Abstract
Hashimoto’s thyroiditis (HT) is the most common autoimmune thyroid disorder, characterized by progressive lymphocytic infiltration, follicular destruction, tissue fibrosis, and an elevated risk of thyroid carcinoma. While the precise mechanisms underlying HT remain incompletely defined, emerging evidence implicates dysregulated sphingolipid (SPL) metabolism, particularly the sphingosine-1-phosphate (S1P) signaling axis, as a central contributor to disease pathogenesis. S1P, a bioactive lipid mediator, integrates metabolic and immunological cues to regulate immune cell trafficking, cytokine production, apoptosis, and fibroblast activation. Aberrant activation of the sphingosine kinase (SPHK)/sphingosine-1-phosphate (S1P)/S1P receptor (S1PR) pathway has been linked to persistent T helper 1 (Th1) cell recruitment, signal transducer and activator of transcription 3 (STAT3)-mediated immune polarization, epithelial–mesenchymal transition, extracellular matrix remodeling, and the establishment of a chronic inflammatory and fibrotic microenvironment. Moreover, S1P signaling may foster a pro-tumorigenic niche, providing a mechanistic explanation for the strong epidemiological association between HT and papillary thyroid carcinoma. This review summarizes current insights into the role of SPL metabolism in HT, highlighting its potential as a mechanistic link between autoimmunity, fibrosis, and carcinogenesis.
1. Introduction
Hashimoto’s thyroiditis (HT) is the most prevalent autoimmune thyroid disorder, with a steadily rising global incidence that underscores its growing public health significance [1,2]. Despite extensive investigation, the pathogenesis of HT remains incompletely understood. Current evidence implicates a multifactorial etiology involving genetic susceptibility, selenium deficiency, and exposure to immunomodulatory agents as key contributors to disease onset and progression [3,4]. Sphingolipids (SPLs), fundamental structural components of cellular membranes, play critical roles in maintaining membrane integrity and functionality [5,6,7]. “SPL metabolism” encompasses the comprehensive network of biosynthetic, catabolic, and interconversion pathways that govern the production and turnover of all SPL species [8,9]. Within this metabolic framework, the S1P pathway represents a distinct and functionally critical branch. S1P, a potent bioactive lipid mediator generated from the catabolism of membrane-bound SPLs, orchestrates a wide array of biological processes [10,11].
The S1P pathway includes the classical SPHK/S1P/S1PR axis in which sphingosine kinases SPHK1 and SPHK2 act as rate-limiting enzymes controlling S1P homeostasis [12,13,14]. Following its generation, S1P undergoes extracellular export and mediates diverse biological effects through five specific G protein-coupled receptors (S1PR1–5), thereby modulating immune cell trafficking, inflammatory responses, and cellular fate decisions [15,16]. Recent studies suggest the pathological relevance of SPL dysregulation in HT. Notably, aberrant SPL metabolism has been associated with severe lymphocytic infiltration in HT, indicating its potential role in pathogenic immune cell recruitment [17]. Moreover, S1P levels have been shown to rise in response to membrane damage, a pathological feature consistently observed in HT thyroid follicles [18]. Although elevated S1P has been detected in both HT patients and animal models, its precise mechanistic contribution to thyroid follicular cell injury remains unclear. This review systematically summarizes current insights linking disruptions in SPL metabolism to the immunopathogenesis of HT, providing theoretical foundation for the development of SPL-based interventions in the treatment of HT.
2. Pathophysiological Overview of HT
HT is an organ-specific autoimmune disorder marked by the presence of thyroid-specific autoantibodies, dense lymphocytic infiltration, progressive tissue fibrosis, and eventual architectural disruption of the thyroid gland, culminating in hypothyroidism [19]. Mounting evidence implicates infiltrating B and T lymphocytes as central mediators in HT pathogenesis, with their accumulation in thyroid tissue serving as a histological hallmark of disease progression [20,21]. Through the release of pro-inflammatory cytokines and chemokines, these immune cell populations orchestrate a sustained autoimmune response that drives thyroidal injury and dysfunction [22]. Among the cytokines involved, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin-1β (IL-1β) have been identified as key effector molecules contributing to thyroid destruction via multiple interrelated mechanisms [3,23,24]. Notably, IL-1β acts as a potent immunostimulatory cytokine by enhancing CD40 ligand (CD40L)-mediated activation of dendritic cells, promoting the antigen-dependent expansion of naïve CD4+ T cells and facilitating their differentiation into T helper 17 (Th17) effector subsets. Additionally, IL-1β has been shown to induce the expression of Fas on thyrocytes, a critical component of the Fas/Fas ligand (FasL) apoptotic signaling cascade [25,26,27], which is widely recognized as a principal mediator of programmed cell death [28]. Fas-dependent apoptosis is thus postulated to constitute a major effector mechanism driving thyrocyte loss and the development of hypothyroidism in HT [29]. Supporting this, comparative histopathological studies have demonstrated significantly elevated Fas expression in thyroid tissues of HT patients relative to those with simple goiter, implicating IL-1β–driven Fas upregulation as a pivotal trigger of immune-mediated thyrocyte apoptosis [30]. Despite extensive investigation since HT was first described, the molecular mechanisms underlying thyroid tissue destruction remain incompletely understood. Recently, dysregulated SPL metabolism has emerged as a potential contributor to HT pathophysiology. Aberrant SPL profiles have been identified in HT patients, suggesting a mechanistic role for lipid signaling perturbations in modulating immune responses and thyroidal inflammation [31]. These findings implicate a previously underexplored dimension of HT pathogenesis, warranting further investigation into this novel mechanistic pathway.
3. Roles of Sphingolipid Metabolism and S1P Signaling in Immune Regulation and Autoimmunity
3.1. Sphingolipid Metabolic Pathways
SPLs constitute a vital class of membrane lipids that play fundamental roles in maintaining cellular structural integrity and regulating critical biological processes [32]. As essential components of cellular organelles and plasma membranes, SPLs participate in a sophisticated metabolic network centered around ceramide (Cer), which serves as the pivotal hub for both biosynthetic and catabolic pathways [33]. The SPL family encompasses several biologically active molecules, including Cer, S1P, sphingosine (Sph), sphingomyelin, and glycosphingolipids, each of which is implicated in the regulation of key cellular functions such as proliferation, differentiation, senescence, and programmed cell death [34]. Ceramide biosynthesis occurs through three major routes. First, the de novo pathway is initiated by serine palmitoyltransferase-mediated condensation of serine and palmitoyl- CoA [35]. Second, the sphingomyelinase pathway involves hydrolysis of sphingomyelin. Third, S1P is dephosphorylated to sphingosine and subsequently re-acylated to regenerate ceramide in a specific salvage pathway [36,37]. A biologically consequential concept emerging from these interrelated pathways is the “sphingolipid rheostat”, a homeostatic balance between the pro-apoptotic lipids Cer and Sph, and the pro-survival lipid S1P [38,39]. This metabolic equilibrium plays a pivotal role not only in fundamental cellular fate decisions but also in diverse pathological processes [40,41], including autoimmune disorders such as HT [31,42], where dysregulation of SPL metabolism contribute to disease pathogenesis through modulation of immune responses and thyrocyte viability.
3.2. The Pivotal Role of Sphingosine-1-Phosphate in Sphingolipid Metabolism
S1P is a critical bioactive lipid mediator derived from the catabolism of membrane SPLs. Following its intracellular biosynthesis, S1P is actively exported into the plasma and lymphatic compartments, where it orchestrates a diverse array of cellular processes including proliferation, differentiation, and migration [43,44]. The synthesis and homeostasis of S1P are tightly regulated by two isoforms of sphingosine kinases, SPHK1 and SPHK2, which exhibit distinct subcellular localization and non-redundant biological functions [45,46]. SPHK1 is predominantly localized in the cytosol and translocates to the plasma membrane upon stimulation, a prerequisite for its enzymatic activation [47]. Elevated SPHK1 expression and consequent intracellular S1P accumulation have been implicated in enhanced proliferation and cell survival [48]. In contrast, SPHK2, which localizes primarily to the nucleus, has been associated with pro-apoptotic signaling [49]. Extracellular export of S1P is mediated by two specialized transporter systems, members of the ATP-binding cassette (ABC) transporter family and the spinster homolog 2 (SPNS2) protein [50,51]. Upon release into the extracellular milieu, S1P engages a family of five G protein–coupled receptors (S1PR1–S1PR5), whose expression is cell- and tissue-specific [52]. These receptors initiate downstream signaling cascades, including the PI3K and ERK1/2 pathways, which modulate essential cellular functions such as cytokine secretion, expression of adhesion molecules, and regulation of proliferation, migration, and apoptosis [53,54]. Thus, the spatially and temporally coordinated regulation of S1P synthesis, secretion, and receptor activation forms an integrated signaling network that links metabolic and extracellular cues to orchestrate complex physiological and pathological responses [55,56].
