Abstract
The aging process is closely linked to cognitive decline, and numerous studies have demonstrated a decrease in both the quality and quantity of sleep in the general population. Melissa officinalis (lemon balm) is a plant rich in bioactive compounds, including flavonoids, phenolic acids, and essential oils, which are responsible for its neuroprotective and antidepressant properties. Its positive effects on the sleep quality are probably, at least in part, attributable to the presence of rosmarinic acid, which modulates γ-aminobutyric acid transaminase activity. This review aimed to investigate the effects of M. officinalis on cognition and sleep quality in human clinical trials. For cognition, studies have shown that the plant improved cognitive performance and mood. In elderly individuals with mild cognitive impairment or early Alzheimer’s disease, extracts standardized in rosmarinic acid stabilized cognitive functions and reduced neuropsychiatric symptoms such as agitation. Regarding sleep, a combination of lemon balm and valerian significantly improved sleep quality in postmenopausal women. Isolated lemon balm extracts also reduced sleep disorders in cardiac patients. When compared to citalopram, lemon balm enhanced the quality of life, including sleep quality. It is concluded that lemon balm has the potential to improve cognition and sleep quality; however, robust evidence is needed, as more rigorous trials are required.
1. Introduction
According to the World Health Organization (WHO), aging is a process that involves biological, psychological, and social changes, which can lead to increased vulnerability to disease and a greater need for healthcare. This process is natural and irreversible, resulting in physiological changes that affect the autonomy and quality of life of older people [1,2,3,4,5]. The increase in life expectancy leads to an irreversible global population aging. As a result, some projections indicate that by 2050, one in six people worldwide will be 65 years of age or older [5,6,7].
Modern society is facing a decline in the quality and quantity of sleep. Age and pre-existing health conditions exacerbate this situation, making older people more susceptible to sleep deprivation and dysregulation [8,9]. Sleep disorders are increasingly being seen as risk factors for dementia and accelerated cognitive impairment [10,11]. The study by Yang et al. shows that sleep quality is negatively correlated with cognitive function, with poor sleep quality potentially accelerating cognitive impairment [12,13,14]. Although changes in memory and sleep quality are often associated with the aging process, these problems are not restricted to the elderly population. Some studies show that younger adults may also experience cognitive deficits and sleep disorders, particularly in situations of intense stress or in individuals with mental health conditions, such as anxiety and depression [15,16,17,18].
Cognitive alterations, especially memory deficits, are closely related to neurodegenerative diseases. These diseases, which include Alzheimer’s disease (AD), Parkinson’s disease (PD), and others, are characterized by the progressive loss of neuronal function and structure, resulting in significant cognitive impairments, including memory loss [19,20,21].
Poor sleep quality results from a combination of factors, including stress, lifestyle habits, underlying health conditions, and environmental influences [22,23,24]. Stress, particularly among college students, has emerged as a significant factor affecting sleep, exacerbated by academic pressure and the uncertainty generated by the COVID-19 pandemic [25,26]. Habits such as excessive consumption of caffeine, alcohol, and tobacco, as well as lack of physical activity, also contribute to sleep disorders [16,27,28]. Health conditions, such as chronic respiratory diseases, psychological disorders, depression, and anxiety, have a significant impact on sleep quality [29,30,31].
Some plants have gained prominence in the medical literature due to their antioxidant effects [32,33,34,35]. Melissa officinalis L., commonly known as lemon balm, is often used to enhance cognitive function and improve sleep quality [36]. This medicinal plant is rich in bioactive compounds (Table 1), including flavonoids, phenolic acids, and essential oils, which are responsible for its neuroprotective and antidepressant properties, and have been studied in relation to its potential health benefits, especially in the treatment of cognitive alterations and neurodegenerative diseases, such as Alzheimer’s and Parkinson’s [37,38,39].

Table 1.
Some bioactive compounds are found in M. officinalis.
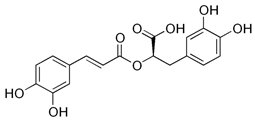
Some studies have shown anxiolytic, antispasmodic, antiviral, mood-modulating, and cognitive-enhancing benefits [39,63,64]. The effects on memory and cognitive function are attributed to its richness in antioxidants, including rosmarinic acid. This compound has the potential to reduce neuroinflammation and enhance mental capacity [65,66]. Its benefits on sleep quality are possibly related to the presence of rosmarinic acid, which modulates γ-aminobutyric acid transaminase (GABA-T) activity. This enzyme converts γ-aminobutyric acid (GABA) to succinate semialdehyde. GABA-T is essential for maintaining the balance of GABAergic neurotransmission, which is fundamental to brain function, including the regulation of anxiety, sleep, and epilepsy [63,67].
In view of the potential benefits of M. officinalis on cognition and sleep quality, this review aims to investigate its role in these areas.
2. Methods
2.1. Focused Question
This review was performed to answer the focused question: Can M. officinalis produce beneficial effects on cognition and sleep quality?
2.2. Language
Only studies in English were selected.
2.3. Databases
This review has included clinical trials published in PubMed (National Library of Medicine, National Institutes of Health), COCHRANE, and EMBASE. The mesh terms used were M. officinalis or Melissa, antioxidant, anti-inflammatory, cognition, memory, Alzheimer’s disease, Parkinson’s disease, neurodegenerative disease, sleep, or sleep quality. The use of these descriptors helped identify studies related to the plant and its health benefits on cognition and sleep quality.
2.4. Study Selection
Abstracts, conferences, reviews, letters to editors, and other sources were evaluated but not included. Furthermore, other relevant studies were included to help in the Introduction and Discussion sections. The inclusion criteria applied to this review were only human interventional studies. The PICO (Population, Intervention, Comparison, and Outcomes) format was used to evaluate the included clinical trials.
2.5. Data Extraction
We did not restrict the period of search.
3. Melissa officinalis and Conditions That Can Be Affected by This Plant
3.1. M. officinalis
M. officinalis L., popularly known as lemon balm, belongs to the Lamiaceae family and the Nepetoideae subfamily. Lemon balm can grow between 60 and 100 cm in height. Its leaves, which range from 2 to 8 cm in length, are dark green, heart-shaped, and have a rough, ribbed surface with serrated edges [64,68,69]. The plant has a hairy root system, which makes it adaptable to different environments, although its upper parts die in winter and grow back in spring; this family is widely recognized for its aromatic herbaceous plants, many of which are valued for their medicinal and nutritional properties, used in culinary and medicinal practices in various cultures. It primarily grows in the Mediterranean and Western Asia regions, but is currently cultivated in different parts of the world, including Europe, North America, and South America, such as Brazil. The plant prefers temperate climates and well-drained soils, and is frequently found in vegetable gardens, orchards, and gardens, due to its ornamental and utilitarian characteristics [37,65,70,71].
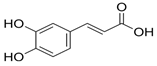
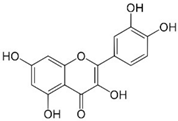
Chemical research on the composition of M. officinalis has revealed that the plant is rich in flavonoids, terpenoids, phenolic acids, tannins, and essential oils. These compounds can be extracted from the fresh or dried flowers, leaves, and twigs using steam distillation or chemical extraction [72,73,74]. In terms of polyphenolic compounds, the plant contains phenolic acids such as caffeic acid, caftaric acid, chlorogenic acid, ferulic acid, gentisic acid, p-coumaric acid, and rosmarinic acid, as well as flavonoids including apigenin, cynaroside, daidzein, hyperoside, isoquercetin, kaempferol, luteolin, myricetin, quercetin, quercetrol, and rutin. Volatile compounds account for (E)-caryophyllene, caryophyllene oxide, citronellal, geranial (citral A), geranyl acetate, neral (citral B), α-cadinol, α-copaene, and β-caryophyllene as major components. Triterpenes are mainly betulinic acid, oleanolic acid, and ursolic acid [37,75,76].
3.2. Cognition and Neurodegenerative Conditions
Human cognition involves large-scale networks that maintain functional coherence at rest and are collectively activated during cognitive processes. Cognition refers to the psychological and other processes involved in acquiring information and the capacity to understand ideas, experiences, and sensations. It is subdivided into social and non-social cognition. Non-social cognition refers to individual mental abilities, including attention, processing speed, problem-solving, reasoning skills, and working memory [77,78]. The psychological processes involved in perceiving, encoding, storing, retrieving, and regulating knowledge about oneself and others are collectively labeled as social cognition. Cognitive efficiency refers to a complex construct that represents the ability to develop learning and problem-solving skills through the optimal use of mental resources [79,80,81].
In light of this, neurodegenerative diseases are a heterogeneous group of complex conditions characterized by neuronal loss and progressive degeneration of various areas of the nervous system, impairing its functions and leading to cognitive decline [3,82,83]. These conditions, which affect the brain, retina, and spinal cord, can manifest at any stage of life. They range from congenital disorders, such as leukodystrophies, which affect the white matter during childhood, to more prevalent pathologies in aging, such as AD, PD, and age-related macular degeneration [34,84,85]. Although the exact pathogenesis of neurodegenerative diseases has not yet been fully elucidated, it is believed to result from a complex interaction between genetic, epigenetic, and environmental factors. To date, no effective therapy has been developed to slow, halt, or prevent these conditions. Therefore, understanding the molecular mechanisms underlying the pathogenesis of neurodegenerative diseases remains in high demand [82,86,87,88].
In this context, one common component among neurodegenerative diseases, such as AD, PD, and amyotrophic lateral sclerosis, is neuroinflammation [89,90,91]. In the central nervous system, microglia and astrocytes play crucial roles as regulators of inflammatory responses [92,93]. The activation of these cell types is heterogeneous and traditionally classified into two categories: neurotoxic (M1 phenotype microglia and A1 phenotype astrocytes) or neuroprotective (M2 phenotype microglia and A2 phenotype astrocytes) [94,95].
The role of the cerebral immune system is crucial in maintaining tissue homeostasis and responding to infections and injuries. Microglia, the brain’s primary resident immune cells, continuously monitor the environment and interact with astrocytes and neurons [96]. Under normal conditions, microglia remain in a state of surveillance, releasing anti-inflammatory and neuroprotective factors. However, in cases of infection or injury, they become activated, triggering an inflammatory response that eliminates pathogens and repairs damaged tissue. Ideally, this response is transient and concludes with the resolution of the pathological event [97,98]. Thereby, in situations of chronic inflammation, resolution mechanisms fail, often due to the persistent presence of inflammatory stimuli, such as misfolded protein aggregates [99,100]. These stimuli create positive feedback loops that exacerbate inflammation, leading to the production of neurotoxic factors that can worsen underlying diseases. Inflammatory responses are mediated by pattern recognition receptors, such as Toll-like receptors (TLRs), which detect foreign molecules or danger signals. For example, TLR4 recognizes bacterial lipopolysaccharides, while TLR3 detects viral RNA. These receptors can also be activated by cellular components released in response to damage, such as ATP, triggering an inflammatory cascade. The activation of these receptors initiates signaling pathways that regulate the expression of inflammatory and antimicrobial genes, such as tumor necrosis factor-α (TNFα) and interleukin (IL)-1β. These cytokines amplify the immune response and recruit other immune cells to the injury site [101,102]. While these responses are critical for defending against infections, they can cause significant collateral damage to tissues, especially in the central nervous system. In the context of neurodegenerative diseases, activated microglia release inflammatory mediators that can damage neurons, contributing to the progression of these pathologies [103,104].
