Gene Expression and Fatty Acid Composition in Sea Buckthorn Seeds and Pulp During Fruit Development of Different Varieties
Abstract
1. Introduction
2. Results and Discussion
2.1. Gene Expression Analysis
2.2. KAS II Expression
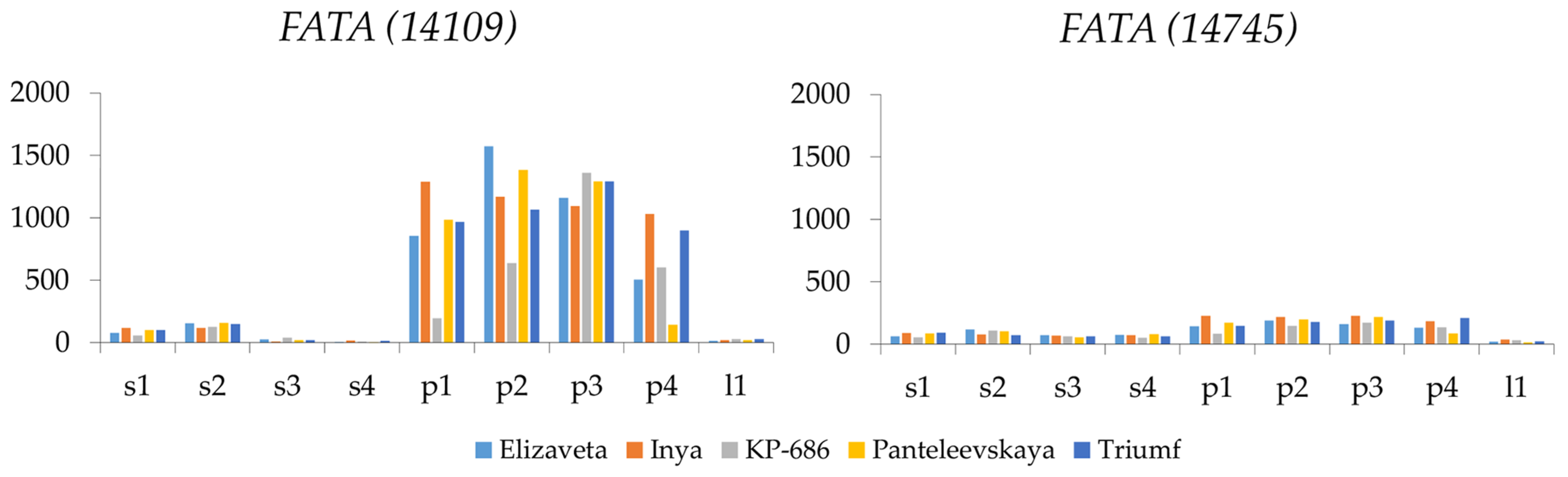
2.3. FAT Expression
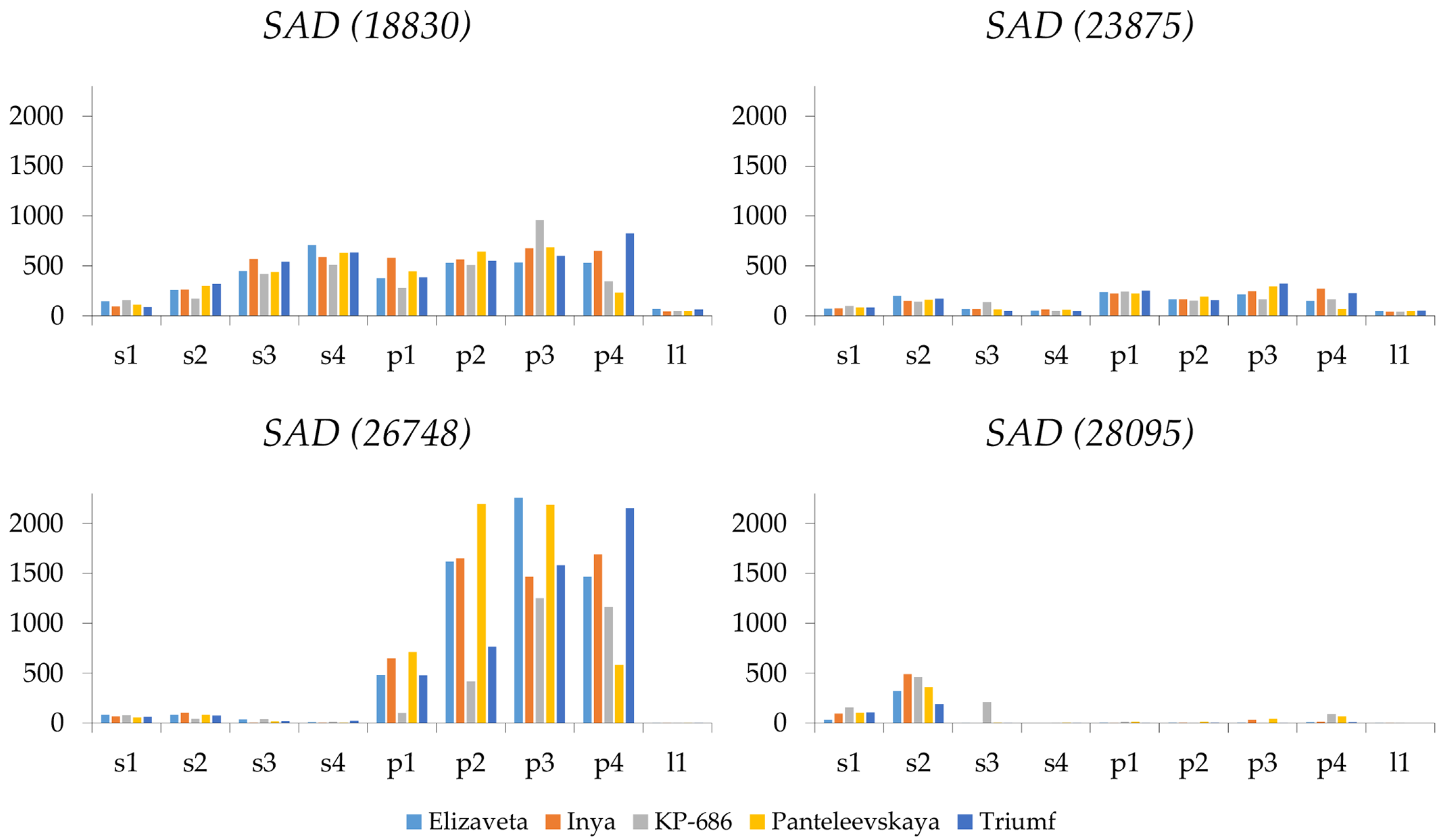
2.4. SAD Expression
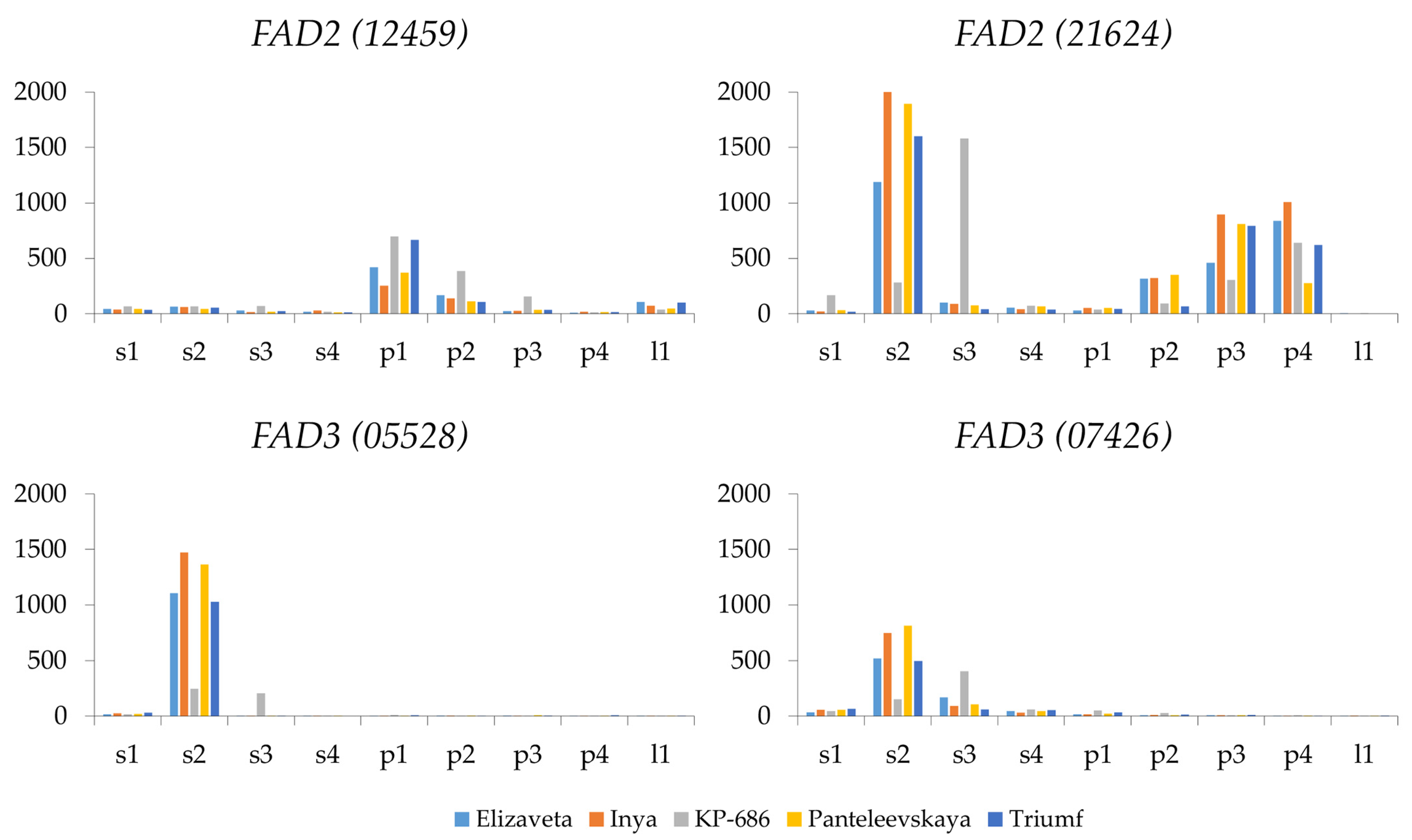
2.5. FAD2 Expression
2.6. FAD3 Expression
2.7. FAD6 and FAD7/8 Expression
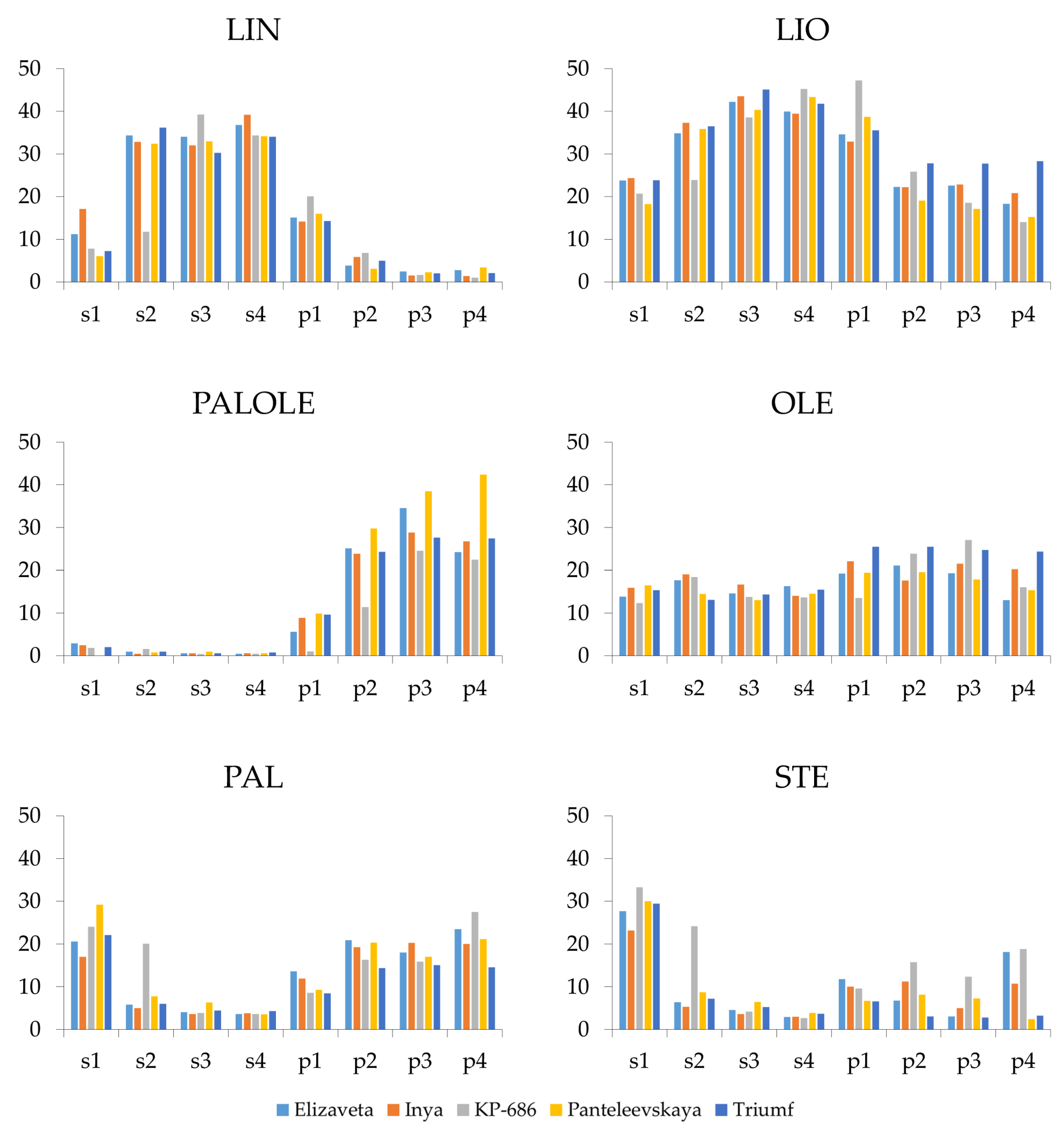
2.8. FA Composition Analysis
2.9. Gene Expression and FA Composition
3. Materials and Methods
3.1. Plant Material and RNA Extraction
3.2. cDNA Sequencing and Data Analysis
3.3. Fatty Acid Composition Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fatima, T.; Snyder, C.L.; Schroeder, W.R.; Cram, D.; Datla, R.; Wishart, D.; Weselake, R.J.; Krishna, P. Fatty Acid Composition of Developing Sea Buckthorn (Hippophae rhamnoides L.) Berry and the Transcriptome of the Mature Seed. PLoS ONE 2012, 7, e34099. [Google Scholar] [CrossRef] [PubMed]
- Cakir, A. Essential oil and fatty acid composition of the fruits of Hippophae rhamnoides L. (Sea Buckthorn) and Myrtus communis L. from Turkey. Biochem. Syst. Ecol. 2004, 32, 809–816. [Google Scholar] [CrossRef]
- Sola Marsinach, M.; Cuenca, A.P. The impact of sea buckthorn oil fatty acids on human health. Lipids Health Dis. 2019, 18, 145. [Google Scholar] [CrossRef]
- Jasniewska, A.; Diowksz, A. Wide Spectrum of Active Compounds in Sea Buckthorn (Hippophae rhamnoides) for Disease Prevention and Food Production. Antioxidants 2021, 10, 1279. [Google Scholar] [CrossRef]
- Ciesarova, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolkova, B.; Koplik, R.; Belajova, E.; Kukurova, K.; Dasko, L.; Panovska, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef]
- Zuchowski, J. Phytochemistry and pharmacology of sea buckthorn (Elaeagnus rhamnoides; syn. Hippophae rhamnoides): Progress from 2010 to 2021. Phytochem. Rev. 2023, 22, 3–33. [Google Scholar] [CrossRef]
