Abstract
MicroRNAs (miRNAs) are key post-transcriptional regulators of plant development and stress responses, with many being conserved across diverse plant lineages. In this study, we investigated the expression profiles of miRNAs and their corresponding target genes in Arachis stenosperma, a wild peanut relative that exhibits robust resistance to root-knot nematodes (RKN). Small RNA sequencing of nematode-infected roots identified 107 miRNA loci, of which 93 corresponded to conserved miRNA families and 14 represented novel candidates, designated as miRNOVO. Among these, 18 miRNAs belonging to 11 conserved families were identified as differentially expressed (DEMs). Notably, miR399 and miR319 showed the highest upregulation (logFC = 4.25 and 4.20), while miR393 and miR477 were the most downregulated (logFC = −0.83 and −0.79). Integrated analysis of miRNA and transcriptome data revealed several regulatory interactions involving key defense-related genes. These included NLR genes targeted by miR393 and miR477, hormone signaling components such as the auxin response factor ARF8 targeted by miR167, and the growth regulator GRF2 targeted by miR396. Additionally, miR408 was predicted to target laccase3, a gene involved in the oxidation of phenolic compounds, lignin biosynthesis, copper homeostasis and defense responses. Remarkably, four immune receptor genes belonging to the nucleotide-binding site leucine-rich repeat (NLR) family displayed inverse expression patterns relative to their regulatory miRNAs, suggesting miRNA-mediated post-transcriptional control during the early stages of nematode infection. These findings reveal both conserved and species-specific miRNA–mRNA modules associated with nematode resistance in A. stenosperma, highlighting promising targets for developing RKN-tolerant peanut cultivars through miRNA-based strategies.
1. Introduction
MicroRNAs (miRNAs) participate in various plant developmental processes, gene expression regulations, and complex cellular mechanisms such as defense strategies, intra- and extracellular stress responses and growth initiation [1]. They are fundamental players in broad-spectrum diseases, as pathogen attack triggers massive miRNA changes in the host causing alteration or silencing of diverse phytohormone pathways, thus regulating plant immunity [2]. miRNAs are single-strand small non-coding RNAs, typically 21–24 nucleotides in length, that originate from miRNA genes and function at the post-transcription level by base pairing with cognate mRNA, degrading or inhibiting mRNA translation [3]. They are evolutionarily highly conserved in plant species, with each species harboring primarily conserved miRNAs, mostly involved in regulating common plant developmental processes, and some species-specific miRNAs, regulating special trait development, such as nodule development and symbiotic nitrogen fixation in legumes [4,5]. The expression of miRNAs can vary depending on cell type, tissue, genotype, or environmental condition, thereby regulating the expression of their target genes. For instance, miRNAs modulate biotic and abiotic stress-responsive genes, including pathogenesis-related (PR) and resistance (R) genes, and those involved in drought, aluminum and salinity tolerance mechanisms [6,7,8,9].
Peanut (Arachis hypogaea) is one of the most important legume crops in the world, grown widely for both oil and protein production (http://www.fao.org/faostat/, accessed on 29 October 2025). Their seeds also offer health benefits, being rich in heart-healthy oleic and linoleic acid, resveratrol, fiber and folic acid, and easily digested protein [9]. Nevertheless, destructive diseases including early and late leaf spot, spotted wilt disease, white mold, and nematode infection affect various plant parts and can drastically reduce peanut yield and quality [10,11]. In contrast, peanut wild relatives (Arachis spp.) have evolved and adapted to a wide range of environments, and harbor high levels of resistance to many pathogens, including the highly damaging root-knot nematode (RKN) Meloidogyne arenaria, and tolerance to drought [12,13,14,15]. Thus, wild Arachis species constitute an important genetic reservoir for resistance and tolerance traits that can be seized and transferred to cultivated peanut or other legume crops by marker-assisted breeding or transgenic approaches [16,17].
Several studies have demonstrated the involvement of peanut miRNAs in the regulation of key developmental processes, including gynophore formation [18], embryo abortion [19], embryogenesis and pod development [20], anthocyanin biosynthesis [21], and seed expansion [22]. Additionally, miRNAs have been implicated in peanut responses to a wide range of biotic stresses, such as bacterial wilt (Ralstonia solanacearum) [23], Aspergillus flavus infection [24,25], Sclerotium rolfsii [26] and RKNs [27], as well as abiotic challenges, including nitrogen and potassium deficiencies [28], drought and heat stress [29], cold [30] and aluminum toxicity [9]. In contrast, relatively few studies have investigated miRNAs and their target genes in wild Arachis species. Notably, Zhao et al. [23] reported contrasting miRNA expression profiles in response to bacterial wilt between the wild species A. glabrata, which shows resistance to soil-borne pathogens, and the susceptible cultivated peanut, and Garg et al. [31] conducted a comprehensive genome-wide analysis of key components involved in miRNA biogenesis and target gene regulation in A. duranensis and A. ipaënsis, the wild progenitors of peanut. Both works provided valuable insights into the small RNA-mediated regulatory networks in these species.
In this study, we analyzed the miRNA transcriptome of A. stenosperma, a wild species highly resistant to M. arenaria, to identify pathogen-responsive miRNAs and predict their corresponding target genes. Our results reveal complex miRNA-mediated regulatory networks underlying RKN resistance in this wild species and offer valuable insights into the molecular mechanisms of its robust defense against nematode infection. As growing evidence shows that pathogen-responsive small RNAs fine-tune plant immunity by silencing negative regulators or activating positive ones, our findings highlight promising candidates for the application of small RNA–based technologies, such as artificial microRNAs (amirRNAs), to develop peanut cultivars with durable resistance to M. arenaria and other nematodes.
2. Results
2.1. miRNA Sequencing and Analysis
Overall, six miRNA libraries were constructed, consisting of three libraries from A. stenosperma roots inoculated with M. arenaria (AsINOC) collected at 6 days after inoculation (DAI) and three of non-inoculated control (AsCTR), with an average number of raw reads of 30 and 32.9 Mb, respectively (Supplementary Table S1). Since a fully annotated genome of A. stenosperma is not yet available, after contaminants removal and size selection (20–24 nt), transcript reads were mapped into A. duranensis reference genome (https://peanutbase.org/, accessed on 29 October 2025; [32]). In average, 2.6% of A. stenosperma miRNA reads, which represents 53.8% of 20–24 nt sequences, mapped uniquely to the heterologous A. duranensis genome (Supplementary Table S1).
2.2. A. stenosperma miRNAs Expression During M. arenaria Infection
The size distribution of miRNA-mapped reads from A. stenosperma revealed prominent peaks at 21 and 24 nucleotides, consistent with canonical plant miRNA profiles. Across all libraries, 24-nt miRNA reads represented the highest proportion of both total and uniquely mapped reads to the reference genome, whereas 21-nt reads were more frequently associated with multi-mapping loci, in line with expectations for plant-derived small RNA datasets (Figure 1A). A subtle trend was observed toward a higher number of 21-nt reads in inoculated samples and 24-nt reads in control samples; however, this difference was not statistically significant. These results are consistent with those reported for other plant species, including Arabidopsis thaliana, Medicago truncatula, Oryza sativa, Populus spp. and Citrus trifoliate, where sequences with 24 nt in length dominated the whole miRNA transcriptome [33].
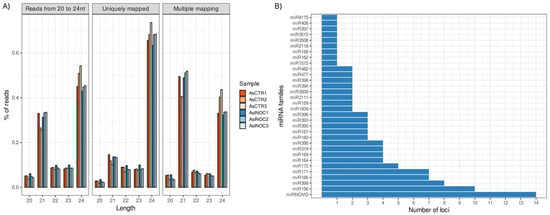
Figure 1.
Overview of sRNA-Seq data in Arachis stenosperma roots infected with Meloidogyne arenaria: (A) Percentage of reads mapped to the A. duranensis reference genome from A. stenosperma infected (AsINOC—shades of blue) and control (AsCTR—shades of red) libraries: (i) total mapped reads between 20 and 24 nt; (ii) uniquely mapped reads; and (iii) ambiguously mapped reads (B) Number of miRNA loci per miRNA family identified in A. stenosperma.
The prediction of miRNA precursors using the A. duranensis reference genome identified 107 distinct miRNA loci, of which 93 belonged to conserved miRNA families and 14 represented novel candidates, designated as miRNOVO (Figure 1B; Supplementary Table S2). These miRNOVO sequences were classified as novel because they exhibited no detectable homology in the miRBase database (release 22.1; e-value < 10−4) [34], yet fulfilled the established structural and biogenetic criteria for authentic miRNAs as outlined by Axtell and Meyers [35].
To ensure that the miRNAs identified in the A. stenosperma libraries, including miRNOVO, were of plant origin and not derived from nematode sequences, we compared all plant miRNA sequences against M. arenaria miRNA transcripts (unpublished data). No identical matches were detected, confirming that the A. stenosperma miRNAs did not result from nematode contamination.
Predicted miRNAs in A. stenosperma were distributed across 31 conserved families, of which 25 are broadly conserved in plants, one is specific to Fabaceae (miR1509), and three are exclusive to the genus Arachis (miR3508, miR3509, and miR3515). Among these, miR156, miR399, miR166, and miR171 showed the highest genomic representation, with 10, 8, 7, and 7 loci, respectively (Figure 1B).
2.3. A. stenosperma Differentially Expressed miRNAs (DEMs) in Response to M. arenaria Infection
A total of 18 miRNAs belonging to 11 miRNA conserved families were identified as differentially expressed miRNAs (DEMs) in A. stenosperma roots upon M. arenaria infection compared to non-inoculated control (FDR < 0.05) (Figure 2A). The majority of these DEMs (15 out of 18) were upregulated in response to nematode infection, while three miRNAs belonging to two families (miR393 and miR477) were downregulated (Figure 2A). Four miR399 loci and miR319 exhibited the highest levels of upregulation (logFC = 4.25, 4.20, 2.66 and 1.40 for miR399 and 1.30 for miR319)), whereas miR393 and miR477 showed the strongest downregulation (logFC = −0.83 and −0.80 for miR393 and −0.79 for miR477)) (Figure 2A; Supplementary Table S3). miR166 was by far the most abundant DEM, with up to 5.7 million reads in infected and control roots, followed by miR396 and miR159 (Supplementary Table S2).
