Thrombosis and Anemia in Pediatric Inflammatory Bowel Disease: Pathophysiology, Clinical Impact and Future Directions
Abstract
1. Introduction
2. Anemia in PIBD
2.1. Epidemiology of Anemia in PIBD
2.2. Pathophysiology
2.3. Clinical Manifestations and Burden of Anemia in Pediatric Patients with IBD
2.4. Management Strategies
2.5. The Role of Predictive Biomarkers in Diagnosis, Risk Stratification and Therapy Response
3. Thrombosis in PIBD
3.1. Incidence of TE in PIBD and Risk Factors
3.2. Pathophysiological Pathways Leading to Thrombosis and Common Mechanisms That Can Cause Both Anemia and TE in PIBD
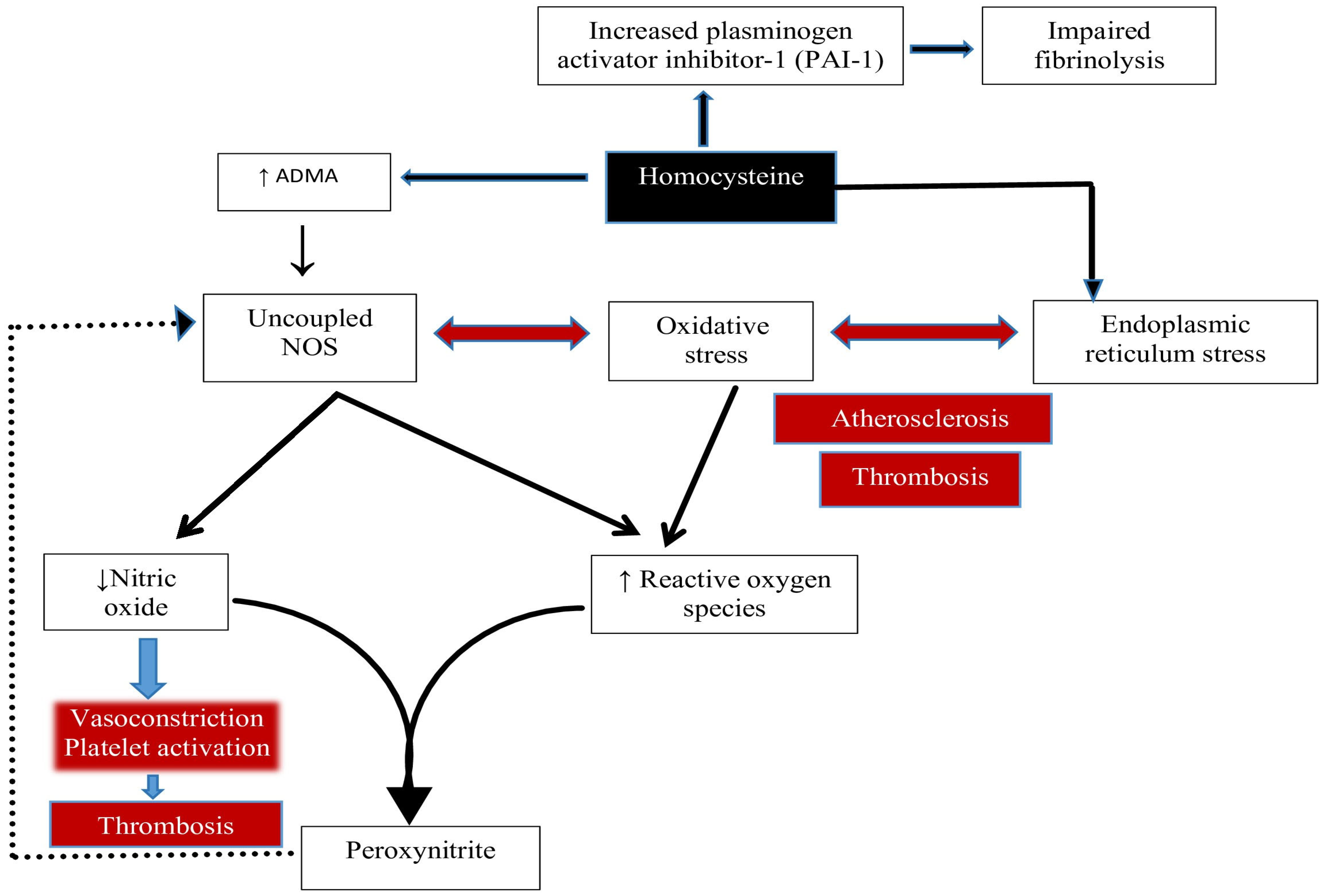
- NO Trapping and Degradation: NO is rapidly inactivated by superoxide radicals via peroxynitrite formation. Elevated Hcy also inhibits glutathione peroxidase (GPx), impairing hydrogen peroxide detoxification and exacerbating oxidative stress [53].
- ADMA-Mediated NOS Inhibition: Hcy promotes production of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase (NOS) inhibitor [56].
