Distinct Gut–Brain Axis Dysregulation in Episodic Versus Chronic Migraine: Insights from NTG-Induced Mouse Models
Abstract
1. Introduction
2. Results
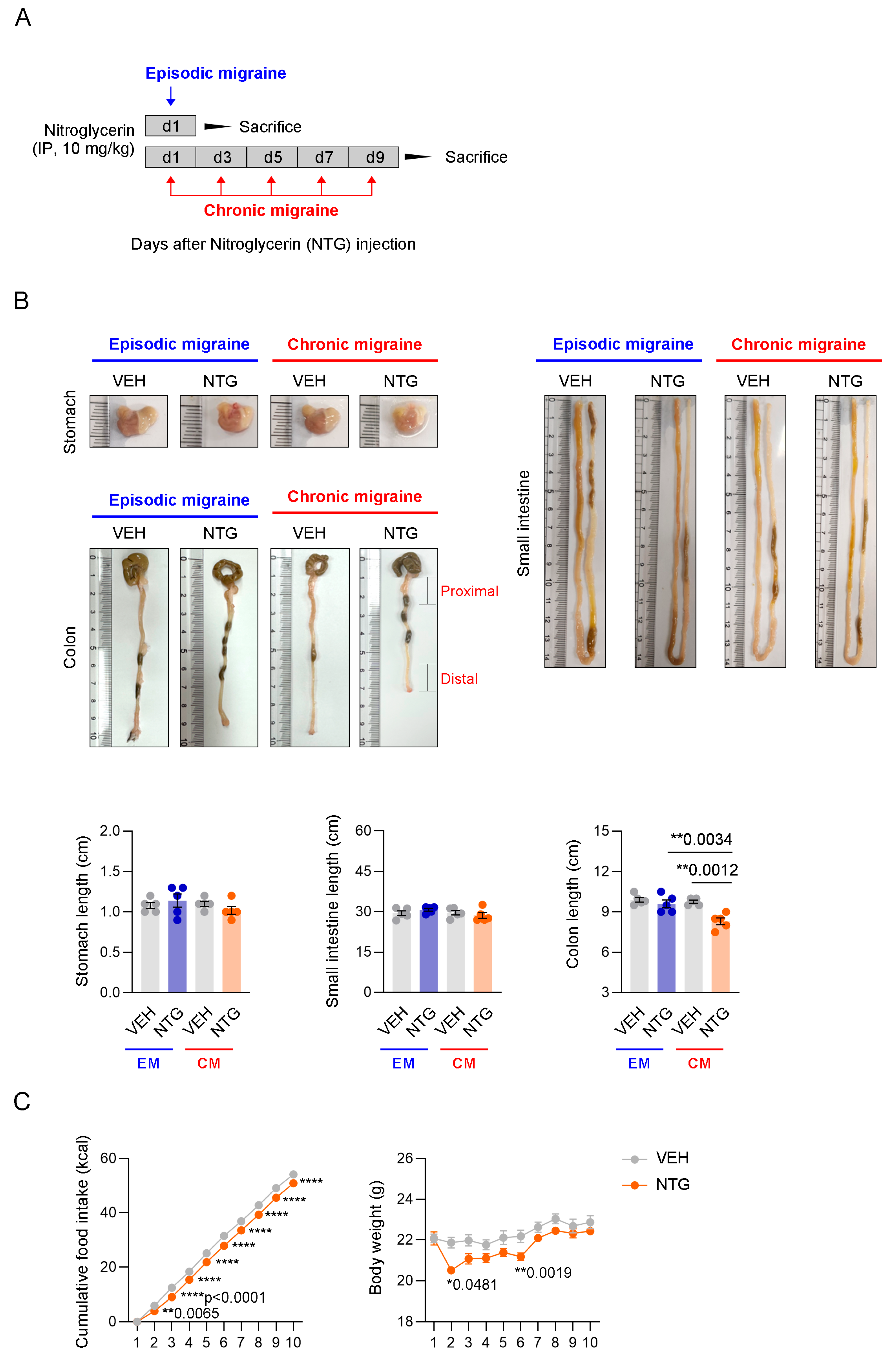
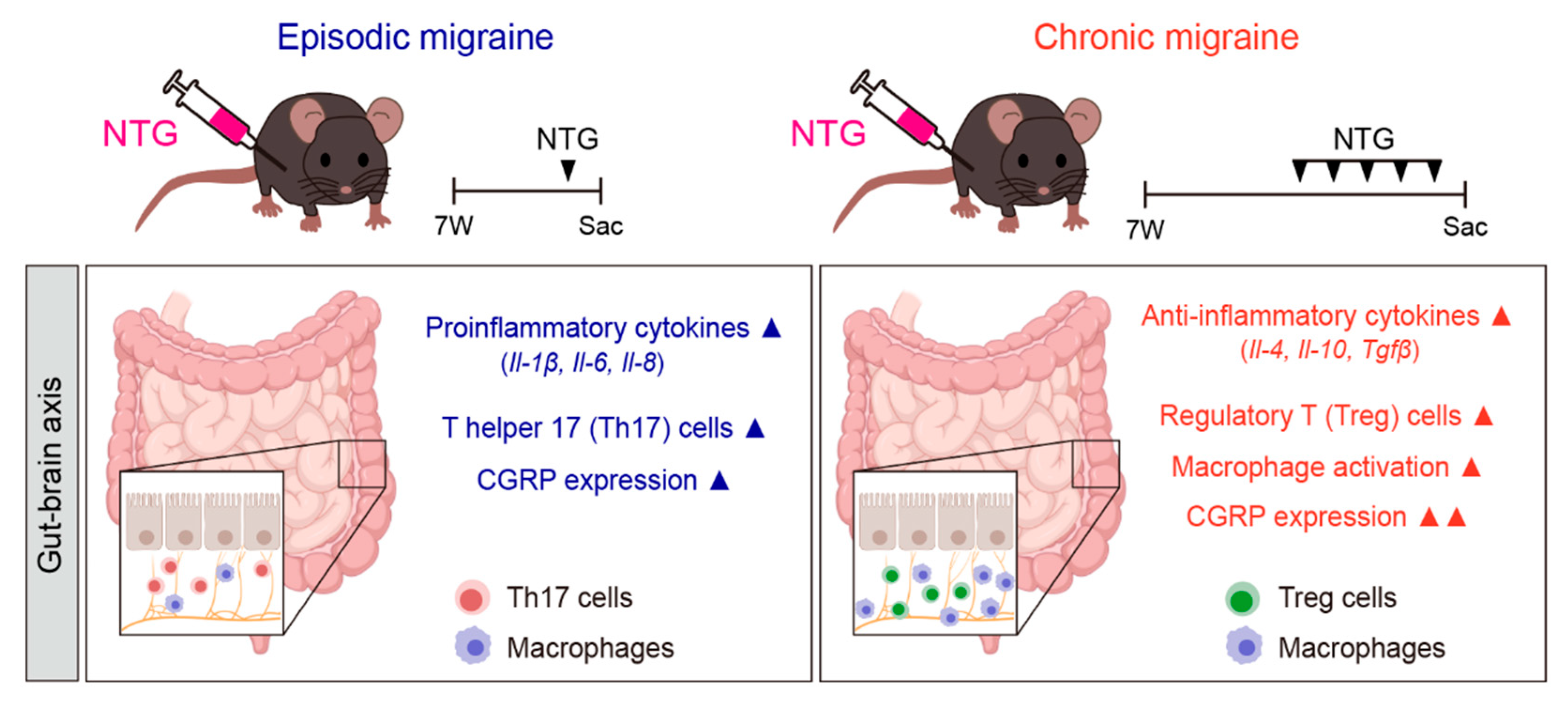
2.1. Changes in the GI Tract in NTG-Induced EM and CM Models
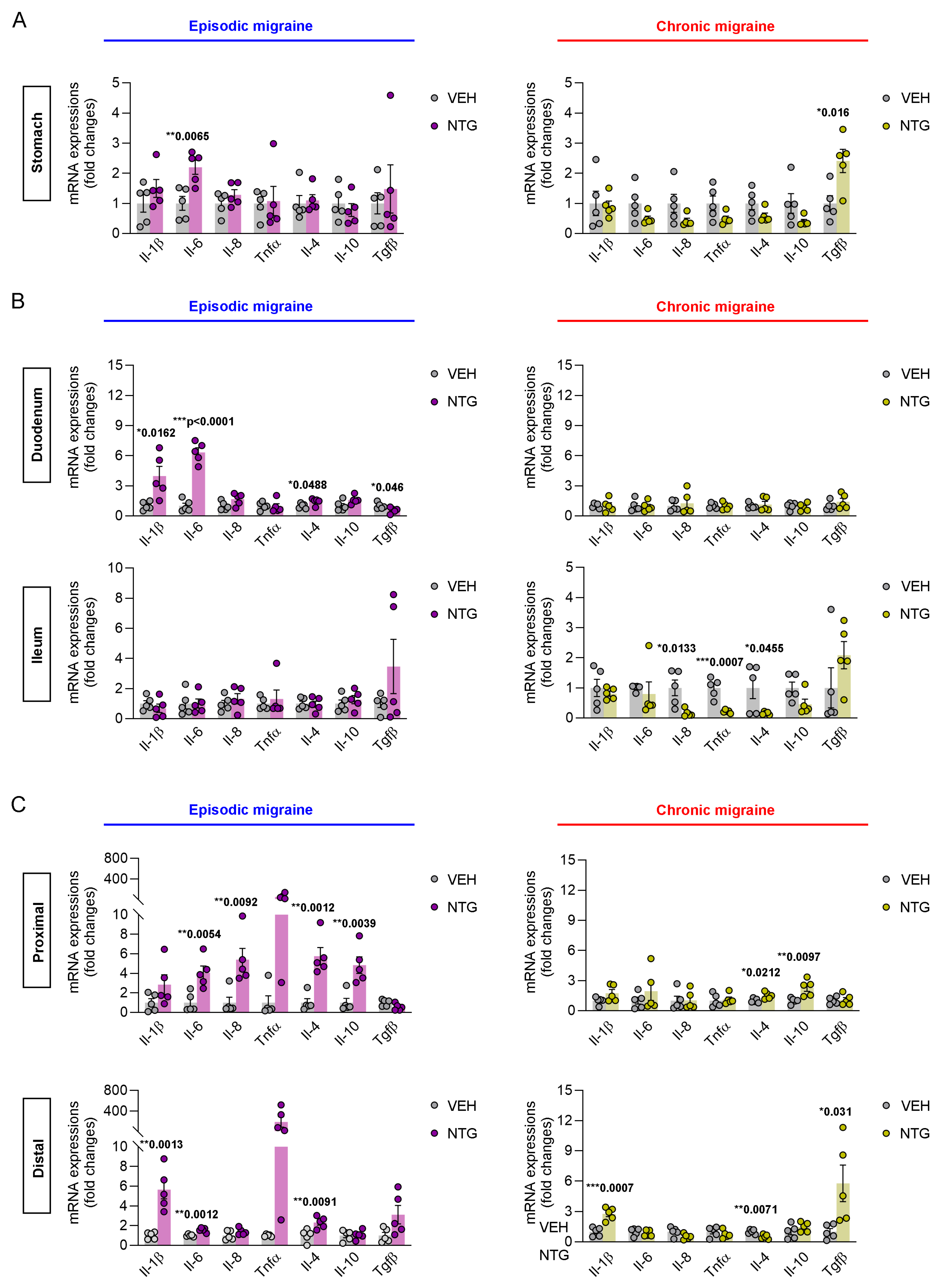
2.2. Differences in Inflammatory Cytokines in the GI Tract Between NTG-Induced EM and CM Models
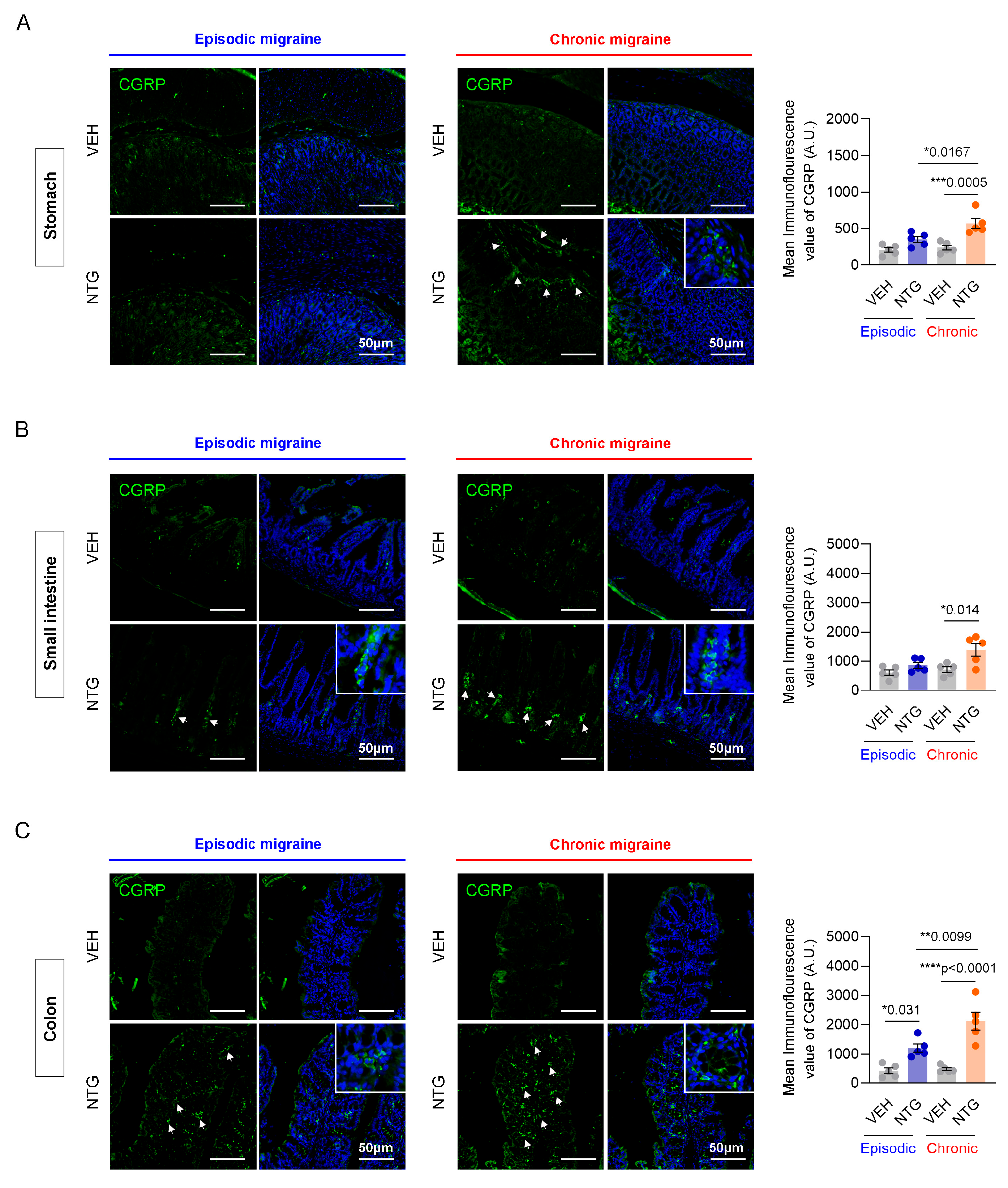
2.3. Differences in CGRP Expression in the GI Tract Between NTG-Induced EM and CM Models
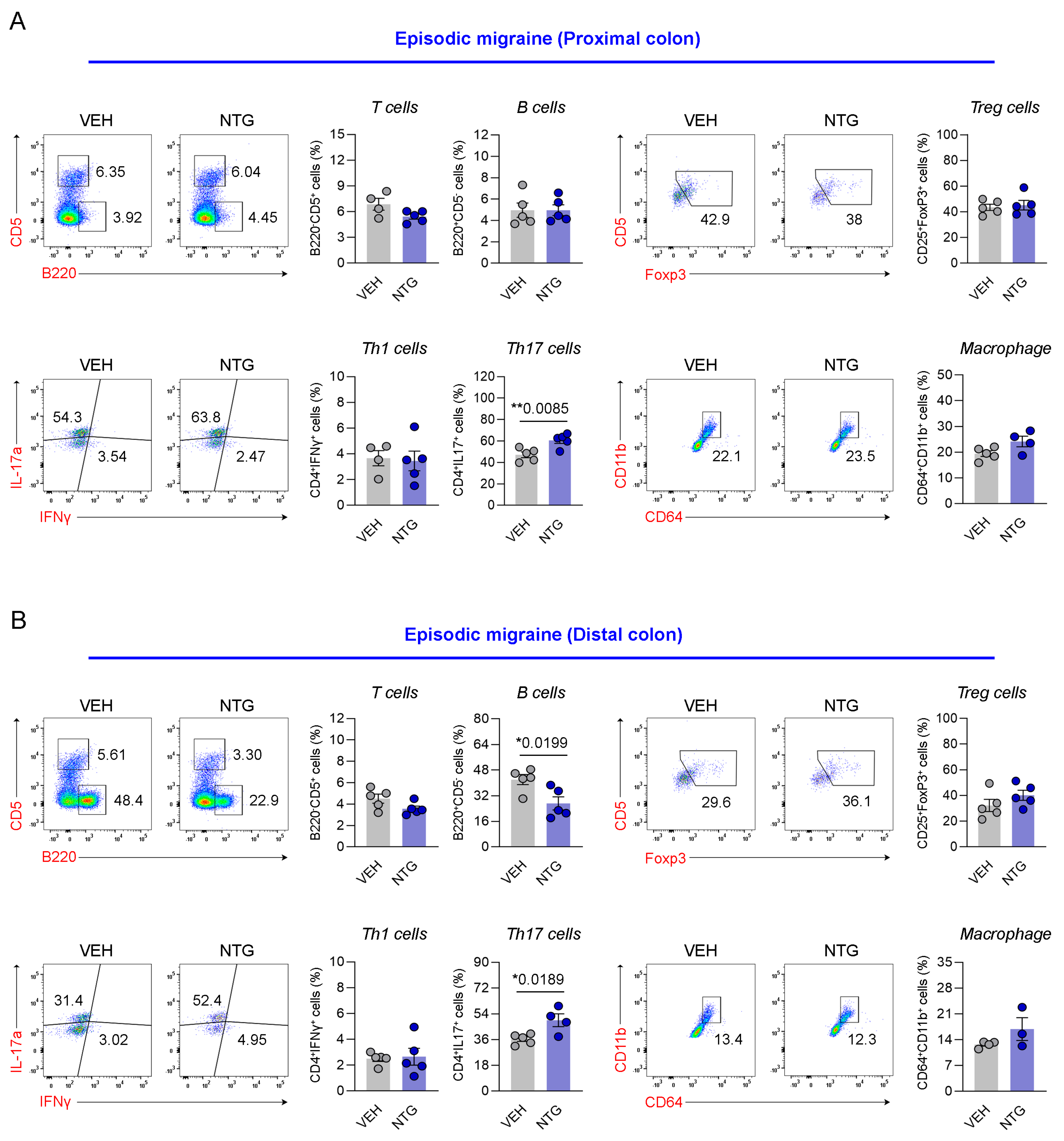
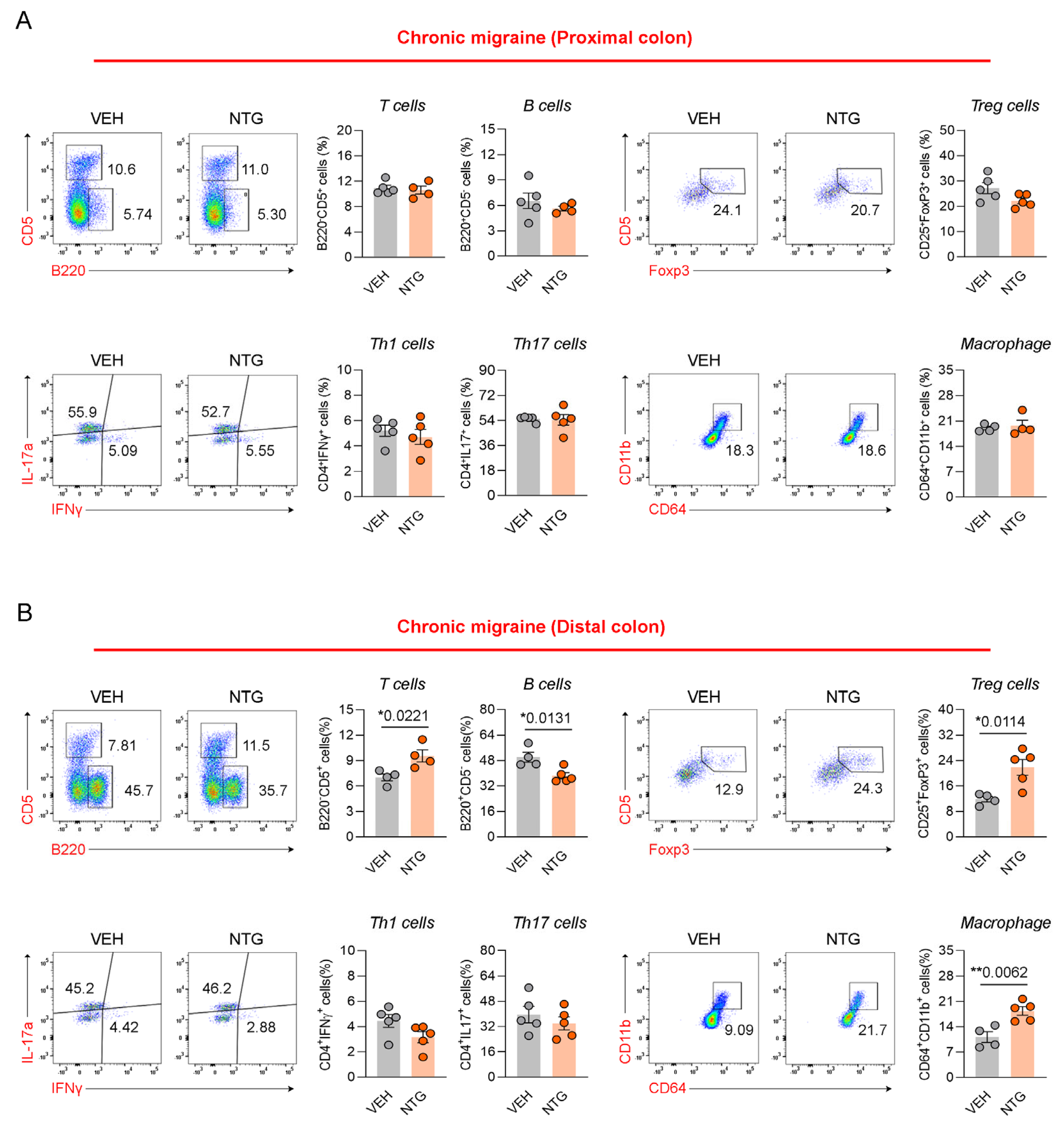
2.4. Differences in Immune Cell Responses in the Colon Between NTG-Induced EM and CM Models
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. NTG-Induced Migraine Model
4.3. Animals Were Divided into Four Groups Based on the Experimental Conditions
4.4. Food Intake and Body Weight Measurement
4.5. Tissue Collection
4.6. Immunohistochemical Staining
4.7. cDNA Synthesis
4.8. Real-Time Quantitative Reverse Transcription PCR
4.9. Cell Preparation
4.10. Flow Cytometric Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NTG | Nitroglycerin; |
| EM | Episodic migraine; |
| CM | Chronic migraine; |
| CGRP | Calcitonin gene-related peptide; |
| GI | Gastrointestinal; |
| TNF-α | Tumor necrosis factor-alpha; |
| TGF-β | Transforming growth factor beta; |
| IL-1β | Interleukin-1β; |
| IL-6 | Interleukin-6; |
| IL-8 | Interleukin-8; |
