Gender and Allergy: Mechanisms, Clinical Phenotypes, and Therapeutic Response—A Position Paper from the Società Italiana di Allergologia, Asma ed Immunologia Clinica (SIAAIC)
Abstract
1. Introduction
2. Methods
3. Immunological Aspects of Allergic Diseases
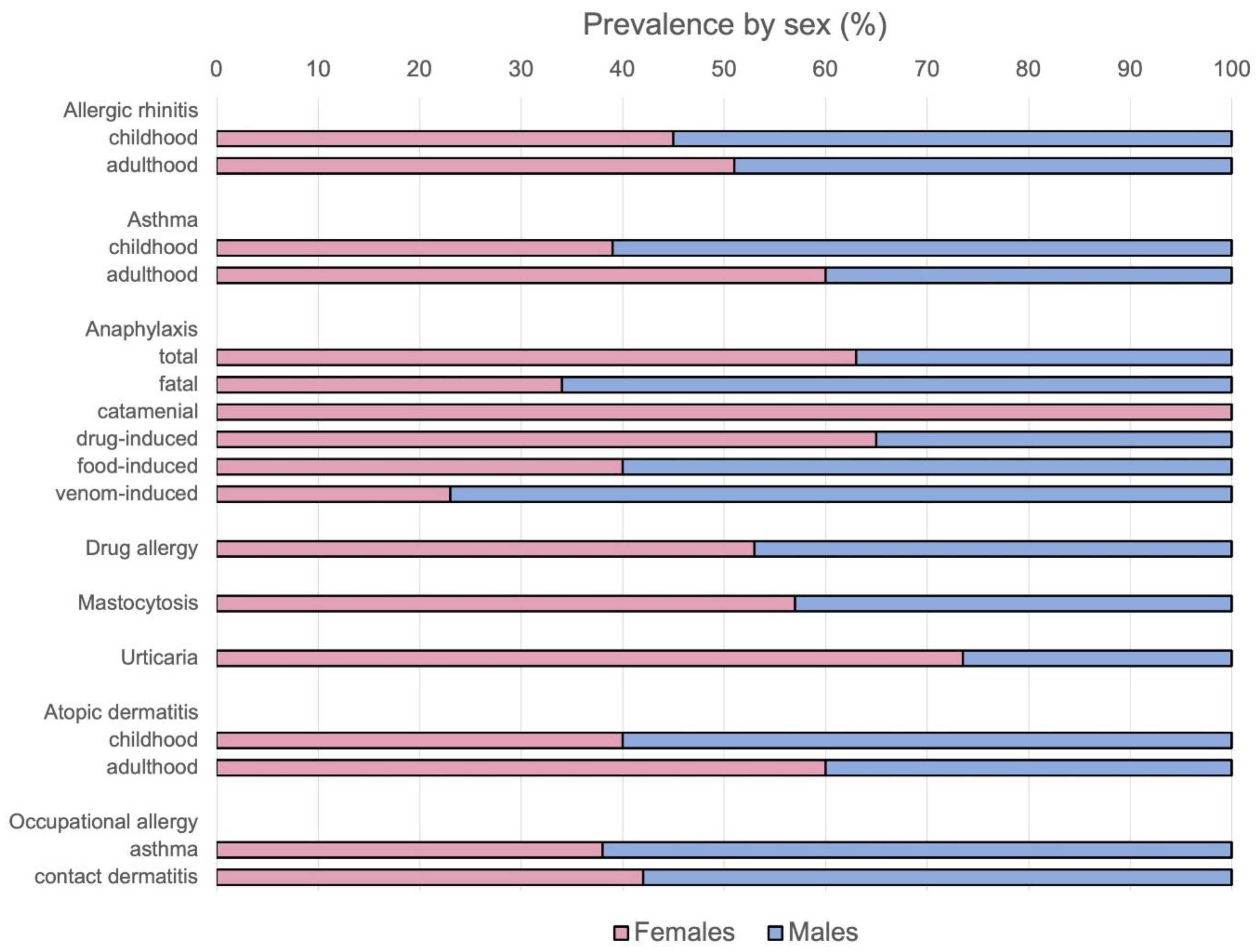
4. Allergic Rhinitis
5. Asthma
6. Anaphylaxis
7. Drug Hypersensitivity
8. Mastocytosis
9. Urticaria
10. Atopic Dermatitis and Allergic Contact Dermatitis
11. Gastrointestinal Allergies
12. Professional Allergies
13. Conclusions
13.1. Gender as a Key Determinant in Allergy Pathophysiology
13.2. The Need for a Gender-Specific Approach in Research
13.3. Implications for Clinical Practice and Personalised Therapy
13.4. Future Research Directions and Policy Recommendations
13.5. Towards Precision Medicine in Allergy and Immunology
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taborda-Barata, L.; Ventura, M.T.; Blain, H.; Brussino, L.; Kvedariene, V.; Larenas-Linneman, D.E.; Pham-Thi, N.; Samolinski, B.; Fonseca, J.A.; Bousquet, J.; et al. MASK-air® real-world data in respiratory allergy in old-age adults. Clin. Transl. Allergy 2023, 13, e12216. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Hickey, P.M.; Best, L.A.; Speed, D. Access to Healthcare and Unmet Needs in the Canadian Lesbian-Gay-Bisexual Population. J. Homosex. 2024, 71, 3276–3294. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.F.M.; Buquicchio, R.; Patella, V.; Bedbrook, A.; Bousquet, J.; Senna, G.; Ventura, M.T. Rediscovering Allergic Rhinitis: The Use of a Novel mHealth Solution to Describe and Monitor Health-Related Quality of Life in Elderly Patients. Int. Arch. Allergy Immunol. 2022, 183, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual Dimorphism in Innate Immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Cittadini, C.; Ortona, E.; Matarrese, P. Sex Disparity in Cancer: Role of Autophagy and Estrogen Receptors. Cells 2025, 14, 273. [Google Scholar] [CrossRef]
- Triggianese, P.; Novelli, L.; Galdiero, M.R.; Chimenti, M.S.; Conigliaro, P.; Perricone, R.; Perricone, C.; Gerli, R. Immune checkpoint inhibitors-induced autoimmunity: The impact of gender. Autoimmun. Rev. 2020, 19, 102590. [Google Scholar] [CrossRef]
- Gutiérrez-Brito, J.A.; Lomelí-Nieto, J.Á.; Muñoz-Valle, J.F.; Oregon-Romero, E.; Corona-Angeles, J.A.; Hernández-Bello, J. Sex hormones and allergies: Exploring the gender differences in immune responses. Front. Allergy 2024, 5, 1483919. [Google Scholar] [CrossRef]
- Trigunaite, A.; Dimo, J.; Jørgensen, T.N. Suppressive effects of androgens on the immune system. Cell. Immunol. 2015, 294, 87–94. [Google Scholar] [CrossRef]
- Triggianese, P.; Perricone, C.; Chimenti, M.S.; De Carolis, C.; Perricone, R. Innate Immune System at the Maternal-Fetal Interface: Mechanisms of Disease and Targets of Therapy in Pregnancy Syndromes. Am. J. Reprod. Immunol. 2016, 76, 245–257. [Google Scholar] [CrossRef]
- Ortona, E.; Pierdominici, M.; Rider, V. Editorial: Sex Hormones and Gender Differences in Immune Responses. Front. Immunol. 2019, 10, 1076. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Peeva, E.; Shoenfeld, Y. Gender and autoimmunity. Autoimmun. Rev. 2007, 6, 366–372. [Google Scholar] [CrossRef]
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef]
- De Vito, P.; Balducci, V.; Leone, S.; Percario, Z.; Mangino, G.; Davis, P.J.; Davis, F.B.; Affabris, E.; Luly, P.; Pedersen, J.Z.; et al. Nongenomic effects of thyroid hormones on the immune system cells: New targets, old players. Steroids 2012, 77, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Triggianese, P.; Perricone, C.; Conigliaro, P.; Chimenti, M.S.; Perricone, R.; De Carolis, C. Peripheral blood natural killer cells and mild thyroid abnormalities in women with reproductive failure. Int. J. Immunopathol. Pharmacol. 2016, 29, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Shelly, S.; Boaz, M.; Orbach, H. Prolactin and autoimmunity. Autoimmun. Rev. 2012, 11, A465–A470. [Google Scholar] [CrossRef]
- Triggianese, P.; Perricone, C.; Perricone, R.; De Carolis, C. Prolactin and natural killer cells: Evaluating the neuroendocrine-immune axis in women with primary infertility and recurrent spontaneous abortion. Am. J. Reprod. Immunol. 2015, 73, 56–65. [Google Scholar] [CrossRef]
- Mackey, E.; Ayyadurai, S.; Pohl, C.S.; D’ Costa, S.; Li, Y.; Moeser, A.J. Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol. Sex Differ. 2016, 7, 60. [Google Scholar] [CrossRef]
- Pinart, M.; Keller, T.; Reich, A.; Fröhlich, M.; Cabieses, B.; Hohmann, C.; Postma, D.S.; Bousquet, J.; Antó, J.M.; Keil, T. Sex-Related Allergic Rhinitis Prevalence Switch from Childhood to Adulthood: A Systematic Review and Meta-Analysis. Int. Arch. Allergy Immunol. 2017, 172, 224–235. [Google Scholar] [CrossRef]
- Fagan, J.K.; Scheff, P.A.; Hryhorczuk, D.; Ramakrishnan, V.; Ross, M.; Persky, V. Prevalence of asthma and other allergic diseases in an adolescent population: Association with gender and race. Ann. Allergy Asthma Immunol. 2001, 86, 177–184. [Google Scholar] [CrossRef]
