thrombosis is also known to be associated with a high risk of mortality, which also emphasizes the importance of this problem [55]. According to the data, the risk of death from PE in women of reproductive age using OCs is 10.5 (6.2–16.6) per million women per year (95% CI: 6.2–16.6) [56]. In this case, PE developed in 78% of patients taking OCs. At the same time, 10% of patients with VTE also developed PE. DVT was noted in 52% of patients, and most of them had thrombosis in the left leg. Rarer thrombotic complications, according to the study, include cerebral venous thrombosis (n = 12), Budd-Chiari syndrome (n = 2), and visceral thrombosis (n = 6). The estimated age of patients with PE and VTE was 34 ± 8.1 years, with a mean duration of COC intake of 13.6 ± 2.7 months. Patients mostly used COCs such as cyproterone acetate 2 mg + ethinylestradiol 0.035 mg (n = 6), gestodene 075 mg + ethinylestradiol 0.03 mg (n = 18), ethinylestradiol 0.035 mg + desogestrel 0.15 mg (n = 14), and drospirenone 3 mg + ethinylestradiol 0.03 mg (n = 8). Additional risk factors for PE and VTE associated with COCs intake, which have a synergistic effect on the occurrence of thrombosis of various causes, were also identified. Such risk factors include morbid obesity (53%), recent surgical interventions (15%), a family history of VTE (9%), thrombophilia (8%), and diabetes mellitus (5%).
Some reports demonstrated that the risk of DVT (OR = 6.6, 95% CI 5.4–8.0) is slightly higher than that of PE (OR = 3.9, 95% CI 3.2–4.8) [57]. The development of VTE is also directly related to associated risk factors, such as increased body mass index (BMI), age, and the presence of hereditary or acquired thrombophilia [56].
Other side effects that are less life-threatening include arm deep vein thrombosis (OR = 1.9, 95% CI 1.1–3.4) [56,57,58]. Visceral venous thrombosis should also be included among the rare localizations of VTE. The risk of cerebral sinus vein thrombosis (CSVT) increases 7-fold when taking OCs [59]. Thrombosis of deep and superficial cerebral veins, as well as cerebral sinuses, should be included in CVT. There are reports on the occurrence of isolated splenic vein thrombosis (ISVT) with the history of OCs use [60]. ISVT is a rare form of venous thrombosis occurring without pancreatic disease. This condition is characterized by acute abdominal pain and chronic left-sided portal hypertension despite a lack of disease control. In this clinical case, the patient was treated with oral rivaroxaban as an anticoagulant and systemic thrombolysis using urokinase. These pathological conditions are relatively uncommon, and the risk of their development depends not only on the use of OCs but also on the presence of concomitant risk factors.
4.2. Risk of Arterial Thrombosis
In addition to venous thrombosis, COC use may increase the risk of arterial thromboses, such as ischemic stroke, by twofold to fourfold [3,61]. However, risk factors for arterial thrombosis differ from those for venous thrombosis. For example, smoking is a risk factor for myocardial infarction associated with OC use but does not significantly affect the risk of venous thrombosis in COC users [62].
The risk of acute ischemic stroke (AIS) associated with high estrogen states arising from exogenous estrogen (COCs, hormone replacement therapy), or in the hyperestrogenic prothrombotic setting of pregnancy and the post-partum period has been well documented [63]. The risk of stroke increases by 2 to 3 times in women with additional risk factors, such as smoking, hypertension, and hypercholesterolemia [63]. The combination of estrogen-progestin hormonal contraception and smoking is associated with an elevated risk of various vascular complications, including stroke and myocardial infarction. These complications are linked to pathological changes in coagulation factors, specifically an increase in the level of the fibrinolysis inhibitor PAI-1 [64].
4.3. Duration of Oral Contraceptive Use and Risk of Thrombosis
The time interval from the start of COC use and the development of VTE/PE was an average of 13.6 ± 2.7 months [65]. In 34% of women, thrombosis developed after less than 3 months of taking OCs, in 24.5% after 3–6 months, in 15.1% after 7–12 months, and in 26.4% the interval was more than 12 months.
According to van Hylckama Vlieg’s research, the risk of VTE development was highest in the first three months of OC use (odds ratio, 12.6; 95% CI, 7.1 to 22.4) [66]. After one year of COC intake, the risk of venous thrombosis had decreased to an overall estimate of a five-fold increased risk compared with non-using women.
The risk of thrombosis decreases over time, possibly because high-risk patients stop using hormonal contraceptives due to thrombosis. In particular, the risk of VTE returns to the background 8–12 weeks after the complete discontinuation of hormonal contraceptive preparations [67], but still, it remains higher than in non-users.
4.4. Composition of Oral Contraceptives and Risk of Thrombosis
The risk of thrombotic complications depends not only on the duration of drug use and associated risk factors but also on the composition of the OCs. The type of oral contraceptives evaluated in the study by AlSheef was known in 46%. In 6%, it was a combination of cyproterone acetate 2 mg/EE 0.035 mg; in 18%—gestodene 0.075 mg/EE 0.03 mg; in 14%—desogestrel 0.15 mg/EE 0.035 mg; in 8%—drospirenone 3 mg/EE 0.03 mg [54]. Reducing the dose of estrogen from 100 µg to 50 µg in COCs decreased the risk of future thrombotic events, but no evidence that lowering the dose of estrogen by less than 50 µg prevents VTE [54]. However, some data showed that when the estrogen dose was reduced to 30–20 µg, the risk of venous thrombosis was 0.8 (95% CI 0.5–1.2), while for a 50 µg estrogen dose, the risk was slightly higher at 1.9 (95% CI 1.1–3.4) [68]. At the same time, higher estrogen doses are directly linked to an increased risk of thrombosis for OCs containing desogestrel and gestodene. When the ethinylestradiol dose was lowered to 20 µg, OCs with LNG in their formulation showed no significant reduction in venous thrombotic risk complications [69].
For a long time, the increased risk of thrombosis was attributed solely to the estrogen component of oral contraceptives. However, subsequent episodes of thromboembolic events have challenged the concept that reducing the dose of estrogen in hormonal contraceptive pills eliminates the risk of VTE. Since low-dose second- and third-generation COCs contain the same constant dose of estrogen component, the differences in thrombotic risk most likely reflect a progestogen-specific effect, indicating that certain progestins may increase the risk of thrombosis [70]. Although progestogens may have estrogen-like effects, including on hemostasis, there is no data on the effect of levonorgestrel or desogestrel on the protein C pathway in the absence of an estrogen component.
Vlieg et al., in the MEGA (Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis) study, which included 1524 patients with VTE, evaluated the risk of thrombotic complications by focusing on the estrogen dose and progestin type of OCs [66]. According to the study, the risk of venous thrombosis was much higher in women taking commercially available OCs than in those who do not use such hormones (OR 5.0, 95% CI 4.2–5.8). Levonorgestrel-containing OCs use increases the risk of VTE almost fourfold (odds ratio 3.6, 95% CI 2.9–4.6), gestodene was associated with a 5.6-fold increase (95% CI 3.7 to 8.4), desogestrel—7.3-fold (5.3 to 10.0), and cyproterone acetate—6.8-fold (4.7 to 10.0) compared with non-use [66]. The most frequently used type of progesterone was levonorgestrel. To assess the risk associated with progesterone type, the study authors directly compared COCs with different types of progesterone with contraceptives containing levonorgestrel. All contraceptives contained the same dose of estrogen. The relative risk of VTE development in women taking COCs containing 30–35 mcg ethinylestradiol and gestodene (OR 1.6, 95% CI 1.0 to 2.4), desogestrel (2.0, 1.4 to 2.8), cyproterone acetate (2.0, 1.3 to 3.0), or drospirenone (1.7, 0.7 to 3.9) was similar and approximately 50–80% higher than for levonorgestrel-containing OCs. Among 3rd-generation COCs, the risk was slightly higher for desogestrel than gestodene (odds ratio 1.3, 95% CI 0.8 to 2.2). Higher doses of EE were associated with a higher risk of thrombosis. This dose–response relationship was observed for gestodene, desogestrel, and levonorgestrel. Even if most women use monophasic pills, the risk of VTE associated with triphasic OCs (levonorgestrel or gestodene with 30–40 mcg EE) has also been assessed. It has shown an increased risk like that of monophasic COCs compared with non-users [64].
