TRPC6 Deficiency Attenuates Mitochondrial and Cardiac Dysfunction in Heart Failure with Preserved Ejection Fraction Induced by High-Fat Diet Plus L-NAME
Abstract
1. Introduction
2. Results
2.1. Metabolic Profiles and Mean Arterial Pressure Before and After Administration of HFD+L-NAME in WT and TRPC6 KO Mice
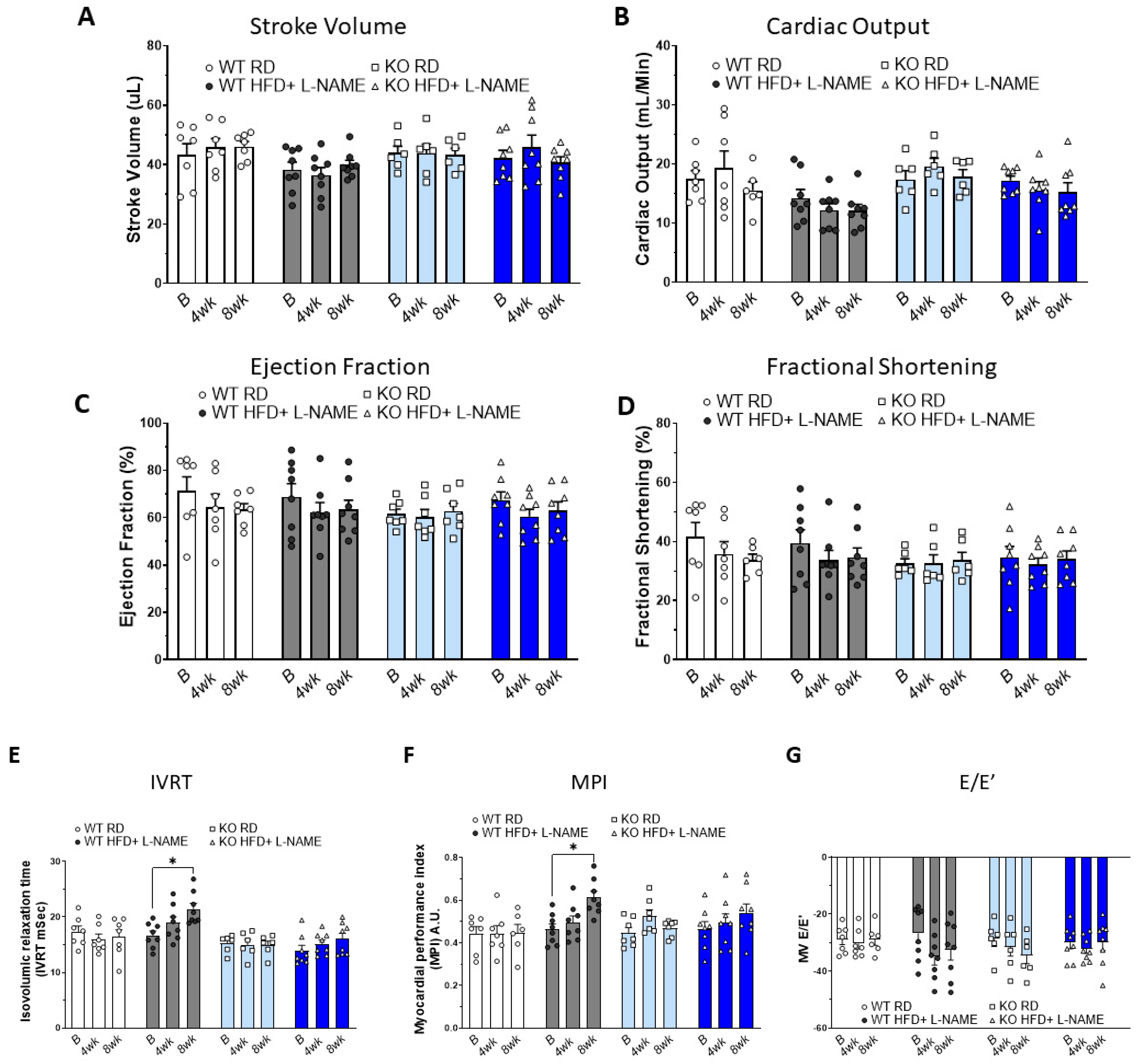
2.2. Effects of HFD+L-NAME on Cardiac Systolic Function in WT and TRPC6 KO Mice
2.3. Effects of HFD+L-NAME on Cardiac Diastolic Function in WT and TRPC6 KO Mice
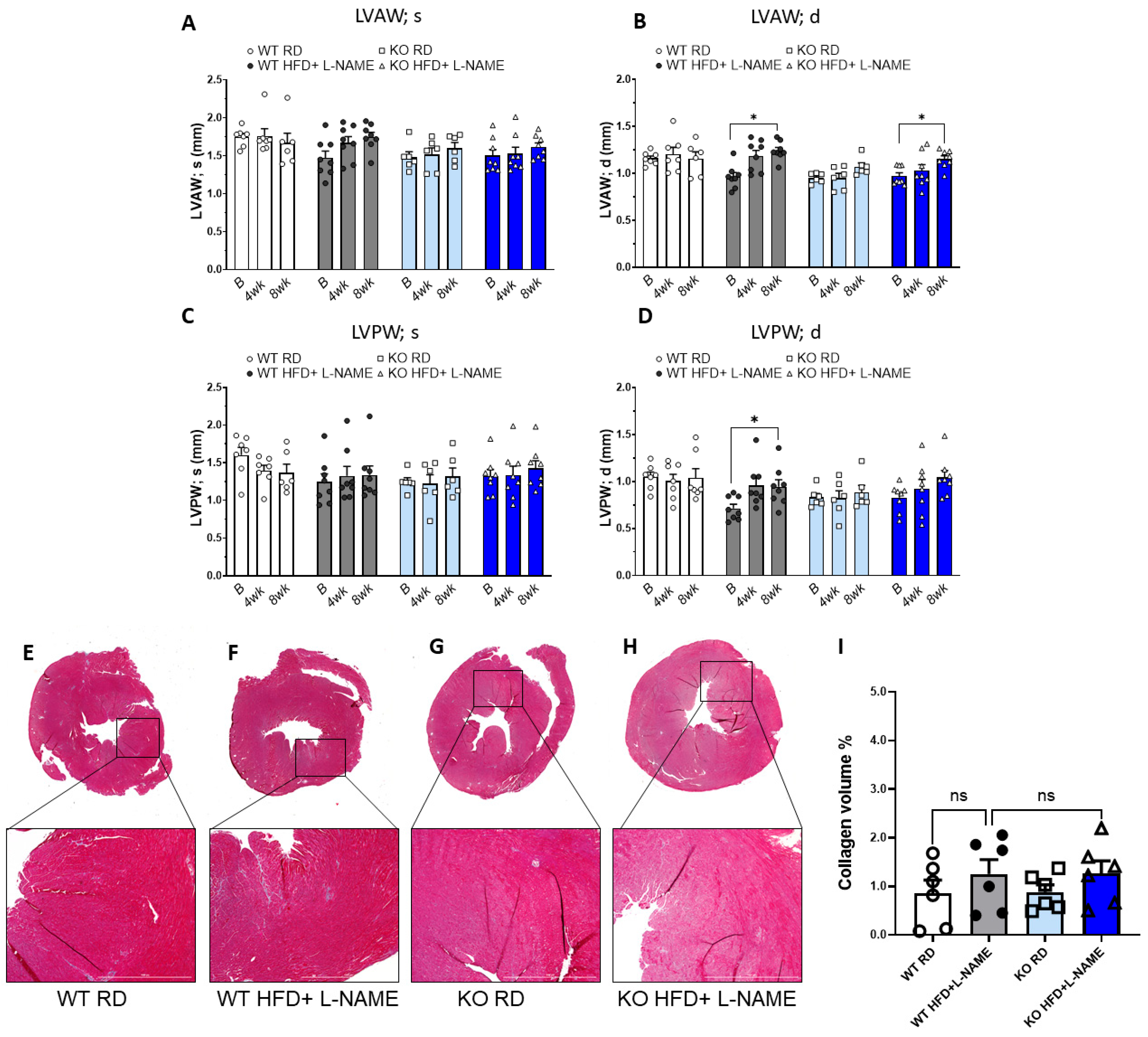
2.4. HFD+L-NAME Induced Left Ventricular Hypertrophy in WT and TRPC6 Mice, but Did Not Cause Significant Fibrosis
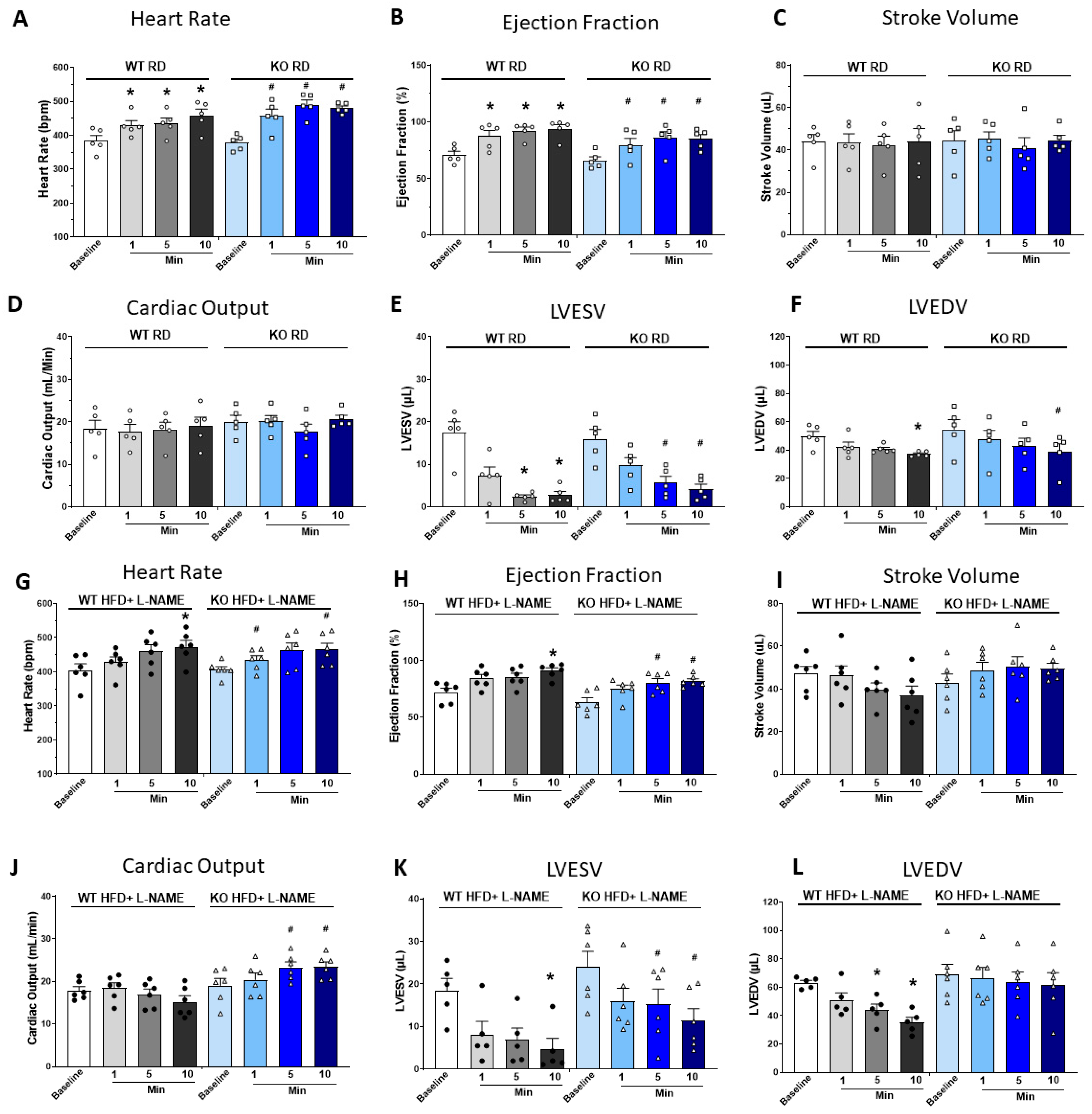
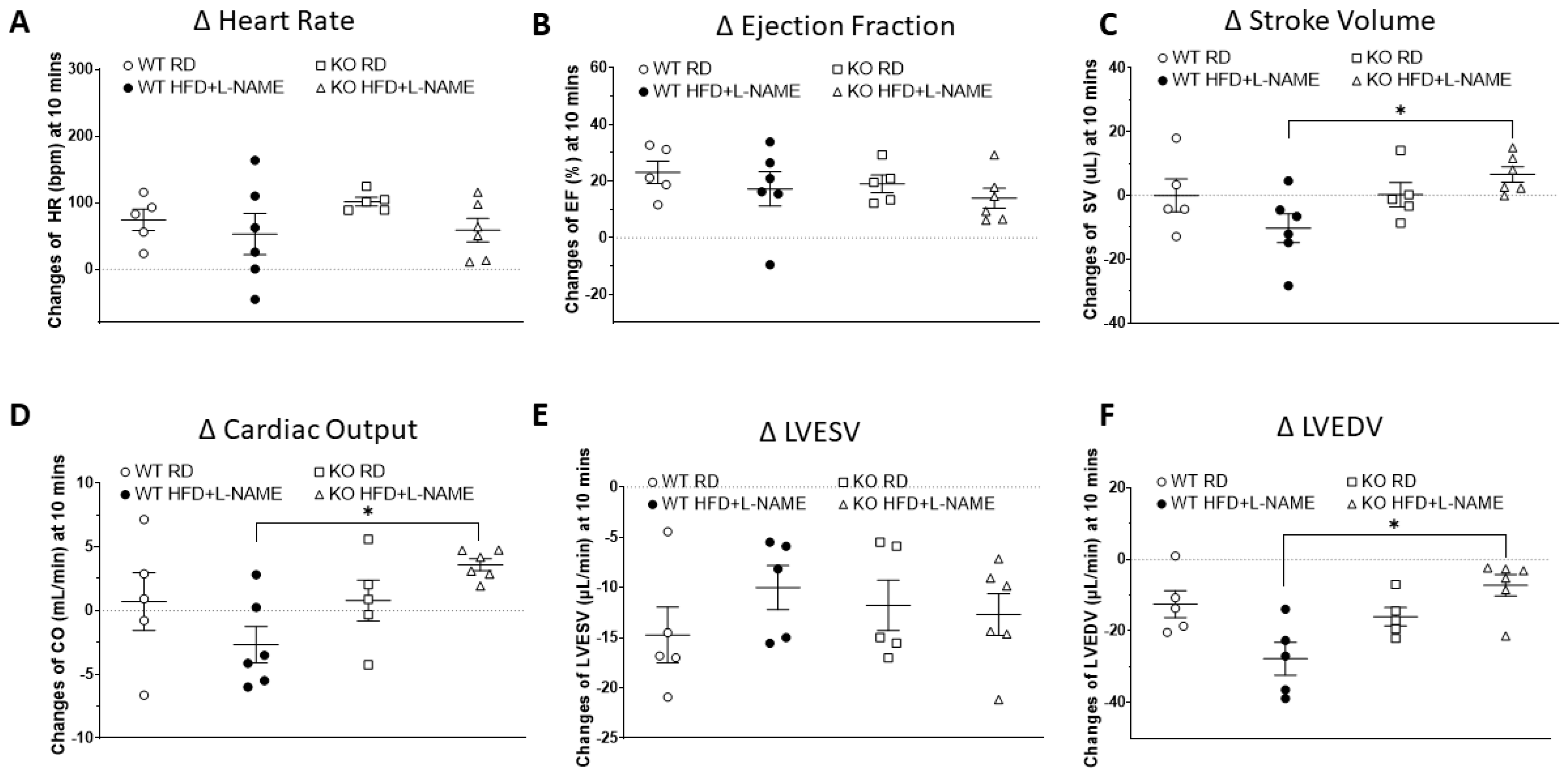
2.5. Dobutamine Stress Test Suggested Reduced Cardiac Reserve Capacity in WT but Not TRPC6 KO Mice After HFD+L-NAME
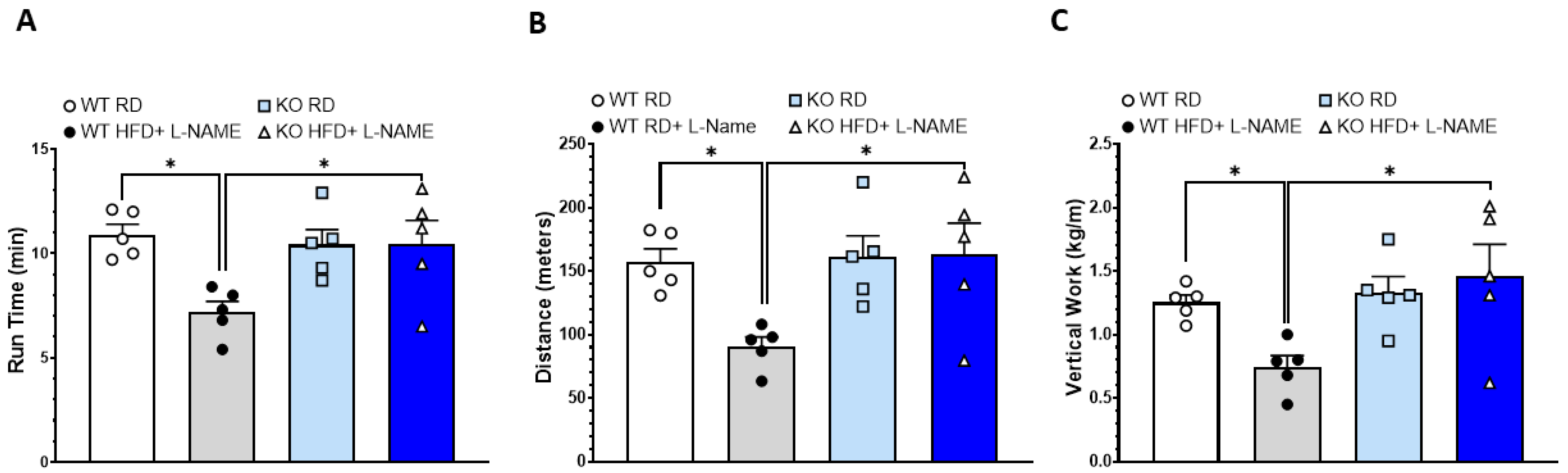
2.6. Reduced Exercise Capacity in WT but Not TRPC6 KO Mice After HFD+L-NAME
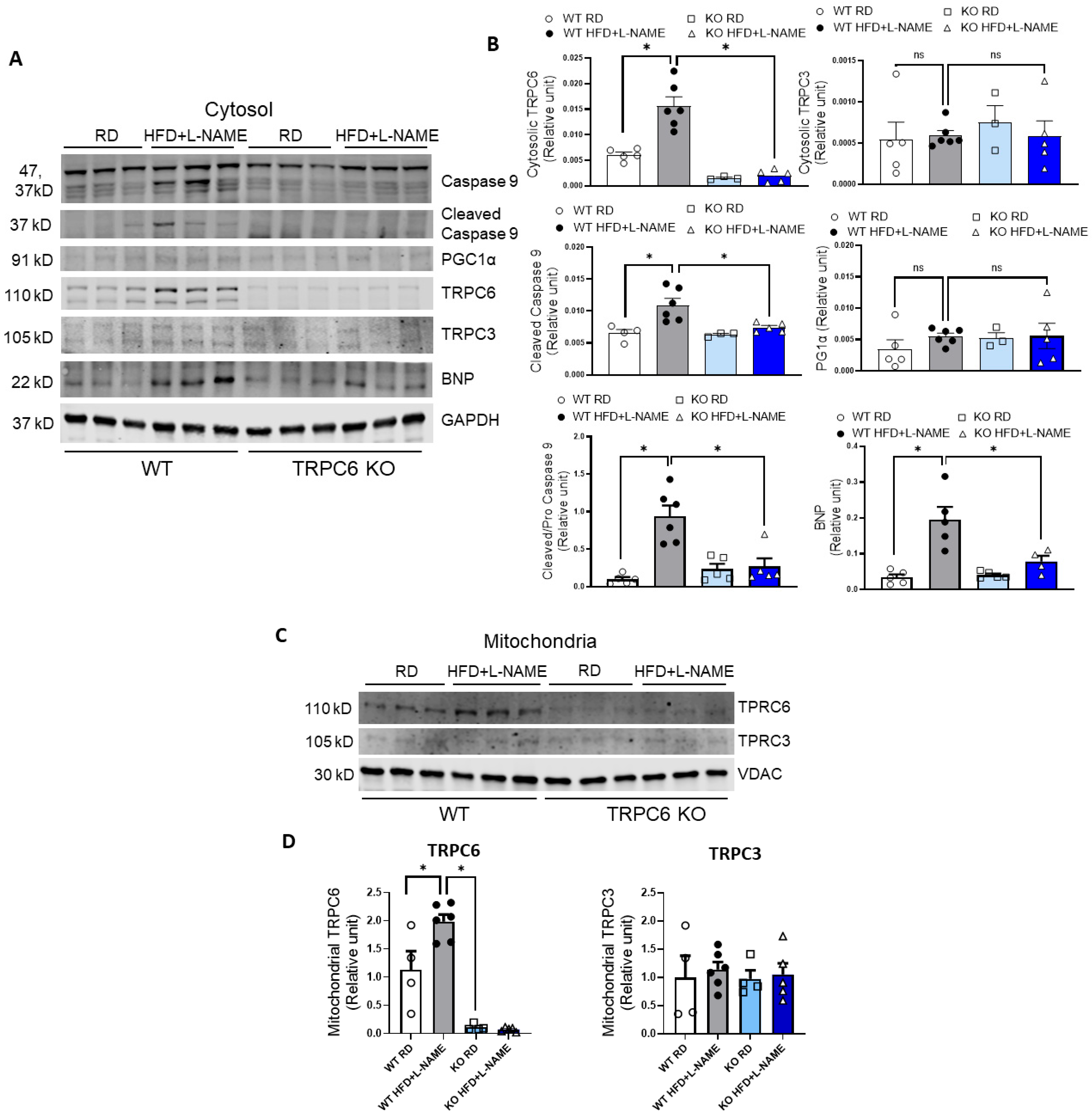
2.7. TRPC6 Gene Expression in Mitochondria and Cytosol of Cardiac Cells in WT and TRPC6 Mice with RD or HFD+L-NAME
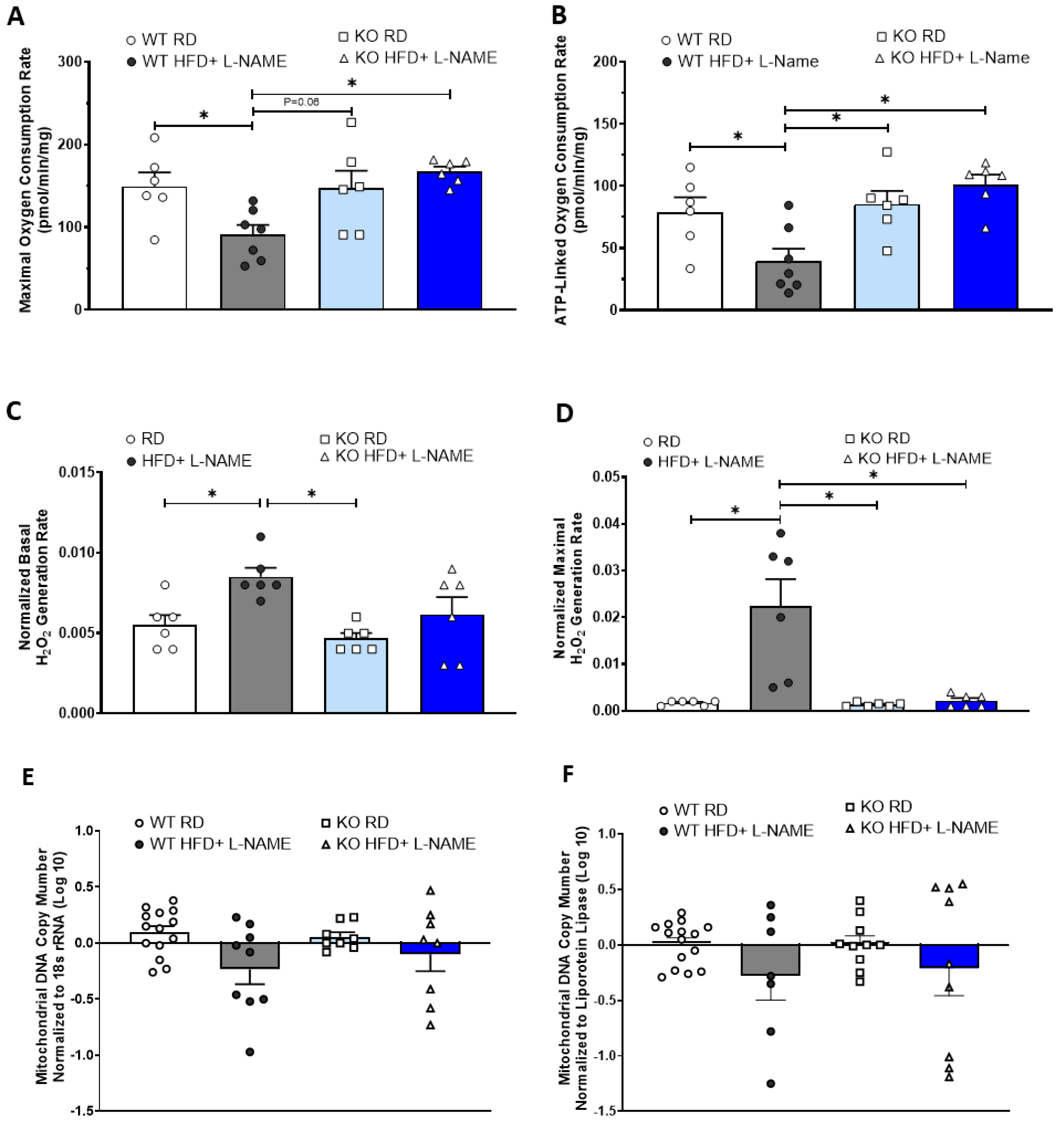
2.8. TRPC6 KO Attenuates Mitochondrial Dysfunction and Reduces Mitochondria-Derived ROS Generation in Mice Treated with HFD+L-NAME
2.9. Cardiac Mitochondria DNA Copy Numbers in Mice with HFD+L-NAME
3. Discussion
3.1. Diastolic Dysfunction and Impaired Cardiac Reserve in the HFpEF Model
3.2. Mitochondrial Dysfunction Is an Early Event in the Development of HFpEF
3.3. The Role of TRPC6 in Development of HFpEF
3.4. Limitations
4. Materials and Methods
4.1. Animals
4.2. Experimental Protocol
4.3. Transthoracic Echocardiography
4.4. Dobutamine Stress Test
4.5. Treadmill Exercise Test
4.6. High-Resolution Respirometry (HRR) Measurement of Mitochondrial Respiration in Permeabilized Heart Samples
4.7. Hydrogen Peroxide Flux Measurement as an Indicator of Mitochondrial Superoxide Generation in Permeabilized Heart Samples
4.8. Fractional Mitochondria and Cytosol Protein Isolation for Western Blot
