Abstract
Mannose is a natural monosaccharide that plays a central role in host–pathogen interactions and has emerged as a versatile scaffold for designing anti-infective agents. This review summarizes recent advances in mannose-based glycoconjugates with antibacterial, antiviral, antifungal, and antiparasitic activity. In bacteria, FimH antagonists prevent Escherichia coli adhesion, while mannose-functionalized materials disrupt Pseudomonas and Burkholderia biofilms or enhance delivery of anti-tubercular drugs. In virology, mannose-containing dendrimers, glycopolymers, and nanoparticles inhibit HIV, SARS-CoV-2, Ebola, HPV, and HSV by targeting viral glycoproteins or blocking lectin-mediated transmission. Mannose-decorated vaccines and nanocarriers also show promise against fungal pathogens and parasites. Continued optimization of presented structures could lead to the promising candidates for clinically applicable therapies.
1. Introduction
Carbohydrates serve essential roles such as modifying biomolecules for specific functions, mediating metabolic processes, acting as biosynthetic intermediates, and most notably, supporting energy generation and storage. Their covalent linkage to proteins provides a structural scaffold necessary for proper protein function. Carbohydrate-based modifications are crucial for processes such as cell–cell communication and adhesion, protein conformation, membrane architecture, and intracellular signaling [1].
Carbohydrate-binding agents (CBAs) exploit these glycan structures to modulate or interfere with biological interactions, making them valuable tools in both therapeutic and diagnostic applications. CBAs refer to natural or synthetic molecules that bind specifically to carbohydrates (sugars) or glycan structures on biomolecules, enabling them to block host–pathogen interactions, label cells, or activate immune responses. Main classes of CBA are lectins, antibodies and synthetic CBAs like glycomimetics [2,3].
d-Mannose is a naturally occurring hexose sugar (Figure 1), primarily found in plant cell walls as a component of mannan. It plays a critical role in glycoprotein synthesis, contributing to immune regulation, wound healing, antimicrobial and antitumor activities [4]. Recent studies highlight mannose’s potential in immunoregulation, suppressing autoimmune and inflammatory conditions. Additionally, mannose-based delivery systems have been developed for targeted therapies [5].
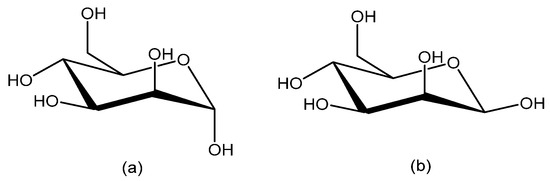
Figure 1.
d-Mannose in cyclic forms; (a) α-mannopyranose and (b) β-mannopyranose.
Since mannose is a key sugar involved in microbial recognition in humans, this review focuses on glycoconjugates containing mannose (mannoconjugates) that act on biological targets exhibiting antiviral, antibacterial, antifungal, and antiparasitic activities. The goal is to consolidate the most significant recent findings related to mannoconjugates as anti-infective agents, highlighting their emerging potential as drug candidates. Emphasis is placed on mannose-targeted pathways, which are receiving growing attention due to their biological and clinical relevance. Glycodendrimers are a notable category of synthetic macromolecules designed to replicate various structural and functional aspects of cell-surface glycoconjugates. The carbohydrate components of these molecules play crucial roles in the processes of bacterial and viral infections, primarily through carbohydrate–protein interactions [6]. Research has demonstrated that the molecular architecture, valency, and spatial arrangement of carbohydrate epitopes significantly influence the specificity and strength of these interactions. Selecting appropriate glycodendrimers can effectively disrupt these interactions, thereby preventing bacterial or viral adhesion and entry into host cells—an approach that offers a promising means of infection control. This review highlights recent advances in the design of glycodendrimers containing mannose developed for anti-infective therapies [7].
2. Antibacterial Activities
2.1. Escherichia coli
Recurrent urinary tract infections (UTIs) represent a significant clinical challenge, particularly among adult women, due to their high prevalence and the limitations associated with antibiotic prophylaxis, including antimicrobial resistance and adverse effects [8]. The widespread reliance on antibiotics as first-line therapy further exacerbates the problem by driving the emergence of antimicrobial resistance, with Escherichia coli constituting a major contributor to the global antimicrobial resistance burden [9]. Consequently, there is a growing demand for novel strategies to prevent and treat UTIs, among which anti-adhesion therapy has emerged as one of the most promising approaches [10,11].
Recent systematic reviews and meta-analyses have evaluated the efficacy of d-mannose, as a non-antibiotic option for preventing and managing UTIs, particularly those caused by uropathogenic E. coli (UPEC). d-mannose significantly lowers the risk of recurrent UTI compared to placebo and shows similar efficacy to commonly used antibiotics like nitrofurantoin [12]. Importantly, d-mannose exhibits a favorable safety profile, with less adverse events reported in comparison to antibiotics. These findings position d-mannose as an effective and well-tolerated prophylactic agent in UTI management, contributing to reduced antibiotic consumption and helping combat the global challenge of antimicrobial resistance [13].
The pathogenesis of UTIs caused by E. coli critically depends on the bacterium’s ability to adhere to the epithelial cells lining the urinary tract. Namely, the initial step in UTI pathogenesis involves the binding of bacterial surface-associated proteins, known as adhesins, to complementary receptors on eukaryotic cells. These adhesins are often presented on filamentous surface structures termed pili or fimbriae. In UPEC, the most prevalent adhesive organelles include type 1, P, S, and F1C fimbriae [10]. Type 1 pili are complex protein assemblies consisting of a linear tip fibrillum at its distal end, composed of the mannose-binding adhesin FimH together with the adaptor subunits FimG and FimF. This fibrillum is anchored to a helical rod made up of several hundred to several thousand FimA subunits, with assembly terminated by a single FimI subunit at the proximal end and capped by the chaperone FimC. The outer membrane usher protein FimD serves as the assembly platform and orchestrates pilus biogenesis [14].
FimH is composed of the N-terminal lectin-binding domain (FimHLD) encompassing the carbohydrate-binding domain (CBD) which recognizes mannose residues on host cell glycoproteins, and C-terminal pilin domain (FimHPD). FimHLD goes through the conformational changes with two endpoint conformations; an “open” conformation that binds mannose weakly and a “closed” conformation that binds with a high affinity [15]. The pilin domain fine-tunes this switch, usually holding the lectin domain in the low-affinity state under static conditions. When mannose derivatives are encountered, however, the lectin domain shifts into its tightly binding form, increasing its affinity up to 100,000-fold [16]. Natural ligand for FimH is a heavily mannosylated oligosaccharide (Man9GlcNAc2). Because this molecular system is central to bacterial adhesion and colonization, FimH has become a prime focus for developing anti-adhesives and vaccines aimed at preventing UTIs [17]. FimH is also crucial factor in the pathogenicity of adherent-invasive E. coli (AIEC). By binding to mannosylated receptors such as CEACAM6 on the intestinal epithelium, FimH enables AIEC to strongly adhere to and colonize the gut mucosa. This interaction promotes bacterial invasion, persistence, and the induction of inflammatory responses characteristic of Crohn’s disease. Because of its central role in AIEC adhesion, FimH is considered a promising therapeutic target for strategies aimed at preventing or treating AIEC-driven intestinal inflammation [18].
Research into FimH antagonists began decades ago. In 1977, Ofek, Mirelman, and Sharon provided a detailed description of mannose’s inhibitory effects on E. coli [19]. Since then, significant progress has been made in designing both mono-glycomimetics and multivalent glycomimetic constructs that exhibit enhanced binding affinity and specificity for FimH. α-d-Mannopyranosides with hydrophobic aglycons are potent FimH inhibitors [10,20,21]. Simple α-d-mannopyranoside, n-heptyl mannoside 1, exhibits strong binding with a KD of 5 nM [22], while simple aryl mannosides displayed weaker binding, among them ortho- and meta-substituted derivatives showed higher potency than their corresponding para analogues.
Namely, based on co-crystal structures of E. coli FimH in complex with mannopyranoside antagonists, researchers identified mannopyranoside derivatives with hydrophobic aglycons as promising candidates due to their strong binding affinity [23,24,25]. This conclusion was drawn from the hydrophobic interactions observed within CRD of FimH. In particular, interactions involving Tyr-48, Tyr-137, and Ile-52 residues from the “tyrosine gate” within the active binding site [25]. Therefore, addition of a second aryl ring increases hydrophobic and π-stacking interactions within the tyrosine gate, as shown in representing O-mannosides 2,4 [26], 3 [27] (Figure 2). Researchers have also explored C-, N-, and S-mannosides because a common limitation of O-mannosides is their poor bioavailability due to the instability of the O-glycosidic bond. C-mannosides 5 [28], and GSK3882347 6 showed great potential and it should be highlighted that GSK3882347 6 was in clinical trial for UTI [29].
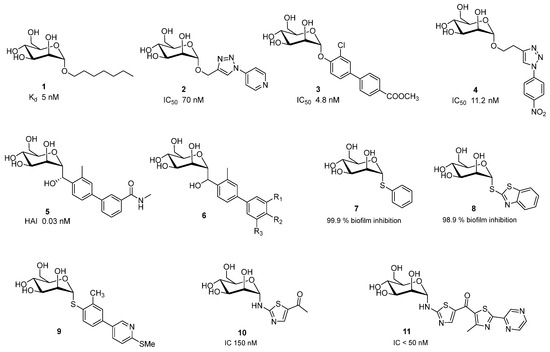
Figure 2.
Potent monovalent FimH antagonist.
Abdu-Allah et al. also evaluated thiomannosides and showed that their activity was primarily influenced by the nature of the aryl or heteroaryl aglycone, and that compounds 7 and 8 (Figure 2) had superior affinity and inhibitory effects then the reference ligand 1 [30].
Nishi et al. have prepared and evaluated thiomannoside 9 with improved metabolic stability and plasma exposures, and potential to inhibit biofilm formation and also to disrupt the preformed biofilm [31].