- Gatlan, A.M.; Gutt, G. Sea Buckthorn in Plant Based Diets. An Analytical Approach of Sea Buckthorn Fruits Composition: Nutritional Value, Applications, and Health Benefits. Int. J. Environ. Res. Public Health 2021, 18, 8986. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.; Zhang, Z.; Rahman, K.; Wang, S.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Hao, W.; He, Z.; Zhu, H.; Liu, J.; Kwek, E.; Zhao, Y.; Ma, K.Y.; He, W.S.; Chen, Z.Y. Sea buckthorn seed oil reduces blood cholesterol and modulates gut microbiota. Food Funct. 2019, 10, 5669–5681. [Google Scholar] [CrossRef]
- Nybom, H.; Ruan, C.; Rumpunen, K. The Systematics, Reproductive Biology, Biochemistry, and Breeding of Sea Buckthorn-A Review. Genes 2023, 14, 2120. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Diao, S.; Zhang, G.; Yu, J.; Zhang, T.; Luo, H.; Duan, A.; Wang, J.; He, C.; Zhang, J. Genome sequence and population genomics provide insights into chromosomal evolution and phytochemical innovation of Hippophae rhamnoides. Plant Biotechnol. J. 2022, 20, 1257–1273. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Luo, S.; Yang, S.; Duoji, C.; Wang, Q.; Chen, Z.; Yang, D.; Yang, T.; Wan, X.; Yang, Y.; et al. Chromosome-level genome assembly of Hippophae rhamnoides variety. Sci. Data 2024, 11, 776. [Google Scholar] [CrossRef]
- Du, W.; Ding, J.; Lu, S.; Wen, X.; Hu, J.; Ruan, C. Identification of the key flavonoid and lipid synthesis proteins in the pulp of two sea buckthorn cultivars at different developmental stages. BMC Plant Biol. 2022, 22, 299. [Google Scholar] [CrossRef]
- Ding, J.; Ruan, C.; Du, W.; Guan, Y. RNA-seq data reveals a coordinated regulation mechanism of multigenes involved in the high accumulation of palmitoleic acid and oil in sea buckthorn berry pulp. BMC Plant Biol. 2019, 19, 207. [Google Scholar] [CrossRef]
- Arkhipov, A.A.; Dvorianinova, E.M.; Turba, A.A.; Novakovskiy, R.O.; Zubarev, Y.A.; Predushchenko, P.A.; Sigova, E.A.; Zhernova, D.A.; Borkhert, E.V.; Pushkova, E.N.; et al. Identification and Analysis of KAS II, FAT, SAD, and FAD Gene Families in Hippophae rhamnoides. Plants 2024, 13, 3486. [Google Scholar] [CrossRef]