Stegeman et al. demonstrated the most comprehensive information on the risk of VTE using OCs in their meta-analysis [53]. They concluded that regardless of the drug composition and duration of therapy, almost all OCs are associated with the risk of venous thrombosis [53]. There is an association between individual generations of OCs and the risk of venous thrombosis. Thus, first-generation OCs had a 3.2-fold increased risk of thrombotic complications compared with women not taking OCs (95% CI 2.0–5.1). Second-generation OCs were characterized by a 2.8-fold (95% CI 2.0–4.1) increased risk of thrombosis, while third-generation OCs resulted in a 3.8-fold (95% CI 2.7–5.4) increased risk compared with patients not using hormonal contraception. This meta-analysis also gives an example of the most dangerous drug in terms of thrombosis, which is 50LNG. At the same time, 20LNG and 20GSD contributed to venous thrombosis in a smaller percentage of cases. A dose-dependent effect was observed for gestodene, desogestrel, and LNG—the risk of thrombosis increased significantly with increasing OC dosage [68].
OCs containing cyproterone acetate, which also has an anti-ovulatory effect similar to that of progestogens, should be categorized as a distinct group of drugs. Data show that the risk of venous thrombosis in women using OCs with cyproterone acetate and/or drospirenone is approximately 50–80% higher compared to those using LNG-containing OCs [70]. However, the use of OCs with LNG is also associated with a fourfold increased risk of VTE (OR = 3.6:2, 9:4.6) [53] compared to women who do not take OCs. Consequently, the safest option regarding thrombosis risk is LNG, with the risk increasing gradually with gestodene, drospirenone, and cyproterone acetate, respectively. Notably, desogestrel is linked to the highest risk of thrombosis [67]. There is no conclusive, reliable data confirming a difference in thrombotic risk between second- and third-generation OCs [71,72,73].
Other studies also confirm that the risk of thrombotic complications depends on the type of progestogen in OCs [69,70]. According to a literature review by Deeksha Khialani et al., new kinds of OCs containing the estrogenic component E2 are safer in terms of thrombotic risk compared to those with EE. EE has been repeatedly shown to increase the risk of thrombosis in the population [65]. Consequently, new OC formulations have been developed that include estradiol valerate (E2V) and 17β-E2 as estrogen components. 17β-E2 is a natural estrogen with the fewest metabolic side effects [71]. Additionally, E2V pairs well with DNG in a four-phase dosing regimen to offer practical and safe contraception. DNG provides a strong progestational effect, suppressing ovulation and reducing menstrual bleeding. The DNG/E2V combination has fewer cardiovascular adverse effects and is also less likely to cause VTE 0.5 (95% Cl: 0.2–1.5) [71]. Also, 17β-E2 combines well with nomegestrol acetate (NOMAC), providing effective contraception and reducing the duration of menstrual bleeding [72]. NOMAC is probably associated with a lower risk of VTE than other OCs, such as those with LNG and EE in the formula.
In recent years, a new combined oral contraceptive containing estetrol (E4) and drospirenone has been introduced [73]. Estetrol is a natural fetal estrogen with selective action on estrogen receptors and has a weaker effect on hepatic synthesis of coagulation factors compared to ethinylestradiol. Early clinical data and postmarketing observations suggest that E4/drospirenone may have a more favorable hemostatic profile and potentially a lower VTE risk than EE-containing preparations with drospirenone, while still offering effective contraception [74,75]. However, these findings still require confirmation in larger real-world study cohorts.
Table 3 compares the relative venous thromboembolism (VTE) risk associated with various COC formulations by estrogen type and progestin generation, providing a visual framework for understanding how formulation differences affect hemostasis. Formulations containing estetrol/drospirenone are highlighted as emerging options with potentially lower thrombotic risk.

Table 3.
Comparative venous thromboembolism (VTE) risk among COC formulations.
4.5. Age-Dependent Risk of Thrombosis
When choosing a particular OC product, each reason for the potential risk of VTE or PE must be balanced against the benefit that a woman may derive from hormonal contraception. The patient’s age seems to play a significant role in the development of thrombotic complications. To assess the age-related risk of VTE, the MEGA study calculated the risk for OC users in different age groups. Data from 1524 women taking COCs who developed thrombosis of various localizations showed that the risk of thrombotic complications increases with age. The relative risk (95% CI) of VTE associated with OC use for women aged ≤30 years was 3.1 (2.2 to 4.6), 30–40 years—5.0 (3.8 to 6.5), and 40–50 years—5.8 (4.6 to 7.3) compared with non-users of OCs [66].
The age-related increase in VTE incidence, from 1.84 per 10,000 woman-years in women aged 15–19 to 6.59 per 10,000 woman-years in women aged 45–49, was confirmed in a Danish National cohort study [76]. The study analyzed 4213 cases of VTE occurring in 3.4 million women per year, of which 2045 cases of thrombosis were among current OC users.
Younger women are more likely to use OCs. In adolescents, OCs may be prescribed for reasons beyond contraception. For instance, they are often used to treat endometriosis and dysmenorrhea, as well as for hormone replacement therapy. However, many adolescents (about 55%) use OCs specifically for effective and safe contraception [77]. The risk of thrombotic complications in adolescents using OCs is 3–5 times higher compared to those not on hormonal therapy. According to 2011 statistics, the annual risk of venous thrombosis in healthy adolescents not taking OCs is 0.05%. The most suitable medications for adolescents are LNG, norgestrel, and norethindrone, which are associated with a lower percentage of venous thrombosis cases. In individuals with thrombophilia, a purely progestin-based contraceptive (PBC) can be used, as PBCs at standard doses generally do not negatively affect hemostasis.
4.6. Personal History of VTE and COC Use
In women with a history of venous thromboembolism (VTE), the use of COCs is contraindicated, whether the event was provoked or unprovoked [78]. Estrogen-containing contraceptives considerably increase the risk of recurrent thrombosis by promoting a hypercoagulable state through increased production of clotting factors, decreased natural anticoagulants, and reduced fibrinolytic activity. According to the World Health Organization (WHO) and CDC Medical Eligibility Criteria, COCs are classified as Category 4 (“unacceptable health risk”) in women with previous VTE who are not on anticoagulant therapy. If a woman with prior VTE is receiving long-term anticoagulation, the use of COCs may be considered Category 3 (“risks generally outweigh benefits”) and should only be prescribed under hematologic and gynecologic supervision.
The recommended contraceptive options for women with a history of VTE include non-hormonal methods (copper IUD, barrier methods) or PBC, such as levonorgestrel-releasing intrauterine devices, progestogen-only pills, or subcutaneous progestogen implants [79]. It is advisable to avoid combined hormonal contraceptives and injectable progesterone in women with a history of thromboembolic events.
