Molecular Impact of Metabolic and Endocrine Disturbance on Endometrial Function in Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Scope and Methodology
3. Endometrial Function in Reproductive-Age Women
3.1. Normal Endometrial Physiology
3.2. Regulation of Hypothalamic–Pituitary Gonadotrophin Hormones
3.3. Hormonal Control of Ovarian Hormones—Indirect Control of the Endometrium
3.3.1. Sex Steroid Regulation of the Endometrium—Systemic Endocrine Control
3.3.2. Intracrine Metabolism in the Endometrium—Local Hormonal Control
3.4. The Endometrium as a Component of the Mucosal Immune System
3.5. Endometrial Inflammation and Leukocyte Function
3.6. Role of Vasoconstriction, Hypoxia, and Hemostasis in Menstruation
3.7. Endometrial Stem Cells
4. Pathophysiology of Dysfunctional Endometrium
4.1. Classification of Abnormal Uterine Bleeding (AUB) and Heavy Menstrual Bleeding (HMB)
4.2. Association Between IR, Hyperinsulinemia, and Menstrual Dysfunction
4.3. Impact of IR and Hyperinsulinemia on Endometrial Dysfunction
4.3.1. Indirect Effect of IR and Hyperinsulinemia on Endometrial Dysfunction via Altered Ovarian Hormone Production
4.3.2. Direct Effect of IR and Hyperinsulinemia on Endometrial Dysfunction
4.4. Chronic Systemic Inflammation (CSI) and Endometrial Dysfunction
4.5. Molecular Changes in the Dysfunctional Endometrium in Ovulatory and Anovulatory PCOS
4.5.1. Endometrial Changes in Ovulatory PCOS
4.5.2. Endometrial Changes in Anovulatory PCOS
4.6. Reduced Vasoconstriction, Angiogenesis, and Matrix Remodeling in HMB
4.7. Genetic Insights into Endometrial Changes in PCOS
4.8. Role of the Microbiome (MB) in PCOS
4.8.1. Role of the Microbiome in PCOS and Endometrial Dysfunction
4.8.2. Mechanistic Links Between the Gut Microbiota and Endometrial Dysfunction
4.8.3. Role of the Endometrial Microbiome in Endometrial Dysfunction
4.9. PCOS Endometrium-Derived Organoids and Endometrial Dysfunction
4.10. Impact of Lifestyle Changes on Endometrial Dysfunction Where No Cause Is Identified
5. Discussion
5.1. Mechanistic Implications of Novel Findings Identified in This Review
5.2. Current Gaps in the Molecular Understanding of Endometrial Dysfunction in PCOS
5.3. Development of Precision Research and Targeted Therapeutic Strategies
6. Limitations of the Current Narrative Review
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Serine/threonine-specific kinase (also known as protein kinase B) |
| AR | Androgen receptor |
| AUB | Abnormal uterine bleeding |
| BMI | Body mass index |
| cAMP | Cyclic adenosine 3’5’-monophosphate |
| CHO | Carbohydrate |
| COEIN | Coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, not otherwise classified |
| COX-2 | Cyclooxygenase-2 |
| CSI | Chronic systemic inflammation |
| CYP19A1 | Aromatase |
| DEG | Differentially expressed genes |
| DHEA | Dehydroepiandrosterone |
| EEO | Endometrial epithelial organoid |
| ER | Estrogen receptor |
| E2 | 17β-estradiol |
| FIGO | International Federation of Gynecology and Obstetrics |
| FOXO | Forkhead box protein O |
| FSH | Follicle-stimulating hormone |
| GLUT4 | Glucose transporter type 4 |
| GnRH | Gonadotropin-releasing hormone |
| HA | Hyperandrogenism |
| HMB | Heavy menstrual bleeding |
| HOXA10 | Homeobox 10 |
| HPO | Hypothalamic–pituitary–ovarian |
| HSD | Hydroxysteroid dehydrogenase |
| IGF-1 | Insulin-like growth factor-1 |
| IL | Interleukin |
| IR | Insulin resistance |
| KNDy | Kisspeptin, neurokinin B, dynorphin-y |
| LH | Luteinizing hormone |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MB | Microbiome |
| MMP | Matrix metalloproteinase |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PALM | Polyp, adenomyosis, leiomyoma, malignancy, or hyperplasia |
| PAX6 | Heterogeneous paired box 6 |
| PCOS | Polycystic ovary syndrome |
| PG | Prostaglandin |
| PI3K | Phosphoinositide 3-kinase |
| PR | Progesterone receptor |
| P4 | Progesterone |
| RNA | Ribonucleic acid |
| STAT3 | Signal transducer and activator of transcription 3 |
| TNF-α | Tumor necrosis factor-α |
| T2DM | Type 2 diabetes mellitus |
| VEGF | Vascular endothelial growth factor |
| WT1 | Wilms tumor-1 |
| Wnt | Wingless-related integration site |
References
- Parker, J.; O’Brien, C.; Hawrelak, J.; Gersh, F.L. Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment. Int. J. Environ. Res. Public Health 2022, 19, 1336. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Abbott, D.H.; Chazenbalk, G.D.; Scholar, G. An Evolutionary Model for the Ancient Origins of Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 6120. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Padmanabhan, V.; Abbott, D.H. Polycystic ovary syndrome: An evolutionary metabolic adaptation. Reproduction 2025, 169, e250021. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur. J. Endocrinol. 2023, 189, G43–G64. [Google Scholar] [CrossRef]
- Wechsung, K.; Neumann, U.; Balint, N.; Wiegand, S.; Klinikum, C.V.; Der Entwicklung, P.; Frauenheilk, G. Polycystic Ovary Syndrome–Support and Prevention in Adolescence. Geburtsh Frauenheilk 2025; August, 1–7. Available online: https://www.thieme-connect.de/products/ejournals/abstract/10.1055/a-2622-6321 (accessed on 15 July 2025).
- Parker, J. Pathophysiological Effects of Contemporary Lifestyle on Evolutionary-Conserved Survival Mechanisms in Polycystic Ovary Syndrome. Life 2023, 13, 1056. [Google Scholar] [CrossRef]
- Balen, A.; Rajkowha, M. Polycystic ovary syndrome—A systemic disorder? Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 263–274. [Google Scholar] [CrossRef]
- Teede, H.J.; Moran, L.J.; Morman, R.; Gibson, M.; Dokras, A.; Berry, L.; Laven, J.S.E.; Joham, A.; Piltonen, T.T.; Costello, M.F.; et al. Polycystic ovary syndrome perspectives from patients and health professionals on clinical features, current name, and renaming: A longitudinal international online survey. eClinicalMedicine 2025, 84, 103287. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zheng, Z.; Zhang, R. Evolution of polycystic ovary syndrome and related infertility in women of child-bearing age: A global burden of disease study 2021 analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 314, 114659. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C.; Yeoh, C.; Gersh, F.L.; Brennecke, S. Reducing the Risk of Pre-Eclampsia in Women with Polycystic Ovary Syndrome Using a Combination of Pregnancy Screening, Lifestyle, and Medical Management Strategies. J. Clin. Med. 2024, 13, 1774. [Google Scholar] [CrossRef]
- Palomba, S.; Piltonen, T.T.; Giudice, L.C. Endometrial function in women with polycystic ovary syndrome: A comprehensive review. Hum. Reprod. Update 2021, 27, 584–618. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Tay, C.T.; Teede, H. Technical Report for the 2023 International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome; Monash University: Clayton, VIC, Australia, 2023. [Google Scholar]
- Giudice, L.C. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 235–244. [Google Scholar] [CrossRef]
- Eriksson, G.; Li, C.; Sparovec, T.G.; Dekanski, A.; Torstensson, S.; Risal, S.; Ohlsson, C.; Hirschberg, A.L.; Petropoulos, S.; Deng, Q.; et al. Single-cell profiling of the human endometrium in polycystic ovary syndrome. Nat. Med. 2025, 30, 1925–1938. [Google Scholar] [CrossRef]
- Wong, F.C.; Kim, C.E.; Garcia-Alonso, L.; Vento-Tormo, R. The human endometrium: Atlases, models, and prospects. Curr. Opin. Genet. Dev. 2025, 92, 102341. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Zhou, Y.; Cao, F.; Fang, H.; Ma, H.; Ren, J.; Huang, C.; Diao, L.; Li, Q.; et al. Molecular subtype of recurrent implantation failure reveals distinct endometrial etiology of female infertility. J. Transl. Med. 2025, 23, 792. [Google Scholar] [CrossRef]
- Lou, L.; Kong, S.; Sun, Y.; Zhang, Z.; Wang, H. Human Endometrial Organoids: Recent Research Progress and Potential Applications. Front. Cell Dev. Biol. 2022, 10, 844623. [Google Scholar] [CrossRef] [PubMed]
- Fornes, R.; Ormazabal, P.; Rosas, C.; Gabler, F.; Vantman, D.; Romero, C.; Vega, M. Changes in the expression of insulin signaling pathway molecules in endometria from polycystic ovary syndrome women with or without hyperinsulinemia. Mol. Med. 2010, 16, 129–136. [Google Scholar] [CrossRef]
- Gonzalez, D.; Thackeray, H.; Lewis, P.D.; Mantani, A.; Brook, N.; Ahuja, K.; Margara, R.; Joels, L.; White, J.O.; Conlan, R.S. Loss of WT1 expression in the endometrium of infertile PCOS patients: A hyperandrogenic effect? J. Clin. Endocrinol. Metab. 2012, 97, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Mehdinejadiani, S.; Amidi, F.; Mehdizadeh, M.; Barati, M. Effects of letrozole and clomiphene citrate on Wnt signaling pathway in endometrium of polycystic ovarian syndrome and healthy women. Biol. Reprod. 2019, 100, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Li, J.; Feng, J.; Han, H.; Zhao, J.; Zhang, J.; Han, Y.; Wu, X.; Zhang, Y. Research Progress on the Mechanism Between Polycystic Ovary Syndrome and Abnormal Endometrium. Front. Physiol. 2021, 12, 788772. [Google Scholar] [CrossRef]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Oróstica, L.; Poblete, C.; Romero, C.; Vega, M. Pro-Inflammatory Markers Negatively Regulate IRS1 in Endometrial Cells and Endometrium from Women with Obesity and PCOS. Reprod. Sci. 2020, 27, 290–300. [Google Scholar] [CrossRef]
- Retis-Resendiz, A.M.; González-García, I.N.; León-Juárez, M.; Camacho-Arroyo, I.; Cerbón, M.; Vázquez-Martínez, E.R. The role of epigenetic mechanisms in the regulation of gene expression in the cyclical endometrium. Clin. Epigenet. 2021, 13, 116. [Google Scholar] [CrossRef]
- Khatun, M.; Meltsov, A.; Lavogina, D.; Loid, M.; Kask, K.; Arffman, R.K.; Rossi, H.R.; Lättekivi, F.; Jääger, K.; Krjutškov, K.; et al. Decidualized endometrial stromal cells present with altered androgen response in PCOS. Sci. Rep. 2021, 11, 16287. [Google Scholar] [CrossRef]
- Ding, N.; Wang, R.; Wang, P.; Wang, F. Metabolism-related proteins as biomarkers for predicting prognosis in polycystic ovary syndrome. Proteome Sci. 2024, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Reavey, J.J.; Walker, C.; Murray, A.A.; Brito-Mutunayagam, S.; Sweeney, S.; Nicol, M.; Cambursano, A.; Critchley, H.O.D.; Maybin, J.A. Obesity is associated with heavy menstruation that may be due to delayed endometrial repair. J. Endocrinol. 2021, 249, 71–82. [Google Scholar] [CrossRef]
- Parker, J.; Hofstee, P.; Brennecke, S. Prevention of Pregnancy Complications Using a Multimodal Lifestyle, Screening, and Medical Model. J. Clin. Med. 2024, 13, 4344. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Insulin–PI3K signalling: An evolutionarily insulated metabolic driver of cancer. Nat. Rev. Endocrinol. 2020, 16, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. The human placenta: New perspectives on its formation and function during early pregnancy. Proc. R. Soc. B Biol. Sci. 2023, 290, 20230191. [Google Scholar] [CrossRef] [PubMed]
- Dias Da Silva, I.; Wuidar, V.; Zielonka, M.; Pequeux, C. Unraveling the Dynamics of Estrogen and Progesterone Signaling in the Endometrium: An Overview. Cells 2024, 13, 1236. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Ph, D.; Jain, V.; Sc, M.; Critchley, H.; Sc, D. From menarche to menopause, heavy menstrual bleeding is the underrated compass in reproductive health. Fertil. Steril. 2022, 118, 625–636. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Committee on Adolescence; American College of Obstetricians and Gynecologists; Committee on Adolescent Health Care. Menstruation in Girls and Adolescents: Using the Menstrual Cycle as a Vital Sign. Pediatrics 2006, 118, 2245–2250. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists Menstruation in Girls and Adolescents: Using the Menstrual Cycle as a Vital Sign. Committee Opinion No. 651. Obstet. Gynecol. 2015, 126, e143–e146. [CrossRef]
- Houghton, L.C. Menstruation as the Next Vital Sign. JAMA Netw. Open 2024, 7, e2412778. [Google Scholar] [CrossRef]
- Vollmar, A.K.R.; Ph, D.; Mahalingaiah, S.; Jukic, A.M.; Ph, D. The menstrual cycle as a vital sign: A comprehensive review. Fertil. Steril. Rev. 2025, 6, 100081. [Google Scholar] [CrossRef]
- Moore, A.M.; Coolen, L.M.; Porter, D.T.; Goodman, R.L.; Lehman, M.N. KNDy cells revisited. Endocrinology 2018, 159, 3219–3234. [Google Scholar] [CrossRef]
- Nagae, M.; Uenoyama, Y.; Okamoto, S.; Tsuchida, H.; Ikegami, K.; Goto, T.; Majarune, S.; Nakamura, S.; Sanbo, M.; Hirabayashi, M.; et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc. Natl. Acad. Sci. USA 2021, 118, e2009156118. [Google Scholar] [CrossRef]
- Marques, P.; Skorupskaite, K.; George, J.T.; Anderson, R.A. Physiology of GNRH and Gonadotropin Secretion. Endotext. 13 October 2024; pp. 1–35. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25905297 (accessed on 25 July 2025).
