Abstract
Four human sialidases (hNEUs, E.C 3.2.1.18) have been identified. Each is an exosialidase identified as either NEU1, NEU2, NEU3, or NEU4. They exhibit differences in structure, subcellular distribution, substrate specificity, and the diseases with which they are associated. Similarly, microbial sialidases (NAs) may catalyze the release of sialyl residues from the same sialoglycoconjugates as hNEUs, even though they have low sequence homology with human NEUs. Use of sequence homology, plus the crystalline structure of human NEU2, has provided researchers with the basis for developing inhibitors that may differentiate between them. While microbial-induced diseases that use sialidase to complete their infectious cycle have been the driving force behind interrogation of possible NA inhibitors, errors affecting expression of functional hNEUs and their correlation with clinical problems has led to study of the sialidases per se. Information gained about sialidase structure, function, mechanism of action, mutations affecting expression, and their role(s) in disease, has provided the information about the different sialidases needed for development of specific therapies.
1. Introduction
Sialidase participates in the maintenance of a cell’s sialoglycoconjugates, and errors affecting its expression contribute to diseases seen phenotypically such as fibrosis, cancer, and diabetes and in neural development/function. Humans express four distinct human exo-sialidases (neuraminidase, NEU, E.C.3.2.1.18), each capable of catalyzing the release of sialic acid residues (hence use of the term sialidase) from sialylated compounds. Each hNEU has been identified, cloned, and named NEU1 [1], NEU2 [2], NEU3 [3], and NEU4 [4]. Although differences in subcellular location, substrate specificity, pH optimum, and association with physiological problems have been ascertained for each, they are not absolute (see Table 1). Expression levels also differ, with NEU1 expression greater than that of NEU3 and NEU4, while NEU2 expression is lowest in human tissues [5]. The close association of sialidase (NEU3) activity with both its ganglioside substrates and caveolin in plasma membrane microdomains [6] supports the concept that it is able to affect cell function by acting on cell surface sialylated moieties that may be involved in cell signaling [7] (for a review, see [8]). Alterations in NEU3 activity underlie its role in diseases such as fibrosis and cancer (e.g., [9,10]), as well as in neuronal development/function [11,12,13]. For a general review about NEU3, see Miyagi and Yamamoto [14]. While the emphasis, when discussing a specific problem, may focus on a specific hNEU, it should be understood that more than one hNEU may contribute to the resultant changes.

Table 1.
General information about human sialidases.
Sialic acid, the compound released by the action of sialidases, was first characterized by Blix and Gottschalk [38] in mucins isolated from salivary glands, hence the name sialic acid. Some years later it was characterized by Klenk [39], who identified it in preparations from brain, where it is found in highest concentration in the gray matter [40], and who called it neuraminic acid. Subsequently the authors agreed [41] that the unsubstituted compound should be called neuraminic acid (Neu, Figure 1) while substituted versions would be called sialic acid. In human glycoconjugates, the sialic acid most commonly found is 5-N-acetyneuraminic acid (Neu5NAc), while small amounts of Neu5NAc9OAc can also be found [42,43]. The Neu5N-glycolyl found in people is presumably due to that obtained from food as people lack the enzyme needed to catalyze hydroxylation of the CMP-NeuAc needed for its synthesis [44]. The development of methods to synthesize specific acetylated sialic acids (e.g., [45]) for use as standards for the identification of specific sialic acids should help researchers define their biological effects.
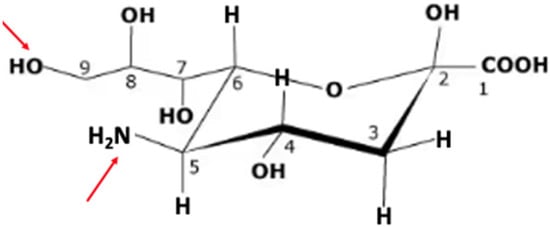
Figure 1.
Neuraminic acid (Neu). The IUPAC name for Neu is (4S,5R,6R)-5-amino-2,4-dihydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid. It does not occur naturally, but a number of its derivatives can be found. The arrows in red indicate the site at C5 that is modified by N-acetylneuraminate synthase in humans to yield 5N-acetylneuraminic acid (Neu5NAc) and C9, which can be O-acetylated by the action of sialic acid O-acetyl-transferase identified as capsule structure 1 domain containing 1 (CASD1), catalyzing formation of Neu5NAc9OAc [46].
Gangliosides, sialylated glycosphingolipids, can be found in lipid rafts [47,48] on the outer surface of cell membranes, with their carbohydrate moiety protruding into the glycocalyx [49], on the outer surface of the plasma membrane [50], and with the lipid portion interacting with the membrane’s lipid and protein components. Changes in either the carbohydrate or lipid composition [51] of the ganglioside can affect its interaction with membrane proteins. Such a change can occur when the carbohydrate portion of the ganglioside interacts with an extracellular component, thereby affecting proteins, including those involved in signal transduction (e.g., [52]). Sialyl residues on gangliosides can, in some instances, block accessibility to receptors. This can be ameliorated by cleaving them with sialidases. The combination of variability in both the carbohydrate and ceramide composition in gangliosides, allows for fine tuning of their behavior in signal transduction [53]. It is in the fine tuning that the ability of hNEUs to catalyze the release of sialic acid moieties from cell surface gangliosides can affect cell behavior [8].
Ganglioside substrates, a major class of sialylated compounds acted upon by hNEUs, include those with terminal α2-3, α2-6, and α2-8 sialic acid residues, as indicated in Table 2. It can be seen that a single sialic acid moiety linked to an internal Gal on gangliosides having additional external sugar residues is not susceptible to hNEU activity [3]. When looking at results obtained in studies of sialidase activity one should note not just type(s) of sialidase(s) expressed and their possible activity overlap, but the animal used, as results obtained may differ from those found in humans. Support for possible animal-related differences was provided by the observation that mice were able to catalyze conversion of both GM1 and GM2 to the corresponding asialo derivatives in the presence of GM2 activator protein [13], a reaction not found in humans lacking either the β-galactosidase or β-N-acetylhexosaminidase A needed for conversion of GM1a and GM2 to GM3 [54].

Table 2.
The carbohydrate sequences of the discussed human gangliosides and potential sites of action for NEU3.
2. Development of Sialidase Inhibitors
The crystal structure for hNEU2 was used in homology modeling [55] to obtain information about possible binding sites of each hNEU, which in turn was used to develop potential inhibitors of each, based, in part, on how modifications to sialic acid might affect its efficacy as a sialidase ligand. While hNEUs 2, 3, and 4 have 34–40% homology, hNEU1 has only 19–24% [14]. Interestingly, protective protein/cathepsin A (ppCA), found in association with hNEU1, serves both as a chaperone/transporter and to protect hNEU1 from the lysosomal environment [56]. The sites identified for modifying neuraminic acid in order to synthesize hNEU ihibitors are shown in Figure 2 [35] and information about examples of actual inhibitors in Table 3. Comparison of the effectiveness of various sialic acid-based inhibitors at blocking the activity of specific hNEUs allowed researchers to draw conclusions about which site’s modification would block which hNEU activity most effectively. For example, a C9 biphenyl carbamate derivative had high selectivity and potency for NEU3 (µM), while activity of NEU1 and NEU4 was inhibited more efficiently by amide and triazole derivatives, respectively.

Figure 2.
Sites on neuraminic acid modified to give sialic acid-based inhibitors. Copied with permission [Bourguet et al. J. Med. Chem. 2022, 65, 4, 3002–3025] [35]. Larger arcs indicate relative size of effective substituents.
The finding that both NEU1 [57] and NEU3 [58] can dimerize supports interrogation of whether blocking dimerization would effectively inhibit their activity [35]. For a general review of sialidase inhibitors see Keil et al. [59].

Table 3.
Examples of inhibitors for specific mammalian sialidases.
Table 3.
Examples of inhibitors for specific mammalian sialidases.
| Examples of hNEU Inhibitors | Sialidase Inhibited | Cell Source of NEU | Substrate | Effect |
|---|---|---|---|---|
| C9-BA-DANA 1 [60] 2 | NEU1 | HEK293 cells | 4-MU-NANA 3 | IC50 = 10 µM |
| Neu5Ac2en- OacOMe [61] | Coronavirus 4 HCoV-OC43- infected epithelial cells | Viral N protein | Inhibited viral replication. | |
| C5-hexanamido-C9- acetamido-DANA [62] | COS-7 for overexpression of NEU1 | 4-MU-NANA | Ki of 53 ± 5 nM | |
| Oseltamivir [63] | 3T3–hEGFR cells | Inhibition of EGF- stimulated sialidase activity | IC50 = 4.86 µM | |
| Neu5AcN39N32en [64] | NEU2 | E. coli-expressed NEU2 | Neu5Acα2–6GalβpNP | IC50 = 13 ± 3 µM |
| DANA [65] | NEU3 | E. coli-expressed NEU3 | 4-MU-NANA | Ki = 30 µM |
| 5-Acetamido-9-(([1,1′-biphenyl]-4-cabamoyl)-oxy)-DANA [66] | E. coli-expressed NEU3 | 4MU-NANA | IC50 = 0.31 µM | |
| 2AP [67] | CCl4-induced liver fibrosis in mice | Effect on liver fibrosis | Reduced liver inflammation NEU3 | |
| MP [68] | Recombinant NEU3 | rhL-TGF-β1 | IC50 = 0.002 µM | |
| AMPCA [68] | Recombinant NEU3 | rhL-TGF-β1 | IC50 = 0.002 µM | |
| C9-4HMT-DANA [69] | NEU4 | E. coli expressed NEU4 A 2011 | 4-MU-NANA | Ki = 30 nM |
1 Abbreviations: C9-BA-DANA, C9-butyl-amide-DANA; DANA: 2-deoxy-2,3-didehydro-N-acetylneuraminic acid; C9-4HMT-DANA (5-acetamido-9-[4-hydroxymethyl[1,2,3]triazol-1-yl]-2,3,5,9-tetradeoxy-D-glycero-D-galacto-2-nonulopyranosonic acid); 4-MU-NANA, 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid; 2AP, 2-acetylpyridine; MP, methylpicolinate; AMPCA, 4-amino-1-methyl-2-piperidinecarboxylic acid; rhL-TGF, recombinant human latent transforming growth factor. 2 References given cite source of information shown for the NEU inhibitor. 3 It should be noted that when 4-MU-NANA is used to measure the Ki or IC50, the results may differ from those obtained using more natural substrates [70]. 4 Desialylation of the coronavirus N protein catalyzed by NEU1 enhances its replication [61].
3. Microbial Sialidases
A number of microbes utilize sialidase during the infectious process for such purposes as providing sialic acid residues as catabolites [71], exposing the appropriate cell surface oligosaccharide binding sites [e.g., V. cholerae for cholera toxin] and catalyzing their release from those binding sites [e.g., influenza]. The ability to act on cell surface ganglioside and/or sialoglycoprotein binding sites on target cells during the infectious process reflects the fact that the outer surface of the cell’s plasma membrane is coated by a glycocalyx [45] comprised of carbohydrate moieties, including gangliosides and sialoglycoproteins with their carbohydrate portions extending away from the outer surface [72]. The smaller glycosylated portion of gangliosides is found closer to the surface of the cell than the larger sialylated glycoproteins. Perhaps the best-studied sialidase-mediated pathogenic microbial infection is that of influenza virus, in which its sialidase activity participates with hemagglutinin in the infectious process as well as in the release of newly formed virions from sialylated binding sites on the cell surface [73]. It should be noted that sialidase behavior can vary depending on viral subtype [73].
Influenza viruses bind α2-3gal/galNAc and α2-6gal/galNAc-linked sialyl residues [73], which indicates that they could bind those on gangliosides and sialoglycoproteins. The finding that sialidase on the newly synthesized virions had to catalyze the cleavage of the cell surface sialyl moieties on the glycoconjugates to which they are adhered in order to be released and infect new host cells [74] led researchers to develop sialic acid derivatives that might inhibit the viral sialidase more efficiently than it did hNEUs. For a review about influenza sialidase see [75]. Results led to the development of Oseltamivir (Tamiflu) [3R,4R,5S)-4-acetylamino-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester, phosphate (1:1)], discovered by Gilead Sciences in 1995, co-developed with F. Hoffmann-La Roche Ltd., and patented in 1996. Zanamivir (Relenza). The 4-guanidino derivative of DANA, was discovered [76], licensed to Glaxo, and it was Glaxo Wellcome (now Glaxo Smith Kline) who obtained FDA approval for its use in 1999 [77]. Confirmation of the poor ability of the two drugs to inhibit activity of hNEUs was provided by Hata et al. [78], who found that, at 1mM, oseltamivir had little effect on any of the human neuraminidases, while µM quantities of Zanamivir were required to inhibit NEU2 and NEU3. Both drugs were effective on the viral sialidase in low nM concentrations. A third inhibitor of influenza sialidase, Peramivir (Rapivab, (1S,2S,3R,4R)-3-[(1S)-1-(acetylamino)-2-ethylbutyl]-4-(carbamimidoylamino)-2hydroxycyclopentanecarboxylic acid, trihydrate), developed by BioCryst Pharmaceuticals, Inc., gained FDA approval in 2014 [79].
Over time, the effectiveness of Oseltamivir and Peramivir has decreased somewhat due to the appearance of a genetic change identified as the H275Y mutation in influenza sialidase [80]. A series of analyses of the susceptability of sialidase associated with cultured samples of the H3N2 variant of influenza to each of the three inhibitors shown in Figure 3, plus a fourth authorized for use in Japan (Laninamivir), was undertaken annually (2010–2020) and again in 2022–2023 in Japan. Results indicate no significant changes in the efficacy of each inhibitor [81]. Despite the reduction in effectiveness seen in some samples from children in the USA, as of September 2024, the CDC was still recommending their use as not all strains of the virus have the mutation and for many people the drugs were still helpful. These drugs may prove useful in the treatment of other ailments, as Zanamivir has been shown to be effective in an animal model of arthritis [82] and Oseltamivir and Peramivir have been seen to reduce mortality in patients with severe COVID-19 [83].