| IL-4 | Interleukin-4; |
| IL-10 | Interleukin-10; |
| FACS | Fluorescence-activated cell sorting; |
| PFA | Paraformaldehyde; |
| Th1 | Type 1 T helper cell; |
| Th17 | Type 17 T helper cell; |
| Treg | Regulatory T cell. |
References
- He, J.; Zhou, M.; Zhao, F.; Cheng, H.; Huang, H.; Xu, X.; Han, J.; Hong, W.; Wang, F.; Xiao, Y.; et al. FGF-21 and GDF-15 are increased in migraine and associated with the severity of migraine-related disability. J. Headache Pain 2023, 24, 28. [Google Scholar] [CrossRef]
- Amiri, P.; Kazeminasab, S.; Nejadghaderi, S.A.; Mohammadinasab, R.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.A.; Safiri, S. Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front. Neurol. 2021, 12, 800605. [Google Scholar] [CrossRef]
- Fan, L.; Wu, Y.; Wei, J.; Xia, F.; Cai, Y.; Zhang, S.; Miao, J.; Zhou, Y.; Liu, C.; Yan, W.; et al. Global, regional, and national time trends in incidence for migraine, from 1990 to 2019: An age-period-cohort analysis for the GBD 2019. J. Headache Pain 2023, 24, 79. [Google Scholar] [CrossRef] [PubMed]
- Imai, N.; Matsumori, Y. Different effects of migraine associated features on headache impact, pain intensity, and psychiatric conditions in patients with migraine. Sci. Rep. 2024, 14, 22611. [Google Scholar] [CrossRef]
- Khan, J.; Asoom, L.I.A.; Sunni, A.A.; Rafique, N.; Latif, R.; Saif, S.A.; Almandil, N.B.; Almohazey, D.; AbdulAzeez, S.; Borgio, J.F. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed. Pharmacother. 2021, 139, 111557. [Google Scholar] [CrossRef] [PubMed]
