Abstract
Ricinus communis is a significant economic crop, where plant height and stress tolerance are critical factors influencing both yield and quality. The variation in plant height is influenced by both genetic and environmental factors, with environmental stresses such as salt, drought, and cold notably affecting plant growth phenotypes. In this study, we utilized transcriptome data from two varieties, DL01 and Hale, which differ in plant height, to systematically identify the RcMYB transcription factor family and screen 12 key RcMYBs associated with height variation. We also analyzed the expression patterns of these genes under various stress conditions, including salt, drought, cold, and heat. Notably, these 12 height/stress-related RcMYB genes such as RcMYB45 and RcMYB27 showed notable expression changes in response to different stress treatments, suggesting their pivotal roles in regulating both plant height and stress tolerance. Through protein–protein interaction (PPI) network analysis, we further discovered that these RcMYBs could interact with several regulatory factors. This study highlights the roles of RcMYB regulators in controlling plant height and stress adaptation in R. communis, providing potential target genes for molecular breeding and offering valuable insights into improving growth performance and stress tolerance.
1. Introduction
As a key structural trait, plant height is an important component of crop improvement, directly influencing lodging tolerance and yield performance []. Shorter plants, by reducing resource consumption and enhancing lodging tolerance, have been found in short varieties of crops such as Triticum aestivum (wheat), Oryza sativa (rice), Zea mays (maize), and Sorghum bicolor (sorghum), as well as in fruit trees like Malus domestica (apple) and Prunus persica (peach) [,,,,]. This is considered one of the results of domestication and selection. However, excessive dwarfing may limit root development and reduce overall stress tolerance. For example, the M9 dwarfing rootstock in apple trees is often less adaptable, with shallow roots that are more susceptible to drought []. Plant height influences planting density, and in Ricinus communis, while plant density had no significant effect on seed yield in temperate climates, it was positively associated with increased productivity in tropical environments []. In addition, the R. communis variety Hale shows field tolerance to Verticillium wilt and leaf spot, but under salt and drought stress conditions, some tolerant varieties yield 30–60% higher than Hale [], highlighting the importance of optimizing plant height and enhancing stress tolerance in R. communis breeding to improve crop productivity and adaptability. Thus, achieving an optimal plant height and stress tolerance has always been a key objective in breeding.
Disruption of hormone synthesis or signal transduction is the primary cause of plant dwarf phenotypes. Gibberellins (GA) and brassinosteroids (BR) directly affect plant height, with reduced GA levels being associated with dwarfism [,,]. For example, the semi-dwarf phenotype in the IR8 rice variety is caused by a mutation in the sd1 gene [], and the dwarfing in the hybrid cultivar DGO24 of Prunus salicina L. (plum) is caused by the overexpression of the PslGA2ox gene []. Additionally, BR plays a crucial role in cell elongation [], and BR-deficient or insensitive mutants exhibit dwarfism, small leaves, delayed flowering, and other characteristics []. GA signaling is a core pathway regulating stem elongation and plant morphology. Studies have shown that specific MYB factors, such as GAMYB, MYB33, and MYB65 in Arabidopsis thaliana, mediate GA responses and influence growth-related traits [,,]. Although there are no related reports in R. communis, a few genes associated with traits such as plant height, main stem diameter, number of nodes, and seed size have been identified in regions linked to flowering regulation, cell wall synthesis, and adaptability, including the RcGA2ox2 and RcMYB46 genes []. AtGA2ox2 controls plant growth and height by regulating GA concentrations [], while AtMYB46 is involved in regulating secondary cell wall thickening []. However, the functions of these genes in R. communis remain unverified. The interaction of these pathways could serve as a promising focus for both experimental research and practical applications.
MYB transcription factors are well-known for their highly conserved MYB domain at the N-terminus, playing a key role in regulating plant height, structural development, lateral organ formation, as well as fruit and seed development [,,]. Previous studies have demonstrated that MYB genes regulate plant height. For example, the SbMYB110 gene was identified at the qHT7.1 plant height locus in sorghum, and mutations in ZmMYB78 in maize significantly affect plant height and inflorescence length []. In addition, OsMYB110 in rice has been found to be induced under low phosphorus stress, and mutations lead to a significant increase in plant height without affecting phosphorus uptake []. Other transcriptome studies have also identified MYBs associated with plant height regulation, such as in Capsicum baccatum [] and paper mulberry (Broussonetia papyrifera) [], where several differentially expressed MYB genes were found. Although direct studies on regulating MYB genes to alter plant height in R. communis and its related species are limited and not well-developed, their successful application in other crops highlights their immense potential. In R. communis, the core gene RcGA2ox4, which was upregulated under polyethylene glycol-induced oxidative stress, has been identified through WGCNA []. This provides important clues for a deeper understanding of the relationship between hormones, plant height, and stress responses in R. communis, opening up new research directions for exploring how these factors interact to regulate plant growth and adaptability.
Plants face biological and abiotic stresses during growth, with abiotic stresses like temperature extremes, drought, salinity, and nutrient deficiencies affecting yield and variety. Many transcription factors and genes are involved in plant responses to stress, improving tolerance by activating specific genes, regulating the abscisic acid (ABA) pathway, and enhancing antioxidant capacity. For example, MdICE1 from the bHLH family, MbWRKY50 and VhWRKY44 from the WRKY family, FvNAC29 from the NAC family, and FvMYB44 and VhMYB2 from the MYB family all enhance transgenic plant tolerance to stress by upregulating antioxidant enzyme activity [,,,,,]. In addition to their crucial role in plant development, MYB proteins are also involved in the plant’s response to environmental stresses, as they not only contribute to the biosynthesis of metabolites like flavonoids and anthocyanins, but also play a key role in coping with abiotic stresses such as drought, salt, and cold []. In the rice varieties Nagina 22 and IR64, 92 MYBs showed significantly differential expression in seedlings under drought stress; while in A. thaliana, more than 40% of MYBs exhibited differential expression in response to drought stress []. In addition, MYB factors could regulate plant salt tolerance through multiple pathways, including redox homeostasis, ion efflux, DNA methylation, and ABA signaling pathways [,,,]. In cold stress, MYB factors have been found to confer cold tolerance by regulating CBF genes, with AtMYB15 interacting with ICE1 to modulate CBF expression, where overexpression reduces freezing tolerance and loss of function enhances it []. However, research on the interaction of MYBs in different signaling pathways, particularly their roles in plant growth and stress response, remains limited. One example is the overexpression of GmMYB14 in soybean (Glycine max), which, by reducing endogenous BR levels, results in a semi-dwarf and compact plant structure and enhanced drought tolerance under field conditions []. These results demonstrate the roles of GmMYB14 in regulating plant structure and drought tolerance. MYBs that regulate both plant height and stress response may have the potential to become a promising gene in molecular breeding.
R. communis, a member of the Euphorbiaceae family, is an oilseed crop whose breeding goal is primarily to increase yield []. It possesses significant advantages, such as drought tolerance, salt and alkali tolerance, and tolerance to heavy metal pollution. Its oil is widely used in the production of high-temperature lubricants, surfactants, and other important industrial products [,,,]. With the release of the high-quality reference genome in R. communis and the establishment of genetic populations, research utilizing forward genetics approaches to uncover the genetic mechanisms controlling targeted breeding traits has been progressively reported [,,]. As a result, most existing research on R. communis focuses on analyzing seed yield, oil content, and other components [,,]. For example, the analysis of the hybrid genetic population between the cultivated variety DL01 and the wild type WH11 identified a major locus, miR396b, which regulates seed size in R. communis by inhibiting the expression of RcGRF4 and modulating Indole-3-acetic acid (IAA) content []. However, in R. communis research, the concept of an “ideal plant type” has been underexplored, with limited in-depth studies and optimization, especially regarding plant height and branching patterns. Since plant height and stress tolerance are critical agronomic traits, their coordinated improvement could significantly enhance both productivity and adaptability, ultimately enabling stable high yields and more efficient cultivation.
