Anticancer Potential of Whey Proteins—A Systematic Review of Bioactivity and Functional Mechanisms
Abstract
1. Introduction
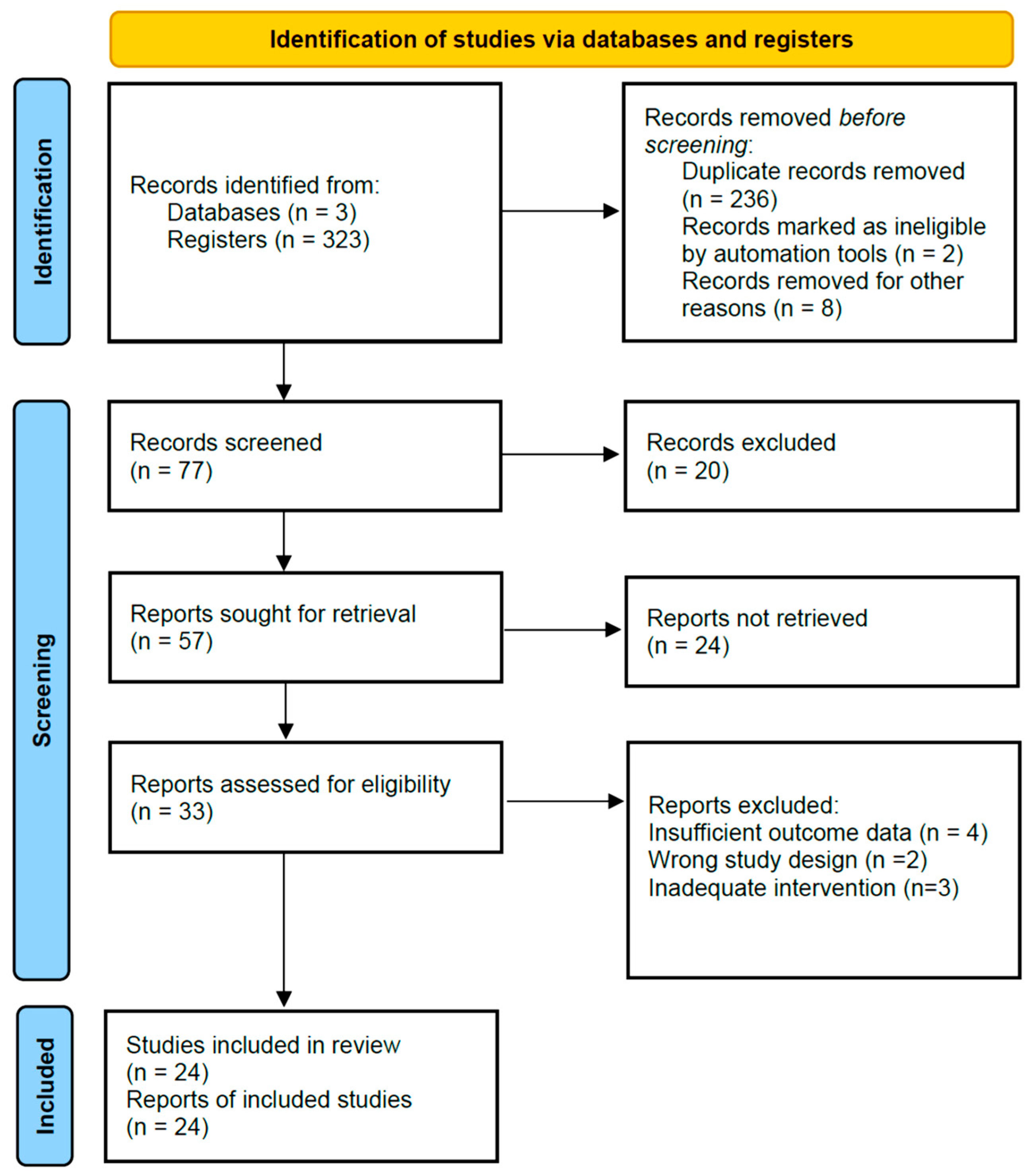
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Van Eycken, L.J.; Giannopoulos, E.; Tittenbrun, Z.; Piñeros, M.; Mery, L.; Rahal, R.; Helliwell, T.R.; Kohler, B.A.; Aitken, J.F.; Rous, B.A.; et al. Future of population-based cancer registries: A global perspective—A survey of population-based cancer registries. Int. J. Cancer 2025, 157, 1566–1576. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Rubio-Zarapuz, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional and Exercise Interventions in Cancer-Related Cachexia: An Extensive Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 4604. [Google Scholar] [CrossRef]
- Braha, A.; Albai, A.; Timar, B.; Negru, Ș.; Sorin, S.; Roman, D.; Popovici, D. Nutritional Interventions to Improve Cachexia Outcomes in Cancer—A Systematic Review. Medicina 2022, 58, 966. [Google Scholar] [CrossRef]
- Thampy, A.; Palani Kumar, M.K.; Serva Peddha, M.; Reddy, M. The effectiveness of whey proteins in prevention and treatment of cancer: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2088–2104. [Google Scholar] [CrossRef]
- Krissansen, G.W. Emerging Health Properties of Whey Proteins and Their Clinical Implications. J. Am. Coll. Nutr. 2007, 26, 713S–723S. [Google Scholar] [CrossRef]
- Cichosz, G.; Czeczot, H.; Bielecka, M. The anticarcinogenic potential of milk fat. Ann. Agric. Environ. Med. 2020, 27, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.S.; Das, S.K.; Mukherjee, S.; Ballard, B.R. Protection Against Dimethylbenz[a] Anthracene-Induced Breast Cancer in Female Rats by α-Lactalbumin. Int. J. Cancer Oncol. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Chen, G.Q.; Qu, Y.; Gras, S.L.; Kentish, S.E. Separation Technologies for Whey Protein Fractionation. Food Eng. Rev. 2023, 15, 438–465. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Wang, M.; She, Y.; Yang, B.; Sheng, Q.; El-Aty, A.A. β-Lactoglobulin separation from whey protein: A comprehensive review of isolation and purification techniques and future perspectives. J. Dairy Sci. 2024, 107, 11785–11795. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Pavadhgul, P.; Nunthanawanich, P.; Sirikanchanarod, A.; Adulbhan, A. Whey Protein Supplementation Improves Nutritional Status, Glutathione Levels, and Immune Function in Cancer Patients: A Randomized, Double-Blind Controlled Trial. J. Med. Food 2018, 21, 612–616. [Google Scholar] [CrossRef]
- Mazzuca, F.; Roberto, M.; Arrivi, G.; Sarfati, E.; Schipilliti, F.M.; Crimini, E.; Botticelli, A.; Di Girolamo, M.; Muscaritoli, M.; Marchetti, P. Clinical Impact of Highly Purified, Whey Proteins in Patients Affected With Colorectal Cancer Undergoing Chemotherapy: Preliminary Results of a Placebo-Controlled Study. Integr. Cancer Ther. 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Herrera-Martínez, A.D.; Navas Romo, A.; León-Idougourram, S.; Muñoz-Jiménez, C.; Rodríguez-Alonso, R.; García, G.M.; Camacho-Cardenosa, M.; Casado-Diaz, A.; Gálvez-Moreno, M.