3.3. Cognition and Inflammatory Processes
Aging brings about progressive and natural changes in cognitive function. These changes can vary from person to person, leading to different courses of aging, which can be categorized as healthy aging (considered normal) or pathological [105,106,107]. Neuroinflammation, which plays a role in correcting and resolving neural tissue damage, is an immune response to infections that can eliminate pathogens and cellular debris from the central nervous system. A priori, they are beneficial to neural tissue; however, in chronic neurological diseases, they become persistent and harmful to neurons [108,109,110].
Neurodegenerative diseases such as AD, amyotrophic lateral sclerosis, and PD have in common neuroinflammation mediated by microglia, which can assume an M1 or M2 phenotype. Depending on which phenotype is activated, these cells may have a neuroprotective or cytotoxic effect. Microglia with the M1 phenotype are associated with the production of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, IL-18, and IL-12, reactive oxygen species (ROS), nitric oxide, glutamate, and prostaglandins, as well as enzymes such as matrix metalloproteinases (MMPs). In contrast, the M2 phenotype is associated with anti-inflammatory effects [110,111,112].
The accumulation of proteins and the dysregulation of mechanisms controlling inflammation are among the various causes of inflammation in neurodegenerative disorders. The primary one is the aggregation of proteins resulting from genetic mutations in specific proteins. For example, it is worth mentioning the mutations in the genes of the human amyloid precursor protein that cause early-onset AD, as well as the mutations in proteins that encode the ubiquitin E3 ligase parkin, which can cause familial PD [113,114,115].
Currently, studies describe the mechanisms associated with neuroinflammation due to the accumulation of misfolded proteins in neurodegenerative diseases [116,117]. The interaction that occurs between microglia, neurons and astrocytes, in the nervous system, during neurodegenerative diseases leads to the activation of the nuclear factor-κB (NF-κB) pathway, which, when translocated to the cell nucleus, promotes the transcription of several cytokines and pro-inflammatory chemokines, including pyrin-containing inflammasome 3 (NLRP3), triggering a cascade of inflammation that leads to behavioral deficits and neuronal death [118,119].
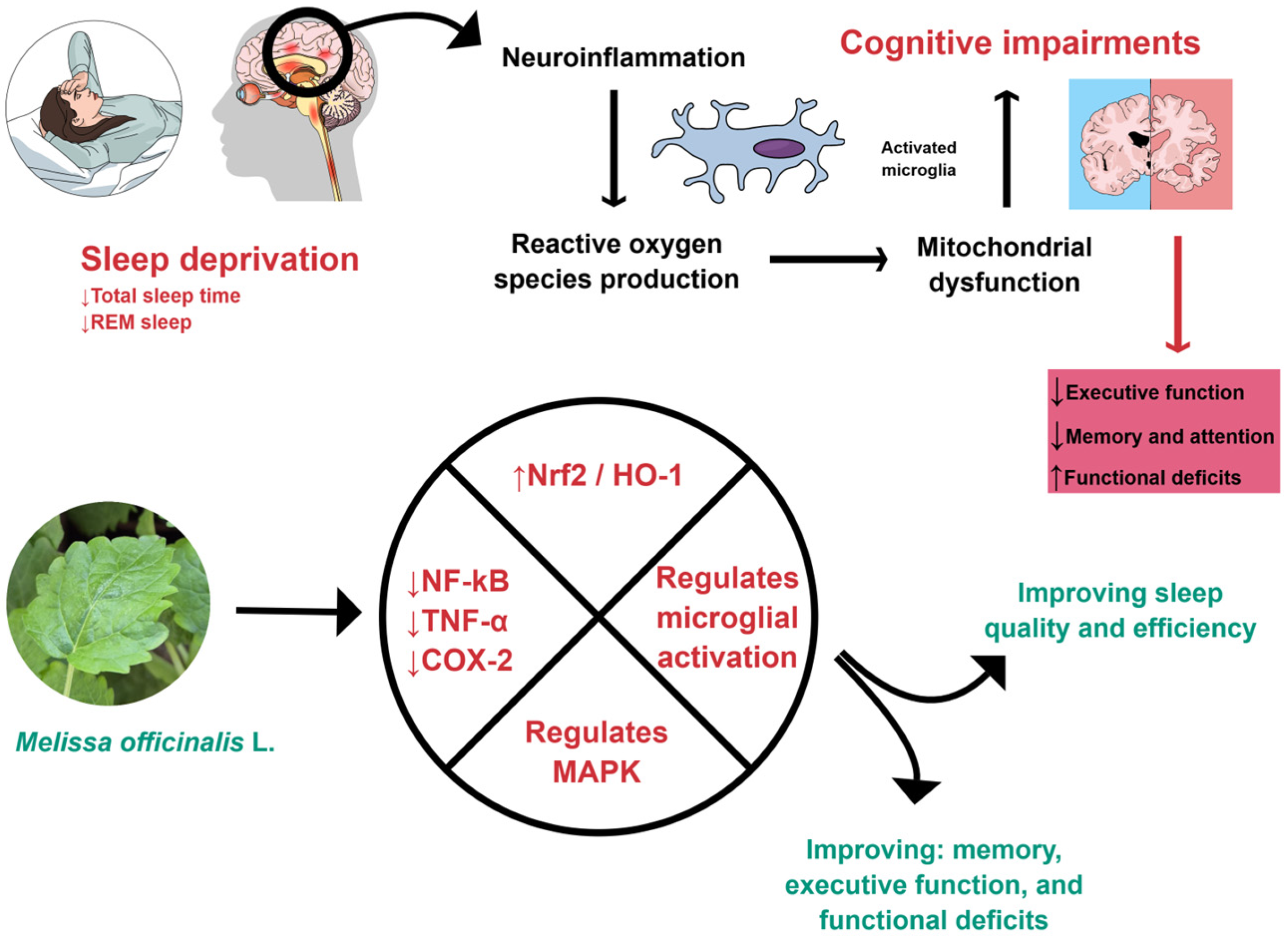
M. officinalis can affect oxidative and inflammatory processes, as shown in Figure 1 and Figure 2. For these reasons, it can improve cognition.
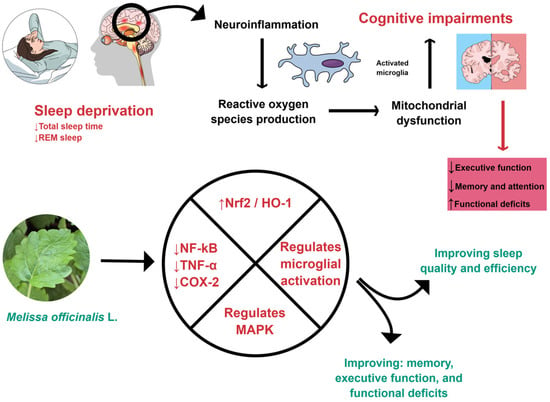
Figure 1.
M. officinalis can interfere with inflammatory processes by down-regulating NF-kB, TNF-α, and COX-2. Furthermore, it can promote an increase in Nrf2 and HO-1, regulate microglial activation, reduce neuroinflammation, and improve sleep. COX-2: Cyclooxygenase-2; HO-1, Heme-oxygenase 1; MAPK, Mitogen-activated protein kinase; NF-kB: Nuclear Factor Kappa Beta; Nrf2: Nuclear factor erythroid 2-related factor 2; REM, Rapid eye movement; TNF-α: Tumor Necrosis Factor-alpha. Created using Mind the Graph (https://mindthegraph.com/), accessed on 17 October 2025.
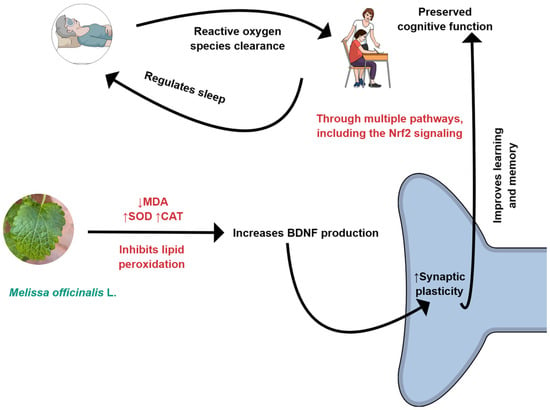
Figure 2.
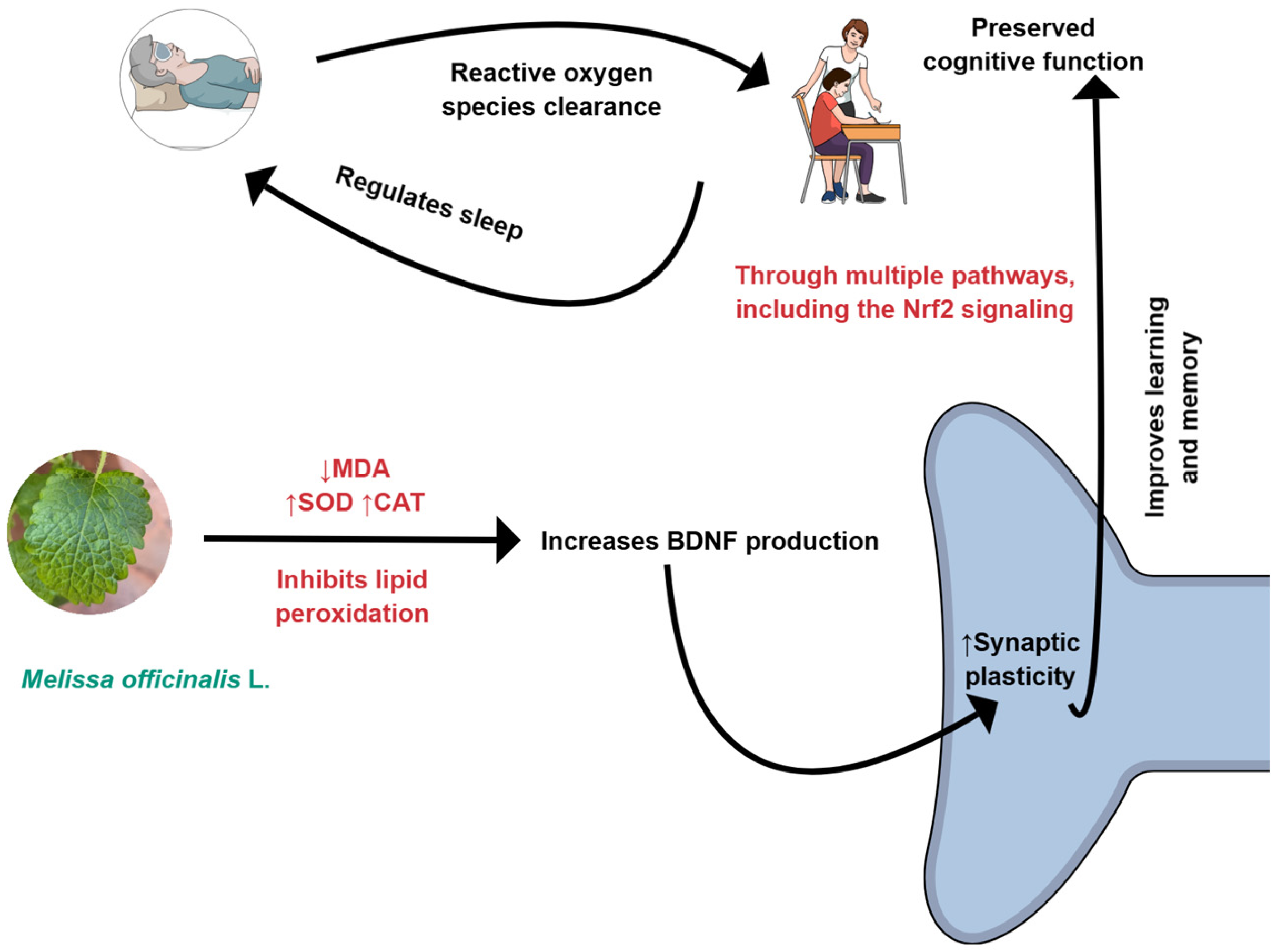
M. officinalis can reduce oxidative stress by decreasing the production of MDA (malondialdehyde) and increasing the levels of CAT (catalase) and SOD (superoxide dismutase). BDNF, Brain-derived neurotrophic factor; Nrf2: Nuclear factor erythroid 2-related factor 2. Created using Mind the Graph (https://mindthegraph.com/), accessed on 17 October 2025.