- Ples, H.; Florian, I.A.; Timis, T.L.; Covache-Busuioc, R.A.; Glavan, L.A.; Dumitrascu, D.I.; Popa, A.A.; Bordeianu, A.; Ciurea, A.V. Migraine: Advances in the Pathogenesis and Treatment. Neurol. Int. 2023, 15, 1052–1105. [Google Scholar] [CrossRef]
- Hainer, B.L.; Matheson, E.M. Approach to acute headache in adults. Am. Fam. Physician 2013, 87, 682–687. [Google Scholar]
- Nguyen, L.; Hindiyeh, N.; Ray, S.; Vann, R.E.; Aurora, S.K. The Gut-brain Connection and Episodic Migraine: An Update. Curr. Pain Headache Rep. 2023, 27, 765–774. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Rodriguez-Gutierrez, R.; Monreal-Robles, R.; Marfil-Rivera, A. Gastrointestinal disorders associated with migraine: A comprehensive review. World J. Gastroenterol. 2016, 22, 8149–8160. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Saleh, C.; Beyenburg, S. Is there an Association between Migraine and Gastrointestinal Disorders? J. Clin. Neurol. 2017, 13, 215–226. [Google Scholar] [CrossRef]
- Buse, D.C.; Reed, M.L.; Fanning, K.M.; Bostic, R.C.; Lipton, R.B. Demographics, Headache Features, and Comorbidity Profiles in Relation to Headache Frequency in People with Migraine: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2020, 60, 2340–2356. [Google Scholar] [CrossRef]
- Buse, D.C.; Manack, A.; Serrano, D.; Turkel, C.; Lipton, R.B. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J. Neurol. Neurosurg. Psychiatry 2010, 81, 428–432. [Google Scholar] [CrossRef]
