CRISPR-Cas12 Application for the Detection of Pneumocystis jirovecii in Immunodepression Patients Through Fluorescent and Lateral Flow Colorimetric Assay
Abstract
1. Introduction
2. Results
2.1. Primers, gRNAs, and Reporter Probes
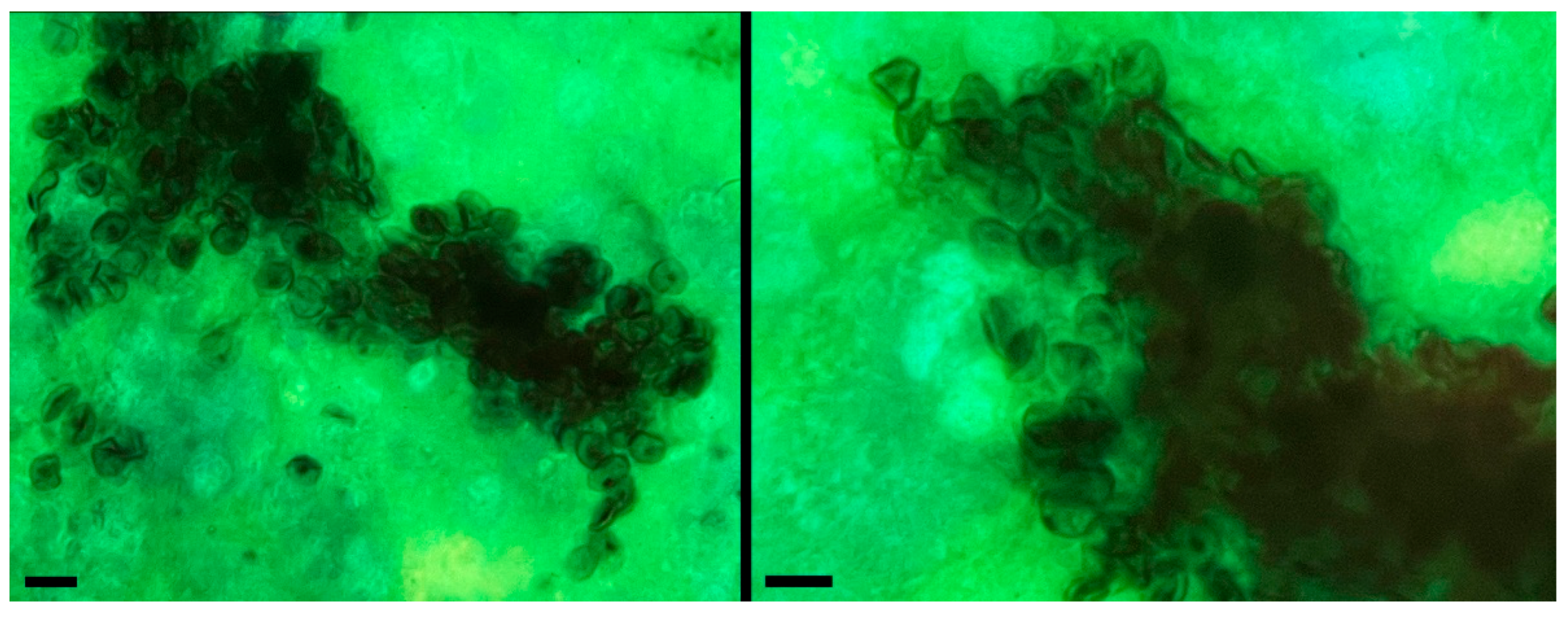
2.2. Detection of P. jirovecii Through Microscopic Examination and PCR Amplification of the Respiratory Sample
2.3. Detection by Fluorescence Using CRISPR-Cas12
2.3.1. Optimal DNA Amount by Fluorescence
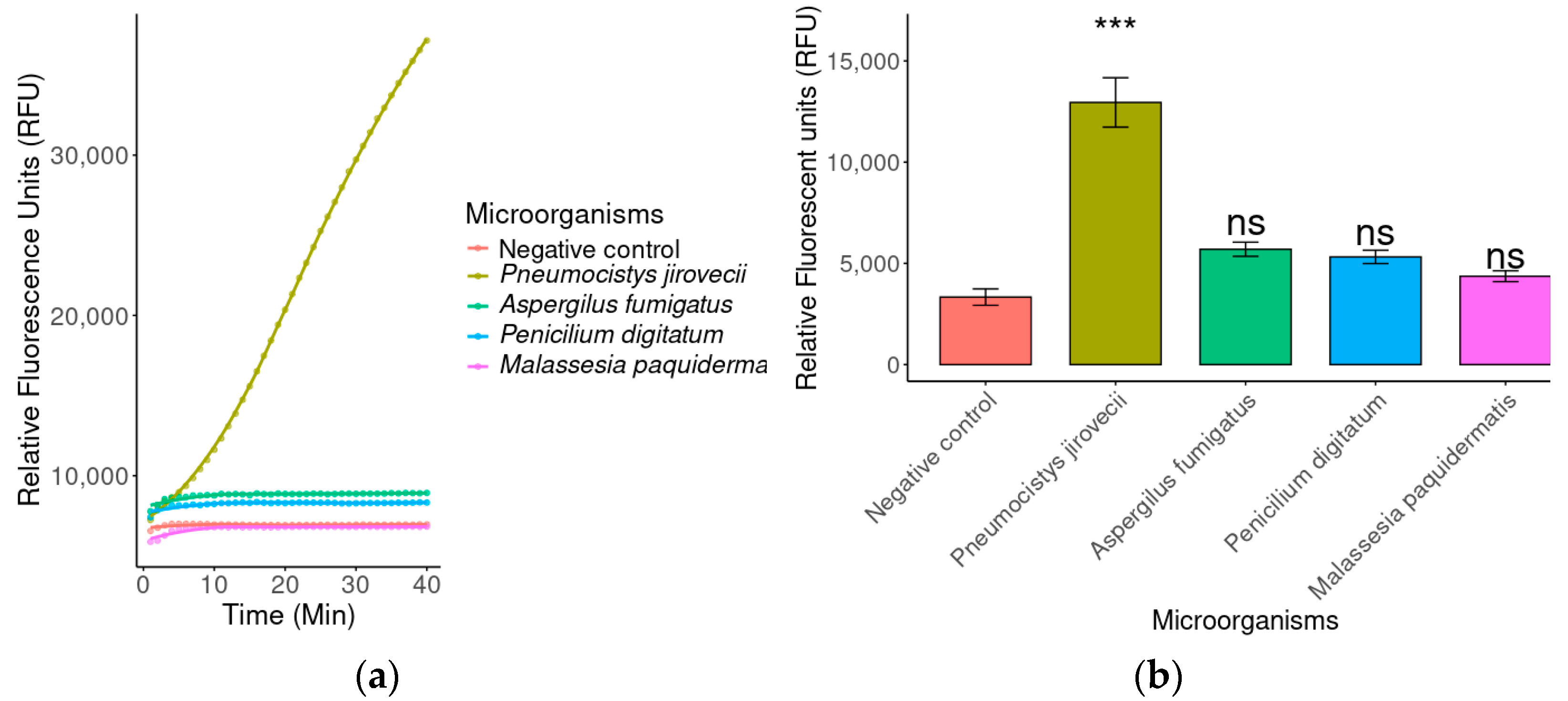
2.3.2. Specificity by Fluorescence
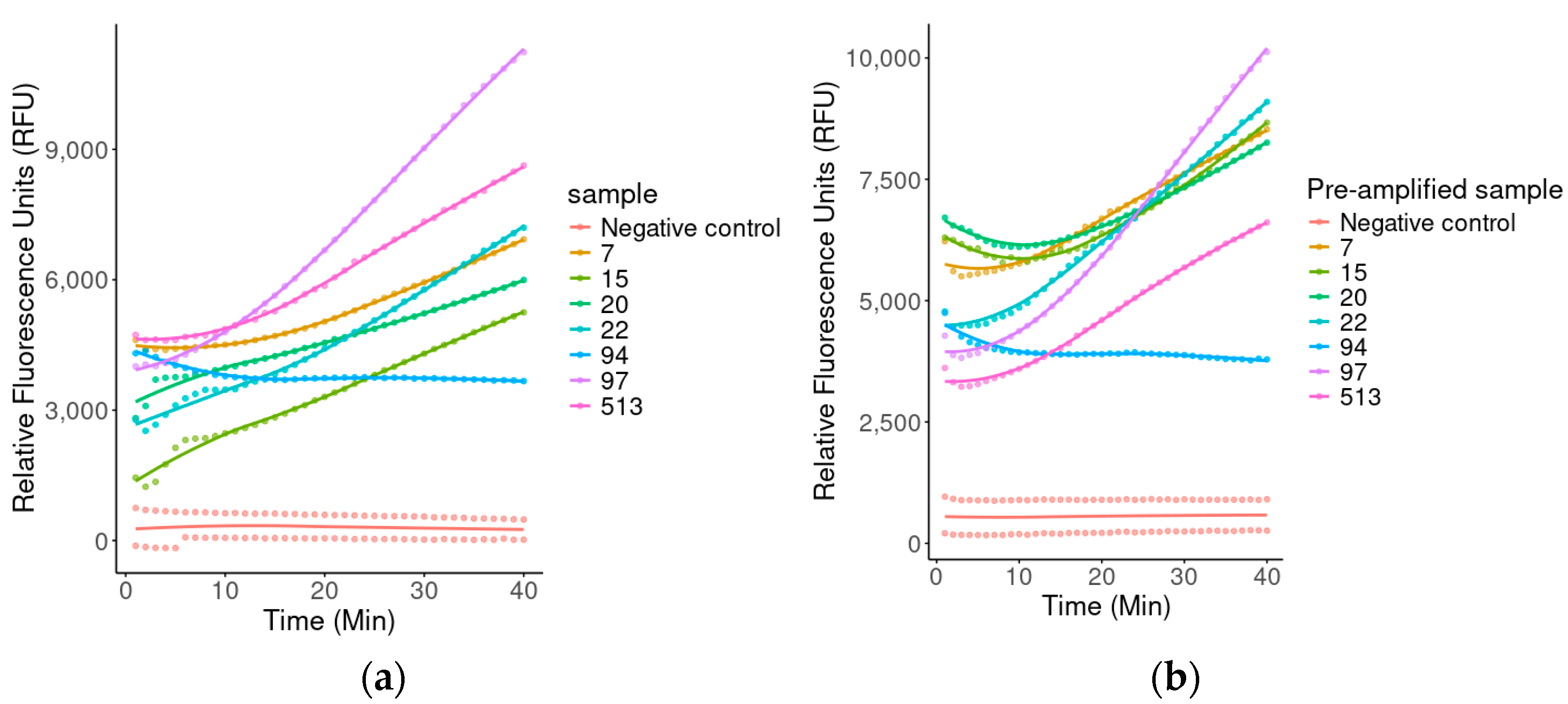
2.3.3. Fluorescence-Assisted Analysis for Biological Diagnosis
2.4. Detection by Lateral Flow Test
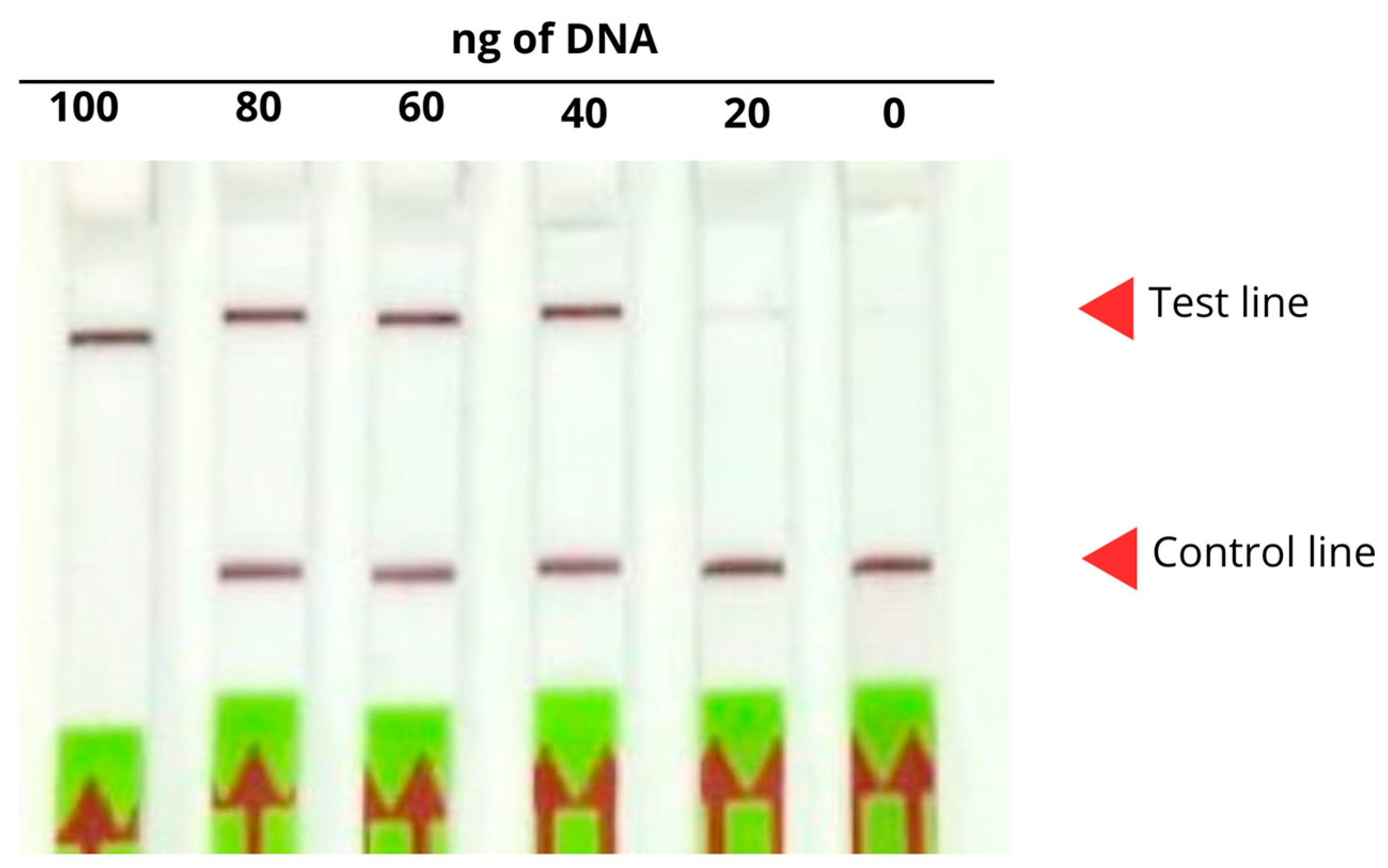
2.4.1. Detection of the Optimal Amount of DNA
2.4.2. Optimal Probe Concentration
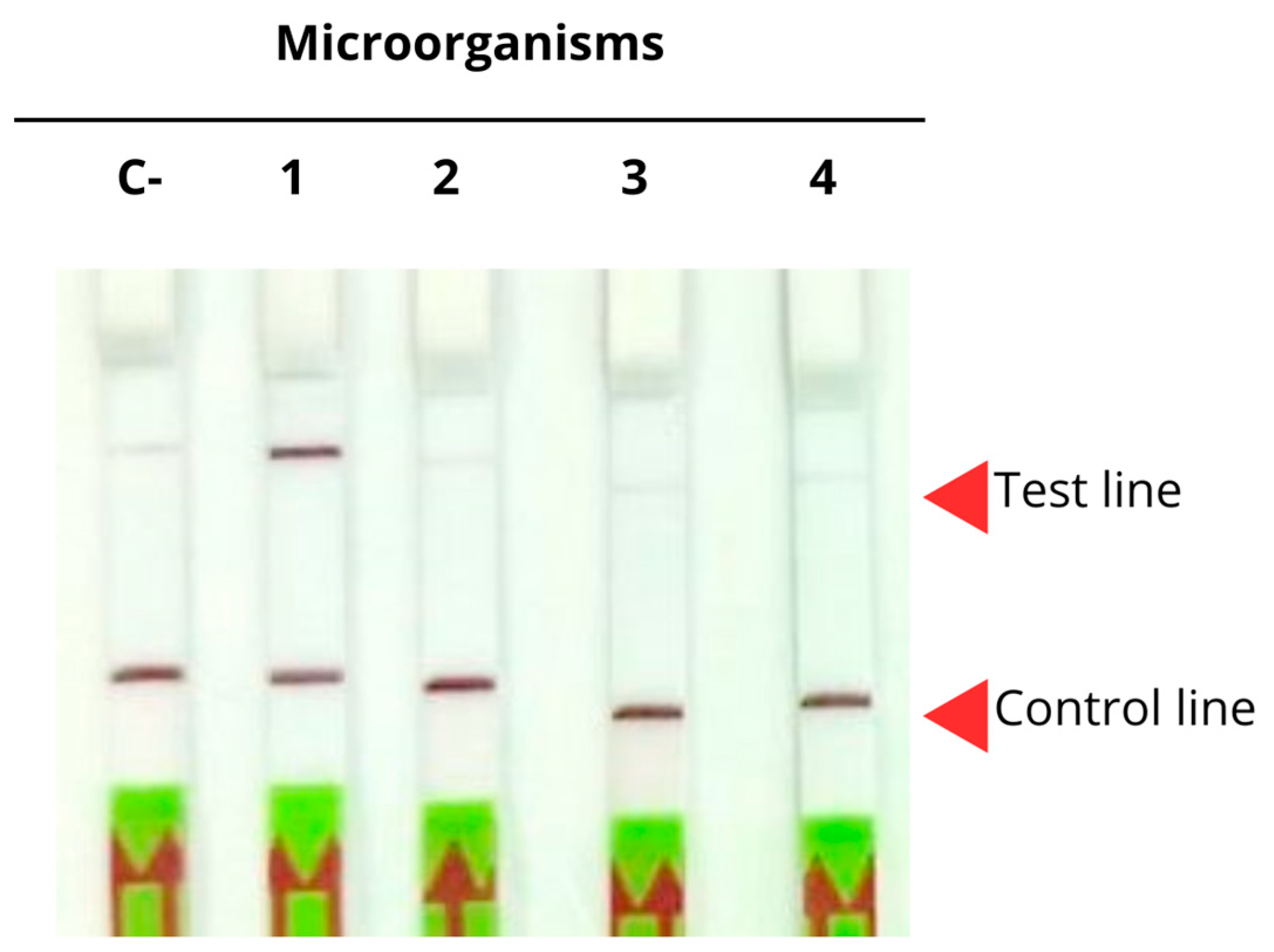
2.4.3. Specificity in Lateral Flow Strips
2.4.4. Optimal DNA Amount
3. Discussion
4. Materials and Methods
4.1. Biological Samples
4.2. Primers, gRNAs, and Probes
4.3. Detection of P. jirovecii Through Microscopic Examination of the Respiratory Sample
4.4. Evaluation of Detection Using Fluorescence Coupled to CRISPR-Cas12
4.4.1. Identification of the Optimal DNA Amounts
4.4.2. Evaluation of Specificity by Fluorescence
4.4.3. Diagnosis of Biological Samples by Fluorescence
4.5. Detection by Lateral Flow Test Coupled to CRISPR-Cas12
4.5.1. Determination of the Optimal DNA Amount
4.5.2. Determination of the Optimal Probe Concentration
4.5.3. Evaluation of Specificity in Lateral Flow Strips
4.5.4. Evaluation of Sensitivity in Amplified and Non-Amplified Clinical Samples
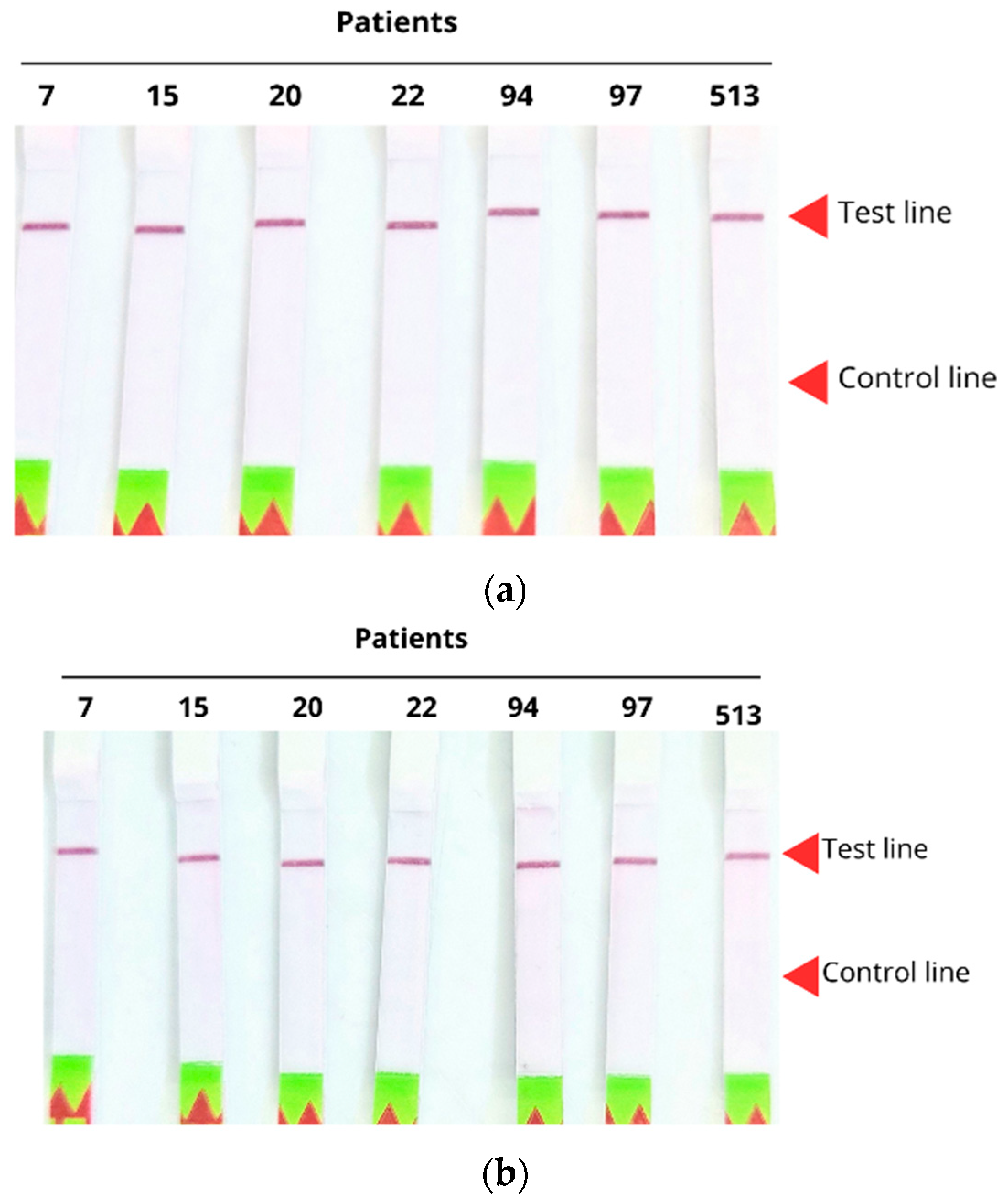
4.5.5. Diagnosis of Biological Samples Through Lateral Flow Strips
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iturrieta-González, I.; Chahin, C.; Cabrera, J.; Concha, C.; Olivares-Ferretti, P.; Briones, J.; Vega, F.; Bustos-Medina, L.; Fonseca-Salamanca, F. Molecular Study of Pneumocystis Jirovecii in Respiratory Samples of HIV Patients in Chile. J. Fungi 2024, 10, 117. [Google Scholar] [CrossRef]
- Ponce, C.A.; Chabé, M.; George, C.; Cárdenas, A.; Durán, L.; Guerrero, J.; Bustamante, R.; Matos, O.; Huang, L.; Miller, R.F.; et al. High Prevalence of Pneumocystis Jirovecii Dihydropteroate Synthase Gene Mutations in Patients with a First Episode of Pneumocystis Pneumonia in Santiago, Chile, and Clinical Response to Trimethoprim-Sulfamethoxazole Therapy. Antimicrob. Agents Chemother. 2017, 61, e01290-16. [Google Scholar] [CrossRef]
- Nevez, G.; Hauser, P.M.; Le Gal, S. Pneumocystis Jirovecii. Trends Microbiol. 2020, 28, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Schildgen, V.; Mai, S.; Khalfaoui, S.; Lüsebrink, J.; Pieper, M.; Tillmann, R.L.; Brockmann, M.; Schildgen, O. Pneumocystis Jirovecii Can Be Productively Cultured in Differentiated CuFi-8 Airway Cells. mBio 2014, 5, e01186-14. [Google Scholar] [CrossRef] [PubMed]
