Abstract
Endometrial cancers increase expression of the renin–angiotensin system (RAS). This study aimed to determine if inhibiting the RAS would reduce the viability and proliferation of endometrial cancer cells. The expression of RAS genes was measured in three endometrial epithelial adenocarcinoma cell lines (Ishikawa, HEC-1-A, AN3CA). Ishikawa cells had the highest expression of REN, ACE, and AGTR1 mRNA. AGT mRNA and protein levels were most abundant in HEC-1-A cells. We then determined the effects of drugs that inhibit the action of renin (VTP-27999 and aliskiren) or angiotensin-converting enzyme (perindoprilat) or block the angiotensin II type 1 receptor (losartan and telmisartan). Overall, VTP-27999, aliskiren, perindoprilat, and losartan had minimal effects on cell viability in all three cell lines, and combinations of these drugs did not have any effect. Telmisartan (a dual angiotensin receptor blocker and PPAR-γ agonist) significantly reduced the viability of all three cell lines and reduced the proliferation of both Ishikawa and AN3CA cells. Telmisartan was more effective than troglitazone (PPAR-γ agonist) in Ishikawa and HEC-1-A cells. RAS inhibitors were most effective in Ishikawa cells, which had the highest levels of RAS expression. Therefore, levels of RAS expression in endometrial cancers might indicate the potential efficacy of RAS drugs.
1. Introduction
Endometrial cancer is the most common gynecological malignancy [1]. Risk factors for endometrial cancer include obesity, hypertension, and diabetes, all of which are associated with increased activity of the renin–angiotensin system (RAS) [2]. The circulating RAS consists of a cascade of enzymes that begins with renin acting on angiotensinogen (the sole source of angiotensin peptides) [3], to produce the biologically active peptides. Angiotensin II (Ang II) is the major end-product of the RAS, playing an important role in the regulation of blood pressure and water and electrolyte balance. Drugs that block the formation of Ang II or its interaction with the Ang II type 1 receptor (AT1R) are used widely in the treatment of hypertension, heart failure, and diabetic nephropathy [4,5,6].
As well as the circulating RAS (Figure 1), there are, in tissues, ‘protease Ang II-producing systems’, which include prorenin (precursor of renin). Tissue RASs appear to play a role in cell growth, differentiation, and angiogenesis [7]. Since prorenin, the inactive precursor of renin, is the only form of renin produced in tissues, it has to be activated by removal of a 28-amino-acid pro-segment that covers its catalytic cleft by tissue proteases or by uncovering the catalytic cleft by binding to the (pro)renin receptor [8]. The decapeptide Ang I, produced by the action of renin, is cleaved by dipeptidase, an angiotensin-converting enzyme (ACE), to produce the biologically active octapeptide Ang II [9].
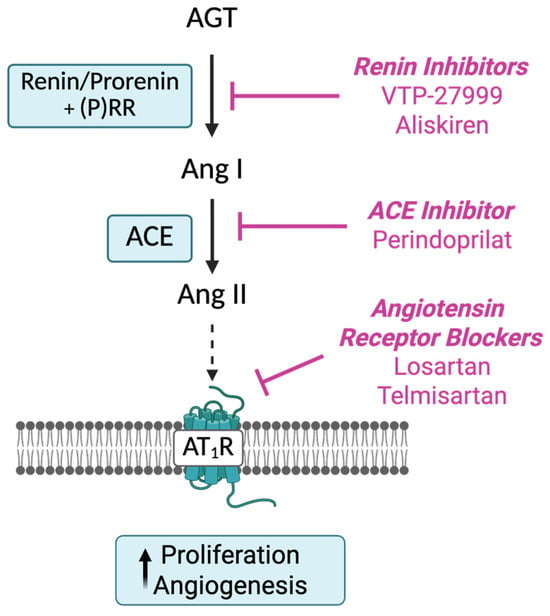
Figure 1.
Sites of action of drugs acting on the RAS cascade. Renin inhibitors, including VTP-27999 and aliskiren, act directly on renin/prorenin to prevent the production of angiotensin (Ang) I from angiotensinogen (AGT). Angiotensin-converting enzyme inhibitors (ACEIs) act by inhibiting the conversion of Ang I to Ang II, resulting in the reduced production of Angiotensin (Ang) II, the main biologically active peptide in the cascade. Ang II receptor blockers (ARBs) act directly to inhibit activation of the Ang II type 1 receptor (AT1R). Created in BioRender. Pringle, K. (2025) https://BioRender.com/0xqzg94.
There is some evidence that long-term use of anti-hypertensives that reduce the activity of the RAS, such as ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), reduces the risk of some cancers, particularly female-specific cancers [10]. A more recent study has, however, shown that RAS inhibitors are associated with a decreased risk of cervical and ovarian cancer but an increased endometrial cancer risk [11]. Thus, the epidemiological associations are still under debate [12]. Previous laboratory studies showed that ARBs inhibited the growth of several cancers both in vivo and in vitro, including colon [13], gastric [14,15,16], breast [17,18], and endometrial cancers [19]. Watanabe et al. showed that endometrial cancer cells treated with the major effector peptide of the RAS, Ang II, had increased vascular endothelial growth factor (VEGF) expression and endothelial cell migration [20]. It should be noted that some ARBs, such as telmisartan, have additional actions including acting as a partial agonist of peroxisome proliferator-activated receptor gamma (PPAR-γ) [21].
Key genes controlling the synthesis and activity of RAS components like prorenin (REN), angiotensinogen (AGT), AT1R (AGTR1), and angiotensin converting enzyme (ACE), as well as Ang II, are present in the normal endometrium and show cyclical changes in levels of expression [22,23,24,25,26]. They are also abundantly expressed in endometrial cancer tissues and could play a role in cancer growth and angiogenesis [27,28,29,30,31]. Therefore, drugs that act on the RAS and that are used to treat hypertension could be repurposed and used in the control of growth and spread of endometrial cancer (Figure 1).
Since we had shown that the RAS is overexpressed in endometrial cancers [27], we measured expression of RAS genes (REN, AGT, ACE, and AGTR1) in three endometrial cancer cell lines and determined if cell viability and cell proliferation were inhibited by drugs that act to block the activity of the RAS at sites shown in Figure 1. These were the renin inhibitors (aliskiren and VTP-27999), a drug that inhibits ACE and blocks the formation of Ang II (perindoprilat), and drugs that block the interaction between Ang II and its AT1R (losartan and telmisartan).