Gouin et al. showed that thiazolylaminomannosides 10 and 11 (Figure 2) have nanomolar affinity for FimH. Especially, compound 11 which have high capacity of to prevent AIEC adhesion in vitro and ex vivo compared to 1 and α-d-mannose [26]. Second generation of homologated C-mannosides with an NH group exhibited anti-adhesive effect lower than the first class of N-linked mannosides [32].
Structures harboring di-, tri-, hepta- and dendrimetic α-mannopyranosides 12–14 shown in Figure 3 are also very potent FimH antagonists [21], while the divalent C-linked mannopyranoside EB8018/Sibofimloc 12 which entered Phase IIa clinical trials against Crohn’s disease [33].
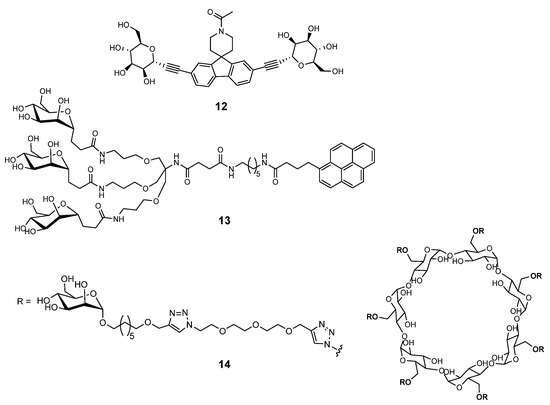
Figure 3.
Multivalent FimH antagonists.
In general, small-molecule inhibitors have been optimized to improve oral bioavailability, metabolic stability, and target selectivity, showing promising anti-adhesive properties in vitro and in vivo models. Multivalent glycomimetics, such as dendrimers and nanoparticles functionalized with mannose residues, demonstrate multivalent interactions with bacterial lectins, greatly increasing their inhibitory potency against E. coli adhesion and biofilm formation [34,35]. These constructs not only improve bacterial clearance but also reduce the risk of resistance development by blocking bacterial colonization rather than killing the bacteria directly. Continued efforts in refining molecular design and delivery methods are essential to advance these agents toward clinical application, offering alternatives to conventional antibiotics and addressing rising antimicrobial resistance [21].
2.2. Pseudomonas aeruginosa and Burkholderia cepacia Complex
Chronic lung colonization by opportunistic pathogens such as P. aeruginosa and members of the B. cepacia complex (Bcc) remains a leading contributor to illness and death in cystic fibrosis patients.
2.2.1. Pseudomonas aeruginosa
Infections caused by P. aeruginosa, particularly in hospitalized and cystic fibrosis patients, are notably difficult to treat due to the bacterium’s ability to form antibiotic-resistant biofilms. P. aeruginosa employs various adhesins, including LecA (PA-IL) and LecB (PA-IIL) lectins, type VI pili, and flagella, as well as iron acquisition systems to establish infection and form protective biofilms. The lectin LecB has been identified as a key factor in biofilm formation, and its inhibition via specific carbohydrate ligands has been shown to reduce biofilm accumulation. LecB is a tetrameric, soluble C-type lectin with affinity for l-fucose, d-mannose, and glycans presenting terminal fucosyl or mannosyl residues. The crystal structure of LecB in complex with fucose, mannose and fructopyranose was determined [36]. A defining characteristic of the carbohydrate-binding site is the presence of two Ca2+ ions. They stabilize the conformation of loop Asn95-Asp104, which forms the core of the carbohydrate-binding site. Upon binding of a monosaccharide, these ions form direct interactions with three hydroxyl groups of the sugar [36]. To unravel the molecular basis of this selectivity, a series of glycomimetic derivatives were designed. Structural and thermodynamic analyses revealed that perturbation of the hydrogen bonding network in mannosides contributes to their lower binding affinity [37].
Extensive research efforts have been directed on developing glycoconjugate-based inhibitors that target LecB lectin [38]. Titz et al. identified d-mannose derivatives bearing sulfonamide 15 and amide 16 substituents at C6 (Figure 4) as potent LecB inhibitors, effectively blocking bacterial adhesion with up to a 20-fold increase in affinity to LecB compared to the natural ligand methyl mannoside. Amide 16 is weaker LecB binder (IC50 37 μM) then compound 15 (3.4 μM) because of dual binding contributions of sulfonamide group; (i) coordination of two to LecB Ca2+ ions with O4 and O5, and (ii) H-bond formed between the sulfonamide and Asp96, in addition to the lipophilic interaction of the aryl group with the binding pocket surface [39]. To optimize cinnamide inhibitors, over 20 derivatives were synthesized with varied aromatic substitutions. SAR analysis revealed that ortho substitutions reduced potency, likely due to steric clash, while meta and para lipophilic groups significantly improved activity. Polar substituents consistently weakened binding, confirming a strong preference for hydrophobic interactions within the LecB pocket. Methoxy emerged as the optimal substituent, and the dimethoxy derivative 17 was the most potent compound (IC50 = 19.9 µM). [40]
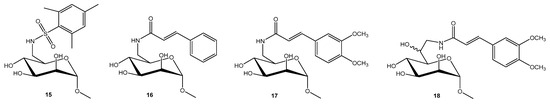
Figure 4.
Potent monovalent LecB inhibitors.
Furthermore, mannoheptose derivatives bearing cinnamide and sulfonamide groups were prepared by elongating the sugar backbone, with the aim of liberating the O6 position for potential hydrogen bonding with Ser23. However, compounds such as 18 did not exhibited improved affinity, even though (6S)-mannoheptose derivatives demonstrated higher inhibitory activity than methyl α-d-mannoside [41].
FThe fucosylated mannose-centered glycocluster also demonstrated good binding activity (in the low micromolar range). Antenna-like scaffolds were preferred over linear or crown-like architectures, and within crown-shaped carbohydrate-centered fucosylated glycoclusters, mannose-based cores outperformed those built on glucose or galactose [42,43].
Biofilms present a major barrier to effective treatment of bacterial infections, shielding pathogens from both host immune responses and antibiotic therapies. This protective matrix enables chronic colonization and contributes significantly to antimicrobial resistance. One strategy for eliminating biofilm involves glycan-targeted polymers that selectively bind biofilm structures. Using a continuous-flow biofilm model with P. aeruginosa PAO1 strain, mannose-functionalized fluorescent polymers exhibited stable and prolonged association with biofilms for over 24 h. Genetic knockout studies further revealed that both the lectin LecB and the adhesin CdrA contribute to polymer retention, highlighting their role in the polymer–biofilm interaction [44].
Another approach is targeting iron uptake while simultaneously interfering with adhesin functions. O’Toole and Mo Benazza with coworkers designed an glycocluster tetrahydroxamic acid iron-chelating calixarene (Figure 5) that disrupts both iron sequestration and glycan-mediated adhesion [45]. In the fluorescence polarization competition assays monovalent mannose ligands (methyl α-d-mannopyranoside (IC50 157 μM) and compound 19 (146 μM) displayed weak affinity relative to fucose analogues, while the mannose-capped multivalent cluster 20 exhibited a dramatic enhancement in binding (IC50 0.66 μM). In contrast, the fucose-based cluster showed diminished potency upon multimerization. Surprisingly, the mannocluster outperformed the fucocluster, despite L-fucose being LecB’s natural ligand. The data suggest a chelating binding mode as the likely origin of this unexpected mannose-driven affinity enhancement [45].
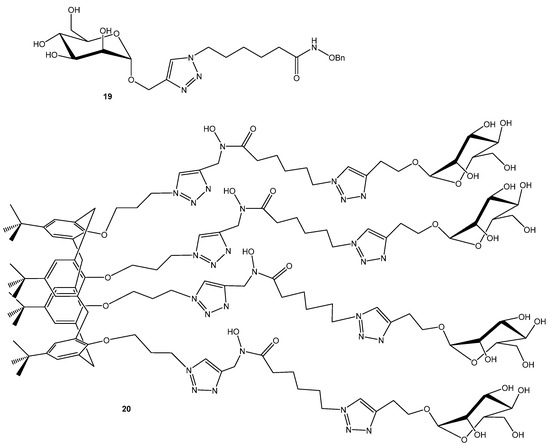
Figure 5.
Multivalent LecB inhibitor.
2.2.2. Burkholderia cepacia Complex
Burkholderia cenocepacia is one of the most dangerous in the B. cepacian complex (Bcc). It is a Gram-negative, rod-shaped bacterium commonly found in soil and water but is also an opportunistic pathogen. In cystic fibrosis patients, it can cause chronic lung infections, and in severe cases, a fatal condition known as “cepacia syndrome”. The bacterium is highly resistant to antibiotics and capable of forming biofilms, making infections difficult to treat. B. cenocepacia produces three soluble lectins; BC2L-A, BC2L-B, and BC2L-C. Each have sequence similarity with PA-IIL. The smallest member of this family, BC2L-A, exhibits mannose specificity [46,47]. Each monomer contains a single CRD that coordinates two Ca2+ ions directly involved in ligand binding. Crystallographic analyses with methyl-α-d-mannopyranoside (αMeMan) [46] and a branched trimannoside (αMan1-3(αMan1-6)Man) [47] revealed that the high affinity results from the coordination of three mannose hydroxyl groups (O-2, O-3, and O-4) by the two Ca2+ ions in the binding site. Trisaccharide (αMan1-3(αMan1-6)Man 21 (Figure 6) was tested by titration microcalorimetry in order to evaluate their affinity for BC2L-A in solution, along with disaccharides (αMan1-2Man, αMan1-3Man, αMan1-6Man), and synthetic mono- and dimannnosides. Natural dimannosides showed only modest variation in LecB affinity (αMan1-3Man was the strongest (Kd = 2.6 μM)). In contrast, synthetic mono-mannoside 22 and dimannoside with flexible linker 23 behaved similarly to αMan1-3Man, with moderate affinity (Kd = 2.8 μM). The rigid analogue 24 exhibited substantially enhanced affinity (Kd = 220 nM) and a stoichiometry of ~0.5, indicating simultaneous engagement of both mannose units via a bridging mode. Thus, ligand rigidity strongly promotes multivalent binding and affinity enhancement [47].
