Abstract
Binge eating disorder (BED) is a prevalent eating disorder lacking adequate pharmacological interventions. This review examines the therapeutic potential of glucagon-like peptide-1 receptor agonists (GLP-1RAs), medications approved for type 2 diabetes and obesity now being investigated for eating disorders through their modulation of metabolic and reward pathways. A narrative review was conducted using PubMed/MEDLINE, through May 2025, to examine GLP-1RA effects on BED, including preclinical and clinical studies, mechanistic investigations, and relevant reviews. GLP-1 receptors (GLP-1Rs) are expressed in hypothalamic nuclei, regulating energy homeostasis and mesolimbic circuits controlling food reward. Preclinical studies demonstrate that GLP-1RAs reduce food-seeking behavior, suppress dopamine signaling in reward circuits, and modulate neural transmission in key brain regions. These effects extend beyond appetite suppression to directly modify reward processing underlying compulsive eating. Emerging clinical evidence with semaglutide and liraglutide report reductions in binge eating episodes, decreased food cravings, and improved symptom scores. However, current studies remain small-scale with methodological limitations, and translating findings from animal models to human eating disorder complexity presents significant challenges. This review integrates preclinical and clinical evidence demonstrating that GLP-1RAs modulate both metabolic and reward pathways. By elucidating the underlying neurobiological mechanisms, GLP-1RAs may offer advantages over current symptom-focused therapies for BED.
Keywords:
GLP-1; binge eating disorder; reward system; dopamine; eating disorders; semaglutide; liraglutide 1. Introduction
Feeding and eating disorders (FEDs) are a growing health burden that significantly impairs both mental and physical health [1]. Among the deadliest of all mental illnesses, they are attributable to severe medical complications and suicide [2,3,4]. As defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and the International Classification of Diseases (ICD-11), FEDs are characterized by persistent disturbances in eating-related behavior that compromise biopsychosocial functioning [5].
Multiple factors contribute to these disorders, including genetic, psychological, social, and biological influences [6]. The most prevalent FEDs are anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED) [7,8].
BED is the most prevalent eating disorder, with lifetime prevalence estimates of approximately 2.8% in women and 1.0% in men, with systematic reviews reporting rates reaching up to 6% [3,9,10]. These estimates exceed those for anorexia nervosa (1.4% in women, 0.2% in men) and bulimia nervosa (1.9% in women, 0.6% in men) [3,9], establishing BED as the most common eating disorder among adults [11]. The global lifetime prevalence of FEDs has increased notably over the past two decades, particularly affecting women [3]. Moreover, BED is now recognized to occur across the lifespan rather than being limited to youth populations, with binge eating rates of 3.5–12% in women aged 50 and older, and up to 26% experiencing weekly episodes in some populations [12,13]. BED is characterized by recurrent episodes of consuming large quantities of food in a short period, accompanied by feelings of loss of control [14,15,16]. While BED is the formal clinical diagnosis, the core behavior of binge eating (BE) can occur across multiple eating disorder presentations. Beyond its high prevalence, BED presents urgent clinical needs. The disorder is associated with significant medical and psychiatric comorbidity, including high rates of anxiety, depression, and obsessive–compulsive disorder, as well as medical complications affecting multiple organ systems from cardiovascular damage and neurological impairment to bone density loss [4,17,18].
Current first-line treatment relies on psycho-behavioral therapy, such as cognitive behavioral therapy (CBT) and interpersonal therapy (IPT) [19,20,21]. However, these interventions are frequently associated with high relapse rates and limited long-term outcomes [22,23]. Furthermore, pharmacological interventions are insufficient, and specialized treatment for BED remains difficult to access with inconsistent efficacy and significant adverse effects [17,21]. These limitations show unmet clinical needs, leading to the investigation of innovative therapeutic strategies that target the underlying neurobiological mechanisms.
The pathophysiology of BED demonstrates notable parallels with substance use disorders, suggesting shared dysregulated brain reward circuits [15]. This mechanistic similarity, combined with BED’s higher prevalence compared to AN and BN, significant comorbidity burden, and limited approved pharmacological treatments, creates urgent clinical needs [3,9,17,18]. Furthermore, BED’s neurobiological profile of dysregulated reward-driven overconsumption aligns with GLP-1RA effects on both appetite regulation and reward circuits [24]. Unlike AN’s complex food avoidance behaviors and body image pathology, BED’s core pathophysiology of reward-driven overconsumption makes it a particularly suitable target for GLP-1RA intervention and novel therapies targeting reward pathways [25]. This has led to the investigation of innovative therapeutic strategies targeting underlying neurobiological mechanisms. The hormone glucagon-like peptide-1 (GLP-1), a key mediator in the gut–brain axis, has emerged as a promising therapeutic target due to its dual role in regulating both energy balance and reward pathways [26,27]. GLP-1 receptors (GLP-1Rs) are uniquely positioned in both hypothalamic nuclei regulating energy balance and reward-related dopaminergic areas, including the nucleus accumbens (NAc) and ventral tegmental area (VTA) [28,29,30].
GLP-1 receptor agonists (GLP-1RAs), including semaglutide and liraglutide, are already approved for treating type 2 diabetes and obesity [31,32,33,34,35,36,37,38,39]. They are known to modulate brain reward pathways and reduce food cravings, which are core features of eating disorders [40,41,42]. Growing evidence supports their potential therapeutic application in BED, with recent attention focused on their ability to reduce binge eating episodes [43].
This narrative review will explore the compelling rationale for investigating the GLP-1 system in BED, detailing its biological basis and analyzing the promising therapeutic evidence from preclinical and clinical studies. Through narrative synthesis of the existing literature, this work aims to provide a comprehensive overview of the current therapeutic implications and potential of GLP-1RAs to combat BED and identify key areas for future research.
A comprehensive literature search was conducted using PubMed/MEDLINE from inception through May 2025 to identify studies examining GLP-1RA effects on reward-related eating behavior and BED. The search strategy employed combinations of GLP-1-related terms (‘GLP-1’, ‘glucagon-like peptide-1’, ‘GLP-1 receptor agonist’, ‘semaglutide’, ‘liraglutide’, ‘exendin-4’, ‘dulaglutide’) with eating behavior and neurobiological terms (‘binge eating disorder’, ‘BED’, ‘eating disorder’, ‘feeding behavior’, ‘reward system’, ‘dopamine’, ‘mesolimbic’, ‘food intake’). We included preclinical studies examining GLP-1RA effects on feeding behavior and reward circuits, clinical trials and pilot studies evaluating GLP-1RAs in eating disorders, and mechanistic studies on GLP-1 receptor signaling in reward-related brain regions. The search was limited to English-language publications, with additional relevant articles identified through citation searching. Data were synthesized using a narrative approach, organizing findings thematically according to neurobiological mechanisms, preclinical evidence, and clinical studies. This integrative synthesis emphasizes mechanistic understanding of how GLP-1RAs modulate reward circuits and eating behavior in BED, providing a comprehensive framework for evaluating their therapeutic potential. This narrative review synthesizes the current evidence on GLP-1RA mechanisms and therapeutic potential in BED through comprehensive analysis of the neurobiological basis and clinical findings.
2. GLP-1 Therapeutic Opportunity in Eating Disorders
The pathophysiology of FEDs is multifactorial, involving complex interactions among genetic, psychological, and environmental factors that contribute to dysregulated eating and loss of control [44,45]. Recent research has revealed additional factors, such as gut microbiome dysbiosis, correlating with dysregulated eating behaviors [46]. A core feature of FEDs involves fundamental disruption of feeding control from imbalances between two primary, interconnected systems, namely the homeostatic pathway, which regulates energy balance, and the hedonic pathway, which controls motivated food consumption [47,48]. Eating disorders are characterized by dysfunction in the integration of homeostatic and hedonic systems, where hedonic drives dominate physiological hunger and satiety signals, contributing to loss of control over eating [1]. BED appears to be common among eating disorders in older populations, with a notable proportion being late-onset cases [49,50], suggesting that these dysfunctional patterns can emerge across the lifespan and may involve distinct etiological pathways.
The homeostatic system, centered in the hypothalamus, regulates energy balance by integrating peripheral signals through key nuclei, including the arcuate nucleus (ARC), paraventricular nucleus (PVN), and lateral hypothalamus (LH) [51,52,53,54]. The ARC contains both appetite-stimulating (orexigenic) neurons that co-express neuropeptide Y (NPY) and agouti-related peptide (AgRP), and appetite-suppressing (anorexigenic) proopiomelanocortin (POMC) neurons [55,56]. These hypothalamic circuits serve as the central hub for the gut–brain axis, integrating both short- and long-term signals [57,58,59]. Long-term energy status is communicated through leptin, secreted by adipose tissue in proportion to fat stores to suppress appetite, and insulin, secreted by pancreatic beta cells to regulate glucose metabolism and signal metabolic state [60]. Meanwhile, short-term satiety is regulated by gastrointestinal hormones released in response to food intake. These include the satiety-promoting L-cell hormones GLP-1 and peptide YY (PYY), cholecystokinin (CCK) released by I-cells to stimulate digestive enzymes and promote satiety, and ghrelin, produced by gastric cells to stimulate hunger and initiate food intake [61,62,63]. These peripheral signals reach the brain through both humoral pathways via circumventricular organs (CVO), which lack a complete blood–brain barrier and allow peripheral hormones direct access to the central nervous system circuit, and direct neural communication via the vagus nerve [64,65,66,67]. Specifically, gut-secreted GLP-1 modulates vagal neural pathways in with signals transmitted to the nucleus tractus solitarius (NTS) in the brainstem and relayed to hypothalamic nuclei, while centrally secreted GLP-1 from the NTS projects widely to create a comprehensive feedback loop that aligns food intake with energy demands [68,69]. In contrast, the hedonic system is a reward-based system driving the compulsive consumption of highly palatable foods, often independent of energy needs through the mesolimbic dopaminergic reward circuit [47,70,71]. This pathway comprises dopaminergic neurons projecting from the VTA of the midbrain to the NAc in the ventral striatum, where dopamine signals food reward value and drives motivation [72]. The reward circuitry interacts closely with the mesocortical dopamine pathway, which connects the VTA to key cortical regions, including the orbitofrontal cortex (OFC), ventromedial prefrontal cortex (vmPFC), medial prefrontal cortex (mPFC), and anterior cingulate cortex (ACC) [73,74]. These regions are essential for higher-order cognitive functions, such as inhibitory control, decision-making, and assessing long-term consequences, all of which are frequently impaired in eating disorders [74,75].
These homeostatic and hedonic systems are interconnected through direct hypothalamic-mesolimbic connections. During fasting states, decreased glucose levels activate glutamate LH neurons that project to and excite VTA dopaminergic neurons, driving food-seeking behavior [76,77]. Conversely, reward signals from highly palatable foods can activate mesolimbic circuits that override normal hypothalamic satiety cues, creating persistent motivation to continue eating despite physical fullness [78]. The NAc sends feedback signals to both the LH and VTA through inhibitory connections, creating bidirectional control that normally stops eating when sufficient reward is received [79,80,81]. This integrated network allows metabolic needs and reward motivation to influence each other [82]. However, in eating disorders, this balance becomes dysregulated, where hedonic drives consistently override physiological hunger and satiety signals [83,84]. Dysregulation of these dopaminergic, glutamatergic, and GABAergic systems plays a significant role in eating disorder pathophysiology. The reward circuit operates through wanting and liking components [85,86]. Dopamine signaling within the mesolimbic pathway mediates motivational drive and craving for palatable food, while endogenous opioids and endocannabinoids mediate the pleasurable experience of consuming food [87,88,89]. In eating disorders, dysregulation occurs where dopamine-driven wanting becomes uncoupled from the liking or satiety response, contributing to compulsive food pursuit without corresponding satisfaction [83].
GLP-1 is uniquely positioned to address feeding dysregulation through its dual role in both homeostatic and hedonic systems [90]. GLP-1 acts centrally by activating POMC neurons in the hypothalamic ARC to promote satiety while simultaneously inhibiting dopamine neurons in the VTA to reduce food reward value [91,92,93]. GLP-1Rs are widely expressed throughout reward-related brain areas, and their activation significantly influences dopamine levels by modulating GABAergic and glutamatergic neurons [82,94]. Importantly, dysregulation of endogenous GLP-1 signaling may contribute to eating disorder pathophysiology, with studies showing altered GLP-1 secretion in eating disorders [95,96,97]. This evidence provides a mechanistic basis for targeting the GLP-1 system to correct feeding dysregulation, offering a comprehensive approach that addresses both metabolic and reward-driven aspects of disordered eating behaviors.