2. Results
2.1. RAS mRNA and Protein Levels in Endometrial Cancer Cell Lines
Three endometrial cancer cell lines (Ishikawa, HEC-1-A, and AN3CA) were cultured, and RAS mRNA abundances and levels of secreted RAS proteins were measured. There were significant differences between these three cell lines in their expression of RAS genes and proteins (Figure 2).
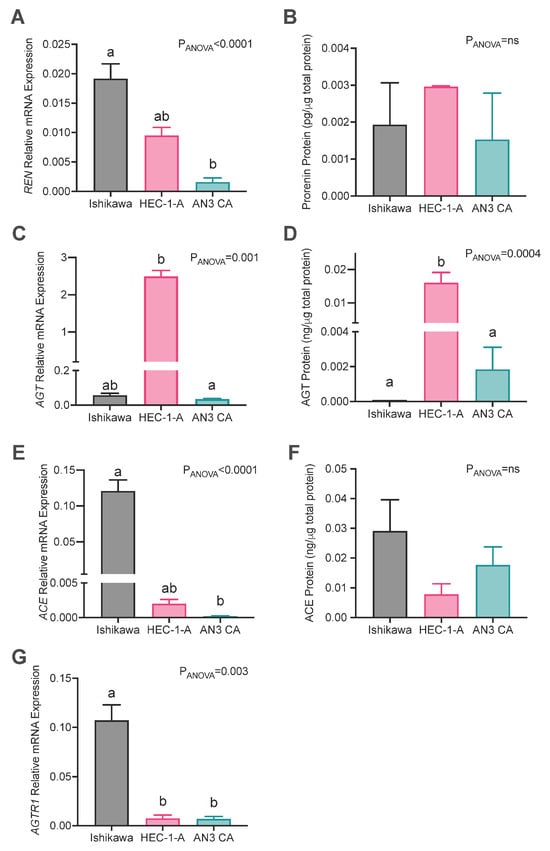
Figure 2.
Expression of RAS genes in endometrial cancer cell lines. Abundances of (A,B) prorenin (REN), (C,D) angiotensinogen (AGT), (E,F) angiotensin-converting enzyme (ACE) mRNA and protein levels, and (G) angiotensin II type 1 receptor (AGTR1) mRNA abundances in Ishikawa, HEC-1-A, and AN3CA cells. Data are expressed as the mean ± SEM. Different superscript letters denote significant differences between cell lines. mRNA: N = 3 experiments performed independently with technical replicates. Protein abundance: N = 3–6 independent experiments.
The expression of REN mRNA was most abundant in Ishikawa cells, which had significantly higher levels of REN mRNA than AN3CA cells (p = 0.003) but not HEC-1A cells (Figure 2A). Conversely, prorenin protein levels in cell culture supernatants were similar in all three cell lines (Figure 2B). AGT mRNA and protein levels were abundant in HEC-1-A cells (Figure 2C,D) and were greater than those in AN3CA (mRNA: p = 0.004; protein: p = 0.038) and Ishikawa cells, although not significantly at the mRNA level for Ishikawa cells (mRNA: p = 0.124; protein: p = 0.003). ACE mRNA expression was highest in Ishikawa cells compared with HEC-1A and AN3CA, although the difference between Ishikawa and HEC-1A cells was not statistically significant (p = 0.17 and p = 0.002, respectively; Figure 2E). Conversely, there was no difference in ACE protein levels in cell culture supernatants from the three cell lines (Figure 2F). AGTR1 expression was significantly higher in Ishikawa cells compared with both HEC-1-A and AN3CA cells (p = 0.025 and 0.038, respectively, Figure 2G). Thus, overall Ishikawa had the greatest levels of expression of REN and ACE and HEC-1-A had the highest expression of AGT. On the other hand, only in HEC-1-A cells were protein levels greater than that measured in other cell lines, specifically AGT.
2.2. Effect of Blockade of the RAS on Cell Viability and Proliferation Rates
2.2.1. Effect of Renin Inhibitors VTP-27999 and Aliskiren
Treatment with VTP-27999 caused a minimal reduction in the cell viability of Ishikawa and HEC-1-A cells by an average of 7–13% at doses of 10 and 100 μM (Supplementary Figure S1). Treatment with aliskiren reduced cell viability in Ishikawa cells by an average of 20% (Supplementary Figure S1). Effects were only significant with high doses (100 μM). There were no effects on the viability of AN3CA cells (Supplementary Figure S1).
2.2.2. Effects of Angiotensin Receptor Blockers (ARBs) Losartan and Telmisartan on Cell Viability and Cell Proliferation
Treatment with losartan had a small stimulatory effect on the viability of Ishikawa, AN3CA, and HEC-1-A cells (Figure 3A) at a dose of 100 μM (p = 0.003, 0.0009, and <0.0001, respectively) and markedly stimulated the proliferation rate of Ishikawa cells with doses of 0.1 and 10 μM (p = 0.018 and 0.002, respectively). However, treatment with 100 μM of losartan was associated with a 65% reduction in Ishikawa cell proliferation (Figure 3B; p = 0.005).
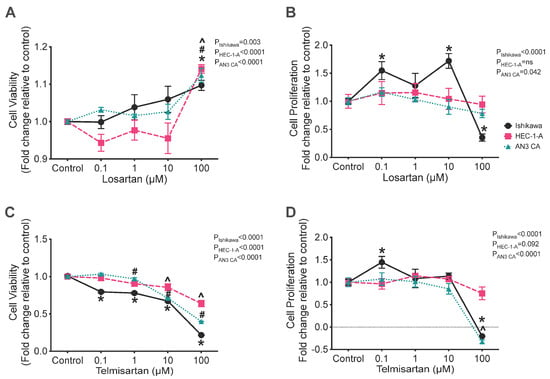
Figure 3.