- Yang, B.; Kallio, H.P. Fatty acid composition of lipids in sea buckthorn (Hippophae rhamnoides L.) berries of different origins. J. Agric. Food Chem. 2001, 49, 1939–1947. [Google Scholar] [CrossRef]
- Dolzhenko, A.I.; Zubarev, Y.A.; Gunin, A.V. Fatty acid composition of fruit pulp oil and seeds of sea buckthorn selection. Bull. NSAU (Novosib. State Agrar. Univ.) 2024, 1, 51–58. [Google Scholar] [CrossRef]
- Zubarev, Y.A.; Gunin, A.V.; Moersel, J.; Zemtsova, A.I. Composition of essential fatty acids in fruit pulp and seeds of sea buckthorn varieties of different ecological origins. In Proceedings of the Collection of Scientific Articles from the International Conference “Lomonosov Readings in Altai: Fundamental Problems of Science and Education”, Barnaul, Russia, 20–24 October 2015; pp. 1588–1594. [Google Scholar]
- Shanklin, J.; Somerville, C. Stearoyl-acyl-carrier-protein desaturase from higher plants is structurally unrelated to the animal and fungal homologs. Proc. Natl. Acad. Sci. USA 1991, 88, 2510–2514. [Google Scholar] [CrossRef]
- Dar, A.A.; Choudhury, A.R.; Kancharla, P.K.; Arumugam, N. The FAD2 Gene in Plants: Occurrence, Regulation, and Role. Front. Plant Sci. 2017, 8, 1789. [Google Scholar] [CrossRef] [PubMed]
- Browse, J.; McConn, M.; James, D., Jr.; Miquel, M. Mutants of Arabidopsis deficient in the synthesis of alpha-linolenate. Biochemical and genetic characterization of the endoplasmic reticulum linoleoyl desaturase. J. Biol. Chem. 1993, 268, 16345–16351. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, N.V.; Arkhipov, A.A.; Zubarev, Y.A.; Novakovskiy, R.O.; Turba, A.A.; Pushkova, E.N.; Zhernova, D.A.; Mazina, A.S.; Dvorianinova, E.M.; Sigova, E.A.; et al. Genetic diversity of Hippophae rhamnoides varieties with different fruit characteristics based on whole-genome sequencing. Front. Plant Sci. 2025, 16, 1542552. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Wang, K.; Li, Z.; Jia, Q.; Zhao, C.; Zhang, M. ABA-INSENSITIVE 3 with or without FUSCA3 highly up-regulates lipid droplet proteins and activates oil accumulation. J. Exp. Bot. 2022, 73, 2077–2092. [Google Scholar] [CrossRef]