4.7. Family History of VTE and COC Use
To assess the risk of hormone-related thrombosis with a positive history of VTE in at least one parent or sibling, a Swedish nationwide case–control study analyzed 2311 cases of thrombosis in women aged 15–49 and did not find any significant links between family history of VTE and COC use (OR 0.92, 0.57–1.46) [80]. A reasonable explanation for this finding is the low prevalence of COC prescription among women with a family history of VTE. So, the authors suspect that they may underestimate the importance of a family history of VTE.
However, another Swedish study revealed that the risk of VTE associated with hormone use was doubled in women with a family history of VTE as compared to women with hormones and a negative family history [81].
Data from the START registry indicated that women of reproductive age are more prone to develop VTE in various situations, especially with COC use and pregnancy. They observed that cerebral vein thrombosis and pulmonary embolism occurred more frequently in the COC user group (14.8% and 36.9%, respectively) [82]. After adjusting for age, body mass index (BMI), smoking, hemoglobin level, cancer, thrombophilia, and family history of VTE, the researchers found that women with a family history had a significantly higher risk of VTE related to COC use than women with VTE who did not use COCs or who were pregnant.
4.8. Thrombophilia and the Risk of Thrombosis
Evaluation of genetic predisposition for thrombosis may identify an additional risk factor for venous thromboembolism development in COC users.
A meta-analysis of 12 case–control and three cohort studies performed in the Netherlands in the 2016 year showed that “mild” (FVL or prothrombin mutation) and “severe” (antithrombin, protein C and S deficiency, homozygosity or double heterozygosity for FVL or prothrombin mutation) thrombophilia increased the risk of VTE almost 6-fold (RR 5.89; 95% confidence interval [CI], 4.21–8.23) and 7-fold (RR, 7.15; 95% CI, 2.93–17.45), respectively, compared with COC users without genetic thrombophilia [83]. However, the authors of the meta-analysis believe that, in the absence of other risk factors, COCs can be offered to women with “mild” thrombophilic defects in situations where other reliable alternative contraceptives are not tolerated [83].
Martinelli et al.’s study found a strong link between oral contraceptive use and venous thrombosis, especially carriers of the prothrombin G20210A mutation [84] (OR 22.1 for cerebral vein thrombosis (CVT), 4.4 for deep vein thrombosis (DVT)).
Results from a prospective UK Biobank study assessing VTE risk factors related to OC use among 244,420 female participants showed that OC use was linked to an increased risk, especially in women with genetic thrombophilia [85]. They noted that women with high-risk genetic thrombophilia face a greater risk of thrombosis during the first two years of OC use (OR 6.35; 95% confidence interval, 4.98–8.09), with a general decline in risk over continued use. The highest risk was observed among carriers of the FV Leiden mutation (OR 5.73; 95% CI, 5.31–6.17) and the prothrombin gene mutation (OR 4.93; 95% CI, 3.16–7.7). Combining these two genetic variants significantly raises the risk of VTE to up to 15 times (OR 14.8; 95% CI, 9.28–23.6) [85].
Even though the risk of DVT is higher in adulthood, Krleza et al. described a case report of a seventeen-year-old girl with a history of OC use who developed DVT [86]. After a thorough examination, several genetic thrombophilic mutations were identified in this patient, including heterozygosity for factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase (MTHFR) C677T gene mutations, as well as a homozygous 4G/4G genotype in the PAI-1 gene. Family history also revealed a few thromboembolic events. Thus, the presented clinical case confirms the multifactorial nature of the occurrence of deep vein thrombosis and allows us to conclude that there is a close relationship between the use of oral contraceptives in adolescents, a family history of thrombosis, and the development of thrombotic complications in the future.
The TREATS (Thrombosis: Risk and Economic Assessment of Thrombophilia Screening) study found that patients with FVL have a high risk of VTE while taking OCs (OR = 15.62; 95% CI 8.66–28.15) [87]. They showed that a multiplicative risk increase occurs when hereditary thrombophilia is combined with COC use for factor V Leiden or prothrombin mutation alone: approximately 4–5 times the risk; with COC use, up to a 30–35 times higher risk of VTE. A direct relationship between the development of VTE in patients taking OCs and deficiencies such as antithrombin (OR = 12.60; 95% CI 1.37–115.79), protein C (OR = 6.33; 95% CI 1.68–23.87), or protein S (OR = 4.88; 95% CI 1.39–17.10), as well as inherited increased factor VIII levels (OR = 8.80; 95% CI 4.13–18.75), was also identified.
Because of the high prevalence of FII G20210A and FV Leiden mutations in the Spanish population (6.5% and 2%, respectively) and the increased risk of VTE in COC users (3 times higher for the prothrombin mutation and 1.4 times higher for the FV mutation), the authors of another study recommend genetic screening in women from families with thrombophilia before prescribing COCs [88].
Dulicek et al., in their study of 770 patients, showed that hereditary thrombophilia, present in 44.5% of patients, is directly linked to the development of VTE in patients taking OCs [58]. In this study, the FVL mutation was the most common cause. Hereditary thrombophilia and antiphospholipid syndrome (APS) were diagnosed in nearly 45% of cases among women who develop OC-related issues and VTE. Reported COC use in APS is associated with markedly higher rates of venous and arterial thromboses [89]. COCs are absolutely contraindicated in women with definite APS (clinical + laboratory criteria), persistently positive lupus anticoagulant (LA), and multiple aPL positivity (LA + aCL + anti-β2GPI). Even asymptomatic carriers of antiphospholipid antibodies (aPL) have a higher baseline thrombosis risk compared with the general population. Safer alternatives are progestin-only pills (POP, desogestrel 75 µg Levonorgestrel IUS (LNG-IUS), copper IUD, barrier methods.
Currently, universal thrombophilia testing is not recommended when prescribing OCs. A simple activated protein C resistance test that detects the presence of the factor V Leiden mutation can provide a quick, affordable way to accurately identify and counsel women who are more prone to VTE before using OC, if a complete thrombophilic profile cannot be performed.
4.9. Smoking in COC Users
A study by Lidegaard in 1993 showed that the risk of cardiovascular diseases exceeds the risk of venous thromboembolic complications in women who smoke and use oral contraceptives [90]. Moreover, smoking and COC use have an additive and synergistic effect in increasing the risk of acute myocardial infarction and cerebral thrombosis (but not VTE), mainly with high doses of EE (50 mcg) [90].
Another extensive case–control study [91] showed that the combination of current smoking, oral contraceptive use, and FV Leiden mutation increased the risk of thrombosis by 5.0 times; for the FII G20210A mutation, this risk resulted in a 6.0-fold increase.
4.10. Obesity and Risk of Thrombosis
The evaluation of the effects of obesity and COC use on the risks of arterial and venous thrombosis identified that obese COC users (BMI) ≥30 kg/m2) had a higher risk for acute myocardial infarction [92], stroke [93], and cerebral venous thrombosis [94] compared with normal-weight nonusers. Regarding VTE, different prospective studies [95] found that obesity and COC use have a 5.0- to 8.0-fold increased risk of VTE compared with obese nonusers and a 10.0-fold increased risk compared with nonobese nonusers.