- Armstrong-James, D.; Meintjes, G.; Brown, G.D. A Neglected Epidemic: Fungal Infections in HIV/AIDS. Trends Microbiol. 2014, 22, 120–127. [Google Scholar] [CrossRef]
- Krajicek, B.J.; Thomas, C.F.; Limper, A.H. Pneumocystis Pneumonia: Current Concepts in Pathogenesis, Diagnosis, and Treatment. Clin. Chest Med. 2009, 30, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Tasaka, S.; Tokuda, H. Pneumocystis Jirovecii Pneumonia in Non-HIV-Infected Patients in the Era of Novel Immunosuppressive Therapies. J. Infect. Chemother. 2012, 18, 793–806. [Google Scholar] [CrossRef]
- Ward, M.M.; Donald, F. Pneumocystis Carinii Pneumonia in Patients with Connective Tissue Diseases: The Role of Hospital Experience in Diagnosis and Mortality. Arthritis Rheum. 1999, 42, 780–789. [Google Scholar] [CrossRef]
- Fan, L.-C.; Lu, H.-W.; Cheng, K.-B.; Li, H.-P.; Xu, J.-F. Evaluation of PCR in Bronchoalveolar Lavage Fluid for Diagnosis of Pneumocystis Jirovecii Pneumonia: A Bivariate Meta-Analysis and Systematic Review. PLoS ONE 2013, 8, e73099. [Google Scholar] [CrossRef]
- Bateman, M.; Oladele, R.; Kolls, J.K. Diagnosing Pneumocystis Jirovecii Pneumonia: A Review of Current Methods and Novel Approaches. Med. Mycol. 2020, 58, 1015–1028. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, C.; Sun, Y.; Chen, X.; Wang, Y.; Xiang, H.; Liang, Y.; Wei, F.; Zhang, Y. Diagnostic Tests Performance in Detecting Pneumocystis Jirovecii: A Systematic Review and Meta-Analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 789–805. [Google Scholar] [CrossRef]
- Flori, P.; Bellete, B.; Durand, F.; Raberin, H.; Cazorla, C.; Hafid, J.; Lucht, F.; Sung, R.T.M. Comparison between Real-Time PCR, Conventional PCR and Different Staining Techniques for Diagnosing Pneumocystis Jiroveci Pneumonia from Bronchoalveolar Lavage Specimens. J. Med. Microbiol. 2004, 53, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Lipschik, G.Y.; Andrawis, V.A.; Ognibene, F.P.; Kovacs, J.A.; Gill, V.J.; Nelson, N.A.; Lundgren, J.D.; Nielsen, J.O. Improved Diagnosis of Pneumocystis Carinii Infection by Polymerase Chain Reaction on Induced Sputum and Blood. Lancet 1992, 340, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Luísa Tomás, A.; Matos, O. Pneumocystis Jirovecii Pneumonia: Current Advances in Laboratory Diagnosis. OBM Genet. 2018, 2, 049. [Google Scholar] [CrossRef]
- Procop, G.W.; Haddad, S.; Quinn, J.; Wilson, M.L.; Henshaw, N.G.; Reller, L.B.; Artymyshyn, R.L.; Katanik, M.T.; Weinstein, M.P. Detection of Pneumocystis Jiroveci in Respiratory Specimens by Four Staining Methods. J. Clin. Microbiol. 2004, 42, 3333–3335, Correction in J. Clin. Microbiol. 2005, 43, 3333–3335. [Google Scholar] [CrossRef]
