Evaluation of Serum FGF21 Levels in Patients with Mitochondrial Aminoacyl-tRNA Synthetase Deficiency
Abstract
1. Introduction
2. Results
2.1. Study Groups
2.1.1. Clinical Findings
2.1.2. Laboratory Findings
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Biochemical Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahin, E.; Alver, A. Mitohormesis and Regulatory Mechanisms. Farabi Med. J. 2020, 1, 21–26. [Google Scholar]
- Zhang, B.; Chang, J.Y.; Lee, M.H.; Ju, S.H.; Yi, H.S.; Shong, M. Mitochondrial Stress and Mitokines: Therapeutic Perspectives for the Treatment of Metabolic Diseases. Diabetes Metab. J. 2024, 48, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gedikbasi, A.; Toksoy, G.; Karaca, M.; Gulec, C.; Balci, M.C.; Uyguner, Z.O. Clinical and Bi-Genomic DNA Findings of Patients Suspected to Have Mitochondrial Diseases. Front. Genet. 2023, 14, 1191159. [Google Scholar] [CrossRef] [PubMed]
- Quirós, P.M.; Mottis, A.; Auwerx, J. Mitonuclear Communication in Homeostasis and Stress. Nat. Rev. Mol. Cell Biol. 2016, 17, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Felhi, R.; Charif, M.; Sfaihi, L.; Mkaouar-Rebai, E.; Desquiret-Dumas, V.; Kallel, R.; Bris, C.; Goudenège, D.; Guichet, A.; Bonneau, D.; et al. Mutations in aARS Genes Revealed by Targeted Next-Generation Sequencing in Patients with Mitochondrial Diseases. Mol. Biol. Rep. 2020, 47, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.S.; Nemeth, C.L.; Kaufman, M.L.; Fatemi, A. Mitochondrial Aminoacyl-tRNA Synthetase Disorders: An Emerging Group of Developmental Disorders of Myelination. J. Neurodev. Disord. 2019, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Wei, P.; Zou, T.; You, J. Fibroblast Growth Factor 21: A Fascinating Perspective on the Regulation of Muscle Metabolism. Int. J. Mol. Sci. 2023, 24, 16951. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, A.; Elo, J.M.; Pietiläinen, K.H.; Hakonen, A.H.; Sevastianova, K.; Tyynismaa, H. FGF-21 as a Biomarker for Muscle-Manifesting Mitochondrial Respiratory Chain Deficiencies: A Diagnostic Study. Lancet Neurol. 2011, 10, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, D. A New Biomarker for Mitochondrial Disease. Lancet Neurol. 2011, 10, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L.; Liang, C.; Edema-Hildebrand, F.; Riley, C.; Needham, M.; Sue, C.M. Fibroblast Growth Factor 21 Is a Sensitive Biomarker of Mitochondrial Disease. Neurology 2013, 81, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L.; Liang, C.; Sue, C.M. A Comparison of Current Serum Biomarkers as Diagnostic Indicators of Mitochondrial Diseases. Neurology 2016, 86, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Morovat, A.; Weerasinghe, G.; Nesbitt, V.; Hofer, M.; Agnew, T.; Poulton, J. Use of FGF-21 as a Biomarker of Mitochondrial Disease in Clinical Practice. J. Clin. Med. 2017, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Scholle, L.M.; Lehmann, D.; Deschauer, M.; Kraya, T.; Zierz, S. FGF-21 as a Potential Biomarker for Mitochondrial Diseases. Curr. Med. Chem. 2018, 25, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.; Davis, R.; Sue, C.M. New Diagnostic Pathways for Mitochondrial Disease. J. Transl. Genet. Genom. 2020, 4, 188–202. [Google Scholar] [CrossRef]
- Shayota, B.J. Biomarkers of Mitochondrial Disorders. Neurotherapeutics 2024, 21, e00325. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, J.M.; Forsström, S.; Bottani, E.; Viscomi, C.; Baris, O.R.; Suomalainen, A. FGF21 Is a Biomarker for Mitochondrial Translation and mtDNA Maintenance Disorders. Neurology 2016, 87, 2290–2299. [Google Scholar] [CrossRef] [PubMed]