Á.; Puertas, M.J.M.; et al. Systemic Systemic Inflammation in Oncologic Patients Undergoing Systemic Treatment and Receiving Whey Protein-Based Nutritional Support. Int. J. Mol. Sci. 2024, 25, 5821. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Xavier Malcata, F. Bovine whey proteins—Overview on their main biological properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Di Patti, M.C.B.; Valenti, P.; Musci, G. Lactoferrin’s anti-cancer properties: Safety, selectivity, and wide range of action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, J.; Khan, M.Z.; Alugongo, G.M.; Chen, T.; Liu, S.; Li, S.; Cao, Z. Unlocking the potential of milk whey protein components in colorectal cancer prevention and therapy. Crit. Rev. Food Sci. Nutr. 2024, 64, 12961–12998. [Google Scholar] [CrossRef] [PubMed]
- Rabie, A.S.I.; Alhomsi, T.; AbouKhatwa, M.M.; Mosilhy, E.A.; Elzahaf, R.A. Impact of whey protein supplementation as adjuvant therapy on malnourished cancer patients: Systematic review and meta-analysis. Discov. Food 2024, 4, 118. [Google Scholar] [CrossRef]
- Nukumi, N.; Iwamori, T.; Kano, K.; Naito, K.; Tojo, H. Reduction of tumorigenesis and invasion of human breast cancer cells by whey acidic protein (WAP). Cancer Lett. 2007, 252, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sahna, K.O.; Cakir, B.; Tunali-Akbay, T. Antiproliferative Activity of Whey and Casein Bioactive Peptides on Breast Cancer: An In Vitro and In Silico Study. Int. J. Pept. Res. Ther. 2022, 28, 128. [Google Scholar] [CrossRef]
- Cheng, S.H.; Tseng, Y.M.; Wu, S.H.; Tsai, S.M.; Tsai, L.Y. Whey Protein Concentrate Renders MDA-MB-231 Cells Sensitive to Rapamycin by Altering Cellular Redox State and Activating GSK3β/mTOR Signaling. Sci. Rep. 2017, 7, 15976. [Google Scholar] [CrossRef]
- Singh, S.; Maurya, P.; Rani, S.; Mishra, N.; Nisha, R.; Singh, P.; Saraf, S.A. Development of doxorubicin hydrochloride–loaded whey protein nanoparticles and its surface modification with N-acetyl cysteine for triple-negative breast cancer. Drug Deliv. Transl. Res. 2022, 12, 3047–3062. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Maitiniyazi, G.; Liu, Y.; Chen, Y.; Guo, M.; He, J.; Tao, W.; Li, Z. Whey protein isolate attenuates depression-like behavior developed in a mouse model of breast tumor. Food Res. Int. 2023, 169, 112849. [Google Scholar] [CrossRef] [PubMed]
- Sabancılar, I.; Durak, M.H. Cytotoxic Activity of Whey Protein on Breast Cancer Cell in Vitro. SAS J. Med. 2022, 8, 543–548. [Google Scholar] [CrossRef]
- Ronis, M.J.; Hakkak, R.; Korourian, S.; Badger, T.M. Whey Protein Hydrolysate but not Whole Whey Protein Protects Against 7,12-Dimethylbenz(a)anthracene-Induced Mammary Tumors in Rats. Nutr. Cancer 2015, 67, 949–953. [Google Scholar] [CrossRef]
- Ramani, A.; Hazra, T.; Mudgil, S.; Mudgil, D. Emerging potential of whey proteins in prevention of cancer. Food Humanit. 2024, 2, 100199. [Google Scholar] [CrossRef]
- Mohammed, M.M.; Ramadan, G.; Zoheiry, M.K.; El-Beih, N.M. Antihepatocarcinogenic activity of whey protein concentrate and lactoferrin in diethylnitrosamine-treated male albino mice. Environ. Toxicol. 2019, 34, 1025–1033. [Google Scholar] [CrossRef]