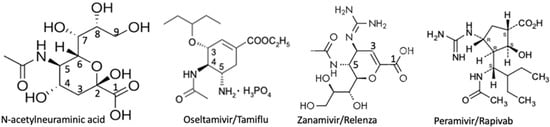
Figure 3.
Sialic acid-based inhibitors for influenza sialidase.
Structures were obtained from the label information for a drug prescribing information insert for Oseltamivir/Tamiflu, GlaxoSmithKline LLC for Zanamivir/Relenza, and accessdata.fda.gov for Peramivir/Rapivab.
Due to the increasing number of influenza subtypes, methods to enhance the effectiveness of Oseltamivir and Zanamivir at inhibiting these subtypes have been, and are being, investigated. One that showed promise was that in which polyglycol was used to link two Oseltamivir molecules together, thereby providing a divalent ligand potentially able to bind more than one active site on the viral sialidase tetramer. The dimers were significantly more effective against three strains of influenza than Oseltamivir alone [84]. The concept of multivalency providing a more effective ligand for a protein that has multiple binding sites for its target ligand is not new [85,86]. The effectiveness of such multivalent carbohydrate ligands as inhibitors for glycoconjugate binding proteins may reflect the fact that, while binding to a single carbohydrate ligand can be weak, binding to the multiple sites that can be found in clusters in the cell’s glycocalyx, can be quite strong [87]. Multivalency has now been used to develop a drug that appears to protect people infected with influenza, as detected by the development of hemagglutinin antibodies, from developing typical flu-associated symptoms. The drug, developed by Cidara Therapeutics and named CD388, is designed to be used prophylactically and to cover a broad spectrum of influenza strains. It is a multivalent derivative in which an average of 4.5 dimers of Zanamivir [88] are linked to lysine residues on the carrier. To prolong the half-life of the multivalent ligand, the surface of the Fc portion of the CH1–Fc hybrid domain of human IgG1, engineered for extended half-life [89], was used as the carrier. The spacing of Zanamivir dimers was such that they could bind to either adjacent sialidase tetramers on the same or nearby virions [88]. Animal tests have indicated that the drug was effective against a number of different strains of viruses, including influenza B. Efficacy was corroborated by results from a small preliminary study in which people were given either CD388 or placebo five days prior to exposure to the virus. A significant reduction in the development of symptomatic infection was seen, with only 17.6%, of those given the drug five days before exposure to virus expressing flu symptoms, while 60% of those given placebo became ill [90]. Cidara plans to start long-term clinical efficacy trials in early 2026 at the start of flu season in the southern hemisphere.
Interestingly, conversion of the NEU2 inhibitor Neu5AcN39N32en to Neu5Gc9N32en produced an inhibitor of V. cholerae neuraminidase [64] that effectively prevented its conversion of intestinal gangliosides to GM1 [91], the receptor for cholera toxin [92]. Influenza virus and V. cholerae are just two of a number of pathogens that utilize sialidase activity during the infectious process (for some examples see Table 4). With the mounting evidence for the efficacy of multivalent Zanamivir for the prevention of influenza, it is anticipated that researchers will determine whether multivalency enhances the effectiveness of monovalent drugs at inhibiting infection by other microbes.
An alternative approach to the use of chemical inhibitors for respiratory infections is the possible use of an inhaled bacterial sialidase to catalyze removal of the sialic acid residues from molecules on the surface of respiratory epithelial cells, thereby blocking adherance of respiratory viruses to their target cells (e.g., [93]). Conditions for the use of an inhaled bacterial sialidase against influenza and parainfluenza viruses have been studied [94] and its use against additional pulmonary problems that can make a person more susceptible to viral infection, such as chronic obstructive pulmonary disease, is also being investigated [95]. This approach could eliminate the possibility that chemical inhibitors developed to treat a microbial infection would inhibit an hNEU, as observed when antibodies prepared against sialidases produced by either C. perfringens or influenza virus were found to inhibit hNEU3 [96].

Table 4.
Examples of pathogens using sialidase during infection and, when known, potential inhibitors.
Table 4.
Examples of pathogens using sialidase during infection and, when known, potential inhibitors.
| Pathogen | Cells Infected | Disease In people | Binding Site(s) | Inhibitor | Inhibitor Efficacy |
|---|---|---|---|---|---|
| NDV 1 HN 2 [97] | Lung epithelia. | Mild flu-like in humans, deadly in birds. | α2-3- and α2-6-linked sialic acid [98]. | 4-trifluoro-acetamido-N-trifluoroacetyl-DANA, | Viral yield reduction assay IC50 = 0.03 µM [99]. |
| MuV HN [100] | Multiple types. | Mumps complications including e.g., deafness, meningitis, and infertility. | Trisaccharide receptors: α2-3SL, α2-3SLN, α2-3sLex, and oligo-GM3 [101]. | Milk-derived sialoglycopeptides with short glycans having α2-3-linked terminal sialic acids. | Inhibited infection by different strains; based on sialic acid concentration IC50 = 0.2–1 mM [100]. |
| Influenza A and B [102] | Pulmonary epithelia. | Flu. | Human strain A, α2-6-, strain B α2-3- and α2-6-linked sialic acid [103]. | Oseltamivir. Zanamivir. Peramivir. | Substrate 4-MU-NANA, IC50 = 0.78 nM, IC50 = 2.08 nM, IC50 = 0.66 nM, and influenza isolate 74 A H3N2 [81]. |
| Dengue serotype 2 [104] | Blood, skin, and liver. | Fever, and possibly headache, muscle or joint pain, nausea, vomiting, pain behind the eyes, swollen glands, rash. | Viral NS1 affects transcription of NEU1-4, suggesting that they help with viral Uncoating, replication and intracellular trafficking [105]. | Not a viral sialidase. | Dengue affected expression of all hNEUs [105]. |
| Vibrio cholerae [106] | Intestinal mucosal cells. | Cholera. | Di-and trisialo-gangliosides terminal sialic acid-linked α2-3 and α2-8 [107]. | Neu5Gc9N32en. | Neu5Acα2-3Galßρ NP, IC50 = ~18 µM Neu5Acα2-6Galßρ NP, IC50 = ~13 µM [64]. |
| Gardnerella vaginalis [108] | Genital tract. | Vaginal irritation and increases probability of pre-term birth. | Mucosal sialoglycans [109]. | 10 mM Zanamivir. | Cell invasion decreased ~50% [110]. |
| C. perfringens type F 3, Nans I, J and H. I provides most of the exosialidase activity [111]. | Intestinal enterocyte type cells | Food poisoning and chronic non foodborne gastrointestinal diseases. | Linkage cleaved: I, α 2-3; J, α 2-6; and H, α 2-8 [112]. | 7-(3,4-dihydroxyphenyl)-5-hydroxy-1-(3- hydroxy-4-methoxy phenyl) hepta-1,4,6- trien-3-one (individual Nan not identified). | Substrate 4MU-NANA IC50 = 0.5 ± 0.07 µM for Nan I [113]. |
| S. pneumoniae Nans A, B, and C [114] | Lung and epithelial cells. | Pneumonia, meningitis, and sepsis. | Surface protein A functions as an adhesive while sialidase degrades mucus, providing sialic acid as a bacterial nutrient [115]. | Zanamivir for NanA. 1 mM Oseltamivir for NanA. | Ki = 0.72mmM using MU-NANA [116]. Inhibited in vivo S. pneumoniae viability [115]. |
| C. sordellii, NanS [117] | Soft tissue and intrauterine infections. | Toxic shock and sepsis; low survival [118]. | Acted on cervical cell sialoglycoconjugates enhancing suscept-ability to bacterial toxins [117]. | ||
| Porphyromonas gingivalis [119] | Mouth. | Severe periodontitis. | Submaxillary glycoproteins. | Zanamivir. | Inhibited attachment and invasion [119]. |
1 Abbreviations: NDV, Newcastle disease virus; MuV, mumps virus; Nan followed by a capitol letter identifies the type of microbial sialidase; bacteria names are italicized; C., Clostridial; and S., Streptococcus. 2 HN indicates a single glycoprotein with both hemagglutinin and sialidase activity. Research indicates that H mediates binding and N cleaves sialic acid from progeny virus, promoting release of newly formed virions during the budding process [120]. The reason for looking at the inhibitors of infection by the mumps virus is the ineffectiveness of the vaccine against some of the strains. Hemagglutinin has 3 sialic acid binding sites [121] so multivalent inhibitors might be used to block its function in binding. 3 C. perfringens type F was previously known as C. perfringens type A [122].
4. Examples of Diseases Involving hNEUs
Characterization of the structure of the four different human sialidases and an understanding of their functions, has led to investigation of how alterations in their specific activity affect disease. Studies of each have shown they function in a variety of diseases, ranging from those caused by exposure to environmental agents such as coaldust or asbestos (e.g., [123]), to mutations that affect expression/activity of the NEU. Examples of the diseases discussed, and for which hNEUs can be a factor, include pulmonary fibrosis [9,23], atherosclerosis [124], diabetes [125], specific cancers [30], and neuronal development/function [126]. Examples of each and of possible therapeutic sites based on a NEU or NEUs follow.
4.1. Fibrosis
Coaldust and asbestos-induced pulmonary fibrosis are examples of environmentally induced diseases in which both NEU1 and NEU3 have been shown to have a role [9,23]. NEU1 presumably acts by catalyzing release of sialic acid from the membrane-tethered mucin, MUC1, thereby enhancing cell adhesion [127] while activated NEU3 may act on ganglioside substrates which in turn can affect tumor growth factor-ß1 (TGF-β1 [128]). This is of interest because TGF-β1 has been reported to be a major driver of lung fibrosis via the promotion of the differentiation of fibroblasts into myofibroblasts that produce excessive extracellular matrix that can contribute to deteriorating lung function [129]. The finding that TGF-ß1 can initiate a series of down-stream events forming a phospho-Smad3/MUC1-CT (MUC1-cytoplasmc tail) and MUC1-CT/β-catenin nuclear complex capable of enhancing conversion of alveolar epithelial type II cells and fibroblasts to myofibroblasts provides an explanation for its function in pulmonary fibrosis [130]. Increased MUC1 is found in both bronchoalveolar lavage and serum from those with pulmonary fibrosis, while it is present in less than 10% of serum samples from patients with chronic lung diseases [128]. After oropharyngeal instillation of silica into the lungs of hMUC1 transgenic and MUC1 KO mice, elevated levels of the covalently linked extracellular glycosylated α-subunit of MUC1 were found in the lungs of the transgenic animals and their lungs were protected from fibrosis [128]. Release of the extracellular domain of MUC1 allows it to act as a decoy barrier to external pathogens, while the bioactivated CT portion can act as an anti-inflammatory molecule in a number of airway infections [131]. Combining these observations indicates how the activities of both NEU1 and 3 could affect the clinical symptoms seen in environmentally induced pulmonary fibroses [132].
Some patients with idiopathic pulmonary fibrosis (IPF) have abnormally high levels of NEU3, which can upregulate active serum TGF-β1. TGF-β1, in turn, can upregulate NEU3 expression by decreasing its degradation and up-regulating its translation [133]. Translation is upregulated by TGF-β1 enhancing the binding of DEAD box helicase 3 (DDX3) to a common 20-nucleotide motif present on a total of 180 mRNAs, including that for NEU3. Reduced DDX3 reduced TGF-β1-enhanced NEU3 synthesis. Mice lacking NEU3 had very little fibrosis when treated with bleomycin to induce it [134].
Using a mouse model of kidney fibrosis, induced using unilateral ureteral obstruction (UUO), Xiao et al. found that NEU4 activity was upregulated, a finding also seen in patients with renal fibrosis [134]. Results from the studies of kidneys from male NEU4−/− mice, UUO-induced to develop kidney fibrosis, showed that the epithelial to mesenchymal cell transition was attenuated, as was the production of pro-fibrotic cytokines. NEU1 appears to have a similar effect on kidney fibrosis [135].
Patients with non-alcoholic fatty liver disease (NAFLD) may also develop fibrosis [136]. The finding that NEU3 was elevated in patients with idiopathic pulmonary fibrosis led to study of its potential role in NAFLD. Using mice treated with CCl4 to induce liver inflammation and fibrosis, treatment with the NEU3 inhibitor 2-acetylpyridine was found to ameliorate the symptoms [67]. Interestingly, the intestines of patients with inflammatory bowel disease, many of whom also develop fibrosis [137], were found to contain 8-fold more NEU3 than controls, while the concentration of GD1a, an NEU3 substrate, was 1/3 [138]. All of these results support the interrogation of whether specific hNEUs, or components in the pathways they affect, might be attractive targets for the development of therapies to treat specific types of fibrosis.