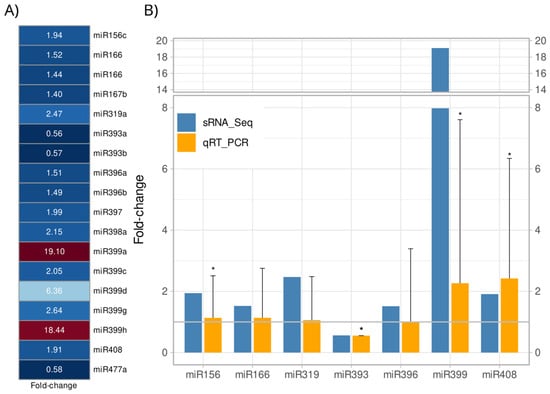
Figure 2.
Differentially expressed miRNAs (DEMs) in Arachis stenosperma roots in response to Meloidogyne arenaria infection. (A) Heatmap showing the relative expression of 18 DEMs (FDR < 0.05) in A. stenosperma based on their in silico average fold change (FC) values between M. arenaria infected and control roots. The red-blue scale indicates upregulation (red) and downregulation (blue); (B) Expression profiles of seven DEMs validated by qRT-PCR (Relative Quantification, RQ) (orange) compared to RNA-Seq-based fold change (FC) values (blue). Relative Quantification (RQ) values were calculated from the mean of three biological replicates (n = 9 total) and analyzed by Tukey’s test (* p ≤ 0.05).
To validate the in silico expression profiles of A. stenosperma DEMs, we analyzed the expression of seven miRNAs by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) using the same miRNA samples extracted for sequencing, and specific primers designed following the stem-loop methodology described by Varkonyi-Gasic et al. [36] (Supplementary Table S4). Overall, the expression trends for the miRNAs in the qRT-PCR analysis supported the accuracy and reliability of the in silico analysis (Figure 2B). Notably, miRNAs previously associated with biotic stress responses, such as miR166, miR399 and miR408 were found to be upregulated in both analyzes. These miRNAs are known to regulate various stress-related pathways in plants, as miR166 is involved in stress signaling and vascular development [37], miR399 plays a central role in phosphate homeostasis and has been linked to defense responses [38], while miR408 is implicated in oxidative stress regulation and copper homeostasis [39]. The validation of these expression patterns through qRT-PCR underscores the potential roles of these miRNAs in mediating A. stenosperma responses to stress, particularly in the context of biotic challenges.
2.4. Correlation of Expression Profiles Between miRNAs and Their Target Genes
We identified 92 miRNA loci with at least one predicted target gene in the A. duranensis reference genome (Supplementary Table S5). These miRNAs belong to 29 conserved families and 14 novel sequences (miRNOVO), collectively targeting 584 loci corresponding to 435 different gene products in A. duranensis. While some miRNA families had numerous predicted target genes in A. duranensis, such as miR156 (78), miR172 (45) and miR396 and miR399 (25 each), miR2118 had only one target.
To further explore the regulatory dynamics of A. stenosperma miRNAs, we compared the expression patterns of the previously identified DEMs and their predicted targets between the resistant A. stenosperma and the susceptible A. duranensis, using our RNA-Seq datasets across three post-inoculation time points (3, 6, and 9 DAI) [12,40]. Overall, 11 DEMs predicted a total of 65 target genes in the transcriptomes of both A. stenosperma and A. duranensis (Figure 3). Most miRNAs (nine) were upregulated in A. stenosperma during nematode infection, including members of the miR399 family, which showed significant expression changes in five isoforms (log2FC ranging from 1.03 to 4.25). In contrast, the most strongly downregulated miRNAs were miR393 and miR477, with log2FC values of −0.83 and −0.79, respectively (Figure 3).
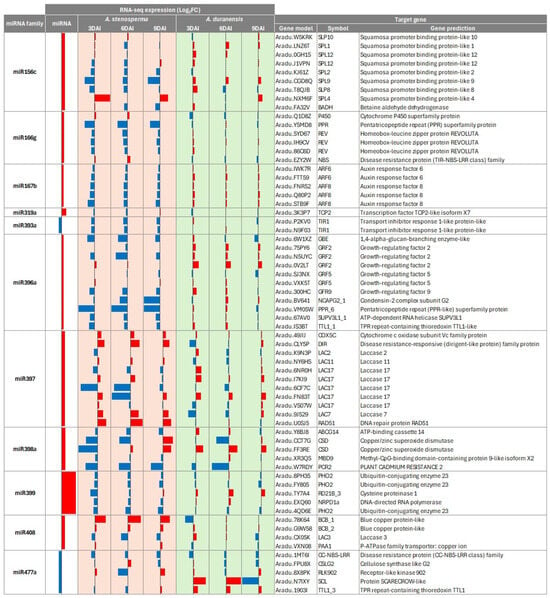
Figure 3.
Heatmap of the RNA-Seq expression (Log2 FC) of A. stenosperma miRNAs and their target genes in the RKN resistant A. stenosperma and the more susceptible A. duranensis. Colum: (1) miRNA identity; (2) miRNA expression (Log2 FC); (3–8) RNA-Seq expression (Log2 FC) of target genes at three time-points (DAI) after M. arenaria inoculation in A. stenosperma (3, 6 and 9 DAI); (8–10) RNA-Seq expression (Log2 FC) of target genes at three time-points after M. arenaria inoculation in A. duranensis (3, 6 and 9 DAI); (9) miRNA target gene identity (ID) in A. duranensis; (10) symbol; (11) target gene annotation (https://www.peanutbase.org/, accessed on 29 October 2025).
Notably, several DEMs, particularly those associated with stress response pathways, exhibited contrasting target gene expression profiles between the RKN-resistant A. stenosperma and the more susceptible A. duranensis. These targets include key transcription factors and stress-related proteins such as SQUAMOSA Promoter Binding Protein-Like (SPL) genes, canonical targets of miR156; the homeobox-leucine zipper protein REVOLUTA, a well-characterized target of miR166; auxin response factors (ARFs), primarily regulated by miR167; transport inhibitor response 1, target by miR393 and growth-regulating factors (GRFs), targeted by miR396. Additionally, copper/zinc superoxide dismutase (Cu/Zn-SOD), a critical enzyme in reactive oxygen species (ROS) detoxification, is regulated by miR398. Predicted target genes encoding disease resistance proteins, such as dirigent-like protein (DIR) targeted by miR397, nucleotide-binding site leucine-rich repeat (NLRs) proteins regulated by miR166, and genes involved in oxidative stress responses, such as aldehyde dehydrogenase (ALDH) and cytochrome C oxidase (COX), which are targets of miR156 and miR397, respectively, also exhibited species-specific opposite expression patterns (Figure 3).
Other miRNA-target pairs, despite not exhibiting huge differences in their expression levels are also worth attention, such as those displayed by some of the laccase genes (LAC), known targets of miR397, and by PHO genes, known targets of miR399. miR399 was the most differentially expressed miRNA, with five isoforms showing induced expression in response to infection, while its target gene PHO2 displayed a mild but consistent downregulation in the resistant A. stenosperma. This comparative expression analysis highlighted several miRNA–target regulatory pairs that may underlie the distinct resistance responses observed between the two species, offering insights into the molecular mechanisms of nematode resistance in wild Arachis.
Representatives of six DEM families in A. stenosperma (miR156, miR166, miR319, miR396, miR399 and miR408) and their respective target genes had their expression behavior in silico validated by qRT-PCR analysis. For that, we used total RNA from A. stenosperma roots infected with M. arenaria at 6 DAI, which is the inflection time point of most RKN-responsive genes [12], and specific primers for each miRNA according to Varkonyi-Gasic et al. [36] (Supplementary Table S4). In addition to belonging to different conserved families, these DEMs were selected as their predicted target genes are well-known to be involved in response to stress, regulating different metabolic and regulatory pathways [41]. For instance, miR156, which target is a squamosa promoter binding protein-like 2 (SPL2), is involved in stress signaling and plant developmental process [42,43], miR166 targeting pentatricopeptide repeat (PPR)-like superfamily protein, is involved in biotic and abiotic stress [44], miR319, targeting transcription factor TCP2-like, is involved in leaf development and biotic stress responses [45,46], miR396, targeting RNA-binding protein (RBP), is implicated in miRNA binding and biogenesis [47], miR399 targeting ubiquitin-conjugating enzyme (PHO2), has a role in phosphate homeostasis and virus defense response [48,49] and miR408 targeting laccase genes (LAC3), which are enzymes involved in oxidation of phenolic compounds, lignin biosynthesis, copper homeostasis, and defense responses [50].
All the six miRNAs selected were upregulated in response to M. arenaria infection, with a contrasting behavior compared to their respective target genes in A. stenosperma that produced lower transcript levels, confirming their in silico expression profiling. The exception was miR319 and its target gene, the transcription factor (TCP2), which were both slight upregulated at similar levels (Figure 4).
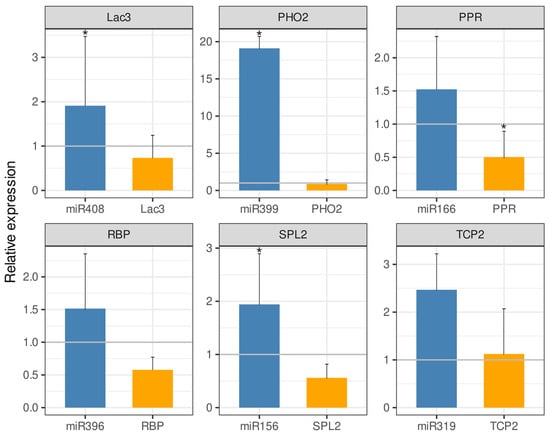
Figure 4.