Here, we performed stem RNA-seq on two R. communis varieties (DL01, tall; Hale, dwarf) and conducted a genome-wide assessment of the RcMYB family, analyzing its subfamilies, physicochemical properties, conserved domains, and promoter elements. Through the DL01-Hale comparison, we identified differential RcMYB expression as a potential contributor to plant height variation and selected 12 key candidates for further investigation. We then evaluated whether these height-related RcMYBs also showed responses under abiotic stresses, including salt, drought, cold, and heat, where the candidates displayed distinct, condition-responsive expression patterns. Therefore, these 12 height/stress-related RcMYBs may play important roles in regulating both plant height and stress tolerance. Further protein–protein interaction (PPI) network analysis revealed interactions between these RcMYBs and several regulatory factors. Taken together, this stepwise progression from the DL01-Hale comparison to the identification of height/stress-related RcMYBs highlights a targeted set of RcMYBs for subsequent functional studies and potential application in R. communis improvement. Although this study mainly relies on bioinformatics analysis, further validation of these RcMYBs will be carried out to explore the molecular mechanisms of RcMYBs and lay the foundation for future research in R. communis height and stress regulation.
2. Results
2.1. Genes Associated with Plant Height Differences Between DL01 and Hale in R. communis
Two R. communis varieties, DL01 (tall plants with long internodes) and Hale (dwarf plants with short internodes), exhibited significant differences in stem morphology (Figure 1A), providing a basis for the identification and functional enrichment analysis of plant height-related genes. Therefore, the stem internodes of these two varieties at the seedling stage were selected for transcriptome sequencing. The results revealed a large number of differentially expressed genes (DEGs) between the two varieties, with 1994 genes downregulated and 2238 genes upregulated in Hale compared to DL01. Gene Ontology (GO) enrichment analysis showed that the downregulated genes were mainly involved in biological processes related to stress responses and cellular structure, including secondary metabolism, response to salicylic acid (SA), defense response, phenylpropanoid biogenesis, cell wall biogenesis, and response to hormone (Figure 1C). In contrast, the upregulated genes were significantly enriched in hormone metabolic processes, particularly in the biosynthesis and homeostasis of brassinosteroids and gibberellins, as well as in light response and lipid homeostasis pathways (Figure 1D).
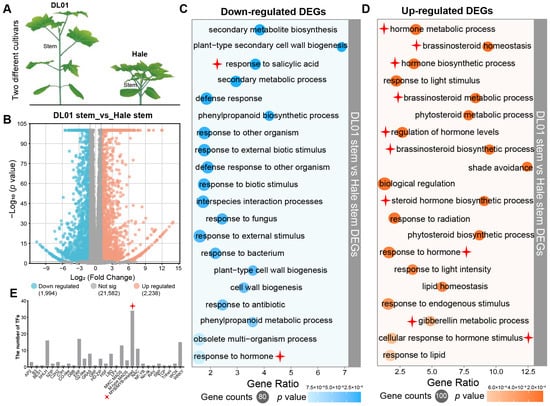
Figure 1.
Identification and screening of genes associated with plant height variation in R. communis. (A), Schematic diagram of the DL01 and Hale varieties in R. communis showing differences in their stem node regions. (B), Volcano plot of differentially expressed genes (DEGs) in the stem node regions of DL01 and Hale. Blue circles indicate downregulated genes in Hale, and orange circles indicate upregulated genes in Hale. (C), The top 20 GO enrichment results for downregulated genes. (D), The top 20 GO enrichment results for upregulated genes. The color of each circle represents the significance level (p value), and the size of the circle reflects the number of enriched genes in each category. (E), The number of transcription factor family members in the DEGs, with MYB transcription factors being the most abundant. The star marks indicate the sections that are the main focus of this study.
Notably, transcription factor family analysis indicated that RcMYB family members were the most abundant among the DEGs, suggesting their potential role in the regulation of plant height differences (Figure 1E). Given the obvious differential expression of RcMYBs between DL01 and Hale and its central role in plant development and adaptation, RcMYB genes were considered key regulators of stem elongation and plant height in R. communis.
2.2. Identification and Characterization of the RcMYB Gene Family in R. communis
By downloading 144 MYB family members of A. thaliana from the PlantTFDB database [] and performing BLASTP alignment along with conserved domain analysis, the RcMYB transcription factor family members in R. communis were identified (Table S1). A total of 99 RcMYB members were identified in the R. communis genome, distributed across 10 chromosomes, with the highest number on Chr05 and the lowest on Chr02 (Figure 2 and Table S1). Among them, RcMYBs on Chr01, Chr03, Chr05, and Chr06 tend to cluster toward the upper arms of the chromosomes, whereas those on Chr02 and Chr10 tend to cluster toward the lower arms. These results suggested that complex gene duplication of RcMYBs and chromosomal rearrangement events may have occurred during evolution. Chromosomal rearrangements such as translocations or inversions may have shaped the present-day organization of RcMYBs during the evolutionary history of R. communis (Figure 2).
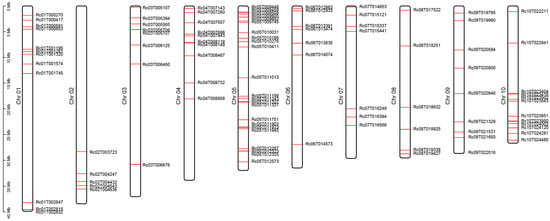
Figure 2.
Chromosomal distribution of RcMYB members. Chromosome names are labeled on the left side of the chromosomes, and gene positions are marked by red lines.
To explore the evolutionary relationships of RcMYBs, a phylogenetic tree was constructed using the full-length MYB protein sequences from R. communis and A. thaliana [] (Figure 3). Based on sequence similarity and clade topology, all MYB proteins were classified into 27 distinct subgroups. The majority of RcMYBs (S1–S26) were assigned to the well-characterized R2R3-MYB class, which is the largest and most functionally diverse subgroup in plants. In addition, smaller but distinct groups corresponding to 3R-MYB and 4R-MYB proteins were also detected, and three novel subgroups (Rc1–Rc3) appeared to be new clades to R. communis.
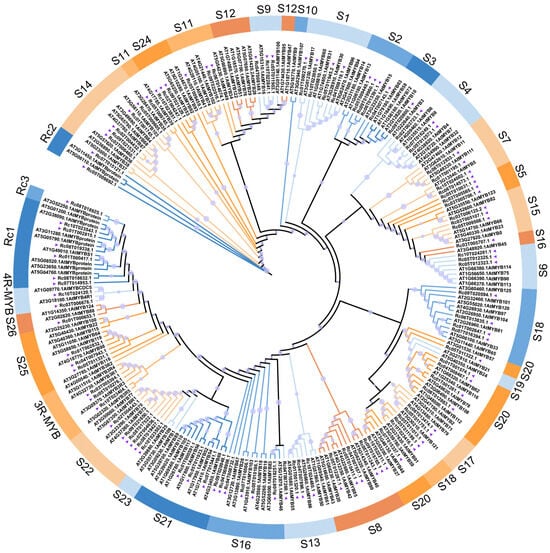
Figure 3.
Maximum likelihood (ML) phylogenetic tree of MYB family proteins from A. thaliana and R. communis. The phylogenetic tree was generated with 1000 bootstraps, and bootstrap values are represented by the size of purple circles and displayed next to the nodes. Different colors represent different subfamilies, with subfamily names based on the Arabidopsis subfamily nomenclature. Arabidopsis proteins are represented by ‘AT-’, while R. communis proteins by ‘Rc-’ and are indicated by purple triangles.
By using the CDD database [] to predict the conserved domains of RcMYB proteins, a total of nine conserved domains were identified (Figure 4). Most RcMYBs contained the Myb_DNA-bind 6, SANT, and Myb_DNA-binding domains, which showed a certain degree of similarity in their distribution across different proteins. These domains are essential for DNA binding and transcriptional regulation, indicating that most RcMYB proteins may function by directly binding to the promoters of target genes. In contrast, several other domains, including Aa_trans, IDnaJ, Myb_Cef, IbpA, COG3536, and GBBH-like N, were detected only in a subset of RcMYBs (Figure 4A–C), indicating that proteins harboring these motifs may have noncanonical functions.
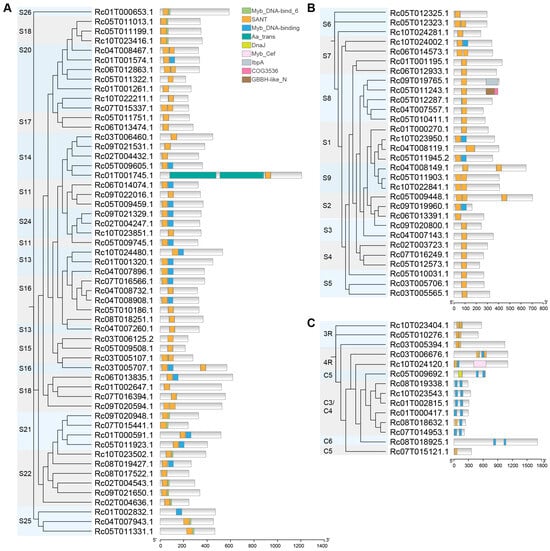
Figure 4.