Effect of angiotensin receptor blockers (losartan and telmisartan) on the cell viability (A,B) and rate of cell proliferation (C,D) in Ishikawa (black), HEC-1-A (pink), and AN3CA (blue) cells. (A) Treatment with 100 μM of losartan significantly enhanced the viability of Ishikawa, HEC-1-A, and AN3CA compared with that of their respective vehicle controls (*, p = 0.003, #, p = 0.0009, and ^, p < 0.0001, respectively). (B) In Ishikawa cells, treatment with 0.1 or 10 μM of losartan significantly enhanced the proliferation of Ishikawa cells compared with that of vehicle control cells (*: p = 0.018 and 0.002, respectively). At 100 μM of losartan, the proliferation of Ishikawa cells was significantly reduced compared with that of vehicle control cells (*: p = 0.005). (C) Treatment with telmisartan significantly reduced cell viability in Ishikawa, HEC-1-A, and AN3CA cells at 10 and 100 μM (*, both p < 0.0001, #, p = 0.001 and <0.0001, and ^, both p < 0.0001, respectively). At 1 μM of telmisartan, the viability of Ishikawa and HEC-1-A cells was reduced compared with that of vehicle control cells (*, p < 0.0001 and #, p = 0.045, respectively). Ishikawa cell viability was also significantly reduced with treatment with 1 μM of telmisartan (*: p < 0.0001). (D) The cell proliferation of Ishikawa cells was enhanced by treatment with 1 μM of telmisartan compared with that of vehicle control cells (*: p = 0.042). Treatment with telmisartan at 100 μM significantly reduced the cell proliferation of Ishikawa and AN3CA cells (*, p < 0.0001 and ^, p < 0.0001, respectively). In HEC-1-A cells, proliferation was unaffected by treatment with telmisartan. Results are expressed as mean fold change relative to respective vehicle control cells ± SEM. N = 3 independent experiments in triplicate.
Treatment with telmisartan caused a consistent reduction in the viability of all three cell lines (Figure 3C). Treatment with telmisartan at a concentration of 0.1, 1, 10, or 100 μM significantly reduced the viability of Ishikawa cells compared with the control (Figure 3C; all p = 0.0001) and treatment with 1, 10, or 100 μM of telmisartan also reduced the viability of HEC-1-A cells (Figure 3C; p = 0.045, 0.001, and 0.0001, respectively). In AN3CA cells, cell viability was also significantly reduced by treatment with 10 or 100 μM of telmisartan (Figure 3C; both p = 0.0001).
Additionally, treatment with telmisartan significantly altered the rates of cell proliferation of both Ishikawa and AN3CA cells, although it had no effect on the proliferation of HEC-1-A cells (Figure 3D). In Ishikawa cells, telmisartan had a biphasic effect on their rate of proliferation; 0.1 μM of telmisartan was associated with a 45% increase in the rate of cell proliferation (Figure 3D; p = 0.04), whereas 100 μM of telmisartan was associated with a 120% reduction in cell proliferation, which was indicative of cell death (Figure 3D; p = 0.0001). Similarly, 100 μM of telmisartan was associated with a significant reduction in cell proliferation of 130% in AN3CA cells (Figure 3D; p = 0.0001).
2.2.3. Comparison of the Effects of Telmisartan with a PPAR-γ Agonist (Troglitazone) and the Efficacy of Combination Treatment with Telmisartan and Troglitazone on Cell Viability and Proliferation
Since telmisartan, which is both an ARB and a PPAR-γ agonist, was effective in inhibiting cell viability and proliferation of all three cell lines, we determined if its effects depended on the fact that it is a PPAR-γ agonist as well as an ARB. Therefore, the three cell lines were cultured with troglitazone or telmisartan alone and both in combination.
Troglitazone alone was the least effective treatment; both telmisartan alone and the combination of telmisartan + troglitazone were more effective at reducing the cell viability of Ishikawa cells compared with troglitazone alone (Figure 4D; p < 0.0001). The viability of Ishikawa cells was reduced by treatment with 0.1, 1, 10, or 100 μM of both telmisartan alone and telmisartan + troglitazone by an average of 10–60% (Figure 4D, all p < 0.01). Troglitazone alone reduced the cell viability of Ishikawa cells by an average of 50% at 10 and 100 μM (Figure 4A, p = 0.022 and <0.0001, respectively).
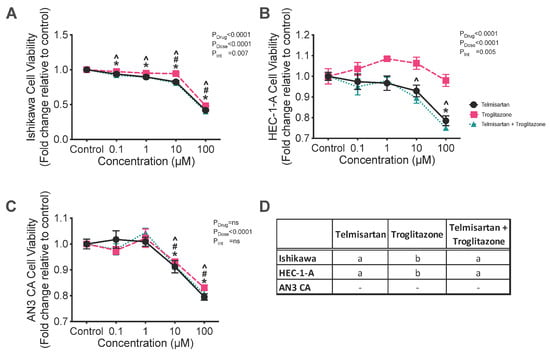
Figure 4.
Effect of the combination of telmisartan, an ARB and partial PPAR-γ agonist, and troglitazone, a PPAR-γ agonist, on cell viability in (A) Ishikawa, (B) HEC-1-A, and (C) AN3CA cells. (A) Ishikawa cell viability was reduced by treatment with 0.1, 1, 10, or 100 μM of telmisartan alone and telmisartan + troglitazone (*, p = 0.0090, <0.0001, <0.0001, and <0.0001 and ^, p = 0.0006, <0.0001, <0.0001, and <0.0001, respectively). Troglitazone reduced the viability of Ishikawa cells at 10 and 100 μM (#: p = 0.022 and <0.0001, respectively). (B) Treatment with telmisartan significantly reduced the viability of HEC-1-A cells at 100 μM (*: p < 0.0001). Combined treatment with telmisartan + troglitazone reduced the viability of HEC-1-A cells at 10 and 100 μM (^: p = 0.035 and <0.0001). (C) Treatment with telmisartan alone, troglitazone alone, or telmisartan + troglitazone reduced the viability of AN3CA cells at 10 and 100 μM (*, p = 0.007 and <0.0001, #, p = 0.050 and <0.0001, and ^, p = 0.01 and <0.0001, respectively). (D) Different letters denote significant differences in the drug treatments within each cell line. Results are expressed as mean fold change relative to respective vehicle control cells ± SEM. N = 3 independent experiments in triplicate.