Expression of six A. stenosperma miRNAs and their respective target genes during M. arenaria infection by qRT-PCR. The relative quantification (RQ) of mRNA levels of small RNAs (miRNAs) and target genes in inoculated plants was normalized with non-stressed control samples using two miRNA reference genes for the miRNAs (U3 and U6) and two Arachis reference genes (60S and GAPDH), with RQ values above or below 1.0 indicating, respectively, up- or downregulated genes (* p ≤ 0.05).
Markedly, 12 miRNAs identified in A. stenosperma during M. arenaria infection, including the differentially expressed miR166g and miR477a, were predicted to target resistance (R) genes from the nucleotide-binding site leucine-rich repeat (NLR) family (Figure 5). Interestingly, three of these miRNAs (miR482a, miRNOVO12, miRNOVO13) showed downregulation, while four of their corresponding target NLRs were upregulated in the resistant A. stenosperma in at least one of the early time points (3 and 6 DAI) of the RKN infection (Figure 5).

Figure 5.
Heatmap of the RNA-Seq expression (Log2FC) of A. stenosperma miRNAs and their target NLR genes in A. stenosperma (SN) and A. duranensis (DN). Column: (1) miRNA identity; (2) miRNA expression (Log2FC); (3–5) RNA-Seq expression (Log2FC) of A. stenosperma target genes at three time-points after M. arenaria inoculation (3, 6, 9 DAI); (6–8) RNA-Seq expression (Log2FC) of A. duranensis target genes at three time-points after M. arenaria inoculation (3, 6 and 9 DAI); (9) target gene annotation (https://www.peanutbase.org/, accessed on 29 October 2025); (10) target NLR gene family. Values are Log2FC between inoculated and control samples (p < 0.05).
This contrasting expression behavior suggests a possible post-transcriptional regulation mechanism contributing to nematode resistance. Interestingly, the gene model Aradu.NL1YQ has previously been identified as a differentially expressed gene (DEG) in response to both M. arenaria infection and UV stress in A. stenosperma, while Aradu.EZY2W was responsive to UV stress [40,51].
These findings suggest that the downregulation of specific miRNAs at the early stages of nematode infection might contribute to the accumulation of their corresponding target NLR transcripts, thereby strengthening the plant’s immune response. This inverse expression pattern highlights a potential regulatory mechanism that contributes to A. stenosperma more robust defense against M. arenaria and possibly other root-knot nematodes.
In general, the correlation of the DEMs and their predicted targets in A. stenosperma revealed their involvement in diverse stress-responsive pathways during M. arenaria infection (Figure 6). These miRNAs appear to regulate genes that are critical components of plant defense, including pathogen recognition via NLR resistance proteins, modulation of hormone signaling pathways such as jasmonic acid and salicylic acid, reinforcement of cell walls through dirigent-like proteins and laccases, mitigation of oxidative stress via oxidoreductases and superoxide dismutases, and maintenance of nutrient homeostasis through regulators such as PHO2 and PPR proteins (Figure 6). The involvement of these miRNA/target modules in diverse biological processes during M. arenaria infection in wild Arachis shows their relevant role in coordinating plant defense responses, highlighting their potential as valuable molecular markers or targets for the development of nematode-resistant cultivars through precision breeding or genetic engineering strategies.
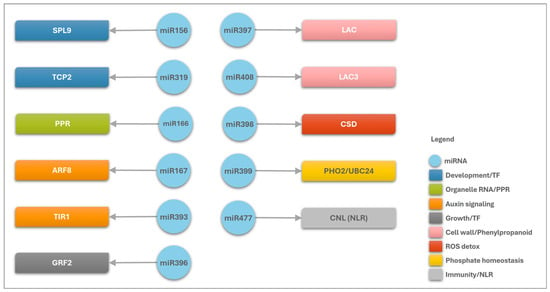
Figure 6.
Schematic representation of A. stenosperma differentially expressed miRNA (DEM)–target interactions involved in the plant’s response to M. arenaria infection. Blue circles represent miRNAs, while colored squares indicate the functional categories of their target genes. Black arrows illustrate the regulatory relationships between miRNAs and their targets.
3. Discussion
High-throughput sequencing and bioinformatics have revealed a diverse set of miRNAs and their targets in peanut, regulating key processes such as growth, development, and stress responses [22,29,52]. Recent studies further demonstrate their roles in modulating disease resistance pathways [24,25,27]. Currently, 11 conserved miRNAs from eight families and 12 peanut-specific sequences are listed in miRBase (http://www.mirbase.org/, accessed on 29 October 2025), underscoring the importance of miRNA-mediated regulation. However, despite the potential of wild Arachis species as sources of stress-resilient traits, their miRNA repertoires remain poorly explored. Although miRNA biogenesis proteins show strong evolutionary conservation, stress-specific regulatory differences suggest the existence of species- or stress-specific mechanisms [31]. Thus, investigating conserved and novel miRNAs and their targets in wild Arachis offers a promising approach to uncover regulatory elements that could enhance stress tolerance in cultivated peanut.
Disruption of specific miRNA biogenesis or function has been linked to increased resistance to both RKN and cyst nematodes (CN) nematodes, underscoring their importance in modulating plant susceptibility to phytonematodes [52,53,54]. Notably, a general downregulation of host miRNAs has been observed in galls induced by Meloidogyne spp. [55] and in CN-induced syncytia [56], with many of these miRNAs associated with stress responses [57]. This widespread repression of plant miRNAs in RKN-induced galls may reflect their role in regulating developmental processes that balance cell proliferation and differentiation within the complex gall structure. These massive miRNA changes triggered by pathogen attack has also been reported in other plant–pathogen interactions [2].
In this study, we identified 18 DEMs, belonging to 11 miRNA families, in the RKN-resistant A. stenosperma of which 15 were upregulated during M. arenaria infection. These included upregulated miRNAs associated with the regulation of cell growth and development, nutrient homeostasis, oxidative stress, and defense-related protein biosynthesis. Seven of these DEM families, miR156, miR167, miR393, miR396, miR398, miR399 and miR408, have previously been implicated in plant responses to RKN infection. miR156 has been reported to regulate Squamosa Promoter Binding Protein-like (SPL) genes in susceptible tomato (Solanum lycopersicum) roots infected with M. incognita [58]. In our study, we observed a negative correlation between miR156 and SPL transcript levels across four loci in A. stenosperma. The miR156/SPL regulatory module plays a central role in modulating immune-related genes, including Jasmonate-ZIM-domain (JAZ) proteins, which act as negative regulators of jasmonate signaling—a key pathway in plant defense [59]. miR156 upregulation leads to lower SPL levels, which in turn weakens SPL-JAZ repression and allows stronger jasmonic acid (JA) responses. Notably, A. stenosperma showed miR156 upregulation together with downregulation of four SPL loci, a pattern absent in susceptible A. duranensis. These data support a model in which RKN-induced miR156 represses SPLs, alleviates JAZ-based inhibition, and thereby enhances JA-mediated defense in the resistant species.
A particularly noteworthy finding is the upregulation of miR167, a microRNA known to target the auxin response factors ARF6 and ARF8. In this study, gene expression analysis reveals that these targets are downregulated in A. stenosperma, a resistant genotype, while showing an elevated expression in A. duranensis, which is susceptible to RKN infection. Previous studies in A. thaliana have established the role of auxin in gall development and nematode feeding site formation [60,61]. More recently, Noureddine et al. [62] demonstrated that miR167 and its targets ARF8A and ARF8B are critical regulators of gall formation in tomato. Their study identified the ARF8/miR167 regulatory module as being actively involved in the plant’s response to nematode infection, with upregulation of ARF8A and ARF8B in giant cells and surrounding gall tissues. This suggests that miR167-mediated repression of ARFs in resistant genotypes may serve as a defense strategy to suppress auxin signaling, thereby inhibiting the formation of nematode-induced feeding structures.
In addition to ARFs, the auxin receptor TIR1 (Transport Inhibitor Response 1), typically targeted by miR393, is also downregulated in the resistant A. stenosperma but not in the susceptible A. duranensis. When auxin binds TIR1 and other auxin F-BOX proteins, it promotes degradation of Aux/IAA repressors, releasing ARFs to activate auxin-responsive genes. This signaling pathway regulates root development, organogenesis, tropic growth, and stress adaptation in plants [63]. Here, we observed concurrent repression of both miR393 and its target gene TIR, likely reflecting restricted auxin signaling in response to nematode infection, a pattern not detected in susceptible species. This apparent decoupling may reflect a fine-tuned regulatory balance, wherein the plant modulates auxin perception independently of miR393 to avoid excessive suppression of auxin signaling. Such regulation could be essential for maintaining normal root development while still restricting nematode-induced cellular reprogramming. In resistant species, this strategy likely contributes to limiting gall formation and nematode establishment, without compromising overall root functionality.
Notably, members of the miR396 family, previously implicated in regulating parasitism by CN and RKN via the GRF (Growth Regulating Factor) pathway [53,64,65,66], were slightly -upregulated in A. stenosperma roots. In contrast, their GRF2 target genes were downregulated in this resistant genotype but upregulated in the more susceptible A. duranensis, suggesting miR396-mediated repression of growth-promoting genes may contribute to enhanced resistance. In a study involving A. thaliana infected with the cyst nematode Heterodera schachtii, elevated levels of miR396—resulting in the repression of GRF1 and GRF3—were associated with a reduction in syncytium size and the induction of nematode resistance [53]. These findings are consistent with the results obtained in the present study for the A. stenosperma-M. arenaria interaction.