- Lu, L.; Wei, W.; Li, Q.T.; Bian, X.H.; Lu, X.; Hu, Y.; Cheng, T.; Wang, Z.Y.; Jin, M.; Tao, J.J.; et al. A transcriptional regulatory module controls lipid accumulation in soybean. New Phytol. 2021, 231, 661–678. [Google Scholar] [CrossRef]
- Yang, Y.; Kong, Q.; Tee, W.T.; Li, Y.; Low, P.M.; Patra, B.; Guo, L.; Yuan, L.; Ma, W. Transcription factor bZIP52 modulates Arabidopsis seed oil biosynthesis through interaction with WRINKLED1. Plant Physiol. 2023, 192, 2628–2639. [Google Scholar] [CrossRef]
- Ben Ayed, R.; Ennouri, K.; Ercisli, S.; Ben Hlima, H.; Hanana, M.; Smaoui, S.; Rebai, A.; Moreau, F. First study of correlation between oleic acid content and SAD gene polymorphism in olive oil samples through statistical and bayesian modeling analyses. Lipids Health Dis. 2018, 17, 74. [Google Scholar] [CrossRef]
- Hernandez, M.L.; Sicardo, M.D.; Belaj, A.; Martinez-Rivas, J.M. The Oleic/Linoleic Acid Ratio in Olive (Olea europaea L.) Fruit Mesocarp Is Mainly Controlled by OeFAD2-2 and OeFAD2-5 Genes Together With the Different Specificity of Extraplastidial Acyltransferase Enzymes. Front. Plant Sci. 2021, 12, 653997. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; Mariotti, R.; Mousavi, S.; Baldoni, L.; Guerrero, C.; Roka, L.; Cultrera, N.; Pierantozzi, P.; Maestri, D.; Gentili, L.; et al. Characterization and validation of olive FAD and SAD gene families: Expression analysis in different tissues and during fruit development. Mol. Biol. Rep. 2020, 47, 4345–4355. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, M.H.; McAssey, E.V.; Dittmar, E.L.; Burke, J.M. Transcriptomics of developing wild sunflower seeds from the extreme ends of a latitudinal gradient differing in seed oil composition. Plant Direct 2022, 6, e423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Song, S.; Segla Koffi Dossou, S.; Zhou, R.; Wei, X.; Wang, Z.; Sheng, C.; Zhang, Y.; You, J.; Wang, L. Genome-wide association analysis and transcriptome reveal novel loci and a candidate regulatory gene of fatty acid biosynthesis in sesame (Sesamum indicum L.). Plant Physiol. Biochem. 2022, 186, 220–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, H.; Cui, X.; Gao, C.; Li, N.; Pu, Y.; Zhang, X.; Zhao, J. Integrated lipidomic and transcriptomic analyses reveal the mechanism of lipid biosynthesis and accumulation during seed development in sesame. Front. Plant Sci. 2023, 14, 1211040. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Huang, Y.; Cui, Y.; Hua, J. Gene network of oil accumulation reveals expression profiles in developing embryos and fatty acid composition in Upland cotton. J. Plant Physiol. 2018, 228, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cheng, X.; Wang, C.; Zhang, X.; Xue, F.; Li, Y.; Zhu, Q.; Sun, J.; Liu, F. Explore the gene network regulating the composition of fatty acids in cottonseed. BMC Plant Biol. 2021, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Pei, W.; Wang, N.; Ma, J.; Xin, Y.; Yang, S.; Wang, W.; Chen, Q.; Zhang, J.; Yu, J.; et al. Transcriptome analysis and identification of genes associated with oil accumulation in upland cotton. Physiol. Plant. 2022, 174, e13701. [Google Scholar] [CrossRef]
- Ting, N.C.; Sherbina, K.; Khoo, J.S.; Kamaruddin, K.; Chan, P.L.; Chan, K.L.; Halim, M.A.A.; Sritharan, K.; Yaakub, Z.; Mayes, S.; et al. Expression of fatty acid and triacylglycerol synthesis genes in interspecific hybrids of oil palm. Sci. Rep. 2020, 10, 16296. [Google Scholar] [CrossRef]
- Wei, L.; Yang, C.; John Martin, J.J.; Li, R.; Zhou, L.; Cheng, S.; Cao, H.; Liu, X. Metabonomics and Transcriptomic Analysis of Free Fatty Acid Synthesis in Seedless and Tenera Oil Palm. Int. J. Mol. Sci. 2024, 25, 1686. [Google Scholar] [CrossRef]