- Stevenson, H.; Bartram, S.; Charalambides, M.M.; Murthy, S.; Petitt, T.; Pradeep, A.; Vineall, O.; Abaraonye, I.; Lancaster, A.; Koysombat, K.; et al. Kisspeptin-neuron control of LH pulsatility and ovulation. Front. Endocrinol. 2022, 13, 951938. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Lin, H. Estrogen disorders: Interpreting the abnormal regulation of aromatase in granulosa cells (Review). Int. J. Mol. Med. 2021, 47, 73. [Google Scholar] [CrossRef]
- Das, S.K. Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction 2009, 137, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.C.; Winuthayanon, W.; Lierz, S.L.; Hamilton, K.J.; Donoghue, L.J.; Ramsey, J.T.; Grimm, S.A.; Arao, Y.; Korach, K.S. Role of ERα in mediating female uterine transcriptional responses to IGF1. Endocrinology 2017, 158, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hou, C.C.; Luo, L.F.; Hu, Y.J.; Yang, W.X. Endometrial stromal cells and decidualized stromal cells: Origins, transformation and functions. Gene 2014, 551, 1–14. [Google Scholar] [CrossRef]
- Vasquez, Y.M.; Wang, X.; Wetendorf, M.; Franco, H.L.; Mo, Q.; Wang, T.; Lanz, R.B.; Young, S.L.; Lessey, B.A.; Spencer, T.E.; et al. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet. 2018, 14, e1007787. [Google Scholar] [CrossRef]
- Mueller, M.D.; Vigne, J.L.; Minchenko, A.; Lebovic, D.I.; Leitman, D.C.; Taylor, R.N. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors α and β. Proc. Natl. Acad. Sci. USA 2000, 97, 10972–10977. [Google Scholar] [CrossRef]
- King, A.E.; Critchley, H.O.D. Oestrogen and progesterone regulation of inflammatory processes in the human endometrium. J. Steroid Biochem. Mol. Biol. 2010, 120, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.D.; Sison, C.A.M.; Yildiz, S.; Miyazaki, K.; Coon, V.J.; Yin, P.; Bulun, S.E. Genome-wide estrogen receptor-α binding and action in human endometrial stromal cells. FS Sci. 2020, 1, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Takakura, M.; Fujii, R.; Maida, Y.; Bono, Y.; Mizumoto, Y.; Zhang, X.; Kiyono, T.; Kyo, S. The PRB-dependent FOXO1/IGFBP-1 axis is essential for progestin to inhibit endometrial epithelial growth. Cancer Lett. 2013, 336, 68–75. [Google Scholar] [CrossRef]
- Itoh, H.; Kishore, A.H.; Lindqvist, A.; Rogers, D.E.; Word, R.A. Transforming growth factor β1 (TGFβ1) and progesterone regulate matrix metalloproteinases (MMP) in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 2012, 97, 888–897. [Google Scholar] [CrossRef][Green Version]
- Konings, G.; Brentjens, L.; Delvoux, B.; Linnanen, T.; Cornel, K.; Koskimies, P.; Bongers, M.; Kruitwagen, R.; Xanthoulea, S.; Romano, A. Intracrine regulation of estrogen and other sex steroid levels in endometrium and non-gynecological tissues; Pathology, physiology, and drug discovery. Front. Pharmacol. 2018, 9, 940. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F. Intracrinology. Mol. Cell. Endocrinol. 1991, 78, 13–18. [Google Scholar] [CrossRef]
- Gibson, D.A.; Simitsidellis, I.; Collins, F.; Saunders, P.T.K. Endometrial intracrinology: Oestrogens, androgens and endometrial disorders. Int. J. Mol. Sci. 2018, 19, 3276. [Google Scholar] [CrossRef]
- Alanazi, S. Recent Advances in Liquid Chromatography–Mass Spectrometry (LC–MS) Applications in Biological and Applied Sciences. Anal. Sci. Adv. 2025, 6, e70024. [Google Scholar] [CrossRef] [PubMed]
- Huhtinen, K.; Desai, R.; Stahle, M.; Salminen, A.; Handelsman, D.J.; Perheentupa, A.; Poutanen, M. Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J. Clin. Endocrinol. Metab. 2012, 97, 4228–4235. [Google Scholar] [CrossRef]
- Huhtinen, K.; Saloniemi-Heinonen, T.; Keski-Rahkonen, P.; Desai, R.; Laajala, D.; Stahle, M.; Häkkinen, M.R.; Awosanya, M.; Suvitie, P.; Kujari, H.; et al. Intra-tissue steroid profiling indicates differential progesterone and testosterone metabolism in the endometrium and endometriosis lesions. J. Clin. Endocrinol. Metab. 2014, 99, E2188–E2197. [Google Scholar] [CrossRef]
- Cloke, B.; Huhtinen, K.; Fusi, L.; Kajihara, T.; Yliheikkilä, M.; Ho, K.K.; Teklenburg, G.; Lavery, S.; Jones, M.C.; Trew, G.; et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology 2008, 149, 4462–4474. [Google Scholar] [CrossRef]
- Ishikawa, T.; Harada, T.; Kubota, T.; Aso, T. Testosterone inhibits matrix metalloproteinase-1 production in human endometrial stromal cells in vitro. Reproduction 2007, 133, 1233–1239. [Google Scholar] [CrossRef]
- Grzechocińska, B.; Dabrowski, F.; Cyganek, A.; Panek, G.; Wielgoś, M. The role of metalloproteinases in endometrial remodelling during menstrual cycle. Ginekol. Pol. 2017, 88, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Staun-Ram, E.; Goldman, S.; Gabarin, D.; Shalev, E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod. Biol. Endocrinol. 2004, 2, 59. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Zhu, Z.; Lu, C.; Zhang, C.; Zeng, L.; Xie, F.; Zhang, L.; Zhou, F. Mucosal immune response in biology, disease prevention and treatment. Signal Transduct. Target. Ther. 2025, 10, 7. [Google Scholar] [CrossRef]
- Kim, S.; Ndwandwe, C.; Devotta, H.; Kareem, L.; Yao, L.; O’Mahony, L. Role of the microbiome in regulation of the immune system. Allergol. Int. 2025, 74, 187–196. [Google Scholar] [CrossRef]
- Choden, T.; Cohen, N.A. The gut microbiome and the immune system. Explor. Med. 2022, 3, 219–233. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C.; Hawrelak, J. A narrative review of the role of gastrointestinal dysbiosis in the pathogenesis of polycystic ovary syndrome. Obstet. Gynecol. Sci. 2022, 65, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Colonetti, T.; Limas Carmo Teixeira, D.; Grande, A.J.; Rodrigues Uggioni, M.L.; Generoso, J.; Harding, S.; Rodriguez-Mateos, A.; Rech, P.; Rosa Silva, F.; Toreti, I.; et al. The role of intestinal microbiota on pre-eclampsia: Systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 291, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Medina-Bastidas, D.; Camacho-Arroyo, I.; García-Gómez, E. Current findings in endometrial microbiome: Impact on uterine diseases. Reproduction 2022, 163, R81–R96. [Google Scholar] [CrossRef]
- Monin, L.; Whettlock, E.M.; Male, V. Immune responses in the human female reproductive tract. Immunology 2020, 160, 106–115. [Google Scholar] [CrossRef]
- Bister, J.; Crona Guterstam, Y.; Strunz, B.; Dumitrescu, B.; Haij Bhattarai, K.; Özenci, V.; Brännström, M.; Ivarsson, M.A.; Gidlöf, S.; Björkström, N.K. Human endometrial MAIT cells are transiently tissue resident and respond to Neisseria gonorrhoeae. Mucosal Immunol. 2021, 14, 357–365. [Google Scholar] [CrossRef]
- Wira, C.R.; Rodriguez-Garcia, M.; Patel, M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 2015, 15, 217–230. [Google Scholar] [CrossRef]
- Chatterjee, S.K.; Saha, S.; Munoz, M.N.M. Activation of mucosal immunity and novel prophylactic and therapeutic strategy in combating COVID-19. Explor. Immunol. 2021, 1, 374–397. [Google Scholar] [CrossRef]
- Evans, J.; Salamonsen, L.A. Inflammation, leukocytes and menstruation. Rev. Endocr. Metab. Disord. 2012, 13, 277–288. [Google Scholar] [CrossRef]
- Azlan, A.; Salamonsen, L.A.; Hutchison, J.; Evans, J. Endometrial inflammasome activation accompanies menstruation and may have implications for systemic inflammatory events of the menstrual cycle. Hum. Reprod. 2020, 35, 1363–1376. [Google Scholar] [CrossRef]
- Maybin, J.A.; Critchley, H.O.D. Menstrual physiology: Implications for endometrial pathology and beyond. Hum. Reprod. Update 2015, 21, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Grzybowska, M.E.; Barcikowska, Z. Inflammatory Markers in Dysmenorrhea and Therapeutic Options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the endometrium and regulation of menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef]