- Limper, A.H.; Offord, K.P.; Smith, T.F.; Martin, W.J. Pneumocystis Carinii Pneumonia: Differences in Lung Parasite Number and Inflammation in Patients with and without AIDS. Am. Rev. Respir. Dis. 1989, 140, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Bossart, S.; Mühlethaler, K.; Garzoni, C.; Furrer, H. Is Real Time PCR Preferable to the Direct Immunofluorescence in the Diagnosis of Pneumocystis Jirovecii Pneumonia in HIV-Infected Patients? BMC Res. Notes 2020, 13, 235. [Google Scholar] [CrossRef]
- Oladele, R.O.; Otu, A.A.; Richardson, M.D.; Denning, D.W. Diagnosis and Management of Pneumocystis Pneumonia in Resource-Poor Settings. J. Health Care Poor Underserved 2018, 29, 107–158. [Google Scholar] [CrossRef]
- Rohner, P.; Jacomo, V.; Studer, R.; Schrenzel, J.; Graf, J.-D. Detection of Pneumocystis Jirovecii by Two Staining Methods and Two Quantitative PCR Assays. Infection 2009, 37, 261–265. [Google Scholar] [CrossRef]
- Jaramillo Cartagena, A.; Asowata, O.E.; Ng, D.; Babady, N.E. An Overview of the Laboratory Diagnosis of Pneumocystis Jirovecii Pneumonia. J. Clin. Microbiol. 2025, 63, e0036124. [Google Scholar] [CrossRef]
- Homayouni, M.M.; Rostami, A.; Gholizadeh, H.; Mehbod, A.S.A.; Ebrahimi, M.; Mehravar, S. Comparison of Three Cost Effective Staining Methods for Detection of Pneumocystis Jirovecii. J. Parasit. Dis. 2017, 41, 298–301. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, S.; Ponce, C.A.; Pesantes, N.; Bustamante, R.; Gatti, G.; San Martin, V.; Gutierrez, M.; Bórquez, P.; Vargas, S.L.; Magne, F.; et al. A Real-Time PCR Assay for Detection of Low Pneumocystis Jirovecii Levels. Front. Microbiol. 2022, 12, 787554. [Google Scholar] [CrossRef] [PubMed]
- Scharmann, U.; Kirchhoff, L.; Schmidt, D.; Buer, J.; Steinmann, J.; Rath, P. Evaluation of a Commercial Loop-mediated Isothermal Amplification (LAMP) Assay for Rapid Detection of Pneumocystis Jirovecii. Mycoses 2020, 63, 1107–1114. [Google Scholar] [CrossRef]
- Tomás, A.L.; Cardoso, F.; Esteves, F.; Matos, O. Serological Diagnosis of Pneumocystosis: Production of a Synthetic Recombinant Antigen for Immunodetection of Pneumocystis Jirovecii. Sci. Rep. 2016, 6, 36287. [Google Scholar] [CrossRef]
- Liu, Q.; Zeng, H.; Wang, T.; Ni, H.; Li, Y.; Qian, W.; Fang, T.; Xu, G. Development of RPA-Cas12a Assay for Rapid and Sensitive Detection of Pneumocystis Jirovecii. BMC Microbiol. 2024, 24, 314. [Google Scholar] [CrossRef]
- Edouard, S.; Million, M.; Lepidi, H.; Rolain, J.-M.; Fournier, P.-E.; La Scola, B.; Grisoli, D.; Raoult, D. Persistence of DNA in a Cured Patient and Positive Culture in Cases with Low Antibody Levels Bring into Question Diagnosis of Q Fever Endocarditis. J. Clin. Microbiol. 2013, 51, 3012–3017. [Google Scholar] [CrossRef]