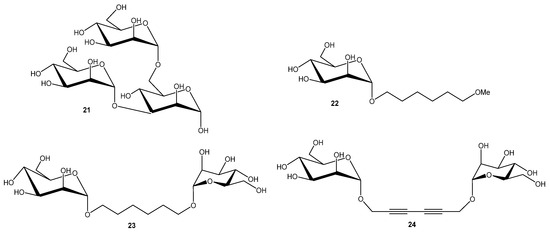
Figure 6.
BC2L-A ligands.
Marchetti with coworkers have demonstrated that revealed that a manno configuration bearing a hydroxyl or glycol group at C6 is essential for BC2L-A recognition [48].
To exploit the cluster glycoside effect, multivalent mannoside derivatives were also developed. Since replacing the glycosidic oxygen with sulfur increases resistance to chemical and enzymatic hydrolysis, Wimmerová, Borbás and co-workers synthesized multivalent thiomannosides (Figure 7) with an (α1→2)-thio-linked mannobioside mimic functionalized with an azide-bearing aglycone, which was subsequently conjugated to various multivalent scaffolds via copper-catalyzed azide–alkyne cycloaddition. The methyl gallate-based cluster 25 and pentaerythritol-based glycoclusters 26 showed only slightly higher inhibition potency than αMeMan (relative potency 1.3), whereas compound 14 exhibited more than double the activity [49].
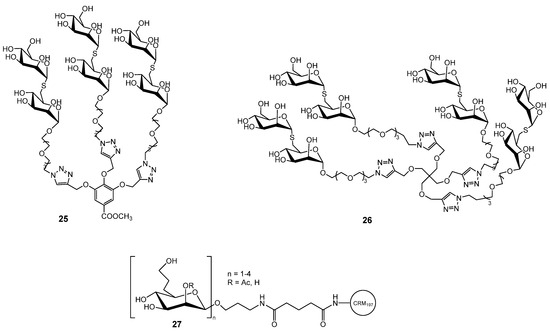
Figure 7.
Multivalent mannose derivatives explored against B. cenocepacia.
Multivalent mannose derivatives were also evaluated for their potential application in anti-B. pseudomallei and anti-B. mallei vaccines. 6-Deoxy-d-manno-heptopyranose and its β-(1→3)-linked oligomers 27 (Figure 7), polysaccharides common to B. pseudomallei and B. mallei, were prepared. Synthetically, the most challenging task was the preparation of β-mannosidic linkages within the oligosaccharides, which was achieved with high stereoselectivity through hydrogen-bond-mediated aglycone delivery. They were used for subsequent conjugation to the carrier protein CRM197, enabling immunological evaluation. These glycoconjugates elicited strong IgG antibody titers and robust T cell–dependent immune responses in mice [50]. Another example of mannose targeted delivery system is amphiphilic diblock copolymer, poly(ethylene oxide)-b-poly(ε-caprolactone) (PEO-b-PCL), functionalized with a terminal mannose group. The mannose moiety was subsequently introduced via quaternization of the PEO chain end with a brominated mannose derivative. These amphiphilic copolymers self-assembled into uniform spherical micelles with the mannose units on the micelle surface that served for the binding of BC2L-A lectin. The binding thermodynamics confirmed selective interaction, highlighting the potential of these micellar systems in targeted drug delivery and vaccine applications [51].
2.3. Mycobacterium tuberculosis
The global health threat posed by tuberculosis (TB) has been intensified by the emergence of multidrug-resistant strains of M. tuberculosis (Mtb) and a long-standing stagnation in the discovery of novel mycobactericidal agents. A key factor underlying the persistence and resilience of Mtb is its unusually impermeable and polysaccharide-rich cell wall, a complex structure that plays a critical role in virulence and drug resistance. Despite its importance, many aspects of mycobacterial cell wall biosynthesis remain incompletely understood. Among the enzymes involved in cell wall construction is glucosyl-3-phosphoglycerate synthase (GpgS), a retaining glycosyltransferase found in Mtb and related species. GpgS is unique in that it belongs to the newly defined GT-81 family and shares low sequence similarity with previously characterized glycosyltransferases. Functional studies have confirmed its essentiality for Mtb survival, highlighting it as a potential drug target. The three-dimensional representations of GpgS, including the apo form and its ternary complex with UDP and 3-phosphoglycerate, was resolved by X-ray crystallography [52]. Importantly, the structural data illuminate the enzyme’s specificity for UDP-based donor substrates and its preference for glucose over mannose, while identifying key residues responsible for acceptor substrate recognition [53,54].
A severe extrapulmonary manifestation of Mtb infection, presents unique therapeutic challenges. Current treatment protocols rely on prolonged administration (8–20 months) of first- and second-line anti-tubercular drugs, typically delivered orally or intravenously at high doses due to limited bioavailability. The long-term multidrug regimen required for TB treatment is often accompanied by the emergence of drug-resistant Mtb strains [55]. Nanocarrier-based drug delivery systems offer notable advantages, including improved bioavailability, reduced systemic toxicity, and lower dosing frequency, which collectively contribute to better patient compliance [56]. However, passive targeting through nanoformulations alone often lacks sufficient specificity. To address this limitation, active targeting strategies have emerged, where nanocarriers are conjugated with ligands that bind selectively to receptors or cellular components of macrophages, the primary host cells for Mtb, or to structures unique to the mycobacterial cell wall [57]. Ligand-mediated targeting not only improves drug accumulation at intracellular sites of infection but can also enhance adhesion to mucosal surfaces such as alveolar or intestinal epithelium, especially through interactions with lectin-binding domains. A wide array of ligands has been investigated for this purpose, including mannose, mycolic acid, lectins, and aptamers, each offering distinct advantages in directing drug-loaded nanocarriers to desired biological targets [58].
Polymeric nanoparticles, particularly those composed of biodegradable materials like chitosan, are readily internalized by macrophages through opsonization and phagocytosis. Targeting can be further enhanced by surface modification with ligands such as mannose, which exploits receptor-mediated uptake mechanisms specific to macrophages. In recent work, an intra-articular injectable, in situ gelling system incorporating mannose-conjugated chitosan nanoparticles was developed for localized and sustained delivery of anti-tubercular drug rifampicin [59].
Another promising strategy for targeted delivery of rifampicin involves the use of mannose-anchored solid lipid nanoparticles (SLNs). Antimicrobial assays revealed a notable enhancement in activity, with MICs reduced 4-fold and 8-fold against wild-type and drug-resistant Mycobacterium smegmatis, respectively, compared to free rifampicin. Fluorescently labeled SLNs (loaded with coumarin-6) further demonstrated improved macrophage uptake following mannose conjugation, confirming receptor-mediated internalization. Importantly, mannose-rifampicin-SLNs exhibited superior intracellular killing of M. tuberculosis H37Ra within macrophages, outperforming both non-functionalized SLNs and free rifampicin [60].
2.4. Methicillin-Resistant Staphylococcus aureus (MRSA)
Methicillin-resistant S. aureus (MRSA) is a leading cause of osteomyelitis. The clinical management of hospital-acquired chronic osteomyelitis caused by MRSA is hindered by two major obstacles: poor drug penetration into deep tissues and the rapid onset of an immunosuppressive microenvironment. MRSA has the ability to evade immune detection and impair both innate and adaptive immune responses [61]. To enhance antibiotic efficacy and eliminate intracellular bacteria, researchers developed different antibiotic delivery system targeting macrophages. The mannose-modified nanotherapeutic system co-delivering Zn2+ and vancomycin (Van) demonstrated potent antibacterial activity against both extracellular and intracellular MRSA, significantly lowering the minimum inhibitory concentration (MIC) of vancomycin. In vivo studies using a murine osteomyelitis model confirmed that Man-Zn2+/Van nanoparticles effectively reduced bacterial burden, restored normal gait, enhanced bone regeneration, and suppressed pro-inflammatory cytokine levels. Mechanistically, the nanotherapeutic acts through multiple antibacterial pathways, including disruption of the MRSA cell membrane, degradation of intracellular proteins and DNA, suppression of glycolysis, and interference with bacterial energy metabolism [62].
Another example is nanovesicle-based system that integrates macrophage-derived microvesicles with an M1 pro-inflammatory phenotype (M1-MW), which encapsulate vancomycin-crosslinked micelles containing the sonosensitizer indocyanine green (termed VCG micelles). The surface of M1-MW was modified with PEGylated mannose, enabling targeted delivery to infected tissues. In the reductive microenvironment typical of infections, these micelles release indocyanine green, which produces reactive oxygen species (ROS) upon ultrasound exposure, enhancing bacterial killing in conjunction with vancomycin. In an osteomyelitis mouse model, treatment with this platform led to improved survival, effective bacterial clearance, and reprogramming of macrophages toward the M1 phenotype [63].
Wang and He with coworkers described exosome-based antibiotic delivery system composed of mannosylated exosomes which are preferentially internalized by macrophages, loaded with lysostaphin (MExoL) and vancomycin (MExoV) [64]. The combined use of MExoL and MExoV effectively eradicated dormant intracellular MRSA. Furthermore, following intravenous administration, mannosylated exosomes rapidly accumulated in the liver and spleen, primary sites of intracellular MRSA infection, highlighting their organ-targeting capability [64].
2.5. Other Bacteria
Studies also highlight the potential of mannose-rich compounds in modulating bacterial adhesion and biofilm formation of Clostridium difficile and Salmonella [65,66]. In the C. difficile study, mannose, along with fructooligosaccharides, was shown to significantly inhibit bacterial adhesion to human epithelial cells, particularly across different strains. However, at sub-inhibitory concentrations, both fructooligosaccharides and mannose unexpectedly promoted biofilm formation, illustrating the complex, context-dependent effects of prebiotics on bacterial behavior [66]. In contrast, the mannose-rich oligosaccharides prevented bacterial adhesion of Salmonella to intestinal epithelial cells by binding to mannose-specific lectins on Salmonella. Moreover, mannose-rich oligosaccharides exhibited immunomodulatory effects, reducing inflammation and regulating energy metabolism in a chicken model of systemic inflammation induced by Salmonella [65]. Although these studies did not identify the exact lectin targeted by mannose structures, they emphasize the potential of mannose-containing compounds as non-antibiotic strategies for mitigating bacterial infections by targeting bacterial adhesion.
