Abstract
Stress is a predisposing factor for pulmonary diseases; however, its effects on the lungs of healthy individuals have not been fully elucidated. Since bovine lactoferrin (bLf) is a powerful immunomodulator, this study aimed to evaluate whether lactoferrin can modulate the effects of chronic stress on humoral and cellular immunity in the lungs. We performed chronic restraint stress (RS) and oral administration of bLf in a BALB/c model, assessing serum corticosterone, body weight, and various lung immunity parameters, including immunoglobulin concentrations in serum and tracheobronchial lavages (TBLs), secretory IgA (S-IgA) levels in TBLs, IgA-secreting plasma cells, relative expression of pIgR, CD4+ lymphocyte Th1 and Th2 populations, and antigen-presenting cell (APC) populations in the lungs. Our results demonstrate that stress increases corticosterone and production of total IgA and IgG, while decreasing levels of IgM and S-IgA, promotes a Th1/Th2 profile imbalance, and decreases APC populations. Interestingly, bLf modulates serum corticosterone levels and stress-induced weight loss, and it also modulates humoral and cellular effects produced by chronic stress. These results demonstrate that bLf should be considered a new therapeutic target for further studies, focusing on prophylactic and co-therapeutic administration to treat and prevent respiratory diseases.
1. Introduction
In recent decades, stress has been studied and defined by various authors, highlighting the importance of the nature of the stressor (physical or psychological), its duration (acute or chronic), and the individual’s response in an attempt to maintain homeostasis [1]. One of the first events in response to a stress stimulus is the activation of the hypothalamic–pituitary–adrenal axis, which culminates in the release of catecholamines, neurotransmitters, and hormones, particularly corticosterone [2]. This hormone is secreted into the bloodstream and reaches the entire body, stimulating various organs and systems, including the immune system and mucosal surfaces [3].
The lungs are one of the mucosal organs that are constantly interacting with the environment, and numerous antigens enter the respiratory airways during breathing. Thus, the lower respiratory airways have various immune mechanisms that play a crucial role in their proper functioning. Although the lungs are considered an immunologically privileged site due to the absence of resident microbiota on their surfaces, a limited number of low-molecular-weight particles still gain access. Immune cells are essential for maintaining optimal conditions in the alveoli, the functional and structural units of the lung, thereby facilitating successful gas exchange [4,5]. When these conditions are not optimal for the physiological functioning of the lung, it leads to the development of diseases and infections. In accordance with this, evidence suggests that stress could be a predisposing factor for respiratory pathologies [6], such as infectious diseases [7], by promoting an increase in the number of inflammatory infiltrate cells and prompting defects in cellular remodeling in models of chronic inflammation similar to bronchitis [8,9]. It has also been reported that stress also produces exacerbation of diseases such as asthma [10], Chronic Obstructive Pulmonary Disease (COPD), and allergic rhinitis [11]. Despite the numerous pharmaceutical therapies and strategies available to treat respiratory illnesses today, there is still a need for further research on their effectiveness, monetary costs, side effects and the risk–benefit balance for patients [12]. Consequently, some researchers have focused on the use of adjuvants in the treatment and prevention of respiratory diseases. Thus, the administration of bovine lactoferrin (bLf) has been proposed due to its promising properties, which have been demonstrated in several models [13,14].
Lactoferrin is a glycoprotein found in the secretions of all mammals, exhibiting structural homology across species and direct receptor recognition in various cell types, which enables its heterologous administration; therefore, bovine lactoferrin is among the most extensively used in experimental and clinical trials [15,16,17]. It has been demonstrated that bLf exhibits antioxidant, antiviral, antifungal, and antimicrobial activity, as well as immunomodulatory effects, including a reduction in inflammatory infiltrates in infected tissues. Studies performed on different models and anatomical sites have reported that bLf promotes the production of IgM and IgA antibodies, exhibits anti-inflammatory properties in sepsis processes, and possesses immunomodulatory properties to promote the Th1–Th2 balance [18,19,20,21,22,23].
Despite this, there are no studies focused on elucidating the effect of chronic stress in the respiratory airways of healthy individuals, and little is currently known about the modulation of bLf on the lung response to stress. This study demonstrated that bLf modulates weight loss-induced stress in serum and tracheobronchial lavages, as well as S-IgA concentration and IgA+ plasma cell populations in the lungs, and affects Th1 and Th2 CD4+ lymphocyte percentages and APC populations, in a corticosterone-dependent manner. This study provides a first overview to elucidate the immunomodulatory effects of lactoferrin on the respiratory immunological response during chronic stress, thereby contributing to the development of a novel therapeutic target for treating and preventing infectious and chronic lung diseases.
2. Results
2.1. Bovine Lactoferrin Counteracts Weight Loss Induced by Stress
Mice were randomly separated into four experimental groups (Figure 1A). They gained weight during the homing week, before the stress protocol (Figure 1B). Once the stress protocol concluded (Figure 1C), RS lost 0.5 ± 0.24 g (p value ≤ 0.01).
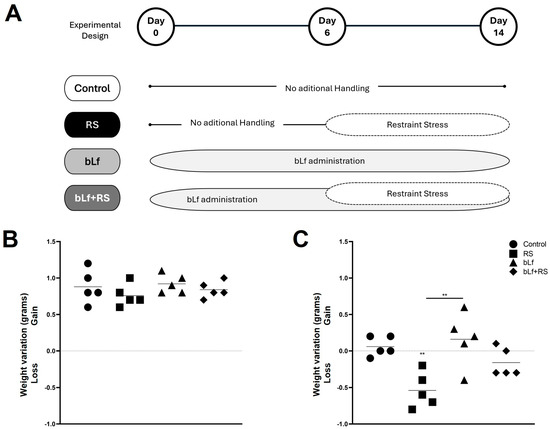
Figure 1.
Experimental design and weight monitoring. Mice were divided into four experimental groups: control (CTL), restraint stress (RS), bLf (bLf administered orally), and bLf + RS. (A) Graphics represent the mean and ±SD of weight variation from arrival to before the experimental model (B) and during the experimental model’s performance (C). Mice groups showed no difference in weight gain or loss during the first week; however, after the stress protocol, the RS group lost approximately 0.5 g (** p ≤ 0.01).
2.2. Bovine Lactoferrin Modulates Corticosterone
Chronic stress increased serum corticosterone (Figure 2) in the RS group (6869.30 ± 684.94 pg/mL; p ≤ 0.001), while in the bLf group, these levels were decreased (561.12 ± 191.36 pg/mL; p ≤ 0.05) compared to CTL (2086.64 ± 214.68 pg/mL). Interestingly, in the bLf + RS mice group, corticosterone was increased (4288.41 ± 680.55 pg/mL; p ≤ 0.01) at a lower concentration than in the RS group. These results suggest that oral bLf administration before and during chronic stress modulates corticosterone release.
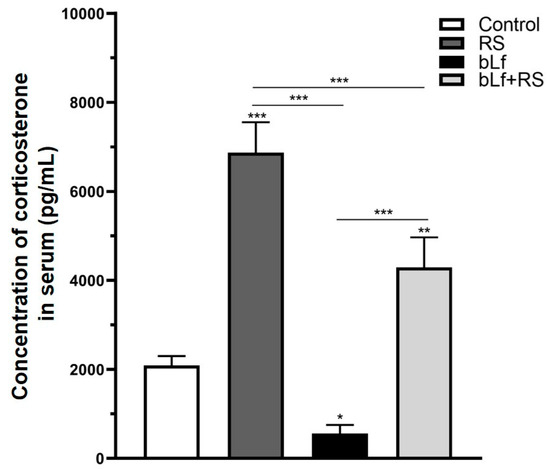
Figure 2.
Effect of stress and bLf administration on serum corticosterone. Corticosterone levels were increased in the RS group, which were almost four times those of baseline (*** p ≤ 0.001), but in the bLf + RS group, levels were barely doubled (** p ≤ 0.01). bLf diminished corticosterone levels by half of those of the control group (* p ≤ 0.05).
2.3. Bovine Lactoferrin Modulates the Effect of Chronic Stress on Immunoglobulin Concentrations
Immunoglobulin concentrations of serum and TBL were modified by chronic stress (Figure 3). Total IgA was increased in serum (Figure 3A; 5.55 ± 0.41 µg/mL, p ≤ 0.01) and TBL (Figure 3D; 5.55 ± 0.41 µg/mL, p ≤ 0.001) versus the CTL group. Serum IgM (Figure 3B) was diminished in RS (8.32 ± 1.49 µg/mL, p ≤ 0.001) and bLf + RS (9.57 ± 0.41 µg/mL, p ≤ 0.01), as well as IgM TBL levels of the RS group (Figure 3E; 0.16 ± 0.01 µg/mL, p ≤ 0.05), in comparison to the CTL group. IgG was increased in the serum of RS (Figure 3C; 19.83 ± 3.57 µg/mL, p ≤ 0.01) and TBL (Figure 3F; 3.58 ± 0.31 µg/mL, p ≤ 0.001) versus the CTL group. These results demonstrate the bLf’s immunomodulatory properties by up- or downregulating immunoglobulin concentrations in both serum and TBL during stress.