3. The Neurobiological and Molecular Basis of GLP-1 Action
3.1. GLP-1 Receptor Biology
The GLP-1R first gained significant attention in the field of endocrinology and diabetes due to its primary function as an incretin hormone, which enhances glucose-dependent insulin secretion and regulates systemic glucose homeostasis [98]. GLP-1 exerts its diverse physiological effects by activating GLP-1R [99]. The GLP-1R belongs to the class B G-protein coupled receptor (GPCR) family, characterized by a seven alpha-helical transmembrane domain and a large N-terminal extracellular domain crucial for binding to its ligands, including both GLP-1 and its pharmacological agonists.
Upon activation, the intracellular C-terminal tail of the receptor couples to the stimulatory G-protein (Gs) subunit, triggering a canonical signaling cascade that involves the activation of adenylyl cyclase (AC) and a subsequent increase in intracellular cyclic adenosine monophosphate (cAMP) [100,101,102,103]. The resulting cAMP increase activates protein kinase A (PKA), a key downstream mediator of GLP-1 signaling that phosphorylates various target proteins, leading to increased glucose-dependent insulin secretion and modulated neuronal excitability [104,105]. GLP-1R activation can also engage non-canonical signaling pathways, including the phospholipase C (PLC) pathway, which triggers protein kinase C (PKC) activation, diacylglycerol (DAG) production, and intracellular calcium mobilization [106,107]. These diverse signaling events allow the GLP-1R to exert its pleiotropic effects across different cell types and tissues [108]. The receptor signaling is also regulated by desensitization and internalization, which are mechanisms that prevent over-stimulation and allow for recycling, which are important for sustained therapeutic effects [109,110].
The wide-ranging physiological effects of GLP-1RAs are fundamentally determined by the widespread distribution of GLP-1Rs throughout both peripheral tissues and the central nervous system. In the periphery, GLP-1Rs are found in multiple key metabolic organs, including the pancreas, gut, heart, and kidneys, where they mediate glucose-dependent insulin secretion, gastric emptying, and cardiovascular and renal regulation [98,111,112,113,114,115]. Within the central nervous system, GLP-1Rs play a pivotal role in both homeostatic and hedonic feeding control [30]. In homeostatic centers, GLP-1Rs are expressed in hypothalamic nuclei, including ARC, PVN, and LH, where they promote satiety and reduce food intake [116]. GLP-1Rs are abundant in the brainstem, particularly in the NTS, which integrates visceral signals from the gut via the vagus nerve, and the area postrema (AP), a CVO that responds directly to circulating GLP-1 [69,117]. Extending to the mesolimbic reward system, GLP-1R expression in the VTA and NAc facilitates direct modulation of dopaminergic signaling and attenuation of food reward [118,119]. Additional receptor distribution in the amygdala, hippocampus, and prefrontal cortex (PFC) allows GLP-1 to influence emotional, memory, and executive control aspects of feeding behavior [120].
3.2. Mechanisms of GLP-1 Receptor Agonist Action on Feeding Behavior
GLP-1RAs exert their therapeutic effects through both peripheral and central mechanisms, acting as a physiological brake on both appetite and reward-driven food consumption. Understanding the distinction between these pathways is crucial for comprehending their therapeutic potential in eating disorders.
3.2.1. Peripheral Mechanisms of Action
The endogenous GLP-1 system originates peripherally in the gastrointestinal tract, primarily from enteroendocrine L-cells located in the ileum and colon in response to nutrient ingestion [121]. These specialized cells release GLP-1 in response to nutrient ingestion, with a major stimulus coming from the fermentation of dietary fiber by the gut microbiome, which produces short-chain fatty acids (SCFAs), such as acetate, butyrate, and propionate [122,123]. These SCFAs activate specific GPCRs on the L-cells, triggering the release of GLP-1 and establishing a crucial link between gut microbiota metabolism and hormonal appetite regulation [124,125,126]. As an incretin hormone, GLP-1 plays a fundamental role in glucose homeostasis by stimulating glucose-dependent insulin secretion from pancreatic beta-cells and inhibiting glucagon release, which is the primary mechanism by which GLP-1RAs treat type 2 diabetes [127,128].
GLP-1RAs are approved by the U.S. Food and Drug Administration for managing type 2 diabetes and obesity through multiple mechanisms, including enhanced insulin secretion, glucagon suppression, delayed gastric emptying, and modulation of central appetite pathways [98]. In type 2 diabetes, GLP-1RAs reduce HbA1c by 0.8–1.5% depending on agent and dosage [129], with semaglutide demonstrating superior glycemic control and greater reductions in body weight compared to other GLP-1RAs [130]. Comparative analyses show GLP-1RAs provide superior metabolic efficacy versus other antidiabetic therapies, with sustained benefits for up to two years [131]. For weight management, liraglutide 3.0 mg induces approximately 5% body weight reduction, while semaglutide 2.4 mg achieves 12–15% weight loss over 52–68 weeks [132,133,134].
Beyond these metabolic effects, GLP-1RAs have multiple peripheral actions that contribute to feeding regulation, including slowing gastric emptying, which promotes prolonged satiety by contributing to feelings of fullness and decreasing the rate of intestinal nutrient absorption with subsequent reduction in post-meal glucose spikes [135,136]. Additionally, GLP-1RAs act on vagal afferent nerve endings in the gut wall, signaling satiety to the brainstem, and influence the myenteric plexus of the enteric nervous system [127,137,138].
3.2.2. Central Mechanisms of Action
While FDA-approved GLP-1RAs, such as semaglutide and liraglutide, do not readily cross the blood–brain barrier, they can access central nervous system regions through CVO [139,140,141]. Studies using fluorescently labeled semaglutide and liraglutide have demonstrated drug accumulation in multiple brain areas, including regions typically protected by the blood–brain barrier, such as the NTS and AP [142,143,144].
In homeostatic centers, they activate anorexigenic POMC neurons in the ARC while inhibiting orexigenic NPY and AgRP neurons [145,146,147]. GLP-1 acts centrally by activating POMC neurons in the hypothalamic ARC to promote satiety while simultaneously inhibiting dopamine neurons in the VTA to reduce homeostatic hunger and decrease the food reward value [91,92,93]. Electrophysiological studies have confirmed direct neuronal responses and corresponding changes in gene expression [91,143,148].
Beyond homeostatic control, GLP-1RAs directly modulate reward processing through the mesolimbic system. The NAc serves as a central integration node, receiving dopaminergic input from the VTA and glutamatergic input from prefrontal cortical regions [149,150]. GLP-1Rs are widely expressed throughout these reward-related brain areas, and their activation significantly influences dopamine levels by modulating GABAergic and glutamatergic neurons [82,94]. This leads to reduced dopamine release in the NAc, thereby attenuating the motivational drive to consume palatable foods [151,152].
In brainstem regions, particularly the NTS and AP, GLP-1RAs reinforce satiety signals transmitted from the gut via the vagus nerve [69]. This comprehensive neuroanatomical distribution permits GLP-1RAs to target both homeostatic hunger signals and hedonic food motivation simultaneously. The integration of peripheral incretin effects, homeostatic appetite control, and central reward processing establishes GLP-1RAs as promising therapeutic tools for eating disorders. This integrated mechanism enables GLP-1RAs to bridge metabolic and neuropsychiatric treatment approaches, targeting both physiological and behavioral aspects of eating disorders.
4. Therapeutic Potential of GLP-1R Agonists for Binge-Eating Disorder
4.1. Preclinical Models and Baseline Findings for Binge Eating Research
Rodent models have been developed to provide insights into reward-related eating behaviors, which play a significant role in eating disorders. To study these complex human conditions, researchers have established animal models that mimic specific behavioral components of BED. According to the DSM-5, BE is defined as consuming an unusually large amount of food in a discrete period of time, such as a 2-h period, coupled with a sense of a lack of control during the episode [153]. BN combines BE episodes with inappropriate compensatory behaviors like self-induced vomiting [154]. As vomiting behavior cannot be replicated, rodent models focus primarily on mimicking binge-type eating and its underlying motivations [155].
Several methods are used to induce binge-eating behavior in rodents, including the manipulation of food availability, diet composition, feeding schedules, and stress exposure. Brief periods of food restriction can trigger binge-eating behavior [155]. Nevertheless, this model may not fully replicate human BED since the motivation is primarily hunger-driven. Another relevant approach provides intermittent access to highly palatable foods, such as sucrose, which drives increased intake patterns similar to binge eating after periods of deprivation [25,156]. Additionally, stress exposure can trigger binge-like episodes in rats, particularly when palatable food is available post-stress [157]. Both acute and chronic stressors, such as fasting or foot shock, can induce these behaviors when rats are given access to palatable food following stress exposure [157]. Among these approaches, stress-induced models most closely resemble human BED [158,159]. These paradigms employ restraint or foot-shock stress combined with palatable food access, producing robust, reproducible binge-like eating patterns [160]. They capture key BED features including stress-triggered episodes and emotional dysregulation, providing superior construct validity compared to other approaches. While intermittent access models induce intake escalation and progressive ratio schedules assess motivation, stress-induced paradigms uniquely integrate the affective disturbances and episodic nature of human BED, making them most relevant for therapeutic evaluation.
To comprehensively understand the mechanisms driving these behaviors, several validated methods assess binge and reward-related paradigms. Quantitative assessments include measuring caloric intake and comparing the amount of palatable food consumed to standard chow [161]. Behavioral measures, such as latency to feed, provide insight into compulsivity, as binge-eating rats often demonstrate increased impulsivity [162]. Since eating disorders involve dysregulated reward pathways, operant conditioning models are used to measure the drive to obtain food [163]. The progressive ratio (PR) schedule of reinforcement is widely used, where rodents have to make an increasing number of responses to earn a reward, such as pressing levers for sucrose. The breakpoint represents the highest ratio an animal completes and indicates how motivated the animal is to obtain the reward [164]. Conditioned place preference (CPP) represents a complementary approach using classical conditioning to assess learned preferences for a rewarding stimulus. In this paradigm, animals learn to associate a chamber with palatable food rewards, and the time subsequently spent in that chamber reflects the learned reward value of the food [165,166]. These models are complementary, as PR focuses on the motivational drive or wanting, while CPP addresses the hedonic component or liking of reward.
These validated preclinical models have revealed important neurobiological alterations that characterize binge-type eating behaviors. In animals exhibiting binge-eating patterns, repeated episodes of palatable food intake are associated with reduced adenosine A2A receptor expression in the amygdala and VTA, as well as downregulation of dopamine D2 receptors in the NAc [167]. Additionally, neural activity in the NAc core and shell becomes hyper-responsive during initial binge episodes but less responsive during chronic episodes [168]. These changes reflect disruption in the mesolimbic dopamine pathway, a crucial circuit regulating reward-related behaviors and motivation [169].
Importantly, these findings parallel functional brain studies in humans, which demonstrate reduced dopamine release and functional disconnection between the frontal cortex and striatum in individuals with binge-eating behaviors [163,170]. These similarities between animal and human studies support the use of these preclinical models for understanding eating disorder mechanisms and testing potential treatments.
4.2. Effects of GLP-1 Receptor Agonists on Eating Behavior and Reward
GLP-1 receptor agonists have demonstrated therapeutic potential beyond their original applications in type 2 diabetes and obesity treatment. These medications are now being investigated for eating disorders characterized by compulsive overeating and dysregulated food reward processing. GLP-1RAs, including exendin-4, semaglutide, liraglutide, and dulaglutide, have shown effects on both homeostatic appetite regulation and hedonic feeding through their action on brain reward circuitry.
4.2.1. Exendin-4
Exendin-4 (Ex-4) is a synthetic analog originally derived from the saliva of the Gila monster [171]. Ex-4 has been extensively studied in preclinical research due to its high binding affinity, prolonged half-life, and brain penetrance, making it especially suitable for investigating central mechanisms of feeding regulation [171,172]. Central administration into brain regions involved in reward and appetite regulation leads to significant suppression of palatable food intake, suggesting its role in modulating hedonic feeding behavior [28,173,174,175]. When administered into the NAc core of rats, Ex-4 could suppress μ-opioid receptor-mediated feeding [176]. Notably, sex-dependent responses were observed, with male rats showing enhanced motivation for palatable food compared to females following intra-LH administration [174]. In contrast, no significant sex differences were observed with intracerebroventricular infusion, suggesting that site-specific neural responses may account for these behavioral differences [175]. Ex-4 also influences dopaminergic activity. Administration into the NTS led to a four-fold increase in tyrosine hydroxylase mRNA expression in rats [28]. In the VTA, Ex-4 modulated dopamine signaling that was transiently activated in response to sucrose cues, indicating that it may attenuate cue-driven dopamine release associated with palatable food rewards [175,177]. Collectively, these findings highlight the therapeutic potential of Ex-4 to regulate maladaptive eating behavior through modulation of reward-related neural circuits.