3.3. Types of TE and Clinical Manifestations
3.4. Management
3.5. Current Biomarkers for Diagnosing Thrombosis and Monitoring Therapeutic Response in PIBD
4. Interplay Between Anemia and Thrombosis
4.1. Role of Anemia in Increasing Thrombosis Risk
4.2. Mechanisms by Which Thrombosis May Worsen Anemia in Patients with IBD
5. Discussion
- -
- development of pediatric-specific diagnostic and therapeutic algorithms for both anemia and thrombosis, as adult guidelines are not directly applicable to the pediatric population;
- -
- exploration of biomarkers—including hepcidin, soluble transferrin receptor, and reticulocyte hemoglobin content—to improve diagnostic accuracy and facilitate early detection of iron metabolism disturbances and thrombotic risk;
- -
- conducting prospective longitudinal and randomized clinical studies to determine the safety and efficacy of iron supplementation and anticoagulation therapies in children with IBD;
- -
- assessing the long-term impact of chronic anemia on growth, cognitive function, and school performance in pediatric populations;
- -
- implementing integrated, multidisciplinary care strategies to enhance screening, prevention, and management of extraintestinal complications in PIBD.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| ADMA | Asymmetric dimethylarginine |
| ASC | Acute severe colitis |
| BMC | BioMed Central |
| BMJ | British Medical Journal |
| CAI | Cerebral arterial infarction |
| CD | Crohn’s disease |
| CVST | Cerebral venous sinus thrombosis |
| DNA | Deoxyribonucleic acid |
| DOACs | Direct oral anticoagulants |
| DVT | Deep vein thrombosis |
| ECCO | European Crohn’s and Colitis Organisation |
| EPO | Erythropoietin |
| ESA | Erythropoiesis-stimulating agent |
| FDP | Fibrin/fibrinogen degradation products |
| HRQoL | Health-related quality of life |
| IBD | Inflammatory bowel disease |
| ICU | Intensive care unit |
| ID | Iron deficiency |
| IDA | Iron deficiency anemia |
| IG-IBD | Italian Group for Inflammatory Bowel Disease |
| IL | Interleukin |
| IV | Intravenous |
| JAMA | Journal of the American Medical Association |
| LDH | Lactate dehydrogenase |
| LMWH | Low molecular weight heparin |
| MCA | Middle cerebral artery |
| NET | Neutrophil extracellular trap |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| PAI | Plasminogen activator inhibitor |
| PE | Pulmonary embolism |
| PIBD | Pediatric inflammatory bowel disease |
| PS | Phosphatidylserine |
| PTS | Post-thrombotic syndrome |
| PVT | Portal vein thrombosis |
| RBC | Red blood cell |
| RET-He | Reticulocyte hemoglobin content |
| ROS | Reactive oxygen species |
| RSH | Sulfhydryl group |
| SIGENP | Società Italiana di Gastroenterologia Epatologia e Nutrizione Pediatrica |
| TE | Thromboembolic event |
| TF | Tissue factor |
| TNF | Tumor necrosis factor |
| TPO | Thrombopoietin |
| UC | Ulcerative colitis |
| VTE | Venous thromboembolism |
| VWF | von Willebrand factor |
| WHO | World Health Organization |
References
- Long, D.; Wang, C.; Huang, Y.; Mao, C.; Xu, Y.; Zhu, Y. Changing epidemiology of inflammatory bowel disease in children and adolescents. Int. J. Color. Dis. 2024, 39, 73. [Google Scholar] [CrossRef]
- Bouhuys, M.; Lexmond, W.S.; van Rheenen, P.F. Pediatric Inflammatory Bowel Disease. Pediatrics 2022, 151, e2022058037. [Google Scholar] [CrossRef]
- Ding, Z.; Sherlock, M.; Chan, A.K.C.; Zachos, M. Venous thromboembolism in pediatric inflammatory bowel disease: An 11-year population-based nested case–control study in Canada. Blood Coagul. Fibrinolysis 2022, 33, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Mages, K.; Kucine, N.; Chien, K. Venous Thromboembolism in Pediatric Inflammatory Bowel Disease: A Scoping Review. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 491–498. [Google Scholar] [CrossRef]
- Klomberg, R.C.W.; Vlug, L.E.; de Koning, B.A.E.; de Ridder, L. Venous Thromboembolic Complications in Pediatric Gastrointestinal Diseases: Inflammatory Bowel Disease and Intestinal Failure. Front. Pediatr. 2022, 10, 885876. [Google Scholar] [CrossRef]
- D’aRcangelo, G.; Distante, M.; Veraldi, S.; Tarani, F.; Musto, F.; Aloi, M. Natural History of Anemia and Efficacy and Safety of Oral Iron Therapy in Children Newly Diagnosed with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 771–775. [Google Scholar] [CrossRef]
- D’ARcangelo, G.; Brecciaroli, M.; Gagliostro, G.; Auletta, D.; Pellegrino, S.; Arrigo, S.; Graziano, F.; Miele, E.; Illiceto, M.T.; Alvisi, P.; et al. Prevalence and trend of anemia in children with inflammatory bowel disease: A national register-based cohort study. J. Pediatr. Gastroenterol. Nutr. 2025, 80, 967–978. [Google Scholar] [CrossRef]
