Production of Organic Acids from Cashew Nut Shell Liquid (CNSL) via Electrochemical Synthesis
Abstract
1. Introduction
2. Results and Discussion
2.1. Spectroelectrochemical Analysis
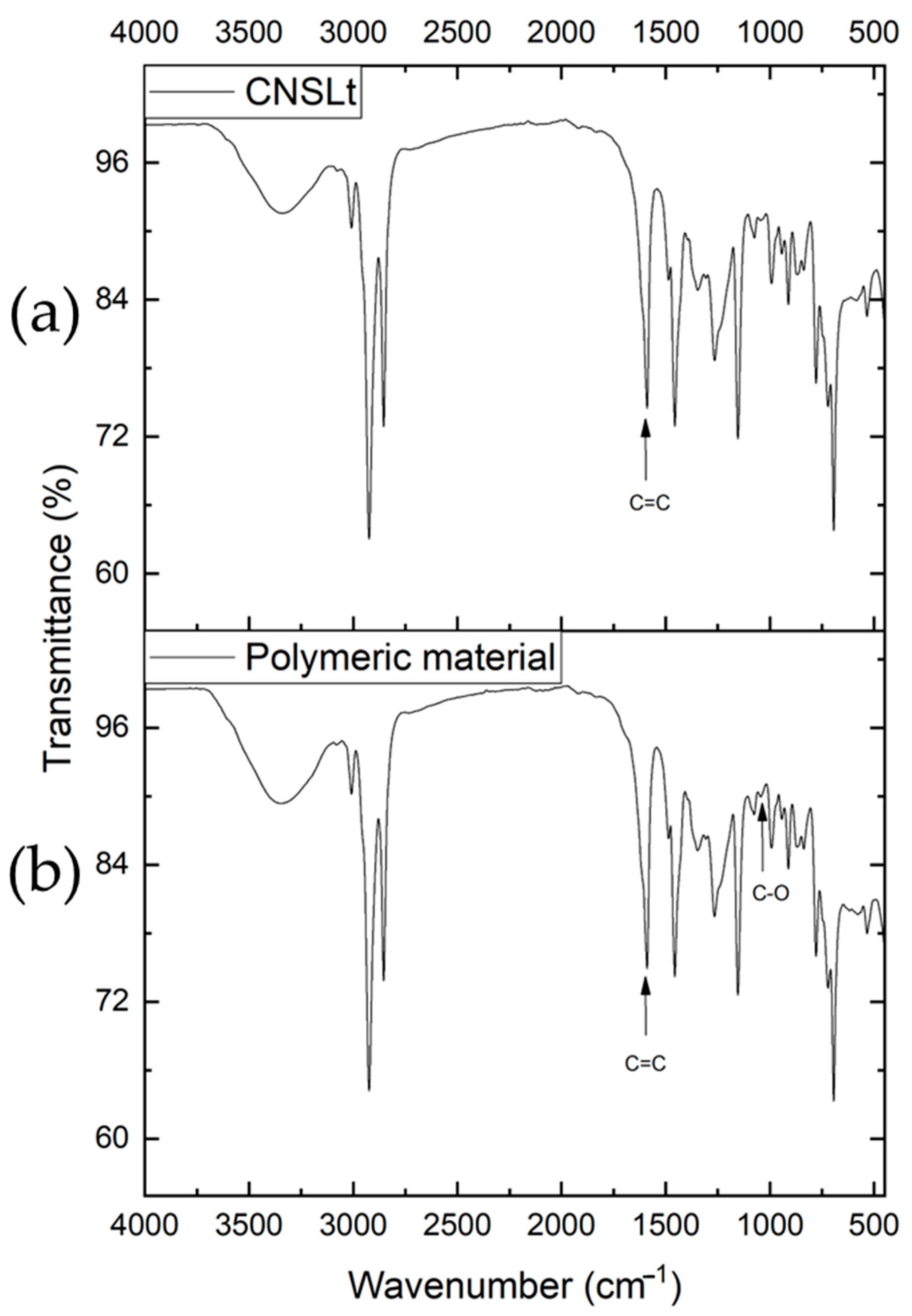
2.2. Infrared Spectroscopy Characterization
3. Materials and Methods
3.1. Reagents
3.2. Electrochemical Synthesis of High-Value-Added Organic Acids
3.3. Detection of Organic Acids by HPLC
3.4. Spectroelectrochemical Analysis
3.5. Cyclic Voltammetry Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brusseau, M.L.; Artiola, J.F. Chemical contaminants. In Environmental and Pollution Science; Academic Press: Cambridge, MA, USA, 2019; pp. 175–190. [Google Scholar]
- Pereira, R.; Díaz-Cruz, M.S.; Barceló, D.; Llorca, M. Human health risk assessment of endocrine disrupting compounds in urban wastewater treatment plants: A comparative study of influent and effluent concentrations in different European countries. Sci. Total Environ. 2019, 685, 556–569. [Google Scholar]
- Baquerizo, M.; Acuña, M.; Solis-Castro, M. Contaminación de los ríos: Caso río Guayas y sus afluentes. Manglar 2019, 16, 63–70. [Google Scholar] [CrossRef]
- Fenga, C.; Gangemi, S.; Costa, C. Health effects of toxicants: Online knowledge support. Interdiscip. Toxicol. 2015, 8, 145–146. [Google Scholar]
- Clark, J.H.; Macquarrie, D. (Eds.) Handbook of Green Chemistry and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Lubi, M.C.; Thachil, E.T. Cashew nut shell liquid (CNSL)—A versatile monomer for polymer synthesis. Des. Monomers Polym. 2000, 3, 123–153. [Google Scholar] [CrossRef]
- Veeramanoharan, A.; Kim, S.C. A comprehensive review on sustainable surfactants from CNSL: Chemistry, key applications and research perspectives. RSC Adv. 2024, 14, 25429–25471. [Google Scholar] [CrossRef]
