Alogliptin Mitigates Methotrexate-Induced Nephrotoxicity in a Rat Model: Antagonizing Oxidative Stress, Inflammation and Apoptosis
Abstract
1. Introduction
2. Results
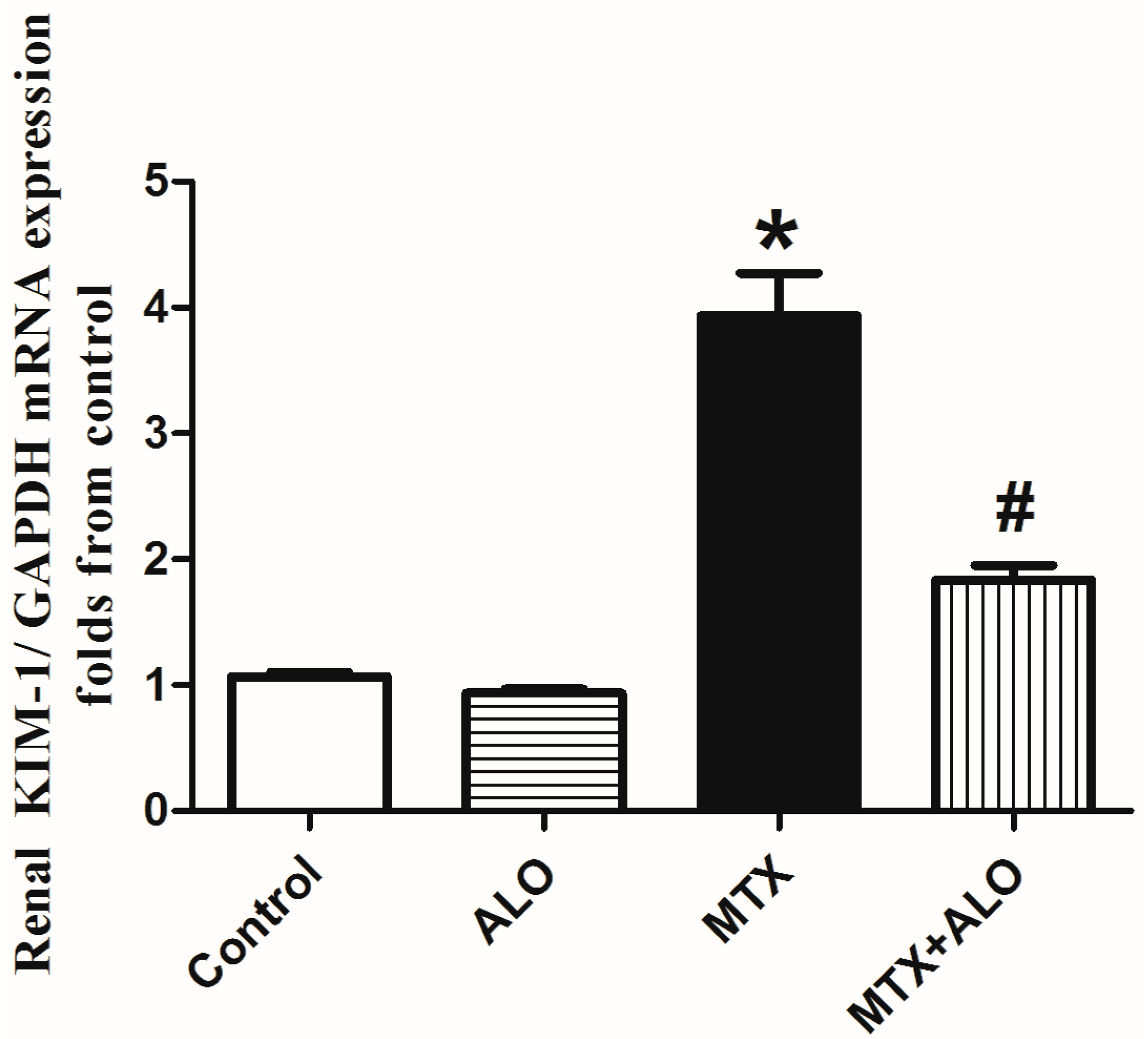
2.1. Effect of ALO on Glycemic Level, Renal Index, Serum Creatinine and Urea Concentration Besides mRNA Expression of KIM-1 in MTX-Induced Renal Damage
2.2. Histopathological Findings of Renal Tissues
2.3. Effect of ALO on Renal MDA, GSH, and Catalase in MTX-Induced Renal Damage
