The cGAS-STING Pathway in Pulmonary Diseases: Mechanisms and Therapeutic Potential
Abstract
1. Introduction
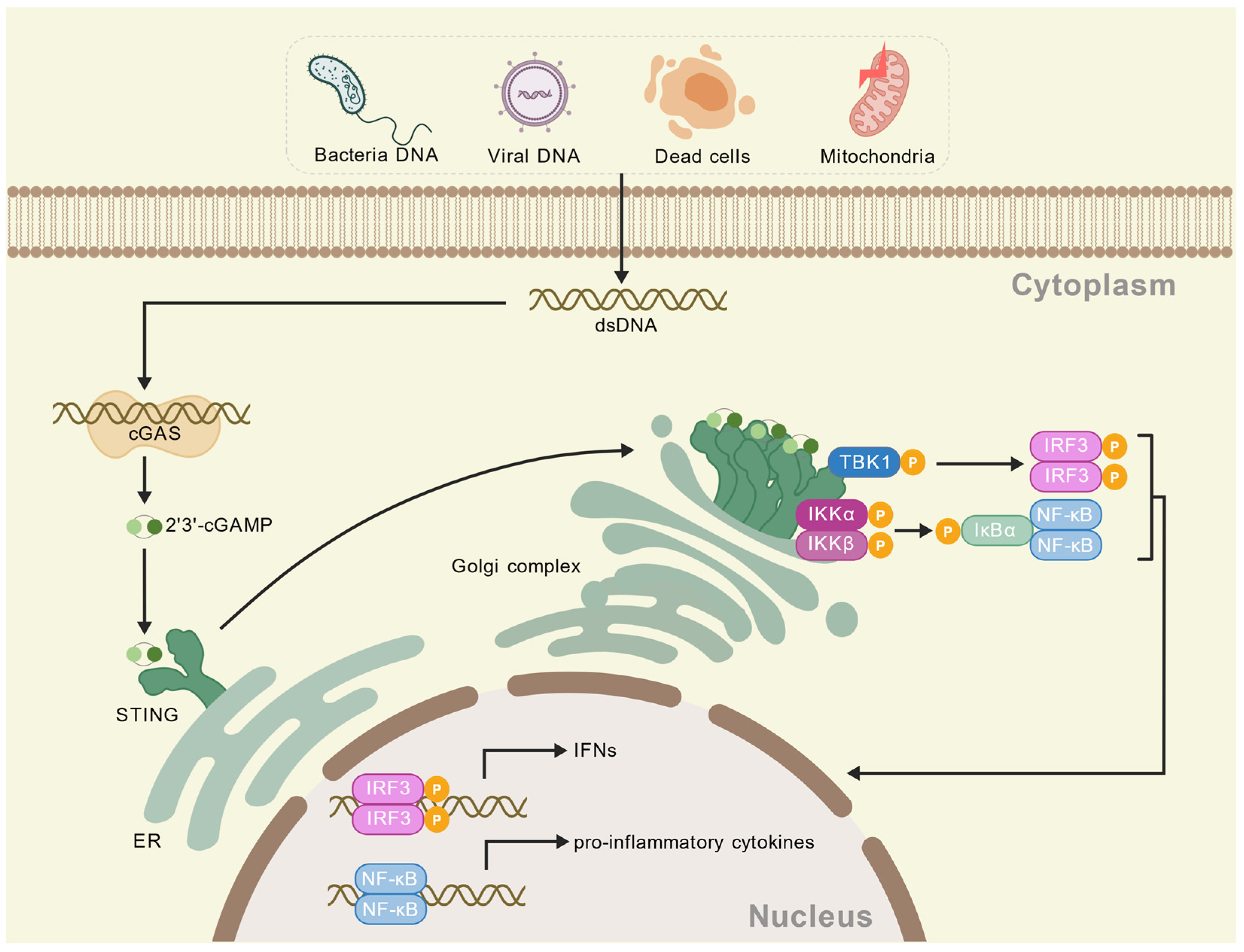
2. cGAS-STING Pathway
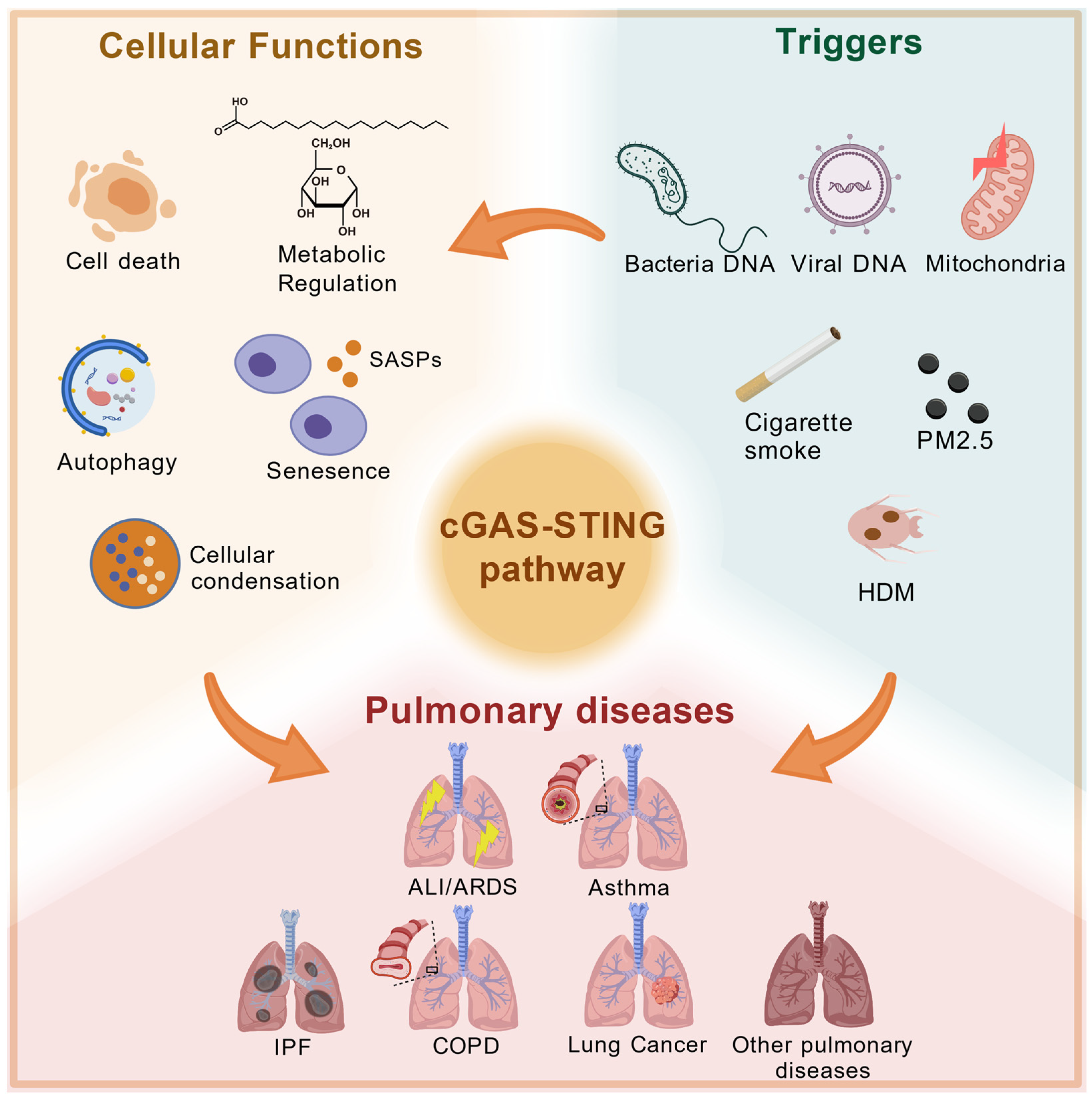
3. Activations and Functions
3.1. Conditions for cGAS-STING Pathway Activation
3.2. Biological Functions of cGAS-STING Pathway
3.2.1. cGAS-STING Pathway and Cell Death
3.2.2. cGAS-STING Pathway and Autophagy
3.2.3. cGAS-STING Pathway and Metabolic Regulation
3.3. Relationship Between cGAS-STING Pathway and Other Inflammatory Pathways
4. The cGAS-STING Pathway in Pulmonary Disease
4.1. Acute Respiratory Distress Syndrome (ARDS)/Acute Lung Injury (ALI)
4.2. Asthma
4.3. Idiopathic Pulmonary Fibrosis (IPF)
4.4. Chronic Obstructive Pulmonary Disease (COPD)
4.5. Lung Cancer
4.6. Coronavirus Disease 2019 (COVID-19)
4.7. Other Pulmonary Diseases
5. Agonists and Inhibitors of cGAS-STING Pathway
5.1. Agonists
5.2. Inhibitors
6. Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Schuliga, M.; Read, J.; Blokland, K.E.C.; Waters, D.W.; Burgess, J.; Prêle, C.; Mutsaers, S.E.; Jaffar, J.; Westall, G.; Reid, A.; et al. Self DNA Perpetuates IPF Lung Fibroblast Senescence in a cGAS-Dependent Manner. Clin. Sci. 2020, 134, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Ren, J.; Chen, Q.; Chen, Z.J. cGAS Is Essential for Cellular Senescence. Proc. Natl. Acad. Sci. USA 2017, 114, E4612–E4620. [Google Scholar] [CrossRef]
- Ishikawa, H.; Barber, G.N. STING Is an Endoplasmic Reticulum Adaptor That Facilitates Innate Immune Signalling. Nature 2008, 455, 674–678, Erratum in Nature 2008, 456, 274. [Google Scholar] [CrossRef]
- Chen, K.; Lai, C.; Su, Y.; Bao, W.D.; Yang, L.N.; Xu, P.-P.; Zhu, L.-Q. cGAS-STING-Mediated IFN-I Response in Host Defense and Neuroinflammatory Diseases. Curr. Neuropharmacol. 2022, 20, 362–371. [Google Scholar] [CrossRef]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP Is an Endogenous Second Messenger in Innate Immune Signaling by Cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef]
- Chen, C.; Xu, P. Cellular Functions of cGAS-STING Signaling. Trends Cell Biol. 2023, 33, 630–648. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Mohammadi, S.; Khorasani, M. Implications of the cGAS-STING Pathway in Diabetes: Risk Factors and Therapeutic Strategies. Int. J. Biol. Macromol. 2024, 278, 134210. [Google Scholar] [CrossRef]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.-P.; Ludwig, J.; Hornung, V. cGAS Produces a 2′-5′-Linked Cyclic Dinucleotide Second Messenger That Activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef]
- Balka, K.R.; Louis, C.; Saunders, T.L.; Smith, A.M.; Calleja, D.J.; D’Silva, D.B.; Moghaddas, F.; Tailler, M.; Lawlor, K.E.; Zhan, Y.; et al. TBK1 and IKKε Act Redundantly to Mediate STING-Induced NF-κB Responses in Myeloid Cells. Cell Rep. 2020, 31, 107492. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Zhu, Y.; Zhang, Q.; Guan, H.; Liu, S.; Chen, S.; Mei, C.; Chen, C.; Liao, Z.; et al. A Non-Canonical cGAS-STING–PERK Pathway Facilitates the Translational Program Critical for Senescence and Organ Fibrosis. Nat. Cell Biol. 2022, 24, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, C. Regulation of cGAS–STING Signalling and Its Diversity of Cellular Outcomes. Nat. Rev. Immunol. 2025, 25, 425–444. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Shadel, G.S. Mitochondrial DNA in Innate Immune Responses and Inflammatory Pathology. Nat. Rev. Immunol. 2017, 17, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular Mechanisms and Physiological Functions of Mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS–STING Pathway as a Therapeutic Target in Inflammatory Diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Ha, S.C.; Kim, D.; Hwang, H.-Y.; Rich, A.; Kim, Y.-G.; Kim, K.K. The Crystal Structure of the Second Z-DNA Binding Domain of Human DAI (ZBP1) in Complex with Z-DNA Reveals an Unusual Binding Mode to Z-DNA. Proc. Natl. Acad. Sci. USA 2008, 105, 20671–20676. [Google Scholar] [CrossRef]