- Wesół-Kucharska, D.; Rokicki, D.; Greczan, M.; Kaczor, M.; Czekuć-Kryśkiewicz, E.; Piekutowska-Abramczuk, D. The Fibroblast Growth Factor 21 Concentration in Children with Mitochondrial Disease Does Not Depend on the Disease Stage, but Rather on the Disease Genotype. Pediatr. Endocrinol. Diabetes Metab. 2022, 28, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Soltany, A.; Visavadiya, N.P.; Burtscher, M.; Millet, G.P.; Khoramipour, K.; Khamoui, A.V. Mitochondrial Stress and Mitokines in Aging. Aging Cell 2023, 22, e13770. [Google Scholar] [CrossRef]
- Domouzoglou, E.M.; Vlahos, A.P.; Vasileios, K.; Cholevas, V.K.; Michail, I.; Papafaklis, M.I.; Chaliasos, N.; Siomou, E. Association of Fibroblast Growth Factor 21 with Metabolic Syndrome and Endothelial Function in Children: A Prospective Cross-Sectional Study on Novel Biomarkers. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 242–251. [Google Scholar] [CrossRef]
- Szczepańska, E.; Gietka-Czernel, M. FGF21: A Novel Regulator of Glucose and Lipid Metabolism and Whole-Body Energy Balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef] [PubMed]
| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Gender | Age (Years) | Weight (kg) | Height (cm) | BMI kg/m2 | Lactate (mg/dL) | Pyruvate (mg/dL) | FGF21 (pg/mL) |
| C1 | M | 3.7 | 9.18 | 81.5 | 13.8 | 49.9 | 0.59 | 3495 |
| C2 | M | 8.1 | 20.1 | 120.5 | 13.8 | 45.7 | 0.75 | 26,661 |
| C3 | F | 3.4 | 13.7 | 96 | 15.1 | 8.7 | 0.37 | 298.44 |
| C4 | M | 15.3 | 46.5 | 162 | 17.7 | 19.8 | 0.45 | 233.8 |
| C5 | F | 6.5 | 19.5 | 111.5 | 15.7 | 19.5 | 0.42 | 2029.45 |