2.4. Effect of ALO on the Renal Nrf2/HO-1 Protein Expression
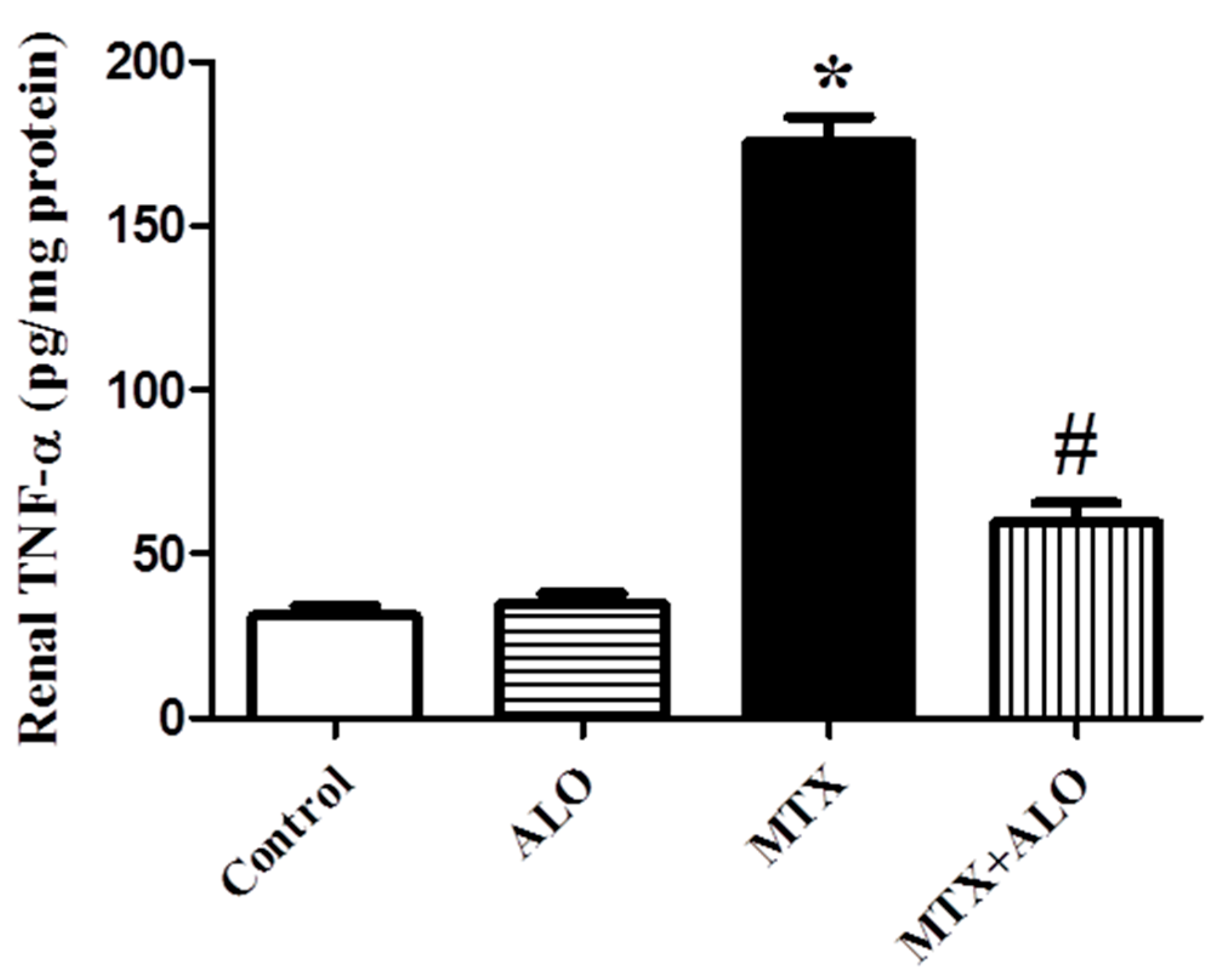
2.5. Effects of ALO on Renal Inflammatory Mediator (TNF-α) Protein Expression
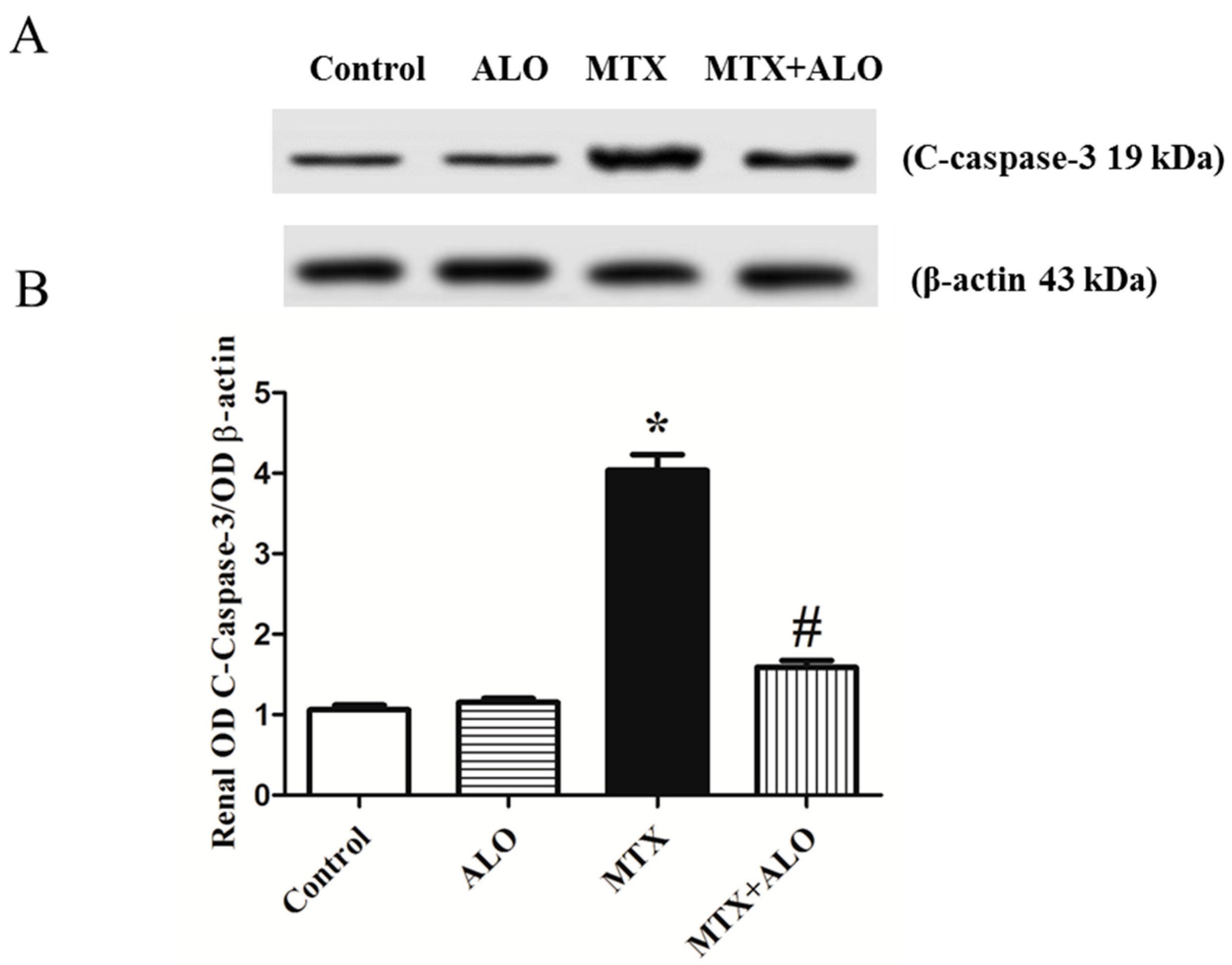
2.6. Effect of ALO on the Renal C-Caspase-3 Protein Expression
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Experimental Design
- The control group was given the vehicle of ALO, 0.5% carboxymethyl cellulose (CMC), orally for ten consecutive days and a single dose of saline via intraperitoneal route on the seventh day.