- Maybin, J.A.; Murray, A.A.; Saunders, P.T.K.; Hirani, N.; Carmeliet, P.; Critchley, H.O.D. Hypoxia and hypoxia inducible factor-1α are required for normal endometrial repair during menstruation. Nat. Commun. 2018, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Kadir, R.A. Endometrial haemostasis and menstruation. Rev. Endocr. Metab. Disord. 2012, 13, 289–299. [Google Scholar] [CrossRef]
- Gelety, T.J.; Chaudhuri, G. Haemostatic mechanism in the endometrium: Role of cyclo-oxygenase products and coagulation factors. Br. J. Pharmacol. 1995, 114, 975–980. [Google Scholar] [CrossRef]
- Cousins, F.L.; Filby, C.E.; Gargett, C.E. Endometrial Stem/Progenitor Cells–Their Role in Endometrial Repair and Regeneration. Front. Reprod. Health 2021, 3, 811537. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Nguyen, H.P.T.; Ye, L. Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 2012, 13, 235–251. [Google Scholar] [CrossRef]
- Kong, Y.; Shao, Y.; Ren, C.; Yang, G. Endometrial stem/progenitor cells and their roles in immunity, clinical application, and endometriosis. Stem Cell Res. Ther. 2021, 12, 474. [Google Scholar] [CrossRef]
- Nair, R.; Agarwal, P.; Gadre, M.A.; Vasanthan, K.S.; Seetharam, R.N. Stem cell treatments for female reproductive disorders: A comprehensive review. J. Ovarian Res. 2025, 18, 161. [Google Scholar] [CrossRef]
- Sarvestani, M.; Rajabzadeh, A.; Mazoochi, T.; Samimi, M.; Navari, M.; Moradi, F. Use of placental-derived mesenchymal stem cells to restore ovarian function and metabolic profile in a rat model of the polycystic ovarian syndrome. BMC Endocr. Disord. 2024, 24, 154. [Google Scholar] [CrossRef] [PubMed]
- Chugh, R.M.; Park, H.S.; Esfandyari, S.; Elsharoud, A.; Ulin, M.; Al-Hendy, A. Mesenchymal stem cell-conditioned media regulate steroidogenesis and inhibit androgen secretion in a PCOS cell model via BMP-2. Int. J. Mol. Sci. 2021, 22, 9184. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Seok, J.; Lim, S.M.; Kim, T.H.; Kim, G.J. Microenvironmental changes induced by placenta-derived mesenchymal stem cells restore ovarian function in ovariectomized rats via activation of the PI3K-FOXO3 pathway. Stem Cell Res. Ther. 2020, 11, 486. [Google Scholar] [CrossRef]
- Jafarzadeh, H.; Nazarian, H.; Ghaffari Novin, M.; Shams Mofarahe, Z.; Eini, F.; Piryaei, A. Improvement of oocyte in vitro maturation from mice with polycystic ovary syndrome by human mesenchymal stromal cell–conditioned media. J. Cell. Biochem. 2018, 119, 10365–10375. [Google Scholar] [CrossRef]
- Xie, Q.; Xiong, X.; Xiao, N.; He, K.; Chen, M.; Peng, J.; Su, X.; Mei, H.; Dai, Y.; Wei, D.; et al. Mesenchymal Stem Cells Alleviate DHEA-Induced Polycystic Ovary Syndrome (PCOS) by Inhibiting Inflammation in Mice. Stem Cells Int. 2019, 2019, 9782373. [Google Scholar] [CrossRef]
- Ciprietti, M.; Bueds, C.; Vankelecom, H.; Vriens, J. Organoids as powerful models of endometrium epithelium in transcriptomic, cellular and functional mimicry. Cell. Mol. Life Sci. 2025, 82, 272. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.D.; Fraser, I.S.; Haththotuwa, R.; Kriplani, A.; Bahamondes, L.; Füchtner, C.; Tonye, R.; Archer, D.; Abbott, J.; et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int. J. Gynecol. Obstet. 2018, 143, 393–408. [Google Scholar] [CrossRef]
- Pouraliroudbaneh, S.; Marino, J.; Riggs, E.; Saber, A.; Jayasinghe, Y.; Peate, M. Heavy menstrual bleeding and dysmenorrhea in adolescents: A systematic review of self-management strategies, quality of life, and unmet needs. Int. J. Gynecol. Obstet. 2024, 167, 16–41. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.D.; Fraser, I.S. The FIGO systems for nomenclature and classification of causes of abnormal uterine bleeding in the reproductive years: Who needs them? Am. J. Obstet. Gynecol. 2012, 207, 259–265. [Google Scholar] [CrossRef]
- Comishen, K.J.; Bhatt, M.; Yeung, K.; Irfan, J.; Zia, A.; Sidonio, R.F.; James, P. Etiology and diagnosis of heavy menstrual bleeding among adolescent and adult patients: A systematic review and meta-analysis of the literature. J. Thromb. Haemost. 2025, 23, 863–876. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Heavy Menstrual Bleeding: Assessment and Management. [NICE Guideline NG88]: Updated 24 May 2021. Available online: http://www.nice.org.uk/guidance/ng88 (accessed on 10 August 2025).
- Australian Commission on Safety and Quality in Health Care. Heavy Menstrual Bleeding Clinical Care Standard: June 2024. Available online: https://www.safetyandquality.gov.au/standards/clinical-care-standards/heavy-menstrual-bleeding-clinical-care-standard (accessed on 10 August 2025).
- Warner, P.; Harriet, L.; Whitaker, R.; Anthony, R.; John, C.; Douglas, A.; Holm, C.; Madhra, M.; Gilbert, S.; Tansy, P.; et al. Low dose dexamethasone as treatment for women with heavy menstrual bleeding: A response-adaptive randomised placebo-controlled dose-finding parallel group trial (DexFEM). eBioMedicine 2021, 69, 103434. [Google Scholar] [CrossRef] [PubMed]
- Mardon, A.K.; White, S.; Howe, D.; O’Shea, M.; Eathorne, A.; Gannott, M.; Schott, A.; Armour, M. Problematic Periods Costing Young Women—The Impact of Menstrual Symptoms on Work and Study. Aust. N. Z. J. Obstet. Gynaecol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bigambo, F.M.; Wang, D.; Zhang, Y.; Mzava, S.M.; Dai, R.; Wang, X. Current situation of menstruation and gynecological diseases prevalence among Chinese women: A cross-sectional study. BMC Women’s Health 2022, 22, 270. [Google Scholar] [CrossRef]
- Ashworth, G. Management of heavy menstrual bleeding in Australian general practice An analysis of BEACH data. Aust. J. Gen. Pract. 2021, 50, 573–579. [Google Scholar] [CrossRef]
- Wilson, L.; Copp, T.; Hickey, M.; Jenkinson, B.; Jordan, S.J.; Thompson, R.; Mishra, G.D.; Doust, J.A. Women who experience heavy menstrual bleeding: Prevalence and characteristics from young adulthood to midlife, Australia, 2000–2021: A longitudinal cohort survey study. Med. J. Aust. 2025, 222, 191–197. [Google Scholar] [CrossRef]
- Serhat, E.; Cogendez, E.; Selcuk, S.; Asoglu, M.R.; Arioglu, P.F.; Eren, S. Is there a relationship between endometrial polyps and obesity, diabetes mellitus, hypertension? Arch. Gynecol. Obstet. 2014, 290, 937–941. [Google Scholar] [CrossRef]
- Keizer, A.L.; Semmler, A.; Kok, H.S.; van Kesteren, P.J.M.; Huirne, J.A.F.; Hehenkamp, W.J.K. Modifiable prognostic factors in uterine fibroid development: A systematic review of literature. J. Obstet. Gynaecol. 2024, 44, 2288225. [Google Scholar] [CrossRef] [PubMed]
- Jasper, E.A.; Mautz, B.S.; Hellwege, J.N.; Piekos, J.A.; Jones, S.H.; Zhang, Y.; Torstenson, E.S.; Pendergrass, S.A.; Lee, M.T.M.; Edwards, T.L.; et al. A phenome-wide association study of uterine fibroids reveals a marked burden of comorbidities. Commun. Med. 2025, 5, 174. [Google Scholar] [CrossRef]
- Palomba, S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? An opinion paper. Hum. Reprod. 2021, 36, 2421–2428. [Google Scholar] [CrossRef]
- Patel, S. Disruption of aromatase homeostasis as the cause of a multiplicity of ailments: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2017, 168, 19–25. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Trojanowski, S.; Kociszewska, A.; Szewczyk, G. Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)—Searching for Epigenetic Factors. Int. J. Mol. Sci. 2022, 23, 14663. [Google Scholar] [CrossRef]
- Hapangama, D.K.; Bulmer, J.N. Pathophysiology of heavy menstrual bleeding. Women’s Health 2016, 12, 3–13. [Google Scholar] [CrossRef]