4.2.2. Semaglutide
Multiple studies demonstrate that semaglutide, a long-acting GLP-1 receptor agonist, suppresses appetite, reduces food cravings, and contributes to significant weight loss across both clinical and preclinical models [143,178,179,180]. In rodent models, peripheral semaglutide administration reliably decreased total food intake and dampened motivation for palatable foods, supporting its potential in addressing binge-type eating patterns [143]. Semaglutide modulates activity within reward-related dopaminergic circuits in complex ways. While semaglutide at a higher dose enhanced dopaminergic signaling during reward collection, the animals obtained fewer rewards [180]. This suggests a possible dissociation between reward signaling and food-seeking behavior. Interestingly, this contrasts with earlier findings from other GLP-1RAs like Ex-4, which suppressed dopamine release in the NAc [28,175]. These findings reflect different reward phases. GLP-1RAs like Ex-4 suppress cue-triggered dopamine surges that drive food-seeking, thereby reducing cravings. However, semaglutide preserved dopamine responses during actual consumption while reducing total rewards obtained. This allows GLP-1RAs to reduce binge eating by blocking food cravings while maintaining the pleasurable aspects of eating. Peripherally administered semaglutide activated neurons in CVO, particularly the NTS and AP, regions regulating both homeostatic and hedonic feeding [143]. This was supported by increased c-Fos expression, a marker of neuronal activation, primarily localized in the hindbrain [180]. Despite increased neural activity in these regions, semaglutide did not stimulate compensatory intake, suggesting its anorexigenic effects involve modulation of central reward and satiety signals without triggering aversive responses [180]. Clinical evidence from a retrospective study showed that both semaglutide monotherapy and combination therapy with anti-obesity medications (AOM) resulted in significantly greater reductions in Binge-Eating Scale (BES) scores compared with AOM alone, with no significant difference between monotherapy and combination approaches [181,182]. At present, only one study has directly examined the effects of semaglutide on binge eating. Additional studies are needed to better define its therapeutic potential and clarify the mechanisms involved.
4.2.3. Liraglutide
Liraglutide is well-documented to produce anorectic effects in both rodent models and humans, leading to reduced caloric intake and body weight in several studies [183,184,185]. Beyond reducing hedonic food value, liraglutide enhances cognitive control over food-related behaviors, potentially through hippocampal-dependent mechanisms involved in behavioral inhibition [186,187,188]. With its ability to access brain regions, liraglutide acts on GLP-1 receptors in mesolimbic and mesocortical reward pathways, including the VTA and NAc, suppressing operant food-seeking behaviors [171,189]. Clinical trials provide mixed but encouraging results. In a randomized trial involving obese, non-diabetic individuals with BED, liraglutide led to significant reductions in BES scores, with 81% shifting to the non-binge category [190]. A pilot double-blind trial showed early reduction in binge episodes for both liraglutide and placebo groups, but liraglutide produced significantly greater weight loss, though interpretation was limited by medication misallocation [191]. A study examining liraglutide combined with behavioral therapy decreased Eating Disorder Examination Questionnaire (EDE-Q) scores and reduced binge episode frequency, although statistical significance declined over extended follow-up periods. The lack of a placebo control limited interpretation [40]. Among these three studies, two showed that higher doses of liraglutide (3 mg/day) were associated with greater reductions in weight, body mass index (BMI), and waist circumference, suggesting dose-dependent effects. Although current evidence is preliminary, liraglutide shows promise for BED management. Larger, well-controlled trials are needed to establish its efficacy and clarify its mechanisms.
4.2.4. Dulaglutide
Dulaglutide has also attracted attention for its potential role in improving BED. Dulaglutide showed promise in a pilot study of patients with type 2 diabetes and BED, demonstrating significant reductions in BES scores accompanied by decreases in body weight, BMI, and body fat mass compared to gliclazide treatment [192].
Collectively, these findings highlight the therapeutic potential of GLP-1RAs across both preclinical and clinical settings. Table 1 and Table 2 summarize the preclinical and clinical evidence, respectively, demonstrating how different GLP-1RAs modulate reward-related feeding behaviors across various animal models and human study populations, administration routes, brain regions, and key findings.

Table 1.
Preclinical Evidence for GLP-1RAs in Reward-Related Feeding Behaviors. Experimental parameters and behavioral/neurochemical outcomes from animal studies examining GLP-1RAs effects and underlying mechanisms.

Table 2.
Clinical Evidence for GLP-1RAs in Reward-Related Feeding Behaviors. Experimental parameters and behavioral/neurochemical outcomes from animal studies examining GLP-1RAs effects and underlying mechanisms.
4.3. Region-Specific GLP-1 Receptor Activation and Behavioral Outcomes
The therapeutic effects of GLP-1RAs on eating behavior are rooted in their region-specific activation within the central nervous system. GLP-1Rs are widely expressed in multiple brain regions that are pivotal in modulating motivation, reward anticipation, and feeding inhibition. Key sites include the NTS, CVOs, several hypothalamic nuclei, and the mesolimbic reward pathway, particularly the VTA and NAc [28,30,194]. The hypothalamus integrates homeostatic hunger signals and interacts with reward circuits through key neurotransmitters, such as dopamine and serotonin [195], while the mesolimbic reward pathway mediates the hedonic aspects of feeding by responding to highly palatable food stimuli [194]. Figure 1 illustrates the anatomical and functional connectivity between GLP-1 signaling pathways and dopaminergic reward circuits involved in appetite regulation.
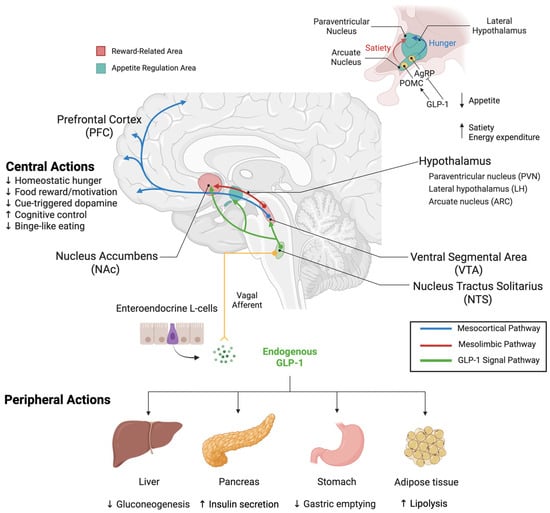
Figure 1.
Integrated Peripheral and Central GLP-1 Modulation of Appetite and Reward Pathways. Peripheral Actions: GLP-1, an incretin hormone secreted by enteroendocrine L-cells in the intestinal tract, acts on multiple peripheral organs. In the pancreas, GLP-1RAs stimulate insulin secretion and inhibit glucagon release. In the stomach, they delay gastric emptying to promote satiety. In the liver, they regulate glucose metabolism. In adipose tissue, they modulate energy expenditure and metabolism. GLP-1 signals via vagal afferent nerves to the nucleus tractus solitarius (NTS) in the brainstem, establishing direct gut–brain communication. Central Actions and Reward Circuits: The NTS serves as a critical integration hub, both receiving peripheral GLP-1 signals and producing GLP-1 centrally. NTS GLP-1 neurons send projections to reward centers, including the VTA and NAc, and appetite-regulating nuclei in the hypothalamus. The mesolimbic reward circuit originates from dopaminergic neurons in the VTA that project to the NAc, regions critical for reward-related behaviors. Within the hypothalamus, key appetite-regulating regions include the LH, PVN, and ARC. In the ARC, GLP-1 activates POMC neurons to promote satiety while inhibiting AgRP neurons to suppress hunger. These integrated signals from reward and homeostatic centers project to the PFC, where feeding behavior and reward processing are coordinated. Through these coordinated peripheral and central mechanisms, GLP-1RAs modulate both metabolic function and reward-driven eating behavior. Color coding: Blue = mesocortical pathway (reward centers to PFC), red = mesolimbic dopaminergic pathway (VTA to NAc), green = central GLP-1 neuronal projections (NTS to hypothalamus and reward regions), yellow = peripheral GLP-1 signaling (intestinal L-cells to brainstem via vagal afferents). Arrows indicate neural projections and signaling pathways; (↑) indicates stimulatory effects, (↓) indicates inhibitory effects. Created with www.BioRender.com (accessed on 15 September 2025). Abbreviations: AgRP, agouti-related peptide; ARC, arcuate nucleus; GLP-1, glucagon-like peptide-1; LH, lateral hypothalamic area; NAc, nucleus accumbens; NTS, nucleus tractus solitarius; PFC, prefrontal cortex; POMC, pro-opiomelanocortin; PVN, paraventricular nucleus; VTA, ventral tegmental area.
Direct administration of Ex-4 into the NTS suppressed palatable food intake in rats without affecting standard chow consumption and reduced operant responses for sucrose rewards, demonstrating selective suppression of hedonic feeding [28,196]. Central GLP-1R stimulation in the NTS also led to upregulation of tyrosine hydroxylase mRNA, a critical enzyme for dopamine synthesis, suggesting that GLP-1 influences dopaminergic tone within downstream reward circuits [28]. The NTS maintains important projections to key mesolimbic structures, including the VTA and NAc, creating an anatomical link through by which brainstem signaling can directly influence reward processing [197,198]. In the LH, which integrates both homeostatic and hedonic signals, acute Ex-4 administration reduced food-motivated behaviors, evident by decreased sucrose rewards earned and reduced lever presses [174]. This effect was sex-dependent, with a more pronounced response observed in male rats compared to females [199]. However, the broader literature reveals that female rodents generally show greater sensitivity to GLP-1RA effects on reward-driven eating [200]. This sex difference may reflect hormonal modulation of GLP-1R expression and function in reward circuits. Sex hormones influence GLP-1R levels [201], and estrogen enhances GLP-1RA effects on food reward behavior [202]. These findings suggest therapeutic responses may vary between sexes, with potential implications for sex-specific dosing or treatment strategies in BED.
GLP-1-dopamine crosstalk creates an integrated network modulating both homeostatic and hedonic feeding behaviors through complex, region-specific mechanisms. The mesolimbic pathway serves as the primary target for GLP-1-mediated reward modulation and is extensively innervated by GLP-1 neurons from the NTS [203]. This interaction extends to peripheral dopamine signaling in adipose tissue, where dopamine functions as a nutrient sensor and correlates with GLP-1R expression, suggesting coordinated metabolic regulation between central reward circuits and peripheral energy metabolism [136,204]. At the molecular level, GLP-1R activation initiates GPCR signaling that increases cAMP production and activates PKA, triggering intracellular cascades that alter neurotransmitter release and synaptic plasticity (Figure 2).
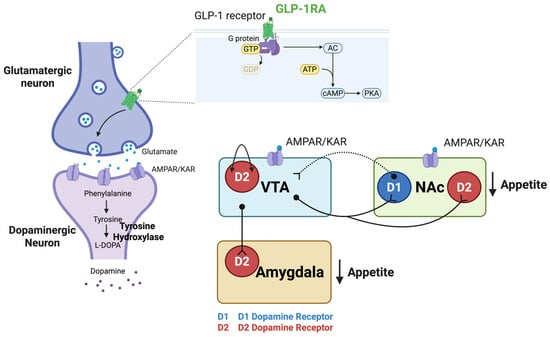
Figure 2.
GLP-1 Receptor Signaling Mechanisms in Reward Circuits. GLP-1R activation modulates feeding behavior in key reward-related brain regions, including the VTA, NAc, and amygdala through GPCR signaling. GLP-1R stimulation increases cAMP and activates PKA, triggering intracellular signaling cascades that result in altered neurotransmitter release and synaptic transmission for appetite regulation. In the VTA and NAc, GLP-1R activation enhances presynaptic glutamate release, which stimulates postsynaptic AMPAR/KAR on target neurons. In the NAc, this glutamatergic mechanism activates D2-MSNs, contributing to appetite suppression through increased downstream inhibition of reward targets. Dopaminergic projections from the VTA to the NAc modulate reward-related behaviors, while GABAergic feedback from the NAc D1-MSNs to the VTA regulates dopaminergic neuron activity and modulates dopamine release to balance reward and motivational signals associated with food intake. D2 autoreceptors on VTA dopamine neurons provide additional negative feedback, further modulating dopamine release. VTA dopaminergic projections also innervate the amygdala, regulating emotional aspects of feeding behavior. Created with www.BioRender.com (accessed on 15 September 2025). Abbreviations: AMPAR/KAR, AMPA/kainate receptors; cAMP, cyclic adenosine monophosphate; D1-MSNs, dopamine D1 receptor-expressing MSNs; D2-MSNs, dopamine D2 receptor-expressing MSNs; GABA, gamma-aminobutyric acid; GLP-1R, glucagon-like peptide-1 receptor; GPCR, G protein-coupled receptor; MSNs, medium spiny neurons; NAc, nucleus accumbens; PKA, protein kinase A; VTA, ventral tegmental area.