3.3. The Association Between Sphingosine-1-Phosphate and Autoimmune Diseases
Accumulating evidence implicates S1P signaling as a pivotal regulator in the immunopathogenesis of autoimmune disorders including multiple sclerosis (MS), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) [57,58,59]. In RA, enhanced synovial S1PR1 expression promotes fibroblast-like synoviocyte proliferation, exacerbating joint destruction [60,61]. Functional studies reveal that S1P–S1PR1 signaling fosters synoviocyte proliferation, contributing to joint pathology. Notably, pharmacological blockade of SPHK using N,N-dimethylsphingosine substantially reduces circulating levels of IL-6, TNF-α, and IFN-γ in RA patients, signifying a suppression of systemic inflammatory responses [62]. Supporting these findings, SPHK1-deficient murine models exhibit attenuated disease activity and decreased inflammation-related morbidity, reinforcing the mechanistic role of S1P signaling in orchestrating pro-inflammatory pathways in RA [63]. In MS, phosphoproteomic analyses of demyelinated brain lesions revealed heightened phosphorylation of S1PR1, which correlated with exacerbated neuroinflammation [64]. Mechanistically, activated S1PR1 potentiates IL-6–dependent STAT3 signaling, thereby promoting Th17 cell polarization and amplifying autoimmune-mediated neuroinflammation [65]. Elevated SPHK1 and S1PR1 expression in patients with primary Sjögren’s syndrome and in models of autoimmune liver injury further illustrates the relevance of the S1P metabolic pathway across autoimmune phenotypes [15,66].
FTY720 (fingolimod), a functional antagonist of S1P receptors, has emerged as a clinically relevant immunomodulator targeting the S1P pathway [67]. In MS, fingolimod has demonstrated therapeutic efficacy, not only dampening neuroinflammation but also contributing to remyelination through direct regulatory actions on central nervous system (CNS) cell populations [68]. Preclinical evidence extends its therapeutic potential to experimental autoimmune myasthenia gravis and thyroiditis models of HT [69]. Moreover, FTY720 suppresses the fibrotic markers transforming growth factor-β (TGF-β) and α-SMA) across multiple organ fibrosis models [70,71]. However, concerns regarding cardiovascular toxicity and infection risk due to broad immunosuppression necessitate cautious clinical application [68,72]. Continued investigation of the S1P pathway dynamics offers promising avenues for precision immunomodulation in diverse autoimmune settings [73,74].
4. Sphingolipid Metabolism and Its Relevance to Hashimoto’s Thyroiditis
4.1. Association Between Sphingosine Kinase and Thyroid Follicular Membrane Disruption in Hashimoto’s Thyroiditis
Recent studies have demonstrated substantial dysregulation of SPL metabolism in both patients with HT and experimental autoimmune thyroiditis models, with a particular emphasis on the aberrant activation of the SPHK/S1P/S1PR signaling axis [18]. This dysregulation is characterized by upregulated expression of SPHK, S1P, and its receptors S1PRs, a profile consistently observed in HT pathology. The inflammatory microenvironment of HT appears to be a principal driver of this metabolic reprogramming, wherein pro-inflammatory cytokines such as TNF-α, IL-1β, TGF-β, and vascular endothelial growth factor (VEGF) potently induce SPHK1 expression and enzymatic activity [75,76,77,78]. Mechanistic insights have demonstrated that TNF-α directly activates SPHK1 through ERK1-dependent phosphorylation events [79]. Elevated concentrations of TNF-α and IL-1β have been consistently reported in the context of autoimmune thyroid diseases [80]. Notably, both cytokines not only enhance SPHK1 activity but concurrently promote transcriptional upregulation of Fas in thyroid follicular cells (thyrocytes) via the NF-κB and AP-1 signaling pathways [81,82,83,84]. This convergence creates a pathogenic nexus whereby SPHK1-derived S1P signaling intersects with Fas/FasL-mediated apoptotic cascades [85].
S1P functions as a crucial modulator of apoptosis by regulating key downstream effectors such as caspase-3 [86]. Elevated intracellular S1P concentrations facilitate the assembly of the death-inducing signaling complex (DISC) at the Fas receptor, thereby sensitizing thyrocytes to Fas-triggered apoptosis under inflammatory conditions [87,88]. Furthermore, engagement of S1P with its receptor S1PR1 activates the JNK signaling pathway, which synergistically amplifies Fas-mediated pro-apoptotic responses [89,90]. This mechanistic interplay suggests that IL-1β-induced SPHK activation, in concert with Fas upregulation, potentiates thyrocyte apoptosis, accelerating autoimmune-driven follicular destruction in HT [18,91,92]. Collectively, these findings position aberrant SPL metabolism, particularly dysregulated S1P signaling, as a central pathogenic mechanism underpinning the structural disintegration of thyroid follicles in HT. The SPHK/S1P/S1PR axis thereby emerges as a critical molecular link between chronic autoimmune inflammation and progressive thyroid tissue injury [44], underscoring its potential as a therapeutic target in autoimmune thyroid disorders (Figure 1).
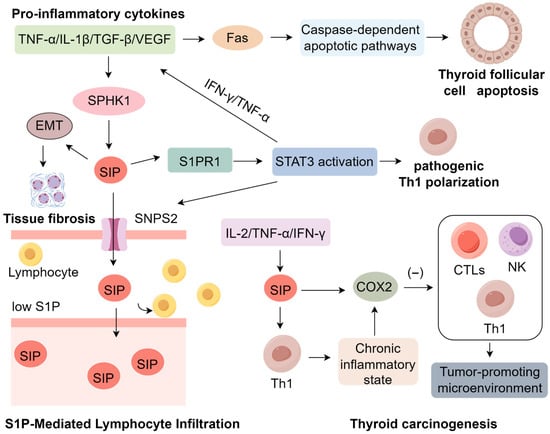
Figure 1.
Mechanisms of S1P Signaling in Inducing Hashimoto’s Thyroiditis.
4.2. Sphingosine-1-Phosphate-Mediated Lymphocyte Infiltration and Immune Dysregulation in the Pathogenesis of Hashimoto’s Thyroiditis
The characteristic lymphocytic infiltration observed in HT has been mechanistically linked to aberrant S1P signaling, which orchestrates both lymphocyte trafficking and immune cell differentiation [93]. Within thyroid tissue, S1P is actively exported via the SPNS2 transporter, establishing a concentration gradient characterized by low levels in secondary lymphoid organs and elevated levels in the peripheral circulation [94]. This chemotactic axis facilitates lymphocyte egress and subsequent infiltration into the thyroid gland [93,95]. In the autoimmune milieu of HT, this process is markedly amplified by upregulation of the S1P receptor 1 (S1PR1), as demonstrated in autoimmune thyroiditis models, where S1PR1 expression serves as a pivotal mediator of S1P-induced activation of signal transducer and activator of transcription 3 (STAT3) [18]. STAT3, a master regulator of CD4+ T cell lineage commitment, undergoes phosphorylation at Tyr705 and Ser727, enhancing its transcriptional activity and promoting the differentiation of pro-inflammatory Th1 cells [96]. However, the immunomodulatory effects of the S1P–S1PR1–STAT3 signaling cascade extend beyond Th1 polarization. This axis critically perturbs the equilibrium between Th17 cells and regulatory T cells (Tregs) [65,97], a skewing differentiation toward a pathogenic Th17 phenotype at the expense of Foxp3+ Treg development. This shift is driven by sustained STAT3 activation, which promotes IL-17 production while concurrently suppressing Treg-mediated immunoregulation [66,98]. The resultant Th17/Treg imbalance fosters a persistent pro-inflammatory microenvironment characterized by elevated IL-17 levels, increased neutrophil and macrophage recruitment, and breakdown of peripheral tolerance mechanisms [99,100,101]. In experimental models of HT, heightened Th17 activity is closely associated with exacerbated follicular cell damage and progressive thyroid gland destruction, whereas compromised Treg function contributes to unchecked autoimmune responses and disease acceleration [102,103]. Collectively, these findings underscore the central role of dysregulated S1P signaling in shaping the Th17/Treg axis and delineate a critical immunopathogenic mechanism driving the chronic inflammation and tissue injury characteristic of HT.