- Danko, I.; Weidkamp, M.; Eickhoff, J.C. Improvement of Health-Related Quality of Life in Children with Inflammatory Bowel Disease Receiving Routine Intravenous Iron Supplementation. J. Pediatr. Pharmacol. Ther. 2019, 24, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, G.; Colombel, J.-F.; Katsanos, K.; Mearin, F.; Stein, J.; Andretta, M.; Antonacci, S.; Arenare, L.; Citraro, R.; Dell’oRco, S.; et al. Iron deficiency anemia impacts disease progression and healthcare resource consumption in patients with inflammatory bowel disease: A real-world evidence study. Ther. Adv. Gastroenterol. 2023, 16, 17562848231177153. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.D.; Cuffari, C.; Akhuemonkhan, E.; Guerrerio, A.L.; Lehmann, H.; Hutfless, S. Anemia Screening, Prevalence, and Treatment in Pediatric Inflammatory Bowel Disease in the United States, 2010–2014. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 152–161. [Google Scholar] [CrossRef]
- Goyal, A.; Zheng, Y.; Albenberg, L.G.; Stoner, N.L.; Hart, L.; Alkhouri, R.; Hampson, K.; Ali, S.; Cho-Dorado, M.; Goyal, R.K.; et al. Anemia in Children With Inflammatory Bowel Disease: A Position Paper by the IBD Committee of the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 563–582. [Google Scholar] [CrossRef]
- Shentova-Eneva, R.; Kofinova, D.; Hadzhiyski, P.; Yaneva, P.; Lazarova, E.; Baycheva, M. Anemia in Newly Diagnosed Pediatric Patients with Inflammatory Bowel Disease. Gastroenterol. Insights 2021, 12, 376–383. [Google Scholar] [CrossRef]
- Rempel, J.; Grover, K.; El-Matary, W. Micronutrient Deficiencies and Anemia in Children with Inflammatory Bowel Disease. Nutrients 2021, 13, 236. [Google Scholar] [CrossRef]
- Woźniak, M.; Borkowska, A.; Jastrzębska, M.; Sochal, M.; Małecka-Wojciesko, E.; Talar-Wojnarowska, R. Clinical and Laboratory Characteristics of Anaemia in Hospitalized Patients with Inflammatory Bowel Disease. J. Clin. Med. 2023, 12, 2447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kern, I.; Rothe, U.; Schoffer, O.; Weidner, J.; Richter, T.; Laass, M.W.; Kugler, J.; Manuwald, U. Growth development of children and adolescents with inflammatory bowel disease in the period 2000–2014 based on data of the Saxon pediatric IBD registry: A population-based study. BMC Gastroenterol. 2024, 24, 25. [Google Scholar] [CrossRef]
- Fiani, D.; Kim, J.-W.; Hu, M.; Salas, R.; Heilbronner, S.; Powers, J.; Haque, M.; Dinh, S.; Huang, X.; Worthy, D.; et al. Iron Deficiency Without Anemia and Reduced Basal Ganglia Iron Content in Youths. JAMA Netw. Open 2025, 8, e2516687. [Google Scholar] [CrossRef] [PubMed]
- Fiani, D.; Chahine, S.; Zaboube, M.; Solmi, M.; Powers, J.M.; Calarge, C. Psychiatric and cognitive outcomes of iron supplementation in non-anemic children, adolescents, and menstruating adults: A meta-analysis and systematic review. Neurosci. Biobehav. Rev. 2025, 178, 106372. [Google Scholar] [CrossRef]
- Gingoyon, A.; Borkhoff, C.M.; Koroshegyi, C.; Mamak, E.; Birken, C.S.; Maguire, J.L.; Fehlings, D.; Macarthur, C.; Parkin, P.C. Chronic Iron Deficiency and Cognitive Function in Early Childhood. Pediatrics 2022, 150, e2021055926. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Obazenu, L.O.; Monis, M.; Mustapha-Adebiyi, H.T.; Wandala, A.; Harischandra, T.V.; Ayuba, P.D.; Thorani, M.; Arshad, M.R.; Al Shamsi, M.; et al. Association Between Iron Deficiency Without Anemia and Cognitive Impairment in Children. Cureus 2025, 17, e89049. [Google Scholar] [CrossRef]
- Gutema, B.T.; Sorrie, M.B.; Megersa, N.D.; Yesera, G.E.; Yeshitila, Y.G.; Pauwels, N.S.; De Henauw, S.; Abbeddou, S. Effects of iron supplementation on cognitive development in school-age children: Systematic review and meta-analysis. PLoS ONE 2023, 18, e0287703. [Google Scholar] [CrossRef]
- Eloi, C.; Foulon, G.; Bridoux-Henno, L.; Breton, E.; Pelatan, C.; Chaillou, E.; Grimal, I.; Darviot, E.; Carré, E.; Gastineau, S.; et al. Inflammatory Bowel Diseases and School Absenteeism. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Jacobson-Kelly, A.M.; Donegan, A.M.; Boyle, B.; Maltz, R.M.; Michel, H.K.; Dotson, J.L. Diagnosis and Treatment of Iron Deficiency and Anemia in Youth with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2022, 76, 313–318. [Google Scholar] [CrossRef]
- Lopes, A.; Azevedo, S.; Cabral, J.; Ferreira, M.; Sande-Lemos, P.; Ferreira, R.; Trindade, E.; Lima, R.; Antunes, H. Portuguese Consensus on Diagnosis, Treatment, and Management of Anemia in Pediatric Inflammatory Bowel Disease. GE-Port. J. Gastroenterol. 2020, 27, 244–254. [Google Scholar] [CrossRef]