- Rojtman, E.; Denis, M.; Sirvent, C.; Lapinte, V.; Caillol, S.; Briou, B. Polyols from Cashew Nutshell Liquid (CNSL), corner-stone building blocks for cutting edge biobased additives and polymers. Polym. Chem. 2024, 15, 4375–4415. [Google Scholar] [CrossRef]
- Gartili, A.; Lapinte, V.; Briou, B.; Caillol, S. Valorization of CNSL as a Sustainable Solution for PVC Plasticization: A Comprehensive Study of Cardanol–Cardol Mixture. J. Am. Oil Chem. Soc. 2025, 102, 1141–1157. [Google Scholar] [CrossRef]
- Preethi, R.; Moses, J.A.; Anandharamakrishnan, C. Development of anacardic acid incorporated biopolymeric film for active packaging applications. Food Packag. Shelf Life 2021, 28, 100656. [Google Scholar] [CrossRef]
- Krishnan, P. Cashew Nut Shell Liquid (CNSL) Based Bio-Derived Resin And Composites for Advanced Structural, Automotive, Electronic Packaging and Medical applications-A Review. Org. Polym. Mater. Res. 2019, 1, 9–13. [Google Scholar] [CrossRef]
- Paramashivappa, R.; Kumar, P.P.; Vithayathil, P.J.; Rao, A.S. Novel method for isolation of major phenolic constituents from cashew (Anacardium occidentale L.) nut shell liquid. J. Agric. Food Chem. 2001, 49, 2548–2551. [Google Scholar] [CrossRef]
- Phani Kumar, P.; Paramashivappa, R.; Vithayathil, P.J.; Subba Rao, P.V.; Srinivasa Rao, A. Process for isolation of cardanol from technical cashew (Anacardium occidentale L.) nut shell liquid. J. Agric. Food Chem. 2002, 50, 4705–4708. [Google Scholar] [CrossRef]
- Rodrigues, F.H.; França, F.C.; Souza, J.R.; Ricardo, N.M.; Feitosa, J. Comparison between physico-chemical properties of the technical Cashew Nut Shell Liquid (CNSL) and those natural extracted from solvent and pressing. Polímeros 2011, 21, 156–160. [Google Scholar] [CrossRef]