Likewise, miR399, the most strongly upregulated miRNA in M. arenaria-infected A. stenosperma roots, exhibited inverse expression with its target gene, PHO2, a ubiquitin-conjugating enzyme involved in phosphate (Pi) homeostasis and protein turnover [67]. The PHO2 downregulation could facilitate increased phosphate mobilization, potentially support the metabolic demands of defense responses or alter nutrient availability in a way that is unfavorable to nematode development. Although miR399 has not been widely reported in RKN-related studies, previous reports have shown differential miR399 expression in tomato during M. incognita infection [58], soybean under CN infection [68], and in peanut exposed to nitrogen and potassium starvation [28], supporting its role in the intersection of nutrient signaling and stress responses [69]. Moreover, Pi accumulation has been shown to enhance A. thaliana defense against fungal pathogens by modulating the salicylic and jasmonic acid dependent defense pathways [70]. In addition, the miR399 family has been linked to broader abiotic stress responses and hormone signaling crosstalk, reinforcing its potential in enhancing plant resilience [29]. These findings suggest a novel mechanism by which nutrient signaling may contribute to nematode resistance, potentially by altering the metabolic environment of the root to deter nematode establishment or development.
Similarly, isoforms of miR408 and miR398 were upregulated in the early stages of M. arenaria infection in A. stenosperma roots. This mirrors findings in A. thaliana and S. lycopersicum, where the SPL7/miR408/miR398 regulatory module modulates both the development of nematode-induced feeding sites and copper deficiency responses [71]. Beyond nematode interactions, both miR408 and miR398 families have been associated with responses to various biotic and abiotic stresses, including fungal pathogens [72], mechanical wounding, and insect herbivory [73], highlighting their central roles in orchestrating adaptive stress responses across multiple environmental challenges.
While miR408 was upregulated, one of its targets, laccase 3, was downregulated in A. stenosperma during the RKN infection. Under stress conditions, miR408 induction can lead to laccase downregulation, thereby reallocating copper to other essential cuproproteins and ensuring sufficient copper for vital processes, such as energy production and defense-related processes, including oxidative stress mitigation and hormone signaling [50]. Similar mechanisms have been described in Arabidopsis under copper deficiency, where miR408-mediated suppression of laccases prioritizes copper for plastocyanin and other vital proteins [74].
The cytochrome P450, a non-canonical target of the miR166 family, is slightly upregulated in the resistant A. stenosperma, while the opposite expression pattern was observed in the more susceptible A. duranensis. Cytochrome P450 enzymes are crucial components of plant biochemical defense pathways and have been implicated in nematode resistance, as shown in expression and functional studies in tomato and soybean challenged with Meloidogyne spp. [75,76]. These enzymes participate primarily in the phenylpropanoid biosynthetic pathway, contributing to the production of lignin and other phenolic compounds that fortify the cell wall and restrict nematode invasion [77].
Additionally, differentially expressed members of the miR166 and miR396 families were found to target genes encoding pentatricopeptide repeat (PPR) proteins downregulated in RKN-infected A. stenosperma roots. PPR proteins are known to regulate RNA processing and play significant roles in plant responses to both biotic and abiotic stresses [44]. Studies have shown that knockout of PPR genes can lead to increased reactive oxygen species (ROS) accumulation, lipid peroxidation, and enhanced superoxide dismutase activity, collectively contributing to improved defense responses and stress adaptation [78,79]. These findings suggest that miRNA-mediated regulation of cytochrome P450s and PPR proteins may be integral to the enhanced resistance observed in A. stenosperma.
Most interestingly, three miRNAs predicted to target NLR-type resistance (R) genes in A. stenosperma (miR482a, miRNOVO12 and miRNOVO 13) were downregulated during M. arenaria infection while their corresponding NLR genes were upregulated. NLRs are central components of plant innate immunity, functioning as intracellular immune receptors, detecting pathogen-associated molecular patterns (PAMPs) or specific effector molecules and initiating robust downstream defense signaling [80]. Several studies have demonstrated that miRNAs contribute to the regulation of immune homeostasis by targeting NLRs and related resistance genes highlighting their role in balancing growth and defense in plants [81,82,83].
Among the A. stenosperma miRNA/R-genes modules identified, miR482/CCNL module is particularly noteworthy, as miR482 family is known to target conserved regions within NLR resistance genes, initiating the production of phased secondary small interfering RNAs (phasiRNAs), which serve to amplify the silencing signal [81]. The miR482-mediated silencing cascade has shown to be suppressed in plants infected with fungi, viruses, bacteria and RKNs [27,84,85], allowing pathogen-inducible expression of NBS-LRR proteins, and therefore contributing to a new layer of defense against pathogen attack. This miRNA-mediated regulation of NLR genes has been described in several plant families, including Solanaceae and Fabaceae, supporting the notion that this is a conserved mechanism of immune modulation across angiosperms [86].
Additionally, both miRNOVO12 and miRNOVO13 were downregulated in A. stenosperma during the early stages of infection (3 and 6 DAI), while their respective targets, TNL and TNX genes, were upregulated. In contrast, these targets were suppressed in the susceptible A. duranensis. These findings suggest that these novel miRNAs identified in A. stenosperma may act as a regulator of NLR-mediated defense responses in the resistant wild species and underscore the diversification of miRNA regulatory networks contributing to nematode resistance in Arachis.
In this study, the comparative analysis of miRNA and mRNA expression profiles between contrasting genotypes for RKN resistance revealed several miRNA–target regulatory pairs that may contribute to the distinct resistance phenotypes observed in the two species. However, despite the significant advances in bioinformatics, accurately predicting genuine miRNAs and their targets remains a major challenge due to the complexity of miRNA–mRNA interactions and context-dependent regulation. To complement computational predictions, small RNA–based approaches such as artificial microRNAs (amiRNAs) have been developed for functional validation and for studying the regulatory control of gene expression associated with plant stress responses [87]. These tools are particularly valuable for fine-tuning the expression of NLR genes, whose excessive accumulation can lead to autoimmunity, or cell death, emphasizing the need for precise regulation to maintain plant fitness [88]. In this study, twelve miRNAs, including four novel ones, were identified as potential regulators of NLRs in wild A. stenosperma. These miRNA–mRNA pairs represent promising candidates for in planta validation and for the development of RNA-based biotechnological strategies to enhance disease resistance in peanut and other legume crops.
4. Materials and Methods
4.1. Plant Materials and Nematode Bioassays
Seeds of the wild RKN-resistant A. stenosperma (accession V10309) were obtained from the Active Germplasm Bank of Embrapa Genetic Resources and Biotechnology (Cenargen, Brasília, Brazil). For the A. stenosperma miRNA libraries, a randomized complete block design of 30 plants (3 blocks of 10) was produced and grown under greenhouse conditions. When four-weeks-old, half of the plants in each block were inoculated with 2500 juveniles (J2) of M. arenaria and the other half with deionised water according to Morgante et al., [89]. Roots from inoculated and mock control were collected six days after inoculation (6DAI). Root samples were immediately frozen in liquid nitrogen and kept at −80 °C for miRNA and total RNA extractions.
4.2. miRNA Extraction and Sequencing
Three biological replicates were prepared for each treatment, consisting of pooled root samples from three individual A. stenosperma plants. These included roots inoculated with M. arenaria (AsINOC) and their corresponding non-stressed controls (AsCTR). Each pooled sample was divided into two groups: one designated for miRNA extraction and the other for total RNA extraction, ensuring parallel analyses of small RNA and transcriptome profiles from the same biological material.
Plant miRNA was extracted using mirVana™ miRNA Isolation Kit (ThermoFischer Scientific, Waltham, MA, USA, Cat. No. AM1560) [90] and used to produce six miRNA libraries (3 AsINOC, 3 AsCTR) using the NEBNext Small RNA Sample Prep SET for Illumina kit (New England BioLabs, Hitchin, UK, Cat. No.50-202-4020). All six libraries and their technical replicates were constructed and single-end sequenced on a HiSeq4000 System using the service of the University of Illinois (EUA). Transcriptomic raw data is available on NCBI Bioproject PRJNA284674 under accession numbers SAMN49651445 to SAMN49651450.
4.3. miRNA Identification and Target Prediction
As there is not yet a fully annotated genome for A. stenosperma available, high-quality, adapter-trimmed reads were aligned to the A. duranensis reference genome (version 1.0; https://peanutbase.org/, accessed on 29 October 2025) using Kallisto v0.46.1 with default parameters [91]. Adapter sequences were removed and reads between 20 and 24 nucleotides in length were selected using Cutadapt v4.6 [92]. To eliminate non-miRNA contaminants, such as rRNAs, tRNAs, snRNAs, and snoRNAs, trimmed reads were mapped against reference sequences using Bowtie v1.2.1 [93].
A. stenosperma miRNA loci were then identified using both Mireap (https://github.com/liqb/mireap, accessed on 29 October 2025) and ShortStack v4.1.2 [94], with quantification and classification performed using the latter tool. Homology-based annotation of predicted miRNAs was carried out using BLASTn v2.15.0 (e-value < 1 × 10−4) against the miRBase database (https://www.mirbase.org/, accessed on 29 October 2025; Release 22.1) [34]. To assess the secondary structures of precursor miRNAs, folding analyzes were performed using StrucVis (https://github.com/MikeAxtell/strucVis, accessed on 29 October 2025) and the RNAfold WebServer [95].
Prediction of miRNA target genes was performed using the psRNATarget web server (http://plantgrn.noble.org/psRNATarget/, accessed on 29 October 2025) [96] with default parameters, using the A. duranensis genome assembly (Aradu1.1; https://peanutbase.org/, accessed on 29 October 2025) as the reference.