- Lee, V.S.; Chiu, R.G.; Dick, A.I.; Nyenhuis, S.M.; Vajaranant, T.S.; Caskey, R.; Eldeirawi, K. Biological sex differences in rhinitis prevalence among adults in the United States: An “All of Us” Research Program database analysis. J. Allergy Clin. Immunol. Pract. 2024, 13, 950–952. [Google Scholar] [CrossRef]
- Dunn, S.E.; Perry, W.A.; Klein, S.L. Mechanisms and consequences of sex differences in immune responses. Nat. Rev. Nephrol. 2024, 20, 37–55. [Google Scholar] [CrossRef] [PubMed]
- LoMauro, A.; Aliverti, A. Sex and gender in respiratory physiology. Eur. Respir. Rev. 2021, 30, 210038. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, L.; Zhang, B.; Xie, S.; Meng, L.; Zhong, W.; Jia, J.; Zhang, H.; Jiang, W.; Xie, Z. Unraveling Sex-Based Differences in Efficacy and Safety of Subcutaneous Immunotherapy for Allergic Rhinitis: A Propensity Score-Matched Cohort Study. Int. Forum. Allergy Rhinol. 2025, 15, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Orban, N.; Maughan, E.; Bleach, N. Pregnancy-induced rhinitis. Rhinology 2013, 51, 111–119. [Google Scholar] [CrossRef]
- Favilli, A.; Laurenti, E.; Stagni, G.M.; Tassi, L.; Ricci, G.; Gerli, S. Effects of Sodium Hyaluronate on Symptoms and Quality of Life in Women Affected by Pregnancy Rhinitis: A Pilot Study. Gynecol. Obstet. Investig. 2019, 84, 159–165. [Google Scholar] [CrossRef]
- Gupta, K.K.; Anari, S. Medical management of rhinitis in pregnancy. Auris Nasus Larynx 2022, 49, 905–911. [Google Scholar] [CrossRef]
- Ridolo, E.; Incorvaia, C.; Martignago, I.; Caminati, M.; Canonica, G.W.; Senna, G. Sex in Respiratory and Skin Allergies. Clin. Rev. Allergy Immunol. 2019, 56, 322–332. [Google Scholar] [CrossRef]
- Yuan, L.; Tao, J.; Wang, J.; She, W.; Zou, Y.; Li, R.; Ma, Y.; Sun, C.; Bi, S.; Wei, S.; et al. Global, regional, national burden of asthma from 1990 to 2021, with projections of incidence to 2050: A systematic analysis of the global burden of disease study 2021. EClinicalMedicine 2025, 80, 103051. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef]
- McConnochie, K.M.; Russo, M.J.; McBride, J.T.; Szilagyi, P.G.; Brooks, A.M.; Roghmann, K.J. Socioeconomic variation in asthma hospitalization: Excess utilization or greater need? Pediatrics 1999, 103, e75. [Google Scholar] [CrossRef]
- Syssoyev, D.; Mussina, K.; Poddighe, D.; Gaipov, A.; Galiyeva, D. All-cause hospital admissions and incidence of asthma in children in Kazakhstan: A population-based retrospective cohort study. Sci. Rep. 2025, 15, 8985. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-C.; Pajak, A.; Teitelbaum, S.L.; Vangeepuram, N.; Galvez, M.; Pinney, S.M.; Windham, G.; Kushi, L.H.; Biro, F.M.; Wolff, M.S.; et al. Younger pubertal age is associated with allergy and other atopic conditions in girls. Pediatr. Allergy Immunol. 2014, 25, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, F.S.; Bonini, M.; Forgione, A.; Melandri, S.; Usmani, O.S. Asthma and gender: The female lung. Pharmacol. Res. 2017, 119, 384–390. [Google Scholar] [CrossRef]
- Reale, C.; Invernizzi, F.; Panteghini, C.; Garavaglia, B. Genetics, sex, and gender. J. Neurosci. Res. 2023, 101, 553–562. [Google Scholar] [CrossRef]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef]
- Shah, S.A.; Tibble, H.; Pillinger, R.; McLean, S.; Ryan, D.; Critchley, H.; Price, D.; Hawrylowicz, C.M.; Simpson, C.R.; Soyiri, I.N.; et al. Hormone replacement therapy and asthma onset in menopausal women: National cohort study. J. Allergy Clin. Immunol. 2021, 147, 1662–1670. [Google Scholar] [CrossRef]
- Foschino Barbaro, M.P.; Costa, V.R.; Resta, O.; Prato, R.; Spanevello, A.; Palladino, G.P.; Martinelli, D.; Carpagnano, G.E. Menopausal asthma: A new biological phenotype? Allergy 2010, 65, 1306–1312. [Google Scholar] [CrossRef]
- Zaibi, H.; Touil, A.; Fessi, R.; Ben Amar, J.; Aouina, H. Asthma in Menopausal Women: Clinical and Functional Particularities. Tanaffos 2020, 19, 216–222. [Google Scholar]
- Haggerty, C.L.; Ness, R.B.; Kelsey, S.; Waterer, G.W. The impact of estrogen and progesterone on asthma. Ann. Allergy Asthma Immunol. 2003, 90, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Lauzon-Joset, J.F. Oestrogen amplifies pre-existing atopy-associated Th2 bias in an experimental asthma model. Clin. Exp. Allergy 2020, 50, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Deering-Rice, C.E.; Aamodt, S.E.; Huecksteadt, T.P.; Myers, E.J.; Sanders, K.A.; Paine, R.; Warren, K.J. Progesterone amplifies allergic inflammation and airway pathology in association with higher lung ILC2 responses. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 327, L65–L78. [Google Scholar] [CrossRef] [PubMed]