- Medeiros, M.C.; dos Santos, E.V.; Martinez-Huitle, C.A.; Fajardo, A.S.; Castro, S.S. Obtaining high-added value products from the technical cashew nut shell liquid using electrochemical oxidation with BDD anodes. Sep. Purif. Technol. 2020, 250, 117099. [Google Scholar] [CrossRef]
- Medeiros, M.; de Medeiros, J.; Martinez-Huitle, C.; Oliveira, T.; Mazzetto, S.; da Silva, F.; Castro, S.S. Long-chain phenols oxidation using a flow electrochemical reactor assembled with a TiO2-RuO2-IrO2 DSA electrode. Sep. Purif. Technol. 2021, 264, 118425. [Google Scholar] [CrossRef]
- Medeiros, M.C.; Castro, S.S.; dos Santos, E.V.; Rodrigo, M.A.; Martínez-Huitle, C.A. A proof of concept for the electro-refinery: Selective electroproduction of acetic acid from t-CNSL waste by using DSA electrode. Electrochem. Commun. 2022, 141, 107356. [Google Scholar] [CrossRef]
- Orduz-Rodríguez, J.O.; Rodríguez-Polanco, E. El marañón (Anacardium occidentale L.) un cultivo con potencial productivo: Desarrollo tecnológico y perspectivas en Colombia. Agron. Mesoam. 2022, 33, 47268. [Google Scholar] [CrossRef]
- Martins, R.I.; Beatriz, A.D.; Santaella, S.T.; Lotufo, L.V. Ecotoxicological analysis of cashew nut industry effluents, specifically two of its major phenolic components, cardol and cardanol. Pan-Am. J. Aquat. Sci. 2009, 4, 363–368. [Google Scholar]
- Sahoo, A.; Ghosh, A.; Dey, A.; Maji, S.; Das, S.; Sultana, I.; Chattopadhyay, S. Assessment of Oxidative Liver Injury Caused by Cashew Nut Shell Liquid (CNSL) via Interacting with Bcl2 Gene in Male Wistar Rats and Basic In-silico Approaches. In Proceedings of the Zoological Society; Springer: New Delhi, India, 2024; Volume 77, pp. 136–146. [Google Scholar]