- Sureda-Gibert, P.; Romero-Reyes, M.; Akerman, S. Nitroglycerin as a model of migraine: Clinical and preclinical review. Neurobiol. Pain 2022, 12, 100105. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Li, J.; Hu, B.; Yang, W.; Zhan, M.; Ma, T.; Xu, S. IL-17 crosses the blood-brain barrier to trigger neuroinflammation: A novel mechanism in nitroglycerin-induced chronic migraine. J. Headache Pain 2022, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Karsan, N.; Gosalia, H.; Goadsby, P.J. Molecular Mechanisms of Migraine: Nitric Oxide Synthase and Neuropeptides. Int. J. Mol. Sci. 2023, 24, 11993. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.A.; Bertels, Z.; Akerman, S. Targeted Nitric Oxide Synthase Inhibitors for Migraine. Neurotherapeutics 2018, 15, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Socala, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Wlodarczyk, M.; Zielinska, A.; Poleszak, E.; Fichna, J.; Wlaz, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Crawford, J.; Liu, S.; Tao, F. Gut microbiota and migraine. Neurobiol. Pain 2022, 11, 100090. [Google Scholar] [CrossRef]
- Sgro, M.; Ray, J.; Foster, E.; Mychasiuk, R. Making migraine easier to stomach: The role of the gut-brain-immune axis in headache disorders. Eur. J. Neurol. 2023, 30, 3605–3621. [Google Scholar] [CrossRef]