- Fuseini, H.; Cephus, J.-Y.; Wu, P.; Davis, J.B.; Contreras, D.C.; Gandhi, V.D.; Rathmell, J.C.; Newcomb, D.C. ERα Signaling Increased IL-17A Production in Th17 Cells by Upregulating IL-23R Expression, Mitochondrial Respiration, and Proliferation. Front. Immunol. 2019, 10, 2740. [Google Scholar] [CrossRef]
- Liu, R.; Lauridsen, H.M.; Amezquita, R.A.; Pierce, R.W.; Jane-Wit, D.; Fang, C.; Pellowe, A.S.; Kirkiles-Smith, N.C.; Gonzalez, A.L.; Pober, J.S. IL-17 Promotes Neutrophil-Mediated Immunity by Activating Microvascular Pericytes and Not Endothelium. J. Immunol. 2016, 197, 2400–2408. [Google Scholar] [CrossRef]
- Zhang, P.; Zein, J. Novel Insights on Sex-Related Differences in Asthma. Curr. Allergy Asthma Rep. 2019, 19, 44. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Di Silvestre, D.; Ginaldi, L. Sex and Gender Aspects for Patient Stratification in Allergy Prevention and Treatment. Int. J. Mol. Sci. 2020, 21, 1535. [Google Scholar] [CrossRef]
- Vrieze, A.; Postma, D.S.; Kerstjens, H.A. m Perimenstrual asthma: A syndrome without known cause or cure. J. Allergy Clin. Immunol. 2003, 112, 271–282. [Google Scholar] [CrossRef]
- Graziottin, A.; Serafini, A. Perimenstrual asthma: From pathophysiology to treatment strategies. Multidiscip. Respir. Med. 2016, 11, 30. [Google Scholar] [CrossRef]
- Yuan, T.; Li, Y. The association between free testosterone and current asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3245. [Google Scholar] [CrossRef]
- Wang, X.; Huang, L.; Jiang, S.; Cheng, K.; Wang, D.; Luo, Q.; Wu, X.; Zhu, L. Testosterone attenuates pulmonary epithelial inflammation in male rats of COPD model through preventing NRF1-derived NF-κB signaling. J. Mol. Cell. Biol. 2021, 13, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Youness, A.; Cenac, C.; Faz-López, B.; Grunenwald, S.; Barrat, F.J.; Chaumeil, J.; Mejía, J.E.; Guéry, J.-C. TLR8 escapes X chromosome inactivation in human monocytes and CD4+ T cells. Biol. Sex Differ. 2023, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.M.; Lieberman, P. Anaphylaxis: A review of 601 cases. Ann. Allergy Asthma Immunol. 2006, 97, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.; Edenharter, G.; Ruëff, F.; Scherer, K.; Pföhler, C.; Mahler, V.; Treudler, R.; Lang, R.; Nemat, K.; Koehli, A.; et al. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy 2012, 67, 691–698. [Google Scholar] [CrossRef]
- González-Pérez, A.; Aponte, Z.; Vidaurre, C.F.; Rodríguez, L.A.G. Anaphylaxis epidemiology in patients with and patients without asthma: A United Kingdom database review. J. Allergy Clin. Immunol. 2010, 125, 1098–1104.e1. [Google Scholar] [CrossRef]
- Roh, M.-H.L.E.-J.; Jung, Y.-M.; Ahn, Y.; Chung, E.H. Characteristics of anaphylaxis patients who visited emergency departments in Korea: Results from a national emergency department information system. PLoS ONE 2022, 17, e0266712. [Google Scholar] [CrossRef]
- Yao, T.-C.; Wu, A.C.; Huang, Y.-W.; Wang, J.-Y.; Tsai, H.-J. Increasing trends of anaphylaxis-related events: An analysis of anaphylaxis using nationwide data in Taiwan, 2001–2013. World Allergy Organ. J. 2018, 11, 23. [Google Scholar] [CrossRef]
- Worm, M.; Höfer, V.; Dölle-Bierke, S.; Bilo, M.B.; Hartmann, K.; Sabouraud-Leclerc, D.; Treudler, R. Occupational anaphylaxis-Data from the anaphylaxis registry. Allergy 2024, 79, 702–710. [Google Scholar] [CrossRef]
- Turner, P.J.; Baumert, J.L.; Beyer, K.; Boyle, R.J.; Chan, C.-H.; Clark, A.T.; Crevel, R.W.R.; DunnGalvin, A.; Fernández-Rivas, M.; Gowland, M.H.; et al. Can we identify patients at risk of life-threatening allergic reactions to food? Allergy 2016, 71, 1241–1255. [Google Scholar] [CrossRef]
- Yu, R.J.; Krantz, M.S.; Phillips, E.J.; Stone, C.A. Emerging Causes of Drug-Induced Anaphylaxis: A Review of Anaphylaxis-Associated Reports in the FDA Adverse Event Reporting System (FAERS). J. Allergy Clin. Immunol. Pract 2021, 9, 819–829.e2. [Google Scholar] [CrossRef]
- Dhopeshwarkar, N.; Sheikh, A.; Doan, R.; Topaz, M.; Bates, D.W.; Blumenthal, K.G.; Zhou, L. Drug-Induced Anaphylaxis Documented in Electronic Health Records. J. Allergy Clin. Immunol. Pract. 2019, 7, 103–111. [Google Scholar] [CrossRef]
- Sugizaki, C.; Sato, S.; Yanagida, N.; Ebisawa, M. Analysis of drug-induced anaphylaxis cases using the Japanese Adverse Drug Event Report (JADER) database-Secondary publication. Allergol. Int. 2023, 72, 580–587. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Dhami, S.; Sheikh, A. Idiopathic Anaphylaxis. Curr. Treat. Options Allergy 2017, 4, 312–319. [Google Scholar] [CrossRef]
- Höfer, V.; Dölle-Bierke, S.; Francuzik, W.; Ruëff, F.; Sabouraud-Leclerc, D.; Treudler, R.; Moeser, A.; Hartmann, K.; Pföhler, C.; Wagner, N.; et al. Fatal and Near-Fatal Anaphylaxis: Data From the European Anaphylaxis Registry and National Health Statistics. J. Allergy Clin. Immunol. Pract. 2024, 12, 96–105.e8. [Google Scholar] [CrossRef] [PubMed]