4.4. Differentially Expressed miRNAs (DEMs)
The counts for mature miRNAs from each of the three biological replicates were used for differential expression analysis using edgeR v3.40.2 [97] package. The reproducibility of the biological replicates of A. stenosperma samples was assessed using normalized count data obtained with the EdgeR package. These data were processed using the DESeq2 package v1.40.2, which generated a distance matrix among the six samples. Data visualization was performed using ClustVis (https://biit.cs.ut.ee/clustvis/, accessed on 29 October 2025) [98].
For the A. stenosperma libraries data (3 AsINOC and 3 AsCTR), miRNAs were considered differentially expressed (DEMs) if they exhibited an adjusted p-value < 0.05 (Benjamini–Hochberg correction) and a false discovery rate (FDR) below 5%.
The expression profiles of miRNA target genes in A. stenosperma were visualized using heatmaps generated with the ggplot2 package v3.5.1 [99], based on transcriptome datasets previously published by our group [12,40] from root samples of A. stenosperma plants infected with M. arenaria.
4.5. Expression Analysis by qRT-PCR
To validate the in silico expression analysis of miRNAs and their putative target genes, quantitative RT-PCR (qRT-PCR) was performed using the same three biological replicates used for miRNA sequencing. Equal amounts of total RNA per sample were pooled from three plants to produce three independent biological replicates.
For miRNA expression analysis, the stem-loop quantitative RT-PCR (qRT-PCR) was conducted essentially as in Varkonyi-Gasic et al. [36]. Stem-loop reverse transcription specific primers were designed based on the miRNA sequences identified here, and Universal primers were designed as downstream primers based on the stem-loop structure (Supplementary Table S4). Reverse transcription reactions were performed using 2 µg of total RNA and M-MLV Reverse Transcriptase kit (Promega, Madison, WI, USA, Cat. No. M1701) at 16 °C for 30 min, followed by 60 cycles at 30 °C for 30 s, 42 °C for 30 s, 50 °C for 1 s and terminated by incubating at 85 °C for 5 min, according to Chen et al. [100].
For target genes expression analysis, total RNA was extracted from root samples using the Quick-RNA Plant Miniprep Kit (Zymo Research, Irvine, CA, USA, Cat. No. R2024), according to the manufacturer’s instructions. Next, DNA contamination was eliminated and cDNA synthesized as previously described [90].
For both miRNAs and mRNA samples, qRT-PCR reactions were performed with three biological replicates and two technical replicates on a StepOne Plus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using specific primers (Supplementary Table S3) as previously described [89]. The online real-time PCR Miner tool [101] was used to estimate primer efficiency and optimal average cycle of quantification (Cq) values. The relative quantification (RQ) of miRNAs and target gene mRNA levels was determined and statistically tested using the REST 2009 v. 2.0.13 software [102]. The RQs of miRNA levels were normalized with the small nuclear RNA (snRNA) U3 and U6 [100,103], and for target genes with the 60S and GAPDH reference genes (Supplementary Table S3), in accordance with [36] and [104], respectively.
5. Conclusions
In summary, our results reveal an intricate miRNA/target regulatory network in A. stenosperma contributing to enhanced resistance against M. arenaria. This network integrates transcription factors, NLR immune receptors, RNA-binding proteins, cell wall-associated proteins, and components of hormone and redox signaling pathways. The modulation of miRNA expression represents a promising strategy to enhance crop resilience to biotic stresses. This can be achieved through overexpression of defense-promoting miRNAs or their targets [105,106], or through the use of artificial miRNAs (amiRNAs) for targeted gene silencing [107,108]. Additionally, CRISPR/Cas9 genome editing allows precise modification of miRNA loci or their target binding sites, enabling fine-tuning miRNA-mediated regulatory networks [109]. Elucidating the miRNA-mediated regulatory mechanisms in stress-resilient wild Arachis species holds great promise for developing peanut cultivars with durable and broad-spectrum resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262210824/s1.
Author Contributions
P.M.G., conceptualization, resources, writing—original draft preparation, writing—review and editing, funding acquisition; A.d.C.Q.M., A.L.M.L. and M.A.d.P.S. methodology, investigation, validation; R.C.T., methodology, software, data curation; A.C.M.B., methodology, investigation, validation, formal analysis, writing—original draft preparation; P.G., methodology, investigation, validation, formal analysis, writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Fundação de Amparo à Pesquisa do Estado de Distrito Federal (FAPDF) (grant number 00.193.00002413/2023-77) and the Instituto Nacional de Ciência e Tecnologia (INCT) (MCTI/CNPq/CAPES/FAPDF) (grant number 465480/2014-4). A.L.M.L and A.d.C.Q.M. were supported by scholarships from INCT and FAPDF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the reported results can be found at the NCBI Sequence Read Archive (SRA) database (NCBI Bioproject PRJNA284674 under accession numbers SAMN49651445 to SAMN49651450).
Acknowledgments
The authors would like to thanks Regina Carneiro for provision of RKN nematodes for the study. The authors also thank the anonymous reviewers for comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| miRNAs | MicroRNAs |
| DEMs | Differentially Expressed MicroRNAs |
| NLR | Nucleotide-binding site Leucine-Rich |
| RKN | Root-Knot Nematode |
| mRNA | Messenger RNA |
| PR | Pathogenesis-related genes |
| R | Resistance genes |
| AsCTR | Control samples (not inoculated) of Arachis stenosperma |
| AsINOC | Inoculated samples of Arachis stenosperma |
| Mare | Sample of Meloidogyne arenaria |
| Mb | Million base pairs |
| nt | Nucleotides |
| miRNOVO | New miRNA candidates |
| qRT-PCR | Quantitative Reverse Transcription-Polymerase Chain Reaction |
| RNA-Seq | RNA sequencing |
| DAI | Days After Inoculation |
| MLP | Major Latex Protein |
| MLO | Mildew Locus O |
| TF | Transcription Factor |
| SPL | Squamosa Promoter-Binding Protein-Like |
| HD-ZIP | Homeodomain-leucine Zipper |
| FC | Fold-change |
| TCP2 | Teosinte branched1/Cycloidea/Proliferating cell factor |
| PHO2 | Phosphatase2 |
| DEG | Differentially Expressed Gene |
| UV | Ultraviolet |
| TIR-NBS-LRR | Toll-Interleukin Receptor /Nucleotide-Binding Site/Leucine-Rich Repeat |
| PPR | Pentatricopeptide Repeat |
| CN | Cyst Nematode |
| GRF | Growth Regulating Factor |
| Pi | Phosphate |
| ROS | Reactive Oxygen Species |
| PAMP | Pathogen-Associated Molecular Patterns |
| CCNL | Cyclin L |
| phasiRNA | Phased secondary small interfering RNAs |
| TNL | TIR-NBS-LRR |
| MYB | Myeloblastosis |
| TNX | Toll-Interleukin Receptor /Nucleotide-Binding Site without LRR domain |
| AP2 | APETALA |
| amiRNA | Artificial miRNA |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| cv | Cultivate |
References
- Islam, W.; Islam, S.U.; Qasim, M.; Wang, L. Host-Pathogen Interactions Modulated by Small RNAs. RNA Biol. 2017, 14, 891–904. [Google Scholar] [CrossRef]
- Balmer, D.; Mauch-Mani, B. Small Yet Mighty—MicroRNAs in Plant-Microbe Interactions. MicroRNA 2013, 2, 73–80. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Zhuang, X.; Yu, Y.; Liu, X.; Cui, X.; Ji, L.; Pan, Z.; Cao, X.; Mo, B.; et al. MicroRNAs Inhibit the Translation of Target MRNAs on the Endoplasmic Reticulum in Arabidopsis. Cell 2013, 153, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Fu, Y.; Sunkar, R.; Barbazuk, W.B.; Zhu, J.K.; Yu, O. Novel and Nodulation-Regulated MicroRNAs in Soybean Roots. BMC Genom. 2008, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Chand Jha, U.; Nayyar, H.; Mantri, N.; Siddique, K.H.M. Non-Coding RNAs in Legumes: Their Emerging Roles in Regulating Biotic/Abiotic Stress Responses and Plant Growth and Development. Cells 2021, 10, 1674. [Google Scholar] [CrossRef]