Due to the stagnation in antibiotic discovery and the rise of multidrug resistance, Helicobacter pylori-associated gastric infections have become increasingly difficult to treat. As an alternative therapeutic approach, chitosan was functionalized with mannose via reductive amination, followed by ionic gelation to produce mannose-functionalized chitosan nanoparticles (Man-CS-Nps). Molecular docking and molecular dynamics (MD) simulations were performed targeting H. pylori lectin, a protein implicated in bacterial adhesion, biofilm formation, and cytotoxicity. The antibacterial potential of Man-CS-Nps was evaluated through time-kill assays, polystyrene adherence tests, and antibiofilm studies, demonstrating significant anti-adhesion and biofilm-disrupting effects against resistant H. pylori strains [67].
2.6. Mannose-Containing Antibiotics
In clinical use for serious Gram-positive infection are natural glycopeptide teicoplanin and semi-synthetic lipoglycopeptides dalbavancin and oritavancin. Teicoplanin from Actinoplanes teichomyceticus, targets cell wall synthesis (d-Ala-d-Ala binding). It is effective in the treatment of Gram-positive infections (skin, bone, endocarditis), as an alternative to vancomycin [68]. Dalbavancin is derived from teicoplanin with dual mechanisms of action: It blocks bacterial cell wall synthesis and anchors to cell membranes. Compared to earlier glycopeptide, it shows enhanced activity against Gram-positive bacteria and has an extended half-life of about one week, persisting even longer in tissues such as skin and bone than in plasma [69]. Oritavancin also related to teicoplanin and dalbavancin, is approved for acute bacterial skin and skin structure infections [70].
Mannose-containing antibiotics which were studied in preclinical/experimental settings are actaplanin, ramoplanin, and mannopeptimycins. Actaplanin (A4696) is a broad-spectrum Gram-positive antibiotic complex produced by Actinoplanes missouriensis. It consists of several related actaplanins (A, B1, B2, B3, C1, G), all sharing the same peptide core and an amino sugar, but differing in the amounts of glucose, mannose, and rhamnose attached [71]. Ramoplanin is a lipoglycopeptide antibiotic complex derived also from Actinoplanes sp. It consists of three related polypeptides featuring chlorinated phenyl groups and d-mannose [72]. Mannopeptimycins are cyclic glycopeptide antibiotics isolated from Streptomyces hygroscopicus, consisting of a heptapeptide core decorated with mannose. Their mode of action includes binding lipid II, a key precursor in bacterial cell wall biosynthesis, thereby blocking peptidoglycan formation [73].
4. Antiparasitic Activities
Glycosylphosphatidylinositols (GPIs) are complex glycolipids located on the surface of Plasmodium parasites and are believed to act as toxins during malaria infection. These molecules can trigger immune responses and stimulate the production of anti-GPI antibodies that help neutralize their harmful effects. As a result, vaccines targeting GPIs through glycoconjugate formulations may offer protection against malaria-related disease. To examine the impact of three specific structural features of Plasmodium GPIs, Lepenies and Varón Silva with co-workers synthesized six distinct GPI fragments from Plasmodium falciparum. These fragments were linked to the CRM197 carrier protein and assessed for their ability to provoke immune responses and provide protection in a cerebral malaria mouse model (C57BL/6JRj). The level of protection appears to be influenced by both antibody-mediated and cellular immune responses, which in turn are shaped by factors such as glycan orientation, mannose content, and the inclusion of phosphoethanolamine and inositol groups [109].
In Entamoeba histolytica, the antiretroviral lectin cyanovirin-N specifically binds α-1,2-linked mannose residues on surface N-glycans, causing aggregation and capping of key glycoproteins including the Gal/GalNAc adherence lectin and O-phosphodiester-linked glycoproteins. This capping inhibits phagocytosis, a crucial virulence mechanism. Mass spectrometry of lectin-enriched proteins identified known virulence factors and numerous previously uncharacterized, abundant glycoproteins, many of which are unique and promising vaccine candidates [110].
5. Antifungal Activities
Fungal infections pose a health challenge, compounded by limited therapeutic options and rising drug resistance. Candida albicans is a common commensal organism capable of causing severe infections, particularly in immunocompromised patients.
Poláková et al. evaluated antifungal activities of selected alkyl and (thio)dodecyl hexopyranosides based on d-glucose, d-galactose, N-acetyl d-glucosamine, and d-mannose. Treatment of C. albicans biofilms with (thio)alkyl glycosides led to a marked reduction in fungal cell proliferation, with activity dependent on glycoside structure and concentration. The relative potency of the tested derivatives against the azole-sensitive strain (GalOC12 > GlcOC12 ≈ ManOC12 > GlcNAcOC12) differed from that observed for the multiazole-resistant strain (GlcOC12 > ManOC12 ≈ GalOC12 > GlcNAcOC12) [111].
C. albicans strains can be classified into serotypes A and B, and further distinguished by antigenic factors 1, 4, 5, 6, and 13b. Most of these antigenic factors are composed of β-mannan chains, which differ in how they are attached to the α-mannan side chains of the cell wall. Consequently, the (1→2)-β-mannans located at the nonreducing ends of phosphomannan oligosaccharide side chains represent major antigenic determinants [112]. A minimal β-mannan disaccharide epitope from C. albicans that can elicit protective antibodies was identified and it was confirmed that a disaccharide or trisaccharide is sufficient for immune recognition and protection. Therefore, simple disaccharide or trisaccharide conjugates were linked to tetanus toxoid (TT) or bovine serum albumin (BSA) (Figure 12) [113].
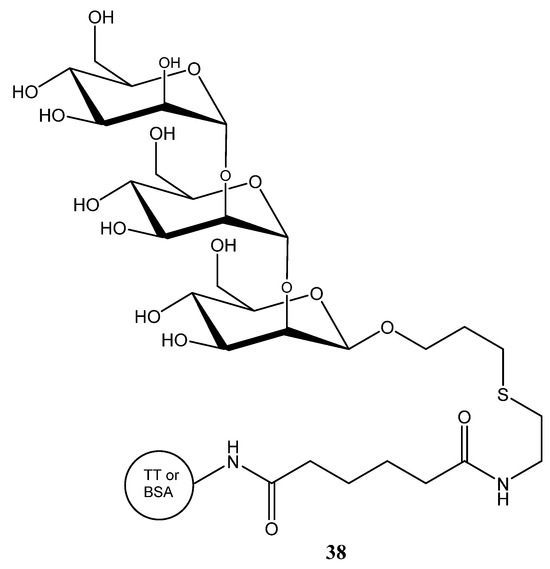
Figure 12.
Trisaccharide conjugates used for the development of antifungal vaccines.
Furthermore, peptide-based glycoconjugates incorporating known Candida T-cell epitopes were prepared [114], including a self-adjuvanting tricomponent vaccine linking β-mannan, T-cell peptide, and TT, which conferred protection in mice without added adjuvant [114]. Immunization in animal models demonstrated that these vaccines reduced fungal burden and improved survival. Notably, the combination of glycan and peptide (targeting both B and T cell responses) proved particularly effective [112].
Recently, Krylov and Nifantiev with coworkers have investigated two monoclonal antibodies, CM532 and FG70, which specifically recognize oligosaccharide fragments of fungal polysaccharides mannan and β-(1→3)-glucan, characteristic markers of fungal pathogens including C. albicans. CM532, developed by immunization with a pentamannoside conjugate, selectively binds a specific trisaccharide epitope in mannan. FG70, raised against a heptaglucan conjugate, interacts with a linear β-(1→3)-linked pentaglucoside fragment; branching in the epitope does not significantly affect this binding. These findings suggest that CM532 and FG70 have potential use for developing effective diagnostic tools for fungal infections, which are currently lacking [115].
6. Conclusions
Mannose represents a powerful and versatile building block in the development of next-generation anti-infective agents. Through its ability to engage both microbial adhesins and host lectin receptors, mannose-based conjugates have shown efficacy across a wide range of pathogens, including bacteria, viruses, fungi, and parasites. Advances in glycomimetic chemistry, nanotechnology, and vaccine design have expanded the scope of mannoconjugates from prophylactic anti-adhesion agents to targeted drug delivery systems and immunotherapeutics. Future efforts should focus on optimizing the structural features, multivalency, and pharmacokinetic properties of mannoconjugates, as well as validating their efficacy in clinically relevant models. Taken together, mannoconjugates hold substantial promise as innovative tools to address persistent challenges in infectious disease therapy and to contribute to the global fight against antimicrobial resistance.