4.11. Arterial Hypertension
Estrogen-progestin drugs can increase BP by 5–7 mmHg, but persistent hypertension is very rare [96]. Prolonged OC use contributes to the occurrence of hypertension, a 1.9 (95% CI, 1.6–2.4) and 1.2 (95% CI, 1.1–1.5) times higher risk of hypertension during four-year follow-up among current and past OC users, respectively [97]. In this case, drospirenone, which eliminates the adverse effects of OCs on BP due to its anti-mineralocorticoid activity, can be recommended to patients for effective and safe contraception.
4.12. Dyslipidemia and COC Use
OCs may slightly raise triglyceride and HDL levels in the blood while lowering LDL levels. This mechanism also introduces some cardiovascular risks for patients, including the development of myocardial infarction or arterial thrombosis in other locations [98]. In women with dyslipidemia or metabolic syndrome, non-estrogen or low-estrogen contraception should be considered.
An alternative method of contraception in this case would be the use of a progestogen-only pill or the use of drospirenone [98].
4.13. Non-O Blood Type and the Risk of Thrombosis in COC Users
Multiple studies demonstrated that having a non-O blood type is considered an independent risk factor for VTE (OR, 1.98, 95% CI 1.57–2.49), (OR, 1.56, 95% CI, 1.43–1.69) [99,100,101] compared with those having the O blood group. It seems that ABO blood groups strongly affect the clearance of VWF. Due to increased VWF elimination, healthy individuals with type O have approximately 20% to 30% lower VWF levels compared to those in the non-O group [101], and according to a recent study, they have a higher susceptibility to ADAMTS13 proteolysis, which affects platelet function [102]. The addition of high-risk genetic thrombophilia to the non-O group increases this risk nearly threefold.
4.14. COVID/Post-COVID and the COC Use
Evidence that hormonal contraceptive users with COVID-19 are at heightened risk for venous or arterial thromboembolism is sparse and of low certainty [103]; no apparent increase in severe COVID outcomes in COC users, but COVID itself is a prothrombotic state, so additive risks must be considered.
4.15. Non-Steroidal Anti-Inflammatory Drugs and COC Use
The use of OCs in combination with other medications may also lead to venous thrombosis. Concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs) and OCs was positively associated with an increased risk of DVT compared with a group of women who took OCs alone (OR = 7.2, 95% confidence interval 6.0–8.5) [104]. For this reason, women who simultaneously require NSAID therapy and hormonal contraception should be given informed counseling about the occurrence of possible risk factors.
4.16. Postpartum Period and COC Use
The risk of VTE is highest immediately after delivery and gradually declines. After 6 weeks (≥42 days) postpartum, in the absence of additional risk factors such as obesity, thrombophilia, cesarean delivery, maternal age over 35 years, or smoking, COCs may be started, provided the woman is not breastfeeding. However, individual VTE risk still needs to be evaluated [105].
4.17. Post-Oncologic Prothrombotic States
Women recovering from oncologic disease frequently exhibit prolonged prothrombotic states characterized by endothelial activation, elevated inflammatory cytokines, platelet hyperreactivity, and persistent coagulation abnormalities [106]. In such settings, the use of COCs is contraindicated or strongly discouraged, as estrogens may exacerbate the residual hypercoagulability and increase the risk of VTE. Progestin-only contraceptives (e.g., desogestrel-only pill, levonorgestrel IUD, or etonogestrel implant) and non-hormonal methods are considered safer alternatives [107].
Table 4 outlines major categories of women at increased thrombotic risk during COC use, together with recommended management strategies and safer contraceptive alternatives.

Table 4.
Women at increased thrombotic risk during COC use and recommended management strategies.
Women with a medical history of endogenous risk factors for venous thrombosis are in a separate category of patients at risk of thrombotic complications. Multiple studies indicate that the risk of thrombosis is increased in women with a personal history of VTE, gross obesity, age over 35 years, intimate partner violence [108], prolonged immobilization, in vitro fertilization [109], stress [110], hereditary thrombophilia, and antiphospholipid syndrome. However, none of these factors increases this risk more than pregnancy (5–20/10,000 woman-years) [111,112,113]; however, all these risks are additive [114]. Therefore, concomitant risk factors for venous thrombosis should be considered before prescribing OCs, and the patient’s family history of VTE and PE should be carefully assessed. Still, this decision also requires an individual approach to the patient. Modifiable risk factors for venous thrombosis, such as smoking and the use of prothrombotic drugs, should also be considered. To prevent the adverse effects of hormonal contraception, it is crucial to select the proper medication and dose. In this context, women at high risk of venous thrombosis might be advised to use microdosed oral agents.
5. Conclusions
Combined oral contraceptives are not uniform regarding thrombotic risk. The highest VTE risk is consistently linked to EE-containing COCs combined with third-generation progestins (desogestrel, gestodene) and cyproterone acetate. Preparations with levonorgestrel show a comparatively lower VTE risk and remain the preferred option for women without additional thrombotic risk factors. Recently introduced combinations with natural estrogens, especially estetrol/drospirenone, seem to have a more favorable hemostatic profile, although evidence is still emerging. An individual risk assessment—considering age, BMI, smoking, thrombophilia, and prior VTE—should guide the choice of COC and the necessity for follow-up.
Author Contributions
Conceptualization, J.K. and A.M.; methodology, V.B., G.G. and M.T.; data curation, D.K., F.Y. and M.K.; software, A.T., I.H. and A.K.; validation, G.S. and G.G.; formal analysis, J.-C.G. and I.E.; writing—original draft preparation, J.K.; visualization, J.K. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manzoli, L.; De Vito, C.; Marzuillo, C.; Boccia, A.; Villari, P. Oral contraceptives and venous thromboembolism: A systematic review and meta-analysis. Drug Saf. 2012, 35, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Stadel, B.V. Oral contraceptives and cardiovascular disease (first of two parts). N. Engl. J. Med. 1981, 305, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Croft, P.; Hannaford, P.C. Risk factors for acute myocardial infarction in women: Evidence from the Royal College of General Practitioners’ oral contraception study. BMJ 1989, 298, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.V.; Dollin, J. Half a century of the oral contraceptive pill: Historical review and view to the future. Can. Fam. Physician 2012, 58, e757–e760. [Google Scholar] [PubMed] [PubMed Central]