- Lanza, M.; Filippone, A.; Ardizzone, A.; Casili, G.; Paterniti, I.; Esposito, E.; Campolo, M. SCFA Treatment Alleviates Pathological Signs of Migraine and Related Intestinal Alterations in a Mouse Model of NTG-Induced Migraine. Cells 2021, 10, 2756. [Google Scholar] [CrossRef]
- Ardizzone, A.; Capra, A.P.; Repici, A.; Lanza, M.; Bova, V.; Palermo, N.; Paterniti, I.; Esposito, E. Rebalancing NOX2/Nrf2 to limit inflammation and oxidative stress across gut-brain axis in migraine. Free Radic. Biol. Med. 2024, 213, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R. Understanding migraine: Potential role of neurogenic inflammation. Ann. Indian Acad. Neurol. 2016, 19, 175–182. [Google Scholar] [CrossRef]
- Biagioli, V.; Mela, F.; Ferraro, P.; Villano, G.; Orsini, A.; Diana, M.C.; Striano, P.; Santangelo, A. The Interplay Between Gut Microbiota, Adipose Tissue, and Migraine: A Narrative Review. Nutrients 2025, 17, 337. [Google Scholar] [CrossRef]
- Lankarani, K.B.; Akbari, M.; Tabrizi, R. Association of Gastrointestinal Functional Disorders and Migraine Headache: A Population Base Study. Middle East J. Dig. Dis. 2017, 9, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.K.; Shrewsbury, S.B.; Ray, S.; Hindiyeh, N.; Nguyen, L. A link between gastrointestinal disorders and migraine: Insights into the gut-brain connection. Headache 2021, 61, 576–589. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.; Kwon, Y.S.; Sohn, J.H. Clinical Implications of the Association between Respiratory and Gastrointestinal Disorders in Migraine and Non-Migraine Headache Patients. J. Clin. Med. 2023, 12, 3434. [Google Scholar] [CrossRef]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M.; School of Advanced Studies of the European Headache, F. Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef]