- Hao, Y.; Ge, X.; Xu, R.; Zhao, X.; Zhai, M. Transcriptome analysis of lipid biosynthesis during kernel development in two walnut (Juglans regia L.) varieties of ‘Xilin 3’ and ‘Xiangling’. BMC Plant Biol. 2024, 24, 828. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, Y.; Zhang, J.; Ji, F.; Jin, F.; Fan, W.; Pei, D. Transcriptome Analysis of Walnut (Juglans regia L.) Embryos Reveals Key Developmental Stages and Genes Involved in Lipid Biosynthesis and Polyunsaturated Fatty Acid Metabolism. J. Agric. Food Chem. 2021, 69, 377–396. [Google Scholar] [CrossRef]
- Shi, T.L.; Ma, H.Y.; Wang, X.; Liu, H.; Yan, X.M.; Tian, X.C.; Li, Z.C.; Bao, Y.T.; Chen, Z.Y.; Zhao, S.W.; et al. Differential gene expression and potential regulatory network of fatty acid biosynthesis during fruit and leaf development in yellowhorn (Xanthoceras sorbifolium), an oil-producing tree with significant deployment values. Front. Plant Sci. 2023, 14, 1297817. [Google Scholar] [CrossRef]
- Lin, P.; Wang, K.; Zhou, C.; Xie, Y.; Yao, X.; Yin, H. Seed Transcriptomics Analysis in Camellia oleifera Uncovers Genes Associated with Oil Content and Fatty Acid Composition. Int. J. Mol. Sci. 2018, 19, 118. [Google Scholar] [CrossRef] [PubMed]
- Dvorianinova, E.M.; Zinovieva, O.L.; Pushkova, E.N.; Zhernova, D.A.; Rozhmina, T.A.; Povkhova, L.V.; Novakovskiy, R.O.; Sigova, E.A.; Turba, A.A.; Borkhert, E.V.; et al. Key FAD2, FAD3, and SAD Genes Involved in the Fatty Acid Synthesis in Flax Identified Based on Genomic and Transcriptomic Data. Int. J. Mol. Sci. 2023, 24, 14885. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yu, R.; Xie, L.H.; Rahman, M.M.; Kilaru, A.; Niu, L.X.; Zhang, Y.L. Fatty Acid and Associated Gene Expression Analyses of Three Tree Peony Species Reveal Key Genes for alpha-Linolenic Acid Synthesis in Seeds. Front. Plant Sci. 2018, 9, 106. [Google Scholar] [CrossRef]
- Li, T.; Sun, Y.; Chen, Y.; Gao, Y.; Gao, H.; Liu, B.; Xue, J.; Li, R.; Jia, X. Characterisation of two novel genes encoding Delta(9) fatty acid desaturases (CeSADs) for oleic acid accumulation in the oil-rich tuber of Cyperus esculentus. Plant Sci. 2022, 319, 111243. [Google Scholar] [CrossRef]