- The ALO group was orally administered ALO at a dosage of 20 mg/kg/day for ten days while being intraperitoneally injected with saline on the 7th day.
- The MTX group received 0.5% CMC orally for ten days and was exposed to a single intraperitoneal injection of MTX at a dosage of 20 mg/kg on day 7.
- The MTX + ALO group was given ALO orally at a dose of 20 mg/kg/day for ten days while receiving an intraperitoneal injection of MTX (20 mg/kg) on day 7 of the experimentation period.
4.3. Tissue and Blood Collection
4.4. Determination of Kidney Function Biomarkers
4.5. Assessment of KIM-1 mRNA Expression by Quantitative Real-Time (RT)-PCR
4.6. Histopathological Examination of Renal Tissues
4.7. Determination of Renal Oxidative Stress Parameters
4.8. Nrf2, HO-1, and C-Caspase-3 Assessment Using Western Blot Analysis
4.9. Determination of Renal TNF-α Protein Expression
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MTX | Methotrexate |
| ALO | Alogliptin |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| HO-1 | Heme oxygenase-1 |
| AKI | Acute kidney injury |
| ROS | Reactive oxygen species |
| GLP-1 | Glucagon-like peptide 1 |
| EGTI | Endothelial, Glomerular, Tubular, and Interstitial |
| RNS | Reactive nitrogen species |
| NF-κB | Nuclear factor-kappa B |
| Cr | Creatinine |
| MDA | Malondialdehyde |
| CMC | Carboxymethyl cellulose |
| GSH | Reduced glutathione |
| H&E | Hematoxylin and eosin |
References
- Ezhilarasan, D.J.T. Hepatotoxic potentials of methotrexate: Understanding the possible toxicological molecular mechanisms. Toxicology 2021, 458, 152840. [Google Scholar] [CrossRef]
- Kumar, S.; Garg, N.K.; Jain, A.; Khopade, A.; Pandey, P.; Sawant, K.K. Nanocarriers mediated delivery of methotrexate is instrumental in treating auto-immune diseases and cancer. J. Drug Deliv. Sci. Technol. 2023, 88, 104969. [Google Scholar] [CrossRef]
- Jacobs, S.A.; Stoller, R.G.; Chabner, B.; Johns, D.-G. 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J. Clin. Investig. 1976, 57, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Smeland, E.; Fuskevåg, O.M.; Nymann, K.; Svendsen, J.S.; Olsen, R.; Lindal, S.; Bremnes, R.M.; Aarbakke, J. High-dose 7-hydroxymethotrexate: Acute toxicity and lethality in a rat model. Cancer Chemother. Pharmacol. 1996, 37, 415–422. [Google Scholar] [CrossRef]
- Arab, H.H.; Abd El-Aal, S.A.; Eid, A.H.; Arafa, E.-S.A.; Mahmoud, A.M.; Ashour, A.M. Targeting inflammation, autophagy, and apoptosis by troxerutin attenuates methotrexate-induced renal injury in rats. Int. Immunopharmacol. 2022, 103, 108284. [Google Scholar] [CrossRef]
- Liang, C.-A.; Su, Y.-C.; Lin, S.-J.; Tsai, T.-H. Risk factors for acute kidney injury after high-dose methotrexate therapy: A single-center study and narrative review. Eur. J. Clin. Pharmacol. 2023, 79, 789–800. [Google Scholar] [CrossRef]
- Thomas, E.; Symmons, D.P.; Brewster, D.H.; Black, R.J.; Macfarlane, G.J. National study of cause-specific mortality in rheumatoid arthritis, juvenile chronic arthritis, and other rheumatic conditions: A 20 year followup study. J. Rheumatol. 2003, 30, 958–965. [Google Scholar] [PubMed]
- Hamed, K.M.; Dighriri, I.M.; Baomar, A.F.; Alharthy, B.T.; Alenazi, F.E.; Alali, G.H.; Alenazy, R.H.; Alhumaidi, N.T.; Alhulayfi, D.H.; Alotaibi, Y.B. Overview of methotrexate toxicity: A comprehensive literature review. Cureus 2022, 14, e29518. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Salama, S.A.; Maghrabi, I.A. Camel milk attenuates methotrexate-induced kidney injury via activation of PI3K/Akt/eNOS signaling and intervention with oxidative aberrations. Food Funct. 2018, 9, 2661–2672. [Google Scholar] [CrossRef]
- Wasfey, E.F.; Shaaban, M.; Essam, M.; Ayman, Y.; Kamar, S.; Mohasseb, T.; Rozik, R.; Khaled, H.; Eladly, M.; Elissawi, M. Infliximab ameliorates methotrexate-induced nephrotoxicity in experimental rat model: Impact on oxidative stress, mitochondrial biogenesis, apoptotic and autophagic machineries. Cell Biochem. Biophys. 2023, 81, 717–726. [Google Scholar] [CrossRef]
- Abraham, P.; Kolli, V.K.; Rabi, S. Melatonin attenuates methotrexate-induced oxidative stress and renal damage in rats. Cell Biochem. Funct. 2010, 28, 426–433. [Google Scholar] [CrossRef]
- Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.N.; Germoush, M.O.; Abd El-Twab, S.M.; Mahmoud, A.M. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO-1 signaling. Environ. Sci. Pollut. Res. 2020, 27, 20725–20735. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Hussein, O.E.; Abukhalil, M.H.; Saghir, S.A.; Bin-Jumah, M.; Alfwuaires, M.A.; Germoush, M.O.; Almaiman, A.A.; Mahmoud, A.M. Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants 2019, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.B.; Cole, S.W. Alogliptin: Safety, efficacy, and clinical implications. J. Pharm. Pract. 2015, 28, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.; D’Alessio, D.; Drucker, D.J.; Flatt, P.; Fritsche, A.; Gribble, F.; Grill, H.; Habener, J. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Lambeir, A.-M.; Durinx, C.; Scharpé, S.; De Meester, I. Dipeptidyl-peptidase IV from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, K. The role of renal dipeptidyl peptidase-4 in kidney disease: Renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin. Clin. Sci. 2018, 132, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Stiller, D.; Bahn, H.; August, C. Demonstration of glomerular DPP IV activity in kidney diseases. Acta Histochem. 1991, 91, 105–109. [Google Scholar] [CrossRef]
- Stefanovic, V.; Ardaillou, N.; Vlahovic, P.; Placier, S.; Ronco, P.; Ardaillou, R. Interferon-gamma induces dipeptidylpeptidase IV expression in human glomerular epithelial cells. Immunology 1993, 80, 465. [Google Scholar]
- Pala, L.; Mannucci, E.; Pezzatini, A.; Ciani, S.; Sardi, J.; Raimondi, L.; Ognibene, A.; Cappadona, A.; Vannelli, B.G.; Rotella, C.M. Dipeptidyl peptidase-IV expression and activity in human glomerular endothelial cells. Biochem. Biophys. Res. Commun. 2003, 310, 28–31. [Google Scholar] [CrossRef]
- Mitic, B.; Lazarevic, G.; Vlahovic, P.; Rajic, M.; Stefanovic, V. Diagnostic value of the aminopeptidase N, N-Acetyl-β-d-glucosaminidase and dipeptidylpeptidase IV in evaluating tubular dysfunction in patients with glomerulopathies. Ren. Fail. 2008, 30, 896–903. [Google Scholar] [CrossRef]
- Sun, A.-l.; Deng, J.-t.; Guan, G.-j.; Chen, S.-h.; Liu, Y.-t.; Cheng, J.; Li, Z.-w.; Zhuang, X.-h.; Sun, F.-d.; Deng, H.-p. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diabetes Vasc. Dis. Res. 2012, 9, 301–308. [Google Scholar] [CrossRef]
- Wolke, C.; Teumer, A.; Endlich, K.; Endlich, N.; Rettig, R.; Stracke, S.; Fiene, B.; Aymanns, S.; Felix, S.B.; Hannemann, A. Serum protease activity in chronic kidney disease patients: The GANI_MED renal cohort. Exp. Biol. Med. 2017, 242, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Baskota, A.; Gao, Y.; Tian, H.; Yang, F. Increased plasma dipeptidyl peptidase 4 activities predict new-onset microalbuminuria in association with its proinflammatory effects in Chinese without diabetes: A four-year prospective study. Nephrol. Dial. Transplant. 2015, 30, 460–466. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, Y.; Qin, S.; Liu, H.; Zhang, X.; Zhao, H. Increased plasma dipeptidyl peptidase-4 activities are associated with high prevalence of diabetic nephropathy in Chinese patients with newly diagnosed type 2 diabetes: A cross-sectional study. Diabetes Vasc. Dis. Res. 2016, 13, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Atkin, S.L.; Montecucco, F.; Jamialahmadi, T.; Sahebkar, A. Renoprotective effects of incretin-based therapy in diabetes mellitus. BioMed Res. Int. 2021, 2021, 8163153. [Google Scholar] [CrossRef] [PubMed]
- Faruqui, A.A. Can DPP-4 Enhibitors and SGLT-2 Inhibitors Pleotropic Effects be Extended to Treat Diabetic Nephropathy? J. Med. 2023, 24, 43–49. [Google Scholar] [CrossRef]
- Esaki, H.; Tachi, T.; Goto, C.; Sugita, I.; Kanematsu, Y.; Yoshida, A.; Saito, K.; Noguchi, Y.; Ohno, Y.; Aoyama, S. Renoprotective effect of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes mellitus. Front. Pharmacol. 2017, 8, 835. [Google Scholar] [CrossRef]
- Mori, H.; Okada, Y.; Arao, T.; Tanaka, Y. Sitagliptin improves albuminuria in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2014, 5, 313–319. [Google Scholar] [CrossRef]