- Song, L.; Fang, Y.; Chen, L.; Wang, J.; Chen, X. Role of Non-Coding RNAs in Plant Immunity. Plant Commun. 2021, 2, 100180. [Google Scholar] [CrossRef]
- Zhang, B. MicroRNA: A New Target for Improving Plant Tolerance to Abiotic Stress. J. Exp. Bot. 2015, 66, 1749. [Google Scholar] [CrossRef]
- Barrera-Figueroa, B.E.; Gao, L.; Diop, N.N.; Wu, Z.; Ehlers, J.D.; Roberts, P.A.; Close, T.J.; Zhu, J.K.; Liu, R. Identification and Comparative Analysis of Drought-Associated MicroRNAs in Two Cowpea Genotypes. BMC Plant Biol. 2011, 11, 127. [Google Scholar] [CrossRef]
- Tong, B.; Shi, Y.; Ntambiyukuri, A.; Li, X.; Zhan, J.; Wang, A.; Xiao, D.; He, L. Integration of Small RNA and Degradome Sequencing Reveals the Regulatory Network of Al-Induced Programmed Cell Death in Peanut. Int. J. Mol. Sci. 2022, 23, 246. [Google Scholar] [CrossRef]
- Anco, D.J.; Thomas, J.S.; Jordan, D.L.; Shew, B.B.; Monfort, W.S.; Mehl, H.L.; Small, I.M.; Wright, D.L.; Tillman, B.L.; Dufault, N.S.; et al. Peanut Yield Loss in the Presence of Defoliation Caused by Late or Early Leaf Spot. Plant Dis. 2020, 104, 1390–1399. [Google Scholar] [CrossRef]
- Biswal, A.K.; Ozias-Akins, P.; Holbrook, C.C. Recent Technological Advancements for Identifying and Exploiting Novel Sources of Pest and Disease Resistance for Peanut Improvement. Agronomy 2024, 14, 3071. [Google Scholar] [CrossRef]
- Guimaraes, P.M.; Guimaraes, L.A.; Morgante, C.V.; Silva, O.B.; Araujo, A.C.G.; Martins, A.C.Q.; Saraiva, M.A.P.; Oliveira, T.N.; Togawa, R.C.; Leal-Bertioli, S.C.M.; et al. Root Transcriptome Analysis of Wild Peanut Reveals Candidate Genes for Nematode Resistance. PLoS ONE 2015, 10, e0140937. [Google Scholar] [CrossRef]
- Mota, A.P.Z.; Vidigal, B.; Danchin, E.G.J.; Togawa, R.C.; Leal-Bertioli, S.C.M.; Bertioli, D.J.; Araujo, A.C.G.; Brasileiro, A.C.M.; Guimaraes, P.M. Comparative Root Transcriptome of Wild Arachis Reveals NBS-LRR Genes Related to Nematode Resistance. BMC Plant Biol. 2018, 18, 159. [Google Scholar] [CrossRef]
- Kumar, D.; Kirti, P.B. The Genus Arachis: An Excellent Resource for Studies on Differential Gene Expression for Stress Tolerance. Front. Plant Sci. 2023, 14, 1275854. [Google Scholar] [CrossRef]
- Brasileiro, A.C.M.; Lacorte, C.; Pereira, B.M.; Oliveira, T.N.; Ferreira, D.S.; Mota, A.P.Z.; Saraiva, M.A.P.; Araujo, A.C.G.; Silva, L.P.; Guimaraes, P.M. Ectopic Expression of an Expansin-like B Gene from Wild Arachis Enhances Tolerance to Both Abiotic and Biotic Stresses. Plant J. 2021, 107, 1681–1696. [Google Scholar] [CrossRef]
- Leal-Bertioli, S.C.M.; Moretzsohn, M.C.; Roberts, P.A.; Ballén-Taborda, C.; Borba, T.C.O.; Valdisser, P.A.; Vianello, R.P.; Araújo, A.C.G.; Guimarães, P.M.; Bertioli, D.J. Genetic Mapping of Resistance to Meloidogyne arenaria in Arachis stenosperma: A New Source of Nematode Resistance for Peanut. G3 Genes Genomes Genet. 2016, 6, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, P.M.; Brasileiro, A.C.M.; Mehta, A.; Araujo, A.C.G. Functional Genomics in Peanut Wild Relatives. In The Peanut Genome; Springer International Publishing: Cham, Switzerland, 2017; pp. 149–164. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, Y.H.; Zhang, X.J.; Sha, Q.; Chen, Z.D. Gynophore MiRNA Analysis at Different Developmental Stages in Arachis duranensis. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, Q.; Chen, K.; Zhao, S.; Zhang, C.; Pan, R.; Cai, T.; Deng, Y.; Wang, X.; Chen, Y.; et al. Integrated MicroRNA and Transcriptome Profiling Reveals a MiRNA-Mediated Regulatory Network of Embryo Abortion under Calcium Deficiency in Peanut (Arachis hypogaea L.). BMC Genom. 2019, 20, 392. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, P.; Zhao, S.; Zhao, C.; Xia, H.; Hou, L.; Ju, Z.; Zhang, Y.; Li, C.; Wang, X. Small RNA Profiling and Degradome Analysis Reveal Regulation of MicroRNA in Peanut Embryogenesis and Early Pod Development. BMC Genom. 2017, 18, 220. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Hu, M.; Zhao, Y.; Liu, B.; Wang, C.; Zhang, M.; Zhang, L.; Yang, X.; Mu, G. Multi-Omics and MiRNA Interaction Joint Analysis Highlight New Insights into Anthocyanin Biosynthesis in Peanuts (Arachis hypogaea L.). Front. Plant Sci. 2022, 13, 818345, Erratum in Front. Plant Sci. 2022, 13, 929085. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Zhao, K.; Li, F.; Li, K.; Ning, L.; He, J.; Xin, Z.; Yin, D. Small RNA and Degradome Deep Sequencing Reveals the Roles of MicroRNAs in Seed Expansion in Peanut (Arachis hypogaea L.). Front. Plant Sci. 2018, 9, 330144. [Google Scholar] [CrossRef]
- Zhao, C.; Xia, H.; Cao, T.; Yang, Y.; Zhao, S.; Hou, L.; Zhang, Y.; Li, C.; Zhang, X.; Wang, X. Small RNA and Degradome Deep Sequencing Reveals Peanut MicroRNA Roles in Response to Pathogen Infection. Plant Mol. Biol. Rep. 2015, 33, 1013–1029. [Google Scholar] [CrossRef]
- Zhao, C.; Li, T.; Zhao, Y.; Zhang, B.; Li, A.; Zhao, S.; Hou, L.; Xia, H.; Fan, S.; Qiu, J.; et al. Integrated Small RNA and MRNA Expression Profiles Reveal MiRNAs and Their Target Genes in Response to Aspergillus flavus Growth in Peanut Seeds. BMC Plant Biol. 2020, 20, 215. [Google Scholar] [CrossRef]
- Joshi, P.; Sharma, V.; Pandey, A.K.; Nayak, S.N.; Bajaj, P.; Sudini, H.K.; Sharma, S.; Varshney, R.K.; Pandey, M.K. Identification of MiRNAs Associated with Aspergillus flavus Infection and Their Targets in Groundnut (Arachis hypogaea L.). BMC Plant Biol. 2025, 25, 345. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, X.; Hou, R.; Zhang, X.; Li, S.; Yue, F.; Zhang, X.; Zhu, X. Multiple MicroRNAs Are Involved in Regulating Peanut (Arachis hypogaea L.) Resistance to Sclerotium rolfsii at the Early Stage. Trop. Plant Biol. 2022, 15, 276–287. [Google Scholar] [CrossRef]
- Xu, P.; Li, H.; Wang, X.; Zhao, G.; Lu, X.; Dai, S.; Cui, X.; Yuan, M.; Liu, Z. Integrated Analysis of the LncRNA/CircRNA-MiRNA-MRNA Expression Profiles Reveals Novel Insights into Potential Mechanisms in Response to Root-Knot Nematodes in Peanut. BMC Genom. 2022, 23, 239. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, Y.; Zhang, Z.; Zhang, B.; Zhou, Y.; Li, L.; Li, Q.; Davis, K.E.; Patterson, C.; Oo, S.; et al. Response of Root Growth and Development to Nitrogen and Potassium Deficiency as Well as MicroRNA-Mediated Mechanism in Peanut (Arachis hypogaea L.). Front. Plant Sci. 2021, 12, 695234. [Google Scholar] [CrossRef]
- Mittal, M.; Dhingra, A.; Dawar, P.; Payton, P.; Rock, C.D. The Role of MicroRNAs in Responses to Drought and Heat Stress in Peanut (Arachis hypogaea). Plant Genome 2023, 16, e20350. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, C.; Xue, Y.; Tian, Y.; Zhang, H.; Li, N.; Sheng, C.; Jiang, H.; Bai, D. Small RNA and Degradome Deep Sequencing Reveals the Roles of MicroRNAs in Peanut (Arachis hypogaea L.) Cold Response. Front. Plant Sci. 2022, 13, 920195. [Google Scholar] [CrossRef]
- Garg, V.; Agarwal, G.; Pazhamala, L.T.; Nayak, S.N.; Kudapa, H.; Khan, A.W.; Doddamani, D.; Sharma, M.; Kavi Kishor, P.B.; Varshney, R.K. Genome-Wide Identification, Characterization, and Expression Analysis of Small RNA Biogenesis Purveyors Reveal Their Role in Regulation of Biotic Stress Responses in Three Legume Crops. Front. Plant Sci. 2017, 8, 238864. [Google Scholar] [CrossRef]
- Bertioli, D.J.; Cannon, S.B.; Froenicke, L.; Huang, G.; Farmer, A.D.; Cannon, E.K.S.; Liu, X.; Gao, D.; Clevenger, J.; Dash, S.; et al. The Genome Sequences of Arachis duranensis and Arachis ipaensis, the Diploid Ancestors of Cultivated Peanut. Nat. Genet. 2016, 48, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yang, Q.; Chen, X.; Wang, J.; Pan, L.; Chen, M.; Yang, Z.; He, Y.; Liang, X.; Yu, S. Identification and Characterization of MicroRNAs from Peanut (Arachis hypogaea L.) by High-Throughput Sequencing. PLoS ONE 2011, 6, e27530. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Axtell, M.J.; Meyers, B.C. Revisiting Criteria for Plant MicroRNA Annotation in the Era of Big Data. Plant Cell 2018, 30, 272–284. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Wu, R.; Wood, M.; Walton, E.F.; Hellens, R.P. Protocol: A Highly Sensitive RT-PCR Method for Detection and Quantification of MicroRNAs. Plant Methods 2007, 3, 12. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, N.; Tian, C.; Wen, S.; Zhang, C.; Zheng, A.; Hu, X.; Fang, J.; Zhang, Z.; Lai, Z.; et al. The MiR166 Targets CsHDZ3 Genes to Negatively Regulate Drought Tolerance in Tea Plant (Camellia sinensis). Int. J. Biol. Macromol. 2024, 264, 130735. [Google Scholar] [CrossRef]