Funding
This research was funded by University North, grant number UNIN-BIOMED-25-1-2.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AZTMP | Azt 5′-Monophosphate |
| BH30 | Boltornh30 |
| BSA | Bovine Serum Albumin |
| CBA | Carbohydrate-Binding Agents |
| DC | Dendritic Cells |
| DC-SIGN | Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-Integrin |
| GPI | Glycosylphosphatidylinositol |
| HIV | Human Immunodeficiency Virus |
| HSA | Human Serum Albumin |
| IC50 | half-maximal inhibitory concentration |
| Man | Mannose |
| αMeMan | methyl α-D-mannopyranoside |
| MIC | minimum inhibitory concentration |
| Mtb | Mycobacterium Tuberculosis |
| PEG | Polyethylene Glycol |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SLN | Solid Lipid Nanoparticles |
| Tb | Tuberculosis |
| TT | Tetanus Toxoid |
| UPEC | Uropathogenic Escherichia Coli |
| UTI | Urinary Tract Infection |
References
- Tian, M.; Li, X.; Yu, L.; Qian, J.; Bai, X.; Yang, J.; Deng, R.; Lu, C.; Zhao, H.; Liu, Y. Glycosylation as an Intricate Post-Translational Modification Process Takes Part in Glycoproteins Related Immunity. Cell Commun. Signal. 2025, 23, 214. [Google Scholar] [CrossRef]
- Leusmann, S.; Ménová, P.; Shanin, E.; Titz, A.; Rademacher, C. Glycomimetics for the Inhibition and Modulation of Lectins. Chem. Soc. Rev. 2023, 52, 3663–3740. [Google Scholar] [CrossRef]
- Francesconi, O.; Roelens, S. Biomimetic Carbohydrate-Binding Agents (CBAs): Binding Affinities and Biological Activities. Chembiochem 2019, 20, 1329–1346. [Google Scholar] [CrossRef]
- Chen, S.; Wang, K.; Wang, Q. Mannose: A Promising Player in Clinical and Biomedical Applications. Curr. Drug Deliv. 2024, 21, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, M.; Sruthi, D.; Jinuraj, K.R.; Das, K.; Dave, S.; Andal, N.M.; Das, J. Mannose: A Potential Saccharide Candidate in Disease Management. Med. Chem. Res. 2023, 32, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Imberty, A.; Chabre, Y.M.; Roy, R. Glycomimetics and Glycodendrimers as High Affinity Microbial Anti-Adhesins. Chem.—A Eur. J. 2008, 14, 7490–7499. [Google Scholar] [CrossRef]
- Hoyos, P.; Perona, A.; Juanes, O.; Rumbero, Á.; Hernáiz, M.J. Synthesis of Glycodendrimers with Antiviral and Antibacterial Activity. Chem.—A Eur. J. 2021, 27, 7593–7624. [Google Scholar] [CrossRef]
- Ribić, R.; Petrović Peroković, V.; Meštrović, T.; Neuberg, M.; Bradić, N. Cranberry-Derived Phenolic Compounds Contribute to the Inhibition of FimH-Mediated Escherichia coli Hemagglutination. Antibiotics 2025, 14, 418. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and Anti-Adhesive Therapeutics: A Disarming Strategy Against Uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef]
- Ribić, R.; Meštrović, T.; Neuberg, M.; Kozina, G. Effective Anti-Adhesives of Uropathogenic Escherichia coli. Acta Pharm. 2018, 68, 1–18. [Google Scholar] [CrossRef]
- Lenger, S.M.; Bradley, M.S.; Thomas, D.A.; Bertolet, M.H.; Lowder, J.L.; Sutcliffe, S. D-Mannose vs Other Agents for Recurrent Urinary Tract Infection Prevention in Adult Women: A Systematic Review and Meta-Analysis. Am. J. Obs. Gynecol. 2020, 223, 265.e1–265.e13. [Google Scholar] [CrossRef]
- Cooper, T.E.; Teng, C.; Howell, M.; Teixeira-Pinto, A.; Jaure, A.; Wong, G. D-Mannose for Preventing and Treating Urinary Tract Infections. Cochrane Database Syst. Rev. 2022, 8, CD013608. [Google Scholar] [CrossRef]
- Bachmann, P.; Afanasyev, P.; Boehringer, D.; Glockshuber, R. Structures of the Escherichia coli Type 1 Pilus during Pilus Rod Assembly and after Assembly Termination. Nat. Commun. 2025, 16, 4988. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.; Fiege, B.; Eris, D.; Silbermann, M.; Jakob, R.P.; Navarra, G.; Maier, T.; Ernst, B. Conformational Switch of the Bacterial Adhesin FimH in the Absence of the Regulatory Domain: Engineering a Minimalistic Allosteric System. J. Biol. Chem. 2018, 293, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Aprikian, P.; Tchesnokova, V.; Kidd, B.; Yakovenko, O.; Yarov-Yarovoy, V.; Trinchina, E.; Vogel, V.; Thomas, W.; Sokurenko, E. Interdomain Interaction in the FimH Adhesin of Escherichia coli Regulates the Affinity to Mannose. J. Biol. Chem. 2007, 282, 23437–23446. [Google Scholar] [CrossRef] [PubMed]
- de Monerri, N.C.S.; Che, Y.; Lees, J.A.; Jasti, J.; Wu, H.; Griffor, M.C.; Kodali, S.; Hawkins, J.C.; Lypowy, J.; Ponce, C.; et al. Structure-Based Design of an Immunogenic, Conformationally Stabilized FimH Antigen for a Urinary Tract Infection Vaccine. PLoS Pathog. 2025, 21, e1012325. [Google Scholar] [CrossRef]
- Bertuccini, L.; Costanzo, M.; Iosi, F.; Tinari, A.; Terruzzi, F.; Stronati, L.; Aloi, M.; Cucchiara, S.; Superti, F. Lactoferrin Prevents Invasion and Inflammatory Response Following E. coli Strain LF82 Infection in Experimental Model of Crohn’s Disease. Dig. Liver Dis. 2014, 46, 496–504. [Google Scholar] [CrossRef]
- Ofek, I.; Mirelman, D.; Sharon, N. Adherence of Escherichia coli to Human Mucosal Cells Mediated by Mannose Receptors. Nature 1977, 265, 623–625. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.K.; Cusumano, Z.T.; Janetka, J.W. Mannose-Derived FimH Antagonists: A Promising Anti-Virulence Therapeutic Strategy for Urinary Tract Infections and Crohn’s Disease. Expert. Opin. Ther. Pat. 2016, 26, 175–197. [Google Scholar] [CrossRef]
- Mousavifar, L.; Roy, R. Recent Development in the Design of Small “drug-like” and Nanoscale Glycomimetics against Escherichia coli Infections. Drug Discov. Today 2021, 26, 2124–2137. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, J.; Berglund, J.; Schembri, M.; De Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.-S.; Pinkner, J.; Slättegård, R.; Zavialov, A.; et al. Receptor Binding Studies Disclose a Novel Class of High-Affinity Inhibitors of the Escherichia coli FimH Adhesin. Mol. Microbiol. 2005, 55, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Wellens, A.; Lahmann, M.; Touaibia, M.; Vaucher, J.; Oscarson, S.; Roy, R.; Remaut, H.; Bouckaert, J. The Tyrosine Gate as a Potential Entropic Lever in the Receptor-Binding Site of the Bacterial Adhesin FimH. Biochemistry 2012, 51, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Gouin, S.G.; Roos, G.; Bouckaert, J. Discovery and Application of FimH Antagonists. In Carbohydrates as Drugs; Seeberger, P.H., Rademacher, C., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 123–168. ISBN 978-3-319-08675-0. [Google Scholar]
- Touaibia, M.; Krammer, E.-M.; Shiao, T.C.; Yamakawa, N.; Wang, Q.; Glinschert, A.; Papadopoulos, A.; Mousavifar, L.; Maes, E.; Oscarson, S.; et al. Sites for Dynamic Protein-Carbohydrate Interactions of O- and C-Linked Mannosides on the E. coli FimH Adhesin. Molecules 2017, 22, 1101. [Google Scholar] [CrossRef]
- Schwardt, O.; Rabbani, S.; Hartmann, M.; Abgottspon, D.; Wittwer, M.; Kleeb, S.; Zalewski, A.; Smieško, M.; Cutting, B.; Ernst, B. Design, Synthesis and Biological Evaluation of Mannosyl Triazoles as FimH Antagonists. Bioorg. Med. Chem. 2011, 19, 6454–6473. [Google Scholar] [CrossRef]
- Klein, T.; Abgottspon, D.; Wittwer, M.; Rabbani, S.; Herold, J.; Jiang, X.; Kleeb, S.; Lüthi, C.; Scharenberg, M.; Bezençon, J.; et al. FimH Antagonists for the Oral Treatment of Urinary Tract Infections: From Design and Synthesis to in Vitro and in Vivo Evaluation. J. Med. Chem. 2010, 53, 8627–8641. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.; Cusumano, Z.; Han, Z.; Binkley, J.; Kostakioti, M.; Hannan, T.; Pinkner, J.S.; Klein, R.; Kalas, V.; Crowley, J.; et al. Antivirulence C-Mannosides as Antibiotic-Sparing, Oral Therapeutics for Urinary Tract Infections. J. Med. Chem. 2016, 59, 9390–9408. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, R.; Fang, Y.; Lv, T.; Liu, J.; Wang, X. Facile Synthesis of FimH Antagonist and Its Analogues: Simple Entry to Complex C-Mannoside Inhibitors of E. coli Adhesion . ACS Med. Chem. Lett. 2024, 15, 1724–1730. [Google Scholar] [CrossRef]
- Mohammed, A.F.; Othman, S.A.; Abou-Ghadir, O.F.; Kotb, A.A.; Mostafa, Y.A.; El-Mokhtar, M.A.; Abdu-Allah, H.H.M. Design, Synthesis, Biological Evaluation and Docking Study of Some New Aryl and Heteroaryl Thiomannosides as FimH Antagonists. Bioorg. Chem. 2024, 145, 107258. [Google Scholar] [CrossRef]
- Sattigeri, J.A.; Garg, M.; Bhateja, P.; Soni, A.; Rauf, A.R.A.; Gupta, M.; Deshmukh, M.S.; Jain, T.; Alekar, N.; Barman, T.K.; et al. Synthesis and Evaluation of Thiomannosides, Potent and Orally Active FimH Inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 2993–2997. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.K.; Hannan, T.J.; Janetka, J.W. Rational Design Strategies for FimH Antagonists: New Drugs on the Horizon for Urinary Tract Infection and Crohn’s Disease. Expert. Opin. Drug Discov. 2017, 12, 711–731. [Google Scholar] [CrossRef]
- asif Enterome Highlights Microbiome Publication Describing Sibofimloc’s Novel Mechanism of Action for the Treatment of Crohn’s Disease. Enterome 2021. Available online: https://www.enterome.com/news-events/enterome-highlights-microbiome-publication-describing-sibofimlocs-novel-mechanism-of-action-for-the-treatment-of-crohns-disease/ (accessed on 7 September 2025).