- Rosa, L.S.; Santos, M.L.; Abreu, J.P.; Balthazar, C.F.; Rocha, R.S.; Silva, H.L.; Esmerino, E.A.; Duarte, M.C.K.; Pimentel, T.C.; Freitas, M.Q.; et al. Antiproliferative and apoptotic effects of probiotic whey dairy beverages in human prostate cell lines. Food Res. Int. 2020, 137, 109450. [Google Scholar] [CrossRef]
- Badr, G.; Sayed, E.A.; Abdel-Ghaffar, W.H.; Badr, B.M.; Sayed, L.H.; Sayed, A.; Mahmoud, M.H.; Alamery, S. Molecular mechanisms underlying antitumor activity of camel whey protein against multiple myeloma cells. Saudi J. Biol. Sci. 2021, 28, 2374–2380. [Google Scholar] [CrossRef]
- Ali, M.; Elsebaie, E. Milk whey proteins and onion extract powder interactions-antimicrobial and anticancer activities. J. Agroaliment. Process. Technol. 2018, 24, 152–160. [Google Scholar]
- Tsai, W.Y.; Chang, W.H.; Chen, C.H.; Lu, F.J. Enhancing effect of patented whey protein isolate (Immunocal) on cytotoxicity of an anticancer drug. Nutr. Cancer 2000, 38, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, D.; Martindale, W.; Romeih, E.; Hebishy, E. Recent advances in whey processing and valorisation: Technological and environmental perspectives. Int. J. Dairy Technol. 2023, 76, 291–312. [Google Scholar] [CrossRef]
- Soumati, B.; Atmani, M.; Benabderrahmane, A.; Benjelloun, M. Whey Valorization—Innovative Strategies for Sustainable Development and Value-Added Product Creation. J. Ecol. Eng. 2023, 24, 86–104. [Google Scholar] [CrossRef]
- Sharma, R. Whey Proteins in Functional Foods. In Whey Proteins: From Milk to Medicine; Academic Press: Cambridge, MA, USA, 2019; pp. 637–663. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, Y.; Xiong, J.; Zeng, Q.; Gan, Y.; Jiang, K.; Xie, N. Anti-breast cancer effects of dairy protein active peptides, dairy products, and dairy protein-based nanoparticles. Front. Pharmacol. 2024, 15, 1486264. [Google Scholar] [CrossRef] [PubMed]
- Murali, C.; Mudgil, P.; Gan, C.Y.; Tarazi, H.; El-Awady, R.; Abdalla, Y.; Amin, A.; Maqsood, S. Camel whey protein hydrolysates induced G2/M cellcycle arrest in human colorectal carcinoma. Sci. Rep. 2021, 11, 7062. [Google Scholar] [CrossRef] [PubMed]
- Sabancılar, İ. Cytoxic Effect Levels Of Sheep Whey Protein in Colorectal Adenocarcinoma Cell Line (Caco-2). Int. Arch. Med. Res. 2022, 14, 24–29. [Google Scholar] [CrossRef]
- De Simone, C.; Ferranti, P.; Picariello, G.; Scognamiglio, I.; Dicitore, A.; Addeo, F.; Chianese, L.; Stiuso, P. Peptides from water buffalo cheese whey induced senescence cell death via ceramide secretion in human colon adenocarcinoma cell line. Mol. Nutr. Food Res. 2011, 55, 229–238. [Google Scholar] [CrossRef]
- Eason, R.R.; Till, S.R.; Frank, J.A.; Badger, T.M.; Korourian, S.; Simmen, F.A.; Simmen, R.C.M. Tumor-protective and tumor-promoting actions of dietary whey proteins in an N-methyl-N-nitrosourea model of rat mammary carcinogenesis. Nutr. Cancer 2006, 55, 171–177. [Google Scholar] [CrossRef]