- Zhan, Y.; Gao, X.; Li, S.; Si, Y.; Li, Y.; Han, X.; Sun, W.; Li, Z.; Ye, F. Development and Evaluation of Rapid and Accurate CRISPR/Cas13-Based RNA Diagnostics for Pneumocystis Jirovecii Pneumonia. Front. Cell. Infect. Microbiol. 2022, 12, 904485. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Maeda, T.; Suzuki, T.; Abe, T.; Mikita, K.; Hamakawa, Y.; Ono, T.; Sonehara, W.; Miyahira, Y.; Kawana, A. Loop-Mediated Isothermal Amplification with the Procedure for Ultra Rapid Extraction Kit for the Diagnosis of Pneumocystis Pneumonia. J. Infect. Chemother. 2015, 21, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liang, Y.; Wang, R.; Wu, Y.; Zhang, X.; Huang, H.; Yu, X.; Hong, M.; Yang, J.; Liao, K.; et al. Metagenomic Next-Generation Sequencing for the Diagnosis of Pneumocystis Jirovecii Pneumonia in Critically Pediatric Patients. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 6. [Google Scholar] [CrossRef]
- Mao, Z.; Lei, H.; Chen, R.; Ren, S.; Liu, B.; Gao, Z. CRISPR Molecular Detection Techniques: Advances from Single to Multiple Detection Methods. TrAC Trends Anal. Chem. 2023, 166, 117198. [Google Scholar] [CrossRef]
- Doudna, J.A.; Mali, P. CRISPR-Cas: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2016. [Google Scholar]
- Park, B.J.; Yoo, J.R.; Heo, S.T.; Kim, M.; Lee, K.H.; Song, Y.-J. A CRISPR-Cas12a-Based Diagnostic Method for Multiple Genotypes of Severe Fever with Thrombocytopenia Syndrome Virus. PLoS Neglected Trop. Dis. 2022, 16, e0010666. [Google Scholar] [CrossRef]
- Lapee-e, V.; Nuanualsuwan, S.; Hongtanee, L.; Yakoh, A. Paper-Based CRISPR-Cas Diagnostics: A Comprehensive Review of Advances and Applications in Disease Detection. Microchem. J. 2025, 211, 113055. [Google Scholar] [CrossRef]
- Song, R.; Chen, Z.; Xiao, H.; Wang, H. The CRISPR-Cas System in Molecular Diagnostics. Clin. Chim. Acta 2024, 561, 119820. [Google Scholar] [CrossRef]
- Xia, Y.; Rao, R.; Xiong, M.; He, B.; Zheng, B.; Jia, Y.; Li, Y.; Yang, Y. CRISPR-Powered Strategies for Amplification-Free Diagnostics of Infectious Diseases. Anal. Chem. 2024, 96, 8091–8108. [Google Scholar] [CrossRef]
- Kostyusheva, A.; Brezgin, S.; Babin, Y.; Vasilyeva, I.; Glebe, D.; Kostyushev, D.; Chulanov, V. CRISPR-Cas Systems for Diagnosing Infectious Diseases. Methods 2022, 203, 431–446. [Google Scholar] [CrossRef]
- Osborn, M.J.; Bhardwaj, A.; Bingea, S.P.; Knipping, F.; Feser, C.J.; Lees, C.J.; Collins, D.P.; Steer, C.J.; Blazar, B.R.; Tolar, J. CRISPR/Cas9-Based Lateral Flow and Fluorescence Diagnostics. Bioengineering 2021, 8, 23. [Google Scholar] [CrossRef]