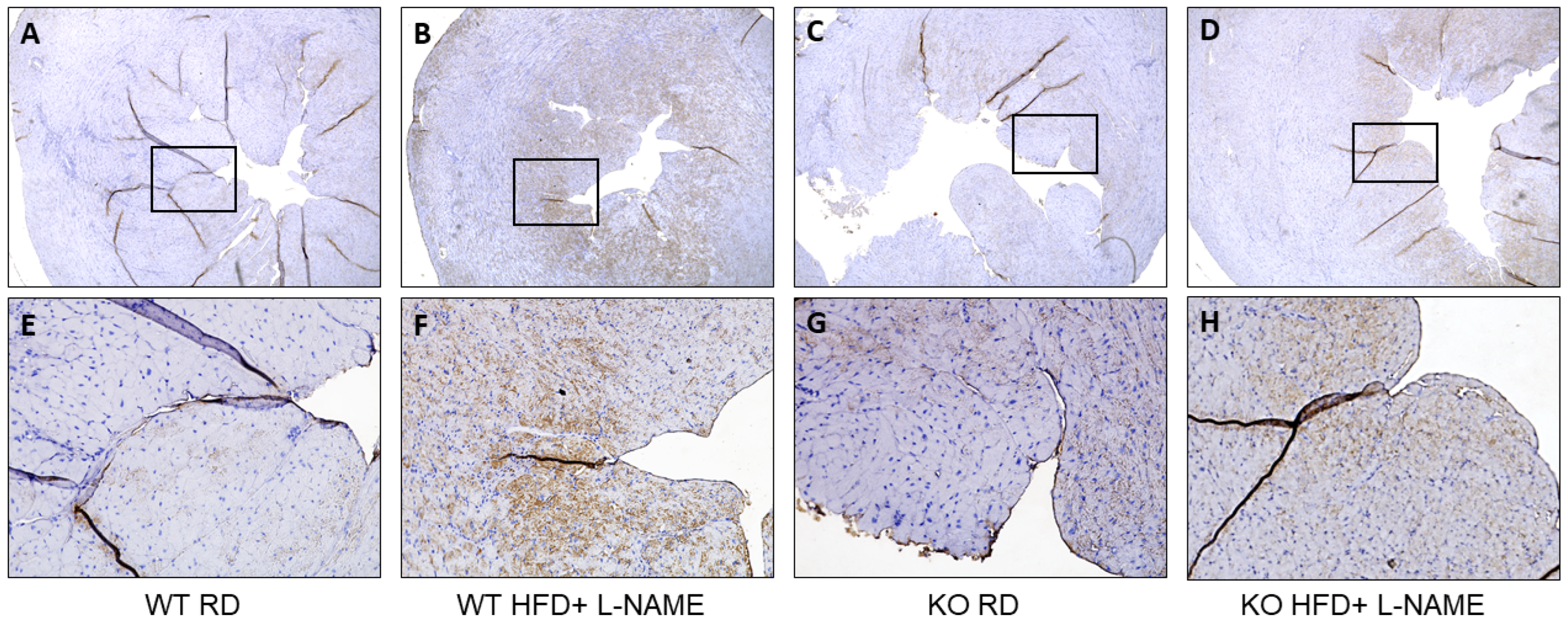
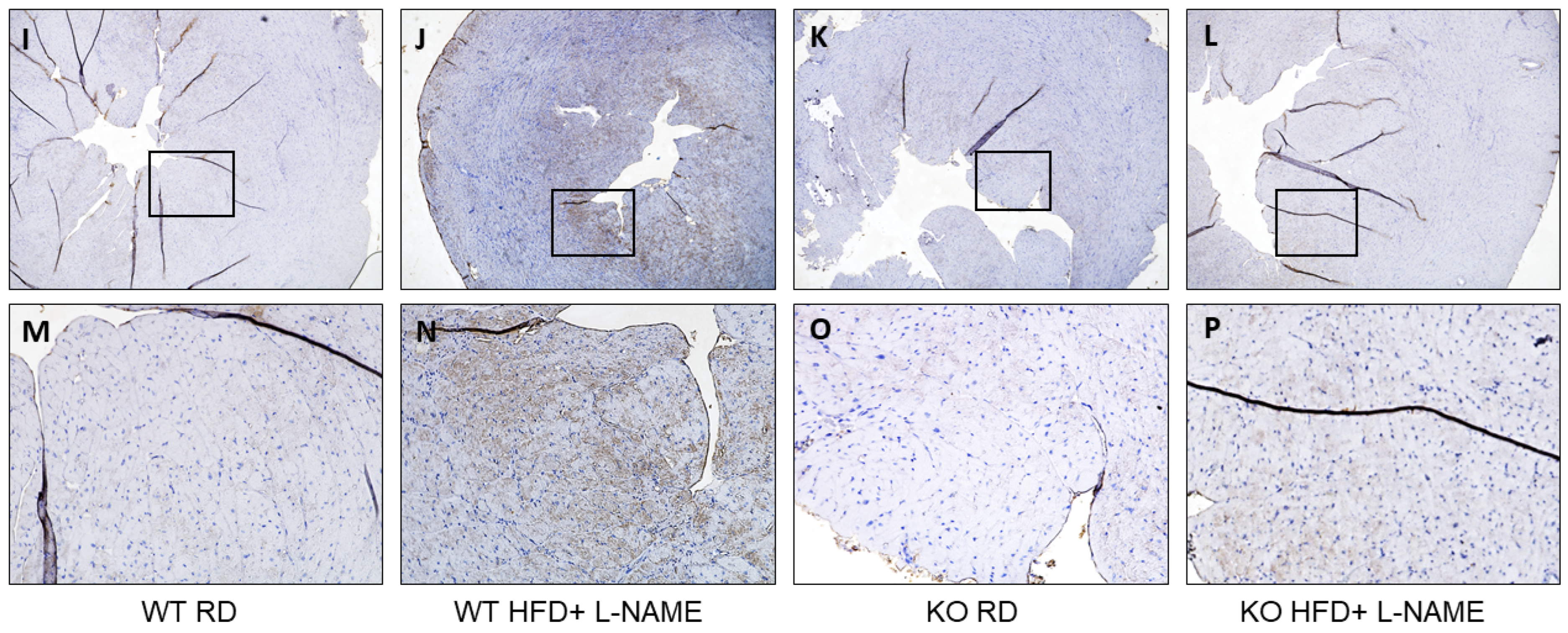
4.9. Immunohistochemistry (IHC)
4.10. Evaluation of Cardiac Fibrosis
4.11. Mitochondrial DNA Content Measurement by Real-Time Quantitative PCR
4.12. Data Representation and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Redfield, M.M.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction: A Review. JAMA 2023, 329, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.A.; Dayer, V.W.; Hansen, R.N.; Du, Y.; Williamson, T.; Kong, S.X.; Singh, R.; Sullivan, S.D. Long-Term Outcomes of Heart Failure with Preserved or Mid-Range Ejection Fraction in the United States. JACC Adv. 2024, 3, 101027. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Abildstrom, S.Z.; Borlaug, B.A.; Butler, J.; Christensen, L.; Davies, M.; Hovingh, K.G.; Kitzman, D.W.; Lindegaard, M.L.; Moller, D.V.; et al. Design and Baseline Characteristics of STEP-HFpEF Program Evaluating Semaglutide in Patients with Obesity HFpEF Phenotype. JACC Heart Fail. 2023, 11, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Hill, J.A.; Singh, A.; Valero-Munoz, M.; Sam, F. Heart Failure with Preserved Ejection Fraction: Heterogeneous Syndrome, Diverse Preclinical Models. Circ. Res. 2022, 130, 1906–1925, Erratum in Circ. Res. 2022, 131, e100. [Google Scholar] [CrossRef]
- Faulkner, J.L. Obesity-associated cardiovascular risk in women: Hypertension and heart failure. Clin. Sci. 2021, 135, 1523–1544. [Google Scholar] [CrossRef]
- Oktay, A.A.; Rich, J.D.; Shah, S.J. The emerging epidemic of heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2013, 10, 401–410. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Shah, A.M.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction In Perspective. Circ. Res. 2019, 124, 1598–1617. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Panjrath, G.S.; Amancherla, K.; Davis, L.L.; Deswal, A.; Dixon, D.L.; Januzzi, J.L., Jr.; Yancy, C.W. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure with Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2023, 81, 1835–1878. [Google Scholar] [CrossRef]