Treatment with telmisartan alone and telmisartan + troglitazone in combination were also more effective at reducing the viability of HEC-1-A cells compared with troglitazone alone (Figure 4D; both p < 0.0001). The combined treatment of telmisartan + troglitazone significantly reduced the viability of HEC-1-A cells at 100 μM by an average of 25% (Figure 4B; both p < 0.0001). Combined treatment with telmisartan + troglitazone also reduced the viability of HEC-1-A cells at 10 μM by an average of 10% (p = 0.035, Figure 4B).
In AN3CA cells, treatment with telmisartan alone, troglitazone alone, or telmisartan + troglitazone reduced the viability of AN3CA cells at 10 and 100 μM by approximately 10 and 20%, respectively (Figure 4C; all p < 0.05). There were no significant differences between these three treatments (Figure 4D).
2.2.4. Combined Effect of Aliskiren and an ACEI (Perindoprilat) or an ARB (Losartan) on Cell Viability
Since the effects of the inhibitors of the circulating RAS were limited in their capacity to reduce cell viability or inhibit cell proliferation, it was decided to see if the combination of a renin inhibitor with either an ACEI or with an ARB was more effective.
The combination treatment of aliskiren + perindoprilat was significantly more effective at reducing cell viability compared with either aliskiren or perindoprilat alone in HEC-1-A cells (Supplementary Figure S2), neither of which had any effect on cell viability. The combined treatment of aliskiren + perindoprilat significantly reduced the cell viability of HEC-1-A cells at 10 and 100 μM by approximately 10–15% (Supplementary Figure S2; p = 0.0004 and <0.0001).
Overall, treatment of AN3CA cells with inhibitors either alone or in combination had no inhibitory effect on the viability of AN3CA cells and had minimal enhancing effects (<5% change from control, Supplementary Figure S2).
The combination of aliskiren and losartan had no synergistic effect on the viability of Ishikawa, HEC-1-A, or AN3CA cells (Supplementary Figure S3). However, in Ishikawa cells, the combination of aliskiren and losartan mitigated the effect of aliskiren alone (Supplementary Figure S3).
3. Discussion
We have shown that genes controlling the expression of key components of the RAS cascade were expressed in the Ishikawa, HEC-1-A, and AN3CA endometrial cancer cell lines. Overall, Ishikawa cells appeared to express REN, ACE, and AGTR1 mRNAs in the greatest abundance. Therefore, it was not surprising that any effects of RAS-blocking drugs on cell viability and proliferation were most evident in the Ishikawa cells. Overall, the AN3CA cell line was most resistant to any inhibitory effects of the drugs studied on cell viability and/or proliferation.
Low levels of mRNA expression of REN in all three cell lines (Figure 2, as determined by a qPCR cycle threshold > 32), as well as low levels of soluble prorenin, may indicate that REN was not expressed in amounts sufficient to activate the RAS. This could account for the low efficacy of the two renin inhibitors studied, i.e., aliskiren and VTP-27999 (Supplementary Figure S1). We have previously shown that endometrial adenocarcinomas express prorenin mRNA and protein abundantly [27], so these low levels of REN mRNA were unexpected. We have also shown that there is a wide range of REN expression in both the cancerous endometrium and adjacent normal endometrium [27]. Therefore, while the drugs are ineffective in inhibiting cancer growth in established cancer cell lines, they may block the increased activity of the RAS in the pre-cancerous stages of endometrial dysfunction associated with endometrial hyperplasia and estrogen stimulation [32]. This may also indicate that REN mRNA is primarily produced in the stroma of the endometrium, rather than the glandular epithelium. Thus, these drugs should be assessed in culture models containing both stromal and glandular cells.
Interestingly, the expression of RAS components tends to correlate negatively with the histological grade the cell line represents. That is, Ishikawa cells, which expressed the highest level of most RAS components, are histologically representative of grade 1 tumours [33], whereas the AN3CA cell line, which expressed the lowest levels of RAS components, is histologically representative of grade 3 tumours (metastatic undifferentiated) [34]. This indicates that the RAS may be inactivated as cancer cells differentiate. This was also shown by Piastowska-Ciesielska et al. who found that the expression of AGTR1 mRNA and protein levels was highest in grade 1 tumours compared with grade 3 tumours [28]. Thus, it is possible that at higher grades, the RAS is no longer regulating endometrial cancer cell growth and RAS-inhibiting drugs are likely to be ineffective. It is also possible that RAS inhibitors may be effective in the prodromal phase of disease, such as during endometrial hyperplasia, prior to the development of endometrial cancer.
The three cell lines used in the study have also been reported to have different levels of estrogen receptors [35] and differing responses to estrogen [36]. Ishikawa cells have high expression of estrogen receptor (ER)-α and ER-β [35] and are responsive to estrogen treatment [36], while the expression of these receptors is low in HEC-1-A and AN3CA cells [35]. Since many components of the RAS are stimulated by estrogens, it is perhaps not surprising that expression of RAS is highest in Ishikawa cells, which have high levels of the estrogen receptors [35] and are estrogen-responsive [36]. Given the different expression of RAS components and estrogen receptors in the cell lines, as well as the interactions between estrogen and the RAS, future studies should assess the impact of estrogens on RAS expression in these cells and the effects of the RAS antagonists in the presence of estrogens.
It is well established that estrogens can cause endometrial hyperplasia and pre-cancerous changes in the endometrium. This is particularly noted in patients taking tamoxifen (a selective estrogen receptor modulator) as an adjunct therapy for breast cancer. Patients undergoing tamoxifen treatment have a significantly increased risk of developing endometrial hyperplasia and a 1.5–6.9-fold increased risk of developing endometrial cancer [37]. Currently, no studies have looked at the expression of the endometrial RAS in women taking tamoxifen for the treatment of breast cancer. Future studies should therefore be undertaken to examine the endometrial RAS in these patients, as RAS inhibitors may be effective at reducing endometrial hyperplasia and the progression to endometrial cancer.