- Kwon, S.; Shin, H.Y. Advanced CRISPR-Cas Effector Enzyme-Based Diagnostics for Infectious Diseases, Including COVID-19. Life 2021, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Liberty, J.T.; Bromage, S.; Peter, E.; Ihedioha, O.C.; Alsalman, F.B.; Odogwu, T.S. CRISPR Revolution: Unleashing Precision Pathogen Detection to Safeguard Public Health and Food Safety. Methods 2025, 240, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Leta, S.; Chibssa, T.R.; Paeshuyse, J. CRISPR-Cas12/Cas13: Bibliometric Analysis and Systematic Review of Its Application in Infectious Disease Detection. J. Infect. Public Health 2024, 17, 741–747. [Google Scholar] [CrossRef]
- Mukama, O.; Wu, J.; Li, Z.; Liang, Q.; Yi, Z.; Lu, X.; Liu, Y.; Liu, Y.; Hussain, M.; Makafe, G.G.; et al. An Ultrasensitive and Specific Point-of-Care CRISPR/Cas12 Based Lateral Flow Biosensor for the Rapid Detection of Nucleic Acids. Biosens. Bioelectron. 2020, 159, 112143. [Google Scholar] [CrossRef] [PubMed]
- Mane, A.; Gujar, P.; Chandra, J.; Lokhande, R.; Dhamgaye, T.; Ghorpade, S.; Risbud, A. Pneumocystis Jirovecii Infection and the Associated Dihydropteroate Synthase (DHPS) and Dihydrofolate Reductase (DHFR) Mutations in HIV-Positive Individuals from Pune, India. Mycopathologia 2015, 179, 141–145. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, H.; Wang, Y.; Cheng, X.; Chen, H.; Yang, X.; Zhang, H.; Rong, Z.; Wang, S. Efficient Magnetic Enrichment Cascade Single-Step RPA-CRISPR/Cas12a Assay for Rapid and Ultrasensitive Detection of Staphylococcus Aureus in Food Samples. J. Hazard. Mater. 2024, 465, 133494. [Google Scholar] [CrossRef]
- Srivastava, S.; Upadhyay, D.J.; Srivastava, A. Next-Generation Molecular Diagnostics Development by CRISPR/Cas Tool: Rapid Detection and Surveillance of Viral Disease Outbreaks. Front. Mol. Biosci. 2020, 7, 582499. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, S.; Zhao, N.; Liu, X.; Cao, Y.; Zhang, G.; Wang, G.; Guo, C. Development and Clinical Application of a Novel CRISPR-Cas12a Based Assay for the Detection of African Swine Fever Virus. BMC Microbiol. 2020, 20, 282. [Google Scholar] [CrossRef]
- Ai, J.-W.; Zhou, X.; Xu, T.; Yang, M.; Chen, Y.; He, G.-Q.; Pan, N.; Cai, Y.; Li, Y.; Wang, X.; et al. CRISPR-Based Rapid and Ultra-Sensitive Diagnostic Test for Mycobacterium Tuberculosis. Emerg. Microbes Infect. 2019, 8, 1361–1369. [Google Scholar] [CrossRef]
- Ding, R.; Long, J.; Yuan, M.; Zheng, X.; Shen, Y.; Jin, Y.; Yang, H.; Li, H.; Chen, S.; Duan, G. CRISPR/Cas12-Based Ultra-Sensitive and Specific Point-of-Care Detection of HBV. Int. J. Mol. Sci. 2021, 22, 4842. [Google Scholar] [CrossRef]