- Martinelli, M.; Fioretti, M.T.; Aloi, M.; Alvisi, P.; Arrigo, S.; Banzato, C.; Bramuzzo, M.; Campanozzi, A.; Civitelli, F.; Knafelz, D.; et al. Diagnosis and management of anemia in pediatric inflammatory bowel diseases: Clinical practice guidelines on behalf of the SIGENP IBD Working group. Dig. Liver Dis. 2024, 56, 1257–1269. [Google Scholar] [CrossRef]
- Sabe, R.; Vatsayan, A.; Mahran, A.; Khalili, A.S.; Ahuja, S.; Sferra, T.J. Safety and Efficacy of Intravenous Iron Sucrose for Iron-Deficiency Anemia in Children and Adolescents With Inflammatory Bowel Disease. Glob. Pediatr. Health 2019, 6, 2333794X19870981. [Google Scholar] [CrossRef]
- Manokaran, K.; Spaan, J.; Cataldo, G.; Lyons, C.; Mitchell, P.D.; Sare, T.; Zimmerman, L.A.; Rufo, P.A. Inpatient management of iron deficiency anemia in pediatric patients with inflammatory bowel disease: A single center experience. World J. Clin. Pediatr. 2024, 13, 89318. [Google Scholar] [CrossRef]
- Bevers, N.; Van de Vijver, E.; Aliu, A.; Ardabili, A.R.; Rosias, P.; Stapelbroek, J.; Maartens, I.A.B.; van de Feen, C.; Escher, H.; Oudshoorn, A.; et al. Ferric Carboxymaltose Versus Ferrous Fumarate in Anemic Children with Inflammatory Bowel Disease: The POPEYE Randomized Controlled Clinical Trial. J. Pediatr. 2022, 256, 113–119.e4. [Google Scholar] [CrossRef]
- Elimeleh, Y.; Zittan, E.; Levy, M.; Rinawi, F. Adherence to ECCO Guidelines for Management of Iron Deficiency and Anemia in Inflammatory Bowel Diseases Among Israeli Adult and Pediatric Gastroenterologists. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Castiglione, F.; D’Incà, R.; Astegiano, M.; Fries, W.; Milla, M.; Ciacci, C.; Rizzello, F.; Saibeni, S.; Ciccocioppo, R.; et al. Follow-up evaluation and management of anemia in inflammatory bowel disease: A study by the Italian Group for Inflammatory Bowel Diseases (IG-IBD). Dig. Liver Dis. 2024, 56, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Alexiadou, S.; Tsigalou, C.; Kourkouni, E.; Tsalkidis, A.; Mantadakis, E. Oral iron-hydroxide polymaltose complex versus sucrosomial iron for children with iron deficiency with or without anemia: A clinical trial with emphasis on intestinal inflammation. Mediterr. J. Hematol. Infect. Dis. 2024, 16, e2024075. [Google Scholar] [CrossRef]
- Bahbah, W.A.; Younis, Y.A.H.S.; Elbelouny, H.S.; Mahmoud, A.A. Liposomal SunActive versus conventional iron for treatment of iron-deficiency anemia in children aged 2–12 years: A prospective randomized controlled trial. Clin. Exp. Pediatr. 2025, 68, 608–615. [Google Scholar] [CrossRef]
- Pantopoulos, K. Oral iron supplementation: New formulations, old questions. Haematologica 2024, 109, 2790–2801. [Google Scholar] [CrossRef]
- Kochar, I.S.; Kaur, D.; Sachdeva, M. Comparative efficacy and tolerability of ferric pyrophosphate vs ferrous sulfate in pediatric iron deficiency anemia: A 6-month randomized controlled trial. Egypt. Pediatr. Assoc. Gaz. 2025, 73, 69. [Google Scholar] [CrossRef]
- Gómez-Ramírez, S.; Brilli, E.; Tarantino, G.; Girelli, D.; Muñoz, M. Sucrosomial® Iron: An Updated Review of Its Clinical Efficacy for the Treatment of Iron Deficiency. Pharmaceuticals 2023, 16, 847. [Google Scholar] [CrossRef]
- Lepus, C.A.; Samela, K.; Mokha, J.S. Efficacy and safety of intravenous iron sucrose in children younger than 2 years with intestinal failure. Nutr. Clin. Pract. 2022, 38, 899–903. [Google Scholar] [CrossRef]
- Roganovic, J. Parenteral iron therapy in children with iron deficiency anemia. World J. Clin. Cases 2024, 12, 2138–2142. [Google Scholar] [CrossRef]
- Zhao, S.; Cheng, J.; Zhang, L.; Yao, J.; Jiang, J. Safety and effectiveness of iron sucrose therapy in paediatric patients with iron deficiency anaemia: A single-institution retrospective study. BMC Pediatr. 2025, 25, 601. [Google Scholar] [CrossRef] [PubMed]
- Loveikyte, R.; Duijvestein, M.; Mujagic, Z.; Goetgebuer, R.L.; Dijkstra, G.; Jong, A.E.v.d.M.-D. Predicting response to iron supplementation in patients with active inflammatory bowel disease (PRIme): A randomised trial protocol. BMJ Open 2024, 14, e077511. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Abhilasha, S. Efficacy of alternate day versus daily oral iron therapy in children with iron deficiency anemia: A randomized controlled trial. Int. J. Hematol. 2025; advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lu, D.; Zhang, Z.; Tao, L. Association of soluble transferrin receptor/log ferritin index with all-cause and cause-specific mortality: National Health and Nutrition Examination Survey. Front. Nutr. 2024, 11, 1275522. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y. Advances in the diagnosis and treatment of inflammatory bowel disease-associated anemia in children. J. Shanghai Jiao Tong Univ. (Med. Sci.) 2025, 45, 1232. [Google Scholar] [CrossRef]
