Impact of Early Postnatal Maternal Separation Stress on Pancreatic Function in Rodents: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Selection Process
2.6. Data Collection Process
2.7. Data Items
2.8. Study Risk of Bias Assessment
2.9. Synthesis Methods
2.10. Assessment of Heterogeneity
2.11. Assessment of Reporting Bias
3. Results
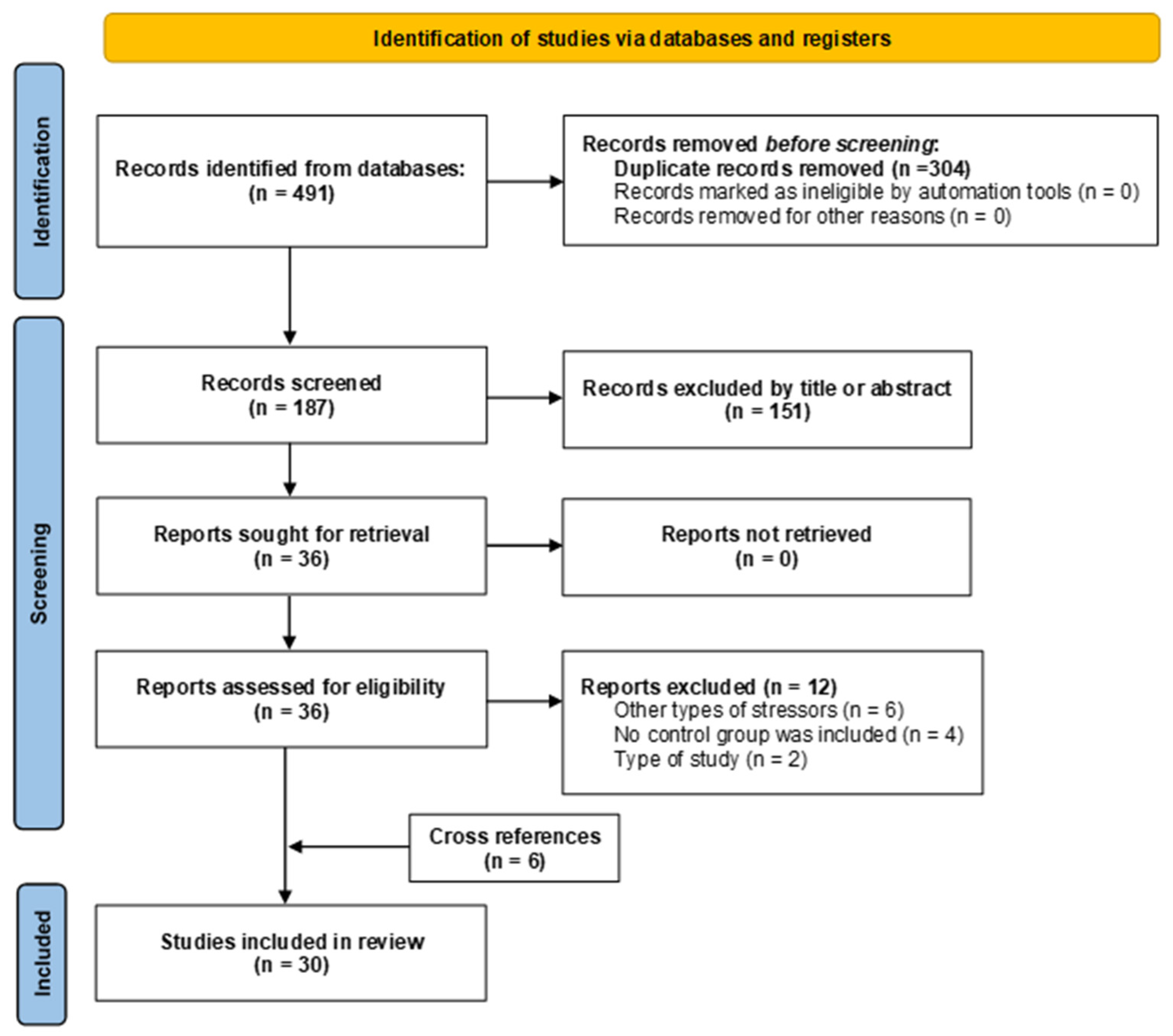
3.1. Study Selection
3.2. Study Characteristics
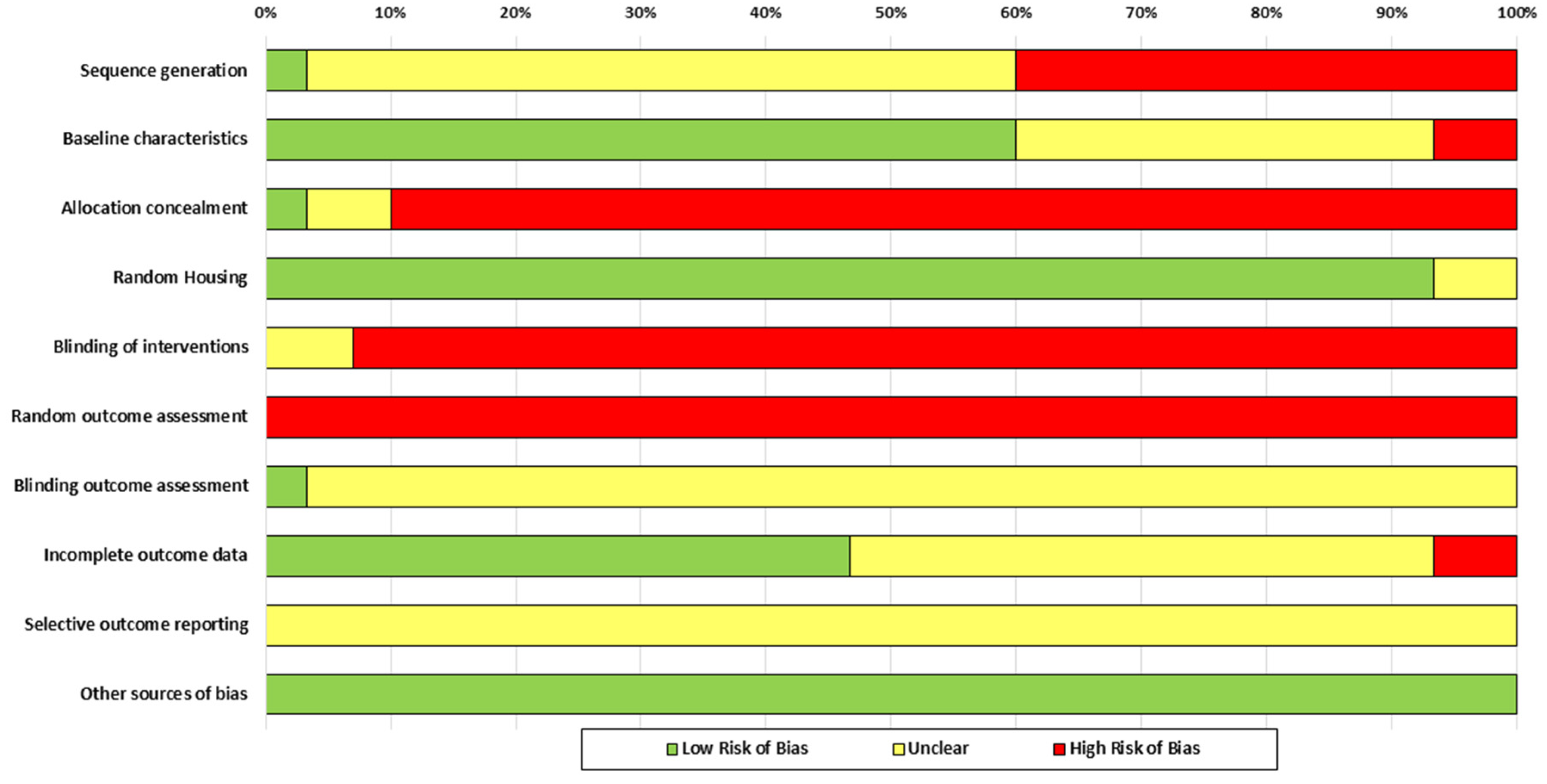
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies
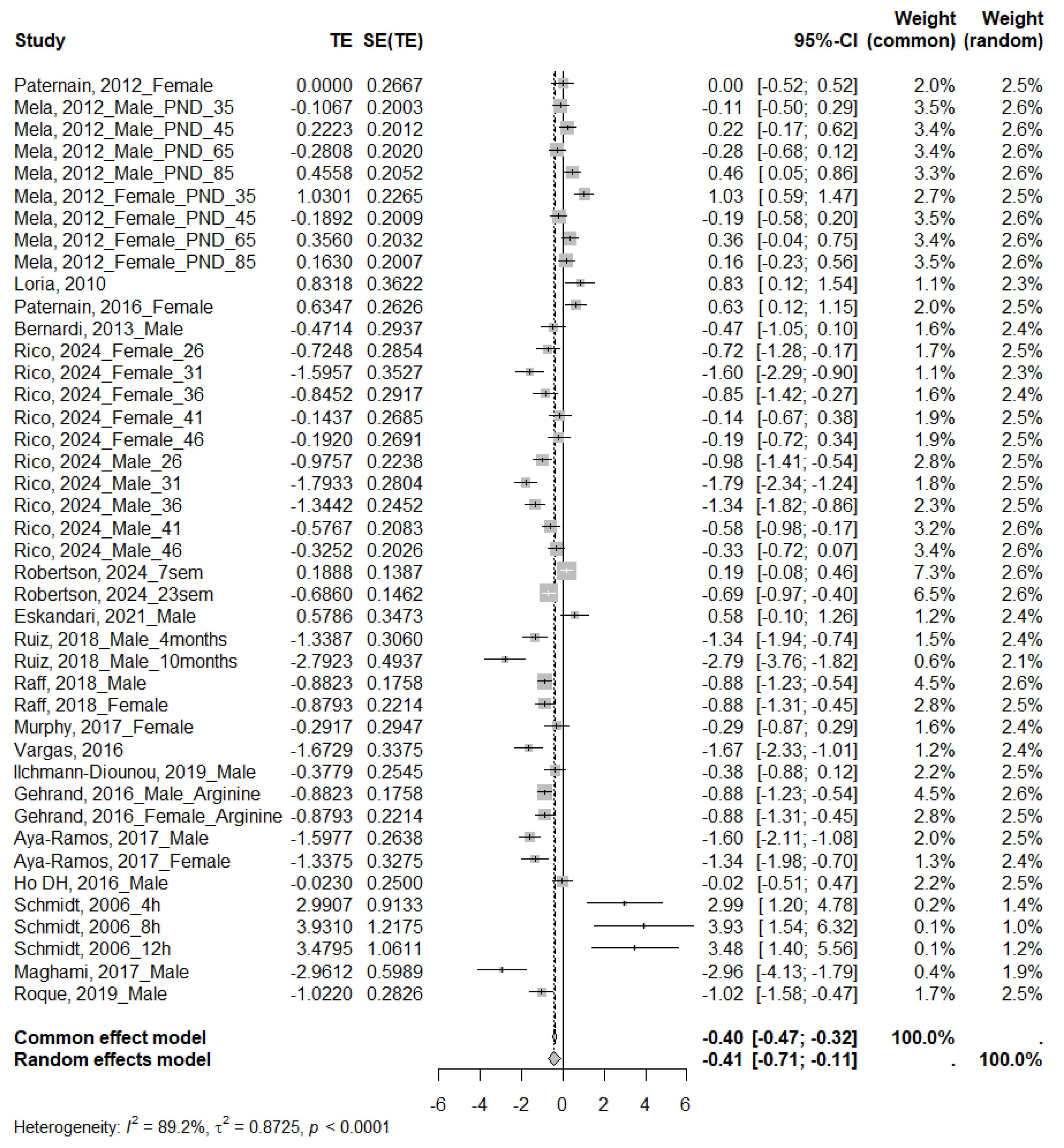
3.4.1. Glucose
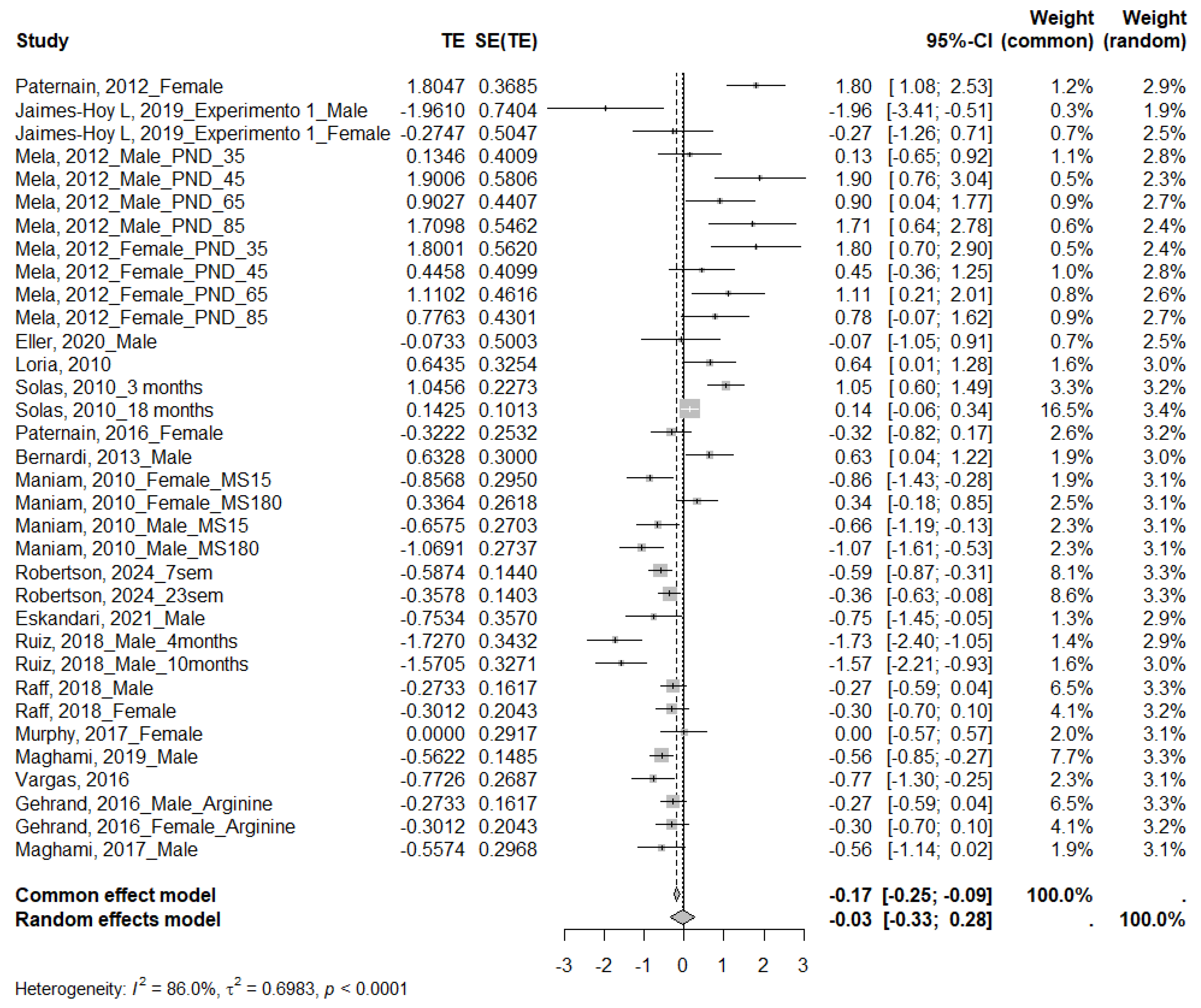
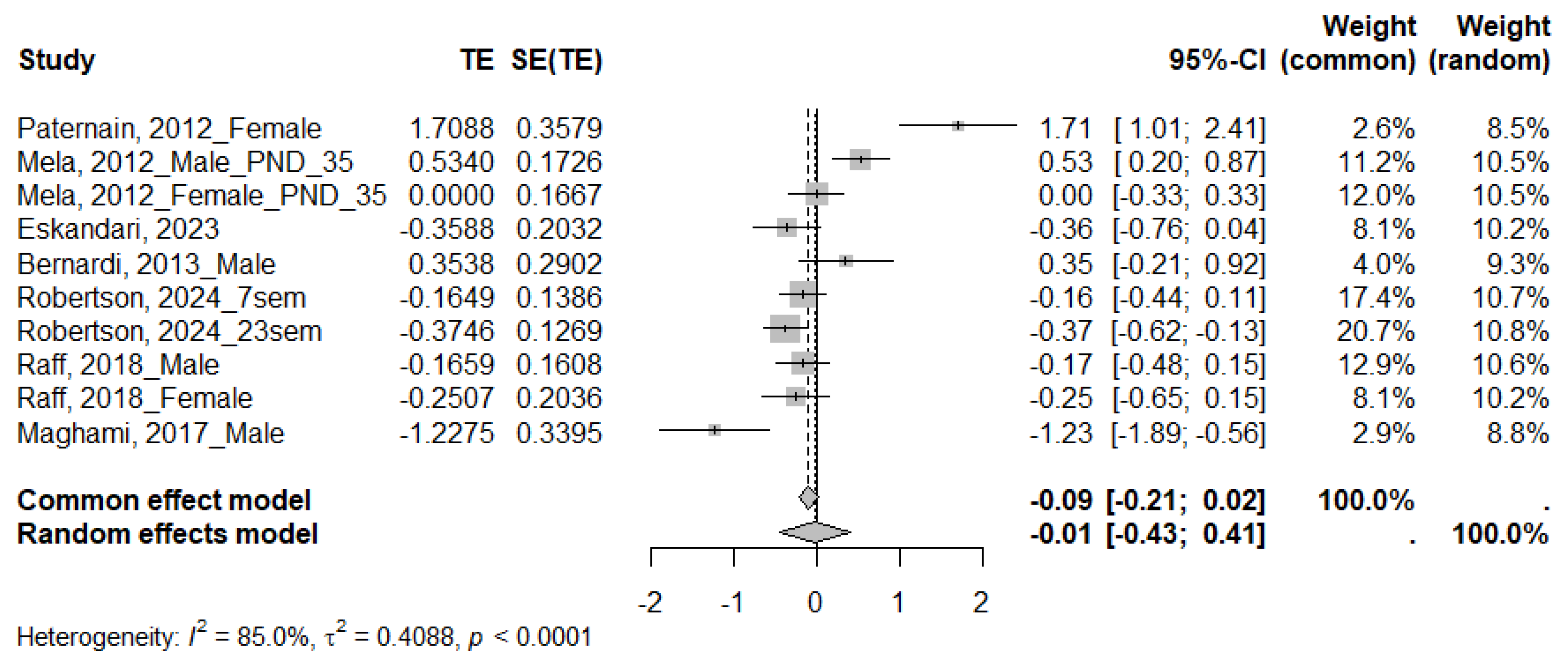
3.4.2. Insulin
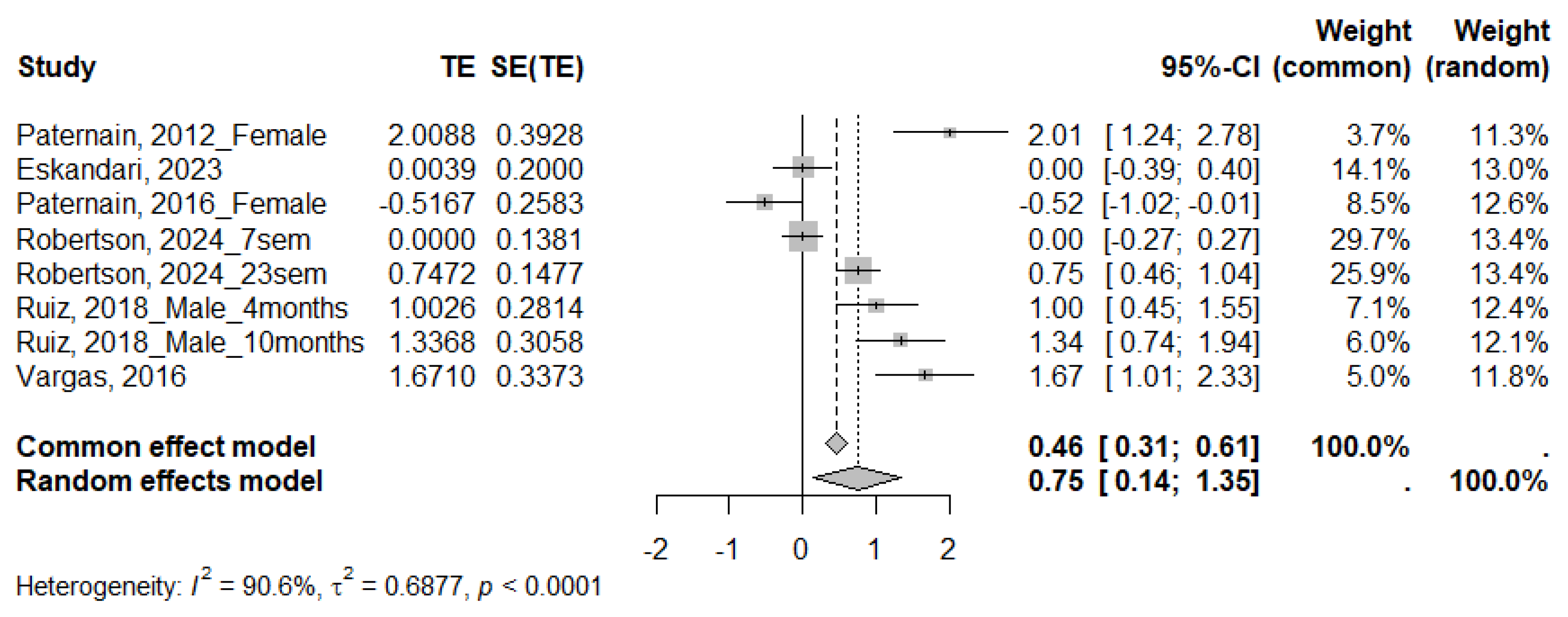
3.4.3. QUICKI
3.4.4. HOMA
3.4.5. GTTs
4. Discussion
4.1. Glucose
4.2. Insulin
4.3. QUICKI
4.4. GTTs
4.5. HOMA
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACEs | Adverse childhood experiences |
| CAT | Catalase |
| FRAP | Ferric-Reducing Ability Potential |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Reduced glutathione |
| GST | Glutathione S-transferase |
| GTTs | Glucose tolerance tests |
| HOMA | Homeostasis model assessment |
| HOMA-B | Homeostasis model assessment of Beta-cell function |
| HPA | Hypothalamic–pituitary–adrenal |
| IGF-1 | Insulin-like growth factor 1 |
| IL-1β | Interleukin-1β |
| ISI | Insulin sensitivity index |
| ITTs | Insulin tolerance tests |
| MDA | Malondialdehyde |
| MS | Maternal separation |
| NOS | Nitric oxide synthase |
| PND | Postnatal day |
| QUICKI | Quantitative insulin sensitivity check index |
| SOD | Superoxide dismutase |
References
- Pace, C.S.; Muzi, S.; Rogier, G.; Meinero, L.L.; Marcenaro, S. The Adverse Childhood Experiences—International Questionnaire (ACE-IQ) in community samples around the world: A systematic review (part I). Child Abus. Negl. 2022, 129, 105640. [Google Scholar] [CrossRef] [PubMed]
- Bauldry, S.; Shanahan, M.J.; Boardman, J.D.; Miech, R.A.; Macmillan, R. A life course model of self-rated health through adolescence and young adulthood. Soc. Sci. Med. 2012, 75, 1311–1320. [Google Scholar] [CrossRef]
- Kalmakis, K.A.; Chandler, G.E. Health consequences of adverse childhood experiences: A systematic review. J. Am. Assoc. Nurse Pract. 2015, 27, 457–465. [Google Scholar] [CrossRef]
- Orso, R.; Creutzberg, K.C.; Kestering-Ferreira, E.; Wearick-Silva, L.E.; Tractenberg, S.G.; Grassi-Oliveira, R. Maternal Separation Combined with Limited Bedding Increases Anxiety-Like Behavior and Alters Hypothalamic-Pituitary-Adrenal Axis Function of Male BALB/cJ Mice. Front. Behav. Neurosci. 2020, 14, 600766. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Perez, A.; West, X.; Brown, M.; Kim, E.; Salih, Z.; Aronoff, S. The Association of Adverse Childhood Experiences and Resilience with Health Outcomes in Adolescents: An Observational Study. Glob. Pediatr. Health 2021, 8, 2333794X20982433. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Bhavnani, S.; Betancourt, T.S.; Tomlinson, M.; Patel, V. Adverse childhood experiences and lifelong health. Nat. Med. 2023, 29, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Hughes, K.; Bellis, M.A.; Hardcastle, K.A.; Sethi, D.; Butchart, A.; Mikton, C.; Jones, L.; Dunne, M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e356–e366. [Google Scholar] [CrossRef]
- Obi, I.E.; McPherson, K.C.; Pollock, J.S. Childhood adversity and mechanistic links to hypertension risk in adulthood. Br. J. Pharmacol. 2019, 176, 1932–1950. [Google Scholar] [CrossRef]
- Guzmán, D.B.; Howell, B.; Sánchez, M. Early life stress and development: Preclinical science. In Posttraumatic Stress Disorder, 1st ed.; Bremner, J.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 61–80. [Google Scholar] [CrossRef]
- Petruccelli, K.; Davis, J.; Berman, T. Adverse childhood experiences and associated health outcomes: A systematic review and meta-analysis. Child Abus. Negl. 2019, 97, 104127. [Google Scholar] [CrossRef]
- Murthy, S.; Gould, E. Early Life Stress in Rodents: Animal Models of Illness or Resilience? Front. Behav. Neurosci. 2018, 12, 157. [Google Scholar] [CrossRef]
- Schmidt, M.V.; Wang, X.D.; Meijer, O.C. Early life stress paradigms in rodents: Potential animal models of depression? Psychopharmacology 2011, 214, 131–140. [Google Scholar] [CrossRef]
- Nishi, M. Effects of Early-Life Stress on the Brain and Behaviors: Implications of Early Maternal Separation in Rodents. Int. J. Mol. Sci. 2020, 21, 7212. [Google Scholar] [CrossRef]