4.3. The Role of Sphingosine-1-Phosphate in Thyroid Tissue Fibrosis in Hashimoto’s Thyroiditis
Beyond lymphocytic infiltration, HT is frequently marked by progressive fibrosis, contributing to thyroid architectural remodeling and dysfunction [104]. The SPHK/S1P/S1PR axis has emerged as a central regulator of fibrotic remodeling across multiple organs including the heart, lungs, and kidneys [105,106]. Mechanistic studies have identified S1P as a key inducer of fibroblast-to-myofibroblast transdifferentiation, a pivotal step in extracellular matrix (ECM) deposition and scar formation [107]. During early stages of fibrosis, S1P synergizes with pro-inflammatory cytokines such as TNF-α, IFN-γ, TGF-β, and IL-6 to promote epithelial–mesenchymal transition (EMT), a reparative cellular reprogramming event that, when unresolved, drives pathological fibrosis and may even predispose cells to malignant transformation [108]. In renal fibrosis models, S1P robustly upregulates α-smooth muscle actin (α-SMA) in fibroblasts, facilitating their conversion into collagen-producing myofibroblasts [109,110,111]. Among S1P receptors, S1PR2 has been implicated in pro-fibrotic signaling via activation of the Rho/ROCK pathway, B cell niche organization, and amplification of cytokine cascades, whereas S1PR3 enhances myofibroblast differentiation and augments TGF-β/Smad signaling during fibrotic remodeling [112].
Recent studies in thyroid tissues from HT patients demonstrate that fibroblast populations expressing classical myofibroblast markers including α-SMA, vimentin, and fibronectin, and preferentially localize to regions with dense interstitial collagen deposition [113]. Concurrently, chronic exposure of thyrocytes to IL-6 and TGF-β induces EMT-like phenotypes via TGF-β/Smad-dependent transcriptional programs, implicating both resident fibroblasts and EMT-derived thyrocytes in the fibrotic matrix in this process [113,114,115]. Furthermore, sustained immune-stromal crosstalk—particularly mediated by IL-1β and TNF-α—perpetuates fibroblast activation, creating a pro-fibrotic microenvironment that, while mechanistically similar to fibrosis in other organs, exhibits thyroid-specific features [116,117]. Although elevated S1P and cytokine levels have been reported in HT patients, the precise mechanisms by which S1P orchestrates thyroid-specific fibrogenesis remain to be fully elucidated [118].
4.4. The Role of Sphingosine-1-Phosphate in HT-Associated Thyroid Carcinogenesis
Emerging evidence indicates a strong pathological link between HT and the development of thyroid malignancies, particularly that of papillary thyroid carcinoma (PTC). The persistent inflammatory microenvironment that defines HT is characterized by elevated levels of pro-inflammatory cytokines including IL-2, TNF-α, and IFN-γ, which collectively promote tumorigenesis through S1P-mediated pathways [119]. A pivotal study demonstrated that the Th1-skewed immune response in HT enhances SPHK1 expression, resulting in S1P overproduction [120]. This S1P-rich milieu not only sustains chronic inflammation but also upregulates cyclooxygenase-2 (COX2), an enzyme whose immunosuppressive properties suppress cytotoxic T lymphocytes (CTLs), Th1 cells, and natural killer (NK) cells—central mediators of anti-tumor immunity. Beyond immunosuppression, S1P–COX2 signaling intersects with oncogenic pathways [121]. Specifically, S1P activates PI3K/AKT cascade via S1PR1 and S1PR3 [122,123], thereby creating a permissive context for the proliferation and survival of genetically altered thyrocytes [122]. Simultaneously, elevated COX2 enhances prostaglandin E2 (PGE2) production, which in turn transactivates the EGFR–RAS–RAF–MEK–ERK pathway [124,125]. This crosstalk may synergize with BRAFV600E or RAS mutations, which are common driver events in PTC, leading to sustained MAPK activation [126,127]. The convergence of inflammatory metabolic signaling with BRAF-driven oncogenic cascades promotes thyrocyte dedifferentiation, genomic instability, and clonal expansion, thereby accelerating malignant transformation [128]. Concurrently, S1P-induced immunosuppression weakens immune surveillance, allowing mutated cells to evade detection while MAPK signaling drives tumor progression [129]. Notably, malignant thyrocytes themselves contribute to increased S1P levels, forming a feed-forward loop that links chronic inflammation to carcinogenesis [130]. Epidemiological studies reveal a striking 23% co-occurrence rate of HT and PTC in Chinese populations, with HT patients exhibiting higher rates of lymph node metastasis compared to PTC patients without HT [131]. Although these epidemiological and pathological associations are well established, the precise mechanistic contribution of S1P to this relationship remains incompletely defined [132,133]. Thus, the study of SPL metabolism represents a promising avenue for elucidating the mechanisms underpinning HT pathogenesis (Table S1).
5. Conclusions
HT is a complex autoimmune disorder marked by chronic lymphocytic infiltration, progressive destruction of thyroid follicles, tissue fibrosis, and an elevated risk of thyroid carcinogenesis. Despite decades of investigation, its underlying mechanisms remain incompletely defined. Recent advances highlight the central role of SPL metabolism—particularly the S1P signaling axis—in orchestrating key pathological events in HT. As a bioactive lipid messenger, S1P integrates metabolic, inflammatory, and immunological signals to regulate lymphocyte trafficking, pro-inflammatory cytokine release, thyrocyte apoptosis, and fibroblast activation. Aberrant activation of the SPHK/S1P/S1PR1 pathway drives persistent Th1 cell recruitment, STAT3-mediated immune polarization, EMT, and extracellular matrix remodeling, thereby amplifying autoimmune destruction and fibrotic progression. Moreover, the chronic inflammatory milieu maintained by S1P signaling may establish a pro-tumorigenic niche that predisposes HT patients to PTC.
Collectively, these observations position S1P dysregulation as a unifying mechanism linking immune activation, tissue injury, fibrogenesis, and oncogenesis in HT. Nevertheless, significant gaps remain regarding receptor subtype specificity, downstream signaling networks, and tissue-contextual effects. Addressing these gaps will be crucial for identifying biomarkers and devising targeted therapies aimed at modulating S1P signaling to preserve thyroid structure and limit disease progression. Future research should exploit single-cell RNA sequencing to delineate the cellular heterogeneity of HT lesions and organoid models to reproduce the thyroid immune microenvironment in vitro. In parallel, pharmacological strategies targeting SPHK/S1P/S1PR signaling, including selective SPHK inhibitors, may offer precision therapeutic approaches to prevent tissue damage and disease progression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262110674/s1.
Author Contributions
J.H.: Writing—original draft. Z.C.: Writing—original draft. Y.W.: Writing—original draft. C.S.: Writing—original draft. Y.F.: Writing—original draft, review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Sichuan Provincial Natural Science Foundation Project [Grant Number: 2025ZNSFSC0631].
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that no competing financial interests or commercial relationships have influenced the research presented herein.