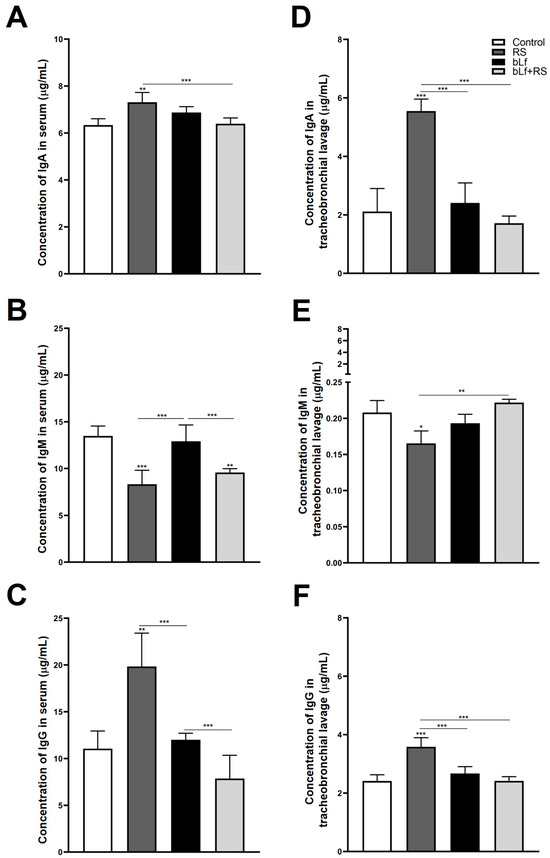
Figure 3.
Levels of immunoglobulins in serum and TBL. Total IgA was increased in serum (A) and TBL (D) of the RS group; total IgM was diminished in serum (B) and TBL (E) of the RS group; and IgG concentrations were increased in serum (C) and TBL (F) of the RS group. These modifications in immunoglobulin levels of stressed mice were modulated by the administration of bLf (bLf + RS groups). (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
2.4. Bovine Lactoferrin Modulates Stress-Induced Diminished S-IgA Release in Tracheobronchial Lavage and Serum
The analysis of S-IgA TBL concentration (Figure 4A; 0.27 ± 0.04 U/A), and the IgA+ plasma cell population (Figure 4B; 3.12% ± 0.25%, p ≤ 0.001) was diminished both in the RS group versus CTL. In addition to this, relative expression of pIgR is expressed four times more in the bLf + RS group (Figure 3C; 4.49 ± 0.24, p ≤ 0.001) versus the CTL group. These results suggest that chronic stress decreases TBL S-IgA levels, which may be a consequence of the decrease in IgA+ plasma cell populations in the lung; however, this decrease does not appear to be related to the relative expression of pIgR. Meanwhile, oral administration of bLf before and during stress modulates S-IgA concentrations and the IgA+ plasma cell population, similar to the CTL group, and promotes the overexpression of pIgR.
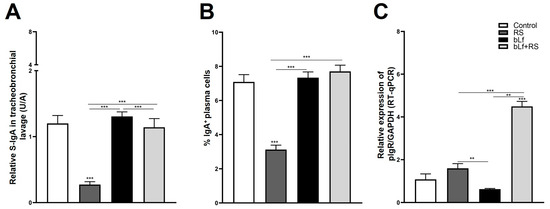
Figure 4.
Analysis of S-IgA levels in TBL, secretion, and transport-related mechanisms. RS decreased S-IgA in TBL (A) and the percentage of IgA+ plasma cell populations (*** p ≤ 0.001) (B), but these levels are not modified in bLf nor bLf + RS groups. Relative expression of pIgR was increased in bLf + RS (*** p ≤ 0.001), and it was decreased in bLf group only compared to RS and bLf+RS groups (** p ≤ 0.01) (C).
2.5. bLf Promotes Th1 Response and Decreases Th2 Established During Stress
Representative dot plots of CD4+ T cell populations are shown in Figure 5A. Analysis of the cytokine profile of lung lymphocytes (Figure 5B) revealed a decrease in the CD4+/IL-12+ population (1.16% ± 0.06%) and an upregulation of CD4+/IL-4+ (1.28% ± 0.01%) and CD4+/IL-10+ (1.34% ± 0.01%) in the RS group. In mice administered with bLf, the CD4+/IL-1β+ population was increased (1.73% ± 0.05%). In the bLf + RS group, CD4+/IL-1β+ (1.67 ± 0.05%) and CD4+/IL-12+ (1.86 ± 0.06%) percentages were increased; remarkably, CD4+/IL-4+ (1.18 ± 0.01) and CD4+/IL-10+ (1.14% ± 0.01%) decreased to levels similar to the CTL group. These results demonstrate that chronic stress upregulates Th2 cytokines, while bLf administration modulates by reestablishing Th1/Th2 balance in the cytokine profile.
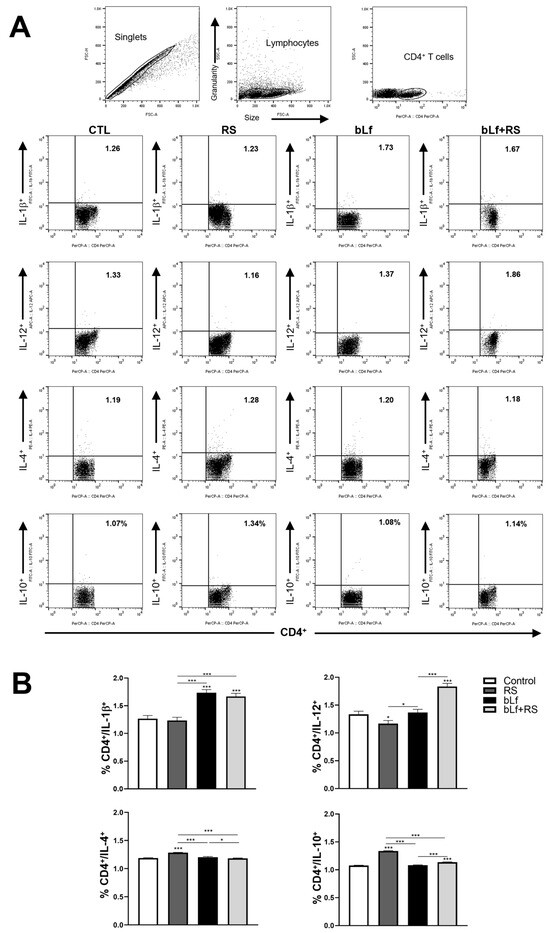
Figure 5.
Effect of stress and bLf administration on Th1 and Th2 populations in the lungs. Representative dot plots of CD4+ cell populations are shown in (A). In the RS group, the CD4+/IL-12+ population decreased, while CD4+/IL-4+ and CD4+/IL-10+ cell percentages increased. bLf administration upregulated CD4+/IL-1β+ populations. In the bLf + RS group, CD4+/IL-1β+ and CD4+/IL-12+ percentages were increased, but CD4+/IL-4+ and CD4+/IL-10+ cell populations decreased (B) (* p ≤ 0.05, *** p ≤ 0.001).
2.6. bLf Modulates CD64+/CD86+ Cell Populations Promoted by Stress
Representative dot plots of APC populations are shown in Figure 6A. The analysis revealed a decrease in CD64+/CD86+ cell populations (1.03% ± 0.01%, p ≤ 0.001) in the RS group and an increase in the bLf + RS group (1.42% ± 0.02%) compared to the control group (Figure 6B). These results demonstrate bLf’s capability to modulate APC percentages during chronic stress.
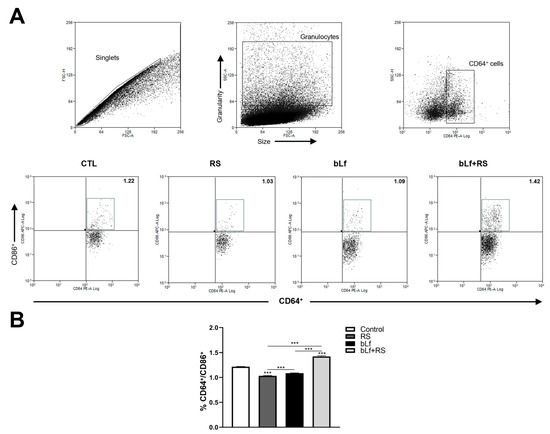
Figure 6.