- Stiefel, S.; Marks, C.; Schmidt, T.; Hanisch, S.; Spalding, G.; Wessling, M. Wessling, Overcoming lignin heterogeneity: Reliably characterizing the cleavage of technical lignin. Green Chem. 2016, 18, 531–540. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; De Lima, M.D.; Da Silva, D.R.; Garcia-Segura, S.; Martínez-Huitle, C.A. Influence of the water hardness on the performance of electro-Fenton approach: Decolorization and mineralization of Eriochrome Black T. Electrochem. Acta 2016, 208, 153–163. [Google Scholar] [CrossRef]
- Alfonta, L.; Bardea, A.; Khersonsky, O.; Katz, E.; Willner, I. Chronopotentiometry and Faradaic impedance spectroscopy as signal transduction methods for the biocatalytic precipitation of an insoluble product on electrode supports: Routes for enzyme sensors, immunosensors and DNA sensors. Biosens. Bioelectron. 2001, 16, 675–687. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J. Electrochemical reduction of CO2 to formate in aqueous solution using electro-deposited Sn catalysts. Chem. Eng. J. 2016, 293, 161–170. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Ducuara, J.A.; Nuñez de la Rosa, Y. Study of Electrochemical Transformation of Anacardic Acid from Cashew (Anacardium occidentale) Nut Shell Liquid. Molecules 2025, 30, 1330. [Google Scholar] [CrossRef] [PubMed]
- Larkin, P.J.; Jackson, A. Interpretation of the Infrared Spectra of Metal Stearate Salts. Appl. Spectrosc. Pract. 2024, 2, 27551857241253834. [Google Scholar] [CrossRef]
- Smith, B.C. The Carbonyl Group, Part V: Carboxylates—Coming Clean. Spectroscopy 2018, 33, 20–23. [Google Scholar]
- Xue, Y.; Hu, X.; Sun, Q.; Wang, H.-Y.; Wang, H.-L.; Hou, X.-M. Review of electrochemical degradation of phenolic compounds. Int. J. Miner. Metall. Mater. 2021, 28, 1413–1428. [Google Scholar] [CrossRef]