- Girling, J.E.; Lockhart, M.G.; Olshansky, M.; Paiva, P.; Woodrow, N.; Marino, J.L.; Hickey, M.; Rogers, P.A.W. Differential Gene Expression in Menstrual Endometrium from Women with Self-Reported Heavy Menstrual Bleeding. Reprod. Sci. 2017, 24, 28–46. [Google Scholar] [CrossRef]
- Li, X.; Wang, F.; Chen, M.; Ling, L.; Zhao, F.; Peng, D. The association between endometrial polyps and insulin resistance from the expression of PI3K and AKT proteins perspective. BMC Women’s Health 2024, 24, 366. [Google Scholar] [CrossRef]
- Oliva, M.M.; Gambioli, R.; Forte, G.; Porcaro, G.; Aragona, C.; Unfer, V. Unopposed estrogens: Current and future perspectives. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2975–2989. [Google Scholar]
- Nijkang, N.P.; Anderson, L.; Markham, R.; Manconi, F. Endometrial polyps: Pathogenesis, sequelae and treatment. SAGE Open Med. 2019, 7, 2050312119848247. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, Y.; Liang, W.; Hu, Q.; Zeng, A.; Ding, M.; Chen, D.; Xie, M. Clinical and metabolic characteristics of endometrial lesions in polycystic ovary syndrome at reproductive age. BMC Women’s Health 2023, 23, 236. [Google Scholar] [CrossRef]
- Cakmak, H.; Taylor, H.S. Implantation failure: Molecular mechanisms and clinical treatment. Hum. Reprod. Update 2011, 17, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Amri, M.F.; Ugusman, A.; Hamid, A.A.; Wahab, N.A.; Mokhtar, M.H. Hyperandrogenism and Its Possible Effects on Endometrial Receptivity: A Review. Int. J. Mol. Sci. 2023, 24, 12026. [Google Scholar] [CrossRef]
- Salamonsen, L.A.; Dimitriadis, E. Infertility and the Endometrium. Clin. Exp. Obstet. Gynecol. 2022, 49, 195. [Google Scholar] [CrossRef]
- Skliutė, G.; Baušytė, R.; Ramašauskaitė, D.; Navakauskienė, R. Characterization of Epigenetic and Molecular Factors in Endometrium of Females with Infertility. Biomedicines 2022, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, M.M.; Noori, Z.; Zare, F.; Meshkin, A. Ferroptosis and recurrent miscarriage: A critical review of pathophysiology and emerging therapeutic targets. Front. Cell Dev. Biol. 2025, 13, 1559300. [Google Scholar] [CrossRef] [PubMed]
- Gusarova, T.A.; Nizyaeva, N.V.; Mikhalev, S.A.; Tikhonova, N.B.; Orgadeeva, D.A.; Mikhaleva, L.M.; Sharapova, O.V. Morphological and molecular features of decidual endometrial cells in miscarriage. Morphology 2023, 161, 37–49. [Google Scholar] [CrossRef]
- Muter, J.; Kong, C.S.; Nebot, M.T.; Tryfonos, M.; Vrljicak, P.; Brighton, P.J.; Dimakou, D.B.; Vickers, M.; Yoshihara, H.; Ott, S.; et al. Stalling of the endometrial decidual reaction determines the recurrence risk of miscarriage. Sci. Adv. 2025, 11, eadv1988. [Google Scholar] [CrossRef]
- Bacon, S.J.; Zhu, Y.; Ghosh, P. Early spiral arteriole remodeling in the uterine–placental interface: A rat model. J. Anat. 2024, 244, 1054–1066. [Google Scholar] [CrossRef]
- Elawad, T.; Scott, G.; Bone, J.N.; Elwell, H.; Lopez, C.E.; Filippi, V.; Green, M.; Khalil, A.; Kinshella, M.L.W.; Mistry, H.D.; et al. Risk factors for pre-eclampsia in clinical practice guidelines: Comparison with the evidence. BJOG Int. J. Obstet. Gynaecol. 2022, 131, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Kibret, K.T.; Chojenta, C.; Gresham, E.; Tegegne, T.K.; Loxton, D. Maternal dietary patterns and risk of adverse pregnancy (hypertensive disorders of pregnancy and gestational diabetes mellitus) and birth (preterm birth and low birth weight) outcomes: A systematic review and meta-analysis. Public Health Nutr. 2019, 22, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Paula, W.O.; Patriota, E.S.O.; Gonçalves, V.S.S.; Pizato, N. Maternal Consumption of Ultra-Processed Foods-Rich Diet and Perinatal Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3242. [Google Scholar] [CrossRef]
- Sanderson, P.A.; Critchley, H.O.D.; Williams, A.R.W.; Arends, M.J.; Saunders, P.T.K. New concepts for an old problem: The diagnosis of endometrial hyperplasia. Hum. Reprod. Update 2017, 23, 232–254. [Google Scholar] [CrossRef]
- Damor, C.B.; Damor, P. Molecular Profiling of Endometrial Hyperplasia and its Progression to Endometrial Carcinoma: A Histopathological and Genomic Correlation Study. Int. J. Life Sci. Biotechnol. Pharma Res. 2025, 14, 150–153. [Google Scholar]
- Bostan, I.S.; Mihaila, M.; Roman, V.; Radu, N.; Neagu, M.T.; Bostan, M.; Mehedintu, C. Landscape of Endometrial Cancer: Molecular Mechanisms, Biomarkers, and Target Therapy. Cancers 2024, 16, 2027. [Google Scholar] [CrossRef]
- Balhara, N.; Yadav, R.; Chauhan, M.B. Role of signaling pathways in endometrial cancer. Mol. Biol. Rep. 2025, 52, 408. [Google Scholar] [CrossRef]
- Brower, M.; Brennan, K.; Pall, M.; Azziz, R. The severity of menstrual dysfunction as a predictor of insulin resistance in PCOS. J. Clin. Endocrinol. Metab. 2013, 98, E1967–E1971. [Google Scholar] [CrossRef]
- Li, X.; Yang, D.; Pan, P.; Azziz, R.; Yang, D. The Degree of Menstrual Disturbance Is Associated With the Severity of Insulin Resistance in PCOS. Front. Endocrinol. 2022, 13, 873726. [Google Scholar] [CrossRef]
- Ezeh, U.; Ezeh, C.; Pisarska, M.D.; Azziz, R. Menstrual dysfunction in polycystic ovary syndrome: Association with dynamic state insulin resistance rather than hyperandrogenism. Fertil. Steril. 2021, 115, 1557–1568. [Google Scholar] [CrossRef]
- Solomon, C.G.; Hu, F.B.; Dunaif, A.; Rich-edwards, J.; Hunter, D.J.; Colditz, G.A.; Speizer, F.E.; Manson, J.E. Long or Highly Irregular Menstrual Cycles as a Marker for Risk of Type 2 Diabetes Mellitus. JAMA 2001, 286, 2421–2426. [Google Scholar] [CrossRef] [PubMed]
- Strowitzki, T.; Capp, E.; Von Eye, H. The degree of cycle irregularity correlates with the grade of endocrine and metabolic disorders in PCOS patients. Eur. J. Obstet. Gynecol. 2010, 149, 178–181. [Google Scholar] [CrossRef]
- Cooper, G.S.; Ephross, S.A.; Sandler, D.P. Menstrual patterns and risk of adult-onset diabetes mellitus. J. Clin. Epidemiol. 2000, 53, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shan, Z.; Arvizu, M.; Pan, A.; Manson, J.E.; Missmer, S.A.; Sun, Q.; Chavarro, J.E. Associations of Menstrual Cycle Characteristics Across the Reproductive Life Span and Lifestyle Factors with Risk of Type 2 Diabetes. JAMA Netw. Open 2020, 3, e2027928, Erratum in JAMA Netw. Open 2021, 4, e2034976. [Google Scholar] [CrossRef] [PubMed]
- Foryś, E.; Baran, A.; Dziurdzia, A.; Jarosz-wójcik, E.; Matusik, P.; Gawlik, A.; Tomaszewski, R.; Zachurzok, A.; Donayeva, A.; Amanzholkyzy, A.; et al. Are menstrual disorders in adolescent girls related to metabolic disorders? Paediatr. Endocrinol. Diabetes, Metab. 2023, 29, 75–82. [Google Scholar] [CrossRef]
- Onalan, R.; Onalan, G.; Tonguc, E.; Ozdener, T. Body mass index is an independent risk factor for the development of endometrial polyps in patients undergoing in vitro fertilization. Fertil. Steril. 2009, 91, 1056–1060. [Google Scholar] [CrossRef]
- Matsuyama, S.; Whiteside, S.; Li, S.Y. Implantation and Decidualization in PCOS: Unraveling the Complexities of Pregnancy. Int. J. Mol. Sci. 2024, 25, 1203. [Google Scholar] [CrossRef]
- Salcedo, A.C.; Yun, J.; Carter, C.; Hart, E. Therapeutic Carbohydrate Restriction as a Metabolic Modality for the Prevention and Treatment of Abnormal Uterine Bleeding. Nutrients 2023, 15, 3760. [Google Scholar] [CrossRef]
- Laughlin-tommaso, S.K.; Fuchs, E.L.; Wellons, M.F.; Lewis, C.E.; Calderon-margalit, R.; Stewart, E.A.; Schreiner, P.J. Uterine Fibroids and the Risk of Cardiovascular Disease in the Coronary Artery Risk Development in Young Adult Women ’s Study. J. Women’s Health 2019, 28, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Final Annual Report RCOG. NHMBA. Final Annual Report: National Heavy Menstrual Bleeding Audit. 2014. Available online: https://www.hqip.org.uk/wp-content/uploads/2018/02/HwNYNM.pdf (accessed on 13 August 2025).