- Attaallah, W.; Yılmaz, A.M.; Erdoğan, N.; Yalçın, A.S.; Aktan, A.Ö. Whey protein versus whey protein hydrolyzate for the protection of azoxymethane and dextran sodium sulfate induced colonic tumors in rats. Pathol. Oncol. Res. 2012, 18, 817–822. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Salzano, A.; D’Onofrio, N.; Venneri, T.; Cicco, P.D.; Vinale, F.; Petillo, O.; Martano, M.; Maiolino, P.; Neglia, G.; et al. Buffalo Milk Whey Activates Necroptosis and Apoptosis in a Xenograft Model of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 8464. [Google Scholar] [CrossRef]
- Hakkak, R.; Korourian, S.; JRonis, M.J.; Johnston, J.M.; Badger, T.M. KPS Dietary Whey Protein Protects against Azoxymethane-induced Colon Tumors in Male Rats 1. Cancer Epidemiol. Biomark. Prev. 2001, 10, 555–558. [Google Scholar]
- Aksoy, F.T.; Yilmaz, A.M.; Bicim, G.; Yalcin, A.S. Effect of whey protein derivatives on cell viability, cell migration and cell cycle phases in MCF-7 cells. Marmara Med. J. 2023, 36, 39–45. [Google Scholar] [CrossRef]
- Dreanca, A.; Sarpataki, O.; Popescu, A.; Toma, A.G.; Moldovan, M.; Prodan, D.; Nagy, A.; Sevastre, B.; Marcus, I. The Study of Nutraceutical Effects of the Whey Zonar and of Lyophilized Concentrate Obtained from Zonar in C26 Colon Carcinoma Grafted Subcutaneously in Balb/C Mice. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2022, 79, 1–11. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Martino, E.; Balestrieri, A.; Mele, L.; Neglia, G.; Balestrieri, M.L.; Campanile, G. SIRT3 and Metabolic Reprogramming Mediate the Antiproliferative Effects of Whey in Human Colon Cancer Cells. Cancers 2021, 13, 5196. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Venneri, T.; Salzano, A.; D’Onofrio, N.; Martano, M.; Saggese, A.; Vinale, F.; Neglia, G.; Campanile, C.; Baccigalupi, L.; et al. Chemopreventive effect of a milk whey by-product derived from Buffalo (Bubalus bubalis) in protecting from colorectal carcinogenesis. Cell Commun. Signal. 2023, 21, 245. [Google Scholar] [CrossRef]
- Taghipour, M.J.; Ezzatpanah, H.; Ghahderijani, M. In vitro and in silico studies for the identification of anti-cancer and antibacterial peptides from camel milk protein hydrolysates. PLoS ONE 2023, 18, e0288260. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Yang, M.; Li, Q.; Cheng, Q.; Wang, Y.; Ye, L.; Tian, F.; Ding, H.; Ling, Y.; Xia, M.; et al. Antitumor activity of a whey peptide-based enteral diet in C26 colon tumor-bearing mice. J. Food Sci. 2023, 88, 4275–4288. [Google Scholar] [CrossRef]
- Duarte, D.C.; Nicolau, A.; Teixeira, J.A.; Rodrigues, L.R. The effect of bovine milk lactoferrin on human breast cancer cell lines. J. Dairy Sci. 2011, 94, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Boukhettala, N.; Ibrahim, A.; Aziz, M.; Vuichoud, J.; Saudan, K.Y.; Blum, S.; Déchelotte, P.; Breuillé, D.; Coëffier, M. A Diet Containing Whey Protein, Free Glutamine, and Transforming Growth Factor-β Ameliorates Nutritional Outcome and Intestinal Mucositis during Repeated Chemotherapeutic Challenges in Rats. J. Nutr. 2010, 140, 799–805. [Google Scholar] [CrossRef]
- Eason, R.R.; Velarde, M.C.; Chatman, L.; Till, S.R.; Geng, Y.; Ferguson, M.; Badger, T.M.; Simmen, R.C.M. Dietary Exposure to Whey Proteins Alters Rat Mammary Gland Proliferation, Apoptosis, and Gene Expression during Postnatal Development. J. Nutr. 2004, 134, 3370–3377. [Google Scholar] [CrossRef][Green Version]