3.4. Cognition and Oxidative Stress
Oxidative stress is characterized by an imbalance between the production of ROS and antioxidant substances, which are responsible for protecting the body from free radicals, leading to oxidative damage to cells [120,121]. The brain is susceptible to neurodegeneration, which is the progressive and irreversible loss of interference in the nervous system due to damage to neurons. This occurs when the brain is exposed to an accumulation of ROS, which have lipid components capable of promoting a cascade of redox reactions, which cause damage to cells and compromise their functions [122,123,124].
Neuronal injuries caused by oxidative stress, which result in the interruption of synaptic transmission and neuronal dysfunction due to changes in DNA, proteins, and lipids, generally occur in more susceptible areas, such as the hippocampus and regions responsible for memory storage [125,126]. Cognitive deficits resulting from neuronal injuries can be classified according to their impact on the person’s quality of life, which can be disabling for both the patient and those around them [127].
3.5. Sleep Quality
Sleep is a physiological component of the human being, with a strong influence on cognitive processing [128]. However, as age progresses, older adults tend to experience more sleep disorders than young people [129,130]. Sleep pattern disorders are considered health problems that interfere with a person’s ability to sleep consistently and without interruptions for an extended period, and can be associated with pathologies, such as depressive disorders, anxiety, post-traumatic events, as well as stressful episodes of everyday life. The main manifestations of sleep disorders are insomnia, sleep apnea, and restlessness [131,132].
Sleep disorders are associated with numerous pathologies, including neurodegenerative diseases, which have a bidirectional association with changes in sleep patterns [133,134]. There are mechanisms by which sleep disorders and the risks of neurodegeneration are associated, among them it is worth mentioning, the production and aggregation of β-amyloid (Aβ), due to the increase in synaptic neuronal activity, which is naturally removed during sleep and when there is a change in the sleep pattern, this removal does not occur, leading to the accumulation of this substance and the activation of the inflammatory process with secretion of pro-inflammatory cytokines, due to the stimulation of inflammatory pathways when there is a change in the sleep pattern. Furthermore, the presence of pro-inflammatory cytokines, such as IL-6 and TNF-α, results in greater stimulation of Aβ production and aggregation, which in turn stimulates the secretion of more inflammatory cytokines and amplifies neurodegeneration [135,136].
In general, sleep disorders affect the functioning of the human body, leading to changes such as a decreased pain threshold, which increases sensitivity and perception of pain stimuli. Moreover, it is related to anxiety, reduction in memory, and neurodegenerative diseases. During sleep, the body produces endorphins and other neurochemical substances that can help control pain [137,138]. M. officinalis can interfere in oxidative and inflammatory processes, as shown in Figure 1 and Figure 2. For these reasons, it can improve sleep quality.
4. Clinical Trials That Evaluated the Effects of M. officinalis on Cognition
As mentioned above, M. officinalis is known to influence cognition and sleep quality. The effects on cognition are shown in Table 2. This table presents a variety of clinical studies that investigated the impact of M. officinalis on cognitive function. These studies vary in design, population, dosage, and outcome measures, but collectively provide insight into the potential mental effects of M. officinalis in both healthy individuals and those with cognitive impairments. Although this review provides an overview of the current evidence regarding the effects of M. officinalis L. on cognition and sleep quality, it is essential to recognize that most of the available clinical trials are small, single-center studies with limited sample sizes, which may restrict the generalizability of their findings. Furthermore, the predominance of studies reporting positive results raises the possibility of publication bias, as trials with null or adverse outcomes may be underrepresented in the literature. Future research should therefore include larger, multicenter randomized controlled trials with standardized methodologies to confirm and extend these preliminary findings.

Table 2.
Clinical trials that evaluated the effects of M. officinalis on cognition.
The randomized, double-blind, placebo-controlled, crossover trial conducted by Kennedy et al. investigated the acute effects of M. officinalis (lemon balm) extract on cognition and mood in 20 healthy young volunteers [139]. Participants received single doses of 600 mg, 1000 mg, or 1600 mg of M. officinalis (encapsulated dried leaf) or a placebo. Cognitive performance and mood were assessed before and after administration. Results demonstrated significant improvements in cognitive task performance and mood, particularly with the 1600 mg dose. No adverse effects were reported. Although this is a critical study, the sample size is too restricted. Furthermore, the study evaluated only the single-dose acute impact, which does not provide information on chronic use effects and long-term safety. These aspects interfere with the conclusions of this study.
The randomized, placebo-controlled trial by Watson et al. investigated the impact of aromatherapy using lavender (Lavandula angustifolia) and lemon balm (M. officinalis) essential oils on agitation in older adults, with and without dementia [140]. Participants received once-daily treatments with lavender, lemon balm, or placebo oil (sunflower oil) for 2 weeks. The study found no statistically significant differences in agitation levels between the treatment groups and the placebo group. No significant adverse effects were reported.
Noguchi-Shinohara et al. conducted a study to evaluate the effects of rosmarinic acid, present in M. officinalis extract, in patients with dementia associated with AD [141]. A total of 23 patients participated in this study, who were randomized into two groups (M. officinalis extract and placebo groups). During 24 weeks, participants received daily doses of either 500 mg of rosmarinic acid in M. officinalis extract or a placebo. The study lasted an additional 24 weeks (washout period), totaling 48 weeks of evaluation, during which all participants were assigned to the M. officinalis extract group. The results suggested that the treatment can help in the prevention of neuropsychiatric symptoms related to AD. Despite the promising results, the study has limitations, including a small sample size, which necessitates a more cautious interpretation of the findings. Moreover, this study may have other limitations, such as the inclusion of heterogeneous patients.
Noguchi-Shinohara et al. conducted a study aiming to evaluate the effects of M. officinalis on cognition in older adults [142]. For this, 323 participants with subjective or mild cognitive impairment were randomized into two groups: the M. officinalis group and the placebo group. The study had two phases. Phase 1, which corresponds to the intervention lasting 96 weeks, and phase 2, which was a washout period of 24 weeks. The results obtained indicate that M. officinalis extract can help prevent cognitive decline in older adults without hypertension. The randomized, double-masked, placebo-controlled study design is its strong point. However, the sample size and the failure to analyze variables such as amyloid and tau protein limited the study’s findings.
The studies included in this narrative review exhibit notable methodological limitations and considerable heterogeneity, which warrant careful interpretation of their findings. First, many of the trials had relatively small sample sizes, which limits the statistical power and generalizability of their results. In some cases, convenience sampling and inadequate randomization procedures further undermine the reliability of the reported outcomes.
Additionally, there was significant variation in study designs, intervention protocols, and outcome measures across the trials. The dosage and duration of M. officinalis administration varied widely, ranging from single acute doses to chronic administration over several weeks, and included both standardized extracts and combinations with other herbal compounds (e.g., valerian). This heterogeneity complicates direct comparisons between studies and precludes the possibility of performing a meta-analysis.
Moreover, most studies relied on subjective assessment tools, such as self-reported questionnaires, to evaluate cognitive performance and sleep quality, which are susceptible to bias. Objective measures, including neuroimaging biomarkers or polysomnography, were rarely employed. The lack of standardized and validated outcome measures contributes to the difficulty in synthesizing results across studies.
Blinding and allocation concealment procedures were insufficiently described in several trials, introducing a potential risk of bias. Furthermore, only a few studies reported monitoring or controlling for confounding variables such as concomitant medication use, lifestyle factors, or comorbid conditions, all of which could have influenced the outcomes.
When comparing trials with positive outcomes to those reporting null results, several methodological and population-related differences emerge. For instance, Kennedy et al. [139] reported acute improvements in cognitive performance and mood following single doses of standardized M. officinalis extract in healthy young adults. In contrast, Noguchi-Shinohara et al. [142] observed no significant cognitive improvements after long-term supplementation in older adults with mild cognitive impairment. These discrepancies may be attributed to differences in the study populations (healthy versus cognitively impaired), duration of intervention (acute versus chronic), and the specific formulations used (standardized extract with defined rosmarinic acid content versus a more variable preparation). Similarly, while aromatherapy trials using M. officinalis essential oil [140] yielded null results, these interventions employed administration routes other than oral administration, which may have limited bioavailability and central nervous system effects. Overall, the variability in study design, extract composition, and participant characteristics seems to partly explain the inconsistent findings across clinical trials evaluating M. officinalis and cognition.
5. Clinical Trials That Evaluated the Effects of M. officinalis on Sleep Quality
The effects of M. officinalis on sleep quality are shown in Table 3. This table showcases a range of clinical studies examining the effects of M. officinalis on sleep quality. As the previous table investigated the effects of M. officinalis on cognitive function, Table 3 also presents studies that differ in their design, population, dosage, and outcome measures. Together, these selected studies shed light on the potential mental benefits of M. officinalis for individuals with sleep disturbances.

Table 3.
Clinical trials showing the effects of M. officinalis on sleep quality.
In a clinical trial involving 13 men and 17 women, the authors used a highly standardized M. officinalis extract, formulated as Phytosome (MOP), and observed a significant improvement in sleep quality [63]. The results of this study are critical because they suggest a beneficial effect of MOP on subjective sleep quality in adults with mild to moderate insomnia. However, this study has limitations, including a small sample size and the need to evaluate different doses and long-term outcomes to confirm these findings.
An interesting study evaluated the effects of M. officinalis combined with valerian on menopausal women [143]. Their results showed significant effects on sleep quality. Nevertheless, some limitations are related to this trial, such as the combination of the plants.
The study conducted by Haybar et al. evaluated the effects of M. officinalis in patients with angina and showed significant amelioration of anxiety, depression, and sleep disorders [144].
Another clinical trial demonstrated that the use of M. officinalis could improve all MENQOL domain scores compared to the citalopram or placebo groups; consequently, it is possible to infer that there was an improvement in sleep quality. However, as a limitation, the study presented a small sample size and a limited intervention duration. Furthermore, lifestyle factors and medications were not controlled [145].