- Maia, F.; Clemente, C.; Oliveira, T.; Lomonaco, D.; Oliveira, T.; Almeida, M.; De Lima, P.; Correia, A.; Mazzeto, S. Electrochemical and computational studies of phenolic antioxidants from cashew nut shell liquid. Electrochim. Acta. 2012, 79, 67–73. [Google Scholar] [CrossRef]
- Tasic, Z.; Gupta, V.K.; Antonijevic, M.M. The mechanism and kinetics of degradation of phenolics in wastewaters using electrochemical oxidation. Int. J. Electrochem. Sci. 2014, 9, 3473–3490. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Yakupova, E. Electrochemical sensors based on the electropolymerized natural phenolic antioxidants and their analytical application. Sensors 2021, 21, 8385. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- Tahar, N.B.; Savall, A. Electropolymerization of phenol on a vitreous carbon electrode in alkaline aqueous solution at different temperatures. Electrochim. Acta 2009, 55, 465–469. [Google Scholar] [CrossRef][Green Version]
- Saravanan, G. Porous Carbon Materials for Electrochemical Energy Storage. Int. J. Energetic Mater. 2018, 4, 13–16. [Google Scholar][Green Version]

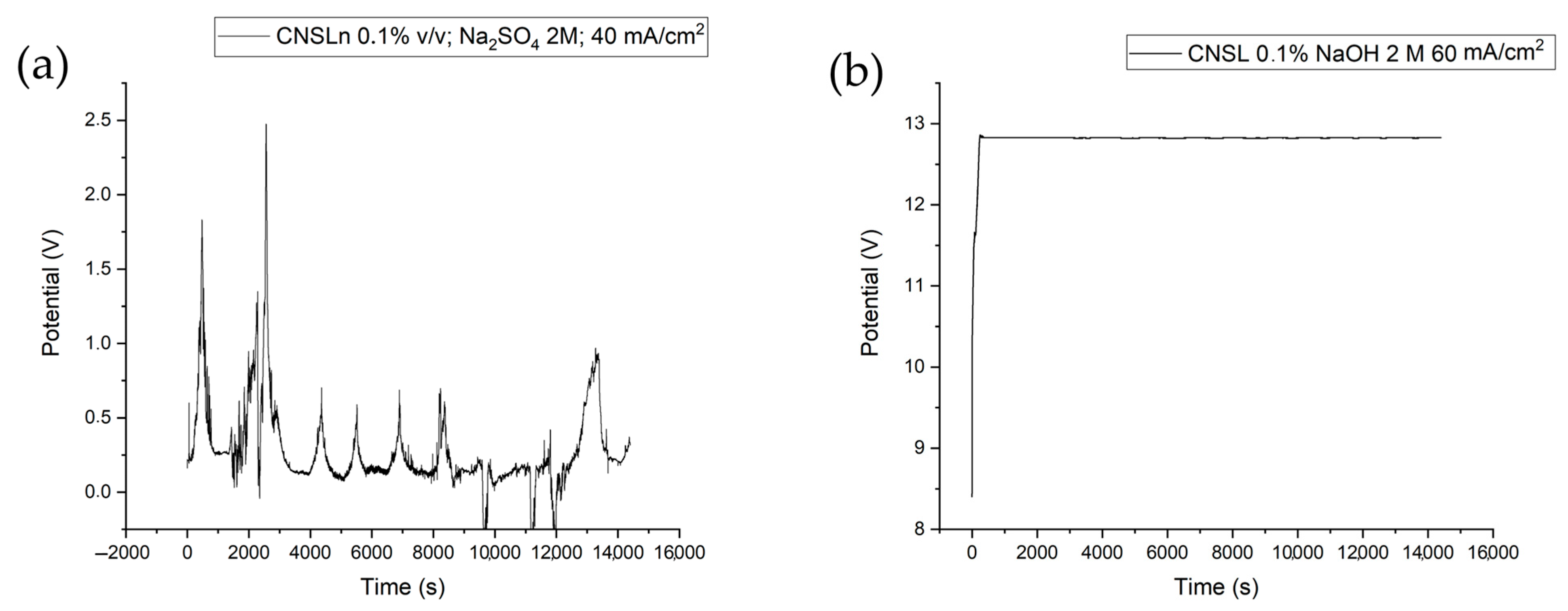
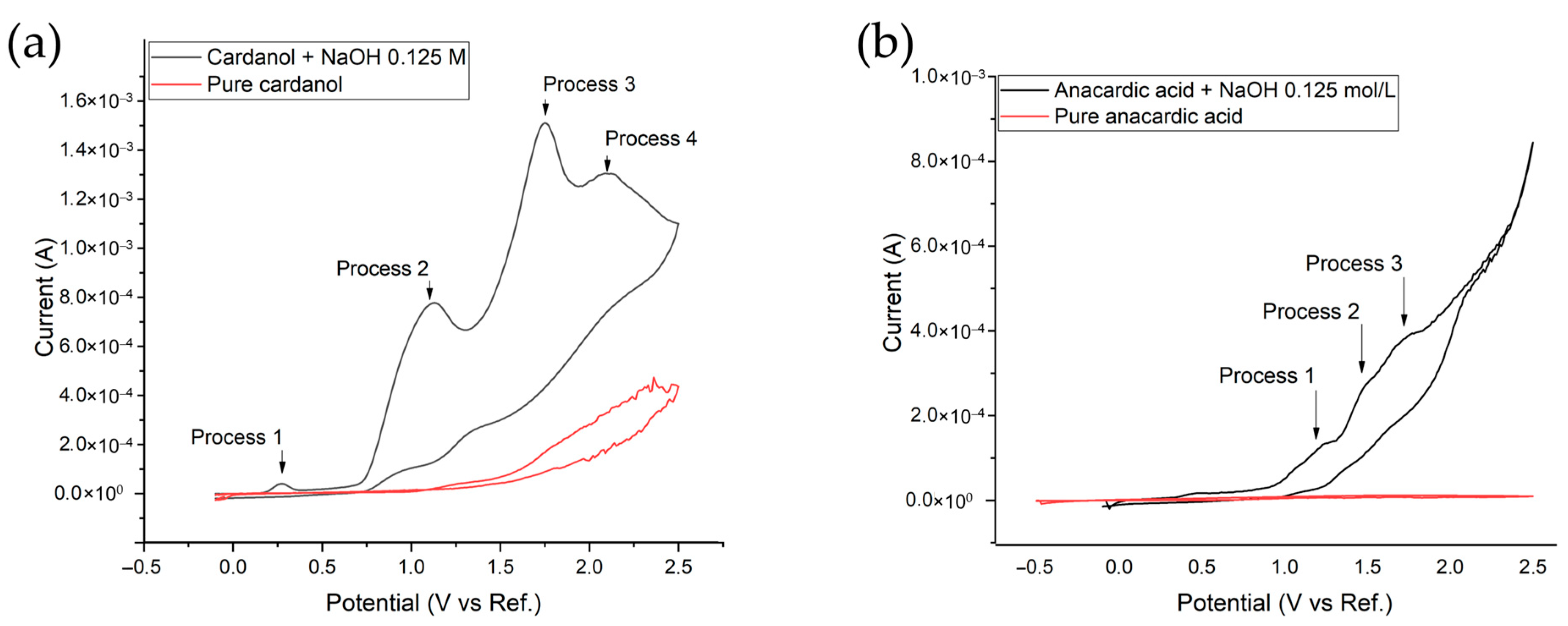


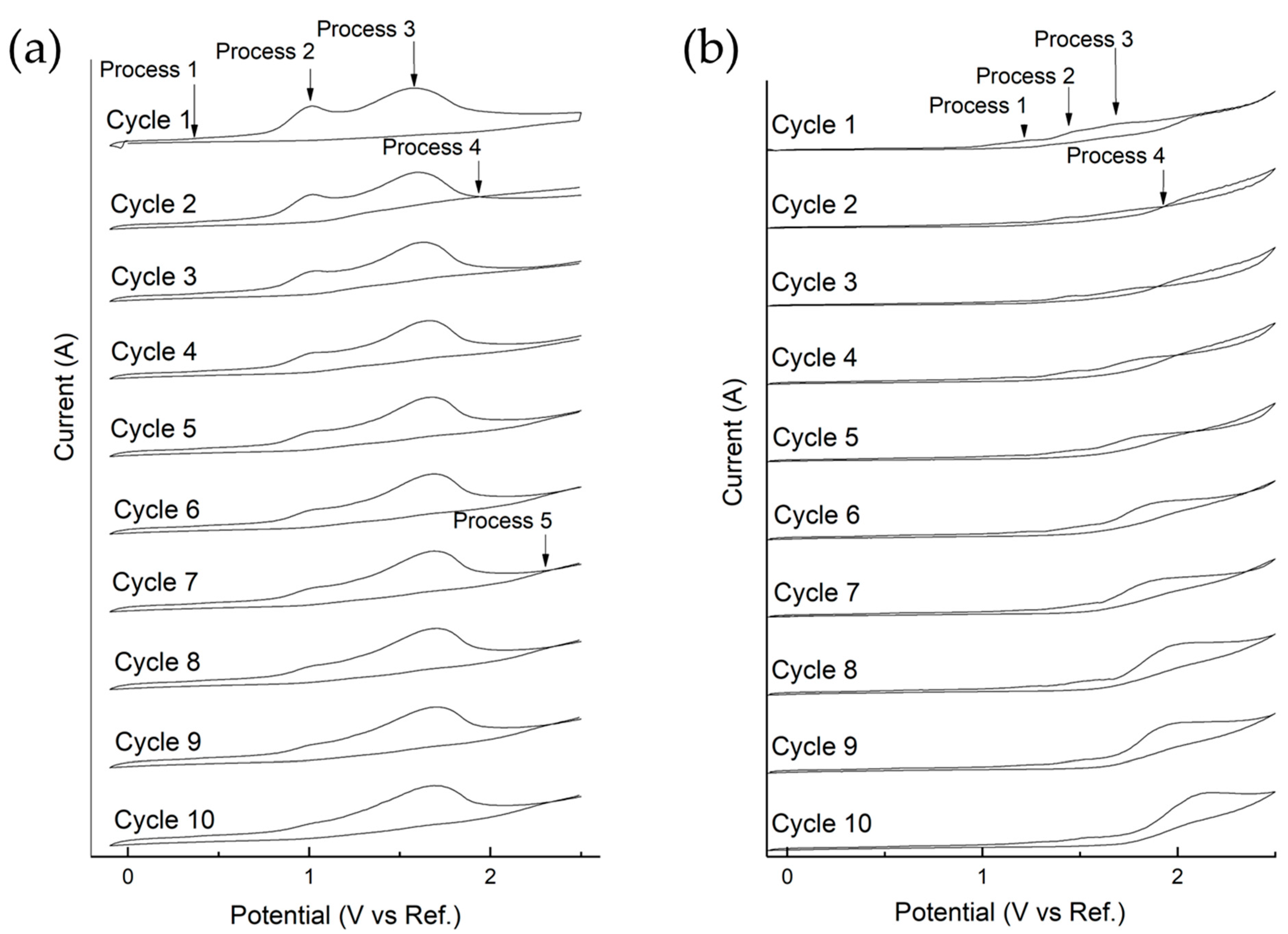
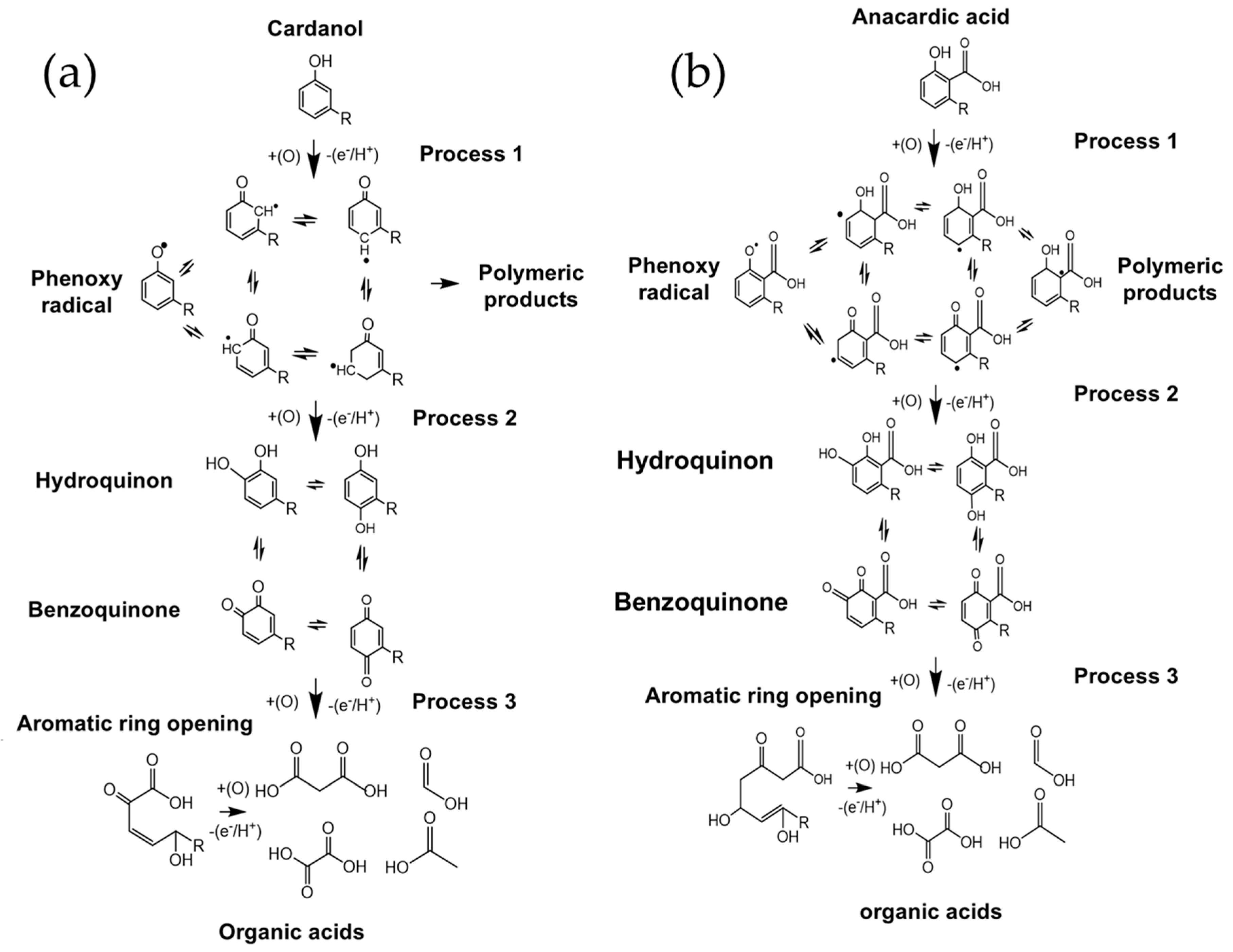
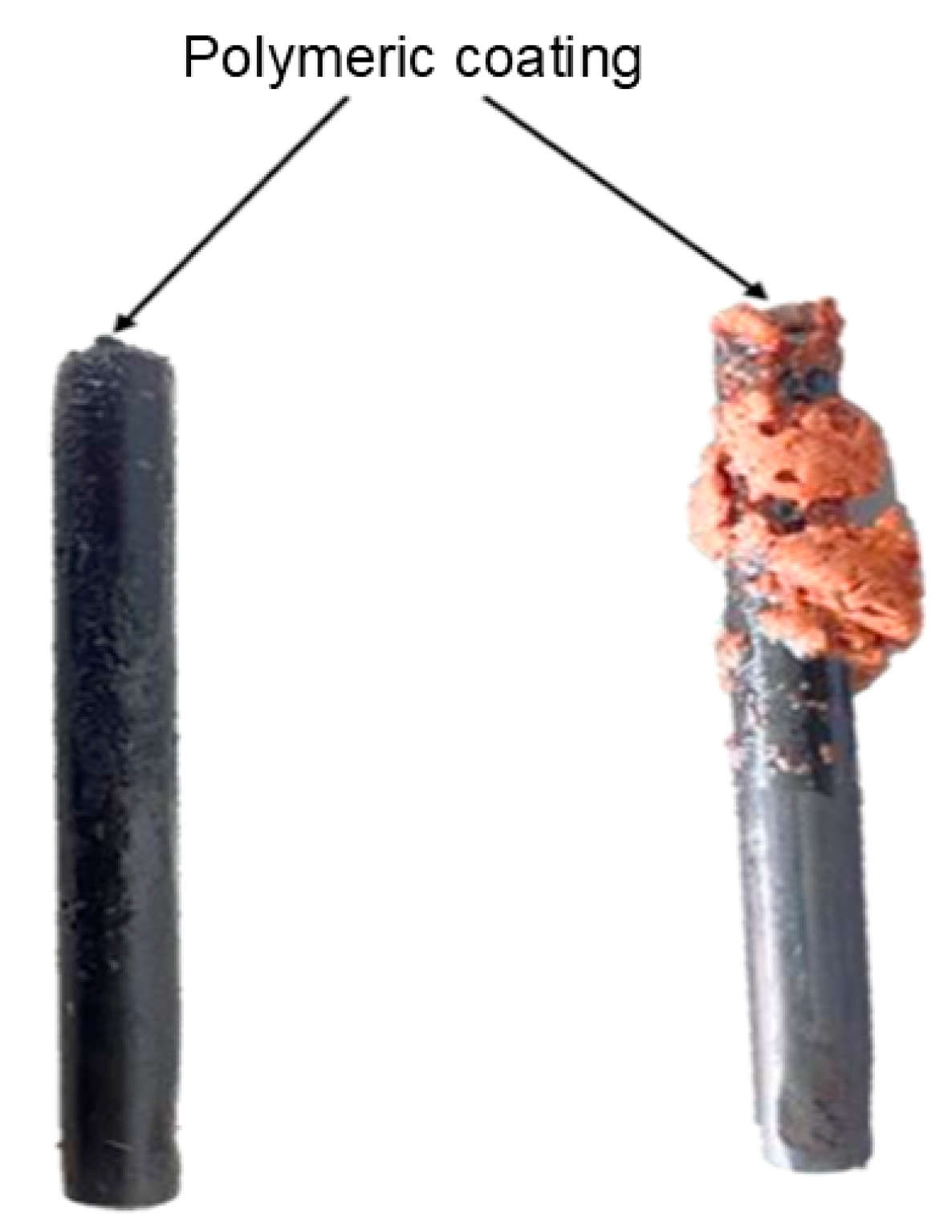

| CNSL-n | CNSL-t | Electroly Salts (M) | Current Density | Organic Acids Concentration (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % v/v | % v/v | Na2SO4 | NaOH | mA cm−2 | Citric Acid | Propionic Acid | Malonic Acid | Oxalic Acid | Lactic Acid | Formic Acid | Acetic Acid |
| 0.01 | - | 0.125 | - | 40 | 9.12 | 10.02 | 6.79 | 1.83 | 33.1 | 11.52 | 38.72 |