- Takaoka, A.; Wang, Z.; Choi, M.K.; Yanai, H.; Negishi, H.; Ban, T.; Lu, Y.; Miyagishi, M.; Kodama, T.; Honda, K.; et al. DAI (DLM-1/ZBP1) Is a Cytosolic DNA Sensor and an Activator of Innate Immune Response. Nature 2007, 448, 501–505. [Google Scholar] [CrossRef]
- Mishra, S.; Dey, A.A.; Kesavardhana, S. Z-Nucleic Acid Sensing and Activation of ZBP1 in Cellular Physiology and Disease Pathogenesis. Immunol. Rev. 2025, 329, e13437. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A Diverse Range of Gene Products Are Effectors of the Type I Interferon Antiviral Response. Nature 2011, 472, 481–485, Erratum in Nature 2015, 525, 144. https://doi.org/10.1038/nature14554. [Google Scholar] [CrossRef]
- Domizio, J.D.; Gulen, M.F.; Saidoune, F.; Thacker, V.V.; Yatim, A.; Sharma, K.; Nass, T.; Guenova, E.; Schaller, M.; Conrad, C.; et al. The cGAS–STING Pathway Drives Type I IFN Immunopathology in COVID-19. Nature 2022, 603, 145–151. [Google Scholar] [CrossRef]
- Sha, H.-X.; Liu, Y.-B.; Qiu, Y.-L.; Zhong, W.-J.; Yang, N.-S.-Y.; Zhang, C.-Y.; Duan, J.-X.; Xiong, J.-B.; Guan, C.-X.; Zhou, Y. Neutrophil Extracellular Traps Trigger Alveolar Epithelial Cell Necroptosis through the cGAS-STING Pathway during Acute Lung Injury in Mice. Int. J. Biol. Sci. 2024, 20, 4713–4730. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jesus, A.A.; Marrero, B.; Yang, D.; Ramsey, S.E.; Montealegre Sanchez, G.A.; Tenbrock, K.; Wittkowski, H.; Jones, O.Y.; Kuehn, H.S.; et al. Activated STING in a Vascular and Pulmonary Syndrome. N. Engl. J. Med. 2014, 371, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, L.; Liu, H.; Jin, Z.; Wu, Y.; Wu, Y.; Li, W.; Ying, S.; Chen, Z.; Shen, H.; et al. Airway Epithelial cGAS Is Critical for Induction of Experimental Allergic Airway Inflammation. J. Immunol. 2020, 204, 1437–1447. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Lu, D.; Fang, Y.; Yan, X.; Chen, J.; Xia, Z.; Yuan, Q.; Chen, L.; Zhang, Y.; et al. GPR84 Regulates Pulmonary Inflammation by Modulating Neutrophil Functions. Acta Pharmacol. Sin. 2023, 44, 1665–1675. [Google Scholar] [CrossRef]
- Zierhut, C.; Yamaguchi, N.; Paredes, M.; Luo, J.-D.; Carroll, T.; Funabiki, H. The Cytoplasmic DNA Sensor cGAS Promotes Mitotic Cell Death. Cell 2019, 178, 302–315.e23. [Google Scholar] [CrossRef]
- Petrasek, J.; Iracheta-Vellve, A.; Csak, T.; Satishchandran, A.; Kodys, K.; Kurt-Jones, E.A.; Fitzgerald, K.A.; Szabo, G. STING-IRF3 Pathway Links Endoplasmic Reticulum Stress with Hepatocyte Apoptosis in Early Alcoholic Liver Disease. Proc. Natl. Acad. Sci. USA 2013, 110, 16544–16549. [Google Scholar] [CrossRef]
- Brault, M.; Olsen, T.M.; Martinez, J.; Stetson, D.B.; Oberst, A. Intracellular Nucleic Acid Sensing Triggers Necroptosis through Synergistic Type I IFN and TNF Signaling. J. Immunol. 2018, 200, 2748–2756. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Ramshorn, K.; Pinci, F.; Zuber, S.; O’Duill, F.; Schmid-Burgk, J.L.; Hoss, F.; Buhmann, R.; et al. The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell 2017, 171, 1110–1124.e18. [Google Scholar] [CrossRef]
- Bouis, D.; Kirstetter, P.; Arbogast, F.; Lamon, D.; Delgado, V.; Jung, S.; Ebel, C.; Jacobs, H.; Knapp, A.-M.; Jeremiah, N.; et al. Severe Combined Immunodeficiency in Stimulator of Interferon Genes (STING) V154M/Wild-Type Mice. J. Allergy Clin. Immunol. 2019, 143, 712–725.e5. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis Is a Type of Autophagy-Dependent Cell Death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Liu, J.; Kang, R.; Klionsky, D.J.; Tang, D. Mitochondrial DNA Stress Triggers Autophagy-Dependent Ferroptotic Death. Autophagy 2021, 17, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Gonugunta, V.K.; Sakai, T.; Pokatayev, V.; Yang, K.; Wu, J.; Dobbs, N.; Yan, N. Trafficking-Mediated STING Degradation Requires Sorting to Acidified Endolysosomes and Can Be Targeted to Enhance Anti-Tumor Response. Cell Rep. 2017, 21, 3234–3242. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Yang, H.; Li, T.; Tan, X.; Shi, P.; Li, M.; Du, F.; Chen, Z.J. Autophagy Induction via STING Trafficking Is a Primordial Function of the cGAS Pathway. Nature 2019, 567, 262–266. [Google Scholar] [CrossRef]