- Syed, S.; Kugathasan, S.; Kumar, A.; Prince, J.; Schoen, B.T.; McCracken, C.; Ziegler, T.R.; Suchdev, P.S. Use of Reticulocyte Hemoglobin Content in the Assessment of Iron Deficiency in Children With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Tung, K.T.S.; Chen, C.; Chiu, Y.T.; So, H.K.; So, C.C.; Ip, P. Reference values for reticulocyte haemoglobin equivalent in healthy Chinese children under 5 years and its associations with various blood parameters. BMJ Paediatr. Open 2024, 8, e002736. [Google Scholar] [CrossRef]
- Harvey, P.R.; McNulty, D.; Coupland, B.; Kemos, P.; Croft, N.M.; Trudgill, N.J. The Risk of Venous Thromboembolism in Children with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2025, 31, 1861–1867. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Dong, H.C.; Wang, W.F.; Zhang, Y. Risk of venous thromboembolism in children and adolescents with inflammatory bowel disease: A systematic review and meta-analysis. World J. Gastroenterol. 2022, 28, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Bitton, A.; Carroll, M.W.; Kaplan, G.G.; Otley, A.R.; Singh, H.; Nguyen, G.C.; Griffiths, A.M.; A Stukel, T.; E Targownik, L.; et al. Inflammatory Bowel Disease Increases the Risk of Venous Thromboembolism in Children: A Population-Based Matched Cohort Study. J. Crohn’s Colitis 2021, 15, 2031–2040. [Google Scholar] [CrossRef]
- Mitchel, E.B.; Rosenbaum, S.; Gaeta, C.; Huang, J.; Raffini, L.J.; Baldassano, R.N.; Denburg, M.R.; Albenberg, L. Venous Thromboembolism in Pediatric Inflammatory Bowel Disease: A Case-Control Study. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 742–747. [Google Scholar] [CrossRef]
- Richard, N.; Leroyer, A.; Ley, D.; Dupont, C.; Bertrand, V.; Wils, P.; Gower-Rousseau, C.; Turck, D.; Guillon, N.; Sarter, H.; et al. Incidence and risk factors for thromboembolic events in pediatric-onset inflammatory bowel disease: A French population-based study. Dig. Liver Dis. 2025, 57, 584–594. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Risk Factors of Venous Thromboembolism in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 693927. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, Y.; Liu, Y.; Zhan, H.; Niu, P.; Chen, H.; Zhang, J. Proportion and risk factors for hospital-acquired venous thromboembolism in children: A systematic review and meta-analysis of data from 20 million individuals in 22 countries. Res. Pract. Thromb. Haemost. 2024, 8, 102541. [Google Scholar] [CrossRef]
- Aardoom, M.A.; Klomberg, R.C.; Kemos, P.; Ruemmele, F.M.; van Ommen, C.H.; de Ridder, L.; Croft, N.M. The Incidence and Characteristics of Venous Thromboembolisms in Paediatric-Onset Inflammatory Bowel Disease: A Prospective International Cohort Study Based on the PIBD-SETQuality Safety Registry. J. Crohn’s Colitis 2021, 16, 695–707. [Google Scholar] [CrossRef]
- van Ommen, C.H. Thromboprophylaxis in Children: Navigating Uncharted Waters. Hamostaseologie 2025, 45, 302–311. [Google Scholar] [CrossRef]
- Yuan, D.; Chu, J.; Lin, H.; Zhu, G.; Qian, J.; Yu, Y.; Yao, T.; Ping, F.; Chen, F.; Liu, X. Mechanism of homocysteine-mediated endothelial injury and its consequences for atherosclerosis. Front. Cardiovasc. Med. 2023, 9, 1109445. [Google Scholar] [CrossRef]
- Li, B.; Kou, Y.; Zhang, L.; Yi, L. Hyperhomocysteinemia-Driven Ischemic Stroke: Unraveling Molecular Mechanisms and Therapeutic Horizons. Food Sci. Nutr. 2025, 13, e70517. [Google Scholar] [CrossRef]
- Nair, A.S.; Tauro, L.; Joshi, H.B.; Makhal, A.; Sobczak, T.; Goret, J.; Dewitte, A.; Kaveri, S.; Chakrapani, H.; Matsuda, M.M.; et al. Influence of homocysteine on regulating immunothrombosis: Mechanisms and therapeutic potential in management of infections. Inflamm. Res. 2025, 74, 86. [Google Scholar] [CrossRef]
- Saija, C.; Currò, M.; Ientile, R.; Caccamo, D.; Bertuccio, M.P. Impact of Alterations in Homocysteine, Asymmetric Dimethylarginine and Vitamins-Related Pathways in Some Neurodegenerative Diseases: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3672. [Google Scholar] [CrossRef] [PubMed]
- Jacovina, A.T.; Deora, A.B.; Ling, Q.; Broekman, M.J.; Almeida, D.; Greenberg, C.B.; Marcus, A.J.; Smith, J.D.; Hajjar, K.A. Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2–dependent fibrinolysis. J. Clin. Investig. 2009, 119, 3384–3394. [Google Scholar] [CrossRef] [PubMed]
- Gala, D.; Newsome, T.; Roberson, N.; Lee, S.M.; Thekkanal, M.; Shah, M.; Kumar, V.; Bandaru, P.; Gayam, V. Thromboembolic Events in Patients with Inflammatory Bowel Disease: A Comprehensive Overview. Diseases 2022, 10, 73. [Google Scholar] [CrossRef]
- Wu, H.; Hu, T.; Hao, H.; A Hill, M.; Xu, C.; Liu, Z. Inflammatory bowel disease and cardiovascular diseases: A concise review. Eur. Heart J. Open 2022, 2, oeab029. [Google Scholar] [CrossRef]
