Special Issue “Molecular Advances in Cancer Immunotherapy”
Acknowledgments
Conflicts of Interest
References
- Awadasseid, A.; Wu, M.; Zhang, F.; Song, Y.; Wu, Y.; Zhang, W. Novel PD-L1 Small-Molecule Inhibitors Advancing Cancer Immunotherapy. Anticancer Agents Med. Chem. 2025. [Google Scholar] [CrossRef]
- Crowther, M.D.; Sohlin, J.E.; Svane, I.M.; Met, Ö. Tumour-infiltrating lymphocyte therapy comes of age in the era of genetic engineering. Lancet Oncol. 2025, 26, e577–e585. [Google Scholar] [CrossRef]
- Qian, J.; Liu, Y. Recent advances in adoptive cell therapy for cancer immunotherapy. Front. Immunol. 2025, 16, 1665488. [Google Scholar] [CrossRef]
- Tuhin, I.J.; Zhu, H.J.; Monty, M.A.; Tan, J.W.; Xu, N.; Ye, J.; Yu, L. From innate power to intelligent design: The evolution of NK cell-based cancer immunotherapy. Crit. Rev. Oncol. Hematol. 2025, 216, 104972. [Google Scholar] [CrossRef]
- Burón, M.; Etxebarria, A.; Álvarez, M.; Romayor, I.; Eguizabal, C. Natural killer cells in adoptive cell therapy: Current landscape of genetic engineering strategies. Oncoimmunology 2025, 14, 2563099. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Chen, Y.; Chi, E.; Li, S.; He, Y.; Wang, B.; Shen, H.; Fan, L.; Wang, J.; Shangguan, T.; et al. New power in cancer immunotherapy: The rise of chimeric antigen receptor macrophage (CAR-M). J. Transl. Med. 2025, 23, 1182. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, C.; Zhang, P.; Shen, L.; Qi, C. Optimizing CAR T cell therapy for solid tumours: A clinical perspective. Nat. Rev. Clin. Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Schaft, N. The Landscape of CAR-T Cell Clinical Trials against Solid Tumors-A Comprehensive Overview. Cancers 2020, 12, 2567. [Google Scholar] [CrossRef]