| 0.01 | - | 2 | - | 17 | - | 10.65 | - | 39.73 | 19.84 | 281.96 | |
| 0.1 | - | 0.125 | - | 12.19 | - | - | 0.46 | - | 9.1 | 199.78 | |
| 0.1 | - | 2 | - | - | - | 8.81 | 58.69 | 29.59 | 21.9 | 28.77 | |
| 0.01 | - | - | 0.125 | 33.88 | - | 3.92 | 10.81 | 60.22 | 58.94 | 121.78 | |
| 0.01 | - | - | 2 | 12.85 | - | - | 21.59 | 269.48 | 58.83 | 132.64 | |
| 0.1 | - | - | 0.125 | - | 13.5 | - | 45.58 | 41.44 | 34.64 | 65.99 | |
| 0.1 | - | - | 2 | - | - | - | 65.93 | 327.25 | 189.09 | 378.1 | |
| - | 0.01 | 0.125 | - | - | 11.3 | 11.14 | 1.4 | 31.51 | 9.98 | 89.98 | |
| - | 0.01 | 2 | - | 17.16 | - | 9.01 | - | 35.2 | 12.28 | 26.99 | |
| - | 0.1 | 0.125 | - | 11.46 | - | 17.46 | - | 13.57 | 15.47 | 189.41 | |
| - | 0.1 | 2 | - | - | - | 8.32 | 62.59 | - | 12.41 | 567 | |
| - | 0.01 | - | 0.125 | - | 38.83 | - | - | 19.32 | 58.96 | 133.09 | |
| - | 0.01 | - | 2 | 13.98 | - | 15.25 | 13.88 | 250.57 | 156.57 | 431.79 | |
| - | 0.1 | - | 0.125 | - | 109.65 | - | 41.42 | 371.68 | 140.29 | 248.88 | |
| - | 0.1 | - | 2 | - | - | - | 66.42 | 531.78 | 261.7 | 573.92 | |
| 0.01 | - | 0.125 | - | - | - | 7.6 | 106.85 | 1.15 | 29.1 | 8.71 | 77.3 |
| 0.01 | - | 2 | - | 14.35 | - | 12.8 | - | 34.39 | 16.37 | 167.61 | |
| 0.1 | - | 0.125 | - | 10.83 | - | - | 1.85 | 5.43 | 14.38 | 205.38 | |
| 0.1 | - | 2 | - | - | 13.13 | 37.68 | |||||
| 0.01 | - | - | 0.125 | - | 34.19 | 10.37 | 9.01 | 49.97 | 51.44 | 154.77 | |
| 0.01 | - | - | 2 | - | 16.17 | - | 18.03 | 395.16 | 266.13 | 572.09 | |
| 0.1 | - | - | 0.125 | - | 68.23 | - | 49.03 | 58.16 | 60.99 | 123.54 | |
| 0.1 | - | - | 2 | - | - | - | 55.46 | 405.88 | 305.24 | 744.3 | |
| - | 0.01 | 0.125 | - | - | - | 11.94 | 1.63 | 35.77 | 8.79 | 17.7 | |
| - | 0.01 | 2 | - | 12.51 | - | 9.73 | 7.13 | 37.4 | 10.46 | 21.4 | |