Characteristics of conserved domains in RcMYB family member proteins. (A), Phylogenetic tree and conserved domain analysis of the S11–S26 subfamilies. (B), Phylogenetic tree and conserved domain analysis of the S1–S9 subfamilies. (C), Phylogenetic tree and conserved domain analysis of the 3R, 4R, C3/C4, C5, and C6 subfamilies. Rectangles in different colors represent different domains. Myb_DNA-bind 6, SANT and Myb_DNA-binding domains contain the DNA binding domains from MYB proteins; Aa trans, transmembrane region; IDnaJ domain plays crucial roles in protein translation, folding, unfolding, translocation, and degradation; Myb_Cef is a region of the Myb-Related Cdc5p/Cef1 proteins, and is part of the pre-mRNA splicing factor complex; IbpA belongs to the small heat shock protein IbpA; COG3536, putative dioxygenase with GBBH-like_N/DUF971 domain; GBBH-like N could facilitate dimer formation.
2.3. RcMYB Gene Expression in DL01 and Hale Stems and Plant Height-Related Candidates
To investigate the expression differences in RcMYB genes, we analyzed their expression patterns in the stems of DL01 and Hale. Based on clustering of expression profiles, the RcMYB genes were divided into 10 distinct clusters (Figure 5A and Figure S1). Each cluster contained 4–27 genes, with members within the same cluster exhibiting similar expression trends. Several clusters, such as clusters 1, 2, 3, and 10, exhibited notable differences between DL01 and Hale stem samples. Therefore, we focused on the RcMYBs that were up- or downregulated between DL01 and Hale, considering them as plant height-related candidates (Figure 5A).
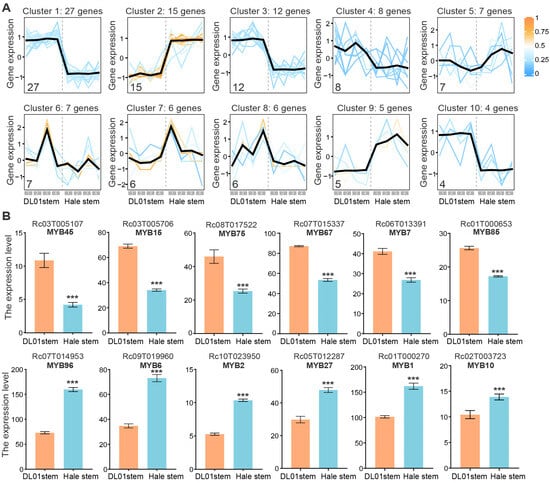
Figure 5.
Expression trends and differential expression analysis of RcMYB genes in DL01 and Hale stem samples. (A), Expression trends of RcMYB members in different clusters. The line chart shows the expression trends of different RcMYB genes in the stem nodes of DL01 and Hale, with genes clustered based on their expression patterns. The number in the lower left corner of each chart indicates the number of RcMYB genes within that cluster. Each line represents the expression trend of each gene, and the color of the line, ranging from blue to yellow, indicates the degree of membership (similarity to the cluster center). The black line represents the average expression trend of all genes within this cluster. The gray boxes at the bottom, labeled 1, 2, 3, and 4, correspond to four sample groups of DL01 and Hale stem samples. (B), The top 6 downregulated and upregulated RcMYB genes in Hale stem samples compared to DL01, ranked by the fold change in expression. The gene IDs and the names assigned in this study are shown at the top. These genes are referred to as height-related RcMYB genes. FPKM values are presented, with error bars representing ± SD of four biological replicates (n = 4). Statistical significance was determined by Student’s t-test: ***, p < 0.001.
By ranking genes by fold change in descending order and filtering for those with a baseline expression level above 4, we identified the 12 most significantly down- or up-regulated RcMYB genes in Hale stems compared to DL01 (Figure 5B). Among them, RcMYB45, RcMYB15, RcMYB75, RcMYB67, RcMYB7, and RcMYB85 were significantly downregulated in Hale, while RcMYB96, RcMYB6, RcMYB2, RcMYB27, RcMYB1, and RcMYB10 were significantly upregulated (p < 0.001). The similar genes in A. thaliana are as follows: RcMYB45–AtMYB123, RcMYB15–AtMYB5, RcMYB75–AtMYB70, RcMYB67–AtMYB48, RcMYB7–AtMYB14, RcMYB85–AtMYB124, RcMYB96–AtDIV2, RcMYB6–AtMYB15, RcMYB2–AtMYB60, RcMYB27–AtMYB43, RcMYB1–AtMYB17, and RcMYB10–AtMYB3. The expression changes in these plant height-related RcMYBs may play a key role in the regulation of plant height in R. communis, particularly in processes such as cell expansion and tolerance responses.
2.4. Analysis of Cis-Regulatory Elements and Expression Patterns of Height/Stress-Related RcMYBs Under Stress
The significant differential expression levels of RcMYB genes between the two R. communis varieties highlight their potential role in regulating plant height and suggest they may also help the plant adapt to the environment by modulating stress responses. To further investigate, cis-regulatory elements were predicted in the 2000 bp upstream sequences of these genes, and a total of 2290 elements were identified (Figure S2 and Figure 6A). These elements include multiple regulatory factors related to plant growth and stress responses. For example, ABA-responsive elements play a role in drought and salt stress, gibberellin (GA) elements regulate growth and development, while salicylic acid (SA) elements are associated with disease tolerance. The presence of drought and low temperature response elements also indicated the regulatory role of RcMYBs under stress conditions (Figure S2). Analysis of the 12 plant-height-related RcMYB genes above revealed that their promoters contain different numbers and types of elements (Figure 6A). Elements such as light, hormone, and stress response elements suggested that these genes may be regulated by multiple signals involved in plant growth and stress responses, with their expression potentially varying under different stress conditions.
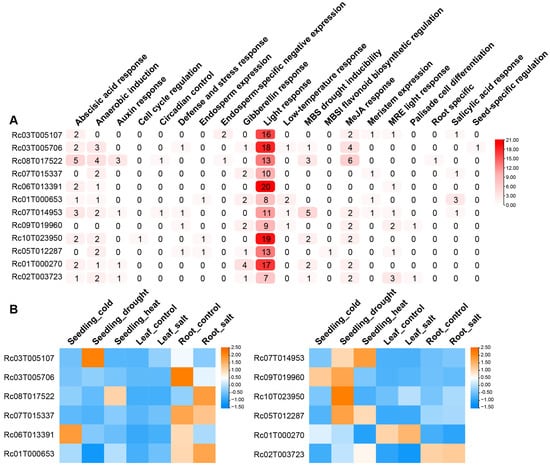
Figure 6.
Cis-regulatory element analysis of height-related RcMYB genes and their expression patterns under stress conditions. (A), Cis-regulatory elements in the promoter regions of height-related RcMYB genes. The numbers represent the count of each element, and the color scale indicates their abundance. The color intensity of each cell, ranging from white to red, reflects the number of elements associated with that gene promoter, as indicated by the color scale on the right. MBS, MYB binding site; MBSI, flavonoid biosynthetic genes regulation, MYB binding site involved in flavonoid biosynthetic genes regulation. (B), Heatmaps show the relative expression levels of the top 6 downregulated (left panel) and top 6 upregulated (right panel) RcMYB genes under different conditions: seedling under cold treatment (seedling_cold), seedling under drought treatment (seedling_drought), seedling under heat treatment (seedling_heat), leaf with no treatment (leaf_control), leaf under salt treatment (leaf_salt), root with no treatment (root_control), and root under salt treatment (root_salt). Rows represent individual RcMYB genes, and columns represent treatments. Relative expression levels in the heatmap are log2-transformed, with blue representing lower expression and orange representing higher expression.