| C6 | M | 2.9 | 9.8 | 88 | 12.6 | 26 | 0.47 | 2117.14 |
| (B) | ||||||||
| Cases | Parental Consanguinity | Zygosity | Gene | Variant in Nucleotide | Variant in Peptide | |||
| C1 | Yes | Hom | AARS2 | c.302G>T | p.(Arg101His) | |||
| C2 | No | Com.het | AARS2 | c.277C>T | p.(Arg93Ter) | |||
| C3 | No | Com.het | EARS2 | c.845C>G | p.(Ser282Cys) | |||
| C4 | No | Com.het | DARS2 | c.319C>T | p.(Arg107Cys) | |||
| C5 | Yes | Hom | SARS2 | c.1283delC | p.(Pro428Leufs*3) | |||
| C6 | Yes | Hom | WARS2 | c.492+2T>C | p.(?) | |||
| Control Group | Patient Group | p | ||||
|---|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 6.73 ± 4.01 | 6.65 ± 4.70 | 0.743 † | ||
| Median (IQR) | 5.6 (3.55–8.13) | 5.1 (3.28–9.9) | ||||
| Gender | Male (n, %) | 7, | 58.33% | 4, | 66.67% | 0.732 + |
| Female (n, %) | 5, | 41.67% | 2, | 33.33% | ||
| Weight (kg) | Mean ± SD | 20.33 ± 13.09 | 19.8 ± 13.88 | 0.682 † | ||
| Median (IQR) | 16.55 (10.73–20.8) | 16.6 (9.65–26.7) | ||||
| Height (cm) | Mean ± SD | 111.69 ± 27.31 | 109.92 ± 29.36 | 0.815 † | ||
| Median (IQR) | 104.8 (90.5–124.73) | 103.75 (86.38–130.88) | ||||
| BMI (kg/m2) | Mean ± SD | 14.91 ± 2.03 | 14.78 ± 1.80 | 0.896 * | ||
| Lactate (mg/dL) | Mean ± SD | 5.68 ± 0.60 | 28.27 ± 16.18 | 0.001 † | ||
| Median (IQR) | 5.75 (5.13–6.18) | 22.9 (16.8–46.75) | ||||
| Pyruvate (mg/dL) | Mean ± SD | 0.38 ± 0.05 | 0.51 ± 0.14 | 0.015 † | ||
| Median (IQR) | 0.375 (0.33–0.42) | 0.46 (0.41–0.63) | ||||
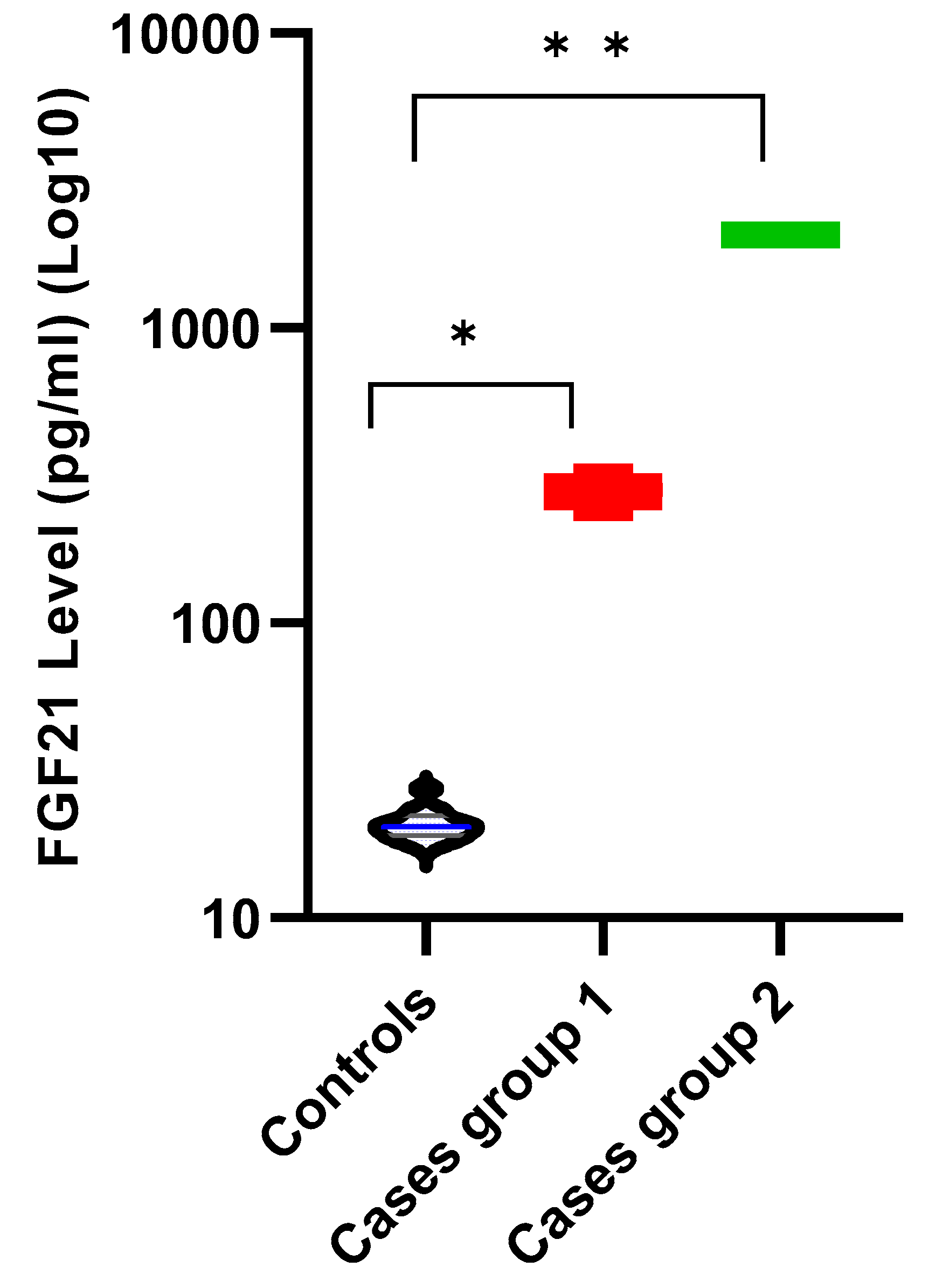
| FGF21 (pg/mL) | Mean ± SD | 20.89 ± 2.63 | 882.49 ± 923.60 | 0.001 † | ||
| Median (IQR) | 20.33 (19.01–22.24) | 323.97 (258.41–2051.37) | ||||
| (A) | |||||||
|---|---|---|---|---|---|---|---|
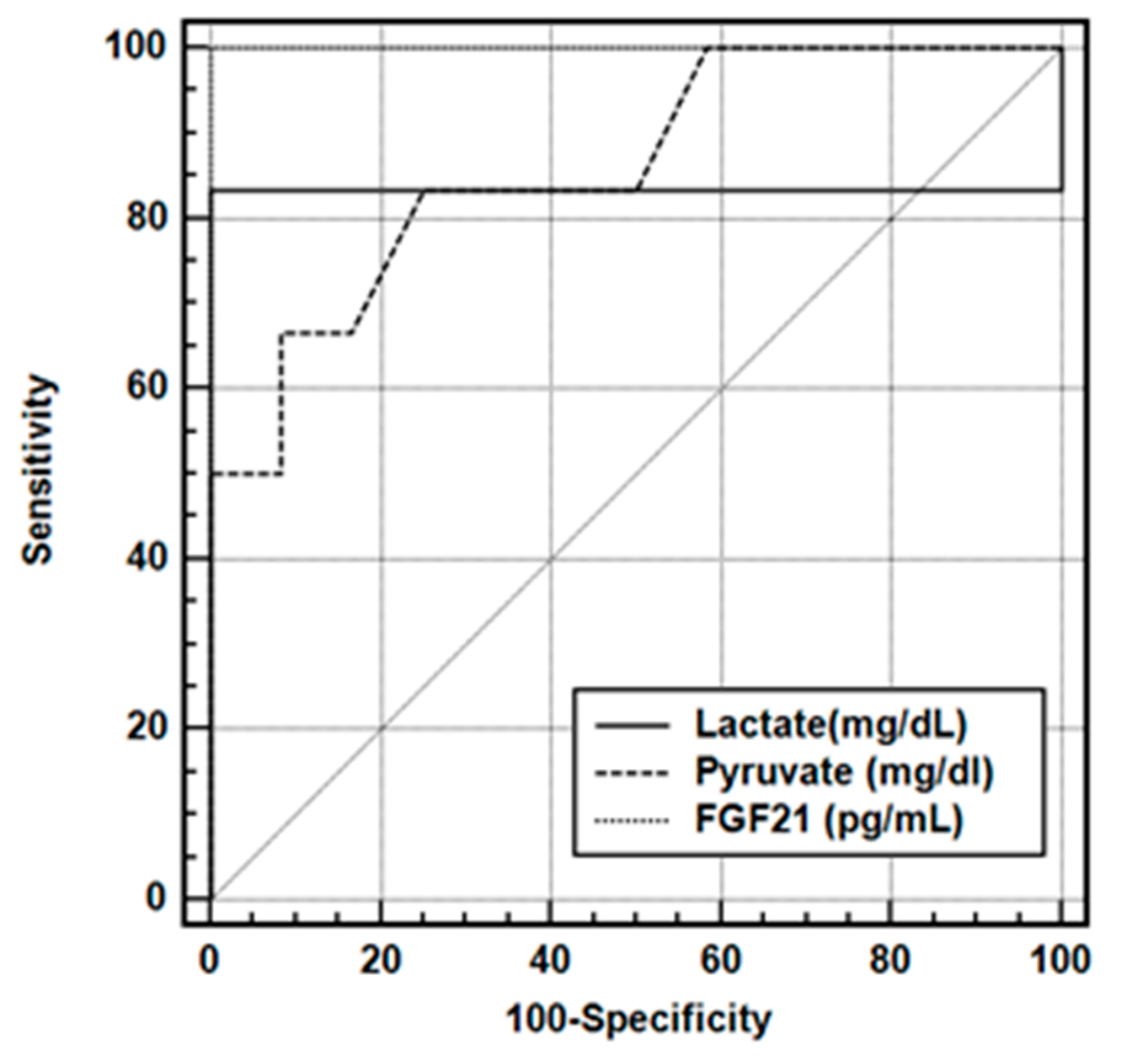
| AUC | SE | 95% CI | |||||