- Park, S.; Jung, H.; Han, S.W.; Lee, S.H.; Sohn, J.H. Differences in Neuropathology between Nitroglycerin-Induced Mouse Models of Episodic and Chronic Migraine. Int. J. Mol. Sci. 2024, 25, 3706. [Google Scholar] [CrossRef]
- Casili, G.; Lanza, M.; Filippone, A.; Campolo, M.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Dimethyl fumarate alleviates the nitroglycerin (NTG)-induced migraine in mice. J. Neuroinflammation 2020, 17, 59. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Z.; Zhai, D.; Yang, C.; Lu, G.; Wang, S.; Xiao, S.; Li, C.; Chen, L.; Lin, X.; et al. Unveiling the therapeutic potential of Dl-3-n-butylphthalide in NTG-induced migraine mouse: Activating the Nrf2 pathway to alleviate oxidative stress and neuroinflammation. J. Headache Pain 2024, 25, 50. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.; Vanden Berghe, P.; Verschueren, S.; Lehmann, A.; Depoortere, I.; Tack, J. Review article: The role of gastric motility in the control of food intake. Aliment. Pharmacol. Ther. 2011, 33, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Covasa, M. Deficits in gastrointestinal responses controlling food intake and body weight. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1423–R1439. [Google Scholar] [CrossRef]