- Rosendaal, F.R.; Helmerhorst, F.M.; Vandenbroucke, J.P. Female hormones and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 201–210. [Google Scholar] [CrossRef]
- García-Sáenz, M.; Ibarra-Salce, R.; Pozos-Varela, F.J.; Mena-Ureta, T.S.; Flores-Villagómez, S.; Santana-Mata, M.; De Los Santos-Aguilar, R.G.; Uribe-Cortés, D.; Ferreira-Hermosillo, A. Understanding Progestins: From Basics to Clinical Applicability. J. Clin. Med. 2023, 12, 3388. [Google Scholar] [CrossRef]
- Bahamondes, L.; Valeria Bahamondes, M.; Shulman, L.P. Non-contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Hum. Reprod. Update 2015, 21, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, O.; Owren, P.A. Oral Contraception and Blood Coagulability. Br. Med. J. 1963, 1, 220–221. [Google Scholar] [CrossRef]
- Meade, T.W. Oral contraceptives, clotting factors, and thrombosis. Am. J. Obs. Gynecol. 1982, 142 Pt 2, 758–761. [Google Scholar] [CrossRef]
- Dahlbäck, B. Pro- and anticoagulant properties of factor V in pathogenesis of thrombosis and bleeding disorders. Int. J. Lab. Hematol. 2016, 38 (Suppl. S1), 4–11. [Google Scholar] [CrossRef]
- Shen, L.; Dahlbäck, B. Factor V and protein S as synergistic cofactors to activated protein C in degradation of factor VIIIa. J. Biol. Chem. 1994, 269, 18735–18738. [Google Scholar] [CrossRef] [PubMed]
- Abou-Ismail, M.Y.; Citla Sridhar, D.; Nayak, L. Estrogen and thrombosis: A bench to bedside review. Thromb. Res. 2020, 192, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Bonnar, J. Coagulation effects of oral contraception. Am. J. Obs. Gynecol. 1987, 157 Pt 2, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; Rosing, J.; Bloemenkamp, K.W.; Middeldorp, S.; Helmerhorst, F.M.; Bouma, B.N.; Rosendaal, F.R. Oral contraceptives and the risk of venous thrombosis. N. Engl. J. Med. 2001, 344, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Burkman, R.T.; Bell, W.R.; Zacur, H.A.; Kimball, A.W. Oral contraceptives and antithrombin III: Variations by dosage and ABO blood group. Am. J. Obs. Gynecol. 1991, 164 Pt 1, 1453–1458; discussion 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Denora, D.; Di Rosa, M.V.; Altamura, N.; Pellicori, F.; Vinci, P.; Sisto, U.G.; Spanò, F.; Di Girolamo, F.G.; Fiotti, N.; Biolo, G. Acquired SERPINC1/antithrombin deficiency during oral contraceptive consumption: A case report. J. Med. Case Rep. 2023, 17, 323. [Google Scholar] [CrossRef] [PubMed]
- Rühl, H.; Schröder, L.; Müller, J.; Sukhitashvili, S.; Welz, J.; Kuhn, W.C.; Oldenburg, J.; Rudlowski, C.; Pötzsch, B. Impact of hormone-associated resistance to activated protein C on the thrombotic potential of oral contraceptives: A prospective observational study. PLoS ONE 2014, 9, e105007. [Google Scholar] [CrossRef]
- Koenen, R.R.; Christella, M.; Thomassen, L.G.D.; Tans, G.; Rosing, S.; Hackeng, T.M. Effect of oral contraceptives on the anticoagulant activity of protein S in plasma. Thromb. Haemost. 2005, 93, 853–859. [Google Scholar] [CrossRef]
- Kemmeren, J.M.; Algra, A.; Meijers, J.C.M.; Tans, G.; Bouma, B.N.; Curvers, J.; Rosing, J.; Grobbee, D.E. Effect of second- and third-generation oral contraceptives on the protein C system in the absence or presence of the factor VLeiden mutation: A randomized trial. Blood 2004, 103, 927–933. [Google Scholar] [CrossRef]
- Hugon-Rodin, J.; Alhenc-Gelas, M.; Hemker, H.C.; Brailly-Tabard, S.; Guiochon-Mantel, A.; Plu-Bureau, G.; Scarabin, P.-Y. Sex hormone-binding globulin and thrombin generation in women using hormonal contraception. Biomarkers 2017, 22, 81–85. [Google Scholar] [CrossRef]
- Panzer, C.; Wise, S.; Fantini, G.; Kang, D.; Munarriz, R.; Guay, A.; Goldstein, I. Original Research—Women’s Sexual Dysfunction: Impact of Oral Contraceptives on Sex Hormone-Binding Globulin and Androgen Levels: A Retrospective Study in Women with Sexual Dysfunction. J. Sex. Med. 2006, 3, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Odlind, V.; Milsom, I.; Persson, I.; Victor, A. Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills? Acta Obstet. Gynecol. Scand. 2002, 81, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Dahm, A.; van Hylckama AVlieg Bendz, B.; Rosendaal, F.; Bertina, R.M.; Sandset, P.M. Low levels of tissue factor pathway inhibitor (TFPI) increase the risk of venous thrombosis. Blood 2003, 101, 4387–4392. [Google Scholar] [CrossRef] [PubMed]
- Dama, A.; Baggio, C.; Trevisi, L.; Bolego, C.; Cignarella, A. Regulation of human endothelial cell migration by oral contraceptive estrogen receptor ligands. Eur. J. Pharmacol. 2023, 945, 175591. [Google Scholar] [CrossRef]
- Bouck, E.G.; Arvanitis, M.; Osburn, W.O.; Sang, Y.; Reventun, P.; Ahmadzia, H.K.; Smith, N.L.; Lowenstein, C.J.; Wolberg, A.S. High risk oral contraceptive hormones do not directly enhance endothelial cell procoagulant activity in vitro. PLoS ONE 2023, 18, e0284333. [Google Scholar] [CrossRef]
- Hvas, C.L.; Larsen, J.B. The Fibrinolytic System and Its Measurement: History, Current Uses and Future Directions for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 14179. [Google Scholar] [CrossRef]
- Meijers, J.C.; Middeldorp, S.; Tekelenburg, W.; Ende, A.E.V.D.; Tans, G.; Prins, M.H.; Rosing, J.; Büller, H.R.; Bouma, B.N. Increased fibrinolytic activity during use of oral contraceptives is counteracted by an enhanced factor XI-independent down regulation of fibrinolysis: A randomized cross-over study of two low-dose oral contraceptives. Thromb. Haemost. 2000, 84, 9–14. [Google Scholar]
- Tian, J.; Adams, M.J.; Tay, J.W.T.; James, I.; Powell, S.; Hughes, Q.W.; Gilmore, G.; Baker, R.I.; Tiao, J.Y.-H. Estradiol-Responsive miR-365a-3p Interacts with Tissue Factor 3′UTR to Modulate Tissue Factor-Initiated Thrombin Generation. Thromb. Haemost. 2021, 121, 1483–1496. [Google Scholar] [CrossRef]
- Loscalzo, J.; Welch, G. Nitric oxide and its role in the cardiovascular system. Prog. Cardiovasc. Dis. 1995, 38, 87–104. [Google Scholar] [CrossRef]
- Nakano, Y.; Oshima, T.; Matsuura, H.; Kajiyama, G.; Kambe, M. Effect of 17beta-estradiol on inhibition of platelet aggregation in vitro is mediated by an increase in NO synthesis. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 961–967. [Google Scholar] [CrossRef]
- Olatunji, L.A.; Olaniyi, K.S.; Usman, T.O.; Abolarinwa, B.A.; Achile, C.J.; Kim, I.K. Combined oral contraceptive and nitric oxide synthesis inhibition synergistically causes cardiac hypertrophy and exacerbates insulin resistance in female rats. Environ. Toxicol. Pharmacol. 2017, 52, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef] [PubMed]