In most instances, the reduction in cell viability and/or proliferation caused by the RAS antagonists was minimal and significant reductions in cell viability and cell growth only occurred when high concentrations of drugs were used. Specifically, treatment with high doses of a renin inhibitor, VTP-27999 or aliskiren, was minimally effective at inhibiting cell viability and proliferation in Ishikawa and HEC-1-A cells and did not affect AN3CA cells. In addition, perindoprilat, an ACE inhibitor, did not have any effect on the viability of any of the three endometrial cancer lines. To the best of our knowledge, this is the first study to examine the effect of renin inhibitors (aliskiren and VTP-27999) and ACE inhibitors (perindopril) in endometrial cancer cells. Surprisingly, at high concentrations, losartan significantly increased the viability of all three cancer cell lines. This finding is similar to that reported by Koyama et al., who also showed that losartan increased the viability of endometrial cancer cells [19]. Contrastingly, the rate of proliferation of Ishikawa cells was significantly reduced when treated with 100 μM losartan. Since aliskiren, VTP-27999, perindopril, and losartan only reduced cell viability and proliferation to a limited extent (~10–15%) and high doses were required to have any effect, only telmisartan would likely be of clinical value as an adjunct treatment for endometrial cancer. These conclusions are supported by the fact that the combination of drug treatments used in this study had no additive effect.
Overall, telmisartan, the combined ARB and PPAR-γ agonist [21], appeared to be the most effective inhibitor of cell viability in all three cell lines (Figure 3 and Figure 4). Telmisartan’s most significant effects were on Ishikawa cells, which expressed REN, ACE, and AGTR1 to a greater extent than the other cell lines. These three genes are the key regulators of Ang II production and signalling. It should be noted that in a previous study, we found an association between the prevalence of endometrial cancer and single nucleotide polymorphisms (SNPs) associated with increased expression of AT1R (rs5186) and ACE (rs4291 and rs4292) [38]. We also showed that the mRNA expression of AGTR1 and ACE were significantly higher in endometrial cancer tissue, further indicating the potential for increased Ang II/AT1R signalling in endometrial cancer [27]. The efficacy of telmisartan in reducing the viability and proliferative capacity of endometrial cancer cell lines is consistent with results obtained by Koyama et al. [19]. The effects of telmisartan, in part, must be a result of AT1R inhibition as troglitazone, a sole PPAR-γ, alone was not as effective at reducing cell viability compared with telmisartan. These results are similar to those observed in human colon cancer cells [13].
Angiogenesis is essential for tumour growth and spread. Activation of AT1R by Ang II stimulates the secretion of VEGF from endometrial cancer cell lines, including HEC-1-A [20]. VEGF is a powerful angiogenic factor, which promotes tumour angiogenesis [39]. Recently, it has been shown that aerobic exercise induced the ACE/Ang II/AT1R/VEGF axis in skeletal muscle and increased the capillary-to-fibre ratio by inhibiting miRNAs miR-27a and b. It was further shown that losartan partially reversed these changes through effects on miR-27a and b [40]. Such an angiogenic pathway in tumorigenesis has not been demonstrated, yet the hypoxia induced by the metabolic demand of rapidly dividing pre-cancerous lesions could induce a similar response to that seen in exercising skeletal muscle. This merits its investigation in endometrial hyperplasia and endometrial cancer.
In conclusion, some of the drugs that limit the formation and actions of Ang II via the AT1R modestly reduce the viability and proliferation of endometrial cancer cells. This study did not examine the effects of these drugs on angiogenesis nor on cell migration, two other properties of cancer cells that promote malignancy. Also, some of the effects we observed could be off-target actions of the drugs. However, their increased efficacy in the cell line that grew most vigorously and had the highest level of expression of the renin–Ang II/AT1R pathway (Ishikawa) suggests that their effects on cell viability and proliferation were at least partially related to their inhibitory effects on the RAS in this tumour cell line.
4. Materials and Methods
4.1. Cell Culture
Three endometrial epithelial adenocarcinoma cell lines were used for this study; these were Ishikawa (confirmed by genotyping), AN3CA, and HEC-1-A cells (American Type Culture Collection, Manassas, VA, USA). Ishikawa and AN3CA cells were cultured in minimum essential medium (MEM; Sigma Aldrich, St. Louis, MO, USA) supplemented with 5% heat-inactivated fetal bovine serum (HI-FBS; Bovogen Biologicals, Keilor East, VIC, Australia) and 1% antibiotic–antimycotic (Thermo Fisher Scientific, Waltham, MA, USA). HEC-1-A cells were cultured in McCoy’s 5A medium (Sigma Aldrich) supplemented with 10% HI-FBS (Bovogen Biologicals), 1% antibiotic–antimycotic, and 2 mM L-glutamine (Thermo Fisher Scientific). For gene expression studies, cells were seeded at a density of 1.5 × 105 in 6-well plates in 2 mL of incubation medium and allowed to settle overnight. After 24 h, the incubation medium was changed, and cells were cultured for 48 h. Cells were then harvested and snap frozen in liquid nitrogen and stored at −80 °C for subsequent mRNA analyses.
4.2. Semi-Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (qPCR)
Total RNA was isolated using the RNeasy mini kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). RNA samples were DNase-treated (Qiagen). RNA quantity was assessed using the Nanodrop spectrophotometer and RNA quality was determined by agarose gel electrophoresis. RNA was then reverse-transcribed using a Superscript III RT kit with random hexamers (Invitrogen, Waltham, MA, USA).
qPCR was performed with a 7500 Real Time PCR System (Applied Biosystems, Waltham, MA, USA) using SYBR Green for detection. Each reaction contained 5 μL of SYBR Green PCR master mix (Applied Biosystems), primers [27], cDNA reversed-transcribed from 10 ng of total RNA, and water to 10 μL. Genes examined were REN, ATP6AP2 AGT, ACE, and AGTR1. The geometric average of the three housekeepers was measured. These housekeepers were β-actin (ACTB; [27]), 18s ribosomal RNA (RNA18S1; Fwd: GTAACCCGTTGAACCCCATT; Rev: CCATCCAATCGGTAGTAGCG), and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ; Fwd: CCTGCATGAAGTCTGTAACTGAG; Rev: GACCTACGGGCTCCTACAACA), which were stably expressed between cell types. Messenger RNA abundance was calculated as described previously, using the 2−ΔΔCT method and expressed relative to the mean of ACTB, RNA18S1, and YWHAZ and a calibrator sample (a term placental sample collected upon elective cesarean section) [19].