- Cuomo, R.; Sarnelli, G. Food intake and gastrointestinal motility. A complex interplay. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 173–179. [Google Scholar] [CrossRef]
- Banks, W.A. The blood-brain barrier: Connecting the gut and the brain. Regul. Pept. 2008, 149, 11–14. [Google Scholar] [CrossRef]
- Levy, D.; Moskowitz, M.A. Meningeal Mechanisms and the Migraine Connection. Annu. Rev. Neurosci. 2023, 46, 39–58. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Wattiez, A.S.; Sowers, L.P.; Russo, A.F. Calcitonin gene-related peptide (CGRP): Role in migraine pathophysiology and therapeutic targeting. Expert. Opin. Ther. Targets 2020, 24, 91–100. [Google Scholar] [CrossRef]
- Schou, W.S.; Ashina, S.; Amin, F.M.; Goadsby, P.J.; Ashina, M. Calcitonin gene-related peptide and pain: A systematic review. J. Headache Pain 2017, 18, 34. [Google Scholar] [CrossRef]
- Kim, Y.J.; Granstein, R.D. Roles of calcitonin gene-related peptide in the skin, and other physiological and pathophysiological functions. Brain Behav. Immun. Health 2021, 18, 100361. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 2017, 158, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.F.; Hay, D.L. CGRP physiology, pharmacology, and therapeutic targets: Migraine and beyond. Physiol. Rev. 2023, 103, 1565–1644. [Google Scholar] [CrossRef]
- Holzer, P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998, 30, 5–11. [Google Scholar] [CrossRef]