- Zou, Z.; Fu, X.; Li, C.; Huang, J.; Zhao, Y. Insights into membrane-bound fatty acid desaturase genes in tigernut (Cyperus esculentus L.), an oil-rich tuber plant in Cyperaceae. BMC Plant Biol. 2025, 25, 382. [Google Scholar] [CrossRef]
- Subedi, U.; Jayawardhane, K.N.; Pan, X.; Ozga, J.; Chen, G.; Foroud, N.A.; Singer, S.D. The Potential of Genome Editing for Improving Seed Oil Content and Fatty Acid Composition in Oilseed Crops. Lipids 2020, 55, 495–512. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, Q.; Yang, Y.; Li, Q.; Li, R.; Ye, J. Regulation of Oil Biosynthesis and Genetic Improvement in Plants: Advances and Prospects. Genes 2024, 15, 1125. [Google Scholar] [CrossRef]
- Zafar, S.; Li, Y.L.; Li, N.N.; Zhu, K.M.; Tan, X.L. Recent advances in enhancement of oil content in oilseed crops. J. Biotechnol. 2019, 301, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.R.; Liu, Q.; Singh, S. Engineering Nutritionally Improved Edible Plant Oils. Annu. Rev. Food Sci. Technol. 2023, 14, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Pushkova, E.N.; Povkhova, L.V.; Dvorianinova, E.M.; Novakovskiy, R.O.; Rozhmina, T.A.; Gryzunov, A.A.; Sigova, E.A.; Zhernova, D.A.; Borkhert, E.V.; Turba, A.A.; et al. Expression of FAD and SAD Genes in Developing Seeds of Flax Varieties under Different Growth Conditions. Plants 2024, 13, 956. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, G.S.; Dmitriev, A.A.; Kudryavtseva, A.V.; Shargunov, A.V.; Karpov, D.S.; Uroshlev, L.A.; Melnikova, N.V.; Blinov, V.M.; Poverennaya, E.V.; Archakov, A.I. PPLine: An automated pipeline for SNP, SAP, and splice variant detection in the context of proteogenomics. J. Proteome Res. 2015, 14, 3729–3737. [Google Scholar] [CrossRef]