- Bi, F.; Zhang, J.; Xie, R.; Yu, D.; Wei, H.; Wang, Y.; Hua, Z.; Qi, X.; Huang, B.; Yang, G. Adenosine Triphosphate-Responsive Glyconanorods through Self-Assembly of β-Cyclodextrin-Based Glycoconjugates for Targeted and Effective Bacterial Sensing and Killing. Biomacromolecules 2023, 24, 1003–1013. [Google Scholar] [CrossRef]
- Sehad, C.; Shiao, T.C.; Sallam, L.M.; Azzouz, A.; Roy, R. Effect of Dendrimer Generation and Aglyconic Linkers on the Binding Properties of Mannosylated Dendrimers Prepared by a Combined Convergent and Onion Peel Approach. Molecules 2018, 23, 1890. [Google Scholar] [CrossRef] [PubMed]
- Loris, R.; Tielker, D.; Jaeger, K.-E.; Wyns, L. Structural Basis of Carbohydrate Recognition by the Lectin LecB from Pseudomonas Aeruginosa. J. Mol. Biol. 2003, 331, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.; Exner, T.E.; Titz, A. A Biophysical Study with Carbohydrate Derivatives Explains the Molecular Basis of Monosaccharide Selectivity of the Pseudomonas Aeruginosa Lectin LecB. PLoS ONE 2014, 9, e112822. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, K.; Byrne, J.P. Structural Considerations for Building Synthetic Glycoconjugates as Inhibitors for Pseudomonas Aeruginosa Lectins. ChemMedChem 2022, 17, e202200081. [Google Scholar] [CrossRef]
- Hauck, D.; Joachim, I.; Frommeyer, B.; Varrot, A.; Philipp, B.; Möller, H.M.; Imberty, A.; Exner, T.E.; Titz, A. Discovery of Two Classes of Potent Glycomimetic Inhibitors of Pseudomonas Aeruginosa LecB with Distinct Binding Modes. ACS Chem. Biol. 2013, 8, 1775–1784. [Google Scholar] [CrossRef]
- Sommer, R.; Hauck, D.; Varrot, A.; Wagner, S.; Audfray, A.; Prestel, A.; Möller, H.M.; Imberty, A.; Titz, A. Cinnamide Derivatives of D-Mannose as Inhibitors of the Bacterial Virulence Factor LecB from Pseudomonas Aeruginosa. ChemistryOpen 2015, 4, 756–767. [Google Scholar] [CrossRef]
- Hofmann, A.; Sommer, R.; Hauck, D.; Stifel, J.; Göttker-Schnetmann, I.; Titz, A. Synthesis of Mannoheptose Derivatives and Their Evaluation as Inhibitors of the Lectin LecB from the Opportunistic Pathogen Pseudomonas Aeruginosa. Carbohydr. Res. 2015, 412, 34–42. [Google Scholar] [CrossRef]
- Gerland, B.; Goudot, A.; Pourceau, G.; Meyer, A.; Dugas, V.; Cecioni, S.; Vidal, S.; Souteyrand, E.; Vasseur, J.-J.; Chevolot, Y.; et al. Synthesis of a Library of Fucosylated Glycoclusters and Determination of Their Binding toward Pseudomonas Aeruginosa Lectin B (PA-IIL) Using a DNA-Based Carbohydrate Microarray. Bioconjug. Chem. 2012, 23, 1534–1547. [Google Scholar] [CrossRef]
- Dupin, L.; Noël, M.; Bonnet, S.; Meyer, A.; Géhin, T.; Bastide, L.; Randriantsoa, M.; Souteyrand, E.; Cottin, C.; Vergoten, G.; et al. Screening of a Library of Oligosaccharides Targeting Lectin LecB of Pseudomonas Aeruginosa and Synthesis of High Affinity Oligoglycoclusters. Molecules 2018, 23, 3073. [Google Scholar] [CrossRef]
- Limqueco, E.; Passos Da Silva, D.; Reichhardt, C.; Su, F.-Y.; Das, D.; Chen, J.; Srinivasan, S.; Convertine, A.; Skerrett, S.J.; Parsek, M.R.; et al. Mannose Conjugated Polymer Targeting P. Aeruginosa Biofilms. ACS Infect. Dis. 2020, 6, 2866–2871. [Google Scholar] [CrossRef]
- Taouai, M.; Chakroun, K.; Sommer, R.; Michaud, G.; Giacalone, D.; Ben Maaouia, M.A.; Vallin-Butruille, A.; Mathiron, D.; Abidi, R.; Darbre, T.; et al. Glycocluster Tetrahydroxamic Acids Exhibiting Unprecedented Inhibition of Pseudomonas Aeruginosa Biofilms. J. Med. Chem. 2019, 62, 7722–7738. [Google Scholar] [CrossRef] [PubMed]
- Lameignere, E.; Malinovská, L.; Sláviková, M.; Duchaud, E.; Mitchell, E.P.; Varrot, A.; Sedo, O.; Imberty, A.; Wimmerová, M. Structural Basis for Mannose Recognition by a Lectin from Opportunistic Bacteria Burkholderia Cenocepacia. Biochem. J. 2008, 411, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Lameignere, E.; Shiao, T.C.; Roy, R.; Wimmerova, M.; Dubreuil, F.; Varrot, A.; Imberty, A. Structural Basis of the Affinity for Oligomannosides and Analogs Displayed by BC2L-A, a Burkholderia Cenocepacia Soluble Lectin. Glycobiology 2010, 20, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, R.; Malinovska, L.; Lameignère, E.; Adamova, L.; de Castro, C.; Cioci, G.; Stanetty, C.; Kosma, P.; Molinaro, A.; Wimmerova, M.; et al. Burkholderia Cenocepacia Lectin A Binding to Heptoses from the Bacterial Lipopolysaccharide. Glycobiology 2012, 22, 1387–1398. [Google Scholar] [CrossRef]
- Csávás, M.; Malinovská, L.; Perret, F.; Gyurkó, M.; Illyés, Z.T.; Wimmerová, M.; Borbás, A. Tri- and Tetravalent Mannoclusters Cross-Link and Aggregate BC2L-A Lectin from Burkholderia Cenocepacia. Carbohydr. Res. 2017, 437, 1–8. [Google Scholar] [CrossRef][Green Version]
- Geng, X.; Wang, G.; Guo, Z.; Gu, G. Synthesis of the Oligosaccharides of Burkholderia Pseudomallei and B. Mallei Capsular Polysaccharide and Preliminary Immunological Studies of Their Protein Conjugates. J. Org. Chem. 2020, 85, 2369–2384. [Google Scholar] [CrossRef]
- Rieger, J.; Stoffelbach, F.; Cui, D.; Imberty, A.; Lameignere, E.; Putaux, J.-L.; Jérôme, R.; Jérôme, C.; Auzély-Velty, R. Mannosylated Poly(Ethylene Oxide)-b-Poly(Epsilon-Caprolactone) Diblock Copolymers: Synthesis, Characterization, and Interaction with a Bacterial Lectin. Biomacromolecules 2007, 8, 2717–2725. [Google Scholar] [CrossRef]
- Gest, P.; Kaur, D.; Pham, H.T.; van der Woerd, M.; Hansen, E.; Brennan, P.J.; Jackson, M.; Guerin, M.E. Preliminary Crystallographic Analysis of GpgS, a Key Glucosyltransferase Involved in Methylglucose Lipopolysaccharide Biosynthesis in Mycobacterium Tuberculosis. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2008, 64, 1121–1124. [Google Scholar] [CrossRef]
- Urresti, S.; Albesa-Jové, D.; Schaeffer, F.; Pham, H.T.; Kaur, D.; Gest, P.; van der Woerd, M.J.; Carreras-González, A.; López-Fernández, S.; Alzari, P.M.; et al. Mechanistic Insights into the Retaining Glucosyl-3-Phosphoglycerate Synthase from Mycobacteria. J. Biol. Chem. 2012, 287, 24649–24661. [Google Scholar] [CrossRef]
- Pereira, P.J.B.; Empadinhas, N.; Albuquerque, L.; Sá-Moura, B.; da Costa, M.S.; Macedo-Ribeiro, S. Mycobacterium Tuberculosis Glucosyl-3-Phosphoglycerate Synthase: Structure of a Key Enzyme in Methylglucose Lipopolysaccharide Biosynthesis. PLoS ONE 2008, 3, e3748. [Google Scholar] [CrossRef] [PubMed]
- Gopalaswamy, R.; Dusthackeer, V.N.A.; Kannayan, S.; Subbian, S. Extrapulmonary Tuberculosis—An Update on the Diagnosis, Treatment and Drug Resistance. J. Respir. 2021, 1, 141–164. [Google Scholar] [CrossRef]
- Kumar, M.; Virmani, T.; Kumar, G.; Deshmukh, R.; Sharma, A.; Duarte, S.; Brandão, P.; Fonte, P. Nanocarriers in Tuberculosis Treatment: Challenges and Delivery Strategies. Pharmaceuticals 2023, 16, 1360. [Google Scholar] [CrossRef] [PubMed]
- Gairola, A.; Benjamin, A.; Weatherston, J.D.; Cirillo, J.D.; Wu, H.-J. Recent Developments in Drug Delivery for Treatment of Tuberculosis by Targeting Macrophages. Adv. Ther. 2022, 5, 2100193. [Google Scholar] [CrossRef]
- Prabhu, P.; Fernandes, T.; Damani, M.; Chaubey, P.; Narayanan, S.; Sawarkar, S. 2 Receptor Specific Ligand Conjugated Nanocarriers: An Effective Strategy for Targeted Therapy of Tuberculosis. Curr. Drug Deliv. 2022, 19, 830–845. [Google Scholar] [CrossRef]
- Prabhu, P.; Fernandes, T.; Chaubey, P.; Kaur, P.; Narayanan, S.; Vk, R.; Sawarkar, S.P. Mannose-Conjugated Chitosan Nanoparticles for Delivery of Rifampicin to Osteoarticular Tuberculosis. Drug Deliv. Transl. Res. 2021, 11, 1509–1519. [Google Scholar] [CrossRef]