- Prazeres, P.; Costa da Silva, G.H.; Azevedo, G.V.; Alves da Silva, N.J.; Carvalho Costa, P.A.; Da Silva, W.N.; Lobo, A.O.; Guimaraes, P.P.G. Advancing Cancer Immunotherapy Using Lipid Nanoparticle-Based Approaches. Int. J. Nanomed. 2025, 20, 12283–12305. [Google Scholar] [CrossRef]
- Yang, H.; Liu, D.; Qiu, L.; Wang, R.; Zhang, C.; Yu, D.; Zhong, Q.; Yuki, N.; Song, Z.; Zhu, T.; et al. Reprogramming cellular senescence and aging clocks for advanced cancer immunotherapy. Mol. Cancer 2025, 24, 237. [Google Scholar] [CrossRef]
- Upadhyay, S.; Upmanyu, K.; Gabr, M.T. Designing immunity with cytokines: A logic-based framework for programmable CAR therapies. Cytokine Growth Factor. Rev. 2025, 86, 40–55. [Google Scholar] [CrossRef]
- Mandal, K.; Barik, G.K.; Santra, M.K. Overcoming resistance to anti-PD-L1 immunotherapy: Mechanisms, combination strategies, and future directions. Mol. Cancer 2025, 24, 246. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, C.; Zhou, H.; Han, R. Transforming cancer immunotherapy: Integration of distinct immune-based approaches as redefined dual immunotherapy with potential third-sensitizer. Exp. Hematol. Oncol. 2025, 14, 114. [Google Scholar] [CrossRef]
- Jandová, M.; Lánská, M.; Sýkorová, A.; Gregor, J.; Rozsívalová, P.; Beková, L.; Ducháčová, Z.W.; Radocha, J.; Stacey, G.N.; Měřička, P.; et al. Current Role of CAR-T Therapy in Haematological Care. Adv. Exp. Med. Biol. 2025, 1486, 193–216. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Robinson, J.; Flanagan, C.; Pawlyn, C.; Jackson, G.; Jones, J.R. The risk of second primary malignancies in patients receiving T-cell directed therapies for multiple myeloma: A systematic review. Leuk. Lymphoma 2025, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Premchandani, T.; Qutub, M.; Tatode, A.; Umekar, M.; Taksande, J.; Hussain, U.M.; Khidkikar, S.R. Engineering CAR-T cells for solid tumors: Bispecific antigen targeting, tumor microenvironment modulation, and toxicity control. Immunol. Res. 2025, 73, 135. [Google Scholar] [CrossRef]