References
- Lu, Q.; Dou, Y.; Yu, T.; Ren, X.; Li, Y.; Zhu, X.; Zhao, Y.; Miao, G. Association of thyroglobulin antibody and thyroid peroxidase antibody status with aggressive features in papillary thyroid carcinoma with Hashimoto’s thyroiditis. Gland. Surg. 2025, 14, 1091–1100. [Google Scholar] [CrossRef]
- Tywanek, E.; Michalak, A.; Świrska, J.; Zwolak, A. Autoimmunity, New Potential Biomarkers and the Thyroid Gland-The Perspective of Hashimoto’s Thyroiditis and Its Treatment. Int. J. Mol. Sci. 2024, 25, 4703. [Google Scholar] [CrossRef]
- Wrońska, K.; Hałasa, M.; Szczuko, M. The Role of the Immune System in the Course of Hashimoto’s Thyroiditis: The Current State of Knowledge. Int. J. Mol. Sci. 2024, 25, 6883. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, Y.; Pei, X.; Wang, W.; Zhang, H. Causal role of immune cells in Hashimoto’s thyroiditis: Mendelian randomization study. Front. Endocrinol. 2024, 15, 1352616. [Google Scholar] [CrossRef]
- Xiao, J.; Lin, H.; Liu, B.; Xia, Z.; Zhang, J.; Jin, J. Decreased S1P and SPHK2 are involved in pancreatic acinar cell injury. Biomark. Med. 2019, 13, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhuge, J.; Liu, J.; Xia, Z.; Wang, H.; Gao, Q.; Jiang, H.; Qu, Y.; Fan, L.; Ma, J.; et al. Prognostic signatures of sphingolipids: Understanding the immune landscape and predictive role in immunotherapy response and outcomes of hepatocellular carcinoma. Front. Immunol. 2023, 14, 1153423. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, Y.; Wei, S.; Chen, J.; Zhong, C.; Cai, W.; Jin, W.; Peng, H. Dissecting the effect of sphingolipid metabolism gene in progression and microenvironment of osteosarcoma to develop a prognostic signature. Front. Endocrinol. 2022, 13, 1030655. [Google Scholar] [CrossRef]
- Zhakupova, A.; Zeinolla, A.; Kokabi, K.; Sergazy, S.; Aljofan, M. Drug Resistance: The Role of Sphingolipid Metabolism. Int. J. Mol. Sci. 2025, 26, 3716. [Google Scholar] [CrossRef]
- Peters, F.; Höfs, W.; Lee, H.; Brodesser, S.; Kruse, K.; Drexler, H.C.A.; Hu, J.; Raker, V.K.; Lukas, D.; von Stebut, E.; et al. Sphingolipid metabolism orchestrates establishment of the hair follicle stem cell compartment. J. Cell Biol. 2025, 224, e202403083. [Google Scholar] [CrossRef] [PubMed]
- Benkhoff, M.; Barcik, M.; Mourikis, P.; Dahlmanns, J.; Kahmann, P.; Wollnitzke, P.; Hering, M.; Huckenbeck, T.; Hoppe, J.; Semleit, N.; et al. Targeting Sphingosine-1-Phosphate Signaling to Prevent the Progression of Aortic Valve Disease. Circulation 2025, 151, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Rufail, M.L.; Bassi, R.; Giussani, P. Sphingosine-1-Phosphate Metabolic Pathway in Cancer: Implications for Therapeutic Targets. Int. J. Mol. Sci. 2025, 26, 1056. [Google Scholar] [CrossRef]
- Xu, X.; Han, Y.; Zhu, T.; Fan, F.; Wang, X.; Liu, Y.; Luo, D. The role of SphK/S1P/S1PR signaling pathway in bone metabolism. Biomed. Pharmacother. 2023, 169, 115838. [Google Scholar] [CrossRef] [PubMed]
- Piao, J.; Su, Z.; He, J.; Zhu, T.; Fan, F.; Wang, X.; Yang, Z.; Zhan, H.; Luo, D. SphK1 deficiency ameliorates the development of atherosclerosis by inhibiting the S1P/S1PR3/Rhoa/ROCK pathway. Cell. Signal. 2024, 121, 111252. [Google Scholar] [CrossRef]
- Gong, L.; Shen, Y.; Wang, S.; Wang, X.; Ji, H.; Wu, X.; Hu, L.; Zhu, L. Nuclear SPHK2/S1P induces oxidative stress and NLRP3 inflammasome activation via promoting p53 acetylation in lipopolysaccharide-induced acute lung injury. Cell Death Discov. 2023, 9, 12. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Zhang, Y.; Liu, P.; Liu, M.; Zhang, M.; Wu, R. S1P/S1PR signaling pathway advancements in autoimmune diseases. Biomol. Biomed. 2023, 23, 922–935. [Google Scholar] [CrossRef]
- Chatzikonstantinou, S.; Poulidou, V.; Arnaoutoglou, M.; Kazis, D.; Heliopoulos, I.; Grigoriadis, N.; Boziki, M. Signaling through the S1P-S1PR Axis in the Gut, the Immune and the Central Nervous System in Multiple Sclerosis: Implication for Pathogenesis and Treatment. Cells 2021, 10, 3217. [Google Scholar] [CrossRef]
- Asakura, T.; Ishii, M.; Namkoong, H.; Suzuki, S.; Kagawa, S.; Yagi, K.; Komiya, T.; Hashimoto, T.; Okamori, S.; Kamata, H.; et al. Sphingosine 1-phosphate receptor modulator ONO-4641 stimulates CD11b(+)Gr-1(+) cell expansion and inhibits lymphocyte infiltration in the lungs to ameliorate murine pulmonary emphysema. Mucosal Immunol. 2018, 11, 1606–1620. [Google Scholar] [CrossRef]
- Han, C.; He, X.; Xia, X.; Guo, J.; Liu, A.; Liu, X.; Wang, X.; Li, C.; Peng, S.; Zhao, W.; et al. Sphk1/S1P/S1PR1 Signaling is Involved in the Development of Autoimmune Thyroiditis in Patients and NOD.H-2(h4) Mice. Thyroid 2019, 29, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Valenti, A. Medical Applications of Molecular Biotechnologies in the Context of Hashimoto’s Thyroiditis. Diagnostics 2023, 13, 2114. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; He, L.; Huang, J.; Liu, J.; Chen, W.; Zhong, J.; Wei, T.; Li, Z.; Zhu, J.; Lei, J. miR-142-3p encapsulated in T lymphocyte-derived tissue small extracellular vesicles induces Treg function defect and thyrocyte destruction in Hashimoto’s thyroiditis. BMC Med. 2023, 21, 206. [Google Scholar] [CrossRef]
- Pereira Vasconcelos, A.; Santos, E.S.J.C.; Simizo, A.; Peña Avila, J.; Nassar Reich Goldstein, G.; Prado de Oliveira, P.H.; Mogollón García, H.; de Carvalho Fraga, C.A.; Nakaya, H.I. Sex-Based Differences in Thyroid Plasma B Cell Infiltration: Implications for Autoimmune Disease Susceptibility. Endocrinology 2024, 165, bqae148. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Ye, X.P.; Zhou, Z.; Zhu, C.F.; Li, R.; Fang, Y.; Zhang, R.J.; Li, L.; Liu, W.; Wang, Z.; et al. Lymphocyte infiltration and thyrocyte destruction are driven by stromal and immune cell components in Hashimoto’s thyroiditis. Nat. Commun. 2022, 13, 775. [Google Scholar] [CrossRef]
- Mirandola, P.; Gobbi, G.; Masselli, E.; Micheloni, C.; Di Marcantonio, D.; Queirolo, V.; Chiodera, P.; Meschi, T.; Vitale, M. Protein kinase Cε regulates proliferation and cell sensitivity to TGF-1β of CD4+ T lymphocytes: Implications for Hashimoto thyroiditis. J. Immunol. 2011, 187, 4721–4732. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Guo, F.; Tan, Y.; Zhang, Y.; Geng, Y.; Yang, G.; Wang, S. Causal relationship between inflammatory cytokines and autoimmune thyroid disease: A bidirectional two-sample Mendelian randomization analysis. Front. Immunol. 2024, 15, 1334772. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Z.; Zhang, C.; Guo, Q.; Chen, C.; Peng, X. Unraveling the connection between Hashimoto’s Thyroiditis and non-alcoholic fatty liver disease: Exploring the role of CD4(+)central memory T cells through integrated genetic approaches. Endocrine 2024, 85, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Nodehi, M.; Ajami, A.; Izad, M.; Asgarian Omran, H.; Esfahanian, F.; Yekaninejad, S.; Hemmatabadi, M.; Amouzegar, A.; Chahardoli, R.; Mansouri, F.; et al. The Frequency of CD4(+) T Cells in Women with Hashimoto’s Thyroiditis. Int. J. Endocrinol. Metab. 2021, 19, e110013. [Google Scholar] [CrossRef]