- Chagovets, V.; Wang, Z.; Kononikhin, A.; Starodubtseva, N.; Borisova, A.; Salimova, D.; Popov, I.; Kozachenko, A.; Chingin, K.; Chen, H.; et al. A comparison of tissue spray and lipid extract direct injection electrospray ionization mass spectrometry for the differentiation of eutopic and ectopic endometrial tissues. J. Am. Soc. Mass Spectrom. 2018, 29, 323–330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikova, N.V.; Arkhipov, A.A.; Zubarev, Y.A.; Novakovskiy, R.O.; Turba, A.A.; Yablokov, A.G.; Vladimirov, G.N.; Osipenko, S.V.; Bashilov, A.A.; Kostyukevich, Y.I.; et al. Gene Expression and Fatty Acid Composition in Sea Buckthorn Seeds and Pulp During Fruit Development of Different Varieties. Int. J. Mol. Sci. 2025, 26, 10396. https://doi.org/10.3390/ijms262110396
Melnikova NV, Arkhipov AA, Zubarev YA, Novakovskiy RO, Turba AA, Yablokov AG, Vladimirov GN, Osipenko SV, Bashilov AA, Kostyukevich YI, et al. Gene Expression and Fatty Acid Composition in Sea Buckthorn Seeds and Pulp During Fruit Development of Different Varieties. International Journal of Molecular Sciences. 2025; 26(21):10396. https://doi.org/10.3390/ijms262110396
Chicago/Turabian StyleMelnikova, Nataliya V., Alexander A. Arkhipov, Yury A. Zubarev, Roman O. Novakovskiy, Anastasia A. Turba, Arthur G. Yablokov, Gleb N. Vladimirov, Sergey V. Osipenko, Anton A. Bashilov, Yury I. Kostyukevich, and et al. 2025. "Gene Expression and Fatty Acid Composition in Sea Buckthorn Seeds and Pulp During Fruit Development of Different Varieties" International Journal of Molecular Sciences 26, no. 21: 10396. https://doi.org/10.3390/ijms262110396
APA StyleMelnikova, N. V., Arkhipov, A. A., Zubarev, Y. A., Novakovskiy, R. O., Turba, A. A., Yablokov, A. G., Vladimirov, G. N., Osipenko, S. V., Bashilov, A. A., Kostyukevich, Y. I., Nikolaev, E. N., Sigova, E. A., Dvorianinova, E. M., Krupskaya, D. A., Barsukov, N. M., Krasnov, G. S., Ruan, C., Pushkova, E. N., & Dmitriev, A. A. (2025). Gene Expression and Fatty Acid Composition in Sea Buckthorn Seeds and Pulp During Fruit Development of Different Varieties. International Journal of Molecular Sciences, 26(21), 10396. https://doi.org/10.3390/ijms262110396