- Khan, U.M.; Selamoglu, Z. Nutritional and Medical Perspectives of Whey Protein: A Historical Overview. J. Pharm. Care 2019, 7, 112–117. [Google Scholar]
- Martino, E.; Balestrieri, A.; Mele, L.; Sardu, C.; Marfella, R.; D’onofrio, N.; Campanile, G.; Balestrieri, M.L. Milk Exosomal miR-27b Worsen Endoplasmic Reticulum Stress Mediated Colorectal Cancer Cell Death. Nutrients 2022, 14, 5081. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Main Mechanisms | Major Cancer Models |
|---|---|---|
| Whey Protein Concentrate (WPC) | Antioxidant activity, Glutathione synthesis, ROS scavenging | Colorectal, Breast |
| Whey Protein Isolate (WPI) | mTOR/PI3K inhibition, Immune activation | Liver, Breast |
| Whey Protein Hydrolysate (WPH) | Apoptosis induction, Caspase-3 activation, Cell cycle arrest | Colon, Mammary |
| Bioactive Peptides | Gut microbiota modulation, Anti-inflammatory effect | Prostate, Multiple myeloma |
| Study | Study Design | Cancer Type | Whey Protein Type | Key Outcomes | Risk of Bias Tool | Risk of Bias Judgment |
|---|---|---|---|---|---|---|
| De Simone et al., 2011 [37] | In vitro | Colon adenocarcinoma | Peptide fractions from buffalo cheese whey | Reduced cell viability, cell cycle arrest | Reproducibility criteria | High |
| Eason et al., 2006 [38] | Experimental animal | Mammary carcinoma | Whey protein hydrolysate | Decreased tumor incidence, delayed tumor appearance | ARRIVE checklist | Some concerns |
| Attaallah et al., 2012 [39] | Experimental animal | Colon cancer | Whey protein concentrate and hydrolysate | Reduced tumor development | ARRIVE checklist | Some concerns |
| Cacciola et al., 2022 [40] | Experimental animal | Colorectal cancer | Delactosed milk whey | Activation of necroptosis and apoptosis pathways | ARRIVE checklist | Some concerns |
| Badr et al., 2021 [28] | In vitro | Multiple myeloma | Camel whey protein | Reduced cell viability, induced apoptosis | Reproducibility criteria | Some concerns |
| Mohammed et al., 2019 [26] | Experimental animal | Hepatocarcinoma | Whey protein concentrate | Alleviated liver carcinoma markers, improved antioxidant defense | ARRIVE checklist | Low |
| Ronis et al., 2015 [24] | Experimental animal | Mammary tumors | Whey protein hydrolysate | Reduced tumor incidence and increased tumor latency | ARRIVE checklist | Low |
| I.Sabancılar & Durak, 2022 [23] | In vitro | Breast cancer | Whey protein (unspecified) | Reduced cell viability | Reproducibility criteria | High |
| Hakkak et al., 2001, [41] | Experimental animal | Colon cancer | Whey protein (unspecified) | Reduced tumor incidence | ARRIVE checklist | Some concerns |
| I.Sabancılar, 2022 [36] | In vitro | Colorectal adenocarcinoma | Sheep whey protein | Reduced cell viability | Reproducibility criteria | Low |
| Aksoy et al., 2023 [42] | In vitro | Breast cancer | Whey protein derivatives | Decreased cell viability and migration | Reproducibility criteria | Some concerns |
| Dreanca et al., [43] | Experimental animal | Colon carcinoma | Whey beverage and concentrate | Reduced tumor volume, increased glutathione levels | ARRIVE checklist | Low |