Effect of stress and bLf administration in APC populations in the lungs. (A) Representative dot plots of CD64+/CD86+ cells. (B) The percentage of APC decreased in RS (*** p ≤ 0.001), but this population increased in the bLf + RS group compared to the control group (*** p ≤ 0.001).
3. Discussion
Among many other effects related to stress exposure, metabolic deregulation is one of the most extensively studied. Despite contradictory effects on weight variation, the response appears to be dependent on the duration of exposure to the stimulus. The weight lost in the group subjected to chronic stress aligns with previous reports [24]. Evidence suggests that this may be attributed to hypothalamic–pituitary axis (HPA) activation, which mediates a reduction in leptin levels [25], resulting in lower body fat [26] and diminished energy metabolism [27], thereby disrupting the metabolism of carbohydrates, lipids, and food intake-related hormones [28]. In accordance with our results regarding the bLf + RS group, studies indicate that lactoferrin administration ameliorates weight loss associated with chronic diseases, such as hypertension [29] and influenza [13]. Controversially, it has been reported that lactoferrin administration facilitates weight loss in patients with obesity [30] or predisposed to obesity [31]. However, in our experimental model, we propose that the main mechanism by which bLf prevents body weight loss is related to the modulation of stress-related hormones.
We demonstrated that prophylactic and therapeutic administration of bLf modulates serum corticosterone during chronic restraint stress. As is known, one of the first events in response to stress stimulus is the activation of the HPA, releasing neurotransmitters and hormones such as norepinephrine and corticosterone [2,32], which in turn promote changes in almost every body tissue, including the immune system [3,33] and the lung [7,8,11]. We employed the chronic restraint stress protocol in male mice to obtain more consistent results and accurately evaluate the effect of bLf on serum corticosterone levels. Many authors have reported discrepancies in the levels of stress mediators and cellular responses between male and female mice [34,35,36], and the evidence strongly suggests that the stress response can be modified by hormones associated with the estrous cycle [37]. In this regard, bLf is capable of modulating corticosterone in a manner that depends on the stressor, dose, and timing [38,39]. It has been proposed that bLf is endocytosed by enterocytes and transported to the plasma, passing through the blood–brain barrier to reach the cerebrospinal fluid of the choroidal plexus [40,41,42]. Afterwards, bLf modulates the increase in nitric oxide production by upregulation of all nitric oxide synthase isoforms [43]. Then, the anti-stress effect of opioid µ receptors decreases HPA activity, specifically the release of corticoid-releasing hormone (CRH). Still, it does not modify other mediators, such as adrenocorticotropic hormone (ACTH) [44], epinephrine, and glucagon [45,46]. Several models of chronic stress and lactoferrin administration in rodents yield similar findings to those of this study, providing strong evidence that oral bLf administration modulates serum corticosterone levels in response to stress stimuli [45,47,48]. bLf is also capable of modulating corticosterone release by regulating intestinal microbiota. Studies performed in germ-free mouse models have shown that microbiota downregulate serum corticosterone [49,50,51]. Although the microbicidal effects of lactoferrin have been documented in in vitro and in vivo models [52], numerous reports highlight the prebiotic ability of bLf, which promotes the growth of Bacteroides and Parasutterella [53,54]. These effects seem to be related to lactoferrin dose and iron saturation [54,55]. In this regard, the effect of stress on the intestinal microbiota is not well understood, as differences in reports exist depending on the strain of mice, stress model, and duration of exposure [56]. However, further studies are required to determine the exact mechanisms by which bLf modulates the microbiota and whether this is related to corticosterone release in this specific model of chronic stress.
On the other hand, one of the main determinants of the correct functioning of the lung immunological system is the presence of immunoglobulins in tracheobronchial secretions. There are numerous studies examining how stress promotes immunoglobulin production through HPA activation in healthy individuals; however, most of the research focuses on the intestine [57]. In accordance with this, it has been proposed that stress can have diverse effects on serum immunoglobulins in a duration- and intensity-dependent manner. The first immunoglobulin secreted by plasma cells is IgM. In the respiratory system, IgM is primarily active due to the deficiency of other immunoglobulins, as it is found in low concentrations due to the infrequent presence of IgM-producing cells in the bronchial tree, and its size complicates its transudation from the bloodstream to the bronchial lavage [58]. Nevertheless, it has been reported that IgM concentrations may be increased or decreased depending on the model disease in which they have been evaluated, some of which include fibrosis (barely detectable), pneumonitis, and transplant rejection (increased) [59]. In this study, chronic stress was found to promote a decrease in IgM concentrations; however, this effect was modulated by bLf administration, which may promote proper functioning of the defense against potential pathogens in the lung.
Furthermore, IgA plays a crucial role in lung pathologies such as asthma and COPD, as it can influence tolerance to certain antigens. Additionally, it has been reported that the concentration of IgA in the bronchial secretions of asthmatic patients is increased, while in patients with COPD, it is decreased [60]. Previously, an increase in IgA concentrations in intestinal lavages from the duodenum and ileum has been reported during chronic stress [61]. In contrast, Jarillo-Luna found decreased IgA concentrations in small intestine lavages in a mouse model of chronic stress [62]. Otherwise, both authors reported that the intestine is one of the sites most resistant to stress. In our model, an increase in IgA levels in serum and TBL suggests that chronic stress may be modulating the isotype change in IgA-producing cells in the lung, favoring their secretion and transudation from the serum, likely as a protective mechanism against potential pathogens or particles that could compromise the homeostasis of this mucosa. To clarify the mechanism by which there is such an increase in IgA concentrations, it would be necessary to determine the difference in the concentrations of monomeric IgA (mIgA) and dimeric IgA (dIgA).
Since there are no studies focused on elucidating the role of bLf on immunoglobulin modulation in respiratory secretions, our results demonstrate that bLf does not modify the total IgA, IgG, or IgM in serum nor TBL concentration, in contrast to the increase reported in the distal small intestine [63]. This could be explained by the nature of both mucous tissues. Strikingly, the modulation of these immunoglobulins in the stressed groups is remarkable. The bLf antibody modulation observed in our results is similar to that found in the intestinal lavage of the chronic immobilization model [48]. In the lower respiratory airways, it has only been previously reported that bLf increases IL-17-producing cells and enhances IFN-γ-mediated responses [64], as well as reduces lung consolidation and infiltration in bronchial lavage during influenza virus infection [13]. It is also capable of regulating cytokine genes related to IgA production [65]. Even in human trials, bLf has been reported to contribute to protection against viral infections and modulate respiratory immunity [66]. Still, there is no further information regarding the mechanisms of IgM and IgG secretion. Since IgG levels in serum and TBL showed a similar pattern to IgA concentrations, these results suggest that chronic stress modifies IgG change in isotype and transudation mechanisms, which are also modulated by bLf. Nowadays, we are far from clearly understanding how allostasis mechanisms are formed, and this study provides an initial overview of how chronic stress modifies immunoglobulin levels in a healthy individual, while demonstrating that lactoferrin modulates the effects of stress on the humoral immunity of the lungs.
It has been well established that one of the most important mechanisms for maintaining mucosal homeostasis is the secretion of S-IgA. The deficiency of this immunoglobulin is associated with pathogen colonization, asthma, allergic diseases, and the progression of chronic obstructive respiratory disease [67,68,69]. In concordance with these reports, our results showed decreased S-IgA levels in the TBL and a decrease in the percentages of IgA+ plasma cells in the RS group. Interestingly, these parameters in the bLf + RS group are similar to those of the control, demonstrating the immunomodulatory effect of bLf. Since S-IgA production and secretion depend on the expression of the polymeric immunoglobulin receptor (pIgR) in lung epithelial cells, we evaluated the expression of the pIgR gene in these cells. pIgR is related to the process of transcytosis and the release of the secretory component (SC) to form S-IgA complex [70]. Several studies have demonstrated that pIgR expression is regulated by some cytokines, such as TNF-α, IFN-γ, TGF-β, as well as signal pathways activated by glucocorticoid receptors [71]. Hence, it has been shown that IL-4 is capable of downregulating bronchial epithelial pIgR expression in asthma [72]. Therefore, the relative expression of the pIgR gene had no difference in the stress group, but it was overexpressed in the bLf + RS group. It has been reported that in an acute stress model, bLf promoted the upregulation of pIgR mRNA, while diminishing protein expression in the proximal intestine. Conversely, in the distal small intestine, pIgR mRNA was not modified, but protein expression increased [47]. Despite this, studies on cultures of primary human bronchial epithelial cells from smokers demonstrated that pIgR protein expression is not necessarily related to its gene expression, and this could be related to post-transcriptional mechanisms [73,74].