- Shen, S.; Wang, W.; Ma, Y.; Wang, S.; Zhang, S.; Cai, X.; Chen, L.; Zhang, J.; Li, Y.; Wu, X.; et al. Affinity Molecular Assay for Detecting Candida Albicans Using Chitin Affinity and RPA-CRISPR/Cas12a. Nat. Commun. 2024, 15, 9304. [Google Scholar] [CrossRef]
- Lin, C.; Zhou, J.; Gao, N.; Liu, R.; Li, G.; Wang, J.; Lu, G.; Shen, J. Establishing a Pulmonary Aspergillus Fumigatus Infection Diagnostic Platform Based on RPA-CRISPR-Cas12a. World J. Microbiol. Biotechnol. 2024, 40, 116. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, S.; Robert-Gangneux, F.; Pépino, Y.; Belaz, S.; Damiani, C.; Guéguen, P.; Pitous, M.; Virmaux, M.; Lissillour, E.; Pougnet, L.; et al. A Misleading False-Negative Result of Pneumocystis Real-Time PCR Assay Due to a Rare Punctual Mutation: A French Multicenter Study. Med. Mycol. 2017, 55, 180–184. [Google Scholar] [CrossRef]
- Hoarau, G.; Le Gal, S.; Zunic, P.; Poubeau, P.; Antok, E.; Jaubert, J.; Nevez, G.; Picot, S. Evaluation of Quantitative FTD-Pneumocystis Jirovecii Kit for Pneumocystis Infection Diagnosis. Diagn. Microbiol. Infect. Dis. 2017, 89, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Grønseth, S.; Rogne, T.; Heggelund, L.; Åsvold, B.O.; Afset, J.E.; Damås, J.K. Role of Fungal Burden in Risk Stratification of HIV-Negative Patients with Pneumocystis Pneumonia: A 12-Year, Retrospective, Observational, Multicenter Cohort. Int. J. Infect. Dis. 2023, 134, 177–186. [Google Scholar] [CrossRef]
- Wu, Y.; Battalapalli, D.; Hakeem, M.J.; Selamneni, V.; Zhang, P.; Draz, M.S.; Ruan, Z. Engineered CRISPR-Cas Systems for the Detection and Control of Antibiotic-Resistant Infections. J. Nanobiotechnology 2021, 19, 401. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, I.; Delforge, M.-L.; Ajjaham, F.; Brancart, F.; Hites, M.; Jacobs, F.; Denis, O. Evaluation of a New Commercial Real-Time PCR Assay for Diagnosis of Pneumocystis Jirovecii Pneumonia and Identification of Dihydropteroate Synthase (DHPS) Mutations. Diagn. Microbiol. Infect. Dis. 2017, 87, 32–36. [Google Scholar] [CrossRef]
- Iliades, P.; Meshnick, S.R.; Macreadie, I.G. Mutations in the Pneumocystis Jirovecii DHPS Gene Confer Cross-Resistance to Sulfa Drugs. Antimicrob. Agents Chemother. 2005, 49, 741–748. [Google Scholar] [CrossRef]
- de la Horra, C.; Friaza, V.; Morilla, R.; Delgado, J.; Medrano, F.J.; Miller, R.F.; de Armas, Y.; Calderón, E.J. Update on Dihydropteroate Synthase (DHPS) Mutations in Pneumocystis Jirovecii. J. Fungi 2021, 7, 856. [Google Scholar] [CrossRef]
- Montesinos, I.; Brancart, F.; Schepers, K.; Jacobs, F.; Denis, O.; Delforge, M.-L. Comparison of 2 Real-Time PCR Assays for Diagnosis of Pneumocystis Jirovecii Pneumonia in Human Immunodeficiency Virus (HIV) and Non-HIV Immunocompromised Patients. Diagn. Microbiol. Infect. Dis. 2015, 82, 143–147. [Google Scholar] [CrossRef]