- Andreou, T.; Neophytou, C.; Kalli, M.; Mpekris, F.; Stylianopoulos, T. Breaking barriers: Enhancing CAR-armored T cell therapy for solid tumors through microenvironment remodeling. Front. Immunol. 2025, 16, 1638186. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Tong, F.; Chen, J.; Kayumov, M.; Lv, Y.; Shi, Y.; Ye, B.; Gao, H. Tumor Microenvironment-Responsive Nanomedicines for Potentiating Cancer Immunotherapy. Adv. Sci. 2025, 12, e13567. [Google Scholar] [CrossRef]
- Lashkajani, S.Z.; Azad, Y.S.; Nami, M.T.; Darzi, A.; Shokouhfar, M.; Faizabadi, S.N.; Diansaei, M.; Aghazadeh-Habashi, K.; Tabrizi, Z.A.; Vanan, A.G. Neurologic complications of immune checkpoint inhibitors: A comprehensive review. Biomed. Pharmacother. 2025, 192, 118669. [Google Scholar] [CrossRef]
- Kungwankiattichai, S.; Desai, A.; Koppinger, S.; Maziarz, R.T. Immune-related adverse events associated with cancer immunotherapy. Med 2025, 6, 100800. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Li, D.; Yang, Y.; Lin, S.; Zhao, R.; Li, Y.; Peng, L. Peripheral blood cell counts as predictors of immune-related adverse events in cancer patients receiving immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2025, 16, 1528084. [Google Scholar] [CrossRef] [PubMed]
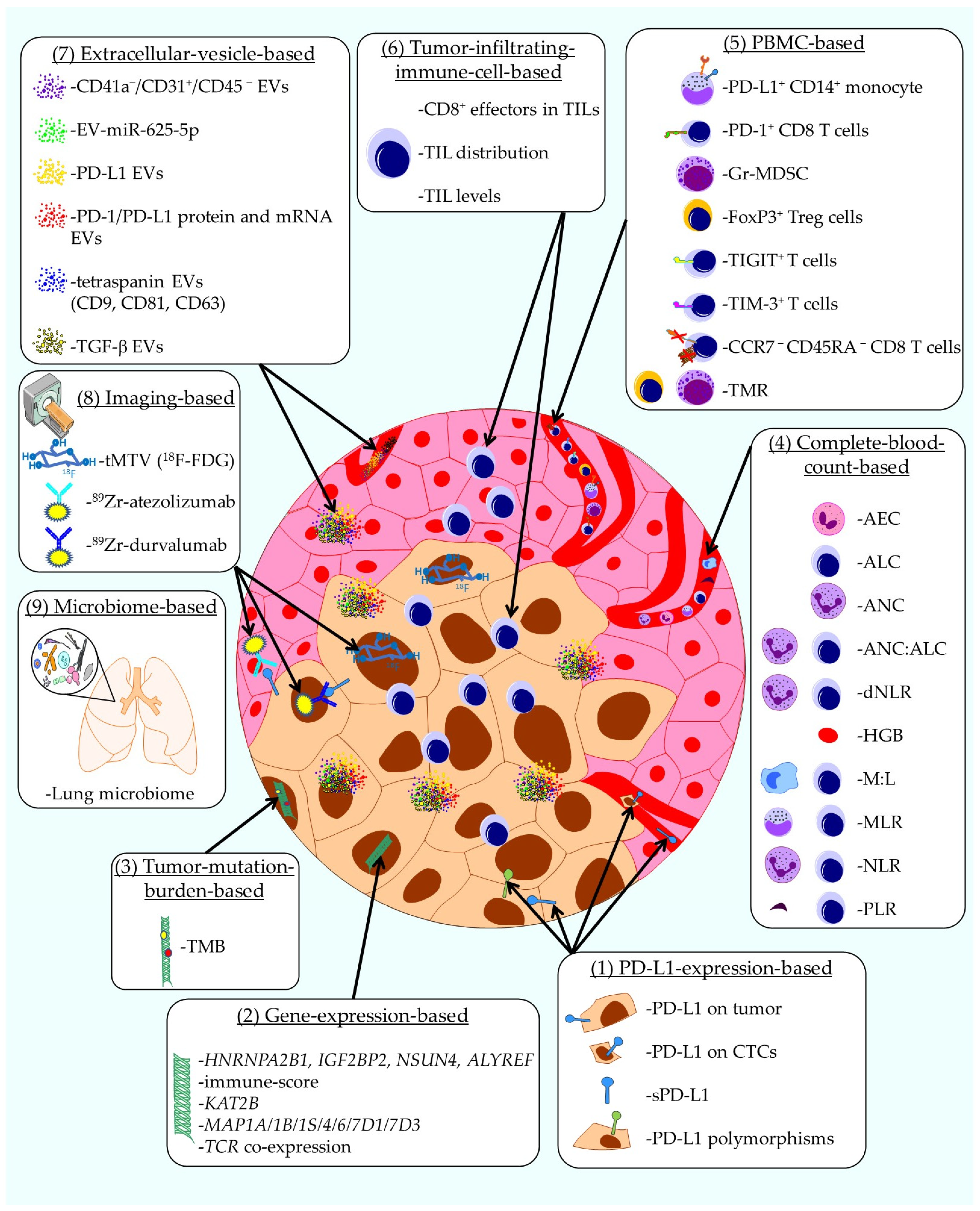
- Tostes, K.; Siqueira, A.P.; Reis, R.M.; Leal, L.F.; Arantes, L. Biomarkers for Immune Checkpoint Inhibitor Response in NSCLC: Current Developments and Applicability. Int. J. Mol. Sci. 2023, 24, 11887. [Google Scholar] [CrossRef]