- Willemsen, J.; Neuhoff, M.-T.; Hoyler, T.; Noir, E.; Tessier, C.; Sarret, S.; Thorsen, T.N.; Littlewood-Evans, A.; Zhang, J.; Hasan, M.; et al. TNF Leads to mtDNA Release and cGAS/STING-Dependent Interferon Responses That Support Inflammatory Arthritis. Cell Rep. 2021, 37, 109977. [Google Scholar] [CrossRef]
- Watson, R.O.; Bell, S.L.; MacDuff, D.A.; Kimmey, J.M.; Diner, E.J.; Olivas, J.; Vance, R.E.; Stallings, C.L.; Virgin, H.W.; Cox, J.S. The Cytosolic Sensor cGAS Detects Mycobacterium Tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell Host Microbe 2015, 17, 811–819. [Google Scholar] [CrossRef]
- Dey, B.; Dey, R.J.; Cheung, L.S.; Pokkali, S.; Guo, H.; Lee, J.-H.; Bishai, W.R. A Bacterial Cyclic Dinucleotide Activates the Cytosolic Surveillance Pathway and Mediates Innate Resistance to Tuberculosis. Nat. Med. 2015, 21, 401–406. [Google Scholar] [CrossRef]
- Kimmey, J.M.; Huynh, J.P.; Weiss, L.A.; Park, S.; Kambal, A.; Debnath, J.; Virgin, H.W.; Stallings, C.L. Unique Role for ATG5 in Neutrophil-Mediated Immunopathology during M. Tuberculosis Infection. Nature 2015, 528, 565–569. [Google Scholar] [CrossRef]
- Margolis, S.R.; Wilson, S.C.; Vance, R.E. Evolutionary Origins of cGAS-STING Signaling. Trends Immunol. 2017, 38, 733–743. [Google Scholar] [CrossRef]
- Bai, J.; Cervantes, C.; Liu, J.; He, S.; Zhou, H.; Zhang, B.; Cai, H.; Yin, D.; Hu, D.; Li, Z.; et al. DsbA-L Prevents Obesity-Induced Inflammation and Insulin Resistance by Suppressing the mtDNA Release-Activated cGAS-cGAMP-STING Pathway. Proc. Natl. Acad. Sci. USA 2017, 114, 12196–12201. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, Z.; Zhang, H.; Hou, S.; Zhou, Y.; Liu, X. Targeting STING: From Antiviral Immunity to Treat Osteoporosis. Front. Immunol. 2023, 13, 1095577. [Google Scholar] [CrossRef]
- Luo, X.; Li, H.; Ma, L.; Zhou, J.; Guo, X.; Woo, S.-L.; Pei, Y.; Knight, L.R.; Deveau, M.; Chen, Y.; et al. Expression of STING Is Increased in Liver Tissues from Patients with NAFLD and Promotes Macrophage-Mediated Hepatic Inflammation and Fibrosis in Mice. Gastroenterology 2018, 155, 1971–1984.e4. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Luo, W.; Zhang, L.; Wu, W.; Yuan, L.; Xu, H.; Song, J.; Fujiwara, K.; Abe, J.; LeMaire, S.A.; et al. STING–IRF3 Triggers Endothelial Inflammation in Response to Free Fatty Acid-Induced Mitochondrial Damage in Diet-Induced Obesity. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 920–929, Erratum in Arterioscler. Thromb. Vasc. Biol. 2018, 38, e60. https://doi.org/10.1161/ATV.0000000000000069. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wu, Z.; Chen, D.; Zhu, L.; Yang, Y. A Role of STING Signaling in Obesity-Induced Lung Inflammation. Int. J. Obes. 2023, 47, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; Homem de Bittencourt, P.I., Jr. Molecular Mechanisms of ROS Production and Oxidative Stress in Diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in Health, Disease, and Aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Luo, T.; Zhou, X.; Qin, M.; Lin, Y.; Lin, J.; Chen, G.; Liu, A.; Ouyang, D.; Chen, D.; Pan, H. Corilagin Restrains NLRP3 Inflammasome Activation and Pyroptosis through the ROS/TXNIP/NLRP3 Pathway to Prevent Inflammation. Oxid. Med. Cell. Longev. 2022, 2022, 1652244. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Wang, H. The cGAS-STING-Mediated NLRP3 Inflammasome Is Involved in the Neurotoxicity Induced by Manganese Exposure. Biomed. Pharmacother. 2022, 154, 113680. [Google Scholar] [CrossRef]
- Wang, W.; Hu, D.; Wu, C.; Feng, Y.; Li, A.; Liu, W.; Wang, Y.; Chen, K.; Tian, M.; Xiao, F.; et al. STING Promotes NLRP3 Localization in ER and Facilitates NLRP3 Deubiquitination to Activate the Inflammasome upon HSV-1 Infection. PLoS Pathog. 2020, 16, e1008335. [Google Scholar] [CrossRef]
- Yee, M.; Gelein, R.; Mariani, T.J.; Lawrence, B.P.; O’Reilly, M.A. The Oxygen Environment at Birth Specifies the Population of Alveolar Epithelial Stem Cells in the Adult Lung. Stem Cells 2016, 34, 1396–1406. [Google Scholar] [CrossRef]