- Fallah, S.; Nouroozi, V.; Seifi, M.; Samadikuchaksaraei, A.; Aghdashi, E.M. Influence of Oral Contraceptive Pills on Homocysteine and Nitric Oxide Levels: As Risk Factors for Cardiovascular Disease. J. Clin. Lab. Anal. 2012, 26, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Brozek, J.; Szczeklik, A. Homocysteine and thrombosis: From basic science to clinical evidence. Thromb. Haemost. 2005, 94, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Merki-Feld, G.S.; Imthurn, B.; Keller, P.J. Effects of two oral contraceptives on plasma levels of nitric oxide, homocysteine, and lipid metabolism. Metabolism 2002, 51, 1216–1221. [Google Scholar] [CrossRef]
- Momeni, Z.; Dehghani, A.; Fallahzadeh, H.; Koohgardi, M.; Dafei, M.; Hekmatimoghaddam, S.H.; Mohammadi, M. The impacts of pill contraceptive low-dose on plasma levels of nitric oxide, homocysteine, and lipid profiles in the exposed vs. non exposed women: As the risk factor for cardiovascular diseases. Contracept. Reprod. Med. 2020, 5, 7. [Google Scholar] [CrossRef]
- Fox, S.W.; Chambers, T.J. The effect of oestrogen on megakaryocyte differentiation and platelet counts in vivo. Int. J. Cardiol. 2006, 109, 359–366. [Google Scholar] [CrossRef]
- Saleh, A.A.; Ginsburg, K.A.; Duchon, T.A.; Dorey, L.G.; Hirata, J.; Alshameeri, R.S.; Dombrowski, M.P.; Mammen, E.F. Hormonal contraception and platelet function. Thromb. Res. 1995, 78, 363–367. [Google Scholar] [CrossRef]
- Bulur, S.; Albayrak, M.; Bulur, S.; Keskin, F.; Köse, S.A.; Aslantaş, Y.; Türker, Y.; Ozhan, H. Effect of combined oral contraceptive use on platelet volume in women at reproductive age. Clin. Exp. Obs. Gynecol. 2012, 39, 314–316. [Google Scholar]
- Beltran, D.A.; Crespo, A.B.; Valle, S.G. Effect of Four Hormonal Contraceptives on Platelet Count and Coagulation Parameters Under Real Conditions of Use After 6 Months of Treatment. J. Food Sci. Nutr. 2020, 6, 066. [Google Scholar] [CrossRef]
- McGregor, L.; Toor, B.; McGregor, J.L.; Renaud, S.; Clemetson, K.J. Effects of oral contraceptives, or lanosterol, on ADP-induced aggregation and binding of 125I-fibrinogen to rat platelets. Thromb. Res. 1984, 33, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Ciavatti, M.; Davenas, E.; Blache, D.; Monnier, M.A.; Renaud, S. Biosynthesis of platelet lipids in relation to aggregation in women using oral contraceptives. Contraception 1982, 25, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Guo, H.; Zhang, Y.; Qiao, R. Procoagulant platelets: Generation, characteristics, and therapeutic target. J. Clin. Lab. Anal. 2021, 35, e23750. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L.; McKee, P.A. Estrogen stimulates von Willebrand factor production by cultured endothelial cells. Blood 1984, 63, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Calippe, B.; Douin-Echinard, V.; Delpy, L.; Laffargue, M.; Lélu, K.; Krust, A.; Pipy, B.; Bayard, F.; Arnal, J.-F.; Guéry, J.-C.; et al. 17β-Estradiol Promotes TLR4-Triggered Proinflammatory Mediator Production through Direct Estrogen Receptor α Signaling in Macrophages In Vivo. J. Immunol. 2010, 185, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Danckwardt, S.; Trégouët, D.A.; Castoldi, E. Post-transcriptional control of haemostatic genes: Mechanisms and emerging therapeutic concepts in thrombo-inflammatory disorders. Cardiovasc. Res. 2023, 119, 1624–1640, Erratum in Cardiovasc Res. 2023, 119, 2015. https://doi.org/10.1093/cvr/cvad090. [Google Scholar] [CrossRef]
- Calippe, B.; Douin-Echinard, V.; Laffargue, M.; Laurell, H.; Rana-Poussine, V.; Pipy, B.; Guéry, J.-C.; Bayard, F.; Arnal, J.-F.; Gourdy, P. Chronic Estradiol Administration In Vivo Promotes the Proinflammatory Response of Macrophages to TLR4 Activation: Involvement of the Phosphatidylinositol 3-Kinase Pathway. J. Immunol. 2008, 180, 7980–7988. [Google Scholar] [CrossRef]
- Mengelkoch, S.; Gassen, J.; Slavich, G.M.; Hill, S.E. Hormonal contraceptive use is associated with differences in women’s inflammatory and psychological reactivity to an acute social stressor. Brain Behav. Immun. 2024, 115, 747–757. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Wilchesky, M.; Mumford, S.L.; Whitcomb, B.W.; Browne, R.W.; Wactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: The BioCycle Study. Am. J. Epidemiol. 2012, 175, 423–431. [Google Scholar] [CrossRef]
- Cauci, S.; Xodo, S.; Buligan, C.; Colaninno, C.; Barbina, M.; Barbina, G.; Francescato, M.P. Oxidative Stress Is Increased in Combined Oral Contraceptives Users and Is Positively Associated with High-Sensitivity C-Reactive Protein. Molecules 2021, 26, 1070. [Google Scholar] [CrossRef]
- Delplanque, B.; Beaumont, V.; Lemort, N.; Beaumont, J.L. Lp(a) levels and antiestrogen antibodies in women with and without thrombosis in the course of oral contraception. Atherosclerosis 1993, 100, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Kronenberg, F. Lipoprotein(A). JAMA 2025, 333, 1918–1919. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, B.H.; De Bastos, M.; Rosendaal, F.R.; Vlieg, A.v.H.; Helmerhorst, F.M.; Stiner, T.; Dekkers, O.M. Different combined oral contraceptives and the risk of venous thrombosis: Systematic review and network meta-analysis. BMJ 2013, 347, f5298. [Google Scholar] [CrossRef] [PubMed]
- AlSheef, M.; Abuzied, Y.; Alzahrani, G.R.; AlAraj, N.; AlAqeel, N.; Aljishi, H.; Alomar, M.J.; Zaidi, A.R.Z.; Alarfaj, O.M. Combined Oral Contraceptives and Vascular Thrombosis: A Single-Center Experience. Cureus 2022, 14, e25865. [Google Scholar] [CrossRef] [PubMed]
- Barcellona, D.; Grandone, E.; Marongiu, F. Hormones and thrombosis: The dark side of the moon: Hormonal therapy and thrombosis. Blood Transfus. 2023, 22, 46–54. [Google Scholar]
- Parkin, L.; Skegg, D.C.; Wilson, M.; Herbison, G.P.; Paul, C. Oral contraceptives and fatal pulmonary embolism. Lancet 2000, 355, 2133–2134. [Google Scholar] [CrossRef]
- Cohen, A.T.; Agnelli, G.; Anderson, F.A.; Arcelus, J.; Bergqvist, D.; Brecht, J.G.; Greer, I.A.; Heit, J.A.; Hutchinson, J.L.; Kakkar, A.K.; et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb. Haemost. 2007, 98, 756–764. [Google Scholar]
- Dulicek, P.; Ivanova, E.; Kostal, M.; Sadilek, P.; Beranek, M.; Zak, P.; Hirmerova, J. Analysis of Risk Factors of Stroke and Venous Thromboembolism in Females with Oral Contraceptives Use. Clin. Appl. Thromb. 2018, 24, 797–802. [Google Scholar] [CrossRef]
- Amoozegar, F.; Ronksley, P.E.; Sauve, R.; Menon, B.K. Hormonal Contraceptives and Cerebral Venous Thrombosis Risk: A Systematic Review and Meta-Analysis. Front. Neurol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Li, Q.; Wang, R.; Qi, X. Systemic Thrombolysis for Isolated Splenic Vein Thrombosis Secondary to Oral Contraceptives: A Case Report. Int. J. Women’s Health 2024, 16, 811–818. [Google Scholar] [CrossRef]