To further explore the relationship between the regulation of plant height and stress responses by plant height-related RcMYB genes, RNA-seq data under different stress conditions in R. communis were downloaded. Then we analyzed the expression levels of 6 downregulated and 6 upregulated plant height-related RcMYB genes under different stresses, including leaf and root salt stress, heat, drought, and cold stresses during the seedling stage, with untreated leaves and roots as controls (Figure 6B and Figure S3). The results showed that the expression patterns of plant height-related RcMYB genes vary considerably under different stress conditions (Figure 6B). Among the downregulated height-related RcMYB genes, RcMYB45 showed increased expression under drought stress during the seedling stage, which may enhance drought tolerance; RcMYB15 had higher expression in untreated roots, possibly related to normal root development; RcMYB75 showed increased expression under heat and salt stress in seedlings, likely involved in these adaptive responses; RcMYB67 exhibited expression changes only under salt stress in roots, potentially regulating root responses to salt accumulation; while RcMYB7 responded to cold stress in seedlings, possibly playing a role under low temperature stress. Among the upregulated height-related RcMYB genes, RcMYB96 and RcMYB27 showed increased expression under drought and heat stress in seedlings, while RcMYB6 exhibited elevated expression under cold and drought stress, indicating the regulatory roles of these genes in response to dual stress. RcMYB2 responded specifically to drought stress in seedlings, suggesting its potential unique role in drought tolerance regulation. RcMYB1 was upregulated in leaves under salt stress, while RcMYB10 showed minor changes in expression under salt stress in roots. Therefore, these plant height-related RcMYB genes are closely linked to the mechanisms of plant stress response, making them key functional genes that may play a central bridging role in both plant height regulation and stress adaptation.
2.5. PPI Network of Plant Height/Stress-Related RcMYBs
We selected these 12 genes as height/stress-related RcMYBs and performed protein–protein interaction (PPI) screening using the STRING database []. The PPI network was optimized by using Cytoscape software v3.8.0 [], and the retrieved protein IDs were mapped to their corresponding genes (Figure 7 and Figure S4A,B). Interacting proteins with expression differences in plant height between DL01 and Hale samples were highlighted in blue within the PPI network, with a particular focus on the relationship between RcMYBs and these proteins. The greater the number of these blue-highlighted proteins among the interacting proteins, the more prominent the position of these genes in the network. Therefore, in the PPI network, we selected RcMYB45 and RcMYB27, as they interacted with the most blue-highlighted proteins (Figure 7A,B and Figure S4).
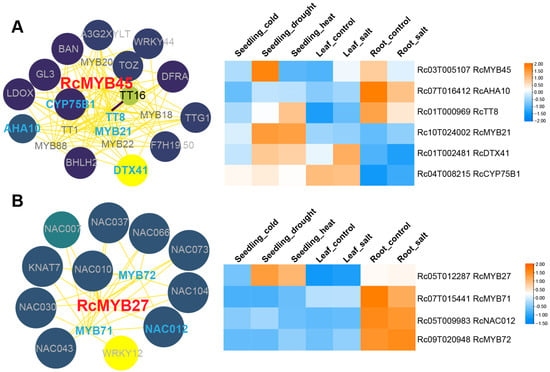
Figure 7.
Protein–protein interaction (PPI) network and stress expression analysis of RcMYB45 and RcMYB27. (A), PPI network of RcMYB45 and expression analysis of its potential interacting proteins under different stress conditions. The heatmap represents the expression patterns of RcMYB45 and its height-related interacting genes (in blue text) under different stresses: seedling under cold treatment (seedling_cold), seedling under drought treatment (seedling_drought), seedling under heat treatment (seedling_heat), leaf with no treatment (leaf_control), leaf under salt treatment (leaf_salt), root with no treatment (root_control), and root under salt treatment (root_salt). (B), PPI network of RcMYB27 and expression analysis of its potential interacting proteins (in blue text) under different stress conditions. Red text represents the RcMYB45 and RcMYB27 genes, while blue text represents their potential interacting genes that show differential expression in DL01 and Hale stem samples. The color intensity and size of the circles represent the confidence of interactions, with darker colors and larger circles indicating higher confidence. The color intensity of the lines represents the strength of interactions, with darker colors indicating a greater potential for interaction.
RcMYB45 had the highest protein similarity with AtMYB123, which is a key determinant in the accumulation of seed proanthocyanidins []. The interacting proteins of RcMYB45 include TT8, RcMYB21, CYP75B1, DTX41/TT12, and AHA10, which are involved in anthocyanin biosynthesis (e.g., TT8, DTX41, AHA10, and CYP75B1), seed coat development (e.g., RcMYB21), and ion transport across the cell membrane (e.g., AHA10) [,,,] (Figure 7A). When seedlings were subjected to drought stress, the expression levels of RcMYB45, CYP75B1, DTX41, and TT8 were increased; when the roots were exposed to salt stress, the expression levels of RcMYB45, AHA10, and TT8 were increased (Figure 7A). These results indicated that RcMYB45 could regulate plant physiological adaptability and stress tolerance through interactions with different factors.
The most similar protein to RcMYB27 is AtMYB43, which negatively regulates freezing tolerance and participates in the formation of secondary cell walls by directly activating the synthesis genes of lignin and phenylalanine [,]. Unlike the PPI network of RcMYB45, factors interacting with RcMYB27 include NAC012/NST3, which is involved in fiber secondary wall biosynthesis; NAC073, KNAT7, RcMYB71 (similar to AtMYB52), and RcMYB72 (similar to AtMYB69), which are associated with secondary cell wall biosynthesis [,,] (Figure 7B). RcMYB27 showed upregulated expression under drought and heat stress conditions during the seedling stages, while its height-related interacting factors exhibited differential expression only under root salt stress (Figure 7B). This difference may be related to the specific regulation of different stress types, stress intensity, or duration. Additionally, the regulatory network of RcMYB27 may involve multiple cross-regulatory factors, so the expression and interaction factor trends could vary under different stress conditions.
3. Discussion
Plant height is an important morphological trait, and its regulatory mechanism is related not only to gene expression but also to plant hormones, environmental conditions, and interactions between plants []. In R. communis, by comparing the stem transcriptomes of the Hale and DL01 varieties, we found that DEGs were predominantly enriched in the BR and GA hormone pathways, suggesting their involvement in the differences in plant height and growth between these varieties (Figure 1C,D). This pattern is similar to the differential expression of some MYBs identified in the long and short varieties of paper mulberry and C. baccatum, although the specific members may differ [,]. Additionally, the enrichment in pathways associated with secondary metabolism, cell wall synthesis, and hormone response (Figure 1B,C) suggests that MYBs may regulate plant height variation across different species by modulating these pathways.
The MYB gene family is one of the largest transcription factor families in plants, with the R2R3-MYB factors being the most abundant type. In A. thaliana, 126 R2R3-MYB genes have been identified, while 244 were found in soybean (Glycine max), 117 in rice, 123 in Salvia nemorosa, 124 in coconut (Cocos nucifera L.), and 65 in quinoa (Chenopodium quinoa Willd.) [,,,,,]. In this study, we systematically identified 99 MYB members in the R. communis genome, including 85 R2R3-MYBs (Table S1 and Figure 2 and Figure 3). The phylogenetic tree divided the RcMYB members of R. communis into 27 subfamilies (Figure 3), all of which clustered with the known functions of similar proteins in A. thaliana []. The conserved domains in RcMYBs suggested their functional conservation, but variations in the types and number of domains, as well as cis-regulatory elements in the promoter regions across subfamilies, may indicate functional differences (Figure 4 and Figure S2).
In this study, 12 height-related RcMYBs were first identified (Figure 5); although these genes have not been previously linked to plant height and stress or experimentally validated, they present new targets for further research. Cis-regulatory elements and stress treatment analyses also suggested that these genes not only participated in plant height regulation but also could respond to stress signals (Figure 6 and Figure S3). These subtle or tissue-specific expression changes in RcMYBs, while not broadly responsive to stress, may indicate localized regulation in response to specific stress conditions. For instance, RcMYB45 was similar to AtMYB123/AtTT2, which has been reported to be involved in anthocyanin accumulation and heat stress response in A. thaliana [,]. While RcMYB45 could contribute to enhanced drought tolerance, its adaptation to heat stress might rely on other regulatory factors (Figure 6). Another gene, RcMYB15, was similar to AtMYB5, which has been shown to enhance heat tolerance when overexpressed [], whereas RcMYB15 may play a negative regulatory role in the roots under salt stress (Figure 6B and Figure S3). AtMYB70 has been reported to regulate seed germination and root development [], while RcMYB75 may play a positive role in enhancing root salt tolerance and develop a new function to cope with heat stress (Figure 6). AtDIV, similar to RcMYB96, negatively regulates salt stress by modulating ABA signaling []; however, RcMYB96 showed a strong response to seedling drought and heat stress, with only a slight response to salt stress in the roots, possibly due to a higher number of drought-responsive elements in its promoter (Figure 6A,B and Figure S3). Another gene, RcMYB6, responds to cold and drought stress, similar to AtMYB15, whose overexpression can enhance ABA sensitivity and improve drought tolerance []. Additionally, AtMYB43 could regulate cold tolerance by modulating the expression of AtCBFs, while RcMYB27 was upregulated under drought and heat stress (Figure 6B), suggesting that despite their similarities, they play distinct roles in adapting to different types of stress. Although these changes may appear subtle or tissue-specific, they could be part of the plant’s adaptive response to complex environmental stresses and hold potential functional significance. These RcMYBs’ functions still need to be validated through further experiments. Future studies should focus on exploring their roles in regulating plant height and stress adaptation, with the goal of uncovering their potential for use in R. communis breeding.