- Carvalho, A.C.; Pinho, J.; Cancela, E.; Vieira, H.M.; Silva, A.; Ministro, P. Inflammatory bowel disease and thromboembolic events: A c’lot to learn. Ther. Adv. Gastroenterol. 2022, 15, 17562848221100626. [Google Scholar] [CrossRef] [PubMed]
- Britzen-Laurent, N.; Weidinger, C.; Stürzl, M. Contribution of Blood Vessel Activation, Remodeling and Barrier Function to Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2023, 24, 5517. [Google Scholar] [CrossRef]
- Yao, M.; Ma, J.; Wu, D.; Fang, C.; Wang, Z.; Guo, T.; Mo, J. Neutrophil extracellular traps mediate deep vein thrombosis: From mechanism to therapy. Front. Immunol. 2023, 14, 1198952. [Google Scholar] [CrossRef] [PubMed]
- Aklilu, A.; Lai, M.S.-L.; Jiang, Z.; Yip, S.P.; Huang, C.-L. Immunothrombosis in Sepsis: Cellular Crosstalk, Molecular Triggers, and Therapeutic Opportunities—A Review. Int. J. Mol. Sci. 2025, 26, 6114. [Google Scholar] [CrossRef] [PubMed]
- Maneta, E.; Aivalioti, E.; Tual-Chalot, S.; Veseli, B.E.; Gatsiou, A.; Stamatelopoulos, K.; Stellos, K. Endothelial dysfunction and immunothrombosis in sepsis. Front. Immunol. 2023, 14, 1144229. [Google Scholar] [CrossRef]
- Bin Park, S.; Lee, D.H.; Kim, S.Y.; So, H.S.; Sung, J.E.; Lee, K.; Yang, G. The Inflammasome-NETosis Axis: A Critical Pathway in Atherosclerosis Pathogenesis and Therapeutic Targeting. Innov. Acupunct. Med. 2025, 18, 12. [Google Scholar] [CrossRef]
- Sleutjes, J.A.M.; van Lennep, J.E.R.; van der Woude, C.J.; de Vries, A.C. Thromboembolic and atherosclerotic cardiovascular events in inflammatory bowel disease: Epidemiology, pathogenesis and clinical management. Ther. Adv. Gastroenterol. 2021, 14, 175628482110321. [Google Scholar] [CrossRef]
- Xu, C.; Song, Z.; Hu, L.-T.; Tong, Y.-H.; Hu, J.-Y.; Shen, H. Abnormal platelet parameters in inflammatory bowel disease: A systematic review and meta-analysis. BMC Gastroenterol. 2024, 24, 214. [Google Scholar] [CrossRef]
- De Laffolie, J.; Ballauff, A.; Wirth, S.; Blueml, C.; Rommel, F.R.; Claßen, M.; Laaß, M.; Lang, T.; Hauer, A.C.; CEDATA-GPGE Study Group. Occurrence of Thromboembolism in Paediatric Patients with Inflammatory Bowel Disease: Data From the CEDATA-GPGE Registry. Front. Pediatr. 2022, 10, 883183. [Google Scholar] [CrossRef]
- Rohani, P.; Taraghikhah, N.; Nasehi, M.M.; Alimadadi, H.; Aghdaei, H.A. Cerebrovascular Events in Pediatric Inflammatory Bowel Disease: A Review of Published Cases. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Shujun, W.; Huijie, Z.; Xia, B.; Hongjian, W. Cerebral venous sinus thrombosis in patients with inflammatory bowel disease: A retrospective study. Sci. Rep. 2021, 11, 17004. [Google Scholar] [CrossRef]
- Engel, E.R.; Nguyen, A.T.H.; Amankwah, E.K.; Albisetti, M.; Brandão, L.R.; Goldenberg, N.A.; Betensky, M. Predictors of postthrombotic syndrome in pediatric thrombosis: A systematic review and meta-analysis of the literature. J. Thromb. Haemost. 2020, 18, 2601–2612. [Google Scholar] [CrossRef]
- Nguyen, M.H.N.; Sieloff, E.M.; Tariq, T.; Bannon, S.F. Portal vein thrombosis as an initial presentation of Crohn’s disease. Clin. J. Gastroenterol. 2021, 14, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Alhalabi, M.; Nasri, D.; Aji, W. Portal vein thrombosis as extraintestinal complications of Crohn’s disease: A case report and review of literature. J. Med. Case Rep. 2024, 18, 246. [Google Scholar] [CrossRef]
- Chien, K.A.; Cooley, V.; Prishtina, F.M.; Grinspan, Z.M.; Gerber, L.M.; Kucine, N. Health and Financial Burdens Associated with Venous Thrombosis in Hospitalized Children with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 748–751. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis—An Evidence-based Consensus Guideline From the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 292–310. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Bernstein, C.N.; Bitton, A.; Chan, A.K.; Griffiths, A.M.; Leontiadis, G.I.; Geerts, W.; Bressler, B.; Butzner, J.D.; Carrier, M.; et al. Consensus Statements on the Risk, Prevention, and Treatment of Venous Thromboembolism in Inflammatory Bowel Disease: Canadian Association of Gastroenterology. Gastroenterology 2014, 146, 835–848.e6. [Google Scholar] [CrossRef] [PubMed]
- Torrente, F.; Meade, S.; I Benchimol, E.; de Ridder, L.; Croft, N.M.; Kammermeier, J.; Mack, D.R.; Klomberg, R.C.W.; Turner, D.; Wilson, D.C.; et al. Thromboprophylaxis Use in Paediatric Inflammatory Bowel Disease: An International RAND Appropriateness Panel. J. Crohn’s Colitis 2022, 16, 1609–1616. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Q.; Liao, J.; Wang, N.; Li, S.; Zhang, Q.; Yu, F.; Luo, J.; Wang, H.; Hu, D.; et al. Addressing challenges in pediatric thrombosis: A comprehensive guideline development. Front. Pediatr. 2025, 13, 1519517. [Google Scholar] [CrossRef]