Some limitations can be reported in the studies related to M. officinalis effects both in cognition and sleep quality; the benefits found are more consistent in specific populations (such as menopause and angina), but are frequently associated with other herbs such as valerian. In addition, the samples are usually small, there are issues with blinding in some studies, and the trials employ subjective outcome measures.
A notable limitation of the current body of evidence on M. officinalis is the potential for publication bias. Many of the included studies are small-scale trials reporting positive outcomes, which raises concerns about the underreporting of negative or inconclusive findings. This bias may overestimate the perceived efficacy of M. officinalis in improving cognition and sleep quality, as studies with unfavorable results are less likely to be published or indexed in major databases. A systematic effort to include unpublished data and gray literature would be essential to mitigate this risk in future reviews.
Furthermore, there is a marked lack of standardization in the dosing regimens of M. officinalis used across studies. Dosages varied widely, with differences in the form of administration (e.g., capsules, extracts, or combined formulations with other herbs such as valerian). These inconsistencies make it difficult to identify an optimal therapeutic dose or to compare results across trials. The absence of standardized extracts and uniform reporting of bioactive compound concentrations, such as rosmarinic acid levels, further limits the ability to draw robust conclusions.
To strengthen the evidence base, future research should prioritize the use of standardized M. officinalis preparations with well-defined concentrations of active constituents. Dose-response studies and trials using harmonized protocols are needed to determine effective and safe dosing strategies.
While several trials examining the effects of M. officinalis on sleep quality report positive results, there is insufficient critical comparison of these findings with those from studies reporting null results. For example, in the trial by Di Pierro et al. [63], significant improvements in sleep quality were observed in the treatment group, with 87% of participants experiencing an improvement in sleep quality compared to just 30% in the placebo group. In contrast, the trial by Shirazi et al. [145] showed that while M. officinalis was more effective than placebo and citalopram in improving various quality-of-life scores in postmenopausal women, the study did not provide a robust comparison of the magnitude of effects across different interventions. Additionally, the trial by Taavoni et al. [143] reported improvements in sleep quality in menopausal women, but without clearly contrasting the effects with studies that failed to show such benefits. A more balanced presentation of both positive and null findings would help contextualize the potential efficacy of M. officinalis in sleep quality improvement and give readers a clearer understanding of the treatment’s effectiveness relative to other interventions.
Although preclinical studies provide robust evidence that M. officinalis can modulate key neurobiological pathways—such as reducing oxidative stress and downregulating pro-inflammatory mediators—these mechanistic effects have not yet been conclusively demonstrated in human populations. Small sample sizes, short durations, heterogeneous populations, and variability in the form and standardization of M. officinalis extracts limit most clinical trials conducted to date. Moreover, most trials rely on subjective outcome measures (e.g., self-reported sleep quality or cognitive assessments) and lack objective biomarkers that could confirm engagement of the proposed molecular targets. No studies to date have measured inflammatory or oxidative stress markers (such as cytokine levels, Nrf2 activation, or oxidative stress biomarkers) in clinical contexts, limiting the ability to establish a transparent mechanistic bridge.
Therefore, while the biological plausibility of M. officinalis as a neuroprotective agent is supported by mechanistic studies in vitro and in vivo, the current clinical evidence remains preliminary. To strengthen the translational link, future trials should incorporate standardized extracts with defined concentrations of bioactive compounds (e.g., rosmarinic acid), utilize objective and validated outcome measures, and assess biological endpoints that align with preclinical mechanisms. This approach would allow for a more rigorous evaluation of the therapeutic potential of M. officinalis in cognitive and sleep-related disorders.
6. Conclusions and Future Perspectives
The available evidence suggests that M. officinalis L. may exert beneficial effects on specific aspects of cognition and sleep quality. This supports its use as a nutraceutical supplement to ameliorate people’s well-being regarding sleep disorders and cognition-related deficits, including those related to anxiety and stress. However, caution is needed due to the included studies’ heterogeneity and methodological limitations.
M. officinalis L.’s bioactive compounds, such as flavonoids and phenolic acids, appear to have a positive influence on brain function and neurodegenerative processes. Studies suggest that lemon balm extract can enhance cognitive performance and mood, particularly at moderate doses, with minimal adverse effects. Additionally, the evidence points to its potential in alleviating neuropsychiatric symptoms associated with neurodegenerative diseases like AD, which may help prevent cognitive decline in older adults without dementia. However, while the results are promising, there are still limitations, such as small sample sizes in some studies and a lack of deeper analyses of biomarkers like amyloid and tau, which are crucial for understanding the underlying mechanisms of the plant’s effects on the brain. Further clinical studies with larger sample sizes and more extended follow-up periods are necessary to confirm these findings and explore the therapeutic potential of M. officinalis. Future studies should also prioritize robust cohorts to enhance the statistical reliability and generalizability of their results. In addition to increasing sample size, it is necessary to investigate the effects in groups of different ethnicities, elderly individuals, and those with comorbidities, and explore the effects in younger populations with chronic stress or anxiety.
Other limitations of the current literature involve a lack of long-term/chronic studies, which could raise concerns about safety and sustained efficacy. Additionally, the included studies often comprised heterogeneous designs and dosing regimens, which limit the identification of optimal doses or the comparison of results with the utmost clarity. Furthermore, some studies combined M. officinalis with other herbs, such as valerian, which makes it unclear whether the effects studied are due to M. officinalis itself.
In this sense, larger and adequately powered randomized clinical studies should be tailored to enhance the generalizability of findings and to confirm efficacy across diverse populations. The cohorts should also be studied under more extended intervention periods to assess sustained effects and safety over chronic use. Standardization of M. officinalis preparations should also be prioritized, including the quantification of key bioactive compounds such as rosmarinic acid, which is crucial to determine dose-response relationships and enable cross-trial comparisons.
Rigorous randomization and blinding procedures are necessary in this regard, along with careful monitoring of potential cofounders such as concomitant medications, lifestyle factors, and comorbid conditions. To overcome the limitations of subjective assessments, future studies should prioritize other methods or objective biomarkers to assess treatment results. These include neuroimaging and electrophysiological measures for cognition, as well as polysomnography or actigraphy for sleep quality.
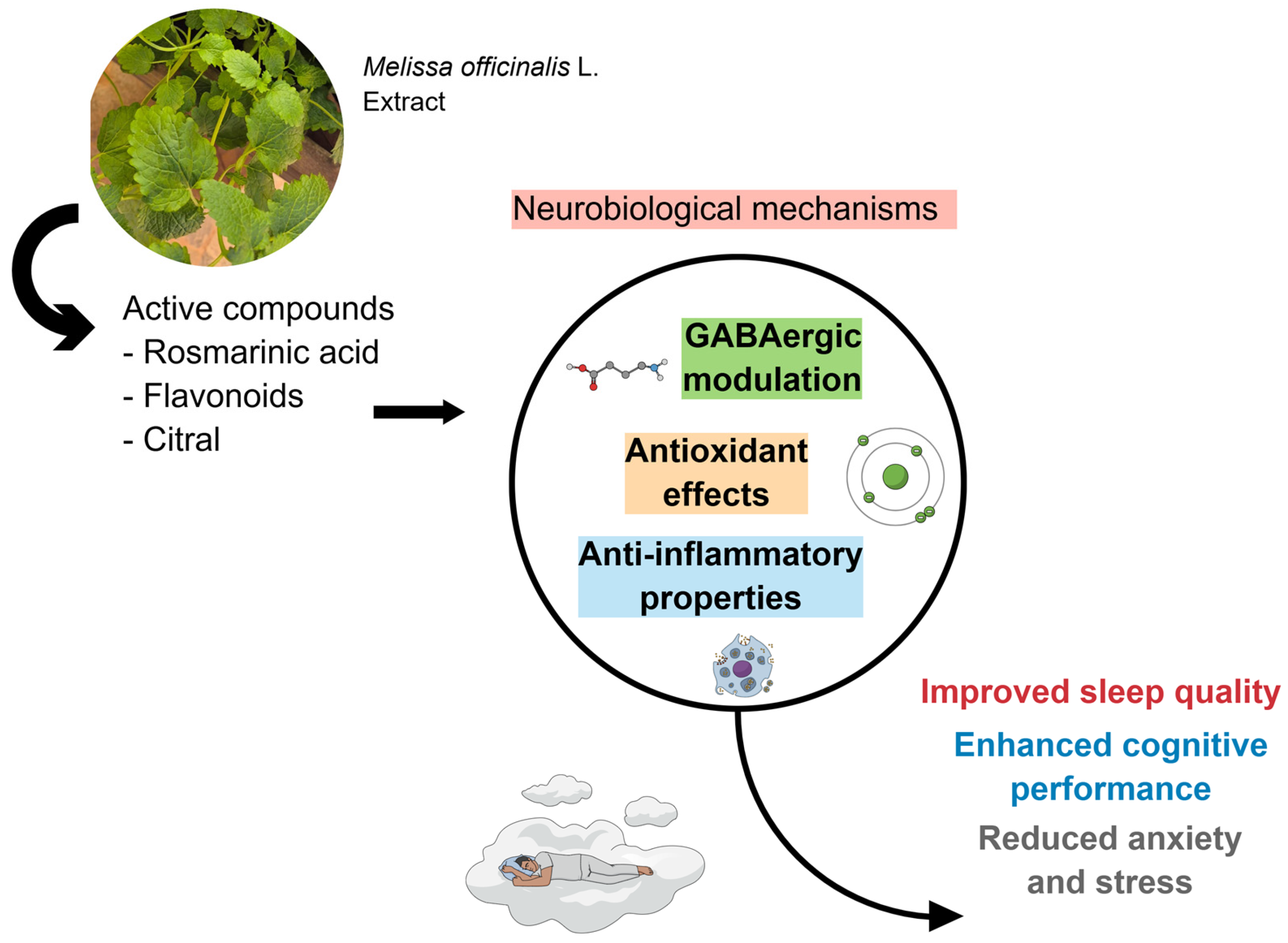
The evaluation of inflammatory and oxidative stress markers, as well as cognitive markers, can significantly help in understanding the effects produced by the plant. Standardization of the strata used, along with objective measures for assessing sleep and cognition, would provide more in-depth explanations of the effects of M. officinalis. Figure 3 shows the results of the included clinical studies, considering the biological effects attributed to M. officinalis.
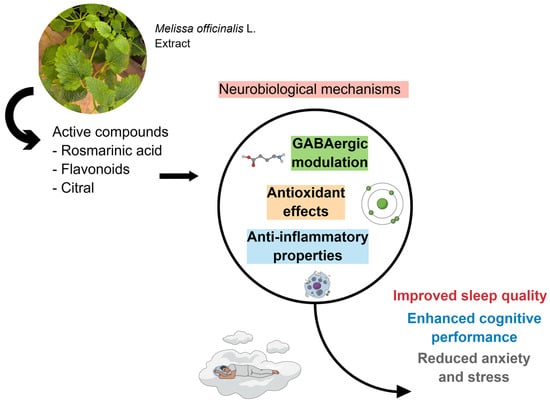
Figure 3.
Due to its bioactive compounds, M. officinalis can effectively trigger GABAergic modulation, promote antioxidant effects, and exert anti-inflammatory properties. These actions modulate sleep quality, enhance cognitive performance, and reduce anxiety and stress. Created using Mind the Graph (https://mindthegraph.com/), accessed on 17 October 2025.