- Kappeter, A.; Sipos, D.; Varga, A.; Vigvari, S.; Halda-Kiss, B.; Peterfi, Z. Migraine as a Disease Associated with Dysbiosis and Possible Therapy with Fecal Microbiota Transplantation. Microorganisms 2023, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L. CGRP and migraine: From bench to bedside. Rev. Neurol. 2021, 177, 785–790. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, D.-C.; Jung, H.; Park, S.; Song, D.-G.; Lee, S.-H.; Sohn, J.-H. Distinct Gut–Brain Axis Dysregulation in Episodic Versus Chronic Migraine: Insights from NTG-Induced Mouse Models. Int. J. Mol. Sci. 2025, 26, 10493. https://doi.org/10.3390/ijms262110493
Shin D-C, Jung H, Park S, Song D-G, Lee S-H, Sohn J-H. Distinct Gut–Brain Axis Dysregulation in Episodic Versus Chronic Migraine: Insights from NTG-Induced Mouse Models. International Journal of Molecular Sciences. 2025; 26(21):10493. https://doi.org/10.3390/ijms262110493
Chicago/Turabian StyleShin, Dae-Chul, Harry Jung, Songyi Park, Dan-Gyeong Song, Sang-Hwa Lee, and Jong-Hee Sohn. 2025. "Distinct Gut–Brain Axis Dysregulation in Episodic Versus Chronic Migraine: Insights from NTG-Induced Mouse Models" International Journal of Molecular Sciences 26, no. 21: 10493. https://doi.org/10.3390/ijms262110493
APA StyleShin, D.-C., Jung, H., Park, S., Song, D.-G., Lee, S.-H., & Sohn, J.-H. (2025). Distinct Gut–Brain Axis Dysregulation in Episodic Versus Chronic Migraine: Insights from NTG-Induced Mouse Models. International Journal of Molecular Sciences, 26(21), 10493. https://doi.org/10.3390/ijms262110493