- Webster, J.F.; Beerens, S.; Wozny, C. Effects of early life stress and subsequent re-exposure to stress on neuronal activity in the lateral habenula. Neuropsychopharmacology 2023, 48, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, D.M. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology 1998, 23, 663–700. [Google Scholar] [CrossRef]
- Bondar, N.P.; Lepeshko, A.A.; Reshetnikov, V.V. Effects of Early-Life Stress on Social and Anxiety-Like Behaviors in Adult Mice: Sex-Specific Effects. Behav. Neurol. 2018, 2018, 1538931. [Google Scholar] [CrossRef]
- Shin, S.; Lee, S. The impact of environmental factors during maternal separation on the behaviors of adolescent C57BL/6 mice. Front. Mol. Neurosci. 2023, 16, 1147951. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, L.; Fu, H.; Xie, Y.; Sun, L.; Li, Y.; Xiao, L.; Zhang, L.; Su, Y.; Wang, G. Maternal separation during lactation affects recognition memory, emotional behaviors, hippocampus and gut microbiota composition in C57BL6J adolescent female mice. Behav. Brain Res. 2025, 476, 115249. [Google Scholar] [CrossRef] [PubMed]
- Maghami, S.; Zardooz, H.; Khodagholi, F.; Binayi, F.; Ranjbar Saber, R.; Hedayati, M.; Sahraei, H.; Ansari, M.A. Correction: Maternal separation blunted spatial memory formation independent of peripheral and hippocampal insulin content in young adult male rats. PLoS ONE 2018, 13, e0204731, Erratum in: PLoS ONE 2019, 14, e0210893. https://doi.org/10.1371/journal.pone.0210893. [Google Scholar] [CrossRef]
- Eskandari, F.; Salimi, M.; Khodagholi, F.; Hedayati, M.; Zardooz, H. Investigation of the effects of maternal separation on the pancreatic oxidative and inflammatory damages along with metabolic impairment in response to chronic social defeat stress in young adult male rats. J. Diabetes Metab. Disord. 2021, 20, 1557–1565. [Google Scholar] [CrossRef]
- Eskandari, F.; Salimi, M.; Binayi, F.; Abdollahifar, M.A.; Eftekhary, M.; Hedayati, M.; Ghanbarian, H.; Zardooz, H. Investigating the Effects of Maternal Separation on Hypothalamic-Pituitary-Adrenal Axis and Glucose Homeostasis under Chronic Social Defeat Stress in Young Adult Male Rat Offspring. Neuroendocrinology 2023, 113, 361–380. [Google Scholar] [CrossRef]
- de Vries, R.B.M.; Hooijmans, C.R.; Langendam, M.W.; van Luijk, J.; Leenaars, M.; Ritskes-Hoitinga, M.; Wever, K.E. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid. Based Preclin. Med. 2015, 2, e00007. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Chapter 13: Assessing Risk of Bias Due to Missing Evidence in a Meta-Analysis. In Cochrane Handbook for Systematic Reviews of Interventions, version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2024; Available online: https://cochrane.org/handbook (accessed on 6 October 2025).
- Nguyen, P.Y.; Kanukula, R.; McKenzie, J.E.; Alqaidoom, Z.; Brennan, S.E.; Haddaway, N.R.; Hamilton, D.G.; Karunananthan, S.; McDonald, S.; Moher, D.; et al. Changing patterns in reporting and sharing of review data in systematic reviews with meta-analysis of the effects of interventions: Cross sectional meta-research study. BMJ 2022, 379, e072428. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.V.; Levine, S.; Alam, S.; Harbich, D.; Sterlemann, V.; Ganea, K.; de Kloet, E.R.; Holsboer, F.; Müller, M.B. Metabolic signals modulate hypothalamic-pituitary-adrenal axis activation during maternal separation of the neonatal mouse. J. Neuroendocrinol. 2006, 18, 865–874. [Google Scholar] [CrossRef]
- George, E.D.; Bordner, K.A.; Elwafi, H.M.; Simen, A.A. Maternal separation with early weaning: A novel mouse model of early life neglect. BMC Neurosci. 2010, 11, 123. [Google Scholar] [CrossRef]
- Loria, A.S.; Pollock, D.M.; Pollock, J.S. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension 2010, 55, 494–499. [Google Scholar] [CrossRef]
- Maniam, J.; Morris, M.J. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology 2010, 35, 717–728. [Google Scholar] [CrossRef]
- Solas, M.; Aisa, B.; Mugueta, M.C.; Del Río, J.; Tordera, R.M.; Ramírez, M.J. Interactions between age, stress and insulin on cognition: Implications for Alzheimer’s disease. Neuropsychopharmacology 2010, 35, 1664–1673. [Google Scholar] [CrossRef]
- Viveros, M.P.; Díaz, F.; Mateos, B.; Rodríguez, N.; Chowen, J.A. Maternal deprivation induces a rapid decline in circulating leptin levels and sexually dimorphic modifications in hypothalamic trophic factors and cell turnover. Horm. Behav. 2010, 57, 405–414. [Google Scholar] [CrossRef]
- Mela, V.; Llorente-Berzal, Á.; Díaz, F.; Argente, J.; Viveros, M.P.; Chowen, J.A. Maternal Deprivation Exacerbates the Response to a High Fat Diet in a Sexually Dimorphic Manner. PLoS ONE 2012, 7, e48915. [Google Scholar] [CrossRef]