- Kjeldsen, S.E.; von Lueder, T.G.; Smiseth, O.A.; Wachtell, K.; Mistry, N.; Westheim, A.S.; Hopper, I.; Julius, S.; Pitt, B.; Reid, C.M.; et al. Medical Therapies for Heart Failure with Preserved Ejection Fraction. Hypertension 2020, 75, 23–32. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef]
- Kumar, A.A.; Kelly, D.P.; Chirinos, J.A. Mitochondrial Dysfunction in Heart Failure with Preserved Ejection Fraction. Circulation 2019, 139, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, A.; McMillen, T.S.; Wang, Y.; Zhou, B.; Chen, H.; Banerjee, D.; Herrero, M.; Wang, P.; Muraoka, N.; Wang, W.; et al. Blunted Cardiac Mitophagy in Response to Metabolic Stress Contributes to HFpEF. Circ. Res. 2024, 135, 1004–1017, Erratum in Circ. Res. 2024, 135, e154. [Google Scholar] [CrossRef]
- Ranjbarvaziri, S.; Kooiker, K.B.; Ellenberger, M.; Fajardo, G.; Zhao, M.; Roest, A.S.V.; Woldeyes, R.A.; Koyano, T.T.; Fong, R.; Ma, N.; et al. Altered Cardiac Energetics and Mitochondrial Dysfunction in Hypertrophic Cardiomyopathy. Circulation 2021, 144, 1714–1731. [Google Scholar] [CrossRef]
- Wang, Z.; Carmo, J.M.D.; da Silva, A.A.; Fu, Y.; Hall, J.E. Mechanisms of Synergistic Interactions of Diabetes and Hypertension in Chronic Kidney Disease: Role of Mitochondrial Dysfunction and ER Stress. Curr. Hypertens. Rep. 2020, 22, 15. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Ma, H.; Wang, Z.; Zhang, R.; Jiao, J. TRPC6 mediates high glucose-induced mitochondrial fission through activation of CDK5 in cultured human podocytes. Front. Physiol. 2022, 13, 984760. [Google Scholar] [CrossRef]
- Nakayama, H.; Wilkin, B.J.; Bodi, I.; Molkentin, J.D. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006, 20, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, K.; Wang, Y.; McAnally, J.; Richardson, J.A.; Bassel-Duby, R.; Hill, J.A.; Olson, E.N. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Investig. 2006, 116, 3114–3126. [Google Scholar] [CrossRef] [PubMed]