In the VTA, GLP-1R activation exhibits complex effects that may differ from expected dopaminergic outcomes. Ex-4 administration enhances presynaptic glutamatergic release via AMPA/kainate receptors (AMPA/KAR), increasing tyrosine hydroxylase expression and dopaminergic neuron firing [177]. However, this increased VTA activity preferentially directs dopamine release toward the amygdala rather than the NAc, where amygdala dopamine D2 receptor activation suppresses food intake [205]. This mechanism explains how increased VTA firing can ultimately suppress feeding behavior despite dopamine’s traditional role in reward enhancement. Within the VTA, D2 autoreceptors on dopamine neurons provide additional negative feedback that regulates dopamine release [206].
The NAc, consisting of core and shell subregions with distinct functional roles, responds differently to GLP-1R activation. The core governs goal-directed decision-making, including operant behaviors for palatable food, while the shell modulates incentive motivation driven by reward-predictive cues [89]. GLP-1R activation in the NAc enhances medium spiny neuron (MSN) activity through presynaptic glutamatergic signaling without directly affecting local dopamine levels [175,177]. This glutamatergic mechanism preferentially activates MSNs expressing dopamine D2 receptors (D2-MSNs) in the indirect pathway, increasing downstream inhibition of reward targets without directly affecting local dopamine levels, ultimately reducing consumption of rewarding substances [40,190,191]. The VTA sends dopaminergic projections to the NAc that regulate reward-related behaviors, while receiving inhibitory feedback from the NAc via GABAergic D1-MSNs, creating a bidirectional circuit that modulates dopaminergic activity [207,208].
Several preclinical studies consistently show that GLP-1RAs suppress dopamine responses to substance exposure. Peripheral Ex-4 reduces extracellular dopamine levels in the NAc after alcohol, nicotine, and cocaine intake, without affecting baseline dopamine levels, indicating that GLP-1R activation selectively inhibits substance-induced dopamine release while preserving normal dopaminergic tone [151,209]. Direct microinjection studies reveal regional specificity in GLP-1’s effects. Ex-4 administration into the VTA produces more potent suppression of motivated behavior than NAc administration, although both regions effectively reduce food intake when activated [173,203,210,211]. VTA injections significantly reduce motivated responding for palatable food and decrease NAc dopamine release, whereas NAc core activation reduces food intake and increases c-fos expression, indicating enhanced neural activity [203,212]. The mechanism extends beyond dopaminergic modulation to include glutamatergic signaling, as GLP-1R activation alters glutamate signaling in the NAc to promote negative energy balance without affecting dopamine activity [177].
Recent clinical studies support the therapeutic potential of GLP-1RAs in treating reward-related disorders. Semaglutide has been shown to suppress alcohol-induced dopamine elevation in the NAc, extending preclinical findings to clinically relevant medications [213]. Advanced formulations like the brain-penetrant agonist NLY01 demonstrate neuroprotective effects by directly targeting microglial GLP-1Rs, preventing dopaminergic neuron loss, and modulating astrocyte calcium signaling [214]. The consistency of dopamine suppression across different substances and GLP-1RA formulations demonstrates robust therapeutic mechanisms that position GLP-1RAs as promising therapeutic agents for disorders characterized by dysregulated reward processing, including BED, where the same mesolimbic circuits drive compulsive consumption of highly palatable foods.
4.4. Limitations of Current Research and Clinical Translation
Although GLP-1RAs have shown promise in preclinical rodent models of BED, several key limitations must be considered for clinical translation. BED is a complex psychiatric disorder involving multiple domains, including emotional regulation, cognitive control, and reward sensitivity, which are difficult to replicate in animal models. Rodent behavioral tests like CPP and operant conditioning offer quantitative insights. However, they cannot fully capture the complex psychological aspects of human BED. Biological and methodological variability further complicates interpretation. Different mouse strains exhibit inherent differences in baseline behaviors, with some strains showing higher anxiety-like behavior that may confound assessments of reward responsiveness [215]. Sex differences play a significant role, as studies using Ex-4 demonstrate that female rats show less potent intake suppression and reward-driven behavior compared to males, with significant effects observed only at higher doses [174]. The hormonal cycle can also modulate behavioral responses and drug efficacy, with effects more pronounced during the estrous phase [216]. Despite BED being more prevalent in females, insights into sex-specific drug responses remain underexplored [216]. Additionally, commonly used models employ lean or diet-induced obesity rodents, which may not fully replicate the neurobehavioral profile of human BED. Additionally, current preclinical research lacks aged animal models. While BED remains prevalent in older populations [12,13], studies evaluating GLP-1RA effects in aged rodents are scarce. Age-related changes in GLP-1R expression and dopaminergic function may influence therapeutic responses. Given BED’s clinical relevance in older adults, future preclinical research should incorporate aged animal models to optimize treatment strategies across the lifespan.
Preliminary clinical observations provide encouraging but limited evidence. A qualitative study of nine individuals prescribed GLP-1RAs reported reduced appetite, decreased consumption quantities, and improved mindfulness around food choices with corresponding decreases in EDE-Q scores. Two participants with compulsive eating behaviors, including one with a prior BED diagnosis, reported marked reductions in eating urges and improved control [193], with the BED participant achieving apparent remission. However, significant limitations exist in the current clinical research. Most studies are pilot trials with a small sample size and limited statistical power [217]. Study design challenges include inadequate blinding, drug misallocation, and study durations ranging from 11 to 52 weeks, which are potentially insufficient to capture long-term efficacy. Inconsistent dosing protocols, particularly for liraglutide, complicate cross-study comparisons. Assessment methodology remains problematic. Studies rely primarily on self-reported questionnaires, such as the EDE-Q and BES, which are subject to recall bias and underreporting [218]. Future research should integrate objective measures, including hormone levels, neuroimaging, or structured clinical assessments. Population heterogeneity, including comorbidities such as type 2 diabetes and psychiatric disorders, further complicates interpretation.
Clinical trial outcomes show considerable variability, with some studies reporting significant reductions in binge eating while others show non-significant trends. This heterogeneity likely reflects multiple factors. Small sample sizes (typically under 50 participants) limit statistical power [217]. Dosing protocols vary considerably, particularly for liraglutide, with higher doses generally showing stronger effects. Treatment duration ranges from 11 to 52 weeks, which may be insufficient for neurobiological adaptations. High placebo response rates occur in eating disorder trials. Population heterogeneity, including comorbidities such as type 2 diabetes and psychiatric disorders, further complicates interpretation [217,218]. These methodological limitations make it difficult to draw definitive conclusions about efficacy. Future research requires adequately powered, randomized controlled trials with standardized dosing protocols, objective assessment measures, and sufficient duration to establish true therapeutic benefits of GLP-1RAs in BED.
Emerging research explores GLP-1RA integration with established treatments. CBT remains the first-line non-pharmacological approach for BED, while lisdexamfetamine (LDX) is the only approved pharmacological agent [219]. A pilot study combining liraglutide with intensive behavioral therapy demonstrated reductions in binge eating episodes and improved metabolic outcomes [40,190]. Similarly, combining semaglutide with AOM improved BES scores, though this study found no significant difference between semaglutide monotherapy and dual therapy, suggesting that semaglutide alone may exert substantial therapeutic effects [181,182]. These findings highlight the potential for multidimensional approaches where GLP-1RAs complement existing therapies, though further trials are needed to explore the efficacy of the combination strategy.
GLP-1RAs and behavioral interventions may work synergistically through complementary mechanisms. GLP-1RAs reduce reward circuit hyperreactivity and hunger, creating favorable conditions for implementing CBT strategies. Prefrontal GLP-1R activation enhances cognitive control, facilitating cognitive restructuring, while hippocampal engagement accelerates extinction of maladaptive associations. Reduced cravings improve capacity to implement behavioral strategies, enhancing self-efficacy [40,190]. Sustained reward modulation may prevent post-CBT relapse [219]. This multi-level synergy provides rationale for combination therapy trials [181,182].
These limitations collectively call for well-designed, larger-scale, standardized studies integrating objective measures to evaluate both efficacy and safety of GLP-1RAs in BED treatment. Future research should prioritize proper study design with adequate sample sizes, standardized dosing protocols, longer follow-up periods, and objective assessment tools while accounting for population heterogeneity and sex-specific responses. Addressing these methodological challenges will be essential for translating promising preclinical and preliminary clinical findings into effective therapeutic interventions for binge eating disorder.
5. Conclusions and Future Perspectives
The GLP-1 system represents a promising therapeutic target for eating disorders through its dual modulation of metabolic and reward-driven behaviors. Preclinical evidence demonstrates that GLP-1RAs act on both homeostatic circuits in the hypothalamus and brainstem, as well as hedonic pathways in the mesolimbic reward system. By targeting key brain regions, including the NTS, VTA, and NAc, these agents effectively suppress operant behaviors for palatable food and attenuate food-motivated drive. This mechanism-based approach addresses core neurobiological drivers rather than isolated symptoms, providing a strong foundation for clinical applications in BED and BN. Despite promising preclinical findings, significant challenges remain. The translational gap between animal models and human eating disorders is substantial, as complex psychological symptoms cannot be fully captured in rodent paradigms. Critical research needs include larger placebo-controlled trials across different eating disorder subtypes, investigation of sex-specific effects given the high prevalence in women, and clarification of optimal dosing and treatment duration.
Future research should prioritize elucidating disorder-specific mechanisms across AN, BN, and BED while exploring combination therapies that integrate GLP-1RAs with behavioral interventions and other pharmacological agents. Additionally, advancing toward personalized medicine using neuroimaging and biomarker analysis could identify patients most likely to benefit from treatment. GLP-1RAs represent a promising new therapeutic avenue in eating disorder treatment by targeting fundamental neurobiological drivers of both homeostatic and hedonic feeding. This neurobiologically targeted approach offers the prospect of complementing existing treatments by addressing core pathophysiological mechanisms, potentially improving therapeutic outcomes in these complex disorders.
Author Contributions
Conceptualization, S.T., T.S. and N.P.; writing—original draft preparation, S.T.; writing—review and editing, N.P. and T.S.; visualization, S.T.; supervision, N.P.; project administration, T.S.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research project is supported by Mahidol University, Thailand (Basic Research Fund: fiscal year 2022, grant number BRF1-021/2565).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable.