- Paternain, L.; Martisova, E.; Milagro, F.I.; Ramírez, M.J.; Martínez, J.A.; Campión, J. Postnatal maternal separation modifies the response to an obesogenic diet in adulthood in rats. Dis. Models Mech. 2012, 5, 691–697. [Google Scholar] [CrossRef]
- Bernardi, J.R.; Ferreira, C.F.; Senter, G.; Krolow, R.; de Aguiar, B.W.; Portella, A.K.; Kauer-Sant’anna, M.; Kapczinski, F.; Dalmaz, C.; Goldani, M.Z.; et al. Early life stress interacts with the diet deficiency of omega-3 fatty acids during the life course increasing the metabolic vulnerability in adult rats. PLoS ONE 2013, 8, e62031. [Google Scholar] [CrossRef]
- Gehrand, A.L.; Hoeynck, B.; Jablonski, M.; Leonovicz, C.; Ye, R.; Scherer, P.E.; Raff, H. Sex differences in adult rat insulin and glucose responses to arginine: Programming effects of neonatal separation, hypoxia, and hypothermia. Physiol. Rep. 2016, 4, e12972. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, K.K.; Vaidya, V.A.; Kolthur-Seetharam, U. Early Stress History Alters Serum Insulin-Like Growth Factor-1 and Impairs Muscle Mitochondrial Function in Adult Male Rats. J. Neuroendocrinol. 2016, 28, 10. [Google Scholar] [CrossRef]
- Ho, D.H.; Burch, M.L.; Musall, B.; Musall, J.B.; Hyndman, K.A.; Pollock, J.S. Early life stress in male mice induces superoxide production and endothelial dysfunction in adulthood. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1267–H1274. [Google Scholar] [CrossRef]
- Paternain, L.; Martisova, E.; Campión, J.; Martínez, J.A.; Ramírez, M.J.; Milagro, F.I. Methyl donor supplementation in rats reverses the deleterious effect of maternal separation on depression-like behaviour. Behav. Brain Res. 2016, 299, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.; Junco, M.; Gomez, C.; Lajud, N. Early Life Stress Increases Metabolic Risk, HPA Axis Reactivity, and Depressive-Like Behavior When Combined with Postweaning Social Isolation in Rats. PLoS ONE 2016, 11, e0162665. [Google Scholar] [CrossRef] [PubMed]
- Aya-Ramos, L.; Contreras-Vargas, C.; Rico, J.L.; Dueñas, Z. Early maternal separation induces preference for sucrose and aspartame associated with increased blood glucose and hyperactivity. Food Funct. 2017, 8, 2592–2600. [Google Scholar] [CrossRef]
- Maghami, S.; Sadeghimahalli, F.; Zardooz, H. Effects of maternal separation stress on glucose homeostasis in pubertal male rats. Koomesh 2017, 19, 887–893. [Google Scholar]
- Murphy, M.O.; Herald, J.B.; Wills, C.T.; Unfried, S.G.; Cohn, D.M.; Loria, A.S. Postnatal treatment with metyrapone attenuates the effects of diet-induced obesity in female rats exposed to early-life stress. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E98–E108. [Google Scholar] [CrossRef] [PubMed]
- Raff, H.; Hoeynck, B.; Jablonski, M.; Leonovicz, C.; Phillips, J.M.; Gehrand, A.L. Insulin sensitivity, leptin, adiponectin, resistin, and testosterone in adult male and female rats after maternal-neonatal separation and environmental stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R12–R21. [Google Scholar] [CrossRef]
- Ruiz, R.; Roque, A.; Pineda, E.; Licona-Limón, P.; Valdéz-Alarcón, J.J.; Lajud, N. Early life stress accelerates age-induced effects on neurogenesis, depression, and metabolic risk. Psychoneuroendocrinology 2018, 96, 203–211. [Google Scholar] [CrossRef]
- Ilchmann-Diounou, H.; Olier, M.; Lencina, C.; Riba, A.; Barretto, S.; Nankap, M.; Sommer, C.; Guillou, H.; Ellero-Simatos, S.; Guzylack-Piriou, L.; et al. Early life stress induces type 2 diabetes-like features in ageing mice. Brain Behav. Immun. 2019, 80, 452–463. [Google Scholar] [CrossRef]
- Jaimes-Hoy, L.; Romero, F.; Charli, J.L.; Joseph-Bravo, P. Sex Dimorphic Responses of the Hypothalamus-Pituitary-Thyroid Axis to Maternal Separation and Palatable Diet. Front. Endocrinol. 2019, 10, 445. [Google Scholar] [CrossRef]
- Roque, A.; Ruiz-González, R.; Pineda-López, E.; Torner, L.; Lajud, N. Prenatal immobilization stress and postnatal maternal separation cause differential neuroendocrine responses to fasting stress in adult male rats. Dev. Psychobiol. 2020, 62, 737–748. [Google Scholar] [CrossRef]
- Eller, O.C.; Morris, E.M.; Thyfault, J.P.; Christianson, J.A. Early life stress reduces voluntary exercise and its prevention of diet-induced obesity and metabolic dysfunction in mice. Physiol. Behav. 2020, 223, 113000. [Google Scholar] [CrossRef]
- Eller, O.C.; Foright, R.M.; Brake, A.D.; Winter, M.K.; Bantis, L.E.; Morris, E.M.; Thyfault, J.P.; Christianson, J.A. An Omega-3-rich Anti-inflammatory Diet Improved Widespread Allodynia and Worsened Metabolic Outcomes in Adult Mice Exposed to Neonatal Maternal Separation. Neuroscience 2021, 468, 53–67. [Google Scholar] [CrossRef]