In previous studies, the bLf administration modulates IgA+ plasma cell populations of the distal small intestine [63]. This is the first report to focus on the effect of stress on lung immunoglobulins, demonstrating bLf’s capability of modulating the decrease in IgA+ plasma cell population and S-IgA low expression induced by stress. Nevertheless, further studies are required to clarify if it is related to pIgR protein expression and the mechanisms and specific signaling pathways implicated. These findings suggest that lactoferrin may serve as another protective mechanism, acting as a prophylactic and adjuvant therapeutic agent in lower respiratory tract diseases.
Numerous studies have investigated the role of stress in pulmonary diseases. It has been widely reported that it induces airway inflammation [9] via HPA activation [10]. Consequently, asthma symptoms are exacerbated by the promotion of an increase in CD4+ Th2 cells [75] and the further secretion of cytokines, such as IL-4, IL-5, and IL-13 [76,77,78,79]. At the same time, Th1 cytokines are diminished [80,81]. Asthma symptoms improve when a glucocorticoid receptor antagonist is administered [82]. There are also findings in infectious diseases. In a model of the influenza virus, which demonstrates that stress promotes a Th2 profile, thereby decreasing antiviral defense and worsening the disease’s development [83]. Lafuse et al. have reported that stress improves IL-10 levels by inducing psychological stress in mice infected with Mycobacterium tuberculosis (MTB), which promotes pathogenicity [84]. We found that T CD4+/IL-4+ and T CD4+/IL-10+ populations were highly increased in the RS group, and our results are consistent with these reports, suggesting that chronic stress might be promoting lung susceptibility to infectious and respiratory diseases.
On the other hand, numerous studies have demonstrated the immunomodulatory effects of lactoferrin in various respiratory pathologies by promoting or downregulating inflammatory responses [85]. It has been reported that oral administration of bLf diminished viral load in BALB/c mice infected with the influenza virus, and it also promotes tissue repair response by decreasing cell infiltration [13]. Furthermore, some findings suggest bLf is capable of downregulating asthma symptoms and ovalbumen-induced lung inflammation [86]. In an MTB infection, bLf demonstrated its ability to increase the number of CD4+ Th1 cytokine-producing cells in the lung, thereby promoting the amelioration of pathological response and preventing the formation of granuloma [64,87]. Some authors have hypothesized that bLf could be a novel prophylactic and therapeutic agent for ameliorating COVID-19 and other infectious lung diseases [88]. Our results demonstrated that bLf modulates the increase in Th2 lymphocyte populations during chronic stress and promotes a Th1 profile, thereby balancing the cytokine profile in the lung. These effects may help prevent the exacerbation of chronic pulmonary or allergic diseases and reduce the risk of infectious diseases of the lower respiratory airways; however, further studies are required to clearly understand the mechanisms involved in signaling these immunological events.
The role of macrophages and antigen-presenting cells is a notable finding in recent studies aimed at elucidating the mechanisms of pulmonary homeostasis management. Among other functions, these cells are the first to protect the lower respiratory tract against pathogens and coordinate and regulate respiratory secretions [89]. Their presence is also associated with the prevention of asthma exacerbation [90]. There is evidence that stress diminishes APC population, such as dendritic cells [82], and this might be related to a decrease in IL-12 release in response to glucocorticoids [91]. In this study, chronic stress diminished CD64+/CD86+ cell populations; however, this effect was mitigated by the prophylactic administration of bLf.
The evidence provided in this study demonstrated that oral administration of bLf modulates the effect of chronic stress in the serum and TBL immunoglobulins, prevents the decrease in S-IgA and IgA+ plasma cell populations promoted by stress, and counterbalances Th1-Th2 lymphocyte profiles. Even when more studies are conducted to clarify these findings, such as the protein expression of pIgR and the role of macrophages in lung immunity, we hypothesize that this may be a protective effect of bLf, maintaining the innate immune system in a state that allows it to mount a response. This assertion is supported by the fact that bLf administration had no changes without exposure to stress stimulus. Thus, this study provides a first overview of the potential prophylactic and co-therapeutic administration of bLf to prevent low respiratory infectious diseases and amelioration of chronic lung diseases.
4. Materials and Methods
4.1. Animals
A total of 40 male BALB/c mice (aged 10–12 weeks old and weighing 25–30 g) were used. Mice were housed in transparent polycarbonate boxes with sterile shavings bed and kept on a 12 h light/dark cycle (lights on at 6:00 a.m.) at room temperature at 20 °C, with relative humidity of 55% and provided with water and Purina Lab Diet 5001. Animals were handled according to a protocol (ESM-CICUAL-03/06-09-2020) in accordance with the Mexican federal regulations for animal experimentation and care (NOM-062-ZOO-1999, Ministry of Agriculture, SAGARPA, Mexico City, Mexico), and the experiments were approved by the Institutional Animal Care and Use Committee of the Escuela Superior de Medicina, Instituto Politécnico Nacional.
4.2. Stress and bLf Administration Protocol
Mice were randomly divided into four experimental groups with n = 10: (a) Control (CTL): kept for 14 days in housing with water and food ad libitum, but this group was devoid of drinking and food intake while RS was performed. (b) Restraint stress (RS): mice were maintained for six more days with minimal manipulation, and then, they were introduced into 9 cm large, 3 cm high, and 3.5 cm diameter cylindrical plexiglass movement restriction chambers with many holes for adequate ventilation [62] for four hours, from 8:00 a.m. to 12:00 p.m., for eight consecutive days [92]. (c) bLf: A total of 5 mg of bovine lactoferrin diluted in 100 µL of vehicle (sterile water) was administered by buccal deposition daily, for a period of 14 consecutive days [63,93]. (d) bLf + RS: A total of 5 mg of bovine lactoferrin diluted in 100 µL of sterile water was administered daily, for a period of six days before stress induction. Administration of bLf continued for eight more days (for a total of 14 days), during which the mice were subjected to movement restriction stress for four hours (Figure 1A).
4.3. Mice Weight
Mice were weighed first upon arrival at the laboratory, before the stress protocol, and after the experimental model, right before euthanasia. The weight registered at the beginning of each week was subtracted from the next measure to analyze weight gain or loss (Figure 1B,C).
4.4. Blood Collection
Each mouse was euthanized by an intraperitoneal injection of a lethal dose of 100 mg/kg body weight pentobarbital sodium salt (cat. P3761, Sigma-Aldrich, Darmstadt, Germany) and exsanguinated by cardiac puncture. Approximately 1 mL of blood was collected and centrifuged at 3000 rpm for 10 min to obtain the serum, and it was stored at −20 °C for further use.
4.5. Corticosterone Assay
The corticosterone concentration in the plasma was determined using a commercially available ELISA kit according to the manufacturer’s instructions (cat. no. 501320 Cayman Chemical, Ann Arbor, MI, USA). Plasma samples’ corticosterone concentrations were calculated based on a standard curve and were expressed in pg/mL (Figure 2).
4.6. Tracheobronchial Lavage
Once exsanguination was performed, the mouse was fixed on a dissection table, and the thoracic cavity was exposed. An incision was made in the trachea by inserting a metal cannula, which was used to introduce 1 mL of 1X PBS into the lungs. Gentle massage and aspiration were then performed, and the lavage was recovered, placed in a microtube, and stored. The lavage was centrifuged at 1500 rpm at 4 °C for 10 min. The supernatant was collected and stored at −20 °C with brief modification [94].
4.7. Immunoglobulin Measurement
Concentrations of total IgM, IgG, and IgA, as well as secretory IgA (S-IgA), were determined in the tracheobronchial lavages by performing a sandwich ELISA with brief modifications [63]. The plate was coated with the capture antibody (anti-IgA, anti-IgM, anti-IgG) and incubated. HRP-coupled antibodies were added: anti-IgA (HRP goat anti-mouse cat. 626720 Life technologies, Carlsbad, CA, USA), anti-IgM (HRP goat anti-mouse cat. M31507 Life technologies, Carlsbad, CA, USA), anti-IgG (HRP goat anti-mouse cat. 626520), and anti-secretory component (HRP goat anti-mouse cat. sc-374343, Santacruz, Dallas, TX, USA). Absorbance was measured at 490 nm using an enzyme-linked immunosorbent assay reader (Sigma).
4.8. Lung Lymphocyte Cells Purification
Lungs were removed and incubated in 15 mL of 1X RPMI 1640 (cat. 23400-062, GIBCO, Carlsbad, CA, USA) supplemented with 1% fetal bovine serum (FBS), with 100 µM ethylenediaminetetraacetic acid (8993-01 J.T. Baker, Phillipsburg, NJ, USA) and 1 mM dithiothreitol (D9779, Sigma-Aldrich, Darmstadt, Germany) for 30 min at 37 °C. After incubation, tissue dissociation was performed using a plunger and a steel mesh, followed by filtration and centrifugation. The cell button was subjected to 75%/40% Percoll gradients to obtain leukocytes and 40%/20% Percoll gradients to obtain epithelial cells. Interphase cell rings were collected and washed to obtain a pellet [95].