Acknowledgments
During the preparation of the manuscript, N.P. used Claude Sonnet 4.0 for the grammar checking. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AC | Adenylyl Cyclase |
| ACC | Anterior Cingulate Cortex |
| AgRP | Agouti-Related Peptide |
| AMPA/KAR | AMPA/Kainate Receptors |
| AN | Anorexia Nervosa |
| AOM | Anti-Obesity Medications |
| AP | Area Postrema |
| ARC | Arcuate Nucleus |
| BE | Binge Eating |
| BED | Binge Eating Disorder |
| BES | Binge-Eating Scale |
| BMI | Body Mass Index |
| BN | Bulimia Nervosa |
| cAMP | Cyclic Adenosine Monophosphate |
| CBT | Cognitive Behavioral Therapy |
| CCK | Cholecystokinin |
| CPP | Conditioned Place Preference |
| CVO | Circumventricular Organs |
| D1-MSN | Dopamine D1 Receptor-expressing Medium Spiny Neuron |
| D2-MSN | Dopamine D2 Receptor-expressing Medium Spiny Neuron |
| DAG | Diacylglycerol |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, 5th Edition |
| EDE-Q | Eating Disorder Examination Questionnaire |
| Ex-4 | Exendin-4 |
| FEDs | Feeding and Eating Disorders |
| GLP-1 | Glucagon-Like Peptide-1 |
| GLP-1R | GLP-1 Receptor |
| GLP-1RA | GLP-1 Receptor Agonist |
| GPCR | G-Protein Coupled Receptor |
| ICD-11 | International Classification of Diseases, 11th Revision |
| IPT | Interpersonal Therapy |
| LDX | Lisdexamfetamine |
| LH | Lateral Hypothalamus |
| mPFC | Medial Prefrontal Cortex |
| MSN | Medium Spiny Neuron |
| NAc | Nucleus Accumbens |
| NPY | Neuropeptide Y |
| NTS | Nucleus Tractus Solitarius |
| OFC | Orbitofrontal Cortex |
| PFC | Prefrontal Cortex |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
| PLC | Phospholipase C |
| POMC | Proopiomelanocortin |
| PR | Progressive Ratio |
| PVN | Paraventricular Nucleus |
| PYY | Peptide YY |
| SCFA | Short-Chain Fatty Acid |
| vmPFC | Ventromedial Prefrontal Cortex |
| VTA | Ventral Tegmental Area |
References
- Feng, B.; Harms, J.; Chen, E.; Gao, P.; Xu, P.; He, Y. Current Discoveries and Future Implications of Eating Disorders. Int. J. Environ. Res. Public Health 2023, 20, 6325. [Google Scholar] [CrossRef]
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef]
- Qian, J.; Wu, Y.; Liu, F.; Zhu, Y.; Jin, H.; Zhang, H.; Wan, Y.; Li, C.; Yu, D. An update on the prevalence of eating disorders in the general population: A systematic review and meta-analysis. Eat. Weight Disord. 2022, 27, 415–428. [Google Scholar] [CrossRef]
- Hambleton, A.; Pepin, G.; Le, A.; Maloney, D.; Touyz, S.; Maguire, S. Psychiatric and medical comorbidities of eating disorders: Findings from a rapid review of the literature. J. Eat. Disord. 2022, 10, 132. [Google Scholar] [CrossRef]
- First, M.B. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J. Nerv. Ment. Dis. 2013, 201, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Barakat, S.; McLean, S.A.; Bryant, E.; Le, A.; Marks, P.; Touyz, S.; Maguire, S. Risk factors for eating disorders: Findings from a rapid review. J. Eat. Disord. 2023, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Claudino, A.M.; Zucker, N. Eating disorders. Lancet 2010, 375, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Duarte, T.A.; Schmidt, U. Eating disorders. Lancet 2020, 395, 899–911. [Google Scholar] [CrossRef]
- Galmiche, M.; Déchelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of eating disorders over the 2000-2018 period: A systematic literature review. Am. J. Clin. Nutr. 2019, 109, 1402–1413. [Google Scholar] [CrossRef]
- Silén, Y.; Keski-Rahkonen, A. Worldwide prevalence of DSM-5 eating disorders among young people. Curr. Opin. Psychiatry 2022, 35, 362–371. [Google Scholar] [CrossRef]
- Smink, F.R.; van Hoeken, D.; Hoek, H.W. Epidemiology of eating disorders: Incidence, prevalence and mortality rates. Curr. Psychiatry Rep. 2012, 14, 406–414. [Google Scholar] [CrossRef]
- Wilfred, S.A.; Becker, C.B.; Kanzler, K.E.; Musi, N.; Espinoza, S.E.; Kilpela, L.S. Binge eating among older women: Prevalence rates and health correlates across three independent samples. J. Eat. Disord. 2021, 9, 132. [Google Scholar] [CrossRef]
- Kilpela, L.S.; Marshall, V.B.; Keel, P.K.; LaCroix, A.Z.; Espinoza, S.E.; Hooper, S.C.; Musi, N. The clinical significance of binge eating among older adult women: An investigation into health correlates, psychological wellbeing, and quality of life. J. Eat. Disord. 2022, 10, 97, Erratum in J. Eat. Disord. 2023, 15, 203. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.A.; Chiu, W.T.; Deitz, A.C.; Hudson, J.I.; Shahly, V.; Aguilar-Gaxiola, S.; Alonso, J.; Angermeyer, M.C.; Benjet, C.; et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol. Psychiatry 2013, 73, 904–914. [Google Scholar] [CrossRef] [PubMed]
- di Giacomo, E.; Aliberti, F.; Pescatore, F.; Santorelli, M.; Pessina, R.; Placenti, V.; Colmegna, F.; Clerici, M. Disentangling binge eating disorder and food addiction: A systematic review and meta-analysis. Eat. Weight Disord. 2022, 27, 1963–1970. [Google Scholar] [CrossRef]
- Abdulla, Z.; Almahmood, H.O.; Alghasra, R.R.; Alherz, Z.A.S.; Alsharifa, H.A.G.; Qamber, S.J.; Alomar, N.A.; Almajed, F.E.; Almahroos, T.R.; Alnajjas, Z.A.; et al. Prevalence and associated factors of binge eating disorder among Bahraini youth and young adults: A cross-sectional study in a self-selected convenience sample. J. Eat. Disord. 2023, 11, 5. [Google Scholar] [CrossRef]
- Himmerich, H.; Kan, C.; Au, K.; Treasure, J. Pharmacological treatment of eating disorders, comorbid mental health problems, malnutrition and physical health consequences. Pharmacol. Ther. 2021, 217, 107667. [Google Scholar] [CrossRef]
- Kowalewska, E.; Bzowska, M.; Engel, J.; Lew-Starowicz, M. Comorbidity of binge eating disorder and other psychiatric disorders: A systematic review. BMC Psychiatry 2024, 24, 556. [Google Scholar] [CrossRef]
- Murphy, R.; Straebler, S.; Cooper, Z.; Fairburn, C.G. Cognitive behavioral therapy for eating disorders. Psychiatr. Clin. N. Am. 2010, 33, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Debar, L.L.; Wilson, G.T.; Yarborough, B.J.; Burns, B.; Oyler, B.; Hildebrandt, T.; Clarke, G.N.; Dickerson, J.; Striegel, R.H. Cognitive Behavioral Treatment for Recurrent Binge Eating in Adolescent Girls: A Pilot Trial. Cogn. Behav. Pract. 2013, 20, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Grilo, C.M.; Juarascio, A. Binge-Eating Disorder Interventions: Review, Current Status, and Implications. Curr. Obes. Rep. 2023, 12, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Zhang, G.; Jin, J.; Zheng, Z. The effect of CBT and its modifications for relapse prevention in major depressive disorder: A systematic review and meta-analysis. BMC Psychiatry 2018, 18, 50. [Google Scholar] [CrossRef]
- Moberg, L.T.; Solvang, B.; Sæle, R.G.; Myrvang, A.D. Effects of cognitive-behavioral and psychodynamic-interpersonal treatments for eating disorders: A meta-analytic inquiry into the role of patient characteristics and change in eating disorder-specific and general psychopathology in remission. J. Eat. Disord. 2021, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Boswell, R.G.; Potenza, M.N.; Grilo, C.M. The Neurobiology of Binge-eating Disorder Compared with Obesity: Implications for Differential Therapeutics. Clin. Ther. 2021, 43, 50–69. [Google Scholar] [CrossRef]
- Brown, R.M.; James, M.H. Binge eating, overeating and food addiction: Approaches for examining food overconsumption in laboratory rodents. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 123, 110717. [Google Scholar] [CrossRef]
- Eren-Yazicioglu, C.Y.; Yigit, A.; Dogruoz, R.E.; Yapici-Eser, H. Can GLP-1 Be a Target for Reward System Related Disorders? A Qualitative Synthesis and Systematic Review Analysis of Studies on Palatable Food, Drugs of Abuse, and Alcohol. Front. Behav. Neurosci. 2020, 14, 614884. [Google Scholar] [CrossRef]
- Badulescu, S.; Tabassum, A.; Le, G.H.; Wong, S.; Phan, L.; Gill, H.; Llach, C.D.; McIntyre, R.S.; Rosenblat, J.; Mansur, R. Glucagon-like peptide 1 agonist and effects on reward behaviour: A systematic review. Physiol. Behav. 2024, 283, 114622. [Google Scholar] [CrossRef]
- Richard, J.E.; Anderberg, R.H.; Göteson, A.; Gribble, F.M.; Reimann, F.; Skibicka, K.P. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS ONE 2015, 10, e0119034. [Google Scholar] [CrossRef]
- Jensen, M.E.; Galli, A.; Thomsen, M.; Jensen, K.L.; Thomsen, G.K.; Klausen, M.K.; Vilsbøll, T.; Christensen, M.B.; Holst, J.J.; Owens, A.; et al. Glucagon-like peptide-1 receptor regulation of basal dopamine transporter activity is species-dependent. Neurochem. Int. 2020, 138, 104772. [Google Scholar] [CrossRef] [PubMed]
- Diz-Chaves, Y.; Herrera-Pérez, S.; González-Matías, L.C.; Lamas, J.A.; Mallo, F. Glucagon-Like Peptide-1 (GLP-1) in the Integration of Neural and Endocrine Responses to Stress. Nutrients 2020, 12, 3304. [Google Scholar] [CrossRef]
- Sorli, C.; Harashima, S.I.; Tsoukas, G.M.; Unger, J.; Karsbøl, J.D.; Hansen, T.; Bain, S.C. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.; Henry, R.; Ratner, R.; Garcia-Hernandez, P.A.; Rodriguez-Pattzi, H.; Olvera-Alvarez, I.; Hale, P.M.; Zdravkovic, M.; Bode, B. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009, 373, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Victoza (Liraglutide) Injection: Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2010/022341s000ltr.pdf (accessed on 19 October 2025).
- U.S. Food and Drug Administration. Saxenda (Liraglutide) Injection: Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/206321Orig1s000ltr.pdf (accessed on 19 October 2025).
- U.S. Food and Drug Administration. Ozempic (Semaglutide) Injection: Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/209637Orig1s000ltr.pdf (accessed on 19 October 2025).
- U.S. Food and Drug Administration. FDA Approves New Medication for Chronic Weight Management. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management (accessed on 19 October 2025).
- U.S. Food and Drug Administration. Wegovy (Semaglutide) Injection: Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/215256Orig1s000ltr.pdf (accessed on 19 October 2025).