4.2. Atherosclerosis
Atherosclerosis, accumulation of plaque within arteries, contributed to by hypoxia-induced autophagy and macrophage inflammation [139], is affected by hNEU1, with the level of NEU1 gene transcripts in lipofibrous plaques significantly increased compared with that in healthy tissue samples [140]. The fact that NEU1 can affect insulin resistance [141], lipid metabolism [142] and inflammatory responses [24], has made NEU1 a likely candidate for study. Results from the analysis of the development of atherosclerosis in Neu1hypoApoe−/− mice indicate a decrease in aortic sinus atherosclerosis when compared with Apoe−/− mice [143]. Additional studies of the possible effect of hypoxia on hNEU1 have indicated that hypoxic conditions enhanced the desialylation of the autophagy protein ATG5 and promoted formation of an ATG5–ATG12–ATG16L complex, enhancing autophagosome formation. Hypoxic conditions were accompanied by translocation of hNEU1 from lysosomes into the cytoplasm and an up-regulation in its activity towards sialylated ATG5. Inhibition or knock-down of hNEU1 inhibited desialylation of ATG5 and inhibited the hypoxia-induced autophagy and cellular inflammation seen in atherosclerosis [144].
mRNAs for hNEU3 and hNEU4 were found to be 20-fold higher in lipofibrous plaques than normal tissue (human thoracic samples) and has been suggested to be a specific marker of atherosclerotic plaque instability [140]. NEUs1 and 3 were shown to trigger atherosclerosis by desialylating ApoB100 in low-density lipoproteins (LDLs), thereby increasing their uptake via the asialoglycoprotein receptor 1 present on human macrophages as well as aortic root lesions in mice [124]. Genetic inactivation or pharmacological inhibition of NEUs1 and 3, but not NEU4, significantly delayed the formation of fatty streaks in the aortic root, without affecting plasma cholesterol and LDL levels in ApoE−/− mouse models of atherosclerosis. Combined, these results support the hypothesis that the action of sialidase on ApoB100 might be the reason that some of the ~50% of people develop atherosclerosis despite having LDL levels thought to be in the normal range [145]. To interrogate this possibility one could (1) test individuals who have an atherosclerotic event, despite having a history of normal LDL levels, for the presence of desialylated ApoB100; (2) develop a simple method to quantify the amount of desialylated circulating ApoB100 in order to identify individuals for whom uptake of LDLs by macrophage could lead to atherosclerosis; and (3) develop an effective protocol for inhibiting desialylation of ApoB100 to retard LDL accumulation by macrophage.
4.3. Diabetes
As early as 2003 Sasaki et al. [146] reported that overexpression of the “plasma membrane associated sialidase” (NEU3) attenuated insulin signaling. Support for this was provided by the observation that transgenic mice overexpressing the human ortholog of NEU3 developed diabetic characteristics, such as hyperinsulinemia, after 18–22 weeks [146]. Analyses indicated that phosphorylation of the insulin receptor (IR) was reduced in response to insulin, while NEU3 tyrosine phosphorylation increased, as did its association with the growth factor receptor-bound protein 2, which activated the NEU3, thus enhancing the negative regulation of insulin signaling. Interestingly, the accumulation of GM1 and GM2, possible products of the transgenic NEU3, inhibited IR phosphorylaton in vitro [146]. Subsequent studies have found that KO of the NEU2 gene in mice altered sialylated glycoproteins needed for lipid metabolism, impaired muscle function, and led to diabetes [26]. NEU1 appears to reverse insulin resistance in type 2 diabetes [141]. Experimental results indicate that insulin receptor ß (IRß) is activated by a G-protein-coupled receptor (GPCR)-signaling platform potentiating NEU1 and matrix metalloproteinase-9 (MMP-9) cross talk on the cell surface. The activated NEU1 catalyzes release of α2-3 linked sialyl resides from IRß, allowing association of IRα and IRß subunits and activation of tyrosine kinase [147]. For an explanation of the structure and function of the insulin receptor see [148].
4.4. Cancer
Evidence for a correlation between sialidase activity and cancer was reported more than 50 years ago [149,150,151,152]. Since then, the four hNEUs have been identified and research is now focusing on their individual contributions to the oncogenic process. As an example, over time, evidence has been found indicating that NEU3 promotes cell invasion by renal cell carcinoma [153], head and neck squamous cell carcinoma [154], and glioblastoma cells [155]. As hNEUs have been studied in more cancers, their possible functions are beginning to be understood, such as those of NEUs1 and 3 in bladder cancer tissue cells [30]. The finding that knock down of NEU3 results in decreased invasiveness, reduced phosphorylation of ERK and P13K and decreased expression of the androgen receptor provides insight into how elevated NEU3 activity may contribute to oncogenicity in the bladder cancer tissues studied [30]. The fact that tissues from different bladder cancers may not express the same growth factors and receptors [30] supports the need for a general data bank in order to optimize the use of personalized medicine in their treatment. Support for this suggestion is provided by the fact that much of the information used by some of the researchers, whose data are cited in Table 5, was drawn from a variety of data bases, such as ONCOMINE [156], the Cancer Genome Atlas (TCGA, accessed through the National Cancer Institute’s Genomic Data Commons (GDC), the Gene Expression Omnibus (GEO, accessed via the NCBI GEO homepage), the Cancer Cell Line Encyclopedia (CCLE, data can be downloaded from the DepMap portal), and the Human Protein Atlas (HPA).

Table 5.
Examples of the effects of hNEUs in different cancers.
5. Sialidase in Neuronal Development/Function
5.1. Development
Analysis of brains from chickens has indicated that sialidase activity is expressed in 13-day old embryonic chicken brains and that its activity towards endogenous substrate increases through 3 months post hatching, while that towards added ganglioside substrate decreases [169]. In people, ganglioside sialidase has been detected between fetal weeks 15 and 20, reaching about half that found in adults at term and increasing thereafter to that found at about five years of age [170]. Neuronal development occurs over time and research has shown that each hNEU contributes to changes that occur. In terms of development, several cell adhesion molecules, including E-cadherin, integrins, and catenins, are sialylated glycoproteins [171] and aberrant sialylation can disrupt interactions with their receptors, such as those needed for stem cell differentiation [36]. Murine NEU4 catalyzes the degradation of the polysialic acid which associates with neural cell adhesion molecule (polySia-NCAM) affecting not just neurite growth but synaptic plasticity, and cell migration as well [172]. Results from studies of fish (Tilapia) indicate that, when they are grown in the absence of sunlight, expression of NEU4 mRNA as well as retinal differentiation markers was upregulated [173]. Addition of Tilapia NEU4 to two different neuroblastoma cell lines decreased sialic acid levels in nuclear glycoproteins and glycolipids, accelerated neurite formation, and enhanced acetylcholinesterase activity, supporting the hypothesis that NEU4 accelerates differentiation. NEU4 activity in Japanese rice fish was relatively low in the brains of embryos and then rose rapidly 3–13 days after birth [174]. In contrast, NEU3 expression was high in embryos and decreased after birth. The decrease in NEU3 expression after birth appears to coincide with neuronal differentiation and the appearance of the more complex gangliosides GD1a, GD1b. and GT1b [175,176,177], known NEU3 substrates [178]. Those gangliosides, plus GM1a, account for ~75% of the sialic acid found in the adult brain [5,179]. The recent development of a method to isolate both neural stem progenitor cells and oligodendrocyte progenitor cells from the brains of live rats [180] means that developmental changes can be studied in each, as the cells mature in tissue culture.
The observation that induced differentiation of pheochromocytoma (PC12) cells was accompanied by an induction of transcription of NEU2 and that the activity of NEU2 disappeared in differentiated PC12 cells indicates a defined time period for the need for NEU2 during their differentiation [181]. Identification of the interaction of NEU2 with α- and ß-actin [182], coupled with the requirement of sialylated glycoproteins for actin to function [183,184] support the hypothesis that NEU2 may act to remodel actin via desialylation of one or more of the sialoglycoproteins needed for its involvement in neuronal differentiation.
NEU1 acts to stop the migration of hippocampal granule cells by catalyzing release of sialyl residues from cell surface polysialic acid when they reach the innermost surface of the granule cell layer [185]. NEU1 influences microglia by modulating the sialylation of full-length Trem2 (Trem2-FL), a multifunctional receptor that regulates microglial survival, phagocytosis, and cytokine production [186]. When NEU1 is deficient/down regulated, Trem2-FL remains sialylated, accumulates intracellularly, and is excessively cleaved into a C-terminal fragment and an extracellular soluble domain, enhancing signaling capacity. Because NEU1 and Trem2 are implicated in neurodegenerative/neuro-inflammatory diseases, including Alzheimer’s disease and sialidosis, modulating NEU1 activity may present a therapeutic approach to broadly regulate microglia-mediated neuroinflammation. Combined, these findings support the hypothesis that expression of each of the hNEUs correlates with changes in the degree of sialylation of differentiation-related sialoglycoconjugates that function during neural development.
5.2. Relation of Sialidase to Neural Transmission
During the neural transmission that is induced by the exposure of rat hippocampal slices to a high-frequency electrical stimulation to induce long-term potentiation, NEU4 activity was found to change within seconds [187] by using a benzothiozolylphenol-based sialic acid derivative to monitor free sialic acid levels [188]. NEU4 can catalyze the cleavage of sialyl residues on the polysialylated neural cell adhesion molecule (polySia-NCAM) which carries a linear homopolymer comprised of 8–90 sialic acid residues linked α2-8 [172]. PolySia-NCAM functions in brain development, synaptic plasticity and learning (for a review see [189]). Genetic or enzymatic manipulations blocking synthesis of polySia-NCAM were found to result in impaired synaptic plasticity and learning that could be ameliorated by the addition of polysialic acid [190].
While not discussed in this review, one must consider not just the expression of hNEUs but the availability of sialic acid, sialyl- and glycosyltransferases and the transport proteins used to synthesize the sialoglycolipids and sialoglycoproteins that NEUs modify as needed (see Figure 4). Some examples are as follows: knockdown of the ST6Gal1 gene or the use of sialyltransferase inhibitors negatively impacts the efficiency of somatic cell reprogramming [191], failure to synthesize the GM3 induced by a homozygous loss-of-function mutation in the gene encoding GM3 synthase (ST3Gal5) results in infantile-onset symptomatic epilepsy syndrome [192], while mutations in the human ST8SIA1 gene are associated with an increased risk of developing multiple sclerosis [193].

Figure 4.
Synthesis of sialic acid and its subsequent incorporation into sialoglycoconjugates.
5.3. Examples of Sialidase Misfunction in Neural Diseases
Sialidosis: Misfunction of hNEU1 has been implicated in sialidosis, Parkinson’s and Alzheimer’s diseases (PD and AD). Lysosomal NEU1 functions to catalyze the release of sialic acid from sialylated glycoproteins and glycolipids and when it fails to do so, the resultant lysosomal storage of those products is evidenced by clinical problems reflective of the degree of substrate accumulation [194]. Sialidosis, a rare, autosomal recessive disease is clinically divided into two types: in type 1, symptoms of progressive neurological and ophthalmologic problems present in late childhood or early adulthood, while in type 2 symptoms are more severe and present earlier [195]. Interestingly, ethnicity was found to correlate with the expressed phenotype, with Asian patients with sialidosis type 1 tending to have less severe symptoms than Caucasians [196]. This, coupled with the finding of a variety of genetic mutations [197], underscores the need for an understanding of the relationship between genotype and phenotype in order to optimize patient care [198]. Treatment of this error requires increasing the amount of functional NEU1 in the lysosome. The need of NEU1 for protective protein/cathepsin A to assist its entry into the lysosome and, once there, to protect it from degradation presents an obstacle to the use of NEU1 alone [199]. For a discussion of possible treatment approaches see [198].
Parkinson’s disease (PD): In contrast to the rare occurrence of sialidosis, PD is the second most common neurodegenerative disease in individuals ≥50 yrs of age, with an estimated 8.5 million globally affected in 2019 (World Health Org. Parkinson’s disease data: https://www.who.int/news-room/fact-sheets/detail/parkinson-disease#:~:text=PD%20is%20a%20clinical%20diagnosis,of%20over%20100%25%20since%202000, accessed on 9 July 2025)—a number that is expected to reach 12.7–17 million by 2040 [200]. A number of studies have indicated that treatment of animals expressing PD symptoms with isolated GM1 showed an increase in striatal dopamine and enhanced dopamine synthetic capability in residual dopaminergic neurons (e.g., [201,202]) and, when used in a monkey model of PD, improvement in cognitive and motor deficits was observed [203]. Lipid analyses have indicated alterations in expression of gangliosides and that the deficiency in GM1 correlated with Parkinson’s [204]. Subsequent studies of the efficacy of GM1 for the treatment of patients with PD have indicated that it is effective but that its use is problematic [205]. A major problem is obtaining enough GM1 that is guaranteed to be prion free, with sialic acid that is solely NeuNAc, and which is available in large quantities. Use of an in vivo method for the synthesis of GM1 [206] may address the problem of large-scale synthesis. The problem of GM1 transport across the blood–brain barrier [207] might be circumvented by infusing it intranasally, a method found to reduce neurotoxic proteins and restore functional neurons when used in a PD mouse model [126]. To circumvent both problems, V. cholerae sialidase was administered intraventricularly to MPTP-treated mice. Its use was based on the premise that the exogenously added sialidase would increase the conversion of di- and trisialogangliosides to GM1. Results indicate that dopaminergic neurons of the substantia nigra pars compacta were also spared by exposure to sialidase, occurred when animals were treated with GM1 [208]. More recently the oligosaccharide portion of GM1 (oligo-GM1) was shown to cross the blood–brain barrier (BBB) and promote recovery in a sporadic PD model in the B4galnt1+/− mouse [209]. Partial loss of expression of B4GALNT1 results in failure to synthesize enough GM2 and the more complex gangliosides, including GM1, causing both motor and non-motor symptoms of PD [210]. Using a cellular model to understand how the oligosaccharide portion might cross the BBB to promote recovery in the B4galnt1+/− mouse, researchers concluded that it was via a paracellular mechanism and that this permitted transport of 20-fold more oligo-GM1 than of intact GM1 [207]. Analysis of the enzymes involved in the metabolism of gangliosides and sialoglycoproteins indicated that NEU4 in lysosomes is decreased in PD and therefore cannot catalyze conversion of more complex gangliosides to GM1. In addition to an increase in GD1a and decrease in GM1, NEU4−/− mice had enhanced astro- and microgliosis as well as motor impairment [211].