- Habib, H.A.; Heeba, G.H.; Khalifa, M.M. Comparative effects of incretin-based therapy on early-onset diabetic nephropathy in rats: Role of TNF-α, TGF-β and c-caspase-3. Life Sci. 2021, 278, 119624. [Google Scholar] [CrossRef]
- Chen, Y.; Wallace, C.; Yang, C.; Chen, C.; Chen, K.; Sung, P.; Chen, Y.; Chai, H.; Chung, S.; Chua, S. DPP-4 enzyme deficiency protects kidney from acute ischemiareperfusion injury: Role for remote intermittent bowel ischemia-reperfusion preconditioning. Oncotarget 2017, 8, 54821–54837. [Google Scholar] [CrossRef] [PubMed]
- Watife, A.T.; Bairam, A.F.; Al-Ghuraibawi, N.H. Vildagliptin Nephroprotective Effect in Rats Model with Cisplatin-Induced Nephrotoxicity. J. Contemp. Med. Sci. 2024, 10, 37–42. [Google Scholar] [CrossRef]
- Ewees, M.G.E.-D.; Mostafa-Hadeab, G.; Saber, S.; Abd El-Meguid, E.A.; Sree, H.T.A.; Rahman, F.E.-Z.S.A.; Mahmoud, N.I. Linagliptin mitigates cisplatin-induced kidney impairment via mitophagy regulation in rats, with emphasis on SIRT-3/PGC-1α, PINK-1 and Parkin-2. Toxicol. Appl. Pharmacol. 2024, 491, 117048. [Google Scholar] [CrossRef]
- Salama, R.M.; Nasr, M.M.; Abdelhakeem, J.I.; Roshdy, O.K.; ElGamal, M.A. Alogliptin attenuates cyclophosphamide-induced nephrotoxicity: A novel therapeutic approach through modulating MAP3K/JNK/SMAD3 signaling cascade. Drug Chem. Toxicol. 2022, 45, 1254–1263. [Google Scholar] [CrossRef]
- Jo, C.H.; Kim, S.; Park, J.-S.; Kim, G.-H. Anti-inflammatory action of sitagliptin and linagliptin in doxorubicin nephropathy. Kidney Blood Press. Res. 2018, 43, 987–999. [Google Scholar] [CrossRef]
- Mostafa, R.E.; Morsi, A.H.; Asaad, G.F. Anti-inflammatory effects of saxagliptin and vildagliptin against doxorubicin-induced nephrotoxicity in rats: Attenuation of NLRP3 inflammasome up-regulation and tubulo-interstitial injury. Res. Pharm. Sci. 2021, 16, 547–558. [Google Scholar] [CrossRef]
- Botros, S.R.; Matouk, A.I.; Amin, A.; Heeba, G.H. Comparative effects of incretin-based therapy on doxorubicin-induced nephrotoxicity in rats: The role of SIRT1/Nrf2/NF-κB/TNF-α signaling pathways. Front. Pharmacol. 2024, 15, 1353029. [Google Scholar] [CrossRef]
- Afkhami Fard, L.; Malekinejad, H.; Esmaeilzadeh, Z.; Jafari, A.; Khezri, M.R.; Ghasemnejad-Berenji, M. Protective effects of sitagliptin on methotrexate-induced nephrotoxicity in rats. J. Environ. Sci. Health Part C 2023, 41, 22–35. [Google Scholar] [CrossRef]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Nagai, J.; Takano, M. Molecular-targeted approaches to reduce renal accumulation of nephrotoxic drugs. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1125–1138. [Google Scholar] [CrossRef]
- El-Saed, M.; El-Sherbini, E.-S.; El-Adl, M. Epigallocatechin Gallate Alleviated Methotrexate-Induced Nephrotoxicity in Rats. Egypt. J. Vet. Sci. 2025, 1–9. [Google Scholar] [CrossRef]
- Olayinka, E.T.; Ore, A.; Adeyemo, O.A.; Ola, O.S. Ameliorative effect of gallic acid on methotrexate-induced hepatotoxicity and nephrotoxicity in rat. J. Xenobiot. 2016, 6, 6092. [Google Scholar] [CrossRef] [PubMed]
- Al-Abkal, F.; Abdel-Wahab, B.A.; El-Kareem, H.F.A.; Moustafa, Y.M.; Khodeer, D.M. Protective effect of pycnogenol against methotrexate-induced hepatic, renal, and cardiac toxicity: An in vivo study. Pharmaceuticals 2022, 15, 674. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.H.; Moselhy, W.A.; Azmy, A.F.; El-Ela, F.I.A. Hesperidin loaded bilosomes mitigate the nephrotoxicity induced by methotrexate; biochemical and molecular in vivo investigations. BMC Nephrol. 2025, 26, 404. [Google Scholar] [CrossRef] [PubMed]
- Kutbi, D.; Almalki, R.S. Valsartan Mitigates the Progression of Methotrexate-Induced Acute Kidney Injury in Rats via the Attenuation of Renal Inflammation and Oxidative Stress. J. Inflamm. Res. 2024, 17, 2233–2243. [Google Scholar] [CrossRef]
- Mishriki, A.A.; Khalifa, A.K.; Ibrahim, D.A.; Hashem, G.M.A.Z.; Rashed, L.A.; Abdelrahman, S.S.; Mahmoud, H.M. Empagliflozin mitigates methotrexate-induced nephrotoxicity in male albino rats: Insights on the crosstalk of AMPK/Nrf2 signaling pathway. Future J. Pharm. Sci. 2024, 10, 95. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Al-Anazi, K.M.; Mahmoud, A.H.; Farah, M.A.; Allam, A.A. Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating Nrf2/ARE/HO-1 signaling. Biomed. Pharmacother. 2018, 106, 499–509. [Google Scholar] [CrossRef]