- Chao, A.M.; Wadden, T.A.; Walsh, O.A.; Gruber, K.A.; Alamuddin, N.; Berkowitz, R.I.; Tronieri, J.S. Effects of Liraglutide and Behavioral Weight Loss on Food Cravings, Eating Behaviors, and Eating Disorder Psychopathology. Obesity 2019, 27, 2005–2010. [Google Scholar] [CrossRef]
- Bettadapura, S.; Dowling, K.; Jablon, K.; Al-Humadi, A.W.; le Roux, C.W. Changes in food preferences and ingestive behaviors after glucagon-like peptide-1 analog treatment: Techniques and opportunities. Int. J. Obes. 2025, 49, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Hironaka, J.; Ushigome, E.; Kondo, Y.; Hashimoto, Y.; Osaka, T.; Majima, S.; Nakanishi, N.; Okada, H.; Senmaru, T.; Hamaguchi, M.; et al. Changes in food preferences after oral semaglutide administration in Japanese patients with type 2 diabetes: KAMOGAWA-DM cohort. Diabetes Vasc. Dis. Res. 2025, 22, 14791641251318309. [Google Scholar] [CrossRef]
- Aoun, L.; Almardini, S.; Saliba, F.; Haddadin, F.; Mourad, O.; Jdaidani, J.; Morcos, Z.; Al Saidi, I.; Bou Sanayeh, E.; Saliba, S.; et al. GLP-1 receptor agonists: A novel pharmacotherapy for binge eating (Binge eating disorder and bulimia nervosa)? A systematic review. J. Clin. Transl. Endocrinol. 2024, 35, 100333. [Google Scholar] [CrossRef]
- Mazzeo, S.E.; Bulik, C.M. Environmental and genetic risk factors for eating disorders: What the clinician needs to know. Child Adolesc. Psychiatr. Clin. N. Am. 2009, 18, 67–82. [Google Scholar] [CrossRef]
- Klump, K.L.; Suisman, J.L.; Burt, S.A.; McGue, M.; Iacono, W.G. Genetic and environmental influences on disordered eating: An adoption study. J. Abnorm. Psychol. 2009, 118, 797–805. [Google Scholar] [CrossRef]
- Butler, M.J.; Perrini, A.A.; Eckel, L.A. The Role of the Gut Microbiome, Immunity, and Neuroinflammation in the Pathophysiology of Eating Disorders. Nutrients 2021, 13, 500. [Google Scholar] [CrossRef]
- Lutter, M.; Nestler, E.J. Homeostatic and hedonic signals interact in the regulation of food intake. J. Nutr. 2009, 139, 629–632. [Google Scholar] [CrossRef]
- Mendoza, J. Food intake and addictive-like eating behaviors: Time to think about the circadian clock(s). Neurosci. Biobehav. Rev. 2019, 106, 122–132. [Google Scholar] [CrossRef]
- Guerdjikova, A.I.; O’Melia, A.M.; Mori, N.; McCoy, J.; McElroy, S.L. Binge eating disorder in elderly individuals. Int. J. Eat. Disord. 2012, 45, 905–908. [Google Scholar] [CrossRef]
- Conceição, E.M.; Gomes, F.V.S.; Vaz, A.R.; Pinto-Bastos, A.; Machado, P.P.P. Prevalence of eating disorders and picking/nibbling in elderly women. Int. J. Eat. Disord. 2017, 50, 793–800. [Google Scholar] [CrossRef]
- Schneeberger, M.; Gomis, R.; Claret, M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol. 2014, 220, T25–T46. [Google Scholar] [CrossRef] [PubMed]
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G. Development of Hypothalamic Circuits That Control Food Intake and Energy Balance. In Appetite and Food Intake: Central Control; Harris, R.B.S., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017; pp. 135–154. [Google Scholar]
- Chen, X.; Wang, Y.; Fu, S.; Wan, Y.; Mao, J.; Cui, K.; Jiang, H. The Integrated Function of the Lateral Hypothalamus in Energy Homeostasis. Cells 2025, 14, 1042. [Google Scholar] [CrossRef] [PubMed]
- Joly-Amado, A.; Cansell, C.; Denis, R.G.; Delbes, A.S.; Castel, J.; Martinez, S.; Luquet, S. The hypothalamic arcuate nucleus and the control of peripheral substrates. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 725–737. [Google Scholar] [CrossRef]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharmacol. 2022, 915, 174611. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef]
- Morton, G.J.; Meek, T.H.; Schwartz, M.W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 2014, 15, 367–378. [Google Scholar] [CrossRef]
- Wren, A.M.; Bloom, S.R. Gut hormones and appetite control. Gastroenterology 2007, 132, 2116–2130. [Google Scholar] [CrossRef]
- Latorre, R.; Sternini, C.; De Giorgio, R.; Greenwood-Van Meerveld, B. Enteroendocrine cells: A review of their role in brain-gut communication. Neurogastroenterol. Motil. 2016, 28, 620–630. [Google Scholar] [CrossRef]
- Yu, M.; Yu, B.; Chen, D. The effects of gut microbiota on appetite regulation and the underlying mechanisms. Gut Microbes 2024, 16, 2414796. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.D.; Xu, Q.J.; Chang, R.B. Vagal sensory neurons and gut-brain signaling. Curr. Opin. Neurobiol. 2020, 62, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef] [PubMed]
- Sammons, M.; Popescu, M.C.; Chi, J.; Liberles, S.D.; Gogolla, N.; Rolls, A. Brain-body physiology: Local, reflex, and central communication. Cell 2024, 187, 5877–5890. [Google Scholar] [CrossRef]
- Ma, L.; Wang, H.B.; Hashimoto, K. The vagus nerve: An old but new player in brain-body communication. Brain Behav. Immun. 2025, 124, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Burcelin, R.; Da Costa, A.; Drucker, D.; Thorens, B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 2001, 50, 1720–1728. [Google Scholar] [CrossRef]
- Jones, L.A.; Brierley, D.I. GLP-1 and the Neurobiology of Eating Control: Recent Advances. Endocrinology 2025, 166, bqae167. [Google Scholar] [CrossRef]
- Tulloch, A.J.; Murray, S.; Vaicekonyte, R.; Avena, N.M. Neural responses to macronutrients: Hedonic and homeostatic mechanisms. Gastroenterology 2015, 148, 1205–1218. [Google Scholar] [CrossRef]
- Dunigan, A.I.; Roseberry, A.G. Actions of feeding-related peptides on the mesolimbic dopamine system in regulation of natural and drug rewards. Addict. Neurosci. 2022, 2, 100011. [Google Scholar] [CrossRef] [PubMed]
- Yapici, N. Eating regulation: How diet impacts food cognition. Curr. Biol. 2023, 33, R153–R156. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Boyle, C.C.; Bower, J.E.; Eisenberger, N.I.; Irwin, M.R. Stress to inflammation and anhedonia: Mechanistic insights from preclinical and clinical models. Neurosci. Biobehav. Rev. 2023, 152, 105307. [Google Scholar] [CrossRef]
- Hou, G.; Hao, M.; Duan, J.; Han, M.H. The Formation and Function of the VTA Dopamine System. Int. J. Mol. Sci. 2024, 25, 3875. [Google Scholar] [CrossRef]
- Sheng, Z.; Santiago, A.M.; Thomas, M.P.; Routh, V.H. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol. Cell. Neurosci. 2014, 62, 30–41. [Google Scholar] [CrossRef]
- Perez-Bonilla, P.; Santiago-Colon, K.; Leinninger, G.M. Lateral hypothalamic area neuropeptides modulate ventral tegmental area dopamine neurons and feeding. Physiol. Behav. 2020, 223, 112986. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.; Woods, S.C.; Pelchat, M.; Grigson, P.S.; Stice, E.; Farooqi, S.; Khoo, C.S.; Mattes, R.D.; Beauchamp, G.K. Food reward system: Current perspectives and future research needs. Nutr. Rev. 2015, 73, 296–307. [Google Scholar] [CrossRef]
- Jennings, J.H.; Ung, R.L.; Resendez, S.L.; Stamatakis, A.M.; Taylor, J.G.; Huang, J.; Veleta, K.; Kantak, P.A.; Aita, M.; Shilling-Scrivo, K.; et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 2015, 160, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Nieh, E.H.; Matthews, G.A.; Allsop, S.A.; Presbrey, K.N.; Leppla, C.A.; Wichmann, R.; Neve, R.; Wildes, C.P.; Tye, K.M. Decoding neural circuits that control compulsive sucrose seeking. Cell 2015, 160, 528–541. [Google Scholar] [CrossRef]
- Barbano, M.F.; Wang, H.L.; Morales, M.; Wise, R.A. Feeding and Reward Are Differentially Induced by Activating GABAergic Lateral Hypothalamic Projections to VTA. J. Neurosci. 2016, 36, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Avena, N.M.; Bocarsly, M.E. Dysregulation of brain reward systems in eating disorders: Neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology 2012, 63, 87–96. [Google Scholar] [CrossRef]
- Campos, A.; Port, J.D.; Acosta, A. Integrative Hedonic and Homeostatic Food Intake Regulation by the Central Nervous System: Insights from Neuroimaging. Brain Sci. 2022, 12, 431. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670–679. [Google Scholar] [CrossRef]
- Nguyen, D.; Naffziger, E.E.; Berridge, K.C. Positive Affect: Nature and brain bases of liking and wanting. Curr. Opin. Behav. Sci. 2021, 39, 72–78. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E.; Aldridge, J.W. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 2009, 9, 65–73. [Google Scholar] [CrossRef]
- Mitchell, M.R.; Berridge, K.C.; Mahler, S.V. Endocannabinoid-Enhanced “Liking” in Nucleus Accumbens Shell Hedonic Hotspot Requires Endogenous Opioid Signals. Cannabis Cannabinoid Res. 2018, 3, 166–170. [Google Scholar] [CrossRef]
- Morales, I.; Berridge, K.C. ‘Liking’ and ‘wanting’ in eating and food reward: Brain mechanisms and clinical implications. Physiol. Behav. 2020, 227, 113152. [Google Scholar] [CrossRef]
- Anandhakrishnan, A.; Korbonits, M. Glucagon-like peptide 1 in the pathophysiology and pharmacotherapy of clinical obesity. World J. Diabetes 2016, 7, 572–598. [Google Scholar] [CrossRef] [PubMed]
- Secher, A.; Jelsing, J.; Baquero, A.F.; Hecksher-Sørensen, J.; Cowley, M.A.; Dalbøge, L.S.; Hansen, G.; Grove, K.L.; Pyke, C.; Raun, K.; et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Investig. 2014, 124, 4473–4488. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The GLP-1 journey: From discovery science to therapeutic impact. J. Clin. Investig. 2024, 134, e175634. [Google Scholar] [CrossRef]
- Merkel, R.; Hernandez, N.S.; Weir, V.; Zhang, Y.; Caffrey, A.; Rich, M.T.; Crist, R.C.; Reiner, B.C.; Schmidt, H.D. An endogenous GLP-1 circuit engages VTA GABA neurons to regulate mesolimbic dopamine neurons and attenuate cocaine seeking. Sci. Adv. 2025, 11, eadr5051. [Google Scholar] [CrossRef]
- Maldonado-Irizarry, C.S.; Swanson, C.J.; Kelley, A.E. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J. Neurosci. 1995, 15, 6779–6788. [Google Scholar] [CrossRef]
- Brambilla, F.; Monteleone, P.; Maj, M. Glucagon-like peptide-1 secretion in bulimia nervosa. Psychiatry Res. 2009, 169, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Moran, T.H. Gastrointestinal peptides in eating-related disorders. Physiol. Behav. 2021, 238, 113456. [Google Scholar] [CrossRef]
- Balantekin, K.N.; Kretz, M.J.; Mietlicki-Baase, E.G. The emerging role of glucagon-like peptide 1 in binge eating. J. Endocrinol. 2024, 262, e230405. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, N.; Wang, M.; Huang, W.; Luo, Y.; Huang, J. GLP-1R in diabetes mellitus: From basic discovery to therapeutics development. Front. Pharmacol. 2025, 16, 1610512. [Google Scholar] [CrossRef] [PubMed]
- Underwood, C.R.; Garibay, P.; Knudsen, L.B.; Hastrup, S.; Peters, G.H.; Rudolph, R.; Reedtz-Runge, S. Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J. Biol. Chem. 2010, 285, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; de Graaf, C.; Yang, L.; Song, G.; Dai, A.; Cai, X.; Feng, Y.; Reedtz-Runge, S.; Hanson, M.A.; Yang, H.; et al. Structural Determinants of Binding the Seven-transmembrane Domain of the Glucagon-like Peptide-1 Receptor (GLP-1R). J. Biol. Chem. 2016, 291, 12991–13004. [Google Scholar] [CrossRef]
- Graaf, C.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol. Rev. 2016, 68, 954–1013. [Google Scholar] [CrossRef]
- Lei, S.; Clydesdale, L.; Dai, A.; Cai, X.; Feng, Y.; Yang, D.; Liang, Y.L.; Koole, C.; Zhao, P.; Coudrat, T.; et al. Two distinct domains of the glucagon-like peptide-1 receptor control peptide-mediated biased agonism. J. Biol. Chem. 2018, 293, 9370–9387. [Google Scholar] [CrossRef]
- Dong, Z.; Chai, W.; Wang, W.; Zhao, L.; Fu, Z.; Cao, W.; Liu, Z. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E222–E228. [Google Scholar] [CrossRef]
- Al-Noshokaty, T.M.; Abdelhamid, R.; Abdelmaksoud, N.M.; Khaled, A.; Hossam, M.; Ahmed, R.; Saber, T.; Khaled, S.; Elshaer, S.S.; Abulsoud, A.I. Unlocking the multifaceted roles of GLP-1: Physiological functions and therapeutic potential. Toxicol. Rep. 2025, 14, 101895. [Google Scholar] [CrossRef]