- Soldani, R.; Cagnacci, A.; Yen, S.S.C. Insulin insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro. Eur. J. Endocrinol. 1994, 131, 641–645. [Google Scholar] [CrossRef]
- Sliwowska, J.H.; Fergani, C.; Gawałek, M.; Skowronska, B.; Fichna, P.; Lehman, M.N. Insulin: Its role in the central control of reproduction. Physiol. Behav. 2014, 133, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Jakubowicz, D.J.; De Vargas, A.F.; Brik, C.; Quintero, N.; Medina, F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J. Clin. Endocrinol. Metab. 1998, 83, 2001–2005. [Google Scholar]
- Baillargeon, J.P.; Nestler, J.E. Commentary: Polycystic ovary syndrome: A syndrome of ovarian hypersensitivity to insulin? J. Clin. Endocrinol. Metab. 2006, 91, 22–24. [Google Scholar] [CrossRef]
- Rice, S.; Khalid, A.; Khan, S.; Lacey, M.; Homburg, R. The effect of hyperinsulinemia on FSH-mediated signalling pathways in granulosa cells-implications for follicle growth in women with PCOS. Hum. Reprod. 2023, 38 (Suppl. S1), P-643. [Google Scholar] [CrossRef]
- Nelson-degrave, V.L.; Wickenheisser, J.K.; Hendricks, K.L.; Asano, T.; Fujishiro, M.; Legro, R.S.; Kimball, S.R.; Strauss, J.F.; Mcallister, J.M. Alterations in Mitogen-Activated Protein Kinase Kinase and Extracellular Regulated Kinase Signaling in Theca Cells Contribute to Excessive Androgen Production in Polycystic Ovary Syndrome. Mol. Endocrinol. 2015, 19, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef] [PubMed]
- Maclean, J.A.; Hayashi, K. Progesterone Actions and Resistance in Gynecological Disorders. Cells 2022, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Merhi, Z. Relationship between Advanced Glycation End Products and Steroidogenesis in PCOS. Reprod. Biol. Endocrinol. 2016, 14, 71. [Google Scholar] [CrossRef]
- Zuo, T.; Zhu, M.; Xu, W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid. Med. Cell. Longev. 2016, 2016, 8589318. [Google Scholar] [CrossRef]
- Zheng, X.; Pan, X.; Zhang, J.; Cao, X. Hyperinsulinemia-induced PAX6 expression promotes endometrial epithelial cell proliferation via negatively modulating p27 signaling. Biomed. Pharmacother. 2018, 97, 802–808. [Google Scholar] [CrossRef]
- Maybin, J.A.; Critchley, H.O.D.; Jabbour, H.N. Inflammatory pathways in endometrial disorders. Mol. Cell. Endocrinol. 2011, 335, 42–51. [Google Scholar] [CrossRef]
- Bremer, A.A.; Miller, W.L. The serine phosphorylation hypothesis of polycystic ovary syndrome: A unifying mechanism for hyperandrogenemia and insulin resistance. Fertil. Steril. 2008, 89, 1039–1048. [Google Scholar] [CrossRef]
- Lee, M.; Yoon, J.; Kim, H.; Kim, Y.S.; Lyu, S.W.; Lee, B.S. Hyperandrogenic Milieu Dysregulates the Expression of Insulin Signaling Factors and Glucose Transporters in the Endometrium of Patients with Polycystic Ovary Syndrome. Reprod. Sci. 2020, 27, 1637–1647. [Google Scholar] [CrossRef]
- Nagao, H.; Cai, W.; Wewer, N.J.; Steger, M.; Batista, T.M.; Pan, H. Distinct signaling by insulin and IGF-1 receptors and their extra- and intracellular domains. Proc. Natl. Acad. Sci. USA 2021, 118, e2019474118. [Google Scholar] [CrossRef]
- Wang, Y.; Hua, S.; Tian, W.; Zhang, L.; Zhao, J.; Zhang, H.; Zhang, W.; Xue, F. Mitogenic and anti-apoptotic effects of insulin in endometrial cancer are phosphatidylinositol 3-kinase/Akt dependent. Gynecol. Oncol. 2012, 125, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhao, W.; Shen, H.; Du, D.; Li, X. Hyperinsulinemia impairs decidualization via AKT-NR4A1 signaling: New insight into polycystic ovary syndrome (PCOS)-related infertility. J. Ovarian Res. 2024, 17, 31. [Google Scholar] [CrossRef]
- Kooijman, R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev. 2006, 17, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.J. Effect of low carbohydrate diets on insulin resistance and the metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 463–468. [Google Scholar] [CrossRef]
- Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. Recent Advances in the Treatment of Insulin Resistance Targeting Molecular and Metabolic Pathways: Fighting a Losing Battle? Medicina 2022, 58, 472. [Google Scholar] [CrossRef]
- Vitale, S.G.; Di Michele, S.; Tassi, A.; Succu, C.; Angioni, S.; Fulghesu, A.M. Sustained Metabolic Improvements with Low-Dose Metformin Combined with Oral Contraceptives in Female Adolescents with PCOS: A Single-Center Retrospective Cohort Study. Adv. Ther. 2025, 42, 3762–3773. [Google Scholar] [CrossRef]
- Robinson, S.; Kiddy, D.; Gelding, S.V.; Willis, D.; Niththyananthan, R.; Bush, A.; Johnston, D.G.; Franks, S. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin. Endocrinol. 1993, 39, 351–355. [Google Scholar] [CrossRef]
- Niu, J.; Lu, M.; Liu, B. Association between insulin resistance and abnormal menstrual cycle in Chinese patients with polycystic ovary syndrome. J. Ovarian Res. 2023, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Bhattacharya, K.; Basak, A.K.; Paul, N.; Bandyopadhyay, R.; Chaudhuri, G.R.; Purkait, M.P.; Bhattacharjee, A.; Bose, C.; Shukla, N. Inflammatory perspectives of polycystic ovary syndrome: Role of specific mediators and markers. Middle East Fertil. Soc. J. 2023, 28, 33. [Google Scholar] [CrossRef]
- Zhai, Y.; Pang, Y. Systemic and ovarian inflammation in women with polycystic ovary syndrome. J. Reprod. Immunol. 2022, 151, 103628. [Google Scholar] [CrossRef]
- Ye, Z.; Zhao, J.; Li, R. Effects of immune cells and cytokines on the endometrial immune microenvironment in polycystic ovary syndrome. Gynecol. Obstet. Clin. Med. 2022, 2, 181–185. [Google Scholar] [CrossRef]
- Wang, J.; Yin, T.; Liu, S. Dysregulation of immune response in PCOS organ system. Front. Immunol. 2023, 14, 1169232. [Google Scholar] [CrossRef]
- Liu, S.; Hong, L.; Mo, M.; Xiao, S.; Chen, C.; Li, Y.; Lian, R.; Wang, X.; Cai, S.; Diao, L.; et al. Evaluation of endometrial immune status of polycystic ovary syndrome. J. Reprod. Immunol. 2021, 144, 103282. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.P.M.; Jabbour, H.N.; Critchley, H.O.D. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum. Reprod. 2007, 22, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Milne, S.A.; Perchick, G.B.; Boddy, S.C.; Jabbour, H.N. Expression, Localization, and Signaling of PGE2 and EP2/EP4 Receptors in Human Nonpregnant Endometrium across the Menstrual Cycle. J. Clin. Endocrinol. Metab. 2015, 86, 4453–4459. [Google Scholar] [CrossRef]
- Van Eijkeren, M.A.; Christiaens, G.C.M.L. Effects of mefenamic acid on menstrual hemostasis in essential menorrhagia. Am. J. Obstet. Gynecol. 1992, 166, 1419–1428. [Google Scholar] [CrossRef]
- Khajehei, M.; Abdali, K.; Tabatabaee, H. The effect of mefenamic acid and naproxen on heavy menstrual bleeding: A placebo-controlled study. S. Afr. J. Obstet. Gynecolgy 2013, 19, 31–34. [Google Scholar] [CrossRef]
- Rees, M.C.P.; Bernal, S.L.; Ca, R.; Turnbull, A.C.; Hospital, J.R.; Ox, O. Effect of Fenamates on Prostaglandin E Receptor Binding. Lancet 1988, 332, 541–542. [Google Scholar] [CrossRef]
- Shetty, S.S.; Deepthi, D.; Harshitha, S.; Sonkusare, S.; Naik, P.B.; N, S.K.; Madhyastha, H. Environmental pollutants and their effects on human health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef]
- Stegehuis, N.; Kotsirilos, V.; Parker, J. The Impact of Microparticulate Air Pollution in Polycystic Ovary Syndrome: A Narrative Review. Clin. Exp. Obstet. Gynecol. 2024, 51, 233. [Google Scholar] [CrossRef]
- Ameho, S.; Klutstein, M. The effect of chronic inflammation on female fertility. Reproduction 2025, 169, e240197. [Google Scholar] [CrossRef]
- Drizi, A.; Djokovic, D.; Laganà, A.S.; Herendael, B. Van Impaired inflammatory state of the endometrium: A multifaceted approach to endometrial inflammation. Current insights and future directions. Menopause Rev. 2020, 19, 90–100. [Google Scholar] [CrossRef]
- Palomba, S.; Daolio, J.; La Sala, G.B. Oocyte Competence in Women with Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2017, 28, 186–198. [Google Scholar] [CrossRef]
- Piltonen, T.T. Polycystic ovary syndrome: Endometrial markers. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 37, 66–79. [Google Scholar] [CrossRef]
- Cicerchi, C.; Li, N.; Kratzer, J.; Garcia, G.; Roncal-Jimenez, C.A.; Tanabe, K.; Hunter, B.; Rivard, C.J.; Sautin, Y.Y.; Gaucher, E.A.; et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: Evolutionary implications of the uricase loss in hominids. FASEB J. 2014, 28, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R.M.; Kim, T.H.; Shin, J.H.; Jeong, J.W. Progesterone and estrogen signaling in the endometrium: What goes wrong in endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, P.H.; Wang, Y.; van der Zee, M.; Burger, C.W.; Blok, L.J. Interaction between sex hormones and WNT/β-catenin signal transduction in endometrial physiology and disease. Mol. Cell. Endocrinol. 2012, 358, 176–184. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Salamun, V.; Rizzo, M.; Lovrecic, L.; Hocevar, K.; Burnik, T.P.; Janez, A.; Jensterle, M.; Bokal, E.V.; Peterlin, B.; Maver, A. The Endometrial Transcriptome of Metabolic and Inflammatory Pathways During the Window of Implantation Is Deranged in Infertile Obese Polycystic Ovarian Syndrome Women. Metab. Syndr. Relat. Disord. 2022, 20, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Savaris, R.F.; Groll, J.M.; Young, S.L.; DeMayo, F.J.; Jeong, J.W.; Hamilton, A.E.; Giudice, L.C.; Lessey, B.A. Progesterone resistance in PCOS endometrium: A microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J. Clin. Endocrinol. Metab. 2011, 96, 1737–1746. [Google Scholar] [CrossRef]