- Onohara, N.; Nishida, M.; Inoue, R.; Kobayashi, H.; Sumimoto, H.; Sato, Y.; Mori, Y.; Nagao, T.; Kurose, H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006, 25, 5305–5316. [Google Scholar] [CrossRef]
- Seo, K.; Rainer, P.P.; Hahn, V.S.; Lee, D.I.; Jo, S.H.; Andersen, A.; Liu, T.; Xu, X.; Willette, R.N.; Lepore, J.J.; et al. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2014, 111, 1551–1556, Erratum in Proc. Natl. Acad. Sci. USA 2014, 111, 6115. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Lira, J.R.; Guymon, A.L.; Yang, L.; Sternburg, J.O.; Giri, S.; Wang, X. The double-hit protocol induces HFpEF and impairs myocardial ubiquitin-proteasome system performance in FVB/N mice. Front. Physiol. 2023, 14, 1208153. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.F.; Moreau, P.; Takase, H.; Luscher, T.F. L-NAME hypertension alters endothelial and smooth muscle function in rat aorta. Prevention by trandolapril and verapamil. Hypertension 1995, 26, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Vrankova, S.; Cebova, M. Chronic L-Name-Treatment Produces Hypertension by Different Mechanisms in Peripheral Tissues and Brain: Role of Central eNOS. Pathophysiology 2020, 27, 46–54. [Google Scholar] [CrossRef]
- Jama, H.A.; Muralitharan, R.R.; Xu, C.; O’Donnell, J.A.; Bertagnolli, M.; Broughton, B.R.S.; Head, G.A.; Marques, F.Z. Rodent models of hypertension. Br. J. Pharmacol. 2022, 179, 918–937. [Google Scholar] [CrossRef]
- Bernatova, I.; Pechanova, O.; Pelouch, V.; Simko, F. Regression of chronic L -NAME-treatment-induced left ventricular hypertrophy: Effect of captopril. J. Mol. Cell. Cardiol. 2000, 32, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Setty, S.; Tune, J.D.; Downey, H.F. Nitric oxide modulates right ventricular flow and oxygen consumption during norepinephrine infusion. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H696–H703. [Google Scholar] [CrossRef][Green Version]