- Edlind, T.D.; Bartlett, M.S.; Weinberg, G.A.; Prah, G.N.; Smith, J.W. The Β-tubulin Gene from Rat and Human Isolates of Pneumocystis Carinii. Mol. Microbiol. 1992, 6, 3365–3373. [Google Scholar] [CrossRef] [PubMed]
- Nasri, T.; Hedayati, M.T.; Abastabar, M.; Pasqualotto, A.C.; Armaki, M.T.; Hoseinnejad, A.; Nabili, M. PCR-RFLP on β-Tubulin Gene for Rapid Identification of the Most Clinically Important Species of Aspergillus. J. Microbiol. Methods 2015, 117, 144–147. [Google Scholar] [CrossRef]
- Jabrodini, A.; Sohrabizdeh, M.; Aboutalebian, S.; Hashemi, S.B.; Zomorodian, K.; Alirezaie, A.; Rasti Jahromi, M.; Shamsdin, S.N.; Motamedi, M. Molecular Strategy for the Direct Detection and Identification of the Most Common Fungal Community in Cerumen Specimens by Multiplex PCR. J. Med. Microbiol. 2023, 72, 001746. [Google Scholar] [CrossRef]
- Qian, J.; Huang, D.; Ni, D.; Zhao, J.; Shi, Z.; Fang, M.; Xu, Z. A Portable CRISPR Cas12a Based Lateral Flow Platform for Sensitive Detection of Staphylococcus Aureus with Double Insurance. Food Control 2022, 132, 108485. [Google Scholar] [CrossRef]
- Ali, Z.; Aman, R.; Mahas, A.; Rao, G.S.; Tehseen, M.; Marsic, T.; Salunke, R.; Subudhi, A.K.; Hala, S.M.; Hamdan, S.M.; et al. ISCAN: An RT-LAMP-Coupled CRISPR-Cas12 Module for Rapid, Sensitive Detection of SARS-CoV-2. Virus Res. 2020, 288, 198129. [Google Scholar] [CrossRef]
- Jirawannaporn, S.; Limothai, U.; Tachaboon, S.; Dinhuzen, J.; Kiatamornrak, P.; Chaisuriyong, W.; Bhumitrakul, J.; Mayuramart, O.; Payungporn, S.; Srisawat, N. Rapid and Sensitive Point-of-Care Detection of Leptospira by RPA-CRISPR/Cas12a Targeting LipL32. PLoS Negl. Trop. Dis. 2022, 16, e0010112. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, A.R.; Talib, W.; Al-Dulaimi, A.; Daoud, S.; Al Maqbali, M. The First Detection of Pneumocystis Jirovecii in Asthmatic Patients Post COVID-19 in Jordan. Bosn. J. Basic Med. Sci. 2022, 22, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, É.; Lemiale, V.; Kouatchet, A.; Vincent, F.; Roux, A.; Bollée, G.; Roux, P. Pneumocystis Pneumonia in Non-AIDS Immunocompromised Patients. In Pulmonary Involvement in Patients with Hematological Malignancies; Springer: Berlin/Heidelberg, Germany, 2011; pp. 313–325. [Google Scholar]
- Mühlethaler, K.; Bögli-Stuber, K.; Wasmer, S.; von Garnier, C.; Dumont, P.; Rauch, A.; Mühlemann, K.; Garzoni, C. Quantitative PCR to Diagnose Pneumocystis Pneumonia in Immunocompromised Non-HIV Patients. Eur. Respir. J. 2012, 39, 971–978. [Google Scholar] [CrossRef]
- Perret, T.; Kritikos, A.; Hauser, P.M.; Guiver, M.; Coste, A.T.; Jaton, K.; Lamoth, F. Ability of Quantitative PCR to Discriminate Pneumocystis Jirovecii Pneumonia from Colonization. J. Med. Microbiol. 2020, 69, 705–711. [Google Scholar] [CrossRef]