- Fenton Navarro, B.; Casimiro Aguayo, A.A.; Torres Gómez, Y.L.; Cervantes Alfaro, M.; Torner, L. Early Life Stress Influences Oxidative Stress Enzyme Activities in Liver, Heart, Kidney, Suprarenal Glands, and Pancreas in Male and Female Rat Pups. Antioxidants 2024, 13, 802. [Google Scholar] [CrossRef] [PubMed]
- Rico, J.L.; Aya-Ramos, L.; Dueñas, Z. Effects of early-life stress followed by access to stevia or sucralose during adolescence on weight gain, glycemia, and anxiety-related behaviors in male and female rats. Physiol. Behav. 2024, 280, 114529. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.; Griffith, T.A.; Helman, T.J.; Hatton-Jones, K.; Naghipour, S.; Robertson, D.A.; Peart, J.N.; Headrick, J.P.; Du Toit, E.F. Early life stress exacerbates the obesogenic and anxiogenic effects of a Western diet without worsening cardiac ischaemic tolerance in male mice. J. Dev. Orig. Health Dis. 2024, 15, e14. [Google Scholar] [CrossRef]
- Miki, T.; Liu, J.Q.; Ohta, K.; Suzuki, S.; Kusaka, T.; Warita, K.; Yokoyama, T.; Jamal, M.; Ueki, M.; Yakura, T.; et al. Early postnatal maternal separation causes alterations in the expression of β3-adrenergic receptor in rat adipose tissue suggesting long-term influence on obesity. Biochem. Biophys. Res. Commun. 2013, 442, 68–71. [Google Scholar] [CrossRef]
- del Sol, M.; Navarrete, J.; García-Orozco, L.; Duque-Colorado, J.; Sócola-Barsallo, Z.; Sandoval, C.; Vásquez, B. When Timing Matters: Effects of Maternal Separation and Post-Weaning High-Fat Diet on Liver Morphology in a Rodent Model. Nutrients 2025, 17, 1619. [Google Scholar] [CrossRef]
- Rakipovski, G.; Raun, K.; Lykkesfeldt, J. Fluctuating hyperglycaemia increases oxidative stress response in lean rats compared to sustained hyperglycaemia despite lower glycaemic exposure. Diab. Vasc. Dis. Res. 2011, 8, 295–298. [Google Scholar] [CrossRef]
- Kawai, K.; Sakairi, T.; Harada, S.; Shinozuka, J.; Ide, M.; Sato, H.; Tanaka, M.; Toriumi, W.; Kume, E. Diet modification and its influence on metabolic and related pathological alterations in the SHR/NDmcr-cp rat, an animal model of the metabolic syndrome. Exp. Toxicol. Pathol. 2012, 64, 333–338. [Google Scholar] [CrossRef]
- Gao, X.; Jansson, L.; Persson, A.E.; Sandberg, M. Short-term glucosamine infusion increases islet blood flow in anesthetized rats. Islets 2013, 5, 201–206. [Google Scholar] [CrossRef]
- Parfitt, D.B.; Walton, J.R.; Corriveau, E.A.; Helmreich, D.L. Early life stress effects on adult stress-induced corticosterone secretion and anxiety-like behavior in the C57BL/6 mouse are not as robust as initially thought. Horm. Behav. 2007, 52, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.; Padow, V.A.; Franco, D.; Hall, B.S.; Park, B.; Klein, Z.A.; Romeo, R.D. Divergent stress-induced neuroendocrine and behavioral responses prior to puberty. Physiol. Behav. 2012, 107, 104–111. [Google Scholar] [CrossRef]
- Campos-Cardoso, R.; Novaes, L.S.; Godoy, L.D.; Dos Santos, N.B.; Perfetto, J.G.; Lazarini-Lopes, W.; Garcia-Cairasco, N.; Padovan, C.M.; Munhoz, C.D. The resilience of adolescent male rats to acute stress-induced delayed anxiety is age-related and glucocorticoid release-dependent. Neuropharmacology 2023, 226, 109385. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Measuring energy metabolism in the mouse—Theoretical, practical, and analytical considerations. Front. Physiol. 2013, 4, 34. [Google Scholar] [CrossRef]
- da Silva Xavier, G.; Hodson, D.J. Mouse models of peripheral metabolic disease. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 299–315. [Google Scholar] [CrossRef]
- Li, L.; Garvey, W.T.; Gower, B.A. Childhood Maltreatment Is an Independent Risk Factor for Prediabetic Disturbances in Glucose Regulation. Front. Endocrinol. 2017, 8, 151. [Google Scholar] [CrossRef]
- Gísladóttir, E.U.; Daníelsdóttir, H.B.; Song, H.; Bjarnardóttir, M.D.; Hauksdóttir, A.; Guðmundsdóttir, A.; Yacamán-Méndez, D.; Thordardottir, E.B.; Tomasson, G.; Rúnarsdóttir, H.; et al. Adverse childhood experiences and prevalence of type 2 diabetes in a nationwide study of women. Eur. J. Public Health 2025, 35, ckaf079. [Google Scholar] [CrossRef]
- Wen, S.; Zhu, J.; Han, X.; Li, Y.; Liu, H.; Yang, H.; Hou, C.; Xu, S.; Wang, J.; Hu, Y.; et al. Childhood maltreatment and risk of endocrine diseases: An exploration of mediating pathways using sequential mediation analysis. BMC Med. 2024, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Antuna-Puente, B.; Faraj, M.; Karelis, A.D.; Garrel, D.; Prud’homme, D.; Rabasa-Lhoret, R.; Bastard, J.P. HOMA or QUICKI: Is it useful to test the reproducibility of formulas? Diabetes Metab. 2008, 34, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Muniyappa, R.; Yan, X.; Chen, H.; Yue, L.Q.; Hong, E.G.; Kim, J.K.; Quon, M.J. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E261–E270. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Chen, H.; Muzumdar, R.H.; Einstein, F.H.; Yan, X.; Yue, L.Q.; Barzilai, N.; Quon, M.J. Comparison between surrogate indexes of insulin sensitivity/resistance and hyperinsulinemic euglycemic clamp estimates in rats. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1023–E1029. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]