- Agoston, G.; Morvai-Illes, B.; Palinkas, A.; Varga, A. The role of stress echocardiography in cardiovascular disorders. Kardiol. Pol. 2019, 77, 1011–1019. [Google Scholar] [CrossRef]
- Rad, A.A.; Tserioti, E.; Magouliotis, D.E.; Vardanyan, R.; Samiotis, I.V.; Skoularigis, J.; Ariff, B.; Xanthopoulos, A.; Triposkiadis, F.; Casula, R.; et al. Assessment of Myocardial Viability in Ischemic Cardiomyopathy with Reduced Left Ventricular Function Undergoing Coronary Artery Bypass Grafting. Clin. Cardiol. 2024, 47, e24307. [Google Scholar] [CrossRef]
- Hughey, C.C.; Hittel, D.S.; Johnsen, V.L.; Shearer, J. Respirometric oxidative phosphorylation assessment in saponin-permeabilized cardiac fibers. J. Vis. Exp. 2011, 48, 2431. [Google Scholar]
- Krajcova, A.; Urban, T.; Megvinet, D.; Waldauf, P.; Balik, M.; Hlavicka, J.; Budera, P.; Janousek, L.; Pokorna, E.; Duska, F. High resolution respirometry to assess function of mitochondria in native homogenates of human heart muscle. PLoS ONE 2020, 15, e0226142. [Google Scholar] [CrossRef]
- Jia, M.; Liu, W.; Zhang, K.; Wang, Z.; Li, R.; Pan, J.; Yang, J.; Wang, D. Larixyl acetate, a TRPC6 inhibitor, attenuates pressure overload-induced heart failure in mice. Mol. Med. Rep. 2024, 29, 49. [Google Scholar] [CrossRef]
- Lin, B.L.; Matera, D.; Doerner, J.F.; Zheng, N.; Del Camino, D.; Mishra, S.; Bian, H.; Zeveleva, S.; Zhen, X.; Blair, N.T.; et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc. Natl. Acad. Sci. USA 2019, 116, 10156–10161. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef]
- Murphy, E.; Liu, J.C. Mitochondrial calcium and reactive oxygen species in cardiovascular disease. Cardiovasc. Res. 2023, 119, 1105–1116. [Google Scholar] [CrossRef]
- Wang, Z.; Carmo, J.M.D.; da Silva, A.A.; Fu, Y.; Jaynes, L.T.; Sears, J.; Li, X.; Mouton, A.J.; Omoto, A.C.M.; Xu, B.P.; et al. Transient receptor potential cation channel 6 deficiency leads to increased body weight and metabolic dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R81–R97. [Google Scholar] [CrossRef]