Accumulating evidence indicates that chronic inflammation contributes to neurodegenerative diseases and that microglia are involved in the process (for a review see [212]). In response to inflammation induced by exposing microglia to lipopolysaccharide, microglia were found to secrete NEU3 associated with extracellular vesicles that are able to fuse with neurons. There, NEU3 can act to catalyze the release of sialic acid from cell surface sialoglycoconjugates, modifying the neuronal glycocalyx and neuronal connectivity [213]. These results support the hypothesis that, over time, remodeling of the glycocalyx induced by inflammation and the attendant effects on network-level activity of neurons could contribute to neuroinflammatory diseases such as Parkinson’s and Alzheimer’s [213]. If this is proven correct, identification of effective anti-inflammatory agents might provide potential drug targets.
Alzheimer’s: The fact that an estimated 7 million Americans age > 65 yrs are currently living with Alzheimer’s dementia and that number is growing (Alzheimer Disease Assoc, 2024: https://www.alz.org/alzheimers-dementia/facts-figures#:~:text=Over%207%20million%20Americans%20have,older%20(11%25)%20has%20Alzheimer’s, accessed on 9 July 2025), makes this the sixth leading cause of death in 2022 for Americans in this age group. It also underscores the need for an understanding of the disease’s development in order to identify targets for drug development.
NEU1 deficiency in mice induces a spontaneous phenotype of AD-like amyloidosis, while overexpression of NEU1 obtained by injecting NEU1 into the brain of an AD mouse model was conducive to a reduction in amyloid plaques [214]. Early work has indicated that ß-amyloid bound cell surface glycolipids or glycoproteins [215] and that that binding required sialic acid [216]. More specifically, evidence indicates that the association of ß-amyloid with GM1 appears to enhance formation of amyloid plaques [217]. These findings led to interrogating the possibility that added NEU1 might catalyze cleavage of non-ganglioside associated clustered sialyl residues present on the cell surface or cytosol that might interfere with binding to GM1. Support for this hypothesis was provided by the observation that ß-amyloid-induced toxicity could be reduced by binding the ß-amyloid to sialic acid-conjugated dendrimers [218]. Exosomes can carry proteins, including glycosidases, from one cell to another [219]. The proteins can act on compounds in the extra cellular space altering, in the case of sialidases, sialic acid distribution on the surface of the glycocalyx and possibly affecting cell adhesion and invasion (modified from Sanderson et al. [220]). It has been postulated that, over time, this remodeling can gradually uncover amyloid binding sites that affect the progression of AD and which eventually cause neurons to die.
The NEU4-catalyzed removal of sialyl residues from hippocampal polySia-NCAM inhibits neurite outgrowth [172] while NEU3 enhances it [174]. As stated in the discussion of Parkinson’s, activity of extravesicular NEU3 on glycocalyx sialoglycoconjugates can modify network connectivity, affecting network-level neuronal activity [213] and contributing to the problems seen in AD.
While amyloid and tau have been hypothesized as the primary components causing Alzheimer’s [221], other factors are being studied. Based on observations that the ratio of toxic to nontoxic microRNAs predicted sensitivity of ovarian cancer cells to platinum [222], this approach was used to determine whether the ratio of nontoxic to toxic sRNAs might correlate with the neurotoxicity seen in AD [223]. Results indicate that a shift to more toxic sRNAs correlates with neuronal cell susceptibility due to the death induced by survivor gene elimination [223]. The reported observations support the hypotheses that (1) high expression of nontoxic miRNAs protects from neurodegeneration and that increasing the biogenesis of nontoxic miRNAs or blocking that of toxic RNA-induced silencing complex sRNAs might be a viable treatment option for many neurodegenerative diseases, including AD, and (2) that identification of the eliminated survivor genes will further our understanding of symptom development in AD and suggest possible therapeutic targets.
6. Conclusions
Research designed to learn about both human and microbial sialidases, their structure, substrate specificity, mechanism of action, behavior in disease, etc. has grown consistently since 1957, the year for which I found the first listing in PubMed using the search word sialidase. This growth in publication has been reasonably steady, with much information now available about sialidase structure, function, cellular location and mechanism of action; however, much is still unknown.
When discussing the various diseases affected by hNEUs, it is evident that more than one isozyme may contribute to the symptoms seen, reflecting limitations in both their substrate specificity as well as their subcellular location. To consider interrogating the best possible treatment for an effect that their expression or lack thereof induces, we need to know more about the primary substrate each NEU acts on, resultant up- and down-stream effects, and how they may alter cell behavior. When looking at cancer, evidence indicates a given hNEU isozyme does not necessarily have the same effect in different tumors. All of these are reasons for establishing a repository for information regarding sialidase, possibly, in part, by combining on-line ones such as those mentioned earlier in this review. Such a repository should be readily accessible and be contributed to by researchers world-wide. In addition to specific facts, it should include connections between observations made. For example, what are the differences that enable an hNEU to induce one response in one type of cancer and a very different response in another, or which enable different results to be seen in animal models than those found in humans.
Early success with the use of multivalency to develop a potentially more effective inhibitor of clinical symptoms induced by the influenza virus supports the suggestion that a similar approach might be effective for inhibiting infection by other microbes that are dependent on sialidase activity for part of the infectious process. However, the effectiveness of a multivalent ligand is dependent upon the sialidase having multiple ligand binding sites and knowledge of their spacing, which underscores the need to continue to gather information about their structure as well as their function(s) in the pathogen’s life cycle.
In terms of development, it is apparent that each hNEU has a role. This is especially true when looking at the central nervous system. In this case, development can be considered to be a lifelong procedure, as changes in sialidase activity and sialoglycoconjugates are continually occurring. The fact that changes that appear minor may be ongoing long before associated clinical changes become apparent will require the ongoing development of tools with which to study these changes and the ability to connect changes induced by hNEU activity with those that may occur in sialoglycoconjugate synthesis, signal transduction efficacy, and more general metabolic changes, such as those seen in lipid metabolism. Ready access to all of this knowledge will optimize the use of personalized approaches in the treatment of individuals with sialidase-related diseases.
Funding
This work received no external support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented is available in the references cited.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Bonten, E.; van der Spoel, A.; Fornerod, M.; Grosveld, G.; d’Azzo, A. Characterization of human lysosomal neuraminidase defines the molecular basis of the metabolic storage disorder sialidosis. Genes Dev. 1996, 10, 3156–3169. [Google Scholar] [CrossRef]
- Monti, E.; Preti, A.; Rossi, E.; Ballabio, A.; Borsani, G. Cloning and characterization of NEU2, a human gene homologous to rodent soluble sialidases. Genomics 1999, 57, 137–143. [Google Scholar] [CrossRef]
- Wada, T.; Yoshikawa, Y.; Tokuyama, S.; Kuwabara, M.; Akita, H.; Miyagi, T. Cloning, expression, and chromosomal mapping of a human ganglioside sialidase. Biochem. Biophys. Res. Commun. 1999, 261, 21–27. [Google Scholar] [CrossRef]
- Monti, E.; Bassi, M.T.; Bresciani, R.; Civini, S.; Croci, G.L.; Papini, N.; Riboni, M.; Zanchetti, G.; Ballabio, A.; Preti, A.; et al. Molecular cloning and characterization of neu4, the fourth member of the human sialidase gene family. Genomics 2004, 83, 445–453. [Google Scholar] [CrossRef]
- Pshezhetsky, A.V.; Ashmarina, M. Keeping it Trim: Roles of neuraminidases in CNS function. Glycoconj. J. 2018, 35, 375–386. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Sun, P.; Paller, A.S. Ganglioside induces caveolin-1 redistribution and interaction with the epidermal growth factor receptor. J. Biol. Chem. 2002, 277, 47028–47034. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Wada, T.; Iwamatsu, A.; Hata, K.; Yoshikawa, Y.; Tokuyama, S.; Sawada, M. Molecular cloning and characterization of a plasma membrane-associated sialidase specific for gangliosides. J. Biol. Chem. 1999, 274, 5004–5011. [Google Scholar] [CrossRef]
- Schengrund, C.-L. The ying and yang of ganglioside function in cancer. Cancers 2023, 15, 5362. [Google Scholar] [CrossRef] [PubMed]
- Karhadkar, T.R.; Chen, W.; Pilling, D.; Gomer, R.H. Inhibitors of the sialidase NEU3 as potential therapeutics for fibrosis. Int. J. Mol. Sci. 2023, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hosono, M.; Sato, I.; Hata, K.; Wada, T.; Yamaguchi, K.; Nitta, K.; Shima, H.; Miyagi, T. Sialidase NEU3 contributes neoplastic potential on colon cancer cells as a key modulator of gangliosides by regulating Wnt signaling. Int. J. Cancer 2015, 137, 1560–1573. [Google Scholar] [CrossRef]
- Kappagantula, S.; Andrews, M.R.; Cheah, M.; Abad-Rodriguez, J.; Dotti, C.G.; Fawcett, J.W. Neu3 Sialidase-mediated ganglioside conversion is necessary for axon regeneration and is blocked in CNS axons. J. Neurosci. 2014, 34, 2477. [Google Scholar] [CrossRef]
- Pan, X.; De Aragão, C.B.P.; Velasco-Martin, J.P.; Priestman, D.A.; Wu, H.Y.; Takahashi, K.; Yamaguchi, K.; Sturiale, L.; Garozzo, D.; Platt, F.M.; et al. Neuraminidases 3 and 4 regulate neuronal function by catabolizing brain gangliosides. FASEB J. 2017, 31, 3467–3483. [Google Scholar] [CrossRef]
- Allende, M.L.; Lee, Y.T.; Byrnes, C.; Li, C.; Tuymetova, G.; Bakir, J.Y.; Nicoli, E.-R.; James, V.K.; Brodbelt, J.S.; Tifft, C.J.; et al. Sialidase NEU3 action on GM1 ganglioside Is neuroprotective in GM1 gangliosidosis. J. Lipid Res. 2023, 64, 100463. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Yamamoto, K. Sialidase NEU3 and its pathological significance. Glycoconj. J. 2022, 39, 677–683, Erratum in Glycoconj. J. 2022, 39, 543. https://doi.org/10.1007/s10719-022-10075-7. [Google Scholar] [CrossRef]
- Bocquet, O.; Tembely, D.; Rioult, D.; Terryn, C.; Romier, B.; Bennasroune, A.; Blaise, S.; Sartelet, H.; Martiny, L.; Duca, L.; et al. Characterization of novel interactions with membrane NEU1 highlights new regulatory functions for the elastin receptor complex in monocyte interaction with endothelial cells. Cell Biosci. 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Smutova, V.; Albohy, A.; Pan, X.; Korchagina, E.; Miyagi, T.; Bovin, N.; Cairo, C.W.; Pshezhetsky, A.V. Structural basis for substrate specificity of mammalian neuraminidases. PLoS ONE 2014, 9, e106320. [Google Scholar] [CrossRef]
- Miyagi, T.; Hata, K.; Hasegawa, A.; Aoyagi, T. Differential effect of various inhibitors on four types of rat sialidase. Glycoconj. J. 1993, 10, 45–49. [Google Scholar] [CrossRef]