4.3. ELISAs
Total protein was isolated from cell culture media by acetone precipitation for both prorenin and AGT ELISA, as previously described [41]. ACE protein from cell culture media was run undiluted. Acetone precipitation was completed by combining the medium supernatant with three times the volume of chilled acetone (−20 °C, UNIVAR, Downers Grove, IL, USA). Samples were then incubated at −20 °C for 4 h before being centrifuged at 14,000× g for 10 min at 4 °C. The precipitated proteins were re-solubilised in RIPA buffer and quantified by BCA assay (Thermo Fisher Scientific).
The levels of prorenin, AGT, and ACE were measured by commercially available ELISA kits (Prorenin ELISA, Abcam, Cambridge, UK; AGT and ACE Duoset ELISAs, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. As all samples were assayed on one plate, there was no inter-assay variability. The intra-assay variability was 8.4% for prorenin, 4.3% for AGT, and 4.2% for ACE.
4.4. Assessment of Cell Viability
Effects of RAS inhibitors on cell viability was assessed using a Resazurin Assay. Cells were seeded in 100 μL of respective medium in 96-well plates and allowed to settle overnight. The cells were seeded at densities determined by individual cell growth rates. The cell densities for Ishikawa, HEC-1-A, and AN3CA were 1.5 × 102 cells/well, 5 × 103 cells/well, and 2.5 × 102 cells/well, respectively. After 24 h the culture medium was replaced with medium containing a 0.1 μM, 1 μM, 10 μM, or 100 μM concentration of one of the following drugs:
- (1)
- A renin inhibitor (either aliskiren or VTP-27999; Medchem Express);
- (2)
- An ACE inhibitor (perindoprilat; Sigma Aldrich);
- (3)
- An angiotensin receptor blocking drug (either losartan or telmisartan; Sigma Aldrich);
- (4)
- A PPAR-γ agonist (troglitazone; Sigma Aldrich).
Or the medium contained a combination of the following (at the doses above):
- (1)
- Aliskiren and perindoprilat;
- (2)
- Aliskiren and losartan;
- (3)
- Telmisartan and troglitazone.
Drug concentrations of 0.1–100 μM were chosen based on previously published studies investigating the effects of these drugs on cell proliferation in other cancer cell types [42,43,44,45,46,47].
At 43 h of culture, 5 h before the endpoint, 20 μL of Resazurin Reagent (BioVision, Milpitas, CA, USA, 0.15 mg/mL) was added to each well and the plates were returned to the incubator. At 48 h, fluorescence was measured at 590 nM emission using the FLUOStar OPTIMA (BMG Labtech, Offenburg, Germany) after 570 nM excitation. Data are expressed as the fold change (in relative fluorescence units (RFU)) relative to the average of the vehicle control for each experiment for each cell line.
4.5. Measurement of Cell Proliferation
The effects of RAS inhibitors on cell proliferation were measured using an xCELLigence RTCA DP instrument (ACEA Biosciences, Hangzhou, China). Sixteen-well E-plates (ACEA Biosciences) were used to study the rate of cell proliferation. Briefly, prior to beginning the experiment, 100 μL of medium was added to each well to allow the E-plate to achieve equilibrium. After 30 min, a background impedance reading was recorded. One hundred microliters of medium containing the densities of each cell type (described above) was added and the E-plates were inserted into the xCELLigence machine and left for 30 min to allow the cells to attach to the plate, after which readings commenced. After 24 h, the culture medium was replaced with fresh medium containing the drugs listed above. Cell proliferation was monitored by measuring the cell index (change in electrical impedance) every 15 min for 48 h. Data are expressed as the fold change in slope of the impedance trace over 48 h, relative to the average of the vehicle control for each experiment for each cell line assessed.
4.6. Statistics
All experiments were performed three times, with each dose being tested at least in duplicate. A Kruskal–Wallis test with Dunn’s multiple-comparisons test was used to determine differences in mRNA expression between cell lines and the effects of the drug treatments within each cell line. GraphPad Prism (Version 10.0) was used for all graphs and statistical analyses. Statistical significance was set at p < 0.05. If a sample had undetectable levels of mRNA expression as measured by qPCR, a value of 1 × 10−5, which was 100 times lower than the lowest value, was allocated so that it could be included in the analyses.
5. Conclusions
Overall, this is the first study to examine the effect of renin inhibitors on endometrial cancer cell lines. Levels of expression of the RAS genes may therefore be biomarkers for drug efficacy. Telmisartan consistently reduced cell proliferation and cell viability in all three endometrial cancer cell lines and was more effective than either a renin inhibitor, a sole ARB (losartan), or a sole PPAR-γ agonist. Future studies examining the efficacy of these RAS blockers in a mouse model of endometrial cancer will shed more light on the potential of RAS blockers to inhibit endometrial cancer growth and vasculogenesis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms262210968/s1.