- Holmstrom, K.M.; Pan, X.; Liu, J.C.; Menazza, S.; Liu, J.; Nguyen, T.T.; Pan, H.; Parks, R.J.; Anderson, S.; Noguchi, A.; et al. Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter. J. Mol. Cell. Cardiol. 2015, 85, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fu, Y.; Carmo, J.M.D.; da Silva, A.A.; Li, X.; Mouton, A.; Omoto, A.C.M.; Sears, J.; Hall, J.E. Transient receptor potential cation channel 6 contributes to kidney injury induced by diabetes and hypertension. Am. J. Physiol. Renal Physiol. 2022, 322, F76–F88. [Google Scholar] [CrossRef]
- Dietrich, A.; Mederos, Y.S.M.; Gollasch, M.; Gross, V.; Storch, U.; Dubrovska, G.; Obst, M.; Yildirim, E.; Salanova, B.; Kalwa, H.; et al. Increased vascular smooth muscle contractility in TRPC6-/-mice. Mol. Cell. Biol. 2005, 25, 6980–6989. [Google Scholar] [CrossRef]
- Li, X.; Lu, Q.; Qiu, Y.; Carmo, J.M.D.; Wang, Z.; da Silva, A.A.; Mouton, A.; Omoto, A.C.M.; Hall, M.E.; Li, J.; et al. Direct Cardiac Actions of the Sodium Glucose Co-Transporter 2 Inhibitor Empagliflozin Improve Myocardial Oxidative Phosphorylation and Attenuate Pressure-Overload Heart Failure. J. Am. Heart Assoc. 2021, 10, e018298. [Google Scholar] [CrossRef]
- Coyle-Asbil, B.; Holjak, E.J.B.; Marrow, J.P.; Alshamali, R.; Ogilvie, L.M.; Edgett, B.A.; Hopkinson, L.D.; Brunt, K.R.; Simpson, J.A. Assessing systolic and diastolic reserves in male and female mice. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H129–H140. [Google Scholar] [CrossRef] [PubMed]
- Puhl, S.L.; Weeks, K.L.; Guran, A.; Ranieri, A.; Boknik, P.; Kirchhefer, U.; Muller, F.U.; Avkiran, M. Role of type 2A phosphatase regulatory subunit B56alpha in regulating cardiac responses to beta-adrenergic stimulation in vivo. Cardiovasc. Res. 2019, 115, 519–529. [Google Scholar] [CrossRef]
- Puhl, S.L.; Weeks, K.L.; Ranieri, A.; Avkiran, M. Assessing structural and functional responses of murine hearts to acute and sustained beta-adrenergic stimulation in vivo. J. Pharmacol. Toxicol. Methods 2016, 79, 60–71. [Google Scholar] [CrossRef]