- Das, S.K. Regional development of uterine decidualization: Molecular signaling by Hoxa-10. Mol. Reprod. Dev. 2010, 77, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Cermik, D.; Selam, B.; Taylor, H.S. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 238–243. [Google Scholar] [CrossRef]
- Ali, R.; Ahmed, T.; Gul, H.; Rehman, R. An interplay of Progesterone, Leukemia Inhibitor Factor and Interleukin-6 in the window of implantation; Impact on fertility. Cytokine 2023, 170, 156332. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.; Avellaira, C.; Johnson, M.C.; Gabler, F.; Fuentes, A.; Vega, M. Evaluation of steroid receptors, coregulators, and molecules associated with uterine receptivity in secretory endometria from untreated women with polycystic ovary syndrome. Fertil. Steril. 2006, 85, 1017–1026. [Google Scholar] [CrossRef]
- Ujvari, D.; Hulchiy, M.; Calaby, A.; Nybacka, A.; Byström, B.; Hirschberg, A.L. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum. Reprod. 2014, 29, 1526–1535. [Google Scholar] [CrossRef]
- Hagenfeldt, K. The role of prostaglandins and allied substances in uterine haemostasis. Contraception 1987, 36, 23–35. [Google Scholar] [CrossRef]
- Smith, S.K.; Abel, M.H.; Kelly, R.W.; Baird, D.T. Prostaglandin Synthesis in the Endometrium of Women with Ovulatory Dysfunctional Uterine Bleeding. Brithish J. Obstet. Gynaecol. 1981, 88, 434. [Google Scholar] [CrossRef]
- Marsh, M.M.; Malakooti, N.; Taylor, N.H. Endothelin and neutral endopeptidase in the endometrium of women with menorrhagia. Hum. Reprod. 1997, 12, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Abberton, K.M.; Healy, D.L.; Rogers, P.A.W. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum. Reprod. 1999, 14, 3095–3100. [Google Scholar] [CrossRef][Green Version]
- Abberton, K.M.; Taylor, N.H.; Healy, D.L.; Rogers, P.A.W. Vascular smooth muscle cell proliferation in arterioles of the human endometrium. Hum. Reprod. 1999, 14, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, N.; Devitt, M.; Sheppard, B.L.; Bonnar, N. Endometrial fibrinolytic enzymes in women with normal menstruation and dysfunctional uterine bleeding. Br. J. Obstet. Gynaecol. 1993, 100, 768–771. [Google Scholar] [CrossRef]
- Sylus, A.M.; Nandeesha, H.; Chitra, T. Matrix metalloproteinase-9 increases and interleukin-10 reduces with increase in body mass index in polycystic ovary syndrome: A cross-sectional study. Int. J. Reprod. Biomed. 2020, 18, 605–610. [Google Scholar]
- Middelkoop, M.A.; Don, E.E.; Hehenkamp, W.J.K.; Polman, N.J.; Griffioen, A.W.; Huirne, J.A.F. Angiogenesis in abnormal uterine bleeding: A narrative review. Hum. Reprod. Update 2023, 29, 457–485. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Ch, M.B.B. Molecular and Cellular Causes of Abnormal Uterine Bleeding of Endometrial Origin. Semin. Reprod. Med. 2011, 29, 400–409. [Google Scholar] [CrossRef]
- Rae, M.; Mohamad, A.; Price, D.; Hadoke, P.W.F.; Walker, B.R.; Mason, J.I.; Hillier, S.G.; Critchley, H.O.D. Cortisol Inactivation by 11β-Hydroxysteroid dehydrogenase-2 May Enhance Endometrial Angiogenesis via Reduced Thrombospondin-1 in Heavy Menstruation. J. Clin. Endocrinol. Metab. 2009, 94, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, A.; Bacallao, K.; Avellaira, C.; Gabler, F.; Fuentes, A.; Vega, M. Androgen and estrogen receptors and co-regulators levels in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia. Gynecol. Oncol. 2006, 103, 307–314. [Google Scholar] [CrossRef]
- Guo, F.; Huang, Y.; Fernando, T.; Shi, Y. Altered Molecular Pathways and Biomarkers of Endometrial Receptivity in Infertile Women with Polycystic Ovary Syndrome. Reprod. Sci. 2022, 29, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Koc, O.; Ozdemirici, S.; Acet, M.; Soyturk, U. Nuclear factor-κB expression in the endometrium of normal and overweight women with polycystic ovary syndrome. J. Obstet. Gynaecol. 2017, 37, 924–930. [Google Scholar] [CrossRef]
- Younas, K.; Quintela, M.; Thomas, S.; Garcia-Parra, J.; Blake, L.; Whiteland, H.; Bunkheila, A.; Francis, L.W.; Margarit, L.; Gonzalez, D.; et al. Delayed endometrial decidualisation in polycystic ovary syndrome; the role of AR-MAGEA11. J. Mol. Med. 2019, 97, 1315–1327. [Google Scholar] [CrossRef]
- James, D.W.; Quintela, M.; Lucini, L.; Alkafri, N.K.; Healey, G.D.; Younas, K.; Bunkheila, A.; Margarit, L.; Francis, L.W.; Gonzalez, D.; et al. Homeobox regulator Wilms Tumour 1 is displaced by androgen receptor at cis-regulatory elements in the endometrium of PCOS patients. Front. Endocrinol. 2024, 15, 1368494, Erratum in Front. Endocrinol. 2024, 15, 1450375. [Google Scholar]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13, Erratum in Nutrition 2019, 62, 213. [Google Scholar] [CrossRef]
- Rizk, M.G.; Thackray, V.G. Intersection of Polycystic Ovary Syndrome and the Gut Microbiome. J. Endocr. Soc. 2021, 5, bvaa177. [Google Scholar]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Parker, J.; Hawrelak, J.; Gersh, F. Nutritional Role of Polyphenols As a Component of a Wholefood Diet in the Management of Polycystic Ovary Syndrome. Australas. Coll. Nutr. Environ. Med. J. 2021, 40, 6–12. [Google Scholar]
- Wu, Y.; Lin, Z.; Li, C.; Lin, X.; Shan, S.; Guo, B. Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal Transduct. Target. Ther. 2023, 8, 98. [Google Scholar] [CrossRef]
- Faraji, J.; Metz, G.A.S. Environmental Epigenetics: New Horizons in Redefining Biological and Health Outcomes. PREPRINT 2025. [Google Scholar] [CrossRef]
- Scarfo, G.; Daniele, S.; Fusi, J.; Gesi, M.; Martini, C.; Franzoni, F.; Cela, V.; Artini, P.G. Metabolic and Molecular Mechanisms of Diet and Physical Exercise in the Management of Polycystic Ovarian Syndrome. Biomedicines 2022, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kikut, J.; Szczuko, U.; Szydłowska, I.; Nawrocka-rutkowska, J.; Zi, M. Nutrition Strategy and Life Style in Polycystic Ovary Syndrome—Narrative Review. Nutrients 2021, 13, 2452. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, G.; Zhou, F.; Li, Y.; Wu, Q.; He, H.; Zhang, Y.; Ma, C.; Ding, J.; Hua, K. Gut and Vaginal Microbiomes in PCOS: Implications for Women’s Health. Front. Endocrinol. 2022, 13, 808508. [Google Scholar] [CrossRef] [PubMed]
- Sola-Leyva, A.; Pérez-Prieto, I.; Molina, N.M.; Vargas, E.; Ruiz-Durán, S.; Leonés-Baños, I.; Canha-Gouveia, A.; Altmäe, S. Microbial composition across body sites in polycystic ovary syndrome: A systematic review and meta-analysis. Reprod. Biomed. Online 2023, 47, 129–150. [Google Scholar] [CrossRef]
- Lin, S.Y.; Yang, Y.C.; Chang, C.Y.Y.; Lin, C.C.; Hsu, W.H.; Ju, S.W.; Hsu, C.Y.; Kao, C.H. Risk of polycystic ovary syndrome in women exposed to fine air pollutants and acidic gases: A nationwide cohort analysis. Int. J. Environ. Res. Public Health 2019, 16, 4816. [Google Scholar] [CrossRef]
- Leceta, J.; Del Campo, R.; Jordan, S.; Klose, C.S.N. Editorial: Immunoregulation at mucosal surfaces. Front. Immunol. 2022, 13, 983201. [Google Scholar] [CrossRef] [PubMed]
- Hampl, R.; Starka, L. Endocrine Disruptors and Gut Microbiome Interactions. Physiol. Res. 2020, 69 (Suppl. 2), S211–S223. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.C.; Bellalta, S.; Basoalto, R.; Gómez-Valenzuela, F.; Jalil, Y.; Lépez, M.; Matamoros, A.; von Bernhardi, R. The Challenge by Multiple Environmental and Biological Factors Induce Inflammation in Aging: Their Role in the Promotion of Chronic Disease. Front. Immunol. 2020, 11, 570083. [Google Scholar] [CrossRef]
- Thin, Z.S.; Chew, J.; Yu, T.; Ong, Y.; Affendi, R.; Ali, R.; Gew, L.T. Impact of microplastics on the human gut microbiome: A systematic review of microbial composition, diversity, and metabolic disruptions. BMC Gastroenterol. 2025, 25, 583. [Google Scholar] [CrossRef]
- Balali, H.; Morabbi, A.; Karimian, M. Concerning influences of micro/nano plastics on female reproductive health: Focusing on cellular and molecular pathways from animal models to human studies. Reprod. Biol. Endocrinol. 2024, 22, 141. [Google Scholar] [CrossRef]
- Sun, J.; Sui, M.; Wang, T.; Teng, X.; Sun, J.; Chen, M. Detection and quantification of various microplastics in human endometrium based on laser direct infrared spectroscopy. Sci. Total Environ. 2024, 906, 167760. [Google Scholar] [CrossRef]
- Kim, N.; Lee, J.H.; Lee, I.; Park, J.H.; Jung, G.S.; Lee, M.J.; Im, W.; Cho, S.H.; Choi, Y.S. Investigation of potential toxic effects of nano- and microplastics on human endometrial stromal cells. Reprod. Toxicol. 2025, 132, 108848. [Google Scholar] [CrossRef]
- Qin, X.; Cao, M.; Peng, T.; Shan, H.; Lian, W.; Yu, Y.; Shui, G.; Li, R. Features, Potential Invasion Pathways, and Reproductive Health Risks of Microplastics Detected in Human Uterus. Environ. Sci. Technol. 2024, 58, 10482–10493. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, V.K.; Gacem, A.; Al-Dossari, M.; Yadav, K.K.; Abd El-Gawaad, N.S.; Ben Khedher, N.; Choudhary, N.; Kumar, P.; Cavalu, S. Deleterious Effect of Air Pollution on Human Microbial Community and Bacterial Flora: A Short Review. Int. J. Environ. Res. Public Health 2022, 19, 15494. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.J.; Chan, C.C.; Su, T.C.; Lee, C.T.; Tang, C.S. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am. J. Respir. Crit. Care Med. 2007, 176, 370–376. [Google Scholar] [CrossRef]