- Shigeto, M.; Ramracheya, R.; Tarasov, A.I.; Cha, C.Y.; Chibalina, M.V.; Hastoy, B.; Philippaert, K.; Reinbothe, T.; Rorsman, N.; Salehi, A.; et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J. Clin. Investig. 2015, 125, 4714–4728. [Google Scholar] [CrossRef]
- Shigeto, M.; Cha, C.Y.; Rorsman, P.; Kaku, K. A role of PLC/PKC-dependent pathway in GLP-1-stimulated insulin secretion. J. Mol. Med. 2017, 95, 361–368. [Google Scholar] [CrossRef]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Fletcher, M.M.; Halls, M.L.; Zhao, P.; Clydesdale, L.; Christopoulos, A.; Sexton, P.M.; Wootten, D. Glucagon-like peptide-1 receptor internalisation controls spatiotemporal signalling mediated by biased agonists. Biochem. Pharmacol. 2018, 156, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Moo, E.V.; Møller, T.C.; Sørensen, F.A.; Inoue, A.; Bräuner-Osborne, H. Arrestin-independent internalization of the GLP-1 receptor is facilitated by a GRK, clathrin, and caveolae-dependent mechanism. FEBS J. 2025, 292, 1675–1695. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Baggio, L.L.; Yusta, B.; Mulvihill, E.E.; Cao, X.; Streutker, C.J.; Butany, J.; Cappola, T.P.; Margulies, K.B.; Drucker, D.J. GLP-1 Receptor Expression Within the Human Heart. Endocrinology 2018, 159, 1570–1584. [Google Scholar] [CrossRef]
- Holst, J.J.; Andersen, D.B.; Grunddal, K.V. Actions of glucagon-like peptide-1 receptor ligands in the gut. Br. J. Pharmacol. 2022, 179, 727–742. [Google Scholar] [CrossRef]
- Hinrichs, G.R.; Hovind, P.; Asmar, A. The GLP-1-mediated gut-kidney cross talk in humans: Mechanistic insight. Am. J. Physiol.-Cell Physiol. 2024, 326, C567–C572. [Google Scholar] [CrossRef]
- Krieger, J.P.; Daniels, D.; Lee, S.; Mastitskaya, S.; Langhans, W. Glucagon-Like Peptide-1 Links Ingestion, Homeostasis, and the Heart. Compr. Physiol. 2025, 15, e7. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Wang, L.; Xia, B.; Liu, J.; Tahiri, A.; El Ouaamari, A.; Wheeler, M.B.; Pang, Z.P. Activation of arcuate nucleus glucagon-like peptide-1 receptor-expressing neurons suppresses food intake. Cell Biosci. 2022, 12, 178. [Google Scholar] [CrossRef]
- Katsurada, K.; Nakata, M.; Saito, T.; Zhang, B.; Maejima, Y.; Nandi, S.S.; Sharma, N.M.; Patel, K.P.; Kario, K.; Yada, T. Central Glucagon-like Peptide-1 Receptor Signaling via Brainstem Catecholamine Neurons Counteracts Hypertension in Spontaneously Hypertensive Rats. Sci. Rep. 2019, 9, 12986. [Google Scholar] [CrossRef]
- Shirazi, R.H.; Dickson, S.L.; Skibicka, K.P. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS ONE 2013, 8, e61965. [Google Scholar] [CrossRef]
- Farkas, E.; Szilvásy-Szabó, A.; Ruska, Y.; Sinkó, R.; Rasch, M.G.; Egebjerg, T.; Pyke, C.; Gereben, B.; Knudsen, L.B.; Fekete, C. Distribution and ultrastructural localization of the glucagon-like peptide-1 receptor (GLP-1R) in the rat brain. Brain Struct. Funct. 2021, 226, 225–245. [Google Scholar] [CrossRef]
- Hsu, T.M.; Hahn, J.D.; Konanur, V.R.; Lam, A.; Kanoski, S.E. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology 2015, 40, 327–337. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Greiner, T.U.; Bäckhed, F. Microbial regulation of GLP-1 and L-cell biology. Mol. Metab. 2016, 5, 753–758. [Google Scholar] [CrossRef]
- Puddu, A.; Sanguineti, R.; Montecucco, F.; Viviani, G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat. Inflamm. 2014, 2014, 162021. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Sun, Y.S.; Zhao, L.Q.; Chen, T.T.; Fan, M.N.; Jiao, H.C.; Zhao, J.P.; Wang, X.J.; Li, F.C.; Li, H.F.; et al. SCFAs-Induced GLP-1 Secretion Links the Regulation of Gut Microbiome on Hepatic Lipogenesis in Chickens. Front. Microbiol. 2019, 10, 2176. [Google Scholar] [CrossRef]
- Hinnen, D. Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr. 2017, 30, 202–210. [Google Scholar] [CrossRef]
- Nauck, M.A.; Müller, T.D. Incretin hormones and type 2 diabetes. Diabetologia 2023, 66, 1780–1795. [Google Scholar] [CrossRef]
- Marx, N.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 Receptor Agonists for the Reduction of Atherosclerotic Cardiovascular Risk in Patients With Type 2 Diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar] [CrossRef]
- Pantea, I.; Repanovici, A.; Andreescu, O. The Influence of GLP1 on Body Weight and Glycemic Management in Patients with Diabetes—A Scientometric Investigation and Visualization Study. Medicina 2024, 60, 1761. [Google Scholar] [CrossRef]
- Siriyotha, S.; Anothaisintawee, T.; Looareesuwan, P.; Nimitphong, H.; McKay, G.; Attia, J.; Thakkinstian, A. Effectiveness of glucagon-like peptide-1 receptor agonists for reduction of body mass index and blood glucose control in patients with type 2 diabetes mellitus and obesity: A retrospective cohort study and difference-in-difference analysis. BMJ Open 2024, 14, e086424. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Marso, S.P.; Neeland, I.J. Liraglutide for weight management: A critical review of the evidence. Obes. Sci. Pract. 2017, 3, 3–14. [Google Scholar] [CrossRef]
- Bergmann, N.C.; Davies, M.J.; Lingvay, I.; Knop, F.K. Semaglutide for the treatment of overweight and obesity: A review. Diabetes Obes. Metab. 2023, 25, 18–35. [Google Scholar] [CrossRef] [PubMed]
- White, G.E.; Shu, I.; Rometo, D.; Arnold, J.; Korytkowski, M.; Luo, J. Real-world weight-loss effectiveness of glucagon-like peptide-1 agonists among patients with type 2 diabetes: A retrospective cohort study. Obesity 2023, 31, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Brierley, D.I.; de Lartigue, G. Reappraising the role of the vagus nerve in GLP-1-mediated regulation of eating. Br. J. Pharmacol. 2022, 179, 584–599. [Google Scholar] [CrossRef]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.; Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation. Am. J. Med. 2025, 138, 934–940. [Google Scholar] [CrossRef]
- Hayes, M.R.; Mietlicki-Baase, E.G.; Kanoski, S.E.; De Jonghe, B.C. Incretins and amylin: Neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu. Rev. Nutr. 2014, 34, 237–260. [Google Scholar] [CrossRef]
- Ren, X.; Hua, H.; Wu, Y.; Zhang, W.; Long, X.; Bai, Y.; Cheng, N. Efficacy and safety of GLP-1 agonists in the treatment of T2DM: A systematic review and network meta-analysis. Sci. Rep. 2025, 15, 24103. [Google Scholar] [CrossRef]
- Buller, S.; Blouet, C. Brain access of incretins and incretin receptor agonists to their central targets relevant for appetite suppression and weight loss. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E472–E480. [Google Scholar] [CrossRef]
- Tipa, R.O.; Balan, D.G.; Georgescu, M.T.; Ignat, L.A.; Vacaroiu, I.A.; Georgescu, D.E.; Raducu, L.; Mihai, D.A.; Chiperi, L.V.; Balcangiu-Stroescu, A.E. A Systematic Review of Semaglutide’s Influence on Cognitive Function in Preclinical Animal Models and Cell-Line Studies. Int. J. Mol. Sci. 2024, 25, 4972. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. Understanding semaglutide action in the brain. Nat. Rev. Drug Discov. 2025, 24, 588. [Google Scholar] [CrossRef]
- Kumari, P.; Nakata, M.; Zhang, B.Y.; Otgon-Uul, Z.; Yada, T. GLP-1 receptor agonist liraglutide exerts central action to induce β-cell proliferation through medulla to vagal pathway in mice. Biochem. Biophys. Res. Commun. 2018, 499, 618–625. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef]
- Hankir, M.K.; Lutz, T.A. Novel neural pathways targeted by GLP-1R agonists and bariatric surgery. Pflugers Arch-Eur. J. Physiol. 2025, 477, 171–185. [Google Scholar] [CrossRef]
- He, Z.; Gao, Y.; Lieu, L.; Afrin, S.; Cao, J.; Michael, N.J.; Dong, Y.; Sun, J.; Guo, H.; Williams, K.W. Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons–Implications for energy balance and glucose control. Mol. Metab. 2019, 28, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Deem, J.D.; Faber, C.L.; Morton, G.J. AgRP neurons: Regulators of feeding, energy expenditure, and behavior. FEBS J. 2022, 289, 2362–2381. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Carty, J.; Goldstein, N.; He, Z.; Hwang, E.; Chau, D.; Wallace, B.; Kabahizi, A.; Lieu, L.; Peng, Y.; et al. Time and metabolic state-dependent effects of GLP-1R agonists on NPY/AgRP and POMC neuronal activity in vivo. Mol. Metab. 2021, 54, 101352. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Vianna, A.R.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Effects of liraglutide in hypothalamic arcuate nucleus of obese mice. Obesity 2016, 24, 626–633. [Google Scholar] [CrossRef]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625, Erratum in Nat. Rev. Neurosci. 2013, 14, 736. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, S.Y.; Kwon, H.; Song, Y.; Yun, B.; Lee, Y.; Cho, Y.; Joo, A.; Han, P.L. A Group of Descending Glutamatergic Neurons Activated by Stress in Corticolimbic Regions Project to the Nucleus Accumbens. Exp. Neurobiol. 2018, 27, 387–396. [Google Scholar] [CrossRef]
- Marquez-Meneses, J.D.; Olaya-Bonilla, S.A.; Barrera-Carreño, S.; Tibaduiza-Arévalo, L.C.; Forero-Cárdenas, S.; Carrillo-Vaca, L.; Rojas-Rodríguez, L.C.; Calderon-Ospina, C.A.; Rodríguez-Quintana, J. GLP-1 Analogues in the Neurobiology of Addiction: Translational Insights and Therapeutic Perspectives. Int. J. Mol. Sci. 2025, 26, 5338. [Google Scholar] [CrossRef] [PubMed]
- Krupa, A.J. Curbing the appetites and restoring the capacity for satisfaction: The impact of GLP-1 agonists on the reward circuitry. Neurosci. Appl. 2025, 4, 105512. [Google Scholar] [CrossRef]
- Lindvall Dahlgren, C.; Wisting, L.; Rø, Ø. Feeding and eating disorders in the DSM-5 era: A systematic review of prevalence rates in non-clinical male and female samples. J. Eat. Disord. 2017, 5, 56. [Google Scholar] [CrossRef]
- Hoek, H.W.; van Elburg, A.A. Feeding and eating disorders in the DSM-5. Tijdschr. Psychiatr. 2014, 56, 187–191. [Google Scholar]
- Kim, S.F. Animal models of eating disorders. Neuroscience 2012, 211, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Corwin, R.L.; Babbs, R.K. Rodent models of binge eating: Are they models of addiction? ILAR J. 2012, 53, 23–34. [Google Scholar] [CrossRef]
- Oswald, K.D.; Murdaugh, D.L.; King, V.L.; Boggiano, M.M. Motivation for palatable food despite consequences in an animal model of binge eating. Int. J. Eat. Disord. 2011, 44, 203–211. [Google Scholar] [CrossRef]
- Czyzyk, T.A.; Sahr, A.E.; Statnick, M.A. A model of binge-like eating behavior in mice that does not require food deprivation or stress. Obesity 2010, 18, 1710–1717. [Google Scholar] [CrossRef]
- Petković, A.; Chaudhury, D. Encore: Behavioural animal models of stress, depression and mood disorders. Front. Behav. Neurosci. 2022, 16, 931964. [Google Scholar] [CrossRef] [PubMed]
- Razzoli, M.; Sanghez, V.; Bartolomucci, A. Chronic subordination stress induces hyperphagia and disrupts eating behavior in mice modeling binge-eating-like disorder. Front. Nutr. 2015, 1, 30. [Google Scholar] [CrossRef]
- Burnett, A.L.; Calvin, D.C.; Silver, R.I.; Peppas, D.S.; Docimo, S.G. Immunohistochemical description of nitric oxide synthase isoforms in human clitoris. J. Urol. 1997, 158, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Vickers, S.P.; Goddard, S.; Brammer, R.J.; Hutson, P.H.; Heal, D.J. Investigation of impulsivity in binge-eating rats in a delay-discounting task and its prevention by the d-amphetamine prodrug, lisdexamfetamine. J. Psychopharmacol. 2017, 31, 784–797. [Google Scholar] [CrossRef]
- Pasquale, E.K.; Boyar, A.M.; Boutelle, K.N. Reward and Inhibitory Control as Mechanisms and Treatment Targets for Binge Eating Disorder. Curr. Psychiatry Rep. 2024, 26, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Ackroff, K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol. Behav. 2003, 79, 663–670. [Google Scholar] [CrossRef]
- Velázquez-Sánchez, C.; Santos, J.W.; Smith, K.L.; Ferragud, A.; Sabino, V.; Cottone, P. Seeking behavior, place conditioning, and resistance to conditioned suppression of feeding in rats intermittently exposed to palatable food. Behav. Neurosci. 2015, 129, 219–224. [Google Scholar] [CrossRef]