- Omoto, A.C.M.; Carmo, J.M.D.; Nelson, B.; Aitken, N.; Dai, X.; Moak, S.; Flynn, E.; Wang, Z.; Mouton, A.J.; Li, X.; et al. Central Nervous System Actions of Leptin Improve Cardiac Function After Ischemia-Reperfusion: Roles of Sympathetic Innervation and Sex Differences. J. Am. Heart Assoc. 2022, 11, e027081. [Google Scholar] [CrossRef]
- Li, X.; Flynn, E.R.; Carmo, J.M.D.; Wang, Z.; da Silva, A.A.; Mouton, A.J.; Omoto, A.C.M.; Hall, M.E.; Hall, J.E. Direct Cardiac Actions of Sodium-Glucose Cotransporter 2 Inhibition Improve Mitochondrial Function and Attenuate Oxidative Stress in Pressure Overload-Induced Heart Failure. Front. Cardiovasc. Med. 2022, 9, 859253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fu, Y.; da Silva, A.A.; Carmo, J.M.D.; Mouton, A.; Omoto, A.C.M.; Li, X.; Sears, J.; Hall, J.E. Mitochondria-Derived Reactive Oxygen Species Contribute to Synergistic Interaction of Diabetes and Hypertension in Causing Chronic Kidney Injury. Am. J. Physiol. Renal Physiol. 2024, 326, F534–F544. [Google Scholar] [CrossRef] [PubMed]
- Makrecka-Kuka, M.; Krumschnabel, G.; Gnaiger, E. High-Resolution Respirometry for Simultaneous Measurement of Oxygen and Hydrogen Peroxide Fluxes in Permeabilized Cells, Tissue Homogenate and Isolated Mitochondria. Biomolecules 2015, 5, 1319–1338. [Google Scholar] [CrossRef] [PubMed]
- Cates, C.; Rousselle, T.; Wang, J.; Quan, N.; Wang, L.; Chen, X.; Yang, L.; Rezaie, A.R.; Li, J. Activated protein C protects against pressure overload-induced hypertrophy through AMPK signaling. Biochem. Biophys. Res. Commun. 2018, 495, 2584–2594. [Google Scholar] [CrossRef]
- Matsiukevich, D.; Kovacs, A.; Li, T.; Kokkonen-Simon, K.; Matkovich, S.J.; Oladipupo, S.S.; Ornitz, D.M. Characterization of a robust mouse model of heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H203–H231. [Google Scholar] [CrossRef]

| WT RD | WT HFD+ L-NAME | TRPC6 KO RD | TRPC6 KO HFD+ L-NAME | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 30 wks | Baseline | 30 wks | Baseline | 30 wks | Baseline | 30 wks | |
| Body Weight (g) | 33.7 ± 4.8 | 37.7 ± 2.1 | 35.1 ± 5.6 | 48.1 ± 3.1 * | 37.5 ± 2.7 | 40.1 ± 1.8 | 39.7 ± 3.0 | 45.8 ± 4.0 * |