- Lenahan, S.F.; Blackmore, A.; Fenchel, M.; Thomas, E.; Palumbo, J.S.; Tarango, C. Recurrent Thrombosis and Major Bleeding in Children Treated for VTE. Blood Adv. 2025, 9, 3824–3831. [Google Scholar] [CrossRef]
- Pelland-Marcotte, M.-C.; Bouchard, V.; Bégin, E.; Bouhêlier, È.; Santiago, R.; Monagle, P. Biomarkers in pediatric venous thromboembolism: A systematic review of the literature. J. Thromb. Haemost. 2023, 21, 1831–1848. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhu, L.; Zhao, S.; Zheng, K.; Xu, L.; Shen, H. Fibrinogen, FDP and D-Dimer as Potential Biomarkers for Disease Severity in Ulcerative Colitis: A Retrospective Study. Int. J. Gen. Med. 2024, 17, 5573–5579. [Google Scholar] [CrossRef]
- Kaz, A.M.; Venu, N. Diagnostic Methods and Biomarkers in Inflammatory Bowel Disease. Diagnostics 2025, 15, 1303. [Google Scholar] [CrossRef]
- Tripodi, A.; Spina, L.; Pisani, L.F.; Padovan, L.; Cavallaro, F.; Chantarangkul, V.; Valsecchi, C.; Peyvandi, F.; Vecchi, M. Anti-TNF-α Treatment Reduces the Baseline Procoagulant Imbalance of Patients With Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, 1901–1908. [Google Scholar] [CrossRef]
- Capecchi, M.; Ciavarella, A.; Artoni, A.; Abbattista, M.; Martinelli, I. Thrombotic Complications in Patients with Immune-Mediated Hemolysis. J. Clin. Med. 2021, 10, 1764. [Google Scholar] [CrossRef] [PubMed]
- Jongerius, I.; Zeerleder, S.S.; Delvasto-Núñez, L.; Roem, D.; Bakhtiari, K.; van Mierlo, G.J.; Meijers, J.C.M. Iron-Driven Alterations on Red Blood Cell-Derived Microvesicles Amplify Coagulation during Hemolysis via the Intrinsic Tenase Complex. Thromb. Haemost. 2022, 122, 80–91. [Google Scholar] [CrossRef]
- Kalff, H.; Cario, H.; Holzhauer, S. Iron deficiency anemia and thrombosis risk in children—Revisiting an old hypothesis. Front. Pediatr. 2022, 10, 926925. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, Y.Y.; Seebeck, U.; Bührlen, M.; Overberg, D. Case Report: Cerebral venous sinus thrombosis in the setting of iron deficiency anemia and a high level of lipoprotein (a) in a child. Front. Pediatr. 2025, 13, 1449323. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Khan, A.N.; Chaudhary, A.J.; Iqbal, S.; Ismail, R. A Rare Case of Recurrent Arterial Thrombosis Secondary to Iron Deficiency Anemia. Cureus 2022, 14, e22117. [Google Scholar] [CrossRef]
- Ezeh, E.; Katabi, A.; Khawaja, I. Iron Deficiency Anemia as a Rare Risk Factor for Recurrent Pulmonary Embolism and Deep Vein Thrombosis. Cureus 2021, 13, e13721. [Google Scholar] [CrossRef]
- Tang, X.; Fang, M.; Cheng, R.; Zhang, Z.; Wang, Y.; Shen, C.; Han, Y.; Lu, Q.; Du, Y.; Liu, Y.; et al. Iron-Deficiency and Estrogen Are Associated with Ischemic Stroke by Up-Regulating Transferrin to Induce Hypercoagulability. Circ. Res. 2020, 127, 651–663. [Google Scholar] [CrossRef]
- Kei, T.; Mistry, N.; Curley, G.; Pavenski, K.; Shehata, N.; Tanzini, R.M.; Gauthier, M.-F.; Thorpe, K.; Schweizer, T.A.; Ward, S.; et al. Efficacy and safety of erythropoietin and iron therapy to reduce red blood cell transfusion in surgical patients: A systematic review and meta-analysis. Can. J. Anesth. 2019, 66, 716–731. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Kessler, C.M.; Auerbach, M. Recognition of thrombotic risk of thrombocytosis in iron deficiency. Haematologica 2021, 106, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Janaszak-Jasiecka, A.; Siekierzycka, A.; Płoska, A.; Dobrucki, I.T.; Kalinowski, L. Endothelial Dysfunction Driven by Hypoxia—The Influence of Oxygen Deficiency on NO Bioavailability. Biomolecules 2021, 11, 982. [Google Scholar] [CrossRef]
- Rossetti, G.M.K.; Oliver, S.J.; Sandoo, A.; Macdonald, J.H. Hypoxia-induced endothelial dysfunction: Could targeting oxidative stress provide protection? Exp. Physiol. 2023, 108, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Chennupati, R.; Solga, I.; Wischmann, P.; Dahlmann, P.; Celik, F.G.; Pacht, D.; Şahin, A.; Yogathasan, V.; Hosen, M.R.; Gerdes, N.; et al. Chronic anemia is associated with systemic endothelial dysfunction. Front. Cardiovasc. Med. 2023, 10, 1099069. [Google Scholar] [CrossRef]
- Zezos, P.; Kouklakis, G.; Saibil, F. Inflammatory bowel disease and thromboembolism. World J. Gastroenterol. 2014, 20, 13863. [Google Scholar] [CrossRef]
- Giannotta, M.; Tapete, G.; Emmi, G.; Silvestri, E.; Milla, M. Thrombosis in inflammatory bowel diseases: What’s the link? Thromb. J. 2015, 13, 14. [Google Scholar] [CrossRef]