- Jeanson, A.; Tomasini, P.; Souquet-Bressand, M.; Brandone, N.; Boucekine, M.; Grangeon, M.; Chaleat, S.; Khobta, N.; Milia, J.; Mhanna, L.; et al. Efficacy of Immune Checkpoint Inhibitors in KRAS-Mutant Non-Small Cell Lung Cancer (NSCLC). J. Thorac. Oncol. 2019, 14, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Hu, X.; Zheng, S.; Yang, A.; Li, X.; Tang, H.; Kong, Y.; Xie, X. Discordance of immunotherapy response predictive biomarkers between primary lesions and paired metastases in tumours: A systematic review and meta-analysis. eBioMedicine 2021, 63, 103137. [Google Scholar] [CrossRef]
- Soliman, H.; Shah, V.; Srkalovic, G.; Mahtani, R.; Levine, E.; Mavromatis, B.; Srinivasiah, J.; Kassar, M.; Gabordi, R.; Qamar, R.; et al. MammaPrint guides treatment decisions in breast Cancer: Results of the IMPACt trial. BMC Cancer 2020, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199, Correction in N. Engl. J. Med. 2018, 379, 2185. [Google Scholar] [CrossRef]
- Laza-Briviesca, R.; Cruz-Bermúdez, A.; Nadal, E.; Insa, A.; García-Campelo, M.d.R.; Huidobro, G.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Blood biomarkers associated to complete pathological response on NSCLC patients treated with neoadjuvant chemoimmunotherapy included in NADIM clinical trial. Clin. Transl. Med. 2021, 11, e491. [Google Scholar] [CrossRef]
- Rotunno, M.; Hu, N.; Su, H.; Wang, C.; Goldstein, A.M.; Bergen, A.W.; Consonni, D.; Pesatori, A.C.; Bertazzi, P.A.; Wacholder, S.; et al. A gene expression signature from peripheral whole blood for stage I lung adenocarcinoma. Cancer Prev. Res. 2011, 4, 1599–1608. [Google Scholar] [CrossRef]
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New horizons in tumor microenvironment biology: Challenges and opportunities. BMC Med. 2015, 13, 45. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Calabrò, D.; Conci, N.; Argalia, G.; Marchese, P.V.; Fabbri, F.; Fragomeno, B.; Ricci, D.; Fanti, S.; Ambrosini, V.; et al. Baseline total metabolic tumour volume on 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography-computed tomography as a promising biomarker in patients with advanced non–small cell lung cancer treated with first-line pembrolizumab. Eur. J. Cancer 2021, 150, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Masuhiro, K.; Tamiya, M.; Fujimoto, K.; Koyama, S.; Naito, Y.; Osa, A.; Hirai, T.; Suzuki, H.; Okamoto, N.; Shiroyama, T.; et al. Bronchoalveolar lavage fluid reveals factors contributing to the efficacy of PD-1 blockade in lung cancer. JCI Insight 2022, 7, e157915. [Google Scholar] [CrossRef]
- Binder, A.K.; Bremm, F.; Dorrie, J.; Schaft, N. Non-Coding RNA in Tumor Cells and Tumor-Associated Myeloid Cells-Function and Therapeutic Potential. Int. J. Mol. Sci. 2024, 25, 7275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mao, C.; Wu, Y.; Wang, Y.; Cong, H. Application of non-coding RNAs in tumors (Review). Mol. Med. Rep. 2025, 31, 164. [Google Scholar] [CrossRef] [PubMed]
- Moeinafshar, A.; Nouri, M.; Shokrollahi, N.; Masrour, M.; Behnam, A.; Tehrani Fateh, S.; Sadeghi, H.; Miryounesi, M.; Ghasemi, M.R. Non-coding RNAs as potential therapeutic targets for receptor tyrosine kinase signaling in solid tumors: Current status and future directions. Cancer Cell Int. 2024, 24, 26. [Google Scholar] [CrossRef]
- Waaga-Gasser, A.M.; Böldicke, T. Genetically Engineered T Cells and Recombinant Antibodies to Target Intracellular Neoantigens: Current Status and Future Directions. Int. J. Mol. Sci. 2024, 25, 13504. [Google Scholar] [CrossRef]
- Haus-Cohen, M.; Reiter, Y. Harnessing antibody-mediated recognition of the intracellular proteome with T cell receptor-like specificity. Front. Immunol. 2024, 15, 1486721. [Google Scholar] [CrossRef]
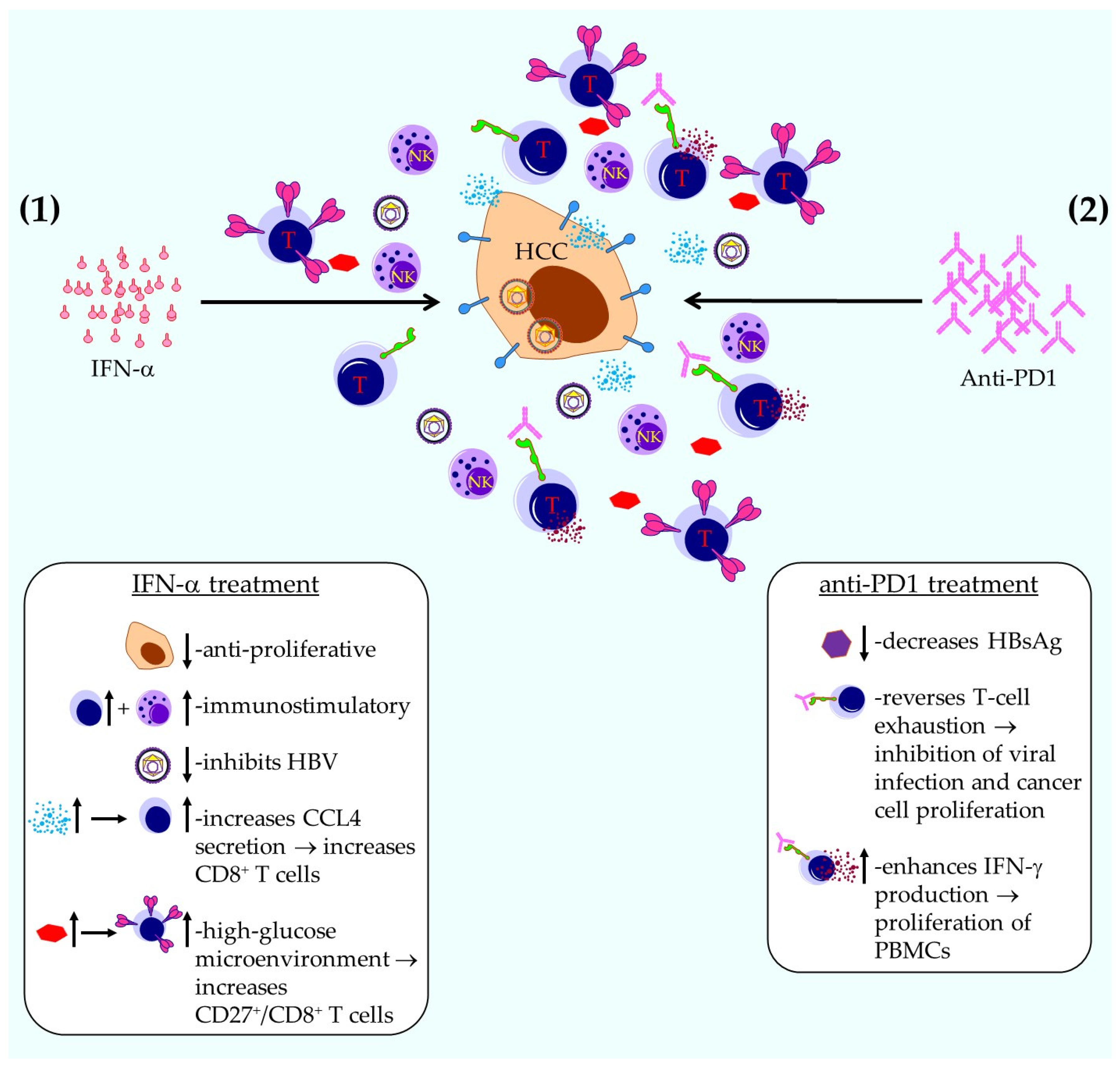