| Authors | Species/ Strain | Total Number (per Group) | Sex | Age of Assessment (PND) | Fasting Before the Tests | Variables Evaluated | Main Findings |
|---|---|---|---|---|---|---|---|
| Schmidt et al. [28] | Mice/CD1 | MS: 8–10, Ctl: 8–10 | M/F | 8 | Yes (4, 8 o 12 h) | Plasma glucose | [↓] Glucose in the MS group at 4, 8 and 12 h vs. Ctl |
| George et al. [29] | Mice/C57BL/6J | NR | M | 10–17 | NR | Serum glucose | MS did not produce significant changes |
| Loria et al. [30] | Rats/Wistar Kyoto | MS: 9–12, Ctl: 9–12 | M | 84 | No | Plasma glucose and insulin | MS did not produce significant changes |
| Maniam & Morris [31] | Rats/Sprague-Dawley | MS: 11–13, Ctl: 11–13 | M/F | 133 | NR | Plasma insulin | MS 15 min [↑] insulin in F of the MS vs. Ctl, while M showed no statistically significant changes. Prolonged MS (180 min) did not produce statistically significant changes in either M or F |
| Solas et al. [32] | Rats/Wistar | MS: 28, Ctl: 32 | M | 84–504 | Yes | Plasma insulin | [↓] insulin in the MS group vs. Ctl |
| Viveros et al. [33] | Rats/Wistar | MS: 12–16, Ctl: 12 | M/F | 9–10 | NR | Circulating glucose | MS 12 h: [↑] glucose in F vs. Ctl0; [↓] in both sexes vs. Ctl12. MS24h and MS36: no change vs. Ctl0, but [↓] vs. Ctl24/36. Ctl36 showed [↑] glucose vs. Ctl0 |
| Mela et al. [34] | Rats/Wistar | MS: 12, Ctl: 12 | M/F | 102 | Yes (12 h) | Plasma glucose and insulin, HOMA | In F with MS: [↓] glucose (PND 35), [↓] insulin (PND 35, 45, 65, 85). In M with MS: [↓] insulin (PND 45, 85); no relevant changes in glucose. HOMA, no significant changes |
| Paternain et al. [35] | Rats/Wistar | MS: 10, Ctl: 6 | F | 95 | NR | Circulating glucose, serum insulin, HOMA, HOMA-B, QUICKI | [↓] Insulin, HOMA, HOMA-B and, QUICKI in the MS group vs. Ctl. No differences in glucose levels |
| Bernardi et al. [36] | Rats/Wistar | MS: 7, Ctl: 7 | M | 126 | Yes (6 h) | Plasma glucose, serum insulin, HOMA | MS did not produce significant changes |
| Gehrand et al. [37] | Rats/Sprague-Dawley | MS: 22, Ctl: 23 | M/F | 105–133 | NR | Plasma glucose, insulin, and glucagon, GTTs, insulin and glucagon, beta cell count | In M and F [↑] glucose. [↑] AUC in M for insulin and glucose, and not in F. No differences in insulin levels, number of β cells, and AUC for glucagon. In M, [↓] glucagon in the MS group vs. Ctl, no differences in F |
| Ghosh et al. [38] | Rats/Sprague-Dawley | MS: 10, Ctl: 10 | M | 112–224 | Yes | Serum glucose, GTTs, and peritoneal insulin | MS did not produce significant changes |
| Ho et al. [39] | Mice/C57BL/6J | MS: 5–7, Ctl: 5–7 | M | 84 | No | Plasma glucose | MS did not produce significant changes |
| Paternain et al. [40] | Rats/Wistar | MS: 8, Ctl: 8 | F | 182 | NR | Serum glucose, plasma insulin, QUICKI | MS did not produce significant changes |
| Vargas et al. [41] | Rats/Sprague-Dawley | MS: 8–12, Ctl: 8–12 | M | 67 | Yes | Plasma glucose and insulin, QUICKI, GTTs | Glucose, insulin, and QUICKI levels were unchanged. Blood glucose levels in the GTTs were [↑] at 30 and 60 min after injection in the MS group vs. Ctl |
| Aya-Ramos et al. [42] | Rats/Wistar | MS: 7–10, Ctl: 8–10 | M/F | 26 | NR | Circulating glucose | [↑] Glucose in both M and F in the MS group vs. Ctl |
| Maghami et al. [43] | Rats/Wistar | MS: 7, Ctl: 7 | M | 53 | NR | Plasma glucose and insulin, HOMA | [↑] Glucose in the MS group vs. Ctl. No differences in insulin levels. [↑] HOMA in the MS group vs. the Ctl |
| Murphy et al. [44] | Rats/Wistar Kyoto | MS: 6–8, Ctl: 6–8 | F | 105 | Yes (16 h) | Circulating glucose and plasma insulin | MS did not produce significant changes |
| Raff et al. [45] | Rats/Sprague-Dawley | MS: 22, Ctl: 23 | M/F | NR | Yes (Night) | Plasma glucose and insulin, HOMA | No differences in glucose and insulin levels. [↑] HOMA in both M and F in the MS group vs. Ctl |
| Ruiz et al. [46] | Rats/Sprague-Dawley | MS: 7–10, Ctl: 7–10 | M | 120–300 | Yes (Night) | Plasma glucose and insulin, QUICKI and GTTs | [↑] Glucose and insulin at both 120 PND and 300 PND in the MS group vs. Ctl. [↓] QUICKI at both 120 PND and 300 PND in the MS group vs. Ctl. [↑] AUC in the MS group 120 PND vs. Ctl, unchanged by 300 PND |