| Lactate (mg/dL) | 0.933 | 0.114 | 0.786–0.962 | ||||
| Pyruvate (mg/dL) | 0.861 | 0.105 | 0.618–0.974 | ||||
| FGF-21 (pg/mL) | 1.000 | 0.000 | 0.813–1.000 | ||||
| (B) | |||||||
| Criterion | Sensitivity | Specificity | PPV | NPV | LR (+) | LR (−) | |
| Lactate (mg/dL) | >6.7 * | 83.33 | 100.00 | 100.0 | 92.3 | N/A | 0.17 |
| Pyruvate (mg/dL) | >0.41 * | 83.33 | 75.00 | 62.5 | 90.0 | 3.33 | 0.22 |
| FGF21 (pg/mL) | >27.4 * | 100.00 | 100.00 | 100.0 | 100.0 | N/A | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekin Neijmann, S.; Gunes, D.; Karaca, M.; Karaman, V.; Balci, M.C.; Gokcay, G.F.; Gedikbasi, A. Evaluation of Serum FGF21 Levels in Patients with Mitochondrial Aminoacyl-tRNA Synthetase Deficiency. Int. J. Mol. Sci. 2025, 26, 9525. https://doi.org/10.3390/ijms26199525
Tekin Neijmann S, Gunes D, Karaca M, Karaman V, Balci MC, Gokcay GF, Gedikbasi A. Evaluation of Serum FGF21 Levels in Patients with Mitochondrial Aminoacyl-tRNA Synthetase Deficiency. International Journal of Molecular Sciences. 2025; 26(19):9525. https://doi.org/10.3390/ijms26199525
Chicago/Turabian StyleTekin Neijmann, Sebnem, Dilek Gunes, Meryem Karaca, Volkan Karaman, Mehmet Cihan Balci, Gulden Fatma Gokcay, and Asuman Gedikbasi. 2025. "Evaluation of Serum FGF21 Levels in Patients with Mitochondrial Aminoacyl-tRNA Synthetase Deficiency" International Journal of Molecular Sciences 26, no. 19: 9525. https://doi.org/10.3390/ijms26199525
APA StyleTekin Neijmann, S., Gunes, D., Karaca, M., Karaman, V., Balci, M. C., Gokcay, G. F., & Gedikbasi, A. (2025). Evaluation of Serum FGF21 Levels in Patients with Mitochondrial Aminoacyl-tRNA Synthetase Deficiency. International Journal of Molecular Sciences, 26(19), 9525. https://doi.org/10.3390/ijms26199525