- Ma, R.; Ortiz Serrano, T.P.; Davis, J.; Prigge, A.D.; Ridge, K.M. The cGAS-STING Pathway: The Role of self-DNA Sensing in Inflammatory Lung Disease. FASEB J. 2020, 34, 13156–13170. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gu, X.; Wang, G.; Zhong, Y.; Ma, F.; Liu, Q.; Xie, J. Regulated Cell Death of Alveolar Macrophages in Acute Lung Inflammation: Current Knowledge and Perspectives. J. Inflamm. Res. 2024, 17, 11419–11436. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A. The Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2000, 18, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Healthy Longevity. Older People and Political Instability. Lancet Healthy Longev. 2021, 2, e528. [Google Scholar] [CrossRef]
- Ning, L.; Wei, W.; Wenyang, J.; Rui, X.; Qing, G. Cytosolic DNA-STING-NLRP3 Axis Is Involved in Murine Acute Lung Injury Induced by Lipopolysaccharide. Clin. Transl. Med. 2020, 10, e228. [Google Scholar] [CrossRef]
- Long, G.; Gong, R.; Wang, Q.; Zhang, D.; Huang, C. Role of Released Mitochondrial DNA in Acute Lung Injury. Front. Immunol. 2022, 13, 973089. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, D.; Liu, F.; Zhou, C.; Shao, Q.; Ding, C.; Qing, C.; Wang, X.; Hu, Z.; Qian, K. Mitochondrial DNA Plays an Important Role in Lung Injury Induced by Sepsis. J. Cell. Biochem. 2019, 120, 8547–8560. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Z.; Sun, H.; Xu, L. Perillaldehyde Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via Suppressing the cGAS/STING Signaling Pathway. Int. Immunopharmacol. 2024, 130, 111641. [Google Scholar] [CrossRef]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma Epidemiology and Risk Factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primer 2015, 1, 15025. [Google Scholar] [CrossRef]
- Tian, M.; Li, F.; Pei, H.; Liu, X.; Nie, H. The Role of the cGAS-STING Pathway in Chronic Pulmonary Inflammatory Diseases. Front. Med. 2024, 11, 1436091. [Google Scholar] [CrossRef]
- Ozasa, K.; Temizoz, B.; Kusakabe, T.; Kobari, S.; Momota, M.; Coban, C.; Ito, S.; Kobiyama, K.; Kuroda, E.; Ishii, K.J. Cyclic GMP-AMP Triggers Asthma in an IL-33-Dependent Manner That Is Blocked by Amlexanox, a TBK1 Inhibitor. Front. Immunol. 2019, 10, 2212. [Google Scholar] [CrossRef]
- Nunokawa, H.; Murakami, Y.; Ishii, T.; Narita, T.; Ishii, H.; Takizawa, H.; Yamashita, N. Crucial Role of Stimulator of Interferon Genes-Dependent Signaling in House Dust Mite Extract-Induced IgE Production. Sci. Rep. 2021, 11, 13157. [Google Scholar] [CrossRef]
- King, T.E.; Pardo, A.; Selman, M. Idiopathic Pulmonary Fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Collard, H.R.; King, T.E., Jr. Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Fell, C.D. Idiopathic Pulmonary Fibrosis: Phenotypes and Comorbidities. Clin. Chest Med. 2012, 33, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Schuliga, M.; Kanwal, A.; Read, J.; Blokland, K.E.C.; Burgess, J.K.; Prêle, C.M.; Mutsaers, S.E.; Grainge, C.; Thomson, C.; James, A.; et al. A cGAS-Dependent Response Links DNA Damage and Senescence in Alveolar Epithelial Cells: A Potential Drug Target in IPF. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L859–L871. [Google Scholar] [CrossRef]
- Wang, X.; Lu, W.; Xia, X.; Zhu, Y.; Ge, C.; Guo, X.; Zhang, N.; Chen, H.; Xu, S. Selenomethionine Mitigate PM2.5-Induced Cellular Senescence in the Lung via Attenuating Inflammatory Response Mediated by cGAS/STING/NF-κB Pathway. Ecotoxicol. Environ. Saf. 2022, 247, 114266. [Google Scholar] [CrossRef]
- Qiu, H.; Weng, D.; Chen, T.; Shen, L.; Chen, S.-S.; Wei, Y.-R.; Wu, Q.; Zhao, M.-M.; Li, Q.-H.; Hu, Y.; et al. Stimulator of Interferon Genes Deficiency in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Front. Immunol. 2017, 8, 1756. [Google Scholar] [CrossRef]
- Picard, C.; Thouvenin, G.; Kannengiesser, C.; Dubus, J.-C.; Jeremiah, N.; Rieux-Laucat, F.; Crestani, B.; Belot, A.; Thivolet-Béjui, F.; Secq, V.; et al. Severe Pulmonary Fibrosis as the First Manifestation of Interferonopathy (TMEM173 Mutation). Chest 2016, 150, e65–e71. [Google Scholar] [CrossRef]
- Sun, S.-C.; Han, R.; Hou, S.-S.; Yi, H.-Q.; Chi, S.-J.; Zhang, A.-H. Juglanin Alleviates Bleomycin-Induced Lung Injury by Suppressing Inflammation and Fibrosis via Targeting Sting Signaling. Biomed. Pharmacother. 2020, 127, 110119. [Google Scholar] [CrossRef] [PubMed]