| D’onofrio et al., 2021 [44] | In vitro | Colorectal cancer | Whey extracts | Inhibited cell proliferation, induced apoptosis | Reproducibility criteria | Low |
| Tsai et al., 2000 [30] | In vitro | Hepatoma | Whey protein isolate | Enhanced cytotoxicity of anticancer drugs | Reproducibility criteria | Some concerns |
| Ali & Elsebaie, 2018 [29] | In vitro | Breast and lung cancer | Whey protein isolate | Cytotoxic activity against cancer cells | ARRIVE and reproducibility | Some concerns |
| Cacciola et al., 2023 [45] | Experimental animal and in vitro | Colorectal cancer | Deactylated buffalo milk whey | Reduced tumor incidence, induced apoptosis | ARRIVE checklist | Some concerns |
| Taghipour et al., 2023 [46] | In vitro | Breast cancer | Whey protein hydrolysates | Reduced cell viability | Reproducibility criteria | Low |
| Rosa et al., 2020 [27] | In vitro | Prostate cancer | Whey beverages | Antiproliferative and apoptotic effects | ARRIVE checklist | Low |
| Liu et al., 2023 [47] | Experimental animal | Colon tumor | Whey peptide-based enteral diet. | Reduced tumor weight, increased apoptosis | ARRIVE checklist | Some concerns |
| Murali et al., 2021 [35] | In vitro | Colon carcinoma | Camel whey protein hydrolysates | Inhibited cell growth, induced cell cycle arrest | Reproducibility criteria | Low |
| Duarte et al., 2011 [48] | In vitro | Breast cancer | Lactoferrin | Decreased cell viability, increased apoptosis | Reproducibility criteria | Low |
| Mazzuca et al., 2019 [12] | Randomized controlled trial | Colorectal cancer | Highly purified whey protein | Improved nutritional status, reduced chemotherapy toxicity | Cochrane RoB 2.0 | Some concerns |
| Boukhettala et al., 2010 [49] | Experimental animal | chemotherapy-induced mucositis | Whey protein (unspecified) | Improved nutritional outcome, reduced intestinal mucositis. | ARRIVE checklist | Some concerns |
| Bumrungpert et al. [11] | Randomized controlled trial | Various cancers | Whey protein isolate | Improved nutritional status, increased glutathione levels | Reproducibility criteria | Low |
| Effect Type | Mechanism | Effectiveness | Study Reference |
|---|---|---|---|
| Antiproliferative | Cell cycle arrest | Significant reduction in cell viability | [37] |
| Apoptosis induction | Activation of apoptotic pathways | Increased apoptosis in cancer cells | [40] |
| Tumor suppression | Reduced tumor incidence and growth | Decreased tumor development in animal models | [50] |
| Metastasis inhibition | Decreased cell migration | Reduced cell migration in vitro | [42] |
| Enhanced drug efficacy | Synergistic effect with anticancer drugs | Improved cytotoxicity of chemotherapy | [30] |
| Effect Type | Mechanism | Effectiveness | Study Reference |
|---|---|---|---|
| Antioxidant activity | Enhanced glutathione production | Improved antioxidant defense | [26] |
| Immune enhancement | Stimulation of the immune response | Potential boost in anticancer immunity | [51] |
| Anti-inflammatory | Modulation of inflammatory markers | Reduced inflammation in cancer models | [45] |