- Lidegaard, Ø.; Løkkegaard, E.; Jensen, A.; Skovlund, C.W.; Keiding, N. Thrombotic stroke and myocardial infarction with hormonal contraception. N. Engl. J. Med. 2012, 366, 2257–2266. [Google Scholar] [CrossRef]
- Fruzzetti, F.; Ricci, C.; Fioretti, P. Haemostasis profile in smoking and nonsmoking women taking low-dose oral contraceptives. Contraception 1994, 49, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Roach, R.E.J.; Helmerhorst, F.M.; Lijfering, W.M.; Stijnen, T.; Algra, A.; Dekkers, O.M. Combined oral contraceptives: The risk of myocardial infarction and ischemic stroke. Cochrane Database Syst. Rev. 2018, 2015, CD011054. [Google Scholar] [CrossRef]
- Agarwal, T.; Mereuta, O.M.; Ghozy, S.; Larco, J.L.A.; Bilgin, C.; Kadirvel, R.; Brinjikji, W.; Kallmes, D.F. High thrombin-activatable fibrinolysis inhibitor expression in thrombi from stroke patients in elevated estrogen states. BMC Neurol. 2024, 24, 90. [Google Scholar] [CrossRef] [PubMed]
- Khialani, D.; Rosendaal, F.; Vlieg, A.V.H. Hormonal Contraceptives and the Risk of Venous Thrombosis. Semin. Thromb. Hemost. 2020, 46, 865–871. [Google Scholar] [CrossRef]
- van Hylckama Vlieg, A.; Helmerhorst, F.M.; Vandenbroucke, J.P.; Doggen, C.J.; Rosendaal, F.R. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: Results of the MEGA case-control study. BMJ 2009, 339, b2921. [Google Scholar] [CrossRef]
- Dinger, J.; Do Minh, T.; Heinemann, K. Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception 2016, 94, 328–339. [Google Scholar] [CrossRef]
- Gaussem, P.; Alhenc-Gelas, M.; Thomas, J.-L.; Bachelot-Loza, C.; Remones, V.; Ali, F.D.; Aiach, M.; Scarabin, P.-Y.; Gaussem, P. Haemostatic effects of a new combined oral contraceptive, nomegestrol acetate/17β-estradiol, compared with those of levonorgestrel/ethinyl estradiol. A double-blind, randomised study. Thromb. Haemost. 2011, 105, 560–567. [Google Scholar]
- Wiegratz, I.; Kuhl, H. Metabolic and clinical effects of progestogens. Eur. J. Contracept. Reprod. Health Care 2006, 11, 153–161. [Google Scholar] [CrossRef]
- Schindler, A.E. Differential effects of progestins on hemostasis. Maturitas 2003, 46, 31–37. [Google Scholar] [CrossRef]
- Kiley, J. Estradiol valerate and dienogest: A new approach to oral contraception. Int. J. Women’s Health 2011, 3, 281. [Google Scholar] [CrossRef] [PubMed]
- Burke, A. Nomegestrol acetate-17b-estradiol for oral contraception. Patient Prefer. Adherence 2013, 7, 607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gemzell-Danielsson, K.; Cagnacci, A.; Chabbert-Buffet, N.; Douxfils, J.; Foidart, J.M.; Kubba, A.; Lasa, L.I.L.; Mansour, D.; Neulen, J.; Neves, J.; et al. A novel estetrol-containing combined oral contraceptive: European expert panel review. Eur. J. Contracept. Reprod. Health Care 2022, 27, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Didembourg, M.; Locquet, M.; Raskin, L.; Tchimchoua, B.T.; Dogné, J.M.; Beaudart, C.; Douxfils, J. Lower reporting of venous thromboembolisms events with natural estrogen-based combined oral contraceptives compared to ethinylestradiol-containing pills: A disproportionality analysis of the Eudravigilance database. Contraception 2025, 142, 110727. [Google Scholar] [CrossRef] [PubMed]
- Douxfils, J.; Raskin, L.; Didembourg, M.; Donis, N.; Dogné, J.M.; Morimont, L.; Beaudart, C. Are natural estrogens used in contraception at lower risk of venous thromboembolism than synthetic ones? A systematic literature review and meta-analysis. Front. Endocrinol. 2024, 15, 1428597. [Google Scholar] [CrossRef]
- Lidegaard, Ø.; Løkkegaard, E.; Svendsen, A.L.; Agger, C. Hormonal contraception and risk of venous thromboembolism: National follow-up study. BMJ 2009, 339, b2890. [Google Scholar] [CrossRef]
- Rott, H. Hormonal contraception in thrombophilic adolescents: Risk of thrombosis and recommendations. Hämostaseologie 2012, 32, 15–21. [Google Scholar] [CrossRef]
- Stam-Slob, M.C.; Lambalk, C.B.; van de Ree, M.A. Contraceptive and hormonal treatment options for women with history of venous thromboembolism. BMJ 2015, 351, h4847. [Google Scholar] [CrossRef]
- Klok, F.A.; Barco, S. Optimal management of hormonal contraceptives after an episode of venous thromboembolism. Thromb. Res. 2019, 181, S1–S5. [Google Scholar] [CrossRef]
- Zöller, B.; Ohlsson, H.; Sundquist, J.; Sundquist, K. Family history of venous thromboembolism is a risk factor for venous thromboembolism in combined oral contraceptive users: A nationwide case-control study. Thromb. J. 2015, 13, 34. [Google Scholar] [CrossRef]
- Sonnevi, K.; Bergendal, A.; Adami, J.; Lärfars, G.; Kieler, H. Self-reported family history in estimating the risk of hormone, surgery and cast related VTE in women. Thromb. Res. 2013, 132, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Grandone, E.; Antonucci, E.; Colaizzo, D.; De Laurenzo, A.; Cosmi, B.; Cini, M.; Legnani, C.; Testa, S.; Margaglione, M.; Palareti, G. Venous Thromboembolism in Women of Childbearing Age: Insights from the START Registry. Thromb. Haemost. 2023, 123, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- van Vlijmen, E.F.; Wiewel-Verschueren, S.; Monster, T.B.; Meijer, K. Combined oral contraceptives, thrombophilia and the risk of venous thromboembolism: A systematic review and meta-analysis. J. Thromb. Haemost. 2016, 14, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Sacchi, E.; Landi, G.; Taioli, E.; Duca, F.; Mannucci, P.M. High risk of cerebral-vein thrombosis in carriers of a prothrombin-gene mutation and in users of oral contraceptives. N. Engl. J. Med. 1998, 338, 1793–1797. [Google Scholar] [CrossRef]
- Lo Faro, V.; Johansson, T.; Johansson, Å. The risk of venous thromboembolism in oral contraceptive users: The role of genetic factors-a prospective cohort study of 240,000 women in the UK Biobank. Am. J. Obs. Gynecol. 2024, 230, 360.e1–360.e13. [Google Scholar] [CrossRef] [PubMed]
- Lenicek Krleza, J.; Jakovljevic, G.; Bronic, A.; Coen Herak, D.; Bonevski, A.; Stepan-Giljevic, J.; Roic, G. Contraception-related deep venous thrombosis and pulmonary embolism in a 17-Year-old girl heterozygous for factor V leiden, prothrombin G20210A mutation, MTHFR C677T and homozygous for PAI-1 mutation: Report of a family with multiple genetic risk factors and review of the literature. Pathophysiol. Haemost. Thromb. 2010, 37, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, O.; Robertson, L.; Langhorne, P.; Twaddle, S.; Lowe, G.D.; Clark, P.; Greaves, M.; Walker, I.D.; Brenkel, I.; Regan, L.; et al. Oral contraceptives, hormone replacement therapy, thrombophilias and risk of venous thromboembolism: A systematic review. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) Study. Thromb. Haemost. 2005, 94, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, A.; Mateo, J.; Oliver, A.; Menéndez, B.; Souto, J.C.; Borrell, M.; Soria, J.M.; Tirado, I.; Fontcuberta, J. Risk of thrombosis associated with oral contraceptives of women from 97 families with inherited thrombophilia: High risk of thrombosis in carriers of the G20210A mutation of the prothrombin gene. Haematologica 2001, 86, 965–971. [Google Scholar]