- Qin, A.; Wu, C.R.; Ho, M.C.; Tsai, C.Y.; Chen, P.J. Sequential Therapy with Ropeginterferon Alfa-2b and Anti-Programmed Cell Death 1 Antibody for Inhibiting the Recurrence of Hepatitis B-Related Hepatocellular Carcinoma: From Animal Modeling to Phase I Clinical Results. Int. J. Mol. Sci. 2023, 25, 433. [Google Scholar] [CrossRef]
- Michaels, E.; Chen, N.; Nanda, R. The Role of Immunotherapy in Triple-Negative Breast Cancer (TNBC). Clin. Breast Cancer 2024, 24, 263–270. [Google Scholar] [CrossRef]
- Heimes, A.S.; Riedel, N.; Almstedt, K.; Krajnak, S.; Schwab, R.; Stewen, K.; Lebrecht, A.; Battista, M.J.; Brenner, W.; Hasenburg, A.; et al. Prognostic Impact of CD38- and IgκC-Positive Tumor-Infiltrating Plasma Cells in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 15219. [Google Scholar] [CrossRef] [PubMed]
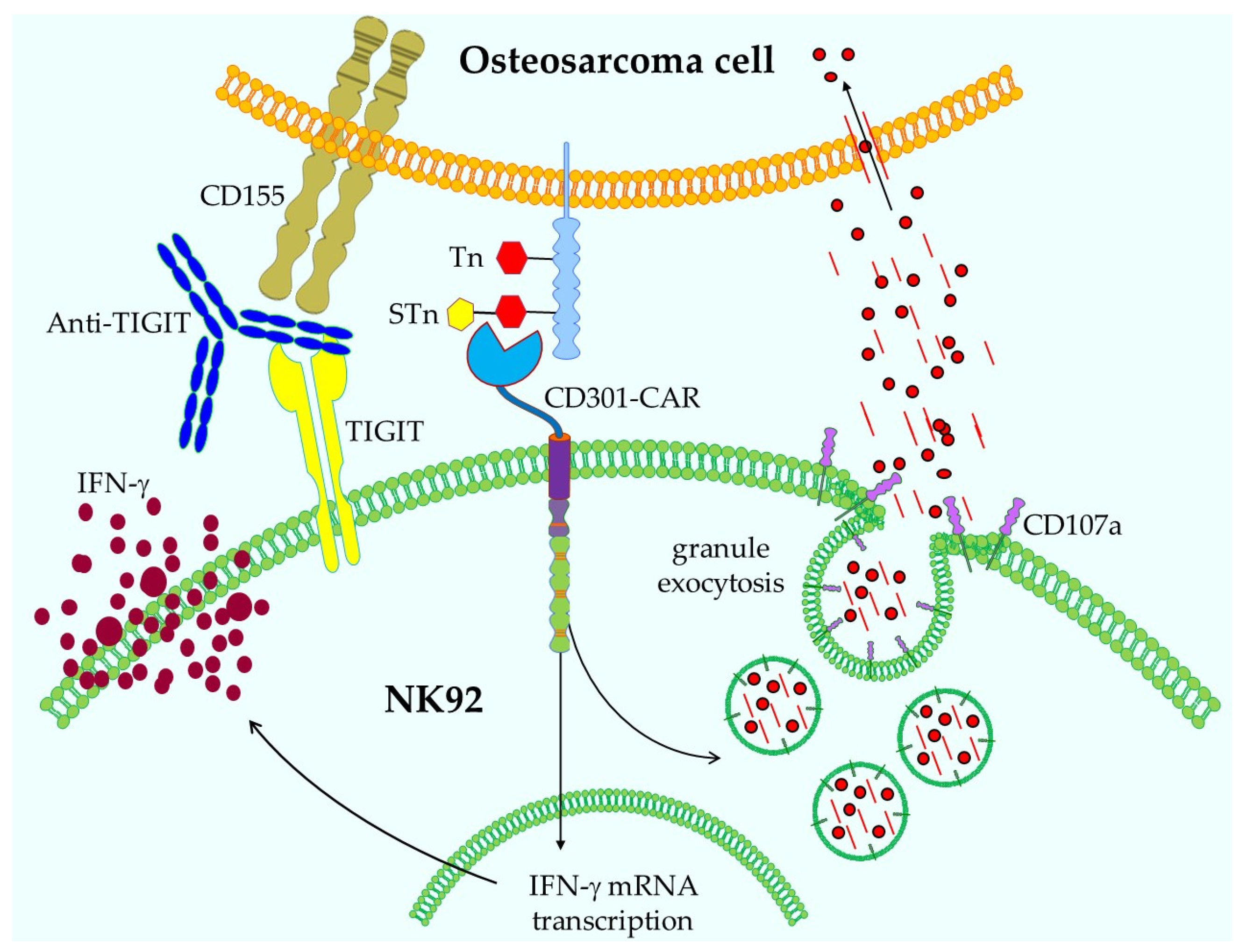
- Prasse, N.; Wessolowski, C.; Müller, I.; Cornils, K.; Franke, A.K. Glycan Structures in Osteosarcoma as Targets for Lectin-Based Chimeric Antigen Receptor Immunotherapy. Int. J. Mol. Sci. 2024, 25, 5344. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaft, N. Special Issue “Molecular Advances in Cancer Immunotherapy”. Int. J. Mol. Sci. 2025, 26, 10839. https://doi.org/10.3390/ijms262210839
Schaft N. Special Issue “Molecular Advances in Cancer Immunotherapy”. International Journal of Molecular Sciences. 2025; 26(22):10839. https://doi.org/10.3390/ijms262210839
Chicago/Turabian StyleSchaft, Niels. 2025. "Special Issue “Molecular Advances in Cancer Immunotherapy”" International Journal of Molecular Sciences 26, no. 22: 10839. https://doi.org/10.3390/ijms262210839
APA StyleSchaft, N. (2025). Special Issue “Molecular Advances in Cancer Immunotherapy”. International Journal of Molecular Sciences, 26(22), 10839. https://doi.org/10.3390/ijms262210839