- Zake, T.; Skuja, S.; Kalere, I.; Konrade, I.; Groma, V. Upregulated tissue expression of T helper (Th) 17 pathogenic interleukin (IL)-23 and IL-1β in Hashimoto’s thyroiditis but not in Graves’ disease. Endocr. J. 2019, 66, 423–430. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Xie, W.; Chen, J.; Liang, S.; Jiang, M.; Wang, J.; Zheng, Z. Potential of gamma/delta T cells for solid tumor immunotherapy. Front. Immunol. 2024, 15, 1466266. [Google Scholar] [CrossRef]
- Blüher, M.; Krohn, K.; Wallaschofski, H.; Braverman, L.E.; Paschke, R. Fas and Fas ligand gene expression in autoimmune thyroiditis in BB/W rats. Eur. J. Endocrinol. 1999, 141, 506–511. [Google Scholar] [CrossRef][Green Version]
- Giordano, C.; Stassi, G.; De Maria, R.; Todaro, M.; Richiusa, P.; Papoff, G.; Ruberti, G.; Bagnasco, M.; Testi, R.; Galluzzo, A. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science 1997, 275, 960–963. [Google Scholar] [CrossRef]
- Bastos, D.; Chiamolera, M.I.; Silva, R.E.; Souza, M.; Antunes, R.A.; Souza, M.M.; Mancebo, A.C.A.; Arêas, P.C.F.; Reis, F.M.; Lo Turco, E.G.; et al. Metabolomic analysis of follicular fluid from women with Hashimoto thyroiditis. Sci. Rep. 2023, 13, 12497. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Wang, C.; Ishaq, H.M.; Zhang, R.; Yang, F. Relationship between sphingolipids-mediated neuroinflammation and alcohol use disorder. Pharmacol. Biochem. Behav. 2024, 235, 173695. [Google Scholar] [CrossRef]
- Schengrund, C.L. Sphingolipids: Less Enigmatic but Still Many Questions about the Role(s) of Ceramide in the Synthesis/Function of the Ganglioside Class of Glycosphingolipids. Int. J. Mol. Sci. 2024, 25, 6312. [Google Scholar] [CrossRef]
- Doll, C.L.; Snider, A.J. The diverse roles of sphingolipids in inflammatory bowel disease. FASEB J. 2024, 38, e23777. [Google Scholar] [CrossRef]
- Sindhu, S.; Leung, Y.H.; Arefanian, H.; Madiraju, S.M.; Al-Mulla, F.; Ahmad, R.; Prentki, M. Neutral sphingomyelinase-2 and cardiometabolic diseases. Obes. Rev. 2021, 22, e13248. [Google Scholar] [CrossRef] [PubMed]
- Wajapeyee, N.; Beamon, T.C.; Gupta, R. Roles and therapeutic targeting of ceramide metabolism in cancer. Mol. Metab. 2024, 83, 101936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, H.; Li, L.; Yu, S.; Liu, M.; Jiang, L. Ceramide on the road to insulin resistance and immunometabolic disorders in transition dairy cows: Driver or passenger? Front. Immunol. 2023, 14, 1321597. [Google Scholar] [CrossRef]
- Woodcock, J. Sphingosine and ceramide signalling in apoptosis. IUBMB Life 2006, 58, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Masuda-Kuroki, K.; Alimohammadi, S.; Di Nardo, A. The Role of Sphingolipids and Sphingosine-1-phosphate-Sphingosine-1-phosphate-receptor Signaling in Psoriasis. Cells 2023, 12, 2352. [Google Scholar] [CrossRef]
- Kleuser, B.; Bäumer, W. Sphingosine 1-Phosphate as Essential Signaling Molecule in Inflammatory Skin Diseases. Int. J. Mol. Sci. 2023, 24, 1456. [Google Scholar] [CrossRef]
- Gaggini, M.; Fenizia, S.; Vassalle, C. Sphingolipid Levels and Signaling via Resveratrol and Antioxidant Actions in Cardiometabolic Risk and Disease. Antioxidants 2023, 12, 1102. [Google Scholar] [CrossRef]
- Park, B.-J.; Kim, J.-H.; Han, J.-S.; Jung, P.-M. Effect of ceramide on apoptosis and phospholipase D activity in FRTL-5 thyroid cells. Exp. Mol. Med. 1999, 31, 142–150. [Google Scholar] [CrossRef]
- Wu, X.; Wabitsch, M.; Yang, J.; Sakharkar, M.K. Effects of adipocyte-conditioned cell culture media on S1P treatment of human triple-negative breast cancer cells. PLoS ONE 2023, 18, e0286111. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hu, B.; Wu, C.; Jiang, T. Targeting the SPHK1/S1P/S1PR2 axis ameliorates GH-secreted pituitary adenoma progression. Eur. J. Clin. Investig. 2024, 54, e14117. [Google Scholar] [CrossRef] [PubMed]
- Khoei, S.G.; Sadeghi, H.; Samadi, P.; Najafi, R.; Saidijam, M. Relationship between Sphk1/S1P and microRNAs in human cancers. Biotechnol. Appl. Biochem. 2021, 68, 279–287. [Google Scholar] [CrossRef]
- Shi, W.; Ma, D.; Cao, Y.; Hu, L.; Liu, S.; Yan, D.; Zhang, S.; Zhang, G.; Wang, Z.; Wu, J.; et al. SphK2/S1P Promotes Metastasis of Triple-Negative Breast Cancer Through the PAK1/LIMK1/Cofilin1 Signaling Pathway. Front. Mol. Biosci. 2021, 8, 598218. [Google Scholar] [CrossRef]
- Qiao, H.; Jiang, T.; Mu, P.; Chen, X.; Wen, X.; Hu, Z.; Tang, S.; Wen, J.; Deng, Y. Cell fate determined by the activation balance between PKR and SPHK1. Cell Death Differ. 2021, 28, 401–418. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, H.; Xiao, F.J.; Shi, X.F.; Zhang, Y.K.; Xu, Q.Q.; Zhang, X.Y.; Ha, X.Q.; Wang, L.S. SIRT1 mediates Sphk1/S1P-induced proliferation and migration of endothelial cells. Int. J. Biochem. Cell Biol. 2016, 74, 152–160. [Google Scholar] [CrossRef]
- Dominguez, G.; Maddelein, M.L.; Pucelle, M.; Nicaise, Y.; Maurage, C.A.; Duyckaerts, C.; Cuvillier, O.; Delisle, M.B. Neuronal sphingosine kinase 2 subcellular localization is altered in Alzheimer’s disease brain. Acta Neuropathol. Commun. 2018, 6, 25. [Google Scholar] [CrossRef]
- Alkafaas, S.S.; Elsalahaty, M.I.; Ismail, D.F.; Radwan, M.A.; Elkafas, S.S.; Loutfy, S.A.; Elshazli, R.M.; Baazaoui, N.; Ahmed, A.E.; Hafez, W.; et al. The emerging roles of sphingosine 1-phosphate and SphK1 in cancer resistance: A promising therapeutic target. Cancer Cell Int. 2024, 24, 89. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Ren, P.; Bian, G.; Wang, J.; Bai, J.; Huang, J.; Ding, Y.; Li, X.; Li, M.; Hou, Z. Enhancing Spns2/S1P in macrophages alleviates hyperinflammation and prevents immunosuppression in sepsis. EMBO Rep. 2023, 24, e56635. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; She, M.; Zeng, Q.; Yi, G.; Zhang, J. Sphingosine 1-phosphate and its receptors in ischemia. Clin. Chim. Acta 2021, 521, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Z.; Liu, C.; Yang, X.; Jiang, J. Sphingosine-1-phosphate Attenuates Endoplasmic Reticulum Stress-induced Cardiomyocyte Apoptosis Through Sphingosine-1-phosphate Receptor 1. Arch. Med. Res. 2022, 53, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Brozowski, J.M.; Timoshchenko, R.G.; Serafin, D.S.; Allyn, B.; Koontz, J.; Rabjohns, E.M.; Rampersad, R.R.; Ren, Y.; Eudy, A.M.; Harris, T.F.; et al. G protein-coupled receptor kinase 3 modulates mesenchymal stem cell proliferation and differentiation through sphingosine-1-phosphate receptor regulation. Stem Cell Res. Ther. 2022, 13, 37. [Google Scholar] [CrossRef]
- Ko, P.; Kim, D.; You, E.; Jung, J.; Oh, S.; Kim, J.; Lee, K.H.; Rhee, S. Extracellular Matrix Rigidity-dependent Sphingosine-1-phosphate Secretion Regulates Metastatic Cancer Cell Invasion and Adhesion. Sci. Rep. 2016, 6, 21564. [Google Scholar] [CrossRef]