PPI network analysis in R. communis identified multiple interacting proteins linked to height- and stress-related RcMYBs, suggesting their involvement in the regulation of plant height and stress responses (Figure 7 and Figure S4). RcMYB45 may regulate anthocyanin biosynthesis under drought stress by controlling the expression of CYP75B1, DTX41, and TT8, a mechanism that has been reported in other species, such as wild tomato (Solanum peruvianum), Chaenomeles speciosa, and common buckwheat (Fagopyrum esculentum) [,,]. Additionally, salt stress could cause ion imbalance in plants [], so RcMYB45 may regulate ion flux to maintain balance and enhance root salt tolerance (Figure 7A). Unlike RcMYB45, the interacting factors of RcMYB27 were primarily associated with secondary cell wall biosynthesis (Figure 7B). AtMYB43 is involved in phenylalanine and lignin biosynthesis, cold stress response, and cadmium tolerance regulation [,,,], while RcMYB27 was upregulated under drought and heat stress, with significant changes in its interacting factors only under salt stress, suggesting they may help R. communis adapt to environmental challenges through specific regulatory networks (Figure 7B).
In addition, the other 10 genes and their interacting proteins exhibited treatment-specific expression patterns: some were induced or repressed under salt stress, some responded markedly to heat or drought stress, while others showed little change under cold conditions (Figure S4A,B,D). The predicted RcMYB-interacting proteins were functionally diverse, also including NAC012, AGL56, LFY, RCMYB52, RCMYB69, RCMYB5, CHX20, AHA10, COR47, RD29A, and XYP7 (Figure S4A,B), playing roles in transcriptional regulation, membrane transport, stress responses, and metabolite trafficking. Although these diverse regulatory networks have not been experimentally validated, they still suggest the functional versatility of these RcMYBs. For instance, the expression patterns of RcMYB6 and its interacting factor RD29A showed that both were upregulated under drought stress, suggesting that they may function in the same drought response mechanism (Figure S4B,D). RD29A, as a typical stress-induced gene, responds more sensitively to drought and cold stress [], and its interaction with RcMYB6 may directly influence drought tolerance in R. communis by modulating key pathways related to stress tolerance (Figure S4A,B). These RcMYBs not only showed differential expression between DL01 and Hale varieties but also exhibited regulation patterns associated with stress conditions (Figure S4). Such tissue-specific and stress-induced expression is common in MYB factors, as observed in related species of R. communis such as cassava (Manihot esculenta), Jatropha curcas, and Hevea brasiliensis [,,], where moderate and targeted transcriptional changes are sufficient to activate downstream responses. Although the PPI network was constructed based on predicted results and had limitations due to its reliance on existing databases and algorithms, it could still provide valuable insights for further research on the interactions of RcMYBs and their interaction relationships. The conclusions need to be confirmed through experimental validation.
In summary, through transcriptomic analysis of two R. communis varieties with different plant heights (DL01 and Hale), we identified 12 RcMYB factors with different expression levels. These factors were also found to be involved in various stress responses and interact with multiple proteins. These height-/stress-related RcMYBs provide a foundation for further research. We aim to explore the relationship between plant height and stress responses in R. communis, particularly by studying these factors, and to uncover their potential mechanisms in regulating plant growth and adaptation to stresses such as salt, cold, drought, and heat. Future work will focus on validating the roles of these RcMYBs through qPCR under different stress conditions, and their functions in plant height and stress responses could be explored using transient overexpression and knockdown experiments. Molecular mechanisms could be further investigated by ChIP-seq for downstream target genes and protein interaction studies using Y2H and BiFC. Due to the lack of a stable transformation system in R. communis, transient expression systems may provide an effective alternative for studying regulatory relationships. Despite this limitation, combining transcriptomic analysis and experimental data provides a solid foundation for gene screening and mechanism exploration, aiming to promote molecular breeding and genetic improvement of R. communis.
4. Materials and Methods
4.1. Plant Materials
Two R. communis lines, DL01 (tall, long-stemmed) and Hale (dwarf, short-stemmed), were grown in field plots at the Tongliao Academy of Agricultural Sciences (Tongliao, Inner Mongolia, China). Seeds were germinated at 28 °C and, upon emergence, seedlings were promptly transplanted to the field. Conditions were a natural long day (~15 h) with early-summer temperatures of 22–26 °C. Seedling stem internodes from both varieties were collected, immediately frozen in liquid nitrogen, and stored at −80 °C in freezers prior to total RNA extraction. Each sample consisted of 4 biological replicates. The seedling stage was selected to capture active internode elongation, when the DL01-Hale height difference is readily observable.
The stress treatment methods and genotypes were described in []. After two weeks of growth in the greenhouse, the seedlings were subjected to one week of drought stress by withholding water. Three-week-old seedlings were used for heat and cold stress treatments. The heat stress group was treated at 45 °C for 12 h, while the cold stress group was treated at 4 °C for 12 h, and whole seedling samples were then collected. For salt stress, two-week-old seedlings were randomly divided into two groups and transferred to a hydroponic system. The control group was continuously supplied with full Hoagland’s solution, while the salt-treated group was supplied with full Hoagland’s solution containing 100 mM NaCl for 24 h. After the treatment, leaves and roots from both the salt-treated and control groups were separately collected.
4.2. RNA-Seq and DEGs Identification
Total RNA was extracted using the RNApure Fast Plant Kit (Beijing Cowin Biotech, Beijing, China), and the RNA quality of all samples met the requirements for transcriptome sequencing. RNA samples were subjected to high-throughput sequencing on the Illumina HiSeq 2000 platform. The raw data obtained were quality-controlled using fastp v0.23.4 and FastQC software v0.12.1 [], then clean reads were aligned using Hisat2 software v2.2.1 and mapped to the R. communis reference genome Rc039 []. The mapped output was processed by Stringtie to generate counts and FPKM values for all genes in each sample []. DEGs were screened using DESeq2, with statistical thresholds set at p-value < 0.05 and |log2FoldChange| > 1 []. Gene functional annotation of all DEGs was further performed using eggNOG, followed by GO enrichment analysis, and visualization was carried out using TBtools software v2.362 []. The classification and quantitative analysis of transcription factors in the DEGs were performed using the PlantTFDB website v5.0 [].
4.3. Identification and Characterization of the RcMYB Family
The MYB family genes and protein sequences of various species were retrieved from the PlantTFDB database []. The BLAST tool v2.17.0 was then used for preliminary screening of RcMYB members within the R. communis reference genome Rc039. To further refine the candidate genes, HMMER was employed to compare the sequences against the known MYB domain from Pfam [,], identifying genes containing the MYB domain in R. communis. Domain verification was carried out using the SMART, CDD, and Pfam databases [,,], leading to the identification of the RcMYB gene family. Proteins’ physicochemical properties were analyzed using the Protein Parameter Calculator in TBtools, gene locations on the chromosomes were visualized using the Gene Location Visualizer, the maximum likelihood (ML)-based phylogenetic tree was constructed using the IQtree Wrapper, while domains of each gene were displayed by using the Gene Structure Viewer in TBtools [].
4.4. Analysis of Expression Trends and Cis-Regulatory Elements of RcMYBs
The Mfuzz package in R v4.5.1 [] was used to analyze the expression trends of RcMYB genes and to classify them into clusters, and the expression levels were visualized through a heatmap by using TBtools. Potential cis-regulatory elements in the promoter regions of RcMYBs were identified using PlantCARE v1.0 [], and the results were visualized using TBtools [].