- Mistry, N.; Bandyopadhyaya, R.; Mehra, S. Enhancement of Antimycobacterial Activity of Rifampicin Using Mannose-Anchored Lipid Nanoparticles against Intramacrophage Mycobacteria. ACS Appl. Bio Mater. 2022, 5, 5779–5789. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus Aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Lv, H.; Yang, M.; Yang, Y.; Tang, Z.; Guo, Y.; Zhou, J.; Gui, Y.; Huang, R.; Cai, J.; Yu, B.; et al. Metal Ion and Antibiotic Co-Loaded Nanoparticles for Combating Methicillin-Rresistant Staphylococcus Aureus-Induced Osteomyelitis. ACS Nano 2025, 19, 5253–5268. [Google Scholar] [CrossRef]
- Yang, X.; Fang, R.; Li, X.; Kong, W.; Jin, Y.; Jiao, R.; Liu, Z.; Zhang, M.; Peng, Q.; Zhang, Y.; et al. Engineered Nanovesicles for the Precise and Noninvasive Treatment of Deep Osteomyelitis Caused by MRSA Infection with Enhanced Immune Response. ACS Appl. Mater. Interfaces 2025, 17, 11795–11810. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xie, B.; Peng, H.; Shi, G.; Sreenivas, B.; Guo, J.; Wang, C.; He, Y. Eradicating Intracellular MRSA via Targeted Delivery of Lysostaphin and Vancomycin with Mannose-Modified Exosomes. J. Control. Release 2021, 329, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Baurhoo, B.; Ferket, P.; Ashwell, C.M.; de Oliviera, J.; Zhao, X. Cell Walls of Saccharomyces Cerevisiae Differentially Modulated Innate Immunity and Glucose Metabolism during Late Systemic Inflammation. PLoS ONE 2012, 7, e30323. [Google Scholar] [CrossRef][Green Version]
- Piotrowski, M.; Wultańska, D.; Obuch-Woszczatyński, P.; Pituch, H. Fructooligosaccharides and Mannose Affect Clostridium Difficile Adhesion and Biofilm Formation in a Concentration-Dependent Manner. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1975–1984. [Google Scholar] [CrossRef]
- Arif, M.; Ahmad, R.; Sharaf, M.; Muhammad, J.; Abdalla, M.; Eltayb, W.A.; Liu, C.-G. Antibacterial and Antibiofilm Activity of Mannose-Modified Chitosan/PMLA Nanoparticles against Multidrug-Resistant Helicobacter pylori. Int. J. Biol. Macromol. 2022, 223, 418–432. [Google Scholar] [CrossRef]
- Shea, K.W.; Cunha, B.A. Teicoplanin. Med. Clin. N. Am. 1995, 79, 833–844. [Google Scholar] [CrossRef]
- Molina, K.C.; Miller, M.A.; Mueller, S.W.; Van Matre, E.T.; Krsak, M.; Kiser, T.H. Clinical Pharmacokinetics and Pharmacodynamics of Dalbavancin. Clin. Pharmacokinet. 2022, 61, 363–374. [Google Scholar] [CrossRef]
- Mattox, J.; Belliveau, P.; Durand, C. Oritavancin: A Novel Lipoglycopeptide. Consult. Pharm. 2016, 31, 86–95. [Google Scholar] [CrossRef]
- Debono, M.; Merkel, K.E.; Molloy, R.M.; Barnhart, M.; Presti, E.; Hunt, A.H.; Hamill, R.L. Actaplanin, New Glycopeptide Antibiotics Produced by Actinoplanes Missouriensis. The Isolation and Preliminary Chemical Characterization of Actaplanin. J. Antibiot. 1984, 37, 85–95. [Google Scholar] [CrossRef]
- O’Hare, M.D.; Ghosh, G.; Felmingham, D.; Grüneberg, R.N. In-Vitro Studies with Ramoplanin (MDL 62,198): A Novel Lipoglycopeptide Antimicrobial. J. Antimicrob. Chemother. 1990, 25, 217–220. [Google Scholar] [CrossRef]
- Singh, M.P.; Petersen, P.J.; Weiss, W.J.; Janso, J.E.; Luckman, S.W.; Lenoy, E.B.; Bradford, P.A.; Testa, R.T.; Greenstein, M. Mannopeptimycins, New Cyclic Glycopeptide Antibiotics Produced by Streptomyces Hygroscopicus LL-AC98: Antibacterial and Mechanistic Activities. Antimicrob. Agents Chemother. 2003, 47, 62–69. [Google Scholar] [CrossRef]
- Lozach, P.-Y.; Burleigh, L.; Staropoli, I.; Navarro-Sanchez, E.; Harriague, J.; Virelizier, J.-L.; Rey, F.A.; Desprès, P.; Arenzana-Seisdedos, F.; Amara, A. Dendritic Cell-Specific Intercellular Adhesion Molecule 3-Grabbing Non-Integrin (DC-SIGN)-Mediated Enhancement of Dengue Virus Infection Is Independent of DC-SIGN Internalization Signals. J. Biol. Chem. 2005, 280, 23698–23708. [Google Scholar] [CrossRef]
- Andreini, M.; Doknic, D.; Sutkeviciute, I.; Reina, J.J.; Duan, J.; Chabrol, E.; Thepaut, M.; Moroni, E.; Doro, F.; Belvisi, L.; et al. Second Generation of Fucose-Based DC-SIGN Ligands: Affinity Improvement and Specificity versus Langerin. Org. Biomol. Chem. 2011, 9, 5778–5786. [Google Scholar] [CrossRef] [PubMed]
- Švajger, U.; Anderluh, M.; Jeras, M.; Obermajer, N. C-Type Lectin DC-SIGN: An Adhesion, Signalling and Antigen-Uptake Molecule That Guides Dendritic Cells in Immunity. Cell. Signal. 2010, 22, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Global HIV & AIDS Statistics—Fact Sheet | UNAIDS. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 7 September 2025).
- Sever, B.; Otsuka, M.; Fujita, M.; Ciftci, H. A Review of FDA-Approved Anti-HIV-1 Drugs, Anti-Gag Compounds, and Potential Strategies for HIV-1 Eradication. Int. J. Mol. Sci. 2024, 25, 3659. [Google Scholar] [CrossRef]
- Wilen, C.B.; Tilton, J.C.; Doms, R.W. HIV: Cell Binding and Entry. Cold Spring Harb. Perspect. Med. 2012, 2, a006866. [Google Scholar] [CrossRef]
- Gamboa Marin, O.J.; Ng, K.; Verma, N.; Flavien Yapi, A.G.; Pantophlet, R.; Gauthier, C. Lewis-X-Containing Triterpenoid Saponins Inhibit DC-SIGN- and L-SIGN-Mediated Transfer of HIV-1 Infection. Chemistry 2025, 31, e202500993. [Google Scholar] [CrossRef]
- Sattin, S.; Daghetti, A.; Thépaut, M.; Berzi, A.; Sánchez-Navarro, M.; Tabarani, G.; Rojo, J.; Fieschi, F.; Clerici, M.; Bernardi, A. Inhibition of DC-SIGN-Mediated HIV Infection by a Linear Trimannoside Mimic in a Tetravalent Presentation. ACS Chem. Biol. 2010, 5, 301–312. [Google Scholar] [CrossRef]
- Berzi, A.; Reina, J.J.; Ottria, R.; Sutkeviciute, I.; Antonazzo, P.; Sanchez-Navarro, M.; Chabrol, E.; Biasin, M.; Trabattoni, D.; Cetin, I.; et al. A Glycomimetic Compound Inhibits DC-SIGN-Mediated HIV Infection in Cellular and Cervical Explant Models. AIDS 2012, 26, 127–137. [Google Scholar] [CrossRef]
- Wells, L.; Vierra, C.; Hardman, J.; Han, Y.; Dimas, D.; Gwarada-Phillips, L.N.; Blackeye, R.; Eggers, D.K.; LaBranche, C.C.; Král, P.; et al. Sulfoglycodendrimer Therapeutics for HIV-1 and SARS-CoV-2. Adv. Ther. 2021, 4, 2000210. [Google Scholar] [CrossRef]
- Kensinger, R.D.; Catalone, B.J.; Krebs, F.C.; Wigdahl, B.; Schengrund, C.-L. Novel Polysulfated Galactose-Derivatized Dendrimers as Binding Antagonists of Human Immunodeficiency Virus Type 1 Infection. Antimicrob. Agents Chemother. 2004, 48, 1614–1623. [Google Scholar] [CrossRef]
- Martínez, C.; Merchán, A.; Perona, A.; Ramírez-López, P.; Suárez, J.R.; Hernáiz, M.J. Design and Sustainable Synthesis of Small Mannose-Based Glycodendrons as Ligands for HIV-1 Envelope Protein Gp120: Toward an Explanation for Their Binding. Catal. Today 2024, 429, 114493. [Google Scholar] [CrossRef]
- Parris, G.E. 2-Deoxy-D-Glucose as a Potential Drug against Fusogenic Viruses Including HIV. Med. Hypotheses 2008, 70, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Dehuyser, L.; Sigwalt, D.; Flacher, V.; Bernacchi, S.; Chaloin, O.; Remy, J.-S.; Mueller, C.G.; Baati, R.; Wagner, A. Dynamic Micelles of Mannoside Glycolipids Are More Efficient than Polymers for Inhibiting HIV-1 Trans-Infection. Bioconjug. Chem. 2013, 24, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Molema, G.; Jansen, R.W.; Pauwels, R.; de Clercq, E.; Meijer, D.K. Targeting of Antiviral Drugs to T4-Lymphocytes. Anti-HIV Activity of Neoglycoprotein-AZTMP Conjugates in Vitro. Biochem. Pharmacol. 1990, 40, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Brigido, L.; Müller, W.E.; Hansen, J.E.; Ezekowitz, R.A.; Mills, J. Screening for Inhibitors of HIV Gp120-CD4 Binding Using an Enzyme-Linked Immunoabsorbent Assay. J. Virol. Methods 1993, 42, 1–12. [Google Scholar] [CrossRef]