| - | 0.1 | 0.125 | - | - | - | - | 1.05 | - | 5.8 | 21.71 | |
| - | 0.1 | 2 | - | - | - | - | - | - | 9.07 | 17.44 | |
| - | 0.01 | - | 0.125 | 6.73 | 6 | 38.45 | 16.4 | 37.14 | |||
| - | 0.01 | - | 2 | - | 17.15 | - | 20.24 | 399.25 | 75.45 | 99.69 | |
| - | 0.1 | - | 0.125 | - | 20.76 | 8.12 | 13.08 | 80.99 | 48.83 | 71.43 | |
| - | 0.1 | - | 2 | - | 60.55 | - | 37.35 | 264.71 | 220.59 | 828.86 | |
| CNSL % v/v | NaOH (M) | Current Density (mA/cm2) | Acetic Acid (mg/L) | Formic Acid (mg/L) | Lactic Acid (mg/L) | Yield (%) | |
|---|---|---|---|---|---|---|---|
| CNSLt | CNSLn | ||||||
| 0.1 | - | 2 | 60 | 828.86 | - | - | 82.9 |
| - | 0.1 | 2 | 60 | 744.3 | - | - | 74.4 |
| 0.1 | - | 2 | 60 | - | 220.59 | - | 22.1 |
| - | 0.1 | 2 | 60 | - | 305.24 | - | 30.5 |
| 0.1 | - | 2 | 40 | - | - | 531.78 | 53.2 |
| - | 0.1 | 2 | 60 | - | - | 405.88 | 40.6 |
| Organic Substrate | Electrolyte | Organic Acids (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anacardic Acid (% v/v) | Cardanol (% v/v) | Na2SO4 (M) | NaOH (M) | Citric Acid | Propionic Acid | Malonic Acid | Tartaric Acid | Oxalic Acid | Lactic Acid | Formic Acid | Acetic Acid |
| 0.1 | 0.125 | 11.8 | 9.27 | 18.45 | 85.72 | ||||||
| 0.1 | 0.125 | 103.69 | 48.43 | 132.25 | 898.74 | ||||||
| Experiment | CNSL-n | CNSL-t | Electrolyte Salts (M) | Current Density | Electrolysis Time | |
|---|---|---|---|---|---|---|
| # | % v/v | % v/v | Na2SO4 | NaOH | mA/cm−2 | Min |
| 1 | 0.01 | - | 0.125 | - | 40 | 240 |
| 2 | 0.01 | - | 2 | - | ||
| 3 | 0.1 | - | 0.125 | - | ||
| 4 | 0.1 | - | 2 | - | ||
| 5 | 0.01 | - | - | 0.125 | ||
| 6 | 0.01 | - | - | 2 | ||
| 7 | 0.1 | - | - | 0.125 | ||
| 8 | 0.1 | - | - | 2 | ||
| 9 | - | 0.01 | 0.125 | - | ||