4.5. Stress Transcriptome Data Processing and Analysis
The raw stress transcriptome data of R. communis were downloaded from the NCBI database [], including leaf control (SRR21177947, SRR21177959, SRR21177958), leaf salt stress (SRR21177957, SRR21177956, SRR21177955), root control (SRR21177953, SRR21177952, SRR21177951), root salt stress (SRR21177950, SRR21177949, SRR21177948), seedling cold stress (SRR21177961), seedling drought stress (SRR21177960), and seedling heat stress (SRR21177954). Tissue types, treatment conditions, and replicates were assigned based on the metadata provided in the corresponding SRA entries. The raw data were quality-controlled with fastp and FastQC [], then clean reads were aligned to the R. communis reference genome Rc039 using Hisat2. The aligned data were processed by Stringtie to generate gene counts and TPM values for each sample []. Gene expression levels were visualized through a heatmap by using TBtools [].
4.6. PPI Network of RcMYBs
The target RcMYB protein sequences were first extracted from the R. communis reference genome Rc039, followed by PPI network prediction using the STRING database [], and potential interacting genes were then identified through BLASTP (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 10 June 2025)). The network was visualized by using Cytoscape software v3.8.0 [].
5. Conclusions
Through stem-internode transcriptome analysis of two contrasting R. communis varieties (DL01, tall; Hale, dwarf), we identified multiple RcMYB transcription factors associated with plant-height regulation. We cataloged the RcMYB gene family, which is widely distributed across the castor genome and exhibits conserved structural features. Those RcMYB genes showing significant differences between DL01 and Hale were prioritized as highly relevant to plant height. Promoter cis-element patterns together with stress-expression analyses indicate regulation by multiple signaling pathways, particularly under abiotic stress. Stress-responsive expression and predicted protein–protein interaction networks suggest that height/stress-related RcMYBs—particularly RcMYB45 and RcMYB27—may interact with multiple regulators. Together, these findings highlight the potential roles of RcMYBs in R. communis plant-height regulation and stress adaptation, with the identified candidates showing condition-responsive patterns across salt, drought, cold, and heat datasets and aligning with interaction neighborhoods linked to secondary cell-wall and anthocyanin/ABA-related pathways. These integrated results offer focused targets for subsequent functional studies and for genetic evaluation in breeding materials of R. communis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262110318/s1.
Author Contributions
J.L. designed the study and provided guidance and suggestions; S.Y. performed sequencing data processing, transcriptome analysis, gene family identification and analysis, and drafted the manuscript; H.W. collected DL01 and Hale materials, performed sequencing data processing, and made modifications to the figures; X.J. collected DL01 and Hale materials, conducted a literature review, and participated in manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Heilongjiang Province Natural Science Foundation (LH2024C036), Opening Project of the State Key Laboratory of Tree Genetics and Breeding (K2021205), and “5211” Research Initiation Funding of Northeast Forestry University (GCC2016-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article and the Supplementary Materials, and the generated raw reads have been uploaded to NCBI with GenBank accession numbers of PRJNA1327410. Further inquiries can be directed to the corresponding author.
Acknowledgments
We greatly thank Song Chen for assistance with the processing of transcriptome sequencing data, and thank Zhibiao He for kindly providing R. communis materials. We would also like to thank Zexin Sun for offering experimental and technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, B.; Smith, S.M.; Li, J. Genetic Regulation of Shoot Architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Ashikari, M.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Swapan, D.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Green revolution: A mutant gibberellin-synthesis gene in rice. Nature 2002, 416, 701–702. [Google Scholar] [CrossRef]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Mu, Q.; Wei, J.; Longest, H.K.; Liu, H.; Char, S.N.; Hinrichsen, J.T.; Tibbs-Cortes, L.E.; Schoenbaum, G.R.; Yang, B.; Li, X.; et al. A MYB transcription factor underlying plant height in sorghum qHT7.1 and maize Brachytic 1 loci. Plant J. 2024, 120, 2172–2192. [Google Scholar] [CrossRef]
- Hollender, C.A.; Hadiarto, T.; Srinivasan, C.; Scorza, R.; Dardick, C. A brachytic dwarfism trait (dw) in peach trees is caused by a nonsense mutation within the gibberellic acid receptor PpeGID1c. New Phytol. 2015, 210, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, W.; Le, L.; Yu, J.; Wu, Y.; Li, D.; Wang, Y.; Wang, H.; Lu, X.; Qiao, H.; et al. Integration of high-throughput phenotyping, GWAS, and predictive models reveals the genetic architecture of plant height in maize. Mol. Plant 2023, 16, 354–373. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, W.; Liu, C.; Tahir, M.M.; Li, X.; Zhou, S.; Ma, F.; Guan, Q. Engineering drought-tolerant apple by knocking down six GH3 genes and potential application of transgenic apple as a rootstock. Hortic. Res. 2022, 9, uhac122. [Google Scholar] [CrossRef] [PubMed]
- Severino, L.S.; Auld, D.L.; Vale, L.S.; Marques, L.F. Plant density does not influence every castor plant equally. Ind. Crop. Prod. 2017, 107, 588–594. [Google Scholar] [CrossRef]
- Anjani, K. Castor genetic resources: A primary gene pool for exploitation. Ind. Crop. Prod. 2012, 35, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Lu, W.; Deng, D. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017, 36, 391–398. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef]
- Tong, H.; Xiao, Y.; Liu, D.; Gao, S.; Liu, L.; Yin, Y.; Jin, Y.; Qian, Q.; Chu, C. Brassinosteroid Regulates Cell Elongation by Modulating Gibberellin Metabolism in Rice. Plant Cell 2014, 26, 4376–4393. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; El Kayal, W.; Prasath, D.; Fernández, H.; Bouzayen, M.; Svircev, A.M.; Jayasankar, S. Identification and genetic characterization of a gibberellin 2-oxidase gene that controls tree stature and reproductive growth in plum. J. Exp. Bot. 2012, 63, 1225–1239. [Google Scholar] [CrossRef]
- Müssig, C.; Fischer, S.; Altmann, T. Brassinosteroid-Regulated Gene Expression. Plant Physiol. 2002, 129, 1241–1251. [Google Scholar] [CrossRef]
- Gocal, G.F.W.; Sheldon, C.C.; Gubler, F.; Moritz, T.; Bagnall, D.J.; MacMillan, C.P.; Li, S.F.; Parish, R.W.; Dennis, E.S.; Weigel, D.; et al. GAMYB-like Genes, Flowering, and Gibberellin Signaling in Arabidopsis. Plant Physiol. 2001, 127, 1682–1693. [Google Scholar] [CrossRef]
- Millar, A.A.; Gubler, F. The Arabidopsis GAMYB-Like Genes, MYB33 and MYB65, Are MicroRNA-Regulated Genes That Redundantly Facilitate Anther Development. Plant Cell 2005, 17, 705–721. [Google Scholar] [CrossRef]
- Alonso-Peral, M.M.; Li, J.; Li, Y.; Allen, R.S.; Schnippenkoetter, W.; Ohms, S.; White, R.G.; Millar, A.A. The MicroRNA159-Regulated GAMYB-like Genes Inhibit Growth and Promote Programmed Cell Death in Arabidopsis. Plant Physiol. 2010, 154, 757–771. [Google Scholar] [CrossRef]