| Ilchmann-Diounou et al. [47] | Mice/C3H/HeN | MS: 8, Ctl: 8 | M | 350 | Yes (6 o 12 h) | Circulating glucose, plasma insulin, GTTs, intraperitoneal insulin tolerance | [↑] Glucose in the MS group vs. Ctl. No differences in insulin levels. The AUC for glucose and insulin [↑] in the MS group vs. the Ctl |
| Jaimes-Hoy et al. [48] | Rats/Wistar | MS: 4, Ctl: 4 | M/F | 90 | No | Serum insulin | [↑] Insulin in M in the MS group vs. Ctl. No changes are evident in F |
| Maghami et al. [20] | Rats/Wistar | MS: 34, Ctl: 34 | M | 53 | Yes (16 h) | Plasma insulin, islet isolation | There were no differences in insulin levels or insulin secretion in isolated islets. Islet insulin content showed a significant reduction in the MS group vs. Ctl |
| Roque et al. [49] | Rats/Sprague-Dawley | MS: 8, Ctl: 8 | M | 126 | Yes (Night) | Circulating glucose, GTTs | [↑] GTTs in MS group vs. Ctl. Glucose without significant changes |
| Eller et al. [50] | Mice/C57BL/6 | MS: 4, Ctl: 4 | M/F | 189 | Yes (6 h) | Serum insulin, GTTs | No differences in insulin levels. [↑] AUC in the MS group vs. Ctl |
| Eller et al. [51] | Mice/C57BL/6 | MS: 6–16, Ctl: 6–16 | M/F | 126–133 | Yes (6 h) | Serum insulin, HOMA, GTTs | MS did not produce significant changes |
| Eskandari et al. [21] | Rats/Wistar | MS: 7, Ctl: 7 | M | 72 | Yes (16–18 h) | Plasma glucose and insulin, HOMA2-IR, GSH, MDA, IL-1β | [↓] CAT and [↑] MDA in the MS group vs. Ctl. The other variables without significant changes |
| Eskandari et al. [22] | Rats/Wistar | MS: 10, Ctl: 10 | M | 70 | Yes (16–18 h) | Plasma insulin, HOMA, HOMA-B, QUICKI, ISI, GTTs, islet area, beta cell number, islet isolation, CRFR1 | MS only produced changes in the percentage of beta cells, where there was a decrease in the MS group vs. Ctl |
| Fenton-Navarro et al. [52] | Rats/Sprague-Dawley | MS: 4–5, Ctl: 4–5 | M/F | 15 | NR | CAT, MDA, SOD, GPX, GR, GST, NOS, FRAP | [↑] CAT, MDA in F and not in M, [↑] SOD, GST in both F and M, [↓] GPX, FRAP in both F and M, [↑] GR in M and not in F, [↑] NOS in F and not in M in the MS group vs. Ctl |
| Rico et al. [53] | Rats/Wistar | MS: 8–10, Ctl: 8–10 | M/F | 26 | No | Circulating glucose | MS did not produce significant changes |
| Robertson et al. [54] | Mice/C57BL/6J | MS: 14, Ctl: 15 | M | 49–161 | Yes (4 h) | Circulating glucose, serum insulin, HOMA, QUICKI | [↑] Glucose in the MS group vs. Ctl. No differences in insulin and HOMA levels. [↓] QUICKI in the MS group vs. Ctl |
| Authors | Beginning of the Separation Period (PND) | Duration of Separation (hours/day) | Length of Separation Period (days) | Conditions of Separation | Weaning (PND) |
|---|---|---|---|---|---|
| Schmidt et al. [28] | 8 | 4, 8 or 12 | 1 | In a group, with heating, the mother is in a separate room | NR |
| George et al. [29] | 2 | 4 (PND 2–5) and 8 (PND 6–16) | 15 | In a group, with heating, the mother is in a separate room | 21 (Ctl), 17 (MS) |
| Loria et al. [30] | 2 | 3 | 13 | Individual, with heating, mother’s position not specified | 28 |
| Maniam & Morris [31] | 2 | 15 min and 3 | 13 | In a group, with heating, the mother is in a separate room | 20 |
| Solas et al. [32] | 2 | 3 | 20 | In a group, without further specifications | 23 |
| Viveros et al. [33] | 9 | 12 or 24 | 12 h o 1 | In a group, the mother is in the same room | 9 or 10 |
| Mela et al. [34] | 9 | 24 | 1 | In a group, the mother is in the same room | 22 |
| Paternain et al. [35] | 2 | 3 | 20 | In a group, with heating, the mother is in the same room | 23 |
| Bernardi et al. [36] | 1 | 3 | 10 | In a group, with heating, the mother is in a separate room | 21 |
| Gehrand et al. [37] | 2 | 1.5 | 5 | In a group, without further specifications | 22 |
| Ghosh et al. [38] | 2 | 3 | 13 | In a group, with heating, the mother’s position is not specified. | 28 |
| Ho et al. [39] | 2 | 4 (PND 2–5) and 8 (PND 6–16) | 15 | In a group, without further specifications | 21 (Ctl), 17 (MS) |
| Paternain et al. [40] | 2 | 3 | 20 | In a group, with heating, the mother is in the same room | 23 |
| Vargas et al. [41] | 1 | 3 | 14 | Individual, with heating, mother in a separate room | 21 |
| Aya-Ramos et al. [42] | 1 | 6 | 21 | In groups, two separations per day, without further specifications | 22 |
| Maghami et al. [43] | 1 | 3 | 21 | In a group, with heating, the mother is in a separate room | 21 |
| Murphy et al. [44] | 2 | 3 | 13 | Individual, with heating, mother’s position not specified | 28 |
| Raff et al. [45] | 2 | 1.5 | 5 | In a group, with heating, the mother’s position is not specified | 22 |
| Ruiz et al. [46] | 1 | 3 | 14 | In a group, with heating, the mother’s position is not specified | 21 |