- Savigny, F.; Schricke, C.; Lacerda-Queiroz, N.; Meda, M.; Nascimento, M.; Huot-Marchand, S.; Da Gama Monteiro, F.; Ryffel, B.; Gombault, A.; Le Bert, M.; et al. Protective Role of the Nucleic Acid Sensor STING in Pulmonary Fibrosis. Front. Immunol. 2021, 11, 588799. [Google Scholar] [CrossRef] [PubMed]
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic Obstructive Pulmonary Disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Respirology 2017, 22, 575–601. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative Stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef]
- Chen, J.; Wang, T.; Li, X.; Gao, L.; Wang, K.; Cheng, M.; Zeng, Z.; Chen, L.; Shen, Y.; Wen, F. DNA of Neutrophil Extracellular Traps Promote NF-κB-Dependent Autoimmunity via cGAS/TLR9 in Chronic Obstructive Pulmonary Disease. Signal Transduct. Target. Ther. 2024, 9, 163. [Google Scholar] [CrossRef]
- Caramori, G.; Ruggeri, P.; Di Stefano, A.; Mumby, S.; Girbino, G.; Adcock, I.M.; Kirkham, P. Autoimmunity and COPD. Chest 2018, 153, 1424–1431. [Google Scholar] [CrossRef]
- Twaddell, S.H.; Baines, K.J.; Grainge, C.; Gibson, P.G. The Emerging Role of Neutrophil Extracellular Traps in Respiratory Disease. Chest 2019, 156, 774–782. [Google Scholar] [CrossRef]
- Pedersen, F.; Marwitz, S.; Holz, O.; Kirsten, A.; Bahmer, T.; Waschki, B.; Magnussen, H.; Rabe, K.F.; Goldmann, T.; Uddin, M.; et al. Neutrophil Extracellular Trap Formation and Extracellular DNA in Sputum of Stable COPD Patients. Respir. Med. 2015, 109, 1360–1362. [Google Scholar] [CrossRef]
- Mdkhana, B.; Saheb Sharif-Askari, N.; Ramakrishnan, R.K.; Al-Sheakly, B.K.; Hafezi, S.; Saheb Sharif-Askari, F.; Bajbouj, K.; Hamid, Q.; Halwani, R. Nucleic Acid Sensor STING Drives Remodeling and Its Inhibition Enhances Steroid Responsiveness in Chronic Obstructive Pulmonary Disease. PLoS ONE 2023, 18, e0284061. [Google Scholar] [CrossRef]
- Nascimento, M.; Gombault, A.; Lacerda-Queiroz, N.; Panek, C.; Savigny, F.; Sbeity, M.; Bourinet, M.; Le Bert, M.; Riteau, N.; Ryffel, B.; et al. Self-DNA Release and STING-Dependent Sensing Drives Inflammation to Cigarette Smoke in Mice. Sci. Rep. 2019, 9, 14848. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Dan, Q.; Yang, Y.; Ge, H. cGAS-STING Pathway as the Target of Immunotherapy for Lung Cancer. Curr. Cancer Drug Targets 2023, 23, 354–362. [Google Scholar] [PubMed]
- Wei, M.; Li, Q.; Li, S.; Wang, D.; Wang, Y. Multifaceted Roles of cGAS-STING Pathway in the Lung Cancer: From Mechanisms to Translation. PeerJ 2024, 12, e18559. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, S.; Chen, X.; Shi, H.; Chen, C.; Sun, L.; Chen, Z.J. cGAS Is Essential for the Antitumor Effect of Immune Checkpoint Blockade. Proc. Natl. Acad. Sci. USA 2017, 114, 1637–1642. [Google Scholar] [CrossRef]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.-R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Xie, M.; Yu, X.; Su, C. cGAS-STING Signaling Pathway in Lung Cancer: Regulation on Antitumor Immunity and Application in Immunotherapy. Chin. Med. J. Pulm. Crit. Care Med. 2024, 2, 257–264. [Google Scholar] [CrossRef]
- Liang, H.; Shen, X. LXR Activation Radiosensitizes Non-Small Cell Lung Cancer by Restricting Myeloid-Derived Suppressor Cells. Biochem. Biophys. Res. Commun. 2020, 528, 330–335. [Google Scholar] [CrossRef]
- Lohinai, Z.; Dora, D.; Caldwell, C.; Rivard, C.J.; Suda, K.; Yu, H.; Rivalland, G.; Ellison, K.; Rozeboom, L.; Dziadziuszko, R.; et al. Loss of STING Expression Is Prognostic in Non–Small Cell Lung Cancer. J. Surg. Oncol. 2022, 125, 1042–1052. [Google Scholar] [CrossRef]
- Xue, A.; Shang, Y.; Jiao, P.; Zhang, S.; Zhu, C.; He, X.; Feng, G.; Fan, S. Increased Activation of cGAS-STIN Pathway Enhances Radiosensitivity of Non-small Cell Lung Cancer Cells. Thorac. Cancer 2022, 13, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, W.; Zhang, L.; Cao, Z.; Cong, X.; Hu, Q.; Hou, J.; Jin, X.; Yuan, Q.; Lin, L.; et al. Arginine Methylation-Dependent cGAS Stability Promotes Non-Small Cell Lung Cancer Cell Proliferation. Cancer Lett. 2024, 586, 216707, Erratum in Cancer Lett. 2024, 595, 217003. https://doi.org/10.1016/j.canlet.2024.217003. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shao, C.; Shi, Y.; Han, W. Lessons Learned from the Blockade of Immune Checkpoints in Cancer Immunotherapy. J. Hematol. Oncol. 2018, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yi, M.; Qin, S.; Song, Y.; Chu, Q.; Wu, K. Activating cGAS-STING Pathway for the Optimal Effect of Cancer Immunotherapy. J. Hematol. Oncol. 2019, 12, 35. [Google Scholar] [CrossRef]