- Xu, W.; Wu, D.; Yang, T.; Sun, C.; Wang, Z.; Han, B.; Wu, S.; Yu, A.; Chapman, M.A.; Muraguri, S.; et al. Genomic insights into the origin, domestication and genetic basis of agronomic traits of castor bean. Genome Biol. 2021, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, L.; Wang, X.; Hu, Z.; Zheng, Y.; Chen, Q.; Hao, X.; Xiao, X.; Wang, X.; Wang, G.; et al. Comprehensive expression analysis of Arabidopsis GA2-oxidase genes and their functional insights. Plant Sci. 2019, 285, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Jeon, H.-W.; Kim, W.-C.; Kim, J.-Y.; Han, K.-H. The MYB46/MYB83-mediated transcriptional regulatory programme is a gatekeeper of secondary wall biosynthesis. Ann. Bot. 2014, 114, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, L.; Zhong, Y. Structure, evolution, and roles of MYB transcription factors proteins in secondary metabolite biosynthetic pathways and abiotic stresses responses in plants: A comprehensive review. Front. Plant Sci. 2025, 16, 1626844. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple Functions of MYB Transcription Factors in Abiotic Stress Responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef]
- Wang, T.; Jin, Y.; Deng, L.; Li, F.; Wang, Z.; Zhu, Y.; Wu, Y.; Qu, H.; Zhang, S.; Liu, Y.; et al. The transcription factor MYB110 regulates plant height, lodging resistance, and grain yield in rice. Plant Cell 2024, 36, 298–323. [Google Scholar] [CrossRef] [PubMed]
- Xing, N.; Li, X.; Wu, S.; Wang, Z. Transcriptome and Metabolome Reveal Key Genes from the Plant Hormone Signal Transduction Pathway Regulating Plant Height and Leaf Size in Capsicum baccatum. Cells 2024, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Y.; Yang, L.; Zhao, T.; Zheng, S.; Peng, X. Genome-wide association study and transcriptome analysis reveal the genetic basis underlying the environmental adaptation of plant height in a woody plant. Plant Physiol. Biochem. 2025, 219, 109361. [Google Scholar] [CrossRef]
- Zhao, Y.; Lei, P.; Zhao, H.; Luo, R.; Li, G.; Di, J.; Wen, L.; He, Z.; Tan, D.; Meng, F.; et al. Physiological, biochemical, and transcriptomic alterations in Castor (Ricinus communis L.) under polyethylene glycol-induced oxidative stress. BMC Plant Biol. 2024, 24, 973. [Google Scholar] [CrossRef]
- Duan, Y.; Han, J.; Guo, B.; Zhao, W.; Zhou, S.; Zhou, C.; Zhang, L.; Li, X.; Han, D. MbICE1 Confers Drought and Cold Tolerance through Up-Regulating Antioxidant Capacity and Stress-Resistant Genes in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 16072. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, Z.; Li, L.; Li, Q.; Geng, Z.; Liu, W.; Hou, R.; Zhang, L.; Han, D. MbWRKY50 confers cold and drought tolerance through upregulating antioxidant capacity associated with ROS scavenging. J. Plant Physiol. 2025, 310, 154526. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.; Wei, Y.; Han, J.; Wang, Y.; Li, X.; Zhang, L.; Han, D. Overexpression of a Fragaria vesca NAM, ATAF, and CUC (NAC) Transcription Factor Gene (FvNAC29) Increases Salt and Cold Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 4088. [Google Scholar] [CrossRef]
- Li, W.; Wei, Y.; Zhang, L.; Wang, Y.; Song, P.; Li, X.; Han, D. FvMYB44, a Strawberry R2R3-MYB Transcription Factor, Improved Salt and Cold Stress Tolerance in Transgenic Arabidopsis. Agronomy 2023, 13, 1051. [Google Scholar] [CrossRef]
- Ren, C.; Li, Z.; Song, P.; Wang, Y.; Liu, W.; Zhang, L.; Li, X.; Li, W.; Han, D. Overexpression of a Grape MYB Transcription Factor Gene VhMYB2 Increases Salinity and Drought Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 10743. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, L.; Dai, J.; Li, Z.; Zhang, A.; Wang, T.; Liu, W.; Li, X.; Han, D. Overexpression of a Grape WRKY Transcription Factor VhWRKY44 Improves the Resistance to Cold and Salt of Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 7437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, H.; Zhang, Y.; Zhao, Y.; Zhang, Y.; Feng, X.; Lin, H. Diverse roles of MYB transcription factors in plants. J. Integr. Plant Biol. 2025, 67, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, A.; Smita, S.; Lenka, S.K.; Rajwanshi, R.; Chinnusamy, V.; Bansal, K.C. Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis. BMC Genom. 2012, 13, 544. [Google Scholar] [CrossRef]
- Gong, Q.; Li, S.; Zheng, Y.; Duan, H.; Xiao, F.; Zhuang, Y.; He, J.; Wu, G.; Zhao, S.; Zhou, H.; et al. SUMOylation of MYB30 enhances salt tolerance by elevating alternative respiration via transcriptionally upregulating AOX1a in Arabidopsis. Plant J. 2020, 102, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, Y.; Zheng, H.; Lu, W.; Wu, C.; Huang, J.; Yan, K.; Yang, G.; Zheng, C. Salt-induced transcription factor MYB74 is regulated by the RNA-directed DNA methylation pathway in Arabidopsis. J. Exp. Bot. 2015, 66, 5997–6008. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Li, X.; Li, Y. E2 conjugases UBC1 and UBC2 regulate MYB42-mediated SOS pathway in response to salt stress in Arabidopsis. New Phytol. 2020, 227, 455–472. [Google Scholar] [CrossRef]
- Cui, M.H.; Yoo, K.S.; Hyoung, S.; Nguyen, H.T.K.; Kim, Y.Y.; Kim, H.J.; Ok, S.H.; Yoo, S.D.; Shin, J.S. An Arabidopsis R2R3-MYB transcription factor, AtMYB20, negatively regulates type 2C serine/threonine protein phosphatases to enhance salt tolerance. FEBS Lett. 2013, 587, 1773–1778. [Google Scholar] [CrossRef]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.-H.; Fujii, H.; Zheng, X.; Zhu, J.-K. A R2R3 Type MYB Transcription Factor Is Involved in the Cold Regulation of CBF Genes and in Acquired Freezing Tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef]
- Chen, L.; Yang, H.; Fang, Y.; Guo, W.; Chen, H.; Zhang, X.; Dai, W.; Chen, S.; Hao, Q.; Yuan, S.; et al. Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway. Plant Biotechnol. J. 2020, 19, 702–716. [Google Scholar] [CrossRef]
- Cousminer, D.L.; Wagley, Y.; Pippin, J.A.; Elhakeem, A.; Way, G.P.; Pahl, M.C.; McCormack, S.E.; Chesi, A.; Mitchell, J.A.; Kindler, J.M.; et al. Genome-wide association study implicates novel loci and reveals candidate effector genes for longitudinal pediatric bone accrual. Genome Biol. 2021, 22, 1. [Google Scholar] [CrossRef]
- Patel, V.R.; Dumancas, G.G.; Viswanath, L.C.K.; Maples, R.; Subong, B.J.J. Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production. Lipid Insights 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Severino, L.S.; Auld, D.L.; Baldanzi, M.; Cândido, M.J.D.; Chen, G.; Crosby, W.; Tan, D.; He, X.; Lakshmamma, P.; Lavanya, C.; et al. A Review on the Challenges for Increased Production of Castor. Agron. J. 2012, 104, 853–880. [Google Scholar] [CrossRef]
- Halek, F.; Delavari, A.; Kavousi-Rahim, A. Production of biodiesel as a renewable energy source from castor oil. Clean Technol. Environ. Policy 2012, 15, 1063–1068. [Google Scholar] [CrossRef]
- Azad, A.; Rasul, M.; Khan, M.; Sharma, S.C.; Mofijur, M.; Bhuiya, M. Prospects, feedstocks and challenges of biodiesel production from beauty leaf oil and castor oil: A nonedible oil sources in Australia. Renew. Sustain. Energy Rev. 2016, 61, 302–318. [Google Scholar] [CrossRef]
- Fan, W.; Lu, J.; Pan, C.; Tan, M.; Lin, Q.; Liu, W.; Li, D.; Wang, L.; Hu, L.; Wang, L.; et al. Sequencing of Chinese castor lines reveals genetic signatures of selection and yield-associated loci. Nat. Commun. 2019, 10, 3418. [Google Scholar] [CrossRef]
- Lu, J.; Pan, C.; Fan, W.; Liu, W.; Zhao, H.; Li, D.; Wang, S.; Hu, L.; He, B.; Qian, K.; et al. A Chromosome-Level Genome Assembly of Wild Castor Provides New Insights into its Adaptive Evolution in Tropical Desert. Genom. Proteom. Bioinform. 2022, 20, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, S.; Li, B.; Liu, Y.; He, Z.; Zhang, Q.; Zheng, Z. A microRNA396b-growth regulating factor module controls castor seed size by mediating auxin synthesis. Plant Physiol. 2024, 196, 916–930. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Nesi, N.; Jond, C.; Debeaujon, I.; Caboche, M.; Lepiniec, L. The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 2001, 13, 2099–2114. [Google Scholar] [CrossRef] [PubMed]