- Astur, R.S.; Palmisano, A.N.; Hudd, E.C.; Carew, A.W.; Deaton, B.E.; Kuhney, F.S.; Niezrecki, R.N.; Santos, M. Pavlovian conditioning to food reward as a function of eating disorder risk. Behav. Brain Res. 2015, 291, 277–282. [Google Scholar] [CrossRef]
- Mercante, F.; Micioni Di Bonaventura, E.; Pucci, M.; Botticelli, L.; Cifani, C.; D’Addario, C.; Micioni Di Bonaventura, M.V. Repeated binge-like eating episodes in female rats alter adenosine A2A and dopamine D2 receptor genes regulation in the brain reward system. Int. J. Eat. Disord. 2024, 57, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.A.; Sinclair, E.B.; Sisk, C.L.; Klump, K.L. Exploring reward system responsivity in the nucleus accumbens across chronicity of binge eating in female rats. Int. J. Eat. Disord. 2018, 51, 989–993. [Google Scholar] [CrossRef]
- Baik, J.H. Dopamine signaling in reward-related behaviors. Front. Neural Circuits 2013, 7, 152. [Google Scholar] [CrossRef]
- Leenaerts, N.; Jongen, D.; Ceccarini, J.; Van Oudenhove, L.; Vrieze, E. The neurobiological reward system and binge eating: A critical systematic review of neuroimaging studies. Int. J. Eat. Disord. 2022, 55, 1421–1458. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Fortin, S.M.; Arnold, M.; Grill, H.J.; Hayes, M.R. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 2011, 152, 3103–3112. [Google Scholar] [CrossRef]
- Liao, S.; Liang, Y.; Zhang, Z.; Li, J.; Wang, J.; Wang, X.; Dou, G.; Zhang, Z.; Liu, K. In vitro metabolic stability of exendin-4: Pharmacokinetics and identification of cleavage products. PLoS ONE 2015, 10, e0116805. [Google Scholar] [CrossRef] [PubMed]
- Dickson, S.L.; Shirazi, R.H.; Hansson, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J. Neurosci. 2012, 32, 4812–4820. [Google Scholar] [CrossRef]
- López-Ferreras, L.; Richard, J.E.; Noble, E.E.; Eerola, K.; Anderberg, R.H.; Olandersson, K.; Taing, L.; Kanoski, S.E.; Hayes, M.R.; Skibicka, K.P. Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Mol. Psychiatry 2018, 23, 1157–1168. [Google Scholar] [CrossRef]
- Konanur, V.R.; Hsu, T.M.; Kanoski, S.E.; Hayes, M.R.; Roitman, M.F. Phasic dopamine responses to a food-predictive cue are suppressed by the glucagon-like peptide-1 receptor agonist Exendin-4. Physiol. Behav. 2020, 215, 112771. [Google Scholar] [CrossRef]
- Pierce-Messick, Z.; Pratt, W.E. Glucagon-like peptide-1 receptors modulate the binge-like feeding induced by µ-opioid receptor stimulation of the nucleus accumbens in the rat. Neuroreport 2020, 31, 1283–1288. [Google Scholar] [CrossRef]
- Mietlicki-Baase, E.G.; Ortinski, P.I.; Reiner, D.J.; Sinon, C.G.; McCutcheon, J.E.; Pierce, R.C.; Roitman, M.F.; Hayes, M.R. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J. Neurosci. 2014, 34, 6985–6992. [Google Scholar] [CrossRef]
- Gibbons, C.; Blundell, J.; Tetens Hoff, S.; Dahl, K.; Bauer, R.; Baekdal, T. Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes. Metab. 2021, 23, 581–588. [Google Scholar] [CrossRef]
- Friedrichsen, M.; Breitschaft, A.; Tadayon, S.; Wizert, A.; Skovgaard, D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes. Metab. 2021, 23, 754–762. [Google Scholar] [CrossRef]
- Kooij, K.L.; Koster, D.I.; Eeltink, E.; Luijendijk, M.; Drost, L.; Ducrocq, F.; Adan, R.A.H. GLP-1 receptor agonist semaglutide reduces appetite while increasing dopamine reward signaling. Neurosci. Appl. 2024, 3, 103925. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Bang, N.; Ratliff, E.L.; Paszkowiak, M.A.; Khorgami, Z.; Khalsa, S.S.; Simmons, W.K. Successful treatment of binge eating disorder with the GLP-1 agonist semaglutide: A retrospective cohort study. Obes. Pillars 2023, 7, 100080. [Google Scholar] [CrossRef] [PubMed]
- Tzoulis, P.; Batavanis, M.; Baldeweg, S. A Real-World Study of the Effectiveness and Safety of Semaglutide for Weight Loss. Cureus 2024, 16, e59558. [Google Scholar] [CrossRef]
- McKay, N.J.; Kanoski, S.E.; Hayes, M.R.; Daniels, D. Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1755–R1764. [Google Scholar] [CrossRef] [PubMed]
- Fortin, S.M.; Lipsky, R.K.; Lhamo, R.; Chen, J.; Kim, E.; Borner, T.; Schmidt, H.D.; Hayes, M.R. GABA neurons in the nucleus tractus solitarius express GLP-1 receptors and mediate anorectic effects of liraglutide in rats. Sci. Transl. Med. 2020, 12, eaay8071. [Google Scholar] [CrossRef]
- Silver, H.J.; Olson, D.; Mayfield, D.; Wright, P.; Nian, H.; Mashayekhi, M.; Koethe, J.R.; Niswender, K.D.; Luther, J.M.; Brown, N.J. Effect of the glucagon-like peptide-1 receptor agonist liraglutide, compared to caloric restriction, on appetite, dietary intake, body fat distribution and cardiometabolic biomarkers: A randomized trial in adults with obesity and prediabetes. Diabetes Obes. Metab. 2023, 25, 2340–2350. [Google Scholar] [CrossRef]
- Jones, S.; Sample, C.H.; Davidson, T.L. The effects of a GLP-1 analog liraglutide on reward value and the learned inhibition of appetitive behavior in male and female rats. Int. J. Obes. 2019, 43, 1875–1879. [Google Scholar] [CrossRef]
- Yan, W.; Pang, M.; Yu, Y.; Gou, X.; Si, P.; Zhawatibai, A.; Zhang, Y.; Zhang, M.; Guo, T.; Yi, X.; et al. The neuroprotection of liraglutide on diabetic cognitive deficits is associated with improved hippocampal synapses and inhibited neuronal apoptosis. Life Sci. 2019, 231, 116566. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, R.; Rigoux, L.; Kuzmanovic, B.; Iglesias, S.; Kretschmer, A.C.; Schlamann, M.; Albus, K.; Edwin Thanarajah, S.; Sitnikow, T.; Melzer, C.; et al. Liraglutide restores impaired associative learning in individuals with obesity. Nat. Metab. 2023, 5, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.R.; Schmidt, H.D. GLP-1 influences food and drug reward. Curr. Opin. Behav. Sci. 2016, 9, 66–70. [Google Scholar] [CrossRef]
- Robert, S.A.; Rohana, A.G.; Shah, S.A.; Chinna, K.; Wan Mohamud, W.N.; Kamaruddin, N.A. Improvement in binge eating in non-diabetic obese individuals after 3 months of treatment with liraglutide–A pilot study. Obes. Res. Clin. Pract. 2015, 9, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.C.; Chao, A.M.; Bruzas, M.B.; McCuen-Wurst, C.; Jones, E.; McAllister, C.; Gruber, K.; Berkowitz, R.I.; Wadden, T.A.; Tronieri, J.S. A pilot randomized controlled trial of liraglutide 3.0 mg for binge eating disorder. Obes. Sci. Pract. 2023, 9, 127–136. [Google Scholar] [CrossRef]
- Da Porto, A.; Casarsa, V.; Colussi, G.; Catena, C.; Cavarape, A.; Sechi, L. Dulaglutide reduces binge episodes in type 2 diabetic patients with binge eating disorder: A pilot study. Diabetes Metab. Syndr. 2020, 14, 289–292. [Google Scholar] [CrossRef]
- Pierret, A.C.S.; Benton, M.; Sen Gupta, P.; Ismail, K. A qualitative study of the mental health outcomes in people being treated for obesity and type 2 diabetes with glucagon-like peptide-1 receptor agonists. Acta Diabetol. 2025, 62, 731–742. [Google Scholar] [CrossRef]
- Menzies, J.R.; Skibicka, K.P.; Dickson, S.L.; Leng, G. Neural substrates underlying interactions between appetite stress and reward. Obes. Facts 2012, 5, 208–220. [Google Scholar] [CrossRef]
- Schiffino, F.L.; Siemian, J.N.; Petrella, M.; Laing, B.T.; Sarsfield, S.; Borja, C.B.; Gajendiran, A.; Zuccoli, M.L.; Aponte, Y. Activation of a lateral hypothalamic-ventral tegmental circuit gates motivation. PLoS ONE 2019, 14, e0219522. [Google Scholar] [CrossRef]
- Alhadeff, A.L.; Grill, H.J. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R465–R470. [Google Scholar] [CrossRef]
- Le Merrer, J.; Becker, J.A.; Befort, K.; Kieffer, B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009, 89, 1379–1412. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci. Biobehav. Rev. 2010, 35, 129–150. [Google Scholar] [CrossRef]
- Funahashi, H.; Takenoya, F.; Guan, J.L.; Kageyama, H.; Yada, T.; Shioda, S. Hypothalamic neuronal networks and feeding-related peptides involved in the regulation of feeding. Anat. Sci. Int. 2003, 78, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Börchers, S.; Skibicka, K.P. GLP-1 and Its Analogs: Does Sex Matter? Endocrinology 2025, 166, bqae165. [Google Scholar] [CrossRef]
- Outeiriño-Iglesias, V.; Romaní-Pérez, M.; González-Matías, L.C.; Vigo, E.; Mallo, F. GLP-1 Increases Preovulatory LH Source and the Number of Mature Follicles, As Well As Synchronizing the Onset of Puberty in Female Rats. Endocrinology 2015, 156, 4226–4237. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Wolf, S.; Rabasa, C.; Rodriguez-Pacheco, F.; Babaei, C.S.; Stöber, F.; Goldschmidt, J.; DiMarchi, R.D.; Finan, B.; Tschöp, M.H.; et al. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology 2016, 110, 396–406. [Google Scholar] [CrossRef]
- Alhadeff, A.L.; Rupprecht, L.E.; Hayes, M.R. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012, 153, 647–658. [Google Scholar] [CrossRef]
- Bu, T.; Sun, Z.; Pan, Y.; Deng, X.; Yuan, G. Glucagon-Like Peptide-1: New Regulator in Lipid Metabolism. Diabetes Metab. J. 2024, 48, 354–372. [Google Scholar] [CrossRef]
- Anderberg, R.H.; Anefors, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior. Physiol. Behav. 2014, 136, 135–144. [Google Scholar] [CrossRef]
- Ford, C.P. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef]
- Juarez, B.; Zweifel, L.S. Disinhibitory feedback loops for reward and aversion. Cell Res. 2022, 32, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Derman, R.C.; Bryda, E.C.; Ferrario, C.R. Role of nucleus accumbens D1-type medium spiny neurons in the expression and extinction of sign-tracking. Behav. Brain Res. 2024, 459, 114768. [Google Scholar] [CrossRef]
- Egecioglu, E.; Engel, J.A.; Jerlhag, E. The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS ONE 2013, 8, e77284. [Google Scholar] [CrossRef]
- Erreger, K.; Davis, A.R.; Poe, A.M.; Greig, N.H.; Stanwood, G.D.; Galli, A. Exendin-4 decreases amphetamine-induced locomotor activity. Physiol. Behav. 2012, 106, 574–578. [Google Scholar] [CrossRef]
- Egecioglu, E.; Engel, J.A.; Jerlhag, E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS ONE 2013, 8, e69010. [Google Scholar] [CrossRef]
- Dossat, A.M.; Lilly, N.; Kay, K.; Williams, D.L. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J. Neurosci. 2011, 31, 14453–14457. [Google Scholar] [CrossRef]
- Aranäs, C.; Edvardsson, C.E.; Shevchouk, O.T.; Zhang, Q.; Witley, S.; Blid Sköldheden, S.; Zentveld, L.; Vallöf, D.; Tufvesson-Alm, M.; Jerlhag, E. Semaglutide reduces alcohol intake and relapse-like drinking in male and female rats. eBioMedicine 2023, 93, 104642. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Dawson, V.L.; Dawson, T.M. From metabolism to mind: The expanding role of the GLP-1 receptor in neurotherapeutics. Neurotherapeutics 2025, 22, e00712. [Google Scholar] [CrossRef]
- Crawley, J.N.; Belknap, J.K.; Collins, A.; Crabbe, J.C.; Frankel, W.; Henderson, N.; Hitzemann, R.J.; Maxson, S.C.; Miner, L.L.; Silva, A.J.; et al. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology 1997, 132, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Breton, É.; Juster, R.P.; Booij, L. Gender and sex in eating disorders: A narrative review of the current state of knowledge, research gaps, and recommendations. Brain Behav. 2023, 13, e2871. [Google Scholar] [CrossRef]
- Faber, J.; Fonseca, L.M. How sample size influences research outcomes. Dental Press. J. Orthod. 2014, 19, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.; Pinto-Gouveia, J.; Ferreira, C. Expanding binge eating assessment: Validity and screening value of the Binge Eating Scale in women from the general population. Eat. Behav. 2015, 18, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Grilo, C.M.; Ivezaj, V.; Yurkow, S.; Tek, C.; Wiedemann, A.A.; Gueorguieva, R. Lisdexamfetamine maintenance treatment for binge-eating disorder following successful treatments: Randomized double-blind placebo-controlled trial. Psychol. Med. 2024, 54, 3334–3344. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).