Author Contributions
Conceptualization, M.V.B.O., J.A.G., C.B.L., L.F.L., V.D.R., K.P.S., L.A.S., E.F.B.C., E.L.G., C.R.P.D., M.A.M., E.d.S.B.M.P., V.E.V., L.R.S. and S.M.B.; methodology, M.V.B.O., J.A.G., C.B.L., L.F.L., V.D.R., K.P.S., L.A.S., E.F.B.C., E.L.G., C.R.P.D., M.A.M., E.d.S.B.M.P., V.E.V., L.R.S. and S.M.B.; investigation, M.V.B.O., J.A.G., C.B.L., L.F.L., V.D.R., K.P.S., L.A.S., E.F.B.C., E.L.G., C.R.P.D., M.A.M., E.d.S.B.M.P., V.E.V., L.R.S. and S.M.B.; data curation, M.V.B.O., J.A.G., C.B.L., L.F.L., V.D.R., K.P.S., L.A.S., E.F.B.C., E.L.G., C.R.P.D., M.A.M., E.d.S.B.M.P., V.E.V., L.R.S. and S.M.B.; writing—original draft preparation, M.V.B.O., J.A.G., C.B.L., L.F.L., V.D.R., K.P.S., L.A.S., E.F.B.C., E.L.G., C.R.P.D., M.A.M., E.d.S.B.M.P., V.E.V., L.R.S. and S.M.B.; writing—review and editing, M.V.B.O., J.A.G., C.B.L., L.F.L., V.D.R., K.P.S., L.A.S., E.F.B.C., E.L.G., C.R.P.D., M.A.M., E.d.S.B.M.P., V.E.V., L.R.S. and S.M.B.; visualization, M.V.B.O., J.A.G., C.B.L., L.F.L., V.D.R., K.P.S., L.A.S., E.F.B.C., E.L.G., C.R.P.D., M.A.M., E.d.S.B.M.P., V.E.V., L.R.S. and S.M.B.; supervision, M.V.B.O., J.A.G., C.B.L., L.F.L., V.D.R., K.P.S., L.A.S., E.F.B.C., E.L.G., C.R.P.D., M.A.M., E.d.S.B.M.P., V.E.V., L.R.S. and S.M.B.; project administration, M.V.B.O., J.A.G., C.B.L., L.F.L., V.D.R., K.P.S., L.A.S., E.F.B.C., E.L.G., C.R.P.D., M.A.M., E.d.S.B.M.P., V.E.V., L.R.S. and S.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to acknowledge Mind the Graph (https://mindthegraph.com/, accessed on 17 October 2025) for their platform, which enabled the creation of the figures. Their high-quality medical illustrations significantly enhanced the readability and analysis of our manuscript. The use of their illustrations is licensed under an active signature on the platform. Last accessed on 17 October 2025.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pacheco, L.M.; da Cunha, B.M.S.d.C.; Queiroz, L.A.Q.; Rocha, S.L.R.; Noleto, T.O.N.; Rodrigues, I.G.; Fernanda Costa Dalla Mutta Resende, C.; Cristian de Souza, H. Doenças cardiovasculares em idosos usuários do SUS: Prevalência e fatores associados: Cardiovascular diseases in elderly sus users: Prevalence and associated factors. Rev. Master Ensino Pesqui. E Extensão 2022, 6, 30–34. [Google Scholar] [CrossRef]
- de Souza, E.; Reis, N.; Reis, S.; Bemvenuto, R.; Ferreira, I.; Rosário, R.; Santos, M.; Reis, S.; Oliveira, A.; Araújo, K. Riscos de quedas em idosos e a COVID-19: Um alerta de saúde e proposta de exercícios funcionais. Rev. Bras. Atividade Física Saúde 2020, 25, 1–7. [Google Scholar] [CrossRef]
- Figueiredo Godoy, A.C.; Frota, F.F.; Araújo, L.P.; Valenti, V.E.; Pereira, E.; Detregiachi, C.R.P.; Galhardi, C.M.; Caracio, F.C.; Haber, R.S.A.; Fornari Laurindo, L.; et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines 2025, 13, 1129. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16, 2721. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525. [Google Scholar] [CrossRef]
- Jee, H. Aging world: Mishap or time for revision. J. Exerc. Rehabil. 2024, 20, 91. [Google Scholar] [CrossRef]
- Gonçalves, L.; Oliveira, J.; Guimarães, A.; Guimarães, B.; Melo Soares, C.E.; Gomes, H.; Queiroz, T. A problemática da epidemia de demência vascular no Brasil: Uma revisão bibliográfica/The problem of the epidemic of vascular dementia in Brazil: A bibliographic review. Braz. J. Health Rev. 2020, 3, 15451–15459. [Google Scholar] [CrossRef]
- Kron, J.O.Z.J.; Keenan, R.J.; Hoyer, D.; Jacobson, L.H. Orexin Receptor Antagonism: Normalizing Sleep Architecture in Old Age and Disease. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 359–386. [Google Scholar] [CrossRef]
- Rigillo, G.; Blom, J.M.C.; Cocchi, A.; Martinucci, V.; Favaro, F.; Baini, G.; Cappellucci, G.; Tascedda, F.; Biagi, M. Medicinal Plants for Child Mental Health: Clinical Insights, Active Compounds, and Perspectives for Rational Use. Children 2025, 12, 1142. [Google Scholar] [CrossRef]
- Wei, J.; Wang, M.; Guo, Y.; Liu, Y.; Dong, X. Sleep structure assessed by objective measurement in patients with mild cognitive impairment: A meta-analysis. Sleep Med. 2024, 113, 397–405. [Google Scholar] [CrossRef]
- Murray, M.E.; Smith, C.; Menon, V.; Keene, C.D.; Lein, E.; Hawrylycz, M.; Aguzzi, A.; Benedetti, B.; Brose, K.; Caetano-Anolles, K.; et al. Accelerating biomedical discoveries in brain health through transformative neuropathology of aging and neurodegeneration. Neuron, 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Yang, H.; Xu, L.; Qin, W.; Hu, F.; Li, L.; Chen, C.; Tang, W. Gender differences in the modifying effect of living arrangements on the association of sleep quality with cognitive function among community-dwelling older adults: A cross-sectional study. Front. Public Health 2023, 11, 1142362. [Google Scholar] [CrossRef]
- Hu, Y.; Zuo, L.; Pan, Y.; Yan, H.; Wang, Y.; Zhao, X. Persisting Short or Long Sleep Duration Predicts Post-Stroke Depression One year After Stroke and Transient Ischemic Attack. Nat. Sci. Sleep 2025, 17, 1507–1519. [Google Scholar] [CrossRef]
- Awlqadr, F.H.; Altemimi, A.B.; Qadir, S.A.; Mohammed, O.A.; Saeed, M.N.; Hesarinejad, M.A.; Lakhssassi, N. Bioactive Compounds, Medicinal Benefits, and Contemporary Extraction Methods for Lemon Balm (Melissa officinalis). Food Sci. Nutr. 2025, 13, e70864. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, L.C.; O’Shea, B.Q.; Joseph, C.; Finlay, J.M. Acute relationships between mental health and cognitive function during the COVID-19 pandemic: Longitudinal evidence from middle-aged and older US adults. SSM Ment. Health 2022, 2, 100097. [Google Scholar] [CrossRef] [PubMed]
- Ayyıldız, M.; Kalafat, Ş. The Relationship between Perceived Stress, Sleep Quality and the Everyday Memory of the Senior Middle School, High School and College Students in Turkey. PsyArXiv 2022. [Google Scholar] [CrossRef]
- Jiahao, W.; Kameyama, J.; Udono, M.; Katakura, Y. Metabolic changes in the one-carbon metabolism-related amino acids during etoposide-induced cellular senescence of neuronal cells. Cytotechnology 2025, 77, 131. [Google Scholar] [CrossRef]
- Piełunowicz, M.; Kotuła, J.; Kotuła, K.; Więckiewicz, M.; Lis, J.; Kawala, B.; Kuc, A.E.; Sarul, M. Effects of rapid maxillary expansion and functional orthodontic treatment in children with sleep disordered breathing: A systematic review. BMC Oral Health 2025, 25, 1059. [Google Scholar] [CrossRef]
- Mandel, N.; Agarwal, N. Role of SUMOylation in Neurodegenerative Diseases. Cells 2022, 11, 3395. [Google Scholar] [CrossRef]
- Pan, X.; Dutta, D.; Lu, S.; Bellen, H.J. Sphingolipids in neurodegenerative diseases. Front. Neurosci. 2023, 17, 1137893. [Google Scholar] [CrossRef]
- Yıldırım, İ.G.; Doğan, F.; Şanlıer, N. Nörodejeneratif Hastalıklar ve Fitokimyasallar. Beslenme Ve Diyet Derg. 2023, 51, 111–118. [Google Scholar] [CrossRef]
- Basiri, R.; Rajanala, Y. Effects of Individualized Nutrition Therapy and Continuous Glucose Monitoring on Dietary and Sleep Quality in Individuals with Prediabetes and Overweight or Obesity. Nutrients 2025, 17, 1507. [Google Scholar] [CrossRef]
- Merchant, A.M.; Gray, S.R.; Gray, C.M.; Finlayson, G.; Manyara, A.M.; Gabler Trisotti, M.F.; Gill, J.M.R. Effect of a cognitive behavioural therapy intervention to improve sleep on food preferences: A randomized controlled trial in adults with overweight and obesity. Appetite 2025, 212, 108022. [Google Scholar] [CrossRef] [PubMed]
- Mathews, I.M.; Eastwood, J.; Lamport, D.J.; Cozannet, R.L.; Fanca-Berthon, P.; Williams, C.M. Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients 2024, 16, 3545. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Oh, J. Factors Affecting Sleep Quality of College Students during the Coronavirus Disease 2019 Pandemic: A Cross-Sectional Study. Medicina 2023, 59, 416. [Google Scholar] [CrossRef] [PubMed]
- Zakiah, N.I.; Syahirah, N. Sleep Disruption and Its Impact on Academic Performance in Medical Students: A Systematic Review. Univers. J. Public Health 2023, 11, 1–7. [Google Scholar]
- Jyothi, R.; Mathangi, D.; Chellaiyan, V. Lifestyle Behaviour and Obstructive Sleep Apnea (OSA): An Association Study Among Young Adults. Natl. J. Community Med. 2022, 13, 400–403. [Google Scholar] [CrossRef]
- Zam, W.; Quispe, C.; Sharifi-Rad, J.; López, M.D.; Schoebitz, M.; Martorell, M.; Sharopov, F.; Fokou, P.V.T.; Mishra, A.P.; Chandran, D.; et al. An Updated Review on The Properties of Melissa officinalis L.: Not Exclusively Anti-anxiety. Front. Biosci. 2022, 14, 16. [Google Scholar] [CrossRef]
- Agustiyaningsih, T.; Ririn, H.; Lilis, S. Factors Affecting the Quality of Sleep in Patients with Chronic Obstructive Pulmonary Disease (COPD). Formosa J. Sci. Technol. 2023, 2, 1105–1114. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H. Factors Affecting the Quality of Sleep and Social Participation of Stroke Patients. Brain Sci. 2023, 13, 1068. [Google Scholar] [CrossRef]
- Wei, W.X.; Meng, L.; Mao, Z.F.; Mo, Z.H.; Yang, L.; Qin, Y.; Huang, J.Y. The association between the incident risk of Parkinson’s disease and depression in middle-aged and older adults, and the moderating role of lifestyle: Evidence from the CHARLS. Front. Psychol. 2025, 16, 1590931. [Google Scholar] [CrossRef]