- Harding, S.M.; Benci, J.L.; Irianto, J.; Discher, D.E.; Minn, A.J.; Greenberg, R.A. Mitotic Progression Following DNA Damage Enables Pattern Recognition within Micronuclei. Nature 2017, 548, 466–470. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6, Erratum in Cell 2020, 183, 1735. https://doi.org/10.1016/j.cell.2020.11.032. [Google Scholar] [CrossRef]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef]
- Moossavi, M.; Rastegar, M.; Moossavi, S.Z.; Khorasani, M. Molecular Function of cGAS-STING in SARS-CoV-2: A Novel Approach to COVID-19 Treatment. BioMed Res. Int. 2022, 2022, 6189254. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Lee, J.H.; Sergi, C.; Kast, R.E.; Kanwar, B.A.; Bourbeau, J.; Oh, S.; Sohn, M.-G.; Lee, C.J.; Coleman, M.D. Aggravating Mechanisms from COVID-19. Virol. J. 2024, 21, 228. [Google Scholar] [CrossRef]
- Cao, D.; Duan, L.; Huang, B.; Xiong, Y.; Zhang, G.; Huang, H. The SARS-CoV-2 Papain-like Protease Suppresses Type I Interferon Responses by Deubiquitinating STING. Sci. Signal. 2023, 16, eadd0082. [Google Scholar] [CrossRef] [PubMed]
- Humphries, F.; Shmuel-Galia, L.; Jiang, Z.; Wilson, R.; Landis, P.; Ng, S.-L.; Parsi, K.M.; Maehr, R.; Cruz, J.; Morales, A.; et al. A Diamidobenzimidazole STING Agonist Protects against SARS-CoV-2 Infection. Sci. Immunol. 2021, 6, eabi9002. [Google Scholar] [CrossRef] [PubMed]
- Zolty, R. Pulmonary Arterial Hypertension Specific Therapy: The Old and the New. Pharmacol. Ther. 2020, 214, 107576. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Huang, J.; Zeng, Y.; Zhong, X.; Fu, Y.; Xiao, H.; Wang, X.; Lian, H.; Luo, H.; Li, D.; et al. CGRP Attenuates Pulmonary Vascular Remodeling by Inhibiting the cGAS-STING-NFκB Pathway in Pulmonary Arterial Hypertension. Biochem. Pharmacol. 2024, 222, 116093. [Google Scholar] [CrossRef]
- Li, J.; Meng, Z.-Y.; Wen, H.; Lu, C.-H.; Qin, Y.; Xie, Y.-M.; Chen, Q.; Lv, J.-H.; Huang, F.; Zeng, Z.-Y. β-Sitosterol Alleviates Pulmonary Arterial Hypertension by Altering Smooth Muscle Cell Phenotype and DNA Damage/cGAS/STING Signaling. Phytomedicine 2024, 135, 156030. [Google Scholar] [CrossRef]
- Dheda, K.; Barry, C.E.; Maartens, G. Tuberculosis. Lancet 2016, 387, 1211–1226, Erratum in Lancet 2016, 387, 1162. https://doi.org/10.1016/S0140-6736(15)00399-2; Erratum in Lancet 2016, 387, 1162. https://doi.org/10.1016/S0140-6736(16)00712-1; Erratum in Lancet 2016, 387, 2092. https://doi.org/10.1016/S0140-6736(16)30544-X. [Google Scholar] [CrossRef]
- Collins, A.C.; Cai, H.; Li, T.; Franco, L.H.; Li, X.-D.; Nair, V.R.; Scharn, C.R.; Stamm, C.E.; Levine, B.; Chen, Z.J.; et al. Cyclic GMP-AMP Synthase Is an Innate Immune DNA Sensor for Mycobacterium Tuberculosis. Cell Host Microbe 2015, 17, 820–828. [Google Scholar] [CrossRef]
- Malik, A.A.; Shariq, M.; Sheikh, J.A.; Fayaz, H.; Srivastava, G.; Thakuri, D.; Ahuja, Y.; Ali, S.; Alam, A.; Ehtesham, N.Z.; et al. Regulation of Type I Interferon and Autophagy in Immunity against Mycobacterium Tuberculosis: Role of CGAS and STING1. Adv. Biol. 2024, 8, 2400174. [Google Scholar] [CrossRef]
- Benmerzoug, S.; Bounab, B.; Rose, S.; Gosset, D.; Biet, F.; Cochard, T.; Xavier, A.; Rouxel, N.; Fauconnier, L.; Horsnell, W.G.C.; et al. Sterile Lung Inflammation Induced by Silica Exacerbates Mycobacterium Tuberculosis Infection via STING-Dependent Type 2 Immunity. Cell Rep. 2019, 27, 2649–2664.e5. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, C.; Lu, D.; Wang, X.; Shang, G. cGAS-STING: Mechanisms and Therapeutic Opportunities. Sci. China Life Sci. 2025, 68, 1309–1323. [Google Scholar] [CrossRef]