4.9. Flow Cytometry Assay
Suspension of 1 × 106 cells was incubated with corresponding extracellular markers for plasma cells (anti-CD19/PE cat. 553786, anti-CD138/APC cat. 558626 BD Biosciences, Franklin Lakes, NJ, USA), APC (anti-CD64/PE cat. 558455, BD Biosciences, anti-CD86 cat. 105012, BioLegend, San Diego, CA, USA), and T CD4+ cells (anti-CD4/PerCP cat. 100538, BioLegend). Subsequently, APCs were fixed with 4% paraformaldehyde. T lymphocytes and plasma cells were fixed and permeabilized by incubating for 20 min in the dark with Cytofix/Cytoperm (cat. 554722, BD Biosciences) and centrifuged at 1500 rpm at 4 °C for 5 min. Then, cells were washed with Perm Wash (cat. 554723, BD Biosciences) and incubated with antibody cocktails, respectively: anti-IgA/FITC (Cat. 559354, BD Biosciences) for plasma cells and Th1 (anti-IL-1β/FITC cat. IC413F, R&D Systems, anti-IL-12/APC cat. 554480, BD Biosciences) and Th2 (anti-IL-4/PE cat. 554435, BD Biosciences, anti-IL-10/FITC cat. 505005, BioLegend) for each analysis of intracellular cytokines. After incubation, the cell pellet was washed with Perm Wash, and then the samples were fixed and filtered [96]. Samples were stored at 4 °C in the dark until analysis on the FACS ARIA flow cytometer (Beckton Dickinson Company, Franklin Lakes, NJ, USA) with BD FACSDIVA™ v6.1 software (BD Biosciences), acquiring 20,000 gated events from each sample. The data were analyzed using FlowJo v10.10.0 (BD Life Sciences, Franklin Lakes, NJ, USA). Cell percentages were reported as the mean ± SD.
4.10. Real-Time qPCR of pIgR
4.10.1. RNA Extraction
The extraction of RNA from leukocytes was carried out by a gradient of TRIzol reagent (cat. Invitrogen™, 15596026, Life Technologies, Carlsbad, CA, USA) and the chloroform technique [97].
4.10.2. cDNA Synthesis
RNA was treated with the RQ1 RNase-Free Dnase Kit (cat. M6101, ThermoScientific, Carlsbad, CA, USA) following the manufacturer’s instructions. The synthesis of the cDNA was made using a commercial kit (RevertAid First Strand cDNA Synthesis kit, cat. K1622, ThermoScientific) by following the manufacturer’s instructions. cDNA samples were stored at −70 °C.
4.10.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The primers for pIgR and GAPDH were designed with Primer Express v.3.0.1 (Applied Biosystems) software and synthesized by UNIPARTS S.A. of C.V. (Table 1). For the PCR reactions, each well was filled with 10 µL of SYBR™ Green PCR Master Mix (cat. 4309155, Applied Biosystems™, Thermo Fischer Scientific, Waltham, MA, USA); 0.2 µL Primer F; 0.2 µL Primer R; 7.6 µL of Water; and 2 µL of the sample. The amplification was carried out in the Step One Real-Time PCR System (Applied Biosystems™, Waltham, MA, USA) and was analyzed with the help of StepOne™ v2.3 software. Data normalization was carried out using the constitutive gene GAPDH for determination.

Table 1.
Primers sequences for pIgR and GAPDH used in the RT-qPCR assay.
4.11. Statistical Analysis
The results were analyzed using a one-way ANOVA and Tukey’s multiple comparisons test with GraphPad Prism v. 8.0.2. Data are presented as the mean ± standard deviation (SD). Significant differences were defined as p ≤ 0.05.
Author Contributions
Conceptualization I.M.A.-M., A.A.R.-A. and M.Y.-O.; methodology M.Y.-O., E.J.Z.-A., C.A.G.-C., B.M.-A., M.V.-T., D.R.-V., M.d.l.Á.G.-R. and U.A.G.-S.; software M.Y.-O., I.M.A.-M., A.A.R.-A. and M.V.-T.; validation I.M.A.-M., A.A.R.-A., M.V.-T. and J.P.-Y.; formal analysis I.M.A.-M., A.A.R.-A. and M.Y.-O.; investigation M.Y.-O., E.J.Z.-A., C.A.G.-C., B.M.-A., M.V.-T., D.R.-V., M.d.l.Á.G.-R. and U.A.G.-S.; resources I.M.A.-M., A.A.R.-A. and J.P.-Y.; data curation I.M.A.-M., A.A.R.-A. and M.Y.-O.; writing—original draft preparation M.Y.-O., I.M.A.-M. and A.A.R.-A.; writing—review and editing M.Y.-O., I.M.A.-M., A.A.R.-A., M.V.-T., D.R.-V.; visualization, M.Y.-O., I.M.A.-M., A.A.R.-A. and M.d.l.Á.G.-R.; supervision I.M.A.-M., A.A.R.-A. and J.P.-Y.; project administration I.M.A.-M. and A.A.R.-A.; funding acquisition I.M.A.-M. and A.A.R.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sistema de Administración de Programa y Proyectos de Investigación (SAPPI-IPN). Multidisciplinary Project, grant number 20230994. Mariazell Yépez-Ortega received financial support through the scholarship 20240519 by BEIFI and the scholarship 993755 granted by the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics in Research Committee of the Escuela Superior de Medicina of the Instituto Politecnico Nacional (ESM-CICUAL-03/06-09-2020) and according to the technical specifications for the production, care, and use of laboratory animals (Regulation-062-ZOO-1999, Ministry of Agriculture, SAGARPA, México City, México.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
None of the authors has any financial or other interest in the products, devices, or methods mentioned in this article.
Abbreviations
The following abbreviations are used in this manuscript:
| bLf | Bovine lactoferrin |
| RS | Restraint Stress |
| TBL | Tracheobronchial lavages |
| S-IgA | Secretory Immunoglobulin A |
| APC | Antigen Presenting Cells |
| COPD | Chronic Obstructive Pulmonary Disease |
| pIgR | Polymeric Immunoglobulin Receptor |
| mIgA | Monomeric ImmunoglobulinA |
| dIgA | Dimeric ImmunoglobulinA |
| SC | Secretory component |
| MTB | Mycobacterium tuberculosis |
References
- Dhabhar, F.S. Effects of Stress on Immune Function: The Good, the Bad, and the Beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Chrousos, G.P. The Hypothalamic–Pituitary–Adrenal Axis and Immune-Mediated Inflammation. N. Engl. J. Med. 1995, 332, 1351–1363. [Google Scholar] [CrossRef]
- Dhabhar, F.S.; Malarkey, W.B.; Neri, E.; McEwen, B.S. Stress-Induced Redistribution of Immune Cells—From Barracks to Boulevards to Battlefields: A Tale of Three Hormones–Curt Richter Award Winner. Psychoneuroendocrinology 2012, 37, 1345–1368. [Google Scholar] [CrossRef]
- Vittwrakis, S.; Gaga, M.; Oikonomidou, E.; Samitas, K.; Xwrianopoulos, D. Immunological Mechanisms in the Lung. Pneumon 2007, 20, 274–278. [Google Scholar]
- Ardain, A.; Marakalala, M.J.; Leslie, A. Tissue-resident Innate Immunity in the Lung. Immunology 2020, 159, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Chrousos, G.P. The Neuroendocrinology of Stress: The Stress-Related Continuum of Chronic Disease Development. Mol. Psychiatry 2022, 27, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S. Psychological Stress and Susceptibility to Upper Respiratory Infections. Am. J. Respir. Crit. Care Med. 1995, 152, S53–S58. [Google Scholar] [CrossRef]
- Leick, E.A.; Reis, F.G.; Honorio-Neves, F.A.; Almeida-Reis, R.; Prado, C.M.; Martins, M.A.; Tibério, I.F.L.C. Effects of Repeated Stress on Distal Airway Inflammation, Remodeling and Mechanics in an Animal Model of Chronic Airway Inflammation. Neuroimmunomodulation 2012, 19, 1–9. [Google Scholar] [CrossRef]
- Reis, F.G.; Marques, R.H.; Starling, C.M.; Almeida-Reis, R.; Vieira, R.P.; Cabido, C.T.; Silva, L.F.F.; Lanças, T.; Dolhnikoff, M.; Martins, M.A.; et al. Stress Amplifies Lung Tissue Mechanics, Inflammation and Oxidative Stress Induced by Chronic Inflammation. Exp. Lung Res. 2012, 38, 344–354. [Google Scholar] [CrossRef]