- Mahajan-Thakur, S.; Böhm, A.; Jedlitschky, G.; Schrör, K.; Rauch, B.H. Sphingosine-1-Phosphate and Its Receptors: A Mutual Link between Blood Coagulation and Inflammation. Mediators Inflamm. 2015, 2015, 831059. [Google Scholar] [CrossRef]
- McGinley, M.P.; Cohen, J.A. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 2021, 398, 1184–1194, Erratum in Lancet 2021, 398, 1132. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Deng, R.; Dai, X.J.; Bu, Y.H.; Sun, M.H.; Zhang, H.; Wang, M.D.; Wang, R.H. Geniposide downregulates the VEGF/SphK1/S1P pathway and alleviates angiogenesis in rheumatoid arthritis in vivo and in vitro. Phytother. Res. 2021, 35, 4347–4362. [Google Scholar] [CrossRef]
- Askanase, A.D.; D’Cruz, D.; Kalunian, K.; Merrill, J.T.; Navarra, S.V.; Cahuzac, C.; Cornelisse, P.; Murphy, M.J.; Strasser, D.S.; Trokan, L.; et al. Cenerimod, a sphingosine-1-phosphate receptor modulator, versus placebo in patients with moderate-to-severe systemic lupus erythematosus (CARE): An international, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Rheumatol. 2025, 7, e21–e32. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Li, C.; Wu, M.; Chen, H.; Li, Y.; You, F.; Zhao, Y.; Jin, J.; Chen, X.; et al. Evaluation of proximod, a selective agonist of sphingosine-1-phosphate receptor-1, in healthy volunteers and patients with rheumatoid arthritis: A phase 1, double-blind, randomised, placebo-controlled, ascending dose trial. Lancet Rheumatol. 2024, 6, e837–e847. [Google Scholar] [CrossRef]
- Wang, M.; Wu, H.; Wang, R.; Dai, X.; Deng, R.; Wang, Y.; Bu, Y.; Sun, M.; Zhang, H. Inhibition of sphingosine 1-phosphate (S1P) receptor 1/2/3 ameliorates biological dysfunction in rheumatoid arthritis fibroblast-like synoviocyte MH7A cells through Gαi/Gαs rebalancing. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1080–1089. [Google Scholar] [CrossRef]
- Lai, W.Q.; Irwan, A.W.; Goh, H.H.; Howe, H.S.; Yu, D.T.; Valle-Oñate, R.; McInnes, I.B.; Melendez, A.J.; Leung, B.P. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J. Immunol. 2008, 181, 8010–8017. [Google Scholar] [CrossRef]
- Baker, D.A.; Obeid, L.M.; Gilkeson, G.S. Impact of sphingosine kinase on inflammatory pathways in fibroblast-like synoviocytes. Inflamm. Allergy-Drug Targets 2011, 10, 464–471. [Google Scholar] [CrossRef]
- Kandjani, O.J.; Yaqoubi, S.; Vahdati, S.S.; Borhannejad, B.; Dastmalchi, S.; Alizadeh, A.A. S1PR1 modulators in multiple sclerosis: Efficacy, safety, comparison, and chemical structure insights. Eur. J. Med. Chem. 2023, 250, 115182. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Wu, L.; Acharya, S.; Arac, A.; Blaho, V.A.; Huang, Y.; Moon, B.S.; Axtell, R.C.; Ho, P.P.; Steinberg, G.K.; et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol. 2013, 14, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, Y.; Feng, M.; Ni, X.; Qiang, L.; Xue, J.; Sun, L.; Gao, C.; Luo, J. Sphingosine-1-phosphate alleviates Sjögren’s syndrome-like symptoms via inducing autophagy and regulating status of Treg cells in NOD mice. Int. Immunopharmacol. 2024, 143, 113514. [Google Scholar] [CrossRef]
- Gan, P.R.; Wang, R.H.; Deng, R.; Wu, H.; Bu, Y.H.; Chen, F.Y.; Dong, X.T.; Ke, J.T. Geniposide inhibits SphK1 membrane targeting to restore macrophage polarization balance in collagen-induced arthritis mice. Eur. J. Pharmacol. 2022, 933, 175271. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.L.; Yang, B.; Du, T.; Li, X.L.; Zhang, P.; Ge, M.R.; Lian, Y.; Li, H.; Liu, Y.D.; et al. Prophylactic administration of fingolimod (FTY720) ameliorated experimental autoimmune myasthenia gravis by reducing the number of dendritic cells, follicular T helper cells and antibody-secreting cells. Int. Immunopharmacol. 2021, 96, 107511. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, W.; Gao, G.; Song, Y.; Liu, H.; Li, L.; Zhou, J.; Yan, G.; Cui, H. Effect of FTY-720 on Pulmonary Fibrosis in Mice via the TGF-β1 Signaling Pathway and Autophagy. Biomol. Ther. 2023, 31, 434–445. [Google Scholar] [CrossRef]
- Hijmans, R.S.; Rasmussen, D.G.; Yazdani, S.; Navis, G.; van Goor, H.; Karsdal, M.A.; Genovese, F.; van den Born, J. Urinary collagen degradation products as early markers of progressive renal fibrosis. J. Transl. Med. 2017, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Budde, K.; Schütz, M.; Glander, P.; Peters, H.; Waiser, J.; Liefeldt, L.; Neumayer, H.H.; Böhler, T. FTY720 (fingolimod) in renal transplantation. Clin. Transplant. 2006, 20 (Suppl. S17), 17–24. [Google Scholar] [CrossRef]
- Stepanovska, B.; Huwiler, A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol. Res. 2020, 154, 104170. [Google Scholar] [CrossRef]
- Bravo, G.; Cedeño, R.R.; Casadevall, M.P.; Ramió-Torrentà, L. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives. Cells 2022, 11, 2058. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Q.; Tian, Y.; Xiong, S.; Li, G.; Xu, L. Expressions of IL-17 and TNF-α in patients with Hashimoto’s disease combined with thyroid cancer before and after surgery and their relationship with prognosis. Clin. Transl. Oncol. 2020, 22, 1280–1287. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, S.E.; Kim, Y.I.; Nam-Goong, I.S.; Jung, H.W.; Kim, E.S. Papillary thyroid cancer with Hashimoto’s thyroiditis attenuates the tumour aggressiveness through the up-regulation of E-cadherin and TGF-β expression. Clin. Exp. Med. 2023, 23, 833–840. [Google Scholar] [CrossRef]
- Hepp, M.; Werion, A.; De Greef, A.; de Ville de Goyet, C.; de Bournonville, M.; Behets, C.; Lengelé, B.; Daumerie, C.; Mourad, M.; Ludgate, M.; et al. Oxidative Stress-Induced Sirtuin1 Downregulation Correlates to HIF-1α, GLUT-1, and VEGF-A Upregulation in Th1 Autoimmune Hashimoto’s Thyroiditis. Int. J. Mol. Sci. 2021, 22, 3806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Gui, B.J.; Liu, J.; Rong, G.X.; Deng, R.; Bu, Y.H.; Zhang, H. Geniposide alleviates VEGF-induced angiogenesis by inhibiting VEGFR2/PKC/ERK1/2-mediated SphK1 translocation. Phytomedicine 2022, 100, 154068. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Paparo, S.R.; Ragusa, F.; Elia, G.; Mazzi, V.; Patrizio, A.; Ghionzoli, M.; Varricchi, G.; Centanni, M.; Ulisse, S.; et al. Chemokines in thyroid autoimmunity. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101773. [Google Scholar] [CrossRef]
- Russo, M.P.; Bennett, B.L.; Manning, A.M.; Brenner, D.A.; Jobin, C. Differential requirement for NF-kappaB-inducing kinase in the induction of NF-kappaB by IL-1beta, TNF-alpha, and Fas. Am. J. Physiol. Cell Physiol. 2002, 283, C347–C357. [Google Scholar] [CrossRef]
- Fang, Y.; Braley-Mullen, H. Cultured murine thyroid epithelial cells expressing transgenic Fas-associated death domain-like interleukin-1beta converting enzyme inhibitory protein are protected from fas-mediated apoptosis. Endocrinology 2008, 149, 3321–3329. [Google Scholar] [CrossRef]