- Burigo, F.E.T.; Salviano, F.d.N.S.; Pincerati, M.R.; Silva, I.S.d. Benefícios das plantas medicinais sobre eventos moleculares associados ao tratamento da Doença de Alzheimer. Rev. DELOS 2024, 17, e1509. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Simili, O.A.G.; Araújo, A.C.; Guiguer, E.L.; Direito, R.; Valenti, V.E.; de Oliveira, V.; de Oliveira, J.S.; Yanaguizawa Junior, J.L.; Dias, J.A.; et al. Melatonin from Plants: Going Beyond Traditional Central Nervous System Targeting-A Comprehensive Review of Its Unusual Health Benefits. Biology 2025, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, G.L.O.; Santos, L.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.; Goulart, R.A.; Catharin, V.; et al. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants 2024, 13, 651. [Google Scholar] [CrossRef]
- Safari, M.; Asadi, A.; Aryaeian, N.; Huseini, H.F.; Shidfar, F.; Jazayeri, S.; Malek, M.; Hosseini, A.F.; Hamidi, Z. The effects of melissa officinalis on depression and anxiety in type 2 diabetes patients with depression: A randomized double-blinded placebo-controlled clinical trial. BMC Complement. Med. Ther. 2023, 23, 140. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef]
- Arshad Ullah, M.; Hassan, A. Medicinal benefits of lemon balm (Melissa officinalis) for human health. World J. Chem. Pharm. Sci. 2022, 1, 028–033. [Google Scholar] [CrossRef]
- Alsahafi, T.; Bouback, T.; Albeshri, A.; Alnhhas, S.; Ali, M.; Moatasim, Y.; Kutkat, O.; Gaballah, M.; Alfasi, F.; Mater, E.H.; et al. Author Correction: Antiviral potential of Melissa officinalis extracts against influenza and emerging coronaviruses. Sci. Rep. 2025, 15, 20076. [Google Scholar] [CrossRef]
- Gutiérrez-Pacheco, M.M.; Torres-Moreno, H.; Flores-Lopez, M.L.; Velázquez Guadarrama, N.; Ayala-Zavala, J.F.; Ortega-Ramírez, L.A.; López-Romero, J.C. Mechanisms and Applications of Citral’s Antimicrobial Properties in Food Preservation and Pharmaceuticals Formulations. Antibiotics 2023, 12, 1608. [Google Scholar] [CrossRef]
- Li, Y.; Mei, J.; Xie, J. Citral: Bioactivity, Metabolism, Delivery Systems, and Food Preservation Applications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70168. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, D.; Xiang, Y.; Jiang, X.; Liu, J.; Bi, K.; Dong, X.; Wu, T.; Zhang, Y. Antifungal activity of essential oils and their potential synergistic effect with amphotericin B. Sci. Rep. 2024, 14, 31125. [Google Scholar] [CrossRef]
- Rokonuzzman, M.; Bhuia, M.S.; Al-Qaaneh, A.M.; El-Nashar, H.A.S.; Islam, T.; Chowdhury, R.; Hasan Shanto, H.; Al Hasan, M.S.; El-Shazly, M.; Torequl Islam, M. Biomedical Perspectives of Citronellal: Biological Activities, Toxicological Profile and Molecular Mechanisms. Chem. Biodivers. 2025, 22, e202401973. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wei, Y.; Yang, Z.; Zhang, L.; Shan, A. Synergism between nisin and citronellal against Fusarium graminearum and their application in maize preservation. Int. J. Food Microbiol. 2025, 441, 111331. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, H.; Krishnatreyya, H. Technological Advancements in Mosquito Repellents: Challenges and Opportunities in Plant-Based Repellents. Acta Parasitol. 2025, 70, 117. [Google Scholar] [CrossRef] [PubMed]
- Godad, A.; Sawant, R.; Pahelkar, A.R.; Pereira, G.; Sathaye, S. Exploring the Potential Mechanism of Action of Ursolic Acid for Parkinson’s Disease: An Integrative Network Pharmacology, Docking and Molecular Dynamics Study. Mol. Neurobiol. 2025, 62, 13636–13649. [Google Scholar] [CrossRef]
- Fajardo, J.B.; Vianna, M.H.; Ferreira, T.G.; de O.Lemos, A.S.; Souza, T.F.; Campos, L.M.; Paula, P.L.; Andrade, N.B.; Gamarano, L.R.; Queiroz, L.S.; et al. Enhanced Antitumor and Antibacterial Activities of Ursolic Acid through β-Cyclodextrin Inclusion Complexation. ACS Omega 2025, 10, 12906–12916. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, F.; Li, C.; Guo, L.; Chen, B.; Wang, F.; Liu, S.; Han, S. Ursolic acid improves growth performance, intestinal health, and antioxidant status in broilers by regulating lipid metabolism and the KEAP1-NRF2 pathway. J. Anim. Sci. 2025, 103, skaf333. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Günther, A.; Sip, S.; Bednarek-Rajewska, K.; Zalewski, P. Enhancing the Pharmacological Properties of Triterpenes Through Acetylation: An Anticancer and Antioxidant Perspective. Molecules 2025, 30, 2661. [Google Scholar] [CrossRef]
- Günther, A.; Bednarczyk-Cwynar, B. Oleanolic Acid: A Promising Antioxidant-Sources, Mechanisms of Action, Therapeutic Potential, and Enhancement of Bioactivity. Antioxidants 2025, 14, 598. [Google Scholar] [CrossRef]
- Qin, K.; Sun, X.; Liu, J.; Wang, R.; Huang, X.; Wang, Y.; Wang, H.; Yang, J.; Wang, S. A rosmarinic acid-fish skin protein-chitosan hybrid nano-delivery system with excellent sustained-release and antioxidant performances. Food Chem. 2025, 491, 145316. [Google Scholar] [CrossRef]
- Uçar-Ekin, C.; Aşır, F.; Şahin, F.; Kaplan, Ş. Rosmarinic acid alleviate hepatotoxicity induced by cyclophosphamide in rats. Cir. Cir. 2025, 93, 546–555. [Google Scholar] [CrossRef]
- Hassler, B.; Mohamed Hizam, V.; Pisani, A.; Sisto, R.; Paciello, F.; Grassi, C.; Fetoni, A.R. Targeting NLRP3 inflammasome activation in styrene-induced ototoxicity: Comparative efficacy of rosmarinic acid and anakinra in mitigating oxidative and inflammatory damage. Int. J. Audiol. 2025; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Al-Hunaiti, A.; Thiab, T.A.; Zihlif, M.; Abdul Majid, A.M.S.; Imraish, A.; Batarseh, Y.; Al Shhab, M. Rosmarinic acid attenuates doxorubicin-induced cardiotoxicity: Bio-nanocarrier system development and an in vitro study using H9c2 rat cardiomyocytes. Nanoscale Adv. 2025. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xu, Z.; Zhu, L.; Zhu, M.; Zhang, W.; Gong, M.; Liu, M.; Wang, M.; Xu, E.; Dai, L. Standardized aqueous extract of Abutilon theophrasti Medic. ameliorates oxidative stress and inflammatory responses against hydrochloric acid/ethanol-induced gastric ulcer in rats. Front. Pharmacol. 2025, 16, 1599810. [Google Scholar] [CrossRef] [PubMed]
- Dinh-Hung, N.; Khang, L.T.P.; Wisetkaeo, S.; Tran, N.T.; Po-Tsang, L.; Brown, C.L.; Sangsawad, P.; Dwinanti, S.H.; Permpoonpattana, P.; Linh, N.V. Caffeic Acid as a Promising Natural Feed Additive: Advancing Sustainable Aquaculture. Biology 2025, 14, 1160. [Google Scholar] [CrossRef]
- Draginic, N.D.; Jakovljevic, V.L.; Jeremic, J.N.; Srejovic, I.M.; Andjic, M.M.; Rankovic, M.R.; Sretenovic, J.Z.; Zivkovic, V.I.; Ljujic, B.T.; Mitrovic, S.L.; et al. Melissa officinalis L. Supplementation Provides Cardioprotection in a Rat Model of Experimental Autoimmune Myocarditis. Oxidative Med. Cell. Longev. 2022, 2022, 1344946. [Google Scholar] [CrossRef]
- Gan, X.; Li, J.; Li, S.; Wang, X.; Wang, Q.; Chen, X.; Huang, Y.; Nie, M.; Kang, H.; Dai, H. Integrating superlubricative nanomaterials with precision drug delivery for advanced osteoarthritis therapy. Mater. Today Bio 2025, 35, 102359. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Coleman, D.T.; Bigelow, R.; Cardelli, J.A. Inhibition of fatty acid synthase by luteolin post-transcriptionally down-regulates c-Met expression independent of proteosomal/lysosomal degradation. Mol. Cancer Ther. 2009, 8, 214–224. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; Yu, T.; Song, R.; Nie, H.; Ding, Y. Anti-Oxidant, Anti-Inflammatory and Antiviral Properties of Luteolin Against SARS-CoV-2: Based on Network Pharmacology. Pharmaceuticals 2025, 18, 1329. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Sisti, D.; Rocchi, M.; Belli, A.; Bertuccioli, A.; Cazzaniga, M.; Palazzi, C.M.; Tanda, M.L.; Zerbinati, N. Effects of Melissa officinalis Phytosome on Sleep Quality: Results of a Prospective, Double-Blind, Placebo-Controlled, and Cross-Over Study. Nutrients 2024, 16, 4199. [Google Scholar] [CrossRef] [PubMed]
- Abbasnia, V.; Foadoddini, M.; Khazdair, M.R. Protective Effect of Melissa officinalis (Lemon Balm) Extract on Cytokine Levels and Oxidative Stress in Ovalbumin Induced Lung Toxicity in Rats. Basic. Clin. Pharmacol. Toxicol. 2025, 137, e70068. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ, Ü.; Macar, O.; Kalefetoğlu Macar, T.; Yalçın, E.; Çavuşoğlu, K. Effect of Melissa officinalis L. leaf extract on manganese-induced cyto-genotoxicity on Allium cepa L. Sci. Rep. 2023, 13, 22110. [Google Scholar] [CrossRef]
- Borgonetti, V.; Pressi, G.; Bertaiola, O.; Guarnerio, C.; Mandrone, M.; Chiocchio, I.; Galeotti, N. Attenuation of neuroinflammation in microglia cells by extracts with high content of rosmarinic acid from in vitro cultured Melissa officinalis L. cells. J. Pharm. Biomed. Anal. 2022, 220, 114969. [Google Scholar] [CrossRef]
- Tokatly Latzer, I.; Pearl, P.L. Update on inherited disorders of GABA metabolism. Eur. J. Paediatr. Neurol. 2025, 56, 10–16. [Google Scholar] [CrossRef]
- Anheyer, M.; Cramer, H.; Ostermann, T.; Längler, A.; Anheyer, D. Herbal Medicine for Treating Herpes Labialis: A Systematic Review. J. Integr. Complement. Med. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Thapa, K.; Kanojia, N.; Khan, H.; Kumar, J. Unveiling the anti-cancer potential of Melissa officinalis. Pharmacol. Res. Nat. Prod. 2025, 9, 100384. [Google Scholar] [CrossRef]