| Gut microbiota modulation | Alteration of microbial composition | Potential indirect anticancer effects | [45] |
| Metastasis inhibition | Decreased cell migration | Reduced cell migration in vitro | [42] |
| Enhanced drug efficacy | Synergistic effect with anticancer drugs | Improved cytotoxicity of chemotherapy | [30] |
| Mechanism | Target Molecules | Observed Effects | Cancer Type |
|---|---|---|---|
| Intrinsic pathway activation | Cytochrome C, Bcl-2 family proteins | Increased apoptosis | Multiple myeloma |
| Extrinsic pathway activation | Caspase-3, PARP-1 | Enhanced apoptotic signaling | Colorectal cancer |
| Cell cycle regulation | Cyclin A, p21, p53 | Cell cycle arrest | Colon adenocarcinoma |
| Endoplasmic Reticulum (ER) stress induction | PERK/IRE1/XBP1, CHOP | Increased ER stress-mediated apoptosis | Colorectal cancer |
| Mechanism | Target Molecules | Observed Effects | Cancer Type |
|---|---|---|---|
| Glutathione synthesis | Glutathione (GSH), Glutathione S-transferase alpha (GST-α) | Enhanced antioxidant defense | Hepatocarcinoma |
| Reactive Oxygen Species (ROS) scavenging | Superoxide dismutase, Catalase | Reduced oxidative stress | Colorectal cancer |
| Iron chelation | Lactoferrin | Prevention of iron-induced oxidative damage | Various cancers |
| Mechanism | Target Molecules | Observed Effects | Cancer Type |
|---|---|---|---|
| Protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway inhibition | AKT, mTOR | Reduced cell proliferation and survival | Multiple myeloma |
| NF-κB pathway modulation | NF-κB, IκB | Altered inflammatory response | Colorectal cancer |
| MAPK pathway regulation | ERK, JNK, p38 | Modified cell growth and apoptosis signaling | Modified cell growth and apoptosis signaling |
| SIRT3 modulation | SIRT3, PPAR-γ, PPAR-α | SIRT3, PPAR-γ, PPAR-α | Colorectal cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmas, S.; Fındık, M.; Kıyak, R.; Taşkın, G.; Cîrțînă, D.; Dîrnu, R.; Guță, N.; Mecu, R.-M.; Bîcă, M.-D. Anticancer Potential of Whey Proteins—A Systematic Review of Bioactivity and Functional Mechanisms. Int. J. Mol. Sci. 2025, 26, 10406. https://doi.org/10.3390/ijms262110406
Elmas S, Fındık M, Kıyak R, Taşkın G, Cîrțînă D, Dîrnu R, Guță N, Mecu R-M, Bîcă M-D. Anticancer Potential of Whey Proteins—A Systematic Review of Bioactivity and Functional Mechanisms. International Journal of Molecular Sciences. 2025; 26(21):10406. https://doi.org/10.3390/ijms262110406
Chicago/Turabian StyleElmas, Selin, Meliha Fındık, Ramazan Kıyak, Gökhan Taşkın, Daniela Cîrțînă, Rodica Dîrnu, Natalia Guță, Roxana-Maria Mecu, and Monica-Delia Bîcă. 2025. "Anticancer Potential of Whey Proteins—A Systematic Review of Bioactivity and Functional Mechanisms" International Journal of Molecular Sciences 26, no. 21: 10406. https://doi.org/10.3390/ijms262110406
APA StyleElmas, S., Fındık, M., Kıyak, R., Taşkın, G., Cîrțînă, D., Dîrnu, R., Guță, N., Mecu, R.-M., & Bîcă, M.-D. (2025). Anticancer Potential of Whey Proteins—A Systematic Review of Bioactivity and Functional Mechanisms. International Journal of Molecular Sciences, 26(21), 10406. https://doi.org/10.3390/ijms262110406