- Miyasaka, T.; Dobashi-Okuyama, K.; Takahashi, T.; Takayanagi, M.; Ohno, I. The Interplay between Neuroendocrine Activity and Psychological Stress-Induced Exacerbation of Allergic Asthma. Allergol. Int. 2018, 67, 32–42. [Google Scholar] [CrossRef]
- Lee, L.; Yu, J. Sensory Nerves in Lung and Airways. Compr. Physiol. 2014, 4, 287–324. [Google Scholar] [CrossRef]
- Kaczyńska, K.; Jampolska, M.; Wojciechowski, P.; Sulejczak, D.; Andrzejewski, K.; Zając, D. Potential of Lactoferrin in the Treatment of Lung Diseases. Pharmaceuticals 2023, 16, 192. [Google Scholar] [CrossRef]
- Shin, K.; Wakabayashi, H.; Yamauchi, K.; Teraguchi, S.; Tamura, Y.; Kurokawa, M.; Shiraki, K. Effects of Orally Administered Bovine Lactoferrin and Lactoperoxidase on Influenza Virus Infection in Mice. J. Med. Microbiol. 2005, 54, 717–723. [Google Scholar] [CrossRef]
- Han, N.; Li, H.; Li, G.; Shen, Y.; Fei, M.; Nan, Y. Effect of Bovine Lactoferrin as a Novel Therapeutic Agent in a Rat Model of Sepsis-Induced Acute Lung Injury. AMB Express 2019, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 550441. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F. Lactoferrin in Milk from Different Species. Comp. Biochem. Physiol. Part B Comp. Biochem. 1971, 39, 119–129. [Google Scholar] [CrossRef]
- Kanwar, J.; Roy, K.; Patel, Y.; Zhou, S.-F.; Singh, M.; Singh, D.; Nasir, M.; Sehgal, R.; Sehgal, A.; Singh, R.; et al. Multifunctional Iron Bound Lactoferrin and Nanomedicinal Approaches to Enhance Its Bioactive Functions. Molecules 2015, 20, 9703–9731. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Flores-Romo, L.; Oliver-Aguillón, G.; Jarillo-Luna, R.A.; Reyna-Garfias, H.; Barbosa-Cabrera, E.; Campos-Rodríguez, R. La Lactoferrina Como Modulador de La Respuesta Inmunitaria. Bioquimia 2008, 33, 71–82. [Google Scholar]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, Function and Applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Franco, D. Actividad Antimicrobiana de La Lactoferrina: Mecanismos y Aplicaciones Clínicas Potenciales. Rev. Latinoam. Microbiol. 2005, 47, 102–111. [Google Scholar] [PubMed]
- Baveye, S.; Elass, E.; Mazurier, J.; Spik, G.; Legrand, D. Lactoferrin: A Multifunctional Glycoprotein Involved in the Modulation of the Inflammatory Process. CCLM 1999, 37, 281–286. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, J.; Chen, G. Role and Mechanism of Chronic Restraint Stress in Regulating Energy Metabolism and Reproductive Function Through Hypothalamic Kisspeptin Neurons. J. Endocr. Soc. 2021, 5, A551–A552. [Google Scholar] [CrossRef]
- Harris, R.B.S.; Mitchell, T.D.; Simpson, J.; Redmann, S.M.; Youngblood, B.D.; Ryan, D.H. Weight Loss in Rats Exposed to Repeated Acute Restraint Stress Is Independent of Energy or Leptin Status. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 282, R77–R88. [Google Scholar] [CrossRef]
- Michel, C.; Duclos, M.; Cabanac, M.; Richard, D. Chronic Stress Reduces Body Fat Content in Both Obesity-Prone and Obesity-Resistant Strains of Mice. Horm. Behav. 2005, 48, 172–179. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Huang, G.; Wen, J.; Chen, G. Hypothalamic Kisspeptin Neurons Regulates Energy Metabolism and Reproduction Under Chronic Stress. Front. Endocrinol. 2022, 13, 844397. [Google Scholar] [CrossRef]
- López López, A. Chronic Unpredictable Mild Stress Progressively Disturbs Glucose Metabolism and Appetite Hormones In Rats. Acta Endocrinol. 2018, 14, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, L.; Zabolian, H. Antioxidant Effects of Bovine Lactoferrin on Dexamethasone-Induced Hypertension in Rat. ISRN Pharmacol. 2014, 2014, 943523. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Murakoshi, M.; Uchiyama, A. Anti-Obesity Effect of Lactoferrin; Subgroup Analysis Excluding Subjects with Obese and/or Hyper-LDL Cholesterolemia. Immunol. Endocr. Metab. Agents Med. Chem. 2018, 18, 105–109. [Google Scholar] [CrossRef]
- Jańczuk-Grabowska, A.; Czernecki, T.; Brodziak, A. Gene–Diet Interactions: Viability of Lactoferrin-Fortified Yoghurt as an Element of Diet Therapy in Patients Predisposed to Overweight and Obesity. Foods 2023, 12, 2929. [Google Scholar] [CrossRef]
- Spencer, R.L.; Deak, T. A Users Guide to HPA Axis Research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar] [CrossRef]
- Zefferino, R.; Di Gioia, S.; Conese, M. Molecular Links between Endocrine, Nervous and Immune System during Chronic Stress. Brain Behav. 2021, 11, e01960. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, H.; Guo, J.; Zhang, X.; Liang, X.; Wei, L.; Wang, Q.; Bi, H.; Gao, T. A Study of Sex Differences in the Biological Pathways of Stress Regulation in Mice. CNS Neurosci. Ther. 2025, 31, e70433. [Google Scholar] [CrossRef] [PubMed]
- Brivio, E.; Kos, A.; Ulivi, A.F.; Karamihalev, S.; Ressle, A.; Stoffel, R.; Hirsch, D.; Stelzer, G.; Schmidt, M.V.; Lopez, J.P.; et al. Sex Shapes Cell-Type-Specific Transcriptional Signatures of Stress Exposure in the Mouse Hypothalamus. Cell Rep. 2023, 42, 112874. [Google Scholar] [CrossRef]
- Mitsushima, D.; Yamada, K.; Takase, K.; Funabashi, T.; Kimura, F. Sex Differences in the Basolateral Amygdala: The Extracellular Levels of Serotonin and Dopamine, and Their Responses to Restraint Stress in Rats. Eur. J. Neurosci. 2006, 24, 3245–3254. [Google Scholar] [CrossRef] [PubMed]
- Heck, A.L.; Handa, R.J. Sex Differences in the Hypothalamic–Pituitary–Adrenal Axis’ Response to Stress: An Important Role for Gonadal Hormones. Neuropsychopharmacology 2019, 44, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Victoria, M.; Cruz-Hernández, T.R.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E.; Sánchez-Gómez, M.C.; Campos-Rodríguez, R. Modulation by Bovine Lactoferrin of Parameters Associated with the IgA Response in the Proximal and Distal Small Intestine of BALB/c Mice. Immunopharmacol. Immunotoxicol. 2017, 39, 66–73. [Google Scholar] [CrossRef]
- Guzmán-Mejía, F.; Vega-Bautista, A.; Molotla-Torres, D.E.; Aguirre-Garrido, J.F.; Drago-Serrano, M.E. Bovine Lactoferrin as a Modulator of Neuroendocrine Components of Stress. Curr. Mol. Pharmacol. 2021, 14, 1037–1045. [Google Scholar] [CrossRef]
- Harada, E.; Itoh, Y.; Sitizyo, K.; Takeuchi, T.; Araki, Y.; Kitagawa, H. Characteristic Transport of Lactoferrin from the Intestinal Lumen into the Bile via the Blood in Piglets. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999, 124, 321–327. [Google Scholar] [CrossRef]
- Talukder, M.J.R.; Takeuchi, T.; Harada, E. Receptor-Mediated Transport of Lactoferrin into the Cerebrospinal Fluid via Plasma in Young Calves. J. Vet. Med. Sci. 2003, 65, 957–964. [Google Scholar] [CrossRef]