Author Contributions
Conceptualization, Y.W., N.M.V., E.R.L., and K.G.P.; Formal analysis, S.J.D. and R.M.; Funding acquisition, S.J.D., E.R.L., and K.G.P.; Investigation, S.J.D., R.M., and T.L.S.; Methodology, S.J.D., R.M., T.L.S., Y.W., N.M.V., E.R.L., and K.G.P.; Supervision, N.M.V. and K.G.P.; Writing—original draft, S.J.D. and R.M.; Writing—review and editing, T.L.S., Y.W., N.M.V., E.R.L., and K.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge funding from the John Hunter Hospital Charitable Trust and the Hunter Cancer Research Alliance. K.G.P and N.V. were supported by ARC Future Fellowships (FT1500100179 and G1601252). S.J.D received funding from the Hunter Medical Research Institute G2200126.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Yu Wang is an employee of and owns stock in Beroni Group Ltd. and its subsidiaries. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hubbard, S.A.; Gargett, C.E. A cancer stem cell origin for human endometrial carcinoma? Reproduction 2010, 140, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.C.; Devine, C.E.; Rao, B.; Sagebiel, T.; Bhosale, P. Imaging and Staging of Endometrial Cancer. Semin. Ultrasound CT MR 2019, 40, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Cassis, L.A.; Kooi, C.W.; Daugherty, A. Structure and functions of angiotensinogen. Hypertens Res. 2016, 39, 492–500, Erratum in Hypertens Res. 2016, 39, 827. https://doi.org/10.1038/hr.2016.106. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Ito, S.; Izzo, J.L.; Januszewicz, A.; Katayama, S.; Menne, J.; Mimran, A.; Rabelink, T.J.; Ritz, E.; Ruilope, L.M.; et al. Olmesartan for the Delay or Prevention of Microalbuminuria in Type 2 Diabetes. N. Engl. J. Med. 2011, 364, 907–917. [Google Scholar] [CrossRef]
- Poole-Wilson, P.A. ACE inhibitors and ARBs in chronic heart failure: The established, the expected, and the pragmatic. Med. Clin. N. Am. 2003, 87, 373–389. [Google Scholar] [CrossRef]
- Abraham, H.A.; White, M.C.; White, W.B. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015, 38, 33–54. [Google Scholar] [CrossRef]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Nguyen, G.; Delarue, F.; Burcklé, C.; Bouzhir, L.; Giller, T.; Sraer, J.D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Investig. 2002, 109, 1417. [Google Scholar] [CrossRef]
- Erdos, E.G. The angiotensin I converting enzyme. Fed. Proc. 1977, 36, 1760–1765. [Google Scholar] [CrossRef]
- Lever, A.F.; Hole, D.J.; Gillis, C.R.; McCallum, I.R.; McInnes, G.T.; MacKinnon, P.L.; Meredith, P.A.; Murray, L.S.; Reid, J.L.; Robertson, J.W. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet 1998, 352, 179–184. [Google Scholar] [CrossRef]
- Nguyen, N.T.H.; Nguyen, P.A.; Huang, C.W.; Wang, C.H.; Lin, M.C.; Hsu, M.H.; Bao, H.B.; Chien, S.C.; Yang, H.C. Renin-Angiotensin-Aldosterone System Inhibitors and Development of Gynecologic Cancers: A 23 Million Individual Population-Based Study. Int. J. Mol. Sci. 2023, 24, 3814. [Google Scholar] [CrossRef]
- Zhang, R.; Yin, H.; Yang, M.; Liu, J.; Zhen, D.; Zhang, Z. Advanced progress of the relationship between renin-angiotensin-aldosterone system inhibitors and cancers. J. Hypertens. 2024, 42, 1862–1873. [Google Scholar] [CrossRef]
- Lee, L.D.; Mafura, B.; Lauscher, J.C.; Seeliger, H.; Kreis, M.E.; Gröne, J. Antiproliferative and apoptotic effects of telmisartan in human colon cancer cells. Oncol. Lett. 2014, 8, 2681–2686. [Google Scholar] [CrossRef]
- Okazaki, M.; Fushida, S.; Harada, S.; Tsukada, T.; Kinoshita, J.; Oyama, K.; Tajima, H.; Ninomiya, I.; Fujimura, T.; Ohta, T. The angiotensin II type 1 receptor blocker candesartan suppresses proliferation and fibrosis in gastric cancer. Cancer Lett. 2015, 355, 46–53. [Google Scholar] [CrossRef]
- Fujita, N.; Fujita, K.; Iwama, H.; Kobara, H.; Fujihara, S.; Chiyo, T.; Namima, D.; Yamana, H.; Kono, T.; Takuma, K.; et al. Antihypertensive drug telmisartan suppresses the proliferation of gastric cancer cells in vitro and in vivo. Oncol. Rep. 2020, 44, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Tsujiya, Y.; Yamamori, M.; Hasegawa, A.I.; Yamamoto, Y.; Yashiro, M.; Okamura, N. Telmisartan Exerts Cytotoxicity in Scirrhous Gastric Cancer Cells by Inducing G(0)/G(1) Cell Cycle Arrest. Anticancer. Res. 2021, 41, 5461–5468. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, Q.; Zhao, Y.; Liu, M.; Li, D.; Yang, Y.; Sun, L.; Sui, G.; Cai, L.; Dong, X. Angiotensin II type 1 receptor antagonists inhibit cell proliferation and angiogenesis in breast cancer. Cancer Lett. 2013, 328, 318–324. [Google Scholar] [CrossRef]
- Kumar, U.; Aich, J.; Devarajan, S. Exploring the repurposing potential of telmisartan drug in breast cancer: An in-silico and in-vitro approach. Anticancer Drugs 2023, 34, 1094–1103. [Google Scholar] [CrossRef]
- Koyama, N.; Nishida, Y.; Ishii, T.; Yoshida, T.; Furukawa, Y.; Narahara, H. Telmisartan induces growth inhibition, DNA double-strand breaks and apoptosis in human endometrial cancer cells. PLoS ONE 2014, 9, e93050. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shibata, K.; Kikkawa, F.; Kajiyama, H.; Ino, K.; Hattori, A.; Tsujimoto, M.; Mizutani, S. Adipocyte-Derived Leucine Aminopeptidase Suppresses Angiogenesis in Human Endometrial Carcinoma via Renin-Angiotensin System. Clin. Cancer Res. 2003, 9, 6497–6503. [Google Scholar]
- Benson, S.C.; Pershadsingh, H.A.; Ho, C.I.; Chittiboyina, A.; Desai, P.; Pravenec, M.; Qi, N.; Wang, J.; Avery, M.A.; Kurtz, T.W. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension 2004, 43, 993–1002. [Google Scholar] [CrossRef]