- Piñana, J.L.; Albert, E.; Gómez, M.D.; Pérez, A.; Hernández-Boluda, J.C.; Montoro, J.; Salavert, M.; González, E.M.; Tormo, M.; Giménez, E.; et al. Clinical Significance of Pneumocystis Jirovecii DNA Detection by Real-Time PCR in Hematological Patient Respiratory Specimens. J. Infect. 2020, 80, 578–606. [Google Scholar] [CrossRef]
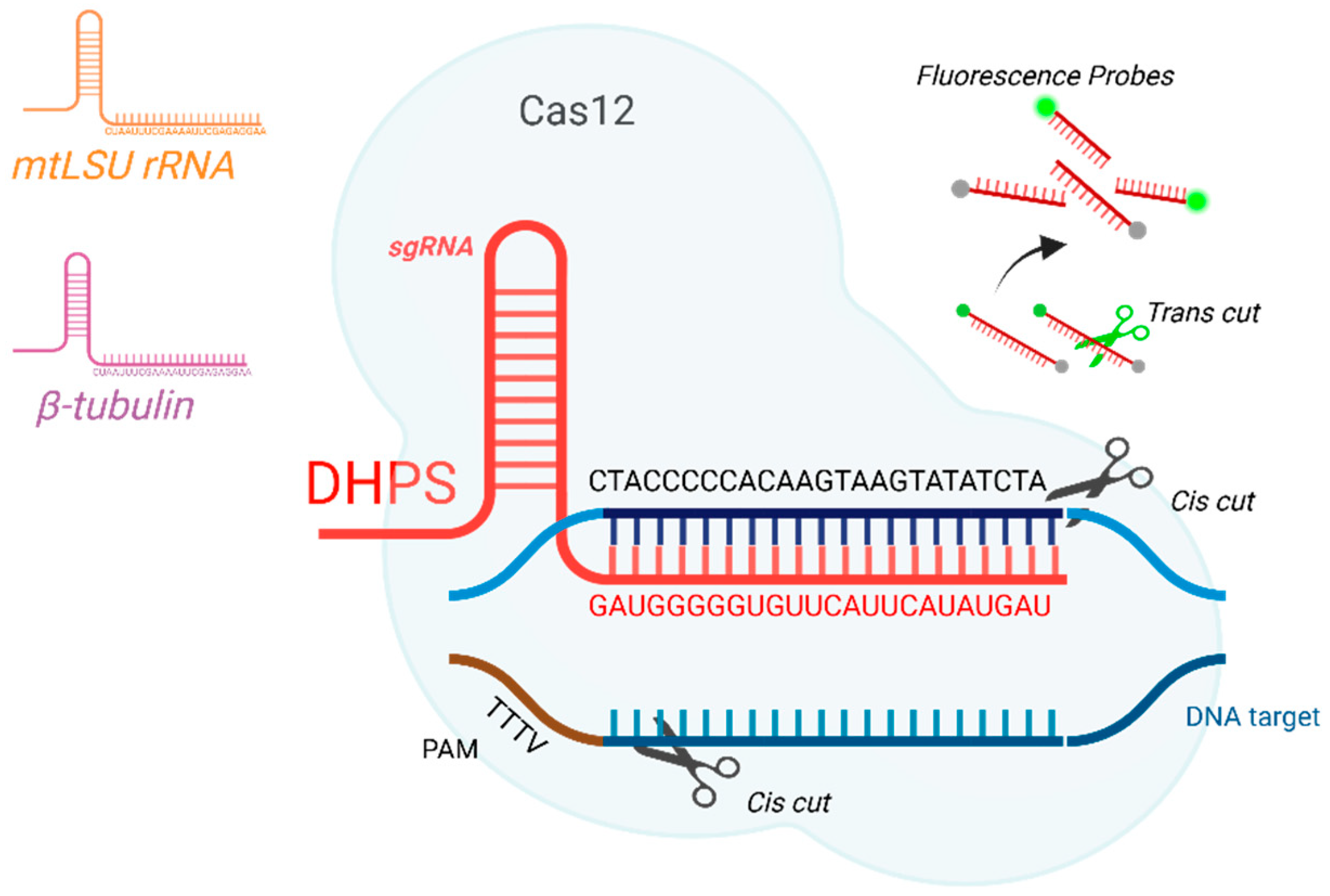



| Target Gene | Oligo | Sequences |
|---|---|---|
| mtLSU rRNA | pAZ102-E | 5′-GATGGCTGTTTCCAAGCCCA-3′ |
| pAZ102-H | 5′-GTGTACGTTGCAAAGTACTC-3′ | |
| gRNA_lsu | UAAUUUCUACUAAGUGUAGAUAAUUAUUAGAAGGGAGUAUGAGAG | |
| DHPS | dhps_3 | 5′-GCGCCTACACATATTATGGCCATTTTAAATC-3′ |
| dhps_4 | 5′-GGAACTTTCAACTTGGCAACCAC-3′ | |
| gRNA_dhps | UAAUUUCUACUAAGUGUAGAUGAUGGGGGUGUUCAUUCAUAUGAU | |
| β-tubulin | tub_f | 5′-TCATTAGGTGGTGGAACGGG-3′ |
| tub_r | 5′-ATCACCATATCCTGGATCCG-3′ | |
| gRNA_tub | UAAUUUCUACUAAGUGUAGAUCUAAUUUCGAAAAUUCGAGAGGAA | |
| Probes | FAM_BHQ1 | 56-FAM/TT TTT TTT TTT T/3BHQ_1 |
| FAM_BIOTIN | 56-FAM/TT TTT TTT TTT T/3Bio |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulloa, D.; Núñez, C.; Matamala, R.; San Martín, A.; Páez-De Ávila, D.; Mercado-Vides, J.; Narváez, J.; Aguirre, J.; Effer, B.; Iturrieta-González, I. CRISPR-Cas12 Application for the Detection of Pneumocystis jirovecii in Immunodepression Patients Through Fluorescent and Lateral Flow Colorimetric Assay. Int. J. Mol. Sci. 2025, 26, 8732. https://doi.org/10.3390/ijms26178732
Ulloa D, Núñez C, Matamala R, San Martín A, Páez-De Ávila D, Mercado-Vides J, Narváez J, Aguirre J, Effer B, Iturrieta-González I. CRISPR-Cas12 Application for the Detection of Pneumocystis jirovecii in Immunodepression Patients Through Fluorescent and Lateral Flow Colorimetric Assay. International Journal of Molecular Sciences. 2025; 26(17):8732. https://doi.org/10.3390/ijms26178732
Chicago/Turabian StyleUlloa, Daniel, Constanza Núñez, Romina Matamala, Aníbal San Martín, Dayana Páez-De Ávila, Jheyson Mercado-Vides, Juan Narváez, Juan Aguirre, Brian Effer, and Isabel Iturrieta-González. 2025. "CRISPR-Cas12 Application for the Detection of Pneumocystis jirovecii in Immunodepression Patients Through Fluorescent and Lateral Flow Colorimetric Assay" International Journal of Molecular Sciences 26, no. 17: 8732. https://doi.org/10.3390/ijms26178732
APA StyleUlloa, D., Núñez, C., Matamala, R., San Martín, A., Páez-De Ávila, D., Mercado-Vides, J., Narváez, J., Aguirre, J., Effer, B., & Iturrieta-González, I. (2025). CRISPR-Cas12 Application for the Detection of Pneumocystis jirovecii in Immunodepression Patients Through Fluorescent and Lateral Flow Colorimetric Assay. International Journal of Molecular Sciences, 26(17), 8732. https://doi.org/10.3390/ijms26178732