- Silva, B.; Cadavez, V.; Caleja, C.; Pereira, E.; Calhelha, R.; Añibarro Ortega, M.; Finimundy, T.; Kostic, M.; Soković, M.; Teixeira, J.; et al. Phytochemical Composition and Bioactive Potential of Melissa officinalis L., Salvia officinalis L. and Mentha spicata L. Extracts. Foods 2023, 12, 947. [Google Scholar] [CrossRef]
- Mabotja, M.B.; Aremu, A.O.; Doležal, K.; Ruzvidzo, O.; Amoo, S.O. Enhancing in vitro propagation of Melissa officinalis L.: Assessing the role of plant growth regulators as a pathway to sustainable production. Ind. Crops Prod. 2025, 233, 121377. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Analysis of Naturally Occurring Phenolic Compounds in Aromatic Plants by RP-HPLC Coupled to Diode Array Detector (DAD) and GC-MS after Silylation. Foods 2013, 2, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Taherianrad, F.; Dehghan, H.; Abbasabadi, N.; Padash, A.; Tehrani, H.J.; Tat, M.; Dayani, A.; Salimi, A. Melissa officinalis extract nanoemulsion, Caffeic acid and Quercetin as a novel inducer for investigating neural differentiation of human Wharton’s jelly mesenchymal stem cells. Tissue Cell 2025, 95, 102815. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, C.; Hiemstra, I.S.A.; Kazbar, A.; Costantino, G.; Righetti, L. Towards eco-metabolomics: NADES-guided extraction enables semi-quantitative metabolomics for Melissa officinalis. Adv. Sample Prep. 2025, 13, 100154. [Google Scholar] [CrossRef]
- Draginic, N.; Jakovljevic, V.; Andjic, M.; Jeremic, J.; Srejovic, I.; Rankovic, M.; Tomovic, M.; Nikolic Turnic, T.; Svistunov, A.; Bolevich, S.; et al. Melissa officinalis L. as a Nutritional Strategy for Cardioprotection. Front. Physiol. 2021, 12, 661778. [Google Scholar] [CrossRef]
- Posłuszny, M.A.; Chłopecka-Słomińska, M.; Cherer, S.S.; Cisse, S.; Benarbia, M.E.A.; Mendel, M. Verification of the Utility of the Standardized Melissa officinalis Extract to Control Gut Contractility in Sheep-Ex Vivo Study. Animals 2025, 15, 626. [Google Scholar] [CrossRef]
- Catalano, L.; Sagliano, L.; Visciglio, A.; Russo, P.; Miniello, S.; Trojano, L.; Panico, F. An integrated multifocal tDCS-EEG protocol for reducing cognitive and affective symptoms in mild cognitive impairment and early stages of dementia: A crossover double-blind randomized controlled trial. Front. Neurol. 2025, 16, 1605970. [Google Scholar] [CrossRef]
- Gavarić, N.; Radovanović, K.; Kladar, N.; Hitl, M.; Čonić, B.S.; Jovin, V.M.; Samojlik, I. Can we use Melissa officinalis (lemon balm) postdistillation waste extracts in pharmacy? In vivo pharmacodynamic studies. S. Afr. J. Bot. 2024, 172, 396–406. [Google Scholar] [CrossRef]
- Srinivas, N.S.; Vimalan, V.; Padmanabhan, P.; Gulyás, B. An Overview on Cognitive Function Enhancement through Physical Exercises. Brain Sci. 2021, 11, 1289. [Google Scholar] [CrossRef]
- Parker, T.D.; Hain, J.A.; Rooney, E.J.; Zimmerman, K.A.; Lee, Y.; Del Giovane, M.; Graham, N.S.N.; Patel, M.; Hampshire, A.; Wilson, M.G.; et al. Brain health concerns in former rugby players: Clinical and cognitive phenotypes. Brain 2025, 148, 2698–2713. [Google Scholar] [CrossRef]
- Lobach, A.R.; Schmidt, F.; Fedrizzi, D.; Müller, S. Toxicological safety evaluation of an aqueous lemon balm (Melissa officinalis) extract. Food Chem. Toxicol. 2024, 187, 114565. [Google Scholar] [CrossRef]
- Agnello, L.; Ciaccio, M. Neurodegenerative Diseases: From Molecular Basis to Therapy. Int. J. Mol. Sci. 2022, 23, 12854. [Google Scholar] [CrossRef]
- Souza-Talarico, J.N.; Perkhounkova, Y.; Hein, M.; Lee, J.; Hefti, M.; Sindi, S. Allostatic load dynamics, Alzheimer’s disease biomarkers, and progression in individuals with mild cognitive impairment: Findings from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 2025, 17, e70140. [Google Scholar] [CrossRef] [PubMed]
- Temple, S. Advancing cell therapy for neurodegenerative diseases. Cell Stem Cell 2023, 30, 512–529. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Pan, G.; Zhang, M.; He, X.; Yao, M.; Yang, Y. The relationship between ageism, loneliness, and anxiety in widowed older adults: Cognitive function as a moderator in the mediated model. Front. Psychol. 2025, 16, 1624197. [Google Scholar] [CrossRef] [PubMed]
- Rommer, P.S.; Fuchs, D.; Leblhuber, F.; Schroth, R.; Greilberger, M.; Tafeit, E.; Greilberger, J. Lowered Levels of Carbonyl Proteins after Vitamin B Supplementation in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Neurodegener. Dis. 2016, 16, 284–289. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals 2025, 18, 133. [Google Scholar] [CrossRef]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Liu, X.; Baxley, S.; Hebron, M.; Turner, R.S.; Moussa, C. Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 5044. [Google Scholar] [CrossRef]
- Ohnishi, A.; Senda, M.; Yamane, T.; Sasaki, M.; Mikami, T.; Nishio, T.; Ikari, Y.; Nishida, H.; Shukuri, M.; Takashima, T.; et al. Human whole-body biodistribution and dosimetry of a new PET tracer, [(11)C]ketoprofen methyl ester, for imagings of neuroinflammation. Nucl. Med. Biol. 2014, 41, 594–599. [Google Scholar] [CrossRef]
- Moon, S.; Sarmento, C.V.M.; Smirnova, I.V.; Colgrove, Y.; Lai, S.M.; Lyons, K.E.; Liu, W. A pilot randomized clinical trial examining the effects of Qigong on inflammatory status and sleep quality in people with Parkinson’s disease. J. Bodyw. Mov. Ther. 2024, 40, 1002–1007. [Google Scholar] [CrossRef]
- Qin, H.; Hussain, L.; Liu, Z.; Yan, X.; Awwad, F.A.; Butt, F.M.; Salaria, U.A.; Ismail, E.A.A. Optimizing deep learning models to combat amyotrophic lateral sclerosis (ALS) disease progression. Digit. Health 2025, 11, 20552076251349719. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, C.H.; Ikawa, M.; Liow, J.S.; Zoghbi, S.S.; Morse, C.L.; Pike, V.W.; Fujita, M.; Innis, R.B.; Kreisl, W.C. Cerebellum Can Serve As a Pseudo-Reference Region in Alzheimer Disease to Detect Neuroinflammation Measured with PET Radioligand Binding to Translocator Protein. J. Nucl. Med. 2015, 56, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- De Wel, B.; Mobach, T.; Pfeffer, G.; Jewett, G. Tofersen treatment normalizes neurofilament levels in autosomal recessive SOD1 Amyotrophic Lateral Sclerosis. Can. J. Neurol. Sci. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Dodd, G.T. Hypothalamic neuronal-glial crosstalk in metabolic disease. NPJ Metab. Health Dis. 2024, 2, 27. [Google Scholar] [CrossRef]
- Fu, X.; Cai, H.; Quan, S.; Ren, Z.; Xu, Y.; Jia, L. Immune cells in Alzheimer’s disease: Insights into pathogenesis and potential therapeutic targets. Med. Rev. 2025, 5, 179–202. [Google Scholar] [CrossRef]
- Rohden, F.; Ferreira, P.C.L.; Bellaver, B.; Ferrari-Souza, J.P.; Aguzzoli, C.S.; Soares, C.; Abbas, S.; Zalzale, H.; Povala, G.; Lussier, F.Z.; et al. Glial reactivity correlates with synaptic dysfunction across aging and Alzheimer’s disease. Nat. Commun. 2025, 16, 5653. [Google Scholar] [CrossRef]
- Arnold, D.L.; Elliott, C.; Martin, E.C.; Hyvert, Y.; Tomic, D.; Montalban, X. Effect of Evobrutinib on Slowly Expanding Lesion Volume in Relapsing Multiple Sclerosis: A Post Hoc Analysis of a Phase 2 Trial. Neurology 2024, 102, e208058. [Google Scholar] [CrossRef]
- Koch, M.W.; Kaur, S.; Sage, K.; Kim, J.; Levesque-Roy, M.; Cerchiaro, G.; Yong, V.W.; Cutter, G.R.; Metz, L.M. Hydroxychloroquine for Primary Progressive Multiple Sclerosis. Ann. Neurol. 2021, 90, 940–948. [Google Scholar] [CrossRef]
- Xiao, M.; Gao, G.; Mu, J.; Sun, Q.; Zhao, Y.; Fan, X. MLKL Modulates Necroptosis and Neuroinflammation in a Mouse Model of MS. Inflammation, 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, A.M.; Hu, T.; Yang, J.; Wang, M.; Xu, Y.; Cao, W.Y. Peripheral administration of the NAT10 inhibitor Remodelin prevents against Lipopolysaccharide induced depression in male mice. Eur. J. Pharmacol. 2025, 1003, 177884. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Kinuthia, U.M.; Möhle, C.; Adams, R.H.; Langmann, T. Immunomodulation of inflammatory responses preserves retinal integrity in murine models of pericyte-depletion retinopathy. JCI Insight 2025, 10, e184465. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, O.; Seghier, M.L. The validity of studying healthy aging with cognitive tests measuring different constructs. Sci. Rep. 2024, 14, 23880. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.Y.; Liang, J.; Chen, Y.J.; Wu, C.C.; Shyu, Y.L. Influences of social support following hip-fracture surgery for older adults with cognitive impairment and their family caregivers. BMC Geriatr. 2025, 25, 469. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Baker, L.D.; Carrillo, M.C.; Snyder, H.M.; Cleveland, M.L.; Gitelman, D.R.; Kivipelto, M.; Leng, X.I.; Lovato, L.; Papp, K.V.; et al. Baseline characteristics of the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER): Successful enrollment of a diverse clinical trial cohort at risk for cognitive decline. Alzheimers Dement. 2025, 21, e70351. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- Alcalá-Lozano, R.; Carmona-Hernández, R.; Ocampo-Romero, A.G.; Sosa-Millán, A.L.; Morelos-Santana, E.D.; Abarca, D.Z.; Castro-de-Aquino, D.V.; Cabrera-Muñoz, E.A.; Ramírez-Rodríguez, G.B.; Sosa Ortiz, A.L.; et al. Predicting the Beneficial Effects of Cognitive Stimulation and Transcranial Direct Current Stimulation in Amnestic Mild Cognitive Impairment with Clinical, Inflammation, and Human Microglia Exposed to Serum as Potential Markers: A Double-Blind Placebo-Controlled Randomized Clinical Trial. Int. J. Mol. Sci. 2025, 26, 1754. [Google Scholar] [CrossRef]