- Kamemori, N.; Takeuchi, T.; Sugiyama, A.; Miyabayashi, M.; Kitagawa, H.; Shimizu, H.; Ando, K.; Harada, E. Trans-Endothelial and Trans-Epithelial Transfer of Lactoferrin into the Brain through BBB and BCSFB in Adult Rats. J. Vet. Med. Sci. 2008, 70, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Kamemori, N.; Takeuchi, T.; Hayashida, K.; Harada, E. Suppressive Effects of Milk-Derived Lactoferrin on Psychological Stress in Adult Rats. Brain Res. 2004, 1029, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Aleshina, G.M.; Yankelevich, I.A.; Zhakharova, E.T.; Kokryakov, V.N. Stress-protective effect of human lactoferrin. Ross. Fiziol. Zhurnal Im. IM Sechenova 2016, 102, 846–851. [Google Scholar]
- Takeuchi, T.; Hayashida, K.; Inagaki, H.; Kuwahara, M.; Tsubone, H.; Harada, E. Opioid Mediated Suppressive Effect of Milk-Derived Lactoferrin on Distress Induced by Maternal Separation in Rat Pups. Brain Res. 2003, 979, 216–224. [Google Scholar] [CrossRef]
- Maekawa, Y.; Sugiyama, A.; Takeuchi, T. Lactoferrin Ameliorates Corticosterone-Related Acute Stress and Hyperglycemia in Rats. J. Vet. Med. Sci. 2017, 79, 412–417. [Google Scholar] [CrossRef]
- Peña-Juárez, M.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Cruz-Hernández, T.R.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E. Effect of Bovine Lactoferrin Treatment Followed by Acute Stress on the IgA-Response in Small Intestine of BALB/c Mice. Immunol. Investig. 2016, 45, 652–667. [Google Scholar] [CrossRef]
- Cruz-Hernández, T.; Gómez-Jiménez, D.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M. Analysis of the Intestinal IgA Response in Mice Exposed to Chronic Stress and Treated with Bovine Lactoferrin. Mol. Med. Rep. 2020, 23, 126. [Google Scholar] [CrossRef]
- Vagnerová, K.; Vodička, M.; Hermanová, P.; Ergang, P.; Šrůtková, D.; Klusoňová, P.; Balounová, K.; Hudcovic, T.; Pácha, J. Interactions Between Gut Microbiota and Acute Restraint Stress in Peripheral Structures of the Hypothalamic–Pituitary–Adrenal Axis and the Intestine of Male Mice. Front. Immunol. 2019, 10, 2655. [Google Scholar] [CrossRef]
- Ergang, P.; Vagnerová, K.; Hermanová, P.; Vodička, M.; Jágr, M.; Šrůtková, D.; Dvořáček, V.; Hudcovic, T.; Pácha, J. The Gut Microbiota Affects Corticosterone Production in the Murine Small Intestine. Int. J. Mol. Sci. 2021, 22, 4229. [Google Scholar] [CrossRef]
- Rabot, S.; Jaglin, M.; Daugé, V.; Naudon, L. Impact of the Gut Microbiota on the Neuroendocrine and Behavioural Responses to Stress in Rodents. OCL 2016, 23, D116. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Tazume, S.; Shimizu, K.; Matsuzawa, H.; Dosako, S.; Isoda, H.; Tsukiji, M.; Fujimura, R.; Muranaka, Y.; Ishida, H. Inhibitory Effects of Bovine Lactoferrin on the Adherence of Enterotoxigenic Escherichia Coli to Host Cells. Biosci. Biotechnol. Biochem. 2000, 64, 348–354. [Google Scholar] [CrossRef]
- Molotla-Torres, D.E.; Hernández-Soto, L.M.; Guzmán-Mejía, F.; Godínez-Victoria, M.; Drago-Serrano, M.E.; Aguirre-Garrido, J.F. Oral Bovine Lactoferrin Modulation on Fecal Microbiota of Mice Underwent Immobilization Stress. J. Funct. Foods 2022, 95, 105153. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Chen, P.-W. Featured Prebiotic Agent: The Roles and Mechanisms of Direct and Indirect Prebiotic Activities of Lactoferrin and Its Application in Disease Control. Nutrients 2023, 15, 2759. [Google Scholar] [CrossRef]
- Griffiths, E.A.; Duffy, L.C.; Schanbacher, F.L.; Dryja, D.; Leavens, A.; Neiswander, R.L.; Qiao, H.; DiRienzo, D.; Ogra, P. In Vitro Growth Responses of Bifidobacteria and Enteropathogens to Bovine and Human Lactoferrin. Dig. Dis. Sci. 2003, 48, 1324–1332. [Google Scholar] [CrossRef]
- Watanabe, Y.; Arase, S.; Nagaoka, N.; Kawai, M.; Matsumoto, S. Chronic Psychological Stress Disrupted the Composition of the Murine Colonic Microbiota and Accelerated a Murine Model of Inflammatory Bowel Disease. PLoS ONE 2016, 11, e0150559. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rodríguez, R.; Godínez-Victoria, M.; Abarca-Rojano, E.; Pacheco-Yépez, J.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E. Stress Modulates Intestinal Secretory Immunoglobulin A. Front. Integr. Neurosci. 2013, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Silberman, D. Acute and Chronic Stress Exert Opposing Effects on Antibody Responses Associated with Changes in Stress Hormone Regulation of T-Lymphocyte Reactivity. J. Neuroimmunol. 2003, 144, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.Y. Immunoglobulin G and Its Function in the Human Respiratory Tract. Mayo Clin. Proc. 1988, 63, 161–174. [Google Scholar] [CrossRef]
- Pilette, C. Mucosal Immunity in Asthma and Chronic Obstructive Pulmonary Disease: A Role for Immunoglobulin A? Proc. Am. Thorac. Soc. 2004, 1, 125–135. [Google Scholar] [CrossRef]
- Reyna-Garfias, H.; Miliar, A.; Jarillo-Luna, A.; Rivera-Aguilar, V.; Pacheco-Yepez, J.; Baeza, I.; Campos-Rodríguez, R. Repeated Restraint Stress Increases IgA Concentration in Rat Small Intestine. Brain Behav. Immun. 2010, 24, 110–118. [Google Scholar] [CrossRef]
- Jarillo-Luna, A.; Rivera-Aguilar, V.; Garfias, H.R.; Lara-Padilla, E.; Kormanovsky, A.; Campos-Rodríguez, R. Effect of Repeated Restraint Stress on the Levels of Intestinal IgA in Mice. Psychoneuroendocrinology 2007, 32, 681–692. [Google Scholar] [CrossRef]
- Arciniega-Martínez, I.M.; Campos-Rodríguez, R.; Drago-Serrano, M.E.; Sánchez-Torres, L.E.; Cruz-Hernández, T.R.; Reséndiz-Albor, A.A. Modulatory Effects of Oral Bovine Lactoferrin on the IgA Response at Inductor and Effector Sites of Distal Small Intestine from BALB/c Mice. Arch. Immunol. Ther. Exp. 2016, 64, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Welsh, K.J.; Hwang, S.-A.; Boyd, S.; Kruzel, M.L.; Hunter, R.L.; Actor, J.K. Influence of Oral Lactoferrin on Mycobacterium Tuberculosis Induced Immunopathology. Tuberculosis 2011, 91, S105–S113. [Google Scholar] [CrossRef]
- Yamauchi, K.; Wakabayashi, H.; Shin, K.; Takase, M. Bovine Lactoferrin: Benefits and Mechanism of Action against Infections. Biochem. Cell Biol. 2006, 84, 291–296. [Google Scholar] [CrossRef]
- Van Splunter, M.E. Immunomodulation by Raw Bovine Milk and Its Ingredients: Effects in Nutritional Intervention, Oral Vaccination and Trained Immunity. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, September 2018. [Google Scholar]
- Corthésy, B. Multi-Faceted Functions of Secretory IgA at Mucosal Surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef]
- de Fays, C.; Carlier, F.M.; Gohy, S.; Pilette, C. Secretory Immunoglobulin A Immunity in Chronic Obstructive Respiratory Diseases. Cells 2022, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wu, Y.; Xu, Y.; Zheng, J. SIgA in Various Pulmonary Diseases. Eur. J. Med. Res. 2023, 28, 299. [Google Scholar] [CrossRef]
- Johansen, F.E.; Kaetzel, C.S. Regulation of the Polymeric Immunoglobulin Receptor and IgA Transport: New Advances in Environmental Factors That Stimulate PIgR Expression and Its Role in Mucosal Immunity. Mucosal Immunol. 2011, 4, 598–602. [Google Scholar] [CrossRef]