- Magesh, S.; Suzuki, T.; Miyagi, T.; Ishida, H.; Kiso, M. Homology modeling of human sialidase enzymes NEU1, NEU3 and NEU4 based on the crystal structure of NEU2: Hints for the design of selective neu3 inhibitors. J. Mol. Graph. Model. 2006, 25, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Maurice, P.; Baud, S.; Bocharova, O.V.; Bocharov, E.V.; Kuznetsov, A.S.; Kawecki, C.; Bocquet, O.; Romier, B.; Gorisse, L.; Ghirardi, M.; et al. New insights into molecular organization of human neuraminidase-1: Transmembrane topology and dimerization ability. Sci. Rep. 2016, 6, 38363. [Google Scholar] [CrossRef]
- Miyagi, T.; Yamaguchi, K. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology 2012, 22, 880–896. [Google Scholar] [CrossRef]
- Seyrantepe, V.; Poupetova, H.; Froissart, R.; Zabot, M.; Maire, I.; Pshezhetsky, A.V. Molecular pathology of NEU1 gene in sialidosis. Hum. Mutat. 2003, 22, 343–352. [Google Scholar] [CrossRef]
- Annunziata, I.; d’Azzo, A. Galactosialidosis: Historic aspects and overview of investigated and emerging treatment options. Exp. Opin. Orph. Drugs 2017, 5, 131–141. [Google Scholar] [CrossRef]
- Luzina, I.G.; Lillehoj, E.P.; Lockatell, V.; Hyun, S.W.; Lugkey, K.N.; Imamura, A.; Ishida, H.; Cairo, C.W.; Atamas, S.P.; Goldblum, S.E. Therapeutic effect of neuraminidase-1–selective inhibition in mouse models of bleomycin-induced pulmonary inflammation and fibrosis. J. Pharmacol. Exp. Ther. 2021, 376, 136–146. [Google Scholar] [CrossRef]
- Du, J.; Shui, H.; Chen, R.; Dong, Y.; Xiao, C.; Hu, Y.; Wong, N.-K. Neuraminidase-1 (NEU1): Biological roles and therapeutic relevance in human disease. CIMB 2024, 46, 8031–8052. [Google Scholar] [CrossRef]
- Tringali, C.; Papini, N.; Fusi, P.; Croci, G.; Borsani, G.; Preti, A.; Tortora, P.; Tettamanti, G.; Venerando, B.; Monti, E. Properties of recombinant human cytosolic sialidase HsNEU2: The enzyme hydrolyzes monomerically dispersed GM1 ganglioside molecules. J. Biol. Chem. 2004, 279, 3169–3179. [Google Scholar] [CrossRef]
- Oh, M.; Ha, D.-I.; Son, C.; Kang, J.G.; Hwang, H.; Moon, S.B.; Kim, M.; Nam, J.; Kim, J.S.; Song, S.Y.; et al. Defect in cytosolic Neu2 sialidase abrogates lipid metabolism and impairs muscle function in Vivo. Sci. Rep. 2022, 12, 3216. [Google Scholar] [CrossRef]
- Mozzi, A.; Forcella, M.; Riva, A.; Difrancesco, C.; Molinari, F.; Martin, V.; Papini, N.; Bernasconi, B.; Nonnis, S.; Tedeschi, G.; et al. NEU3 Activity Enhances EGFR Activation without Affecting EGFR Expression and Acts on Its Sialylation Levels. Glycobiology 2015, 25, 855–868. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zanchetti, G.; Colombi, P.; Manzoni, M.; Anastasia, L.; Caimi, L.; Borsani, G.; Venerando, B.; Tettamanti, G.; Preti, A.; Monti, E.; et al. Sialidase NEU3 is a peripheral membrane protein localized on the cell surface and in endosomal structures. Biochem. J. 2007, 408, 211–219. [Google Scholar] [CrossRef][Green Version]
- Kakugawa, Y.; Wada, T.; Yamaguchi, K.; Yamanami, H.; Ouchi, K.; Sato, I.; Miyagi, T. Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc. Natl. Acad. Sci. USA 2002, 99, 10718–10723. [Google Scholar] [CrossRef] [PubMed]
- Tatsuta, T.; Ito, J.; Yamamoto, K.; Sugawara, S.; Hosono, M.; Sato, M.; Miyagi, T. Sialidase NEU3 contributes to the invasiveness of bladder cancer. Biomedicines 2024, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Seyrantepe, V.; Landry, K.; Trudel, S.; Hassan, J.A.; Morales, C.R.; Pshezhetsky, A.V. Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells. J. Biol. Chem. 2004, 279, 37021–37029. [Google Scholar] [CrossRef]
- Bigi, A.; Morosi, L.; Pozzi, C.; Forcella, M.; Tettamanti, G.; Venerando, B.; Monti, E.; Fusi, P. Human Sialidase NEU4 long and short are extrinsic proteins bound to outer mitochondrial membrane and the endoplasmic reticulum, respectively. Glycobiology 2010, 20, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Okun, S.; Peek, A.; Igdoura, S.A. Neuraminidase 4 (NEU4): New biological and physiological player. Glycobiology 2023, 33, 182–187. [Google Scholar] [CrossRef]
- Shiozaki, K.; Yamaguchi, K.; Takahashi, K.; Moriya, S.; Miyagi, T. Regulation of Sialyl Antigen expression in colon cancer cells by sialidase NEU4. J. Biol. Chem. 2011, 286, 21052–21061. [Google Scholar] [CrossRef] [PubMed]
- Bourguet, E.; Figurska, S.; Fra̧czek, M.M. Human neuraminidases: Structures and stereoselective inhibitors. J. Med. Chem. 2022, 65, 3002–3025. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, Y.; Guo, L.; Feng, S. Biological function of sialic acid and sialylation in human health and disease. Cell Death Discov. 2024, 10, 415. [Google Scholar] [CrossRef]
- Aljohani, M.A.; Sasaki, H.; Sun, X.-L. Cellular translocation and secretion of sialidases. J. Biol. Chem. 2024, 300, 107671. [Google Scholar] [CrossRef]
- Blix, G. Über die kohlenhydratgruppen des submaxillarismucins. Z. Physiol. Chem. 1936, 240, 43–54. [Google Scholar] [CrossRef]
- Klenk, E. Neuraminsäure, Das spaltprodukt eines neuen gehirnlipoids. Z. Physiol. Chem. 1941, 268, 50–58. [Google Scholar] [CrossRef]
- Schnaar, R.L. Glycolipid-mediated cell–cell recognition in inflammation and nerve regeneration. Arch. Biochem. Biophys. 2004, 426, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Blix, F.G.; Gottschalk, A.; Klenk, E. Proposed nomenclature in the field of neuraminic and sialic acids. Nature 1957, 179, 1088. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, J.; Veh, R.W.; Sander, M.; Schauer, R.; Kamerling, J.P.; Vliegenthart, J.G.F. Demonstration of 9-O-acetyl-N-acetylneuraminic acid in brain gangliosides from various vertebrates including man. Z. Physiol. Chem. 1977, 358, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Sonnino, S. Alkali-labile gangliosides. Glycoconj. J. 2023, 40, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Genetic basis for the lack of n-glycolylneuraminic acid expression in human tissues and its implication to human evolution. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2006, 82, 93–103. [Google Scholar] [CrossRef]
- Cheeseman, J.; Badia, C.; Thomson, R.I.; Kuhnle, G.; Gardner, R.A.; Spencer, D.I.R.; Osborn, H.M.I. Quantitative standards of 4-O-acetyl- and 9-O-acetyl-N-acetylneuraminic acid for the analysis of plasma and serum. ChemBioChem 2022, 23, e202100662. [Google Scholar] [CrossRef]
- Visser, E.A.; Moons, S.J.; Timmermans, S.B.P.E.; de Jong, H.; Boltje, T.J.; Büll, C. Sialic Acid O-Acetylation: From Biosynthesis to Roles in Health and Disease. J. Biol. Chem. 2021, 297, 100906. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Hakomori, S.; Handa, K.; Iwabuchi, K.; Yamamura, S.; Prinetti, A. New insights in glycosphingolipid function: “glycosignaling domain,” a cell surface assembly of glycosphingolipids with signal transducer molecules, involved in cell adhesion coupled with signaling. Glycobiology 1998, 8, xi–xviii. [Google Scholar] [CrossRef]
- Luft, J.H. Fine Structures of capillary and endocapillary layer as revealed by ruthenium red. FASEB J. 1966, 25, 1773–1783. [Google Scholar] [PubMed]
- Martínez-Palomo, A. The surface coats of animal cells part of the personal work mentioned in this review was performed at the Institut de Recherches Scientifiques Sur Le Cancer, Villejuif, France. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Jeon, K.W., Eds.; Academic Press: Cambridge, MA, USA, 1970; Volume 29, pp. 29–75. ISBN 0074-7696. [Google Scholar]
- Lunghi, G.; Fazzari, M.; Di Biase, E.; Mauri, L.; Chiricozzi, E.; Sonnino, S. The structure of gangliosides hides a code for determining neuronal functions. FEBS Open Bio 2021, 11, 3193–3200. [Google Scholar] [CrossRef]
- Bremer, E.G.; Hakomori, S.; Bowen-Pope, D.F.; Raines, E.; Ross, R. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J. Biol. Chem. 1984, 259, 6818–6825. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, G.; Fazzari, M.; Ciampa, M.G.; Mauri, L.; Di Biase, E.; Chiricozzi, E.; Sonnino, S. Regulation of signal transduction by gangliosides in lipid rafts: Focus on GM3–IR and GM1–TrkA interactions. FEBS Lett. 2022, 596, 3124–3132. [Google Scholar] [CrossRef]
- LI, S.-C.; LI, Y.-T.; MORIYA, S.; MIYAGI, T. Degradation of GM1 and GM2 by mammalian sialidases. Biochem. J. 2001, 360, 233–237. [Google Scholar] [CrossRef]
- Chavas, L.M.; Tringali, C.; Fusi, P.; Venerando, B.; Tettamanti, G.; Kato, R.; Monti, E.; Wakatsuki, S. Crystal structure of the human cytosolic sialidase Neu2: Evidence for the dynamic nature of substrate recognition. J. Biol. Chem. 2005, 280, 469–475. [Google Scholar] [CrossRef]
- Bonten, E.J.; Campos, Y.; Zaitsev, V.; Nourse, A.; Waddell, B.; Lewis, W.; Taylor, G.; d’Azzo, A. Heterodimerization of the sialidase NEU1 with the chaperone protective protein/Cathepsin A prevents Its premature oligomerization. J. Biol. Chem. 2009, 284, 28430–28441. [Google Scholar] [CrossRef]
- Albrecht, C.; Kuznetsov, A.S.; Appert-Collin, A.; Dhaideh, Z.; Callewaert, M.; Bershatsky, Y.V.; Urban, A.S.; Bocharov, E.V.; Bagnard, D.; Baud, S.; et al. Transmembrane peptides as a new strategy to inhibit neuraminidase-1 activation. Front. Cell Dev. Biol. 2020, 8, 611121. [Google Scholar] [CrossRef]
- Rodriguez-Walker, M.; Daniotti, J.L. Human Sialidase Neu3 Is S-Acylated and Behaves Like an Integral Membrane Protein. Sci. Rep. 2017, 7, 4167. [Google Scholar] [CrossRef]
- Keil, J.M.; Rafn, G.R.; Turan, I.M.; Aljohani, M.A.; Sahebjam-Atabaki, R.; Sun, X.-L. Sialidase Inhibitors with Different Mechanisms. J. Med. Chem. 2022, 65, 13574–13593. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.W.; Liu, A.; Liu, Z.; Cross, A.S.; Verceles, A.C.; Magesh, S.; Kommagalla, Y.; Kona, C.; Ando, H.; Luzina, I.G.; et al. The NEU1-selective sialidase inhibitor, C9-butyl-amide-DANA, blocks sialidase activity and NEU1-mediated bioactivities in human lung in Vitro and murine lung in Vivo. Glycobiology 2016, 26, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wu, Y.; Turan, I.; Keil, J.; Li, K.; Chen, M.H.; Liu, R.; Wang, L.; Sun, X.-L.; Chen, G.-Y. Targeting intracellular Neu1 for coronavirus infection treatment. Iscience 2023, 26, 106037. [Google Scholar] [CrossRef]
- Guo, T.; Héon-Roberts, R.; Zou, C.; Zheng, R.; Pshezhetsky, A.V.; Cairo, C.W. Selective Inhibitors of Human Neuraminidase 1 (NEU1). J. Med. Chem. 2018, 61, 11261–11279. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, A.M.; Abdulkhalek, S.; Cheng, T.S.; Alghamdi, F.; Jayanth, P.; O’Shea, L.K.; Geen, O.; Arvizu, L.A.; Szewczuk, M.R. A novel epidermal growth factor receptor-signaling platform and its targeted translation in pancreatic cancer. Cell Sig. 2013, 25, 2587–2603. [Google Scholar] [CrossRef]
- Khedri, Z.; Li, Y.; Cao, H.; Qu, J.; Yu, H.; Muthana, M.M.; Chen, X. Synthesis of selective inhibitors against V. cholerae sialidase and human cytosolic sialidase NEU2. Org. Biomol. Chem. 2012, 10, 6112–6120. [Google Scholar] [CrossRef]
- Albohy, A.; Mohan, S.; Zheng, R.B.; Pinto, B.M.; Cairo, C.W. Inhibitor selectivity of a new class of oseltamivir analogs against viral neuraminidase over human neuraminidase enzymes. Bioorg. Med. Chem. 2011, 19, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.; Guo, T.; Carvajal, E.G.; Bekkema, B.A.R.; Cairo, C.W. Bioisosteres at C9 of 2-deoxy-2,3-didehydro-N-acetyl neuraminic acid identify selective inhibitors of NEU3. J. Med. Chem. 2024, 67, 13594–13603. [Google Scholar] [CrossRef]
- Pilling, D.; Martinez, T.C.; Gomer, R.H. Inhibition of CCl4-induced liver inflammation and fibrosis by a NEU3 inhibitor. PLoS ONE 2024, 19, e0308060. [Google Scholar] [CrossRef]
- Karhadkar, T.R.; Meek, T.D.; Gomer, R.H. Inhibiting sialidase-induced TGF-Β1 activation attenuates pulmonary fibrosis in mice. J. Pharmacol. Exp. Ther. 2021, 376, 106–117. [Google Scholar] [CrossRef]
- Albohy, A.; Zhang, Y.; Smutova, V.; Pshezhetsky, A.V.; Cairo, C.W. Identification of selective nanomolar inhibitors of the human neuraminidase, NEU4. ACS Med. Chem. Lett. 2013, 4, 532–537. [Google Scholar] [CrossRef]
- Parker, R.B.; McCombs, J.E.; Kohler, J.J. Sialidase specificity determined by chemoselective modification of complex sialylated glycans. ACS Chem Biol. 2012, 7, 1509–1514. [Google Scholar] [CrossRef]