- Baudry, A.; Heim, M.A.; Dubreucq, B.; Caboche, M.; Weisshaar, B.; Lepiniec, L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 2004, 39, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Baxter, I.; Young, J.C.; Armstrong, G.; Foster, N.; Bogenschutz, N.; Cordova, T.; Peer, W.; Hazen, S.; Murphy, A.S.; Harper, J.F. A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2005, 102, 2649–2654. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.-M.; Debeaujon, I.; Klein, M. TheArabidopsisMATE Transporter TT12 Acts as a Vacuolar Flavonoid/H+-Antiporter Active in Proanthocyanidin-Accumulating Cells of the Seed Coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef]
- Gonzalez, A.; Mendenhall, J.; Huo, Y.; Lloyd, A. TTG1 complex MYBs, MYB5 and TT2, control outer seed coat differentiation. Dev. Biol. 2009, 325, 412–421. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, S.; Liu, J.; Zhao, C.; Wu, J.; Cao, Y.; Fu, C.; Han, X.; He, H.; Zhao, Q. MYB20, MYB42, MYB43, and MYB85 Regulate Phenylalanine and Lignin Biosynthesis during Secondary Cell Wall Formation. Plant Physiol. 2020, 182, 1272–1283. [Google Scholar] [CrossRef]
- Zheng, P.; Cao, L.; Zhang, C.; Fang, X.; Wang, L.; Miao, M.; Tang, X.; Liu, Y.; Cao, S. The transcription factor MYB43 antagonizes with ICE1 to regulate freezing tolerance in Arabidopsis. New Phytol. 2023, 238, 2440–2459. [Google Scholar] [CrossRef]
- Tamadaddi, C.; Choi, J.; Ghasemi, M.; Kim, S.H.; Gomez, E.D.; Gomez, E.W.; Anderson, C.T. NST3 induces ectopic transdifferentiation, forming secondary walls with diverse patterns and composition in Arabidopsis thaliana. Ann. Bot. 2024, 134, 1097–1111. [Google Scholar] [CrossRef]
- Li, E.; Bhargava, A.; Qiang, W.; Friedmann, M.C.; Forneris, N.; Savidge, R.A.; Johnson, L.A.; Mansfield, S.D.; Ellis, B.E.; Douglas, C.J. The Class II KNOX gene KNAT7 negatively regulates secondary wall formation in Arabidopsis and is functionally conserved in Populus. New Phytol. 2012, 194, 102–115. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, R.L.; Ye, Z.-H. A Battery of Transcription Factors Involved in the Regulation of Secondary Cell Wall Biosynthesis in Arabidopsis. Plant Cell 2008, 20, 2763–2782. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, X.; Yu, C.; Ye, C.; Yan, Y.; Wang, H. What factors control plant height? J. Integr. Agric. 2024, 23, 1803–1824. [Google Scholar] [CrossRef]
- Yanhui, C.; Xiaoyuan, Y.; Kun, H.; Meihua, L.; Jigang, L.; Zhaofeng, G.; Zhiqiang, L.; Yunfei, Z.; Xiaoxiao, W.; Xiaoming, Q.; et al. The MYB Transcription Factor Superfamily of Arabidopsis: Expression Analysis and Phylogenetic Comparison with the Rice MYB Family. Plant Mol. Biol. 2006, 60, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, S.-S.; Liang, Z.; Feng, B.-R.; Liu, L.; Huang, Y.-B.; Tang, Y.-X. Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC Plant Biol. 2012, 12, 106. [Google Scholar] [CrossRef]
- Yang, H.; Chen, C.; Han, L.; Zhang, X.; Yue, M. Genome-Wide Identification and Expression Analysis of the MYB Transcription Factor Family in Salvia nemorosa. Genes 2024, 15, 110. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Huang, Y.; Zhu, P.; Qian, G.; Zhang, Y.; Li, L. Genome-Wide Identification and Analysis of R2R3-MYB Genes Response to Saline–Alkali Stress in Quinoa. Int. J. Mol. Sci. 2023, 24, 9132. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Gong, Y.-H.; Tao, T.; Lu, S.; Zhou, W.-Y.; Xia, H.; Zhang, X.-Y.; Yang, Q.-Q.; Zhang, M.-Q.; Hong, L.-M.; et al. Genome-wide identification of R2R3-MYB transcription factor subfamily genes involved in salt stress in rice (Oryza sativa L.). BMC Genom. 2024, 25, 797. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, S.; Htwe, Y.M.; Sun, X.; Zhou, L.; Wang, F.; Zeng, C.; Chen, S.; Iqbal, A.; Yang, Y. Genome-wide identification, classification and expression analysis of MYB gene family in coconut (Cocos nucifera L.). Front. Plant Sci. 2024, 14, 1263595. [Google Scholar]
- Jacob, P.; Brisou, G.; Dalmais, M.; Thévenin, J.; van der Wal, F.; Latrasse, D.; Suresh Devani, R.; Benhamed, M.; Dubreucq, B.; Boualem, A.; et al. The Seed Development Factors TT2 and MYB5 Regulate Heat Stress Response in Arabidopsis. Genes 2021, 12, 746. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, R.; Zhang, P.; Sun, L.; Ju, Q.; Huang, H.; Lü, S.; Tran, L.-S.; Xu, J. MYB70 modulates seed germination and root system development in Arabidopsis. iScience 2021, 24, 103228. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, Q.; Mao, H.; Xu, J.; Wang, Y.; Hu, H.; He, S.; Tu, J.; Cheng, C.; Tian, G.; et al. AtDIV2, an R-R-type MYB transcription factor of Arabidopsis, negatively regulates salt stress by modulating ABA signaling. Plant Cell Rep. 2018, 37, 1499–1511. [Google Scholar] [CrossRef]
- Ding, Z.; Li, S.; An, X.; Liu, X.; Qin, H.; Wang, D. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J. Genet. Genom. 2009, 36, 17–29. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, S.; Zhao, Q.; Wu, Y.; Li, H. The CsMYB123 and CsbHLH111 are involved in drought stress-induced anthocyanin biosynthesis in Chaenomeles speciosa. Mol. Hortic. 2023, 3, 25. [Google Scholar] [CrossRef]
- Tapia, G.; Castro, M.; Gaete-Eastman, C.; Figueroa, C.R. Regulation of Anthocyanin Biosynthesis by Drought and UV-B Radiation in Wild Tomato (Solanum peruvianum) Fruit. Antioxidants 2022, 11, 1639. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, X.; Yang, L.; Zhu, X.; Du, Y.; Fang, Z. A R2R3-MYB transcription factor, FeR2R3-MYB, positively regulates anthocyanin biosynthesis and drought tolerance in common buckwheat (Fagopyrum esculentum). Plant Physiol. Biochem. 2024, 217, 109254. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Jiang, J.; Liao, X.; Jin, X.; Tan, L.; Lu, Q.; Yuan, C.; Xue, Y.; Yin, N.; Lin, N.; Chai, Y. MYB43 in Oilseed Rape (Brassica napus) Positively Regulates Vascular Lignification, Plant Morphology and Yield Potential but Negatively Affects Resistance to Sclerotinia sclerotiorum. Genes 2020, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Cao, L.; Zhang, C.; Pan, W.; Wang, W.; Yu, X.; Li, Y.; Fan, T.; Miao, M.; Tang, X.; et al. MYB43 as a novel substrate for CRL4PRL1 E3 ligases negatively regulates cadmium tolerance through transcriptional inhibition of HMAs in Arabidopsis. New Phytol. 2022, 234, 884–901. [Google Scholar] [CrossRef] [PubMed]
- Msanne, J.; Lin, J.S.; Stone, J.M.; Awada, T. Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes. Planta 2011, 234, 97–107. [Google Scholar] [CrossRef]
- Ruan, M.-B.; Guo, X.; Wang, B.; Yang, Y.-L.; Li, W.-Q.; Yu, X.-L.; Zhang, P.; Peng, M. Genome-wide characterization and expression analysis enables identification of abiotic stress-responsive MYB transcription factors in cassava (Manihot esculenta). J. Exp. Bot. 2017, 68, 3657–3672. [Google Scholar] [CrossRef]
- Peng, X.; Liu, H.; Wang, D.; Shen, S. Genome-wide identification of the Jatropha curcas MYB family and functional analysis of the abiotic stress responsive gene JcMYB2. BMC Genom. 2016, 17, 251. [Google Scholar] [CrossRef]
- Qin, B.; Fan, S.-L.; Yu, H.-Y.; Lu, Y.-X.; Wang, L.-F. HbMYB44, a Rubber Tree MYB Transcription Factor With Versatile Functions in Modulating Multiple Phytohormone Signaling and Abiotic Stress Responses. Front. Plant Sci. 2022, 13, 893896. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, P.; Yu, A.; Chapman, M.A.; Liu, A. Genome-wide characterization and evolutionary analysis of linker histones in castor bean (Ricinus communis). Front. Plant Sci. 2022, 13, 1014418. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; López, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Futschik, M.E. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5–7. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).