- Hoorelbeke, B.; Xue, J.; LiWang, P.J.; Balzarini, J. Role of the Carbohydrate-Binding Sites of Griffithsin in the Prevention of DC-SIGN-Mediated Capture and Transmission of HIV-1. PLoS ONE 2013, 8, e64132. [Google Scholar] [CrossRef]
- Nabatov, A.A.; de Jong, M.A.W.P.; de Witte, L.; Bulgheresi, S.; Geijtenbeek, T.B.H. C-Type Lectin Mermaid Inhibits Dendritic Cell Mediated HIV-1 Transmission to CD4+ T Cells. Virology 2008, 378, 323–328. [Google Scholar] [CrossRef]
- Chiba, H.; Inokoshi, J.; Nakashima, H.; Omura, S.; Tanaka, H. Actinohivin, a Novel Anti-Human Immunodeficiency Virus Protein from an Actinomycete, Inhibits Viral Entry to Cells by Binding High-Mannose Type Sugar Chains of Gp120. Biochem. Biophys. Res. Commun. 2004, 316, 203–210. [Google Scholar] [CrossRef]
- Malvy, D.; McElroy, A.K.; de Clerck, H.; Günther, S.; van Griensven, J. Ebola Virus Disease. Lancet 2019, 393, 936–948. [Google Scholar] [CrossRef]
- Barrientos, L.G.; O’Keefe, B.R.; Bray, M.; Sanchez, A.; Gronenborn, A.M.; Boyd, M.R. Cyanovirin-N Binds to the Viral Surface Glycoprotein, GP1,2 and Inhibits Infectivity of Ebola Virus. Antivir. Res. 2003, 58, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Rojo, J.; Delgado, R. Glycodendritic Structures: Promising New Antiviral Drugs. J. Antimicrob. Chemother. 2004, 54, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Lasala, F.; Arce, E.; Otero, J.R.; Rojo, J.; Delgado, R. Mannosyl Glycodendritic Structure Inhibits DC-SIGN-Mediated Ebola Virus Infection in Cis and in Trans. Antimicrob. Agents Chemother. 2003, 47, 3970–3972. [Google Scholar] [CrossRef] [PubMed]
- Luczkowiak, J.; Sattin, S.; Sutkevičiūtė, I.; Reina, J.J.; Sánchez-Navarro, M.; Thépaut, M.; Martínez-Prats, L.; Daghetti, A.; Fieschi, F.; Delgado, R.; et al. Pseudosaccharide Functionalized Dendrimers as Potent Inhibitors of DC-SIGN Dependent Ebola Pseudotyped Viral Infection. Bioconjug. Chem. 2011, 22, 1354–1365. [Google Scholar] [CrossRef]
- Muñoz, A.; Sigwalt, D.; Illescas, B.M.; Luczkowiak, J.; Rodríguez-Pérez, L.; Nierengarten, I.; Holler, M.; Remy, J.-S.; Buffet, K.; Vincent, S.P.; et al. Synthesis of Giant Globular Multivalent Glycofullerenes as Potent Inhibitors in a Model of Ebola Virus Infection. Nat. Chem. 2016, 8, 50–57. [Google Scholar] [CrossRef]
- Ning, X.; Budhadev, D.; Pollastri, S.; Nehlmeier, I.; Kempf, A.; Manfield, I.; Turnbull, W.B.; Pöhlmann, S.; Bernardi, A.; Li, X.; et al. Polyvalent Glycomimetic-Gold Nanoparticles Revealing Critical Roles of Glycan Display on Multivalent Lectin-Glycan Interaction Biophysics and Antiviral Properties. JACS Au 2024, 4, 3295–3309. [Google Scholar] [CrossRef]
- Casalino, L.; Gaieb, Z.; Goldsmith, J.A.; Hjorth, C.K.; Dommer, A.C.; Harbison, A.M.; Fogarty, C.A.; Barros, E.P.; Taylor, B.C.; McLellan, J.S.; et al. Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein. ACS Cent. Sci. 2020, 6, 1722–1734. [Google Scholar] [CrossRef]
- Thépaut, M.; Luczkowiak, J.; Vivès, C.; Labiod, N.; Bally, I.; Lasala, F.; Grimoire, Y.; Fenel, D.; Sattin, S.; Thielens, N.; et al. DC/L-SIGN Recognition of Spike Glycoprotein Promotes SARS-CoV-2 Trans-Infection and Can Be Inhibited by a Glycomimetic Antagonist. PLoS Pathog. 2021, 17, e1009576. [Google Scholar] [CrossRef]
- Cramer, J.; Lakkaichi, A.; Aliu, B.; Jakob, R.P.; Klein, S.; Cattaneo, I.; Jiang, X.; Rabbani, S.; Schwardt, O.; Zimmer, G.; et al. Sweet Drugs for Bad Bugs: A Glycomimetic Strategy against the DC-SIGN-Mediated Dissemination of SARS-CoV-2. J. Am. Chem. Soc. 2021, 143, 17465–17478. [Google Scholar] [CrossRef]
- Delaunay, C.; Pollastri, S.; Thépaut, M.; Cavazzoli, G.; Belvisi, L.; Bouchikri, C.; Labiod, N.; Lasala, F.; Gimeno, A.; Franconetti, A.; et al. Unprecedented Selectivity for Homologous Lectin Targets: Differential Targeting of the Viral Receptors L-SIGN and DC-SIGN. Chem. Sci. 2024, 15, 15352–15366. [Google Scholar] [CrossRef]
- Lokhande, K.B.; Apte, G.R.; Shrivastava, A.; Singh, A.; Pal, J.K.; Swamy, K.V.; Gupta, R.K. Sensing the Interactions between Carbohydrate-Binding Agents and N-Linked Glycans of SARS-CoV-2 Spike Glycoprotein Using Molecular Docking and Simulation Studies. J. Biomol. Struct. Dyn. 2022, 40, 3880–3898. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Ferruti, P.; Duncan, R. Poly(Amidoamine)s as Potential Endosomolytic Polymers: Evaluation In Vitro and Body Distribution in Normal and Tumour-Bearing Animals. J. Drug Target. 1999, 6, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Bisazza, A.; Sessa, R.; Primo, L.; Fenili, F.; Manfredi, A.; Ranucci, E.; Ferruti, P. Amphoteric Agmatine Containing Polyamidoamines as Carriers for Plasmid DNA In Vitro and In Vivo Delivery. Biomacromolecules 2010, 11, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Ferruti, P.; Ranucci, E.; Manfredi, A.; Berzi, A.; Clerici, M.; Cagno, V.; Lembo, D.; Palmioli, A.; Sattin, S. Linear Biocompatible Glyco-Polyamidoamines as Dual Action Mode Virus Infection Inhibitors with Potential as Broad-Spectrum Microbicides for Sexually Transmitted Diseases. Sci. Rep. 2016, 6, 33393. [Google Scholar] [CrossRef]
- Soria-Martinez, L.; Bauer, S.; Giesler, M.; Schelhaas, S.; Materlik, J.; Janus, K.; Pierzyna, P.; Becker, M.; Snyder, N.L.; Hartmann, L.; et al. Prophylactic Antiviral Activity of Sulfated Glycomimetic Oligomers and Polymers. J. Am. Chem. Soc. 2020, 142, 5252–5265. [Google Scholar] [CrossRef]
- Malik, A.; Steinbeis, F.; Carillo, M.A.; Seeberger, P.H.; Lepenies, B.; Varón Silva, D. Immunological Evaluation of Synthetic Glycosylphosphatidylinositol Glycoconjugates as Vaccine Candidates against Malaria. ACS Chem. Biol. 2020, 15, 171–178. [Google Scholar] [CrossRef]
- Carpentieri, A.; Ratner, D.M.; Ghosh, S.K.; Banerjee, S.; Bushkin, G.G.; Cui, J.; Lubrano, M.; Steffen, M.; Costello, C.E.; O’Keefe, B.; et al. The Antiretroviral Lectin Cyanovirin-N Targets Well-Known and Novel Targets on the Surface of Entamoeba Histolytica Trophozoites. Eukaryot. Cell 2010, 9, 1661–1668. [Google Scholar] [CrossRef][Green Version]
- Paulovičová, E.; Paulovičová, L.; Poláková, M. In Vitro Assessment of Immunobiological Effectivity of Synthetic Non-Ionic Glycolipids. Chem. Biodivers. 2025, 22, e202401368. [Google Scholar] [CrossRef]
- Johnson, M.A.; Bundle, D.R. Designing a New Antifungal Glycoconjugate Vaccine. Chem. Soc. Rev. 2013, 42, 4327–4344. [Google Scholar] [CrossRef]
- Wu, X.; Bundle, D.R. Synthesis of Glycoconjugate Vaccines for Candida Albicans Using Novel Linker Methodology. J. Org. Chem. 2005, 70, 7381–7388. [Google Scholar] [CrossRef]
- Xin, H.; Cartmell, J.; Bailey, J.J.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Self-Adjuvanting Glycopeptide Conjugate Vaccine against Disseminated Candidiasis. PLoS ONE 2012, 7, e35106. [Google Scholar] [CrossRef]
- Gening, M.L.; Polyanskaya, A.V.; Kuznetsov, A.N.; Titova, A.D.; Yudin, V.I.; Yashunskiy, D.V.; Tsvetkov, Y.E.; Yudina, O.N.; Krylov, V.B.; Nifantiev, N.E. Characterization of Carbohydrate Specificity of Monoclonal Antibodies to Fungal Antigenic Markers Using Biotinylated Oligosaccharides as Coating Antigens. Biochemistry 2024, 89, 2194–2203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).