- Fang, K.; Zhang, H.; Kong, Q.; Ma, Y.; Xiong, T.; Qin, T.; Li, S.; Zhu, X. Recent Progress in Photothermal, Photodynamic and Sonodynamic Cancer Therapy: Through the cGAS-STING Pathway to Efficacy-Enhancing Strategies. Molecules 2024, 29, 3704. [Google Scholar] [CrossRef]
- Le Naour, J.; Zitvogel, L.; Galluzzi, L.; Vacchelli, E.; Kroemer, G. Trial Watch: STING Agonists in Cancer Therapy. OncoImmunology 2020, 9, 1777624. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.; Burdette, D.L.; Sharma, S.; Bhat, N.; Thompson, M.; Jiang, Z.; Rathinam, V.A.K.; Monks, B.; Jin, T.; Xiao, T.S.; et al. Mouse, but Not Human STING, Binds and Signals in Response to the Vascular Disrupting Agent 5,6-Dimethylxanthenone-4-Acetic Acid. J. Immunol. 2013, 190, 5216–5225. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, E.; Van Moer, K.; Janji, B. Improving STING Agonist-Based Cancer Therapy by Inhibiting the Autophagy-Related Protein VPS34. OncoImmunology 2024, 13, 2364958. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Chen, Y.; Li, Z.; Liu, S.; Guan, W.; Lin, Y.; Cao, C.; Zheng, W.; Wu, J. Novel Emerging Nano-Assisted Anti-Cancer Strategies Based on the STING Pathway. Acta Mater. Medica 2023, 2, 323–341. [Google Scholar] [CrossRef]
- Vincent, J.; Adura, C.; Gao, P.; Luz, A.; Lama, L.; Asano, Y.; Okamoto, R.; Imaeda, T.; Aida, J.; Rothamel, K.; et al. Small Molecule Inhibition of cGAS Reduces Interferon Expression in Primary Macrophages from Autoimmune Mice. Nat. Commun. 2017, 8, 750, Erratum in Nat. Commun. 2017, 8, 1827. https://doi.org/10.1038/s41467-017-01770-3. [Google Scholar] [CrossRef]
- Lama, L.; Adura, C.; Xie, W.; Tomita, D.; Kamei, T.; Kuryavyi, V.; Gogakos, T.; Steinberg, J.I.; Miller, M.; Ramos-Espiritu, L.; et al. Development of Human cGAS-Specific Small-Molecule Inhibitors for Repression of dsDNA-Triggered Interferon Expression. Nat. Commun. 2019, 10, 2261. [Google Scholar] [CrossRef]
- An, J.; Woodward, J.J.; Sasaki, T.; Minie, M.; Elkon, K.B. Cutting Edge: Antimalarial Drugs Inhibit IFN-β Production through Blockade of Cyclic GMP-AMP Synthase–DNA Interaction. J. Immunol. 2015, 194, 4089–4093. [Google Scholar] [CrossRef]
- Hall, J.; Brault, A.; Vincent, F.; Weng, S.; Wang, H.; Dumlao, D.; Aulabaugh, A.; Aivazian, D.; Castro, D.; Chen, M.; et al. Discovery of PF-06928215 as a High Affinity Inhibitor of cGAS Enabled by a Novel Fluorescence Polarization Assay. PLoS ONE 2017, 12, e0184843. [Google Scholar] [CrossRef]
- Steinhagen, F.; Zillinger, T.; Peukert, K.; Fox, M.; Thudium, M.; Barchet, W.; Putensen, C.; Klinman, D.; Latz, E.; Bode, C. Suppressive Oligodeoxynucleotides Containing TTAGGG Motifs Inhibit cGAS Activation in Human Monocytes. Eur. J. Immunol. 2018, 48, 605–611. [Google Scholar] [CrossRef]
- Xu, P.; Liu, Y.; Liu, C.; Guey, B.; Li, L.; Melenec, P.; Ricci, J.; Ablasser, A. The CRL5–SPSB3 Ubiquitin Ligase Targets Nuclear cGAS for Degradation. Nature 2024, 627, 873–879. [Google Scholar] [CrossRef]
- Radziszewska, A.; Peckham, H.; Restuadi, R.; Kartawinata, M.; Moulding, D.; De Gruijter, N.M.; Robinson, G.A.; Butt, M.; Deakin, C.T.; Wilkinson, M.G.L.; et al. Type I Interferon and Mitochondrial Dysfunction Are Associated with Dysregulated Cytotoxic CD8+ T Cell Responses in Juvenile Systemic Lupus Erythematosus. Clin. Exp. Immunol. 2025, 219, uxae127. [Google Scholar] [CrossRef]
- Kang, J.; Wu, J.; Liu, Q.; Wu, X.; Zhao, Y.; Ren, J. Post-Translational Modifications of STING: A Potential Therapeutic Target. Front. Immunol. 2022, 13, 888147. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cai, L.; Yao, J.; Li, C.; Wang, X. Agonists and Inhibitors of the cGAS-STING Pathway. Molecules 2024, 29, 3121. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.M.; Gulen, M.F.; Reymond, L.; Gibelin, A.; Abrami, L.; Decout, A.; Heymann, M.; Van Der Goot, F.G.; Turcatti, G.; Behrendt, R.; et al. Targeting STING with Covalent Small-Molecule Inhibitors. Nature 2018, 559, 269–273. [Google Scholar] [CrossRef]