- Abd El-Twab, S.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Mahmoud, A.M. Chicoric acid prevents methotrexate-induced kidney injury by suppressing NF-κB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflamm. Res. 2019, 68, 511–523. [Google Scholar] [CrossRef]
- Kamel, M.A.; Mohamed, S.S. Nephroprotective effect of Alogliptin and L-Carnitine in Gentamicin-induced Toxicity in male Albino rats. J. Adv. Vet. Res. 2022, 12, 736–742. [Google Scholar]
- Devrim, E.; Çetin, R.; Kılıçoğlu, B.; Imge Ergüder, B.; Avcı, A.; Durak, İ. Methotrexate causes oxidative stress in rat kidney tissues. Ren. Fail. 2005, 27, 771–773. [Google Scholar] [CrossRef]
- Abd El-Twab, S.M.; Hozayen, W.G.; Hussein, O.E.; Mahmoud, A.M. 18 β-Glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the Nrf2/ARE/HO-1 pathway and endogenous antioxidants. Ren. Fail. 2016, 38, 1516–1527. [Google Scholar] [CrossRef]
- Kolli, V.K.; Abraham, P.; Isaac, B.; Selvakumar, D. Neutrophil infiltration and oxidative stress may play a critical role in methotrexate-induced renal damage. Chemotherapy 2009, 55, 83–90. [Google Scholar] [CrossRef]
- Abdel-Raheem, I.T.; Khedr, N.F. Renoprotective effects of montelukast, a cysteinyl leukotriene receptor antagonist, against methotrexate-induced kidney damage in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 341–353. [Google Scholar] [CrossRef]
- Heidari, R.; Ahmadi, A.; Mohammadi, H.; Ommati, M.M.; Azarpira, N.; Niknahad, H. Mitochondrial dysfunction and oxidative stress are involved in the mechanism of methotrexate-induced renal injury and electrolytes imbalance. Biomed. Pharmacother. 2018, 107, 834–840. [Google Scholar] [CrossRef]
- Hejazian, S.M.; Khatibi, S.M.H.; Barzegari, A.; Pavon-Djavid, G.; Soofiyani, S.R.; Hassannejhad, S.; Ahmadian, E.; Ardalan, M.; Vahed, S.Z. Nrf-2 as a therapeutic target in acute kidney injury. Life Sci. 2021, 264, 118581. [Google Scholar] [CrossRef]
- Özgün, E.; Atakişi, E.; Başer, L. Determination of Oxidative Damage Caused by Methotrexate and 5-Fluorouracil in Liver, Heart and Kidney Tissue in Rats: MTX and 5-FU-induced Oxidative Stress in Liver, Heart and Kidney Tissue in Rats. Rats 2024, 2, 15–21. [Google Scholar]
- Mega, C.; Teixeira de Lemos, E.; Vala, H.; Fernandes, R.; Oliveira, J.; Mascarenhas-Melo, F.; Teixeira, F.; Reis, F. Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). J. Diabetes Res. 2011, 2011, 162092. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.J.; Chaware, V.J.; Redasani, V.K. Evaluation of nephro-protective effect of dpp4 inhibitor and antioxidant against gentamycin induced nephrotoxicity in albino rats. World J. Pharm. Res. 2022, 11, 1640–1651. [Google Scholar]
- Mayer, A.L.; Scheitacker, I.; Ebert, N.; Klein, T.; Amann, K.; Daniel, C. The DPP4 inhibitor linagliptin ameliorated renal injury and accelerated resolution in a rat model of crescentic nephritis. Br. J. Pharmacol. 2021, 178, 2026. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.M.; Alqulaly, M.; Elsaeed, M.Y.; Elshora, S.Z.; Atwa, A.H.; Wasfey, E.F. L-carnitine reverses methotrexate-induced nephrotoxicity in experimental rat model: Insight on SIRT1/PGC-1α/Nrf2/HO-1 axis. J. Appl. Toxicol. 2023, 43, 1667–1675. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Abd El-Twab, S.M.; Hozayen, W.G. Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct. 2019, 10, 4593–4607. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free. Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N.; Yamamoto, M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am. J. Nephrol. 2017, 45, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.-Y.; Lee, A.Y.; Song, J.-H.; Lee, M.Y.; Lim, J.-O.; Lee, S.-J.; Ko, J.-W.; Shin, N.-R.; Kim, J.-C.; Shin, I.-S. Scrophularia koraiensis Nakai attenuates allergic airway inflammation via suppression of NF-κB and enhancement of Nrf2/HO-1 signaling. Antioxidants 2020, 9, 99. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of Nrf2 and PPARγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmacother. 2017, 86, 297–306. [Google Scholar] [CrossRef]
- Jarmi, T.; Agarwal, A. Heme oxygenase and renal disease. Curr. Hypertens. Rep. 2009, 11, 56–62. [Google Scholar] [CrossRef]