| Ilchmann-Diounou et al. [47] | 2 | 3 | 10 | With heating, no further specifications | 21 |
| Jaimes-Hoy et al. [48] | 2 | 3 | 20 | In a group, with heating, the mother’s position is not specified | 22 |
| Maghami et al. [20] | 1 | 3 | 21 | In a group, with heating, the mother is in a separate room | 22 |
| Roque et al. [49] | 1 | 3 | 14 | In a group, with heating, the mother’s position is not specified | 21 |
| Eller et al. [50] | 1 | 3 | 21 | In a group, with heating, the mother’s position is not specified | 22 |
| Eller et al. [51] | 1 | 3 | 21 | In a group, with heating, the mother’s position is not specified | 22 |
| Eskandari et al. [21] | 1 | 3 | 14 | No information is provided | 21 |
| Eskandari et al. [22] | 1 | 3 | 14 | Individual, mother in a separate room | 21 |
| Fenton-Navarro et al. [52] | 1 | 3 | 14 | Individual, with heating, mother’s position not specified | 15 |
| Rico et al. [53] | 1 | 6 | 21 | In a group, with heating, two separations per day, the mother’s position is not specified. | 22 |
| Robertson et al. [54] | 2 | 3 | 16 | In a group, with heating, the mother’s position is not specified | 21 (Ctl), 17 (MS) |
| Authors | Sequence Generation | Baseline Characteristics | Allocation Concealment | Random Housing | Blinding of Interventions | Random Outcome Assessment | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Schmidt et al. [28] | Unclear | Low | High | Low | High | High | Unclear | Unclear | Unclear | Low |
| George et al. [29] | Unclear | Low | High | Low | High | High | Unclear | Unclear | Unclear | Low |
| Loria et al. [30] | High | High | High | Low | High | High | Unclear | High | Unclear | Low |
| Maniam & Morris [31] | High | Unclear | High | Low | High | High | Unclear | Low | Unclear | Low |
| Solas et al. [32] | Unclear | Low | High | Low | Unclear | High | Unclear | Unclear | Unclear | Low |
| Viveros et al. [33] | High | Unclear | High | Low | High | High | Unclear | Unclear | Unclear | Low |
| Mela et al. [34] | High | Unclear | High | Low | High | High | Unclear | Low | Unclear | Low |
| Paternain et al. [35] | Low | Low | High | Low | High | High | Unclear | Low | Unclear | Low |
| Bernardi et al. [36] | High | Unclear | High | Low | High | High | Unclear | Unclear | Unclear | Low |
| Gehrand et al. [37] | Unclear | Low | High | Low | High | High | Unclear | High | Unclear | Low |
| Ghosh et al. [38] | Unclear | Low | High | Low | High | High | Unclear | Low | Unclear | Low |
| Ho et al. [39] | High | Unclear | High | Low | High | High | Unclear | Unclear | Unclear | Low |
| Paternain et al. [40] | Unclear | Low | High | Low | High | High | Unclear | Low | Unclear | Low |
| Vargas et al. [41] | Unclear | Low | High | Low | High | High | Unclear | Low | Unclear | Low |
| Aya-Ramos et al. [42] | Unclear | Low | High | Low | High | High | Unclear | Low | Unclear | Low |
| Maghami et al. [43] | Unclear | Low | High | Low | High | High | Unclear | Low | Unclear | Low |
| Murphy et al. [44] | High | Unclear | High | Low | High | High | Unclear | Unclear | Unclear | Low |
| Raff et al. [45] | High | Unclear | High | Unclear | High | High | Unclear | Low | Unclear | Low |
| Ruiz et al. [46] | Unclear | Low | High | Low | High | High | Unclear | Unclear | Unclear | Low |
| Ilchmann-Diounou et al. [47] | High | High | High | Low | High | High | Unclear | Unclear | Unclear | Low |
| Jaimes-Hoy et al. [48] | Unclear | Low | High | Low | High | High | Unclear | Low | Unclear | Low |
| Maghami et al. [20] | Unclear | Low | Unclear | Low | High | High | Unclear | Low | Unclear | Low |
| Roque et al. [49] | High | Unclear | High | Low | High | High | Unclear | Low | Unclear | Low |
| Eller et al. [50] | High | Unclear | High | Low | High | High | Unclear | Low | Unclear | Low |
| Eller et al. [51] | High | Unclear | Unclear | Low | High | High | Unclear | Unclear | Unclear | Low |
| Eskandari et al. [21] | Unclear | Low | High | Unclear | High | High | Unclear | Unclear | Unclear | Low |
| Eskandari et al. [22] | Unclear | Low | High | Low | High | High | Unclear | Unclear | Unclear | Low |
| Fenton-Navarro et al. [52] | Unclear | Low | High | Low | High | High | Unclear | Unclear | Unclear | Low |
| Rico et al. [53] | Unclear | Low | High | Low | High | High | Unclear | Low | Unclear | Low |
| Robertson et al. [54] | Unclear | Low | Low | Low | Unclear | High | Low | Unclear | Unclear | Low |
| Glucose | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | N Studies | SMD | 95% CI | p-Value | I2 (%) | τ2 | Subgroup p | |
| Species | Rat | 15 | −0.59 | [−0.87; −0.30] | <0.0001 | 89.2 | 0.6698 | 0.0206 |
| Mice | 4 | 1.09 | [−0.30; 2.47] | <0.0001 | 89.1 | 3.0760 | ||