- Corfield, T. Bacterial Sialidases—Roles in Pathogenicity and Nutrition. Glycobiology 1992, 2, 509–521. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, M.L.; Woods, R.J. Atomic-Resolution Conformational Analysis of the GM3 Ganglioside in a Lipid Bilayer and Its Implications for Ganglioside–Protein Recognition at Membrane Surfaces. Glycobiology 2009, 19, 344–355. [Google Scholar] [CrossRef][Green Version]
- Matrosovich, M.; Herrler, G.; Klenk, H.D. Sialic acid receptors of viruses. In SialoGlyco Chemistry and Biology II: Tools and Techniques to Identify and Capture Sialoglycans; Gerardy-Schahn, R., Delannoy, P., von Itzstein, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–28. [Google Scholar] [CrossRef]
- Palese, P.; Tobita, K.; Ueda, M.; Compans, R.W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology 1974, 61, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Shtyrya, Y.A.; Mochalova, L.V.; Bovin, N.V. Influenza virus neuraminidase: Structure and function. Acta Naturae 2009, 1, 26–32. [Google Scholar] [CrossRef]
- von Itzstein, M.; Wu, W.-Y.; Kok, G.B.; Pegg, M.S.; Dyason, J.C.; Jin, B.; Van Phan, T.; Smythe, M.L.; White, H.F.; Oliver, S.W.; et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 1993, 363, 418–423, Erratum in Nature 1993, 363, 863. https://doi.org/10.1038/365863a0. [Google Scholar] [CrossRef]
- von Itzstein, M. The war against influenza: Discovery and development of sialidase inhibitors. Nat. Rev. Drug Discov. 2007, 6, 967–974. [Google Scholar] [CrossRef]
- Hata, K.; Koseki, K.; Yamaguchi, K.; Moriya, S.; Suzuki, Y.; Yingsakmongkon, S.; Hirai, G.; Sodeoka, M.; von Itzstein, M.; Miyagi, T. Limited inhibitory effects of oseltamivir and zanamivir on human sialidases. Antimicrob. Agents Chemother. 2008, 52, 3484–3491. [Google Scholar] [CrossRef]
- Alame, M.M.; Massaad, E.; Zaraket, H. Peramivir: A Novel Intravenous Neuraminidase Inhibitor for Treatment of Acute Influenza Infections. Front. Microbiol. 2016, 7, 450. [Google Scholar] [CrossRef] [PubMed]
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kawai, N.; Bando, T.; Tani, N.; Chong, Y.; Ikematsu, H. In Vitro neuraminidase inhibitory concentrations (IC50) of four neuraminidase inhibitors in the Japanese 2022–23 season: Comparison with the 2010–11 to 2019–20 seasons. J. Infect. Chemother. 2024, 30, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Sehnert, B.; Mietz, J.; Rzepka, R.; Buchholz, S.; Maul-Pavicic, A.; Schaffer, S.; Nimmerjahn, F.; Voll, R.E. Neuraminidase inhibitor zanamivir ameliorates collagen-induced arthritis. Int. J. Mol. Sci. 2021, 22, 1428. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, M.; Wei, H.; Li, C.; Hu, D.; Zheng, L.; Wang, D.W. Neuraminidase inhibitor treatment is associated with decreased mortality in COVID-19 patients: A retrospective analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 392–401. [Google Scholar] [CrossRef]
- Yan, Z.-L.; Liu, A.-Y.; Wei, X.-X.; Zhang, Z.; Qin, L.; Yu, Q.; Yu, P.; Lu, K.; Yang, Y. Divalent oseltamivir analogues as potent influenza neuraminidase inhibitors. Carbohydr. Res. 2019, 477, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.P.; Schengrund, C.-L. Oligosaccharide-derivatized dendrimers: Defined multivalent inhibitors of the adherence of the cholera toxin b subunit and the heat labile enterotoxin of E. Coli to GM1. Glycoconj. J. 1997, 14, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Kitov, P.I.; Sadowska, J.M.; Mulvey, G.; Armstrong, G.D.; Ling, H.; Pannu, N.S.; Read, R.J.; Bundle, D.R. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature 2000, 403, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, L.L.; Lamanna, A.C. Multivalency in biologicals. In Chemical Probes in Biology; Schneider, M.P., Ed.; Springer: Dordrecht, The Netherlands, 2003; pp. 345–357. [Google Scholar] [CrossRef]
- Döhrmann, S.; Levin, J.; Cole, J.N.; Borchardt, A.; Amundson, K.; Almaguer, A.; Abelovski, E.; Grewal, R.; Zuill, D.; Dedeic, N.; et al. Drug–Fc conjugate CD388 targets influenza virus neuraminidase and is broadly protective in mice. Nat. Microbiol. 2025, 10, 912–926. [Google Scholar] [CrossRef]
- Foss, S.; Sakya, S.A.; Aguinagalde, L.; Lustig, M.; Shaughnessy, J.; Cruz, A.R.; Scheepmaker, L.; Mathiesen, L.; Ruso-Julve, F.; Anthi, A.K.; et al. Human IgG Fc-engineering for enhanced plasma half-life, mucosal distribution and killing of cancer cells and bacteria. Nat. Commun. 2024, 15, 2007. [Google Scholar] [CrossRef]
- Equils, O.; Flanagan, S.; Wang, S.-S.; Vingerhoets, J.; van Duijnhoven, W.; James Mann, A.; Rojas, R.E.; Sandison, T. 573. CD388, a novel drug-fc conjugate (dfc), demonstrates prophylactic activity in an influenza human challenge model. Open Forum Infect. Dis. 2025, 12, ofae631.174. [Google Scholar] [CrossRef]
- Mann, M.C.; Thomson, R.J.; Dyason, J.C.; McAtamney, S.; Von Itzstein, M. Modelling, synthesis and biological evaluation of novel glucuronide-based probes of Vibrio cholerae sialidase. Bioorg. Med. Chem. A 2006, 14, 1518–1537. [Google Scholar] [CrossRef]
- van Heyningen, W.E.; Carpenter, C.C.J.; Pierce, N.F.; Greenough, W.B., III. Deactivation of cholera toxin by ganglioside. J. Infect. Dis. 1971, 124, 415–418. [Google Scholar] [CrossRef]
- Nicholls, J.M.; Moss, R.B.; Haslam, S.M. The use of sialidase therapy for respiratory viral infections. Antivir. Res. 2013, 98, 401–409. [Google Scholar] [CrossRef]
- Zenilman, J.M.; Fuchs, E.J.; Hendrix, C.W.; Radebaugh, C.; Jurao, R.; Nayak, S.U.; Hamilton, R.G.; McLeod Griffiss, J. Phase 1 clinical trials of DAS181, an inhaled sialidase, in healthy adults. Antivir. Res. 2015, 123, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Armstrong, D.; Knight, J.M.; Gale, T.V.; Hawley, S.; Wang, M.; Chang, N.; Corry, D.B.; Kheradmand, F. Sialidase fusion protein protects against influenza infection in a cigarette smoke-induced model of COPD. Mucosal Immunol. 2025, 18, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Li, J.; Snyder, G.; Huang, W.; Goldblum, S.E.; Chen, W.H.; Wang, L.-X.; McClane, B.A.; Cross, A.S. Antibody against microbial neuraminidases recognizes human sialidase 3 (NEU3): The neuraminidase/sialidase superfamily revisited. mBio 2017, 8, e00078-17. [Google Scholar] [CrossRef]
- Ganar, K.; Das, M.; Sinha, S.; Kumar, S. Newcastle Disease Virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Felipe, L.; Villar, E.; Muñoz-Barroso, I. A2-3-and A2-6-N-Linked sialic acids allow efficient interaction of Newcastle Disease virus with target cells. Glycoconj. J. 2012, 29, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Rota, P.; La Rocca, P.; Bonfante, F.; Pagliari, M.; Piccoli, M.; Cirillo, F.; Ghiroldi, A.; Franco, V.; Pappone, C.; Allevi, P.; et al. Design, synthesis, and antiviral evaluation of sialic acid derivatives as inhibitors of Newcastle Disease virus hemagglutinin-neuraminidase: A translational study on human Parainfluenza viruses. ACS Infect. Dis. 2023, 9, 617–630. [Google Scholar] [CrossRef]
- Takahashi, T.; Kurebayashi, Y.; Suzuki, S.; Konagaya, K.; Narimichi, Y.; Kobatake, E.; Fukudome, H.; Yamaguchi, T.; Sakai, F.; Arai, T.; et al. Bovine milk-derived sialylglycopeptide concentrate suppresses mumps virus infection. J. Funct. Foods 2025, 124, 106656. [Google Scholar] [CrossRef]
- Kubota, M.; Hashiguchi, T. Unique tropism and entry mechanism of mumps virus. Viruses 2021, 13, 1746. [Google Scholar] [CrossRef]
- Barberis, I.; Myles, P.; Ault, S.K.; Bragazzi, N.L.; Martini, M. History and evolution of influenza control through vaccination: From the first monovalent vaccine to universal vaccines. J. Prev. Med. Hyg. 2016, 57, E115–E120. [Google Scholar] [PubMed Central]
- Gambaryan, A.; Yamnikova, S.; Lvov, D.; Tuzikov, A.; Chinarev, A.; Pazynina, G.; Webster, R.; Matrosovich, M.; Bovin, N. Receptor specificity of influenza viruses from birds and mammals: New data on involvement of the inner fragments of the carbohydrate chain. Virology 2005, 334, 276–283. [Google Scholar] [CrossRef]
- Brathwaite Dick, O.; San Martín, J.L.; Montoya, R.H.; del Diego, J.; Zambrano, B.; Dayan, G.H. The history of Dengue outbreaks in the Americas. Amer. Soc. Trop. Med. Hyg. 2012, 87, 584–593. [Google Scholar] [CrossRef] [PubMed]
- St Clair, L.; Pujari, P.; Perera, R. Human Sialidase Activity Is Vital for Dengue Virus Serotype 2 Infection. bioRxiv 2022. [Google Scholar] [CrossRef]
- Lippi, D.; Gotuzzo, E.; Caini, S. Cholera. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Almagro-Moreno, S.; Boyd, E.F. Sialic acid catabolism confers a competitive advantage to pathogenic vibrio cholerae in the mouse intestine. Infect. Immun. 2009, 77, 3807–3816. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Xiao, B. The role of sialidases in the pathogenesis of bacterial vaginosis and their use as a promising pharmacological target in bacterial vaginosis. Front. Cell. Infect. Microbiol. 2024, 14, 1367233. [Google Scholar] [CrossRef]
- Lewis, W.G.; Robinson, L.S.; Gilbert, N.M.; Perry, J.C.; Lewis, A.L. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella Vaginalis. J. Biol. Chem. 2013, 288, 12067–12079. [Google Scholar] [CrossRef]
- Govinden, G.; Parker, J.L.; Naylor, K.L.; Frey, A.M.; Anumba, D.O.C.; Stafford, G.P. Inhibition of sialidase activity and cellular invasion by the bacterial vaginosis pathogen Gardnerella Vaginalis. Arch. Microbiol. 2018, 200, 1129–1133. [Google Scholar] [CrossRef]
- Navarro, M.A.; Li, J.; McClane, B.A.; Morrell, E.; Beingesser, J.; Uzal, F.A. NanI sialidase Is an important contributor to Clostridium Perfringens Type F Strain F4969 intestinal colonization in mice. Infect. Immun. 2018, 86, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McClane, B.A. Contributions of NanI sialidase to Caco-2 cell adherence by Clostridium Perfringens Type A and C strains causing human intestinal disease. Infect. Immun. 2014, 82, 4620–4630. [Google Scholar] [CrossRef]
- Kim, B.R.; Park, J.-Y.; Jeong, H.J.; Kwon, H.-J.; Park, S.-J.; Lee, I.-C.; Ryu, Y.B.; Lee, W.S. Design, synthesis, and evaluation of curcumin analogues as potential inhibitors of bacterial sialidase. J. Enzym. Inhib. Med. Chem. 2018, 33, 1256–1265. [Google Scholar] [CrossRef]
- Jennings, M.P.; Day, C.J.; Atack, J.M. How bacteria utilize sialic acid during interactions with the host: Snip, snatch, dispatch, match and attach. Microbiology 2022, 168, 001157. [Google Scholar] [CrossRef] [PubMed]
- Shizukuishi, S.; Ogawa, M.; Kuroda, E.; Hamaguchi, S.; Sakuma, C.; Kakuta, S.; Tanida, I.; Uchiyama, Y.; Akeda, Y.; Ryo, A.; et al. Pneumococcal sialidase promotes bacterial survival by fine-tuning of pneumolysin-mediated membrane disruption. Cell Rep. 2024, 43, 113962. [Google Scholar] [CrossRef] [PubMed]
- Gut, H.; Xu, G.; Taylor, G.L.; Walsh, M.A. Structural basis for Streptococcus Pneumoniae NanA inhibition by influenza antivirals zanamivir and oseltamivir carboxylate. J. Mol. Biol. 2011, 409, 496–503. [Google Scholar] [CrossRef]
- Awad, M.M.; Singleton, J.; Lyras, D. The sialidase NanS enhances non-TcsL mediated cytotoxicity of Clostridium sordellii. Toxins 2016, 8, 189. [Google Scholar] [CrossRef]
- Aldape, M.; Bryant, A.; Stevens, D. Clostridium Sordellii infection: Epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin. Infect. Dis. 2006, 43, 1436–1446. [Google Scholar] [CrossRef]
- Frey, A.M.; Satur, M.J.; Phansopa, C.; Honma, K.; Urbanowicz, P.A.; Spencer, D.I.R.; Pratten, J.; Bradshaw, D.; Sharma, A.; Stafford, G. Characterization of Porphyromonas gingivalis sialidase and disruption of its role in host-pathogen interactions. Microbiol. Read. 2019, 165, 1181–1197. [Google Scholar] [CrossRef]
- Frost, J.R.; Shaikh, S.; Severini, A. Exploring the mumps virus glycoproteins: A review. Viruses 2022, 14, 1335. [Google Scholar] [CrossRef] [PubMed]
- Rustmeier, N.H.; Strebl, M.; Stehle, T. The symmetry of viral sialic acid binding sites–implications for antiviral strategies. Viruses 2019, 11, 947. [Google Scholar] [CrossRef]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium Perfringens toxin-based typing scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef]