- Sera, N.; Kawakami, A.; Nakashima, T.; Nakamura, H.; Imaizumi, M.; Koji, T.; Abe, Y.; Usa, T.; Tominaga, T.; Ejima, E.; et al. Fas/FasL mediated apoptosis of thyrocytes in Graves’ disease. Clin. Exp. Immunol. 2001, 124, 197–207. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, M.; An, H.; Chen, W.; Liu, S.; Guo, J.; Yu, Y.; Cao, X. Fas ligation induces IL-1beta-dependent maturation and IL-1beta-independent survival of dendritic cells: Different roles of ERK and NF-kappaB signaling pathways. Blood 2003, 102, 4441–4447. [Google Scholar] [CrossRef] [PubMed]
- Hutami, I.R.; Izawa, T.; Mino-Oka, A.; Shinohara, T.; Mori, H.; Iwasa, A.; Tanaka, E. Fas/S1P(1) crosstalk via NF-κB activation in osteoclasts controls subchondral bone remodeling in murine TMJ arthritis. Biochem. Biophys. Res. Commun. 2017, 490, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Li, M.; Zhang, L.; Zhang, K.; An, Y.; Feng, M.; Wang, F.; Yeh, C.T.; Wang, J.; Guo, F. Sphingosine-1-phosphate receptor 3 promotes neuronal apoptosis via the TNF-α/caspase-3 signaling pathway after acute intracerebral hemorrhage. Mol. Cell. Neurosci. 2024, 131, 103979. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, X.; Kong, X.; Li, J.; Huang, B.; Li, H.; Ji, Z.; Wei, X.; Tao, S.; Shan, Z.; et al. S1P regulates intervertebral disc aging by mediating endoplasmic reticulum-mitochondrial calcium ion homeostasis. JCI Insight 2024, 9, e177789. [Google Scholar] [CrossRef]
- Fosuah, E.; Shen, Z.; Xie, J.; Wang, C.; Lin, Q.; Fu, T.M. Assembly and activation of the death-inducing signaling complex. Proc. Natl. Acad. Sci. USA 2025, 122, e2504819122. [Google Scholar] [CrossRef]
- Dixit, D.; Hallisey, V.M.; Zhu, E.Y.; Okuniewska, M.; Cadwell, K.; Chipuk, J.E.; Axelrad, J.E.; Schwab, S.R. S1PR1 inhibition induces proapoptotic signaling in T cells and limits humoral responses within lymph nodes. J. Clin. Investig. 2024, 134, e174984. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Q.; Hou, T.; Xiang, R.; Li, X.; Li, J.; Wang, T.T.; Liu, W.J.; Hou, S.; Wang, D.; Zhao, Q.H.; et al. PANX1-mediated ATP release confers FAM3A’s suppression effects on hepatic gluconeogenesis and lipogenesis. Mil. Med. Res. 2024, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Plöhn, S. The Crucial Role of Sphingosine-1-Phosphate and the Therapeutic Potential of Fingolimod for Graves Disease and Associated Orbitopathy. Ph.D. Thesis, Universität Duisburg-Essen, Duisburg, Germany, Essen, Germany, 2019. [Google Scholar]
- Zhou, Z.; Zuo, C.L.; Li, X.S.; Ye, X.P.; Zhang, Q.Y.; Wang, P.; Zhang, R.X.; Chen, G.; Yang, J.L.; Chen, Y.; et al. Uterus globulin associated protein 1 (UGRP1) is a potential marker of progression of Graves’ disease into hypothyroidism. Mol. Cell. Endocrinol. 2019, 494, 110492. [Google Scholar] [CrossRef]
- Plöhn, S.; Hose, M.; Schlüter, A.; Michel, L.; Diaz-Cano, S.; Hendgen-Cotta, U.B.; Banga, J.P.; Bechrakis, N.E.; Hansen, W.; Eckstein, A.J.T. Fingolimod improves the outcome of experimental Graves’ disease and associated orbitopathy by modulating the autoimmune response to the thyroid-stimulating hormone receptor. Thyroid 2019, 29, 1286–1301. [Google Scholar] [CrossRef]
- Baeyens, A.A.L.; Schwab, S.R. Finding a Way Out: S1P Signaling and Immune Cell Migration. Annu. Rev. Immunol. 2020, 38, 759–784. [Google Scholar] [CrossRef]
- He, B.; Zhao, R.; Zhang, B.; Pan, H.; Liu, J.; Huang, L.; Wei, Y.; Yang, D.; Liang, J.; Wang, M.; et al. Endothelial OX40 activation facilitates tumor cell escape from T cell surveillance through S1P/YAP-mediated angiogenesis. J. Clin. Invest. 2025, 135, e186291. [Google Scholar] [CrossRef]
- Shiah, J.V.; Grandis, J.R.; Johnson, D.E. Targeting STAT3 with Proteolysis Targeting Chimeras and Next-Generation Antisense Oligonucleotides. Mol. Cancer Ther. 2021, 20, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Kudira, R.; Yang, Z.F.; Osuji, I.; Damen, M.; Yang Vom Hofe, A.; Singh, M.; Karns, R.; Bariya, P.; Pfuhler, L.; Mullen, M.; et al. Bile acids engage the SIPR-STAT3 signaling axis to modulate regulatory T cell responses in fibrosing cholangiopathies. J. Hepatol. 2025, 83, 1128–1141. [Google Scholar] [CrossRef]
- Coulombeau, R.; Selck, C.; Giang, N.; Al-Mohammad, A.; Ng, N.; Maher, A.K.; Argüello, R.; Scalfari, A.; Varley, J.; Nicholas, R.; et al. Sphingosine-1-Phosphate Signalling Inhibition Suppresses Th1-Like Treg Generation by Reversing Mitochondrial Uncoupling. Immunology 2025, 174, 153–166. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, R.; An, Y.; Liu, W.; Zheng, M.; Ma, S.; Li, Y.; Ren, X.; He, H. Yunnan Baiyao regulates Th17/Treg cell homeostasis to inhibit periodontitis through the miR-155-5p/ATG5 pathway. Int. Immunopharmacol. 2025, 163, 115253. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, X.; Wang, Y.; Yu, X.; Guo, X.; Wang, C.; Wang, F. Corynebacterium striatum drives neutrophilic asthma via IL-17 signaling activation. Int. Immunopharmacol. 2025, 163, 115255. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Q.; Li, L.; Liu, J. Overexpression of SERPINA3 inhibits castration-resistant prostate cancer progression by enhancing M1 macrophage recruitment via CXCL2 upregulation. Braz. J. Med. Biol. Res. 2025, 58, e14445. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Luo, T.; Zhao, X.; Li, P.; Gu, X.; Wan, C.; Xie, X.; Liu, H. PD-1 Regulates the Glycolytic Pathway to Reverse Abnormal CD4(+) T Cell Differentiation and Alleviate Hashimoto’s Thyroiditis. Immunology 2025, 176, 132–144. [Google Scholar] [CrossRef]
- Xu, Q.; Lin, X.; Song, L.; Ren, Y.; Bai, X.; Zhao, X.; Wang, Y.; Si, X.; Huang, R.; Li, J.; et al. Trichinella spiralis excretory-secretory protein alleviates autoimmune thyroiditis by modulating Th17/Treg balance via the STAT3/STAT5 pathway. Acta Trop. 2025, 268, 107706. [Google Scholar] [CrossRef]
- Mincer, D.L.; Jialal, I. Hashimoto thyroiditis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Huang, L.S.; Sudhadevi, T.; Fu, P.; Punathil-Kannan, P.K.; Ebenezer, D.L.; Ramchandran, R.; Putherickal, V.; Cheresh, P.; Zhou, G.; Ha, A.W.; et al. Sphingosine Kinase 1/S1P Signaling Contributes to Pulmonary Fibrosis by Activating Hippo/YAP Pathway and Mitochondrial Reactive Oxygen Species in Lung Fibroblasts. Int. J. Mol. Sci. 2020, 21, 2064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Ji, X.Y.; Ritter, J.K.; Li, N. Knockout of Sphingosine Kinase 1 Attenuates Renal Fibrosis in Unilateral Ureteral Obstruction Model. Am. J. Nephrol. 2019, 50, 196–203. [Google Scholar] [CrossRef]