- Bari, R.; Pant, B.D.; Stitt, M.; Scheible, W.R. PHO2, MicroRNA399, and PHR1 Define a Phosphate-Signaling Pathway in Plants. Plant Physiol. 2006, 141, 988–999. [Google Scholar] [CrossRef]
- Pan, J.; Huang, D.; Guo, Z.; Kuang, Z.; Zhang, H.; Xie, X.; Ma, Z.; Gao, S.; Lerdau, M.T.; Chu, C.; et al. Overexpression of MicroRNA408 Enhances Photosynthesis, Growth, and Seed Yield in Diverse Plants. J. Integr. Plant Biol. 2018, 60, 323–340. [Google Scholar] [CrossRef]
- Mota, A.P.Z.; Brasileiro, A.C.M.; Vidigal, B.; Oliveira, T.N.; da Cunha Quintana Martins, A.; Saraiva, M.A.D.P.; de Araújo, A.C.G.; Togawa, R.C.; Grossi-de-Sá, M.F.; Guimaraes, P.M. Defining the Combined Stress Response in Wild Arachis. Sci. Rep. 2021, 11, 11097. [Google Scholar] [CrossRef]
- Samynathan, R.; Venkidasamy, B.; Shanmugam, A.; Ramalingam, S.; Thiruvengadam, M. Functional Role of MicroRNA in the Regulation of Biotic and Abiotic Stress in Agronomic Plants. Front. Genet. 2023, 14, 1272446. [Google Scholar] [CrossRef]
- Xu, M.; Hu, T.; Zhao, J.; Park, M.Y.; Earley, K.W.; Wu, G.; Yang, L.; Poethig, R.S. Developmental Functions of MiR156-Regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) Genes in Arabidopsis thaliana. PLoS Genet. 2016, 12, e1006263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Zhang, Y.; Wu, C.; Wang, S.; Hao, L.; Wang, S.; Li, T. Md-MiR156ab and Md-Mir395 Target WRKY Transcription Factors to Influence Apple Resistance to Leaf Spot Disease. Front. Plant Sci. 2017, 8, 256407. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zou, Y.; Hu, J.; Ding, Y. Genome-Wide Analysis of the Rice PPR Gene Family and Their Expression Profiles under Different Stress Treatments. BMC Genom. 2018, 19, 720. [Google Scholar] [CrossRef] [PubMed]
- Bresso, E.G.; Chorostecki, U.; Rodriguez, R.E.; Palatnik, J.F.; Schommer, C. Spatial Control of Gene Expression by MiR319-Regulated TCP Transcription Factors in Leaf Development. Plant Physiol. 2018, 176, 1694–1708. [Google Scholar] [CrossRef]
- Jeyaraj, A.; Elango, T.; Li, X.; Guo, G. Utilization of MicroRNAs and Their Regulatory Functions for Improving Biotic Stress Tolerance in Tea Plant [Camellia sinensis (L.) O. Kuntze]. RNA Biol. 2020, 17, 1365–1382. [Google Scholar] [CrossRef]
- Vaucheret, H.; Mallory, A.C.; Bartel, D.P. AGO1 Homeostasis Entails Coexpression of MIR168 and AGO1 and Preferential Stabilization of MiR168 by AGO1. Mol. Cell 2006, 22, 129. [Google Scholar] [CrossRef]
- Aung, K.; Lin, S.I.; Wu, C.C.; Huang, Y.T.; Su, C.L.; Chiou, T.J. Pho2, a Phosphate Overaccumulator, Is Caused by a Nonsense Mutation in a MicroRNA399 Target Gene. Plant Physiol. 2006, 141, 1000–1011. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Wang, S.; Hao, L.; Wang, S.; Xu, C.; Jiang, F.; Li, T. Characterization of Genome-Wide MicroRNAs and Their Roles in Development and Biotic Stress in Pear. Planta 2019, 249, 693–707. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.E.; Pilon, M. MicroRNA-Mediated Systemic down-Regulation of Copper Protein Expression in Response to Low Copper Availability in Arabidopsis. J. Biol. Chem. 2008, 283, 15932–15945. [Google Scholar] [CrossRef]
- Martins, A.C.Q.; Mota, A.P.Z.; Carvalho, P.A.S.V.; Passos, M.A.S.; Gimenes, M.A.; Guimaraes, P.M.; Brasileiro, A.C.M. Transcriptome Responses of Wild Arachis to UV-C Exposure Reveal Genes Involved in General Plant Defense and Priming. Plants 2022, 11, 408. [Google Scholar] [CrossRef]
- Medina, C.; Da Rocha, M.; Magliano, M.; Ratpopoulo, A.; Revel, B.; Marteu, N.; Magnone, V.; Lebrigand, K.; Cabrera, J.; Barcala, M.; et al. Characterization of MicroRNAs from Arabidopsis Galls Highlights a Role for MiR159 in the Plant Response to the Root-Knot Nematode Meloidogyne incognita. New Phytol. 2017, 216, 882–896. [Google Scholar] [CrossRef]
- Hewezi, T.; Maier, T.R.; Nettleton, D.; Baum, T.J. The Arabidopsis MicroRNA396-GRF1/GRF3 Regulatory Module Acts as a Developmental Regulator in the Reprogramming of Root Cells during Cyst Nematode Infection. Plant Physiol. 2012, 159, 321–335. [Google Scholar] [CrossRef]
- Ruiz-Ferrer, V.; Cabrera, J.; Martinez-Argudo, I.; Artaza, H.; Fenoll, C.; Escobar, C. Silenced Retrotransposons Are Major RasiRNAs Targets in Arabidopsis Galls Induced by Meloidogyne javanica. Mol. Plant Pathol. 2018, 19, 2431–2445. [Google Scholar] [CrossRef]
- Cabrera, J.; Barcala, M.; García, A.; Rio-Machín, A.; Medina, C.; Jaubert-Possamai, S.; Favery, B.; Maizel, A.; Ruiz-Ferrer, V.; Fenoll, C.; et al. Differentially Expressed Small RNAs in Arabidopsis Galls Formed by Meloidogyne javanica: A Functional Role for MiR390 and Its TAS3-Derived TasiRNAs. New Phytol. 2016, 209, 1625–1640. [Google Scholar] [CrossRef]
- Hewezi, T.; Howe, P.; Maier, T.R.; Baum, T.J. Arabidopsis Small RNAs and Their Targets during Cyst Nematode Parasitism. Mol. Plant-Microbe Interact. 2008, 21, 1622–1634. [Google Scholar] [CrossRef] [PubMed]
- Guleria, P.; Mahajan, M.; Bhardwaj, J.; Yadav, S.K. Plant Small RNAs: Biogenesis, Mode of Action and Their Roles in Abiotic Stresses. Genom. Proteom. Bioinform. 2012, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Shukla, N.; Joshi, G.; VijayaKumar, C.; Jagannath, A.; Agarwal, M.; Goel, S.; Kumar, A. Genome-Wide Identification and Characterization of MiRNAome from Tomato (Solanum lycopersicum) Roots and Root-Knot Nematode (Meloidogyne incognita) during Susceptible Interaction. PLoS ONE 2017, 12, e0175178. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.B.; Liu, Y.Q.; Chen, D.Y.; Chen, F.Y.; Fang, X.; Hong, G.J.; Wang, L.J.; Wang, J.W.; Chen, X.Y. Jasmonate Response Decay and Defense Metabolite Accumulation Contributes to Age-Regulated Dynamics of Plant Insect Resistance. Nat. Commun. 2017, 8, 13925. [Google Scholar] [CrossRef]
- Kyndt, T.; Goverse, A.; Haegeman, A.; Warmerdam, S.; Wanjau, C.; Jahani, M.; Engler, G.; De Almeida Engler, J.; Gheysen, G. Redirection of Auxin Flow in Arabidopsis thaliana Roots after Infection by Root-Knot Nematodes. J. Exp. Bot. 2016, 67, 4559–4570. [Google Scholar] [CrossRef]
- Suzuki, R.; Kanno, Y.; Abril-Urias, P.; Seo, M.; Escobar, C.; Tsai, A.Y.L.; Sawa, S. Local Auxin Synthesis Mediated by YUCCA4 Induced during Root-Knot Nematode Infection Positively Regulates Gall Growth and Nematode Development. Front. Plant Sci. 2022, 13, 1019427. [Google Scholar] [CrossRef]
- Noureddine, Y.; Da Rocha, M.; An, J.; Médina, C.; Mejias, J.; Mulet, K.; Quentin, M.; Abad, P.; Zouine, M.; Favery, B.; et al. AUXIN RESPONSIVE FACTOR8 Regulates Development of the Feeding Site Induced by Root-Knot Nematodes in Tomato. J. Exp. Bot. 2023, 74, 5752–5766. [Google Scholar] [CrossRef]
- Kepinski, S.; Leyser, O. The Arabidopsis F-Box Protein TIR1 Is an Auxin Receptor. Nature 2005, 435, 446–451. [Google Scholar] [CrossRef]
- Noon, J.B.; Hewezi, T.; Baum, T.J. Homeostasis in the Soybean MiRNA396–GRF Network Is Essential for Productive Soybean Cyst Nematode Infections. J. Exp. Bot. 2019, 70, 1653. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Jiang, Y.; Wang, C.; Roberts, P.A. Expression Analysis of MicroRNAs and Their Target Genes in Cucumis metuliferus Infected by the Root-Knot Nematode Meloidogyne incognita. Physiol. Mol. Plant Pathol. 2020, 111, 101491. [Google Scholar] [CrossRef]
- Ye, D.; Qi, Y.; Cao, S.; Duan, Y.; Huynh, B.L. Identification and Characterization of MicroRNA396 and Its Targets in Cucumis metuliferus Infected with Meloidogyne incognita. South Afr. J. Bot. 2024, 165, 417–427. [Google Scholar] [CrossRef]
- Kim, W.; Ahn, H.J.; Chiou, T.J.; Ahn, J.H. The Role of the MiR399-PHO2 Module in the Regulation of Flowering Time in Response to Different Ambient Temperatures in Arabidopsis thaliana. Mol. Cells 2011, 32, 83–88. [Google Scholar] [CrossRef]