- Jang, Y.S.; Seo, G.-Y.; Lee, J.-M.; Seo, H.-Y.; Han, H.-J.; Kim, S.-J.; Jin, B.-R.; Kim, H.-J.; Park, S.-R.; Rhee, K.-J.; et al. Lactoferrin Causes IgA and IgG2b Isotype Switching through Betaglycan Binding and Activation of Canonical TGF-β Signaling. Mucosal Immunol. 2015, 8, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Ladjemi, M.Z.; Gras, D.; Dupasquier, S.; Detry, B.; Lecocq, M.; Garulli, C.; Fregimilicka, C.; Bouzin, C.; Gohy, S.; Chanez, P.; et al. Bronchial Epithelial IgA Secretion Is Impaired in Asthma. Role of IL-4/IL-13. Am. J. Respir. Crit. Care Med. 2018, 197, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Gohy, S.T.; Detry, B.R.; Lecocq, M.; Bouzin, C.; Weynand, B.A.; Amatngalim, G.D.; Sibille, Y.M.; Pilette, C. Polymeric Immunoglobulin Receptor Down-Regulation in Chronic Obstructive Pulmonary Disease. Persistence in the Cultured Epithelium and Role of Transforming Growth Factor-β. Am. J. Respir. Crit. Care Med. 2014, 190, 509–521. [Google Scholar] [CrossRef]
- Carlier, F.M.; Detry, B.; Lecocq, M.; Collin, A.M.; Planté-Bordeneuve, T.; Gérard, L.; Verleden, S.E.; Delos, M.; Rondelet, B.; Janssens, W.; et al. The Memory of Airway Epithelium Damage in Smokers and COPD Patients. Life Sci. Alliance 2024, 7, e202302341. [Google Scholar] [CrossRef]
- Cameron, L.; Palikhe, N.S.; Laratta, C.; Vliagoftis, H. Elevated Circulating Th2 Cells in Women With Asthma and Psychological Morbidity: A New Asthma Endotype? Clin. Ther. 2020, 42, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, R.; Kawano, T.; Yoshida, H.; Ishii, M.; Miyasaka, T.; Ohkawara, Y.; Takayanagi, M.; Takahashi, T.; Ohno, I. Maternal Separation as Early-Life Stress Causes Enhanced Allergic Airway Responses by Inhibiting Respiratory Tolerance in Mice. Tohoku J. Exp. Med. 2018, 246, 155–165. [Google Scholar] [CrossRef]
- Rinsho, B. Abstract of a presentation. In Proceedings of the 57th Meeting of the Japanese Society of Clinical Pathology, Tokyo, Japan, 9–12 September 2010. [Google Scholar]
- Dragunas, G.; De Oliveira, M.A.; Tavares-De-Lima, W.; Gosens, R.; Munhoz, C.D. Chronic Unpredictable Stress Exacerbates Allergic Airway Inflammation in Mice. In Proceedings of the Airway pharmacology and treatment. Eur. Respir. Soc. 2022, 8, 28. [Google Scholar]
- Okuyama, K.; Ohwada, K.; Sakurada, S.; Sato, N.; Sora, I.; Tamura, G.; Takayanagi, M.; Ohno, I. The Distinctive Effects of Acute and Chronic Psychological Stress on Airway Inflammation in a Murine Model of Allergic Asthma. Allergol. Int. 2007, 56, 29–35. [Google Scholar] [CrossRef]
- Okuyama, K.; Dobashi, K.; Miyasaka, T.; Yamazaki, N.; Kikuchi, T.; Sora, I.; Takayanagi, M.; Kita, H.; Ohno, I. The Involvement of Glucocorticoids in Psychological Stress-Induced Exacerbations of Experimental Allergic Asthma. Int. Arch. Allergy Immunol. 2014, 163, 297–306. [Google Scholar] [CrossRef]
- Sato, S.; Kawano, T.; Ike, E.; Takahashi, K.; Sakurai, J.; Miyasaka, T.; Miyauchi, Y.; Ishizawa, F.; Takayanagi, M.; Takahashi, T. IL-1β Derived Th17 Immune Responses Are a Critical Factor for Neutrophilic-Eosinophilic Airway Inflammation on Psychological Stress-Induced Immune Tolerance Breakdown in Mice. Int. Arch. Allergy Immunol. 2023, 184, 797–807. [Google Scholar] [CrossRef]
- Kawano, T.; Ouchi, R.; Ishigaki, T.; Masuda, C.; Miyasaka, T.; Ohkawara, Y.; Ohta, N.; Takayanagi, M.; Takahashi, T.; Ohno, I. Increased Susceptibility to Allergic Asthma with the Impairment of Respiratory Tolerance Caused by Psychological Stress. Int. Arch. Allergy Immunol. 2018, 177, 1–15. [Google Scholar] [CrossRef]
- Dobbs, C.M.; Feng, N.; Beck, F.M.; Sheridan, J.F. Neuroendocrine Regulation of Cytokine Production during Experimental Influenza Viral Infection: Effects of Restraint Stress-Induced Elevation in Endogenous Corticosterone. J. Immunol. 1996, 157, 1870–1877. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Wu, Q.; Kumar, N.; Saljoughian, N.; Sunkum, S.; Ahumada, O.S.; Turner, J.; Rajaram, M.V.S. Psychological Stress Creates an Immune Suppressive Environment in the Lung That Increases Susceptibility of Aged Mice to Mycobacterium Tuberculosis Infection. Front. Cell Infect. Microbiol. 2022, 12, 990402. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; Delagarza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef]
- Artym, J.; Kocięba, M.; Zaczyńska, E.; Adamik, B.; Kübler, A.; Zimecki, M.; Kruzel, M. Immunomodulatory Properties of Human Recombinant Lactoferrin in Mice: Implications for Therapeutic Use in Humans. Adv. Clin. Exp. Med. 2018, 27, 391–399. [Google Scholar] [CrossRef]
- Actor, J.K. Lactoferrin: A Modulator for Immunity against Tuberculosis Related Granulomatous Pathology. Mediat. Inflamm. 2015, 2015, 409596. [Google Scholar] [CrossRef]
- Zimecki, M.; Actor, J.K.; Kruzel, M.L. The Potential for Lactoferrin to Reduce SARS-CoV-2 Induced Cytokine Storm. Int. Immunopharmacol. 2021, 95, 107571. [Google Scholar] [CrossRef]
- Bain, C.C.; MacDonald, A.S. The Impact of the Lung Environment on Macrophage Development, Activation and Function: Diversity in the Face of Adversity. Mucosal Immunol. 2022, 15, 223–234. [Google Scholar] [CrossRef] [PubMed]
- de Heer, H.J.; Hammad, H.; Soullié, T.; Hijdra, D.; Vos, N.; Willart, M.A.M.; Hoogsteden, H.C.; Lambrecht, B.N. Essential Role of Lung Plasmacytoid Dendritic Cells in Preventing Asthmatic Reactions to Harmless Inhaled Antigen. J. Exp. Med. 2004, 200, 89–98. [Google Scholar] [CrossRef] [PubMed]
- DeKruyff, R.H.; Fang, Y.; Umetsu, D.T. Corticosteroids Enhance the Capacity of Macrophages to Induce Th2 Cytokine Synthesis in CD4+ Lymphocytes by Inhibiting IL-12 Production. J. Immunol. 1998, 160, 2231–2237. [Google Scholar] [CrossRef]
- Oros-Pantoja, R.; Jarillo-Luna, A.; Rivera-Aguilar, V.; Sánchez-Torres, L.E.; Godinez-Victoria, M.; Campos-Rodríguez, R. Effects of Restraint Stress on NALT Structure and Nasal IgA Levels. Immunol. Lett. 2011, 135, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Rivera-Aguilar, V.; Reséndiz-Albor, A.A.; Campos-Rodríguez, R. Lactoferrin Increases Both Resistance to Salmonella Typhimurium Infection and the Production of Antibodies in Mice. Immunol. Lett. 2010, 134, 35–46. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Job, E.R.; Saelens, X.; Roose, K. Bronchoalveolar Lavage of Murine Lungs to Analyze Inflammatory Cell Infiltration. J. Vis. Exp. 2017, 123, 55398. [Google Scholar] [CrossRef]
- Reséndiz-Albor, A.A.; Reina-Garfias, H.; Rojas-Hernández, S.; Jarillo-Luna, A.; Rivera-Aguilar, V.; Miliar-García, A.; Campos-Rodríguez, R. Regionalization of PIgR Expression in the Mucosa of Mouse Small Intestine. Immunol. Lett. 2010, 128, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Arciniega-Martínez, I.; Reséndiz Albor, A.; Cárdenas Jaramillo, L.; Gutiérrez-Meza, J.; Falfán-Valencia, R.; Arroyo, B.; Yépez-Ortega, M.; Pacheco-yépez, J.; Abarca-rojano, E. CD4+/IL-4+ Lymphocytes of the Lamina Propria and Substance P Promote Colonic Protection during Acute Stress. Mol. Med. Rep. 2021, 25, 63. [Google Scholar] [CrossRef]
- Velásquez-Torres, M.; Trujillo-Ferrara, J.G.; Godínez-Victoria, M.; Jarillo-Luna, R.A.; Tsutsumi, V.; Sánchez-Monroy, V.; Posadas-Mondragón, A.; Cuevas-Hernández, R.I.; Santiago-Cruz, J.A.; Pacheco-Yépez, J. Riluzole, a Derivative of Benzothiazole as a Potential Anti-Amoebic Agent against Entamoeba Histolytica. Pharmaceuticals 2023, 16, 896. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).