- Shalaby, Y.M.; Menze, E.T.; Azab, S.S.; Awad, A.S. Involvement of Nrf2/HO-1 antioxidant signaling and NF-κB inflammatory response in the potential protective effects of vincamine against methotrexate-induced nephrotoxicity in rats: Cross talk between nephrotoxicity and neurotoxicity. Arch. Toxicol. 2019, 93, 1417–1431. [Google Scholar] [CrossRef]
- Guo, K.; Jin, F. Dipeptidyl peptidase-4 (DPP-4) inhibitor saxagliptin alleviates lipopolysaccharide-induced acute lung injury via regulating the Nrf-2/HO-1 and NF-κ B pathways. J. Investig. Surg. 2021, 34, 695–702. [Google Scholar] [CrossRef]
- Si, J.; Meng, R.; Gao, P.; Hui, F.; Li, Y.; Liu, X.; Yang, B. Linagliptin protects rat carotid artery from balloon injury and activates the NRF2 antioxidant pathway. Exp. Anim. 2019, 68, 81–90. [Google Scholar] [CrossRef]
- Ahmad, A.; Alkharfy, K.M.; Bin Jardan, Y.A.; Shahid, M.; Ansari, M.A.; Alqahtani, S.; Jan, B.L.; Al-Jenoobi, F.I.; Raish, M. Sinapic acid mitigates methotrexate-induced hepatic injuries in rats through modulation of Nrf-2/HO-1 signaling. Environ. Toxicol. 2021, 36, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Alum, E.U.; Famurewa, A.C.; Orji, O.U.; Aja, P.M.; Nwite, F.; Ohuche, S.E.; Ukasoanya, S.C.; Nnaji, L.O.; Joshua, D.; Igwe, K.U. Nephroprotective effects of Datura stramonium leaves against methotrexate nephrotoxicity via attenuation of oxidative stress-mediated inflammation and apoptosis in rats. Avicenna J. Phytomed. 2023, 13, 377. [Google Scholar] [PubMed]
- Kabel, A.M. Zinc/alogliptin combination attenuates testicular toxicity induced by doxorubicin in rats: Role of oxidative stress, apoptosis and TGF-β1/NF-κB signaling. Biomed. Pharmacother. 2018, 97, 439–449. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, S.; Huang, J.; Liu, K. Alogliptin inhibits IL-1β-induced inflammatory response in fibroblast-like synoviocytes. Int. Immunopharmacol. 2020, 83, 106372. [Google Scholar] [CrossRef]
- Guerrero-Hue, M.; Rayego-Mateos, S.; Vázquez-Carballo, C.; Palomino-Antolín, A.; García-Caballero, C.; Opazo-Rios, L.; Morgado-Pascual, J.L.; Herencia, C.; Mas, S.; Ortiz, A. Protective role of Nrf2 in renal disease. Antioxidants 2020, 10, 39. [Google Scholar] [CrossRef]
- Kobayashi, E.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Su, Z.-y.; Kong, A.-N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.; Shalkami, A.-G.S.; Khalaf, M.M.; Mohamed, W.R.; Hemeida, R.A. The impact of Keap1/Nrf2, P38MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. [Google Scholar] [CrossRef]
- Hassanein, E.H.; Mohamed, W.R.; Shalkami, A.-G.S.; Khalaf, M.M.; Hemeida, R.A. Renoprotective effects of umbelliferone on methotrexate-induced renal injury through regulation of Nrf-2/Keap-1, P38MAPK/NF-κB, and apoptosis signaling pathways. Food Chem. Toxicol. 2018, 116, 152–160. [Google Scholar] [CrossRef]
- Kızıl, H.E.; Caglayan, C.; Darendelioğlu, E.; Ayna, A.; Gür, C.; Kandemir, F.M.; Küçükler, S. Morin ameliorates methotrexate-induced hepatotoxicity via targeting Nrf2/HO-1 and Bax/Bcl2/Caspase-3 signaling pathways. Mol. Biol. Rep. 2023, 50, 3479–3488. [Google Scholar] [CrossRef]
- El-Kashef, D.H.; Sewilam, H.M. Empagliflozin mitigates methotrexate-induced hepatotoxicity: Targeting ASK-1/JNK/Caspase-3 pathway. Int. Immunopharmacol. 2023, 114, 109494. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, M.; Farag, A.; Elfadadny, A.; Alkafafy, M.; Soliman, A.; Elbadawy, M. Antioxidant and anti-apoptotic potency of allicin and lycopene against methotrexate-induced cardiac injury in rats. Environ. Sci. Pollut. Res. 2023, 30, 88724–88733. [Google Scholar] [CrossRef]
- Al-Taher, A.Y.; Morsy, M.A.; Rifaai, R.A.; Zenhom, N.M.; Abdel-Gaber, S.A. Paeonol attenuates methotrexate-induced cardiac toxicity in rats by inhibiting oxidative stress and suppressing TLR4-induced NF-κB inflammatory pathway. Mediat. Inflamm. 2020, 2020, 8641026. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.M.; Mohamed, A.M.; Hamed, N.S.; Ata, R.M.; NourelDeen, A.S.; Hassan, M.A. Alogliptin: A novel approach against cyclophosphamide-induced hepatic injury via modulating SIRT1/FoxO1 pathway. Toxicol. Res. 2020, 9, 561–568. [Google Scholar] [CrossRef]