- Pawlikowski, M.; Meleń-Mucha, G.; Mucha, S. The involvement of the renin-angiotensin system in the regulation of cell proliferation in the rat endometrium. Cell. Mol. Life Sci. 1999, 55, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Ahmed, A. Dual role of angiotensin II in the human endometrium. Hum. Reprod. 1996, 11, 95–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.F.; Ahmed, A. Compartmentalization and cyclic variation of immunoreactivity of renin and angiotensin converting enzyme in human endometrium throughout the menstrual cycle. Hum. Reprod. 1997, 12, 2804–2809. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Li, X.F.; Shams, M.; Gregory, J.; Rollason, T.; Barnes, N.M.; Newton, J.R. Localization of the angiotensin II and its receptor subtype expression in human endometrium and identification of a novel high-affinity angiotensin II binding site. J. Clin. Investig. 1995, 96, 848–857. [Google Scholar] [CrossRef]
- Qi, R.; Li, T.C.; Chen, X. The role of the renin-angiotensin system in regulating endometrial neovascularization during the peri-implantation period: Literature review and preliminary data. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820920560. [Google Scholar] [CrossRef]
- Delforce, S.J.; Lumbers, E.R.; Corbisier de Meaultsart, C.; Wang, Y.; Proietto, A.; Otton, G.; Scurry, J.; Verrills, N.M.; Scott, R.J.; Pringle, K.G. Expression of renin-angiotensin system (RAS) components in endometrial cancer. Endocr. Connect. 2017, 6, 9–19. [Google Scholar] [CrossRef]
- Piastowska-Ciesielska, A.W.; Płuciennik, E.; Wójcik-Krowiranda, K.; Bieńkiewicz, A.; Bednarek, A.; Ochędalski, T. Analysis of the expression of angiotensin II type 1 receptor and VEGF in endometrial adenocarcinoma with different clinicopathological characteristics. Tumor Biol. 2012, 33, 767–774. [Google Scholar] [CrossRef]
- Shibata, K.; Kikkawa, F.; Mizokami, Y.; Kajiyama, H.; Ino, K.; Nomura, S.; Mizutani, S. Possible involvement of adipocyte-derived leucine aminopeptidase via angiotensin II in endometrial carcinoma. Tumor Biol. 2005, 26, 9–16. [Google Scholar] [CrossRef]
- Nowakowska, M.; Matysiak-Burzynska, Z.; Kowalska, K.; Pluciennik, E.; Dominska, K.; Piastowska-Ciesielska, A.W. Angiotensin II promotes endometrial cancer cell survival. Oncol. Rep. 2016, 36, 1101–1110. [Google Scholar] [CrossRef]
- Martin, J.H.; Mohammed, R.; Delforce, S.J.; Skerrett-Byrne, D.A.; de Meaultsart, C.C.; Almazi, J.G.; Stephens, A.N.; Verrills, N.M.; Dimitriadis, E.; Wang, Y.; et al. Role of the prorenin receptor in endometrial cancer cell growth. Oncotarget 2022, 13, 587–599. [Google Scholar] [CrossRef]
- Hapangama, D.K.; Bulmer, J.N. Pathophysiology of heavy menstrual bleeding. Womens Health 2016, 12, 3–13. [Google Scholar] [CrossRef]
- Nishida, M.; Kasahara, K.; Kaneko, M.; Iwasaki, H.; Hayashi, K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi 1985, 37, 1103–1111. [Google Scholar]
- Van Nyen, T.; Moiola, C.P.; Colas, E.; Annibali, D.; Amant, F. Modeling Endometrial Cancer: Past, Present, and Future. Int. J. Mol. Sci. 2018, 19, 2348. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Zhao, Y.; Wang, X.; Qi, Y.; Zhou, C.; Hua, Y.; Hou, J.; Jiang, S.W. Culture characters, genetic background, estrogen/progesterone receptor expression, and tumorigenic activities of frequently used sixteen endometrial cancer cell lines. Clin. Chim. Acta 2019, 489, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Pavlic, R.; Pusic, M.N.; Rizner, T.L. Evaluation of the effects of estrogens on endometrial cancer cells of different grades. J. Steroid Biochem. Mol. Biol. 2025, 251, 106762. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol. Oncol. 2004, 94, 256–266. [Google Scholar] [CrossRef]
- Pringle, K.G.; Delforce, S.J.; Wang, Y.; Ashton, K.A.; Proietto, A.; Otton, G.; Blackwell, C.C.; Scott, R.J.; Lumbers, E.R. Renin-angiotensin system gene polymorphisms and endometrial cancer. Endocr. Connect. 2016, 5, 128–135. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. 3), 4–10. [Google Scholar] [CrossRef]
- Rodrigues, L.F.; Pelozin, B.R.A.; da Silva Junior, N.D.; Soci, U.P.R.; do Carmo, E.C.; da Mota, G.d.F.A.; Cachofeiro, V.; Lahera, V.; Oliveira, E.M.; Fernandes, T. Angiotensin II Promotes Skeletal Muscle Angiogenesis Induced by Volume-Dependent Aerobic Exercise Training: Effects on miRNAs-27a/b and Oxidant–Antioxidant Balance. Antioxidants 2022, 11, 651. [Google Scholar]
- Morosin, S.K.; Delforce, S.J.; Lumbers, E.R.; Pringle, K.G. Cleavage of the soluble (pro)renin receptor (sATP6AP2) in the placenta. Placenta 2020, 101, 49–56. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.C.; Song, X.; Lu, J.R.; Jin, Z. KRT6 interacting with notch1 contributes to progression of renal cell carcinoma, and aliskiren inhibits renal carcinoma cell lines proliferation in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 9182–9188. [Google Scholar]
- Yasumatsu, R.; Nakashima, T.; Masuda, M.; Ito, A.; Kuratomi, Y.; Nakagawa, T.; Komune, S. Effects of the angiotensin-I converting enzyme inhibitor perindopril on tumor growth and angiogenesis in head and neck squamous cell carcinoma cells. J. Cancer Res. Clin. Oncol. 2004, 130, 567–573. [Google Scholar] [CrossRef]
- Yoshiji, H.; Kuriyama, S.; Kawata, M.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Nakatani, T.; Tsujinoue, H.; Fukui, H. The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: Possible role of the vascular endothelial growth factor. Clin. Cancer Res. 2001, 7, 1073–1078. [Google Scholar] [PubMed]
- Woo, Y.; Jung, Y.J. Angiotensin II receptor blockers induce autophagy in prostate cancer cells. Oncol. Lett. 2017, 13, 3579–3585. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Chou, C.H.; Li, Y.F.; Huang, L.C.; Kao, Y.; Hueng, D.Y.; Tsai, C.K. Antiproliferative and apoptotic effects of telmisartan in human glioma cells. Cancer Cell Int. 2023, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Tsujiya, Y.; Hasegawa, A.; Yamamori, M.; Okamura, N. Troglitazone-Induced Autophagic Cytotoxicity in Lung Adenocarcinoma Cell Lines. Biol. Pharm. Bull. 2022, 45, 276–283. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).