- Andreoli, L.; Bertsias, G.K.; Agmon-Levin, N.; Brown, S.; Cervera, R.; Costedoat-Chalumeau, N.; Doria, A.; Fischer-Betz, R.; Forger, F.; Moraes-Fontes, M.F.; et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann. Rheum. Dis. 2017, 76, 476–485. [Google Scholar] [CrossRef]
- Lidegaard, O. Smoking and use of oral contraceptives: Impact on thrombotic diseases. Am. J. Obs. Gynecol. 1999, 180 Pt 2, S357–S363. [Google Scholar] [CrossRef]
- Pomp, E.R.; Rosendaal, F.R.; Doggen, C.J. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. Am. J. Hematol. 2008, 83, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Su, X.; Li, G.; Chen, J.; Tang, B.; Yang, Y. The incidence of acute myocardial infarction in relation to overweight and obesity: A meta-analysis. Arch. Med. Sci. 2014, 10, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, I. Obesity may increase risk of rare type of stroke in women taking oral contraceptives. BMJ 2016, 352, i1554. [Google Scholar] [CrossRef]
- Zuurbier, S.M.; Arnold, M.; Middeldorp, S.; Broeg-Morvay, A.; Silvis, S.M.; Heldner, M.R.; Meisterernst, J.; Nemeth, B.; Meulendijks, E.R.; Stam, J.; et al. Risk of Cerebral Venous Thrombosis in Obese Women. JAMA Neurol. 2016, 73, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, A.L.; Lawrenson, R.A.; Simpson, E.L.; Williams, T.J.; MacRae, K.D.; Farmer, R.D. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur. J. Contracept. Reprod. Health Care 2000, 5, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.A.; Blyler, C.A.; Bello, N.A. Oral Contraceptive Pills and Hypertension: A Review of Current Evidence and Recommendations. Hypertension 2023, 80, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.M.; Mohllajee, A.P.; Martins, S.L.; Peterson, H.B. Combined oral contraceptive use among women with hypertension: A systematic review. Contraception 2006, 73, 179–188. [Google Scholar] [CrossRef]
- Dragoman, M.; Curtis, K.M.; Gaffield, M.E. Combined hormonal contraceptive use among women with known dyslipidemias: A systematic review of critical safety outcomes. Contraception 2016, 94, 280–287. [Google Scholar] [CrossRef]
- Gallinaro, L.; Cattini, M.G.; Sztukowska, M.; Padrini, R.; Sartorello, F.; Pontara, E.; Bertomoro, A.; Daidone, V.; Pagnan, A.; Casonato, A. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood 2008, 111, 3540–3545. [Google Scholar] [CrossRef]
- Groot, H.E.; Villegas Sierra, L.E.; Said, M.A.; Lipsic, E.; Karper, J.C.; van der Harst, P. Genetically Determined ABO Blood Group and its Associations with Health and Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 830–838. [Google Scholar] [CrossRef]
- Franchini, M.; Makris, M. Non-O blood group: An important genetic risk factor for venous thromboembolism. Blood Transfus. 2013, 11, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.E.; O’Sullivan, J.M.; O’Donnell, J.S. The relationship between ABO blood group, von Willebrand factor, and primary hemostasis. Blood 2020, 136, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.A.; Edelman, A.; Paynter, R.; Henderson, J.T. Risk of thromboembolism in patients with COVID-19 who are using hormonal contraception. Cochrane Database Syst. Rev. 2023, 1, CD014908, Correction in Cochrane Database Syst. Rev. 2023, 5, CD014908. https://doi.org/10.1002/14651858.CD014908.pub3. [Google Scholar] [CrossRef] [PubMed]
- Meaidi, A.; Mascolo, A.; Sessa, M.; Toft-Petersen, A.P.; Skals, R.; Gerds, T.A.; Torp-Pedersen, C. Venous thromboembolism with use of hormonal contraception and non-steroidal anti-inflammatory drugs: Nationwide cohort study. BMJ 2023, 382, e074450. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Appendix D: Classifications for Combined Hormonal Contraceptives. In U.S. Medical Eligibility Criteria for Contraceptive Use; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2024. [Google Scholar]
- Leiva, O.; Newcomb, R.; Connors, J.M.; Al-Samkari, H. Cancer and thrombosis: New insights to an old problem. J. Med. Vasc. 2020, 45, 6S8–6S16. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Schwarz, E.B. Society of Family Planning. Cancer and contraception. Release Date May 2012. SFP Guideline #20121. Contraception 2012, 86, 191–198. [Google Scholar] [CrossRef]
- Chéa, M.; Bourguignon, C.; Bouvier, S.; Nouvellon, E.; Laurent, J.; Perez-Martin, A.; Mousty, E.; Ripart, S.; Nikolaeva, M.G.; Khizroeva, J.; et al. Intimate partner violence as a risk factor for venous thromboembolism in women on combined oral contraceptives: An international matched case-control study. Eur. J. Intern. Med. 2024, 122, 47–53. [Google Scholar] [CrossRef]
- Grandone, E.; Bitsadze, V.; Khizroeva, J.; Chinni, E.; Mastroianno, M.; Nappi, L.; Tretyakova, M.; Makatsariya, N.; Grigoreva, K.; Gashimova, N.; et al. Risk of Thrombosis in Women Undergoing In Vitro Fertilization: A Narrative Review. J. Clin. Med. 2025, 14, 1053. [Google Scholar] [CrossRef]
- Bitsadze, V.; Nikolaeva, M.G.; Mousty, È.; Khizroeva, J.; Laurent, J.; Ripart, S.; Kudryavtseva, E.; Collen, L.L.; Shatilina, A.; Allal, S.; et al. Venous Thromboembolism at Low Risk of Recurrence in Young Women: Stress and Violence Associated with Recurrence. An International Case-Control Study. Thromb. Haemost. 2025, 125, 643–656. [Google Scholar] [CrossRef]
- Brenner, B.; Grandone, E.; Makatsariya, A.; Khizroeva, J.; Bitsadze, V.; Tretyakova, M. Approach to the Evaluation and Treatment of Venous Thromboembolism in Pregnancy. Semin. Reprod. Med. 2021, 39, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Gris, J.C.; Bourguignon, C.; Bouvier, S.; Nouvellon, E.; Laurent, J.; Perez-Martin, A.; Mousty, E.; Nikolaeva, M.; Khizroeva, J.; Bitsadze, V.; et al. Pregnancy after Combined Oral Contraceptive-Associated Venous Thromboembolism: An International Retrospective Study of Outcomes. Thromb. Haemost. 2022, 122, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Bitsadze, V.; Khizroeva, J.; Alexander, M.; Elalamy, I. Venous thrombosis risk factors in pregnant women. J. Perinat. Med. 2022, 50, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Makatsariya, A.; Khizroeva, J.; Bitsadze, V.; Kapanadze, D. The Basic Principles of Pathophysiology of Venous Thrombosis. Int. J. Mol. Sci. 2024, 25, 11447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).