| Blood Glucose (mg/dL) | 121 ± 6 | 130 ± 8 | 127 ± 11 | 142 ± 9 * | 134 ± 8 | 138 ± 10 | 142 ± 7 | 155 ± 10 * |
| Heart Weight (mg) | N/A | 179 ± 5 | N/A | 237 ± 9 + | N/A | 173± 7 | N/A | 235 ± 11 + |
| Mean arterial pressure (mmHg) | N/A | N/A | 102 ± 3 | 119 ± 4 * | N/A | N/A | 106 ± 8 | 120 ± 8 * |
| Heart Rate (beat/min) | N/A | N/A | 541 ± 23 | 515 ± 18 | N/A | N/A | 578 ± 22 | 562 ± 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Fu, Y.; Dai, X.; do Carmo, J.M.; da Silva, A.A.; Mouton, A.J.; Omoto, A.C.M.; Spitz, R.W.; Wang, L.; Hall, J.E.; et al. TRPC6 Deficiency Attenuates Mitochondrial and Cardiac Dysfunction in Heart Failure with Preserved Ejection Fraction Induced by High-Fat Diet Plus L-NAME. Int. J. Mol. Sci. 2025, 26, 9383. https://doi.org/10.3390/ijms26199383
Li X, Fu Y, Dai X, do Carmo JM, da Silva AA, Mouton AJ, Omoto ACM, Spitz RW, Wang L, Hall JE, et al. TRPC6 Deficiency Attenuates Mitochondrial and Cardiac Dysfunction in Heart Failure with Preserved Ejection Fraction Induced by High-Fat Diet Plus L-NAME. International Journal of Molecular Sciences. 2025; 26(19):9383. https://doi.org/10.3390/ijms26199383
Chicago/Turabian StyleLi, Xuan, Yiling Fu, Xuemei Dai, Jussara M. do Carmo, Alexandre A. da Silva, Alan J. Mouton, Ana C. M. Omoto, Robert W. Spitz, Lucas Wang, John E. Hall, and et al. 2025. "TRPC6 Deficiency Attenuates Mitochondrial and Cardiac Dysfunction in Heart Failure with Preserved Ejection Fraction Induced by High-Fat Diet Plus L-NAME" International Journal of Molecular Sciences 26, no. 19: 9383. https://doi.org/10.3390/ijms26199383
APA StyleLi, X., Fu, Y., Dai, X., do Carmo, J. M., da Silva, A. A., Mouton, A. J., Omoto, A. C. M., Spitz, R. W., Wang, L., Hall, J. E., & Wang, Z. (2025). TRPC6 Deficiency Attenuates Mitochondrial and Cardiac Dysfunction in Heart Failure with Preserved Ejection Fraction Induced by High-Fat Diet Plus L-NAME. International Journal of Molecular Sciences, 26(19), 9383. https://doi.org/10.3390/ijms26199383