- Lavisse, S.; Goutal, S.; Wimberley, C.; Tonietto, M.; Bottlaender, M.; Gervais, P.; Kuhnast, B.; Peyronneau, M.A.; Barret, O.; Lagarde, J.; et al. Increased microglial activation in patients with Parkinson disease using [(18)F]-DPA714 TSPO PET imaging. Park. Relat. Disord. 2021, 82, 29–36. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Akimbekov, N.S.; Grant, W.B.; Dean, C.; Fang, X.; Razzaque, M.S. Neuroprotective effects of magnesium: Implications for neuroinflammation and cognitive decline. Front. Endocrinol. 2024, 15, 1406455. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in neurodegenerative disease—A double-edged sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Franklin, E.E.; Li, Y.; Joseph-Mathurin, N.; Burns, A.L.; Hobbs, D.A.; McCullough, A.A.; Schultz, S.A.; Xiong, C.; Wang, G.; et al. Immunohistochemical evaluation of a trial of gantenerumab or solanezumab in dominantly inherited Alzheimer disease. Acta Neuropathol. 2025, 149, 57. [Google Scholar] [CrossRef]
- Dubbelman, M.A.; Liu, A.; Donohue, M.C.; Langford, O.; Raman, R.; Rentz, D.M.; Amariglio, R.; Sperling, R.A.; Aisen, P.S.; Marshall, G.A. Changes in Daily Functioning in Association With Tau and Amyloid Among Unimpaired Older Adults With and Without Elevated Amyloid. Neurology 2025, 104, e213775. [Google Scholar] [CrossRef]
- Safety and efficacy of trehalose in amyotrophic lateral sclerosis (HEALEY ALS Platform Trial): An adaptive, phase 2/3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2025, 24, 500–511. [CrossRef]
- Fleisher, A.S.; Munsie, L.M.; Perahia, D.G.S.; Andersen, S.W.; Higgins, I.A.; Hauck, P.M.; Lo, A.C.; Sims, J.R.; Brys, M.; Mintun, M. Assessment of Efficacy and Safety of Zagotenemab: Results From PERISCOPE-ALZ, a Phase 2 Study in Early Symptomatic Alzheimer Disease. Neurology 2024, 102, e208061. [Google Scholar] [CrossRef]
- Risen, S.J.; Boland, S.W.; Sharma, S.; Weisman, G.M.; Shirley, P.M.; Latham, A.S.; Hay, A.J.D.; Gilberto, V.S.; Hines, A.D.; Brindley, S.; et al. Targeting Neuroinflammation by Pharmacologic Downregulation of Inflammatory Pathways Is Neuroprotective in Protein Misfolding Disorders. ACS Chem. Neurosci. 2024, 15, 1533–1547. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, Z.; Zheng, J.; Deng, C.; Liu, D.; Chen, Y.; Zhou, R.; Zou, J.; Huang, G.; Zeng, Q.; et al. Electroacupuncture Attenuates High-Fat Diet-Exacerbated Alzheimer’s Pathology by Enhancing TFEB/TFE3-Mediated Autophagic Clearance of Tau and NLRP3 Inflammasome in 3xTg Mice. CNS Neurosci. Ther. 2025, 31, e70497. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Behrouz, V.; Zahroodi, M.; Clark, C.C.T.; Mir, E.; Atashi, N.; Rivaz, R. Effects of Garlic Supplementation on Cardiovascular Risk Factors in Adults: A Comprehensive Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. Oxidative damage in neurodegeneration: Roles in the pathogenesis and progression of Alzheimer disease. Physiol. Rev. 2023, 104, 103–197. [Google Scholar] [CrossRef]
- De Falco, A.; Cukierman, D.; Hauser-Davis, R.; Rey, N.A. Alzheimer’s disease: Etiological hypotheses and treatment perspectives. Química Nova 2015, 39. [Google Scholar] [CrossRef]
- Qin, Y.; Qiu, Y.; Zhong, J.; Ouyang, Z.; Jin, L.; Wu, H.; Zeng, Y. Neuroprotective Effects of Anisodine Hydromide in a Rat Model of Vascular Dementia and the Antioxidative Stress Mechanisms Involved. Sichuan Da Xue Xue Bao Yi Xue Ban 2025, 56, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Mulè, S.; Ferrari, S.; Rosso, G.; Galla, R.; Battaglia, S.; Curti, V.; Molinari, C.; Uberti, F. The Combined Effect of Green Tea, Saffron, Resveratrol, and Citicoline against Neurodegeneration Induced by Oxidative Stress in an In Vitro Model of Cognitive Decline. Oxidative Med. Cell. Longev. 2024, 2024, 7465045. [Google Scholar] [CrossRef] [PubMed]
- Piñar-Morales, R.; Durán, R.; Bautista-García, A.; García-Mansilla, M.J.; Aliaga-Gaspar, P.; Vives-Montero, F.; Barrero-Hernández, F.J. The impact of oxidative stress on symptoms associated with multiple sclerosis. Sci. Rep. 2025, 15, 22983. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, A.R.; Lee, J.Y.; Kim, H.S.; Yang, C.; Kim, J.K.; Go, Y.; Jung, I.C. Herbal Medicine for Patients with Cognitive Impairment: An Observational Study. Neuropsychiatr. Dis. Treat. 2021, 17, 3183–3194. [Google Scholar] [CrossRef]
- Kim, C.; Lee, Y.; Kang, S.G.; Lee, S.H. Effectiveness of Information and Communication Technology-Based Cognitive Behavioral Therapy Using the Smart Sleep App on Insomnia in Older Adults: Randomized Controlled Trial. J. Med. Internet Res. 2025, 27, e67751. [Google Scholar] [CrossRef]
- Shi, L.; Chen, S.J.; Ma, M.Y.; Bao, Y.P.; Han, Y.; Wang, Y.M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep. Med. Rev. 2018, 40, 4–16. [Google Scholar] [CrossRef]
- Pavlova, M.K.; Latreille, V. Sleep Disorders. Am. J. Med. 2019, 132, 292–299. [Google Scholar] [CrossRef]
- Kim, J.; Ben-Umeh, K.C.; Weir, R.; Manotas, K.; Kleinschmit, K.; Fischer, A.; Weir, P.; Wilson, F. Evaluating the risk of sleep disorders in subjects with a prior COVID-19 infection. PLoS ONE 2024, 19, e0311929. [Google Scholar] [CrossRef]
- Te, T.T.; Boland, M.R.; Ghadimi, S.; Dzierzewski, J.M.; Alessi, C.; Martin, J.L.; Kremen, S.; Bui, A.A.T.; Naeim, A.; Fung, C.H. Predicting subjective sleepiness during auditory cognitive testing using voice signaling analysis. Sleep. Sci. Pract. 2025, 9, 19. [Google Scholar] [CrossRef]
- Rowe, R.K.; Schulz, P.; He, P.; Mannino, G.S.; Opp, M.R.; Sierks, M.R. Acute sleep deprivation in mice generates protein pathology consistent with neurodegenerative diseases. Front. Neurosci. 2024, 18, 1436966. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Xiao, L.D.; Guo, X.; Hu, Y.; Wang, N.; Wang, Y. The impact of social support and social constraints on sleep disturbances in patients with lung cancer undergoing chemotherapy: Serial mediators of sleep cognition and anxiety-depression. Asia Pac. J. Oncol. Nurs. 2025, 12, 100740. [Google Scholar] [CrossRef] [PubMed]
- Versace, S.; Pellitteri, G.; Sperotto, R.; Tartaglia, S.; Da Porto, A.; Catena, C.; Gigli, G.L.; Cavarape, A.; Valente, M. A State-of-Art Review of the Vicious Circle of Sleep Disorders, Diabetes and Neurodegeneration Involving Metabolism and Microbiota Alterations. Int. J. Mol. Sci. 2023, 24, 10615. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Xu, Y.; Hu, L.; Wei, Y.; Wang, Z. Machine learning-based brain magnetic resonance imaging radiomics for identifying rapid eye movement sleep behavior disorder in Parkinson’s disease patients. BMC Med. Imaging 2025, 25, 227. [Google Scholar] [CrossRef]
- Seiger, A.N.; Penzel, T.; Fietze, I. Chronic pain management and sleep disorders. Cell Rep. Med. 2024, 5, 101761. [Google Scholar] [CrossRef]
- Qiu, Y.; Duan, X.; Zhang, Z.; Zhao, L.; Yuan, Q.; Wang, M.; Xiao, S.; Sun, L. Sleep disturbances and language function impairment in the elderly: Evidence of limbic and prefrontal tracts involvement. J. Alzheimer’s Dis. JAD 2025, 106, 1261–1271. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wake, G.; Savelev, S.; Tildesley, N.T.J.; Perry, E.K.; Wesnes, K.A.; Scholey, A.B. Modulation of Mood and Cognitive Performance Following Acute Administration of Single Doses of Melissa Officinalis (Lemon Balm) with Human CNS Nicotinic and Muscarinic Receptor-Binding Properties. Neuropsychopharmacology 2003, 28, 1871–1881. [Google Scholar] [CrossRef]
- Watson, K.; Hatcher, D.; Good, A. A randomised controlled trial of Lavender (Lavandula Angustifolia) and Lemon Balm (Melissa Officinalis) essential oils for the treatment of agitated behaviour in older people with and without dementia. Complement. Ther. Med. 2019, 42, 366–373. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Nagai, T.; Kobayashi, S.; Komatsu, J.; Samuraki-Yokohama, M.; Iwasa, K.; Yokoyama, K.; Nakamura, H.; et al. Safety and efficacy of Melissa officinalis extract containing rosmarinic acid in the prevention of Alzheimer’s disease progression. Sci. Rep. 2020, 10, 18627. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Hamaguchi, T.; Sakai, K.; Komatsu, J.; Iwasa, K.; Horimoto, M.; Nakamura, H.; Yamada, M.; Ono, K. Effects of Melissa officinalis Extract Containing Rosmarinic Acid on Cognition in Older Adults Without Dementia: A Randomized Controlled Trial. J. Alzheimers Dis. 2023, 91, 805–814. [Google Scholar] [CrossRef]
- Taavoni, S.; Nazem Ekbatani, N.; Haghani, H. Valerian/lemon balm use for sleep disorders during menopause. Complement. Ther. Clin. Pract. 2013, 19, 193–196. [Google Scholar] [CrossRef]
- Haybar, H.; Javid, A.; Haghighizadeh, M.H.; Valizadeh, E.; Mohaghegh, S.M.; Mohammadzadeh, A. The effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable angina. Clin. Nutr. ESPEN 2018, 26, 47–52. [Google Scholar] [CrossRef]
- Shirazi, M.; Jalalian, M.N.; Abed, M.; Ghaemi, M. The Effectiveness of Melissa Officinalis L. versus Citalopram on Quality of Life of Menopausal Women with Sleep Disorder: A Randomized Double-Blind Clinical Trial. Rev. Bras. Ginecol. Obs. 2021, 43, 126–130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).