- Tian, B.; Wang, S.; Todd, T.C.; Johnson, C.D.; Tang, G.; Trick, H.N. Genome-Wide Identification of Soybean MicroRNA Responsive to Soybean Cyst Nematodes Infection by Deep Sequencing. BMC Genom. 2017, 18, 572. [Google Scholar] [CrossRef]
- Val-Torregrosa, B.; Bundó, M.; San Segundo, B. Crosstalk between Nutrient Signalling Pathways and Immune Responses in Rice. Agriculture 2021, 11, 747. [Google Scholar] [CrossRef]
- Val-Torregrosa, B.; Bundó, M.; Martín-Cardoso, H.; Bach-Pages, M.; Chiou, T.J.; Flors, V.; Segundo, B.S. Phosphate-Induced Resistance to Pathogen Infection in Arabidopsis. Plant J. 2022, 110, 452–469. [Google Scholar] [CrossRef]
- Noureddine, Y.; Mejias, J.; da Rocha, M.; Thomine, S.; Quentin, M.; Abad, P.; Favery, B.; Jaubert-Possamai, S. Copper MicroRNAs Modulate the Formation of Giant Feeding Cells Induced by the Root Knot Nematode Meloidogyne incognita in Arabidopsis thaliana. New Phytol. 2022, 236, 283–295. [Google Scholar] [CrossRef]
- Xu, M.; Li, Y.; Zhang, Q.; Xu, T.; Qiu, L.; Fan, Y.; Wang, L. Novel MiRNA and PhasiRNA Biogenesis Networks in Soybean Roots from Two Sister Lines That Are Resistant and Susceptible to SCN Race 4. PLoS ONE 2014, 9, e110051. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.W.; Lin, J.S.; Li, Y.C.; Jhu, M.Y.; King, Y.C.; Jeng, S.T. MicroR408 Regulates Defense Response upon Wounding in Sweet Potato. J. Exp. Bot. 2019, 70, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Pilon, M. Conserved Cu-MicroRNAs in Arabidopsis thaliana Function in Copper Economy under Deficiency. Plants 2019, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Klink, V.P.; Overall, C.C.; Alkharouf, N.W.; MacDonald, M.H.; Matthews, B.F. A Time-Course Comparative Microarray Analysis of an Incompatible and Compatible Response by Glycine max (Soybean) to Heterodera glycines (Soybean Cyst Nematode) Infection. Planta 2007, 226, 1423–1447. [Google Scholar] [CrossRef]
- Shukla, L.I.; Chinnusamy, V.; Sunkar, R. The Role of MicroRNAs and Other Endogenous Small RNAs in Plant Stress Responses. Biochim. Biophys. Acta 2008, 1779, 743–748. [Google Scholar] [CrossRef]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.V.; Jugulam, M. Role of Cytochrome P450 Enzymes in Plant Stress Response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Zsigmond, L.; Rigó, G.; Szarka, A.; Székely, G.; Ötvös, K.; Darula, Z.; Medzihradszky, K.F.; Koncz, C.; Koncz, Z.; Szabados, L. Arabidopsis PPR40 Connects Abiotic Stress Responses to Mitochondrial Electron Transport. Plant Physiol. 2008, 146, 1721–1737. [Google Scholar] [CrossRef]
- Laluk, K.; Abuqamar, S.; Mengiste, T. The Arabidopsis Mitochondria-Localized Pentatricopeptide Repeat Protein PGN Functions in Defense against Necrotrophic Fungi and Abiotic Stress Tolerance. Plant Physiol. 2011, 156, 2053–2068. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Lou, Y. PRRs and NB-LRRs: From Signal Perception to Activation of Plant Innate Immunity. Int. J. Mol. Sci. 2019, 20, 1882. [Google Scholar] [CrossRef]
- Shivaprasad, P.V.; Chen, H.M.; Patel, K.; Bond, D.M.; Santos, B.A.C.M.; Baulcombe, D.C. A MicroRNA Superfamily Regulates Nucleotide Binding Site-Leucine-Rich Repeats and Other MRNAs. Plant Cell 2012, 24, 859–874, Erratum in Plant Cell 2019, 31, 1665–1668. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.Q.; Zhang, J.; Wu, L.; Qi, Y.; Zhou, J.M. Identification of MicroRNAs Involved in Pathogen-Associated Molecular Pattern-Triggered Plant Innate Immunity. Plant Physiol. 2010, 152, 2222–2231. [Google Scholar] [CrossRef]
- Zhai, J.; Jeong, D.H.; de Paoli, E.; Park, S.; Rosen, B.D.; Li, Y.; González, A.J.; Yan, Z.; Kitto, S.L.; Grusak, M.A.; et al. MicroRNAs as Master Regulators of the Plant NB-LRR Defense Gene Family via the Production of Phased, Trans-Acting SiRNAs. Genes Dev. 2011, 25, 2540–2553. [Google Scholar] [CrossRef]
- De Vries, S.; Kloesges, T.; Rose, L.E. Evolutionarily Dynamic, but Robust, Targeting of Resistance Genes by the MiR482/2118 Gene Family in the Solanaceae. Genome Biol. Evol. 2015, 7, 3307–3321. [Google Scholar] [CrossRef]
- Zhang, Y.; Waseem, M.; Zeng, Z.; Xu, J.; Chen, C.; Liu, Y.; Zhai, J.; Xia, R. MicroRNA482/2118, a MiRNA Superfamily Essential for Both Disease Resistance and Plant Development. New Phytol. 2022, 233, 2047–2057. [Google Scholar] [CrossRef]
- Liao, L.; Xie, B.; Guan, P.; Jiang, N.; Cui, J. New Insight into the Molecular Mechanism of MiR482/2118 during Plant Resistance to Pathogens. Front. Plant Sci. 2022, 13, 1026762. [Google Scholar] [CrossRef]
- Tang, J.; Gu, X.; Liu, J.; He, Z. Roles of small RNAs in crop disease resistance. Stress Biol. 2021, 1, 6. [Google Scholar] [CrossRef]
- López-Márquez, D.; Del-Espino, A.; Ruiz-Albert, J.; Bejarano, E.R.; Brodersen, P.; Beuzón, C.R. Regulation of plant immunity via small RNA-mediated control of NLR expression. J. Exp. Bot. 2023, 74, 6052–6068. [Google Scholar] [CrossRef] [PubMed]
- Morgante, C.V.; Brasileiro, A.C.M.; Roberts, P.A.; Guimaraes, L.A.; Araujo, A.C.G.; Fonseca, L.N.; Leal-Bertioli, S.C.M.; Bertioli, D.J.; Guimaraes, P.M. A Survey of Genes Involved in Arachis stenosperma Resistance to Meloidogyne arenaria Race 1. Funct. Plant Biol. 2013, 40, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.M.; Arraes, F.; Quintana Martins, A.C.; Freitas Alves, N.S.; Melo, B.P.; Morgante, C.V.; Passos Saraiva, M.A.; Grossi-De-Sá, M.F.; Guimaraes, P.M.; Miranda Brasileiro, A.C. A Novel Soybean Hairy Root System for Gene Functional Validation. PLoS ONE 2023, 18, e0285504. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-Optimal Probabilistic RNA-Seq Quantification. Nat. Biotechnol. 2016, 34, 525–527, Erratum in Nat. Biotechnol. 2016, 34, 888. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and Memory-Efficient Alignment of Short DNA Sequences to the Human Genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Johnson, N.R.; Yeoh, J.M.; Coruh, C.; Axtell, M.J. Improved Placement of Multi-Mapping Small RNAs. G3 Genes Genomes Genet. 2016, 6, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.R.; Bernhart, S.H.; Lorenz, R. The ViennaRNA Web Services. Methods Mol. Biol. 2015, 1269, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-Time Quantification of MicroRNAs by Stem-Loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative Expression Software Tool (REST) for Group-Wise Comparison and Statistical Analysis of Relative Expression Results in Real-Time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Santos, L.S.; Maximiano, M.R.; Megias, E.; Pappas, M.; Ribeiro, S.G.; Mehta, A. Quantitative Expression of MicroRNAs in Brassica oleracea Infected with Xanthomonas campestris pv. campestris. Mol. Biol. Rep. 2019, 46, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Morgante, C.V.; Guimarães, P.M.; Martins, A.C.Q.; Araújo, A.C.G.; Leal-Bertioli, S.C.M.; Bertioli, D.J.; Brasileiro, A.C.M. Reference Genes for Quantitative Reverse Transcription-Polymerase Chain Reaction Expression Studies in Wild and Cultivated Peanut. BMC Res. Notes 2011, 4, 339. [Google Scholar] [CrossRef] [PubMed]
- Teotia, S.; Wang, X.; Zhou, N.; Wang, M.; Liu, H.; Qin, J.; Han, D.; Li, C.; Li, C.E.; Pan, S.; et al. A High-Efficiency Gene Silencing in Plants Using Two-Hit Asymmetrical Artificial MicroRNAs. Plant Biotechnol. J. 2023, 21, 1799–1811. [Google Scholar] [CrossRef]
- Zhou, Z.; Cao, Y.; Li, T.; Wang, X.; Chen, J.; He, H.; Yao, W.; Wu, J.; Zhang, H. MicroRNAs Are Involved in Maize Immunity Against Fusarium Verticillioides Ear Rot. Genom. Proteom. Bioinform. 2020, 18, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, S.; Schwab, R.; Weigel, D. Gene Silencing in Plants Using Artificial MicroRNAs and Other Small RNAs. Plant J. 2008, 53, 674–690. [Google Scholar] [CrossRef]
- Bravo-Vázquez, L.A.; Castro-Pacheco, A.M.; Pérez-Vargas, R.; Velázquez-Jiménez, J.F.; Paul, S. The Emerging Applications of Artificial MicroRNA-Mediated Gene Silencing in Plant Biotechnology. Non-Coding RNA 2025, 11, 19. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, J.; Zhang, N.; Wu, J.; Si, H. Roles of MicroRNAs in Abiotic Stress Response and Characteristics Regulation of Plant. Front. Plant Sci. 2022, 13, 919243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).