- Bilò, M.B.; Corsi, A.; Martini, M.; Penza, E.; Grippo, F.; Bignardi, D. Fatal anaphylaxis in Italy: Analysis of cause-of-death national data, 2004–2016. Allergy 2020, 75, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Salciccioli, I.; Bhatt, P.; Shalhoub, J.; Marshall, D.; Salciccioli, J.; Blumenthal, K. Persistent sex and race disparities in United States anaphylaxis mortality from 1999 to 2020. Allergy 2024, 79, 2255–2258. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, D.; Foran, T.; Richards, S.; Grover, S.R. Catamenial anaphylaxis in adolescents and young adults: A case series. J. Allergy Clin. Immunol. Pract. 2025, 13, 220–224. [Google Scholar] [CrossRef]
- Lavery, W.J.; Bernstein, J.A. Cyclical hypersensitivity, anaphylaxis, and related hormonal reaction. Ann. Allergy Asthma. Immunol. 2019, 122, 140–147. [Google Scholar] [CrossRef]
- Foer, D.; Buchheit, K.M.; Gargiulo, A.R.; Lynch, D.M.; Castells, M.; Wickner, P.G. Progestogen Hypersensitivity in 24 Cases: Diagnosis, Management, and Proposed Renaming and Classification. J. Allergy. Clin. Immunol. Pract. 2016, 4, 723–729. [Google Scholar] [CrossRef]
- Shank, J.; Olney, S.; Li, F.; McNamara, M. Recurrent post partum anaphylaxis with breast feeding. Obstetr. Gynecol. 2009, 114, 415–416. [Google Scholar] [CrossRef]
- Mayou, S.C.; Charles-Holmes, R.; Kenney, A.; Black, M.M. A premenstrual urticarial eruption treated with bilateral oophorectomy and hysterectomy. Clin. Exp. Dermatol. 1988, 13, 114–116. [Google Scholar] [CrossRef]
- Arroyo, A.C.; Sanchez, D.A.; Camargo, C.A.; Wickner, P.G.; Foer, D. Evaluation of Allergic Diseases in Transgender and Gender-Diverse Patients: A Case Study of Asthma. J. Allergy. Clin. Immunol. Pract 2022, 10, 352–354. [Google Scholar] [CrossRef]
- Omenaas, E.; Bakke, P.; Elsayed, S.; Hanoa, R.; Gulsvik, A. Total and specific serum IgE levels in adults: Relationship to sex, age and environmental factors. Clin. Exp. Allergy 1994, 24, 530–539. [Google Scholar] [CrossRef]
- Barbee, R.A.; Halonen, M.; Lebowitz, M.; Burrows, B. Distribution of IgE in a community opulation sample: Correlations with age, sex, and allergen skin test reactivity. JACI 1981, 68, 106. [Google Scholar]
- Melén, E.; Bergstrom, A. I.K. Male sex is strongly associated with IgE sensitization to airborne but not food allergens: Results up to age 24 years from the BAMSE birth cohort. Clin. Transl. Allergy 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Piccinni, M.P.; Giudizi, M.G.; Biagiotti, R.; Beloni, L.; Giannarini, L.; Sampognaro, S.; Parronchi, P.; Manetti, R.; Annunziato, F.; Livi, C. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J. Immunol. 1995, 155, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.L.; Silva, M.; Barbosa, M.J.; Ferreira, F.; Fragoso, A.S.; Azenha Rama, T. Defining hereditary alpha-tryptasemia as a risk/modifying factor for anaphylaxis: Are we there yet? Eur. Ann. Allergy. Clin. Immunol. 2023, 55, 152–160. [Google Scholar] [CrossRef]
- Korošec, P.; Lyons, J.J.; Svetina, M.; Koudová, M.; Bittóová, M.; Zidarn, M.; Sedláčková, L.; Rijavec, M.; Kopač, P. Hereditary α-tryptasemia is Associated With Anaphylaxis to Antibiotics and Monoclonal Antibodies. J. Allergy Clin. Immunol. Pract. 2025, 13, 1449–1456.e4. [Google Scholar] [CrossRef]
- Puel, M.; Rossignol, J. C.D. Redefining tryptase norms in the pediatric population reveals sex-based differences: Clinical implicantions. Allergy 2024, 80, 335–338. [Google Scholar] [CrossRef]
- Jensen, F.; Woudwyk, M.; Teles, A.; Woidacki, K.; Taran, F.; Costa, S.; Malfertheiner, S.F.; Zenclussen, A.C. Estradiol and progesterone regulate the migration of mast cells from the periphery to the uterus and induce their maturation and degranulation. PLoS ONE 2010, 5, e14409. [Google Scholar] [CrossRef]
- Guhl, S.; Artuc, M.; Zuberbier, T.; Babina, M. Testosterone exerts selective anti-inflammatory effects on human skin mast cells in a cell subset dependent manner. Exp. Dermatol. 2012, 21, 878–880. [Google Scholar] [CrossRef]
- Lee, E.Y.; Copaescu, A.M.; Trubiano, J.A.; Phillips, E.J.; Wolfson, A.R.; Ramsey, A. Drug Allergy in Women. J. Allergy Clin. Immunol. Pract. 2023, 11, 3615–3623. [Google Scholar] [CrossRef]
- Hanschmann, T.; Francuzik, W.; Dölle-Bierke, S.; Hofmeier, K.S.; Grabenhenrich, L.; Ruëff, F.; Renaudin, J.-M.; Pföhler, C.; Treudler, R.; Bilò, M.B.; et al. Different phenotypes of drug-induced anaphylaxis-Data from the European Anaphylaxis Registry. Allergy 2023, 78, 1615–1627. [Google Scholar] [CrossRef]
- Taylor, M.G.; Joerger, T.; Li, Y.; Scheurer, M.E.; Russo, M.E.; Gerber, J.S.; Palazzi, D.L. Factors Associated With Penicillin Allergy Labels in Electronic Health Records of Children in 2 Large US Pediatric Primary Care Networks. JAMA Netw. Open 2022, 5, e222117. [Google Scholar] [CrossRef] [PubMed]
- Orlando, V.; Mucherino, S.; Guarino, I.; Guerriero, F.; Trama, U.; Menditto, E. Gender Differences in Medication Use: A Drug Utilization Study Based on Real World Data. Int. J. Environ. Res. Public Health 2020, 17, 3926. [Google Scholar] [CrossRef] [PubMed]