- Schellenberg, C.; Lagrange, J.; Ahmed, M.U.; Arnone, D.; Campoli, P.; Louis, H.; Touly, N.; Caron, B.; Plénat, F.; Perrin, J.; et al. The Role of Platelets and von Willebrand Factor in the Procoagulant Phenotype of Inflammatory Bowel Disease. J. Crohn’s Colitis 2023, 18, 751–761. [Google Scholar] [CrossRef]
- McNeil, R.; Fredman, D.; Eldar, O.; Gafter-Gvili, A.; Avni, T. Venous Thromboembolism Prophylaxis in Inflammatory Bowel Disease Inpatients: Systematic Review and Meta-Analysis. Acta Haematol. 2024, 147, 702–715. [Google Scholar] [CrossRef]
- Sharma, N.; Tewatia, P.; Harvey, P.R.; Kumar, A. Controversies in Venous Thromboembolism Risk Assessment in Inflammatory Bowel Disease: A Narrative Review. Diagnostics 2024, 14, 2112. [Google Scholar] [CrossRef]
| Mechanism | Pathophysiology | Clinical Relevance/Notes [12,14] |
|---|---|---|
| Iron deficiency anemia | Chronic blood loss from inflamed gastrointestinal mucosa, reduced iron absorption due to inflammation (especially in CD affecting the duodenum) | The most common cause; it may coexist with anemia of chronic disease. |
| Anemia of chronic disease | Inflammation → ↑ Hepcidin → ↓ Iron release from stores and ↓ absorption | normocytic or microcytic anemia; poor response to oral iron. |
| Vitamin B12 deficiency | Terminal ileum involvement or resection impairs B12 absorption | macrocytic anemia; especially in CD with ileal disease/resection. |
| Folate deficiency | Malabsorption, poor intake, or medication-related (methotrexate, sulfasalazine) | macrocytic anemia; less common than B12 deficiency. |
| Acute post-hemorrhagic anemia | Anemia caused by sudden and significant blood loss | accompanied by severe bleeding manifested by symptoms such as melena, hematemesis, rectal bleeding |
| Bone marrow suppression | Inflammatory cytokines suppress erythropoiesis (e.g., IL-6, TNF-α). | It can result in pancytopenia or isolated anemia; associated with low reticulocyte count. |
| Medication-induced anemia | Sulfasalazine, azathioprine, and methotrexate can impair hematopoiesis. | It requires monitoring of the complete blood count (CBC) regularly. |
| Hemolysis | Autoimmune hemolytic anemia or secondary to drugs (rare in IBD) | Consider in cases with elevated lactate dehydrogenase (LDH), reticulocytes, and low haptoglobin; Coombs-positive anemia possible (especially in UC) |
| Mechanism | Description |
|---|---|
| Endothelial dysfunction | Chronic anemia and hypoxia damage endothelium, promoting a prothrombotic state. |
| Increased platelet reactivity | Certain anemia types cause platelet activation or enhanced aggregation. |
| Elevated EPO | High EPO levels stimulate platelet production and activation. |
| Turbulent blood flow | Reduced hematocrit increases shear stress and turbulence, promoting endothelial activation. |
| Free hemoglobin/heme | In hemolysis, free hemoglobin scavenges NO, inducing vasoconstriction and platelet activation. |
| Microparticle release | RBC- and platelet-derived microparticles exhibit strong procoagulant activity. |
| Iron deficiency-induced thrombocytosis | Reactive thrombocytosis in IDA increases thrombotic risk. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesoi, D.-F.; Hancianu, M.; Trandafir, L.M.; Ciocoiu, M.; Vladeanu, M.C.; Barbosu, L.-I.; Bozomitu, L.; Frasinariu, O.E.; Bararu-Bojan, I.; Badulescu, O.-V. Thrombosis and Anemia in Pediatric Inflammatory Bowel Disease: Pathophysiology, Clinical Impact and Future Directions. Int. J. Mol. Sci. 2025, 26, 10407. https://doi.org/10.3390/ijms262110407
Tesoi D-F, Hancianu M, Trandafir LM, Ciocoiu M, Vladeanu MC, Barbosu L-I, Bozomitu L, Frasinariu OE, Bararu-Bojan I, Badulescu O-V. Thrombosis and Anemia in Pediatric Inflammatory Bowel Disease: Pathophysiology, Clinical Impact and Future Directions. International Journal of Molecular Sciences. 2025; 26(21):10407. https://doi.org/10.3390/ijms262110407
Chicago/Turabian StyleTesoi, Dragos-Florin, Monica Hancianu, Laura Mihaela Trandafir, Manuela Ciocoiu, Maria Cristina Vladeanu, Larisa-Ioana Barbosu, Laura Bozomitu, Otilia Elena Frasinariu, Iris Bararu-Bojan, and Oana-Viola Badulescu. 2025. "Thrombosis and Anemia in Pediatric Inflammatory Bowel Disease: Pathophysiology, Clinical Impact and Future Directions" International Journal of Molecular Sciences 26, no. 21: 10407. https://doi.org/10.3390/ijms262110407
APA StyleTesoi, D.-F., Hancianu, M., Trandafir, L. M., Ciocoiu, M., Vladeanu, M. C., Barbosu, L.-I., Bozomitu, L., Frasinariu, O. E., Bararu-Bojan, I., & Badulescu, O.-V. (2025). Thrombosis and Anemia in Pediatric Inflammatory Bowel Disease: Pathophysiology, Clinical Impact and Future Directions. International Journal of Molecular Sciences, 26(21), 10407. https://doi.org/10.3390/ijms262110407