- Alsemeh, A.E.; Abdullah, D.M. Protective effect of alogliptin against cyclophosphamide-induced lung toxicity in rats: Impact on PI3K/Akt/FoxO1 pathway and downstream inflammatory cascades. Cell Tissue Res. 2022, 388, 417–438. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Xiang, X.; Zhu, Y.; Men, J.; He, M. Estimation of the normal range of blood glucose in rats. Wei Sheng Yan Jiu = J. Hyg. Res. 2010, 39, 133–137, 142. [Google Scholar]
- Karakuyu, N.F.; Ertunc, O.; Bedir, M.; Dogan, H.; Taner, R.; Sevuk, M.; İmeci, O.B.; Ergonul, E. Protective role of nebivolol via AKT1/Hif-1α/eNOS signaling pathway: Nephrotoxicity caused by methotrexate in a rat model. Can. J. Physiol. Pharmacol. 2023, 101, 393–399. [Google Scholar] [CrossRef]
- Kirkwood, J.K. AVMA Guidelines for the euthanasia of animals. Anim. Welf. 2013, 22, 412. [Google Scholar] [CrossRef]
- Eleazu, C.; Iroaganachi, M.; Okafor, P.; Ijeh, I.; Eleazu, K. Ameliorative potentials of ginger (Z. officinale Roscoe) on relative organ weights in streptozotocin induced diabetic rats. Int. J. Biomed. Sci. IJBS 2013, 9, 82. [Google Scholar] [CrossRef]
- Schirmeister, J. Determination of creatinine in serum. Dtsch. Med. Wschr. 1964, 89, 796. [Google Scholar]
- Fawcett, J.; Scott, J. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960, 13, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.T.; Khalil, S.R.; Mahmoud, F.A.; Elmowalid, G.A.; Ali, H.A.; El-Serehy, H.A.; Abdel-Daim, M.M. The role of sulpiride in attenuating the cardiac, renal, and immune disruptions in rats receiving clozapine: mRNA expression pattern of the genes encoding Kim-1, TIMP-1, and CYP isoforms. Environ. Sci. Pollut. Res. 2020, 27, 25404–25414. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Jablonski, P.; Howden, B.O.; Rae, D.A.; Birrell, C.S.; Marshall, V.C.; Tange, J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation 1983, 35, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. [30] Microsomal lipid peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R. Catalase activity. In Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985; pp. 1–447. [Google Scholar]



| Groups | Random Blood Glucose Level (mg/dL) | Kidney Index | Serum Creatinine Level (mg/dL) | Serum Urea Level (mg/dL) |
|---|---|---|---|---|
| Control | 120.50 ± 7.18 | 0.51 ± 0.01 | 0.86 ± 0.02 | 29.69 ± 1.50 |
| ALO | 129.30 ± 7.60 | 0.53 ± 0.01 | 0.89 ± 0.04 | 31.25 ± 1.55 |
| MTX | 150.20 ± 9.15 | 0.64 ± 0.03 * | 4.00 ± 0.18 * | 62.33 ± 1.97 * |
| MTX + ALO | 138.20 ± 8.61 | 0.52 ± 0.02 # | 0.82 ± 0.06 # | 46.56 ± 3.04 # |
| Lesion | Tubular | Glomerular | Tubulo/Interstitial | |
|---|---|---|---|---|
| Groups | ||||
| Control | 0 | 0 | 0 | |
| ALO | 0 | 0 | 0 | |
| MTX | 3 | 3 | 3 | |
| MTX + ALO | 1 | 1 | 0 | |
| Forwarded | Reverse | ||
|---|---|---|---|
| KIM-1 | 5′-TTCAGATGTGCTGCTGCTGT-3′ | 5′-AAGGGAAAGGCTGGCAAGTC-3′ | NM_001003336.2 |
| GAPDH | 5′-CATCACTGCCACCCAAAGACTG-3′ | 5′-TGCCAGTGAGCTTCCCGTTCAG-3 | NM_008084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahmy, M.M.; Habib, H.A.; Zeidan, E.M.; Jardan, Y.A.B.; Heeba, G.H. Alogliptin Mitigates Methotrexate-Induced Nephrotoxicity in a Rat Model: Antagonizing Oxidative Stress, Inflammation and Apoptosis. Int. J. Mol. Sci. 2025, 26, 9608. https://doi.org/10.3390/ijms26199608
Fahmy MM, Habib HA, Zeidan EM, Jardan YAB, Heeba GH. Alogliptin Mitigates Methotrexate-Induced Nephrotoxicity in a Rat Model: Antagonizing Oxidative Stress, Inflammation and Apoptosis. International Journal of Molecular Sciences. 2025; 26(19):9608. https://doi.org/10.3390/ijms26199608
Chicago/Turabian StyleFahmy, Marwa M., Heba A. Habib, Esraa M. Zeidan, Yousef A. Bin Jardan, and Gehan H. Heeba. 2025. "Alogliptin Mitigates Methotrexate-Induced Nephrotoxicity in a Rat Model: Antagonizing Oxidative Stress, Inflammation and Apoptosis" International Journal of Molecular Sciences 26, no. 19: 9608. https://doi.org/10.3390/ijms26199608
APA StyleFahmy, M. M., Habib, H. A., Zeidan, E. M., Jardan, Y. A. B., & Heeba, G. H. (2025). Alogliptin Mitigates Methotrexate-Induced Nephrotoxicity in a Rat Model: Antagonizing Oxidative Stress, Inflammation and Apoptosis. International Journal of Molecular Sciences, 26(19), 9608. https://doi.org/10.3390/ijms26199608