- Eaddy Norton, A.; Broyles, A.D. Drug allergy in children and adults: Is it the double X chromosome? Ann. Allergy Asthma Immunol. 2019, 122, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Jutel, M.; Agache, I.; Zemelka-Wiacek, M.; Akdis, M.; Chivato, T.; Del Giacco, S.; Gajdanowicz, P.; Gracia, I.E.; Klimek, L.; Lauerma, A.; et al. Nomenclature of allergic diseases and hypersensitivity reactions: Adapted to modern needs: An EAACI position paper. Allergy 2023, 78, 2851–2874. [Google Scholar] [CrossRef]
- Foer, D.; Wien, M.; Karlson, E.W.; Song, W.; Boyce, J.A.; Brennan, P.J. Patient Characteristics Associated With Reactions to Mrgprx2-Activating Drugs in an Electronic Health Record-Linked Biobank. J. Allergy Clin. Immunol. Pract. 2023, 11, 492–499.e2. [Google Scholar] [CrossRef]
- Gonçalo, M.; Coutinho, I.; Teixeira, V.; Gameiro, A.R.; Brites, M.M.; Nunes, R.; Martinho, A. HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br. J. Dermatol. 2013, 169, 660–665. [Google Scholar] [CrossRef]
- Wolfson, A.R.; Schatz, M.X. Management of the Pregnant Patient with Beta-Lactam Allergy. Curr. Allergy Asthma Rep. 2023, 23, 189–194. [Google Scholar] [CrossRef]
- Romano, A.; Atanaskovic-Markovic, M.; Barbaud, A.; Bircher, A.J.; Brockow, K.; Caubet, J.-C.; Celik, G.; Cernadas, J.; Chiriac, A.-M.; Demoly, P.; et al. Towards a more precise diagnosis of hypersensitivity to beta-lactams—An EAACI position paper. Allergy 2020, 75, 1300–1315. [Google Scholar] [CrossRef]
- Niedoszytko, M.; Gorska, A.; Brockow, K.; Bonadonna, P.; Lange, M.; Kluin-Nelemans, H.; Oude-Elberink, H.; Sabato, V.; Shoumariyeh, K.; von Bubnoff, D.; et al. Prevalence of hypersensitivity reactions in various forms of mastocytosis: A pilot study of 2485 adult patients with mastocytosis collected in the ECNM registry. Allergy 2024, 79, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Gülen, T.; Ljung, C.; Nilsson, G.; Akin, C. Risk Factor Analysis of Anaphylactic Reactions in Patients With Systemic Mastocytosis. J. Allergy Clin. Immunol. Pract. 2017, 5, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Bergström, A.; Hägglund, H.; Berglund, A.; Nilsson, G.; Lambe, M. Epidemiology of mastocytosis: A population-based study (Sweden). Acta. Oncol. 2024, 63, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, R.; Bonifacio, M.; Isolan, C.; Tanasi, I.; Crosera, L.; Olivieri, F.; Orsolini, G.; Schena, D.; Bonadonna, P. A Multidisciplinary Diagnostic Approach Reveals a Higher Prevalence of Indolent Systemic Mastocytosis: 15-Years’ Experience of the GISM Network. Cancers 2021, 13, 6380. [Google Scholar] [CrossRef]
- Trizuljak, J.; Sperr, W.R.; Nekvindová, L.; Elberink, H.O.; Gleixner, K.V.; Gorska, A.; Lange, M.; Hartmann, K.; Illerhaus, A.; Bonifacio, M.; et al. Clinical features and survival of patients with indolent systemic mastocytosis defined by the updated WHO classification. Allergy 2020, 75, 1927–1938. [Google Scholar] [CrossRef]
- Cohen, S.S.; Skovbo, S.; Vestergaard, H.; Kristensen, T.; Møller, M.; Bindslev-Jensen, C.; Fryzek, J.P.; Broesby-Olsen, S. Epidemiology of systemic mastocytosis in Denmark. Br. J. Haematol. 2014, 166, 521–528. [Google Scholar] [CrossRef]
- Ellingwood, S.S.; Kovalszki, A. Effect of Gender and Special Considerations for Women in Mastocytosis and Anaphylaxis. Immunol. Allergy Clin. N. Am. 2023, 43, 763–776. [Google Scholar] [CrossRef]
- Pardanani, A.; Shah, S.; Mannelli, F.; Elala, Y.C.; Guglielmelli, P.; Lasho, T.L.; Patnaik, M.M.; Gangat, N.; Ketterling, R.P.; Reichard, K.K.; et al. Mayo alliance prognostic system for mastocytosis: Clinical and hybrid clinical-molecular models. Blood Adv. 2018, 2, 2964–2972. [Google Scholar] [CrossRef]
- Kluin-Nelemans, H.C.; Jawhar, M.; Reiter, A.; van Anrooij, B.; Gotlib, J.; Hartmann, K.; Illerhaus, A.; Oude Elberink, H.N.G.; Gorska, A.; Niedoszytko, M.; et al. Cytogenetic and molecular aberrations and worse outcome for male patients in systemic mastocytosis. Theranostics 2021, 11, 292–303. [Google Scholar] [CrossRef]
- Alvarez-Twose, I.; Matito, A. Mastocytosis presenting as insect anaphylaxis: Gender differences and natural history. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Van der Veer, E.; Arends, S.; van der Hoek, S.; Versluijs, J.B.; de Monchy, J.G.R.; Oude Elberink, J.N.G.; van Doormaal, J.J. Predictors of new fragility fractures after diagnosis of indolent systemic mastocytosis. J. Allergy Clin. Immunol. 2014, 134, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Tanasi, I.; Crosera, L.; Taus, F.; Orsolini, G.; Adami, G.; Olivieri, F.; Bernardelli, A.; Bonadonna, P.; Nalin, F.; Sella, S.; et al. Underlying systemic mastocytosis in patients with unexplained osteoporosis: Score proposal. Bone 2024, 186, 117141. [Google Scholar] [CrossRef] [PubMed]
- Salvati, L.; Vitiello, G.; Parronchi, P. Gender differences in anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 417–424. [Google Scholar] [CrossRef]