- Wang, D.; Han, S.; Lv, G.; Hu, Y.; Zhuo, W.; Zeng, Z.; Tang, J.; Huang, Y.; Wang, F.; Wang, J.; et al. Pancreatic Acinar Cells-Derived Sphingosine-1-Phosphate Contributes to Fibrosis of Chronic Pancreatitis via Inducing Autophagy and Activation of Pancreatic Stellate Cells. Gastroenterology 2023, 165, 1488–1504.e20. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, Y.; Ikeda, H.; Okamoto, Y.; Takuwa, N.; Yoshioka, K. Sphingosine-1-phosphate as a mediator involved in development of fibrotic diseases. Biochim. Biophys. Acta. 2013, 1831, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shi, D.; Cao, K.; Ru, D.; Ren, J.; Rao, Z.; Chen, Y.; You, Q.; Dai, C.; Liu, L.; et al. Sphingosine kinase 2 cooperating with Fyn promotes kidney fibroblast activation and fibrosis via STAT3 and AKT. Biochim. Et Biophys. Acta Mol. Basis Dis. 2018, 1864, 3824–3836. [Google Scholar] [CrossRef]
- Tanaka, S.; Zheng, S.; Kharel, Y.; Fritzemeier, R.G.; Huang, T.; Foster, D.; Poudel, N.; Goggins, E.; Yamaoka, Y.; Rudnicka, K.P.; et al. Sphingosine 1-phosphate signaling in perivascular cells enhances inflammation and fibrosis in the kidney. Sci. Transl. Med. 2022, 14, eabj2681. [Google Scholar] [CrossRef]
- Liu, J.; Guan, L.; Wang, E.; Schuchman, E.H.; He, X.; Zeng, M. SiO(2) stimulates macrophage stress to induce the transformation of lung fibroblasts into myofibroblasts and its relationship with the sphingomyelin metabolic pathway. J. Appl. Toxicol. 2021, 41, 1584–1597. [Google Scholar] [CrossRef]
- Cruz-Orengo, L.; Daniels, B.P.; Dorsey, D.; Basak, S.A.; Grajales-Reyes, J.G.; McCandless, E.E.; Piccio, L.; Schmidt, R.E.; Cross, A.H.; Crosby, S.D.; et al. Enhanced sphingosine-1-phosphate receptor 2 expression underlies female CNS autoimmunity susceptibility. J. Clin. Investig. 2014, 124, 2571–2584. [Google Scholar] [CrossRef]
- Sacristán-Gómez, P.; Serrano-Somavilla, A.; Castro-Espadas, L.; Sánchez de la Blanca Carrero, N.; Sampedro-Núñez, M.; Muñoz-De-Nova, J.L.; Molina-Jiménez, F.; Rosell, A.; Marazuela, M.; Martínez-Hernández, R. Evaluation of Epithelial-Mesenchymal Transition Markers in Autoimmune Thyroid Diseases. Int. J. Mol. Sci. 2023, 24, 3359. [Google Scholar] [CrossRef]
- Toda, S.; Matsumura, S.; Fujitani, N.; Nishimura, T.; Yonemitsu, N.; Sugihara, H. Transforming growth factor-beta1 induces a mesenchyme-like cell shape without epithelial polarization in thyrocytes and inhibits thyroid folliculogenesis in collagen gel culture. Endocrinology 1997, 138, 5561–5575. [Google Scholar] [CrossRef][Green Version]
- Tajmiri, S.; Farhangi, M.A.; Dehghan, P. Nigella Sativa treatment and serum concentrations of thyroid hormones, transforming growth factor β (TGF-β) and interleukin 23 (IL-23) in patients with Hashimoto’s Thyroiditis. Eur. J. Integr. Med. 2016, 8, 576–580. [Google Scholar] [CrossRef]
- Wei, L.; Huang, Q.; Tu, Y.; Song, S.; Zhang, X.; Yu, B.; Liu, Y.; Li, Z.; Huang, Q.; Chen, L.; et al. Plasma exosomes from patients with active thyroid-associated orbitopathy induce inflammation and fibrosis in orbital fibroblasts. J. Transl. Med. 2024, 22, 546. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, T.; Mao, C.; Dong, X.; Mou, X.; Xu, C.; Lu, Q.; Liu, B.; Wang, S.; Xiao, Y. Aberrant MRP14 expression in thyroid follicular cells mediates chemokine secretion through the IL-1β/MAPK pathway in Hashimoto’s thyroiditis. Endocr. Connect. 2018, 7, 850–858. [Google Scholar] [CrossRef]
- Vukovic, R.; Zeljkovic, A.; Bufan, B.; Spasojevic-Kalimanovska, V.; Milenkovic, T.; Vekic, J. Hashimoto thyroiditis and dyslipidemia in childhood: A review. Front. Endocrinol. 2019, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, J. Sphingosine-1-phosphate induces the migration of thyroid follicular carcinoma cells through the microRNA-17/PTK6/ERK1/2 pathway. PLoS ONE 2015, 10, e0119148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagahashi, M.; Abe, M.; Sakimura, K.; Takabe, K.; Wakai, T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018, 109, 3671–3678. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Peng, X.; Naqvi, S.M.A.S.; Yang, Y.; Zhang, J.; Chen, M.; Chen, Y.; Chen, H.; Yan, H. The tumorigenic effect of sphingosine kinase 1 and its potential therapeutic target. Cancer Control. 2020, 27, 1073274820976664. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, J.; Li, Y.; Du, L.; Cao, Q.; Yang, L.; Zhou, Y.; Chen, P.; Guo, Y.; Zeng, G.; et al. Broad and diverse roles of sphingosine-1-phosphate/sphingosine-1-phosphate receptors in the prostate. iScience 2024, 27, 111290. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Ding, S.F. Sphingosine-1-phosphate promotes the proliferation and attenuates apoptosis of Endothelial progenitor cells via S1PR1/S1PR3/PI3K/Akt pathway. Cell Biol. Int. 2018, 42, 1492–1502. [Google Scholar] [CrossRef]
- Finetti, F.; Paradisi, L.; Bernardi, C.; Pannini, M.; Trabalzini, L. Cooperation between Prostaglandin E2 and Epidermal Growth Factor Receptor in Cancer Progression: A Dual Target for Cancer Therapy. Cancers 2023, 15, 2374. [Google Scholar] [CrossRef]
- Barretto, A.J.B.; Orda, M.A.; Tsai, P.W.; Tayo, L.L. Analysis of Modular Hub Genes and Therapeutic Targets across Stages of Non-Small Cell Lung Cancer Transcriptome. Genes 2024, 15, 1248. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiao, B.; Li, S.; Zhao, L.; Zheng, W.; Wang, K.; Xu, J.; Tian, Y.; Liu, C.; Gui, Z.; et al. Oestrogen receptor alpha in papillary thyroid carcinoma: Association with clinical features and BRAFV600E mutation. Jpn. J. Clin. Oncol. 2021, 51, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fuchs, T.; Dogan, S.; Landa, I.; Katabi, N.; Fagin, J.A.; Tuttle, R.M.; Sherman, E.; Gill, A.J.; Ghossein, R. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid 2020, 30, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Qu, N.; Zhu, R.; Hu, J.; Han, P.; Wu, J.; Tan, L.; Gan, H.; He, C.; Fang, C.; et al. TERT accelerates BRAF mutant-induced thyroid cancer dedifferentiation and progression by regulating ribosome biogenesis. Sci. Adv. 2023, 9, eadg7125. [Google Scholar] [CrossRef]
- Rostami, N.; Nikkhoo, A.; Ajjoolabady, A.; Azizi, G.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Yousefi, B.; Yousefi, M.; Jadidi-Niaragh, F. S1PR1 as a Novel Promising Therapeutic Target in Cancer Therapy. Mol. Diagn. Ther. 2019, 23, 467–487. [Google Scholar] [CrossRef]
- McGowan, E.M.; Lin, Y.; Chen, S. Targeting Chronic Inflammation of the Digestive System in Cancer Prevention: Modulators of the Bioactive Sphingolipid Sphingosine-1-Phosphate Pathway. Cancers 2022, 14, 535. [Google Scholar] [CrossRef]
- Xu, S.; Huang, H.; Qian, J.; Liu, Y.; Huang, Y.; Wang, X.; Liu, S.; Xu, Z.; Liu, J. Prevalence of Hashimoto Thyroiditis in Adults With Papillary Thyroid Cancer and Its Association With Cancer Recurrence and Outcomes. JAMA Netw. Open 2021, 4, e2118526. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, H. Papillary thyroid carcinoma with Hashimoto’s thyroiditis: Impact and correlation. Front. Endocrinol. 2025, 16, 1512417. [Google Scholar] [CrossRef]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).