- Pilling, D.; Sahlberg, K.; Karhadkar, T.R.; Chen, W.; Gomer, R.H. The sialidase NEU3 promotes pulmonary fibrosis in mice. Resp. Res. 2022, 23, 215. [Google Scholar] [CrossRef]
- Demina, E.P.; Smutova, V.; Pan, X.; Fougerat, A.; Guo, T.; Zou, C.; Chakraberty, R.; Snarr, B.D.; Shiao, T.C.; Roy, R.; et al. Neuraminidases 1 and 3 trigger atherosclerosis by desialylating low-density lipoproteins and increasing their uptake by macrophages. J. Amer. Heart Assoc. 2021, 10, e018756. [Google Scholar] [CrossRef]
- Lipina, C.; Nardi, F.; Grace, H.; Hundal, H.S. NEU3 sialidase as a marker of insulin sensitivity: Regulation by fatty acids. Cell. Signal. 2015, 27, 1742–1750. [Google Scholar] [CrossRef]
- Itokazu, Y.; Fuchigami, T.; Morgan, J.C.; Yu, R.K. Intranasal infusion of GD3 and GM1 gangliosides downregulates alpha-synuclein and controls tyrosine hydroxylase gene in a PD model mouse. Mol. Ther. 2021, 29, 3059–3071. [Google Scholar] [CrossRef] [PubMed]
- Tamada, Y.; Nomura, H.; Aoki, D.; Irimura, T. A possible inhibitory role of sialic acid on MUC1 in peritoneal dissemination of clear cell-type ovarian cancer cells. Molecules 2021, 26, 5962. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Zemskova, M.A.; Hanss, A.D.; Kim, M.M.; Summer, R.; Kim, K.C. Muc1 deficiency exacerbates pulmonary fibrosis in a mouse model of silicosis. Biochem. Biophys. Res. Commun. 2017, 493, 1230–1235. [Google Scholar] [CrossRef]
- Fernandez, I.E.; Eickelberg, O. The impact of TGF-β on lung fibrosis: From targeting to biomarkers. Proc. Amer. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Ballester, B.; Montero, P.; Escriva, J.; Artigues, E.; Alós, M.; Pastor-Clerigues, A.; Morcillo, E.; Cortijo, J. MUC1 intracellular bioactivation mediates lung fibrosis. Thorax 2020, 75, 132. [Google Scholar] [CrossRef]
- Ballester, B.; Milara, J.; Cortijo, J. The role of mucin 1 in respiratory diseases. Eur. Respir. Rev. 2021, 30, 200149. [Google Scholar] [CrossRef]
- Mei, S.; Li, D.; Wang, A.; Zhu, G.; Zhou, B.; Li, N.; Qin, Y.; Zhang, Y.; Jiang, S. The role of sialidase Neu1 in respiratory diseases. Resp. Res. 2024, 25, 134. [Google Scholar] [CrossRef]
- Chen, W.; Pilling, D.; Gomer, R.H. The mRNA-binding protein DDX3 mediates TGF-Β1 upregulation of translation and promotes pulmonary fibrosis. JCI Insight 2023, 8, e167566. [Google Scholar] [CrossRef]
- Xiao, P.-T.; Hao, J.-H.; Kuang, Y.-J.; Dai, C.-X.; Rong, X.-L.; Jiang, L.-L.; Xie, Z.-S.; Zhang, L.; Chen, Q.-Q.; Liu, E.-H. Targeting neuraminidase 4 attenuates kidney fibrosis in mice. Adv. Sci. 2024, 11, 2406936. [Google Scholar] [CrossRef]
- Chen, Q.-Q.; Liu, K.; Shi, N.; Ma, G.; Wang, P.; Xie, H.-M.; Jin, S.-J.; Wei, T.-T.; Yu, X.-Y.; Wang, Y.; et al. Neuraminidase 1 promotes renal fibrosis development in male mice. Nat. Commun. 2023, 14, 1713. [Google Scholar] [CrossRef]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, B.; Jin, T.; Ocansey, D.K.W.; Jiang, J.; Mao, F. Intestinal fibrosis in inflammatory bowel disease and the prospects of mesenchymal stem cell therapy. Front. Immunol. 2022, 13, 835005. [Google Scholar] [CrossRef] [PubMed]
- Miklavcic, J.J.; Hart, T.D.; Lees, G.M.; Shoemaker, G.K.; Schnabl, K.L.; Larsen, B.M.K.; Bathe, O.F.; Thomson, A.B.T.; Mazurak, V.C.; Clandinin, M.T. Increased catabolism and decreased unsaturation of ganglioside in patients with inflammatory bowel disease. World Gastroenterol. 2015, 21, 10080–10090. [Google Scholar] [CrossRef]
- Finsterwalder, R.; Ganesan, M.K.; Leb, H.; Habertheuer, A.; Basilio, J.; Lang, I.; Krunic, M.; Wiedemann, D.; Petzelbauer, P. Hypoxia/reperfusion predisposes to atherosclerosis. PLoS ONE 2018, 13, e0205067. [Google Scholar] [CrossRef] [PubMed]
- Kashirskikh, D.A.; Markin, A.M.; Sobenin, I.A.; Orekhov, A.N. Study of the role of human endogenous neuraminidases in atherosclerotic lesions of the thoracic aorta. Atherosclerosis 2020, 315, e114–e115. [Google Scholar] [CrossRef]
- Fougerat, A.; Pan, X.; Smutova, V.; Heveker, N.; Cairo, C.W.; Issad, T.; Larrivée, B.; Medin, J.A.; Pshezhetsky, A.V. Neuraminidase 1 activates insulin receptor and reverses insulin resistance in obese mice. Mol. Metab. 2018, 12, 76–88. [Google Scholar] [CrossRef]
- Yujin, N.; Miwako, N.; Fumiko, K.-N. Neu1 sialidase interacts with perilipin 1 on lipid droplets and inhibits lipolysis in 3T3-L1 Adipocytes. Genes Cells 2017, 22, 485–492. [Google Scholar] [CrossRef]
- White, E.J.; Gyulay, G.; Lhoták, Š.; Szewczyk, M.M.; Chong, T.; Fuller, M.T.; Dadoo, O.; Fox-Robichaud, A.E.; Austin, R.C.; Trigatti, B.L.; et al. Sialidase down-regulation reduces non-hdl cholesterol, inhibits leukocyte transmigration, and attenuates atherosclerosis in ApoE knockout mice. J. Biol. Chem. 2018, 293, 14689–14706. [Google Scholar] [CrossRef]
- Zeng, S.; Wen, Y.; Yu, C. Desialylation of ATG5 by sialidase (NEU1) promotes macrophages autophagy and exacerbates inflammation under hypoxia. Cell. Signal. 2023, 112, 110927. [Google Scholar] [CrossRef] [PubMed]
- Packard, R.R.S.; Libby, P. Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clinical Chem. 2008, 54, 24–38. [Google Scholar] [CrossRef]
- Sasaki, A.; Hata, K.; Suzuki, S.; Sawada, M.; Wada, T.; Yamaguchi, K.; Obinata, M.; Tateno, H.; Suzuki, H.; Miyagi, T. Overexpression of plasma membrane-associated sialidase attenuates insulin signaling in transgenic mice. J. Biol. Chem. 2003, 278, 27896–27902. [Google Scholar] [CrossRef]
- Alghamdi, F.; Guo, M.; Abdulkhalek, S.; Crawford, N.; Amith, S.R.; Szewczuk, M.R. A Novel Insulin Receptor-Signaling Platform and Its Link to Insulin Resistance and Type 2 Diabetes. Cell. Signal. 2014, 26, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Bai, X.-C. The Activation Mechanism of the Insulin Receptor: A Structural Perspective. Annu. Rev. Biochem. 2023, 92, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.A.; Bagshawe, K.D. Tumour Specific Immunogenicity of Methylcholanthrene-Induced Sarcoma Cells after Incubation in Neuraminidase. Br. J. Cancer 1969, 23, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.L.; Rios, A.; Ray, P.K. Regression of established methylcholanthrene tumors by intratumor injections of Vibrio Cholerae neuraminidase. J. Surg. Oncol. 1972, 4, 298–305. [Google Scholar] [CrossRef]
- Schengrund, C.-L.; Duff, R.; Rosenberg, A. Sialidase activity of oncogenic cells transformed by Herpes Simplex virus. Virology 1974, 58, 595–599. [Google Scholar] [CrossRef]
- Yogeeswaran, G.; Hakomori, S. Cell contact-dependent ganglioside changes in mouse 3t3 fibroblasts and a suppressed sialidase activity on cell contact. Biochemistry 1975, 14, 2151–2156. [Google Scholar] [CrossRef]
- Tringali, C.; Lupo, B.; Silvestri, I.; Papini, N.; Anastasia, L.; Tettamanti, G.; Venerando, B. The plasma membrane sialidase neu3 regulates the malignancy of renal carcinoma cells by controlling Β1 integrin internalization and recycling. J. Biol. Chem. 2012, 287, 42835–42845. [Google Scholar] [CrossRef]
- Shiga, K.; Takahashi, K.; Sato, I.; Kato, K.; Saijo, S.; Moriya, S.; Hosono, M.; Miyagi, T. Upregulation of sialidase NEU3 in head and neck squamous cell carcinoma associated with lymph node metastasis. Cancer Sci. 2015, 106, 1544–1553. [Google Scholar] [CrossRef]
- Takahashi, K.; Proshin, S.; Yamaguchi, K.; Yamashita, Y.; Katakura, R.; Yamamoto, K.; Shima, H.; Hosono, M.; Miyagi, T. Sialidase NEU3 defines invasive potential of human glioblastoma cells by regulating calpain-mediated proteolysis of focal adhesion proteins. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 2778–2788. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pander, A.; Chinnaiyan, A.M. ONCOMINE: A Cancer Microarray Database and Integrated Data-Mining Platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Zhou, X.; Zhai, Y.; Liu, C.; Yang, G.; Guo, J.; Li, G.; Sun, C.; Qi, X.; Li, X.; Guan, F. Sialidase NEU1 suppresses progression of human bladder cancer cells by inhibiting fibronectin-integrin A5β1 interaction and Akt signaling pathway. Cell Commun. Signal. 2020, 18, 44. [Google Scholar] [CrossRef]
- Wu, Z.; He, L.; Yang, L.; Fang, X.; Peng, L. Potential role of NEU1 in hepatocellular carcinoma: A study based on comprehensive bioinformatical analysis. Front. Mol. Biosci. 2021, 8, 651525. [Google Scholar] [CrossRef]
- Peng, Q.; Gao, L.; Cheng, H.B.; Wang, J.S.; Wang, J. Sialidase Neu1 may serve as a potential biomarker of proliferation, migration and prognosis in melanoma. World J. Oncol. 2022, 13, 222–234. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, L.; Huang, S.; Zhu, Y.; Li, W.; Fang, S.; Shen, L.; Gao, Y. Effects of sialidase NEU1 siRNA on proliferation, apoptosis, and invasion in human ovarian cancer. Mol. Cell. Biochem. 2016, 411, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Tringali, C.; Lupo, B.; Anastasia, L.; Papini, N.; Monti, E.; Bresciani, R.; Tettamanti, G.; Venerando, B. Expression of sialidase Neu2 in leukemic K562 cells induces apoptosis by impairing Bcr-Abl/Src kinases signaling. J. Biol. Chem. 2007, 282, 14364–14372. [Google Scholar] [CrossRef]
- Satyavarapu, E.M.; Nath, S.; Mandal, C. Desialylation of Atg5 by sialidase (Neu2) enhances autophagosome formation to induce anchorage-dependent cell death in ovarian cancer cells. Cell Death Discov. 2021, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Mandal, C.; Chatterjee, U.; Mandal, C. Association of cytosolic sialidase Neu2 with plasma membrane enhances fas-mediated apoptosis by impairing PI3K-Akt/mTOR-mediated pathway in pancreatic cancer cells. Cell Death Dis. 2018, 9, 210, Erratum in Cell Death Dis. 2022, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Koseki, K.; Wada, T.; Hosono, M.; Hata, K.; Yamaguchi, K.; Nitta, K.; Miyagi, T. Human cytosolic sialidase NEU2-low general tissue expression but involvement in PC-3 prostate cancer cell survival. Biochem. Biophys. Res. Commun. 2012, 428, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Saito, S.; Wada, T.; Yamaguchi, K.; Satoh, M.; Arai, Y.; Miyagi, T. Plasma membrane-associated sialidase is up-regulated in renal cell carcinoma and promotes interleukin-6-induced apoptosis suppression and cell motility. J. Biol. Chem. 2006, 281, 7756–7764. [Google Scholar] [CrossRef]
- Silvestri, I.; Testa, F.; Zappasodi, R.; Cairo, C.W.; Zhang, Y.; Lupo, B.; Galli, R.; Di Nicola, M.; Venerando, B.; Tringali, C. Sialidase NEU4 is involved in glioblastoma stem cell survival. Cell Death Dis. 2014, 5, e1381. [Google Scholar] [CrossRef][Green Version]
- Tringali, C.; Cirillo, F.; Lamorte, G.; Papini, N.; Anastasia, L.; Lupo, B.; Silvestri, I.; Tettamanti, G.; Venerando, B. NEU4L sialidase overexpression promotes Β-catenin signaling in neuroblastoma cells, enhancing stem-like malignant cell growth. Int. J. Cancer 2012, 131, 1768–1778. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, R.; Wang, S.; Liu, Y.; Tian, B.; Liu, Y.; Chen, Y.; Hu, T.; Mu, Y.; Wang, S.; et al. NEU4-mediated desialylation enhances the activation of the oncogenic receptors for the dissemination of ovarian carcinoma. Oncogene 2024, 43, 3556–3569. [Google Scholar] [CrossRef]
- Rosenberg, A.; Schengrund, C.L. Gangliosides, glycosidases, and sialidase in the brain and eyes of developing chickens. Biochemistry 1971, 10, 2424–2428. [Google Scholar] [CrossRef] [PubMed]
- Ohman, R.; Svennerholm, L. The activity of ganglioside sialidase in the developing human brain. J. Neurochem. 1971, 18, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Melo-Braga, M.N.; Schulz, M.; Liu, Q.; Swistowski, A.; Palmisano, G.; Engholm-Keller, K.; Jakobsen, L.; Zeng, X.; Larsen, M.R. Comprehensive quantitative comparison of the membrane proteome, phosphoproteome, and sialiome of human embryonic and neural stem cells. Mol. Cell. Proteomics 2014, 13, 311–328. [Google Scholar] [CrossRef]
- Takahashi, K.; Mitoma, J.; Hosono, M.; Shiozaki, K.; Sato, C.; Yamaguchi, K.; Kitajima, K.; Higashi, H.; Nitta, K.; Shima, H.; et al. Sialidase NEU4 hydrolyzes polysialic acids of neural cell adhesion molecules and negatively regulates neurite formation by hippocampal neurons. J. Biol. Chem. 2012, 287, 14816–14826. [Google Scholar] [CrossRef]