- Matito, A.; Álvarez-Twose, I.; Morgado, J.M.; Sánchez-Muñoz, L.; Orfao, A.; Escribano, L. Clinical impact of pregnancy in mastocytosis: A study of the Spanish Network on Mastocytosis (REMA) in 45 cases. Int. Arch Allergy Immunol. 2011, 156, 104–111. [Google Scholar] [CrossRef]
- Ferrari, J.; Benvenuti, P.; Bono, E.; Fiorelli, N.; Elena, C. Mastocytosis: Fertility and Pregnancy Management in a Rare Disease. Front. Oncol. 2022, 12, 874178. [Google Scholar] [CrossRef]
- Arora, N.; Akin, C.; Kovalszki, A. Mastocytosis in Pregnancy. Immunol. Allergy Clin. N. Am. 2023, 43, 159–168. [Google Scholar] [CrossRef]
- Lei, D.; Akin, C.; Kovalszki, A. Management of Mastocytosis in Pregnancy: A Review. J. Allergy Clin. Immunol. Pract. 2017, 5, 1217–1223. [Google Scholar] [CrossRef]
- Bonadonna, P.; Scaffidi, L.; Boni, E. Tryptase values in anaphylaxis and insect allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 462–467. [Google Scholar] [CrossRef]
- Watson, K.D.; Arendt, K.W.; Watson, W.J.; Volcheck, G.W. Systemic mastocytosis complicating pregnancy. Obstet. Gynecol. 2012, 119, 486–489. [Google Scholar] [CrossRef]
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef]
- Cassano, N.; Colombo, D.; Bellia, G.; Zagni, E.; Vena, G.A. Gender-related differences in chronic urticaria. G. Ital. Di Dermatol. E Venereol. 2016, 151, 544–552. [Google Scholar]
- Kaplan, A.P. Chronic Spontaneous Urticaria: Pathogenesis and Treatment Considerations. Allergy Asthma Immunol. Res. 2017, 9, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Preis, S.; Claussen, C.; Ziehfreund, S.; Biedermann, T.; Horster, S.; Zink, A. Is there a difference between women and men in chronic spontaneous urticaria? A systematic review on gender and sex differences in CSU patients. World Allergy Organ. J. 2024, 17, 100974. [Google Scholar] [CrossRef] [PubMed]
- Erol, K.; Ertaş, Ş.K.; Ertaş, R. Fatigue Is Common and Predicted by Female Gender and Sleep Disturbance in Patients with Chronic Spontaneous Urticaria. J. Allergy Clin. Immunol. Pract. 2021, 9, 469–476. [Google Scholar] [CrossRef]
- Ertaş, R.; Erol, K.; Hawro, T.; Yılmaz, H.; Maurer, M. Sexual Functioning Is Frequently and Markedly Impaired in Female Patients with Chronic Spontaneous Urticaria. J. Allergy Clin. Immunol. Pract. 2020, 8, 1074–1082. [Google Scholar] [CrossRef]
- Amsler, E.; Augey, F.; Soria, A.; Boccon-Gibod, I.; Doutre, M.S.; Mathelier-Fusade, P.; Nicolas, J.F.; Rayson-Peyron, N.; Gompel, A. Chronic urticaria and hormones: Is there a link? J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1527–1530. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Bouillet, L.; Caballero, T.; Staevska, M. Hormonal Effects on Urticaria and Angioedema Conditions. J. Allergy Clin. Immunol. Pract. 2021, 9, 2209–2219. [Google Scholar] [CrossRef]
- Kim, Y.S.; Han, K.; Lee, J.H.; Kim, N.I.; Roh, J.Y.; Seo, S.J.; Song, H.J.; Lee, M.G.; Choi, J.H.; Park, Y.M. Increased Risk of Chronic Spontaneous Urticaria in Patients With Autoimmune Thyroid Diseases: A Nationwide, Population-based Study. Allergy Asthma. Immunol. Res. 2017, 9, 373–377. [Google Scholar] [CrossRef]
- Kocatürk, E.; Al-Ahmad, M.; Krause, K.; Gimenez-Arnau, A.M.; Thomsen, S.F.; Conlon, N.; Marsland, A.; Savk, E.; Criado, R.F.; Danilycheva, I.; et al. Effects of pregnancy on chronic urticaria: Results of the PREG-CU UCARE study. Allergy 2021, 76, 3133–3144. [Google Scholar] [CrossRef]
- Kocatürk, E.; Thomsen, S.F.; Al-Ahmad, M.; Gimenez Arnau, A.M.; Conlon, N.; Şavk, E.; Jardim Criado, R.F.; Danilycheva, I.; Fomina, D.; Khoshkhui, M.; et al. Total IgE levels are linked to the course of chronic spontaneous urticaria during pregnancy. J. Allergy Clin. Immunol. Pract. 2023, 11, 350–353. [Google Scholar] [CrossRef]
- Patruno, C.; Guarneri, F.; Nettis, E.; Bonzano, L.; Filippi, F.; Ribero, S.; Foti, C.; Rubegni, P.; Balato, A.; Miniello, A.; et al. Safety of omalizumab in chronic urticaria during pregnancy: A real-life study. Clin. Exp. Dermatol. 2024, 49, 344–347. [Google Scholar] [CrossRef]
- Liao, S.-L.; Yu, M.; Zhao, Z.-T.; Maurer, M. Case Report: Omalizumab for Chronic Spontaneous Urticaria in Pregnancy. Front. Immunol. 2021, 12, 652973. [Google Scholar] [CrossRef]
- Ornek, S.A.; Suroji Alkilinc, A.; Kızıltac, U.; Kızıltac, K.; Kocaturk, E. Effect of Puberty, Menstruation, Pregnancy, Lactation, and Menopause on Chronic Urticaria Activity. J. Cutan. Med. Surg. 2023, 27, 466–471. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Bassino, E.M.; De Pietro, F.; Ginaldi, L.; De Martinis, M. Sex differences in the efficacy of omalizumab in the treatment of chronic spontaneous urticaria. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211065870. [Google Scholar] [CrossRef] [PubMed]