- Zhang, S.; Mwiberi, S.; Pickford, R.; Breitner, S.; Huth, C.; Koenig, W.; Rathmann, W.; Herder, C.; Roden, M.; Cyrys, J.; et al. Longitudinal associations between ambient air pollution and insulin sensitivity: Results from the KORA cohort study. Lancet Planet. Health 2021, 5, e39–e49. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Wu, X.K.; Zhou, Z.M.; Fu, P.; Chen, X.H.; Yan, Y.; Wang, X.; Yang, Z.W.; Li, W.L.; et al. Effect of exposure to second-hand smoke from husbands on biochemical hyperandrogenism, metabolic syndrome and conception rates in women with polycystic ovary syndrome undergoing ovulation induction. Hum. Reprod. 2018, 33, 617–625. [Google Scholar] [CrossRef]
- Mahalingaiah, S.; Missmer, S.E.; Cheng, J.J.; Chavarro, J.; Laden, F.; Hart, J.E. Perimenarchal air pollution exposure and menstrual disorders. Hum. Reprod. 2018, 33, 512–519. [Google Scholar] [CrossRef]
- Liang, Y.; Lu, Q.; Chen, M.; Zhao, X.; Chu, C.; Zhang, C.; Yuan, J.; Liu, H.; Lash, G.E. Impact of endocrine disrupting chemicals (EDCs) on epigenetic regulation in the uterus: A narrative review. Reprod. Biol. Endocrinol. 2025, 23, 80. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.S.; Damdimopoulou, P.; Johansson, H.K.L.; Bouftas, N.; Draskau, M.K.; Franssen, D.; Fudvoye, J.; van Duursen, M.; Svingen, T. Endocrine-disrupting chemicals and female reproductive health: A growing concern. Nat. Rev. Endocrinol. 2025, 21, 593–607. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef] [PubMed]
- Ashonibare, V.J.; Akorede, B.A.; Ashonibare, P.J.; Akhigbe, T.M.; Akhigbe, R.E. Gut microbiota-gonadal axis: The impact of gut microbiota on reproductive functions. Front. Immunol. 2024, 15, 1346035. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Stavros, S.; Katopodis, P.; Potiris, A.; Drakakis, P.; Dafopoulos, S.; Zachariou, A.; Dafopoulos, K.; Zikopoulos, K.; Zikopoulos, A. Gut Microbiome Dysbiosis and Its Impact on Reproductive Health: Mechanisms and Clinical Applications. Metabolites 2025, 15, 390. [Google Scholar] [CrossRef]
- Kong, F.S.; Huang, P.; Chen, J.H.; Ma, Y. The Novel Insight of Gut Microbiota from Mouse Model to Clinical Patients and the Role of NF-κB Pathway in Polycystic Ovary Syndrome. Reprod. Sci. 2024, 31, 3323–3333. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Roberts, M.H. Toll-like receptor 4 mediates the response of epithelial and stromal cells to lipopolysaccharide in the endometrium. PLoS ONE 2010, 5, e12906. [Google Scholar]
- Krikun, G.; Trezza, J.; Shaw, J.; Rahman, M.; Guller, S.; Abrahams, V.M.; Lockwood, C.J. LPS appears to activate human endometrial endothelial cells through TLR-4-dependent and TLR-4-independent mechanisms. Am. J. Reprod. Immunol. 2012, 68, 233–237. [Google Scholar] [CrossRef]
- Yu, J.; Berga, S.L.; Zou, W.; Taylor, R.N. Interleukin-1β inhibits estrogen receptor-α, progesterone receptors A and B and biomarkers of human endometrial stromal cell differentiation: Implications for endometriosis. Mol. Hum. Reprod. 2019, 25, 625–637. [Google Scholar]
- Gholizadeh Shamasbi, S.; Dehgan, P.; Mohammad-Alizadeh Charandabi, S.; Aliasgarzadeh, A.; Mirghafourvand, M. The effect of resistant dextrin as a prebiotic on metabolic parameters and androgen level in women with polycystic ovarian syndrome: A randomized, triple-blind, controlled, clinical trial. Eur. J. Nutr. 2019, 58, 629–640. [Google Scholar] [CrossRef]
- Martinez Guevara, D.; Vidal Cañas, S.; Palacios, I.; Gómez, A.; Estrada, M.; Gallego, J.; Liscano, Y. Effectiveness of Probiotics, Prebiotics, and Synbiotics in Managing Insulin Resistance and Hormonal Imbalance in Women with Polycystic Ovary Syndrome (PCOS): A Systematic Review of Randomized Clinical Trials. Nutrients 2024, 16, 3916. [Google Scholar] [CrossRef] [PubMed]
- Kaluanga Bwanga, P.; Tremblay-Lemoine, P.L.; Timmermans, M.; Ravet, S.; Munaut, C.; Nisolle, M.; Henry, L. The Endometrial Microbiota: Challenges and Prospects. Medicina 2023, 59, 1540. [Google Scholar] [CrossRef]
- Balla, B.; Illés, A.; Tobiás, B.; Pikó, H.; Beke, A.; Sipos, M.; Lakatos, P.; Kósa, J.P. The Role of the Vaginal and Endometrial Microbiomes in Infertility and Their Impact on Pregnancy Outcomes in Light of Recent Literature. Int. J. Mol. Sci. 2024, 25, 13227. [Google Scholar] [CrossRef]
- Abdalla, W.; Nabeel, W.; Atiyeh, I. The role of the microbiome in endometrial carcinoma: Pathogenesis, biomarkers, and therapeutic prospects. J. Obs. Gynaecol. Res. 2025, 51, e70070. [Google Scholar]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, K.A.; Campbell, R.E.; Benrick, A.; Giacobini, P.; Dumesic, D.A.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41, 538–576. [Google Scholar] [CrossRef] [PubMed]
- Hibaoui, Y.; Feki, A. Organoid Models of Human Endometrial Development and Disease. Front. Cell Dev. Biol. 2020, 8, 84. [Google Scholar] [CrossRef]
- Miyazaki, K.; Dyson, M.T.; Coon, V.J.S.; Furukawa, Y.; Yilmaz, B.D.; Maruyama, T.; Bulun, S.E. Generation of Progesterone-Responsive Endometrial Stromal Fibroblasts from Human Induced Pluripotent Stem Cells: Role of the WNT/CTNNB1 Pathway. Stem Cell Rep. 2018, 11, 1136–1155. [Google Scholar] [CrossRef]
- Gnecco, J.S.; Brown, A.; Buttrey, K.; Ives, C.; Goods, B.A.; Baugh, L.; Hernandez-Gordillo, V.; Loring, M.; Isaacson, K.B.; Griffith, L.G. Organoid co-culture model of the human endometrium in a fully synthetic extracellular matrix enables the study of epithelial-stromal crosstalk. Med 2023, 4, 554–579.e9. [Google Scholar] [CrossRef]
- Filby, C.E.; Wyatt, K.A.; Mortlock, S.; Cousins, F.L.; McKinnon, B.; Tyson, K.E.; Montgomery, G.W.; Gargett, C.E. Comparison of organoids from menstrual fluid and hormone-treated endometrium: Novel tools for gynecological research. J. Pers. Med. 2021, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, L.; Wei, M.; Saarela, U.; Myllykangas, M.; Kinnunen, J.; Arffman, R.; Lie Fong, S.; Vriens, J.; Vankelecom, H.; Piltonen, T.T. PCOS endometrium-derived epithelial organoids as a novel model to study endometrial dysfunction. Hum. Reprod. 2025, 40, 1535–1549. [Google Scholar] [CrossRef] [PubMed]
- Piltonen, T.T.; Chen, J.; Erikson, D.W.; Spitzer, T.L.B.; Barragan, F.; Rabban, J.T.; Huddleston, H.; Irwin, J.C.; Giudice, L.C. Mesenchymal stem/progenitors and other endometrial cell types from women with polycystic ovary syndrome (PCOS) display inflammatory and oncogenic potential. J. Clin. Endocrinol. Metab. 2013, 98, 3765–3775. [Google Scholar] [CrossRef]
- Shishehgar, F.; Ramezani Tehrani, F.; Mirmiran, P.; Hajian, S.; Baghestani, A.R.; Moslehi, N. Comparison of Dietary Intake between Polycystic Ovary Syndrome Women and Controls. Glob. J. Health Sci. 2016, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Gakidou, E.; Afshin, A.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422, Erratum in Lancet 2017, 390, 1736. [Google Scholar] [CrossRef]
- Sabag, A.; Patten, R.K.; Moreno-Asso, A.; Colombo, G.E.; Dafauce Bouzo, X.; Moran, L.J.; Harrison, C.; Kazemi, M.; Mousa, A.; Tay, C.T.; et al. Exercise in the management of polycystic ovary syndrome: A position statement from Exercise and Sports Science Australia. J. Sci. Med. Sport 2024, 27, 668–677. [Google Scholar] [CrossRef]
- Marsh, K.A.; Steinbeck, K.S.; Atkinson, F.S.; Petocz, P.; Brand-miller, J.C. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am. J. Clin. Nutr. 2010, 92, 83–92. [Google Scholar] [CrossRef]
- Moran, L.J.; Ko, H.; Misso, M.; Marsh, K.; Noakes, M.; Talbot, M.; Frearson, M.; Thondan, M.; Stepto, N.; Teede, H.J. Dietary Composition in the Treatment of Polycystic Ovary Syndrome: A Systematic Review to Inform Evidence-Based Guidelines. J. Acad. Nutr. Diet. 2013, 113, 520–545. [Google Scholar] [CrossRef]
- Medeiros, F.L.; Fernandes, A.C.; Padovan, M.; Kraemer, M.V.; Bernardo, G.L.; Uggioni, P.L.; Proença, R.P. The evolution of carbohydrate-restricted diets for type 2 diabetes mellitus: A scoping review. Acad. Nutr. Diet. 2025, 2, 1–13. [Google Scholar] [CrossRef]
- Nicholas, A.P.; Soto-Mota, A.; Lambert, H.; Collins, A.L. Restricting carbohydrates and calories in the treatment of type 2 diabetes: A systematic review of the effectiveness of “low-carbohydrate” interventions with differing energy levels. J. Nutr. Sci. 2021, 10, e76. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Timothy Garvey, W.; Karen Lau, K.H.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Stranks; Stephen, N.; Lawlor-Smith, L. Managing Type 2 Diabetes with Therapeutic Carbohydrate Reduction. 2023; pp. 1–9. Available online: https://www.diabetessociety.com.au/wp-content/uploads/2023/11/Managing-Type-2-Diabetes-with-Therapeutic-Carbohydrate-reduction-TCR-November-2023_Final.pdf (accessed on 1 September 2025).
- Hite, A.; Cavan, D.; Cucuzella, M.; Cywes, R.; Ede, G. Clinical Guidelines for Therapeutic Carbohydrate Restriction. Society of Metabolic Health Practitioners. 2025; pp. 1–23. Available online: https://thesmhp.org/clinical-guidelines/ (accessed on 1 September 2025).