- Zhao, J.; Zhen, N.; Zhou, Q.; Lou, J.; Cui, W.; Zhang, G.; Tian, B. NETs Promote Inflammatory Injury by Activating cGAS-STING Pathway in Acute Lung Injury. Int. J. Mol. Sci. 2023, 24, 5125. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Wang, F.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. DNA Damage-Triggered Activation of cGAS-STING Pathway Induces Apoptosis in Human Keratinocyte HaCaT Cells. Mol. Immunol. 2021, 131, 180–190, Erratum in Mol. Immunol. 2023, 164, 123. https://doi.org/10.1016/j.molimm.2023.11.003. [Google Scholar] [CrossRef]
- Frémond, M.; Uggenti, C.; Van Eyck, L.; Melki, I.; Bondet, V.; Kitabayashi, N.; Hertel, C.; Hayday, A.; Neven, B.; Rose, Y.; et al. Brief Report: Blockade of TANK-binding Kinase 1/IKKε Inhibits Mutant Stimulator of Interferon Genes (STING)–Mediated Inflammatory Responses in Human Peripheral Blood Mononuclear Cells. Arthritis Rheumatol. 2017, 69, 1495–1501. [Google Scholar] [CrossRef]
- Hong, Z.; Mei, J.; Li, C.; Bai, G.; Maimaiti, M.; Hu, H.; Yu, W.; Sun, L.; Zhang, L.; Cheng, D.; et al. STING Inhibitors Target the Cyclic Dinucleotide Binding Pocket. Proc. Natl. Acad. Sci. USA 2021, 118, e2105465118. [Google Scholar] [CrossRef]
- Humphries, F.; Shmuel-Galia, L.; Jiang, Z.; Zhou, J.Y.; Barasa, L.; Mondal, S.; Wilson, R.; Sultana, N.; Shaffer, S.A.; Ng, S.-L.; et al. Targeting STING Oligomerization with Small-Molecule Inhibitors. Proc. Natl. Acad. Sci. USA 2023, 120, e2305420120. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, P.; Rivara, S.; Liu, C.; Ricci, J.; Ren, X.; Hurley, J.H.; Ablasser, A. Clathrin-Associated AP-1 Controls Termination of STING Signalling. Nature 2022, 610, 761–767. [Google Scholar] [CrossRef]
- Shen, A.; Chen, M.; Chen, Q.; Liu, Z.; Zhang, A. Recent Advances in the Development of STING Inhibitors: An Updated Patent Review. Expert Opin. Ther. Pat. 2022, 32, 1131–1143. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type I Interferon Is Selectively Required by Dendritic Cells for Immune Rejection of Tumors. J. Exp. Med. 2011, 208, 1989–2003. [Google Scholar] [CrossRef]
- Grabosch, S.; Bulatovic, M.; Zeng, F.; Ma, T.; Zhang, L.; Ross, M.; Brozick, J.; Fang, Y.; Tseng, G.; Kim, E.; et al. Cisplatin-Induced Immune Modulation in Ovarian Cancer Mouse Models with Distinct Inflammation Profiles. Oncogene 2019, 38, 2380–2393. [Google Scholar] [CrossRef]
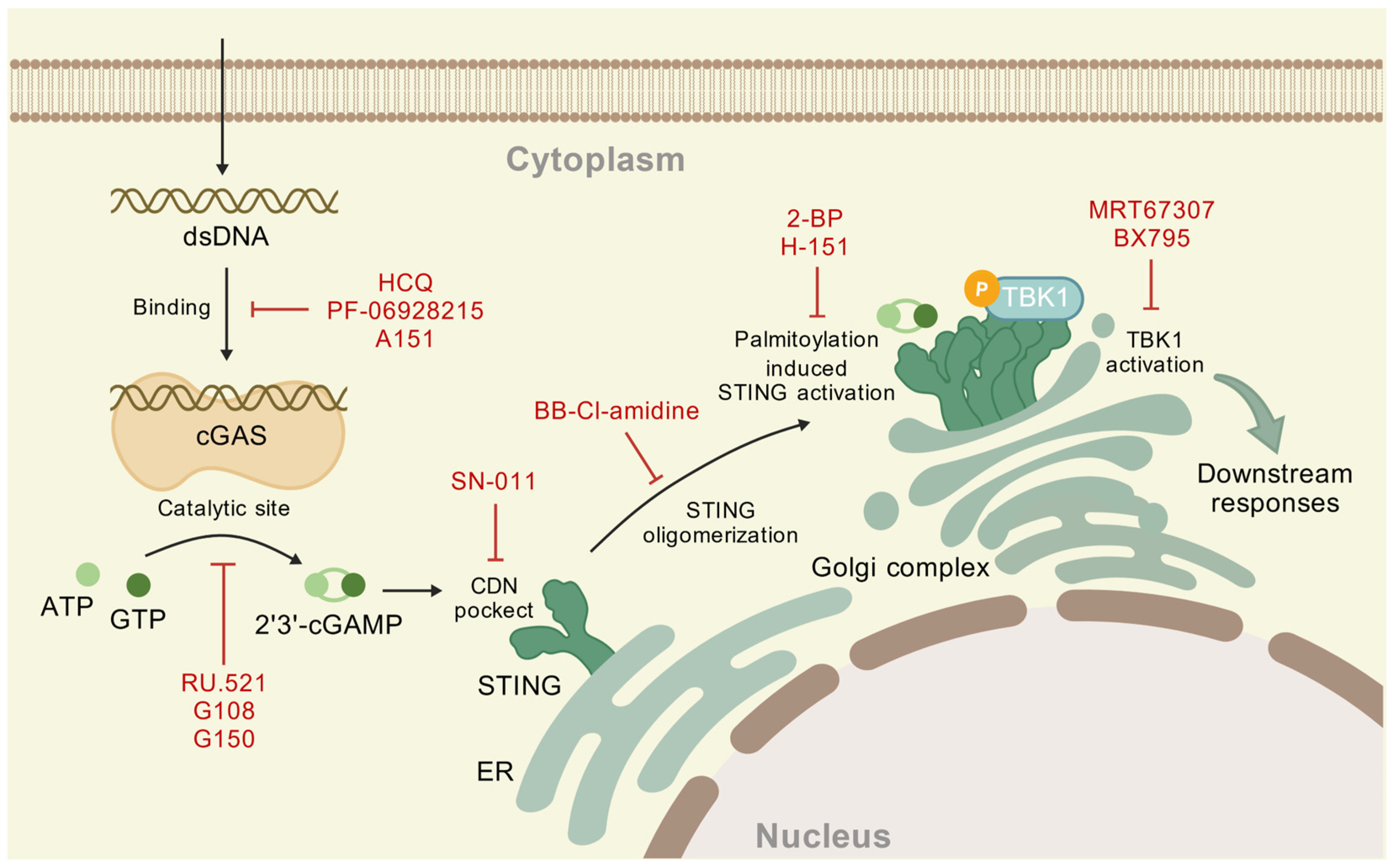
| Function | Compound | Potential Mechanism | Reference |
|---|---|---|---|
| Disrupt DNA binding | hydroxychloroquine (HCQ) | binds to DNA minor groove | [118] |
| PF-06928215 | binds to the cGAS active site | [119] | |
| A151 | competitive with DNA | [120] | |
| Inhibit catalytic site | RU.521 | occupies the catalytic site of m-cGAS | [116] |
| G108 | occupies ATP binding location | [117] | |
| G150 | occupies the catalytic site of h-cGAS | [117] |
| Function | Compound | Potential Mechanism | Reference |
|---|---|---|---|
| CDNs pocket inhibitors | SN-011 | compete for the STING binding pocket | [129] |
| STING oligomerization inhibitors | BB-Cl-amidine | modify Cys148 | [130] |
| Palmitoylation inhibitors | 2-BP | blocking S-palmitoylation of STING | [123] |
| H-151 | modifying Cys91 to block palmitoylation | [125] | |
| TBK1 inhibitors | MRT67307 | dual inhibitor of TBK1/IKKε inhibits IRF3 and NF-κB pathways by blocking kinase activity | [127] |
| BX795 | blocking of STING-TBK1 signal | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Jiang, J.; Wu, G.; Wei, X.; Weng, Y.; Huang, L.S. The cGAS-STING Pathway in Pulmonary Diseases: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 10423. https://doi.org/10.3390/ijms262110423
Zhang Z, Jiang J, Wu G, Wei X, Weng Y, Huang LS. The cGAS-STING Pathway in Pulmonary Diseases: Mechanisms and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(21):10423. https://doi.org/10.3390/ijms262110423
Chicago/Turabian StyleZhang, Zhuo, Jiacheng Jiang, Guodong Wu, Xueping Wei, Yakun Weng, and Long Shuang Huang. 2025. "The cGAS-STING Pathway in Pulmonary Diseases: Mechanisms and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 21: 10423. https://doi.org/10.3390/ijms262110423
APA StyleZhang, Z., Jiang, J., Wu, G., Wei, X., Weng, Y., & Huang, L. S. (2025). The cGAS-STING Pathway in Pulmonary Diseases: Mechanisms and Therapeutic Potential. International Journal of Molecular Sciences, 26(21), 10423. https://doi.org/10.3390/ijms262110423