- Bork, K.; Wulff, K.; Witzke, G.; Hardt, J. Treatment for hereditary angioedema with normal C1-INH and specific mutations in the F12 gene (HAE-FXII). Allergy 2017, 72, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Bouillet, L.; Boccon-Gibod, I.; Gompel, A.; Floccard, B.; Martin, L.; Blanchard-Delaunay, C.; Launay, D.; Fain, O. Hereditary angioedema with normal C1 inhibitor: Clinical characteristics and treatment response with plasma-derived human C1 inhibitor concentrate (Berinert®) in a French cohort. Eur. J. Dermatol. 2017, 27, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Piñero-Saavedra, M.; González-Quevedo, T.; Saenz de San Pedro, B.; Alcaraz, C.; Bobadilla-González, P.; Fernández-Vieira, L.; Hinojosa, B.; García-Lozano, R. Hereditary angioedema with F12 mutation: Clinical features and enzyme polymorphisms in 9 Southwestern Spanish families. Ann. Allergy Asthma. Immunol. 2016, 117, 520–526. [Google Scholar] [CrossRef]
- Firinu, D.; Bafunno, V.; Vecchione, G.; Barca, M.P.; Manconi, P.E.; Santacroce, R.; Margaglione, M.; Del Giacco, S.R. Characterization of patients with angioedema without wheals: The importance of F12 gene screening. Clin. Immunol. 2015, 157, 239–248. [Google Scholar] [CrossRef]
- Maurer, M.; Magerl, M.; Betschel, S.; Aberer, W.; Ansotegui, I.J.; Aygören-Pürsün, E.; Banerji, A.; Bara, N.-A.; Boccon-Gibod, I.; Bork, K.; et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2021 revision and update. Allergy 2022, 77, 1961–1990. [Google Scholar] [CrossRef]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. The Roles of Sex Hormones in the Course of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 4660. [Google Scholar] [CrossRef]
- Dirven-Meijer, P.C.; Glazenburg, E.J.; Mulder, P.G.H.; Oranje, A.P. Prevalence of atopic dermatitis in children younger than 4 years in a demarcated area in central Netherlands: The West Veluwe Study Group. Br. J. Dermatol. 2008, 158, 846–847. [Google Scholar] [CrossRef]
- Saeki, H.; Tsunemi, Y.; Fujita, H.; Kagami, S.; Sasaki, K.; Ohmatsu, H.; Watanabe, A.; Tamaki, K. Prevalence of atopic dermatitis determined by clinical examination in Japanese adults. J. Dermatol. 2006, 33, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Harrop, J.; Chinn, S.; Verlato, G.; Olivieri, M.; Norbäck, D.; Wjst, M.; Janson, C.; Zock, J.-P.; Leynaert, B.; Gislason, D.; et al. Eczema, atopy and allergen exposure in adults: A population-based study. Clin. Exp. Allergy 2007, 37, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Möhrenschlager, M.; Schäfer, T.; Huss-Marp, J.; Eberlein-König, B.; Weidinger, S.; Ring, J.; Behrendt, H.; Krämer, U. The course of eczema in children aged 5-7 years and its relation to atopy: Differences between boys and girls. Br. J. Dermatol. 2006, 154, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.; Stanzel, S.; Ocklenburg, C.; Merk, H.-F.; Baron, J.M.; Lehmann, S. Total serum IgE as a parameter to differentiate between intrinsic and extrinsic atopic dermatitis in children. Acta. Derm. Venereol. 2009, 89, 257–261. [Google Scholar] [CrossRef][Green Version]
- Garg, S.; McDonagh, A.J.G.; Gawkrodger, D.J. Age- and sex-related variations in allergic contact dermatitis to common allergens. Contact. Dermatitis 2009, 61, 46–47. [Google Scholar] [CrossRef]
- Boonchai, W.; Likittanasombat, S.; Viriyaskultorn, N.; Kanokrungsee, S. Gender differences in allergic contact dermatitis to common allergens. Contact. Dermatitis 2024, 90, 458–465. [Google Scholar] [CrossRef]
- Aitella, E.; De Martinis, M.; Romano, C.; Azzellino, G.; Ginaldi, L. Neurogenic Inflammation in Allergic Contact Dermatitis. Biomedicines 2025, 13, 656. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Kilinc, E.; Camsari, C.; Ogun, M.N. Effects of estrogen and progesterone on the neurogenic inflammatory neuropeptides: Implications for gender differences in migraine. Exp. Brain. Res. 2020, 238, 2625–2639. [Google Scholar] [CrossRef]
- Holm, E.A.; Esmann, S.; Jemec, G.B.E. Does visible atopic dermatitis affect quality of life more in women than in men? Gend. Med. 2004, 1, 125–130. [Google Scholar] [CrossRef]
- Osmana, M.; Hansellb, A.L.; Simpsona, C.R.; Hollowellc, J.; Helmsa, P.J. Gender-specific presentations for asthma, allergic rhinitis and eczema in primary care. Prim. Care Respir. J. 2007, 16, 28–35. [Google Scholar] [CrossRef]
- Moniaga, C.S.; Tominaga, M.; Takamori, K. An Altered Skin and Gut Microbiota Are Involved in the Modulation of Itch in Atopic Dermatitis. Cells 2022, 11, 3930. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Pali-Scholl, I. Adverse reactions to food: The female dominance—A secondary publication and update. World Allergy Organ. J. 2017, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Pali-Scholl, I. Adverse reactions to food: The female dominance. Allergologie 2017, 40, 101–110. [Google Scholar]
- Untersmayr, E.; Jensen, A.N.; Walch, K. Sex hormone allergy: Clinical aspects, causes and therapeutic strategies—Update and secondary publication. World Allergy Organ. J. 2017, 10, 45. [Google Scholar] [CrossRef]
- Jensen-Jarolim, E.; Untersmayr, E. Gender-medicine aspects in allergology. Allergy 2008, 63, 610–615. [Google Scholar] [CrossRef]