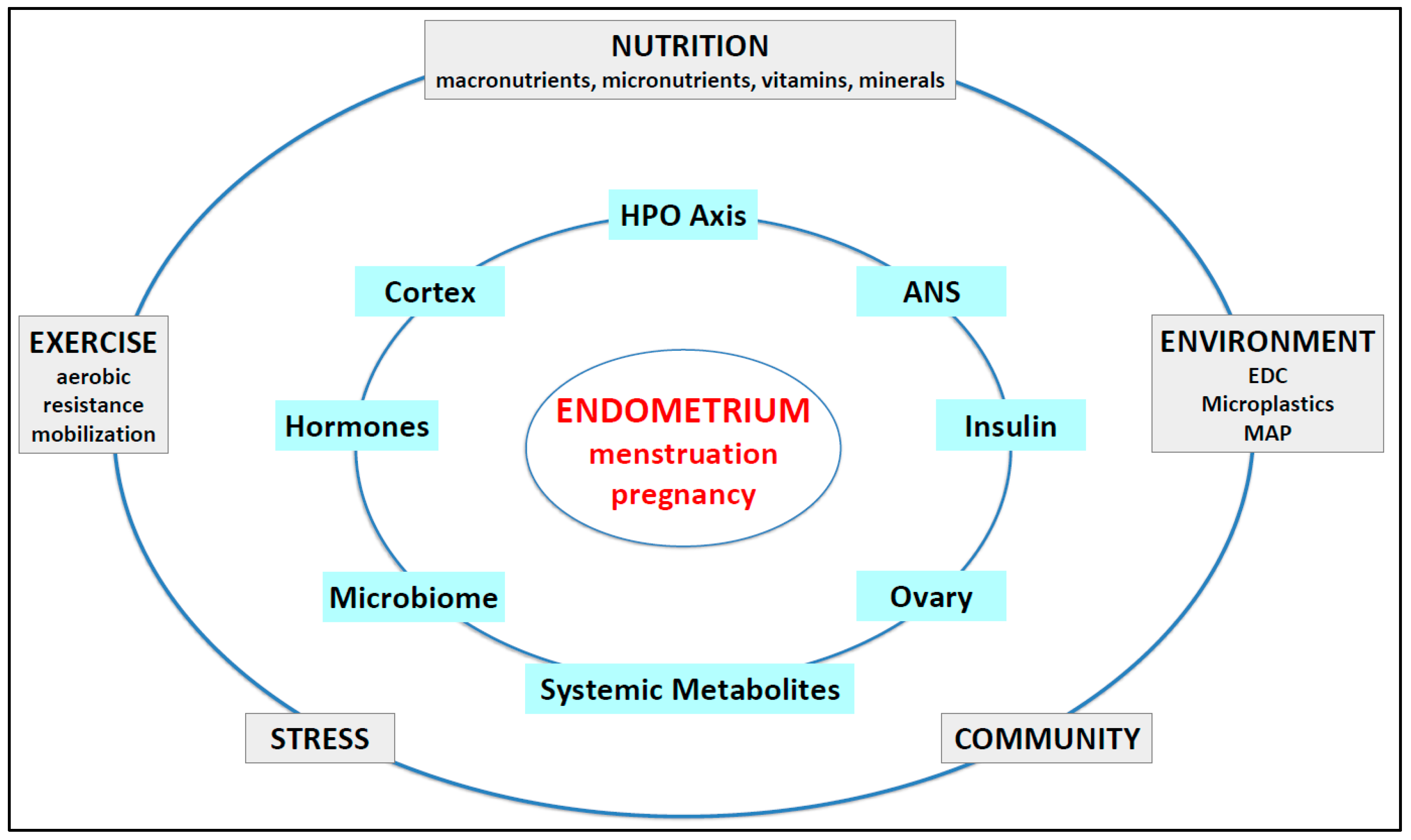
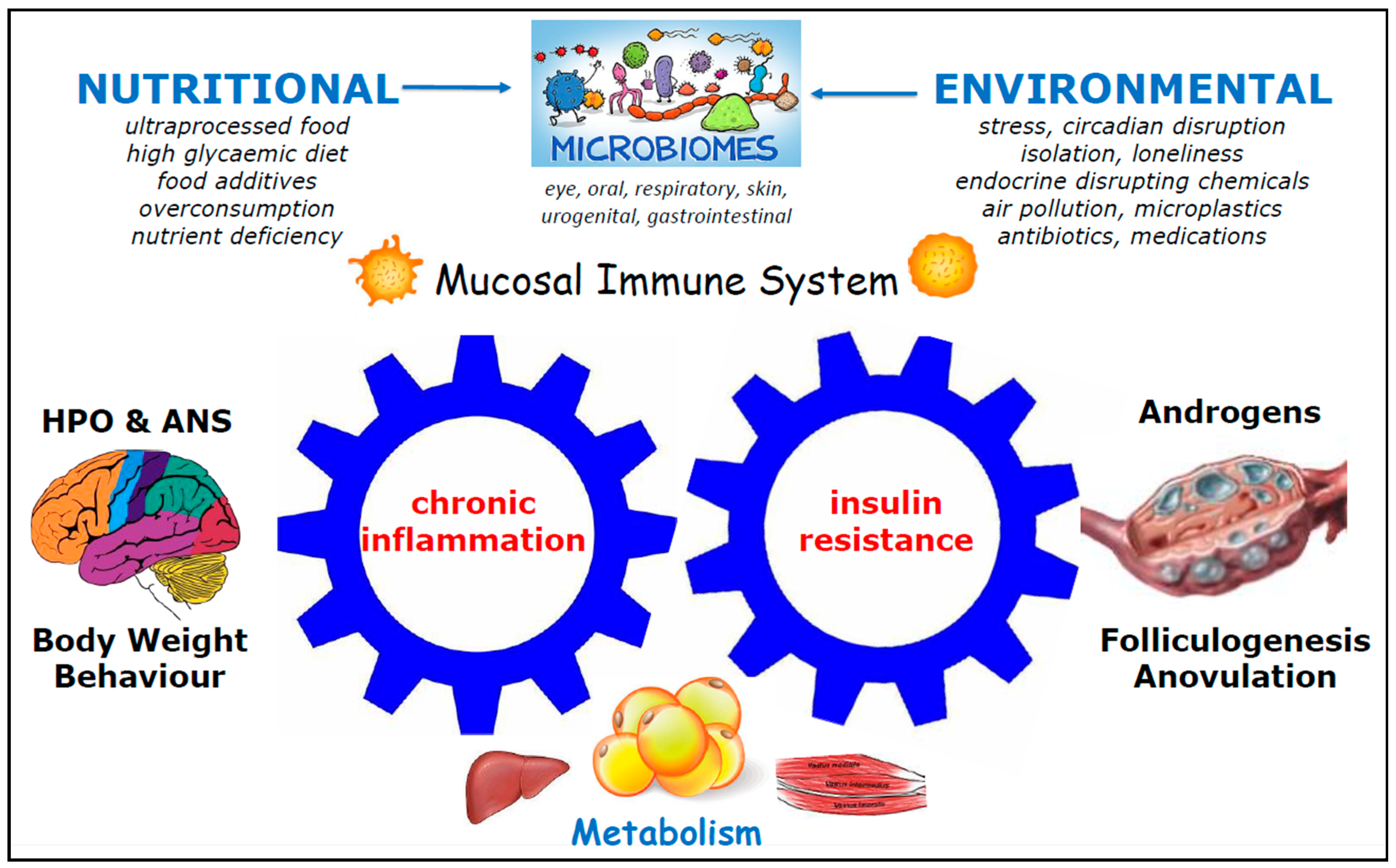
| Endometrial Problem | Pathological Findings and Mechanisms | References |
|---|---|---|
| Heavy menstrual bleeding | Irregular breakdown of a thickened, hyperplastic endometrium due to unopposed E2 and/or P4 deficiency, vascular fragility, and low-grade inflammation. Alterations in inflammatory mediators, hemostasis, fibrinolysis, tissue, and vascular remodeling. | [107,108] |
| Polyps | Chronic unopposed E2 from anovulatory cycles, together with insulin-mediated growth factors (VEGF, TGFβ-1)—and possibly HA—drive excess inflammation and focal endometrial proliferation, cystic glandular changes, stromal fibrosis, and increased vascularity. | [109,110,111,112] |
| Implantation failure | Defective decidualization, altered epithelium, inadequate spiral artery remodeling, immune cell imbalance, and defective extracellular matrix remodeling. Altered endometrial receptivity markers (reduced LIF, HOXA10, αvβ3 integrin, and pinopode formation) driven by HA, IR, inflammation, and obesity. Lifestyle strategies (weight loss, diet, physical activity, and circadian alignment) targeting obesity and IR play an important role. | [16,113,114] |
| Infertility | Combined effects of chronic anovulation and impaired endometrial receptivity reduces conception rates. Key drivers include P4 resistance, low LIF/HOXA10/αvβ3-integrin, defective pinopodes, IR, inflammation, and HA. Upregulated genes involving decidualization (HAND2, MUC1, CSF2), angiogenesis (PDGFA), and inflammation (RELA, CXCL10). Altered epigenetic expression of microRNAs. Lifestyle factors, particularly elevated BMI, are significantly associated with infertility. | [115,116] |
| Miscarriage | Impaired decidualization and trophoblast invasion resulting from P4 resistance, imbalanced cytokines, chronic inflammation, and metabolic dysfunction. Endometrial cells have heightened oxidative stress and dysregulated iron metabolism, leading to increased ferroptosis. Depleted antioxidant defenses (impaired glutathione peroxidase 4) compromise endometrial cell viability and placental development. | [117,118,119] |
| Pregnancy complications | Elevated risk of GDM, PE, FGR, PTB, and stillbirth. Placental abnormalities include defective spiral artery remodeling, spiral artery thrombosis, atherosis of basal arterioles, and failure of deep placentation, with co-existing maternal endothelial dysfunction. Underlying maternal CSI, IR, and HA, alter placental physiology and development. Lifestyle factors modify risk of pregnancy complications. | [10,28,120,121,122,123] |
| Hyperplasia | Prolonged estrogen exposure (unopposed by P4), obesity, decreased SHBG, dyslipidemia, elevated FAI, and IR promote abnormal growth of endometrial glands in relation to stroma +/− cytological atypia (EIN). Loss of PTEN expression, PI3K3CA mutations, and MMR deficiencies. Modifiable risks include obesity, diet, and exercise. | [107,124,125] |
| Endometrial Cancer | Progression from untreated atypical hyperplasia under chronic E2 stimulation, compounded by hyperinsulinemia, inflammation, and genetic mutations. Dysregulated signaling pathways (Notch, Wnt/β-catenin, PI3K/AKT/mTOR, MAPK, JAK/STAT, HER2). | [107,126,127] |
| Study (Year) | Study Design Population (Country) | Key Findings | Insulin Resistance Metrics | Menstrual Cycle Length | Citation |
|---|---|---|---|---|---|
| Robinson et al. (1993) | Cross-sectional 72 PCOS 31 Controls (UK) | ↓Insulin sensitivity in PCOS with oligomenorrhea cw controls (p < 0.01), but normal in PCOS with eumenorrhea | IV Insulin Tolerance Test | >35 days | [162] |
| Strowitzki et al. (2010) | Cross-sectional 118 HA PCOS (Germany) | ↑HOMA-IR with amenorrhea (4.6) cw eumenorrhea (2.8) (p = 0.019) | HOMA-IR | >35 days | [132] |
| Brower et al. (2013) | Cross-sectional 494 PCOS 138 Controls (USA) | Higher mean HOMA-IR (2.2) in PCOS cw controls (1.41), after adjusting for age, BMI, and race | HOMA-IR fasting insulin | >35 days | [128] |
| Ezeh et al. (2021) | Cross-sectional 57 HA PCOS 57 Controls (USA) | ↑Plasma glucose disappearance rate constant (kITT) in amenorrhea (1.98 +/− 0.28) cw eumenorrhea (3.33 +/− 0.51), after adjusting for age, BMI, and ethnicity | Short Insulin Tolerance Test | >35 days | [130] |
| Li et al. (2022) | Cross-sectional 527 PCOS 565 Controls (China) | ↑HOMA-IR, ↑HOMA-β, and ↓QUICKI in women with cycles of 45–90 days cw cycles > 90 days and controls. No significant difference between cycles < 45 and 45–90 days | HOMA-IR HOMA-β QUICKI | 45–90 days | [129] |
| Niu et al. (2023) | Retrospective 140 PCOS (China) | Dose–response relationship between ↑HOMA-IR and cycle length: eumenorrhea (1.61: CI 1.3–1.85), oligomenorrhea (2.02: CI 1.61–2.445), and amenorrhea (2.35: CI 1.96–2.75) | HOMA-IR QUICKI ISI | >35 days | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, J.; O’Brien, C.; Uppal, T.; Tremellen, K. Molecular Impact of Metabolic and Endocrine Disturbance on Endometrial Function in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2025, 26, 9926. https://doi.org/10.3390/ijms26209926
Parker J, O’Brien C, Uppal T, Tremellen K. Molecular Impact of Metabolic and Endocrine Disturbance on Endometrial Function in Polycystic Ovary Syndrome. International Journal of Molecular Sciences. 2025; 26(20):9926. https://doi.org/10.3390/ijms26209926
Chicago/Turabian StyleParker, Jim, Claire O’Brien, Talat Uppal, and Kelton Tremellen. 2025. "Molecular Impact of Metabolic and Endocrine Disturbance on Endometrial Function in Polycystic Ovary Syndrome" International Journal of Molecular Sciences 26, no. 20: 9926. https://doi.org/10.3390/ijms26209926
APA StyleParker, J., O’Brien, C., Uppal, T., & Tremellen, K. (2025). Molecular Impact of Metabolic and Endocrine Disturbance on Endometrial Function in Polycystic Ovary Syndrome. International Journal of Molecular Sciences, 26(20), 9926. https://doi.org/10.3390/ijms26209926