| Sex | Male | 12 | −0.77 | [−1.15; −0.39] | <0.0001 | 88.4 | 0.6588 | 0.0812 |
| Female | 8 | −0.31 | [−0.67; 0.05] | <0.0001 | 86.8 | 0.4383 | ||
| Age | Infant | 1 | 3.38 | [2.20; 4.56] | 0.8207 | 0 | 0 | <0.0001 |
| Adolescent | 6 | −0.74 | [−1.13; −0.34] | <0.0001 | 90.8 | 0.6866 | ||
| Young adult | 9 | −0.14 | [−0.48; 0.19] | <0.0001 | 83.8 | 0.3099 | ||
| Adult | 5 | −0.82 | [−1.86; 0.21] | <0.0001 | 91.0 | 1.2968 | ||
| Duration of MS | Moderate | 14 | −0.65 | [−1.11; −0.19] | <0.0001 | 89.0 | 0.8466 | 0.1280 |
| Severe | 5 | −0.20 | [−0.62; 0.22] | <0.0001 | 89.6 | 0.9820 | ||
| Insulin | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | N Studies | SMD | 95% CI | p-Value | I2 (%) | τ2 | Subgroup p | |
| Species | Rat | 16 | 0.01 | [−0.32; 0.34] | <0.0001 | 86.6 | 0.7771 | 0.0224 |
| Mice | 2 | −0.45 | [−0.66; −0.24] | 0.3855 | 0 | 0.0045 | ||
| Sex | Male | 11 | −0.40 | [−0.86; 0.06] | <0.0001 | 82.7 | 0.6964 | 0.0381 |
| Female | 8 | 0.29 | [−0.18; 0.76] | <0.0001 | 81.8 | 0.5638 | ||
| Age | Adolescent | 5 | 0.12 | [−0.57; 0.81] | <0.0001 | 83.4 | 0.8611 | 0.1541 |
| Young adult | 9 | 0.21 | [−0.36; 0.78] | <0.0001 | 88.4 | 1.0375 | ||
| Adult | 6 | −0.44 | [−0.88; 0.00] | <0.0001 | 81.2 | 0.3760 | ||
| Duration of MS | Mild | 1 | −0.75 | [−1.14; −0.36] | 0.6184 | 0 | 0 | <0.0001 |
| Moderate | 17 | −0.29 | [−0.65; 0.07] | <0.0001 | 86.8 | 0.6169 | ||
| Severe | 1 | 1.01 | [0.56; 1.45] | 0.076 | 45.5 | 0.1830 | ||
| QUICKI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | N Studies | SMD | 95% CI | p-Value | I2 (%) | τ2 | Subgroup p | |
| Species | Rat | 5 | 0.89 | [0.10; 1.68] | <0.0001 | 91.4 | 0.8788 | 0.3456 |
| Mice | 1 | 0.37 | [−0.36; 1.10] | 0.0002 | 92.7 | 0.2587 | ||
| Sex | Male | 1 | 1.16 | [0.75; 1.56] | 0.4213 | 0 | 0 | 0.7385 |
| Female | 2 | 0.73 | [−1.75; 3.20] | <0.0001 | 96.5 | 3.0787 | ||
| Age | Adolescent | 2 | 0.81 | [−0.83; 2.44] | <0.0001 | 95.2 | 1.3298 | 0.8469 |
| Young adult | 3 | 0.97 | [−0.16; 2.09] | <0.0001 | 91.6 | 0.9037 | ||
| Adult | 3 | 0.52 | [−0.53; 1.57] | <0.0001 | 92.1 | 0.8068 | ||
| Duration of MS | Moderate | 6 | 0.75 | [0.14; 1.35] | <0.0001 | 90.6 | 0.6877 | NA |
| HOMA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | N Studies | SMD | 95% CI | p-Value | I2 (%) | τ2 | Subgroup p | |
| Species | Rat | 6 | 0.06 | [−0.48; 0.60] | <0.0001 | 86.7 | 0.5501 | 0.2484 |
| Mice | 1 | −0.28 | [−0.48; −0.07] | 0.2644 | 19.7 | 0.0043 | ||
| Sex | Male | 4 | −0.10 | [−0.84; 0.64] | <0.0001 | 87.9 | 0.5082 | 0.4362 |
| Female | 3 | 0.45 | [−0.72; 1.62] | <0.0001 | 91.5 | 1.0070 | ||
| Age | Adolescent | 3 | −0.17 | [−0.84; 0.50] | <0.0001 | 87.5 | 0.4252 | 0.7479 |
| Young adult | 2 | 0.65 | [−1.37; 2.68] | <0.0001 | 96.0 | 2.0527 | ||
| Adult | 2 | −0.06 | [−0.77; 0.65] | 0.0215 | 81.1 | 0.2151 | ||
| Duration of MS | Moderate | 6 | −0.03 | [−0.77; 0.72] | <0.0001 | 88.6 | 0.7987 | 0.5290 |
| Severe | 1 | 0.27 | [−0.26; 0.79] | 0.0260 | 79.8 | 0.1138 | ||
| Glucose Tolerance Test | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | N Studies | SMD | 95% CI | p-Value | I2 (%) | τ2 | Subgroup p | |
| Species | Rat | 4 | −1.01 | [−1.22; −0.80] | 0.4867 | 0 | 0 | 0.4632 |
| Mice | 1 | −1.49 | [−2.74; −0.24] | – | NA | NA | ||
| Sex | Male | 4 | −1.14 | [−1.40; −0.87] | 0.7522 | 0 | 0 | 0.0977 |
| Female | 1 | −0.72 | [−1.14; −0.29] | – | NA | NA | ||
| Age | Adolescent | 1 | −1.08 | [−1.64; −0.51] | – | NA | NA | 0.5634 |
| Young adult | 2 | −0.96 | [−1.23; −0.69] | 0.2683 | 23.9 | 0.0085 | ||
| Adult | 2 | −1.28 | [−1.81; −0.75] | 0.7262 | 0 | 0 | ||
| Duration of MS | Moderate | 5 | −1.02 | [−1.23; −0.82] | 0.5522 | 0 | 0 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Orozco, L.; Rivadeneira, J.; Vásquez, B. Impact of Early Postnatal Maternal Separation Stress on Pancreatic Function in Rodents: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 9927. https://doi.org/10.3390/ijms26209927
García-Orozco L, Rivadeneira J, Vásquez B. Impact of Early Postnatal Maternal Separation Stress on Pancreatic Function in Rodents: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(20):9927. https://doi.org/10.3390/ijms26209927
Chicago/Turabian StyleGarcía-Orozco, Laura, Josue Rivadeneira, and Bélgica Vásquez. 2025. "Impact of Early Postnatal Maternal Separation Stress on Pancreatic Function in Rodents: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 20: 9927. https://doi.org/10.3390/ijms26209927
APA StyleGarcía-Orozco, L., Rivadeneira, J., & Vásquez, B. (2025). Impact of Early Postnatal Maternal Separation Stress on Pancreatic Function in Rodents: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(20), 9927. https://doi.org/10.3390/ijms26209927



.jpg)