- Kelly, C.; Gangur, V. Sex Disparity in Food Allergy: Evidence from the PubMed Database. J. Allergy 2009, 2009, 159845. [Google Scholar] [CrossRef]
- Conner, T.S.; Mirosa, M.; Bremer, P.; Peniamina, R. The Role of Personality in Daily Food Allergy Experiences. Front. Psychol. 2018, 9, 29. [Google Scholar] [CrossRef]
- Agache, I.; Annesi-Maesano, I.; Cecchi, L.; Biagioni, B.; Chung, F.; D’Amato, G.; Damialis, A.; Del Giacco, S.; Dominguez Ortega, J.; Galán, C.; et al. EAACI Guidelines on Environmental Science for Allergy and Asthma-Recommendations on the Impact of Indoor Air Pollutants on the Risk of New-Onset Asthma and on Asthma-Related Outcomes. Allergy 2025, 80, 651–676. [Google Scholar] [CrossRef]
- Tarlo, S.M.; Lemiere, C. Occupational asthma. N. Engl. J. Med. 2014, 370, 640–649. [Google Scholar] [CrossRef]
- Chan, F.L.; Lipszyc, J.; Dekoven, B.; Nguyen, V.; Ribeiro, M.; Tarlo, S.M. Occupational asthma in Ontario, Canada (2000–2022): A retrospective, clinic-based study evaluating sex differences. J. Allergy Clin. Immunol. Pract. 2024, 12, 1073–1076.e2. [Google Scholar] [CrossRef]
- Moscato, G.; Vandenplas, O.; Van Wijk, R.G.; Malo, J.-L.; Perfetti, L.; Quirce, S.; Walusiak, J.; Castano, R.; Pala, G.; Gautrin, D.; et al. EAACI position paper on occupational rhinitis. Respir. Res. 2009, 10, 16. [Google Scholar] [CrossRef]
- Saito, K.; Orimo, K.; Kubo, T.; Tamari, M.; Yamada, A.; Motomura, K.; Sugiyama, H.; Matsuoka, R.; Nagano, N.; Hayashi, Y.; et al. Laundry detergents and surfactants-induced eosinophilic airway inflammation by increasing IL-33 expression and activating ILC2s. Allergy 2023, 78, 1878–1892. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Akdis, C.A.; Akdis, M.; Damialis, A.; Esposito, G.; Fergadiotou, I.; Goroncy, C.; Guitton, P.; Gotua, M.; Erotokritou, K.; et al. Addressing adverse synergies between chemical and biological pollutants at schools-The “SynAir-G” hypothesis. Allergy 2024, 79, 294–301. [Google Scholar] [CrossRef]
- Moscato, G.; Apfelbacher, C.; Brockow, K.; Eberle, C.; Genuneit, J.; Mortz, C.G.; Quecchia, C.; Quirce, S.; Siracusa, A.; Tarlo, S.M.; et al. Gender and occupational allergy: Report from the task force of the EAACI Environmental and Occupational Allergy Interest Group. Allergy 2020, 75, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
- Fishwick, D. New occupational and environmental causes of asthma and extrinsic allergic alveolitis. Clin. Chest. Med. 2012, 33, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Karagounis, T.K.; Cohen, D.E. Occupational Hand Dermatitis. Curr. Allergy Asthma. Rep. 2023, 23, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Salvati, L.; Vanni, E.; Acciai, M.C.; Parronchi, P. A gender-specific approach to occupational allergic contact dermatitis. Allergo. J. Int. 2021, 30, 109–114. [Google Scholar] [CrossRef]
- Lund, T.; Flachs, E.M.; Ebbehøj, N.E.; Bonde, J.P.; Agner, T. Wet work exposure: Comparison of observed and self-reported data. Int. Arch. Occup. Environ. Health 2019, 92, 317–326. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura, M.T.; Giuliano, A.F.M.; Boni, E.; Brussino, L.; Buquicchio, R.; Conte, M.; Costantino, M.T.; Crivellaro, M.A.; Giuliani, I.M.R.; Losa, F.; et al. Gender and Allergy: Mechanisms, Clinical Phenotypes, and Therapeutic Response—A Position Paper from the Società Italiana di Allergologia, Asma ed Immunologia Clinica (SIAAIC). Int. J. Mol. Sci. 2025, 26, 9605. https://doi.org/10.3390/ijms26199605
Ventura MT, Giuliano AFM, Boni E, Brussino L, Buquicchio R, Conte M, Costantino MT, Crivellaro MA, Giuliani IMR, Losa F, et al. Gender and Allergy: Mechanisms, Clinical Phenotypes, and Therapeutic Response—A Position Paper from the Società Italiana di Allergologia, Asma ed Immunologia Clinica (SIAAIC). International Journal of Molecular Sciences. 2025; 26(19):9605. https://doi.org/10.3390/ijms26199605
Chicago/Turabian StyleVentura, Maria Teresa, Antonio Francesco Maria Giuliano, Elisa Boni, Luisa Brussino, Rosalba Buquicchio, Mariaelisabetta Conte, Maria Teresa Costantino, Maria Angiola Crivellaro, Irene Maria Rita Giuliani, Francesca Losa, and et al. 2025. "Gender and Allergy: Mechanisms, Clinical Phenotypes, and Therapeutic Response—A Position Paper from the Società Italiana di Allergologia, Asma ed Immunologia Clinica (SIAAIC)" International Journal of Molecular Sciences 26, no. 19: 9605. https://doi.org/10.3390/ijms26199605
APA StyleVentura, M. T., Giuliano, A. F. M., Boni, E., Brussino, L., Buquicchio, R., Conte, M., Costantino, M. T., Crivellaro, M. A., Giuliani, I. M. R., Losa, F., Nicola, S., Parronchi, P., Peveri, S., Ridolo, E., Triggianese, P., & Patella, V. (2025). Gender and Allergy: Mechanisms, Clinical Phenotypes, and Therapeutic Response—A Position Paper from the Società Italiana di Allergologia, Asma ed Immunologia Clinica (SIAAIC). International Journal of Molecular Sciences, 26(19), 9605. https://doi.org/10.3390/ijms26199605