- Honda, A.; Hayasaka, O.; Mio, K.; Fujimura, K.; Kotani, T.; Komatsu, M.; Shiozaki, K. The involvement of Nile Tilapia (Oreochromis niloticus) Neu4 sialidase in neural differentiation during early ontogenesis. Biochimie 2021, 185, 105–116. [Google Scholar] [CrossRef]
- Shiozaki, K.; Koseki, K.; Yamaguchi, K.; Shiozaki, M.; Narimatsu, H.; Miyagi, T. Developmental change of sialidase Neu4 expression in murine brain and its involvement in the regulation of neuronal cell differentiation. J. Biol. Chem. 2009, 284, 21157–21164. [Google Scholar] [CrossRef]
- Yu, R.K.; Macala, L.J.; Taki, T.; Weinfeld, H.M.; Yu, F.S. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J. Neurochem. 1988, 50, 1825–1829. [Google Scholar] [CrossRef]
- Yu, R.K.; Tsai, Y.-T.; Ariga, T. Functional Roles of Gangliosides in Neurodevelopment: An Overview of Recent Advances. Neurochem. Res. 2012, 37, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Kracun, I.; Rosner, H.; Drnovsek, V.; Vukelic, Z.; Cosovic, C.; Trbojevic-Cepe, M.; Kubat, M. Gangliosides in the human brain development and aging. Neurochem. Int. 1992, 20, 421–431. [Google Scholar] [CrossRef]
- Ha, K.-T.; Lee, Y.-C.; Cho, S.-H.; Kim, J.-K.; Kim, C.-H. Molecular characterization of membrane type and ganglioside-specific sialidase (Neu3) expressed in E. coli. Mol. Cells 2004, 17, 267–273. [Google Scholar] [CrossRef]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the brain: Physiology, pathophysiology and therapeutic applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef]
- McClenahan, F.; Dimitriou, C.; Koutsakis, C.; Dimitrakopoulos, D.; Arampatzis, A.; Kakouri, P.; Kourla, M.; Oikonomou, S.; Andreopoulou, E.; Patsonis, M.; et al. Isolation of neural stem and oligodendrocyte progenitor cells from the brain of live rats. Stem Cell Rep. 2021, 16, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Fanzani, A.; Colombo, F.; Giuliani, R.; Preti, A.; Marchesini, S. Cytosolic sialidase Neu2 upregulation during PC12 cells differentiation. FEBS Lett. 2004, 566, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Akyildiz Demir, S.; Seyrantepe, V. Identification of cytoplasmic sialidase NEU2-associated proteins by LC-MS/MS. Turk. J. Biochem. 2019, 44, 462–472. [Google Scholar] [CrossRef]
- Bachir, A.I.; Horwitz, A.R.; Nelson, W.J.; Bianchini, J.M. Actin-Based adhesion modules mediate cell interactions with the extracellular matrix and neighboring cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a023234. [Google Scholar] [CrossRef]
- Gentile, J.E.; Carrizales, M.G.; Koleske, A.J. Control of synapse structure and function by actin and its regulators. Cells 2022, 11, 603. [Google Scholar] [CrossRef]
- Sajo, M.; Sugiyama, H.; Yamamoto, H.; Tanii, T.; Matsuki, N.; Ikegaya, Y.; Koyama, R. Neuraminidase-dependent degradation of polysialic acid is required for the lamination of newly generated neurons. PLoS ONE 2016, 11, e0146398. [Google Scholar] [CrossRef]
- Fremuth, L.E.; Hu, H.; van de Vlekkert, D.; Annunziata, I.; Weesner, J.A.; d’Azzo, A. Neuraminidase 1 Regulates neuropathogenesis by governing the cellular state of microglia via modulation of Trem2 sialylation. Cell Rep. 2025, 44, 115204. [Google Scholar] [CrossRef]
- Minami, A.; Meguro, Y.; Ishibashi, S.; Ishii, A.; Shiratori, M.; Sai, S.; Horii, Y.; Shimizu, H.; Fukumoto, H.; Shimba, S.; et al. Rapid regulation of sialidase activity in response to neural activity and sialic acid removal during memory processing in rat hippocampus. J. Biol. Chem. 2017, 292, 5645–5654. [Google Scholar] [CrossRef]
- Minami, A.; Otsubo, T.; Ieno, D.; Ikeda, K.; Kanazawa, H.; Shimizu, K.; Ohata, K.; Yokochi, T.; Horii, Y.; Fukumoto, H.; et al. Visualization of sialidase activity in mammalian tissues and cancer detection with a novel fluorescent sialidase substrate. PLoS ONE 2014, 9, e81941. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, H.; Dityatev, A. Polysialic acid in brain development and synaptic plasticity. In SialoGlyco Chemistry and Biology I: Biosynthesis, Structural Diversity and Sialoglycopathologies; Gerardy-Schahn, R., Delannoy, P., von Itzstein, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 55–96. ISBN 978-3-662-47940-7. [Google Scholar] [CrossRef]
- Varbanov, H.; Jia, S.; Kochlamazashvili, G.; Bhattacharya, S.; Buabeid, M.A.; El Tabbal, M.; Hayani, H.; Stoyanov, S.; Sun, W.; Thiesler, H.; et al. Rescue of synaptic and cognitive functions in polysialic acid-deficient mice and dementia models by short polysialic acid fragments. Neurobiol. Dis. 2023, 180, 106079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Stein, J.W.; Lynch, C.L.; Tran, H.T.; Lee, C.-Y.; Coleman, R.; Hatch, A.; Antontsev, V.G.; Chy, H.S.; O’Brien, C.M.; et al. Glycosyltransferase ST6GAL1 contributes to the regulation of pluripotency in human pluripotent stem cells. Sci. Rep. 2015, 5, 13317. [Google Scholar] [CrossRef]
- Simpson, M.A.; Cross, H.; Proukakis, C.; Priestman, D.A.; Neville, D.C.A.; Reinkensmeier, G.; Wang, H.; Wiznitzer, M.; Gurtz, K.; Verganelaki, A.; et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat. Genet. 2004, 36, 1225–1229. [Google Scholar] [CrossRef]
- Husain, S.; Yildirim-Toruner, C.; Rubio, J.P.; Field, J. Variants of ST8SIA1 are associated with risk of developing multiple sclerosis. PLoS ONE 2008, 3, e2653. [Google Scholar] [CrossRef] [PubMed]
- Pshezhetsky, A.V.; Richard, C.; Michaud, L.; Igdoura, S.; Wang, S.; Elsliger, M.-A.; Qu, J.; Leclerc, D.; Gravel, R.; Dallaire, L. Cloning, expression and chromosomal mapping of human lysosomal sialidase and characterization of mutations in sialidosis. Nat. Genet. 1997, 15, 316–320. [Google Scholar] [CrossRef]
- Lowden, J.; O’brien, J. Sialidosis: A review of human neuraminidase deficiency. Amer. J. Hum. Genet. 1979, 31, 1–18. [Google Scholar] [PubMed Central]
- Fan, S.-P.; Lee, N.-C.; Lin, C.-H. Clinical and electrophysiological characteristics of a type 1 sialidosis patient with a novel deletion mutation in NEU1 gene. J. Formos. Med. Assoc. 2020, 119, 406–412. [Google Scholar] [CrossRef]
- Canafoglia, L.; Robbiano, A.; Pareyson, D.; Panzica, F.; Nanetti, L.; Giovagnoli, A.R.; Venerando, A.; Gellera, C.; Franceschetti, S.; Zara, F. Expanding sialidosis spectrum by genome-wide screening. Neurology 2014, 82, 2003–2006. [Google Scholar] [CrossRef]
- Peng, M.L.; Chau, S.F.; Chien, J.Y.; Woon, P.Y.; Chen, Y.C.; Cheang, W.M.; Tsai, H.Y.; Huang, S.P. Genetic insights and clinical implications of NEU1 mutations in sialidosis. Genes 2025, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- van der Spoel, A.; Bonten, E.; d’Azzo, A. Transport of human lysosomal neuraminidase to mature lysosomes requires protective protein/cathepsin A. EMBO J. 1998, 17, 1588–1597. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The emerging evidence of the parkinson pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Hadjiconstantinou, N.; Rossetti, Z.; Paxton, R.; Neff, N. Administration of GMI ganglioside restores the dopamine content in striatum after chronic treatment with MPTP. Neuropharmacology 1986, 25, 1075–1077. [Google Scholar] [CrossRef]
- Herrero, M.; Perez-Otan, I.; Oset, C.; Kastner, A.; Hirsch, E.; Luquin, M.; Obes, J.; Del Rio, J. GM-1 Ganglioside promotes the recovery of surviving midbrain dopaminergic neurons in MPTP-treated monkeys. J. Neurosci. 1993, 56, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Pope-Coleman, A.; Schneider, J. Effects of chronic GM1 ganglioside treatment on cognitieve and motor deficits in a slowly progressing model of parkinsonism in non-human primates. Restor. Neurol. Neurosci. 1998, 12, 255–266. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.; Kulkarni, N.; Ledeen, R.W. Deficiency of ganglioside GM1 correlates with parkinson’s disease in mice and humans. J. Neurosci. Res. 2012, 90, 1997–2008. [Google Scholar] [CrossRef]
- Schneider, J.S. GM1 ganglioside as a disease-modifying therapeutic for parkinson’s disease: A multi-functional glycosphingolipid that targets multiple parkinson’s disease-relevant pathogenic mechanisms. Int. J. Mol. Sci. 2023, 24, 9183. [Google Scholar] [CrossRef]
- Mata, C.S.V.; Mitchell, J.C. In-Vivo synthesis of GM1 ganglioside through CRISPR-Cas9 mutation in CHO-K1, chinese hamster ovary cells (Cricetulus griseus). FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Di Biase, E.; Lunghi, G.; Maggioni, M.; Fazzari, M.; Pomè, D.Y.; Loberto, N.; Ciampa, M.G.; Fato, P.; Mauri, L.; Sevin, E.; et al. GM1 oligosaccharide crosses the human blood–brain barrier in vitro by a paracellular route. Int. J. Mol. Sci. 2020, 21, 2858. [Google Scholar] [CrossRef]
- Schneider, J.S.; Seyfried, T.N.; Choi, H.-S.; Kidd, S.K. Intraventricular sialidase administration enhances GM1 ganglioside expression and is partially neuroprotective in a mouse model of parkinson’s disease. PLoS ONE 2015, 10, e0143351. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Mauri, L.; Lunghi, G.; Di Biase, E.; Fazzari, M.; Maggioni, M.; Valsecchi, M.; Prioni, S.; Loberto, N.; Pomè, D.Y. Parkinson’s disease recovery by GM1 oligosaccharide treatment in the B4galnt1+/− mouse model. Sci. Rep. 2019, 9, 19330. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.-H.; Seo, J.H.; Alselehdar, S.K.; DeFrees, S.; Ledeen, R.W. Mice deficient in GM1 manifest both motor and non-motor symptoms of parkinson’s disease; successful treatment with synthetic GM1 ganglioside. Exper. Neurol. 2020, 329, 113284. [Google Scholar] [CrossRef]
- Timur, Z.K.; Inci, O.K.; Demir, S.A.; Seyrantepe, V. Sialidase Neu4 deficiency is associated with neuroinflammation in mice. Glycoconj. J. 2021, 38, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 2013, 61, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Delaveris, C.S.; Wang, C.L.; Riley, N.M.; Li, S.; Kulkarni, R.U.; Bertozzi, C.R. Microglia mediate contact-independent neuronal network remodeling via secreted neuraminidase-3 associated with extracellular vesicles. ACS Cent. Sci. 2023, 9, 2108–2114. [Google Scholar] [CrossRef]
- Annunziata, I.; Patterson, A.; Helton, D.; Hu, H.; Moshiach, S.; Gomero, E.; Nixon, R.; d’Azzo, A. Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nat. Commun. 2013, 4, 2734. [Google Scholar] [CrossRef]
- Choo-Smith, L.-P.; Garzon-Rodriguez, W.; Glabe, C.G.; Surewicz, W.K. Acceleration of amyloid fibril formation by specific binding of abeta-(1-40) peptide to ganglioside-containing membrane vesicles. J. Biol. Chem. 1997, 272, 22987–22990. [Google Scholar] [CrossRef]
- Williamson, M.P.; Suzuki, Y.; Bourne, N.T.; Asakura, T. Binding of amyloid β-peptide to ganglioside micelles is dependent on histidine-13. Biochem. J. 2006, 397, 483–490. [Google Scholar] [CrossRef]
- Yanagisawa, K. Role of gangliosides in alzheimer’s disease. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.A.; Henry, J.E.; Good, T.A. Attenuation of β-amyloid-induced toxicity by sialic-acid-conjugated dendrimers: Role of sialic acid attachment. Brain Res. 2007, 1161, 95–105. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Ughetto, S.; Mahjoum, S.; Nair, A.V.; Breakefield, X.O. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep. 2022, 39, 110651. [Google Scholar] [CrossRef]
- Sanderson, R.D.; Bandari, S.K.; Vlodavsky, I. Proteases and glycosidases on the surface of exosomes: Newly discovered mechanisms for extracellular remodeling. Matrix Biol. 2019, 7576, 160–169. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Patel, M.; Wang, Y.; Bartom, E.T.; Dhir, R.; Nephew, K.P.; Matei, D.; Murmann, A.E.; Lengyel, E.; Peter, M.E. The ratio of toxic-to-nontoxic mirnas predicts platinum sensitivity in ovarian cancer. Cancer Res. 2021, 81, 3985–4000. [Google Scholar] [CrossRef]
- Paudel, B.; Jeong, S.-Y.; Martinez, C.P.; Rickman, A.; Haluck-Kangas, A.; Bartom, E.T.; Fredriksen, K.; Affaneh, A.; Kessler, J.A.; Mazzulli, J.R.; et al. Death induced by survival gene elimination (DISE) correlates with neurotoxicity in alzheimer’s disease and aging. Nat. Commun. 2024, 15, 264. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).