| 10 | - | 0.01 | 2 | - | ||
| 11 | - | 0.1 | 0.125 | - | ||
| 12 | - | 0.1 | 2 | - | ||
| 13 | - | 0.01 | - | 0.125 | ||
| 14 | - | 0.01 | - | 2 | ||
| 15 | - | 0.1 | - | 0.125 | ||
| 16 | - | 0.1 | - | 2 | ||
| 17 | 0.01 | - | 0.125 | - | 60 | |
| 18 | 0.01 | - | 2 | - | ||
| 19 | 0.1 | - | 0.125 | - | ||
| 20 | 0.1 | - | 2 | - | ||
| 21 | 0.01 | - | - | 0.125 | ||
| 22 | 0.01 | - | - | 2 | ||
| 23 | 0.1 | - | - | 0.125 | ||
| 24 | 0.1 | - | - | 2 | ||
| 25 | - | 0.01 | 0.125 | - | ||
| 26 | - | 0.01 | 2 | - | ||
| 27 | - | 0.1 | 0.125 | - | ||
| 28 | - | 0.1 | 2 | - | ||
| 29 | - | 0.01 | - | 0.125 | ||
| 30 | - | 0.01 | - | 2 | ||
| 31 | - | 0.1 | - | 0.125 | ||
| 32 | - | 0.1 | - | 2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ducuara, J.A.; Arrieta, A.A.; Calabokis, O.P. Production of Organic Acids from Cashew Nut Shell Liquid (CNSL) via Electrochemical Synthesis. Int. J. Mol. Sci. 2025, 26, 10821. https://doi.org/10.3390/ijms262210821
Ducuara JA, Arrieta AA, Calabokis OP. Production of Organic Acids from Cashew Nut Shell Liquid (CNSL) via Electrochemical Synthesis. International Journal of Molecular Sciences. 2025; 26(22):10821. https://doi.org/10.3390/ijms262210821
Chicago/Turabian StyleDucuara, Jorge A., Alvaro A. Arrieta, and Oriana Palma Calabokis. 2025. "Production of Organic Acids from Cashew Nut Shell Liquid (CNSL) via Electrochemical Synthesis" International Journal of Molecular Sciences 26, no. 22: 10821. https://doi.org/10.3390/ijms262210821
APA StyleDucuara, J. A., Arrieta, A. A., & Calabokis, O. P. (2025). Production of Organic Acids from Cashew Nut Shell Liquid (CNSL) via Electrochemical Synthesis. International Journal of Molecular Sciences, 26(22), 10821. https://doi.org/10.3390/ijms262210821







